Abstract
Despite advances in the pathological understanding of eosinophilic esophagitis (EoE), as of yet no single agent has been approved by the Food and Drug Agency to treat EoE. Off-label, EoE is currently treated by the three D’s, which are drugs (particularly swallowed topical corticosteroids), dietary restriction and endoscopic dilation. In the recent past, considerable progress in terms of EoE treatment has been made: 1) new EoE-specific steroid formulations optimizing mucosal deposition have been developed, which has culminated in the recent approval of a budesonide effervescent tablet (BET) in Europe; 2) biologics used for other Th2-mediated diseases such as allergic asthma and atopic eczema, as well as purpose-developed biologics, have been studied in phase II trials in EoE; and 3) novel dietary restriction strategies have evolved. Finally, further insights into the pathogenesis of EoE have revealed several novel disease mediators that might be targeted in the future. In the following article, we will discuss recent advances in EoE treatment with regards to swallowed topical steroids, biological agents, dietary approaches and novel molecular targets.
Keywords: Eosinophilic esophagitis, treatment, steroids, diet, biologics
INTRODUCTION
Eosinophilic esophagitis (EoE) is a chronic T-helper (Th) 2-mediated inflammatory disorder of the esophagus defined clinically by symptoms of esophageal dysfunction and histologically by an eosinophil-predominant infiltration of the esophageal mucosa.(1) Mechanistically, food and environmental factors interact with the esophageal epithelium stimulating the release of proinflammatory cytokines such as interleukin (IL)-33 and thymic stromal lymphopoietin (TSLP).(2) This leads to a Th2-predominent response that orchestrates the production and release of IL-4, IL-5, IL-13 and transforming growth factor beta (TGFb), which results in disruption of epithelial barrier function, tissue remodeling and eosinophil/mast cell infiltration. Intriguingly, next generation single-cell RNA sequencing has recently revealed T cell heterogeneity with two subtypes, putative T regulatory cells and effector Th2-like cells, being specifically enriched in active EoE.(3) The short-chain fatty acid receptor FFAR3 on these effector Th2-like cells has been identified as a key mediator for the amplification of local Th2 response in EoE.(3)
Despite major advances in the understanding of EoE, medications have not yet been approved by the United States (US) Food and Drug Agency (FDA) to treat EoE. Off-label, EoE is currently treated by drugs (particularly swallowed topical corticosteroids) and dietary restrictions that address the inflammatory response and esophageal dilation that ameliorates fibrostenotic consequences of disease. In the recent past, considerable progress in terms of EoE treatment has been made: 1) new EoE-specific steroid formulations optimizing mucosal deposition have been developed with recent approval of a budesonide effervescent tablet (BET) in Europe; 2) biologics approved for Th2-mediated diseases including allergic asthma and atopic eczema are undergoing phase II and III trials in EoE; and 3) novel dietary restriction strategies have evolved. Furthermore, insights into the pathogenesis of EoE have revealed novel disease mediators that might be targeted in the future.
In the following article, we will discuss recent advances in EoE treatment with regards to swallowed topical steroids, biological agents, dietary restriction strategies, and novel molecular targets.
NEW STEROID FORMULATIONS
Efficacy of swallowed topical corticosteroids in the short-term treatment of EoE has long been proven.(4) Steroids are one of the mainstays of EoE management and considered first-line treatment by many EoE specialists. Topical corticosteroids act through various effects. For example, IL-13-induced pathways and genes – key pathogenic mechanisms in EoE – are largely reversible by steroid treatment.(5) Further effects are reduction of eosophageal eosinophilia, epithelial cell apoptosis and mast cell infiltration, downregulation of mast cell genes, reduction in T-cells and proinflammatory cytokines such as tumor necrosis factor (TNF).(6) Topical corticosteroids can restore epithelial barrier function and positively affect tissue remodeling.(4, 7) Steroid compounds may have similar anti-eosinophil effects, as shown in a recent trial comparing outcomes in patients treated with oral viscous budesonide slurry 1mg b.i.d. or swallowed fluticasone powder 880mcg b.i.d. (histological response of 71 and 64% after 8 weeks of treatment, respectively).(8) However, to exhibit beneficial properties, correct deposition on the epithelial surface layer is crucial: viscous budesonide results in higher and longer exposure to the esophageal mucosa than does budesonide in a nebulized formulation.(9) In addition – despite steroids’ efficacy in the long-term – medication non-adherence appears to be an issue.(10) Therefore, novel formulations have been developed in the recent past in order to increase mucosal contact time and to simplify daily intake (Table 1).
