Abstract
Cannabis exposure during the perinatal period results in varied and significant consequences in affected offspring. The prevalence of detrimental outcomes of perinatal cannabis exposure is likely to increase in tandem with the broadening of legalization and acceptance of the drug. As such, it is crucial to highlight the immediate and protracted consequences of cannabis exposure on pre- and post-natal development. Here, we identify lasting changes in neurons’ learning flexibility (synaptic plasticity) and epigenetic misregulation in animal models of perinatal cannabinoid exposure (using synthetic cannabinoids or active components of the cannabis plant) in addition to significant alterations in social behavior and executive functions. These findings are supported by epidemiological data indicating similar behavioral outcomes throughout life in human offspring exposed to cannabis during pregnancy. Further, we indicate important lingering questions regarding accurate modeling of perinatal cannabis exposure as well as the need for sex- and age-dependent outcome measures in future studies.
Keywords: cannabis, perinatal
Global trends in cannabis use and perinatal cannabis exposure
The current global reassessment of cannabis, exemplified by the legalization and decriminalization of the drug in large swaths of North America and Europe, as well as in other parts of the world, demands in kind an appraisal of concerns regarding its impact on health and well-being. While the economic and sociopolitical push towards increased cannabis acceptance has accelerated, the scientific accompaniment has lagged in addressing concerns regarding increased consumption and widespread use. As with other self-inflicted maladies of modern age, preventative measures including basic education remain favorable options compared to the post-hoc correction of aberrant outcomes.
Cannabis is the world’s most widely used (illicit) drug among the global population, and it is similarly so among the global women population [1-3]. Indeed, the rate of cannabis use by young, pregnant women has been consistently increasing over time[4]. The majority (>70%) of pregnant women consider use of the drug once or twice per week to be of little to no risk [5]. Further, a recent trend of cannabis providers actively encouraging the use of the drug during the perinatal period underscores the urgency of information dissemination with regards to the known negative impacts of cannabis use during this time [6]. Given the known transfer of the primary active constituent in cannabis, Δ9-THC (THC), to the developing fetus at levels equal to maternal plasma [7], the importance of understanding the consequences of maternal cannabis use on development are paramount.
The aim of this review is thus threefold: 1/ provide background for the determinant role of the endocannabinoid system (ECS) during the perinatal period (PNP) for the proper development of brain and behavior; 2/ summarize the current state of knowledge regarding the impact of perinatal cannabis exposure (PCE) as inferred from both human and animal studies and 3/ highlight new findings on PCE and identify critical future directions for this area of research.
The extended perinatal period
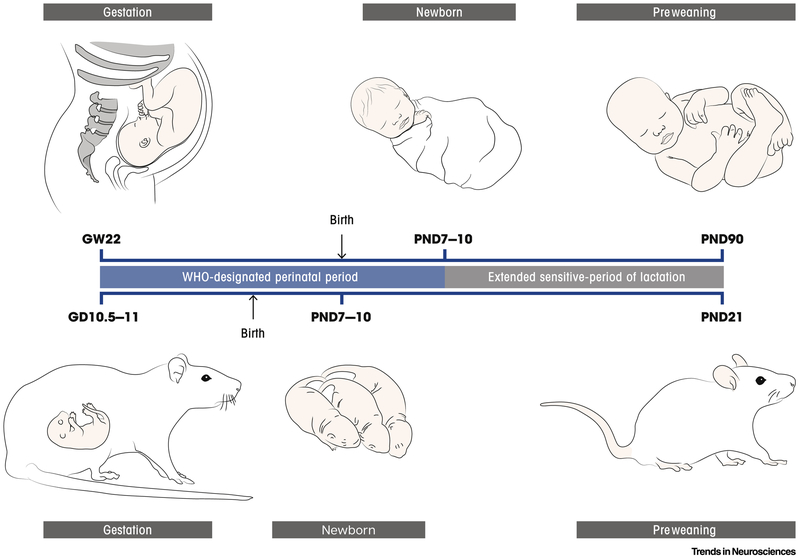
According to the World Health Organization (WHO), the perinatal period (PNP) in humans begins at 22 weeks of gestation and ends 7 days after birth. Biological processes of neurodevelopment linked to specific behavioral outcomes are temporally correlated with the maturation of discrete neuronal networks. Key stages of neurodevelopment during fetal brain formation are remarkably conserved in mammalian species ([8,9] and references therein) which has allowed for the implementation of an online database of early neurodevelopmental milestones among species [10]. For instance, in rodents, the PNP ranges from neural tube formation (gestational day, GD 10.5–11 and 9–9.5 in rats and mice, respectively) to white matter development (postnatal day, PND 7-10) [11].
In the literature, boundaries between prenatal, perinatal and early postnatal stages are rather blurry (Figure 1). Several factors contribute to this imprecision and inconsistency in the definition of the PNP, including the inability to correlate precise timing of specific human brain regions and comparable events in rodents (e.g. absence of gyrification) as well as inconsistency in time-keeping mechanisms underlying comparable developmental paces of separated processes and regions ([12], and references therein). Indeed, the duration of breastfeeding often (~50% of cases) reaches 6 months of age [13], significantly extending the period when child and maternal health are closely tied and of potential transfer of cannabinoids. Breast milk of cannabis-consuming mothers has been shown to contain significant quantities of cannabinoids [14-16], thus indicating that during the pivotal early weeks and months of postnatal development, the influence of maternal cannabis use remains a concern. Therefore, here we discuss the vulnerable period as extending beyond the WHO definition in order to include both early gestational (i.e. first trimester) as well as early postnatal (i.e. breastfeeding) impacts of cannabinoid exposure.
Figure 1: The perinatal period of sensitivity extends from early pregnancy until weaning.
The perinatal period of sensitivity to cannabis exposure in humans and rodents extends from early gestation through the pre-weaning period. In humans (top), the WHO defines the perinatal period as between gestational week (GW) 22 and 7 days after birth. Robust data indicate significant transfer of cannabinoids through lactation, extending this period of vulnerability to the end of breastfeeding, which varies widely among individuals. For the sake of simplified comparison with animal models, and as a conservative estimate, the breastfeeding window can be considered three months post-birth, which approximately corresponds to the weaning age in rodents. In rats and mice (bottom) the perinatal period begins at neural tube formation (GD10.5-11 and GD9-9.5 in rats and mice, respectively) and extends to white matter development (PND07-10). In rodents, the gestational period approximately corresponds to the first two trimesters of human in utero development, whereas the first week of postnatal life corresponds to the third trimester of human in utero development. Cannabinoid transfer to the developing offspring extends until weaning at PND21.
The perinatal period is a critical period
Windows of heightened vulnerability during brain development overlap with critical and sensitive periods of plasticity. A critical period of plasticity is an optimal time for brain changes because it coincides with necessary environmental inputs for proper neural circuitry development (e.g., primary sensory circuits [17]). Since during PNP the diverse developmental processes (e.g., dendritogenesis, spine and synaptic formation, and ion channel compositions) have different paces, this period represents a critical time-window of plasticity with high susceptibility to discrete environmental influences. In fact, any divergence from the orderly sequelae of developmental events (i.e. developmental trajectory, [18]) during PNP imbues serious functional consequences revealed later in life: any delay, stall or acceleration, change in excitatory to inhibitory balance, or synchronization of critical events may uncouple a developmental progression of processes, circuits and brain regions and lead in turn to mental illness. Given the long half-life of cannabinoids in blood and breast milk [19]and the rapid pace of neurodevelopment during this period, even seemingly small levels of exposure to cannabinoids may imbue significant consequences on the developing brain.
Lasting changes in gene expression patterns (epigenetic programming) take place during the PNP to establish correct cell- and tissue- specific gene expression. Perturbation of the epigenomic trajectory during the PNP can derail normal brain development and induce long-lasting effects on brain function and, subsequently, behavior [20,21]. Alternatively, environmental challenges may lead to incorrect epigenetic programming of molecular machinery, resulting in neuropsychiatric (endo)phenotypes dependent on when the hit occurs [22].
