Abstract
N-methyl-d-aspartate (NMDA) receptor (NMDAR) hypofunction plays a key role in pathophysiology of schizophrenia. Since NMDAR hypofunction has also been reported in autism, Alzheimer’s disease and cognitive dementia, it is crucial to identify the location, timing, and mechanism of NMDAR hypofunction for schizophrenia for better understanding of disease etiology and for novel therapeutic intervention. In this review, we first discuss the shared underlying mechanisms of NMDAR hypofunction in NMDAR antagonist models and the anti-NMDAR autoantibody model of schizophrenia and suggest that NMDAR hypofunction could occur in GABAergic neurons in both models. Preclinical models using transgenic mice have shown that NMDAR hypofunction in cortical GABAergic neurons, in particular parvalbumin-positive fast-spiking interneurons, in the early postnatal period confers schizophrenia-related phenotypes. Recent studies suggest that NMDAR hypofunction can also occur in PV-positive GABAergic neurons with alterations of NMDAR–associated proteins, such as neuregulin/ErbB4, α7nAChR, and serine racemase. Furthermore, several environmental factors, such as oxidative stress, kynurenic acid and hypoxia, may also potentially elicit NMDAR hypofunction in GABAergic neurons in early postnatal period. Altogether, the studies discussed here support a central role for GABAergic abnormalities in the context of NMDAR hypofunction. We conclude by suggesting potential therapeutic strategies to improve the function of fast-spiking neurons.
Keywords: NMDA antagonist, anti-NMDAR encephalitis, serine racemase, α7 nicotinic acetylcholine receptor, oxidative stress, kynurenic acid
1. Introduction
Schizophrenia is a major psychotic disorder characterized by positive symptoms (hallucinations and delusions), negative symptoms (avolition and anhedonia), and cognitive dysfunction. Decades of intensive studies have identified excessive striatal presynaptic dopamine release as a final common pathway to evoke positive symptoms (Howes and Kapur, 2009). However, pathophysiology underlying the negative and cognitive symptoms, which often precedes positive symptoms, remains elusive. Multiple routes of genetic, neurodevelopmental, environmental, and social events are suggested to lead to striatal hyperdopaminergia or psychosis (Howes and Kapur, 2009). Among them, one major upstream mechanism leading to striatal hyperdopaminergia is N-methyl-d-aspartate (NMDA) receptor (NMDAR) hypofunction.
NMDARs are heterotetrameric receptor complexes which are composed of one or two GluN1 indispensable subunits (encoded by GRIN1 gene) and two or three GluN2 (A-D; encoded by GRIN2 (A-D) and/or GluN3 (A-B; encoded by GRIN3 (A-B) subunits (Beesley et al., 2019; Hollmann and Heinemann, 1994). These are ligand-gated cationic channels, activation of which requires simultaneous binding of the agonist L-glycine or D-serine (to GluN1 or GluN3) and glutamate (to GluN2 subunits) as well as membrane depolarization to remove the Mg2+ block. In mature cortical principal neurons, NMDARs mediate glutamatergic excitatory neurotransmission and play an important role in learning and memory through induction of synaptic plasticity (Martin et al., 2000; Nakazawa et al., 2004). However, NMDARs in immature glutamatergic neurons are also involved in dendritic maturation and proper synapse refinement, presumably by controlling intrinsic excitability (Frangeul et al., 2017; Gu and Lu, 2018; Hou and Zhang, 2017).
The NMDAR hypofunction hypothesis of schizophrenia originated from the observation that a sub-class of non-competitive NMDAR antagonists, phencyclidine (PCP) and ketamine, induces behaviors reminiscent of all three (positive, negative, and cognitive) symptoms of schizophrenia in human subjects (Coyle et al., 2003; Javitt and Zukin, 1991; Krystal et al., 1994). A compound in the same subclass of non-competitive antagonists, MK-801, shows higher affinity and specificity to NMDARs compared to PCP and ketamine, and produces PCP-like behavioral effects in various species (Koek et al., 1988; Kovacic and Somanathan, 2010). Notably, systemic administration of PCP, ketamine, and MK-801 induces excessive release of dopamine in the striatum of rodents (Balla et al., 2001; Miller and Abercrombie, 1996), monkeys (Adams et al., 2002) and humans (Kegeles et al., 2000; Kokkinou et al., 2018). Conversely, prefrontal dopamine release is diminished by chronic PCP treatment in monkeys (Jentsch et al., 1997) and rats (Jentsch et al., 1998). Such pathway-specific dopamine abnormalities are characteristic of schizophrenia pathophysiology (Slifstein et al., 2015; Weinstein et al., 2017).
Recently, other evidence for NMDAR hypofunction in schizophrenia has emerged from clinical neurology. Clinical expression of anti-NMDAR encephalitis, in which the antibodies cross-react with NMDARs (i.e., autoantibodies) to cause internalization of the receptors from the plasma membrane, first presents as psychotic symptoms akin to first-episode psychosis in schizophrenia (Dalmau et al., 2011). The presence of pronounced psychiatric symptoms, as well as severe cognitive disturbances with high negative symptom scores, can often result in a misdiagnosis as idiopathic schizophrenia (Al-Diwani et al., 2019). It is noted, however, patients with anti-NMDAR encephalitis also frequently exhibit neurological symptoms, such as movement disorders, seizures, and autonomic instability (Dalmau et al., 2008; Gibson et al., 2019). It remains debated whether anti-NMDAR antibody is pathogenic in idiopathic schizophrenia (Masopust et al., 2015; Pearlman and Najjar, 2014; Pollak et al., 2014; Tsutsui et al., 2012). Nonetheless, since both NMDAR antagonism and anti-NMDAR encephalitis share the cardinal schizophrenia-like symptoms, NMDAR hypofunction could be one major convergent point of schizophrenia pathophysiology (Kantrowitz and Javitt, 2010).
In this review, we first discuss the similarity in the underlying mechanism of NMDAR hypofunction induced by NMDAR antagonists and by anti-NMDAR autoantibodies. That is, both antagonists and auto-antibodies appear to preferentially bind to open state of NMDAR channels. Since open-channel probability of NMDAR channels is directly related to firing rate, constantly depolarizing neurons would be predicted to be a target of NMDAR hypofunction, such as fast-spiking (FS) GABAergic interneurons. If their binding to the open-state of NMDARs is the origin of psychotic symptoms, it is tempting to speculate that NMDAR hypofunction takes places in cortical and hippocampal GABAergic neurons in idiopathic schizophrenia (Cohen et al., 2015; Coyle, 2006; Inan et al., 2013; Ju and Cui, 2016; Lisman et al., 2008; Moreau and Kullmann, 2013; Nakazawa et al., 2017; Nakazawa et al., 2012; Olney and Farber, 1995). While clinical evidence supporting this idea is highly limited, recent preclinical literature presents several candidates of preceding molecular and cellular events, which could elicit NMDAR hypofunction in cortical GABAergic neurons, leading to schizophrenia-like phenotypes.
2. Cellular source of NMDAR hypofunction—Lessons from NMDAR antagonist models
The psychotomimetic effect of NMDAR antagonists is not limited to PCP, ketamine, or MK-801. Some competitive (i.e., compete with endogenous ligand for binding) NMDAR antagonists such as CPP (Herrling, 1994) and CGS 19755 (Yenari et al., 1998) are also reported to induce psychotic symptoms in humans. Thus, psychotomimetic effects of NMDAR antagonists could be due to the disruption of normal synaptic transmission (Vyklicky et al., 2014). It should be noted that PCP-like behavioral effects produced by competitive antagonists occurs only at high doses and are of much lesser magnitude than those of non-competitive antagonists in animal studies (Löscher and Hönack, 1991; Tricklebank et al., 1989). The unique mechanism of action of non-competitive antagonists is to directly block the ion flow coupled to NMDAR channels by binding to the channel pore-region. This binding requires agonist-induced channel opening and sufficient cellular membrane depolarization to expel the Mg2+ ion from the channel pore (Halliwell et al., 1989; Huettner and Bean, 1988). Thus, non-competitive NMDAR antagonists act as open channel blockers of the NMDA channels (Lodge and Mercier, 2015). Notably, not all open channel blockers have PCP-like psychotomimetic properties. For example, memantine is an open channel blocker and display similar basic characteristics of NMDAR inhibition to that of ketamine; however, memantine does not exhibit psychotomimetic action and is well tolerated (Johnson et al., 2015; Kornhuber and Weller, 1997; Xia et al., 2010).
The differential effects of memantine may be explained by its lower affinity and more rapid dissociation rates than other NMDAR channel blockers. Open channel blockers can be classified into three subgroups based on their interaction with the channel (Hansen et al., 2018). Importantly, PCP, ketamine and MK-801, all belong to the “trapping blockers”, which are trapped inside the channel pore and the blocked channel can return to the closed state (Sobolevsky and Yelshansky, 2000). These blockers can bind to NMDARs with high affinity, with strong use-dependency and slow off-rate kinetics from channel. Thus, these antagonists would block NMDAR channels during depolarization due to their slow unblocking rate. This would prevent the synaptic NMDAR from reactivation during a fast train of action potentials, thereby robustly disrupting normal synaptic transmission of NMDARs. On the other hand, memantine shows low-affinity binding, with 100-fold faster off-rate than that of MK-801 (“partial trapping blocker”) (Blanpied et al., 1997). Due to this fast dissociation rate, memantine may not affect phasic synaptic responses of NMDARs (Chen and Lipton, 2006; Vyklicky et al., 2014). Thus, memantine appears to inhibit tonic (extrasynaptic) NMDARs in concentrations that do not affect synaptic NMDAR-mediated transmission (Riebe et al., 2016).
Importantly, the specific neuron types most affected by NMDAR antagonists has been identified in cortical and hippocampal networks. In the mPFC of awake rats, systemic injection of MK-801 predominantly decreased the firing of GABAergic interneurons, while the firing rates of pyramidal neurons rather increased by disinhibition, suggesting that the primary target of MK-801 is the GABAergic neurons (Homayoun and Moghaddam, 2007). In the cultured hippocampal neurons, the impact of ketamine on excitatory postsynaptic current (EPSC) amplitudes of excitatory neurons and inhibitory neurons was also measured by somatic voltage recording technique (Fan et al., 2018). Ketamine induced a greater suppression of the EPSCs in GABAergic neurons than in pyramidal neurons, with negligible effect on inhibitory postsynaptic currents (IPSCs) in pyramidal neurons and on intrinsic excitability of both cell-types. These results suggest that the disinhibitory action of ketamine is primarily via blockade of synaptic (but not tonic) NMDARs at the inhibitory neurons receiving excitatory inputs from pyramidal neurons. The finding is also consistent with the previous report that NMDARs contribute more to the EPSPs in FS interneurons than in pyramidal neurons in the cortex (Jones and Buhl, 1993).
Additional evidence supports the hypothesis that ketamine and PCP preferentially block NMDARs in GABAergic interneurons. Parvalbumin (PV)-positive neuron-specific genetic ablation of GluN2A, which is highly expressed in GABA neurons, was also sufficient to ablate ketamine-induced changes in pyramidal cell activity in mouse visual cortex, suggesting that ketamine binds to GluN2A-containing PV neurons (Picard et al., 2019). On the other hand, the GluN2D subunit of the NMDARs is expressed exclusively in GABAergic neurons but not pyramidal neurons in the cerebral cortex in adulthood (Alsaad et al., 2019; von Engelhardt et al., 2015). Interestingly, administration of ketamine or PCP failed to show psychotomimetic action in conventional GluN2D knockout mice (Sapkota et al., 2016; Yamamoto et al., 2016); but see (Shelkar et al., 2019), suggesting that ketamine/PCP may induce schizophrenia-like phenotypes via GluN2D-containing NMDARs in adulthood. On the other hand, GluN2C/GluN2D subunit-selective potentiator CIQ reverses MK-801-induced schizophrenia-related behavioral impairment (Suryavanshi et al., 2014). Taken together, it appears that the psychotomimetic effect of ketamine/PCP/MK-801 is attributable to preferential inhibition of synaptic NMDARs, presumably containing GluN2A and/or GluN2D, on PV-positive GABAergic interneurons, leading to schizophrenia-like psychotomimetic effects.
3. Cellular source of NMDAR hypofunction—Lessons from NMDAR antibody model
The etiology of anti-NMDAR encephalitis is highly debated. In NMDAR antibody-mediated encephalopathy, the antibodies (mostly IgG) present in patients are cross-reactive to the N-terminal region of GluN1 subunit. One group (Pan et al., 2019) has suggested that NMDAR-antibodies are part of a pre-existing autoimmune repertoire. While antibodies themselves do not cause brain inflammation, upon entry to the brain or via intrathecal production by the meningeal inflammation, CSF NMDAR antibodies can precipitate the symptoms by inducing NMDAR hypofunction. This hypothesis may well explain the prodromal signs of this disorder, which can include headache, fever, nausea, and vomiting (Dalmau et al., 2008), possibly triggered by meningeal inflammation. Subsequently, psychotic and cognitive symptoms may quickly develop, with ongoing intrathecal synthesis of specific NMDAR antibodies.