Table 1:
New steroid formulations specifically developed for the treatment of EoE. DSQ, dysphagia symptom questionnaire; RCT, randomized-controlled trial.
| Compound | Studies | Outcome |
|---|---|---|
| Budesonide effervescent tablet | Short-term: | |
| Double blind RCT (n=88), 6- week treatment course with 1mg b.i.d. or placebo. | Clinico-histological remission 58% vs. 0% Histological remission 93% vs. 0% | |
| Long-term: | ||
| Double blind RCT (n=204), 48- week maintenance treatment with 1mg b.i.d., 0.5mg b.i.d. or placebo | Clinico-histological remission 75.0% vs. 73.5% vs. 4.4%. Histological relapse 10.3% vs. 13.2% vs. 89.7% | |
| Budesonide oral suspension | Short-term: | |
| Double blind RCT (n=93), 12- week treatment course with 2mg b.i.d. or placebo | Change in DSQ score −14.3 vs. −7.5. Histological response rates 39% vs. 3%. | |
| Long-term: | ||
| Open-label extension study for 24 weeks (2mg once daily for 12 weeks and optional dose increase 1.5-2.0mg b.i.d. for 12 weeks) | Maintenance of remission in 42%, 4% of short-term non-responders gained response |
In Europe, Miehlke et al. studied two different formulations of budesonide, effervescent tablets for orodispersable use (BET, 1mg b.i.d. and 2mg b.i.d.) and a viscous suspension (BVS, 2mg b.i.d.) in a randomized controlled phase II trial.(11) Histological remission rates after 2 weeks of treatment were high with each formulation (100% BET 2×1mg, 94.7% BET 2×2mg and 94.7% BVS 2×2mg) compared to placebo (0%). However, the orodispersable tablets (BET) were preferred by 80% of patients.(11) Based on these findings, the orodispersable compound was further evaluated in two phase III trials (short-term study in 88 adult patients; long-term study in 204 adult patients). A 6-week course with BET 1mg b.i.d. resulted in 58% clinico-histological remission compared to 0% in the placebo group.(12) Extension for another 6 weeks in initial non-responders increased the remission rates up to 85%.(12) In the long-term, a 48-week treatment with BET 1mg b.i.d. and BET 0.5mg b.i.d. was able to maintain clinico-histological remission in 75.0 and 73.5% compared to 4.4% in the placebo group, respectively.(13) In the US, a novel muco-adherent budesonide formulation (budesonide oral suspension, BOS) has been studied in a multicenter phase II trial (93 adolescent and adult patients). A 12-week treatment with BOS 2mg b.i.d. resulted in significant symptomatic improvement and induced histological remission in 39% of patients compared to 3% in the placebo group.(14) Efficacy was proven in the long-term also: An open-label extension study for 24 weeks revealed maintenance of histological remission in 42%.(15) Very recently, the phase III data were published in abstract form demonstrating histological and clinical response in 53.1 and 52.6%, respectively (compared to placebo rates of 1 and 39.1%).(16) In both the US and European studies, side-effects were negligible with no significant impact on serum cortisol levels and occurrence of esophageal candidiasis in only 2–11%. Finally, a dissolvable fluticasone tablet has also shown promising results in an early phase 1b/2a study.(17)
Recent insights on how long to treat EoE patients comes from the Swiss group. Cessation of steroid treatment in patients with deeply controlled disease (combination of clinical, endoscopic and histological remission for at least 6 months) resulted in a relapse in 81% within a median of 22 weeks.(18) Similar results were seen in an observation phase of the above-mentioned budesonide vs fluticasone study.(19) Long-term maintenance treatment should therefore be recommended. Dose reduction might be reasonable as suggested by Butz and colleagues where a 50% dose reduction in fluticasone was able to maintain complete remission in 73%, (20) although dose finding trials are still lacking in the long-term.
