Abstract
Background
Children HIV exposed uninfected (CHEU) experience higher morbidity and mortality despite safer breastfeeding and improved maternal health with maternal antiretroviral therapy. We present the first global estimates of the CHEU population (age 0–14 years) describing geographic and temporal trends in HIV high-burden countries.
Methods
Avenir Health, UNAIDS and partners developed the Spectrum AIDS Impact Module to estimate key HIV epidemic indicators from mathematical models. In 2019 UNAIDS published the estimated number of CHEU age 0–14 years for the period 2000–2018. For six UNAIDS regions and 21 HIV high-burden countries we used 2019 UNAIDS CHEU estimates and 2017 UN Population Division estimates of the number of all children in each region/country to further estimate regional/national CHEU prevalence, regional/national contribution to global CHEU population, proportion of CHEU antiretroviral exposed, and percentage change in CHEU population between 2000–2018.
Findings
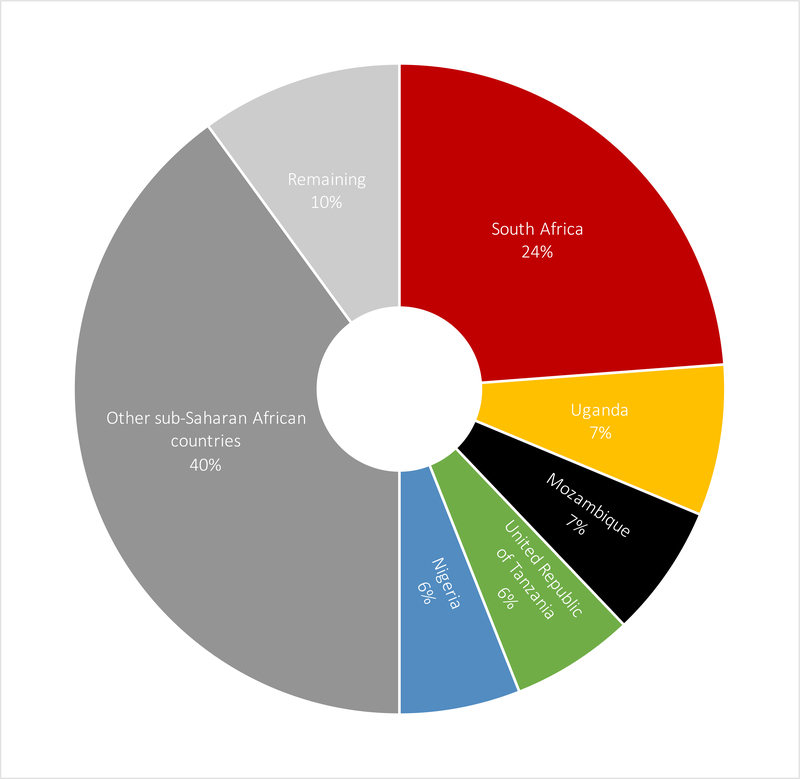
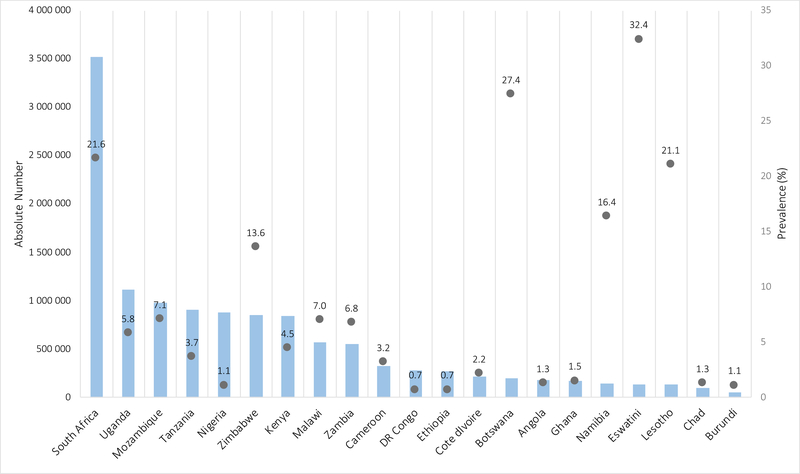
In 2018 there were 14.8 million (lower estimate 11.1 million; upper estimate 18.3 million) CHEU, 90% in sub-Saharan Africa and 5% in Asia and the Pacific. Five countries accounted for 50% of CHEU globally: South Africa (3·5 million; 23·8%), Uganda (1·1 million; 7·5%), Mozambique (1·0 million; 6·6%), Tanzania (0·9 million; 6·1%); and Nigeria (0·9 million; 6·0%). In five southern African countries CHEU prevalence exceeded 15% of the general child population: Eswatini (32·4%), Botswana (27·4%), South Africa (21·6%), Lesotho (21·1%); and Namibia (16·4%).
Interpretation
The CHEU population is substantial, requiring a coordinated strategy to reduce HIV exposure in children and ensure optimal health and well-being of CHEU and their families. Going forward, research and programmatic funding investments must be aligned with the geographic distribution of CHEU.