Epigenetic insults resulting from cannabinoid exposure during the PNP have been briefly explored in rodents as well as humans. However, additional evidence suggests that the epigenome remains sensitive to cannabis at least through adolescence (for a review, see [23]) when even minimal exposure to THC may significantly alter brain structure [24]. The first preclinical investigation into genetic dysregulation induced by PCE observed significant alterations in the mRNA of the opioid peptide hormone, proenkephalin, in the nucleus accumbens of rats exposed to THC, from GD5 to PND2, during both early development and adulthood (decreased in the former while increased at the latter time point), as well as in the amygdala at adulthood [23]. Such an alteration in dopamine function was earlier predicted by Rodriguez de Fonseca and colleagues [25]. Importantly, these epigenetic changes were linked with increased heroin seeking behavior and sensitivity to the drug at adulthood. In another pioneering study, decreased D2 receptor mRNA was observed in human fetal tissue (at 18-22 gestational weeks, GW) collected from cannabis-using mothers [26]. These results were then replicated in a corresponding rat model (THC exposure from GD5 to PND2) in which D2 receptor mRNA was similarly decreased in the nucleus accumbens at both PND2 and at PND62. Interestingly, this effect appears subtype specific as no similar changes were observed in D4 receptors in the offspring of cannabis-using mothers [27]. Finally, epigenetic regulation of the immune system following PCE has been briefly documented and summarized most recently by Dong and colleagues [28] as well as in a previous review [29].
The eCB system during the perinatal period
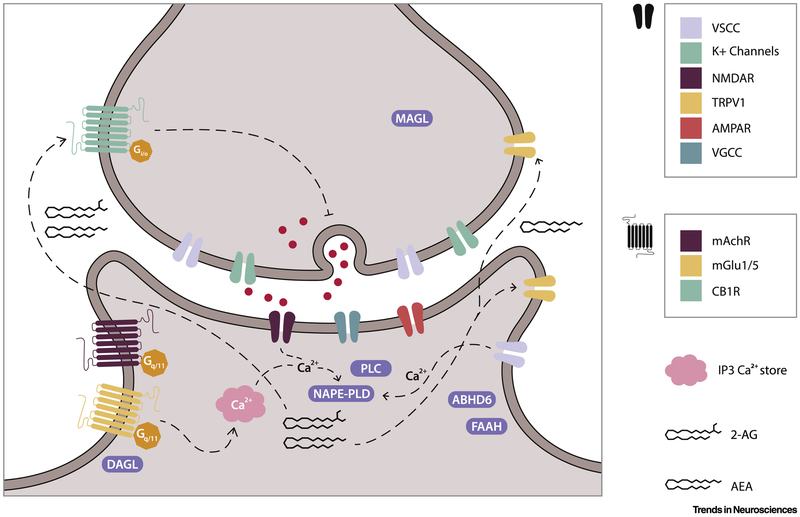
The endocannabinoid system (ECS; Figure 2) is comprised of endocannabinoids (eCBs, primarily AEA and 2-AG), the cannabinoid receptors (CB1R and CB2R) and the enzymes responsible for the synthesis of eCBs (namely NAPE, DGL) and its degradation (namely FAAH, MGL). In addition to these components, it must be considered that the eCBs interact with additional targets (Transient receptor potential vanilloid 1 [TRPV1], peroxisome proliferator-activated receptor [PPAR], G-protein coupled receptor 55, amongst others) and that both CB1R and CB2R co-localize and form heterodimers with a large variety of additional receptor types including μ-opioid[30], 5HT2A serotonin [31] and D2 dopamine [32] amongst others.
Figure 2: The endocannabinoid system (ECS).
The ECS is a complex array of both pre- and post-synaptic receptors, synthesizing and degrading enzymes, and the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG). Post-synaptic synthesis of AEA and 2-AG by the enzyme NAPE-PLD occurs via a Ca2+-dependent process derived from IP3-mediated Ca2+ stores, NMDA receptors and voltage-sensitive Ca2+ channels (VSCC). AEA and 2-AG both act as agonists at the presynaptic CB1R, while AEA acts as an agonist at both pre- and post-synaptic Transient Receptor Potential Vanniloide 1 (TRPV1) channels. While AEA degradation mediated by FAAH occurs post-synaptically, 2-AG hydrolysis is mediated by MAGL and ABHD6 at the pre- and post-synaptic sites, respectively.
The obvious targets of PCE are, therefore, these components of the ECS. Indeed, CB1R and CB2R are both present at nearly all stages of the PNP. The role of the ECS in development is pivotal beginning as early as ontogenesis where it regulates diverse phases of fertility (e.g., ovulation, spermatogenesis, implantation) as well as pregnancy outcomes (e.g., delay in embryo development, poor blastocyst implantation, miscarriage and compromised placentation) [33,34]. The ECS has a precise role in each neurodevelopmental step [35-39] with the expression of CBRs during development following discrete trajectories dependent on species, strain, sex and age [40-42]. For instance, CB2Rs are expressed in the mouse embryo where they are important for stem-cell derived hematopoietic cell proliferation and lineage differentiation [43]. CB1R is expressed during development of the CNS early on in both chick and zebrafish embryo [44,45] and its expression follows neural differentiation [44]. Given the close association between DAGL and CB1Rs, axonal growth and fasciculation appear to be the first functions played by 2-AG during neurodevelopment [45].
Brain distribution of CB1Rs during development is atypical as their activation has different effects when compared to post-natal life. Developmental CB1R expression is regulated both temporally and spatially. In particular, in cholinergic components of the mouse basal forebrain CB1Rs appear to be limited to prenatal life (GD14.5-birth) [45], whereas in cortical regions their expression fluctuates, reaching the highest levels at GD16.5 in mice and during the second trimester in fetal telencephalon [46]. This latter case appear to be more common as CB1Rs are present beginning at GD11 in rodents [46] with similar patterns observed in human fetal brain tissue (GW 14, [47,48]). At GW20, a marked and selective increase in CB1R expression is detected in the limbic portion of the hippocampus and in the basolateral amygdala (BLA) [47], thus highlighting a plausible sensitive period of vulnerability for programming of emotional processing in fetal brains.
CB1R expression peaks as synaptic connectivity is established by cortical pyramidal cells [49] and GABA interneurons [50]. CB1Rs influence neuronal progenitor proliferation [49,51,52], axonal growth and guidance, and fasciculation [45]. In both humans and rodents, this peak in CB1R expression is tightly correlated with 2-AG levels [46,53-55]. The integral role of CB1R in proper neurodevelopment is revealed in genetically modified mice lacking these receptors, which have been used to model the “double hit” hypothesis of psychiatric illness: CB1R lacking mice are indeed susceptible to depression-like disorders and exhibit features of aberrant brain development, such as diminished levels of neurotrophic factors, alterations in the HPA axis and in the levels of pro-inflammatory cytokines [56].
Among the eCBs, 2-AG appears to play a particularly prominent role during PNP not only because its levels are much higher than the other eCBs throughout this period [46,57]. The 2-AG synthesizing enzymes, DGLα and DGLβ, control neurite outgrowth [58] and their expression begins around GD 14.5-6.5 and remains high until the moment of birth, when it decreases abruptly [55]. Moreover, 2-AG gradients modulate growth cone maturation and axonal elongation through a specific spatial and temporal distribution of its main degrading enzyme, MGL. Furthermore, 2-AG regulates morphogenesis and interneuron migration [59]. Finally, a developmental change in spatial distribution of DGL expression reveals 2-AG’s diverse functions in the embryo, where it acts in an autocrine fashion in the axonal tract to promote axonal growth and guidance via CB1R [60]. This is in sharp contrast with its canonical retrograde signaling from the post- to the pre- synaptic compartment in regulating synaptic plasticity throughout post-natal life [61,62]. Conversely, changes in AEA during mid-gestation in mice affects neither axonal development nor neurogenesis [63].
