Abstract
IgA nephropathy is the most common primary glomerulonephritis worldwide. Its frequent coexistence with inflammatory, infectious, or malignant processes raises the possibility of a pathologic rather than coincidental association. Major strides have been made to elucidate the underlying pathophysiologic events that culminate in the development of primary IgA nephropathy. Whether secondary forms of the disease share common pathways triggered by underlying disorders or different mechanisms leading to similar pathologic findings remains to be determined. In this article, we describe the most frequent etiologies for secondary IgA nephropathy and review the available literature for the pathophysiology.
Keywords: Liver disease, post-infectious glomerulonephritis, inflammatory bowel disease, autoimmune diseases, glomerulonephritis
Since the initial description of IgA nephropathy (IgAN) in 1968, significant progress has been made in understanding the pathogenesis of the most common primary glomerulonephritis in the world.1 The diagnosis is established by examination of renal tissue showing IgA as the dominant or co-dominant immunoglobulin in glomeruli, usually accompanied by complement C3 and frequently with IgG and IgM.1 The IgA is exclusively of the IgA1 subclass, with galactose content less than that of most circulatory IgA1.2 The tissue origin of the galactose-deficient IgA1 (Gd-IgA1) is still debatable, with a mucosal origin suspected3,4 Alternatively, polymeric IgA1 may be produced in the bone marrow due to altered homing of Gd-IgA1-producing cells (Figure 1).5 Patients with IgA vasculitis with nephritis (IgAV-N; formerly termed Henoch-Schoenlein purpura nephritis) have histological features indistinguishable from IgAN, suggestive of a systemic form of the disease that causes IgAN.1 Confocal microscopy imaging shows co-localization of IgA with complement C3, consistent with an immune complex. The presence of C3 co-deposits without C1q in the mesangium indicates that complement activation is through the alternative pathway, lectin pathway, or both.6
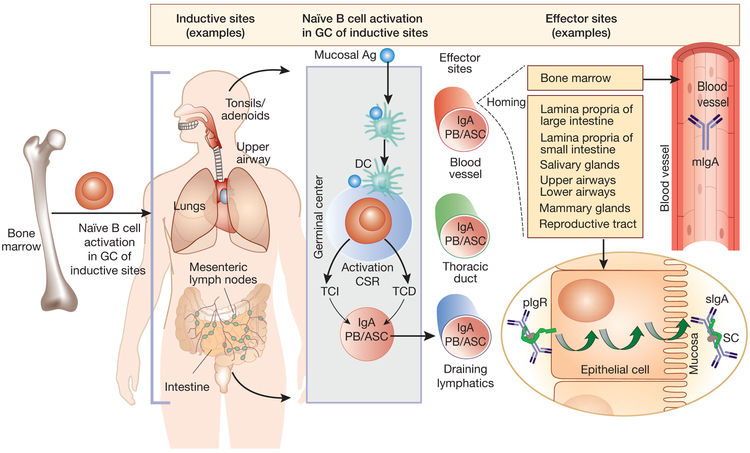
Figure 1. Mucosal immune system and circulatory and mucosal IgA.
The mucosal immune system consists of inductive and effector sites. Inductive sites are the tissues where naïve B cells are exposed to antigen. Inductive sites include Peyer’s patches (small and large intestine), bronchus-associated lymphoid tissue, tonsils and adenoids. The interaction of antigens (mucosa-derived) with B cells occurs in the germinal centers (GC) of the inductive sites. T-cell dependent (TCD) and T-cell-independent (TCI) activation of B cells (IgM+, IgD+) can result in isotype class-switch to IgA; the IgA+ plasmablasts (PB) and antibody-secreting cells (ASC) express tissue-specific homing receptors.56,57 These activated B cells enter the thoracic duct via the draining lymphatics, and then recirculate to the lamina propria of the intestine and other mucosal epithelium (effector sites).57 In primary IgAN, the tissue origin of galactose-deficient IgA1 (Gd-IgA1) is still debated, but evidence indicates its mucosal origin: i) Gd-IgA1 in mesangial deposits is polymeric, typical of IgA1 produced in mucosal tissues; ii) macroscopic hematuria frequently manifests during an active respiratory tract and gastrointestinal tract infection; and iii) polymeric IgA1 produced at mucosal sites has higher capacity for binding to a lectin specific for N-acetylgalactosamine (the terminal sugar in galactose-deficient glycans of Gd-IgA1) than does serum IgA1 in healthy individuals.58, 59 In contrast, other studies support the concept that polymeric IgA1 is produced in the bone marrow in patients with IgAN.58 Thus, it has been postulated that patients with IgAN have IgA1-producing B cells with altered homing receptors when migrating from an inductive site to an effector site in gut mucosa, and therefore mistakenly “home” to the bone marrow.5
TCD mechanisms involve two pathways: 1) CD40L on activated effector T cells and its interaction with CD40 on B cells and 2) involvement of cytokines, transforming growth factor, and interleukins. TCI mechanisms involve dendritic cells (DC) expressing B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL).56 IgA plasmablasts destined for the intestinal lamina propria express chemokine receptors CCR9 (small-intestine homing) and CCR10 (large -intestine homing), the cognate ligands of which are expressed by respective intestinal epithelial cells. The IgA plasmablasts mature into IgA-secreting plasma cells in the lamina propria, to secrete polymeric IgA, composed of two or more monomeric units (either IgA1 or IgA2) joined by disulfide bonds between C-terminal tail pieces of each monomer and a single J chain.