Keywords: HIV exposed uninfected, HEU, CHEU, HIV, UNAIDS, sub-Saharan Africa
Introduction
Pediatric HIV has undergone a substantial epidemiologic transformation, with the number of new HIV infections in children (age 0–14 years) declining by more than 60% from an annual peak of 450,000 (lower estimate (LE) 300,000; upper estimate (UE) 700,000) in 2000 to 160,000 (LE 110,000; UE 260,000) in 2018.1 Simultaneously, the number of women living with HIV (WLHIV) who experience pregnancy annually has remained static since 1999 at 1·3 million (LE 980,000; UE 1·6 million).1 Reduction in child HIV infections has been achieved through substantial global and national investment in effective perinatal and postnatal HIV transmission prevention interventions offered to pregnant and breastfeeding WLHIV, particularly in countries with the highest burden of HIV in East and Southern Africa.2 Consequently there is an expanding population of HIV-uninfected children exposed to HIV in utero and during breastfeeding, referred to as children HIV exposed uninfected (CHEU), and an increasing subset also exposed to antiretroviral drugs.
To universally eliminate perinatal and postnatal HIV acquisition it is essential that all pregnant and breastfeeding WLHIV and their infants who are HIV-exposed receive the preventive benefits of effective antiretroviral (ARV) drugs, resulting in an increasing population of CHEU also ARV-exposed. However, even when CHEU have avoided HIV acquisition, they remain HIV-affected, born to a WLHIV and living in an HIV-affected household.3,4 Prior to availability of universal maternal antiretroviral therapy (ART) the risk of infant and early childhood mortality in CHEU was almost double that of children HIV unexposed uninfected (CHUU).5 Current evidence suggests that increased mortality risk in CHEU persists despite maternal ART and safer breastfeeding, however the effect appears to be less strong.6 CHEU are also at increased risk of experiencing more severe illness in response to common viral respiratory and invasive bacterial infections.7 Similarly, the probability of preterm birth is 50%−100% greater in HIV-exposed than HIV-unexposed newborns, despite mothers receiving effective ART, with consequences of preterm birth extending throughout the life course.8,9 Additionally, there have been signals of concern related to suboptimal CHEU behavioral and neurological development with some evidence that this is associated with preterm birth.10–12.Recent emphasis has been placed on evaluating birth outcomes, particularly possible teratogenicity, of in utero ARV exposure, however longer-term safety of in utero ARVs and combination ART is currently poorly understood.13–15
CHEU may experience multiple early life exposures in addition to HIV and ARVs, including an immunologically perturbed in utero environment, potential maternal physical and mental ill health, amplified infectious pathogen exposure, challenged socioeconomic circumstances and suboptimal infant nutrition.3,16 Appropriately attributing the source of CHEU outcome disparities to these multiple concurrent biological and social exposures, and the associated attributable fractions, has been challenging but necessary to develop appropriate strategies. Irrespective of the pathways to adverse outcomes, it is clear that the CHEU population is not surviving and thriving as well as CHUU.
To better understand the global size and geographic distribution of CHEU and how this population has evolved as the HIV epidemic has matured, we present the first estimates of the CHEU population globally and describe geographic and temporal trends in this population in HIV high-burden countries between 2000 and 2018.
Methods
Avenir Health, The Joint United Nations Program on HIV/AIDS (UNAIDS), and partners have developed software used by individual countries to map their HIV epidemic.17 The Spectrum AIDS Impact Module generates estimates of key epidemic indicators from mathematical models informed by region-specific epidemic assumptions and national program measures. The model assumptions are reviewed annually by the UNAIDS Reference Group on Estimates, Modelling and Projections comprising a multi-disciplinary and multi-institutional group of experts. Annually, individual countries generate national or sub-national epidemic indicator estimates using the Spectrum software.18 These national estimates are validated and collated by UNAIDS to develop regional and global estimates. All previous years’ historic estimates are updated with the current model incorporating the most up-to-date knowledge of the HIV epidemic. Indicators include the number of people living with HIV, the number of new HIV infections, number of pregnant WLHIV, AIDS mortality, and ART coverage.
For the first time in 2018, UNAIDS published annualized estimates of the CHEU population aged 0–14 years for the period 2000–2017 and updated these estimates with the 2019 Spectrum model that includes the period 2000–2018. CHEU included all HIV-uninfected children born to WLHIV whether maternal HIV was diagnosed or not, based on modelled estimates of the total number of pregnant WLHIV.19 Children who are HIV exposed are calculated by first estimating the number of births to WLHIV. This is done by multiplying HIV prevalence in WLHIV by five-year age group against fertility among five-year age groups to estimate births among pregnant WLHIV. This value is then adjusted based on routine testing data from antenatal clinics. Determining what proportion of children remain uninfected requires the perinatal and postnatal HIV transmission rates, which depend on the timing of initiating different maternal ART regimens, maternal CD4, and breastfeeding duration. Children only exposed to HIV during breastfeeding by WLHIV who acquired HIV following delivery were not included. CHEU also ARV-exposed included all children with any in utero exposure to ARVs either as ART for maternal treatment or mono, dual or triple ARVs for HIV transmission prophylaxis. The estimates of CHEU also ARV-exposed do not include children exposed only postnatally to ARVs through breastfeeding or infant prophylaxis. The CHEU estimates are informed by the number of WLHIV and the number of children living with HIV, with the uncertainty in the estimates being most influenced by assumptions regarding the number of births among WLHIV and rates of perinatal and postnatal HIV acquisition in children.19
For six of the UNAIDS regions (Asia and the Pacific, Caribbean, Eastern and Southern Africa, Latin America, Middle East and North Africa, Western and Central Africa,) and 21 sub-Saharan African countries with the highest burden of pediatric HIV, we further analysed the 2019 UNAIDS estimates of the number of CHEU, and incorporated the 2017 UN Population Division estimates of the number of all children in each country for 2018 to derive the following estimates1,2:
Regional or national CHEU prevalence = number of CHEU/number of all children
Regional or national prevalence of CHEU also ARV-exposed = number of CHEU also ARV-ex posed/number of all children
Regional or national contribution to global CHEU population = region or country number of CHEU/global number of CHEU
Proportion of CHEU also ARV-exposed = number of CHEU also ARV-exposed/number of all CHEU
Percent change in CHEU population between 2000 and 2018 = (CHEU number in 2018 - CHEU number in 2000)/(CHEU number in 2000)
For the five countries with the highest absolute number and highest prevalence of CHEU we present these same estimates (1–4) over time from 2000 to 2018. Estimates were not available for the regions of Eastern Europe and central Asia, Western and central Europe and North America (regions that make up less than 2% of children living with HIV). Individual country estimates for India and Indonesia, included in the 23 countries with the highest burden of pediatric HIV, were also not available.2
The Spectrum files, including all inputs and outputs, on which this analysis was based, are available from UNAIDS. The Spectrum software is freely available for download at www.AvenirHealth.org and the model code can be requested from Avenir Health. Results are presented in accordance with the GATHER Statement for global estimates reporting.20 As the estimates presented rely upon publicly available modelled estimates, research ethics approval was not sought. The funding sources had no role in design, analysis, interpretation of data, writing of the report or decision to submit for publication.
