Abstract
Objective:
This study was conducted to determine if the morphology and biochemistry of the mouse submandibular gland is affected by microgravity and the spaceflight environment.
Design:
Tissues from female mice flown on the US space shuttle missions Space Transportation System (STS)-131 and STS-135 for 15 and 13 d, respectively, and from male mice flown on the 30 d Russian Bion-M1 biosatellite, were examined using transmission electron microscopy and light and electron microscopic immunohistochemistry.
Results:
In contrast to the parotid gland, morphologic changes were not apparent in the submandibular gland. No significant changes in protein expression, as assessed by quantitative immunogold labeling, occurred in female mice flown for 13-15 d. In male mice, however, increased labeling for salivary androgen binding protein alpha (in acinar cell secretory granules), and epidermal growth factor and nerve growth factor (in granular convoluted duct cell granules) was seen after 30 d in space.
Conclusion:
These results indicate that spaceflight alters secretory protein expression in the submandibular gland and suggest that the sex of the animals and the length of the flight may affect the response. These findings also show that individual salivary glands respond differently to spaceflight. Saliva contains proteins secreted from salivary glands and is easily collected, therefore is a useful biofluid for general medical analyses and in particular for monitoring the physiology and health of astronauts.
Keywords: salivary glands, microgravity, electron microscopy, immunohistochemistry
Introduction
Spaceflight exposes organisms to numerous environmental stressors, such as gravitational changes, radiation, temperature fluctuations, vibration, noise, confinement, and disruption of sleep and circadian rhythms. Previous studies of animals and humans after travel in space have documented alterations in several tissues and organ systems, including skeletal, immune, cardiovascular, nervous and gastrointestinal (e.g., Borchers et al., 2002; Hargens and Richardson, 2009; Nagaraja and Risin, 2013; Latchney et al., 2014; Porseva et al., 2017; Demontis et al., 2017; Garrett-Bakelman et al., 2019). Early and more recent studies have demonstrated that oral hard and soft tissues, including the mandible (Simmons et al., 1983; Ghosh et al., 2016; Dagdeviren et al., 2018b), teeth (Rosenberg et al., 1984; Dagdeviren et al., 2018b), salivary glands (Groza et al., 1981, 1983; Mednieks and Hand, 1985, 1987; Mednieks et al., 2014, 2015; Dagdeviren et al., 2018a) and saliva (Brown et al., 1977), are significantly affected by spaceflight.
The response of rodent salivary glands to spaceflight varies among glands and cell types. An increase in autophagy and apoptosis occurs in parotid acinar cells (Mednieks et al., 2014, 2015). Large endocytic vacuoles containing acinar cell proteins are present in intercalated and striated duct cells. Changes also occur in the expression of several acinar cell secretory proteins. In contrast, the sublingual gland shows few morphological alterations, but changes do occur in serous demilune cell protein expression (Dagdeviren et al., 2018a).
The aims of the present study were to determine if spaceflight affects the morphology and secretory protein expression of the mouse submandibular gland. Glands from mice flown on two US space shuttle flights and from the Russian Bion-M1 satellite, along with ground control mice, were examined using transmission electron microcopy, immunohistochemistry and immunogold labeling. The findings indicate that the three major salivary glands respond differently to spaceflight and suggest that the age and sex of the animals and the length of the flight affect the response.
Materials and Methods
Animals:
Eight 16-23 wk old female C57BL/6J mice were flown for 15 d on space shuttle Discovery (Space Transportation System [STS]-131), and seven 9 wk old female C57BL/6J mice were flown for 13 d on space shuttle Atlantis (STS-135). The mice were housed in Animal Enclosure Modules, specially designed cages that provide ventilation, lighting, waste collection, food and water (Morey-Holton et al., 2000). Eight mice housed in Animal Enclosure Modules for 15 d served as habitat ground controls for the STS-131 flight, and 5 mice housed in Animal Enclosure Modules for 13 d served as habitat ground controls for the STS-135 flight. Six 15-16 wk old male C57BL/6N mice were flown for 30 d on the Bion-M1 satellite. The mice were housed in “BOS” flight habitats (Andreev-Andrievskiy et al., 2014). Four age-matched male C57BL/6N mice housed in the same flight habitats served as asynchronous ground controls. The experimental procedures were approved by the National Aeronautics and Space Administration (NASA) Institutional Animal Care and Use Committee, the Institutional Animal Care and Use Committee of Moscow State University Institute of Mitoengineering (Protocol No-35, 1 November, 2012), and the Biomedical Ethics Commission of the Institute of Biomedical Problems (Moscow) (protocol No–319, 4 April, 2013). All procedures conformed to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes. The samples were made available through the NASA Biospecimen Sharing Program.
Flight and habitat ground control mice on the space shuttle missions were provided with NASA rodent food bars (Sun et al., 2014) attached to the sides of the Animal Enclosure Modules and water ad libitum. Five mice housed in standard vivarium cages, with rodent chow and water available ad libitum, served as additional ground control animals for the STS-135 mission. Flight and habitat ground control mice on the Bion-M1 mission were fed a paste diet based on standard rodent chow, with water and casein added as a gelling agent (Andreev-Andrievskiy et al., 2014). The paste food was available at 4 h intervals. Four additional ground control mice were housed in standard vivarium cages and fed rodent chow and water ad libitum. Pre- and post-flight body weight and food consumption data have been published (Mednieks et al., 2014; Dagdeviren et al., 2018b).
Sample Collection:
Dissection of the mice from the space shuttle flights began about 3 h after landing of the shuttles at the Kennedy Space Center in Florida; dissection of the habitat ground control mice took place 48 h after the flight mice. The vivarium-housed mice were dissected 48 h prior to dissection of the STS-135 flight mice. The mice were anesthetized with isoflurane and killed by cardiac puncture and exsanguination. Dissection of the Bion-M1 flight mice took place at the Institute of Biomedical Problems in Moscow, beginning about 13 h after landing in the Orenburg region of Russia. The mice were killed by cervical dislocation followed by decapitation. The vivarium control mice were dissected immediately after the flight mice, and the asynchronous habitat ground control mice were dissected 99 days after the flight mice.
Submandibular glands were removed, rinsed briefly in cold phosphate buffered saline and prepared for morphological and immunohistochemical examination. Small pieces of tissue for morphologic analysis were fixed in 2.5% glutaraldehyde/2% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.4. Samples for immunohistochemistry and immunogold labeling were fixed in 4% paraformaldehyde in 0.1 M cacodylate buffer. After overnight fixation at 4°C, the samples were rinsed in 0.1 M cacodylate buffer, and stored in either buffer (morphology) or 1% paraformaldehyde in 0.1 M cacodylate buffer (immunohistochemistry). The samples were shipped cold by express service from the dissection sites to UConn Health.
