Abstract
Background:
Fabry disease is caused by α-galactosidase A deficiency. Substrates of this lysosomal enzyme accumulate, resulting in cellular dysfunction. Patients experience neuropathic pain, kidney failure, heart disease, and strokes.
Scope of review:
The clinical picture and molecular features of Fabry disease are described, along with updates on disease mechanisms, animal models, and therapies.
Major conclusions:
How the accumulation of α-galactosidase A substrates, mainly glycosphingolipids, leads to organ damage is incompletely understood. Enzyme replacement and chaperone therapies are clinically available to patients, while substrate reduction, mRNA-based, and gene therapies are on the horizon. Animal models exist to optimize these therapies and elucidate disease mechanisms for novel treatments.
General significance:
Recent newborn screening studies demonstrate that Fabry disease is the most common lysosomal storage disease. As many countries now include Fabry disease in their screening panels, the number of identified patients is expected to increase significantly. Better knowledge of disease pathogenesis is needed to improve treatment options.
Keywords: Fabry disease, lysosomal storage disease, glycosphingolipids, enzyme replacement therapy, chaperone therapy, rodent models
Introduction
Fabry disease (OMIM #301500) was first described in 1898 by two dermatologists, William Anderson [1] and Johannes Fabry [2]. Sixty-five years later, Sweeley and Klionsky discovered that patients with Fabry disease accumulate the glycosphingolipid, globotriaosylceramide (Gb3) [3]. Roscoe Brady then showed that the lysosomal enzyme, α-galactosidase A (α-Gal A), was deficient in these patients [4]. Since that time, much has been learned about the clinical manifestations and molecular features of Fabry disease, now known to be the most common lysosomal storage disease. Two treatments, intravenous enzyme replacement therapy and oral molecular chaperone therapy, are clinically available to patients. Mouse and more recently rat models are now available to test new treatments and elucidate mechanisms of disease pathogenesis. This mini review provides an update to our understanding of Fabry disease mechanisms and treatments.
Clinical picture
The clinical picture of Fabry disease is characterized by progressive signs and symptoms that impact multiple organ systems (Table 1). The “classic” presentation of patients with no or minimal residual α-Gal A activity will be described here. However, it is important to note that the presentation is often heterogeneous, with a subset of patients experiencing phenotypes in only one (e.g., cardiac variant) or few organ systems. As Fabry disease is X-linked, males present with the classical manifestations more frequently than females.
Table 1:
Fabry disease signs and symptoms and animal model correlations
| Sign or symptoma | Usual age of onset | Gla KO mouse | Gla KO rat |
|---|---|---|---|
| Acroparesthesia | Childhood | Yes [7,8] | Yes [9] |
| Chronic pain | Unknown | Unknown | |
| Sweating abnormalities | Nob | Nob | |
| Corneal & lenticular opacities | Unknown | Yes [10] | |
| Hearing loss | No [11,12] | Yes (unpublishedc) | |
| Gastrointestinal distress | Adolescence | Unknown | Unknown |
| Angiokeratomas | No | Nod | |
| Renal insufficiency | Adulthood | No [13] | Yes – tubular disease [14] |
| Cerebrovascular disease | Unknown | Unknown | |
| Cardiac dysfunction/Cardiac arrhythmias | Debatable hypertrophy reported [15] | Yes – mitral valve disease [14] | |
| Bone demineralization | Unknown | Yes (unpublishede ) | |
Some patients, particularly those with substantial residual α-Gal A activity, may experience attenuated signs and symptoms or manifestations in only one organ system.
Rodents do not sweat
Beltrame, Runge, and Dahms
While Fabry rats do not develop cutaneous vascular anomalies, evidence of macrophage infiltration and lipoatrophy is evident in Fabry rat skin [14]
Miller and Dahms
In childhood, patients develop acroparesthesia, which consists of neuropathic pain in their distal extremities. Diffuse pain attacks and crises can also occur, lasting from minutes to days and are often precipitated by rising body temperature due to exercise, fever, or warm ambient environments [5]. Compounding this problem, patients frequently have sweating abnormalities, with the most frequent being anhidrosis or hypohydrosis. Ophthalmologic opacities, such as cornea verticillata and cataracts, are detected in childhood, but many patients retain intact vision (reviewed in [6]).
During their teenage years, patients develop angiokeratomas in the “bathing trunk” region. Proteinuria, a sign of kidney disease, may be detected at this time. Additionally, gastrointestinal distress, such as frequent and painful bowel movements, begins to affect patients. In adulthood, patients are at significant risk of end-stage renal disease, heart dysfunction (e.g., hypertrophic cardiomyopathy, cardiac arrhythmias, valvular disease), and cerebrovascular events (e.g., transient ischemic attacks, ischemic strokes). Patients may also develop osteopenia or osteoporosis [16,17]. With nephropathy being a major complication of Fabry disease, frequent dialysis treatments become a necessity. Neuropathic pain subsides in some adult patients, but many adults continue to live with debilitating pain (reviewed in [18]). Some adult patients display a unique neuropsychiatric phenotype, characterized by subtle movement impairment and depression [19]. Together, these numerous signs and symptoms significantly reduce quality of life [20].
Fabry disease is considered an attenuated lysosomal storage disease because patients survive into adulthood. However, patients lacking α-Gal A activity exhibit an ~10–20 year shortened life span: male patients with Fabry disease have a median survival of 57 years, and the female median survival is 72 years [21]. Many patients with Fabry disease do not develop symptoms until their teenage or adulthood years. Moreover, as neuropathic pain is a prominent symptom, patients are frequently misdiagnosed with more common diseases, such as fibromyalgia or rheumatologic pain diseases [22]. Although cornea verticillata and angiokeratomas are useful clinical signs, unfortunately many patients experience what they describe as a “diagnostic odyssey” in that while symptoms are prevalent and debilitating, diagnosis is delayed for long periods of time.
The prevalence of Fabry disease was once believed to be rare: approximately 1:50,000 (reviewed in [23]). However, recent newborn screening efforts reveal that the incidence is much more common. In Italy, an incidence of 1:3100 was found [24] and in Taiwan, an incidence as high as 1:1250 was documented [25]. Studies in the United States report incidences of 1:5495 and 1:8454 in Washington and Illinois, respectively [26,27]. The overall discrepancy in estimated prevalence and measured newborn screening incidence is likely caused by the heterogeneity of clinical presentation, suggesting that newborn screening would improve the diagnosis and treatment of patients with Fabry disease. Given these findings, the overall patient population will increase as more states and countries include Fabry disease in their newborn screening panels.
Enzymatic defect
Fabry disease is caused by deficiency of α-Gal A, a lysosomal enzyme encoded by the GLA gene on the X-chromosome (region Xq22.1). Currently, there are 967 different GLA mutations, including 671 missense/nonsense mutations, listed on the Human Gene Mutation Database [28]. Many of these mutations are “private” in that they are only seen in one family. Having only one X-chromosome, males that are hemizygous for a pathogenic GLA mutation usually develop signs and symptoms, and males with completely deficient α-Gal A activity are impacted most severely, resulting in the “classic” presentation of symptoms. However, unlike other X-linked disorders, heterozygous females may experience significant disease symptoms depending on their residual α-Gal A activity [22,29]. Females homozygous for GLA mutations are especially rare but have been reported [30].
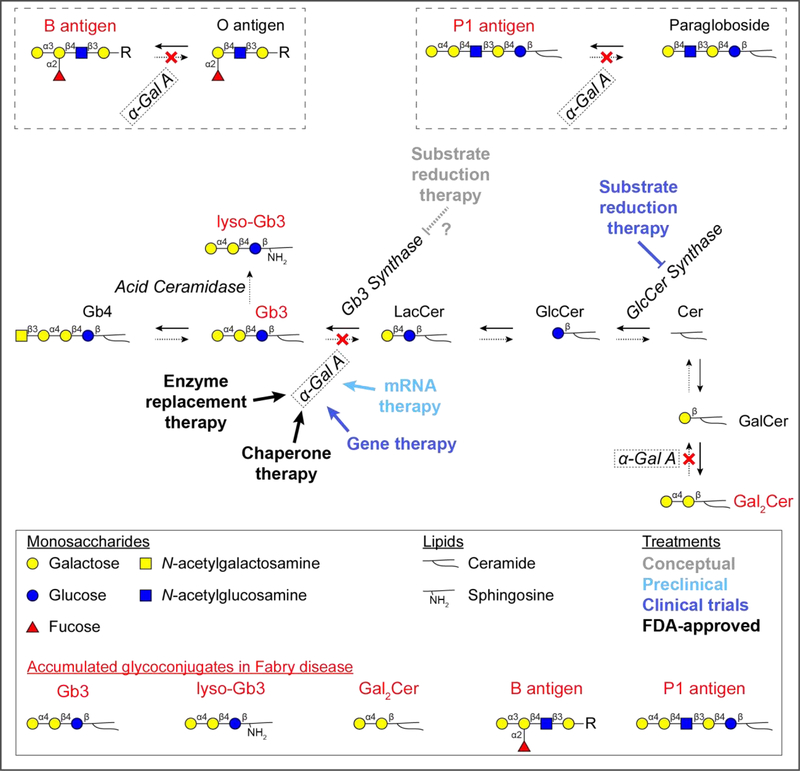
Through hydrolysis, α-Gal A removes terminal α1,3- and α1,4-linked galactosyl residues from various glycoconjugates within lysosomes. The primary substrate is Gb3, which accumulates to a major extent when α-Gal A is deficient. Minor α-Gal A substrates include deacylated Gb3 called globotriaosylsphingosine (lyso-Gb3), digalactosylceramide (Gal2Cer), and blood group B and P1 glycosphingolipids (Figure 1). While lyso-Gb3 accumulates to a lesser extent compared to Gb3, it serves as a biomarker for monitoring therapeutic efficacy and contributes to the pathology of disease [31,32].
