Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is a substantial cause of comorbidity in people living with HIV (PLWH), without proven pharmacologic treatments in this population. We assessed the effects of tesamorelin on liver fat and histology in PLWH with NAFLD.
Methods
61 men and women with HIV-infection and hepatic fat fraction (HFF) ≥5% by proton magnetic resonance spectroscopy were randomized to receive tesamorelin 2mg daily vs. identical placebo for 12 months at two academic centers. The primary endpoint was change in HFF. The primary safety endpoint was glucose. The study was randomized and double-blind, with identical placebo as a comparator. A random treatment sequence was generated by the statistician and provided to the research pharmacies. Analysis was by intention to treat using all available data.
Findings
Tesamorelin (N=26) reduced HFF compared to placebo (N=28), with an absolute effect size of −4.1% (95% CI −7.6, −0.7, P=0.02), corresponding to a −37% (CI −67, −7, P=0.02) relative reduction from baseline. After 12 months, 35% of individuals receiving tesamorelin vs. 4% receiving placebo had a reduction in HFF to <5% (P=0.007). Glucose parameters did not differ between groups. More individuals in the tesamorelin group experienced localized injection site complaints.
Interpretation
Tesamorelin may be beneficial in PLWH with NAFLD; further study is needed to determine the long-term effects of tesamorelin on liver histology.
Trial registration
Introduction
Nonalcoholic fatty liver disease (NAFLD) is defined by excess storage of triglyceride in hepatocytes (steatosis) and is often accompanied by inflammation, cellular damage, and fibrosis. The development of ballooning hepatocellular injury indicates progression to nonalcoholic steatohepatitis (NASH). NASH may progress to cirrhosis and is an increasingly important cause of end-stage liver disease in the general population. NAFLD may be more prevalent in people living with HIV (PLWH) than in the general population, and studies suggest that progression of fibrosis is more likely in HIV (1–3). In contrast to many HIV-associated comorbidities that worsen with increased HIV-disease severity, NAFLD may occur more commonly in those with well-treated HIV and higher CD4+ T-cell counts and weight gain, often in association with central adiposity (4–6). In PLWH, weight gain, abdominal fat accumulation, and increases in visceral fat are common and seen even with newer antiretrovirals (7). There are no proven therapies for NAFLD in HIV, nor is it known how such strategies to reduce liver fat would affect progression of histological changes over time, and thus alter the natural history such changes among PLWH.
Growth hormone (GH) secretory dynamics are perturbed with reduced pulsatile GH among PLWH. The degree of perturbation closely parallels abdominal fat accumulation and weight gain (8–10). Tesamorelin is a growth hormone releasing hormone (GHRH) analog that restores endogenous pulsatile GH secretion and reduces visceral fat in individuals with HIV infection (11–13). Tesamorelin is thought to stimulate lipolysis via increased endogenous GH while maintaining feedback inhibition and limiting toxicity compared to GH per se (13). We previously demonstrated that tesamorelin reduces liver fat content in a preliminary study of PLWH chosen for abdominal obesity (14). The current study was designed to significantly extend these data and address a critical question for the HIV population, assessing for the first time a therapeutic strategy specifically for PLWH with NAFLD, simultaneously assessing changes in liver histopathology, inflammatory and metabolic indices, including specific indices of insulin sensitivity by euglycemic hyperinsulinemic clamp.
Methods
Study Design and Participants
The study consisted of a 12-month, double-blind treatment phase during which participants were randomized in a 1:1 ratio to receive tesamorelin 2mg daily or identical placebo, followed by a 6-month open label phase during which all participants received tesamorelin. The pre-specified primary analysis, comparing changes over twelve months in tesamorelin vs. placebo treatment, is reported here.
Participants were recruited at the Massachusetts General Hospital (MGH, Boston, MA, USA) and the National Institutes of Health (NIH, Bethesda, MD, USA). Potentially eligible participants at both sites were identified through referrals from local physicians and local outreach including advertisements on research websites. Sixty-one men and women with HIV-infection met eligibility criteria and participated in a baseline assessment. The first patient was enrolled on August 20, 2015, and the final patient completed the 12-month assessment on January 16, 2019. All participants provided written informed consent, and the study was approved by the institutional review boards at the MGH and NIH.
Participants were eligible for the study if they were between 18–70 years of age and had confirmed HIV infection as well as hepatic steatosis as demonstrated by liver fat fraction of ≥5% on proton magnetic resonance spectroscopy (MRS). Participants with heavy alcohol use (>20g daily for women or >30g daily for men) were excluded, as were participants with hepatitis B, active hepatitis C, alpha-1 antitrypsin deficiency, Wilson’s disease, hemochromatosis, or autoimmune hepatitis. Participants with history of Hepatitis C were required to have completed treatment more than 1 year prior to study entry and to have cure verified with HCV viral load of 0. Participants with known cirrhosis, Stage 4 fibrosis on biopsy, or other severe chronic illness were also excluded. Participants with mild diabetes were eligible as long as hemoglobin A1c was ≤7%, anti-diabetic agents were stable for ≥6 months, and they were not using insulin or thiazoledinediones. Participants were also required to have a stable antiviral regimen for ≥3 months, stable use of any antihypertensives or lipid-lowering medications for ≥3 months, and, if applicable, stable use of vitamin E for ≥6 months prior to study entry. Participants using chronic systemic corticosteroids, methotrexate, amiodarone, tamoxifen, or growth hormone were excluded, as were participants with any active malignancy. Women 50 years or older were required to have a negative mammogram within 1 year of the baseline visit, and men with history of prostate cancer were excluded. Participants with history of hypopituitarism or other conditions known to affect the GH axis were also ineligible. Other exclusionary labs for safety reasons were as follows: hemoglobin < 11 g/dL, CD4+ count < 100 cells/mm3, HIV viral load > 400 copies/mL, and prostate specific antigen >5 ng/mL.
The National Institute of Allergy and Infectious Diseases, Division of AIDS (DAIDS), received monthly reports on study progress and safety and provided study monitoring. Additionally, a Data Safety Monitoring Board comprised by DAIDS performed study oversight.
Randomization and Masking
Subjects were randomized in a 1:1 ratio to receive tesamorelin 2mg daily vs. identical placebo. Both tesamorelin and placebo (mannitol) were provided in identical vials of lyophilized power which subjects reconstituted with sterile water prior to injection. Randomization was stratified by site (NIH and MGH) and vitamin E use, defined as consistent use of ≥400 international units daily. The randomization list was prepared by the study statistician using a permuted block algorithm within each stratum with randomly varying block sizes.