Results
In 2018, there were an estimated 14.8 million (LE 11.1 million; UE 18.3 million) CHEU aged 0–14 years and 1·7 million (LE 1·3 million; UE 2·2 million) children living with HIV, with a global CHEU prevalence of 0·8% (LE 0.6%; UE 0.9%). Of the 14.8 million CHEU, 10·5 million were also exposed to ARVs in utero. Ninety percent, or 13·2 million CHEU live in sub-Saharan Africa, with an additional 760,000 in the Asia and Pacific region, representing 5% of the global population of CHEU, and 270,000 in the Latin American region (Table 1). In 2018, five countries accounted for 50% of all CHEU globally (Figure 1). These were South Africa with 3·5 million (23·8%) CHEU, Uganda with 1·1 million (7·5%), Mozambique with 1·0 million (6·6%), Tanzania with 0·9 million (6·1%), and Nigeria with 0·9 million (6·0%) CHEU. Zimbabwe, Kenya, Malawi, and Zambia also had large populations of between 500,000 and 850,000 CHEU in 2018 (Table 1). The countries with the highest national prevalence of CHEU differ from countries with the highest absolute numbers, with southern African countries of smaller population size but higher maternal HIV prevalence experiencing the highest prevalence of CHEU, including Eswatini at 32·4% and Botswana at 27·4%, followed by South Africa (21·6%), Lesotho (21·1%) and Namibia (16·4%) (Figure 2).
Table 1:
2019 UNAIDS estimates of the CHEU population size and prevalence in 2018 for 6 UNAIDS regions and 21 sub-Saharan African HIV high-burden countries
| Total number of CHEU (lower bound; upper bound) | Prevalence (%) of CHEU (lower bound; upper bound) | Contribution to global population of CHEU (%) | Proportion of CHEU also ARV-exposed (%) | Change in CHEU population size 2000–2018 (%) | |
|---|---|---|---|---|---|
| Global | 14,800,000 (11,100,000; 18,300,000) | 0·8 (0·6; 0·9) | -- | 71·0 | 119 |
| UNAIDS regions* | |||||
| Asia and the Pacific | 760,000 (640,000; 970,000) | 0·1 (0·1; 0·1) | 5·2 | 45·8 | 126 |
| Caribbean | 97,000 (78,000; 115,000) | 1·0 (0·8; 1·1) | 0·7 | 73·8 | 8 |
| Eastern & southern Africa | 10,600,000 (8,000,000;12,900,000) | 5·5 (4·1; 6·7) | 71·9 | 77·7 | 130 |
| Latin America | 270,000 (220,000; 340,000) | 0·2 (0·1; 0·2) | 1·8 | 59·9 | 1 |
| Middle East & North Africa | 40,000 (22,000; 66,000) | 0·03 (0·02; 0·05) | 0·3 | 22·5 | 47 |
| Western & central Africa | 2,600,000 (1,800,000; 3,400,000) | 1·1 (0·8; 1·5) | 17·6 | 50·3 | 7 |
| HIV high-burden countries | |||||
| Angola | 180,000 (130,000; 220,000) | 1·3 (1·0; 1·7) | 1·2 | 32·0 | 582 |
| Botswana | 200,000 (160,000; 220,000) | 27.4 (21·5; 30·9) | 1·3 | 80·1 | 104 |
| Burundi | 58,000 (45,000; 70,000) | 1·1 (0·9; 1·4) | 0·4 | 57·9 | −22 |
| Cameroon | 320,000 (250,000; 380,000) | 3·2 (2·4; 3·7) | 2·2 | 63·6 | 228 |
| Chad | 94,000 (67,000; 120,000) | 1·3 (0·9; 1·7) | 0·6 | 35·5 | 100 |
| Côte d’Ivoire | 220,000 (160,000; 290,000) | 2·2 (1·6; 2·9) | 1·5 | 64·7 | 16 |
| Democratic Republic of Congo | 280,000 (210,000; 340,000) | 0·7 (0·5; 0·8) | 1·9 | 31·4 | 20 |
| Eswatini | 140,000 (110,000; 150,000) | 32·4 (26·9; 36·3) | 0·9 | 84·1 | 309 |
| Ethiopia | 270,000 (190,000; 380,000) | 0·7 (0·5; 1·0) | 1·8 | 55·8 | −3 |