Figure 1: Accumulating glycosphingolipids and treatment targets in Fabry disease.
Glycosphingolipids are synthesized and degraded by sequential monosaccharide addition and removal, respectively. In Fabry disease, the lysosomal enzyme, α-Gal A, is deficient and glycosphingolipids with terminal α-galactosyl residues accumulate (red font). The major accumulating molecule is Gb3, but lyso-Gb3, Gal2Cer, and blood group B and P1 antigens accumulate to a minor degree. Current and future therapies are aimed at replacing or promoting deficient α-Gal A activity. The FDA-approved therapies are enzyme replacement therapy and chaperone therapy. Clinical trials are currently underway to evaluate gene therapy and substrate reduction therapy. mRNA therapy is in preclinical development. Substrate reduction therapy trials are currently evaluating inhibitors of GlcCer synthase, which catalyzes a biosynthetic reaction prior to formation of Gb3, thereby reducing overall Gb3 load. However, this therapeutic strategy also inhibits the formation of GlcCer and LacCer, glycosphingolipids key to critical cellular processes, which ultimately results in an unfavorable side effect profile. Further, substrate reduction therapy inhibits the formation of other glycosphingolipids, such as gangliosides. Specific inhibition of Gb3 synthase may be more tolerable to patients with Fabry disease, but this approach is conceptual and remains to be developed and tested. The –R group represents that the B antigen glycan can be conjugated to either a protein or lipid. Abbreviations: globotetraosylceramide (Gb4), globotriaosylceramide (Gb3), globotriaosylsphingosine (lyso-Gb3), lactosylceramide (LacCer), glucosylceramide (GlcCer), ceramide (Cer), galactosylceramide (GalCer), digalactosylceramide (Gal2Cer), α-galactosidase A (α-Gal A), glucosylceramide synthase (GlcCer synthase).
The crystal structure of α-Gal A was solved in 2004 by Scott Garman’s group [33]. α-Gal A exists as a dimer, and each monomeric unit of α-Gal A is composed of two domains: an N-terminal (β/α)8 domain and a C-terminal β-domain [33]. The active site of α-Gal A is located in the N-terminal domain and requires two key aspartate residues, D170 and D231, for hydrolysis of terminal α-linked galactose residues from the glycosphingolipid substrates. Three N-linked glycosylation sites are also present on each α-Gal A monomer, and two of these glycans are predominantly modified with mannose 6-phosphate [34,35], which is essential for transport to lysosomes by mannose 6-phosphate receptors (reviewed in [36]).
There may be mechanisms independent of α-Gal A capable of clearing α-galactosyl glycoconjugates. One example is the lysosomal enzyme, α-N-acetylgalactosaminidase (α-NAGA). In addition to hydrolyzing α-N-acetylgalactosamine from glycoconjugates, α-NAGA also contains α-galactosidase activity [37]. In fact, α-Gal A and α-NAGA are the only enzymes with α-galactosidase activity known to exist in humans [38]. Thus, endogenous α-NAGA may be able to partially compensate for α-Gal A deficiency. Supporting this possibility is the observation that α-NAGA is able to hydrolyze Gb3 in vitro [37,39]. Further, GSL loading experiments demonstrate that Fabry patient fibroblasts have a ~50% capacity to digest blood group B and a ~15% capacity to digest Gb3 [40], which is probably accomplished by α-NAGA. Although α-NAGA may partially compensate for α-Gal A, this putative compensation is clearly overwhelmed with age in patients with Fabry disease.
Biological significance of Gb3
Gb3 is a glycosphingolipid that is known by several names, including ceramide trihexoside, CD77, Pk blood group antigen, and Burkitt lymphoma antigen. Synthesis of Gb3 occurs in the Golgi with the addition of α-galactose to lactosylceramide by Gb3 synthase [41] (Figure 1). This enzyme is also known as lactosylceramide 4-α-galactosyltransferase (encoded by A4GALT). Once synthesized, Gb3 is localized to the outer leaflet of the plasma membrane, where it is clustered in lipid rafts with its glycan portion facing the extracellular environment. Upon endocytosis and delivery to lysosomes, the glycan portion faces the lysosomal lumen, which contains the exoglycosidases, including α-Gal A, that are essential for turnover of this glycosphingolipid (reviewed in [42]). Humans deficient in Gb3 synthase are phenotypically healthy but have an increased risk of miscarriage (reviewed in [43]), suggesting that Gb3 may play a role in embryogenesis. Gb3 synthase mRNA is highly abundant in human heart and kidney [44,45], which explains, in part, why patients with Fabry disease experience dysfunction of these organs as they contain high levels of this glycosphingolipid. Despite its potential importance in Fabry disease treatment, no structures have been reported for Gb3 synthase.
Cell surface Gb3 is involved in infectious processes, such as Shiga toxin infection, which may result in hemolytic-uremic syndrome (reviewed in [46]). Gb3 is the cell surface receptor that the Shiga toxin family uses for cellular entry [47]. Shiga toxins include Shiga toxin itself, which is produced by Shigella dysenteriae, and Shiga-like toxins (i.e., verotoxins), which are produced by enterohemorrhagic E. coli serotypes O157:H7 and O104:H4. Shiga toxins bind Gb3 molecules on the plasma membrane where they are subsequently endocytosed and transported to the endoplasmic reticulum. Following transport to the endoplasmic reticulum, these toxins localize to the cytosol and inhibit the 28S subunit of rRNA (reviewed in [48]). Ultimately, ribosomal protein synthesis is inhibited, leading to apoptosis. Humans infected with Shiga or Shiga-like toxins may experience hemolytic-uremic syndrome. This disorder is initially characterized by bloody diarrhea, followed by hemolytic anemia, thrombocytopenia, and acute renal failure. Because they do not express Gb3, mice deficient in Gb3 synthase are insensitive to Shiga toxin infection [49]. Paradoxically, Fabry mice that overexpress Gb3 are protected from Shiga toxin compared to wild type mice [50]. This may be because excess Gb3 acts as a “toxin sink,” where toxin-insensitive cells that normally do not express high levels of Gb3 absorb Shiga toxin, directing this toxin away from toxin-sensitive cells (e.g., renal cells) [50].
Mechanisms of pathogenesis
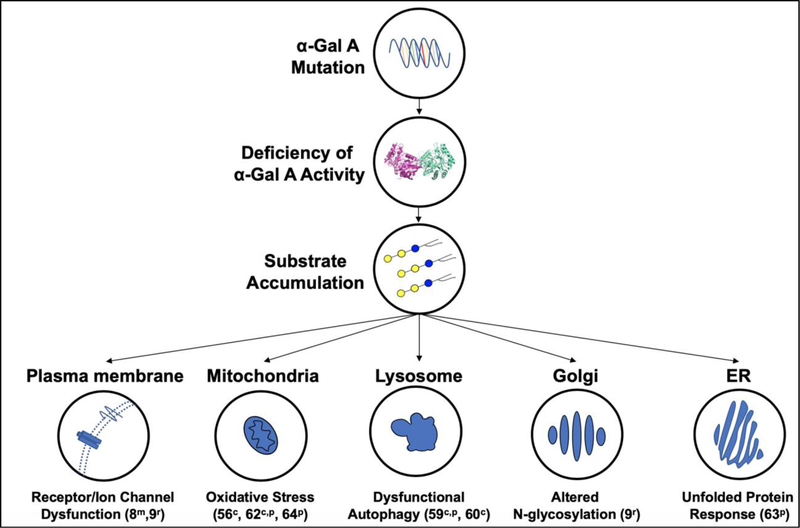
While much is known about the molecular defects and clinical sequelae of Fabry disease, the mechanisms by which substrate accumulation leads to cellular dysfunction remain less defined [51]. Because Gb3 is turned over in lysosomes, the inability to digest Gb3 results in its lysosomal accumulation. However, the impact of α-Gal A substrate accumulation on the development of disease symptoms has increasingly been shown to involve cellular structures beyond strictly the lysosome (Figure 2). Downstream effects, such as fibrosis (reviewed in [52]), inflammation (reviewed in [53]), and the generation of reactive oxygen species [54–56], also seem to play key roles in pathogenesis. Pathogenic mechanisms of Fabry disease are discussed briefly below.