Procedures
Tesamorelin was administered at the FDA-approved dose of 2mg subcutaneously daily; participants were trained in reconstitution and self-injection at the baseline visit and administered the injections at home, returning used vials to assess compliance. IGF-1 Z-scores were monitored throughout the study by an independent endocrinologist at MGH otherwise unaffiliated with the study. A pre-specified threshold of IGF-1 Z-score of ≥3 was included in the protocol as a trigger to decrease tesamorelin dose to 1mg, along with a dummy dose-reduction in the placebo group, but this was not required for any participant.
All subjects received nutritional counseling from clinical research nutritionists at baseline, six months and 12 months. Visits were conducted in the fasting state. The screening visit included history and physical examination, laboratory investigations for eligibility, MRS and magnetic resonance imaging (MRI), with the latter used to assess cross-sectional area of visceral adipose tissue (VAT) at the level of the L4 vertebra. The MRI/MRS, HbA1c, CD4+ count, and HIV viral load from the screening visit were used as the baseline measures. The baseline assessment included an ultrasound-guided percutaneous liver biopsy; whole body dual energy x-ray absorptiometry (DXA); fasting assessment of liver function tests, lipids, serum inflammatory markers, and IGF-1; and bionutrition assessment including 4-day food record, Modifiable Activity Questionnaire, and anthropometric measures performed in triplicate. These assessments, including liver biopsy, were repeated at 12 months along with repeat MRI/MRS, HbA1c, and immunologic parameters. An interim MRI/MRS was performed at the 6-month visit.
Histological scoring was performed by a blinded central pathologist (DEK) for all liver biopsy samples using the Nonalcoholic Steatohepatitis Clinical Research Network (NAS CRN) scoring system (15). The sum of grades for steatosis (grades 0–3), hepatocellular ballooning (grades 0–2), and lobular inflammation (0–3) comprise the NAS score, and fibrosis is independently staged between 0–4 (15). Any presence of features of steatohepatitis, including borderline disease, was determined by histological review (DEK). Progression of fibrosis was considered any increase in fibrosis stage between baseline and 12 months. This included advancement from stage 1a to 1b, which was present in 1 participant. Cross-sectional MRI of the abdomen at the L4 vertebra was performed using a T2-weighted Half-Fourier-Acquired Single-shot Turbo spin Echo (HASTE) pulse sequence, with images read centrally by a blinded radiologist (MT). Visceral (VAT) and subcutaneous (SAT) adipose tissue areas were calculated using semi-automated pixel thresholding to separate each compartment and provide measures in cm2.
Participants at MGH also underwent a euglycemic hyperinsulinemic clamp procedure to assess insulin sensitivity. After a 14-hour overnight fast, a low-dose (insulin 20mU/m2/min) clamp for 2 hours was followed by a high-dose (insulin 80mU/m2/min) clamp for 2 hours as previously described (16). Insulin stimulated glucose disposal (M) was calculated during the last 20 minutes of low-dose and high-dose clamp using the DeFronzo method as the primary indices of hepatic and whole-body insulin sensitivity, respectively.
Laboratory analyses were conducted using standard methodologies. Clinical labs were measured at the NIH Clinical Laboratory and, for MGH, at LabCorp and Quest. IGF-1 was measured centrally at Quest Laboratories. CRP was measured using electrochemiluminescence (Meso Scale Discovery, Rockville, MD), and adiponectin was measured using ELISA (R&D Systems, Minneapolis, MN).
Outcome measures
The primary outcome was hepatic fat fraction (HFF) as measured by MRS, performed in the morning following an 8-hour fast. Fat fraction was calculated as the area under the spectroscopic lipid peak divided by the total area under the water and lipid peaks. Image acquisition followed a standard protocol at MGH and NIH, and liver fat content was quantified by blinded radiologists. The diagnostic accuracy of MRS for liver steatosis has an area under the receiver operating characteristic curve of 0.94 (95% CI 0.88–1.0) compared to assessment of liver biopsy by an experienced pathologist (17). Subjects were eligible if HFF was ≥5%.
Pre-specified secondary endpoints were as follows: histological assessment of hepatic fibrosis using fibrosis stage; histologic assessment of inflammation and cellular ballooning using the NAS Score; alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT) levels; visceral fat area as measured by MRI scan; total body fat and lean mass as measured by DXA, and waist circumference by anthropometry; fasting lipids; c-reactive protein (CRP); adiponectin. Pre-specified endpoints related to safety included measures of glucose homeostasis (fasting glucose, hemoglobin A1c (HbA1c), and, at MGH, insulin-stimulated glucose uptake) as well as CD4+ and CD8+ cell counts and HIV viral load.
Statistical Analysis
The pre-specified primary endpoint was change in HFF between baseline and 12 months. A sample size of 60 was chosen based on 80% power to detect a treatment difference of ≥ 0.85 standard deviation change in hepatic fat fraction over 12 months, assuming a discontinuation rate of 25%, i.e., 45 evaluable patients, at a two-sided alpha of 0.05. After one patient at the MGH site discontinued at the conclusion of the baseline visit, IRB permission was obtained to enroll a 61st participant.
For all endpoints, an intention to treat analysis was performed using all available data; subjects without any follow-up data for an endpoint were not included in the analysis of that endpoint. Per the pre-specified analysis plan, change in HFF was assessed by random intercept mixed effects modeling for continuous repeated measures using restricted maximum likelihood to assess the effect estimate for the time×randomization interaction. All available repeated measures data were used in the analysis, which was based on intention to treat, including available interim data. The sample size for the primary endpoint analysis was 26 in the tesamorelin group and 28 in the placebo group, as shown in Figure 1. In the model for the primary endpoint, residual plots appeared to have a normal distribution with homogeneity across groups, suggesting no violations of assumptions of linearity, normality, or homogeneity of variance. The same analysis was used for other endpoints measured at multiple timepoints during the double-blind period, including VAT, SAT, ALT, BMI, and glucose. Hepatic fat fraction, VAT, SAT, and ALT were available at baseline, 6 months, and 12 months. BMI and glucose were available at every visit. For secondary endpoints measured at only baseline and 12-months, a paired t-test was performed. Data are presented as mean ± standard deviation or, for categorical variables, number and percent. Between group comparisons at baseline were assessed using Student’s t-test for continuous variables and Pearson’s Chi Square statistic for categorical variables. Pearson’s correlation coefficient was used to assess relationships between continuous variables. Two data points – one baseline ALT value and one baseline CRP value – were excluded due to being more than 5 standard deviations above the sample mean. A two-sided alpha of 0.05 was the pre-defined threshold for statistical significance. Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at Partners HealthCare. Sensitivity analyses were performed to address missing data, which were assumed to be missing at random. Data augmentation for missing observations was carried out by way of multiple imputation (MI) using PROC MI in SAS; this method was used to impute missing data for all randomized subjects (N=61). MI was performed for100 iterations, discarding the first 10 iterations. All data analysis was overseen by the study statistician (HL). Statistical analyses were performed in SAS 9.4 or in JMP 12.0. The study was registered on clinicaltrials.gov, .
