Abstract
Advances in neonatology have led to unprecedented improvements in neonatal survival such that those born as early as 22 weeks of gestation now have some chance of survival, and over 70% of those born at 24 weeks of gestation survive. Up to 50% of infants born extremely preterm develop poor outcomes involving long-term neurodevelopmental impairments affecting cognition and learning, or motor problems such as cerebral palsy. Poor outcomes arise because the preterm brain is vulnerable both to direct injury (by events such as intracerebral hemorrhage, infection and/or hypoxia), or indirect injury due to disruption of normal development. This neonatal brain injury and/or dysmaturation is called “encephalopathy of prematurity”. Current and future strategies to improve outcomes in this population include prevention of preterm birth, and pre-, peri-, and postnatal approaches to protect the developing brain. This review will describe mechanisms of preterm brain injury, and current and upcoming therapies in the antepartum and postnatal period to improve preterm encephalopathy.
Introduction
Preterm birth (birth before 37 weeks of gestation) accounts for 35% of neonatal deaths and is the second most common cause of death under 5 years of age.1, 2 The rates of preterm delivery vary by country, and in general, are inversely related to its wealth. Of the 1.2 million preterm births that occur in high income countries, 42% occur in the United States, where the preterm birth rate was 9.85% in 2016.3 In 2005, the U.S. annual economic burden caring for preterm infants up to age 5 years was estimated to be $26 billion by the National Academy of Medicine.4
Advances in neonatology have led to unprecedented improvements in preterm survival disproportionately in high-income countries such that 70% of those born at 24 weeks of gestation, and over 90% of infants born at 27 weeks or later survive.5 With these successes, the limit of viability has continued to decrease, and is now considered to be 22–23 weeks of gestation in most developed countries. Survival of these increasingly smaller and sicker babies has led to a corresponding increase in the prevalence of neurodevelopmental disabilities. Forty to sixty percent of survivors born at less than 28 weeks of gestation still develop moderate or severe impairments (cerebral palsy (CP), intellectual disability, deafness, blindness), with the smallest children at highest risk.6 Preterm survivors are also at higher risk of autism spectrum disorder, attention deficit hyperactivity disorder, and psychiatric disorders than their term-born peers. The brain injury experienced by preterm survivors has changed as neonatology has evolved: severe intracranial hemorrhage and cystic white matter injury (WMI) were much more prevalent in past decades. Modern preterm brain injury is more likely to include dysmaturation of neuronal and glial progenitor cells.7 Despite this more subtle injury, overall developmental outcomes have changed little over the decades. Our challenge now is to develop neuroprotective approaches to improve these outcomes in preterm survivors.
Brain Development in the Third Trimester
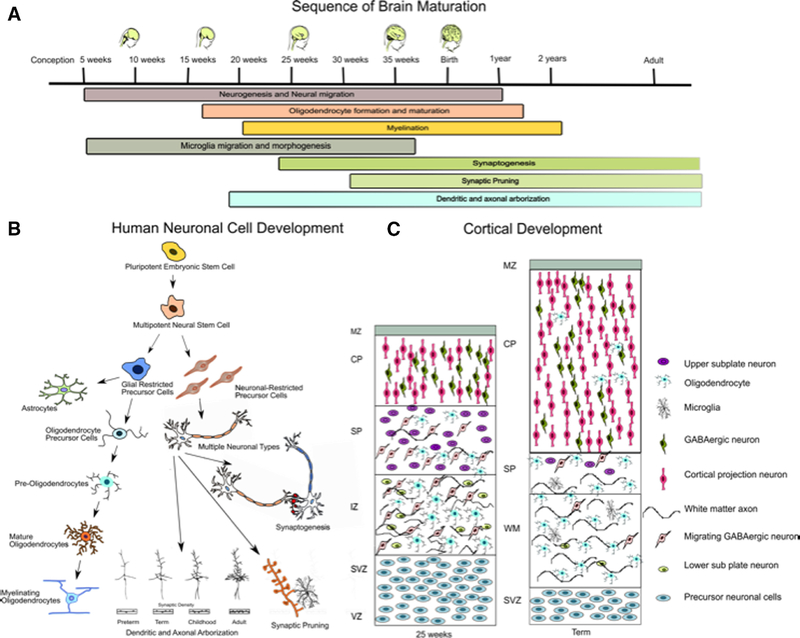
Preterm infants are born during a time of active brain development with a tripling of surface area occurring between 25 and 40 weeks as gyrification occurs.8 Key events occurring during this period include continued birth of neurons in the subventricular zone and ganglionic eminence migrating to the thalamus, basal ganglia, and deeper cortical layers, new active growth of axons in periventricular regions, development of the oligodendrocytes, astrocytes and microglia, synaptogenesis, myelination, and finally, pruning (Figure 1). During this critical period of development, the brains of infants born preterm are exposed to exogenous and endogenous stimuli including hypoxia, hypoxia-ischemia, hyperoxia, inflammation, excitotoxicity, and an excess of free-radicals. Nutritional deficiencies, (both macro and micro), exposure to pain, light, noise, drugs and other factors in the neonatal intensive care environment also play a role in modifying extra-uterine development.
Figure 1. Schematic of human brain development.
(A) Sequence of brain maturation from conception to adulthood. (B) Human neuronal cell development during the life time. (C) Cortical Development in the third trimester of preterm and term infants. MZ: marginal zone, CP: cortical plate, SP: sub plate layer, IZ: intermediate zone, WM: white matter, SVZ: subventricular zone, VZ: ventricular zone.
Oligodendrocytes are the myelinating cells in the brain, and they play a critical role in the maturation of white matter. Oligodendrocytes develop and mature between 24 to 32 weeks of gestation, a period in which they are particularly vulnerable to injury. Oligodendrocyte progenitor cells (OPCs) give rise to preoligodendrocytes (preOL), which develop into mature myelinating oligodendrocytes (OL).9 Developing oligodendrocytes are highly vulnerable to injury caused by glutamate, free radicals, and inflammation. Current data suggests that injury to the OPCs and preOLs results in proliferation of OPCs, but due to a block in maturation, there remains a deficit in mature OLs with myelinating capability, which is associated with axonal injury and WMI.10,11 Mechanisms postulated to contribute to the developmental arrest of OPC and preOLs include dysregulation of Notch and WNT-beta catenin signaling. This is mediated by microglial activation and subsequent hyaluronidase production with resulting hyaluronan break down products that are specific inhibitors of OL maturation.12–14
Microglia are vital to synaptogenesis and pruning, inflammation and tissue repair. These cells reach peak abundance in cerebral white matter in the third trimester. These cells can have both beneficial and injurious effects and are often noted to be activated in areas of oligodendrocyte injury.
Subplate neurons are a transient population of excitatory neurons located just below cortical layer 6.15 These cells, which are most prominent between 24–32 weeks of gestation, play a critical role in the establishment of cortical lamination and thalamocortical connectivity by serving as an intermediary synaptic contact.16 After 33 weeks of gestation when thalamocortical axons establish synapses with cortical plate layer IV neurons, the subplate neurons undergo apoptosis. Damage to developing subplate neurons has been postulated to disrupt thalamocortical white matter axons, as well as disrupt cortical neuronal connectivity, which can lead to long term motor and cognitive deficits.17
Mechanism of Preterm Encephalopathy
There are many antenatal and postnatal factors (Figure 2) that can contribute to injury of the developing brain. Events such as intraparenchymal hemorrhage can damage specific structures that previously developed normally, but most factors interfere with the normal unfolding of development. Since brain development is an intricate unfolding of many events simultaneously, damage to one aspect can adversely affect others.
Figure 2. Flow diagram outlining risk factor and underlying mechanism contributing to preterm encephalopathy.
Mentioned risk factors lead to hypoxia-ischemia, inflammation and oxidative stress, which causes white and gray matter injury by different mechanisms and ultimately lead to long term cognitive and motor impairment.
White Matter Injury (WMI)
Periventricular WMI is the hallmark of preterm brain injury. This ranges from focal cystic necrosis, to focal microscopic necrosis, to diffuse non-necrotic lesions. The large necrotic cystic periventricular leukomalacia that used to be quite common is now seen less frequently, whereas diffuse, microcystic WMI is more common.18 Axonal degeneration occurs in focal necrotic WMI, but is generally spared in diffuse WMI.19 Focal necrotic WMI often contains axonal spheroids and dystrophic axons, which degenerate during the early phase of coagulative necrosis,20 while diffuse WMI is characterized by persistent astrogliosis and microgliosis.9, 21, 22 Factors associated with WMI include perinatal hemodynamic instability, inflammatory exposures, as well as exposures to pain, hypoxia-ischemia, hypoxia, hyperoxia, hypocarbia, hypo and hyperglycemia. Other clinical experiences such as multiple attempts at intubation also are associated with brain injury.23
Gray Matter Injury
Human neuroimaging studies have identified reduction in cerebral cortex volume in preterm survivors. Studies have also shown varying degrees of abnormal cortical folding, reduced gyral complexity, and increased ventricular and extra-axial volume.24 Cerebellar structures and subcortical gray matter including basal ganglia, thalamus, and hippocampus are often adversely affected.25–29 During weeks 24–32 of gestation, dendritogenesis and experience-driven synaptogenesis is active, and largely responsible for the four-fold increase in cortical volume that occurs during this period. These maturational processes are highly vulnerable to injury. Preterm neurons have variable susceptibility to hypoxia-ischemia and other insults. Vulnerability is dependent on the type of neuron, extent of prematurity, severity of the insult, and severity of WMI. Immature axons are susceptible to glutamate-mediated excitotoxicity at sites of contact with OL processes, and axon degeneration identified by fraction immunostaining is often seen in regions of periventricular leukomalacia. Dean et al. in their sheep model of preterm injury showed significant reduction in dendritic arborization and spine densities rather than loss of neurons as a cause of gray matter mass.30 These changes correlate with an increase in functional anisotropy (FA), which usually decreases as the complexity of gray matter in the cerebral cortex increases.30 In addition to altered arborization, there may be a reduction in cortical neurogenesis. Malik et al showed a reduced number of Tbr2+ progenitor cells and increased number of Sox2+ radial glia, suggestive of reduction in glutamatergic neurogenesis.31 A similar reduction in neurogenesis is also observed in GABAergic interneurons.