| Ghana | 170,000 (130,000; 220,000) | 1·5 (1·1; 2·0) | 1·2 | 51·0 | 79 |
| Kenya | 840,000 (650,000; 1,100,000) | 4·5 (3·4; 5·7) | 5·7 | 76·6 | 14 |
| Lesotho | 140,000 (110,000; 160,000) | 21·1 (16·2; 24·4) | 0·9 | 70·9 | 159 |
| Malawi | 580,000 (440,000; 680,000) | 7·0 (5·4; 8·3) | 3·9 | 66·6 | 63 |
| Mozambique | 980,000 (700,000; 1,300,000) | 7·1 (5·1; 9·4) | 6·6 | 86·3 | 443 |
| Namibia | 140,000 (110,000; 160,000) | 16·4 (13·0; 18·8) | 1·0 | 81·1 | 179 |
| Nigeria | 880,000 (570,000; 1,300,000) | 1·1 (0·7; 1·6) | 6.0 | 48·4 | 123 |
| South Africa | 3,500,000 (2,600,000; 4,300,000) | 21·6 (15·7; 26·1) | 23·8 | 78·2 | 739 |
| Uganda | 1,100,000 (880,000; 1,300,000) | 5·8 (4·6; 6·6) | 7·5 | 80·9 | 39 |
| United Republic of Tanzania | 910,000 (680,000; 1,100,000) | 3·7 (2·8; 4·5) | 6·1 | 88·3 | 102 |
| Zambia | 560,000 (440,000; 660,000) | 6·8 (5·5; 8·1) | 3·8 | 98·3 | 111 |
| Zimbabwe | 850,000 (640,000; 1,000,000) | 13·6 (10·3; 16·2) | 5·7 | 65·0 | 14 |
Estimates not available for Eastern Europe and central Asia, Western and central Europe and North America
ARV – antiretroviral; CHEU – children HIV exposed uninfected; UNAIDS – Joint United Nations Program on HIV/AIDs
Figure 1: 2019 UNAIDS estimates of individual country contributions (%) to global population of children HIV exposed uninfected in 2018.
Note: Estimates were not available for the Eastern Europe and Central Asia region, the Western and central Europe and North America region and the high burden countries of India and Indonesia
Figure 2: 2019 UNAIDS estimates of absolute number and prevalence of children HIV exposed uninfected (age 0–14 years) in 21 HIV high-burden countries in 2018.
Left Y-axis represented by bars; right Y-axis represented by circle markers; DR Congo – Democratic Republic of the Congo
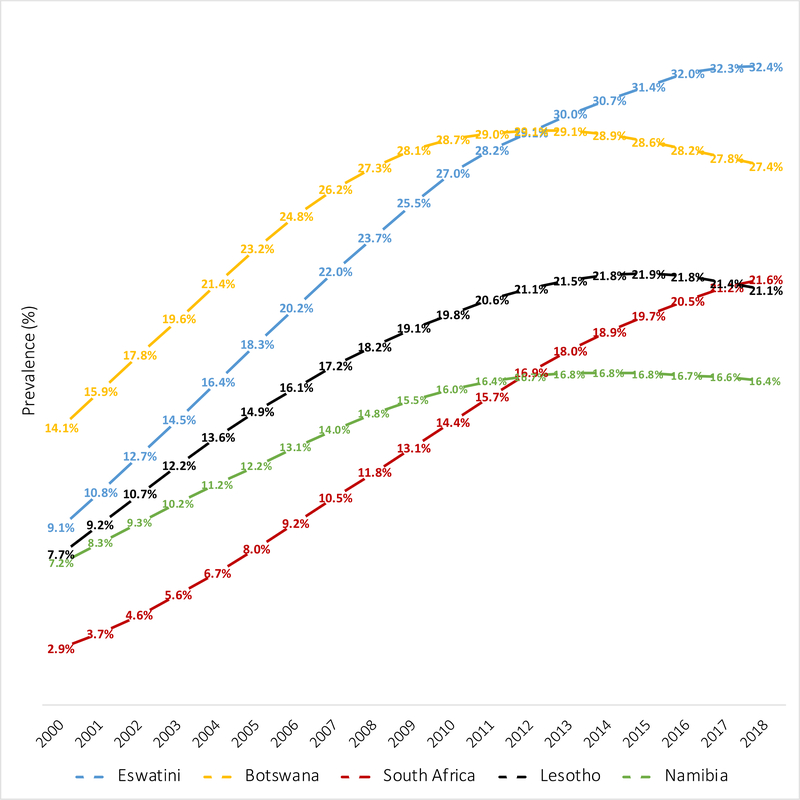
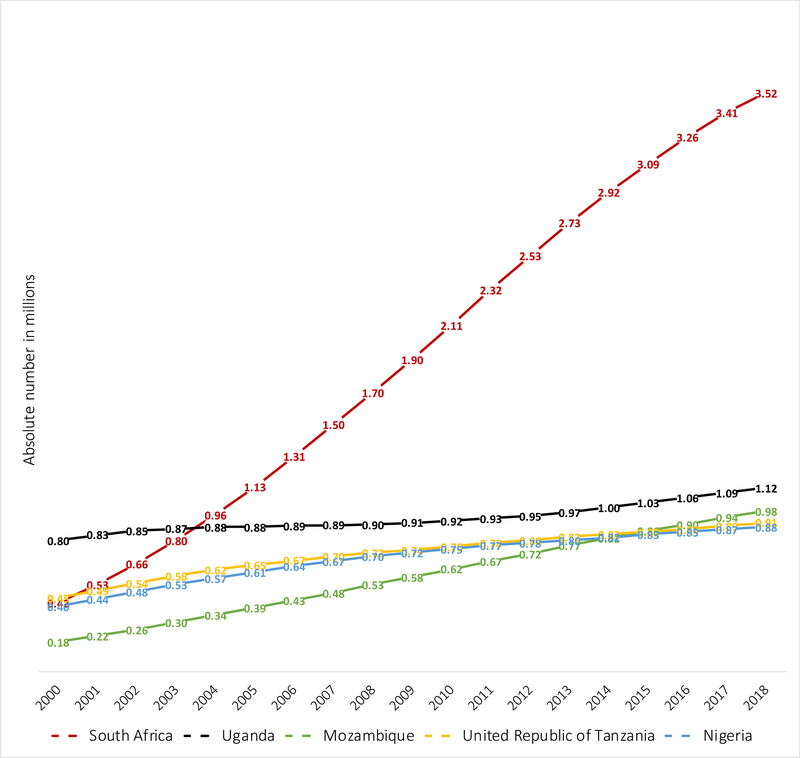
Between 2000 and 2018 the prevalence of CHEU in Eastern and Southern Africa increased from 3·6% to 5·5% of the child population. Asia and the Pacific, as well as Eastern and Southern Africa are regions that have experienced doubling in their CHEU population size between 2000 and 2018. Of the ten countries with a CHEU prevalence above 5%, three southern African countries continue to experience annualized increases in CHEU prevalence (Eswatini, South Africa and Mozambique). The CHEU prevalence between 2000 and 2018 has tripled in Eswatini from 9·1% to 32·4% and increased more than 6-times in South Africa from 2·9% to 21·6%, with recent evidence of stabilizing prevalence in both countries (Figure 3). The CHEU prevalence in Lesotho and Namibia has more than doubled between 2000 and 2018, Lesotho increasing from 7·7% to 21·1% and Namibia increasing from 7·2% to 16·4%. Botswana, with the second highest CHEU prevalence in 2018, started at a higher prevalence than Eswatini and South Africa in 2000 at 14·9%, almost doubling to 27·4% in 2018 but with decline from a peak of 29·1% in 2013. In South Africa the CHEU population has increased seven-times in size from 0·4 million in 2000 to 3·5 million CHEU in 2018 (Table 1, Figure 4). Although Angola is one of the lower CHEU prevalence countries at 1·3%, Angola has experienced a more than five-times increase in CHEU population size between 2000 and 2018.