Morphologic Analysis:
The samples were postfixed in 1% osmium tetroxide/0.8% potassium ferricyanide in 0.1 M cacodylate buffer, stained in block with 1% uranyl acetate, and processed by standard methods (Hand, 1995) for embedding in PolyBed epoxy resin (Polysciences, Inc., Warrington, PA, USA). Thin sections (~70-80 nm) were collected on copper grids, stained with uranyl acetate and lead citrate, and examined and photographed in a Hitachi H7650 transmission electron microscope equipped with an AMT digital camera.
Immunohistochemistry:
Paraformaldehyde-fixed samples were embedded in paraffin, sections were cut at 5 μm thickness, and immunohistochemistry was carried out as described previously (Le et al., 2011), using the Vectastain Elite kits for rabbit IgG (Vector Laboratories, Burlingame, CA, USA). Controls included omission of the primary antibody and substitution of non-immune serum or non-immune IgG for the primary antibody. The sections were observed and photographed using a Nikon Eclipse Ci microscope (Nikon Instruments Inc., Melville, NY, USA) equipped with an Accu-Scope Excelis digital camera (Accu-Scope, Commack, NY, USA).
Immunogold Labeling:
Postfixation was omitted, and the paraformaldehyde-fixed samples were rinsed briefly in 0.1 M cacodylate buffer, dehydrated in cold methanol solutions, embedded in LR Gold resin (Electron Microscopy Sciences, Hatfield, PA, USA) and polymerized in UV light at −20°C. Thin sections from each mouse of the flight and ground control groups from the three missions were collected on formvar-coated nickel grids. Immunolabeling was carried out overnight at 4°C as described previously (Dagdeviren et al., 2018a), using primary antibodies (Table 1) diluted in phosphate buffered saline containing 1% bovine serum albumin and 5% normal goat serum. Immunolabeling controls included omission of the primary antibody and substitution of non-immune serum or non-immune IgG for the primary antibody. Bound antibodies were detected with gold-labeled goat anti-rabbit IgG (Amersham Int., Little Chalfont, Bucks, UK, or Aurion, Electron Microscopy Sciences). The labeled grids were stained with uranyl acetate and lead citrate and examined in the transmission electron microscope.
Table 1:
Antibodies Used for Immunohistochemistry.
| Antibody (Species) |
LM Dilution | EM Dilution | Reference/Source |
|---|---|---|---|
| SABPα (Rabbit) |
1:2000 – 1:10000 | 1:25 - 1:200 | L. Mirels (unpublished) |
| PRP (Chicken) |
--- | 1:50 | D.M. Carlson (unpublished) |
| NGF (Rabbit) |
1:200 – 1:1000 | 1:10 – 1:100 | Santa Cruz (sc-549) |
| EGF (Rabbit) |
--- | 1:25 | Sigma-Aldrich (E2635) |
| SMGC (Rabbit) |
1:3000 – 1:10000 | 1:300 – 1:500 | Zinzen et al., 2004 |
Quantitative Analyses:
Electron micrographs of the labeled sections were obtained and analyzed in an unbiased manner as described previously (Mednieks et al., 2015; Dagdeviren et al., 2018a). Labeling densities of secretory granules of acinar cells, terminal tubule/granular intercalated duct cells, and granular convoluted duct cells were calculated for each mouse as the average number of gold particles/μm2. For direct comparison between different immunogold labeling experiments, the labeling densities were converted to percentages of the corresponding ground control averages. Differences in expression of each protein between the flight and ground control groups were evaluated using ANOVA and t-test functions in Microsoft Excel or StatPlus (v.6.1.60, AnalystSoft, Walnut, CA, USA).
Antibodies:
Primary antibodies used were to salivary androgen binding protein alpha (SABPα), proline-rich protein (PRP), submandibular gland protein C (SMGC), nerve growth factor (NGF) and epidermal growth factor (EGF) (Table 1). A rabbit anti-chicken antibody (Abcam, Cambridge, MA, USA; Cat No. ab97136) was used as a secondary antibody with the chicken anti-PRP primary.
Results
The flight and habitat ground control mice from both space shuttle missions lost approximately 1-2 g of body weight (Mednieks et al., 2014), but the differences were not statistically significant. Consumption of food and water were similar for the flight and habitat ground control mice. The flight and habitat ground control mice from the Bion-M1 mission gained approximately 2-2.5 g body weight; the differences were significant for the flight mice (p = 0.0010) and habitat ground control mice (p = 0.0456) (Andreev-Andrievskiy et al., 2014). Food consumption for the flight and habitat ground control mice, determined in preliminary experiments, was identical.