Dysmaturation and maturational arrest are emerging as key factors in preterm encephalopathy. Further studies are needed to understand the mechanisms leading to maturational arrest and whether there are pathways that can be modified to provide neuroprotection in preterm infants.
Cerebellar Injury
The cerebellum undergoes a 5-fold increase in volume between 24 and 40 weeks of gestation, with an estimated 30-fold increase in surface area as foliation occurs.32 Like the cerebral cortex, the cerebellum is subject both to injury (hemorrhage or infaction) and abnormalities of development.32, 33 Recent data suggest that up to 19% of premature infants born before 32 weeks of gestation have cerebellar abnormalities,34 with higher rates among extremely low birth weight infants.35 Cerebellar injury is now being recognized as one of the important complications of preterm birth leading to poor neurodevelopmental outcome. Cerebellar injury most commonly occurs as direct injury in the form of cerebellar hemorrhage.
Four categories of cerebellar hemorrhage have been described: 1) Primary cerebellar hemorrhage, 2) Venous infarction 3) Extension of intraventricular of subarachnoid blood into cerebellum, and 4) Traumatic laceration of cerebellum or rupture of major veins or occipital sinus. The range of severity of primary cerebellar hemorrhage includes mild punctate lesions, focal unilateral lesions, to extensive bihemispheric and vermian hemorrhages. Cerebellar atrophy can be seen as soon as 2 months after hemorrhage. Associated reduction in contralateral cerebral volumes occurs most likely due to impairment of transynaptic trophic effects.36, 37 Factors that are associated with white matter injury (exposure to hypoxia-ischemia, oxidative stress and infection/inflammation) are also associated with cerebellar underdevelopment, and similar pathologic findings are seen (neuronal loss and gliosis).38 Cerebellar injury is associated with cognitive deficits, language impairment and autism spectrum disorder.39, 40 Cerebellar underdevelopment and injury can be an important contributer to preterm encephalopathy, and additional studies are needed to further define the long term effects in preterm infants.
Antenatal Therapies
Docosahexaenoic acid (DHA)
Long chain polyunsaturated fatty acid (LC-PUFA), including docosahexaenoic acid (DHA) and arachidonic acid (AA), are incorporated into membrane phospholipids and play an important role in brain development, including roles in neurogenesis and neurotransmission. DHA is derived from α-linolenic acid, which is an essential omega-3 fatty acid. The accumulation of DHA in the brain takes place during the brain growth spurt in the third trimester and continues up to two years of age. The high levels of DHA in the brain are maintained throughout life. The Organization of the United Nations agrees that pregnant and lactating women should have a minimal intake of 200–300 mg of DHA per day. A study in 2017 by Nordgren et al showed that in United States on average, DHA intake was only 66% of recommended in pregnant women, and only 9% of women took DHA supplements during pregnancy.41 The neonatal dietary source for DHA is breast milk or formula. DHA content in breast milk varies with maternal diet and obesity. Studies have shown that supplementing DHA in maternal diet during pregnancy increase DHA levels in breast milk. Donor breast milk DHA content varies, but is significantly lower than the fetal accretion rate of 42–67mg/day in the third trimester. As accretion of DHA occurs in third trimester, preterm infants become deficient in DHA over time, particularly if nourished by total parenteral nutrition and intralipids, as they are deficient in DHA.42 A randomized trial of different doses of DHA in preterms showed a DHA dose of 120mg/kg/day prevented the drop of DHA in preterm infants.43 Early studies have shown association of DHA levels and cognitive outcomes. A small study by Tam et al showed an association between higher DHA levels and a decrease incidence in intraventricular hemorrhage (IVH).44 A recent randomized controlled trial (DINO trial) evaluated infants of mothers on high DHA and standard DHA diet until 40 weeks corrected age.45 At 18 months there was no overall significant difference between group, but girls showed significant improvement in Mental development index (MDI) scores. Follow up of these infants at 7 years of age did not showed any significant difference between groups. In contrast, a randomized trial done in Norway, which supplemented DHA in milk for 9 weeks showed significantly higher performance on the problem solving section of the Ages and Stages Questionnaire in treated infants compared to control infants.46 In 2017 Cochrane review analyzed infants with LCPUFAs supplementation (median DHA concentration of 0.33%).47 The author concluded that premature infants did not obtain a benefit from receiving formula milk supplemented with LCPU- FAs. However, the doses used were different and the optimal dose for neuroprotection is still to be determined.
Choline
Choline is an essential component of the phospholipid, phosphatidylcholine, and sphingomyelin membranes. It is an important nutrient for methyl metabolism, acetylcholine synthesis and cell signaling. Choline plays an important role in neurogenesis and neural migration during fetal development, and deficiency is associated with spinal bifida.48 Animal studies have shown that choline supplements during pregnancy increases neuronal size and cell proliferation, reduces hippocampal apoptosis, and improves learning and memory in the offspring.49 Choline is actively transported to the fetus via placenta, and choline concentration in fetal plasma is 3 to 10 times higher than in plasma of pregnant women.50 Wu et al conducted a prospective cohort study of 154 mother-term infant pairs. Maternal plasma choline levels at 16 and 36 weeks were measured and neurodevelopment assessments were done at 18 months. Significant positive associations were found between infant cognitive test scores and maternal plasma free choline.51 A double blinded randomized controlled trial was done comparing nutritional supplement (DHA (1% of total daily estimated fatty-acid intake), eicosapentaenoic acid, arachidonic acid, choline, UMP, cytidine monophosphate, vitamin B12, zinc, and iodine) to placebo. The supplements were given for up to 2 years. No significant difference in cognitive composite score of the Bayley Scales of Infant Development, Third Edition was shown.52 Further studies are needed to evaluate the optimal dose and effect of choline on neurodevelopmental outcome of preterm infants.
Antenatal corticosteroid
The effectiveness of antenatal corticosteroids for neuroprotection was established early in 1972 by Liggins and colleagues. They demonstrated a reduction in respiratory distress syndrome and IVH among preterm infants given antenatal glucocorticoids.53 Over 20 years and 12 randomized controlled trials later, the National Institute of Health published a consensus statement recommending use of antenatal corticosteroids for mothers at risk for preterm birth between 24 and 34 weeks.54 A Cochrane review in 2006 evaluated 21 studies (3885 women and 4269 infants) and showed an overall reduction in neonatal death (relative risk (RR) 0.69, 95% confidence interval (CI) 0.58 to 0.81, 18 studies, 3956 infants), respiratory distress syndrome (RR 0.66, 95% CI 0.59 to 0.73, 21 studies, 4038 infants), cerebroventricular hemorrhage (RR 0.54, 95% CI 0.43 to 0.69, 13 studies, 2872 infants), necrotizing enterocolitis (RR 0.46, 95% CI 0.29 to 0.74, eight studies, 1675 infants), respiratory support, intensive care admissions (RR 0.80, 95% CI 0.65 to 0.99, two studies, 277 infants) and systemic infections in the first 48 hours of life (RR 0.56, 95% CI 0.38 to 0.85, five studies, 1319 infants).55 Despite mounting evidence, it has taken a long time to change clinical practice such that antenatal steroids are routinely given when preterm delivery is likely. The long-term outcomes for infants whose mothers received antenatal corticosteroids vary. In a 30-year follow-up of the Auckland steroid trial, there was no difference in cognitive outcomes between infants exposed to antenatal steroids and those exposed to placebo.56 A more recent meta-analysis, which included 14 studies on long term neurodevelopmental outcome showed reduction in CP in infants less than 34 weeks of gestation.57 In contrast, a recent Cochrane review looking at antenatal and intrapartum interventions showed a low level of evidence in reduction of CP in infants born to women received antenatal corticosteroid.58 The use of antenatal steroids globally is controversial as a large cluster randomized trial in six low- and middle-income countries showed increased perinatal mortality, possibly due to maternal and neonatal infection.59
Magnesium sulfate
Magnesium has been used in obstetrics as a tocolytic and for prophylaxis of eclampsia. An early case control study showed that very low birth weight (less than 1500 grams) infants whose mothers were treated with magnesium sulfate for maternal indications had a lower rate of CP compared to healthy matched controls.60 Several subsequent observational studies and randomized trials showed a similar trend in reduced incidence of CP. A Cochrane review published in 2009 evaluated 6145 children (5 prospective randomized trials). The effect of antenatal magnesium sulfate as a neuroprotective agent was studied in patients at risk of preterm birth. No difference in infant mortality was noted, but a 31% decrease in the overall incidence of CP among infants whose mothers were treated with magnesium sulfate (RR 0.68: 95% CL 0.54 to 0.87) was found. The risk of motor dysfunction was decreased by 39% in those infants whose mother received magnesium sulfate (RR 0.61, 95% Cl 0.44–0.85). There was no difference in IVH, WMI, deafness, intellectual impairment, bronchopulmonary dysplasia at 28 days or 36 weeks, or length of hospital stay. There was also no difference in maternal mortality or obstetric complications.61 The estimated number of pregnant women at risk of preterm birth who need to be treated with MgSO4 to prevent one case of CP (number needed to treat, NNT) is 63 women (95% Cl 43 – 155).62
Mechanisms of magnesium sulfate neuroprotection are still under investigation. One proposed mechanism includes modulation of N-methyl-D-aspartate(NMDA) glutamate channels thus reducing NMDA receptor mediated injury.63 There is also evidence of it stabilizing cell membranes, inhibiting oxidative stress and reducing secondary inflammation. A recent study of preterm infants using near infrared spectroscopy (NIRS) showed that antenatal magnesium sulfate treatment reduced cerebral fractional tissue oxygen extraction in the first 24 hours of life. The authors speculated that the neuroprotective effect was mediated by reduced cerebral metabolic demand.64
Doyle and colleagues followed infants from the ACTOMgSO4 trial at school age. Effects of magnesium on rate of abnormal motor function and CP were not significant at this age. The authors speculated that additional therapeutic interventions in the control group may have improved the outcomes in these patients65
Overall evidence supports magnesium sulfate as a neuroprotectant in preterm infant, thus, the American College of Obstetricians and Gynecologists (ACOG) with the Society for Maternal Fetal Medicine issued a statement in support of magnesium sulfate for neuroprotection in women at risk for delivery under 32 weeks’ gestation.66
Perinatal Therapies
Delayed cord clamping
The optimal timing for umbilical cord clamping has long been controversial, going back to the time of Hippocrates. It is estimated that one third of the fetal-placental blood volume resides in the placenta, and immediate cord clamping prevents the newborn from receiving this blood volume, resulting in increased early physiologic instability, and decreased iron stores later. Delayed cord clamping (DCC) has been of increasing interest in the past decades, as evidence of benefit to newborns accumulates. DCC has been variably defined as 30 seconds up to 1 or 5 minutes after delivery. ACOG and the American Academy of Pediatrics published a Committee opinion in 2012, revised in 2017, recommending DCC up to 60 seconds for vigorous term and preterm infants.67 It was further recommended that future research was needed to evaluate placental transfusion for preterm infants requiring resuscitation, continuous placental transfusion during active resuscitation and long-term effects of potential transfer of regenerative cells. A meta-analysis in 2012 reviewed 15 eligible studies, which included 738 infants born between 24 weeks and 36 weeks’ gestation. DCC was associated with lower incidence of IVH (RR, 0.59; 95% CI, 0.41–0.85), necrotizing enterocolitis (RR, 0.59; 95% CI, 0.41–0.85) and transfusion for anemia (RR, 0.61; 95% CI, 0.46–0.81). Small prospective follow up studies showed long term benefits in preterm infants with DCC compared to early cord clamping at 18 to 22 months.68 This suggests that placental transfusion from DCC has short term and long term benefits in preterm infant.