Hence, PCE, by interfering with physiological regulation operated by eCB signaling [36,37,39,57], disrupts its role in developmental programming and alters synaptogenesis and the correct developmental trajectory of neuronal circuitries [64].
Perinatal cannabis in humans
Compared to the wealth of clinical studies documenting behavioral effects of prenatal exposure to cigarettes or alcohol, only three main longitudinal studies have tracked neurobehavioral consequences of maternal Cannabis use: the Ottawa Prenatal Prospective Study (OPPS), initiated in 1978 by Fried and colleagues, included a low-risk, European-American, middle-class population of pregnant women and followed the offspring until the age of 18-22 years [65-72]; the Maternal Health Practices and Child Development Study (MHPCD), which began in 1982, focused on high-risk pregnant women of mixed ethnicity and low socioeconomic status, with follow-up of the offspring until the age of 14 [73-78]; and the more recent Generation R study, initiated in 2001, which enrolled multi-ethnic pregnant women of middle to high socioeconomic status, with follow-up for most measures until the age of 6 [79-81,81-83]. The results of these studies have been extensively reviewed elsewhere [84-91] and therefore we will only summarize the main findings, controversies and methodological limitations here.
In contrast to prenatal cigarette or alcohol exposure, the consequences of PCE in the human offspring upon growth or behavior immediately after birth are rather subtle [89] and appear to manifest with age. At infancy, the MHPCD study found lower mental development scores in 9 month-old babies exposed to cannabis, although this effect disappeared by 19 months [92]. At the age of 3–4 years, both the OPPS and MHPCD studies reported a negative association between PCE and verbal and memory function [68,74]. While the more recent Generation R study did not confirm these earlier findings, it reported an effect of prenatal cannabis on girls’ aggression and inattention at the age of 18 months [93].
Both the OPPS and MHPCD studies reported impulsivity and hyperactivity in cannabis-exposed children at 6 and 10 years [70,75,94]. Very recently, the Generation R study found that maternal cannabis use during pregnancy was associated with externalizing symptoms (i.e., aggressive and rule-breaking behaviors) at 7–10 years [95]. Because eCBs control emotional states, PCE might negatively impact emotional reactivity of the offspring. Earlier reports from the MHPCD cohort showed that heavy prenatal cannabis consumption during the first trimester predicted levels of self-reported anxiety and depressive symptoms in children aged 10 [76,96,97]. These results were not replicated in the more recent Generation R study [93], perhaps due to the different demographic characteristics of the women enrolled in the two studies (e.g., low versus slightly higher socioeconomic status in the MHPCD and Generation R cohorts, respectively) or concurrent environmental events. Importantly, a significant correlation has been noted between psychiatric diseases and cannabis use, indicating that while positive associations between disturbed emotional and psychiatric health in offspring and maternal cannabis use have been found, a genetic driver for both cannot be ruled out [98,99].
PCE has consequences beyond infancy: data from the OPPS study indicate that PCE does not affect global IQ but may impair performance on tasks that require cognitive performance in the domain of “executive functions”, such as cognitive flexibility, sustained attention, planning, working memory and goal-directed behavior (for a review, see [85]). Both the MHPCD and OPPS have identified PCE as a risk factor for later cannabis use in adolescent and young adults [100-102]. Further, fMRI experiments showed that 18-22 year-old PCE offspring exhibit altered neuronal function in several brain areas during both visuospatial working memory processing and response inhibition [103]. Thus, while PCE appears to induce subtle and sometimes inconsistent effects at infancy, it more consistently results in long-term behavioral deficits that appear at childhood, persist throughout adolescence and last at least into early adulthood.
However, some intrinsic limitations of these clinical data must be carefully considered. First, different population characteristics, patterns of use and content of THC in cannabis preparations may account for discrepancies in the findings. Second, it cannot be excluded that genetic and environmental variables also contribute to the relationship between maternal cannabis use and long-term behavioral outcomes in the offspring. Third, to date only the OPPS and MHPCD studies provided long-term follow-up data, and therefore a comparison across studies cannot always be made. The relatively small subsamples studied also present some difficulty in drawing conclusive determinations. Finally, clinical studies only provide limited information about the specific molecular and cellular consequences that underlie behavioral and neural changes induced by PCE (for a more detailed overview of these methodological caveats, see [79]).
A key strategy to overcome these limitations is to use animal models, which allow a measure of control over the confounding variables that characterize human studies and permit the careful dissection of the roles of specific brain areas and neural pathways in the impact of PCE.
Perinatal cannabis in animal models
Different protocols have been used to model PCE in rodents with multiple variables including the cannabinoid agonist used (i.e., THC or synthetic cannabinoid receptor agonists, primarily WIN55,212-2), doses and routes of administration, time window of exposure and outcome measures. Here, we consider only studies using doses of cannabinoid compounds devoid of overt signs of toxicity and/or gross malformations, and that are equivalent to moderate cannabis consumption in humans. In the rat, an oral dose of 5 mg/kg of THC or a subcutaneous dose of 0.5 mg/kg of WIN55,212-2, given daily through pregnancy/and or lactation, are thought to correspond to moderate levels of cannabis exposure in humans after correcting for differences in route of administration and body weight surface area [104,105]. For this reason, these doses have been widely used in preclinical studies. Of important note, recent advances in the delivery of smoke- or vapor-based cannabis in animal models aimed at replicating human use have thus far indicated similar effects to those of systemic administration [106].
As for the time window of PCE, most protocols are based upon either prenatal (most often GD 0 to GD 20) or perinatal (interpreted as through pregnancy and lactation) cannabinoid administration, with the rat prenatal period being approximately equivalent to the first and second trimesters of human pregnancy and the first 10 postnatal days in the rat approximately equivalent to the third trimester [107].
Because comprehensive reviews about the behavioral consequences of PCE in preclinical models are available [86,88,91,108], here we will only focus on rodent studies with translational relevance to the human findings outlined above.
A wide range of cognitive deficits has consistently been reported in rats exposed to cannabinoid agonists either during prenatal or perinatal period, from early deficits in olfaction-based social discrimination [109] to disrupted memory retention in the inhibitory avoidance task at adolescence and adulthood [105,110,111], as well as impaired discrimination abilities in both social discrimination [110] and object recognition [112]. These cognitive deficits have been correlated with alterations in hippocampal long-term potentiation [105] and changes in both hippocampal and cortical glutamatergic neurotransmission [110,113-116]. In addition, Tortoriello et al. [117] observed a cortical reorganization of axonal morphology in both mouse and human fetuses downstream of SCG10 expression that might induce a predisposition to “circuit failure” upon a second insult [23,104,118,119].
As for the impact of PCE on emotional-related behaviors in rodents, both increased [91] and decreased [109,120] rates of ultrasonic vocalizations (USVs) have been found in infant rats exposed to cannabinoids. Our most recent data illustrate a change in the quality rather than quantity of USV following exposure to cannabinoids via lactation during the first 10 days of postnatal life [121]. Changes in the USV mean dominant frequency were correlated with a delayed trajectory of GABA maturation in the prefrontal cortex following cannabinoid exposure from PND1-10.
At later ages, an interesting behavioral domain that seems to be consistently affected by PCE in rodents is the social repertoire. Both adolescent and adult rats and mice exposed to cannabinoid drugs during pregnancy and/or lactation show specific deficits in social interaction [91,112,122,123]. We recently found that PCE impacts the social repertoire of offspring in a sex-dependent manner: male but not female rats prenatally exposed to cannabinoid drugs showed social deficits associated with disrupted long-term depression (LTD) and heightened excitability of prefrontal cortex pyramidal neurons [122]. Interestingly, positive allosteric modulation of mGlu5 receptors and enhancement of AEA levels through pharmacological inhibition of AEA hydrolysis restored LTD and social interaction in cannabis-exposed males.