Figure 2: Cellular mechanisms of Fabry disease pathogenesis.
A pathogenic genetic mutation in the gene encoding α-Gal A causes decreased activity of this lysosomal enzyme. Subsequently α-Gal A substrates accumulate and lead to cellular dysfunction through multiple pathways. Substrate accumulation has been shown to alter the normal function of several subcellular components that are listed along with the corresponding effects observed in studies involving cellsc, Fabry patientsp, Fabry micem, and Fabry ratsr.
α-Gal A deficiency may lead to the dysfunction of multiple cellular components (Figure 2). Gb3 accumulation has been observed in the plasma membrane of α-Gal A-deficient cells [57,58]. An increase in plasma membrane Gb3 has the potential to alter the activities of various membrane proteins and channels, likely impacting cellular function in a tissue dependent manner. For example, Gb3 accumulation correlated with increased levels of transient receptor potential vanilloid 1 and altered neuronal Ih and Nav 1.7 currents, suggesting that alteration of these ion channels contributes to the development of pain sensitivity [8]. Additionally, altered activity of cation channel transient receptor potential ankyrin 1 has been reported in Fabry sensory neurons [9]. As in many other lysosomal storage diseases, autophagy impairment is also observed in Fabry patient cells [59,60]. Because autophagy is highly dependent on the formation and fusion of membrane-rich autophagosomes with lysosomes, the alteration of autophagic membranes resulting from α-Gal A substrate accumulation may contribute to the impairment of autophagic flux [61]. In addition, mitochondrial dysfunction may be a pathogenic feature as the activities of respiratory chain enzymes are decreased in fibroblasts from Fabry patients [62]. This raises the possibility that Gb3 accumulation affects mitochondrial function, either directly through accumulation within the mitochondrial membrane or indirectly by preventing mitophagy. Finally, induction of the unfolded protein response has been implicated in Fabry disease [63], which suggests dysfunction of the endoplasmic reticulum.
Substrate accumulation and organelle damage ultimately results in oxidative stress, inflammation, and apoptosis. The induction of oxidative stress results in part by the decoupling eNOS, which increases the generation of reactive oxygen species [56,64]. Gb3 accumulation also correlates with an increase in the release of the inflammatory cytokines IL-1β, IL-6, and TNF-α in patient plasma [65,66], with TNF-α accumulation being especially pronounced in patients experiencing pain crises [67]. Together, the increased oxidant burden and proinflammatory state associated with substrate accumulation likely contributes to symptom development through multiple mechanisms. Proinflammatory cytokines have been implicated as a predisposing factor for thrombosis [68], stimulating the release of soluble prothrombotic markers, such as von Willebrand factor, and upregulating the expression of endothelial adhesion molecules [69,70]. In fact, both increases in von Willebrand factor and the expression of endothelial adhesion molecules have been observed in patients and mouse models of the disease [71,72]. Gb3 accumulation also promotes cell death pathways [73]. In cell types with limited regenerative abilities (e.g., neurons, podocytes), the Gb3-mediated stimulation of apoptotic pathways may contribute to the characteristic symptoms (e.g., pain, podocyte disease) experienced by patients. As mesenchymal stem cells in particular have increased rates of apoptosis and senescence [73], Gb3 accumulation in the bone marrow could also alter normal hematopoiesis.
The Gb3 metabolite, lyso-Gb3, has been shown to play important roles in disease pathology. Lyso-Gb3 is formed by the deacylation of Gb3 by acid ceramidase [74] (Figure 1). The accumulation of lyso-Gb3 exacerbates disease pathology as it both inhibits α-Gal A activity and promotes the proliferation of smooth muscle cells [31], a factor that likely contributes to the increased intima-media thickness observed in Fabry patients [75]. Lyso-Gb3 has been shown to promote inflammatory signaling in cultured podocytes [76,77] and may also directly sensitize nociceptors [78], contributing to the renal disease and neuropathic pain experienced by patients, respectively. Overall, the deleterious mechanisms of α-Gal A substrate storage outlined here are likely both substrate and cell-type specific, complicating our understanding of the molecular pathways by which Gb3 storage initiates and maintains the signs and symptoms observed in patients.
Rodent models
α-Gal A-deficient rodent models have been generated to study Fabry disease pathogenesis (Table 1). The first published report of a Fabry mouse (Gla KO) model was in 1997 by the groups of Roscoe Brady and Ashok Kulkarni at the National Institutes of Health [79]. In their first study, the authors reported that while Fabry mice appeared clinically normal at 10 weeks of age, microscopic and biochemical evidence of glycosphingolipid storage was evident [79]. The authors subsequently examined aging Fabry mice; however, they concluded that no clinical signs of Fabry disease or organ failure were evident in aged (80-week-old) Fabry mice [13]. Another group independently generated a Gla KO mouse model and also found no organ failure in aged mice [7]. Subsequent Fabry mouse studies documented neuronal glycosphingolipid storage and altered somatosensory phenotypes in Fabry mice, which are indicative of a neuropathic pain. These studies, however, show conflicting results [80–83]. The unavailability of strain-matched, wild type control mice likely contributes to the discrepancies. Nevertheless, the presence of biochemical and microscopic phenotypes in Fabry mice was critical in the development of enzyme replacement [84] and chaperone therapies [85].
A hypothesis to explain the asymptomatic Fabry mouse is that glycosphingolipid profiles are different between mice and humans. In fact, levels of red blood cell globosides, including globotetraosylceramide (Gb4), Gb3, and lactosylceramide, are much lower in mice than humans [13]. In an attempt to elicit phenotypes, one group crossed a transgenic mouse overexpressing Gb3 synthase (Gb3Stg) [86] with the Gla KO mouse. The resulting Gla KO/Gb3Stg mice developed a significant neurological phenotype characterized by spontaneous tremors, slowed movements, gait disturbances, and a rounded back [87]. Further, Gla KO/Gb3Stg mice had a median survival of 27 weeks, with all mice spontaneously dying by 35 weeks of age. Given that patients do not experience the observed neurological phenotypes to the same degree and do not suffer from an extremely reduced life expectancy, it appears that the phenotypes in Gla KO/Gb3Stg mice may limit the translational relevance of this Gla KO/Gb3Stg mouse model in therapeutic studies.
Given the limitations of Fabry mouse models, our lab developed a Fabry rat model. This rat model was generated using CRISPR/Cas9 technology to delete the rat Gla gene. As such, littermate-matched wild type controls are available to compare with KO rats. Fabry rats are completely deficient in α-Gal A activity and demonstrate accumulation of α-galactosyl glycosphingolipids in all tissues analyzed. We showed that Fabry rats develop pain-like behavior and that alterations in the cation channel, transient receptor potential ankyrin 1, may contribute to the development of neuropathic pain [9]. In addition, we found a significant decrease in various complex and hybrid N-glycans, including sulfated and sialated hybrid N-glycans, in Fabry rat dorsal root ganglia [9]. This observation suggests α-Gal A deficiency affects N-glycan processing within the Golgi, possibly by substrate accumulation within the membranes of this organelle (Figure 2). We also demonstrated that Fabry rats recapitulate cardiorenal phenotypes documented in patients, such as renal tubule dysfunction and mitral valve thickening [14]. Fabry rats develop corneal and lenticular opacities, a common finding in patients [10]. Our preliminary studies also reveal that Fabry rats experience progressive hearing loss as they age. Studies are currently underway to further characterize phenotypes in Fabry rats and understand how glycosphingolipid accumulation results in these phenotypes. The symptomatic Fabry rat model is a useful adjunct to existing Fabry mouse models in continuing efforts to understand disease pathogenesis and to test new and existing therapies.
Therapies
Currently, two therapeutic modalities are available clinically for the treatment of Fabry disease: enzyme replacement therapy and chaperone therapy. Other strategies, such as substrate reduction therapy, mRNA-based therapy, and gene therapy are in development (Figure 1). Enzyme replacement therapy, which consists of systemic α-Gal A infusion, was the first approved treatment for Fabry disease. There are two available pharmaceutical preparations of recombinant human α-Gal A: 1) agalsidase alfa (Replagal by Shire) is produced by overexpression in human fibroblasts, and 2) agalsidase beta (Fabrazyme by Sanofi Genzyme) is produced by overexpression in CHO cells. Both preparations have similar glycosylation patterns, specific activities, and enzyme kinetics [34], and both have been shown to be clinically efficacious [88,89]. However, there is a 5-fold dose discrepancy as agalsidase alfa is approved at 0.2 mg/kg biweekly, and agalsidase beta is approved at 1 mg/kg biweekly [90]. Only agalsidase beta is currently available in the United States, initially approved by the FDA in 2003. Agalsidase alfa is available in several locations outside of the United States, such as the European Union, Canada, Australia, Mexico, and South American countries. Currently, pegunigalsidase alfa (PRX-102; a covalently crosslinked, PEGylated form of α-Gal A), is being evaluated for safety and efficacy in clinical trials (, active; , active; , recruiting). There remains an ongoing debate in the field concerning the optimal age of enzyme replacement therapy initiation, but it is suggested that earlier treatment results in better outcomes [91]. Additionally, a recent 5-year study of male patients aged 5–18 years supports the efficacy of agalsidase beta at the FDA-approved 1 mg/kg biweekly treatment rather than at a reduced dosage [92].