Accumulating evidence suggests that the nature of antibody-mediated NMDAR hypofunction is the internalization of NMDARs, through the process of antibody-mediated cross-linking followed by a reduction of NMDAR clusters from the neuronal surface (Hughes et al., 2010; Jezequel et al., 2018; Mikasova et al., 2012; Moscato et al., 2014). Recently, super-resolution microscopy in cultured hippocampal pyramidal neurons was used to visualize the clustering and internalization of synaptic receptors with antibody stimulation. Subsequently, the remaining receptors clustered and diffused to extra-synaptic sites, causing an overall reduction in synaptic receptor density (Ladepeche et al., 2018). Therefore, the main synaptic effect of the autoantibody appears to be receptor internalization and its re-distribution on the cell surface, leading to a reduction of NMDAR cluster density in the synapses.
Notably, NMDAR auto-antibodies do not affect the ionotropic function of NMDAR channels before internalization. For example, 30 minutes after treatment with autoantibody-containing patients’ cerebrospinal fluid (CSF), a time point before which significant receptor internalization occurs, there is no reduction in NMDA-mediated current amplitudes (Mikasova et al., 2012; Moscato et al., 2014). Therefore, a large reduction in NMDA currents 24 hours after the treatment with patient CSF (Hughes et al., 2010) is attributed to the receptor internalization/lateral diffusion, but not an acute blockade of channel function by the autoantibody. Conversely, antibody-mediated NMDAR internalization can occur in the presence of competitive antagonist AP-5 (Moscato et al., 2014), suggesting ion flux-independent (metabotropic) action of NMDARs (Dore et al., 2016; Montes de Oca Balderas, 2018). It has been shown that the autoantibodies disrupt the interaction between the NMDAR extracellular domain and its synaptic anchoring protein Ephrin-B2 receptor, a member of a family of receptor tyrosine kinases (Mikasova et al., 2012). The disruption of this interaction results in mislocalization of NMDARs to extrasynaptic sites followed by internalization. Receptor internalization is known to occur gradually in a similar fashion for both excitatory and inhibitory neurons, reaching plateau 12 hours after autoantibody treatment in cultured hippocampal neurons (Moscato et al., 2014). NMDAR-mediated miniature-EPSC amplitudes in pyramidal neurons are significantly reduced 24 hours after the antibody is added to the cultured neurons. While the impact of the autoantibody on the synaptic inputs to GABAergic neurons has not been tested, initial presentation of psychotic symptoms could be associated with the initial internalization of NMDARs on GABAergic neurons. Quantitative immunogold electron microscopic study revealed that GluN1 density is normally lowest in the dendrites of PV-positive FS neurons and highest in pyramidal cell spines in rat hippocampus (Nyiri et al., 2003). Therefore, it is tempting to speculate that NMDARs are depleted initially from the cell surface of FS neurons through antibody-mediated NMDAR internalization (Nakazawa et al., 2017). This may explain the slow onset of frank psychosis from prodromal symptom in the auto-NMDAR encephalitis. In contrast, since non-competitive antagonists directly inhibit ion current flow, the onset of PCP’s effects is quite rapid; onset of action occurs in 2–5 minutes, and effects may take 15 to 60 minutes when ingested orally (Cook et al., 1982).
It is notable that anti-NMDAR antibodies found in patients with anti-NMDAR encephalitis preferentially bind to the NMDARs in its open state, like open channel blockers. Lynch’s group showed that patients’ CSF containing NMDAR autoantibodies prolongs the open duration of NMDA channels in the presence of its agonist, while autoantibody itself does not promote channel opening. These results suggest that patients’ antibodies bind to NMDAR channels which are more prone to opening and stabilized in the open state (Gleichman et al., 2012). In a recent paper, they also found that patients’ autoantibodies bind to the GluN1 subunit in the presence of MK-801, but not AP5 (Sharma et al., 2018). Since MK-801 stabilizes the NMDAR in the open state (whereas the AP5 prevents the NMDAR opening), the autoantibody can bind to NMDAR when it is opened by depolarization.
Overall, the mechanism by which NMDAR autoantibodies induce psychotic symptoms, may involve the cross-linking of NMDAR channels by antibody’s binding to their open-state, similar to the binding of PCP/ketamine/MK-801. The similarities and differences between non-competitive antagonists and NMDAR autoantibodies are listed in Table 1.
Table 1:
Comparison between non-competitive NMDAR antagonists and NMDAR autoantibody
| Similarities |
| • Bind when the channel is in open state |
| • Cause symptoms similar to schizophrenia (psychosis, hallucinations, memory deficit etc) |
| Differences |
| • NMDAR antagonists block channel function whereas autoantibodies do not have effect on channel function. |
| • NMDAR antagonists reduce NMDAR function by inhibiting the channel whereas autoantibodies reduce NMDAR function by receptor internalization, thus reducing surface receptors, as well by diffusing receptors from synaptic to extra-synaptic sites. |
| • Most autoantibodies bind to N-terminus of GluN1 subunit, whereas NMDAR antagonists bind to the pore region of the channel and block ion permeation. |
It is noted that NMDAR autoantibody exposure not only promotes internalization of NMDARs from the cell membrane, but also impairs NMDAR-dependent synaptic plasticity at the synapses (Dupuis et al., 2014; Hughes et al., 2010; Wurdemann et al., 2016; Zhang et al., 2012). Patients’ CSF containing the autoantibody has also shown to acutely suppress global functional network activity in neuronal culture in vitro (Jantzen et al., 2013). These additional effects elicited by the autoantibodies could potentially contribute to the emergence of psychosis in this disorder.
While there is substantial evidence for the existence of antibodies which bind directly to the NMDARs, autoantibodies may also exist which target proteins upstream of NMDAR function. For example, mGluR5 is known to up-regulate the NMDARs via the Gq/11 protein and phospholipase C (Ellaithy et al., 2015), and mGluR5-NMDAR association is disrupted in schizophrenia (Gray et al., 2009). A recent report of anti-mGluR5 autoantibody encephalitis indicated that the most frequent neurologic manifestations are personality/mood change, full-blown psychosis, abnormal thought processes, and/or hallucinations (Spatola et al., 2018); somewhat similar to those observed in patients with anti-NMDAR encephalitis. This auto-antibody may internalize mGluR5 proteins, which could down-regulate NMDAR function. This finding suggests that not only NMDAR subunits themselves but also their interacting proteins that preserve NMDAR function could be targeted by their auto-antibodies, which may lead to auto-immune psychosis (Masdeu et al., 2016). The same logic could be applied to idiopathic schizophrenia, the onset of which may be triggered by dysfunction of NMDAR-interacting proteins.
4. Mouse models for NMDAR hypofunction in GABAergic interneurons
Functional NMDAR blockade appears to occur in cortical GABAergic interneurons in both PCP/ketamine drug abuse and anti-NMDAR encephalitis. Preclinically, mouse genetic approaches have been taken to test whether NMDAR deletion in GABAergic neurons confers schizophrenia-like phenotypes. Several groups have reported the effect of NMDAR disruption selectively in a subset of GABAergic interneurons in the brain (reviewed in (Nakazawa et al., 2017). Our group disrupted GRIN1 gene alleles in ~50% of cortical and hippocampal interneurons, the majority of which (>70%) were PV containing, from the 2nd postnatal week using mice expressing Cre recombinase under control of the Ppp1r2 promoter (Belforte et al., 2010; Nakao et al., 2019). According to the Allen Mouse Brain Atlas, endogenous expression of PPP1R2 transcripts is detected in neurons sparsely distributed throughout the entire cortex including mPFC, while significant expression is also observed in olfactory mitral cell layer, olfactory tubercle, piriform cortex, dorsomedial and ventral striatum, hippocampal CA1–3 pyramidal cells, and cerebellar Purkinje cells. Despite the expression pattern of endogenous PPP1R2, little Cre expression is observed in the striatum, olfactory tubercle, and cerebellum of the Ppp1r2Cre line, although aberrant Cre expression is detected in piriform cortex, tenia tecta and lateral septum. Some delayed Cre expression in CA1 pyramidal neurons is also detected after 15–16 weeks of age. Our GluN1 mutant mice grow and behave normally. However, when the animal is socially isolated from 8 weeks of age for over one week, the mutant mice start showing agitation-like hyperactivity, anxiety, anhedonia, impaired nest building, and altered social interaction. They also exhibit schizophrenia-typical behaviors, such as impaired prepulse inhibition (PPI) of startle reflex, deficits of spatial working memory as measured by Y-maze, and exacerbation of psychostimulant-induced hyperactivity. Under the group-housing condition, such mutant behavioral phenotypes appear mostly after 12 weeks of age. The mutants at 8 week-old also show social isolation-induced robust increase in reactive oxygen species (ROS) particularly in cortical PV neurons, suggesting that NMDAR hypofunction in FS neurons generates abnormally high concentrations of ROS (Jiang et al., 2013). These mutant mice are also impaired in evoked auditory steady-state responses at low gamma frequency (Nakao and Nakazawa, 2014), a measure of tone-evoked gamma oscillations that is robustly impaired in schizophrenia (Thune et al., 2016). Finally, in vivo brain microdialysis uncovered that striatal dopamine is excessively released in response to amphetamine in transgenic mice whereas dopamine release in medial prefrontal cortex (mPFC) is disrupted, similar to dopamine abnormalities in patients with schizophrenia (Slifstein et al., 2015; Weinstein et al., 2017). Interestingly, PV neuron-specific GluN1 knockout mice also showed similar deficits of dopamine release. Conversely, genetic GRIN1 deletion from somatostatin-positive interneurons did not show abnormality in amphetamine-induced dopamine release in either striatum or mPFC (Nakao et al., 2019). This indicates that NMDAR hypofunction selectively in PV neurons, but not in somatostatin-positive GABAergic neurons, is sufficient to produce presynaptic dopamine abnormalities seen in schizophrenia. Based on findings from our in vivo microdialysis study, we suggest that NMDAR hypofunction in GABAergic neurons, particularly in PV neurons, could be one of upstream events leading to schizophrenia-typical dopamine abnormality, a final common pathway to psychosis (Howes and Kapur, 2009). Notably, genetic GRIN1 deletion introduced in neurogliaform cells shows little schizophrenia-related phenotypes (Chittajallu et al., 2017), suggesting that the impact of NMDAR hypofunction for schizophrenia is cell-type specific.
It is recently proposed that NMDAR hypofunction in PV neurons does not play a role in schizophrenia (Bygrave et al., 2018), by demonstrating fewer behavioral phenotypes in conditional GluN1 knockout mice, in which the recombination was driven by the same Ppp1r2Cre-driver as in our studies. The observation of weaker behavioral phenotypes, such as PPI, of this study could be due to animal husbandry in less-stressful, well-enriched environments until behavioral testing. Alternatively, it could be due to the use of a different floxed-GRIN1 line, with reduced efficacy of recombination. Two other groups utilized a PV-Cre mouse line to genetically delete GluN1 selectively in PV neurons (Carlen et al., 2011; Korotkova et al., 2010). Although their PV-GluN1 KO mice showed an impairment in spatial and working memory, they were otherwise normal behaviorally. Apparently normal behavior may be due to the slow onset of GluN1 knockout mediated by PVALB gene promoter driven cre lines (PV-cre). Indeed, a robust increase in endogenous PV mRNA level is observed after postnatal 3rd week in rat visual cortex (Patz et al., 2004) and in mouse cortex (Lin et al., 2014). We also observed no schizophrenia-like phenotypes when GluN1 is genetically deleted from cortical GABA neurons after adolescence (Belforte et al., 2010). These findings suggest the existence of a critical period (i.e., postnatal first two weeks in rodent development, which corresponds to the third trimester of pregnancy to early postnatal period in human (Semple et al., 2013), during which NMDA hypofunction leads to the full spectrum of the schizophrenia illness (Nakazawa et al., 2017). Nonetheless, the studies using PV-GRIN1 KO mice also reported impairment in gamma oscillations (Carlen et al., 2012; Korotkova et al., 2010). We also showed dopamine abnormalities reminiscent of schizophrenia in our PV-GRIN1 KO mice (Nakao et al., 2019). Considering all of this evidence, we conclude that NMDAR hypofunction in PV neurons causes schizophrenia-like dopamine abnormalities and deficits in gamma oscillation and spatial working memory, implying that PV neuron-specific NMDAR hypofunction could constitute a major etiological cause of circuit dysfunction in schizophrenia.