Umbilical cord milking (UCM) is an alternative to DCC. In UCM, the umbilical cord is stripped toward the infant several times and then clamped. This procedure can be performed within 20 seconds. A meta-analysis of 6 studies, which included 292 preterm infants with UCM and equal number of DCC concluded that similarly to DCC, umbilical cord milking decreases IVH, necrotizing enterocolitis, death and decreased the incidence of transfusion, with a pooled RR of 0.74 (95% Cl 0.61 – 0.90).69 A recent prospective study looking at neurodevelopmental outcome at 2 and 3.5 years of age showed no difference between infant who underwent DCC and infant who underwent UCM.70
Postnatal Therapies
Breast milk
Human breast milk (HM) has essential nutrients and other bioactive molecules including lactoferrin, lysozyme, and secretory IgA. Numerous studies have showed beneficial effects of HM on preterm outcomes. The mechanism by which HM provides neuroprotection is potentially via direct effects of high concentration of LCPUFAs and other micronutrients like choline. HM improves outcome indirectly by reducing preterm morbidities including sepsis and necrotizing enterocolitis. The National Institute of Child Health and Human Development (NICHD) Neonatal Research Network study of 1035 infants showed that children in the HM group were more likely to have a Bayley MDI greater than or equal to 85, higher mean Bayley PDI, and higher Bayley Behavior Rating Scale percentile scores for orientation/engagement, motor regulation, and total score. Multivariate analyses, adjusting for confounders, confirmed a significant independent association of HM on all four primary outcomes: (1) the mean Bayley MDI, (2) PDI, (3) Behavior Rating Scale, and (4) incidence of rehospitalization.71 A follow up study of this cohort at 30 months showed persistent significant improvement in HM group versus non-HM group.72 Analysis of the EPIPAGE cohort, demonstrated that the odds for mild and severe cognitive deficiency at 5 years of age are decreased in children who received HM at hospital discharge.73 A recent study by Belfort et al studied 180 infants born < 30 weeks of gestation and showed that HM feeding in the first 28 days of life was associated with a greater deep nuclear gray matter volume at term equivalent age and better IQ (0.5 points/d; 95% CI, 0.2–0.8), mathematics (0.5; 95% CI, 0.1–0.9) working memory (0.5; 95% CI, 0.1–0.9), and motor function (0.1; 95% CI, 0.0–0.2) at 7 years of age in very preterm infants.74
Caffeine
Caffeine, a selective adenosine A2a receptor antagonist and non-selective adenosine A1 receptor antagonist, is used to treat apnea of prematurity. Preclinical studies have shown that caffeine is neuroprotective by modulating adenosine A1 receptor, which leads to improvement in hypoxia-induced WMI and ventriculomegaly.75–77 Caffeine also potentiates synaptic plasticity by modifying neural synapses and increasing the size of dendritic spines. Several studies have shown short term neuroprotective effects of caffeine.78
Small studies have shown that caffeine-treated infants have improvement in auditory processing.79 Other studies reported that caffeine treated infants had enhanced cerebral cortical activity in brain.80 In a study of over 2000 infants with birth weights 500–1,250 g, the Caffeine for Apnea of Prematurity trial (CAP trial) showed that in addition to reducing apnea and the need for mechanical ventilation, caffeine treatment decreased the outcome of death or survival with one or more of the following impairments: cognitive deficit, deafness, CP and blindness. Caffeine use nearly halved the rate of CP.81 A follow-up study of 1640 infants enrolled in CAP trial, at the age of 5 years, showed no significant difference between the two groups. Further analysis showed a significant reduction of the incidence of developmental coordination disorder in the caffeine treated group (11.3% vs 15.2% adjusted OR: 0.70, 95%CI: 0.51–0.95).82 While caffeine has been shown to be beneficial at usual doses (10–20 mg/kg), a pilot study looking at the efficacy of high dose caffeine (80 mg/kg) vs standard dose showed a higher incidence of cerebellar injury with subsequent alterations in early motor performance.83
Family Centered Developmental Care
Fetal maturation is disrupted due to preterm birth. Preterm infants in the neonatal intensive care unit (NICU) are exposed to excessive sensory stimuli which are not present in utero. They are continuously exposed to fluctuations of sound, light, temperature, and oxygen. They experience a multitude of painful procedures, and their sleep is constantly interrupted. During this time, the developing brain continues to modulate synaptic neuronal connections, both predetermined, and affected by environmental stimuli. Epigenetic changes (DNA methylation, acetylation and microRNA production) occur as a result of both environmental stimuli, leading to specific patterns of RNA and protein production, and ultimately to the end product of brain development. Thus, encouraging normal patterns of development, minimizing the abnormal sensory stimuli in this critical period of brain growth can lead to improvement in long-term neurodevelopmental outcomes. Consistent and effective neurodevelopmental care given to preterm infants from moment of birth is important in order to support the optimal brain development and protect it from abnormal external stimuli.
One approach to neurodevelopmental care is based upon the Synaptic Theory of Development.84 The core of this approach is individualized, cue-based caregiving, plus interventions designed to reduce stress and promote behavioral organization and physiological stability. Many developmental care strategies have been implemented in NICU to improve neurodevelopmental outcomes. The most studied and implemented model is “Seven Core Measures of Neuroprotective Family-Centered Developmental Care” developed by Altimier and Phillips.85 Core measures include: Safeguarding Sleep, Positioning and Handling, Minimizing Pain and Stress, Protecting Skin, Nutrition, Partnering with Families, and Healing Environment. Different family centered neuroprotective strategies have been shown to improve health outcomes, improve family satisfaction, decrease length of stay and hospital costs.86–88 Kangaroo Mother Care (skin to skin contact between parent and infant) is an important part of family centered care, relevant in both resource sufficient and low resource settings. Short term benefits include decreased maternal anxiety and depression, improved breastfeeding, increased physiologic stability, increase pain tolerance, improved growth and decreased mortality.89–94 Long term benefits of kangaroo mother care are being recognized, with studies showing improved attention and quality of movements95 and enhanced child cognitive developmental and executive functions for up to 10 years.96
Erythropoietin
Erythropoietin (Epo) is a 30.4 kDa cytokine originally recognized for its role in erythropoiesis. Epo and its receptor (EpoR) are required for normal brain development. Astrocytes are the main source of brain Epo, and many cell types express the receptor, including neurons, OL and epithelial cells. The EpoR is expressed in hippocampal and cerebral cortical neurons, and expression of both Epo and EpoR decline postnatally in brain but remain present at lower levels throughout adulthood.97, 98 Prolonged exposure to hypoxia leads to upregulation of EpoR gene expression. In the absence of Epo to bind these upregulated EpoRs, neurons and OL have a higher risk of apoptotic cell death.99, 100
Mechanisms of Epo neuroprotection include phosphorylation of JAK2, activation of mitogen-activated protein kinase (MAPK), extracellular related kinase (ERK1/2), phosphatidylinositol 3-kinase (PI3K/Akt) and signal transducer and transcriptional activator 5 (STAT5) pathways, which are critical for cell survival.101 Acutely, Epo’s effects are anti-apoptotic, anti-inflammatory, neurotrophic, and anti-oxidant. Long term effects that may promote brain development and healing include angiogenesis, neurogenesis, and oligodendrogenesis. Recent preclinical studies have shown that Epo administration as late as 72 hours or one week after injury leads to improved behavioral outcomes, reduced WMI, enhanced neurogenesis and axonal sprouting, emphasizing the importance of the regenerative and reparative effects of Epo.102 Ferroptosis is a mechanism of regulated cell death triggered by free iron in cells. As an important effect of Epo is to increase erythropoiesis, which in turn increases iron utilization and decreases unbound free iron, Epo may also provide neuroprotection by modulating ferroptosis in neuronal cells.
