Abstract
The central nervous system maintains the potential for molecular and cellular plasticity throughout life. This flexibility underlies fundamental features of neural circuitry including the brain’s ability to sense, store, and properly adapt to everchanging external stimuli on time scales from seconds to years. Evidence for most forms of plasticity are centered around changes in neuronal structure and synaptic strength, however recent data suggests that myelinating oligodendrocytes exhibit certain forms of plasticity in the adult. This plasticity ranges from the generation of entirely new myelinating cells to more subtle changes in myelin sheath length, thickness, and distribution along axons. The extent to which these changes dynamically modify axonal function and neural circuitry and whether they are directly related to mechanisms of learning and memory remains an open question. Here we describe different forms of myelin plasticity, highlight some recent evidence for changes in myelination throughout life, and discuss how defects in these forms of plasticity could be associated with cognitive decline in aging.
Introduction
A principal regulator of axonal conduction in the central nervous system is myelination, a multi-layered extension of compacted cell membrane formed by glial cells called oligodendrocytes. The tight wrapping of oligodendrocyte cell membrane around neuronal axons forms a structure that decreases axonal membrane capacitance and allows for saltatory conduction of action potentials. Myelin sheath generation, stability, length and thickness are all tightly regulated through molecular and biophysical cues arising from the axon and the local microenvironment [1-3]. Changes to any of these elements has the potential to modulate conduction velocity, an often overlooked but critical feature that can be used to precisely modify the timing and synchrony of signal arrival at distinct postsynaptic targets [4-6]. Differential conduction velocity accounts for a range of emergent neural circuit functions including, coincidence detection for sound localization in the auditory brainstem [7,8], electric organ discharge in electric fish [9], olivocerebellar processing in the cerebellum [10], thalamocortical processing in the somatosensory cortex [11], and timing of retinal ganglion cell inputs to the brain [12]. These examples likely reflect a widespread feature exploited by neural circuits to accomplish particular tasks by tuning conduction velocity to match circuit demands. Importantly, even with these examples, evidence for functional plasticity at the level of conduction velocity and how these types of signals are disrupted in disease is largely lacking. Understanding how fixed or flexible these structures are is critical to fully appreciate the mechanisms of neural network plasticity.
Oligodendrocytes are generated from a population of resident progenitor cells called NG2 glia (also commonly referred to as oligodendrocyte progenitor cells or OPCs) [13-15]. NG2 glia are resident in the adult brain and maintain the capacity to differentiate into mature myelinating oligodendrocytes. Oligodendrocyte differentiation is tightly regulated in different brain regions and at different developmental stages via a number of mechanisms, including differences in local axonal subtypes, neuronal activity, and phenotypic differences between local NG2 glia populations [2,16,17]. It is likely that these factors directly contribute to differences in the plasticity of myelin between gray and white matter regions.
Here we discuss and define the different types of plasticity exhibited by myelinating oligodendrocytes (summarized in Figure 1). We outline what is currently known and yet to be discovered about these different forms of plasticity and we discuss how defects in myelination and mechanisms of plasticity could be involved in age-related cognitive decline.
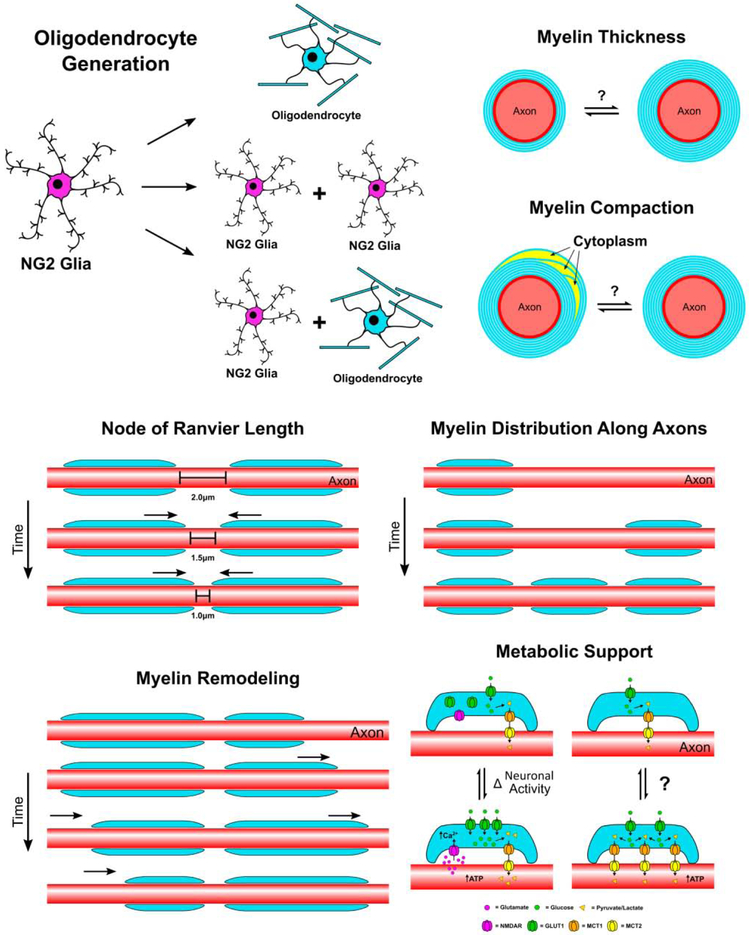
Figure 1: Forms of myelin plasticity.
Myelinating oligodendrocytes maintain the capacity for multiple forms of plasticity in the adult central nervous system. First NG2 glia act as an endogenous source of new oligodendrocytes while maintaining a resident population via self-renewal. This allows for population homeostasis as NG2 glia would otherwise be depleted over time through direct differentiation into mature oligodendrocytes. In addition to direct differentiation, recently divided NG2 glia receive signals to differentiate or remain at the progenitor stage. After terminal differentiation, the myelin sheath is potentially capable of additional forms of structural and molecular plasticity including changes in myelin thickness, degrees of myelin compaction, length and positioning of the myelin sheath, and length of individual nodes of Ranvier. In addition to myelin structural changes, expression and distribution of molecules and transporters involved in providing metabolic support to myelinated axons have the potential to change in response to neuronal activity and throughout life. Each of these forms of plasticity, ranging from the generation of new oligodendrocytes do more subtle changes in node length, have the potential to modulate conduction velocity, neural circuitry, and axonal health.
Oligodendrocyte generation
The predominant form of myelin plasticity stems from the ability of oligodendrocytes to continuously be generated via terminal differentiation of NG2 glia (Figure 1). Because of this, oligodendrocyte production and subsequent formation of new myelin internodes is not limited to a specific developmental window, potentially allowing for the myelination of previously unmyelinated or partially myelinated axons. Pulse chase fate-mapping experiments using thymidine analogue labeling of dividing cells and transgenic labeling of NG2 glia using the cre/lox technique have provided the most direct evidence for the continued differentiation of oligodendrocytes in vivo [18-23]. Overall, these studies have found that in the postnatal brain NG2 glia differentiate exclusively into myelinating oligodendrocytes and patterns of differentiation vary between different white matter tracts (i.e. optic nerve and corpus callosum) and between white and gray matter regions. Differentiation sustains throughout development but declines somewhat in adulthood. It is thought that the differences between brain region and across various ages are due to differences in ion channel expression, epigenetic signatures, resident glial phenotypes and axonal populations, and sensitivity to various growth factors [24-28].