A sexual divergence in the long-term functional and behavioral consequences of PCE has also been found when the offspring were tested for drug self-administration later in life. Indeed, female but not male rats perinatally exposed to THC showed an increase in the rate of acquisition of morphine self-administration, in parallel with changes in mu opioid receptor binding in several brain regions [124], but this vulnerability to morphine disappeared if animals had to work harder to get the drug [125]. Conversely, male rats prenatally exposed to THC showed enhanced heroin seeking only during mild stress and extinction [23]. Concerning the effects of PCE on adult sensitivity to other drugs of abuse, no effects of perinatal THC exposure on alcohol self-administration in the adult male offspring have been found [126]. However, one could plausibly argue that since in humans, early experiences with alcohol consumption in humans are associated with deficits in sociability, this study failed to find an association between PCE and alcohol self-administration because it has been assessed at the wrong age (i.e., not during a sensitive developmental window). Accordingly, juvenile THC-exposed male rats are sensitive to an ineffective dose of THC that induces a hyperlocomotion and deficits in sensorimotor gating functions, and both these effects are dependent upon a biased dopamine system function [119]. In contrast, THC-exposed rats of both sexes exhibit a blunted locomotor response to amphetamine at adulthood [111]. Despite the large clinical evidence, the effects of PCE on sensitivity to drugs like hallucinogens and other drugs commonly experimented before and at adulthood remain unknown.
The quick development of drugs that interfere with ECS signaling not by binding CB receptors but rather by interfering with eCB metabolism is an additional and rising source of concern. Future research needs to be performed to evaluate how pharmacologically-induced changes in eCB levels impact neuronal development and offspring behavior throughout life. In developing such pharmacological tools, it is crucial to consider that perinatal inhibition of eCB degrading enzymes alters the levels of multiple bioactive lipids, which may ultimately act through other targets, including PPARα and TRPV1 [127,128].
Currently, only two studies (two our knowledge) have investigated the effects of perinatal eCB elevation. The pioneering study by Wu et al. [63] addressed the use of FAAH inhibitors, which show promise as a treatment for anxiety, depression and pain, all of which are conditions commonly observed in pregnant women and young mothers. The adult progeny of mouse dams treated with the FAAH inhibitor URB597 (GD 10.5 to 16.5), exhibited reduced cocaine-conditioned preference, increased depressive behaviors and impaired working memory. In this study, URB597 did not affect dam weight gain, neurogenesis or axonal development suggesting that the consequences of perinatal AEA imbalance may be significantly protracted. The observation that anxiety levels, motor function and sensory-motor gating were not significantly altered further indicate that the manifestations of eCB elevation differ from that of direct agonist stimulation. However, a humanized mouse model of a common SNP in the FAAH gene (C385A; [129]) exhibits biochemical and behavioral traits (i.e., heightened fear extinction learning and reduced anxiety-like behaviors [130]) suggestive of differences in developmental alterations resulting from enhanced AEA levels consequent to indirect agonist administration (i.e., FAAH inhibitors) or carrying the 385A SNP. Alpar and colleagues [131] employed JZL184 to inhibit MGL between E12.5-18.5. The data show that 2-AG acting on CB1R facilitated Slit2 accumulation in oligodendrocyte end-feet and Robo1 accumulation in axonal growth cones. Unfortunately, the behavioral consequences of prenatal 2-AG elevation were not evaluated in this paper and thus remain so far elusive. However, in humans, cannabis use disorder is associated with genetic variation of MGLL (i.e., rs604300; [132]) and early childhood adversities, corroborating the “double hit hypothesis” of developmental derangement and vulnerable (endo)phenotypes. Additional studies are needed to address the effects of blocking ABHD6, a serine hydrolase that degrades 2-AG [133] and the behavioral consequences of MGL inhibition.
Double hit: how many tokes does it take to deprogram neurodevelopment?
Cannabis use during the PNP is rarely an isolated developmental insult. The aberrations found in humans following prenatal cannabis exposure are further confounded by several additional variables, including higher rates of cigarette, alcohol and illicit drug use by cannabis users [134-138] and lower educational and socioeconomic status amongst cannabis users during pregnancy [5,139]. Moreover, intoxication from cannabis use is further associated with deficits in maternal care [140].
Indeed, one of the most pressing current questions (and a major future challenge) for cannabis researchers is how to incorporate the “Double Hit Hypothesis” [87,141] when examining the effects of PNP cannabis exposure. In their 2016 review, Richardson and colleagues [142] make the case that PCE, “similar to other neurodevelopmental teratogens, delivers a ‘first hit’ to the endogenous cannabinoid signaling system, which is compromised in such a way that a ‘second hit’ (i.e., postnatal environmental stressors) may precipitate the emergence of a specific phenotype.”
Stressors qualifying for the second hit include environmental, genetic/epigenetic and socioeconomic influences. Thus, environmental factors, drugs, alcohol, tobacco, abused illegal drugs (psychostimulant, opiates…) or prescription drugs (e.g. anxiolytics, benzodiazepines, SSRI, opioid pain killers, AINS), environment-polluting chemicals, in-utero and early life infections and malnutrition may combine with cannabis and result in synergistic or additive deficits. This emphasizes the urgency to design and validate “high translational value” rodent models that incorporate factors interacting with cannabinoids.
In light of the current opioid epidemic, a striking illustration of the double hit theory is that maternal cannabis increases morphine place conditioning [143] and self-administration (in female rats only [124]; for additional references [144]). The mechanistic underpinnings are not clear, but one can speculate a role for the functional interactions between μ-opioid receptors and CB1R [145,146] as well as epigenetic alterations of the dopamine reward pathway.
Both hits may occur at the same time/during the same developmental period. Maternal consumption of cannabis and tobacco has been linked to poor autonomic regulation in infancy [147,148]. In both studies, harsh parenting and reduced parent-infant interactions participate and potentially aggravate the deficits. While simultaneous use of both alcohol and cannabis is high in females (8.7%, [149]) the consequences on their progeny are largely unknown. Brief postnatal (PND 4-9) exposure to a cannabimimetic and alcohol produced more severe alterations of locomotor activity than either drug alone [150].
How chemical pollutants such as pesticides or Bisphenol A (BPA) and cannabis combine and affect neurodevelopment has not been studied. For example, a theoretical study of the neurodevelopmental neurotoxicity of clorpyrifos (an organophosphate pesticide) exposure with maternal cannabis use suggests multiple adverse outcome pathways [151]. Furthermore, perinatal BPA exposure downregulates CB1R in mice and it is therefore tempting to speculate on the consequences of fetal CB1R activation followed by early life BPA exposure [152]. Finally, it is not known how perinatal infection and cannabis combine to affect fetal developmental programming. Nonetheless, maternal infection in rats and adolescent cannabinoid exposure synergize to elevate 5HT1a receptors in the hippocampus [153] and we hypothesize that perinatal infection concomitant to cannabis will perturb offspring development.
Concluding remarks
As cannabis use in the general population changes in accordance with cultural and legal shifts in the drug’s status, the advancement of scientific evaluation of the potential consequences of its use becomes paramount. Here, we have presented the current state of research indicating significant and lasting consequences of maternal cannabis use on offspring health and well-being during the critical and sensitive periods of perinatal development. Clearly, while a wide range of effects has been thus far described both in humans and rodent models, a number of important questions linger (see Outstanding Questions). As improved non-invasive techniques become readily available, the confirmation of thus far observed effects in rodent models must be confirmed in humans. Above all, we would argue, further research will shed light on the molecular underpinnings of PCE and the clear and rapid dissemination of such information as a means of preventative education presents a challenging but valuable approach to addressing many of the concerns herein described.