Despite the availability of enzyme replacement therapy, challenges remain for its use in Fabry disease. Enzyme replacement therapy is time consuming (i.e., hours required for infusion) and expensive (~$200,000 per patient annually), placing a significant burden on patients and the healthcare system. Further, enzyme replacement therapy may cause infusion reactions and is not effective in all patients, such as those with end-stage organ disease [93] or with antibodies to the recombinant enzyme [94]. Therefore, the development of improved treatment options is an important goal for many researchers in academia and industry.
Molecular chaperones are another promising therapeutic avenue for Fabry disease treatment. Some patients have single point mutations that result in misfolded α-Gal A. While the mutated enzyme may possess some residual activity, it may be prematurely destroyed by ER-associated protein degradation. Thus, promoting enzyme stability, such as by a small molecule chaperone, may serve as a treatment. The concept is that a ligand (i.e., molecular chaperone) of α-Gal A may occupy its active site, thereby promoting enzyme folding and stability. Once the α-Gal A-chaperone complex enters the lysosome, the chaperone is dissociated from the enzyme due to pH-sensitive conformational changes and α-Gal A is free to act on glycosphingolipid substrates [95]. In order for chaperone therapy to be effective, patients must have amenable mutations (i.e., non-null α-Gal A activity that can be improved by the chaperone). Currently, the chaperone, migalastat (Galafold by Amicus Therapeutics) shows clinical efficacy in patients with amenable GLA mutations [96,97] and this orally administered small molecule drug was recently approved for use in the United States.
Substrate reduction therapy is another option currently under investigation. Two small molecules are being or are about to be tested in clinical trials: ibiglustat (; completed) and lucerastat (; recruiting) (reviewed in [98]). Both ibiglustat and lucerastat inhibit glucosylceramide synthase, the enzyme that adds glucose to ceramide (Figure 1). Because glucosylceramide is a common precursor in the synthesis of many glycosphingolipids (e.g., globosides, gangliosides, lactosides, sulfatides), glucosylceramide synthase inhibition results in decreased Gb3 synthesis.
Glucosylceramide synthase inhibitors have been tested preclinically in Fabry cell and mouse models and in clinical trials for patients with Gaucher disease (glucosylceramidase deficiency). In patient-derived lymphoblasts, glucosylceramide synthase inhibitors depleted Gb3 by 70–80% and reduced Gb3 levels below those of controls in α-Gal A deficient mice [99,100]. Clinical studies with miglustat, an inhibitor of glucosylceramide synthase, reported significant adverse events, such as weight loss, diarrhea, poor appetite, and tremor [101,102]. These side effects are probably due to the fact that miglustat more potently inhibits other enzymes, such as lysosomal and non-lysosomal β-glucosylceramidase and intestinal disaccharidases (reviewed in [103]). Recent clinical trials using the more selective glucosylceramide synthase inhibitor, eliglustat, provide support for the safety and efficacy of substrate reduction using more specific inhibitors; however, some patients experienced mild-to-moderate abdominal pain, diarrhea, and abnormal nerve conduction studies [104]. As gastrointestinal distress and peripheral neuropathy are dominant symptoms in Fabry disease, the effects of glucosylceramide synthase inhibition need to be further evaluated in patients with Fabry disease.
Glucosylceramide synthase inhibition also depletes glucosylceramide and lactosylceramide, glycosphingolipids which do not accumulate in Fabry disease. Moreover, gangliosides are highly abundant in the nervous system and are important for neuron function (reviewed in [105]). Because lactosylceramide serves as the critical precursor to gangliosides, lactosylceramide depletion might contribute to the neurological side effects (i.e., tremor, abnormal nerve conduction studies) observed with these drugs. Therefore, substrate reduction therapy that specifically targets Gb3 synthesis and avoids depletion of lactosylceramide may be a better option in these patients (Figure 1).
There are several other Fabry disease therapies on the horizon. Gene therapy will likely be a future therapeutic option for patients with Fabry disease (reviewed in [106]). In two trials ( and ), CD34+ stem cells are obtained from a patient and are engineered to express α-Gal A using a lentivirus vector. The transduced cells are then transplanted back into the same patient (i.e., autologous stem cell transplantation) with the goal that secreted α-Gal A will be taken up by the patient’s other cells. Adeno-associated virus capsids are also being evaluated for gene therapy, and novel capsids are in development to improve α-Gal A expression in kidney, heart, and brain [107]. Moving forward, gene therapy for Fabry disease has the potential to systemically express α-Gal A in a manner that would be effective for the large number of genetic mutations that cause Fabry disease.
mRNA-based therapies are also likely to become available for Fabry disease as studies in Fabry mice and non-human primates were recently reported [108,109]. This is an attractive option because the translated α-Gal A would express native post-translational modifications, such as mannose 6-phosphate on N-glycans for lysosomal targeting. With new therapeutic options becoming available, we may discover that combination therapies provide maximal benefit. For example, recent studies show that chaperone therapy coupled with enzyme replacement therapy is more efficacious than either option alone [110,111].
Conclusion
The field has made remarkable progress in our understanding and treatment of Fabry disease. However, an incomplete understanding of disease pathogenesis still limits our ability to effectively treat patients with this debilitating disease. Enzyme replacement therapy and chaperone therapy are currently approved for Fabry disease; however, these treatments are not curative and at best slow progression of the disease. Improvements to these therapies and many other therapeutic options are in the pipeline. Existing treatments can be optimized in animal models, which are now available in both the mouse and rat. Further, new treatments can be conceived and developed as we continue to learn about disease pathogenesis using these models. These efforts offer hope to the patients who suffer.
Highlights.
Fabry disease is now recognized as the most common lysosomal storage disease
Patients experience neuropathic pain and kidney, heart, and cerebrovascular disease
The mechanisms of disease pathogenesis are incompletely understood
Mouse and rat models are available to understand pathogenesis and test therapies
Enzyme and chaperone therapies are approved, and new treatments are in trials
Acknowledgements
This work was supported by National Institutes of Health grants R01DK042667, K12HL141954, and R21NS095627 (to NMD). JJM is supported by a National Research Service Award (F30DK113641) and is a member of the Medical Scientist Training Program, which is partially supported by T32GM080202. AJK is supported by a career development program in translational glycosciences (K12HL141954). The Fabry rat model was generated under NIH resource grant, R24HL114474. The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1.].Anderson W. A case of “angio-keratoma”. Br J Dermatol. 1898;10(4):113–7. doi: doi: 10.1111/j.1365-2133.1898.tb16317.x. [DOI] [Google Scholar]
- [2.].Fabry J. Ein Beitrag zur Kenntnis der Purpura haemorrhagica nodularis (Purpura papulosa haemorrhagica Hebrae). Archiv für Dermatologie und Syphilis, Berlin. 1898;43:187–200. [Google Scholar]
- [3.].Sweeley CC, Klionsky B. Fabry’s Disease: Classification as a Sphingolipidosis and Partial Characterization of a Novel Glycolipid. J Biol Chem. 1963;238:3148–50. [PubMed] [Google Scholar]
- [4.].Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276(21):1163–7. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- [5.].Uceyler N, Ganendiran S, Kramer D, Sommer C. Characterization of pain in fabry disease. Clin J Pain. 2014;30(10):915–20. doi: 10.1097/AJP.0000000000000041. [DOI] [PubMed] [Google Scholar]
- [6.].Samiy N. Ocular features of Fabry disease: diagnosis of a treatable life-threatening disorder. Surv Ophthalmol. 2008;53(4):416–23. doi: 10.1016/j.survophthal.2008.04.005. [DOI] [PubMed] [Google Scholar]