A major question remaining from these studies is whether the generation of new cells is for the replacement of dying oligodendrocytes or results in long-term accumulation of new myelination in the adult. One way to test this is to analyze the generation of oligodendrocytes in the adult optic nerve, a white matter tract that is almost completely myelinated during development [29]. Tracking the fate of NG2 glia in adult mice revealed that approximately 6% of all oligodendrocytes were generated between 4 and 5.5 months [21] despite evidence that 99% of all axons would already be myelinated at 4 months [29]. Is this indicative of replacement of dying oligodendrocytes or de-novo myelination of the small population of unmyelinated axons? A subsequent study analyzed the long-term stability of oligodendrocytes generated in the optic nerve starting at 2 months and found a significant loss of oligodendrocytes over the next 4 months [30]. Taken together these studies provide evidence for oligodendrocyte replacement in the adult in a region that is almost completely myelinated. Interestingly the oligodendrocytes generated after 4 months of age produced more myelin sheaths that were shorter on average compared to those generated at 2 months [21]. Similar differences in internode length have been found in aging [31] as discussed below. Thus, in order to remyelinate or replace the myelin originally made by the dying cell, multiple internodes (from multiple oligodendrocytes?) may be required. As internode length and distribution are critically important for conduction velocity these changes could theoretically result in altered axonal conduction after remyelination has occurred.
In contrast to the optic nerve, fate mapping analyses of oligodendrocyte generation and death in the corpus callosum and cortex, regions which are incompletely myelinated, have shown continued production of new oligodendrocytes at least extending to 1 year of age [32] coincident with the long-term stability of the cells generated in early adulthood [18,20,23,30]. Consistent with this, recent work in human tissue has demonstrated continued generation of new oligodendrocyte in the cerebral cortex [33]. These data suggest that oligodendrocyte generation persists and potentially results in myelination of previously unmyelinated axons. Intravital time-lapse imaging of oligodendrocytes, myelin, and axons in the adult mouse cortex has now directly shown the long-term stability of mature oligodendrocytes in addition to the continued generation of new oligodendrocytes and myelin on previously unmyelinated axons [34,35]. These live imaging experiments did not reveal any evidence for oligodendrocyte or internode replacement, however they were limited to the upper layers of the cerebral cortex and extended for shorter time periods compared to the transgenic fate mapping experiments conducted in fixed tissue. It has been suggested that mature oligodendrocytes can produce new myelinating internodes however direct evidence of this is currently lacking and might occur only in disease contexts [36,37].
Signals directly inducing the formation of new oligodendrocytes in the adult brain are not well defined however there is growing evidence to suggest that new cells are not produced stochastically but rather in a targeted manner based on environmental cues. Neuronal activity [38-43], social isolation [44,45], and sensory stimulation [46,35,19,47,48] have all been shown to influence NG2 glia proliferation and oligodendrogenesis. The precise molecular cues and signaling pathways involved in these changes are not clear and future studies will likely reveal how neuronal activity precisely modulates the differentiation of NG2 glia and formation of new myelinating oligodendrocytes and what consequence the formation of new myelin has on the neural circuitry.
Myelin distribution
Different locations of the CNS display differing degrees of myelination, depending on the timing and extent of oligodendrocyte generation. Axons in some white matter regions, such as the optic nerve, are rapidly and almost completely myelinated early in development while axons in other regions, like the cerebral cortex, display a greater degree of coverage heterogeneity with myelination proceeding into adulthood [29,49,50]. Fully myelinated axons can be in close proximity to other partially or fully unmyelinated axons and these differences can reflect distinct subtypes of both excitatory and inhibitory neurons. For example, significant diversity exists between pyramidal excitatory neurons within different cortical layers with upper layer neurons generally having patchier myelination compared to deeper layer neurons [50]. Moreover, distinct interneuron populations display variable myelination with fast-spiking parvalbumin expressing interneurons being the most heavily myelinated [51]. Recent data also suggests that single oligodendrocytes can exhibit preferential myelination of axons from either inhibitory or excitatory neurons [52]. This raises the possibility that there could be specific signals arising from subsets of neurons which signal to subpopulations of oligodendrocytes to initiate their myelination. The universality of these observations to multiple brain regions and in larger data sets must be determined. Furthermore, whether or not these data represent oligodendrocyte heterogeneity and how dynamic these myelin profiles are throughout life is yet to be resolved.
Intravital imaging of individual partially myelinated excitatory axons in layer I of the cortex has revealed that new internodes are continuously generated throughout development and into late adulthood, potentially signifying an ever-changing pattern of myelination across some axons [34,53]. Importantly, even at peak myelination in late adulthood some axons maintain their partial myelination patterns. This suggests that in some cases the action potential conduction down partially myelinated axons changes and in others it remains constant. It may also point to alternative functions of intermittent myelination, such as inhibiting axonal branch or synapse formation. Signals inducing these differential myelination patterns and how an oligodendrocyte decides to myelinate one portion of an axon and not another are unknown.
Axon diameter is one mechanism that is used to instruct myelination of some axons and not others. Experimentally increasing axon diameter via neuronal genetic deletion of Pten can induce myelination of axons that would not normally ever become myelinated [54]. In addition to increasing axon diameter, this manipulation also increased secretion of promyelinating factors such as brain derived neurotrophic factor. Thus, while there is clearly a diameter threshold and correlation between axonal diameter and propensity for myelination [55,56], direct evidence for diameter alone influencing myelin plasticity and distribution along axons is lacking. Likewise, there is no evidence that partially myelinated axons exhibit differential diameter at myelinated vs nonmyelinated regions.
Neuronal activity and local vesicular release from the axon could also potentially be used to construct a specific myelin distribution pattern. Consistent with this, chemogenetic modulation of activity in a subset of parvalbumin interneurons resulted in changes in myelin patterning specifically along the activated axons [57]. Importantly, however, these manipulations also resulted in changes in axonal arborization. Thus, at least in this study, it is not clear if the change in myelination was simply a secondary effect of greater axonal arborization that was permissive to myelination or specific activity-dependent signaling directly to oligodendrocytes initiating the formation of new myelin sheaths. This provides an important example showing that axonal structural plasticity must be considered when performing and interpreting experiments correlating changes in neuronal activity with myelin plasticity. While secondary changes in myelination would have an effect, a primary change in axonal structure is likely more significant for influencing neural circuitry.