Figure 1:
CONSORT Flow Diagram
Role of the Funding Source
The authors of this manuscript were solely responsible for the hypothesis, study design, data analysis, manuscript preparation, and decision to submit the manuscript. Theratechnologies, Inc., provided study drug at a reduced cost and, per contract, received serious adverse event reports as well as notification of intent to submit for publication; Theratechnologies had no other role in the design or conduct of the research or in manuscript preparation or submission.
Results
Of 143 total participants screened, 61 entered the randomized treatment portion of the trial. Fifteen participants were recruited at NIH and 46 at MGH. Participant flow and reasons for participant exclusion are shown in Figure 1. Four participants in the tesamorelin group and two in the placebo group discontinued before any follow-up imaging, as shown in Figure 1. Five participants in the tesamorelin group and two in the placebo group discontinued after obtaining follow-up imaging that was used in the primary analysis. The overall discontinuation rate was not significantly different between groups (P=0.12).
Clinical characteristics (Table 1) and measures of body composition and metabolism (Table 2) were similar between groups at baseline. Mean hepatic fat fraction among the entire cohort was 13.8 ± 8.4%, with a range of 5% to 45.4%. At baseline, 33% of the cohort had a histologic diagnosis of NASH. Forty-three percent of the cohort had fibrosis Stage 1 or higher; per protocol, none had Stage 4 fibrosis (cirrhosis) at baseline. These rates were similar in the treatment groups (Table 1). ART regimen was similar between groups (Table 1).
Table 1:
Baseline Demographics and Clinical Characteristics
| Tesamorelin (n = 31) | Placebo (n = 30) | |
|---|---|---|
| Sex (N [%]) | ||
| Male | 24 [77.4%] | 24 [80.0%] |
| Female | 7 [22.6%] | 6 [20.0%] |
| Age (years) | 52 ± 8 | 54 ± 7 |
| Race (N [%]) | ||
| White | 21 [67.7%] | 19 [63.3%] |
| Black | 8 [25.8%] | 10 [33.3%] |
| Other | 2 [6.5%] | 1 [3.3%] |
| Ethnicity (N [%]) | ||
| Hispanic | 6 [19.4%] | 3 [10.0%] |
| Non-Hispanic | 25 [80.7%] | 27 [90.0%] |
| Smoking Status (N [%]) | ||
| Never Smoker | 13 [41.9%] | 11 [36.7%] |
| Previous Smoker | 14 [45.2%] | 12 [40.0%] |
| Current Smoker | 4 [12.9%] | 7 [23.3%] |
| Alcohol Use (drinks/week) | 0.3 ± 1.3 | 0.9 ± 2.0 |
| Duration of HIV Infection (years) | 16 ± 9 | 18 ± 8 |
| Current Antiretroviral Use (N [%]) | ||
| NRTI | 27 [87.1%] | 29 [96.7%] |
| PI | 9 [29.0%] | 6 [20.0%] |
| NNRTI | 12 [38.7%] | 11 [36.7%] |
| Integrase Inhibitors | 21 [67.7%] | 18 [60.0%] |
| Entry Inhibitor | 1 [3.2%] | 0 [0%] |
| Type 2 Diabetes (N [%]) | ||
| Known diabetes | 4 [12.9%] | 4 [13.3%] |
| Current use of anti-diabetics | 3 [9.7%] | 3 [10.0%] |
| Current metformin use | 3 [9.7%] | 2 [6.7%] |
| No known diabetes | 27 [87.1%] | 26 [86.7%] |
| Current Lipid Lowering Medications (N [%]) | ||
| Any current lipid-lowering medication use | 13 [41.9%] | 15 [50.0%] |
| Current statin use | 10 [32.3%] | 14 [46.7%] |
| No current lipid-lowering medication use | 18 [58.1%] | 15 [50.0%] |
| Current Vitamin E Use (N [%]) | ||
| Yes | 2 [6.5%] | 1 [3.3%] |
| No | 29 [93.6%] | 29 [96.7%] |
| Hepatic Fat (%) | 12.9 ± 7.7 | 14.7 ± 9.0 |
| NASH (N [% of total with data]) | ||
| Histological NASH | 10 [34.5%] | 9 [31.0%] |
| No Histological NASH | 29 [65.5%] | 20 [69.0%] |
| Data not available | 2 | 1 |
| Fibrosis (N [% of total with data]) | ||
| Stage 0 | 15 [51.7%] | 18 [62.1%] |
| Any fibrosis (Stage 1–3) | 14 [48.3%] | 11 [37.9%] |
| Stage 1 | 4 [13.8%] | 5 [17.2%] |
| Stage 2 | 6 [20.7%] | 4 [13.8%] |
| Stage 3 | 4 [13.8%] | 2 [6.9%] |
| Data not available | 2 | 1 |
There were no statistically significant differences between groups at baseline for any of the variables shown above. Continuous variables are presented as mean ± standard deviation. No data are missing except as noted.
Vitamin E use defined as regular use of ≥ 400 international units daily.