- [7.].Bangari DS, Ashe KM, Desnick RJ, Maloney C, Lydon J, Piepenhagen P, Budman E, Leonard JP, Cheng SH, Marshall J, Thurberg BL. alpha-Galactosidase A Knockout Mice: Progressive Organ Pathology Resembles the Type 2 Later-Onset Phenotype of Fabry Disease. Am J Pathol. 2015;185(3):651–65. doi: 10.1016/j.ajpath.2014.11.004. [DOI] [PubMed] [Google Scholar]
- [8.].Hofmann L, Hose D, Griesshammer A, Blum R, Doring F, Dib-Hajj S, Waxman S, Sommer C, Wischmeyer E, Uceyler N. Characterization of small fiber pathology in a mouse model of Fabry disease. Elife. 2018;7 Epub 2018/10/18. doi: 10.7554/eLife.39300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9.].Miller JJ, Aoki K, Moehring F, Murphy CA, O’Hara CL, Tiemeyer M, Stucky CL, Dahms NM. Neuropathic pain in a Fabry disease rat model. JCI Insight. 2018;3(6):e99171. doi: 10.1172/jci.insight.99171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10.].Miller JJ, Aoki K, Reid CA, Tiemeyer M, Dahms NM, Kassem IS. Rats deficient in alpha-galactosidase A develop ocular manifestations of Fabry disease. Scientific reports. 2019;9(1):9392 Epub 2019/06/30. doi: 10.1038/s41598-019-45837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11.].Noben-Trauth K, Neely H, Brady RO. Normal hearing in alpha-galactosidase A-deficient mice, the mouse model for Fabry disease. Hear Res. 2007;234(1–2):10–4. Epub 2007/10/16. doi: 10.1016/j.heares.2007.08.009. [DOI] [PubMed] [Google Scholar]
- [12.].Sakurai Y, Suzuki R, Yoshida R, Kojima H, Watanabe M, Manome Y, Ohashi T, Eto Y, Moriyama H. Inner ear pathology of alpha-galactosidase A deficient mice, a model of Fabry disease. Auris Nasus Larynx. 2010;37(3):274–80. Epub 2009/11/11. doi: 10.1016/j.anl.2009.08.005. [DOI] [PubMed] [Google Scholar]
- [13.].Ohshima T, Schiffmann R, Murray GJ, Kopp J, Quirk JM, Stahl S, Chan CC, Zerfas P, Tao-Cheng JH, Ward JM, Brady RO, Kulkarni AB. Aging accentuates and bone marrow transplantation ameliorates metabolic defects in Fabry disease mice. Proc Natl Acad Sci U S A. 1999;96(11):6423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14.].Miller JJ, Aoki K, Mascari CA, Beltrame AK, Sokumbi O, North PE, Tiemeyer M, Kriegel AJ, Dahms NM. alpha-Galactosidase A-deficient rats accumulate glycosphingolipids and develop cardiorenal phenotypes of Fabry disease. FASEB J. 2019;33(1):418–29. Epub 2018/07/07. doi: 10.1096/fj.201800771R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15.].Nguyen Dinh Cat A, Escoubet B, Agrapart V, Griol-Charhbili V, Schoeb T, Feng W, Jaimes E, Warnock DG, Jaisser F. Cardiomyopathy and response to enzyme replacement therapy in a male mouse model for Fabry disease. PLoS One. 2012;7(5):e33743. doi: 10.1371/journal.pone.0033743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16.].Germain DP, Benistan K, Boutouyrie P, Mutschler C. Osteopenia and osteoporosis: previously unrecognized manifestations of Fabry disease. Clin Genet. 2005;68(1):93–5. doi: 10.1111/j.1399-0004.2005.00457.x. [DOI] [PubMed] [Google Scholar]
- [17.].Mersebach H, Johansson JO, Rasmussen AK, Bengtsson BA, Rosenberg K, Hasholt L, Sorensen SA, Sorensen SS, Feldt-Rasmussen U. Osteopenia: a common aspect of Fabry disease. Predictors of bone mineral density. Genet Med. 2007;9(12):812–8. doi: 10.1097GIM.0b013e31815cb197. [DOI] [PubMed] [Google Scholar]
- [18.].Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;5:30. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19.].Lohle M, Hughes D, Milligan A, Richfield L, Reichmann H, Mehta A, Schapira AH. Clinical prodromes of neurodegeneration in Anderson-Fabry disease. Neurology. 2015;84(14):1454–64. doi: 10.1212/WNL.0000000000001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20.].Gold KF, Pastores GM, Botteman MF, Yeh JM, Sweeney S, Aliski W, Pashos CL. Quality of life of patients with Fabry disease. Qual Life Res. 2002;11(4):317–27. [DOI] [PubMed] [Google Scholar]
- [21.].Vedder AC, Linthorst GE, van Breemen MJ, Groener JE, Bemelman FJ, Strijland A, Mannens MM, Aerts JM, Hollak CE. The Dutch Fabry cohort: diversity of clinical manifestations and Gb3 levels. J Inherit Metab Dis. 2007;30(1):68–78. doi: 10.1007/s10545-006-0484-8. [DOI] [PubMed] [Google Scholar]
- [22.].MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001;38(11):769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23.].Mehta A, Hughes DA. Fabry Disease. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K Seattle (WA): University of Washington; 2002. August 5 [Updated 2017 Jan 5]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20301469. [Google Scholar]
- [24.].Spada M, Pagliardini S, Yasuda M, Tukel T, Thiagarajan G, Sakuraba H, Ponzone A, Desnick RJ. High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet. 2006;79(1):31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25.].Hwu WL, Chien YH, Lee NC, Chiang SC, Dobrovolny R, Huang AC, Yeh HY, Chao MC, Lin SJ, Kitagawa T, Desnick RJ, Hsu LW. Newborn screening for Fabry disease in Taiwan reveals a high incidence of the later-onset GLA mutation c.936+919G>A (IVS4+919G>A). Hum Mutat. 2009;30(10):1397–405. doi: 10.1002/humu.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26.].Elliott S, Buroker N, Cournoyer JJ, Potier AM, Trometer JD, Elbin C, Schermer MJ, Kantola J, Boyce A, Turecek F, Gelb MH, Scott CR. Pilot study of newborn screening for six lysosomal storage diseases using Tandem Mass Spectrometry. Mol Genet Metab. 2016;118(4):304–9. doi: 10.1016/j.ymgme.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27.].Burton BK, Charrow J, Hoganson GE, Waggoner D, Tinkle B, Braddock SR, Schneider M, Grange DK, Nash C, Shryock H, Barnett R, Shao R, Basheeruddin K, Dizikes G. Newborn Screening for Lysosomal Storage Disorders in Illinois: The Initial 15-Month Experience. J Pediatr. 2017;190:130–5. doi: 10.1016/j.jpeds.2017.06.048. [DOI] [PubMed] [Google Scholar]
- [28.].Cooper DN, Ball EV, Stenson PD, Phillips AD, Evans K, Heywood S, Hayden MJ, Azevedo L, Mort ME, Hussain M. Human Gene Mutation Database [Last accessed: 09/08/19]. Available from: http://www.hgmd.cf.ac.uk/ac/index.php.
- [29.].Oder D, Vergho D, Ertl G, Wanner C, Nordbeck P. Case report of a 45-year old female Fabry disease patient carrying two alpha-galactosidase A gene mutation alleles. BMC Medical Genetics. 2016;17(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30.].Rodriguez-Mari A, Coll MJ, Chabas A. Molecular analysis in Fabry disease in Spain: fifteen novel GLA mutations and identification of a homozygous female. Hum Mutat. 2003;22(3):258. doi: 10.1002/humu.9172. [DOI] [PubMed] [Google Scholar]
- [31.].Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, Ottenhoff R, van Roomen C, Mirzaian M, Wijburg FA, Linthorst GE, Vedder AC, Rombach SM, Cox-Brinkman J, Somerharju P, Boot RG, Hollak CE, Brady RO, Poorthuis BJ. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A. 2008;105(8):2812–7. doi: 10.1073/pnas.0712309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32.].Sakuraba H, Togawa T, Tsukimura T, Kato H. Plasma lyso-Gb3: a biomarker for monitoring fabry patients during enzyme replacement therapy. Clin Exp Nephrol. 2018;22(4):843–9. Epub 2017/12/31. doi: 10.1007/s10157-017-1525-3. [DOI] [PubMed] [Google Scholar]
- [33.].Garman SC, Garboczi DN. The molecular defect leading to Fabry disease: structure of human alpha-galactosidase. J Mol Biol. 2004;337(2):319–35. doi: 10.1016/j.jmb.2004.01.035. [DOI] [PubMed] [Google Scholar]
- [34.].Lee K, Jin X, Zhang K, Copertino L, Andrews L, Baker-Malcolm J, Geagan L, Qiu H, Seiger K, Barngrover D, McPherson JM, Edmunds T. A biochemical and pharmacological comparison of enzyme replacement therapies for the glycolipid storage disorder Fabry disease. Glycobiology. 2003;13(4):305–13. doi: 10.1093/glycob/cwg034. [DOI] [PubMed] [Google Scholar]
- [35.].Caval T, Zhu J, Tian W, Remmelzwaal S, Yang Z, Clausen H, Heck AJR. Targeted Analysis of Lysosomal Directed Proteins and Their Sites of Mannose-6-phosphate Modification. Mol Cell Proteomics. 2019;18(1):16–27. Epub 2018/09/22. doi: 10.1074/mcp.RA118.000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36.].Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4(3):202–12. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- [37.].Dean KJ, Sung SS, Sweeley CC. The identification of alpha-galactosidase B from human liver as an alpha-N-acetylgalactosaminidase. Biochem Biophys Res Commun. 1977;77(4):1411–7. [DOI] [PubMed] [Google Scholar]
- [38.].Carbohydrate Active enZYmes Database [Last accessed: 09/06/19]. Available from: http://www.cazy.org/GH27.html.