Incomplete myelination along stretches of single axons could play several functional roles. One possibility is that it may permit overall circuit flexibility by balancing plasticity potential while utilizing the speed and efficiency benefits of myelination at distinct locations. A fully myelinated axon would have insufficient capacity for the generation of new axonal branches, synapses, and myelin internodes and thus a decreased ability to respond to environmental stimuli via structural modifications and remodeling. Another possibility is that the deposition of new myelin sheaths on previously unmyelinated axons offsets changes in axonal metabolic demands. Myelination has been shown to provide metabolic substrates to axons [58]. This support has been shown to be modulated, at least in part, by changes in neuronal activity and neurotransmitter release. Oligodendrocytes are able to sense glutamate and traffic GLUT-1 transporters to the plasma membrane, increasing intracellular glucose and lactate production which can then be shuttled to ensheathed axons (Figure 1) [59]. It is possible that over time the demands or intrinsic capability of the axon to generate its own substrates declines and deposition of new myelin can compensate and maintain proper axonal function.
Myelin thickness
Myelin’s ability to reduce the membrane capacitance of an axon is directly related to its thickness. Increased thickness bolsters the ability of the internode to insulate the covered axon and facilitate conduction. Axon caliber has long been thought to directly dictate sheath thickness in order to optimize functionality across morphologically distinct axons [60]. However, evidence has suggested that thickness may not be static and thus is not wholly dictated by axon diameter. Many of the factors shown to increase NG2 glia proliferation and oligodendrocyte formation, such as changes in neuronal activity or social environment, also result in changes in myelin sheath thickness [39,40,45]. Because thickness measurements are generally obtained through electron microscopy, it is unclear whether these factors influence thickness of newly produced internodes, established internodes, or a combination of the two. Even so, all cases could result in modulation of axonal conduction and thus contribute to overall myelin plasticity.
One possible mechanism governing changes in myelin thickness is a change in neuronal activity. Optogenetic and chemogenetic induced neuronal hyperstimulation have both been shown to result in increased myelin thickness [39,40]. Chronic light stimulation of cortical layer V projection neurons expressing the excitatory opsin ChR2 elicited a significant increase in myelin thickness [39]. Likewise, chronic chemogenetic neuronal stimulation via the activating modified g-protein coupled receptor, hM3Dq, also resulted in an increase in myelin thickness, as seen by a decrease in average g-ratio [40]. This effect was observed in both 3-week-old and 10-week-old mice, despite being slightly less pronounced in the older cohort. Furthermore, hM3Dq activated axons were more likely to be myelinated as compared to control in both age groups. Importantly, for both studies it is not clear if preexisting myelin sheaths increased their thickness or if newly generated myelin from recently differentiated NG2 glia accounted for the thicker myelin sheaths. A separate study attempted to investigate this question by examining the effects of upregulated ERK1/2 signaling specifically in mature oligodendrocytes [61]. Conditionally expressing constitutively active MEK1 protein in Proteolipid Protein (PLP) positive cells significantly increased myelin thickness in the corpus collosum and spinal cord, suggesting that mature oligodendrocytes may be able to regulate pre-existing sheath thickness. In contrast to increases in activity, decreases in neuronal activity in the optic nerve via monocular deprivation did not cause a decrease in myelin thickness [48], while social isolation did result in decreased myelin thickness in the prefrontal cortex [44,45]. Reasons for these discrepancies are not clear, other than the obvious differences in brain region and sensory manipulation. Also, it is not clear if the decrease in thickness in the social isolation experiments reflects a difference in newly formed vs preexisting myelin. Thus, direct physiological evidence for dynamic changes in myelin thickness in a compact myelin sheath is not yet available.
Myelin compaction
In addition to thickness, membrane compaction between individual layers and extrusion of the oligodendrocyte cytoplasm are also critically important for the function of myelin. Direct evidence for this comes from myelin basic protein deficient mice which have a defect in establishing compact myelin and develop a severe shivering phenotype [62]. In the peripheral nervous system, the myelin sheath maintains distinct cytoplasmic channels called Schmidt-Lanterman Incisures within an otherwise compact myelin sheath. These channels are thought to be important for transport of molecules throughout the myelin sheath and potentially also for transport and signaling between the Schwann cell and underlying axon. It was previously believed that such channels did not exist in CNS myelin, however recent studies using electron microscopy of high-pressure frozen tissue in order to maintain cytoarchitecture have provided evidence of these channels throughout the CNS [63-65] and confirm previous studies suggesting the presence of these channels via dye injections in the spinal cord [66]. The precise function of these channels is not clear but their maintenance is critical as transgenic mice with 2,’3’-cyclicnucleotide 3’-phosphodiesterase (CNP) deletions that fail to form and/or maintain these channels exhibit pronounced myelin pathology and axonal degeneration [65,67].
Whether or not the cytoplasmic channels in CNS myelin are sites of plasticity is not known, although there is some evidence for age-dependent changes in their density and prevalence [64,65]. It is likely that newly formed myelin contains more non-compacted regions as the sheath is lengthening but it is also possible that a mature myelin sheath could maintain these channels and utilize them for communication with the axon or sheath remodeling as described in the next section. Development of methodologies for dynamic visualization and manipulation of these noncompact cytoplasmic channels is necessary in order to directly address these questions.
Myelin internode length
New myelin sheath formation occurs during a defined period, lasting several hours, during which time pre-myelinating oligodendrocytes sample surrounding axons and begin sheath extension [41,43,68,69]. A proportion of oligodendrocyte cell processes which initiate extension subsequently mature into a stable myelin sheath while the rest retract shortly after formation and are lost. This suggests the existence of multiple checkpoints regulating myelin production from initial axon selection through maturation and elongation. Physical characteristics of axons, such as axon diameter (as discussed above), have been implicated as larger diameter axons tend to be myelinated more quickly and to a greater extent than smaller axons. There is also some evidence to suggest that axon derived molecular cues, in the form of pro-/inhibitory cell surfaces markers and/or secreted molecules, may also play a role [70].
Activity-dependent vesicular release is one mode thought to be involved in the formation and stability of newly forming myelin segments [71]. Work in zebrafish has demonstrated that blocking vesicular release in spinal cord axons decreases the stability and length of newly forming myelin sheaths [41,43]. Interestingly, this appears to be important for some axonal populations and not others [72]. Subsequent work has identified calcium signaling as one important mechanism involved nascent sheath elongation. Two concurrent zebrafish studies, using the genetically encoded fluorescent calcium indicator GCaMP6, described high frequency calcium transients within newly formed sheaths promoted elongation, while long lasting, low frequency transients led to sheath retraction [73,74]. Regulation of such calcium fluctuations may be related to neuronal activity. Increasing activity via electrical stimulation was able to induce calcium fluctuations within oligodendrocytes and increased sheath extension by 60% while tetrodotoxin induced neuronal silencing decreased calcium fluctuations and sheath extension by 43% [74].