5. Genetic mutations in NMDAR subunit genes leading to schizophrenia
A fundamental question in the field is whether NMDAR hypofunction in GABAergic neurons takes place in idiopathic schizophrenia. Recent genetic studies suggest that genetic mutations in NMDAR subunits can give rise to various neurodevelopmental disorders, including schizophrenia. Whole-exome sequencing revealed a variety of mutations in each GRIN subunit gene (GRIN1, GRIN2A-2D, GRIN3A-3B). Deleterious missense, nonsense, or frameshift mutations in those genes have been reported to cause encephalopathies (named as “GRIN encephalopathy”) that are often first diagnosed as intellectual disability, epilepsy, autism, or schizophrenia (Hardingham and Do, 2016; Lemke et al., 2016; XiangWei et al., 2018). In certain cases, the mutations cause a reduction or loss of function, while in others, the function is enhanced. Both situations produce aberrant synaptic transmission of neurons and therefore affect the development of the brain and its proper functioning. Generally, epilepsy and intellectual disability are most commonly associated with mutations in GRIN1, GRIN2A, or GRIN2B subunit genes (Hu et al., 2016; Strehlow et al., 2019; XiangWei et al., 2018). Interestingly, out of 7 cases of GRIN2D rare variants, four patients were diagnosed with schizophrenia (XiangWei et al., 2018). An exome-sequencing of six GRIN subunits (except GRIN2B) also identified rare mutations in 25 out of 370 Japanese patients with schizophrenia, indicating that about 7% of patients with idiopathic schizophrenia carry a mutation in GRIN subunit genes (Yu et al., 2018). These findings suggest that ultra-rare variants in GRIN genes contributes to the genetic risk of schizophrenia. Since a majority of GRIN rare variants found in mental disorders are loss-of-function mutations (Genovese et al., 2016; Kataoka et al., 2016; MacArthur et al., 2012), these mutations may elicit, or exacerbate the risk of, NMDAR hypofunction in some cell-types, depending on the mutation types. However, overall contribution of ultra-rare genetic mutations of NMDAR and their interacting proteins may be small among idiopathic schizophrenia cases.
6. NMDAR auto-antibodies detectable in idiopathic schizophrenia
Discovery of anti-NMDAR encephalitis raises the possibility that autoantibodies could be directly responsible for the psychotic symptoms of idiopathic schizophrenia. However, the prevalence of circulating NMDAR autoantibodies is considerably variable between studies. An initial meta-analysis revealed three times greater serum positivity in patients with mental disorders including schizophrenia, compared with healthy controls (Pearlman and Najjar, 2014). In another meta-analysis study of seven reports comprising 1441 patients with idiopathic schizophrenia, 115 (8%) of patients were seropositive for anti-NMDAR antibodies (Pollak et al., 2014). From the recent OPTIMISE project, approximately 5% of NMDAR autoantibody seropositive patients were identified among first-episode psychosis patients with minimal or no exposure to antipsychotics (Jezequel et al., 2018). Another single large study emphasized that NMDAR antibodies are significantly more prevalent in patients with first-episode psychosis patients (seven [3%] of 228 participants) than in controls (0 of 105 participants)(Lennox et al., 2017). In these studies, cell-based immunofluorescent staining was used to qualitatively detect the antibody binding to the cell surface NMDARs expressed in HEK293 cells. A recently developed ELISA assay has enabled the measurement of the NMDAR antibody quantitatively (Sharma et al., 2018). Using this assay, an elevated level of serum anti-NMDAR antibody has been detected in first-episode patients with schizophrenia (Tong et al., 2019). Interestingly, the serum antibody levels were positively correlated with PANSS scores, suggesting that symptom severity is associated with antibody levels. However, lack of sero-positivity in first-episode schizophrenia patients has also been reported (Chen et al., 2017; Mantere et al., 2018; Nakagami et al., 2018). Overall, these results suggest that anti-NMDAR autoantibody may play a role in a minor fraction of patients with schizophrenia. In the future, more sensitive diagnostic tests may provide a better estimate of the prevalence of anti-NMDAR antibodies in idiopathic schizophrenia.
These examples of anti-NMDAR antibodies in patients raise the possibility of an involvement of the immune system in schizophrenia. In support of this, an epidemiological study in Denmark showed that prior autoimmune disease increased the risk of schizophrenia by 30%, while a history of hospitalization with infection increased the risk of schizophrenia by 60% (Benros et al., 2011). In addition, immune abnormalities, such as increased CSF levels of pro-inflammatory cytokines and blood-CSF barrier disturbances, have been detected in a subset of patients with schizophrenia or affective disorders (Cai et al., 2018; Endres et al., 2015; Goldsmith et al., 2016).
A possible connection between immune abnormalities and the generation of anti-NMDAR antibodies is the Toxoplasma gondii infection. It is suspected that toxoplasmosis during gestation affects neurodevelopment and contributes to later onset of schizophrenia (Tyebji et al., 2019). Recent studies demonstrate that exposure to live Toxoplasma parasite in mice produces autoantibody to GluN2 and schizophrenia-related impairments (Kannan et al., 2017). Generation of GluN2 autoantibodies in this study may be elicited by T. gondii-induced cross-reactive immune attacks, because sequence-matching analysis showed a massive peptide sequence overlap between Toxoplasma and GluN2 subunits of NMDARs, in particular, GluN2D (Lucchese, 2017). Indeed, elevated anti-T. gondii antibodies are detected more frequently in human psychiatric patients than in control subjects (Torrey et al., 2007). It is plausible that T. gondii-induced anti-GluN2D cross reactivity may be elicited, at least transiently, during development, which may elicit NMDAR hypofunction, particularly in the GluN2D-expressing GABAergic neurons. In addition, other viral infections, such as herpes simplex virus-1 (HSV-1) injection, also cause NMDAR antibody synthesis (Leypoldt et al., 2013; Salovin et al., 2018). Notably, antibodies against NMDARs have been reported to be detectable in the early course of illness in schizophrenia but not in its chronic stage (Zandi et al., 2011), which could be due to disappearance of NMDAR antibodies over time. Even if the NMDAR auto-antibody was transiently produced by the infection-induced cross-reactivity during development, the antibody may not be detectable when the symptoms of schizophrenia appear in young adulthood. Overall, it is not surprising if NMDAR-autoantibodies could contribute to schizophrenia, by intercurrent neuroinflamation or infection during the critical period in development. Screening of individuals for anti-NMDAR autoantibodies during premormid/prodromal period might be informative.
7. Evidence for NMDAR hypofunction in postmortem brains
The results in postmortem brain studies to measure the expression of GluN subunits are mixed, depending upon the brain region examined and methodology used. GluN1 mRNA/protein levels have been repeatedly examined in postmortem brains of patients with schizophrenia. A recent meta-analysis reported overall decrease in GluN1 mRNA/protein level in the cortex (Catts et al., 2016). Another report concluded no consistent alterations of GluN mRNA expression (Hu et al., 2015). Few postmortem studies have been performed focusing on the GABAergic neurons. Woo’s group showed that the expression of GluN2A mRNA was decreased to a level that is no longer experimentally detectable in 49–73% of the GABAergic neurons in both the prefrontal cortex (Bitanihirwe et al., 2009) and anterior cingulate cortex (Woo et al., 2004) of subjects with schizophrenia. To obtain the better cellular resolution using postmortem brains, it may be crucial to assess the transcript levels at single-cell resolution (Arion et al., 2017; Wang et al., 2018).
8. NMDAR-interacting proteins that diminish NMDAR function in GABAergic neurons
The evidence for altered expression of GluN subunits in GABAergic neurons has been limited in postmortem brain studies. Even in the absence of changes in the expression of NMDAR subunits, NMDAR function can be affected by changes in the expression of proteins involved in its upstream or downstream signaling. Several underlying mechanisms have been proposed to compromise NMDAR function (Harrison, 2015; Ross et al., 2006), and a few studies suggest that the hypofunction resides in the GABAergic interneurons (see Figure 1). For example, altered signaling of a cell adhesion molecule neuregulin 1 (NRG1) and its receptor ErbB4 has been implicated in schizophrenia (Harrison and Law, 2006; Mei and Nave, 2014; Mostaid et al., 2016). Several postmortem studies showed an increase in expression of NRG1 (Hashimoto et al., 2004; Law et al., 2006) and hyper-phosphorylation (over-activation) of ErbB4 (Hahn et al., 2006) in schizophrenia patients. Interestingly, NRG1-ErbB4 signaling inhibits Src kinase activity, leading to the blockade of NMDAR function (Banerjee et al., 2015; Pitcher et al., 2011). Since ErbB4 is highly expressed in PV-positive GABAergic neurons, but not pyramidal neurons (Fazzari et al., 2010; Vullhorst et al., 2009), excessive NRG1-ErbB4 signaling could elicit NMDAR hypofunction in GABA neurons. Indeed, thy1 promotor-driven overexpression mice of NRG1 have been generated, which show reduced synaptic NMDA currents in PV- or cholecystokinin (CCK)-expressing hippocampal interneurons, but not in pyramidal cells (Kotzadimitriou et al., 2018). Notably, overexpression of NRG1 in mice elicits schizophrenia-relevant behavioral phenotypes, including deficits in social preference, impaired fear memory, reduced prepulse inhibition, and altered locomotor sensitivity to MK-801 (Olaya et al., 2018a; Olaya et al., 2018b). It has also been shown that neuregulin 2 (NRG2), a close relative of NRG1, is expressed in cortical GABAergic neurons and activates ErbB4-positive GABAergic neurons cell-autonomously, leading to internalization of NMDARs in those interneurons, but not in pyramidal neurons (Vullhorst et al., 2015).
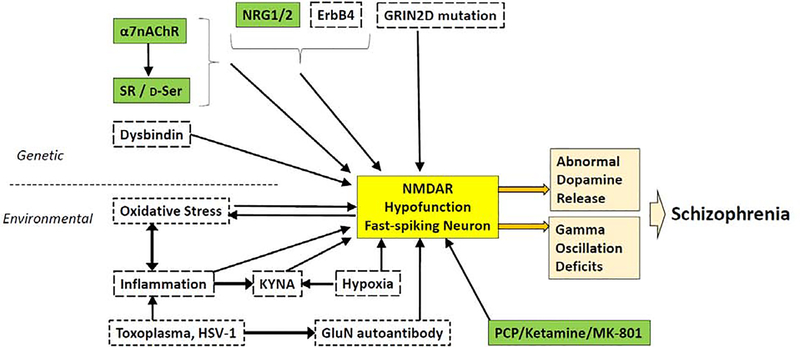
Figure 1:
NMDAR hypofunction in corticolimbic fast-spiking (FS) neurons is a convergent mechanism of major genetic and/or environmental risks, leading to schizophrenia. Genetic and environmental risk factors which can cause NMDAR hypofunction (NMDA currents or NMDAR subunit expression levels) are presented. Factors shown in green boxes are likely to elicit NMDAR hypofunction in GABAergic neurons. Factors in dotted boxes may produce NMDAR hypofunction but remain unexplored in GABAergic neurons. There is ample evidence demonstrating that NMDAR antagonists (ketamine/MK-801) bind to NMDARs in parvalbumin (PV)-positive FS neurons. Overexpression of neuregulin (NRG)-1 produces NMDAR hypofunction in GABAergic neurons, including PV neurons, and exposure to NRG-2 elicits down-regulation of NMDARs in GABAergic neurons. Null mutant mice of α7 nicotinic acetylcholine receptor (α7AChR) show NMDAR hypofunction of GABAergic neurons in which α7 receptors are expressed. α7 receptor null mutant mice also display a robust reduction of D-serine and its synthesizing enzyme SR; otherwise, SR and D-serine are abundantly expressed by GABAergic neurons including PV neurons, suggesting that SR/D-serine deficiency may cause NMDAR hypofunction in FS neurons (also see Figure 2). NMDAR autoantibodies from patients with anti-NMDAR encephalitis may preferentially bind to NMDARs to down-regulate activity when the channel is in open state (see Table 1). Prevalence of schizophrenia appears to be high with rare mutations of the GRIN2D gene, which is predominantly expressed in FS neurons in the cortex and hippocampus. Finally, FS interneurons are highly susceptible to oxidative stress evoked by NMDAR hypofunction, while oxidative stress itself diminishes NMDAR function, coupling oxidative stress and NMDAR hypofunction in FS neurons and synergistically contributing to schizophrenia phenotypes.
Another way that NMDAR hypofunction can occur in PV-containing cortico-hippocampal GABAergic neurons is via modulation of the α7 nicotinic acetylcholine receptor (α7nAChR, forming homopentameric receptor). α7nAChR is highly expressed in the PV-containing interneurons in the cortex (Murakami et al., 2013) and hippocampus (Adams et al., 2001). A homozygous microdeletion of human chromosome 15q13.3, including CHRNA7 gene encoding α7nAChR, is associated with several neurodevelopmental disorders, including schizophrenia (Lowther et al., 2015). In human postmortem studies, reduced levels of α7nAChR are repeatedly reported in the PFC and hippocampus of schizophrenia patients (Ross et al., 2010). Genetic deletion of α7nAChR gene in mice (null mice) produces deficits in attention and working memory (Fernandes et al., 2006). A series of experiments led by Lin H et al. showed a reduced expression of GABAergic markers (PV and Gad67), resulting in a robust reduction of spontaneous IPSC frequency (i.e., presynaptic GABA release reduction) in α7nAChR null mutant mice (Lin et al., 2014). More interestingly, GluN1-immunoreactivity (IR) was also robustly reduced in the GABAergic neurons in mutant cortical cell culture, suggesting a reduction and hypofunction of NMDARs in cortical GABAergic interneurons (potential mechanism addressed below).