Morphology:
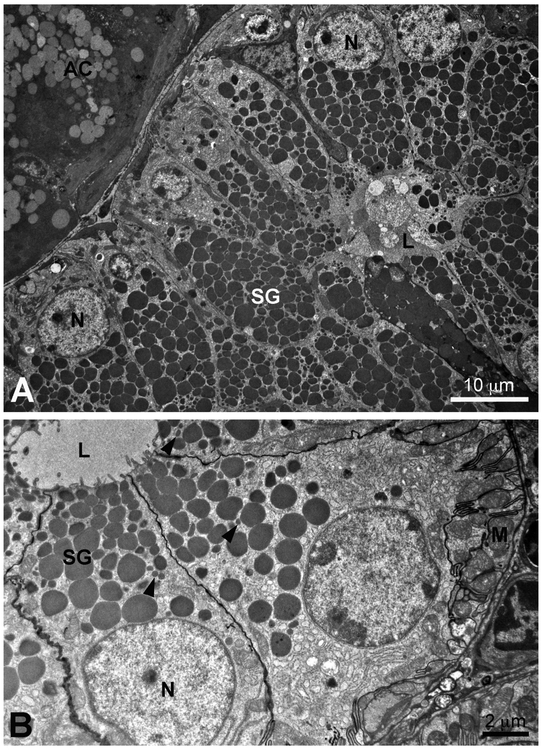
The acinar cells of the mouse submandibular gland are seromucous in nature, with a basally located nucleus, abundant rough endoplasmic reticulum in the basal cytoplasm, a large Golgi complex, and secretory granules with a granular content of moderate density located in the supranuclear cytoplasm (Fig. 1 A, B; see review by Denny et al., 1997). In adult female glands, terminal tubule cells, also called granular intercalated duct cells, retained from prenatal and early postnatal stages, often are observed at the acinar-intercalated duct junction (Caramia, 1966a; Gresik and MaRae, 1975; Hayashi and Sasa, 1989; Yamamoto et al., 2018) (Fig. 1C). These cells contain numerous small dense secretory granules ranging in size from 0.6 to 1.3 μm. In males, the terminal tubule cells undergo androgen-dependent apoptosis and are completely lost by about 1 month of age (Hayashi et al., 2000; Hecht et al., 2000; Yamamoto et al., 2018). The intercalated ducts, consisting of cuboidal cells that occasionally contain a few small granules, connect to a short striated granular duct (Caramia, 1966a; Gresik and MacRae, 1975; Denny et al., 1990) which is confluent with the granular convoluted duct. The granular convoluted ducts consist of tall columnar cells, with basal nuclei, variably infolded basal cell membranes with adjacent mitochondria, and numerous large dense granules in the supranuclear cytoplasm. (Fig. 2) As described by Watson et al. (1985), smaller dense granules often are present in the cytoplasm between the large granules. These ducts are well-developed in adult male glands, constituting about 45% to 60% of the gland parenchyma (Caramia, 1966a; Gresik, 1994; Jayasinghe et al., 1990; Pardini and Taga, 1996) (Fig. 2A). In female glands, the granular convoluted duct cells are smaller than those of males, contain fewer secretory granules (Fig. 2B), and constitute about 25% of the parenchyma. The granular convoluted ducts are confluent with the striated ducts, which become excretory ducts as they enter the connective tissue septa dividing the gland into lobules and lobes.
Figure 1:
A, Space Transportation System (STS)-135. Low magnification transmission electron micrograph of several submandibular gland acinar cells, a terminal tubule/granular intercalated duct cell (TT), and intercalated duct cells (ID) of a female habitat control mouse. Acinar cell nuclei (N); acinar cell secretory granules (SG); lumen (L). B, STS-131. Transmission electron micrograph of an acinar cell of a female flight mouse. Nucleus (N); secretory granules (SG); intercellular canaliculi (arrows). C, STS-131. Transmission electron micrograph of terminal tubule/granular intercalated duct cell (TT) with dense secretory granules adjacent to acinar (AC) and intercalated duct (ID) cells of a female habitat control mouse. Lumen (L).
Figure 2:
A, Bion-M1. Transmission electron micrograph of a submandibular gland granular convoluted duct of a male flight mouse. The tall columnar duct cells are packed with dense secretory granules (SG) in the supranuclear cytoplasm. Nuclei (N); lumen (L); acinar cell (AC). B, STS-131. Transmission electron micrograph of granular convoluted duct cells of a female flight mouse. Note the small granules (arrowheads) scattered among the larger secretory granules (SG). Nuclei (N); lumen (L); basal mitochondria (M) and infolded basal cell membranes.
There were no apparent differences in the morphology of the acinar or duct cells of flight mice from those of their respective habitat ground control or vivarium ground control mice for any of the three missions. All of the cellular organelles exhibited a normal appearance, and the acinar and duct cells had a full complement of secretory granules.
Immunohistochemistry:
In paraffin sections, antibodies to SABPα labeled all of the submandibular gland acinar cells (Fig. 3A), whereas no reactivity was present in duct cells. Antibody to NGF labeled the granular convoluted duct cells (Fig. 3B). In female mice, the residual terminal tubule/granular intercalated duct cells were labeled with antibody to SMGC (Fig. 3C, D). No labeling was seen in control sections that were incubated without primary antibody or with non-immune serum or IgG substituted for the primary antibody (Fig. 3E).
Figure 3:
Immunohistochemical localization of secretory proteins in the submandibular gland of female mice from the STS-131 mission. A, Salivary androgen binding protein alpha (SABPα) in acinar cells of a flight mouse. B, Nerve growth factor (NGF) in granular convoluted duct cells of a habitat ground control mouse. C and D, submandibular gland protein C (SMGC) in terminal tubule cells (arrowheads) of a habitat ground control mouse. E, Immunohistochemical control, non-immune IgG substituted for the primary antibody.
Immunogold labeling:
In the submandibular gland acinar cells, electron microscopic immunogold labeling with antibodies to SABPα and PRP resulted in labeling of the secretory granules (Fig. 4A, B). A few gold particles were also present over the rough endoplasmic reticulum. The labeling density for SABPα in female mice flown on STS-131 and STS-135 was not significantly different than in habitat ground control mice. In male mice flown on the Bion-M1 biosatellite, labeling for SABPα was significantly increased in flight mice compared to habitat control mice (Fig. 5). The labeling density for PRP was unchanged from controls in both female and male flight mice.
Figure 4:
Electron microscopic immunogold labeling. A, Bion-M1. Salivary androgen binding protein alpha (SABPα) in acinar secretory granules of a male flight mouse. B, STS-135. Proline-rich protein (PRP) in acinar secretory granules of a female flight mouse. C, Bion-M1. Epidermal growth factor (EGF) in convoluted granular duct cell secretory granules of a male flight mouse. Note the small labeled granules (arrowheads). D, STS-135. Nerve growth factor (NGF) in convoluted granular duct cell secretory granules of a female vivarium ground control mouse. Note the small labeled granule (arrowhead). E, STS-131. Submandibular gland protein C (SMGC) in terminal tubule/granular intercalated duct cell granules of a female flight mouse. Nucleus (N).
Figure 5:
Quantitative analysis of immunogold labeling for salivary androgen binding protein alpha (SABPα) and proline-rich protein (PRP) in the seromucous acinar cell secretory granules, submandibular gland protein C (SMGC) in the terminal tubule/granular intercalated duct cell granules, and epidermal growth factor (EGF) and nerve growth factor (NGF) in granular duct cell granules of the submandibular gland of flight mice from the STS-131 and STS-135 missions and the Bion-M1 biosatellite. Results are expressed as a percentage ± standard error of the mean of the habitat-housed ground control mice. *, p<0.01.