Once formed, some internodes retain the ability to extend or retract along the axon, changing the total length of the sheath. One study done in embryonic and larval zebrafish found that, while the majority of internode growth was completed within the 3 days of formation, sheaths were able to continue extending in a comparatively slow fashion after initial production. This slow growth was correlated with an increase in size of the developing fish, suggesting that internode extension could be compensating for increases in total axonal length [75]. Two separate studies, done in mice, have both described long-term evidence for myelin remodeling in layer I cortex via intravital fluorescence optical imaging [34,35]. By monitoring individual internodes over days to months a recent study revealed that some sheaths in mice between 2 and 3 months old exhibited length plasticity well after their initial formation While 81% of the sheaths monitored remained stable, 15% lengthened, extending outwards along unmyelinated sections of each axon and 4% retracted from an established position, decreasing their total length. Partially myelinated axons, where many internodes are not directly adjacent to each other, were more likely to exhibit this behavior, as 25% extended and 7% retracted [34]. Individual oligodendrocytes also displayed heterogeneous internode remodeling, with some extending, retracting or remaining stable, indicating that such plasticity is modulated locally, not across the whole cell. A parallel study characterized similar properties of myelin remodeling in 1-year old mice with differing results. In the aged animals, less than 1% of observed internodes underwent extension or retraction [35]. This difference in remodeling frequency described by the two studies may be explained by the age difference in the mice used. The cortex of a 2-month old mouse has a greater percentage of partially myelinated axons compared to 1-year old mouse [34] which likely has an effect on the dynamics of myelin plasticity. The precise mechanisms that govern these changes are unclear, however it seems likely that they would have a direct impact on axon membrane capacitance and conduction.
Node of Ranvier length
While myelin length, thickness, and compaction all play roles in facilitating axon conduction, structural changes at nodes of Ranvier may also play a role in tuning action potential velocity. A key characteristic of myelinated axons is the localization of voltage gated sodium channels to nodes of Ranvier [76]. In contrast, the same sodium channels are evenly distributed throughout the plasma membrane of unmyelinated axons. As the channels are confined to the nodes in myelinated axons, the number and density of channels at each node modulate the ability of the membrane to depolarize. Moreover, different node lengths, i.e. different sized gaps between adjacent internodes, would allow for differential densities of local sodium channels.
Experimental evidence has shown that there is heterogeneity in node length across different axons. Simulations modeling the effect of node length on AP propagation suggest that increasing node length from the shortest observed (0.5μm in the optic nerve and 0.43μm in the cortex) to the longest (1.7μm in both the optic nerve and cortex) can alter conduction speeds by up to 20% in the optic nerve or as much as 24% in cortical axons [77]. Interestingly, there is less length heterogeneity amongst nodes found on the same axon, suggesting a mechanism for regulation along individual axons. Because changes in node length can have substantial effects on conduction speeds, node remodeling in response to external stimuli would be a ready-made way of tuning neuronal circuitry. In fact, it has also been suggested that remodeling in this way could offer a more metabolically efficient means of tuning conduction, as retracting or extending two paranodes would require orders of magnitude less membrane flux than modifying internode length or thickness [77]. While direct evidence for such remodeling is not available, several studies have found significant alterations in nodal length after adverse stimuli including chronic stress [78], high frequency neuronal activity [79], acoustic over exposure [80], and cerebral vascular hypoperfusion [81]. Thus, future work, likely using live imaging, will reveal the extent to which nodes of Ranvier can remodel and if this influences action potential conduction in vivo.
Myelin plasticity in aging
Evidence for myelin defects can be found in disorders that present during development and adolescence such as schizophrenia, bipolar disorder, and autism [82,83] in addition to diseases associated with adulthood and aging such as Alzheimer’s disease [84,85] and amyotrophic lateral sclerosis [86,87]. However, even in normal aging there is significant evidence that myelin defects are an early pathological hallmark that occurs in oligodendrocytes before other cells in the brain exhibit signs of degeneration [31,88-90]. Age-related disruptions in white matter tracts have been reported using low-resolution magnetic resonance imaging (MRI) based approaches in humans [91,92] and specific myelin defects have been found in postmortem tissue from aged humans and other primates [93-96] in addition to rodents [31,97].
If myelin plasticity, as defined in the previous sections, is necessary for neural network homeostasis than any interruption in these processes would result in a degenerative cascade ultimately resulting in severe cognitive decline [98]. Reasons for specific oligodendrocyte vulnerability in aging are not clear however several possibilities have been proposed including decreased remyelination capacity from reduced NG2 glia differentiation [99], unique metabolic demands specific to myelinating oligodendrocytes [100,101], and differential vulnerability of oligodendrocytes to oxidative damage [102] among other potential mechanisms [103].
Aged NG2 glia exhibit decreased oligodendrocyte differentiation in several contexts. First, baseline homeostatic oligodendrocyte generation declines with age in a region-dependent manner [18,21,22]. If myelin maintenance relies on oligodendrocyte replacement, as suggested by some studies in the optic nerve [21], then decreased oligodendrocyte production in advanced aging would result in net myelin loss. On top of this, there is evidence that oligodendrocytes generated in older animals produce shorter and fewer myelin sheaths [31]. This means that the loss of a single oligodendrocyte in the aged brain might require the production of multiple oligodendrocytes to reestablish the myelin pattern. This effect would be compounded in situations where myelin is damaged. If the regenerative capacity of oligodendrocytes is compromised in aging, then incomplete myelin repair could occur in both acute brain injury and in chronic myelin diseases. Chronic stages of multiple sclerosis are characterized by incomplete remyelination [104] presumably due to the inability of NG2 glia to differentiate [99]. Direct age effects on oligodendrocyte differentiation capacity in animal models have been demonstrated via parabiosis [105] and cell transplant [27] experiments suggesting that the brain microenvironment and current state of the cell influence the age-dependent changes in NG2 glia behavior. Cellular state mechanisms may include alteration in the epigenetic status of NG2 glia [28,106,107] while microenvironmental factors could include both changes in growth factor signaling, inflammatory state of other glia [108], tissue stiffness [109], and accumulation of myelin debris [34,97,110]. There is evidence that each of these signals can influence oligodendrocyte generation. Identification of the molecular mechanisms involved in the effects of age on NG2 glia differentiation will likely provide useful therapeutic targets in the future.
Outside oligodendrocyte differentiation not much is known about the effects of age on myelin plasticity. Accumulation of myelin debris in the aging brain has the potential to impact myelin sheath length remodeling, axonal plasticity, and node of Ranvier stability. Moreover, myelin decompaction could occur without immediate oligodendrocyte loss but with similar functional consequences in terms of action potential conduction. Finally, even if structural defects are not present in the myelin sheath there could be age-related dysfunction of oligodendrocyte metabolic support to axons. Increasing evidence suggests that this often-underappreciated myelin function could be disrupted in aging [58,59]. It is possible that the ability of oligodendrocytes to shuttle lactate/pyruvate to axons is disrupted overtime due to changes in GLUT-1/MCT-1 density within myelin sheaths (Figure 1). Much work is needed to precisely dissect how age impacts these other forms of myelin plasticity and whether these cellular and molecular mechanisms can be translated to other neuropathological states.