Lin et al. also found that the levels of both D-serine, endogenous co-agonist of synaptic NMDARs, and its synthesizing enzyme serine racemase (SR) are reduced in the α7nAChR null mutant mice (Lin et al., 2014). Their in-depth immunocytochemistry analysis further revealed that D-serine and SR are both co-localized with PSD-95, a postsynaptic marker at glutamatergic synapses on cortical glutamatergic and GABAergic neurons, but are not colocalized with a glutamatergic presynaptic marker, vesicular glutamate transporter 1 (Lin et al., 2016), suggesting both D-serine and SR are located in the postsynaptic density of excitatory synapses, but not in the presynaptic terminals. These novel findings provided two important implications (see Figure 2). First, contrary to the notion that NMDAR co-agonist D-serine is released from presynaptic terminals acting on the postsynaptic NMDARs, an emerging idea is that D-serine is synthesized and released from the postsynaptic neurons and acting on the postsynaptic NMDARs in an autocrine fashion (Coyle and Balu, 2018). Furthermore, since the levels of D-serine and SR are reduced in α7nAChR null mutant mice, it is conceivable that α7nAChR somehow maintains postsynaptic SR and D-serine levels, contributing to maturation of NMDAR-containing glutamatergic synapses. Indeed, synaptic NMDA currents in mPFC pyramidal neurons were diminished in the α7nAChR null mutant mice (Lin et al., 2014).
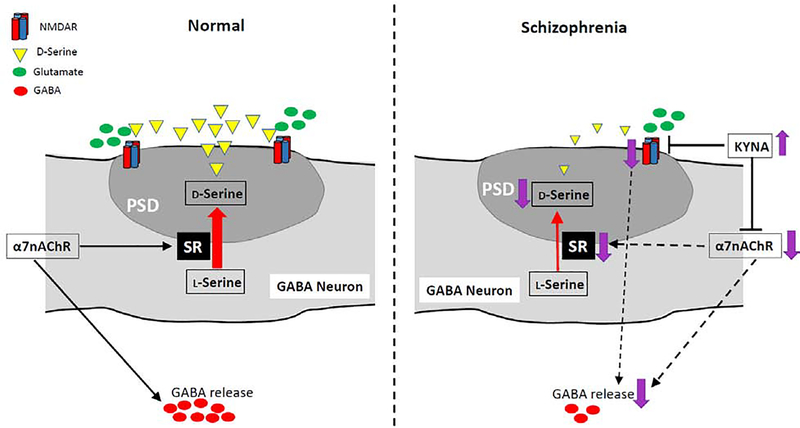
Figure 2:
A hypothetical mechanism of NMDAR hypofunction in GABAergic neurons via reductions in serine racemase (SR) and α7nAChR activity, and/or an increase in brain kynurenic acid (KYNA). The colocalization of SR and its product D-serine in the PSD (postsynaptic density) of GABAergic neurons suggests that the co-agonist D-serine binds to postsynaptic NMDARs in an autocrine fashion and that α7nAChR maintains SR/D-serine levels in GABAergic neurons. Dysregulation of α7nAChR activity can downregulate SR, leading to D-serine deficiency. Thus, hypofunction of α7nAChR and/or SR itself can elicit NMDAR hypofunction in GABAergic neurons, leading to a deficit in synchronized GABA release. GABA release may also be directly disrupted by α7nAChR downregulation. Neuroinflammation-mediated increase in brain KYNA, an antagonist of both NMDARs and α7nAChR, may further inhibit these two receptors, thereby exacerbating the symptoms of schizophrenia.
Notably, α7nAChR appears to maintain the levels of SR and D-serine located in GABAergic neurons, because, as mentioned above, GluN1-immunoreactivity in GABAergic neurons is diminished in α7nAChR null mutants. Indeed, it has been reported that more than half of the D-serine positive neurons are GABAergic interneurons, with a majority of these neurons containing PV and/or somatostatin, while SR-IR is detected in only ~25–40 % of interneurons in the neocortex and hippocampus (Balu et al., 2014). These findings suggest that D-serine is synthesized by SR and locally released at least from a subset of GABAergic neurons, thereby activating NMDARs on GABAergic neurons. It is tempting to speculate that NMDAR hypofunction occurs in PV neurons by the reduction of SR activity, regardless of α7nAChR action. Actually, both SR and D-serine levels are reduced in schizophrenia (Bendikov et al., 2007; Hashimoto et al., 2003). Analysis of genetic variants of SRR gene (encoding SR) in humans identified a robust association with schizophrenia (Labrie et al., 2009). SR null mutant mice (Basu et al., 2009) or SR inhibitor-treated mice (Hagiwara et al., 2013) has been presented as a mouse model of NMDAR hypofunction, due to their striking similarities to the neuropathologic, neurochemical and cognitive deficits associated with schizophrenia
Besides NRGs, ErbB4, α7nAChR and SR, there are many more schizophrenia susceptibility genes known to be linked to NMDAR-mediated glutamatergic neurotransmission in the brain (Pocklington et al., 2014). For example, the dysbindin complex is shown to regulate in NMDA currents in prefrontal pyramidal neurons, as well as impair GluN1 expression and degradation (Karlsgodt et al., 2011; Larimore et al., 2014). Further studies are necessary to assess whether genetic variants of such genes could alter NMDAR function particularly in GABAergic neurons.
9. Environmental factors which elicit NMDAR hypofunction
An extensive body of research links schizophrenia pathogenesis to prenatal events and obstetric complications, such as hypoxia, maternal infection, and autoimmune disease (Boksa, 2004; Brown, 2011). Many of them are associated with oxidative stress and inflammation, which are mutually dependent (Fraguas et al., 2019). Oxidative stress, characterized by an imbalance between the ROS/reactive nitrogen species (RNS) production and their elimination by protective mechanisms, can lead to chronic inflammation. Notably, NMDAR hypofunction and redox imbalance are reciprocally linked. In schizophrenia, multiple studies have found lower levels of glutathione (GSH) (Nucifora et al., 2017), a cofactor of multiple enzymes whose reduction-oxidation (“redox”) reactions convert harmful free-radicals (ROSs) into benign, less-reactive molecules. Oxidizing agents or reducing GSH decrease NMDA currents in cortical (Aizenman et al., 1990; Aizenman et al., 1989) and hippocampal pyramidal neurons (Bernard et al., 1997; Do et al., 2009; Steullet et al., 2006), presumably through oxidation of extracellular cysteine residues on the GluN1 and GluN2A (Lipton et al., 2002). Modulation of intracellular redox state has also been shown to alter the NMDA responses at hippocampal synapses in the aged, but not young rats (Bodhinathan et al., 2010), which may be relevant for the study of age-dependent cognitive decline. Conversely, NMDAR hypofunction, itself, can lead to an increase in oxidative stress. At the cellular level, synaptic NMDARs activity has been shown to boost the capacity of two anti-oxidant pathways, GSH and thioredoxin/peroxiredoxin, through the transcriptional control of several key antioxidant genes (Baxter et al., 2015; Papadia et al., 2008). Consistently, the NMDAR antagonist ketamine induces a persistent increase in ROS through NADPH oxidase 2 (Nox2) activation, and the increase in oxidative stress causes the reduction of PV and Gad67 expression (Behrens et al., 2007). Genetic deletion of GRIN1 from cortical GABAergic neurons produced massive ROS in PV neurons particularly when mice were socially isolated (Jiang et al., 2013). Furthermore, GRIN2A null mutant mice displayed delayed maturation of PV neurons in the cortex, which appears to be caused by a disruption of antioxidant system (Cardis et al., 2018). The unique properties of PV neurons may render them particularly vulnerable to oxidative stress. PV neurons need to produce abundant ATP from mitochondria to maintain high-frequency firing and higher metabolism. As a consequence, increased production of ATP results in a massive ROS production as byproducts (Kann et al., 2014). Therefore, oxidative stress and NMDAR hypofunction in PV interneurons may be mechanistically inter-dependent, synergistically contributing to schizophrenia pathophysiology (Hardingham and Do, 2016).
Neuroinflammation also appears to diminish NMDAR activity. For example, systemic injection of methylazoxymethanol (MAM) to rats on gestational day 17 is known to produce schizophrenia-like phenotypes in adulthood (Modinos et al., 2015). It has been shown that MAM treatment decreases synaptic GluN2B protein levels and NMDA component-spontaneous EPSCs in mPFC Layer 5 pyramidal neurons at postnatal day (PND)-21 (Gulchina et al., 2017). Interestingly, epigenetic transcriptional repressor (RE1-Silencing Transcription factor; REST) and the repressive histone marker H3K27me3 were unusually enriched at the GluN2B promotor, suggesting that NMDAR hypofunction is induced by epigenetic mechanisms (Snyder and Gao, 2019).
Another potential route of neuroinflammation leading to NMDAR hypofunction may be mediated by endogenous NMDAR antagonist, kynurenic acid (KYNA). KYNA is a metabolite in the neuroprotective branch of the kynurenine pathway of tryptophan degradation (Erhardt et al., 2017; Schwarcz et al., 2012). Activated microglia release inflammatory mediators, such as interleukin (IL)-1β and IL-6, which induce synthesis of KYNA in brain astrocytes (Schwieler et al., 2015; Sellgren et al., 2016; Urata et al., 2014). Indeed, recent meta-analysis studies reported that IL-1β, IL-6 and IL-8 (Gallego et al., 2018; Orlovska-Waast et al., 2019) as well as KYNA and its precursor kynurenine (Plitman et al., 2017; Wang and Miller, 2018) are all elevated in the CSF of patients with schizophrenia. This makes the kynurenine pathway of great interest in mental illnesses such as schizophrenia, where a low-grade inflammation has been hypothesized to give rise to symptoms (Muller et al., 2015), possibly through an elevation of brain KYNA (Erhardt et al., 2017).
Dysregulation of kynurenine pathway, particularly, elevation of central KYNA can induce cognitive impairments in rodents. For example, exposure to KYNA during adolescence impairs PPI, spatial working memory, attention, social interaction, and reward learning (Alexander et al., 2012; Chess et al., 2007; Erhardt et al., 2004; Trecartin and Bucci, 2011). Genetic and pharmacological manipulations to elicit increased KYNA level in the brain also cause increased firing rates and burst firing of midbrain dopamine neurons (Erhardt and Engberg, 2002; Linderholm et al., 2016; Tufvesson-Alm et al., 2018). These effects could be explained by the KYNA’s antagonist action on NMDARs on mPFC GABA neurons, leading to disinhibition of mPFC pyramidal neurons (for cognitive dysfunction), or NMDARs on ventral tegmental area (VTA) GABAergic neurons disinhibiting dopamine neurons (for positive symptom) (Erhardt et al., 2017).
Excessive activation of dopamine neurons by the increased KYNA may also be elicited by the disinhibition of two remote areas in the brain. One is infralimbic region of the mPFC; neurons in this region inhibit VTA interneurons via the activation of the basolateral amygdala (Patton et al., 2013; Tufvesson-Alm et al., 2018). Another is the ventral subicular neurons, which has suggested to trigger mid brain dopamine neuron activation via nucleus accumbens and ventral pallidum (Grace, 2017). Disinhibition of mPFC, ventral subiculum, or VTA dopamine neurons could all be attributed to a decrease in GABA release triggered by KYNA’s NMDAR antagonistic action on local GABAergic interneurons. However, it appears that KYNA can also directly inhibit GABA release by antagonizing presynaptic α7nAChRs on the GABAergic axon terminals, allowing disinhibition in the mPFC (Flores-Barrera et al., 2017). Furthermore, it is conceivable that chronic blockade of α7nAChRs by KYNA may down-regulate SR and D-serine in the GABA neurons, thereby inducing GABAergic NMDAR hypofunction and attenuation of GABAergic neuron function. In any case, the action of central KYNA is still debated, in particular, whether it antagonizes NMDARs and/or α7nAChR. Further research is warranted to delineate the action of KYNA inducing disinhibition in cortical area and VTA.
Finally, hypoxia during development is also known to compromise NMDAR function (Waters and Machaalani, 2004). Prenatal hypoxia causes a decrease in the number of NMDARs (Tingley et al., 1997), and decreased glutamate- and glycine-dependent activation of NMDARs (Mishra and Delivoria-Papadopoulos, 1992). Neonatal hypoxia can cause decreased NMDAR binding in the frontal cortex of new-born piglets (Hoffman et al., 1994). Rats that received chronic, repeated hypoxia during PND 4–8 exhibited a deficit in PPI of startle reflex in adulthood. Those rats in PND11 showed a reduction of MK-801 binding to NMDARs in frontal and temporal cortex, hippocampus and nucleus accumbens, while GluN1 mRNA levels were elevated (Schmitt et al., 2007). In cultured cortical neurons, hypoxia also enhances nitric oxide-mediated redox modulation of NMDARs, resulting in increased attenuation of NMDA-evoked currents (Takahashi et al., 2007). From the study of anoxia-tolerated western painted turtle, Ca2+ release from the mitochondria during anoxia appears to trigger the calmodulin binding and dephosphorylation of NMDAR subunits, causing the changes in protein confirmation that inhibits NMDAR redox sites and decrease the NMDAR activity (Dukoff et al., 2014). However, these studies only investigated changes in pyramidal neurons; it is unclear whether NMDAR activity in immature GABAergic neurons is also diminished.