The polymeric IgA secreted by IgA-producing cells in mucosa can bind to polymeric immunoglobulin receptors (pIgR) on the baso-lateral surface of the mucosal epithelial cells, are internalized and then transcytosed into vesicles to the mucosal apical surface. The extracellular portion of the receptor (the secretory component, SC) is cleaved and remains attached to the pIgA that is secreted as secretory IgA (sIgA). Most of the IgA in serum originates from bone marrow and is monomeric IgA1 (mIgA; IgA1~84%, IgA2 ~16%; monomers >90%, polymers <10%), In contrast, mucosal IgA consists mainly of secretory IgA (>95%) with distribution of the two subclasses differing, depending on the mucosal site. sIgA is present in circulation at low concentrations, possibly due to mucosal retrograde transport.60
We have proposed that the glomerular IgA represents deposits of circulating complexes comprised of Gd-IgA1 bound by auto-antibody (IgG or IgA) specific for Gd-IgA1 hinge-region O-linked glycans.7, 8 An alternative hypothesis postulates that latent mesangial deposits of Gd-IgA1 may be bound by autoantibodies from the circulation, forming immune complexes in situ.9 Although mesangial cells can degrade immunodeposits,7 it is possible that, when this system is “overwhelmed”, alternative processes cause pathologic cellular activation and tissue injury.
IgAN may be discovered in patients with many non-renal diseases. In these circumstances, IgA-immune complex-mediated renal injury may develop secondarily due to pathogenic steps similar to those of IgAN or markedly different mechanisms of disease. Herein, we review the more commonly encountered causes of secondary IgAN.
Secondary IgAN
IgAN has been documented in patients with various co-morbidities, ranging from chronic liver disease and inflammatory states to chronic infections and neoplasms (Table 1). Although IgAN in those cases is often referred to as being secondary to the underlying systemic disorder, there is no consistent definition of secondary IgAN in the literature. No specific histologic features differentiate primary from the so-called secondary cases. Mesangial deposits of IgA, sometimes with proliferative glomerulonephritis, are present in as many as 4-16% of the general population in some regions.10 Therefore, an association between IgAN and any co-existing disease may be simply due to exacerbation or recognition of coincidental primary IgAN. Nonetheless, the increased frequency of concurrence with select conditions, suggests a potential causative relationship. Those conditions represent the focus of this review.
Table 1∣.
Systemic disorders associated with secondary IgA nephropathy
| Gastrointestinal and Liver disorders |
|
| Viral Infections |
|
| Other Infections |
|
| Autoimmune disorders |
|
| Respiratory tract |
|
| Neoplasia |
|
Liver disease
Liver disease is the leading cause of secondary IgAN. The prevalence varies, depending on the cohort size, etiology of liver disease, demographics, and criteria for kidney biopsy. IgAN has been found in 9-25% of patients biopsied at the time of liver transplantation.11-14 Conversely, in a French cohort of 356 patients with biopsy-proven IgAN, 9% had cirrhosis.15 The frequencies of hematuria and proteinuria range from 10-90% in patients with IgAN and cirrhosis, depending on size of study population and light microscopy findings of the renal biopsy specimen. Patients with endo- and extra-capillary proliferative lesions in the glomeruli often have hematuria, proteinuria, and renal insufficiency. In contrast, patients with non-proliferative lesions, with deposits of IgA restricted to mesangial areas, may have minimal or no urinary abnormality.16 Some patients with liver disease and IgAN progress to chronic kidney disease.16 In patients with end-stage liver disease, proteinuria and hematuria may persist after liver transplantation.13 IgAN has been discovered within several months after combined liver and kidney transplantation, raising the question whether the renal disease was primary rather than secondary in origin.13
In patients with alcoholic cirrhosis, no histologic feature distinguishes the accompanying IgAN from primary IgAN. Post-mortem studies of patients with IgAN and alcoholic liver disease confirmed that mesangial IgA is predominantly IgA1. Staining for J chain in the absence of IgM confirmed its polymeric nature.17 Among cirrhotic patients, serum total IgA levels are two- to four-fold higher than normal, with 25-45% being polymeric.18 Serum IgA1 in patients with alcoholic cirrhosis is abnormally glycosylated, with fewer galactose and sialic acid residues in O-linked glycans and a different pattern of N-glycosylation. This defective glycosylation becomes more pronounced in patients with decompensated cirrhosis.19 Unlike primary IgAN, aberrant O-glycosylation occurs in monomeric and polymeric IgA1.19 Gd-IgA1 is also in circulating immune complexes, bound mainly to IgG, with levels of these complexes increased in patients with decompensated cirrhosis.19 In patients with biopsy-proven IgAN in the setting of alcoholic cirrhosis, mesangial proliferation was not prominent when assessed by immunohistochemistry using Ki67-specific staining. Additionally, serum IgA1 from patients with alcoholic cirrhosis did not induce proliferation of human mesangial cells in vitro whereas serum IgA1 from patients with primary IgAN did.19