- [39.].Dean KJ, Sweeley CC. Studies on human liver alpha-galactosidases. II. Purification and enzymatic properties of alpha-galactosidase B (alpha-N-acetylgalactosaminidase). J Biol Chem. 1979;254(20):10001–5. [PubMed] [Google Scholar]
- [40.].Asfaw B, Ledvinova J, Dobrovolny R, Bakker HD, Desnick RJ, van Diggelen OP, de Jong JG, Kanzaki T, Chabas A, Maire I, Conzelmann E, Schindler D. Defects in degradation of blood group A and B glycosphingolipids in Schindler and Fabry diseases. J Lipid Res. 2002;43(7):1096–104. [DOI] [PubMed] [Google Scholar]
- [41.].Keusch JJ, Manzella SM, Nyame KA, Cummings RD, Baenziger JU. Cloning of Gb3 synthase, the key enzyme in globo-series glycosphingolipid synthesis, predicts a family of alpha 1, 4-glycosyltransferases conserved in plants, insects, and mammals. J Biol Chem. 2000;275(33):25315–21. doi: 10.1074/jbc.M002630200. [DOI] [PubMed] [Google Scholar]
- [42.].Schnaar RL, Kinoshita T. Glycosphingolipids In: rd, Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH, editors. Essentials of Glycobiology. Cold Spring Harbor; (NY: )2015. p. 125–35. [PubMed] [Google Scholar]
- [43.].Hellberg A, Westman JS, Thuresson B, Olsson ML. P1PK: the blood group system that changed its name and expanded. Immunohematology. 2013;29(1):25–33. [PubMed] [Google Scholar]
- [44.].Kojima Y, Fukumoto S, Furukawa K, Okajima T, Wiels J, Yokoyama K, Suzuki Y, Urano T, Ohta M, Furukawa K. Molecular cloning of globotriaosylceramide/CD77 synthase, a glycosyltransferase that initiates the synthesis of globo series glycosphingolipids. J Biol Chem. 2000;275(20):15152–6. doi: 10.1074/jbc.M909620199. [DOI] [PubMed] [Google Scholar]
- [45.].Steffensen R, Carlier K, Wiels J, Levery SB, Stroud M, Cedergren B, Nilsson Sojka B, Bennett EP, Jersild C, Clausen H. Cloning and expression of the histo-blood group Pk UDP-galactose: Ga1beta-4G1cbeta1-cer alpha1, 4-galactosyltransferase. Molecular genetic basis of the p phenotype. J Biol Chem. 2000;275(22):16723–9. doi: 10.1074/jbc.M000728200. [DOI] [PubMed] [Google Scholar]
- [46.].Lingwood CA, Binnington B, Manis A, Branch DR. Globotriaosyl ceramide receptor function - where membrane structure and pathology intersect. FEBS Lett. 2010;584(9):1879–86. doi: 10.1016/j.febslet.2009.11.089. [DOI] [PubMed] [Google Scholar]
- [47.].Lindberg AA, Brown JE, Stromberg N, Westling-Ryd M, Schultz JE, Karlsson KA. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987;262(4):1779–85. [PubMed] [Google Scholar]
- [48.].Nizet V, Varki A, Aebi M. Microbial Lectins: Hemagglutinins, Adhesins, and Toxins In: rd, Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH, editors. Essentials of Glycobiology. Cold Spring Harbor; (NY: )2015. p. 481–91. [PubMed] [Google Scholar]
- [49.].Okuda T, Tokuda N, Numata S, Ito M, Ohta M, Kawamura K, Wiels J, Urano T, Tajima O, Furukawa K, Furukawa K. Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. J Biol Chem. 2006;281(15):10230–5. doi: 10.1074/jbc.M600057200. [DOI] [PubMed] [Google Scholar]
- [50.].Cilmi SA, Karalius BJ, Choy W, Smith RN, Butterton JR. Fabry disease in mice protects against lethal disease caused by Shiga toxin-expressing enterohemorrhagic Escherichia coli. J Infect Dis. 2006;194(8):1135–40. doi: 10.1086/507705. [DOI] [PubMed] [Google Scholar]
- [51.].Elleder M. Subcellular, cellular, and organ pathology of Fabry disease In: Elstein D, Altarescu G, Beck M, editors. Fabry disease: Springer; 2010. p. 39–79. [Google Scholar]
- [52.].Weidemann F, Sanchez-Nino MD, Politei J, Oliveira JP, Wanner C, Warnock DG, Ortiz A. Fibrosis: a key feature of Fabry disease with potential therapeutic implications. Orphanet J Rare Dis. 2013;8:116. doi: 10.1186/1750-1172-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53.].Rozenfeld P, Feriozzi S. Contribution of inflammatory pathways to Fabry disease pathogenesis. Mol Genet Metab. 2017;122(3):19–27. doi: 10.1016/j.ymgme.2017.09.004. [DOI] [PubMed] [Google Scholar]
- [54.].Shen JS, Meng XL, Moore DF, Quirk JM, Shayman JA, Schiffmann R, Kaneski CR. Globotriaosylceramide induces oxidative stress and up-regulates cell adhesion molecule expression in Fabry disease endothelial cells. Molecular Genetics & Metabolism. 2008;95(3):163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55.].Meng XL, Arning E, Wight-Carter M, Day TS, Jabbarzadeh-Tabrizi S, Chen S, Ziegler RJ, Bottiglieri T, Schneider JW, Cheng SH, Schiffmann R, Shen JS. Priapism in a Fabry disease mouse model is associated with upregulated penile nNOS and eNOS expression. J Inherit Metab Dis. 2018;41(2):231–8. doi: 10.1007/s10545-017-0107-6. [DOI] [PubMed] [Google Scholar]
- [56.].Shu L, Vivekanandan-Giri A, Pennathur S, Smid BE, Aerts JM, Hollak CE, Shayman JA. Establishing 3-nitrotyrosine as a biomarker for the vasculopathy of Fabry disease. Kidney Int. 2014;86(1):58–66. doi: 10.1038/ki.2013.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57.].Shu L, Shayman JA. Caveolin-associated accumulation of globotriaosylceramide in the vascular endothelium of alpha-galactosidase A null mice. J Biol Chem. 2007;282(29):20960–7. doi: 10.1074/jbc.M702436200. [DOI] [PubMed] [Google Scholar]
- [58.].Thomaidis T, Relle M, Golbas M, Brochhausen C, Galle PR, Beck M, Schwarting A. Downregulation of alpha-galactosidase A upregulates CD77: functional impact for Fabry nephropathy. Kidney Int. 2009;75(4):399–407. doi: 10.1038/ki.2008.576. [DOI] [PubMed] [Google Scholar]
- [59.].Chevrier M, Brakch N, Celine L, Genty D, Ramdani Y, Moll S, Djavaheri-Mergny M, Brasse-Lagnel C, Annie Laquerriere AL, Barbey F, Bekri S. Autophagosome maturation is impaired in Fabry disease. Autophagy. 2010;6(5):589–99. doi: 10.4161/auto.6.5.11943. [DOI] [PubMed] [Google Scholar]
- [60.].Liebau MC, Braun F, Hopker K, Weitbrecht C, Bartels V, Muller RU, Brodesser S, Saleem MA, Benzing T, Schermer B, Cybulla M, Kurschat CE. Dysregulated autophagy contributes to podocyte damage in Fabry’s disease. PLoS One. 2013;8(5):e63506. doi: 10.1371/journal.pone.0063506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61.].Kaushal GP. Autophagy protects proximal tubular cells from injury and apoptosis. Kidney Int. 2012;82(12):1250–3. Epub 2012/12/04. doi: 10.1038/ki.2012.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62.].Lucke T, Hoppner W, Schmidt E, Illsinger S, Das AM. Fabry disease: reduced activities of respiratory chain enzymes with decreased levels of energy-rich phosphates in fibroblasts. Mol Genet Metab. 2004;82(1):93–7. doi: 10.1016/j.ymgme.2004.01.011. [DOI] [PubMed] [Google Scholar]
- [63.].Heo SH, Kang E, Kim YM, Go H, Kim KY, Jung JY, Kang M, Kim GH, Kim JM, Choi IH, Choi JH, Jung SC, Desnick RJ, Yoo HW, Lee BH. Fabry disease: characterisation of the plasma proteome pre- and post-enzyme replacement therapy. J Med Genet. 2017;54(11):771–80. Epub 2017/08/25. doi: 10.1136/jmedgenet-2017-104704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64.].Moore DF, Scott LT, Gladwin MT, Altarescu G, Kaneski C, Suzuki K, Pease-Fye M, Ferri R, Brady RO, Herscovitch P, Schiffmann R. Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: reversal by enzyme replacement therapy. Circulation. 2001;104(13):1506–12. Epub 2001/09/26. doi: 10.1161/hc3801.096352. [DOI] [PubMed] [Google Scholar]
- [65.].De Francesco PN, Mucci JM, Ceci R, Fossati CA, Rozenfeld PA. Fabry disease peripheral blood immune cells release inflammatory cytokines: role of globotriaosylceramide. Mol Genet Metab. 2013;109(1):93–9. Epub 2013/03/05. doi: 10.1016/j.ymgme.2013.02.003. [DOI] [PubMed] [Google Scholar]