10. Future therapeutic strategies
Given the substantial evidence presented above that NMDAR activity is impaired in GABAergic neurons in schizophrenia, these neurons could be considered as novel cellular targets for therapeutic intervention. One possible strategy would be to selectively augment NMDAR function of GABAergic neurons by targeting the GluN subunits preferentially expressed in cortical GABA neurons in adulthood. One potential candidate is GluN2D; CIQ is a positive allosteric modulator of GluN2C/D-containing NMDARs. However, CIQ increases phasic release of dopamine through presynaptic activation of GluN2D-containing NMDARs located on dopaminergic terminals (Zhang et al., 2014). Therefore, any benefit by enhancing GluN2D-containing NMDAR function of cortical GABAergic neurons could potentially be complicated by increase in striatal dopamine release, exacerbating positive symptoms.
NMDAR hypofunction in PV neurons appears to cause reduced activity of PV neurons. Prolonged exposure to MK-801 in adolescence is associated with a reduction in AMPAR-mediated synaptic currents in FS neurons in rat mPFC (Wang and Gao, 2012). Subacute blockade of GluN2C/2D-containing NMDARs by DQP-1105 from PND7–9 causes a robust decrease in GABA release and an impaired maturation of interneuron dendritic arborization in mouse sensorimotor cortex (Hanson et al., 2019). One potential approach to rescue the hypofunction of PV neurons would be to boost up the spiking rate of GABAergic neurons. For instance, positive allosteric modulation of Kv3.1/3.2 channels is known to increase intrinsic firing frequency of FS neurons (Boddum et al., 2017). Systemic injections of AUT00063 in vivo can improve auditory synchronization and promote more accurate decoding of sounds in the inferior colliculus and auditory cortex in adult mice (Chambers et al., 2017). A small molecule activator AA43279 of Nav1.1 channels also increases FS neuron excitability and GABAergic transmission in vitro and has anti-convulsive effects in vivo (Frederiksen et al., 2017).
Finally, neural stem cell technology has been developed to the level that makes embryonic stem cell line to generate enriched populations of PV- or somatostatin (SST)-positive interneurons. Previous studies showed that transplantation of cortical interneuron progenitor cells can prevent the induction of PCP-induced cognitive deficits (Tanaka et al., 2011). Stem cell-derived interneuron transplantation into the ventral hippocampus of MAM-treated mice causes an increase in PV neuron number (without affecting on SST-enriched neurons) and normalizes all the behaviors examined (Donegan et al., 2017). It will be interesting to explore whether NMDAR function in PV neurons is altered in this model.
11. Conclusions
NMDARs play important roles in many aspects of neurobiological processes, including brain development, learning and memory, and neuroplasticity. Consequently, abnormal expression levels and altered NMDAR function have been implicated in numerous neurological disorders and pathological conditions. Whereas NMDAR overstimulation causes excitotoxicity and subsequent neurodegeneration, such as in stroke, head trauma, neuropathic pain, Huntington’s and Alzheimer’s disease (Zhou and Sheng, 2013), NMDAR hypofunction is also found in autism (Won et al., 2012), early-phase AD (Liu et al., 2019) and cognitive dementia (Lin and Lane, 2019). Here we suggest in schizophrenia and related neurodevelopmental disorders that NMDAR hypofunction takes place in GABAergic neurons in early postnatal period, while the mechanisms inducing NMDAR hypofunction could be highly heterogeneous. Future research to delineate the upstream and downstream pathways of NMDAR hypofunction is warranted to elucidate the pathophysiological process in schizophrenia.
Acknowledgement
We apologize to the authors whose excellent studies were not cited in this review because of space limitations. We thank Dr. Rita Cowell for critical review of the manuscript. This review was supported by NIH research grants including R01MH110681.
Abbreviations
- α7nAChR
α7 nicotinic acetylcholine receptor
- CCK
cholecystokinin
- CSF
cerebrospinal fluid
- EPSC
excitatory postsynaptic current
- FS
fast spiking
- GABA
gamma-aminobutyric acid
- Gad67
glutamic acid decarboxylase-67
- GSH
glutathione
- IR
immunoreactivity
- IPSC
inhibitory postsynaptic current
- IL
Interleukin
- KO
knockout
- KYNA
kynurenic acid
- MAM
methylazoxymethanol
- MK-801
dizocilpine
- mPFC
medial prefrontal cortex
- NRG
neuregulin
- NMDA
N-Methyl-d-Aspartate
- NMDAR
NMDA receptor
- PPI
prepulse inhibition
- PV
parvalbumin
- PCP
phencyclidine
- PND
postnatal day
- PSD-95
postsynaptic density protein 95
- ROS
reactive oxygen species
- SR
serine racemase
- SST
somatostatin
- VTA
ventral tegmental area
Footnotes
Conflict of interest statement
The authors declare that there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams BW, Bradberry CW and Moghaddam B (2002) NMDA antagonist effects on striatal dopamine release: microdialysis studies in awake monkeys. Synapse 43:12–18. [DOI] [PubMed] [Google Scholar]
- Adams CE, Stitzel JA, Collins AC and Freedman R (2001) Alpha7-nicotinic receptor expression and the anatomical organization of hippocampal interneurons. Brain Res 922:180–190. [DOI] [PubMed] [Google Scholar]
- Aizenman E, Hartnett KA and Reynolds IJ (1990) Oxygen free radicals regulate NMDA receptor function via a redox modulatory site. Neuron 5:841–846. [DOI] [PubMed] [Google Scholar]
- Aizenman E, Lipton SA and Loring RH (1989) Selective modulation of NMDA responses by reduction and oxidation. Neuron 2:1257–1263. [DOI] [PubMed] [Google Scholar]
- Al-Diwani A, Handel A, Townsend L, Pollak T, Leite MI, Harrison PJ, Lennox BR, Okai D, Manohar SG and Irani SR (2019) The psychopathology of NMDAR-antibody encephalitis in adults: a systematic review and phenotypic analysis of individual patient data. The lancet Psychiatry 6:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KS, Wu HQ, Schwarcz R and Bruno JP (2012) Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berl) 220:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaad HA, DeKorver NW, Mao Z, Dravid SM, Arikkath J and Monaghan DT (2019) In the Telencephalon, GluN2C NMDA Receptor Subunit mRNA is Predominately Expressed in Glial Cells and GluN2D mRNA in Interneurons. Neurochem Res 44:61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arion D, Huo Z, Enwright JF, Corradi JP, Tseng G and Lewis DA (2017) Transcriptome Alterations in Prefrontal Pyramidal Cells Distinguish Schizophrenia From Bipolar and Major Depressive Disorders. Biological psychiatry 82:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla A, Koneru R, Smiley J, Sershen H and Javitt DC (2001) Continuous phencyclidine treatment induces schizophrenia-like hyperreactivity of striatal dopamine release. Neuropsychopharmacology 25:157–164. [DOI] [PubMed] [Google Scholar]
- Balu DT, Takagi S, Puhl MD, Benneyworth MA and Coyle JT (2014) D-serine and serine racemase are localized to neurons in the adult mouse and human forebrain. Cell Mol Neurobiol 34:419–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Wang HY, Borgmann-Winter KE, MacDonald ML, Kaprielian H, Stucky A, Kvasic J, Egbujo C, Ray R, Talbot K, Hemby SE, Siegel SJ, Arnold SE, Sleiman P, Chang X, Hakonarson H, Gur RE and Hahn CG (2015) Src kinase as a mediator of convergent molecular abnormalities leading to NMDAR hypoactivity in schizophrenia. Mol Psychiatry 20:1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, Snyder SH, Bergeron R and Coyle JT (2009) Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry 14:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter PS, Bell KF, Hasel P, Kaindl AM, Fricker M, Thomson D, Cregan SP, Gillingwater TH and Hardingham GE (2015) Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nature communications 6:6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley S, Sullenberger T, Pilli J, Abbasi S, Gunjan A and Kumar SS (2019) Colocalization of distinct NMDA receptor subtypes at excitatory synapses in the entorhinal cortex. J Neurophysiol 121:238–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL and Dugan LL (2007) Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 318:1645–1647. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM and Nakazawa K (2010) Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci 13:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendikov I, Nadri C, Amar S, Panizzutti R, De Miranda J, Wolosker H and Agam G (2007) A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr Res 90:41–51. [DOI] [PubMed] [Google Scholar]
- Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO and Mortensen PB (2011) Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry 168:1303–1310. [DOI] [PubMed] [Google Scholar]
- Bernard CL, Hirsch JC, Khazipov R, Ben-Ari Y and Gozlan H (1997) Redox modulation of synaptic responses and plasticity in rat CA1 hippocampal neurons. Exp Brain Res 113:343–352. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe B, Lim M, Kelley J, Kaneko T and Woo T (2009) Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry 9:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied TA, Boeckman FA, Aizenman E and Johnson JW (1997) Trapping channel block of NMDA-activated responses by amantadine and memantine. J Neurophysiol 77:309–323. [DOI] [PubMed] [Google Scholar]
- Boddum K, Hougaard C, Xiao-Ying Lin J, von Schoubye NL, Jensen HS, Grunnet M and Jespersen T (2017) Kv3.1/Kv3.2 channel positive modulators enable faster activating kinetics and increase firing frequency in fast-spiking GABAergic interneurons. Neuropharmacology 118:102–112. [DOI] [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A and Foster TC (2010) Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J Neurosci 30:1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P (2004) Animal models of obstetric complications in relation to schizophrenia. Brain research Brain research reviews 45:1–17. [DOI] [PubMed] [Google Scholar]
- Brown AS (2011) The environment and susceptibility to schizophrenia. Prog Neurobiol 93:23–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave AM, Masiulis S, Kullmann DM, Bannerman DM and Katzel D (2018) Gene-Environment Interaction in a Conditional NMDAR-Knockout Model of Schizophrenia. Frontiers in behavioral neuroscience 12:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai HQ, Catts VS, Webster MJ, Galletly C, Liu D, O’Donnell M, Weickert TW and Weickert CS (2018) Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardis R, Cabungcal JH, Dwir D, Do KQ and Steullet P (2018) A lack of GluN2A-containing NMDA receptors confers a vulnerability to redox dysregulation: Consequences on parvalbumin interneurons, and their perineuronal nets. Neurobiol Dis 109:64–75. [DOI] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI and Tsai LH (2011) A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI and Tsai LH (2012) A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry 17:537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts VS, Lai YL, Weickert CS, Weickert TW and Catts SV (2016) A quantitative review of the postmortem evidence for decreased cortical N-methyl-d-aspartate receptor expression levels in schizophrenia: How can we link molecular abnormalities to mismatch negativity deficits? Biological Psychology 116:57–67. [DOI] [PubMed] [Google Scholar]
- Chambers AR, Pilati N, Balaram P, Large CH, Kaczmarek LK and Polley DB (2017) Pharmacological modulation of Kv3.1 mitigates auditory midbrain temporal processing deficits following auditory nerve damage. Sci Rep 7:17496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Cheng MC, Liu CM, Liu CC, Lin KH and Hwu HG (2017) Seroprevalence survey of selective anti-neuronal autoantibodies in patients with first-episode schizophrenia and chronic schizophrenia. Schizophr Res 190:28–31. [DOI] [PubMed] [Google Scholar]
- Chen HS and Lipton SA (2006) The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem 97:1611–1626. [DOI] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE and Bucci DJ (2007) Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull 33:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Wester JC, Craig MT, Barksdale E, Yuan XQ, Akgul G, Fang C, Collins D, Hunt S, Pelkey KA and McBain CJ (2017) Afferent specific role of NMDA receptors for the circuit integration of hippocampal neurogliaform cells. Nature communications 8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Tsien RW, Goff DC and Halassa MM (2015) The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res 167:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CE, Brine DR, Jeffcoat AR, Hill JM, Wall ME, Perez-Reyes M and Di Guiseppi SR (1982) Phencyclidine disposition after intravenous and oral doses. Clinical pharmacology and therapeutics 31:625–634. [DOI] [PubMed] [Google Scholar]
- Coyle JT (2006) Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol 26:365–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT and Balu DT (2018) The Role of Serine Racemase in the Pathophysiology of Brain Disorders. Advances in pharmacology (San Diego, Calif) 82:35–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Tsai G and Goff D (2003) Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann N Y Acad Sci 1003:318–327. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R and Lynch DR (2008) Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. The Lancet Neurology 7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR and Balice-Gordon R (2011) Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. The Lancet Neurology 10:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P and Cuenod M (2009) Redox dysregulation, neurodevelopment, and schizophrenia. Current Opinion in Neurobiology 19:220–230. [DOI] [PubMed] [Google Scholar]