Abbreviations: HIV, human immunodeficiency virus; NASH, nonalcoholic steatohepatitis; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor
Table 2:
Effects of tesamorelin on hepatic fat, metabolic, and immunologic indices
| Baseline | Change at 12 months | Treatment Effect* | P Value* | |||
|---|---|---|---|---|---|---|
| Tesamorelin (n = 31) | Placebo (n = 30) | Tesamorelin (n = 21) | Placebo (n = 26) | |||
| Liver Endpoints | ||||||
| Primary | ||||||
| Hepatic fat, absolute change, % | 12.9 ± 7.7 | 14.7 ± 9.0 | −4.7 ± 6.6 | −0.0 ± 4.1 | −4.1 (−7.6, −0.7) | 0.02 |
| Hepatic fat, relative change, % | N/A | N/A | −32 ± 54 | 5 ± 42 | −37 (−67, −7) | 0.02 |
| Hepatic fat at 12 months <5%, % | N/A | N/A | 35 | 4 | 31 | 0.007 |
| Secondary Liver Endpoints | ||||||
| ALT, U/L | 33 ± 25 | 26 ± 18 | −2 ± 11 | 5 ± 15 | −7 (−15, 1) | 0.09 |
| GGT, U/L | 55 ± 54 | 65 ± 76 | −12 ± 31 | 7 ± 46 | −19 (−43, 4) | 0.10 |
| Adipose tissue (Secondary Endpoints) | ||||||
| VAT, cm2 | 232 ± 91 | 250 ± 104 | −21 ± 77 | 14 ± 40 | −35 (−66, −4) | 0.03 |
| SAT, cm2 | 290 ± 164 | 333 ± 156 | 24 ± 72 | 12 ± 46 | 11 (−19, 41) | 0.46 |
| BMI, kg/m2 | 30.1 ± 6.0 | 32.9 ± 6.2 | 0.9 ± 2.3 | 0.4 ± 1.1 | 0.3 (−0.3, 0.9) | 0.37 |
| Waist, cm | 107 ± 15 | 114 ± 12 | 0 ± 9 | 1 ± 3 | −1 (−5, 3) | 0.59 |
| Total body fat (kg) | 30.3 ± 10.5 | 34.4 ± 12.0 | 0.5 ± 4.6 | 1.2 ± 4.0 | −0.7 (−3.3, 2.0) | 0.61 |
| Total lean body mass (kg)** | 57.2 ± 10.2 | 63.8 ± 10.6 | 1.9 ± 3.7 | −0.0 ± 2.6 | 1.9 (−0.0, 3.8) | 0.05 |
| Metabolic Indices (Secondary Endpoints) | ||||||
| IGF-1, ng/mL | 132 ± 43 | 115 ± 43 | 116 ± 84 | −1 ± 39 | 117 (76, 157) | < 0.0001 |
| Triglycerides, mg/dL | 151 ± 84 | 128 ± 46 | 19 ± 52 | −4 ± 48 | 23 (−7, 53) | 0.12 |
| HDL-C, mg/dL | 47 ± 13 | 45 ± 11 | 2 ± 6 | −1 ± 6 | 3 (−1, 6) | 0.17 |
| LDL-C, mg/dL | 113 ± 36 | 102 ± 25 | −1 ± 28 | 4 ± 27 | −5 (−21, 11) | 0.54 |
| CRP, mg/L | 7.8 ± 9.9 | 4.2 ± 3.5 | −3.3 ± 9.2 | 1.4 ± 4.2 | −4.7 (−9.2, −0.2) | 0.04 |
| Adiponectin, ng/mL | 2042 ± 1450 | 1638 ± 875 | −118 ± 706 | −340 ± 798 | 222 (−225, 669) | 0.32 |
| Glucose homeostasis and Immunological Parameters (Safety Endpoints) | ||||||
| Fasting glucose, mg/dL | 96 ± 20 | 97 ± 16 | 7 ± 13 | 4 ± 13 | 4 (−5, 13) | 0.40 |
| HbA1c, % | 5.7 ± 0.5 | 5.8 ± 0.5 | 0.2 ± 0.6 | 0.1 ± 0.4 | 0.2 (−0.1, 0.5) | 0.29 |
| CD4+ T-cells (cells/per μL) | 733 ± 290 | 798 ± 260 | −11 ± 141 | −28 ± 118 | 17 (−60, 95) | 0.65 |
| CD8+ T-cells (cells/per μL) | 865 ± 380 | 967 ± 374 | −68 ± 165 | −49 ± 154 | −18 (−113, 77) | 0.70 |
| log HIV viral load (copies per mL) | 0.34 ± 0.59 | 0.50 ± 0.74 | 0.16 ± 0.72 | −0.02 ± 0.75 | 0.18 (−0.25, 0.62) | 0.41 |
For continuous variables, treatment effect and p-values are from repeated measures analysis for primary and secondary endpoints with interim measures (hepatic fat absolute change, ALT, VAT, SAT, BMI, and glucose) and from between-group comparison of change at 12 months by Student’s t-test for other variables. For resolution of NAFLD, P-value by Pearson Chi-Square.
Tesamorelin and placebo group different at baseline.
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; CRP, C-reactive protein; GGT, gamma-glutamyl transferase; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; IGF-1, insulin-like growth factor 1; LDL-C, low-density lipoprotein cholesterol; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
SI Unit Conversions: IGF-1, μg/L equivalent to ng/mL; Triglycerides, multiply by 0.0113 for mmol/L; HDL-C and LDL-C, multiply by 0.0259 for mmol/L; glucose, divide by 18 for mmol/L.
Adherence to daily injections by count of returned empty vials was similar between treatment groups: 87±16% for placebo and 80±15% for tesamorelin (P=0.11). Change in IGF-1 values, shown in Table 2, demonstrate the expected effect of tesamorelin to increase IGF-1, with an effect size of 117 ng/mL (95% CI [76, 157], P < 0.0001). No subjects had IGF-1 Z-scores over the pre-specified dose-reduction threshold (Z-score > 3), but one subject received an IRB-approved dose reduction to 1 mg for symptoms potentially related to growth hormone. This subject self-discontinued from the study soon after the dose reduction.