Figure 3:
2019 UNAIDS estimates of national prevalence of children HIV exposed and uninfected in the 5 highest prevalence countries: 2000 to 2018
Figure 4:
2019 UNAIDS estimates of the number of children HIV exposed and uninfected for the 5 countries with the largest populations of children HIV exposed and uninfected: 2000 to 2018
The proportion of all CHEU that are also ARV-exposed has increased from almost no CHEU children with in utero ARV exposure in the early 2000s to 71% of all CHEU globally in 2018 (Table 1). By region in 2018, less than 60% of CHEU were also ARV-exposed in Asia and the Pacific, Latin America, Middle East and North Africa, and West and Central Africa. By 2018, seven sub-Saharan African high HIV prevalence countries had 80% or more of their CHEU exposed to ARVs, these were Zambia (98%), Tanzania (88%), Mozambique (86%), Eswatini (84%), Namibia (81%), Uganda (81%), and Botswana (80%). Yet, in four high HIV burden countries, less than half of CHEU were ARV-exposed in 2018, including Nigeria (48%), Chad (36%), Angola (32%), and Democratic Republic of the Congo (31%).
Discussion
According to modelled estimates, globally there were 14.8 million CHEU in 2018, 50% living in just five sub-Saharan African countries including South Africa, Uganda, Mozambique, Tanzania, and Nigeria. CHEU prevalence exceeded 25% in Eswatini and Botswana and was above 15% in South Africa, Lesotho and Namibia. There were 1·5 million CHEU outside of sub-Saharan Africa who should also not be overlooked. Although ARV coverage for pregnant WLHIV in 2018 was 82% (LE 62%; UE >95%), in the current cohort of CHEU age 0–14 years and born between 2004 and 2018, only 71% globally were also ARV-exposed, the older children among the cohort less likely to have experienced in utero ARV exposure in the early 2000s.2
The increasing prevalence of CHEU seen between 2000 and 2018 has been driven predominantly by rising and then static prevalence of HIV among pregnant women in HIV high-burden countries in parallel with the success of programs to prevent perinatal and postnatal HIV transmission, resulting in an increasing proportion of children HIV exposed who remained HIV-uninfected. Southern African countries with later onset of their HIV epidemics particularly Eswatini, Mozambique, and South Africa, compared to other African countries, have experienced steep increases in CHEU population size and only recently started experiencing a stabilizing prevalence of CHEU as maternal HIV prevalence has begun to stabilize. At a population level in countries with high CHEU prevalence, infant HIV exposure has been associated with a substantial contribution to infant mortality.21 In the years to come, child HIV exposure will only decline substantially through successful HIV prevention interventions and programming to reduce HIV acquisition in adolescent girls and women, combined with early diagnosis of HIV and access to effective family planning options among adolescent girls and women living with HIV to curtail unintended pregnancies (Table 2).