Outstanding Questions.
Cannabis use during the PNP is rarely an isolated insult, underscoring the importance of a double/multiple hit hypothesis in the context of PCE. How does the presence of multiple “hits” to development during or after PCE alter the outcomes of PCE?
What are the short- and long-term effects of PCE within individual models/cohorts?
What effects of PCE (both established, and ones not previously investigated) show sexual divergence?
How does PCE alter the epigenome in a transgenerational manner?
Certain drugs, such as eCB hydrolysis inhibitors, interfere with the ECS indirectly, e.g., by acting on eCB metabolism rather than binding CB receptors. What are the consequences of perinatal exposure to such indirect cannabinoid drugs?
How can pharmacotherapies for the treatment of PCE be developed in an effect-, age-, and sex-specific manner?
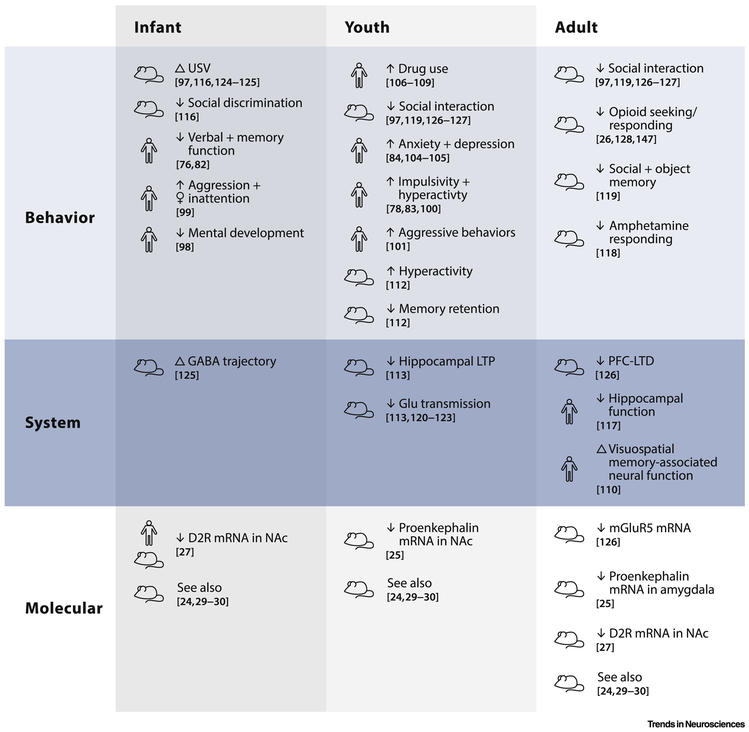
Figure 3: Consequences of perinatal cannabis exposure in humans and rodents.
The table summarizes consequences of perinatal cannabis exposure in both humans and rodents highlighted in this review. Behavioral outcomes (top row) throughout life (left to right) consistently indicate detrimental effects of perinatal cannabis exposure on cognitive and executive functions and social interactions along with increased risk-taking and impulsive behaviors in rodents and humans. Early onset of drug abuse and increased drug seeking behavior are similarly noted starting in adolescence and into adulthood in both models. Systems-level outcomes (middle row) show similar consistency across age groups. Namely, perinatal cannabis exposure appears to result in a loss of plasticity at adolescence and adulthood in multiple brain regions. Molecular outcomes (bottom row) thus far identified appear to impact largely the opioidergic and dopaminergic systems across all age groups, with the latter being similarly misregulated in humans and rodents at infancy.
Highlights.
Perinatal cannabis exposure during pregnancy and breastfeeding alters the developmental trajectory of multiple brain regions and results in lasting functional consequences in both humans and rodent models
Alterations in social behavior and executive functions are correlated with these changes throughout life
Epigenetic regulation by perinatal cannabis exposure unveils potential mechanisms for lasting changes
The detrimental outcomes of perinatal cannabis exposure can be exacerbated by compounding effects of additional environmental and chemical insults
Acknowledgements:
The authors are grateful to Laura F. Scheyer for the illustrations and to members of their laboratories for helpful discussions.
Funding: This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM; O.J.M. & A.F.S.); the INSERM-NIH exchange program (A.F.S.); Agence National de la Recherche (ANR Cannado, O.J.M.); Fondation pour la Recherche Médicale (O.J.M.), the NIH (R01DA043982 & R01DA046196-02 O.J.M.) ; Marie Curie Career Reintegration Grant PCIG09-GA-2011-293589 (V.T.), Jerome Lejeune Foundation Research (O.M & V.T.), University of Cagliari (RICCAR 2017 and 2018 to MM) and Region of Sardinia (L.R. 7 8/2007, RASSR32909 to MM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Azofeifa A et al. (2016) Monitoring Marijuana Use in the United States: Challenges in an Evolving Environment. JAMA 316, 1765–1766 [DOI] [PubMed] [Google Scholar]
- 2.Fantasia HC (2017) Pharmacologic Implications of Marijuana Use During Pregnancy. Nurs Womens Health 21, 217–223 [DOI] [PubMed] [Google Scholar]
- 3.Metz TD and Stickrath EH (2015) Marijuana use in pregnancy and lactation: a review of the evidence. Am. J. Obstet. Gynecol 213, 761–778 [DOI] [PubMed] [Google Scholar]
- 4.Young-Wolff KC et al. (2017) Trends in Self-reported and Biochemically Tested Marijuana Use Among Pregnant Females in California From 2009-2016. JAMA 318, 2490–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko JY et al. (2015) Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am. J. Obstet. Gynecol 213, 201.e1–201.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickson B et al. (2018) Recommendations From Cannabis Dispensaries About First-Trimester Cannabis Use. Obstet Gynecol 131, 1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey JR et al. (1987) Fetal disposition of delta 9-tetrahydrocannabinol (THC) during late pregnancy in the rhesus monkey. Toxicol. Appl. Pharmacol 90, 315–321 [DOI] [PubMed] [Google Scholar]
- 8.Ellenbroek B and Youn J (2016) Rodent models in neuroscience research: is it a rat race? Dis Model Mech 9, 1079–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semple BD et al. (2013) Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol 106–107, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Workman AD et al. (2013) Modeling Transformations of Neurodevelopmental Sequences across Mammalian Species. The Journal of Neuroscience 33, 7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagberg H et al. (2002) Animal models of developmental brain injury: relevance to human disease. A summary of the panel discussion from the Third Hershey Conference on Developmental Cerebral Blood Flow and Metabolism. Dev. Neurosci 24, 364–366 [DOI] [PubMed] [Google Scholar]
- 12.Ohmura Y and Kuniyoshi Y (2017) A translational model to determine rodent’s age from human foetal age. Scientific Reports 7, 17248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC, N. 2016 National Immunization Survey. . (2017)
- 14.Baker T et al. (2018) Transfer of Inhaled Cannabis Into Human Breast Milk. Obstet Gynecol 131, 783–788 [DOI] [PubMed] [Google Scholar]
- 15.Marchei E et al. (2011) Simultaneous analysis of frequently used licit and illicit psychoactive drugs in breast milk by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal 55, 309–316 [DOI] [PubMed] [Google Scholar]
- 16.Perez-Reyes M and Wall ME (1982) Presence of Δ9-Tetrahydrocannabinol in Human Milk. New England Journal of Medicine 307, 819–820 [DOI] [PubMed] [Google Scholar]
- 17.Hensch TK and Bilimoria PM (2012) Re-opening Windows: Manipulating Critical Periods for Brain Development. Cerebrum 2012, [PMC free article] [PubMed] [Google Scholar]
- 18.Hensch TK (2004) Critical period regulation. Annu. Rev. Neurosci 27, 549–579 [DOI] [PubMed] [Google Scholar]
- 19.Bertrand KA et al. (2018) Marijuana Use by Breastfeeding Mothers and Cannabinoid Concentrations in Breast Milk. Pediatrics 142, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bale TL et al. (2010) Early life programming and neurodevelopmental disorders. Biol. Psychiatry 68, 314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jirtle RL and Skinner MK (2007) Environmental epigenomics and disease susceptibility. Nat. Rev. Genet 8, 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kundakovic M and Champagne FA (2015) Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology 40, 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spano MS et al. (2007) Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol. Psychiatry 61, 554–563 [DOI] [PubMed] [Google Scholar]
- 24.Orr C et al. (2019) Grey Matter Volume Differences Associated with Extremely Low Levels of Cannabis Use in Adolescence. J. Neurosci 39, 1817–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez de Fonseca F et al. (1992) Early changes in the development of dopaminergic neurotransmission after maternal exposure to cannabinoids. Pharmacol. Biochem. Behav 41, 469–474 [DOI] [PubMed] [Google Scholar]