Phase I and II studies using doses that vary from 400 U/kg three times a week to 3000 U/kg for 3 doses have demonstrated safety and the potential for neuroprotection.103–106 A recent meta-analysis of neuroprotective effects of prophylactic Epo in preterm infant included 1133 patients from 4 randomized controlled trials. Despite differences in the dosage and timing of Epo treatment, they showed that prophylactic Epo administration decreased the incidence of infants with an MDI < 70 at 18–24 months, with an odds ratio of 0.51 (0.31 – 0.81), P <0.005. The number needed to treat was 14. Significance was not present in the subgroup analysis of infants < 28 weeks of gestation, however, there were significant size limitations, as the subgroup analysis included only 60 Epo treated and 57 placebo treated subjects.107 To evaluate the effectiveness of Epo in the most vulnerable preterm infants (< 28 weeks of gestation) a large prospective, randomized, placebo controlled, double blind study of Preterm Erythropoietin neuroprotection (PENUT trial, ) is being conducted. 941 subjects of gestational age of 24–0/7 weeks to 27–6/7 weeks have been enrolled at 19 sites across the United States. All subjects will be evaluated at 22–26 months’ corrected age. Subjects were randomized to either Epo treatment (1000 U/kg/dose x 6 doses every other day), or placebo, and maintenance treatment (400 U/kg/dose three times a week) was continued until 32–6/7 weeks postmenstrual age. The primary outcome is neurodevelopment at two years of age, which consists of a standardized neuro exam, Gross Motor Function Classification Scale and standardized Bayley III. This study is adequately powered to provide definitive results regarding the neuroprotective potential of Epo in this population. Results should be available in early 2019.108
Future Neuroprotective Strategies
Stem Cells
Stem cells have the ability to differentiate into multiple cell types and have self-regenerative properties. These properties allow them to repair, regenerate and modulate cell death and tissue damage. Stem cells can be of embryonic, fetal or adult in origin. Embryonic stem cells (ESCs) are pluripotent and regenerate but have tumorigenic potential. There are ethical issues in procuring embryonic stem cells from tissues. Adult stem cells include mesenchymal stem cells that can be obtained from a variety of sources (bone marrow, adipose tissue and dental pulp) and neural progenitor cells (NPCs) found in the subventricular zone of the brain. Lastly, fetal derived stem like cells can be obtained from placental tissue and umbilical cord blood (UCB). They include umbilical cord MSCs, endothelial progenitor cells (EPCs), hematopoietic stem cells (HSCs), and amnion epithelia stem cells. Stem cell therapy is an established first line or adjunctive therapy for a variety of neonatal diseases including inborn errors of metabolism. Stem cell treatments have been effective in providing significant neuroprotection and improvement in functional outcomes in animal models of neonatal hypoxia-ischemia, CP, and stroke.109–111
In recent years, focus has shifted to fetal derived MSCs and umbilical cord blood. Preclinical studies have shown the effectiveness of stem cells in improving outcomes in models of preterm encephalopathy. These studies have elucidated potential mechanism by which MSCs exert neuroprotective effects. MSCs down regulated pro-inflammatory cytokines, reducing inflammation and apoptosis.112, 113 In a preterm brain injury model of IVH, UCB-derived MSCs suppressed the upregulation of cytokines IL-1α, IL-1β, IL-6, and TNF-α within the CSF and periventricular brain region. This resulted in reduction in edema and improvement in behavioral outcomes.114 MSCs also alter immune cell programming and proliferation. Preclinical studies have shown that MSCs do not engraft, but rather respond to signals of local injury by secreting trophic factors including fibroblast growth factor-2, Brain-derived neurotrophic factor (BDNF), epidermal growth factor, glial cell line-derived neurotrophic factor, and sonic hedgehog, thereby increasing progenitor cell proliferation and survival of neurons and neuronal stem cells.112, 113, 115 MSCs also stimulate axonal sprouting and proliferation of oligodendrocyte precursors, and promote mature OL fate.112, 116, 117
Numerous open-label clinical trials are ongoing or have been completed for treatment of children with established CP. A recent randomized double blinded placebo-controlled study had subjects with CP aged 10 months to 10 years divided in three groups: intravenously UCB + Epo, Epo only, and controls. Compared with the Epo and control groups, the UCB group + Epo had significantly higher developmental scores at 6 months. Diffusor Tensor Imaging (DTI) revealed significant correlations between the gross motor performance measure increment and changes in FA in the UCB + Epo group.118 A safety trial conducted at Duke University assessed the safety and feasibility of therapeutic hypothermia with concomitant intravenous administration of non-cryopreserved, autologous UCB cells for term infants with hypoxic ischemic encephalopathy (HIE). They concluded that transfusion of up to four doses of 1–5×107 cells per dose did not result in significant adverse reactions, cardiopulmonary compromise, or infections. Seventy four percent of UCB recipients were alive at 1 year with total Bayley scores >85, compared to only 41% in the concurrent cooled infants who received only therapeutic hypothermia.119 Ongoing phase I and II trials include; autologous cord blood and human placental derived stem cells in neonates with severe HIE (HPDSC+HIE) (), umbilical cord milking for neonates with HIE (), and neural progenitor cell and paracrine factors to treat HIE (). There are no clinical trials testing the safety and efficacy of stem cells for treatment of preterm encephalopathy. However, a recent phase 1 trial of intratracheal MSCs for treatment of broncopulmonary dysplasia appeared to be safe and with no adverse respiratory, growth, and neurodevelopmental effects at 2 years’ corrected age.120
There remain many questions regarding the use of stem cells for preterm encephalopathy including the following: (1) The type of most effective stem cell type, (2) The optimal number, timing (early or late), duration and route of administration, (3) Selection of infants who would benefit from therapy.
Melatonin
Melatonin (N-acetyl-5-methoxytryptamine), a neurohormone derived from the amino acid tryptophan. It is a strong antioxidant capable of scavenging free radicals and stimulating several antioxidative enzymes including glutathione, glutathione reductase, peroxidase, and superoxide dismutase.121 Melatonin exerts its effect through both receptor-mediated and receptor-independent mechanisms. Melatonin can directly stimulate cell membrane G protein-coupled high-affinity melatonin receptors that activate numerous second messenger cascades, which vary in cell, tissue, and species-specific ways. Melatonin can also induce receptor-independent intracellular activities by targeting calcium-binding proteins, cytoskeletal and scaffold proteins, and components of mitochondrial signaling.122 Melatonin’s safety profile, antioxidant, anti-inflammatory and anti-apoptotic properties have made it an attractive neuroprotective candidate for treating neonates with preterm encephalopathy.123, 124
Melatonin has been shown to be neuroprotective in preterm and near-term fetal sheep by reducing oxidative stress, apoptosis and decreasing inflammation.125, 126 Similar findings were seen in fetal brain with prophylactic systemic administration of melatonin in pregnant rats.127, 128 Maternal administration one hour before hypoxic-ischemic insult results in improved outcome, but melatonin administration > 3hours did not result in improvement. This suggests there is a critical window of time to administer melatonin in mothers for acute neuroprotection of the fetus. Similar to antenatal treatment, preclinical postnatal studies have shown improvement in short term and long-term outcomes of preterm encephalopathy. Melatonin had a dose-dependent protective effect on the developing white matter in a mouse model of excitotoxic WMI. Systemic administration of melatonin following germinal matrix hemorrhage in rats led to improved cognitive and sensorimotor dysfunction at juvenile age.
The pharmacokinetic profile of melatonin has been studied by Merchant et al.129 The study showed that neonatal dosing of melatonin cannot be extrapolated from adults because the half-life and clearance rate of melatonin are prolonged in preterm infants, and its volume of distribution is decreased.
Melatonin also holds potential as an antenatal therapy that could be administered to pregnant mothers with at-risk fetuses since it appears safe, crosses the placenta,130 and crosses the blood brain barrier.131 A multicenter trial ‘PREMELIP’ is ongoing to test the neuroprotective effects of melatonin administration in immediate peripartum period on preterm infants. The primary outcome will evaluate white matter damage detected by MRI at term equivalent age. A phase 2 exploratory; multi-center double-blinded randomized placebo-controlled 2-arm trial ‘MINT study’, evaluating the neuroprotective effect of melatonin in preterm infants less than 31 weeks’ gestation. In this trial melatonin (0.1 mcg/kg/hr for 2 hrs) was given for 7 days starting by 48 hours after birth, targeting adult melatonin levels. This trial did not show any difference in FA on term corrected MRI between two groups. Results from the above trial indicate that the optimal dose of melatonin for preterm infants still needs further investigation.
Melatonin and Epo may provide synergistic benefit when given in tandem, as melatonin may provide acute downregulation of inflammation and oxidative stress while Epo may provide long term brain repair, enhancing neuronal and oligodendroglia survival and differentiation after CNS injury. This combined therapy is now being studied in preclinical models with the potential for clinical studies for preterm encephalopathy.
Therapies in preclinical stages
Exosomes
Exosomes are small extracellular nano-size vesicles (50–100 nm in diameter) from cells that contain cytoplasmic proteins, micro ribonucleic acid (miRNA), messenger RNA (mRNA) and cytokines. Exosomes are presumed to originate from multivesicular bodies of all cells. The transfer of exosomes between cells is recognized as an important cell-to-cell communication mechanism. Current evidence suggests that the therapeutic potential of stem cells is due to secretion of paracrine factors, mainly being mediated by these extracellular vesicles (EVs).132 Exosomes derived from various MSc have shown to have reparative and regenerative properties in different organs systems, including the brain.133–135 A recent preclinical study showed exosomes derived from bone marrow MSCs improved neurodevelopment in preterm injured animals.132 The study showed that exosomes treatment significantly reduced inflammation induced neuronal cellular degeneration, reduced microgliosis and prevented reactive astrogliosis. MSCs exosomes also improved myelination and provided long lasting improvement in cognitive function. Exosome therapy has potential advantages over stem cells as the risk of grafting and potential malignant transformation is reduce because exosomes are non self-replicating, also exosomes are small in size thus has better potential to reach injured areas and it can be sterilized by filtration. Stem cells modify themselves according to the surrounding environment and their secretome is affected by the environment. In contrast, exosomes are static and have fixed cargo, but there is potential for in-vitro alteration of exosomal cargo by subjecting MSCs to the disease specific microenvironment. EVs like exosomes hold promise as a potential alternative to stem cell therapy.
Polyphenols
Polyphenols are natural molecules with variable phenolic structures that are enriched in foods like fruits, tea, vegetables, wine and other foods. Many known polyphenols have antioxidant, anti-inflammatory, and anti-apoptotic properties. Hence, they are ideal candidates to be evaluated as neuroprotective agents. They are classified into groups depending on the number of phenol rings, and chemical groups bound to the rings.
Resveratrol.