In the granular convoluted duct cells, immunogold labeling with antibodies to EGF and NGF resulted in labeling of the secretory granules (Fig. 4C, D). In our samples fixed only in paraformaldehyde, the secretory granules exhibited variable electron density. The immunogold labeling density is reported only for the electron dense and moderately electron dense granules. No differences were seen in the labeling densities for EGF and NGF between female flight and habitat ground control mice in the STS-131 and STS-135 missions. Although the EGF labeling was decreased in STS-135 flight mice, it was not significantly different from the habitat ground control mice due to substantial variability among the individual mice. Labeling for both EGF and NGF, however, was significantly increased in male mice flown on the Bion-M1 biosatellite compared to habitat ground control mice (Fig. 5).
The labeling of terminal tubule/granular intercalated duct cells in female mice from the STS-131 and STS-135 missions with antibody to SMGC (Fig. 4E) showed no difference between flight and habitat ground controls (Fig. 5).
Ground control vivarium mice were available for study for the STS-135 and Bion-M1 missions. Labeling of female vivarium control mice from the STS-135 mission with antibodies to PRP, EGF, NGF and SMGC was not significantly different from the flight and habitat control mice. Labeling for SABPα, however, was greater in vivarium control mice than in flight mice (p<0.01) and habitat control mice (p<0.05). Labeling of male vivarium control mice from the Bion-M1 mission with antibodies to PRP and EGF was not significantly different from the flight and habitat control mice. Labeling of vivarium control mice for SABPα and NGF was significantly less than in flight mice (p<0.05), but no different from habitat control mice.
Non-specific labeling of sections incubated without primary antibody or with non-immune IgG instead of primary antibody was generally very low, in most cases in the range of 0% to 6% of the specific antibody labeling of secretory granules. Non-specific binding of primary antibodies to plastic in interstitial areas ranged from 0.37% to less than 5%.
Discussion
In the submandibular gland, as in the parotid and sublingual glands of these mice, spaceflight resulted in specific changes in secretory protein expression. Male mice flown for 30 d on the Bion-M1 biosatellite had an increased content of SABPα in acinar cell secretory granules, and EGF and NGF in granular convoluted duct cell secretory granules, when compared to that in the habitat ground control mice. This appears to be a specific effect of microgravity. The shorter 13 d and 15 d flights on the space shuttle, however, did not cause increased expression of these proteins in female glands. This suggests that longer exposure to microgravity may result in more profound changes. Other factors also may play a role, such as the age and sex of the mice, stresses during landing given the different space craft, and the time elapsed between landing and dissection. It is possible that the different diets consumed by the mice on the space shuttle flights compared to those on the Bion-M1 flight could have resulted in differences in protein expression. The comparisons in the present study, therefore, are made between the flight mice and habitat ground control mice that were fed the same diets.
SABPα, also known as SCGB1B27, is a member of the secretoglobin protein family. Secretoglobins bind lipophilic ligands and are present in mammals and birds (Laukaitis et al., 2005; Jackson et al., 2011). In the mouse submandibular gland, SABPα forms heterotetramers with β and γ subunits; the complex binds androgens and acts as a pheromone involved in mate selection (Laukaitis et al., 1997). Previous studies have shown that its expression is similar in male and female mice (Wickliffe et al., 2002), although some paralogues expressed in the female submandibular gland are not present in the male submandibular gland (Laukaitis et al., 2005). Although the pathways regulating SABPα expression are not known, the spaceflight environment, and specifically microgravity, significantly upregulates its expression in male mice after 30 d in space.
The PRPs are expressed in the submandibular and parotid glands of humans, rodents and other species, are secreted into saliva, and have bacterial, calcium, tannin and polyphenol binding functions (Oppenheim et al., 1971; Bennick 1982, 2002; Gibbons and Hay, 1988, 1989; Takano et al., 1993). Their synthesis is enhanced by dietary tannins and by the administration of isoproterenol (Fernandez-Sorensen and Carlson, 1974; Mehansho and Carlson, 1983; Mehansho et al., 1985; Divecha et al., 1989; Matsuura and Hand, 1991). Regulation of PRP gene expression by tannins and β-adrenergic agonists occurs via the cyclic AMP/protein kinase A signaling system (Carlson, 1993; Ann and Lin, 1998), whose responses vary with the amount of stress imposed on the system. Long-term spaceflight appears to have minimal effects on components of the cyclic AMP/protein kinase A system (Mednieks et al., 2014, 2015; Dagdeviren et al., 2018a, 2018b). Relatively modest changes in the levels of salivary and plasma corticosteroids and catecholamines occur as a result of spaceflight in both animals and humans (Kvetnansky et al., 1981, 1991; Macho et al., 1996, 2001, 2004; Larina et al., 1997; Strollo et al., 1997; Blottner et al., 2009; Buchheim et al., 2019). This suggests that the mice from these missions experienced minimal stress due to the spaceflight environment. Stress associated with landing and post-flight readaptation to gravity potentially could affect the salivary glands. However, the relatively short interval between landing and tissue collection for the STS-131 and STS-135 flights may have minimized such effects. This is supported by the lack of significant changes in PRP expression.
SMGC, a secretory protein of unknown function, is produced by the terminal tubule cells of neonatal rats and mice (Ball et al., 1988; Zinzen et al., 2004). As the terminal tubule cells decline in number beyond 20 d of age in females, and disappear in males (Hayashi et al., 2000; Hecht et al., 2000; Yamamoto et al., 2018), the levels of SMGC transcripts and protein detectable in the submandibular gland are greatly diminished (Hecht et al., 2000; Zinzen et al., 2004). SMGC expression in females is decreased after ovariectomy and increased by β-estradiol administration (Kusakabe et al., 2016). No differences in immunogold labeling were observed for the residual terminal tubule/granular intercalated duct cells present in the submandibular gland of the female mice flown on the two space shuttle missions. This suggests that SMGC expression is not affected by microgravity. Alternatively, the length of exposure to microgravity (13-15 d) may have been insufficient to induce changes in SMGC expression.
Numerous studies have demonstrated that granular convoluted duct structure and protein expression are dependent on androgens and thyroid hormones, and that the expression of EGF and NGF is much greater in the adult male submandibular gland than in the female submandibular gland (Caramia, 1966b; Chretien, 1977; Aloe and Levi-Montalcini, 1980; Matsuura et al., 1984; Gubits et al., 1986; Kasayama et al., 1989; Gresik, 1994; Adthapanyawanich et al., 2015; Yamanoto et al., 2018). While plasma levels of androgens and thyroid hormones were not determined in the male mice from the Bion-M1 mission, previous studies have shown decreased plasma testosterone in rats (Amann et al., 1992; Macho et al., 2001), and decreased plasma, salivary and urinary testosterone in astronauts (Strollo et al., 1998; Strollo, 1999). Decreases in thyroid function and plasma levels of thyroid hormones after spaceflight have been observed in rodents and humans (Plakhuta-Plakutina et al., 1990; Strollo, 1999; Macho et al., 2001; Smith et al., 2011). The increase in EGF and NGF expression in the granular convoluted duct cells of the Bion-M1 flight mice thus was unexpected. However, androgens and thyroid hormones also act through non-classic nongenomic pathways (Foradori et al., 2008; Cheng et al., 2010; Hammes and Davis, 2015); it is possible that microgravity may influence EGF and NGF expression via signaling intermediates that intersect with these pathways. The increased expression of both EGF and NGF by approximately 60% in flight mice compared to habitat ground control mice suggests that the same regulatory pathway(s) was activated for both genes.