Conclusions
Myelin plasticity encompasses a range of potential changes in myelin structure, density, and function. These include the generation of new oligodendrocytes from their endogenous progenitors NG2 glia, to changes in myelin length, distribution, thickness, and compaction (summarized in Figure 1). Each feature has the potential to modulate neural network function via alteration in synchronous arrival of signals at postsynaptic targets. The in vivo temporal dynamics and the distinct signals that induce each form of plasticity are just now being revealed. Notably the direct functional consequences of such changes are largely unknown.
Most evidence for myelin plasticity thus far comes from cell culture and transgenic fate mapping in fixed tissue. However, with the development of new label-free optical imaging approaches, such as Coherent Anti-Stokes Raman Scattering (CARS) , Optical Coherence Microscopy (OCM), Third Harmonic Generation (THG) , and Spectral Confocal Reflectance (SCoRe) microscopy, combined with new fluorescent labeling approaches for cellular observation and manipulation in vivo [111-116], we are at the early stages of understanding the extent to which myelin plasticity plays a role in homeostatic maintenance of brain function in addition to its potential mechanisms related to learning and memory. While more work is necessary to directly observe, define, and describe myelin plasticity in vivo, the next exciting challenge is to connect these cellular observations with neural circuit function and ultimately behavior.
Highlights.
Myelin plasticity occurs throughout development and adulthood
Myelin plasticity ranges from the generation of new oligodendrocytes to fine structural changes in the myelin sheath over extended periods
Various environmental cues can induce myelin plasticity
Defects in myelin maintenance are likely involved in age-related cognitive decline
Acknowledgements
This work was supported by the following grants from the National Institutes of Health: R00-NS099469 and P20-GM113132 and by a New Vision Award through the Donors Cure Foundation and a Fay/Frank Seed Grant from the Brain Research Foundation to R.A.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Osso LA, Chan JR, Architecting the myelin landscape., Curr. Opin. Neurobiol 47 (2017) 1–7. 10.1016/j.conb.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Emery B, Regulation of oligodendrocyte differentiation and myelination., Science. 330 (2010) 779–782. 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- [3].Almeida RG, Lyons DA, On myelinated axon plasticity and neuronal circuit formation and function., J. Neurosci 37 (2017) 10023–10034. 10.1523/JNEUROSCI.3185-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zalc B, Goujet D, Colman D, The origin of the myelination program in vertebrates., Curr. Biol 18 (2008) R511–2. 10.1016/j.cub.2008.04.010. [DOI] [PubMed] [Google Scholar]
- [5].Waxman SG, Axon-glia interactions: building a smart nerve fiber., Curr. Biol 7 (1997) R406–10. 10.1016/S0960-9822(06)00203-X. [DOI] [PubMed] [Google Scholar]
- [6].Fields RD, A new mechanism of nervous system plasticity: activity-dependent myelination., Nat. Rev. Neurosci 16 (2015) 756–767. 10.1038/nrn4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Seidl AH, Rubel EW, Harris DM, Mechanisms for adjusting interaural time differences to achieve binaural coincidence detection., J. Neurosci 30 (2010) 70–80. 10.1523/JNEUROSCI.3464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Seidl AH, Rubel EW, Barría A, Differential conduction velocity regulation in ipsilateral and contralateral collaterals innervating brainstem coincidence detector neurons., J. Neurosci 34 (2014) 4914–4919. 10.1523/JNEUROSCI.5460-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bennett MV, Comparative physiology: electric organs., Annu. Rev. Physiol 32 (1970) 471–528. 10.1146/annurev.ph.32.030170.002351. [DOI] [PubMed] [Google Scholar]
- [10].Sugihara I, Lang EJ, Llinás R, Uniform olivocerebellar conduction time underlies Purkinje cell complex spike synchronicity in the rat cerebellum., J. Physiol 470 (1993) 243–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Salami M, Itami C, Tsumoto T, Kimura F, Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex., Proc. Natl. Acad. Sci. U. S. A 100 (2003) 6174–6179. 10.1073/pnas.0937380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stanford LR, Conduction velocity variations minimize conduction time differences among retinal ganglion cell axons, Science. 238 (1987) 358–360. [DOI] [PubMed] [Google Scholar]
- [13].Nishiyama A, Komitova M, Suzuki R, Zhu X, Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity., Nat. Rev. Neurosci 10 (2009) 9–22. 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- [14].Bergles DE, Richardson WD, Oligodendrocyte Development and Plasticity, Cold Spring Harb. Perspect. Biol 8 (2015) a020453 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hill RA, Nishiyama A, NG2 cells (polydendrocytes): listeners to the neural network with diverse properties., Glia. 62 (2014) 1195–1210. 10.1002/glia.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zuchero JB, Barres BA, Intrinsic and extrinsic control of oligodendrocyte development., Curr. Opin. Neurobiol 23 (2013) 914–920. 10.1016/j.conb.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dimou L, Götz M, Glial cells as progenitors and stem cells: new roles in the healthy and diseased brain, Physiol. Rev 94 (2014) 709–737. 10.1152/physrev.00036.2013. [DOI] [PubMed] [Google Scholar]
- [18].Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A, Age-dependent fate and lineage restriction of single NG2 cells., Development. 138 (2011) 745–753. 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Simon C, Götz M, Dimou L, Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury., Glia. 59 (2011) 869–881. 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- [20].Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE, NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration., Neuron. 68 (2010) 668–681. 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Young KM, Psachoulia K, Tripathi RB, Dunn S-J, Cossell L, Attwell D, Tohyama K, Richardson WD, Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling., Neuron. 77 (2013) 873–885. 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dawson MRL, Polito A, Levine JM, Reynolds R, NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS., Mol. Cell. Neurosci 24 (2003) 476–488. 10.1016/S1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- [23].Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M, Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex., J. Neurosci 28 (2008) 10434–10442. 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dimou L, Simons M, Diversity of oligodendrocytes and their progenitors, Curr. Opin. Neurobiol 47 (2017) 73–79. 10.1016/j.conb.2017.09.015. [DOI] [PubMed] [Google Scholar]
- [25].Viganò F, Möbius W, Götz M, Dimou L, Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain., Nat. Neurosci 16 (2013) 1370–1372. 10.1038/nn.3503. [DOI] [PubMed] [Google Scholar]