Hypergammaglobulinemia in patients with cirrhosis may result from increased synthesis and/or decreased clearance of immunoglobulins and may play a role in the development of secondary IgAN in this setting. The innate and adaptive immune systems of the liver participate in clearing pathogens, particularly those from the gastrointestinal tract. As a first-line defense, hepatic macrophages phagocytose and clear microbes. Subsequently, other immune cells (neutrophils, monocytes) are recruited to assist. The adaptive immune system prevents infections from recurring.20 Due to altered mucosal integrity and impaired hepatic immune function in cirrhosis, translocation may account for bacterial products in the circulation. Bacterial pathogen-associated molecular patterns, including lipopolysaccharides, peptidoglycans and bacterial DNA with un-methylated cytosine-guanine dinucleotide (CpG) motifs, act as ligands for Toll-like receptors (TLRs) that play a major role in the innate immune responses to microbial pathogens. TLRs, in turn, induce a cascade of events leading to secretion of pro-inflammatory cytokines and increased immunoglobulin production.20 Specifically, activation of B cells and dendritic cells TLR7 and 9 enhances immunoglobulin production. Peripheral-blood mononuclear cells (PBMC) derived from patients with alcoholic cirrhosis express less TLR-9 compared with PBMC from healthy donors. In ex-vivo studies, PBMC of alcoholic-cirrhosis patients demonstrated a 10- to 20-fold higher basal production of IgA compared with cells from healthy controls (correlating positively with increased serum IgA levels). However, when PBMC were stimulated with CpG, the increase in IgA production was dampened. Thus, priming of PBMC by bacterial products in vivo leads to decreased TLR-9 expression and, consequently, attenuated capacity to increase IgA production ex vivo when stimulated by CpG.20 In other experiments with PBMC from alcoholic cirrhosis patients, soluble polymeric IgA stimulated PBMC to increase production of IL-6. IL-6 in turn, stimulated PBMC to increase IgA synthesis. This positive feedback loop may explain the sustained amplification of IgA production in alcoholic cirrhosis.21
Hypergammaglobulinemia in cirrhotic patients may also result from decreased clearance of immunoglobulins. Asialoglycoprotein receptor (ASGP-R) on hepatocytes binds desialylated glycoproteins through recognition of glycans with terminal galactose or N-acetylgalactosamine (Figure 2).22-24 Thus, under-galactosylation of IgA1 alone should not hamper its clearance by ASGP-R. However, access to this receptor may be reduced. Aberrant distribution of ASGP-R, loss of hepatocytes, decreased number and/or reduced size of endothelial fenestrae, and portal hypertension with portosystemic shunting dampen hepatic clearance of IgA and IgA-containing immune complexes in cirrhotic patients.16 Additionally, IFN-γ (increased by bacterial translocation) may cause defective circulatory monocyte FcαR endocytosis and internalization of IgA, resulting in decreased clearance of IgA and IgA-containing immune complexes by myeloid cells.25
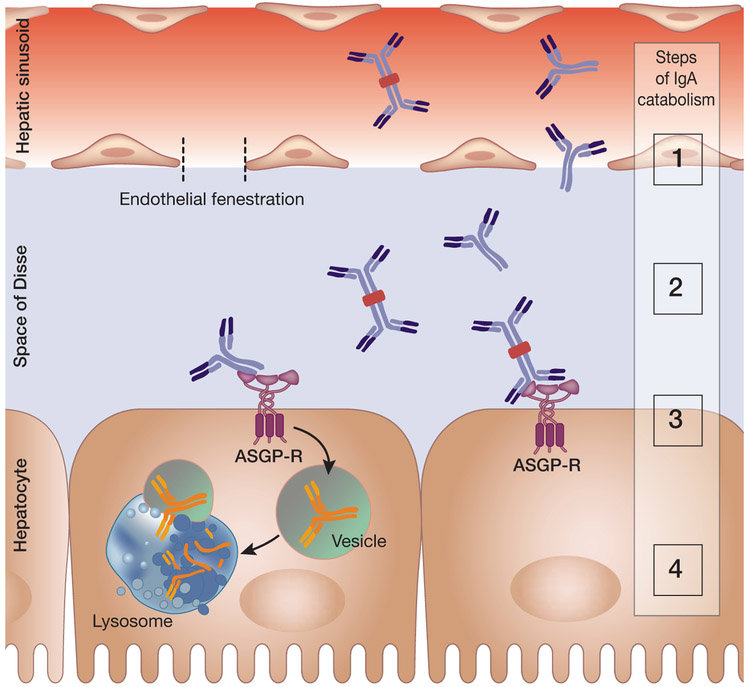
Figure 2. Hepatic catabolism of circulatory IgA.
Liver is the major site of catabolism of circulatory IgA. Asialoglycoprotein receptor (ASGP-R) is expressed on the sinusoidal surface of hepatocytes. It binds terminal galactose or N-acetylgalactosamine of glycoproteins without sialic acid.61 Catabolism of IgA occurs through a series of steps noted in the figure by numbers: 1) Circulatory IgA has to pass through the endothelial fenestration to reach the hepatic sinusoids and enter the space of Disse; 2) IgA binds to ASGP-R on hepatocytes in a calcium-dependent manner; 3) IgA bound to ASGP-R is internalized in vesicles; and 4) vesicles containing IgA (and other asialoglycoproteins) fuse with lysosomes, resulting in IgA catabolic degradation. Although IgA is the most abundantly produced immunoglobulin (66 mg/kg/day), it constitutes only 15% of the total circulatory immunoglobulins due to its short half-life (4-6 days) related the dispersal of IgA polymeric forms into various mucosal secretions and liver catabolism.62 Notably, immune complexes consisting of IgA are often too large to pass through the liver endothelial fenestrations (~148 ± 38 nm)63 and are thus not effectively catabolized in the liver.