Resveratrol (3,5,4′-trihydroxystilbene) is a non-flavonoid polyphenolic compound consisting of two aromatic rings attached by a methylene bridge. Resveratrol is present commonly in grapes, soybeans and pomegranates.136 Preclinical studies have shown resveratrol as a neuroprotective agent in models of ischemia, brain and spinal cord injury. Resveratrol provides neuroprotection through multiple pathways.137–139 Several preclinical studies have demonstrated the efficacy of resveratrol in hypoxic-ischemic brain injury in neonatal rats, preserving neocortical and subcortical brain areas in the short term,140, 141 with long term improvement in motor and behavioral functions.142 Resveratrol has also been investigated as prenatal preventive treatment for neonatal brain injury, as it crosses the placenta and maternal supplementation can lead to reduction in tissue loss and decreased apoptosis in neonatal brains.143, 144
Curcumin.
Curcumin (diferuloyl methane) is a polyphenol present in the rhizomes of Curcuma longa (zingiberaceae). It possesses many therapeutic properties including anti-inflammatory,145, 146 anti-oxidant,147, 148 and anti-cancer effects.149 The neuroprotective effect are due to its modulation of neuronal apoptosis, oxidative stress and pro-inflammatory cytokines.150–152 Curcumin is a potent inhibitor of NFkB signaling which leads a reduction in pro-inflammatory cytokines and enzymes.146, 152 In-vitro and in-vivo studies have shown that curcumin imparts anti-oxidative effects by direct scavenging of free radical with its phenolic structure, and modulation of Nrf2 pathway.148 Curcumin also inhibits activation of NMDA, which is an important mechanism for excitotoxic brain injury.153 Curcumin induces neurogenesis by modulating Canonical Wnt/β-Catenin Pathway, with reversal of cognitive deficits in Alzheimer’s disease.154 Recently we showed curcumin-loaded nanoparticles were neuroprotective in a model of neonatal hypoxic ischemic encephalopathy. Curcumin loaded nanoparticles reduced microglial activation, infarct size, and improved gross injury scores.155 Thus curcumin, like Epo and melatonin works on multiple pathways affecting brain injury and has a potential to be an effective treatment for neonatal brain injury.129
Drug Delivery and Cell-specific Targeting.
A major limitation of polyphenols and other potentially protective compounds is poor bioavailability and cell specificity. Alternative delivery methods are being studied to circumvent this issue. Nanomedicine has emerged as an important field for delivering drugs to specific targets. Nanoparticles, such as polymeric dendrimers, can bind drugs, target their uptake by specific cell types, thereby increasing the drug concentration where it is needed, and they can also modulate drug delivery by modifying speed of release.155 This can increase the bioavailability of drugs and decrease the dose needed for effect, thereby decreasing dose-dependent side effects. While this has been intensively studied for delivery of cancer therapeutic agents, attention is now turning to using these nanoparticles for other purposes such as neuroprotection. Curcumin-loaded nanoparticles have been shown to be more effective than free curcumin in ameliorating cerebral ischemic reperfusion injury in rats.156 Polyamidoamine dendrimers-NAC, which localize in activated microglia and astrocytes, have been used to treat newborn rabbits with CP.157 Nanomedicine has great potential to deliver drugs across the blood brain barrier targeting the site of injury and repair to improve neurologic outcomes.
Conclusions
The success of neonatology has led to an increased population of NICU survivors. The challenge for the next generation of neonatal researchers will be to improve the developmental outcomes of these survivors. Currently, 50% of children born < 1500 gm (~ 31 weeks of gestation) require special educational services, and 15% have repeated at least one grade in school.158 For those born before 28 weeks of gestation, on average, 9% develop CP, 25% intellectual disability, 3% hearing loss, 2% blindness, and 10% autism. We can and must do better.
Acknowledgments
Supported by the National Institutes of Health 1U01NS092764 to SJ and 1U01NS077953 to SJ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no competing interests
References
- 1.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–40. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–61. 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017(287):1–8. [PubMed] [Google Scholar]
- 4.In: Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC)2007. [PubMed] [Google Scholar]
- 5.Hintz SR, Kendrick DE, Wilson-Costello DE, Das A, Bell EF, Vohr BR, et al. Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks’ gestational age. Pediatrics. 2011;127(1):62–70. 10.1542/peds.2010-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younge N, Goldstein RF, Bann CM, Hintz SR, Patel RM, Smith PB, et al. Survival and Neurodevelopmental Outcomes among Periviable Infants. N Engl J Med. 2017;376(7):617–28. doi: 10.1056/NEJMoa1605566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Back SA. Brain Injury in the Preterm Infant: New Horizons for Pathogenesis and Prevention. Pediatr Neurol. 2015;53(3):185–92. 10.1016/j.pediatrneurol.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapellou O, Counsell SJ, Kennea N, Dyet L, Saeed N, Stark J, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3(8):e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71(1):93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Back SA. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 2017;134(3):331–49. 10.1007/s00401-017-1718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, et al. Systemic inflammation disrupts the developmental program of white matter. Ann Neurol. 2011;70(4):550–65. 10.1002/ana.22489. [DOI] [PubMed] [Google Scholar]
- 12.Feigenson K, Reid M, See J, Crenshaw EB 3rd, Grinspan JB Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci. 2009;42(3):255–65. 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12(7):829–38. 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClain CR, Sim FJ, Goldman SA. Pleiotrophin suppression of receptor protein tyrosine phosphatase-beta/zeta maintains the self-renewal competence of fetal human oligodendrocyte progenitor cells. J Neurosci. 2012;32(43):15066–75. 10.1523/JNEUROSCI.1320-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kostovic I, Judas M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatrica. 2010;99(8):1119–27. 10.1111/j.1651-2227.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- 16.Kanold PO, Shatz CJ. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron. 2006;51(5):627–38. 10.1016/j.neuron.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Volpe JJ. Subplate neurons--missing link in brain injury of the premature infant? Pediatrics. 1996;97(1):112–3. [PubMed] [Google Scholar]
- 18.Gano D, Andersen SK, Glass HC, Rogers EE, Glidden DV, Barkovich AJ, et al. Impaired cognitive performance in premature newborns with two or more surgeries prior to term-equivalent age. Pediatr Res. 2015;78(3):323–9. doi: 10.1038/pr.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddle A, Maire J, Gong X, Chen KX, Kroenke CD, Hohimer AR, et al. Differential susceptibility to axonopathy in necrotic and non-necrotic perinatal white matter injury. Stroke. 2012;43(1):178–84. 10.1161/STROKEAHA.111.632265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirayama A, Okoshi Y, Hachiya Y, Ozawa Y, Ito M, Kida Y, et al. Early immunohistochemical detection of axonal damage and glial activation in extremely immature brains with periventricular leukomalacia. Clinical neuropathology. 2001;20(2):87–91. [PubMed] [Google Scholar]
- 21.Riddle A, Dean J, Buser JR, Gong X, Maire J, Chen K, et al. Histopathological correlates of magnetic resonance imaging-defined chronic perinatal white matter injury. Ann Neurol. 2011;70(3):493–507. 10.1002/ana.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sauer CW, Kong JY, Vaucher YE, Finer N, Proudfoot JA, Boutin MA, et al. Intubation Attempts Increase the Risk for Severe Intraventricular Hemorrhage in Preterm Infants-A Retrospective Cohort Study. J Pediatr. 2016;177:108–13. 10.1016/j.jpeds.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 24.Engelhardt E, Inder TE, Alexopoulos D, Dierker DL, Hill J, Van Essen D, et al. Regional impairments of cortical folding in premature infants. Ann Neurol. 2015;77(1):154–62. 10.1002/ana.24313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tam EW, Ferriero DM, Xu D, Berman JI, Vigneron DB, Barkovich AJ, et al. Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr Res. 2009;66(1):102–6. 10.1203/PDR.0b013e3181a1fb3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keunen K, Kersbergen KJ, Groenendaal F, Isgum I, de Vries LS, Benders MJNL. Brain tissue volumes in preterm infants: prematurity, perinatal risk factors and neurodevelopmental outcome: A systematic review. The Journal of Maternal-Fetal & Neonatal Medicine. 2012;25(sup1):89–100. doi: 10.3109/14767058.2012.664343. [DOI] [PubMed] [Google Scholar]
- 27.Nossin-Manor R, Chung AD, Whyte HEA, Shroff MM, Taylor MJ, Sled JG. Deep Gray Matter Maturation in Very Preterm Neonates: Regional Variations and Pathology-related Age-dependent Changes in Magnetization Transfer Ratio. Radiology. 2012;263(2):510–7. doi: 10.1148/radiol.12110367. [DOI] [PubMed] [Google Scholar]
- 28.Vinall J, Grunau RE, Brant R, Chau V, Poskitt KJ, Synnes AR, et al. Slower Postnatal Growth Is Associated with Delayed Cerebral Cortical Maturation in Preterm Newborns. Sci Transl Med. 2013;5(168):168ra8–ra8. doi: 10.1126/scitranslmed.3004666. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasan L, Dutta R, Counsell SJ, Allsop JM, Boardman JP, Rutherford MA, et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics. 2007;119(4):759–65. [DOI] [PubMed] [Google Scholar]