- 26.DiNieri JA et al. (2011) Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol Psychiatry 70, 763–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fransquet PD et al. (2017) Cannabis use by women during pregnancy does not influence infant DNA methylation of the dopamine receptor DRD4. Am J Drug Alcohol Abuse 43, 671–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong C et al. (2019) Cannabinoid exposure during pregnancy and its impact on immune function. Cell. Mol. Life Sci 76, 729–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zumbrun EE et al. (2015) Epigenetic Regulation of Immunological Alterations Following Prenatal Exposure to Marijuana Cannabinoids and its Long Term Consequences in Offspring. J Neuroimmune Pharmacol 10, 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hojo M et al. (2008) mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: electrophysiological and FRET assay analysis. J. Pharmacol. Sci 108, 308–319 [DOI] [PubMed] [Google Scholar]
- 31.Viñals X et al. (2015) Cognitive Impairment Induced by Delta9-tetrahydrocannabinol Occurs through Heteromers between Cannabinoid CB1 and Serotonin 5-HT2A Receptors. PLoS Biol 13, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Przybyla JA and Watts VJ (2010) Ligand-Induced Regulation and Localization of Cannabinoid CB1 and Dopamine D2L Receptor Heterodimers. J Pharmacol Exp Ther 332, 710–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Correa F et al. (2016) Endocannabinoid system and pregnancy. Reproduction 152, R191–R200 [DOI] [PubMed] [Google Scholar]
- 34.Taylor AH et al. (2007) The role of the endocannabinoid system in gametogenesis, implantation and early pregnancy. Hum. Reprod. Update 13, 501–513 [DOI] [PubMed] [Google Scholar]
- 35.Fride E (2008) Multiple roles for the endocannabinoid system during the earliest stages of life: pre- and postnatal development. J. Neuroendocrinol 20 Suppl 1, 75–81 [DOI] [PubMed] [Google Scholar]
- 36.Galve-Roperh I et al. (2013) Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lipid Res 52, 633–650 [DOI] [PubMed] [Google Scholar]
- 37.Harkany T et al. (2007) The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci 28, 83–92 [DOI] [PubMed] [Google Scholar]
- 38.Harkany T et al. (2008) Endocannabinoid functions controlling neuronal specification during brain development. Mol. Cell. Endocrinol 286, S84–90 [DOI] [PubMed] [Google Scholar]
- 39.Jutras-Aswad D et al. (2009) Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. Eur Arch Psychiatry Clin Neurosci 259, 395–412 [DOI] [PubMed] [Google Scholar]
- 40.Heng L et al. (2011) Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse 65, 278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Waes V et al. (2012) CB1 Cannabinoid Receptor Expression in the Striatum: Association with Corticostriatal Circuits and Developmental Regulation. Front Pharmacol 3, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verdurand M et al. (2011) Comparison of Cannabinoid CB1 Receptor Binding in Adolescent and Adult Rats: A Positron Emission Tomography Study Using [18F]MK-9470. Int J Mol Imaging 2011, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang S et al. (2007) Expression and Function of Cannabinoid Receptors CB1 and CB2 and Their Cognate Cannabinoid Ligands in Murine Embryonic Stem Cells. PLoS One 2, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Begbie J et al. (2004) Cannabinoid receptor, CB1, expression follows neuronal differentiation in the early chick embryo. J. Anat 205, 213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson S et al. (2008) The endocannabinoid receptor, CB1, is required for normal axonal growth and fasciculation. Mol. Cell. Neurosci 38, 89–97 [DOI] [PubMed] [Google Scholar]
- 46.Berrendero F et al. (1999) Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse 33, 181–191 [DOI] [PubMed] [Google Scholar]
- 47.Wang X et al. (2003) Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience 118, 681–694 [DOI] [PubMed] [Google Scholar]
- 48.Biegon A and Kerman IA (2001) Autoradiographic study of pre- and postnatal distribution of cannabinoid receptors in human brain. Neuroimage 14, 1463–1468 [DOI] [PubMed] [Google Scholar]
- 49.Mulder J et al. (2008) Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc. Natl. Acad. Sci. U.S.A 105, 8760–8765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berghuis P et al. (2007) Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science 316, 1212–1216 [DOI] [PubMed] [Google Scholar]
- 51.Zurolo E et al. (2010) CB1 and CB2 cannabinoid receptor expression during development and in epileptogenic developmental pathologies. Neuroscience 170, 28–41 [DOI] [PubMed] [Google Scholar]
- 52.Saez TMM et al. (2014) Prenatal exposure to the CB1 and CB2 cannabinoid receptor agonist WIN 55,212–2 alters migration of early-born glutamatergic neurons and GABAergic interneurons in the rat cerebral cortex. J. Neurochem 129, 637–648 [DOI] [PubMed] [Google Scholar]
- 53.Mato S et al. (2003) Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur. J. Neurosci 17, 1747–1754 [DOI] [PubMed] [Google Scholar]
- 54.Long LE et al. (2012) Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neurosci 13, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keimpema E et al. (2010) Differential subcellular recruitment of monoacylglycerol lipase generates spatial specificity of 2-arachidonoyl glycerol signaling during axonal pathfinding. J. Neurosci 30, 13992–14007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valverde O and Torrens M (2012) CB1 receptor-deficient mice as a model for depression. Neuroscience 204, 193–206 [DOI] [PubMed] [Google Scholar]
- 57.Fernandez JW et al. (2014) Postpartum depression in rats: Differences in swim test immobility, sucrose preference and nurturing behaviors. Behavioural Brain Research 272, 75–82 [DOI] [PubMed] [Google Scholar]
- 58.Jung K-M et al. (2011) Diacylglycerol lipase-α and -β control neurite outgrowth in Neuro-2a cells through distinct molecular mechanisms. Mol Pharmacol DOI: 10.1124/mol.110.070458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berghuis P et al. (2005) Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc. Natl. Acad. Sci. U.S.A 102, 19115–19120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bisogno T et al. (2003) Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol 163, 463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu H-C and Mackie K (2016) An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 79, 516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castillo PE et al. (2012) Endocannabinoid signaling and synaptic function. Neuron 76, 70–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu C-S et al. (2011) Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol 6, 459–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernard C et al. (2005) Altering cannabinoid signaling during development disrupts neuronal activity. Proc. Natl. Acad. Sci. U.S.A 102, 9388–9393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fried PA (1980) Marihuana use by pregnant women: neurobehavioral effects in neonates. Drug Alcohol Depend 6, 415–424 [DOI] [PubMed] [Google Scholar]
- 66.Fried PA (1989) Postnatal consequences of maternal marijuana use in humans. Ann. N. Y. Acad. Sci 562, 123–132 [DOI] [PubMed] [Google Scholar]
- 67.Fried PA et al. (1992) 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: cognitive and language assessment. J Dev Behav Pediatr 13, 383–391 [PubMed] [Google Scholar]
- 68.Fried PA and Watkinson B (1990) 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J Dev Behav Pediatr 11, 49–58 [PubMed] [Google Scholar]
- 69.Fried PA and Watkinson B (2000) Visuoperceptual functioning differs in 9- to 12- year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 22, 11–20 [DOI] [PubMed] [Google Scholar]
- 70.Fried PA et al. (1992) A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol Teratol 14, 299–311 [DOI] [PubMed] [Google Scholar]
- 71.Fried PA (2002) Adolescents prenatally exposed to marijuana: examination of facets of complex behaviors and comparisons with the influence of in utero cigarettes. J Clin Pharmacol 42, 97S–102S [DOI] [PubMed] [Google Scholar]
- 72.Fried PA et al. (2003) Differential effects on cognitive functioning in 13- to 16- year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 25, 427–436 [DOI] [PubMed] [Google Scholar]