Infections associated with IgAN
Several systemic infections have been associated with IgAN (Table 1).16, 26 Some associations have been limited to case reports.
The concurrence of IgAN and hepatitis B infection has long been recognized, especially in endemic areas of southeastern Asia. Kidney biopsies show IgAN in about 30% of patients with active hepatitis B infection. Conversely, about 4.2% of IgAN patients test positive for hepatitis B surface antigen.27 Viral DNA has been detected in glomeruli and renal tubular epithelial cells.28 The significance of this finding in terms of pathogenesis of IgAN remains to be determined. 28
The prevalence of IgAN in patients infected with hepatitis C virus (HCV) is not known. Kidney biopsies of 30 patients with HCV-related cirrhosis at liver transplantation showed immune-complex proliferative glomerulonephritis in 25 patients, of whom seven had IgAN.12 Subendothelial deposits were more common than in patients with primary IgAN.12 Several patients had an inactive urinary sediment, suggesting that IgAN may go unrecognized in patients with HCV cirrhosis.12 In another study, investigators used using laser microdissection, to isolate glomeruli and tubules from kidney biopsies of 20 HCV-positive patients with glomerulonephritis, of whom three had IgAN. HCV RNA and core proteins were in glomeruli whereas tubules had mostly viral core proteins. IgM, IgG, IgA, and complement distribution mirroring that of HCV in glomeruli and tubules supports an immune-mediated pathogenesis for kidney involvement.29 More recently, Suzuki et al. reported that a monoclonal antibody to Gd-IgA1 (KM55) stained renal biopsies from patients with primary IgAN or IgAV-N but not tissue from patients with HCV-nephritis with IgA deposits. While this lack of staining may be due to absence of Gd-IgA1 in the glomerular deposits, another basis could be subtle differences in epitope specificity or inaccessible epitopes during antigen retrieval techniques for immunohistochemistry. Future studies using alternative methods of glomerular Gd-IgA1 assessment (lectin or mass spectrometry based) are needed to confirm this novel finding.30 Overall, these findings suggest that the pathogenesis of HCV-nephritis with IgA deposits typical of IgAN in some patients differs from that of primary IgAN. Whether treatment with direct-acting antiviral agents to eradicate HCV viremia will clear the associated renal disease has not been evaluated.
IgA-dominant infection-associated glomerulonephritis (IAGN) is an entity increasingly recognized. Most patients have been in Asia or United States and have hematuria, proteinuria (50% are nephrotic), acute kidney injury, hypo-complementemia, and hypertension.31 They are generally elderly and diabetic. Renal disease typically manifests about four weeks after an active infection, frequently skin associated or deep-seated Staphylococcus aureus and less commonly with Gram-negative organisms.31, 32
The pathophysiology of IAGN is likely distinct from that of primary IgAN, based on several renal pathological findings, including C3 immunofluorescence staining that is stronger than that for IgA, staining for kappa light chains equivalent or more intense than that for lambda light chains, and more frequent sub-epithelial electron-dense humps on electron microscopy.31 Diabetic patients, who are particularly susceptible to develop IgA-dominant IAGN, have elevated levels of serum IgA and IgA-containing circulating immune complexes when compared with healthy individuals.26, 31 Cell-surface antigen of S. aureus, known as “probable adhesin”, has been detected in about 75% of IgA-dominant IAGN kidney-biopsy specimens and co-localizes with IgA deposits, suggesting immune complexes.33
Antibiotics and supportive treatments are the mainstay of therapy of patients with IAGN. Use of immunosuppressants is not recommended. Prognosis is overall guarded; in one case series, 19% and 14% of patients progressed to ESRD or died, respectively, with older age and diabetes being independent risk factors for both outcomes.32
Mucosal inflammation
In primary IgAN, the occurrence of macroscopic hematuria with acute infection of the respiratory or gastrointestinal tract suggests a mucosa-kidney axis in the pathogenesis of disease. The discoveries that Gd-IgA1 secreted by cells of patients with IgAN is dimeric or polymeric and that mesangial IgA1 is mostly polymeric support a mucosal origin for mesangial Gd-IgA1 because polymeric IgA1 is predominantly produced at mucosal sites. Some cytokines released during mucosal infections, such as IL-6, alter the activity of glycosyltransferases to favor synthesis of Gd-IgA1 whereby more Gd-IgA1 enters the circulation.34
An association between IgAN and inflammatory bowel disease (ulcerative colitis and Crohn’s disease) has been reported for many years. The timing of diagnosis of IgAN in relation to activity of the bowel disease is variable, ranging from onset of disease to exacerbation of colitis, or even later during remission.35,36 In a review of 33,713 renal biopsies processed at one US nephropathology center, 83 were from patients with inflammatory bowel disease (45 Crohn’s disease; 38 ulcerative colitis).36 IgAN was diagnosed in 19 (24%) patients. Biopsies were done primarily for acute or chronic renal insufficiency (63%), nephrotic (16%) and sub-nephrotic-range proteinuria (14%) but rarely for isolated hematuria. Frequency of IgAN among patients with inflammatory bowel disease was higher than that among the remaining patients, suggesting a shared pathophysiology for the two diseases.36 A causal relationship is strengthened by biopsy-proven clearance of mesangial IgA deposits in some patients after treatment with sulfasalazine and corticosteroids.36