- [66.].Biancini GB, Vanzin CS, Rodrigues DB, Deon M, Ribas GS, Barschak AG, Manfredini V, Netto CB, Jardim LB, Giugliani R, Vargas CR. Globotriaosylceramide is correlated with oxidative stress and inflammation in Fabry patients treated with enzyme replacement therapy. Biochim Biophys Acta. 2012;1822(2):226–32. Epub 2011/11/17. doi: 10.1016/j.bbadis.2011.11.001. [DOI] [PubMed] [Google Scholar]
- [67.].Uceyler N, Urlaub D, Mayer C, Uehlein S, Held M, Sommer C. Tumor necrosis factor-alpha links heat and inflammation with Fabry pain. Mol Genet Metab. 2019. Epub 2019/06/22. doi: 10.1016/j.ymgme.2019.05.009. [DOI] [PubMed]
- [68.].Mosevoll KA, Johansen S, Wendelbo O, Nepstad I, Bruserud O, Reikvam H. Cytokines, Adhesion Molecules, and Matrix Metalloproteases as Predisposing, Diagnostic, and Prognostic Factors in Venous Thrombosis. Front Med (Lausanne). 2018;5:147 Epub 2018/06/07. doi: 10.3389/fmed.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69.].Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104(1):100–6. Epub 2004/03/18. doi: 10.1182/blood-2004-01-0107. [DOI] [PubMed] [Google Scholar]
- [70.].Xia P, Gamble JR, Rye KA, Wang L, Hii CS, Cockerill P, Khew-Goodall Y, Bert AG, Barter PJ, Vadas MA. Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci U S A. 1998;95(24):14196–201. Epub 1998/11/25. doi: 10.1073/pnas.95.24.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71.].Kang JJ, Kaissarian NM, Desch KC, Kelly RJ, Shu L, Bodary PF, Shayman JA. alpha-galactosidase A deficiency promotes von Willebrand factor secretion in models of Fabry disease. Kidney Int. 2019;95(1):149–59. Epub 2018/11/25. doi: 10.1016/j.kint.2018.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72.].DeGraba T, Azhar S, Dignat-George F, Brown E, Boutiere B, Altarescu G, McCarron R, Schiffmann R. Profile of endothelial and leukocyte activation in Fabry patients. Ann Neurol. 2000;47(2):229–33. [PubMed] [Google Scholar]
- [73.].Squillaro T, Antonucci I, Alessio N, Esposito A, Cipollaro M, Melone MAB, Peluso G, Stuppia L, Galderisi U. Impact of lysosomal storage disorders on biology of mesenchymal stem cells: Evidences from in vitro silencing of glucocerebrosidase (GBA) and alpha-galactosidase A (GLA) enzymes. J Cell Physiol. 2017;232(12):3454–67. doi: 10.1002/jcp.25807. [DOI] [PubMed] [Google Scholar]
- [74.].Ferraz MJ, Marques AR, Appelman MD, Verhoek M, Strijland A, Mirzaian M, Scheij S, Ouairy CM, Lahav D, Wisse P, Overkleeft HS, Boot RG, Aerts JM. Lysosomal glycosphingolipid catabolism by acid ceramidase: formation of glycosphingoid bases during deficiency of glycosidases. FEBS Lett. 2016;590(6):716–25. doi: 10.1002/1873-3468.12104. [DOI] [PubMed] [Google Scholar]
- [75.].Kalliokoski RJ, Kantola I, Kalliokoski KK, Engblom E, Sundell J, Hannukainen JC, Janatuinen T, Raitakari OT, Knuuti J, Penttinen M, Viikari J, Nuutila P. The effect of 12-month enzyme replacement therapy on myocardial perfusion in patients with Fabry disease. J Inherit Metab Dis. 2006;29(1):112–8. Epub 2006/04/08. doi: 10.1007/s10545-006-0221-3. [DOI] [PubMed] [Google Scholar]
- [76.].Sanchez-Nino MD, Sanz AB, Carrasco S, Saleem MA, Mathieson PW, Valdivielso JM, Ruiz-Ortega M, Egido J, Ortiz A. Globotriaosylsphingosine actions on human glomerular podocytes: implications for Fabry nephropathy. Nephrol Dial Transplant. 2011;26(6):1797–802. doi: 10.1093/ndt/gfq306. [DOI] [PubMed] [Google Scholar]
- [77.].Sanchez-Nino MD, Carpio D, Sanz AB, Ruiz-Ortega M, Mezzano S, Ortiz A. Lyso-Gb3 activates Notch1 in human podocytes. Hum Mol Genet. 2015;24(20):5720–32. doi: 10.1093/hmg/ddv291. [DOI] [PubMed] [Google Scholar]
- [78.].Choi L, Vernon J, Kopach O, Minett MS, Mills K, Clayton PT, Meert T, Wood JN. The Fabry disease-associated lipid Lyso-Gb3 enhances voltage-gated calcium currents in sensory neurons and causes pain. Neurosci Lett. 2015;594:163–8. doi: 10.1016/j.neulet.2015.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79.].Ohshima T, Murray GJ, Swaim WD, Longenecker G, Quirk JM, Cardarelli CO, Sugimoto Y, Pastan I, Gottesman MM, Brady RO, Kulkarni AB. alpha-Galactosidase A deficient mice: a model of Fabry disease. Proc Natl Acad Sci U S A. 1997;94(6):2540–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80.].Rodrigues LG, Ferraz MJ, Rodrigues D, Pais-Vieira M, Lima D, Brady RO, Sousa MM, Sa-Miranda MC. Neurophysiological, behavioral and morphological abnormalities in the Fabry knockout mice. Neurobiol Dis. 2009;33(1):48–56. doi: 10.1016/j.nbd.2008.09.001. [DOI] [PubMed] [Google Scholar]
- [81.].Lakoma J, Rimondini R, Donadio V, Liguori R, Caprini M. Pain related channels are differentially expressed in neuronal and non-neuronal cells of glabrous skin of fabry knockout male mice. PLoS One. 2014;9(10):e108641. doi: 10.1371/journal.pone.0108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82.].Lakoma J, Rimondini R, Ferrer Montiel A, Donadio V, Liguori R, Caprini M. Increased expression of Trpv1 in peripheral terminals mediates thermal nociception in Fabry disease mouse model. Mol Pain. 2016;12. doi: 10.1177/1744806916663729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83.].Uceyler N, Biko L, Hose D, Hofmann L, Sommer C. Comprehensive and differential long-term characterization of the alpha-galactosidase A deficient mouse model of Fabry disease focusing on the sensory system and pain development. Mol Pain. 2016;12:1744806916646379. doi: 10.1177/1744806916646379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84.].Ioannou YA, Zeidner KM, Gordon RE, Desnick RJ. Fabry disease: preclinical studies demonstrate the effectiveness of alpha-galactosidase A replacement in enzyme-deficient mice. Am J Hum Genet. 2001;68(1):14–25. doi: 10.1086/316953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85.].Khanna R, Soska R, Lun Y, Feng J, Frascella M, Young B, Brignol N, Pellegrino L, Sitaraman SA, Desnick RJ, Benjamin ER, Lockhart DJ, Valenzano KJ. The pharmacological chaperone 1-deoxygalactonojirimycin reduces tissue globotriaosylceramide levels in a mouse model of Fabry disease. Mol Ther. 2010;18(1):23–33. doi: 10.1038/mt.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86.].Shiozuka C, Taguchi A, Matsuda J, Noguchi Y, Kunieda T, Uchio-Yamada K, Yoshioka H, Hamanaka R, Yano S, Yokoyama S, Mannen K, Kulkarni AB, Furukawa K, Ishii S. Increased globotriaosylceramide levels in a transgenic mouse expressing human alpha1,4-galactosyltransferase and a mouse model for treating Fabry disease. J Biochem. 2011;149(2):161–70. doi: 10.1093/jb/mvq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87.].Taguchi A, Maruyama H, Nameta M, Yamamoto T, Matsuda J, Kulkarni AB, Yoshioka H, Ishii S. A symptomatic Fabry disease mouse model generated by inducing globotriaosylceramide synthesis. Biochem J. 2013;456(3):373–83. doi: 10.1042/BJ20130825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88.].Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ, International Collaborative Fabry Disease Study G. Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N Engl J Med. 2001;345(1):9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- [89.].Schiffmann R, Kopp JB, Austin HA 3rd, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285(21):2743–9. [DOI] [PubMed] [Google Scholar]