- 30.Dean JM, McClendon E, Hansen K, Azimi-Zonooz A, Chen K, Riddle A, et al. Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci Transl Med. 2013;5(168):168ra7. 10.1126/scitranslmed.3004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malik S, Vinukonda G, Vose LR, Diamond D, Bhimavarapu BB, Hu F, et al. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci. 2013;33(2):411–23. doi: 10.1523/JNEUROSCI.4445-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpe JJ. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. 2009;24(9):1085–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limperopoulos C, Soul JS, Haidar H, Huppi PS, Bassan H, Warfield SK, et al. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics. 2005;116(4):844–50. 10.1542/peds.2004-2282. [DOI] [PubMed] [Google Scholar]
- 34.Steggerda SJ, Leijser LM, Wiggers-de Bruine FT, van der Grond J, Walther FJ, van Wezel-Meijler G. Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology. 2009;252(1):190–9. [DOI] [PubMed] [Google Scholar]
- 35.Steggerda SJ, De Bruine FT, van den Berg-Huysmans AA, Rijken M, Leijser LM, Walther FJ, et al. Small cerebellar hemorrhage in preterm infants: perinatal and postnatal factors and outcome. Cerebellum. 2013;12(6):794–801. 10.1007/s12311-013-0487-6. [DOI] [PubMed] [Google Scholar]
- 36.Limperopoulos C, Chilingaryan G, Sullivan N, Guizard N, Robertson RL, du Plessis AJ. Injury to the premature cerebellum: outcome is related to remote cortical development. Cereb Cortex. 2014;24(3):728–36. 10.1093/cercor/bhs354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Limperopoulos C, Chilingaryan G, Guizard N, Robertson RL, du Plessis AJ. Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions Pediatr Res. 2010. 10.1203/PDR.0b013e3181e1d032. [DOI] [PubMed] [Google Scholar]
- 38.Shah DK, Anderson PJ, Carlin JB, Pavlovic M, Howard K, Thompson DK, et al. Reduction in cerebellar volumes in preterm infants: relationship to white matter injury and neurodevelopment at two years of age. Pediatr Res. 2006;60(1):97–102. [DOI] [PubMed] [Google Scholar]
- 39.Limperopoulos C, Bassan H, Gauvreau K, Robertson RL Jr., Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120(3):584–93. [DOI] [PubMed] [Google Scholar]
- 40.Allin M, Matsumoto H, Santhouse AM, Nosarti C, AlAsady MH, Stewart AL, et al. Cognitive and motor function and the size of the cerebellum in adolescents born very pre-term. Brain. 2001;124(Pt 1):60–6. [DOI] [PubMed] [Google Scholar]
- 41.Nordgren TM, Lyden E, Anderson-Berry A, Hanson C. Omega-3 Fatty Acid Intake of Pregnant Women and Women of Childbearing Age in the United States: Potential for Deficiency? Nutrients. 2017;9(3). 10.3390/nu9030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baack ML, Puumala SE, Messier SE, Pritchett DK, Harris WS. What is the relationship between gestational age and docosahexaenoic acid (DHA) and arachidonic acid (ARA) levels? Prostaglandins Leukot Essent Fatty Acids. 2015;100:5–11. 10.1016/j.plefa.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins CT, Sullivan TR, McPhee AJ, Stark MJ, Makrides M, Gibson RA. A dose response randomised controlled trial of docosahexaenoic acid (DHA) in preterm infants. Prostaglandins Leukot Essent Fatty Acids. 2015;99:1–6. 10.1016/j.plefa.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Tam EW, Chau V, Barkovich AJ, Ferriero DM, Miller SP, Rogers EE, et al. Early postnatal docosahexaenoic acid levels and improved preterm brain development. Pediatr Res. 2016;79(5):723–30. 10.1038/pr.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. Jama. 2009;301(2):175–82. [DOI] [PubMed] [Google Scholar]
- 46.Henriksen C, Haugholt K, Lindgren M, Aurvag AK, Ronnestad A, Gronn M, et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics. 2008;121(6):1137–45. 10.1542/peds.2007-1511. [DOI] [PubMed] [Google Scholar]
- 47.Jasani B, Simmer K, Patole SK, Rao SC. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev. 2017;3:CD000376 10.1002/14651858.CD000376.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeisel SH. Nutrition in pregnancy: the argument for including a source of choline. Int J Womens Health. 2013;5:193–9. 10.2147/IJWH.S36610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeisel SH. The fetal origins of memory: the role of dietary choline in optimal brain development. J Pediatr. 2006;149(5 Suppl):S131–6. 10.1016/j.jpeds.2006.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozarda Ilcol Y, Uncu G, Ulus IH. Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery and in newborns. Arch Physiol Biochem. 2002;110(5):393–9. 10.1076/apab.110.5.393.11832. [DOI] [PubMed] [Google Scholar]
- 51.Wu BT, Dyer RA, King DJ, Richardson KJ, Innis SM. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS One. 2012;7(8):e43448 10.1371/journal.pone.0043448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrew MJ, Parr JR, Montague-Johnson C, Laler K, Holmes J, Baker B, et al. Nutritional intervention and neurodevelopmental outcome in newborn infants at risk of neurodevelopmental impairment: the Dolphin neonatal double-blind randomized controlled trial Dev Med Child Neurol. 2018. 10.1111/dmcn.13914. [DOI] [PubMed] [Google Scholar]
- 53.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–25. [PubMed] [Google Scholar]
- 54.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273(5):413–8. [DOI] [PubMed] [Google Scholar]
- 55.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006(3):CD004454 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 56.Dalziel SR, Lim VK, Lambert A, McCarthy D, Parag V, Rodgers A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665 10.1136/bmj.38576.494363.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sotiriadis A, Tsiami A, Papatheodorou S, Baschat AA, Sarafidis K, Makrydimas G. Neurodevelopmental Outcome After a Single Course of Antenatal Steroids in Children Born Preterm: A Systematic Review and Meta-analysis. Obstet Gynecol. 2015;125(6):1385–96. 10.1097/AOG.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 58.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454 10.1002/14651858.CD004454.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Althabe F, Belizan JM, McClure EM, Hemingway-Foday J, Berrueta M, Mazzoni A, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. 2015;385(9968):629–39. doi: 10.1016/S0140-6736(14)61651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson KB, Grether JK. Can magnesium sulfate reduce the risk of cerebral palsy in very low birthweight infants? Pediatrics. 1995;95(2):263–9. [PubMed] [Google Scholar]
- 61.Doyle LW, Crowther CA, Middleton P, Marret S. Antenatal magnesium sulfate and neurologic outcome in preterm infants: a systematic review. Obstet Gynecol. 2009;113(6):1327–33. 10.1097/AOG.0b013e3181a60495. [DOI] [PubMed] [Google Scholar]
- 62.Doyle LW. Antenatal magnesium sulfate and neuroprotection. Curr Opin Pediatr. 2012;24(2):154–9. 10.1097/MOP.0b013e3283504da1. [DOI] [PubMed] [Google Scholar]
- 63.Hallak M, Hotra JW, Custodio D, Kruger ML. Magnesium prevents seizure-induced reduction in excitatory amino acid receptor (kainate and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid) binding in pregnant rat brain. Am J Obstet Gynecol. 2000;183(4):793–8. 10.1067/mob.2000.109491. [DOI] [PubMed] [Google Scholar]
- 64.Stark MJ, Hodyl NA, Andersen CC. Effects of antenatal magnesium sulfate treatment for neonatal neuro-protection on cerebral oxygen kinetics. Pediatr Res. 2015;78(3):310–4. 10.1038/pr.2015.96. [DOI] [PubMed] [Google Scholar]
- 65.Doyle LW, Anderson PJ, Haslam R, Lee KJ, Crowther C, Australasian Collaborative Trial of Magnesium Sulphate Study G. School-age outcomes of very preterm infants after antenatal treatment with magnesium sulfate vs placebo. JAMA. 2014;312(11):1105–13. 10.1001/jama.2014.11189. [DOI] [PubMed] [Google Scholar]
- 66.American College of O, Gynecologists Committee on Obstetric P, Society for Maternal-Fetal M. Committee Opinion No. 455: Magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet Gynecol. 2010;115(3):669–71. 10.1097/AOG.0b013e3181d4ffa5. [DOI] [PubMed] [Google Scholar]
- 67.Committee on Obstetric P Committee Opinion No. 684: Delayed Umbilical Cord Clamping After Birth. Obstet Gynecol. 2017;129(1):e5–e10. [DOI] [PubMed] [Google Scholar]
- 68.Garofalo M, Abenhaim HA. Early versus delayed cord clamping in term and preterm births: a review. J Obstet Gynaecol Can. 2012;34(6):525–31. 10.1016/S1701-2163(16)35268-9. [DOI] [PubMed] [Google Scholar]
- 69.Dang D, Zhang C, Shi S, Mu X, Lv X, Wu H. Umbilical cord milking reduces need for red cell transfusions and improves neonatal adaptation in preterm infants: Meta-analysis J Obstet Gynaecol Res. 2015. doi: 10.1111/jog.12657. [DOI] [PubMed] [Google Scholar]
- 70.Rabe H, Sawyer A, Amess P, Ayers S, Brighton Perinatal Study G. Neurodevelopmental Outcomes at 2 and 3.5 Years for Very Preterm Babies Enrolled in a Randomized Trial of Milking the Umbilical Cord versus Delayed Cord Clamping. Neonatology. 2016;109(2):113–9. 10.1159/000441891. [DOI] [PubMed] [Google Scholar]
- 71.Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Wright LL, Langer JC, et al. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics. 2006;118(1):e115–23. [DOI] [PubMed] [Google Scholar]
- 72.Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Higgins RD, Langer JC, et al. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics. 2007;120(4):e953–9. 10.1542/peds.2006-3227. [DOI] [PubMed] [Google Scholar]