Mechanisms responsible for glomerular deposition of IgA in patients with inflammation of the intestinal mucosa have not been elucidated. Recent genome-wide association studies identified several risk loci for primary IgAN with genes involved with mucosal immunity, including some associated with inflammatory bowel disease.37 However, no direct sequencing of genes at these loci has been undertaken to define an IgAN-specific allele. Unlike patients with primary IgAN, most patients with inflammatory bowel disease have increased numbers of IgA-secreting cells in the intestinal mucosa that may be due to loss of mucosal integrity as a barrier to antigenic stimulation. Furthermore, mucosal production of IgA is stimulated by cytokines/growth factors (IL-6, IL-4, IL-10, TGF-β, BAFF, and APRIL) released in inflammatory states. Whether synthesis of Gd-IgA1 is locally augmented under these conditions has not been assessed. Nonetheless, increased production of Gd-IgA1 within the intestinal mucosa may not be the simple answer. For patients with primary IgAN compared to healthy controls, synthesis of polymeric IgA is increased in the bone marrow but decreased in intestinal mucosae.38 These findings may reflect mucosa-primed Gd-IgA1-secreting cells erroneously locating in bone marrow due to the influence of T cells expressing the α4β1integrin-homing receptor.39 Cytokines released during mucosal inflammation may have a systemic effect to increase production of polymeric Gd-IgA1 in the bone marrow.38 Future studies of patients with colitis are required to clarify the glycosylation pattern of circulatory and mesangial IgA1.
The postulated overproduction of Gd-IgA1 in intestinal Peyer’s patches, culminating in IgAN, was the basis for a clinical trial evaluating the effect of targeted-release budesonide in patients with primary IgAN and significant proteinuria, and therefore at risk for progressive decline in clearance function.40 In a double-blind placebo-controlled trial, they received budesonide in oral capsules designed to provide sustained release of active compound upon reaching the distal ileum. A 16-mg/day dose reduced proteinuria and stabilized glomerular filtration rate in patients on optimized blockade of the renin-angiotensin system. Proteinuria continued to decrease three months after treatment, consistent with a disease-modifying effect.
Some investigators have shown an increased prevalence of IgAN in patients with celiac disease. A meta-analysis of 491,815 patients found a 2.62 pooled relative risk for IgAN.41 This analysis did not account for genetic factors that may increase the risk for IgAN. In a population-based prospective cohort study in Sweden, patients with biopsy-proven celiac disease had a three-fold increased risk for future development of IgAN, independent of the frequencies of human leukocyte antigens DQ2 and DQ8 that are associated with celiac disease. Nevertheless, the absolute risk was very low.16 Some studies have shown decreased oral tolerance in celiac disease and altered mucosal immunity in celiac disease and IgAN. Shared mechanisms of disease have been suggested also by animal models noting increased intestinal permeability of gluten and related peptides; production of Gd-IgA1 specific for gluten and gliadin-mediated immune-complex formation resulting in the glomerular deposition.41 In a murine model of IgAN, transglutaminase-2 on mesangial cells amplified renal injury.42 Circulating gliadin-, reticulin-, and endomysial-specific antibodies, typical of celiac disease, have not been consistently found in patients with IgAN. A gluten-free diet for six months reduced proteinuria and, in some patients, microscopic hematuria but did not stabilize renal function in patients with progressive IgAN. The possibility that the pathogenesis of IgAN in patients with celiac disease may differ from that of primary IgAN stems from the frequent absence of C3 in the renal deposits.43
IgAN has been associated with several lung diseases. In a series of 1100 consecutive autopsy cases, the histology of the 70 cases with renal pathology and a variety of lung diseases (chronic obstructive bronchiolitis, bronchopneumonia, and idiopathic pulmonary fibrosis with and without cancer) was compared to that of 25 control cases.44 Mesangial IgA deposition was noted in 25 cases but in only 9 cases was the immunofluorescence intensity strong enough to diagnose to IgAN.44 Cystic fibrosis (CF), an autosomal recessive disorder due to mutations in the cystic fibrosis transmembrane conductance regulator, was until recently a highly lethal disease. Advances in medical treatment have translated into significant improvement in life expectancy, and, consequently, renal complications have become increasingly recognized. Several glomerulonephritides have been reported among CF patients. While IgAN in early case series was thought to represent the predominant pathology,45 more recent and larger cohort studies suggest that the renal glomerular pathology is more heterogeneous but includes IgAN.46,47 Elevation in total serum IgA levels in patients with CF due to recurrent mucosal infections has been proposed as a mechanism for IgAN development. However, the glycosylation pattern of IgA1 has not been investigated.48
Autoimmune and inflammatory disorders
The association of IgAN with multiple autoimmune disorders, including ankylosing spondylitis, Sjogren’s syndrome, and dermatitis herpetiformis,49 has been limited to case reports. In other autoimmune disorders, such as rheumatoid arthritis (RA) and psoriasis, the association has been substantiated by larger cohort studies. In a series of 100 Japanese patients with RA, IgAN was diagnosed in 12 patients.50 A prevalence of 5-7% had been previously reported for Northern Europeans, perhaps reflecting a lower frequency of disease in that population. Clinically, most patients had isolated hematuria, followed by combined hematuria and sub-nephrotic proteinuria. Renal function was typically preserved.50 Although RA patients have elevated levels of serum IgA, Nakano et al. did not find significant correlation between IgA rheumatoid factors and IgAN.51 IgG anti-citrullinated-protein antibodies in RA displayed less galactose and sialic acid on their Fc portion, rendering them pro-inflammatory. This altered glycosylation pattern antedates the onset of RA.52 Whether a similar change in glycosylation occurs for IgA1 remains to be determined.