- [26].Hill RA, Patel KD, Medved J, Reiss AM, Nishiyama A, NG2 cells in white matter but not gray matter proliferate in response to PDGF., J. Neurosci 33 (2013) 14558–14566. 10.1523/JNEUROSCI.2001-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Spitzer SO, Sitnikov S, Kamen Y, Evans KA, Kronenberg-Versteeg D, Dietmann S, de Faria O, Agathou S, Káradóttir RT, Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age, Neuron. 101 (2019) 459–471.e5. 10.1016/j.neuron.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJM, Casaccia-Bonnefil P, Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency., Nat. Neurosci 11 (2008) 1024–1034. 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Honjin R, Sakato S, Yamashita T, Electron microscopy of the mouse optic nerve: a quantitative study of the total optic nerve fibers, Arch. Histol. Jpn. Nihon Soshikigaku Kiroku 40 (1977) 321–332. [DOI] [PubMed] [Google Scholar]
- [30].Tripathi RB, Jackiewicz M, McKenzie IA, Kougioumtzidou E, Grist M, Richardson WD, Remarkable Stability of Myelinating Oligodendrocytes in Mice, Cell Rep. 21 (2017) 316–323. 10.1016/j.celrep.2017.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lasiene J, Matsui A, Sawa Y, Wong F, Horner PJ, Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes., Aging Cell. 8 (2009) 201–213. 10.1111/j.1474-9726.2009.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huang W, Zhao N, Bai X, Karram K, Trotter J, Goebbels S, Scheller A, Kirchhoff F, Novel NG2-CreERT2 knock-in mice demonstrate heterogeneous differentiation potential of NG2 glia during development., Glia. 62 (2014) 896–913. 10.1002/glia.22648. [DOI] [PubMed] [Google Scholar]
- [33].Fard MK, van der Meer F, Sánchez P, Cantuti-Castelvetri L, Mandad S, Jäkel S, Fornasiero EF, Schmitt S, Ehrlich M, Starost L, Kuhlmann T, Sergiou C, Schultz V, Wrzos C, Brück W, Urlaub H, Dimou L, Stadelmann C, Simons M, BCAS1 expression defines a population of early myelinating oligodendrocytes in multiple sclerosis lesions., Sci. Transl. Med 9 (2017). 10.1126/scitranslmed.aam7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hill R, Li A, Grutzendler J, Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain., Nat. Neurosci 21 (2018) 683–695. 10.1038/s41593-018-0120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE, Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex., Nat. Neurosci 21 (2018) 696–706. 10.1038/s41593-018-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yeung MSY, Djelloul M, Steiner E, Bernard S, Salehpour M, Possnert G, Brundin L, Frisén J, Dynamics of oligodendrocyte generation in multiple sclerosis, Nature. 566 (2019) 538–542. 10.1038/s41586-018-0842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Duncan ID, Radcliff AB, Heidari M, Kidd G, August BK, Wierenga LA, The adult oligodendrocyte can participate in remyelination, Proc. Natl. Acad. Sci. U. S. A 115 (2018) E11807–E11816. 10.1073/pnas.1808064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Barres BA, Raff MC, Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons., Nature. 361 (1993) 258–260. 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- [39].Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M, Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain., Science. 344 (2014) 1252304 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mitew S, Gobius I, Fenlon LR, McDougall SJ, Hawkes D, Xing YL, Bujalka H, Gundlach AL, Richards LJ, Kilpatrick TJ, Merson TD, Emery B, Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner, Nat. Commun 9 (2018) 306 10.1038/s41467-017-02719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B, Neuronal activity biases axon selection for myelination in vivo., Nat. Neurosci 18 (2015) 683–689. 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Stevens B, Tanner S, Fields RD, Control of myelination by specific patterns of neural impulses., J. Neurosci 18 (1998) 9303–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA, Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo., Nat. Neurosci 18 (2015) 628–630. 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Makinodan M, Rosen KM, Ito S, Corfas G, A Critical Period for Social Experience–Dependent Oligodendrocyte Maturation and Myelination, Science. 337 (2012) 1357–1360. 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P, Impaired adult myelination in the prefrontal cortex of socially isolated mice, Nat. Neurosci 15 (2012) 1621–1623. 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hill RA, Patel KD, Goncalves CM, Grutzendler J, Nishiyama A, Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division., Nat. Neurosci 17 (2014) 1518–1527. 10.1038/nn.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hill RA, Natsume R, Sakimura K, Nishiyama A, NG2 cells are uniformly distributed and NG2 is not required for barrel formation in the somatosensory cortex., Mol. Cell. Neurosci 46 (2011) 689–698. 10.1016/j.mcn.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Etxeberria A, Hokanson KC, Dao DQ, Mayoral SR, Mei F, Redmond SA, Ullian EM, Chan JR, Dynamic modulation of myelination in response to visual stimuli alters optic nerve conduction velocity., J. Neurosci 36 (2016) 6937–6948. 10.1523/JNEUROSCI.0908-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Stadelmann C, Timmler S, Barrantes-Freer A, Simons M, Myelin in the Central Nervous System: Structure, Function, and Pathology, Physiol. Rev 99 (2019) 1381–1431. 10.1152/physrev.00031.2018. [DOI] [PubMed] [Google Scholar]
- [50].Tomassy GS, Berger DR, Chen H-H, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, Arlotta P, Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex., Science. 344 (2014) 319–324. 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Micheva KD, Wolman D, Mensh BD, Pax E, Buchanan J, Smith SJ, Bock DD, A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons., ELife. 5 (2016). 10.7554/eLife.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zonouzi M, Berger D, Jokhi V, Kedaigle A, Lichtman J, Arlotta P, Individual Oligodendrocytes Show Bias for Inhibitory Axons in the Neocortex, Cell Rep. 27 (2019) 2799–2808.e3. 10.1016/j.celrep.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hill RA, Grutzendler J, Uncovering the biology of myelin with optical imaging of the live brain, Glia. (2019). 10.1002/glia.23635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Goebbels S, Wieser GL, Pieper A, Spitzer S, Weege B, Yan K, Edgar JM, Yagensky O, Wichert SP, Agarwal A, Karram K, Renier N, Tessier-Lavigne M, Rossner MJ, Káradóttir RT, Nave K-A, A neuronal PI(3,4,5)P3-dependent program of oligodendrocyte precursor recruitment and myelination., Nat. Neurosci 20 (2017) 10–15. 10.1038/nn.4425. [DOI] [PubMed] [Google Scholar]
- [55].Friede RL, Control of myelin formation by axon caliber (with a model of the control mechanism), J. Comp. Neurol 144 (1972) 233–252. 10.1002/cne.901440207. [DOI] [PubMed] [Google Scholar]