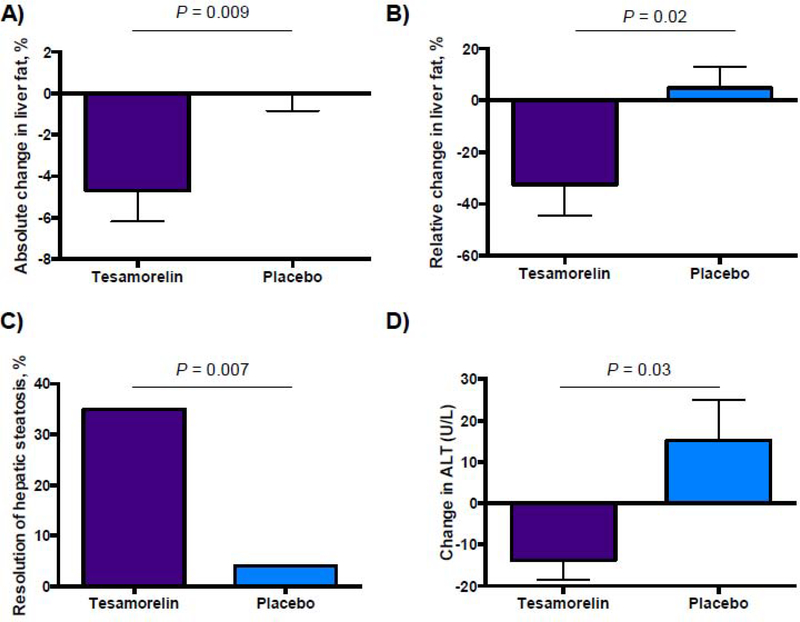
Tesamorelin significantly reduced HFF compared to placebo (effect size −4.1% (95% CI −7.6, −0.7), P=0.02). There was no change in effect size or p-value when adjusting independently for baseline measures of race, antiretroviral use, statin use, or smoking. The change between baseline and 12 months, shown in Figure 2A, corresponded to a −37% (95% CI −67, −7) relative change in liver fat (Figure 2B). In the tesamorelin group, 35% of individuals had a reduction in HFF to <5%, whereas this occurred in 4% of individuals in the placebo group (Figure 2C, P=0.007 for comparison). Changes in HFF for each individual by group are shown in Supplemental Figure 1 (Appendix page 2). Among tesamorelin treated individuals, there was not a significant relationship between change in IGF-1 over 12 months and change in HFF (r=0.10, P=0.67).
Figure 2:
Change in absolute (A) and relative (B) liver fat content between baseline and 12 months, with p-values shown for t-test comparing change between groups. Data are mean ± SEM. C: Percent resolution of steatosis, defined as 12-month hepatic fat fraction <5%, with P-value for Pearson Chi-Square. D: Change in ALT between baseline and 12 months for those with ALT ≥ 30 U/L at baseline, with p-value for t-test comparing change between groups. Data are mean ± SEM.
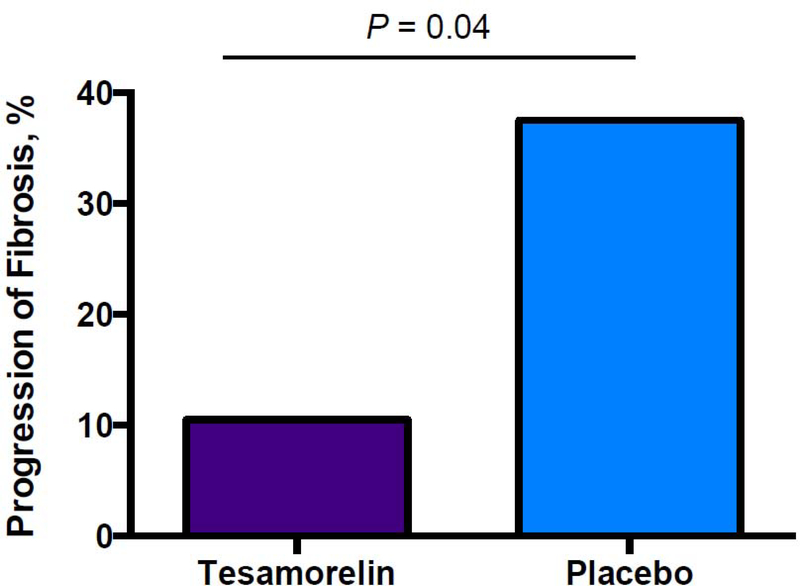
Among the entire cohort, tesamorelin prevented the progression of fibrosis during the treatment period, with 2 individuals (10.5%) showing progression in the tesamorelin group versus 9 (37.5%) in the placebo group (P=0.04) (Figure 3). Tesamorelin did not improve existing fibrosis in this cohort: among those with fibrosis stage ≥ 1 at baseline, fibrosis improved in 2 individuals in the tesamorelin group vs. 3 in the placebo group (P=0.71). Changes in fibrosis during the study were positively associated with changes in NAS score (p-value for ANOVA = 0.0003). Among the entire cohort, compared to placebo, tesamorelin did not significantly change NAS score (effect size −0.3, 95% CI −1.0, 0.5), lobular inflammation score (effect size −0.3, 95% CI −0.7, 0.2), or hepatocellular ballooning (effect size −0.1, 95% CI −0.4, 0.2). In exploratory analyses, the higher the baseline NAS score, the more change was seen among the tesamorelin-treated individuals (r=−0.48, P=0.04), whereas a similar relationship was not observed in the placebo group (r=−0.14, P=0.52).
Figure 3:
Percent of patients with any progression of fibrosis at 12 months, with P-value for Pearson Chi-Square.
Compared to placebo, tesamorelin did not significantly reduce ALT or GGT over the treatment period, although both effect sizes suggest a modest reduction (Table 2). Restricting the cohort to those with elevated ALT (>30 U/L) at baseline, tesamorelin did significantly decrease ALT after 12 months (effect size −29 U/L [95% CI −55, −3], P=0.03, Figure 2D) compared to placebo. Tesamorelin reduced CRP (effect size −4.7 mg/L [95% CI −9.2, −0.2], P=0.04, Table 2) compared to placebo but did not have any effect on adiponectin.
In the current study we did not see any effect of tesamorelin on low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), or triglycerides (Table 2). Tesamorelin did not significantly affect fasting glucose or hemoglobin A1c during the treatment period (Table 2). Fasting glucose at each study visit for each group is shown in Supplemental Figure 2 (Appendix page 3). In the subset of patients at MGH who underwent euglycemic hyperinsulinemic clamp, tesamorelin did not affect the glucose infusion rate required during the low-dose clamp (treatment effect for low-dose M 0.0 mg/kg/min [95% CI −1.1, 1.1], P>0.99) nor the insulin stimulated glucose uptake during the high-dose clamp (treatment effect for high-dose M −0.9 mg/kg/min [95% CI −2.4, 0.7], P=0.27).
As expected, tesamorelin significantly reduced VAT area compared to placebo, with no effect on SAT area (Table 2). Tesamorelin modestly increased lean body mass by DXA, with no significant effect on total body fat mass or body weight (Table 2). BMI and waist circumference did not change.