- Donegan JJ, Tyson JA, Branch SY, Beckstead MJ, Anderson SA and Lodge DJ (2017) Stem cell-derived interneuron transplants as a treatment for schizophrenia: preclinical validation in a rodent model. Mol Psychiatry 22:1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore K, Aow J and Malinow R (2016) The Emergence of NMDA Receptor Metabotropic Function: Insights from Imaging. Frontiers in synaptic neuroscience 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukoff DJ, Hogg DW, Hawrysh PJ and Buck LT (2014) Scavenging ROS dramatically increase NMDA receptor whole-cell currents in painted turtle cortical neurons. The Journal of experimental biology 217:3346–3355. [DOI] [PubMed] [Google Scholar]
- Dupuis JP, Ladepeche L, Seth H, Bard L, Varela J, Mikasova L, Bouchet D, Rogemond V, Honnorat J, Hanse E and Groc L (2014) Surface dynamics of GluN2B-NMDA receptors controls plasticity of maturing glutamate synapses. The EMBO journal 33:842–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaithy A, Younkin J, Gonzalez-Maeso J and Logothetis DE (2015) Positive allosteric modulators of metabotropic glutamate 2 receptors in schizophrenia treatment. Trends Neurosci 38:506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres D, Perlov E, Baumgartner A, Hottenrott T, Dersch R, Stich O and Tebartz Van Elst L (2015) Immunological findings in psychotic syndromes: a tertiary care hospital’s CSF sample of 180 patients. Frontiers in Human Neuroscience 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S and Engberg G (2002) Increased phasic activity of dopaminergic neurones in the rat ventral tegmental area following pharmacologically elevated levels of endogenous kynurenic acid. Acta physiologica Scandinavica 175:45–53. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Emanuelsson C and Geyer M (2004) Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry 56:255–260. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Imbeault S and Engberg G (2017) The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 112:297–306. [DOI] [PubMed] [Google Scholar]
- Fan LZ, Nehme R, Adam Y, Jung ES, Wu H, Eggan K, Arnold DB and Cohen AE (2018) All-optical synaptic electrophysiology probes mechanism of ketamine-induced disinhibition. Nature methods 15:823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Lerma J, Marin O and Rico B (2010) Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature 464:1376–1380. [DOI] [PubMed] [Google Scholar]
- Fernandes C, Hoyle E, Dempster E, Schalkwyk LC and Collier DA (2006) Performance deficit of alpha7 nicotinic receptor knockout mice in a delayed matching-to-place task suggests a mild impairment of working/episodic-like memory. Genes, brain, and behavior 5:433–440. [DOI] [PubMed] [Google Scholar]
- Flores-Barrera E, Thomases DR, Cass DK, Bhandari A, Schwarcz R, Bruno JP and Tseng KY (2017) Preferential Disruption of Prefrontal GABAergic Function by Nanomolar Concentrations of the α7nACh Negative Modulator Kynurenic Acid. The Journal of Neuroscience 37:7921–7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraguas D, Diaz-Caneja CM, Ayora M, Hernandez-Alvarez F, Rodriguez-Quiroga A, Recio S, Leza JC and Arango C (2019) Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-analysis. Schizophr Bull 45:742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangeul L, Kehayas V, Sanchez-Mut JV, Fievre S, Krishna KK, Pouchelon G, Telley L, Bellone C, Holtmaat A, Graff J, Macklis JD and Jabaudon D (2017) Input-dependent regulation of excitability controls dendritic maturation in somatosensory thalamocortical neurons. Nature communications 8:2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen K, Lu D, Yang J, Jensen HS, Bastlund JF, Larsen PH, Liu H, Crestey F, Dekermendjian K, Badolo L, Laursen M, Hougaard C, Yang C, Svenstrup N and Grunnet M (2017) A small molecule activator of Nav 1.1 channels increases fast-spiking interneuron excitability and GABAergic transmission in vitro and has anti-convulsive effects in vivo. Eur J Neurosci 46:1887–1896. [DOI] [PubMed] [Google Scholar]
- Gallego JA, Blanco EA, Husain-Krautter S, Madeline Fagen E, Moreno-Merino P, Del Ojo-Jimenez JA, Ahmed A, Rothstein TL, Lencz T and Malhotra AK (2018) Cytokines in cerebrospinal fluid of patients with schizophrenia spectrum disorders: New data and an updated meta-analysis. Schizophr Res 202:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese G, Fromer M, Stahl EA, Ruderfer DM, Chambert K, Landen M, Moran JL, Purcell SM, Sklar P, Sullivan PF, Hultman CM and McCarroll SA (2016) Increased burden of ultra-rare protein-altering variants among 4,877 individuals with schizophrenia. Nat Neurosci 19:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson LL, Pollak TA, Blackman G, Thornton M, Moran N and David AS (2019) The Psychiatric Phenotype of Anti-NMDA Receptor Encephalitis. The Journal of neuropsychiatry and clinical neurosciences 31:70–79. [DOI] [PubMed] [Google Scholar]
- Gleichman AJ, Spruce LA, Dalmau J, Seeholzer SH and Lynch DR (2012) Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J Neurosci 32:11082–11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH and Miller BJ (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA (2017) Dopamine System Dysregulation and the Pathophysiology of Schizophrenia: Insights From the Methylazoxymethanol Acetate Model. Biological Psychiatry 81:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L, van den Buuse M, Scarr E, Dean B and Hannan AJ (2009) Clozapine reverses schizophrenia-related behaviours in the metabotropic glutamate receptor 5 knockout mouse: association with N-methyl-D-aspartic acid receptor up-regulation. Int J Neuropsychopharmacol 12:45–60. [DOI] [PubMed] [Google Scholar]
- Gu X and Lu W (2018) Genetic deletion of NMDA receptors suppresses GABAergic synaptic transmission in two distinct types of central neurons. Neurosci Lett 668:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulchina Y, Xu S-J, Snyder MA, Elefant F and Gao W-J (2017) Epigenetic mechanisms underlying NMDA receptor hypofunction in the prefrontal cortex of juvenile animals in the MAM model for schizophrenia. Journal of neurochemistry 143:320–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara H, Iyo M and Hashimoto K (2013) Neonatal Disruption of Serine Racemase Causes Schizophrenia-Like Behavioral Abnormalities in Adulthood: Clinical Rescue by D-Serine. PLOS ONE 8:e62438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ and Arnold SE (2006) Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med 12:824–828. [DOI] [PubMed] [Google Scholar]
- Halliwell RF, Peters JA and Lambert JJ (1989) The mechanism of action and pharmacological specificity of the anticonvulsant NMDA antagonist MK-801: a voltage clamp study on neuronal cells in culture. Br J Pharmacol 96:480–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Yi F, Perszyk RE, Furukawa H, Wollmuth LP, Gibb AJ and Traynelis SF (2018) Structure, function, and allosteric modulation of NMDA receptors. The Journal of General Physiology 150:1081–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E, Armbruster M, Lau LA, Sommer ME, Klaft ZJ, Swanger SA, Traynelis SF, Moss SJ, Noubary F, Chadchankar J and Dulla CG (2019) Tonic Activation of GluN2C/GluN2D-Containing NMDA Receptors by Ambient Glutamate Facilitates Cortical Interneuron Maturation. J Neurosci 39:3611–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE and Do KQ (2016) Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci 17:125–134. [DOI] [PubMed] [Google Scholar]
- Harrison PJ (2015) Recent genetic findings in schizophrenia and their therapeutic relevance. Journal of Psychopharmacology 29:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ and Law AJ (2006) Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry 60:132–140. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai K and Iyo M (2003) Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry 60:572–576. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE and Weinberger DR (2004) Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry 9:299–307. [DOI] [PubMed] [Google Scholar]
- Herrling P (1994) D-CPPene (SDZ EAA 494), a competitive NMDA antagonist. Results from animal models and first results in humans. Neuropsychopharmacplogy 10:591S. [Google Scholar]
- Hoffman DJ, McGowan JE, Marro PJ, Mishra OP and Delivoria-Papadopoulos M (1994) Hypoxia-induced modification of the N-methyl-D-aspartate receptor in the brain of the newborn piglet. Neurosci Lett 167:156–160. [DOI] [PubMed] [Google Scholar]
- Hollmann M and Heinemann S (1994) Cloned glutamate receptors. Annu Rev Neurosci 17:31–108. [DOI] [PubMed] [Google Scholar]
- Homayoun H and Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27:11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G and Zhang ZW (2017) NMDA Receptors Regulate the Development of Neuronal Intrinsic Excitability through Cell-Autonomous Mechanisms. Frontiers in cellular neuroscience 11:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD and Kapur S (2009) The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull 35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Chen W, Myers SJ, Yuan H and Traynelis SF (2016) Human GRIN2B variants in neurodevelopmental disorders. Journal of pharmacological sciences 132:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, MacDonald ML, Elswick DE and Sweet RA (2015) The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies. Ann N Y Acad Sci 1338:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner JE and Bean BP (1988) Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A 85:1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, Parsons TD, Lynch DR, Dalmau J and Balice-Gordon RJ (2010) Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 30:5866–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan M, Petros TJ and Anderson SA (2013) Losing your inhibition: linking cortical GABAergic interneurons to schizophrenia. Neurobiol Dis 53:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen SU, Ferrea S, Wach C, Quasthoff K, Illes S, Scherfeld D, Hartung HP, Seitz RJ and Dihne M (2013) In vitro neuronal network activity in NMDA receptor encephalitis. BMC neuroscience 14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC and Zukin SR (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148:1301–1308. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Redmond DE Jr., Elsworth JD, Taylor JR, Youngren KD and Roth RH (1997) Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science 277:953–955. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR and Roth RH (1998) Subchronic Phencyclidine Administration Increases Mesolimbic Dopaminergic System Responsivity and Augments Stress- and Psychostimulant-Induced Hyperlocomotion. Neuropsychopharmacology 19:105–113. [DOI] [PubMed] [Google Scholar]
- Jezequel J, Johansson EM, Leboyer M and Groc L (2018) Pathogenicity of Antibodies against NMDA Receptor: Molecular Insights into Autoimmune Psychosis. Trends Neurosci 41:502–511. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Rompala GR, Zhang S, Cowell RM and Nakazawa K (2013) Social isolation exacerbates schizophrenia-like phenotypes via oxidative stress in cortical interneurons. Biol Psychiatry 73:1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Glasgow NG and Povysheva NV (2015) Recent insights into the mode of action of memantine and ketamine. Current opinion in pharmacology 20:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RS and Buhl EH (1993) Basket-like interneurones in layer II of the entorhinal cortex exhibit a powerful NMDA-mediated synaptic excitation. Neurosci Lett 149:35–39. [DOI] [PubMed] [Google Scholar]
- Ju P and Cui D (2016) The involvement of N-methyl-d-aspartate receptor (NMDAR) subunit NR1 in the pathophysiology of schizophrenia. Acta biochimica et biophysica Sinica 48:209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann O, Papageorgiou IE and Draguhn A (2014) Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J Cereb Blood Flow Metab 34:1270–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan G, Gressitt KL, Yang S, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CLG, Adamos MB, Sweeney KM, Origoni AE, Khushalani S, Bahn S, Leweke FM, Dickerson FB, Yolken RH, Pletnikov MV and Severance EG (2017) Pathogen-mediated NMDA receptor autoimmunity and cellular barrier dysfunction in schizophrenia. Translational psychiatry 7:e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT and Javitt DC (2010) N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull 83:108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Robleto K, Trantham-Davidson H, Jairl C, Cannon TD, Lavin A and Jentsch JD (2011) Reduced dysbindin expression mediates N-methyl-D-aspartate receptor hypofunction and impaired working memory performance. Biol Psychiatry 69:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka M, Matoba N, Sawada T, Kazuno AA, Ishiwata M, Fujii K, Matsuo K, Takata A and Kato T (2016) Exome sequencing for bipolar disorder points to roles of de novo loss-of-function and protein-altering mutations. Mol Psychiatry 21:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL, Cooper TB, Carlsson A and Laruelle M (2000) Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry 48:627–640. [DOI] [PubMed] [Google Scholar]
- Koek W, Woods JH and Winger GD (1988) MK-801, a proposed noncompetitive antagonist of excitatory amino acid neurotransmission, produces phencyclidine-like behavioral effects in pigeons, rats and rhesus monkeys. J Pharmacol Exp Ther 245:969–974. [PubMed] [Google Scholar]
- Kokkinou M, Ashok AH and Howes OD (2018) The effects of ketamine on dopaminergic function: meta-analysis and review of the implications for neuropsychiatric disorders. Mol Psychiatry 23:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J and Weller M (1997) Psychotogenicity and N-methyl-D-aspartate receptor antagonism: implications for neuroprotective pharmacotherapy. Biol Psychiatry 41:135–144. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J and Monyer H (2010) NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68:557–569. [DOI] [PubMed] [Google Scholar]