- 73.Day N et al. (1992) The effects of prenatal tobacco and marijuana use on offspring growth from birth through 3 years of age. Neurotoxicol Teratol 14, 407–414 [DOI] [PubMed] [Google Scholar]
- 74.Day NL et al. (1994) Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol 16, 169–175 [DOI] [PubMed] [Google Scholar]
- 75.Goldschmidt L et al. (2000) Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol 22, 325–336 [DOI] [PubMed] [Google Scholar]
- 76.Goldschmidt L et al. (2004) Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol Teratol 26, 521–532 [DOI] [PubMed] [Google Scholar]
- 77.Goldschmidt L et al. (2008) Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry 47, 254–263 [DOI] [PubMed] [Google Scholar]
- 78.Goldschmidt L et al. (2012) School achievement in 14-year-old youths prenatally exposed to marijuana. Neurotoxicol Teratol 34, 161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El Marroun H et al. (2008) Demographic, emotional and social determinants of cannabis use in early pregnancy: the Generation R study. Drug Alcohol Depend 98, 218–226 [DOI] [PubMed] [Google Scholar]
- 80.Hofman A et al. (2004) Growth, development and health from early fetal life until young adulthood: the Generation R Study. Paediatr Perinat Epidemiol 18, 61–72 [DOI] [PubMed] [Google Scholar]
- 81.Jaddoe VWV et al. (2010) The Generation R Study: design and cohort update 2010. Eur J Epidemiol 25, 823–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kooijman MN et al. (2016) The Generation R Study: design and cohort update 2017. Eur J Epidemiol 31, 1243–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kruithof CJ et al. (2014) The Generation R Study: Biobank update 2015. Eur J. Epidemiol 29, 911–927 [DOI] [PubMed] [Google Scholar]
- 84.Fried PA (2002) Conceptual issues in behavioral teratology and their application in determining long-term sequelae of prenatal marihuana exposure. J Child Psychol Psychiatry 43, 81–102 [DOI] [PubMed] [Google Scholar]
- 85.Fried PA and Smith AM (2001) A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol 23, 1–11 [DOI] [PubMed] [Google Scholar]
- 86.Alpár A et al. (2016) At the Tip of an Iceberg: Prenatal Marijuana and Its Possible Relation to Neuropsychiatric Outcome in the Offspring. Biol. Psychiatry 79, e33–45 [DOI] [PubMed] [Google Scholar]
- 87.Calvigioni D et al. (2014) Neuronal substrates and functional consequences of prenatal cannabis exposure. Eur Child Adolesc Psychiatry 23, 931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Higuera-Matas A et al. (2015) Long-term consequences of perinatal and adolescent cannabinoid exposure on neural and psychological processes. Neurosci Biobehav Rev 55, 119–146 [DOI] [PubMed] [Google Scholar]
- 89.Huizink AC (2014) Prenatal cannabis exposure and infant outcomes: overview of studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 52, 45–52 [DOI] [PubMed] [Google Scholar]
- 90.Huizink AC and Mulder EJH (2006) Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev 30, 24–41 [DOI] [PubMed] [Google Scholar]
- 91.Trezza V et al. (2012) Altering endocannabinoid neurotransmission at critical developmental ages: impact on rodent emotionality and cognitive performance. Front Behav Neurosci 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Richardson GA et al. (1995) Prenatal alcohol, marijuana, and tobacco use: infant mental and motor development. Neurotoxicol Teratol 17, 479–487 [DOI] [PubMed] [Google Scholar]
- 93.El Marroun H et al. (2011) Intrauterine cannabis exposure leads to more aggressive behavior and attention problems in 18-month-old girls. Drug Alcohol Depend 118, 470–474 [DOI] [PubMed] [Google Scholar]
- 94.Leech SL et al. (1999) Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol 21, 109–118 [DOI] [PubMed] [Google Scholar]
- 95.Bolhuis K et al. (2018) Maternal and paternal cannabis use during pregnancy and the risk of psychotic-like experiences in the offspring. Schizophr. Res 202, 322–327 [DOI] [PubMed] [Google Scholar]
- 96.Gray KA et al. (2005) Prenatal marijuana exposure: effect on child depressive symptoms at ten years of age. Neurotoxicol Teratol 27, 439–448 [DOI] [PubMed] [Google Scholar]
- 97.Leech SL et al. (2006) Predictors and correlates of high levels of depression and anxiety symptoms among children at age 10. J Am Acad Child Adolesc Psychiatry 45, 223–230 [DOI] [PubMed] [Google Scholar]
- 98.Carey CE et al. (2016) Associations between Polygenic Risk for Psychiatric Disorders and Substance Involvement. Front Genet 7, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Power RA et al. (2014) Genetic predisposition to schizophrenia associated with increased use of cannabis. Mol. Psychiatry 19, 1201–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Day NL et al. (2006) Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction 101, 1313–1322 [DOI] [PubMed] [Google Scholar]
- 101.Porath AJ and Fried PA (2005) Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicol Teratol 27, 267–277 [DOI] [PubMed] [Google Scholar]
- 102.Sonon KE et al. (2015) Prenatal marijuana exposure predicts marijuana use in young adulthood. Neurotoxicol Teratol 47, 10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smith AM et al. (2004) Effects of prenatal marijuana on response inhibition: an fMRI study of young adults. Neurotoxicol Teratol 26, 533–542 [DOI] [PubMed] [Google Scholar]
- 104.García L et al. (1996) Perinatal Δ9-tetrahydrocannabinol exposure in rats modifies the responsiveness of midbrain dopaminergic neurons in adulthood to a variety of challenges with dopaminergic drugs. Drug and Alcohol Dependence 42, 155–166 [DOI] [PubMed] [Google Scholar]
- 105.Mereu G et al. (2003) Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc. Natl. Acad. Sci. U.S.A 100, 4915–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nguyen JD et al. (2016) Inhaled delivery of Δ(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology 109, 112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spear LP and File SE (1996) Methodological considerations in neurobehavioral teratology. Pharmacol. Biochem. Behav 55, 455–457 [DOI] [PubMed] [Google Scholar]
- 108.Navarro M et al. (1995) Behavioural consequences of maternal exposure to natural cannabinoids in rats. Psychopharmacology (Berl.) 122, 1–14 [DOI] [PubMed] [Google Scholar]
- 109.Antonelli T et al. (2005) Prenatal exposure to the CB1 receptor agonist WIN 55,212–2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb. Cortex 15, 2013–2020 [DOI] [PubMed] [Google Scholar]
- 110.Campolongo P et al. (2007) Perinatal exposure to delta-9-tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addict Biol 12, 485–495 [DOI] [PubMed] [Google Scholar]
- 111.Silva L et al. (2012) Prenatal tetrahydrocannabinol (THC) alters cognitive function and amphetamine response from weaning to adulthood in the rat. Neurotoxicol Teratol 34, 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O’Shea M et al. (2006) Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J. Psychopharmacol. (Oxford) 20, 611–621 [DOI] [PubMed] [Google Scholar]
- 113.Antonelli T et al. (2004) Long-term effects on cortical glutamate release induced by prenatal exposure to the cannabinoid receptor agonist (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinyl-methyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone: an in vivo microdialysis study in the awake rat. Neuroscience 124, 367–375 [DOI] [PubMed] [Google Scholar]
- 114.Castaldo P et al. (2010) Altered regulation of glutamate release and decreased functional activity and expression of GLT1 and GLAST glutamate transporters in the hippocampus of adolescent rats perinatally exposed to Delta(9)-THC. Pharmacol. Res 61, 334–341 [DOI] [PubMed] [Google Scholar]