Watson et al. (1985), using double labeling with different size gold particles, showed that all moderate to large size granules of the granular convoluted duct cells contain both EGF and NGF. They also observed small unreactive granules and suggested that they may contain other granular convoluted duct cell proteins, or perhaps precursors to EGF and NGF that did not react with their antibodies. Similar small granules, occasionally labeled with EGF and NGF antibodies, were observed in the present study. It was not clear whether these labeled granules represent a separate population from the medium to large size granules, or whether they were tangential sections through the larger granules.
Autophagic vacuoles and apoptotic acinar cells were observed more frequently in the parotid glands of the flight mice from these three missions than in the corresponding ground controls (Mednieks et al., 2014, 2015). In other studies an increase in autophagy and apoptosis in various tissues and in cultured cells was observed after spaceflight, as well as increased expression of genes involved in these processes (Schatten et al., 2001; Gridley et al., 2013, 2015; Novoselova et al., 2015; Blaber et al., 2017). In contrast, the morphology of the submandibular gland appeared to be unaffected by either the 13-15 d or 30 d flights, and autophagic vacuoles and apoptotic cells were rare in both flight and ground control mice. The basis for the different responses seen in the parotid and submandibular gland is not known. However, the responses of the two glands to spaceflight parallel those seen in rats in experimental conditions such as feeding a liquid diet and streptozotocin diabetes, which result in increased autophagy and apoptosis in the parotid (Hand and Ho, 1981; Hand and Weiss, 1984; Takahashi et al., 2012), but few morphological changes in the submandibular gland (Mednieks et al., 2009; Takahashi et al., 2014).
In conclusion, exposure to the spaceflight environment, and specifically microgravity, results in alterations in secretory protein expression in acinar and granular convoluted duct cells of the mouse submandibular gland. The effects appear to be related to the length of the spaceflight as they were seen after a 30 d flight but not after 13-15 d flights. They also may be influenced by the animal’s sex as they occurred in males but not females. Unlike the response of the parotid gland, no obvious changes in cell structure were observed in the submandibular gland. Our findings suggest that evaluating changes in the levels of specific proteins in saliva may be useful for monitoring astronaut physiology and health during long-term spaceflights.
Highlights:
Thirty days of spaceflight alters protein expression in the mouse submandibular gland.
The effects appear to be age, sex and flight length dependent.
The individual major salivary glands respond differently to spaceflight.
Evaluating changes in saliva may be useful for monitoring astronaut physiology and health.
Acknowledgements
We thank Ms. Maya Yankova for her expert technical assistance, and UConn Health for the use of the instruments of the Central Electron Microscope Facility. This work was supported by the National Aeronautics and Space Administration (grant number NNX09AP13G [MIM]), the Connecticut Space Grant College Consortium (grant number P-536 [ARH]), and the National Institutes of Health (Shared Instrumentation grant number 1S10RR024742 [ARH]).
Abbreviations:
- STS
space transportation system
- NASA
National Aeronautics and Space Administration
- SABPα
salivary androgen binding protein alpha
- PRP
proline-rich protein
- SMGC
submandibular gland protein C
- EGF
epidermal growth factor
- NGF
nerve growth factor
Footnotes
Declarations of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adthapanyawanich K, Kumchantuek T, Nakata H, Yamamoto M, Wakayama T, Nishiuchi T, et al. (2015). Morphology and gene expression profile of the submandibular gland of androgen receptor-deficient mice. Archives of Oral Biology, 60, 320–332. [DOI] [PubMed] [Google Scholar]
- Aloe L, & Levi-Montalcini R (1980). Comparative studies on testosterone and L-thyroxine effects on the synthesis of nerve growth factor in mouse submaxillary salivary glands. Experimental Cell Research, 125, 15–22. [DOI] [PubMed] [Google Scholar]
- Amann RP, Deaver DR, Zirkin BR, Grills GS, Sapp WJ, Veeramachaneni DN, et al. (1992). Effects of microgravity or simulated launch on testicular function in rats. Journal of Applied Physiology (1985), 73 (2 Suppl), 174S–185S. [DOI] [PubMed] [Google Scholar]
- Andreev-Andrievskiy A, Popova A, Boyle R, Alberts J, Shenkman B, Vinogradova O, et al. (2014). Mice in Bion-M1 space mission: training and selection. PLoS One, 9, e104830. doi: 10.1371/journal.pone.0104830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ann DK, & Lin H,H. (1998). Transcriptional regulation of salivary proline rich protein gene expression. Annals of the New York Academy of Sciences, 842, 108–114. [DOI] [PubMed] [Google Scholar]
- Ball WD, Hand AR, Moreira JE, & Johnson AO (1988). A secretory protein restricted to type I cells of neonatal rat submandibular glands. Developmental Biology, 129, 464–475. [DOI] [PubMed] [Google Scholar]
- Bennick A (1982). Salivary proline-rich proteins. Molecular and Cellular Biochemistry, 45, 83–99. [DOI] [PubMed] [Google Scholar]
- Bennick A (2002). Interaction of plant polyphenols with salivary proteins. Critical Reviews in Oral Biology and Medicine, 13, 184–196. [DOI] [PubMed] [Google Scholar]
- Blaber EA, Pecaut M,J, & Jonscher KR (2017). Spaceflight activates autophagy programs and the proteasome in mouse liver. International Journal of Molecular Sciences, 18, 2062. doi: 10.3390/ijms18102062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blottner D, Serradj N, Salanova M, Touma C, Palme R, Silva M, et al. (2009). Morphological, physiological and behavioural evaluation of a ‘Mice in Space’ housing system. Journal of Comparative Physiology B, 179, 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers AT, Keen C,L, & Gershwin ME (2002). Microgravity and immune responsiveness: implications for space travel. Nutrition 18, 889–898. [DOI] [PubMed] [Google Scholar]