- Kotzadimitriou D, Nissen W, Paizs M, Newton K, Harrison PJ, Paulsen O and Lamsa K (2018) Neuregulin 1 Type I Overexpression Is Associated with Reduced NMDA Receptor-Mediated Synaptic Signaling in Hippocampal Interneurons Expressing PV or CCK. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic P and Somanathan R (2010) Clinical physiology and mechanism of dizocilpine (MK-801): electron transfer, radicals, redox metabolites and bioactivity. Oxidative medicine and cellular longevity 3:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB and Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Arch Gen Psychiatry 51. [DOI] [PubMed] [Google Scholar]
- Labrie V, Fukumura R, Rastogi A, Fick LJ, Wang W, Boutros PC, Kennedy JL, Semeralul MO, Lee FH, Baker GB, Belsham DD, Barger SW, Gondo Y, Wong AH and Roder JC (2009) Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum Mol Genet 18:3227–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladepeche L, Planaguma J, Thakur S, Suarez I, Hara M, Borbely JS, Sandoval A, Laparra-Cuervo L, Dalmau J and Lakadamyali M (2018) NMDA Receptor Autoantibodies in Autoimmune Encephalitis Cause a Subunit-Specific Nanoscale Redistribution of NMDA Receptors. Cell reports 23:3759–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimore J, Zlatic SA, Gokhale A, Tornieri K, Singleton KS, Mullin AP, Tang J, Talbot K and Faundez V (2014) Mutations in the BLOC-1 subunits dysbindin and muted generate divergent and dosage-dependent phenotypes. J Biol Chem 289:14291–14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE and Weinberger DR (2006) Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5’ SNPs associated with the disease. Proc Natl Acad Sci U S A 103:6747–6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke JR, Geider K, Helbig KL, Heyne HO, Schutz H, Hentschel J, Courage C, Depienne C, Nava C, Heron D, Moller RS, Hjalgrim H, Lal D, Neubauer BA, Nurnberg P, Thiele H, Kurlemann G, Arnold GL, Bhambhani V, Bartholdi D, Pedurupillay CR, Misceo D, Frengen E, Stromme P, Dlugos DJ, Doherty ES, Bijlsma EK, Ruivenkamp CA, Hoffer MJ, Goldstein A, Rajan DS, Narayanan V, Ramsey K, Belnap N, Schrauwen I, Richholt R, Koeleman BP, Sa J, Mendonca C, de Kovel CG, Weckhuysen S, Hardies K, De Jonghe P, De Meirleir L, Milh M, Badens C, Lebrun M, Busa T, Francannet C, Piton A, Riesch E, Biskup S, Vogt H, Dorn T, Helbig I, Michaud JL, Laube B and Syrbe S (2016) Delineating the GRIN1 phenotypic spectrum: A distinct genetic NMDA receptor encephalopathy. Neurology 86:2171–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox BR, Palmer-Cooper EC, Pollak T, Hainsworth J, Marks J, Jacobson L, Lang B, Fox H, Ferry B, Scoriels L, Crowley H, Jones PB, Harrison PJ and Vincent A (2017) Prevalence and clinical characteristics of serum neuronal cell surface antibodies in first-episode psychosis: a case-control study. The lancet Psychiatry 4:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypoldt F, Titulaer MJ, Aguilar E, Walther J, Bönstrup M, Havemeister S, Teegen B, Lütgehetmann M, Rosenkranz M, Magnus T and Dalmau J (2013) Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology 81:1637–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH and Lane HY (2019) The Role of N-Methyl-D-Aspartate Receptor Neurotransmission and Precision Medicine in Behavioral and Psychological Symptoms of Dementia. Front Pharmacol 10:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Hsu FC, Baumann BH, Coulter DA, Anderson SA and Lynch DR (2014) Cortical parvalbumin GABAergic deficits with alpha7 nicotinic acetylcholine receptor deletion: implications for schizophrenia. Mol Cell Neurosci 61:163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Jacobi AA, Anderson SA and Lynch DR (2016) D-Serine and Serine Racemase Are Associated with PSD-95 and Glutamatergic Synapse Stability. Frontiers in cellular neuroscience 10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderholm KR, Alm MT, Larsson MK, Olsson SK, Goiny M, Hajos M, Erhardt S and Engberg G (2016) Inhibition of kynurenine aminotransferase II reduces activity of midbrain dopamine neurons. Neuropharmacology 102:42–47. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Choi YB, Takahashi H, Zhang D, Li W, Godzik A and Bankston LA (2002) Cysteine regulation of protein function--as exemplified by NMDA-receptor modulation. Trends Neurosci 25:474–480. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S and Grace AA (2008) Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 31:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chang L, Song Y, Li H and Wu Y (2019) The Role of NMDA Receptors in Alzheimer’s Disease. Frontiers in neuroscience 13:43. doi: 10.3389/fnins.2019.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge D and Mercier MS (2015) Ketamine and phencyclidine: the good, the bad and the unexpected. Br J Pharmacol 172:4254–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W and Hönack D (1991) Anticonvulsant and behavioral effects of two novel competitive N-methyl-D-aspartic acid receptor antagonists, CGP 37849 and CGP 39551, in the kindling model of epilepsy. Comparison with MK-801 and carbamazepine. Journal of Pharmacology and Experimental Therapeutics 256:432–440. [PubMed] [Google Scholar]
- Lowther C, Costain G, Stavropoulos DJ, Melvin R, Silversides CK, Andrade DM, So J, Faghfoury H, Lionel AC, Marshall CR, Scherer SW and Bassett AS (2015) Delineating the 15q13.3 microdeletion phenotype: a case series and comprehensive review of the literature. Genetics in medicine : official journal of the American College of Medical Genetics 17:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchese G (2017) From Toxoplasmosis to Schizophrenia via NMDA Dysfunction: Peptide Overlap between Toxoplasma gondii and N-Methyl-d-Aspartate Receptors As a Potential Mechanistic Link. Frontiers in psychiatry 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur DG, Balasubramanian S, Frankish A, Huang N, Morris J, Walter K, Jostins L, Habegger L, Pickrell JK, Montgomery SB, Albers CA, Zhang ZD, Conrad DF, Lunter G, Zheng H, Ayub Q, DePristo MA, Banks E, Hu M, Handsaker RE, Rosenfeld JA, Fromer M, Jin M, Mu XJ, Khurana E, Ye K, Kay M, Saunders GI, Suner MM, Hunt T, Barnes IH, Amid C, Carvalho-Silva DR, Bignell AH, Snow C, Yngvadottir B, Bumpstead S, Cooper DN, Xue Y, Romero IG, Wang J, Li Y, Gibbs RA, McCarroll SA, Dermitzakis ET, Pritchard JK, Barrett JC, Harrow J, Hurles ME, Gerstein MB and Tyler-Smith C (2012) A systematic survey of loss-of-function variants in human protein-coding genes. Science 335:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantere O, Saarela M, Kieseppa T, Raij T, Mantyla T, Lindgren M, Rikandi E, Stoecker W, Teegen B and Suvisaari J (2018) Anti-neuronal anti-bodies in patients with early psychosis. Schizophr Res 192:404–407. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD and Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711. [DOI] [PubMed] [Google Scholar]
- Masdeu JC, Dalmau J and Berman KF (2016) NMDA Receptor Internalization by Autoantibodies: A Reversible Mechanism Underlying Psychosis? Trends Neurosci 39:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust J, Andrys C, Bazant J, Vysata O, Kuca K and Valis M (2015) Anti-NMDA receptor antibodies in patients with a first episode of schizophrenia. Neuropsychiatric disease and treatment 11:619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L and Nave KA (2014) Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron 83:27–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikasova L, De Rossi P, Bouchet D, Georges F, Rogemond V, Didelot A, Meissirel C, Honnorat J and Groc L (2012) Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain 135:1606–1621. [DOI] [PubMed] [Google Scholar]
- Miller DW and Abercrombie ED (1996) Effects of MK-801 on spontaneous and amphetamine-stimulated dopamine release in striatum measured with in vivo microdialysis in awake rats. Brain Res Bull 40:57–62. [DOI] [PubMed] [Google Scholar]
- Mishra OP and Delivoria-Papadopoulos M (1992) Modification of modulatory sites of NMDA receptor in the fetal guinea pig brain during development. Neurochem Res 17:1223–1228. [DOI] [PubMed] [Google Scholar]
- Modinos G, Allen P, Grace AA and McGuire P (2015) Translating the MAM model of psychosis to humans. Trends Neurosci 38:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes de Oca Balderas P (2018) Flux-Independent NMDAR Signaling: Molecular Mediators, Cellular Functions, and Complexities. International Journal of Molecular Sciences 19:3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau AW and Kullmann DM (2013) NMDA receptor-dependent function and plasticity in inhibitory circuits. Neuropharmacology 74:23–31. [DOI] [PubMed] [Google Scholar]
- Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J and Balice-Gordon RJ (2014) Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol 76:108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostaid MS, Lloyd D, Liberg B, Sundram S, Pereira A, Pantelis C, Karl T, Weickert CS, Everall IP and Bousman CA (2016) Neuregulin-1 and schizophrenia in the genome-wide association study era. Neurosci Biobehav Rev 68:387–409. [DOI] [PubMed] [Google Scholar]
- Muller N, Weidinger E, Leitner B and Schwarz MJ (2015) The role of inflammation in schizophrenia. Frontiers in neuroscience 9:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Ishikawa Y and Sato F (2013) Localization of alpha7 nicotinic acetylcholine receptor immunoreactivity on GABAergic interneurons in layers I-III of the rat retrosplenial granular cortex. Neuroscience 252:443–459. [DOI] [PubMed] [Google Scholar]
- Nakagami Y, Sugihara G, Ikeda A and Murai T (2018) Is the prevalence of anti-N-methyl-d-aspartate receptor antibodies in schizophrenia overestimated? Schizophr Res 197:591–592. [DOI] [PubMed] [Google Scholar]
- Nakao K, Jeevakumar V, Jiang SZ, Fujita Y, Diaz NB, Pretell Annan CA, Eskow Jaunarajs KL, Hashimoto K, Belforte JE and Nakazawa K (2019) Schizophrenia-Like Dopamine Release Abnormalities in a Mouse Model of NMDA Receptor Hypofunction. Schizophr Bull 45:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K and Nakazawa K (2014) Brain state-dependent abnormal LFP activity in the auditory cortex of a schizophrenia mouse model. Frontiers in neuroscience 8:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Jeevakumar V and Nakao K (2017) Spatial and temporal boundaries of NMDA receptor hypofunction leading to schizophrenia. NPJ schizophrenia 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA and Tonegawa S (2004) NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci 5:361–372. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S and Belforte JE (2012) GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 62:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora LG, Tanaka T, Hayes LN, Kim M, Lee BJ, Matsuda T, Nucifora FC Jr., Sedlak T, Mojtabai R, Eaton W and Sawa A (2017) Reduction of plasma glutathione in psychosis associated with schizophrenia and bipolar disorder in translational psychiatry. Translational psychiatry 7:e1215–e1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Stephenson FA, Freund TF and Somogyi P (2003) Large variability in synaptic N-methyl-D-aspartate receptor density on interneurons and a comparison with pyramidal-cell spines in the rat hippocampus. Neuroscience 119:347–363. [DOI] [PubMed] [Google Scholar]
- Olaya JC, Heusner CL, Matsumoto M, Shannon Weickert C and Karl T (2018a) Schizophrenia-relevant behaviours of female mice overexpressing neuregulin 1 type III. Behav Brain Res 353:227–235. [DOI] [PubMed] [Google Scholar]
- Olaya JC, Heusner CL, Matsumoto M, Sinclair D, Kondo MA, Karl T and Shannon Weickert C (2018b) Overexpression of Neuregulin 1 Type III Confers Hippocampal mRNA Alterations and Schizophrenia-Like Behaviors in Mice. Schizophr Bull 44:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW and Farber NB (1995) Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 52:998–1007. [DOI] [PubMed] [Google Scholar]
- Orlovska-Waast S, Kohler-Forsberg O, Brix SW, Nordentoft M, Kondziella D, Krogh J and Benros ME (2019) Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry 24:869–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Oliveira B, Saher G, Dere E, Tapken D, Mitjans M, Seidel J, Wesolowski J, Wakhloo D, Klein-Schmidt C, Ronnenberg A, Schwabe K, Trippe R, Mätz-Rensing K, Berghoff S, Al-Krinawe Y, Martens H, Begemann M, Stöcker W, Kaup F-J, Mischke R, Boretius S, Nave K-A, Krauss JK, Hollmann M, Lühder F and Ehrenreich H (2019) Uncoupling the widespread occurrence of anti-NMDAR1 autoantibodies from neuropsychiatric disease in a novel autoimmune model. Molecular Psychiatry 24:1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C and Hardingham GE (2008) Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci 11:476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton MH, Bizup BT and Grace AA (2013) The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. J Neurosci 33:16865–16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz S, Grabert J, Gorba T, Wirth MJ and Wahle P (2004) Parvalbumin expression in visual cortical interneurons depends on neuronal activity and TrkB ligands during an Early period of postnatal development. Cereb Cortex 14:342–351. [DOI] [PubMed] [Google Scholar]
- Pearlman DM and Najjar S (2014) Meta-analysis of the association between N-methyl-d-aspartate receptor antibodies and schizophrenia, schizoaffective disorder, bipolar disorder, and major depressive disorder. Schizophr Res 157:249–258. [DOI] [PubMed] [Google Scholar]