- 73.Beaino G, Khoshnood B, Kaminski M, Marret S, Pierrat V, Vieux R, et al. Predictors of the risk of cognitive deficiency in very preterm infants: the EPIPAGE prospective cohort. Acta Paediatrica. 2011;100(3):370–8. 10.1111/j.1651-2227.2010.02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belfort MB, Anderson PJ, Nowak VA, Lee KJ, Molesworth C, Thompson DK, et al. Breast Milk Feeding, Brain Development, and Neurocognitive Outcomes: A 7-Year Longitudinal Study in Infants Born at Less Than 30 Weeks’ Gestation. J Pediatr. 2016;177:133–9 e1. 10.1016/j.jpeds.2016.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Back SA, Craig A, Luo NL, Ren J, Akundi RS, Ribeiro I, et al. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol. 2006;60(6):696–705. 10.1002/ana.21008. [DOI] [PubMed] [Google Scholar]
- 76.Rivkees SA, Wendler CC. Adverse and protective influences of adenosine on the newborn and embryo: implications for preterm white matter injury and embryo protection. Pediatr Res. 2011;69(4):271–8. doi: 10.1203/PDR.0b013e31820efbcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner CP, Yan H, Schwartz M, Othman T, Rivkees SA. A1 adenosine receptor activation induces ventriculomegaly and white matter loss. Neuroreport. 2002;13(9):1199–204. [DOI] [PubMed] [Google Scholar]
- 78.Yoshimura H The potential of caffeine for functional modification from cortical neuron networks in the brain. Curr Neuropharmacol. 2005;3(4):309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maitre NL, Chan J, Stark AR, Lambert WE, Aschner JL, Key AP. Effects of caffeine treatment for apnea of prematurity on cortical speech-sound differentiation in preterm infants. J Child Neurol. 2015;30(3):307–13. 10.1177/0883073814538500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Supcun S, Kutz P, Pielemeier W, Roll C. Caffeine increases cerebral cortical activity in preterm infants. J Pediatr. 2010;156(3):490–1. 10.1016/j.jpeds.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357(19):1893–902. 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV, et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307(3):275–82. 10.1001/jama.2011.2024. [DOI] [PubMed] [Google Scholar]
- 83.McPherson C, Neil JJ, Tjoeng TH, Pineda R, Inder TE. A pilot randomized trial of high-dose caffeine therapy in preterm infants. Pediatr Res. 2015;78(2):198–204. 10.1038/pr.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Als H Individualized Developmental Care for the Very Low-Birth-Weight Preterm Infant. JAMA. 1994;272(11):853. doi: 10.1001/jama.1994.03520110033025. [DOI] [PubMed] [Google Scholar]
- 85.Altimier L, Phillips RM. The Neonatal Integrative Developmental Care Model: Seven Neuroprotective Core Measures for Family-Centered Developmental Care. Newborn and Infant Nursing Reviews. 2013;13(1):9–22. doi: 10.1053/j.nainr.2012.12.002. [DOI] [Google Scholar]
- 86.Als H, Duffy FH, McAnulty GB, Rivkin MJ, Vajapeyam S, Mulkern RV, et al. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–57. [DOI] [PubMed] [Google Scholar]
- 87.Melnyk BM, Feinstein NF, Alpert-Gillis L, Fairbanks E, Crean HF, Sinkin RA, et al. Reducing Premature Infants’ Length of Stay and Improving Parents’ Mental Health Outcomes With the Creating Opportunities for Parent Empowerment (COPE) Neonatal Intensive Care Unit Program: A Randomized, Controlled Trial. PEDIATRICS. 2006;118(5):e1414–e27. doi: 10.1542/peds.2005-2580. [DOI] [PubMed] [Google Scholar]
- 88.Cooper LG, Gooding JS, Gallagher J, Sternesky L, Ledsky R, Berns SD. Impact of a family-centered care initiative on NICU care, staff and families. Journal of Perinatology. 2007;27(S2):S32–S7. doi: 10.1038/sj.jp.7211840. [DOI] [PubMed] [Google Scholar]
- 89.Athanasopoulou E, Fox JR. Effects of kangaroo mother care on maternal mood and interaction patterns between parents and their preterm, low birth weight infants: a systematic review. Infant Ment Health J. 2014;35(3):245–62. 10.1002/imhj.21444. [DOI] [PubMed] [Google Scholar]
- 90.Johnston CC, Filion F, Campbell-Yeo M, Goulet C, Bell L, McNaughton K, et al. Kangaroo mother care diminishes pain from heel lance in very preterm neonates: a crossover trial. BMC Pediatr. 2008;8:13 10.1186/1471-2431-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bera A, Ghosh J, Singh AK, Hazra A, Mukherjee S, Mukherjee R. Effect of kangaroo mother care on growth and development of low birthweight babies up to 12 months of age: a controlled clinical trial. Acta Paediatr. 2014;103(6):643–50. 10.1111/apa.12618. [DOI] [PubMed] [Google Scholar]
- 92.Bera A, Ghosh J, Singh AK, Hazra A, Som T, Munian D. Effect of kangaroo mother care on vital physiological parameters of the low birth weight newborn. Indian journal of community medicine : official publication of Indian Association of Preventive & Social Medicine. 2014;39(4):245–9. 10.4103/0970-0218.143030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Conde-Agudelo A, Diaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2014(4):CD002771 10.1002/14651858.CD002771.pub3. [DOI] [PubMed] [Google Scholar]
- 94.Boundy EO, Dastjerdi R, Spiegelman D, Fawzi WW, Missmer SA, Lieberman E, et al. Kangaroo Mother Care and Neonatal Outcomes: A Meta-analysis. Pediatrics. 2016;137(1). doi: 10.1542/peds.2015-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Silva MG, Barros MC, Pessoa UM, Guinsburg R. Kangaroo-mother care method and neurobehavior of preterm infants. Early Hum Dev. 2016;95:55–9. doi: 10.1016/j.earlhumdev.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 96.Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol Psychiatry. 2014;75(1):56–64. 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 97.Yu X, Shacka JJ, Eells JB, Suarez-Quian C, Przygodzki RM, Beleslin-Cokic B, et al. Erythropoietin receptor signalling is required for normal brain development. Development. 2002;129(2):505–16. [DOI] [PubMed] [Google Scholar]
- 98.Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007;64(2):159–71. doi: 10.1016/j.critrevonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jantzie LL, Miller RH, Robinson S. Erythropoietin signaling promotes oligodendrocyte development following prenatal systemic hypoxic-ischemic brain injury. Pediatr Res. 2013;74(6):658–67. doi: 10.1038/pr.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jantzie LL, Corbett CJ, Firl DJ, Robinson S. Postnatal Erythropoietin Mitigates Impaired Cerebral Cortical Development Following Subplate Loss from Prenatal Hypoxia-Ischemia. Cereb Cortex. 2015;25(9):2683–95. doi: 10.1093/cercor/bhu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412(6847):641–7. [DOI] [PubMed] [Google Scholar]
- 102.Larpthaveesarp A, Georgevits M, Ferriero DM, Gonzalez FF. Delayed erythropoietin therapy improves histological and behavioral outcomes after transient neonatal stroke. Neurobiol Dis. 2016;93:57–63. doi: 10.1016/j.nbd.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Juul SE, McPherson RJ, Bauer LA, Ledbetter KJ, Gleason CA, Mayock DE. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics. 2008;122(2):383–91. [DOI] [PubMed] [Google Scholar]
- 104.Fauchere JC, Dame C, Vonthein R, Koller B, Arri S, Wolf M, et al. An approach to using recombinant erythropoietin for neuroprotection in very preterm infants. Pediatrics. 2008;122(2):375–82. 10.1542/peds.2007-2591. [DOI] [PubMed] [Google Scholar]
- 105.McAdams RM, McPherson RJ, Mayock DE, Juul SE. Outcomes of extremely low birth weight infants given early high-dose erythropoietin. J Perinatol. 2013;33(3):226–30. doi: 10.1038/jp.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ohls RK, Kamath-Rayne BD, Christensen RD, Wiedmeier SE, Rosenberg A, Fuller J, et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics. 2014;133(6):1023–30. doi: 10.1542/peds.2013-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fischer Reibel N, Bührer C, Dame C Prophylactic Early Erythropoietin for Neuroprotection in Preterm Infants: A Meta-analysis. PEDIATRICS. 2017;139(5):e20164317. doi: 10.1542/peds.2016-4317. [DOI] [PubMed] [Google Scholar]
- 108.Juul SE, Mayock DE, Comstock BA, Heagerty PJ. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Maternal Health, Neonatology and Perinatology 2015;1:27. doi: DOI: 10.1186/s40748-015-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yasuhara T, Matsukawa N, Yu G, Xu L, Mays RW, Kovach J, et al. Behavioral and Histological Characterization of Intrahippocampal Grafts of Human Bone Marrow-Derived Multipotent Progenitor Cells in Neonatal Rats With Hypoxic-Ischemic Injury. Cell Transplant. 2006;15(3):231–8. doi: 10.3727/000000006783982034. [DOI] [PubMed] [Google Scholar]
- 110.van Velthoven CTJ, Kavelaars A, van Bel F, Heijnen CJ. Regeneration of the ischemic brain by engineered stem cells: Fuelling endogenous repair processes. Brain Res Rev. 2009;61(1):1–13. doi: 10.1016/j.brainresrev.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 111.Lee JA, Kim BI, Jo CH, Choi CW, Kim E-K, Kim H-S, et al. Mesenchymal Stem-Cell Transplantation for Hypoxic-Ischemic Brain Injury in Neonatal Rat Model. Pediatr Res. 2010;67(1):42–6. doi: 10.1203/pdr.0b013e3181bf594b. [DOI] [PubMed] [Google Scholar]
- 112.van Velthoven CTJ, Kavelaars A, van Bel F, Heijnen CJ. Repeated Mesenchymal Stem Cell Treatment after Neonatal Hypoxia-Ischemia Has Distinct Effects on Formation and Maturation of New Neurons and Oligodendrocytes Leading to Restoration of Damage, Corticospinal Motor Tract Activity, and Sensorimotor Function. J Neurosci. 2010;30(28):9603–11. doi: 10.1523/jneurosci.1835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain Behav Immun. 2011;25(7):1342–8. doi: 10.1016/j.bbi.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 114.Ahn SY, Chang YS, Sung DK, Sung SI, Yoo HS, Lee JH, et al. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke. 2013;44(2):497–504. 10.1161/STROKEAHA.112.679092. [DOI] [PubMed] [Google Scholar]
- 115.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 116.Rivera FJ, Couillard-Despres S, Pedre X, Ploetz S, Caioni M, Lois C, et al. Mesenchymal stem cells instruct oligodendrogenic fate decision on adult neural stem cells. Stem Cells. 2006;24(10):2209–19. doi: 10.1634/stemcells.2005-0614. [DOI] [PubMed] [Google Scholar]