- Brown LR, Frome WJ, Wheatcroft MG, Riggan LJ, Bussell NE, & Johnston DA (1977). The effect of Skylab on the chemical composition of saliva. Journal of Dental Research, 56, 1137–1143. [DOI] [PubMed] [Google Scholar]
- Buchheim J-I, Matzel S, Rykova M, Vassilieva G, Ponomarev S, Nichiporuk I, et al. (2019). Stress related shift toward inflammaging in cosmonauts after long-duration space flight. Frontiers in Physiology, 10, 85. doi: 10.3389/fphys.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramia F (1966a). Ultrastructure of mouse submaxillary gland. I. Sexual differences. Journal of Ultrastructure Research, 16, 505–523. [DOI] [PubMed] [Google Scholar]
- Caramia F (1966b). Ultrastructure of mouse submaxillary gland. II. Effect of castration in the male. Journal of ultrastructure Research, 16, 524–536. [DOI] [PubMed] [Google Scholar]
- Carlson DM (1993). Salivary proline-rich proteins: biochemistry, molecular biology, and regulation of expression. Critical Reviews in Oral Biology and Medicine, 4, 495–502. [DOI] [PubMed] [Google Scholar]
- Cheng S-Y, Leonard JL, & Davis PJ (2010). Molecular aspects of thyroid hormone actions. Endocrine Reviews, 31, 139–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien M, (1977). Action of testosterone on the differentiation and secretory activity of a target organ: the submaxillary gland of the mouse. International Review of Cytology, 50, 333–396. [DOI] [PubMed] [Google Scholar]
- Dagdeviren D, Beallias J, Khan I, Mednieks MI, & Hand AR (2018a). Response of the mouse sublingual gland to spaceflight. European Journal of Oral Sciences, 126, 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagdeviren D, Kalajzic Z, Adams DJ, Kalajzic I, Lurie A, Mednieks MI, et al. (2018b). Responses to spaceflight of mouse mandibular bone and teeth. Archives of Oral Biology, 93, 163–176. [DOI] [PubMed] [Google Scholar]
- Demontis GC, Germani MM, Caiani EG, Barravecchia I, Passino C, & Angeloni D (2017). Human pathophysiological adaptations to the space environment. Frontiers in Physiology, 8, 547. doi: 10.3389/fphys.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny PC, Ball WD, & Redman RS (1997). Salivary glands: A paradigm for diversity of gland development. Critical Reviews in Oral Biology and Medicine, 8, 51–75. [DOI] [PubMed] [Google Scholar]
- Denny PC, Chai Y, Pimprapaiporn W, & Denny PA (1990). Three-dimensional reconstruction of adult female mouse submandibular gland secretory structures. Anatomical Record, 226, 489–500. [DOI] [PubMed] [Google Scholar]
- Divecha N, Mansouri H, Peat D, Cope G, Partridge L, & McDonald CJ (1989). Isoprenaline-induced and constitutive members of a proline-rich protein sub-group from mouse parotid glands studied with monoclonal antibody NAL1. Journal of Molecular Endocrinology, 3, 7–14. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sorensen A, & Carlson DM (1974). Isolation of a “proline-rich” protein from rat parotid glands following isoproterenol treatment. Biochemical and Biophysical Research Communications, 60, 249–256. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, & Handa RJ (2008). Non-genomic actions of androgens. Frontiers in Neuroendocrinology, 29, 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Bakelman FE, Darshi M, Green SJ, Gur RC, Ling Lin L, Macias BR, et al. (2019). The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science, 364, 10.1126/science.aau8650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P, Stabley JN, Behnke BJ, Allen MR, & Delp MD (2016). Effects of spaceflight on the murine mandible: Possible factors mediating skeletal changes in non-weight bearing bones of the head. Bone, 83, 156–161. [DOI] [PubMed] [Google Scholar]
- Gibbons RJ, & Hay DI (1988). Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infection and Immunity, 56, 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RJ, & Hay DI (1989). Adsorbed salivary acidic proline-rich proteins contribute to the adhesion of Streptococcus mutans JBP to apatitic surfaces. Journal of Dental Research, 68, 1303–1307. [DOI] [PubMed] [Google Scholar]
- Gresik EW (1994). The granular convoluted tubule (GCT) cell of rodent submandibular glands. Microscopy Research and Technique, 27, 1–24. [DOI] [PubMed] [Google Scholar]
- Gresik EW, & MacRae EK (1975). The postnatal development of the sexually dimorphic duct system and of amylase activity in the submandibular glands of mice. Cell and Tissue Research, 157, 411–422. [DOI] [PubMed] [Google Scholar]
- Gridley DS, Mao XW, Stodieck LS, Ferguson VL, Bateman TA, Moldovan M, et al. (2013). Changes in mouse thymus and spleen after return from the STS-135 mission in space. PLoS One, 8, e75097. doi: 10.1371/journal.pone.0075097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley DS, Mao XW, Tian J, Cao JD, Perez C, Stodieck LS, et al. (2015). Genetic and apoptotic changes in lungs of mice flown on the STS-135 mission in space. In Vivo, 29, 423–434. [PubMed] [Google Scholar]
- Groza P, Bordeianu A, Cananáu S, Boca A, Petrescu A, & Lungu D (1981). The action of simulated and true weightlessness on the digestive tract of rats. Advances in Space Research, 1, 179–185. [DOI] [PubMed] [Google Scholar]
- Groza P, Bordeianu A, & Boca A (1983). Modifications of the digestive tract in rats submitted to an orbital flight aboard the Soviet satellite Cosmos 1129. Physiologie, 20, 35–44. [PubMed] [Google Scholar]
- Gubits RM, Shaw PA, Gresik EW, Onetti-Muda A, & Barka T (1986). Epidermal growth factor gene expression is regulated differently in mouse kidney and submandibular gland. Endocrinology, 119, 1382–1387. [DOI] [PubMed] [Google Scholar]
- Hammes SR, & Davis PJ (2015). Overlapping nongenomic and genomic actions of thyroid hormone and steroids. Best Practices & Research. Clinical Endocrinology & Metabolism, 29, 581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand AR (1995) Electron microscopy In: J. A. Glaser, & M. P. Deutscher MP (Eds.), Introduction to biophysical methods for protein and nucleic acid research (pp. 205–260). San Diego: Academic Press. [Google Scholar]