Table 2:
Coordinated global strategy for improved outcomes in children who are HIV exposed uninfected
| Foundation: Coordinated dialogue with and advocacy for HIV-affected families | |
|---|---|
| Priority | Action Level |
| 1. Prevention: | • Multilateral organizations & partners |
| a. Prevention of new HIV infections in adolescent girls and women | • National Ministries |
| b. Prevention of unintended pregnancies in girls and women living with HIV | • Communities |
| 2. ART coverage coupled with pharmacovigilance systems: | • Multilateral organizations & partners |
| a. Universal coverage of sustained ART in all pregnant and breastfeeding WLHIV and appropriate postnatal ARV prophylaxis to infants HIV-exposed to prevent perinatal and postnatal HIV acquisition and improve maternal and infant survival | • National Ministries |
| • Pharmaceutical regulatory authorities | |
| • Pharmaceutical industry | |
| b. Comprehensive, sustainable pregnancy pharmacovigilance surveillance in HIV high-burden countries | • Communities |
| 3. Research and monitoring partnerships: | • Researchers and funders |
| a. Partnering of established CHEU research cohorts with researchers in countries with the highest CHEU prevalence to design and establish contextually relevant approaches to long-term CHEU research with harmonization of exposure and outcome measures enabling collaboration and comparison across settings | • National Ministries |
| • UNAIDS supporting countries | |
| • Partners supporting routine electronic data systems | |
| b. National cohort monitoring through data disaggregation by HIV exposure and infection status in national child mortality surveillance and vital statistics registration systems | • Communities |
ART – antiretroviral therapy; ARV – antiretroviral; CHEU – children HIV exposed uninfected; WLHIV – women living with HIV; UNAIDS – Joint United Nations Program on HIV/AIDS
The first priority in children who are HIV exposed is prevention of HIV acquisition and essential to achieving this is exposure in utero and postnatally to effective ARV regimens. Several HIV high-burden countries have made remarkable strides towards achieving an HIV-free start for children who are HIV-exposed through maternal scale-up of ART and infant ARV prophylaxis, reaching 80% of WLHIV and their infants over the past 14 years. In contrast, it is evident that in the lower burden and lower prevalence sub-Saharan African countries as well as the regions of Asia and the Pacific, and Middle East and North Africa, where less than half of CHEU are ARV-exposed, perinatal and postnatal HIV prevention has been challenging. This is possibly a result of less national prioritization and international partner support for HIV programs in countries of lower HIV prevalence and possibly less rigorous HIV testing schedules or poor compliance with recommended national HIV testing schedules for pregnant and breastfeeding women, to identify all pregnant and breastfeeding WLHIV and provide appropriate maternal ART and infant prophylaxis. Recognizing the focus and investment still required to reach zero perinatal and postnatal HIV transmissions globally, the responsibility for the well-being of children born to WLHIV does not stop at an HIV free start, but extends to actively monitoring the safety of early-life ARV and HIV exposure through long-term sustainable systems in CHEU high-prevalence countries (Table 2). 13,14
In a number of middle-to-high resource but low HIV prevalence settings there has been considerable investment in establishing long-term research cohorts and national monitoring systems to understand the effect of in utero HIV and ARV exposure.22–25 These cohorts have made tremendous contributions evaluating the safety of ARVs in pregnancy for the woman, fetus and child, establishing novel methods for measurement of medium to long-term outcomes. However, these cohorts exist in contextually different settings where the HIV epidemic is primarily reflective of key populations, where exclusive formula feeding of infants who are HIV exposed is promoted, and death from prematurity, infectious diseases and malnutrition among infants and children is negligible. The research investment is not unwarranted in these middle-to-high resource settings but the contextual challenges in sub-Saharan Africa, home to 90% of children who are HIV and ARV-exposed, provides a clear investment mandate on this sub-continent.26
The CHEU model-based estimates presented here come with uncertainty, one source of which is the assumption related to fertility in women on ART.27 Furthermore, these estimates do not assume higher mortality rates in CHEU compared to CHUU; mortality estimates are based on general country-specific child mortality rates for HIV-uninfected children. As survival in HIV-uninfected children in absolute terms is high in general, this assumption of equivalent CHEU and CHUU mortality is unlikely to substantially overestimate the CHEU population size. These estimates do not deal with the heterogeneity of HIV and ARV exposure experienced by CHEU, and furthermore do not include children only postnatally exposed to HIV or ARVs. A more nuanced understanding of the timing and extent of in utero and early life exposure to HIV and ARVs may aid in elucidating mechanisms of adverse outcomes in the CHEU population.
Recognizing the limitations in these modelled estimates, and the sometimes-limited generalizability of research cohorts, is it necessary and possible for HIV high-burden countries to invest in national longitudinal cohort monitoring measuring the prevalence of their CHEU population, along with key outcomes? National monitoring of child mortality, if disaggregated by HIV-infection and HIV-exposure status i.e. children living with HIV, CHEU and CHUU, will demonstrate ecological temporal trends within countries of whether CHEU survival at a population level is equivalent to CHUU in the same settings in the current context of expansion of universal ART and prolonged safer breastfeeding. Being able to disaggregate longitudinal data at a national level by HIV-infection and HIV-exposure status goes hand-in-hand with the aim of early infant diagnosis and perinatal and postnatal HIV transmission prevention programs, to ensure that the final HIV status of every child who is HIV-exposed is ascertained after breastfeeding completion. Similarly, the value of monitoring the ARVs to which CHEU have been exposed in utero or during breastfeeding extends beyond the knowledge gained about the short- and long-term implications of this exposure, but synergistically facilitates identification of gaps in provision of perinatal and postnatal HIV transmission prevention interventions. It is recognized that for such individual-level monitoring to be achieved there are numerous information privacy considerations, the risks and benefits of which will need to be continuously evaluated.28
South Africa is one example where identifying all CHEU nationally and disaggregating child mortality surveillance data by HIV-infection and HIV-exposure status could be possible in the future. The Western Cape Province of South Africa has implemented unique patient identifiers across all public health facilities with integration of individual-level routine healthcare data from a variety of electronic platforms in an emerging patient information exchange.29 Maternal and infant unique identifiers, regardless of a woman’s HIV status, are linked at birth allowing ascertainment of HIV and ARV-exposure history as well as HIV-infection status at the level of individual children.30 As national electronic medical record systems emerge across Africa, unique patient identifiers with linkage of maternal and infant identifiers at birth can provide a pivotal and value-added platform for monitoring short, medium and long-term outcomes of all children including those HIV-exposed, with sufficient investment and strategic planning. Such population-based national systems would synergistically allow validation of model-based estimates of the CHEU population size, improved monitoring of perinatal and postnatal HIV transmission prevention programs, and evaluation of longer-term CHEU outcomes.