With regard to nutrition and physical activity of study participants, baseline and 12-month estimates of daily caloric and macronutrient intake by four-day food record, self-reported alcoholic drinks per week, and hours of activity per week as assessed by Modifiable Activity Questionnaire are shown in the Supplemental Table 1 (Appendix page 1). There were no significant changes in any of these variables.
Sensitivity analyses for the primary endpoint were performed using multiple imputation for missing data. These data confirmed the primary results, with estimated effect size of −3.8% (95% coverage −5.5, −2.2) reduction in HFF.
Adverse events by study group are shown in Table 3. A limited number of SAE’s were seen, which did not differ by treatment arm. None were judged as related to study drug. In the placebo group, two individuals were hospitalized with SAEs: one following a suicide attempt, and one following a hematoma post liver biopsy. In the tesamorelin group, four individuals were hospitalized with SAEs: one with transient hemiplegia for which a cause was not found, one due to suicidal ideation, one for urosepsis, and one for pneumonia and separately for influenza.
Table 3:
Adverse Events:
| Tesamorelin (n = 31) | Placebo (n = 30) | P-value for comparison* | |
|---|---|---|---|
| Any adverse event | 29 | 29 | 0.57 |
| Serious adverse event | 4 | 2 | 0.41 |
| Event meeting criteria for discontinuation by investigator | 4 | 1 | 0.17 |
| Hyperglycemia | 12 | 11 | |
| Arthralgia | 3 | 3 | |
| Myalgia | 2 | 0 | |
| Paresthesia | 2 | 2 | |
| Injection site bruising | 11 | 11 | |
| Injection site erythema | 3 | 0 | |
| Injection site stinging | 4 | 1 | |
| Other injection site complaints | 10 | 1 | |
| URI | 5 | 5 | |
| Other infection | 7 | 12 | |
| Other | 25 | 24 |
Numbers refer to numbers of patients with events.
P-value for comparison of numbers of events by group by Pearson Chi-Square. The study was not powered to detect differences in adverse events, and p-values are shown only for aggregate events.
One individual in the placebo group and four in the tesamorelin group had events that met a priori protocol criteria for investigator discontinuation (P=0.17), including two discontinuations for hyperglycemia (2-week and 6-month visits) in the tesamorelin group. One had known diabetes at baseline. Other reasons for discontinuation are outlined in Figure 1.
Discussion
Our data demonstrate that tesamorelin reduces liver fat content in PLWH with NAFLD and further suggest it prevents a high rate of progression of fibrosis, and improves inflammatory indices, such as CRP. In this group recruited for NAFLD, we saw a broad range of baseline NAS scores, and tesamorelin-treated patients with higher NAS scores at baseline had greater reductions in NAS score with tesamorelin. Additionally, in those with elevated ALT at baseline, ALT decreased with tesamorelin. Importantly, tesamorelin did not worsen insulin sensitivity. Collectively, these data suggest a significant benefit of tesamorelin among PLWH with NAFLD.
In the larger context of non-AIDS-related comorbidities, chronic liver disease causes substantial morbidity and mortality in PLWH (18). Although estimates of prevalence vary from 13–60%, NAFLD is likely present in 25% or more of PLWH, with an increased prevalence in those with visceral or generalized obesity (6, 19, 20). NAFLD is expected to soon become the leading cause of liver-related morbidity and mortality in PLWH (21). In the general population, lifestyle modification leads to reduction in liver fat content and amelioration of steatohepatitis and is currently the cornerstone of therapy for NAFLD (22). However, weight loss of up to 5–10% may be needed in this regard, and data from the general population may not be generalizable to the HIV population, in whom ectopic adipose tissue, dysfunctional subcutaneous adipose tissue, and excessive immune activation are all seen (22, 23). At present, there are no widely accepted pharmacologic strategies to treat NAFLD and NASH. Vitamin E reduces ALT and improves histologic features of inflammation, and some Societies recommend it for non-diabetic adults with NASH in the general population, but there is concern from meta-analyses that long-term use may modestly increase all-cause mortality (22, 24). Pioglitazone, a peroxisome proliferator-activated receptor (PPAR) gamma agonist, also improves features of steatohepatitis, but use is typically accompanied by weight gain (24). Obeticholic acid is a farnesoid X receptor (FXR) agonist that improved steatohepatitis in earlier trials but also increased LDL-C and decreased HDL-C (25). Recent Phase 3 results are reportedly consistent with earlier trials, both in terms of improvement in steatohepatitis and increase in LDL-C (26). Other agents with varying mechanisms of action are being investigated, but none is thus far approved. Moreover, with few exceptions, these studies have been performed in the general population, and data for the HIV population are extremely limited.
Considering our results in the context of existing and emerging treatment strategies, tesamorelin is the only agent thus far to show efficacy in reducing liver fat and preventing fibrosis progression specifically in HIV. To our knowledge, the only other pharmacologic strategy that has been specifically tested for NAFLD in PLWH is the bile acid conjugate arachidyl amido cholanoic acid (Aramchol), which did not reduce liver fat in 50 PLWH with lipodystrophy treated for 12 weeks (27). Tesamorelin is currently FDA approved to reduce visceral fat in PLWH with central adiposity. Our results now suggest that it may be beneficial among the larger group of PLWH with NAFLD.
The mechanisms whereby liver steatosis in NAFLD progresses to fibrosis and steatohepatitis are not known, nor is the natural history of these changes well defined in longitudinal studies among PLWH. The data from our study show for the first time that a strategy aimed mechanistically at reducing liver fat has significant effect to prevent changes in liver histopathology in PLWH. Tesamorelin may reduce liver fat via multiple mechanisms. One of the most important physiological effects of GH is to increase the activity of hormone sensitive lipase, a key lipolytic enzyme present in adipose tissue, liver, muscle, and other tissues. With present data, it is unclear if increased lipolysis in the liver, in adipose tissue, or both are responsible for net reductions in liver fat, and the tissue-specific mechanisms of tesamorelin require further study.
Fibrosis stage is the strongest predictor of mortality in patients with NAFLD, and FDA guidance on treatments for NAFLD stresses the importance not only of liver fat reduction, but also of prevention of fibrosis and reduction of inflammation (28). We performed liver biopsies before and after treatment and show that tesamorelin prevented progression of fibrosis. Moreover, reductions in liver fat were associated with reductions in fibrosis in a population with strictly defined NAFLD. Such an effect might prevent the development of cirrhosis in PLWH. Future studies are needed to further explore clinical outcomes in the context of liver fat reduction and progression of fibrosis in this population. Uniquely, our data suggest a large percentage, 38%, of HIV patients with NAFLD in the placebo group, demonstrate progression of fibrosis over 1 year. This is an important finding and highlights the critical need for a therapy to prevent fibrosis progression in this population.