- [56].Lee S, Leach MK, Redmond SA, Chong SYC, Mellon SH, Tuck SJ, Feng Z-Q, Corey JM, Chan JR, A culture system to study oligodendrocyte myelination processes using engineered nanofibers., Nat. Methods 9 (2012) 917–922. 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Stedehouder J, Brizee D, Shpak G, Kushner SA, Activity-Dependent Myelination of Parvalbumin Interneurons Mediated by Axonal Morphological Plasticity, J. Neurosci. Off. J. Soc. Neurosci 38 (2018) 3631–3642. 10.1523/JNEUROSCI.0074-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nave K-A, Myelination and the trophic support of long axons., Nat. Rev. Neurosci 11 (2010) 275–283. 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- [59].Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Móbius W, Goetze B, Jahn HM, Huang W, Steffens H, Schomburg ED, Pérez-Samartín A, Pérez-Cerdá F, Bakhtiari D, Matute C, Löwel S, Griesinger C, Hirrlinger J, Kirchhoff F, Nave K-A, Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism, Neuron. 91 (2016) 119–132. 10.1016/j.neuron.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hildebrand C, Hahn R, Relation between myelin sheath thickness and axon size in spinal cord white matter of some vertebrate species, J. Neurol. Sci 38 (1978) 421–434. 10.1016/0022-510X(78)90147-8. [DOI] [PubMed] [Google Scholar]
- [61].Jeffries MA, Urbanek K, Torres L, Wendell SG, Rubio ME, Fyffe-Maricich SL, ERK1/2 Activation in Preexisting Oligodendrocytes of Adult Mice Drives New Myelin Synthesis and Enhanced CNS Function, J. Neurosci 36 (2016) 9186–9200. 10.1523/JNEUROSCI.1444-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chernoff GF, Shiverer: an autosomal recessive mutant mouse with myelin deficiency., J. Hered 72 (1981) 128 10.1093/oxfordjournals.jhered.a109442. [DOI] [PubMed] [Google Scholar]
- [63].Möbius W, Cooper B, Kaufmann WA, Imig C, Ruhwedel T, Snaidero N, Saab AS, Varoqueaux F, Chapter 20 - Electron Microscopy of the Mouse Central Nervous System, in: Müller-Reichert T (Ed.), Methods Cell Biol., Academic Press, 2010: pp. 475–512. 10.1016/S0091-679X(10)96020-2. [DOI] [PubMed] [Google Scholar]
- [64].Snaidero N, Möbius W, Czopka T, Hekking LHP, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, Nave K-A, Simons M, Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue., Cell. 156 (2014) 277–290. 10.1016/j.cell.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Snaidero N, Velte C, Myllykoski M, Raasakka A, Ignatev A, Werner HB, Erwig MS, Möbius W, Kursula P, Nave K-A, Simons M, Antagonistic Functions of MBP and CNP Establish Cytosolic Channels in CNS Myelin, Cell Rep. 18 (2017) 314–323. 10.1016/j.celrep.2016.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Velumian AA, Samoilova M, Fehlings MG, Visualization of cytoplasmic diffusion within living myelin sheaths of CNS white matter axons using microinjection of the fluorescent dye Lucifer Yellow, Neuroimage. 56 (2011) 27–34. 10.1016/j.neuroimage.2010.11.022. [DOI] [PubMed] [Google Scholar]
- [67].Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave K-A, Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination., Nat. Genet 33 (2003) 366–374. 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- [68].Czopka T, Ffrench-Constant C, Lyons DA, Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo., Dev. Cell 25 (2013) 599–609. 10.1016/j.devcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Almeida RG, Czopka T, Ffrench-Constant C, Lyons DA, Individual axons regulate the myelinating potential of single oligodendrocytes in vivo., Development. 138 (2011) 4443–4450. 10.1242/dev.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Elazar N, Vainshtein A, Golan N, Vijayaragavan B, Schaeren-Wiemers N, Eshed-Eisenbach Y, Peles E, Axoglial Adhesion by Cadm4 Regulates CNS Myelination, Neuron. 101 (2019) 224–231.e5. 10.1016/j.neuron.2018.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wake H, Lee PR, Fields RD, Control of local protein synthesis and initial events in myelination by action potentials, Science. (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Koudelka S, Voas MG, Almeida RG, Baraban M, Soetaert J, Meyer MP, Talbot WS, Lyons DA, Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release., Curr. Biol 26 (2016) 1447–1455. 10.1016/j.cub.2016.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Baraban M, Koudelka S, Lyons DA, Ca 2+ activity signatures of myelin sheath formation and growth in vivo., Nat. Neurosci 21 (2018) 19–23. 10.1038/s41593-017-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Krasnow AM, Ford MC, Valdivia LE, Wilson SW, Attwell D, Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo., Nat. Neurosci 21 (2018) 24–28. 10.1038/s41593-017-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Auer F, Vagionitis S, Czopka T, Evidence for Myelin Sheath Remodeling in the CNS Revealed by In Vivo Imaging, Curr. Biol 28 (2018) 549–559.e3. 10.1016/j.cub.2018.01.017. [DOI] [PubMed] [Google Scholar]
- [76].Rasband MN, Peles E, The nodes of ranvier: molecular assembly and maintenance., Cold Spring Harb. Perspect. Biol 8 (2015) a020495 10.1101/cshperspect.a020495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Arancibia-Cárcamo IL, Ford MC, Cossell L, Ishida K, Tohyama K, Attwell D, Node of Ranvier length as a potential regulator of myelinated axon conduction speed., ELife. 6 (2017). 10.7554/eLife.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Miyata S, Taniguchi M, Koyama Y, Shimizu S, Tanaka T, Yasuno F, Yamamoto A, Iida H, Kudo T, Katayama T, Tohyama M, Association between chronic stress-induced structural abnormalities in Ranvier nodes and reduced oligodendrocyte activity in major depression, Sci. Rep 6 (2016) 23084 10.1038/srep23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Trigo D, Smith KJ, Axonal morphological changes following impulse activity in mouse peripheral nerve in vivo: the return pathway for sodium ions, J. Physiol 593 (2015) 987–1002. 10.1113/jphysiol.2014.279331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tagoe T, Barker M, Jones A, Allcock N, Hamann M, Auditory Nerve Perinodal Dysmyelination in Noise-Induced Hearing Loss, J. Neurosci 34 (2014) 2684–2688. 10.1523/JNEUROSCI.3977-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Reimer MM, McQueen J, Searcy L, Scullion G, Zonta B, Desmazieres A, Holland PR, Smith J, Gliddon C, Wood ER, Herzyk P, Brophy PJ, McCulloch J, Horsburgh K, Rapid Disruption of Axon–Glial Integrity in Response to Mild Cerebral Hypoperfusion, J. Neurosci 31 (2011) 18185–18194. 10.1523/JNEUROSCI.4936-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Stedehouder J, Kushner SA, Myelination of parvalbumin interneurons: a parsimonious locus of pathophysiological convergence in schizophrenia., Mol. Psychiatry 22 (2017) 4–12. 10.1038/mp.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Xekardaki A, Giannakopoulos P, Haller S, White Matter Changes in Bipolar Disorder, Alzheimer Disease, and Mild Cognitive Impairment: New Insights from DTI., J. Aging Res 2011 (2011) 286564 10.4061/2011/286564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bartzokis G, Alzheimer’s disease as homeostatic responses to age-related myelin breakdown., Neurobiol. Aging 32 (2011) 1341–1371. 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Dean DC, Hurley SA, Kecskemeti SR, O’Grady JP, Canda C, Davenport-Sis NJ, Carlsson CM, Zetterberg H, Blennow K, Asthana S, Sager MA, Johnson SC, Alexander AL, Bendlin BB, Association of amyloid pathology with myelin alteration in preclinical alzheimer disease., JAMA Neurol. 74 (2017) 41–49. 10.1001/jamaneurol.2016.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, Rothstein JD, Bergles DE, Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis, Nat. Neurosci 16 (2013) 571–579. 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ferraiuolo L, Meyer K, Sherwood TW, Vick J, Likhite S, Frakes A, Miranda CJ, Braun L, Heath PR, Pineda R, Beattie CE, Shaw PJ, Askwith CC, McTigue D, Kaspar BK, Oligodendrocytes contribute to motor neuron death in ALS via SOD1-dependent mechanism, Proc. Natl. Acad. Sci. U. S. A 113 (2016) E6496–E6505. 10.1073/pnas.1607496113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS, Neurobiological bases of age-related cognitive decline in the rhesus monkey., J. Neuropathol. Exp. Neurol 55 (1996) 861–874. 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- [89].Peters A, Sethares C, Killiany RJ, Effects of age on the thickness of myelin sheaths in monkey primary visual cortex., J. Comp. Neurol 435 (2001) 241–248. 10.1002/cne.1205. [DOI] [PubMed] [Google Scholar]