- 115.Castaldo P et al. (2007) Prenatal exposure to the cannabinoid receptor agonist WIN 55,212–2 increases glutamate uptake through overexpression of GLT1 and EAAC1 glutamate transporter subtypes in rat frontal cerebral cortex. Neuropharmacology 53, 369–378 [DOI] [PubMed] [Google Scholar]
- 116.Ferraro L et al. (2009) Short- and long-term consequences of prenatal exposure to the cannabinoid agonist WIN55,212–2 on rat glutamate transmission and cognitive functions. J Neural Transm (Vienna) 116, 1017–1027 [DOI] [PubMed] [Google Scholar]
- 117.Tortoriello G et al. (2014) Miswiring the brain: Δ9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 33, 668–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Keimpema E et al. (2011) Molecular model of cannabis sensitivity in developing neuronal circuits. Trends Pharmacol Sci 32, 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frau R et al. Prenatal THC produces a hyperdopaminergic phenotype accompanied by maladaptive behavioral susceptibility. Nature Neuroscience [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Manduca A et al. Sex-specific behavioral deficits induced at early life by prenatal exposure to the CB1 cannabinoid receptor agonist WIN 55,212-2 depend on mGlu5 receptor signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scheyer AF et al. (2019) Cannabinoid exposure via lactation disrupts perinatal programming of the GABA trajectory and select early-life behaviors. In Revision, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bara A et al. (2018) Sex-dependent effects of in utero cannabinoid exposure on cortical function. Elife 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vargish GA et al. (2017) Persistent inhibitory circuit defects and disrupted social behaviour following in utero exogenous cannabinoid exposure. Mol. Psychiatry 22, 56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vela G et al. (1998) Maternal exposure to delta9-tetrahydrocannabinol facilitates morphine self-administration behavior and changes regional binding to central mu opioid receptors in adult offspring female rats. Brain Res. 807, 101–109 [DOI] [PubMed] [Google Scholar]
- 125.González B et al. (2003) Effects of perinatal exposure to delta 9-tetrahydrocannabinol on operant morphine-reinforced behavior. Pharmacol. Biochem. Behav 75, 577–584 [DOI] [PubMed] [Google Scholar]
- 126.Economidou D et al. (2007) Role of cannabinoidergic mechanisms in ethanol self-administration and ethanol seeking in rat adult offspring following perinatal exposure to Delta9-tetrahydrocannabinol. Toxicol. Appl. Pharmacol 223, 73–85 [DOI] [PubMed] [Google Scholar]
- 127.Zygmunt PM et al. (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457 [DOI] [PubMed] [Google Scholar]
- 128.Lo Verme J et al. (2005) The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol 67, 15–19 [DOI] [PubMed] [Google Scholar]
- 129.Sipe JC et al. (2002) A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc. Natl. Acad. Sci. U.S.A 99, 8394–8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dincheva I et al. (2015) FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun 6, 6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alpár A et al. (2014) Endocannabinoids modulate cortical development by configuring Slit2/Robo1 signalling. Nature Communications 5, 4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carey CE et al. (2015) Monoacylglycerol lipase (MGLL) polymorphism rs604300 interacts with childhood adversity to predict cannabis dependence symptoms and amygdala habituation: Evidence from an endocannabinoid system-level analysis. J Abnorm Psychol 124, 860–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Marrs WR et al. (2010) The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nature Neuroscience 13, 951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grant KS et al. (2018) Cannabis use during pregnancy: Pharmacokinetics and effects on child development. Pharmacol. Ther 182, 133–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gunn JKL et al. (2016) Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open 6, e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Noland JS et al. (2003) Executive Functioning in Preschool-Age Children Prenatally Exposed to Alcohol, Cocaine, and Marijuana. Alcohol Clin Exp Res 27, 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schauer GL and Peters EN (2018) Correlates and trends in youth co-use of marijuana and tobacco in the United States, 2005–2014. Drug Alcohol Depend 185, 238–244 [DOI] [PubMed] [Google Scholar]
- 138.Secades-Villa R et al. (2015) Probability and predictors of the cannabis gateway effect: a national study. Int. J. Drug Policy 26, 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.El-Mohandes A et al. (2003) Prenatal care reduces the impact of illicit drug use on perinatal outcomes. J Perinatol 23, 354–360 [DOI] [PubMed] [Google Scholar]
- 140.Jansson LM et al. (2018) Perinatal Marijuana Use and the Developing Child. JAMA 320, 545–546 [DOI] [PubMed] [Google Scholar]
- 141.Maccarrone M et al. (2014) Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat. Rev. Neurosci 15, 786–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Richardson KA et al. (2016) Prenatal cannabis exposure - The “first hit” to the endocannabinoid system. Neurotoxicology and Teratology 58, 5–14 [DOI] [PubMed] [Google Scholar]
- 143.Rubio P et al. (1998) Maternal exposure to low doses of delta9-tetrahydrocannabinol facilitates morphine-induced place conditioning in adult male offspring. Pharmacol Biochem Behav 61, 229–238 [DOI] [PubMed] [Google Scholar]
- 144.Campolongo P et al. (2011) Developmental consequences of perinatal cannabis exposure: behavioral and neuroendocrine effects in adult rodents. Psychopharmacology 214, 5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Manduca A et al. (2016) Interacting Cannabinoid and Opioid Receptors in the Nucleus Accumbens Core Control Adolescent Social Play. Front. Behav. Neurosci 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rios C et al. (2006) μ opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. British Journal of Pharmacology 148, 387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Eiden RD et al. (2018) Prenatal exposure to tobacco and cannabis: Effects on autonomic and emotion regulation. Neurotoxicology and Teratology 68, 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schuetze P et al. Prenatal exposure to tobacco and marijuana and child autonomic regulation and reactivity: An analysis of indirect pathways via maternal psychopathology and parenting. Developmental Psychobiology 0, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Subbaraman MS and Kerr WC (2015) Simultaneous versus concurrent use of alcohol and cannabis in the National Alcohol Survey. Alcohol. Clin. Exp. Res 39, 872–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Breit KR et al. The effects of alcohol and cannabinoid exposure during the brain growth spurt on behavioral development in rats. Birth Defects Research 0, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leung MCK et al. (2019) Adverse outcome pathway of developmental neurotoxicity resulting from prenatal exposures to cannabis contaminated with organophosphate pesticide residues. Reproductive Toxicology 85, 12–18 [DOI] [PubMed] [Google Scholar]
- 152.Suglia A et al. (2016) Bisphenol A induces hypothalamic down-regulation of the the cannabinoid receptor 1 and anorexigenic effects in male mice. Pharmacological Research 113, 376–383 [DOI] [PubMed] [Google Scholar]
- 153.Dalton VS et al. (2012) , Synergistic Effect between Maternal Infection and Adolescent Cannabinoid Exposure on Serotonin 5HT1A Receptor Binding in the Hippocampus: Testing the “Two Hit” Hypothesis for the Development of Schizophrenia. , International Scholarly Research Notices. [Online]. Available: https://www.hindawi.com/journals/isrn/2012/451865/. [Accessed: 19-Apr-2019] [DOI] [PMC free article] [PubMed] [Google Scholar]