These CHEU estimates bring into focus the need for a multi-pronged strategy, built on a foundation of coordinated dialogue with and advocacy for HIV-affected families, to ensure the health and well-being of the CHEU population (Table 2). Reducing the number of children HIV exposed, that stands at 14.8 million in 2018, requires intensified efforts on multiple fronts. Prevention of new HIV infections and unintended pregnancies in adolescent girls and women is essential. A key priority in ensuring the health and well-being of the global CHEU population is securing universal maternal ART coverage to improve both maternal and child survival, coupled with sustainable pregnancy and early-life pharmacovigilance surveillance systems. Geographically aligned investment in research cohorts and national monitoring to comprehensively evaluate the implications of in utero and postnatal ARV exposure in HIV high-burden countries is required. There is an opportunity for multilateral organizations, ministries of health, healthcare providers, researchers and the community of families living with HIV to partner in new ways to secure a healthy future for the substantial global population of children who are HIV exposed but uninfected.
Research in context.
Evidence before this study
Three previous systematic reviews and meta-analyses have estimated an almost two-times increase in mortality in children HIV exposed uninfected (CHEU) born to women living with HIV compared to children HIV unexposed uninfected (CHUU) born to women living without HIV. We searched the scientific literature through PubMed, the grey literature through Google Scholar and United Nations (UN) agency websites, including UNICEF, WHO, and the UN Population Division for reports of the estimated size of the CHEU population without date restrictions up until 29 September 2019 and using the following search strategy: (global OR world OR regional OR national) AND ((HIV AND exposed AND uninfected) OR HEU) AND (child* OR infant*) AND (burden OR estimate OR size OR level OR trend). The search did not reveal any reports of the estimated size of the CHEU population. Studies frequently referenced national estimates of pregnant women living with HIV or the UNAIDS estimates for the numbers of pregnant women living with HIV annually and the number of newly infected children living with HIV, informally extrapolating from these annual numbers of HIV-uninfected infants born to women living with HIV. There were no reports however of either measured or modelled national, regional or global estimates of the size of the accumulating CHEU population.
Added value of this study
To our knowledge, the 2019 UNAIDS estimates are the only modelled global estimates of CHEU population size, with longitudinal estimates providing insight into how this population has evolved in the maturing HIV epidemic between 2000 and 2018. This is also the first report of country-specific modelled estimates of CHEU population size in 21 countries with a high burden of HIV. With five sub-Saharan African countries estimated to account for 50% of all CHEU (South Africa, Uganda, Mozambique, Tanzania, and Nigeria) and five countries with CHEU prevalence estimated at 15% or more of the general child population (Eswatini, Botswana, South Africa, Lesotho and Namibia), this report brings into sharp focus the need for alignment of research and programmatic investment with the geographic location of the majority of CHEU, who are not surviving and thriving as well as their child peers who are HIV unexposed uninfected.
Implications of all the available evidence
At 14.8 million, the size of the global CHEU population is substantial. Persisting disparities for CHEU in birth outcomes, early childhood survival, growth and neurodevelopment, despite improving maternal health with antiretroviral therapy, forewarns of potential later life challenges for adolescents and adults who are HIV exposed and uninfected. These outcome disparities are particularly relevant to the human capital for countries with a high prevalence of CHEU where directed research and programmatic investment has been limited. Going forward a multi-pronged strategy, built on a foundation of coordinated dialogue with and advocacy for HIV-affected families is required to ensure the health and well-being of the CHEU population, that includes: 1) reducing HIV exposure in children through intensified efforts to prevent new HIV infections in adolescent girls and women and prevent unintended pregnancies in adolescent girls and women living with HIV; 2) ensuring optimal health for all adolescent girls and women living with HIV and their children through realization of global universal coverage of sustained maternal ART, coupled with pharmacovigilance for pregnancy and early-life exposures in HIV high-burden countries to ensure minimal consequences of HIV and ARV exposure to CHEU and 3) establishing research and monitoring partnerships aligned with the geographic distribution of CHEU, across high and low resourced settings and leveraging expansion of emerging electronic health data systems in Africa.
Acknowledgments
Funding sources: Research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health under Award Number 1K43TW010683 to ALS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development under Award Number 1R21HD093531 to KMP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. ALS receives salary support through the CIPHER Grantee Programme of the International AIDS Society (2017/518-SLO). The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication
Funding: National Institutes of Health, International AIDS Society
Footnotes
Declaration of interests
All authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). 2019 UNAIDS Estimates. 2019. www.aidsinfo.unaids.org (accessed July 16, 2019).
- 2.UNAIDS. Start Free Stay Free AIDS Free 2019 report. 2019. https://www.unaids.org/en/resources/documents/2019/20190722_UNAIDS_SFSFAF_2019 (accessed July 31, 2019).