In a population-based study of 205,815 psoriasis patients in the United Kingdom those with moderate to severe disease (n=12,809) had a significantly increased risk of developing glomerular diseases (Hazard Ratio=2.05), particularly IgAN (Hazard Ratio=4.75).53
Neoplasia
There are rare associations of IgAN with cutaneous T-cell lymphoma, Hodgkin’s disease, mucosa-associated lymphoid tissue, and extra-nodal T-cell lymphoma. Most patients present with hematuria (occasionally macroscopic), proteinuria, and renal insufficiency that may include crescentic disease.54 Despite markedly elevated serum IgA levels in patients with IgA myeloma, IgAN and IgAV-N have been rarely documented, limited to patients with decreased sialylation or galactosylation of the hinge-region O-glycans of IgA1. 55 In a series of 60 patients with renal cell carcinoma, IgAN was diagnosed in 11 patients by immunohistochemistry staining of the uninvolved tissue in their surgical specimens.
Treatment
The best treatment strategy for secondary IgAN has not been established. Most reports suggest addressing the underlying disease, with additional supportive and non-disease-specific measures for chronic kidney disease. As described above, in a few settings, such as celiac disease and inflammatory bowel disease, treatment of the associated disease in some patients has led to resolution of the urinary abnormalities or even clearance of the histological findings. In patients with other conditions, a treatment response is not well documented. Further studies are needed to establish a plan for optimal care.
Conclusion
Prevalence of secondary IgAN is difficult to estimate because some associations are undoubtedly coincidental newly discovered primary IgAN. Nonetheless, several diseases, including cirrhosis, inflammatory bowel disease, infections and autoimmune diseases, can induce glomerulonephritis with mesangial deposits of IgA diagnostic of IgAN, sometimes with other immunohistological features less typical of primary disease. The pathogenic mechanisms have not been fully established. Additional studies are necessary to determine whether Gd-IgA1 and anti-glycan autoantibodies are central for these processes as in primary IgAN.
Acknowledgements:
This work was supported in part by NIH grants: T32 DK 07545 (MS), DK078244 (JN and BAJ) and DK082753 (JN, BAJ, and DVR).
Footnotes
Financial disclosure: BAJ, JN, and DVR are co-founders of Reliant Glycosciences, L.L.C., Birmingham, AL, USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med 2013; 368: 2402–2414. [DOI] [PubMed] [Google Scholar]
- 2.Hiki Y, Odani H, Takahashi M, et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int 2001; 59: 1077–1085. [DOI] [PubMed] [Google Scholar]
- 3.Novak J, Rizk D, Takahashi K, et al. New insights into the pathogenesis of IgA nephropathy. Kidney Dis (Basel) 2015; 1: 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knoppova B, Reily C, Maillard N, et al. The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front Immunol 2016; 7: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buren M, Yamashita M, Suzuki Y, et al. Altered expression of lymphocyte homing chemokines in the pathogenesis of IgA nephropathy. Contrib Nephrol 2007; 157: 50–55. [DOI] [PubMed] [Google Scholar]
- 6.Maillard N, Wyatt RJ, Julian BA, et al. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol 2015; 26: 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki H, Kiryluk K, Novak J, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol 2011; 22: 1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novak J, Julian BA, Mestecky J, et al. Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin Immunopathol 2012; 34: 365–382. [DOI] [PubMed] [Google Scholar]
- 9.Glassock RJ. The pathogenesis of IgA nephropathy. Curr Opin Nephrol Hypertens 2011; 20: 153–160. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki K, Honda K, Tanabe K, et al. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int 2003; 63: 2286–2294. [DOI] [PubMed] [Google Scholar]
- 11.Axelsen RA, Crawford DH, Endre ZH, et al. Renal glomerular lesions in unselected patients with cirrhosis undergoing orthotopic liver transplantation. Pathology 1995; 27: 237–246. [DOI] [PubMed] [Google Scholar]
- 12.McGuire BM, Julian BA, Bynon JS Jr., et al. Brief communication: Glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med 2006; 144: 735–741. [DOI] [PubMed] [Google Scholar]