- [90].Peters A, Kemper T, A review of the structural alterations in the cerebral hemispheres of the aging rhesus monkey., Neurobiol. Aging 33 (2012) 2357–2372. 10.1016/j.neurobiolaging.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall KA, Finn JP, Villablanca P, Thompson PM, Mintz J, Lifespan trajectory of myelin integrity and maximum motor speed., Neurobiol. Aging 31 (2010) 1554–1562. 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Salat DH, Tuch DS, Greve DN, van der Kouwe AJW, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM, Age-related alterations in white matter microstructure measured by diffusion tensor imaging, Neurobiol. Aging 26 (2005) 1215–1227. 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- [93].Lintl P, Braak H, Loss of intracortical myelinated fibers: a distinctive age-related alteration in the human striate area, Acta Neuropathol. (Berl.) 61 (1983) 178–182. [DOI] [PubMed] [Google Scholar]
- [94].Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ, Age-induced white matter changes in the human brain: a stereological investigation, Neurobiol. Aging 18 (1997) 609–615. [DOI] [PubMed] [Google Scholar]
- [95].Peters A, The effects of normal aging on myelin and nerve fibers: a review, J. Neurocytol 31 (2002) 581–593. [DOI] [PubMed] [Google Scholar]
- [96].Marner L, Nyengaard JR, Tang Y, Pakkenberg B, Marked loss of myelinated nerve fibers in the human brain with age, J. Comp. Neurol 462 (2003) 144–152. 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- [97].Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, Edinger AL, Jung S, Rossner MJ, Simons M, Age-related myelin degradation burdens the clearance function of microglia during aging., Nat. Neurosci 19 (2016) 995–998. 10.1038/nn.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Haroutunian V, Katsel P, Roussos P, Davis KL, Altshuler LL, Bartzokis G, Myelination, oligodendrocytes, and serious mental illness., Glia. 62 (2014) 1856–1877. 10.1002/glia.22716. [DOI] [PubMed] [Google Scholar]
- [99].Sim FJ, Zhao C, Penderis J, Franklin RJM, The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation., J. Neurosci 22 (2002) 2451–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Rone MB, Cui Q-L, Fang J, Wang L-C, Zhang J, Khan D, Bedard M, Almazan G, Ludwin SK, Jones R, Kennedy TE, Antel JP, Oligodendrogliopathy in Multiple Sclerosis: Low Glycolytic Metabolic Rate Promotes Oligodendrocyte Survival, J. Neurosci. Off. J. Soc. Neurosci 36 (2016) 4698–4707. 10.1523/JNEUROSCI.4077-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave K-A, Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity, Nature. 485 (2012) 517–521. 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ, Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion., J. Neurosci 18 (1998) 6241–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Bartzokis G, Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease, Neurobiol. Aging 25 (2004) 5–18. 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- [104].Franklin RJM, Ffrench-Constant C, Regenerating CNS myelin - from mechanisms to experimental medicines., Nat. Rev. Neurosci 18 (2017) 753–769. 10.1038/nrn.2017.136. [DOI] [PubMed] [Google Scholar]
- [105].Ruckh JM, Zhao J-W, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJM, Rejuvenation of regeneration in the aging central nervous system., Cell Stem Cell. 10 (2012) 96–103. 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zuo H, Wood WM, Sherafat A, Hill RA, Lu QR, Nishiyama A, Age-Dependent Decline in Fate Switch from NG2 Cells to Astrocytes After Olig2 Deletion, J. Neurosci. Off. J. Soc. Neurosci 38 (2018) 2359–2371. 10.1523/JNEUROSCI.0712-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Liu J, Moyon S, Hernandez M, Casaccia P, Epigenetic control of oligodendrocyte development: adding new players to old keepers, Curr. Opin. Neurobiol 39 (2016) 133–138. 10.1016/j.conb.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Miron VE, Microglia-driven regulation of oligodendrocyte lineage cells, myelination, and remyelination., J. Leukoc. Biol 101 (2017) 1103–1108. 10.1189/jlb.3RI1116-494R. [DOI] [PubMed] [Google Scholar]
- [109].Segel M, Neumann B, Hill MFE, Weber IP, Viscomi C, Zhao C, Young A, Agley CC, Thompson AJ, Gonzalez GA, Sharma A, Holmqvist S, Rowitch DH, Franze K, Franklin RJM, Chalut KJ, Niche stiffness underlies the ageing of central nervous system progenitor cells, Nature. 573 (2019) 130–134. 10.1038/s41586-019-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil M-T, Su M, Sen P, Ruhwedel T, Mitkovski M, Trendelenburg G, Lütjohann D, Möbius W, Simons M, Defective cholesterol clearance limits remyelination in the aged central nervous system, Science. 359 (2018) 684–688. 10.1126/science.aan4183. [DOI] [PubMed] [Google Scholar]
- [111].Schain AJ, Hill RA, Grutzendler J, Label-free in vivo imaging of myelinated axons in health and disease with spectral confocal reflectance microscopy., Nat. Med 20 (2014) 443–449. 10.1038/nm.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hill RA, Grutzendler J, In vivo imaging of oligodendrocytes with sulforhodamine 101., Nat. Methods 11 (2014) 1081–1082. 10.1038/nmeth.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Bin JM, Lyons DA, Imaging Myelination In Vivo Using Transparent Animal Models, Brain Plast. Amst. Neth 2 (2016) 3–29. 10.3233/BPL-160029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Farrar MJ, Wise FW, Fetcho JR, Schaffer CB, In vivo imaging of myelin in the vertebrate central nervous system using third harmonic generation microscopy., Biophys. J 100 (2011) 1362–1371. 10.1016/j.bpj.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Fu Y, Huff TB, Wang H-W, Wang H, Cheng J-X, Ex vivo and in vivo imaging of myelin fibers in mouse brain by coherent anti-Stokes Raman scattering microscopy., Opt. Express 16 (2008) 19396–19409. 10.1364/OE.16.019396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ben Arous J, Binding J, Léger J-F, Casado M, Topilko P, Gigan S, Boccara AC, Bourdieu L, Single myelin fiber imaging in living rodents without labeling by deep optical coherence microscopy., J. Biomed. Opt 16 (2011) 116012 10.1117/1.3650770. [DOI] [PubMed] [Google Scholar]