- Hand AR, & Weiss RE (1984). Effects of streptozotocin-induced diabetes on the rat parotid gland. Laboratory Investigation, 51, 429–440. [PubMed] [Google Scholar]
- Hand AR, & Ho B (1981). Liquid-diet-induced alterations of rat parotid acinar cells studied by electron microscopy and enzyme cytochemistry. Archives of Oral Biology, 26, 369–380. [DOI] [PubMed] [Google Scholar]
- Hargens AR, & Richardson S (2009). Cardiovascular adaptations, fluid shifts, and countermeasures related to space flight. Respiratory Physiology and Nenrobiology, 169 (Suppl 1), S30–S33. [DOI] [PubMed] [Google Scholar]
- Hayashi H, & Sasa S (1989). Terminal tubule cells of submandibular gland in prepubertal rat and mouse. Bulletin of the Kanagawa Dental College, 17, 151–154. [PubMed] [Google Scholar]
- Hayashi H, Ozono S, Watanabe K, Nagatsu I, & Onozuka M (2000). Morphological aspects of the postnatal development of submandibular glands in male rats: involvement of apoptosis. Journal of Histochemistry and Cytochemistry, 48, 695–698. [DOI] [PubMed] [Google Scholar]
- Hecht R, Connelly M, Marchetti L, Ball WD, & Hand AR (2000). Cell death during development of intercalated ducts in the rat submandibular gland. Anatomical Record, 258, 349–358. [DOI] [PubMed] [Google Scholar]
- Jackson BC, Thompson DC, Wright MW, McAndrews M, Bernard A, Nebert DW, et al. (2011). Update of the human secretoglobin (SCGB) gene superfamily and an example of ‘evolutionary bloom’ of androgen binding protein genes within the mouse Scgb gene superfamily. Human Genomics, 5, 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasinghe NR, Cope GH, & Jacob S (1990). Morphometric studies on the development and sexual dimorphism of the submandibular gland of the mouse. Journal of Anatomy, 172, 115–127. [PMC free article] [PubMed] [Google Scholar]
- Kasayama S, Yoshimura M, & Oka T (1989). The regulation by thyroid hormones and androgen of epidermal growth factor synthesis in the submandibular gland and its plasma concentrations in mice. Journal of Endocrinology, 121, 269–275. [DOI] [PubMed] [Google Scholar]
- Kusakabe Y, Shindo Y, Kawai T, Takahashi Y, Kobori M, Inoue H, et al. (2016). Sex-based differences in Smgc expression in the submandibular gland of C57BL/6 mice. Pathobiology, 83, 287–294. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Torda T, Macho L, Tigranian RA, Serova L, & Genin AM (1981).Effect of weightlessness on sympathetic-adrenomedullary activity of rats. Acta Astronautica, 8, 469–481. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Noskov VB, Blazicek P, Gharib C, Popova IA, Gauquelin G, et al. (1991). Activity of the sympathoadrenal system in cosmonauts during 25-day space flight on station Mir. Acta Astronautica, 23, 109–116. [DOI] [PubMed] [Google Scholar]
- Larina IM, Bystritzkaya AF, & Smirnova TM (1997). Psycho-physiological monitoring in real and simulated space flight conditions. Journal of Gravitational Physiology, 4, P113–P114. [PubMed] [Google Scholar]
- Latchney SE, Rivera PD, Mao XW, Ferguson VL, Batemen TA, Stodieck LS, et al. (2014). The effect of spaceflight on mouse olfactory bulb volume, neurogenesis, and cell death indicates the protective effect of novel environment. Journal of Applied Physiology (1985), 116, 1593–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukaitis CM, Critser ES, & Karn RC (1997). Salivary androgen-binding protein (ABP) mediates sexual isolation in Mus musculus. Evolution, 51, 2000–2005. [DOI] [PubMed] [Google Scholar]
- Laukaitis CM, Dlouhy SR, Emes RD, Ponting CP, & Karn RC (2005). Diverse spatial, temporal, and sexual expression of recently duplicated androgen-binding protein genes in Mus musculus. BMC Evolutionary Biology, 5, 40. doi: 10.1186/1471-2148-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Saverin M, & Hand AR (2011). Distribution of dendritic cells in normal human salivary glands. Acta Histochemica et Cytochemica, 44, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho L, Kvetnansky R, Fickova M, Popova IA, & Grigoriev A (2001). Effects of exposure to space flight on endocrine regulations in experimental animals. Endocrine Regulations, 35, 101–114. [PubMed] [Google Scholar]
- Macho L, Kvetnansky R, Nemeth S, Fickova M, Popova I, Serova L, et al. (1996). Effects of space flight on endocrine system function in experimental animals. Environmental Medicine, 40, 95–111. [PubMed] [Google Scholar]
- Macho L, Koska J, Ksínantová L, Vigas M, Blazícek P, Noskov VB, et al. (2004). Effects of real and simulated microgravity on response of sympathoadrenal system to various stress stimuli. Annals of the New York Academy of Sciences, 1018, 550–561. [DOI] [PubMed] [Google Scholar]
- Matsuura S, & Hand AR (1991). Quantitative immunocytochemistry of rat submandibular secretory proteins during chronic isoproterenol administration and recovery. Journal of Histochemistry and Cytochemistry, 39, 945–954. [DOI] [PubMed] [Google Scholar]
- Matsuura S, Sahara N, & Suzuki K (1984). Fine structure of submandibular glands of mice with testicular feminization (Tfm/Y). Cell and Tissue Research, 235, 295–301. [DOI] [PubMed] [Google Scholar]
- Mednieks MI, & Hand AR (1985). Biochemical and morphological evaluation of the effects of space flight on rat salivary glands. Physiologist, 28 (6 Suppl), S215–S216. [PubMed] [Google Scholar]
- Mednieks MI, & Hand AR (1987). Salivary gland ultrastructure and cyclic AMP-dependent reactions in Spacelab 3 rats. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 252, R233–R239. [DOI] [PubMed] [Google Scholar]
- Mednieks M, Khatri A, & Hand AR (2015). Salivary gland protein expression after Bion-M1 and Space Shuttle STS-135 missions. Gravitational and Space Research, 3, 2–19. [Google Scholar]
- Mednieks M, Khatri A, Rubenstein R, Burleson JA, & Hand AR (2014). Microgravity alters the expression of salivary proteins. Oral Health and Dental Management, 13, 211–216. [PubMed] [Google Scholar]