- 117.van Velthoven CTJ, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun. 2010;24(3):387–93. doi: 10.1016/j.bbi.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 118.Min K, Song J, Kang JY, Ko J, Ryu JS, Kang MS, et al. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: a double-blind, randomized, placebo-controlled trial. Stem Cells. 2013;31(3):581–91. doi: 10.1002/stem.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr. 2014;164(5):973–9 e1. doi: 10.1016/j.jpeds.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ahn SY, Chang YS, Kim JH, Sung SI, Park WS. Two-Year Follow-Up Outcomes of Premature Infants Enrolled in the Phase I Trial of Mesenchymal Stem Cells Transplantation for Bronchopulmonary Dysplasia. J Pediatr. 2017;185:49–54 e2. 10.1016/j.jpeds.2017.02.061. [DOI] [PubMed] [Google Scholar]
- 121.Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7(6):444–58. [DOI] [PubMed] [Google Scholar]
- 122.Luchetti F, Canonico B, Betti M, Arcangeletti M, Pilolli F, Piroddi M, et al. Melatonin signaling and cell protection function. FASEB J. 2010;24(10):3603–24. doi: 10.1096/fj.10-154450. [DOI] [PubMed] [Google Scholar]
- 123.Alonso-Alconada D, Alvarez A, Arteaga O, Martinez-Ibarguen A, Hilario E. Neuroprotective effect of melatonin: a novel therapy against perinatal hypoxia-ischemia. International journal of molecular sciences. 2013;14(5):9379–95. doi: 10.3390/ijms14059379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Biran V, Phan Duy A, Decobert F, Bednarek N, Alberti C, Baud O. Is melatonin ready to be used in preterm infants as a neuroprotectant? Dev Med Child Neurol. 2014;56(8):717–23. doi: 10.1111/dmcn.12415. [DOI] [PubMed] [Google Scholar]
- 125.Miller SL, Yan EB, Castillo-Melendez M, Jenkin G, Walker DW. Melatonin provides neuroprotection in the late-gestation fetal sheep brain in response to umbilical cord occlusion. Dev Neurosci. 2005;27(2–4):200–10. 10.1159/000085993. [DOI] [PubMed] [Google Scholar]
- 126.Welin AK, Svedin P, Lapatto R, Sultan B, Hagberg H, Gressens P, et al. Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatric Research. 2007;61(2):153–8. 10.1203/01.pdr.0000252546.20451.1a. [DOI] [PubMed] [Google Scholar]
- 127.Watanabe K, Hamada F, Wakatsuki A, Nagai R, Shinohara K, Hayashi Y, et al. Prophylactic administration of melatonin to the mother throughout pregnancy can protect against oxidative cerebral damage in neonatal rats. J Matern Fetal Neonatal Med. 2012;25(8):1254–9. 10.3109/14767058.2011.636094. [DOI] [PubMed] [Google Scholar]
- 128.Watanabe K, Wakatsuki A, Shinohara K, Ikenoue N, Yokota K, Fukaya T. Maternally administered melatonin protects against ischemia and reperfusion-induced oxidative mitochondrial damage in premature fetal rat brain. J Pineal Res. 2004;37(4):276–80. 10.1111/j.1600-079X.2004.00167.x. [DOI] [PubMed] [Google Scholar]
- 129.Merchant NM, Azzopardi DV, Hawwa AF, McElnay JC, Middleton B, Arendt J, et al. Pharmacokinetics of melatonin in preterm infants. Br J Clin Pharmacol. 2013;76(5):725–33. doi: 10.1111/bcp.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Okatani Y, Okamoto K, Hayashi K, Wakatsuki A, Tamura S, Sagara Y. Maternal-fetal transfer of melatonin in pregnant women near term. J Pineal Res. 1998;25(3):129–34. [DOI] [PubMed] [Google Scholar]
- 131.Vitte PA, Harthe C, Lestage P, Claustrat B, Bobillier P. Plasma, cerebrospinal fluid, and brain distribution of 14C-melatonin in rat: a biochemical and autoradiographic study. J Pineal Res. 1988;5(5):437–53. [DOI] [PubMed] [Google Scholar]
- 132.Drommelschmidt K, Serdar M, Bendix I, Herz J, Bertling F, Prager S, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun. 2017;60:220–32. 10.1016/j.bbi.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 133.Rose RA, Jiang H, Wang X, Helke S, Tsoporis JN, Gong N, et al. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26(11):2884–92. 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- 134.Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28(8):1446–55. 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Janas AM, Sapon K, Janas T, Stowell MH, Janas T. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim Biophys Acta. 2016;1858(6):1139–51. 10.1016/j.bbamem.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 136.Liu BL, Zhang X, Zhang W, Zhen HN. New enlightenment of French Paradox: resveratrol’s potential for cancer chemoprevention and anti-cancer therapy. Cancer Biol Ther. 2007;6(12):1833–6. [DOI] [PubMed] [Google Scholar]
- 137.Kesherwani V, Atif F, Yousuf S, Agrawal SK. Resveratrol protects spinal cord dorsal column from hypoxic injury by activating Nrf-2. Neuroscience. 2013;241:80–8. doi: 10.1016/j.neuroscience.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 138.Agrawal M, Kumar V, Kashyap MP, Khanna VK, Randhawa GS, Pant AB. Ischemic insult induced apoptotic changes in PC12 cells: protection by trans resveratrol. Eur J Pharmacol. 2011;666(1–3):5–11. doi: 10.1016/j.ejphar.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 139.Gao ZB, Chen XQ, Hu GY. Inhibition of excitatory synaptic transmission by transresveratrol in rat hippocampus. Brain Res. 2006;1111(1):41–7. doi: 10.1016/j.brainres.2006.06.096. [DOI] [PubMed] [Google Scholar]
- 140.West T, Atzeva M, Holtzman DM. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev Neurosci. 2007;29(4–5):363–72. doi: 10.1159/000105477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shin JY, Seo MA, Choi EJ, Kim JK, Seo ES, Lee JH, et al. Neuroprotective effects of resveratrol via anti-apoptosis on hypoxic-ischemic brain injury in neonatal rats. Korean journal of pediatrics. 2008;51(10):1102–11. [Google Scholar]
- 142.Karalis F, Soubasi V, Georgiou T, Nakas CT, Simeonidou C, Guiba-Tziampiri O, et al. Resveratrol ameliorates hypoxia/ischemia-induced behavioral deficits and brain injury in the neonatal rat brain. Brain Res. 2011;1425:98–110. doi: 10.1016/j.brainres.2011.09.044. [DOI] [PubMed] [Google Scholar]
- 143.Loren DJ, Seeram NP, Schulman RN, Holtzman DM. Maternal Dietary Supplementation with Pomegranate Juice Is Neuroprotective in an Animal Model of Neonatal Hypoxic-Ischemic Brain Injury. Pediatr Res. 2005;57(6):858–64. [DOI] [PubMed] [Google Scholar]
- 144.Bourque SL, Dolinsky VW, Dyck JR, Davidge ST. Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta. 2012;33(5):449–52. doi: 10.1016/j.placenta.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 145.Lin J-K. Molecular Targets of Curcumin Adv Exp Med Biol: Springer Nature; p. 227–43. [DOI] [PubMed] [Google Scholar]
- 146.Zhu H-t, Bian C, Yuan J-c, Chu W-h, Xiang X, Chen F, et al. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-κ signaling pathway in experimental traumatic brain injury. J Neuroinflammation. 2014;11(1):59. doi: 10.1186/1742-2094-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–41. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 148.Esatbeyoglu T, Huebbe P, Ernst IMA, Chin D, Wagner AE, Rimbach G. Curcumin-From Molecule to Biological Function. Angewandte Chemie International Edition. 2012;51(22):5308–32. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- 149.Singh S, Khar A. Biological Effects of Curcumin and Its Role in Cancer Chemoprevention and Therapy. Anti-Cancer Agents in Medicinal Chemistry. 2006;6(3):259–70. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- 150.Dohare P, Garg P, Jain V, Nath C, Ray M. Dose dependence and therapeutic window for the neuroprotective effects of curcumin in thromboembolic model of rat. Behav Brain Res. 2008;193(2):289–97. doi: 10.1016/j.bbr.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 151.Zhao J, Yu S, Zheng W, Feng G, Luo G, Wang L, et al. Curcumin Improves Outcomes and Attenuates Focal Cerebral Ischemic Injury via Antiapoptotic Mechanisms in Rats. Neurochem Res. 2009;35(3):374–9. doi: 10.1007/s11064-009-0065-y. [DOI] [PubMed] [Google Scholar]
- 152.Liu Z-J, Liu W, Liu L, Xiao C, Wang Y, Jiao J-S. Curcumin Protects Neuron against Cerebral Ischemia-Induced Inflammation through Improving PPAR-Gamma Function. Evidence-Based Complementary and Alternative Medicine. 2013;2013:1–10. doi: 10.1155/2013/470975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huang H-C, Chang P, Lu S-Y, Zheng B-W, Jiang Z-F. Protection of curcumin against amyloid-beta-induced cell damage and death involves the prevention from NMDA receptor-mediated intracellular Ca2+elevation. J Recept Signal Transduct Res. 2015;35(5):450–7. doi: 10.3109/10799893.2015.1006331. [DOI] [PubMed] [Google Scholar]
- 154.Tiwari SK, Agarwal S, Seth B, Yadav A, Nair S, Bhatnagar P, et al. Curcumin-Loaded Nanoparticles Potently Induce Adult Neurogenesis and Reverse Cognitive Deficits in Alzheimer’s Disease ModelviaCanonical Wnt-beta-Catenin Pathway. ACS Nano. 2014;8(1):76–103. doi: 10.1021/nn405077y. [DOI] [PubMed] [Google Scholar]
- 155.Joseph A, Wood T, Chen C-C, Corry K, Snyder JM, Juul SE, et al. Curcumin-loaded polymeric nanoparticles for neuroprotection in neonatal rats with hypoxic-ischemic encephalopathy Nano Research. 2018. doi: 10.1007/s12274-018-2104-y. [DOI] [Google Scholar]
- 156.Kakkar V, Muppu SK, Chopra K, Kaur IP. Curcumin loaded solid lipid nanoparticles: an efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur J Pharm Biopharm. 2013;85(3 Pt A):339–45. doi: 10.1016/j.ejpb.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 157.Nance E, Porambo M, Zhang F, Mishra MK, Buelow M, Getzenberg R, et al. Systemic dendrimer-drug treatment of ischemia-induced neonatal white matter injury. J Control Release. 2015;214:112–20. doi: 10.1016/j.jconrel.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Raju TN, Pemberton VL, Saigal S, Blaisdell CJ, Moxey-Mims M, Buist S, et al. Long-Term Healthcare Outcomes of Preterm Birth: An Executive Summary of a Conference Sponsored by the National Institutes of Health J Pediatr. 2016. doi: 10.1016/j.jpeds.2016.10.015. [DOI] [PubMed] [Google Scholar]