- [90.].Warnock DG, Mauer M. Fabry disease: dose matters. J Am Soc Nephrol. 2014;25(4):653–5. doi: 10.1681/ASN.2013121322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91.].Germain DP, Charrow J, Desnick RJ, Guffon N, Kempf J, Lachmann RH, Lemay R, Linthorst GE, Packman S, Scott CR, Waldek S, Warnock DG, Weinreb NJ, Wilcox WR. Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet. 2015;52(5):353–8. doi: 10.1136/jmedgenet-2014-102797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92.].Ramaswami U, Bichet DG, Clarke LA, Dostalova G, Fainboim A, Fellgiebel A, Forcelini CM, An Haack K, Hopkin RJ, Mauer M, Najafian B, Scott CR, Shankar SP, Thurberg BL, Tondel C, Tylki-Szymanska A, Benichou B, Wijburg FA. Low-dose agalsidase beta treatment in male pediatric patients with Fabry disease: A 5-year randomized controlled trial. Molecular genetics and metabolism. 2019. Epub 2019/04/17. doi: 10.1016/j.ymgme.2019.03.010. [DOI] [PubMed]
- [93.].Biegstraaten M, Arngrimsson R, Barbey F, Boks L, Cecchi F, Deegan PB, Feldt-Rasmussen U, Geberhiwot T, Germain DP, Hendriksz C, Hughes DA, Kantola I, Karabul N, Lavery C, Linthorst GE, Mehta A, van de Mheen E, Oliveira JP, Parini R, Ramaswami U, Rudnicki M, Serra A, Sommer C, Sunder-Plassmann G, Svarstad E, Sweeb A, Terryn W, Tylki-Szymanska A, Tondel C, Vujkovac B, Weidemann F, Wijburg FA, Woolfson P, Hollak CE. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: the European Fabry Working Group consensus document. Orphanet J Rare Dis. 2015;10:36. doi: 10.1186/s13023-015-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94.].van der Veen SJ, van Kuilenburg ABP, Hollak CEM, Kaijen PHP, Voorberg J, Langeveld M. Antibodies against recombinant alpha-galactosidase A in Fabry disease: Subclass analysis and impact on response to treatment. Mol Genet Metab. 2019;126(2):162–8. Epub 2018/11/27. doi: 10.1016/j.ymgme.2018.11.008. [DOI] [PubMed] [Google Scholar]
- [95.].Fan JQ, Ishii S, Asano N, Suzuki Y. Accelerated transport and maturation of lysosomal alpha-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med. 1999;5(1):112–5. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- [96.].Germain DP, Hughes DA, Nicholls K, Bichet DG, Giugliani R, Wilcox WR, Feliciani C, Shankar SP, Ezgu F, Amartino H, Bratkovic D, Feldt-Rasmussen U, Nedd K, Sharaf El Din U, Lourenco CM, Banikazemi M, Charrow J, Dasouki M, Finegold D, Giraldo P, Goker-Alpan O, Longo N, Scott CR, Torra R, Tuffaha A, Jovanovic A, Waldek S, Packman S, Ludington E, Viereck C, Kirk J, Yu J, Benjamin ER, Johnson F, Lockhart DJ, Skuban N, Castelli J, Barth J, Barlow C, Schiffmann R. Treatment of Fabry’s Disease with the Pharmacologic Chaperone Migalastat. N Engl J Med. 2016;375(6):545–55. doi: 10.1056/NEJMoa1510198. [DOI] [PubMed] [Google Scholar]
- [97.].Hughes DA, Nicholls K, Shankar SP, Sunder-Plassmann G, Koeller D, Nedd K, Vockley G, Hamazaki T, Lachmann R, Ohashi T, Olivotto I, Sakai N, Deegan P, Dimmock D, Eyskens F, Germain DP, Goker-Alpan O, Hachulla E, Jovanovic A, Lourenco CM, Narita I, Thomas M, Wilcox WR, Bichet DG, Schiffmann R, Ludington E, Viereck C, Kirk J, Yu J, Johnson F, Boudes P, Benjamin ER, Lockhart DJ, Barlow C, Skuban N, Castelli JP, Barth J, Feldt-Rasmussen U. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J Med Genet. 2017;54(4):288–96. doi: 10.1136/jmedgenet-2016-104178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98.].Platt FM. Emptying the stores: lysosomal diseases and therapeutic strategies. Nat Rev Drug Discov. 2018;17(2):133–50. doi: 10.1038/nrd.2017.214. [DOI] [PubMed] [Google Scholar]
- [99.].Abe A, Gregory S, Lee L, Killen PD, Brady RO, Kulkarni A, Shayman JA. Reduction of globotriaosylceramide in Fabry disease mice by substrate deprivation. J Clin Invest. 2000;105(11):1563–71. Epub 2000/06/07. doi: 10.1172/JCI9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100.].Abe A, Arend LJ, Lee L, Lingwood C, Brady RO, Shayman JA. Glycosphingolipid depletion in fabry disease lymphoblasts with potent inhibitors of glucosylceramide synthase. Kidney Int. 2000;57(2):446–54. Epub 2000/01/29. doi: 10.1046/j.1523-1755.2000.00864.x. [DOI] [PubMed] [Google Scholar]
- [101.].Hollak CE, Hughes D, van Schaik IN, Schwierin B, Bembi B. Miglustat (Zavesca) in type 1 Gaucher disease: 5-year results of a post-authorisation safety surveillance programme. Pharmacoepidemiol Drug Saf. 2009;18(9):770–7. doi: 10.1002/pds.1779. [DOI] [PubMed] [Google Scholar]
- [102.].Machaczka M, Hast R, Dahlman I, Lerner R, Klimkowska M, Engvall M, Hagglund H. Substrate reduction therapy with miglustat for type 1 Gaucher disease: a retrospective analysis from a single institution. Ups J Med Sci. 2012;117(1):28–34. doi: 10.3109/03009734.2011.641609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103.].Shayman JA, Larsen SD. The development and use of small molecule inhibitors of glycosphingolipid metabolism for lysosomal storage diseases. Journal of lipid research. 2014;55(7):1215–25. Epub 2014/02/19. doi: 10.1194/jlr.R047167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104.].Lukina E, Watman N, Dragosky M, Lau H, Avila Arreguin E, Rosenbaum H, Zimran A, Foster MC, Gaemers SJM, Peterschmitt MJ. Outcomes after 8 years of eliglustat therapy for Gaucher disease type 1: Final results from the Phase 2 trial. Am J Hematol. 2019;94(1):29–38. Epub 2018/09/29. doi: 10.1002/ajh.25300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105.].Jennemann R, Grone HJ. Cell-specific in vivo functions of glycosphingolipids: lessons from genetic deletions of enzymes involved in glycosphingolipid synthesis. Prog Lipid Res. 2013;52(2):231–48. doi: 10.1016/j.plipres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- [106.].Nagree MS, Scalia S, McKillop WM, Medin JA. An update on gene therapy for lysosomal storage disorders. Expert Opin Biol Ther. 2019:1–16. Epub 2019/05/07. doi: 10.1080/14712598.2019.1607837. [DOI] [PubMed]
- [107.].Kevany BK S; Padegimas L; Miller TJ. AAV gene therapy for the treatment of Fabry disease: A novel capsid with improved tropism to heart, kidney and CNS and improved GLA expression. Molecular Genetics and Metabolism. 2019;126:S83 Epub February 2019. doi: 10.1016/j.ymgme.2018.12.203. [DOI] [Google Scholar]
- [108.].DeRosa F, Smith L, Shen Y, Huang Y, Pan J, Xie H, Yahalom B, Heartlein MW. Improved Efficacy in a Fabry Disease Model Using a Systemic mRNA Liver Depot System as Compared to Enzyme Replacement Therapy. Mol Ther. 2019;27(4):878–89. Epub 2019/03/19. doi: 10.1016/j.ymthe.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109.].Zhu X, Yin L, Theisen M, Zhuo J, Siddiqui S, Levy B, Presnyak V, Frassetto A, Milton J, Salerno T, Benenato KE, Milano J, Lynn A, Sabnis S, Burke K, Besin G, Lukacs CM, Guey LT, Finn PF, Martini PGV. Systemic mRNA Therapy for the Treatment of Fabry Disease: Preclinical Studies in Wild-Type Mice, Fabry Mouse Model, and Wild-Type Non-human Primates. American journal of human genetics. 2019;104(4):625–37. Epub 2019/03/19. doi: 10.1016/j.ajhg.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110.].Warnock DG, Bichet DG, Holida M, Goker-Alpan O, Nicholls K, Thomas M, Eyskens F, Shankar S, Adera M, Sitaraman S, Khanna R, Flanagan JJ, Wustman BA, Barth J, Barlow C, Valenzano KJ, Lockhart DJ, Boudes P, Johnson FK. Oral Migalastat HCl Leads to Greater Systemic Exposure and Tissue Levels of Active alpha-Galactosidase A in Fabry Patients when Co-Administered with Infused Agalsidase. PLoS One. 2015;10(8):e0134341. doi: 10.1371/journal.pone.0134341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111.].Xu S, Lun Y, Brignol N, Hamler R, Schilling A, Frascella M, Sullivan S, Boyd RE, Chang K, Soska R, Garcia A, Feng J, Yasukawa H, Shardlow C, Churchill A, Ketkar A, Robertson N, Miyamoto M, Mihara K, Benjamin ER, Lockhart DJ, Hirato T, Fowles S, Valenzano KJ, Khanna R. Coformulation of a Novel Human alpha-Galactosidase A With the Pharmacological Chaperone AT1001 Leads to Improved Substrate Reduction in Fabry Mice. Mol Ther. 2015;23(7):1169–81. doi: 10.1038/mt.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]