- Picard N, Takesian AE, Fagiolini M and Hensch TK (2019) NMDA 2A receptors in parvalbumin cells mediate sexspecific rapid ketamine response on cortical activity. Molecular Psychiatry 24:828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher GM, Kalia LV, Ng D, Goodfellow NM, Yee KT, Lambe EK and Salter MW (2011) Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat Med 17:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitman E, Iwata Y, Caravaggio F, Nakajima S, Chung JK, Gerretsen P, Kim J, Takeuchi H, Chakravarty MM, Remington G and Graff-Guerrero A (2017) Kynurenic Acid in Schizophrenia: A Systematic Review and Meta-analysis. Schizophr Bull 43:764–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocklington AJ, O’Donovan M and Owen MJ (2014) The synapse in schizophrenia. Eur J Neurosci 39:1059–1067. [DOI] [PubMed] [Google Scholar]
- Pollak TA, McCormack R, Peakman M, Nicholson TR and David AS (2014) Prevalence of anti-N-methyl-D-aspartate (NMDA) receptor [corrected] antibodies in patients with schizophrenia and related psychoses: a systematic review and meta-analysis. Psychological medicine 44:2475–2487. [DOI] [PubMed] [Google Scholar]
- Riebe I, Seth H, Culley G, Dosa Z, Radi S, Strand K, Frojd V and Hanse E (2016) Tonically active NMDA receptors--a signalling mechanism critical for interneuronal excitability in the CA1 stratum radiatum. Eur J Neurosci 43:169–178. [DOI] [PubMed] [Google Scholar]
- Ross CA, Margolis RL, Reading SA, Pletnikov M and Coyle JT (2006) Neurobiology of schizophrenia. Neuron 52:139–153. [DOI] [PubMed] [Google Scholar]
- Ross RG, Stevens KE, Proctor WR, Leonard S, Kisley MA, Hunter SK, Freedman R and Adams CE (2010) Research review: Cholinergic mechanisms, early brain development, and risk for schizophrenia. Journal of child psychology and psychiatry, and allied disciplines 51:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salovin A, Glanzman J, Roslin K, Armangue T, Lynch DR and Panzer JA (2018) Anti-NMDA receptor encephalitis and nonencephalitic HSV-1 infection. Neurol Neuroimmunol Neuroinflamm 5:e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota K, Mao Z, Synowicki P, Lieber D, Liu M, Ikezu T, Gautam V and Monaghan DT (2016) GluN2D N-Methyl-d-Aspartate Receptor Subunit Contribution to the Stimulation of Brain Activity and Gamma Oscillations by Ketamine: Implications for Schizophrenia. J Pharmacol Exp Ther 356:702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Fendt M, Zink M, Ebert U, Starke M, Berthold M, Herb A, Petroianu G, Falkai P and Henn FA (2007) Altered NMDA receptor expression and behavior following postnatal hypoxia: potential relevance to schizophrenia. Journal of neural transmission (Vienna, Austria : 1996) 114:239–248. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ and Wu HQ (2012) Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 13:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S, Finn A, Bhat M, Samuelsson M, Lundberg K, Dahl ML, Sellgren C, Schuppe-Koistinen I, Svensson C, Erhardt S and Engberg G (2015) Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia--significance for activation of the kynurenine pathway. J Psychiatry Neurosci 40:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellgren CM, Kegel ME, Bergen SE, Ekman CJ, Olsson S, Larsson M, Vawter MP, Backlund L, Sullivan PF, Sklar P, Smoller JW, Magnusson PK, Hultman CM, Walther-Jallow L, Svensson CI, Lichtenstein P, Schalling M, Engberg G, Erhardt S and Landen M (2016) A genome-wide association study of kynurenic acid in cerebrospinal fluid: implications for psychosis and cognitive impairment in bipolar disorder. Mol Psychiatry 21:1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM and Noble-Haeusslein LJ (2013) Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106–107:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Al-Saleem FH, Panzer J, Lee J, Puligedda RD, Felicori LF, Kattala CD, Rattelle AJ, Ippolito G, Cox RH, Lynch DR and Dessain SK (2018) Monoclonal antibodies from a patient with anti-NMDA receptor encephalitis. Annals of clinical and translational neurology 5:935–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelkar GP, Pavuluri R, Gandhi PJ, Ravikrishnan A, Gawande DY, Liu J, Stairs DJ, Ugale RR and Dravid SM (2019) Differential effect of NMDA receptor GluN2C and GluN2D subunit ablation on behavior and channel blocker-induced schizophrenia phenotypes. Sci Rep 9:7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, Hackett E, Girgis R, Ojeil N, Moore H, D’Souza D, Malison RT, Huang Y, Lim K, Nabulsi N, Carson RE, Lieberman JA and Abi-Dargham A (2015) Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry 72:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder MA and Gao WJ (2019) NMDA receptor hypofunction for schizophrenia revisited: Perspectives from epigenetic mechanisms. Schizophr Res. doi: 10.1016/j.schres.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI and Yelshansky MV (2000) The trapping block of NMDA receptor channels in acutely isolated rat hippocampal neurones. J Physiol 526 Pt 3:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatola M, Sabater L, Planaguma J, Martinez-Hernandez E, Armangue T, Pruss H, Iizuka T, Caparo Oblitas RL, Antoine JC, Li R, Heaney N, Tubridy N, Munteis Olivas E, Rosenfeld MR, Graus F and Dalmau J (2018) Encephalitis with mGluR5 antibodies: Symptoms and antibody effects. Neurology 90:e1964–e1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P, Neijt HC, Cuenod M and Do KQ (2006) Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: relevance to schizophrenia. Neuroscience 137:807–819. [DOI] [PubMed] [Google Scholar]
- Strehlow V, Heyne HO, Vlaskamp DRM, Marwick KFM, Rudolf G, de Bellescize J, Biskup S, Brilstra EH, Brouwer OF, Callenbach PMC, Hentschel J, Hirsch E, Kind PC, Mignot C, Platzer K, Rump P, Skehel PA, Wyllie DJA, Hardingham GE, van Ravenswaaij-Arts CMA, Lesca G and Lemke JR (2019) GRIN2A-related disorders: genotype and functional consequence predict phenotype. Brain 142:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryavanshi PS, Ugale RR, Yilmazer-Hanke D, Stairs DJ and Dravid SM (2014) GluN2C/GluN2D subunit-selective NMDA receptor potentiator CIQ reverses MK-801-induced impairment in prepulse inhibition and working memory in Y-maze test in mice. Br J Pharmacol 171:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Shin Y, Cho SJ, Zago WM, Nakamura T, Gu Z, Ma Y, Furukawa H, Liddington R, Zhang D, Tong G, Chen HS and Lipton SA (2007) Hypoxia enhances S-nitrosylation-mediated NMDA receptor inhibition via a thiol oxygen sensor motif. Neuron 53:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka DH, Toriumi K, Kubo K, Nabeshima T and Nakajima K (2011) GABAergic precursor transplantation into the prefrontal cortex prevents phencyclidine-induced cognitive deficits. J Neurosci 31:14116–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thune H, Recasens M and Uhlhaas PJ (2016) The 40-Hz Auditory Steady-State Response in Patients With Schizophrenia: A Meta-analysis. JAMA Psychiatry 73:1145–1153. [DOI] [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT and Huganir RL (1997) Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem 272:5157–5166. [DOI] [PubMed] [Google Scholar]
- Tong J, Huang J, Luo X, Chen S, Cui Y, An H, Xiu M, Tan S, Wang Z, Yuan Y, Zhang J, Yang F, Li CR, Hong LE and Tan Y (2019) Elevated serum anti-NMDA receptor antibody levels in first-episode patients with schizophrenia. Brain, behavior, and immunity 81:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Lun ZR and Yolken RH (2007) Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull 33:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecartin KV and Bucci DJ (2011) Administration of kynurenine during adolescence, but not during adulthood, impairs social behavior in rats. Schizophr Res 133:156–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricklebank MD, Singh L, Oles RJ, Preston C and Iversen SD (1989) The behavioural effects of MK-801: a comparison with antagonists acting non-competitively and competitively at the NMDA receptor. Eur J Pharmacol 167:127–135. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Kanbayashi T, Tanaka K, Boku S, Ito W, Tokunaga J, Mori A, Hishikawa Y, Shimizu T and Nishino S (2012) Anti-NMDA-receptor antibody detected in encephalitis, schizophrenia, and narcolepsy with psychotic features. BMC Psychiatry 12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufvesson-Alm M, Schwieler L, Schwarcz R, Goiny M, Erhardt S and Engberg G (2018) Importance of kynurenine 3-monooxygenase for spontaneous firing and pharmacological responses of midbrain dopamine neurons: Relevance for schizophrenia. Neuropharmacology 138:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyebji S, Seizova S, Hannan AJ and Tonkin CJ (2019) Toxoplasmosis: A pathway to neuropsychiatric disorders. Neurosci Biobehav Rev 96:72–92. [DOI] [PubMed] [Google Scholar]
- Urata Y, Koga K, Hirota Y, Akiyama I, Izumi G, Takamura M, Nagai M, Harada M, Hirata T, Yoshino O, Kawana K, Fujii T and Osuga Y (2014) IL-1beta increases expression of tryptophan 2,3-dioxygenase and stimulates tryptophan catabolism in endometrioma stromal cells. American journal of reproductive immunology (New York, NY : 1989) 72:496–503. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Bocklisch C, Tonges L, Herb A, Mishina M and Monyer H (2015) GluN2D-containing NMDA receptors-mediate synaptic currents in hippocampal interneurons and pyramidal cells in juvenile mice. Frontiers in cellular neuroscience 9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vullhorst D, Mitchell RM, Keating C, Roychowdhury S, Karavanova I, Tao-Cheng JH and Buonanno A (2015) A negative feedback loop controls NMDA receptor function in cortical interneurons via neuregulin 2/ErbB4 signalling. Nature communications 6:7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ and Buonanno A (2009) Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci 29:12255–12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, Lichnerova K, Cerny J, Krusek J, Dittert I, Horak M and Vyklicky L (2014) Structure, function, and pharmacology of NMDA receptor channels. Physiological research / Academia Scientiarum Bohemoslovaca 63 Suppl 1:S191–203. [DOI] [PubMed] [Google Scholar]
- Wang AK and Miller BJ (2018) Meta-analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr Bull 44:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-X and Gao W-J (2012) Prolonged exposure to NMDAR antagonist induces cell-type specific changes of glutamatergic receptors in rat prefrontal cortex. Neuropharmacology 62:1808–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Allen WE, Wright MA, Sylwestrak EL, Samusik N, Vesuna S, Evans K, Liu C, Ramakrishnan C, Liu J, Nolan GP, Bava F-A and Deisseroth K (2018) Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science (New York, NY) 361:eaat5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters KA and Machaalani R (2004) NMDA receptors in the developing brain and effects of noxious insults. Neuro-Signals 13:162–174. [DOI] [PubMed] [Google Scholar]
- Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H and Abi-Dargham A (2017) Pathway-Specific Dopamine Abnormalities in Schizophrenia. Biological Psychiatry 81:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J, Ha S, Chung C, Jung ES, Cho YS, Park SG, Lee JS, Lee K, Kim D, Bae YC, Kaang BK, Lee MG and Kim E (2012) Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 486:261–265. [DOI] [PubMed] [Google Scholar]
- Woo TU, Walsh JP and Benes FM (2004) Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry 61:649–657. [DOI] [PubMed] [Google Scholar]
- Wurdemann T, Kersten M, Tokay T, Guli X, Kober M, Rohde M, Porath K, Sellmann T, Bien CG, Kohling R and Kirschstein T (2016) Stereotactic injection of cerebrospinal fluid from anti-NMDA receptor encephalitis into rat dentate gyrus impairs NMDA receptor function. Brain Res 1633:10–18. [DOI] [PubMed] [Google Scholar]
- Xia P, Chen HS, Zhang D and Lipton SA (2010) Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci 30:11246–11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XiangWei W, Jiang Y and Yuan H (2018) De Novo Mutations and Rare Variants Occurring in NMDA Receptors. Current opinion in physiology 2:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Nakayama T, Yamaguchi J, Matsuzawa M, Mishina M, Ikeda K and Yamamoto H (2016) Role of the NMDA receptor GluN2D subunit in the expression of ketamine-induced behavioral sensitization and region-specific activation of neuronal nitric oxide synthase. Neurosci Lett 610:48–53. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Bell TE, Kotake AN, Powell M and Steinberg GK (1998) Dose escalation safety and tolerance study of the competitive NMDA antagonist selfotel (CGS 19755) in neurosurgery patients. Clinical neuropharmacology 21:28–34. [PubMed] [Google Scholar]
- Yu Y, Lin Y, Takasaki Y, Wang C, Kimura H, Xing J, Ishizuka K, Toyama M, Kushima I, Mori D, Arioka Y, Uno Y, Shiino T, Nakamura Y, Okada T, Morikawa M, Ikeda M, Iwata N, Okahisa Y, Takaki M, Sakamoto S, Someya T, Egawa J, Usami M, Kodaira M, Yoshimi A, Oya-Ito T, Aleksic B, Ohno K and Ozaki N (2018) Rare loss of function mutations in N-methyl-D-aspartate glutamate receptors and their contributions to schizophrenia susceptibility. Translational psychiatry 8:12–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi MS, Irani SR, Lang B, Waters P, Jones PB, McKenna P, Coles AJ, Vincent A and Lennox BR (2011) Disease-relevant autoantibodies in first episode schizophrenia. Journal of neurology 258:686–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Tanaka K, Sun P, Nakata M, Yamamoto R, Sakimura K, Matsui M and Kato N (2012) Suppression of synaptic plasticity by cerebrospinal fluid from anti-NMDA receptor encephalitis patients. Neurobiol Dis 45:610–615. [DOI] [PubMed] [Google Scholar]
- Zhang X, Feng ZJ and Chergui K (2014) Allosteric modulation of GluN2C/GluN2D-containing NMDA receptors bidirectionally modulates dopamine release: implication for Parkinson’s disease. Br J Pharmacol 171:3938–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q and Sheng M (2013) NMDA receptors in nervous system diseases. Neuropharmacology 74:69–75. [DOI] [PubMed] [Google Scholar]