- 3.Evans C, Jones CE, Prendergast AJ. Review HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis 2016; 3099: 1–16. [DOI] [PubMed] [Google Scholar]
- 4.Ramokolo V, Goga AE, Slogrove AL, Powis KM. Unmasking the vulnerabilities of uninfected children exposed to HIV. Br Med J 2019; 366: doi: 10.1136/bmj.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan AT, Bonawitz R, Gill CJ, et al. A meta-analysis assessing all-cause mortality in HIV-exposed uninfected compared to HIV-unexposed uninfected infants and children. AIDS 2016; 30: 2351–60. [DOI] [PubMed] [Google Scholar]
- 6.Evans C, Chasekwa B, Ntozini R, et al. Surviving and thriving? Outcomes of HIV-exposed children in rural Zimbabwe. In: Conference on Retroviruses and Opportunistic Infections. Seattle, Washington.4–7 March 2019; Abstract #790 [Google Scholar]
- 7.Slogrove AL, Esser MM, Cotton MF, et al. A Prospective cohort study of common childhood infections in South African HIV-exposed uninfected and HIV-unexposed infants. Pediatr Infect Dis J 2017; 36: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao P-L, Zhou Y-B, Chen Y, et al. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy Childbirth 2015; 15: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malaba TR, Phillips T, Le Roux S, et al. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int J Epidemiol 2017; 46: 1678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McHenry MS, McAteer CI, Oyungu E, et al. Neurodevelopment in Young Children Born to HIV-Infected Mothers: A Meta-analysis. Pediatrics 2018; 141: e20172888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budd MA, Calli K, Samson L, et al. Blood mitochondrial DNA content in HIV-exposed uninfected children with autism spectrum disorder. Viruses 2018; 10: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wedderburn CJ, Yeung S, Rehman AM, et al. Neurodevelopment of HIV-exposed uninfected children in South Africa: outcomes from an observational birth cohort study. Lancet Child Adolesc Heal 2019; 4642: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zash RM, Williams PL, Sibiude J, Lyall H, Kakkar F. Surveillance monitoring for safety of in utero antiretroviral therapy exposures: current strategies and challenges. Expert Opin Drug Saf 2016; 0338: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mofenson LM. In-utero ART exposure and the need for pharmacovigilance. Lancet Glob Heal 2018; 6: e716–7. [DOI] [PubMed] [Google Scholar]
- 15.Zash R, Holmes L, Diseko M, et al. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. N Engl J Med 2019; 381: 827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slogrove AL, Frigati L, Gray DM. Maternal HIV and Paediatric Lung Health. Paediatr Respir Rev 2017; 21: 47–53. [DOI] [PubMed] [Google Scholar]
- 17.Joint United Nations Programme on HIV/AIDS (UNAIDS). Quick Start Guide for Spectrum 2019. 2019. https://www.unaids.org/sites/default/files/media_asset/QuickStartGuide_Spectrum_en.pdf (accessed July 16, 2019).
- 18.Mahy M, Brown T, Stover J, et al. Producing HIV estimates: From global advocacy to country planning and impact measurement. Glob Health Action 2017; 10 DOI: 10.1080/16549716.2017.1291169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahy M, Penazzato M, Ciaranello A, et al. Improving estimates of children living with HIV from the Spectrum AIDS Impact Model. AIDS 2017; 31: S13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens GA, Alkema L, Black RE, et al. Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet 2016; 388: e19–23. [DOI] [PubMed] [Google Scholar]
- 21.Slogrove AL, Johnson LF, Powis KM. Population-level Mortality Associated with HIV Exposure in HIV-uninfected Infants in Botswana and South Africa: A Model-based Evaluation. J Trop Pediatr 2019; 65: 373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Dyke RB, Chadwick EG, Hazra R, Williams PL, Seage GR. The PHACS SMARTT study: Assessment of the safety of in utero exposure to antiretroviral drugs. Front Immunol 2016; 7: doi 10.3389/fimmu.2016.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazra R, Stoszek SK, Freimanis Hance L, et al. Cohort Profile: NICHD international site development initiative (NISDI): A prospective, observational study of HIV-exposed and HIV-infected children at clinical sites in Latin American and caribbean countries. Int J Epidemiol 2009; 38: 1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorne C, Tookey P. Strategies for Monitoring Outcomes in HIV-Exposed Uninfected Children in the United Kingdom. Front Immunol 2016; 7: doi 10.3389/mmu.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC) Study Group. NRTI backbones and pregnancy outcomes. AIDS 2019; 33: 295–304.30562172 [Google Scholar]
- 26.Slogrove AL, Archary M, Cotton MF. Optimizing research methods to understand HIV-exposed uninfected infant and child morbidity: Report of the second HEU infant and child workshop. Front Immunol 2016; 7 DOI: 10.3389/fimmu.2016.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson LF, Mutemaringa T, Heekes A, Boulle A. Effect of HIV Infection and Antiretroviral Treatment on Pregnancy Rates in the Western Cape Province of South Africa. J Infect Dis 2019; epub: doi: 10.1093/infdis/jiz362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jao J, Hazra R, Mellins CA, Remien RH, Abrams EJ. Debate article Disclosing in utero HIV/ARV exposure to the HIV-exposed uninfected adolescent: is it necessary? J Int AIDS Soc 2016; 19: e21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heekes A, Tiffin N, Dane P, et al. Self-enrolment antenatal health promotion data as an adjunct to maternal clinical information systems in the Western Cape Province of South Africa. BMJ Glob Heal 2018; 3: e000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalk E, Kroon M, Boulle A, et al. Neonatal and infant diagnostic HIV-PCR uptake and associations during three sequential policy periods in Cape Town, South Africa: a longitudinal analysis. J Int AIDS Soc 2018; 21: e25212. [DOI] [PMC free article] [PubMed] [Google Scholar]