- 13.Hommos MS, El-Zoghby ZM. Renal outcomes in patients with IgA nephropathy undergoing liver transplant: A retrospective cohort study. Transplant Direct 2017; 3: e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calmus Y, Conti F, Cluzel P, et al. Prospective assessment of renal histopathological lesions in patients with end-stage liver disease: effects on long-term renal function after liver transplantation. J Hepatol 2012; 57: 572–576. [DOI] [PubMed] [Google Scholar]
- 15.Berthoux FC, Mohey H, Afiani A. Natural history of primary IgA nephropathy. Semin Nephrol 2008; 28: 4–9. [DOI] [PubMed] [Google Scholar]
- 16.Pouria S, Barratt J. Secondary IgA nephropathy. Semin Nephrol 2008; 28: 27–37. [DOI] [PubMed] [Google Scholar]
- 17.Lomax-Smith JD, Zabrowarny LA, Howarth GS, et al. The immunochemical characterization of mesangial IgA deposits. Am J Pathol 1983; 113: 359–364. [PMC free article] [PubMed] [Google Scholar]
- 18.Kutteh WH, Prince SJ, Phillips JO, et al. Properties of immunoglobulin A in serum of individuals with liver diseases and in hepatic bile. Gastroenterology 1982; 82: 184–193. [PubMed] [Google Scholar]
- 19.Tissandie E, Morelle W, Berthelot L, et al. Both IgA nephropathy and alcoholic cirrhosis feature abnormally glycosylated IgA1 and soluble CD89-IgA and IgG-IgA complexes: common mechanisms for distinct diseases. Kidney Int 2011; 80: 1352–1363. [DOI] [PubMed] [Google Scholar]
- 20.Massonnet B, Delwail A, Ayrault JM, et al. Increased immunoglobulin A in alcoholic liver cirrhosis: exploring the response of B cells to Toll-like receptor 9 activation. Clin Exp Immunol 2009; 158: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deviere J, Content J, Denys C, et al. Immunoglobulin A and interleukin 6 form a positive secretory feedback loop: a study of normal subjects and alcoholic cirrhotics. Gastroenterology 1992; 103: 1296–1301. [DOI] [PubMed] [Google Scholar]
- 22.Baenziger JU, Fiete D. Galactose and N-acetylgalactosamine-specific endocytosis of glycopeptides by isolated rat hepatocytes. Cell 1980; 22: 611–620. [DOI] [PubMed] [Google Scholar]
- 23.Baenziger JU, Maynard Y. Human hepatic lectin. Physiochemical properties and specificity. J Biol Chem 1980; 255: 4607–4613. [PubMed] [Google Scholar]
- 24.Tomana M, Kulhavy R, Mestecky J. Receptor-mediated binding and uptake of immunoglobulin A by human liver. Gastroenterology 1988; 94: 762–770. [DOI] [PubMed] [Google Scholar]
- 25.Silvain C, Patry C, Launay P, et al. Altered expression of monocyte IgA Fc receptors is associated with defective endocytosis in patients with alcoholic cirrhosis. Potential role for IFN-gamma. J Immunol 1995; 155: 1606–1618. [PubMed] [Google Scholar]
- 26.Rollino C, Vischini G, Coppo R. IgA nephropathy and infections. J Nephrol 2016; 29: 463–468. [DOI] [PubMed] [Google Scholar]
- 27.Sun IO, Hong YA, Park HS, et al. Clinical characteristics and treatment of patients with IgA nephropathy and hepatitis B surface antigen. Ren Fail 2013; 35: 446–451. [DOI] [PubMed] [Google Scholar]
- 28.Wang NS, Wu ZL, Zhang YE, et al. Existence and significance of hepatitis B virus DNA in kidneys of IgA nephropathy. World J Gastroenterol 2005; 11: 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sansonno D, Lauletta G, Montrone M, et al. Hepatitis C virus RNA and core protein in kidney glomerular and tubular structures isolated with laser capture microdissection. Clin Exp Immunol 2005; 140: 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki H, Yasutake J, Makita Y, et al. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int 2018. [DOI] [PubMed] [Google Scholar]
- 31.Nasr SH, D'Agati VD. IgA-dominant postinfectious glomerulonephritis: a new twist on an old disease. Nephron Clin Pract 2011; 119: c18–25; discussion c26. [DOI] [PubMed] [Google Scholar]
- 32.Bu R, Li Q, Duan ZY, et al. Clinicopathologic features of IgA-dominant infection-associated glomerulonephritis: a pooled analysis of 78 cases. Am J Nephrol 2015; 41: 98–106. [DOI] [PubMed] [Google Scholar]
- 33.Koyama A, Sharmin S, Sakurai H, et al. Staphylococcus aureus cell envelope antigen is a new candidate for the induction of IgA nephropathy. Kidney Int 2004; 66: 121–132. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, Raska M, Yamada K, et al. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem 2014; 289: 5330–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filiopoulos V, Trompouki S, Hadjiyannakos D, et al. IgA nephropathy in association with Crohn's disease: a case report and brief review of the literature. Ren Fail 2010; 32: 523–527. [DOI] [PubMed] [Google Scholar]