- Mednieks MI, Szczepanski A, Clark B, & Hand AR (2009). Protein expression in salivary glands of rats with streptozotocin diabetes. International Journal of Experimental Pathology, 90, 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehansho H, & Carlson DM (1983). Induction of protein and glycoprotein synthesis in rat submandibular glands by isoproterenol. Journal of Biological Chemistry, 258, 6616–6620 [PubMed] [Google Scholar]
- Mehansho H, Clements S, Sheares BT, Smith S, & Carlson DM (1985). Induction of proline-rich glycoprotein synthesis in mouse salivary glands by isoproterenol and by tannins. Journal of Biological Chemistry, 260, 4418–4423. [PubMed] [Google Scholar]
- Morey-Holton ER, Halloran BP, Garetto LP, & Doty SB (2000). Animal housing influences the response of bone to spaceflight in juvenile rats. Journal of Applied Physiology (1985), 88, 1303–1309. [DOI] [PubMed] [Google Scholar]
- Nagaraja MP, & Risin D (2013), The current state of bone loss research: data from spaceflight and microgravity simulators. Journal of Cellular Biochemistry, 114, 1001–1008. [DOI] [PubMed] [Google Scholar]
- Novoselova EG, Lunin SM, Khrenov MO, Parfenyuk SB, Novoselova TV, Shenkman BS, et al. (2015). Changes in immune cell signalling, apoptosis and stress response functions in mice returned from the BION-M1 mission in space. Immunobiology 220, 500–509. [DOI] [PubMed] [Google Scholar]
- Oppenheim FG, Hay DI, & Franzblau C (1971). Proline-rich proteins from human parotid saliva. I. Isolation and partial characterization. Biochemistry, 10, 4233–4238. [DOI] [PubMed] [Google Scholar]
- Pardini LC, & Taga R (1996). Stereological study of the sexual dimorphism in mouse submandibular glands. Okajimas Folia Anatomica Japonica, 73, 119–124. [DOI] [PubMed] [Google Scholar]
- Plakhuta-Plakkutina GI, Kabitskiĭ EN, Dmitrieva NP, & Amirkhanian EA (1990). [Studies of the morphology of the thyroid gland and thyroid hormone levels in the blood of rats in experiments on “Kosmos-1667” and “Kosmos-1887”]. Kosmicheskaia Biologiia i Aviakosmicheskaia Meditsina, 24, 25–27. [PubMed] [Google Scholar]
- Porseva VV, Shilkin VV, Strelkov AA, Krasnov IB, & Masliukov PM (2017). Changes in the neurochemical composition of motor neurons of the spinal cord in mice under conditions of spaceflight. Bulletin of Experimental Biology and Medicine, 162, 336–339. [DOI] [PubMed] [Google Scholar]
- Rosenberg GD, Campbell SC, & Simmons DJ (1984). The effects of spaceflight on the mineralization of rat incisor dentin. Proceedings of the Society for Experimental Biology and Medicine, 175, 429–437. [DOI] [PubMed] [Google Scholar]
- Schatten H, Lewis ML, & Chakrabarti A (2001). Spaceflight and clinorotation cause cytoskeleton and mitochondria changes and increases in apoptosis in cultured cells. Acta Astronautica, 49, 399–418. [DOI] [PubMed] [Google Scholar]
- Simmons DJ, Russell J,E, Winter F, Tran Van P, Vignery A, Baron R, et al. (1983). Effect of spaceflight on the non-weight-bearing bones of rat skeleton. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 244, R319–R326. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zwart SR, McMonigal KA, & Huntoon CL (2011). Thyroid status of space shuttle crewmembers: effects of iodine removal. Aviation Space and Environmental Medicine, 82, 49–51. [DOI] [PubMed] [Google Scholar]
- Strollo F (1999). Hormonal changes in humans during spaceflight. Advances in Space Biology and Medicine, 7, 99–129. [DOI] [PubMed] [Google Scholar]
- Strollo F, Strollo G, More M, Ferretti C, Mangrossa N, Casarosa E, et al. (1997). Changes in human adrenal and gonadal function onboard SpaceLab. Journal of Gravitational Physiology, 4, P103–P104. [PubMed] [Google Scholar]
- Strollo F, Riondino G, Harris B, Strollo G, Casarosa E, Mangrossa N, et al. (1998). The effect of microgravity on testicular androgen secretion. Aviation Space and Environmental Medicine, 69, 133–136. [PubMed] [Google Scholar]
- Sun GS, Tou JC, Yu D, Girten BE, & Cohen J (2014). The past, present, and future of National Aeronautics and Space Administration spaceflight diet in support of microgravity rodent experiments. Nutrition, 30, 125–130. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Uekita H, Kato T, Yuge F, Ushijima N, Inoue K, et al. (2012). Involvement of apoptosis and proliferation of acinar cells in atrophy of rat parotid glands induced by liquid diet. Journal of Molecular Histology, 43, 761–766. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Uekita H, Kato T, Yuge F, Ushijima N, Inoue K, et al. (2014). Immunohistochemical and ultrastructural investigation of acinar cells in submandibular and sublingual glands of rats fed a liquid diet. Tissue and Cell, 46 136–143. [DOI] [PubMed] [Google Scholar]
- Takano K, Malamud D, Bennick A, Oppenheim F, Hand AR (1993). Localization of salivary proteins in granules of human parotid and submandibular acinar cells. CRC Critical Reviews in Oral Biology and Medicine, 4, 399–405. [DOI] [PubMed] [Google Scholar]
- Watson AY, Anderson JK, Siminoski K, Mole JE, & Murphy RA (1985). Cellular and subcellular colocalization of nerve growth factor and epidermal growth factor in mouse submandibular glands. Anatomical Record, 213, 365–376. [DOI] [PubMed] [Google Scholar]
- Wickliffe JK, Lee VH, Smith E, Tandler B, & Philips CJ (2002). Gene expression, cell localization, and evolution of rodent submandibular gland androgen-binding protein. European Journal of Morphology, 40, 257–260. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Nakata H, Kumchantuek T, Adhapanyawanich K, & Iseki S (2018).Distinct hormonal regulation of two types of sexual dimorphism in submandibular gland of mice. Cell and Tissue Research, 371, 261–272. [DOI] [PubMed] [Google Scholar]
- Zinzen KM, Hand AR, Yankova M, Ball WD, & Mirels L (2004). Molecular cloning and characterization of the neonatal rat and mouse submandibular gland protein SMGC. Gene, 334, 23–33. [DOI] [PubMed] [Google Scholar]