In the current study, two individuals in the tesamorelin group, one with baseline diabetes, required discontinuation for worsening hyperglycemia. Among the entire cohort, however, tesamorelin was neutral to glucose homeostasis over the 12-month study period, and clinically relevant hyperglycemia events were well-balanced between the placebo and tesamorelin group. These results are consistent with our previous studies, which show a modest worsening of insulin resistance in the first few weeks to months of tesamorelin treatment, followed by a return to baseline over longer term therapy (14, 29). In prior studies aggregating Phase III clinical trial data, the degree of VAT reduction was associated with lowering of HbA1c (29), suggesting that ongoing reduction in ectopic fat, as well as increasing lean body mass, may ultimately counterbalance initial increases in glucose. Nonetheless, caution should be used in patients with NAFLD and significant baseline dysglycemia, and a minority of patients may not be able to continue tesamorelin due to hyperglycemia. Clinically tesamorelin has been safely used in the HIV population for almost a decade, but patients with NAFLD may have more severe glucose derangements, and assessment of glucose early after intervention in such patients is prudent. Importantly, we did not test whether simultaneous antidiabetic strategies to lower glucose and insulin might be useful to allow the small subset experiencing dysglycemia to continue with tesamorelin and maintain normoglycemia. Future studies should address this issue, given the potential value of reducing fibrosis progression in such patients.
This study was randomized, placebo-controlled, and included gold standard imaging and pre and post biopsy sampling, as well the performance of euglycemic hyperinsulinemic clamp. We recruited for NAFLD as prespecified in the study protocol, and the study was powered a priori to detect changes in liver fat. In contrast, the study was not designed as a therapeutic trial for NASH, not all patients had significant steatohepatitis, and there was a range of baseline NAS scores. Therefore, we could not definitively assess effects on endpoints recommended by the FDA as appropriate for late-stage studies of NASH therapeutics, namely improvements in histological evidence of fibrosis, or inflammation, or both. Further studies specifically recruiting for NASH are now indicated. Our data demonstrate an effect of tesamorelin to prevent progression of fibrosis among PLWH with NAFLD, with strong relationships between reductions in liver fat and fibrosis. Importantly, this suggests that the strategy of tesamorelin, aimed mechanistically at reducing liver fat, may be useful to prevent liver fibrosis in PLWH. Studies of individuals achieving visceral fat reduction with tesamorelin demonstrate that visceral fat reaccumulates after discontinuation of treatment (30), and studies investigating the utility of tesamorelin in NAFLD/NASH will need to investigate whether effects are durable beyond the treatment period.
In conclusion, our data demonstrate that tesamorelin, currently FDA approved for reduction of abdominal fat accumulation in PLWH, robustly decreases liver fat, while also preventing fibrosis progression, in association with improvement in indices of liver inflammation among PLWH with NAFLD. The treatment is generally well tolerated, and future studies are needed to further define the clinical role of tesamorelin with respect to NAFLD in PLWH, and potentially other populations.
Supplementary Material
Research in Context.
Evidence before this study
We searched PUBMED without date restriction or language restriction for the terms “NAFLD and HIV” and “tesamorelin and HIV”. Nonalcoholic fatty liver disease (NAFLD) is highly prevalent in people living with HIV (PLWH) and may have a more aggressive natural history than in those without HIV-infection. There are currently no approved pharmacotherapies for NAFLD in HIV. Growth hormone (GH) increases lipolysis and suppresses de novo lipogenesis in the liver. Tesamorelin, a GH releasing hormone analog, increases endogenous pulsatile GH production and reduces visceral fat in people with HIV. A previous study demonstrated that tesamorelin reduces liver fat content in people with HIV and abdominal adiposity, but its effects on liver fat content and liver histopathology in PLWH chosen based on strictly defined NAFLD are unknown.
Added value of this study
This study demonstrates that tesamorelin reduces liver fat content in men and women with HIV and NAFLD. Further, tesamorelin significantly attenuated the high rate of fibrosis progression that was seen in the placebo-treated group and was well-tolerated. Tesamorelin improved systemic inflammation as measured by c-reactive protein, and reductions in liver fat with tesamorelin were associated with improvements in fibrosis.
Implications of all the available evidence
In individuals with HIV and NAFLD, tesamorelin decreases liver fat content and reduces progression of fibrosis. These data have a number of implications for research and clinical practice. This study suggests that a strategy mechanistically targeting liver fat reduction may provide a key long-term clinical benefit in terms of preventing fibrosis progression in HIV. Tesamorelin may be an important new strategy for people with HIV and NAFLD, simultaneously reducing liver and visceral fat, and improving critical inflammatory indices in this population. Future studies are needed to further define effects of tesamorelin on the histopathological features of steatohepatitis.
Acknowledgements
We would like to thank the participants of the study for their willingness to volunteer their time and effort and their dedication to research.
Funding
The study was funded by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (U01 AI115711). This research was also supported in part by the Intramural Research Program of the NIH, National Cancer Institute and the Nutrition Obesity Research Center at Harvard (P30 DK040561), and by Grant Numbers 1UL1TR002541-01 and 1UL1TR001102. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Theratechnologies provided study material under a material transfer agreement and had no role in the drafting of the manuscript.
Takara L. Stanley has received funding from Novo Nordisk for an investigator-initiated grant unrelated to the current project.
Kathleen Corey has received grant funding from BMS and Boehringer Ingelheim as well as fees for consulting from Gilead and Novo Nordisk, unrelated to the current project.
Raymond Chung has received funding to the institution from Gilead, Merck, BMS, Janssen, Boehringer, and Roche unrelated to the current project.
Steven K. Grinspoon has served as a member of the Scientific Advisory Board and as a consultant to Theratechnologies. He has received research support, for an investigator-initiated project-unrelated to this project from Theratechnologies. Dr. Grinspoon is the named inventor on a patent application on the effects of tesamorelin in the treatment of hepatic disease.
Footnotes
Declaration of Interests
Lindsay T. Fourman has nothing to declare.
Meghan N. Feldpausch has nothing to declare.
Julia Purdy is an employee of NIH and has nothing to declare.
Isabel Zheng has nothing to declare.
Chelsea Pan has nothing to declare.
Julia Aepfelbacher is an employee of NIH and has nothing to declare.