- 36.Ambruzs JM, Walker PD, Larsen CP. The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin J Am Soc Nephrol 2014; 9: 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiryluk K, Li Y, Scolari F, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet 2014; 46: 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harper SJ, Allen AC, Layward L, et al. Increased immunoglobulin A and immunoglobulin A1 cells in bone marrow trephine biopsy specimens in immunoglobulin A nephropathy. Am J Kidney Dis 1994; 24: 888–892. [DOI] [PubMed] [Google Scholar]
- 39.Batra A, Smith AC, Feehally J, et al. T-cell homing receptor expression in IgA nephropathy. Nephrol Dial Transplant 2007; 22: 2540–2548. [DOI] [PubMed] [Google Scholar]
- 40.Fellstrom BC, Barratt J, Cook H, et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet 2017; 389: 2117–2127. [DOI] [PubMed] [Google Scholar]
- 41.Boonpheng B, Cheungpasitporn W, Wijarnpreecha K. Renal disease in patients with celiac disease: a review. Minerva Med 2017. [DOI] [PubMed] [Google Scholar]
- 42.Berthelot L, Papista C, Maciel TT, et al. Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med 2012; 209: 793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasternack A, Collin P, Mustonen J, et al. Glomerular IgA deposits in patients with celiac disease. Clin Nephrol 1990; 34: 56–60. [PubMed] [Google Scholar]
- 44.Endo Y, Hara M. Glomerular IgA deposition in pulmonary diseases. Kidney Int 1986; 29: 557–562. [DOI] [PubMed] [Google Scholar]
- 45.Stirati G, Antonelli M, Fofi C, et al. IgA nephropathy in cystic fibrosis. J Nephrol 1999; 12: 30–31. [PubMed] [Google Scholar]
- 46.Yahiaoui Y, Jablonski M, Hubert D, et al. Renal involvement in cystic fibrosis: diseases spectrum and clinical relevance. Clin J Am Soc Nephrol 2009; 4: 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santoro D, Postorino A, Lucanto C, et al. Cystic Fibrosis: A risk condition for renal disease. J Ren Nutr 2017; 27: 470–473. [DOI] [PubMed] [Google Scholar]
- 48.Stephens SE, Rigden SP. Cystic fibrosis and renal disease. Paediatr Respir Rev 2002; 3: 135–138. [PubMed] [Google Scholar]
- 49.Gaboardi F, Perletti L, Cambie M, et al. Dermatitis herpetiformis and nephrotic syndrome. Clin Nephrol 1983; 20: 49–51. [PubMed] [Google Scholar]
- 50.Makino H, Yoshinaga Y, Yamasaki Y, et al. Renal involvement in rheumatoid arthritis: analysis of renal biopsy specimens from 100 patients. Mod Rheumatol 2002; 12: 148–154. [DOI] [PubMed] [Google Scholar]
- 51.Nakano M, Ueno M, Nishi S, et al. Determination of IgA- and IgM-rheumatoid factors in patients with rheumatoid arthritis with and without nephropathy. Ann Rheum Dis 1996; 55: 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernard NJ. Rheumatoid arthritis: Changes in ACPA Fc glycosylation patterns prior to RA onset. Nat Rev Rheumatol 2013; 9: 697. [DOI] [PubMed] [Google Scholar]
- 53.Grewal SK, Wan J, Denburg MR, et al. The risk of IgA nephropathy and glomerular disease in patients with psoriasis: a population-based cohort study. Br J Dermatol 2017; 176: 1366–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson FP, Nasr SH, Markowitz GS, et al. A destructive nasal lesion and glomerulonephritis. Kidney Int 2006; 69: 1699–1703. [DOI] [PubMed] [Google Scholar]
- 55.Van Der Helm-Van Mil AH, Smith AC, Pouria S, et al. Immunoglobulin A multiple myeloma presenting with Henoch-Schonlein purpura associated with reduced sialylation of IgA1. Br J Haematol 2003; 122: 915–917. [DOI] [PubMed] [Google Scholar]
- 56.Macpherson AJ, McCoy KD, Johansen FE, et al. The immune geography of IgA induction and function. Mucosal Immunol 2008; 1: 11–22. [DOI] [PubMed] [Google Scholar]
- 57.Mora JR, von Andrian UH. Differentiation and homing of IgA-secreting cells. Mucosal Immunol 2008; 1: 96–109. [DOI] [PubMed] [Google Scholar]
- 58.Mestecky J, Novak J, Moldoveanu Z, et al. IgA nephropathy enigma. Clin Immunol 2016; 172: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith AC, Molyneux K, Feehally J, et al. O-glycosylation of serum IgA1 antibodies against mucosal and systemic antigens in IgA nephropathy. J Am Soc Nephrol 2006; 17: 3520–3528. [DOI] [PubMed] [Google Scholar]
- 60.Woof JM, Mestecky J. Mucosal immunoglobulins. Immunol Rev 2005; 206: 64–82. [DOI] [PubMed] [Google Scholar]
- 61.Mestecky J, Lue C, Russell MW. Selective transport of IgA. Cellular and molecular aspects. Gastroenterol Clin North Am 1991; 20: 441–471. [PubMed] [Google Scholar]
- 62.Lechner SM, Papista C, Chemouny JM, et al. Role of IgA receptors in the pathogenesis of IgA nephropathy. J Nephrol 2016; 29: 5–11. [DOI] [PubMed] [Google Scholar]
- 63.Zapotoczny B, Szafranska K, Kus E, et al. Quantification of fenestrations in liver sinusoidal endothelial cells by atomic force microscopy. Micron 2017; 101: 48–53. [DOI] [PubMed] [Google Scholar]