Colleen Buckless has nothing to declare.
Andrew Tsao has nothing to declare.
Anela Kellogg was an employee of NIH at the time of the work and has nothing to declare.
Karen Branch has nothing to declare.
Hang Lee has nothing to declare.
Chia-Ying Liu has nothing to declare.
Martin Torriani has nothing to disclose.
David E. Kleiner is an employee of NIH and has nothing to declare.
Colleen M. Hadigan is an employee of NIH and has nothing to declare.
Data Sharing Statement
Research data, with all patient identifiers removed, will be available as per NIH policy to other researchers through request to the PI.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rockstroh JK. Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH) in HIV. Curr HIV/AIDS Rep. 2017;14(2):47–53. [DOI] [PubMed] [Google Scholar]
- 2.Vodkin I, Valasek MA, Bettencourt R, Cachay E, Loomba R. Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: a case-control study. Aliment Pharmacol Ther. 2015;41(4):368–78. [DOI] [PubMed] [Google Scholar]
- 3.Chamroonkul N, Bansal MB. HIV and the liver. Nat Rev Gastroenterol Hepatol. 2019;16(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guaraldi G, Squillace N, Stentarelli C, Orlando G, D’Amico R, Ligabue G, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47(2):250–7. [DOI] [PubMed] [Google Scholar]
- 5.van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48(2):449–57. [DOI] [PubMed] [Google Scholar]
- 6.Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS. 2017;31(11):1621–32. [DOI] [PubMed] [Google Scholar]
- 7.Lake JE, Stanley TL, Apovian CM, Bhasin S, Brown TT, Capeau J, et al. Practical Review of Recognition and Management of Obesity and Lipohypertrophy in Human Immunodeficiency Virus Infection. Clin Infect Dis. 2017;64(10):1422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rietschel P, Hadigan C, Corcoran C, Stanley T, Neubauer G, Gertner J, et al. Assessment of growth hormone dynamics in human immunodeficiency virus-related lipodystrophy. J Clin Endocrinol Metab. 2001;86(2):504–10. [DOI] [PubMed] [Google Scholar]
- 9.Koutkia P, Canavan B, Breu J, Grinspoon S. Growth hormone (GH) responses to GH-releasing hormone-arginine testing in human immunodeficiency virus lipodystrophy. J Clin Endocrinol Metab. 2005;90(1):32–8. [DOI] [PubMed] [Google Scholar]
- 10.Makimura H, Stanley T, Mun D, You SM, Grinspoon S. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab. 2008;93(11):4254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falutz J, Allas S, Blot K, Potvin D, Kotler D, Somero M, et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med. 2007;357(23):2359–70. [DOI] [PubMed] [Google Scholar]
- 12.Falutz J, Mamputu JC, Potvin D, Moyle G, Soulban G, Loughrey H, et al. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. J Clin Endocrinol Metab. 2010;95(9):4291–304. [DOI] [PubMed] [Google Scholar]
- 13.Stanley TL, Chen CY, Branch KL, Makimura H, Grinspoon SK. Effects of a Growth Hormone-Releasing Hormone Analog on Endogenous GH Pulsatility and Insulin Sensitivity in Healthy Men. J Clin Endocrinol Metab. 2011;96(1):150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanley TL, Feldpausch MN, Oh J, Branch KL, Lee H, Torriani M, et al. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA. 2014;312(4):380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. [DOI] [PubMed] [Google Scholar]
- 16.Braun LR, Feldpausch MN, Czerwonka N, Weiss J, Branch K, Lee H, et al. Effects of Pitavastatin on Insulin Sensitivity and Liver Fat: A Randomized Clinical Trial. J Clin Endocrinol Metab. 2018;103(11):4176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgoff P, Thomasson D, Louie A, Fleischman E, Dutcher L, Mani H, et al. Hydrogen-1 MR spectroscopy for measurement and diagnosis of hepatic steatosis. AJR Am J Roentgenol. 2012;199(1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384(9939):241–8. [DOI] [PubMed] [Google Scholar]
- 19.Iogna Prat L, Roccarina D, Lever R, Lombardi R, Rodger A, Hall A, et al. Etiology and Severity of Liver Disease in HIV-Positive Patients With Suspected NAFLD: Lessons From a Cohort With Available Liver Biopsies. J Acquir Immune Defic Syndr. 2019;80(4):474–80. [DOI] [PubMed] [Google Scholar]
- 20.Price JC, Seaberg EC, Latanich R, Budoff MJ, Kingsley LA, Palella FJ Jr., et al. Risk factors for fatty liver in the Multicenter AIDS Cohort Study. Am J Gastroenterol. 2014;109(5):695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tafesh ZH, Verna EC. Managing nonalcoholic fatty liver disease in patients living with HIV. Curr Opin Infect Dis. 2017;30(1):12–20. [DOI] [PubMed] [Google Scholar]
- 22.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver isease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–609. [DOI] [PubMed] [Google Scholar]
- 23.Erlandson KM, Lake JE. Fat Matters: Understanding the Role of Adipose Tissue in Health in HIV Infection. Curr HIV/AIDS Rep. 2016;13(1):20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanyal AJ. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younossi Z, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. , editors. Positive results from REGENERATE: a Phase 3 international, randomized, placebo-controlled study evaluating obeticholic acid treatment for NASH. The International Liver Congress, 2019; 2019; Vienna, Austria: Journal of Hepatology; 2019. [Google Scholar]
- 27.Ajmera VH, Cachay E, Ramers C, Vodkin I, Bassirian S, Singh S, et al. MRI Assessment of Treatment Response in HIV-associated NAFLD: A Randomized Trial of a Stearoyl-Coenzyme-A-Desaturase-1 Inhibitor (ARRIVE Trial). Hepatology. 2019. Epub ahead of print 23 Apr 2019. Doi: 10.1002/hep.30674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–54. [DOI] [PubMed] [Google Scholar]
- 29.Stanley TL, Falutz J, Marsolais C, Morin J, Soulban G, Mamputu JC, et al. Reduction in visceral adiposity is associated with an improved metabolic profile in HIV-infected patients receiving tesamorelin. Clin Infect Dis. 2012;54(11):1642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falutz J, Allas S, Mamputu JC, Potvin D, Kotler D, Somero M, et al. Long-term safety and effects of tesamorelin, a growth hormone-releasing factor analogue, in HIV patients with abdominal fat accumulation. AIDS. 2008;22(14):1719–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.