BIOLOGICS
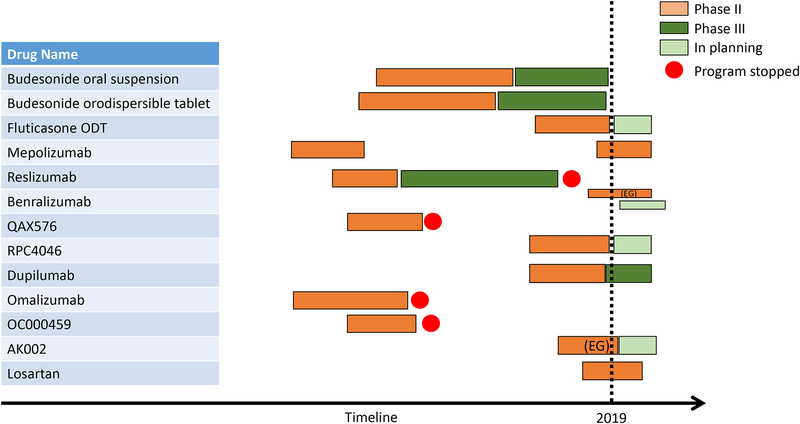
Despite emerging data about the high efficacy of novel steroid formulations, several issues remain: a considerable proportion of steroid-refractory patients can still be observed; adherence rates to daily medication, particularly in the long-term, are low; and response to steroids might be lost over time. In light of these limitations, biological agents targeting key cytokines such as IL-4, −5, and −13, as well as Immunoglobulin (Ig) E and TNF have been studied for the treatment of EoE. Some of the drug development programs have successfully progressed to phase III trials (Figure 1).
FIGURE 1:
Development pipeline of EoE-specific programs over the last two decades with progression from phase I/II to phase III stage. EG, eosinophilic gastritis.
Anti-IL5
IL-5 is involved in eosinophil trafficking and eosinophil survival. Two anti-IL5 antibodies, mepolizumab and reslizumab, have been studied for the treatment of active EoE with various degree of success. While a first study involving 4 adult patients suggested histological and symptomatic response with mepolizumab, a subsequent randomized-controlled trial in 11 adult patients demonstrated a significant decrease in esophageal eosinophilia by 54% and reduced levels of TGFb as a marker of tissue remodeling, but only minor symptomatic improvement.(21, 22) Similarly, a larger randomized-controlled trial evaluating three different doses of mepolizumab in 59 children revealed histological response (89.5% with achieving a mean eosinophil count <20eos/hpf), but very limited clinical improvement (though it should be noted symptoms were not a primary outcome nor were validated measures used in this set of studies).(23) Similarly to mepolizumab, reslizumab treatment significantly reduced esophageal eosinophilia in a randomized controlled trial including 227 children and adolescents compared to placebo, but without an effect on clinical activity judged by the blinded treating physician.(24) However, at one participating center 6 patients continued with reslizumab as part of an open-label extension and 4 continued through compassionate use. Over a follow-up of 9 years, Markowitz and colleagues demonstrated both histological response (median eosinophil count of 35 vs. 3 per hpf) and considerable symptomatic improvement in terms of dysphagia, abdominal pain, heartburn, vomiting and reflux.(25) These data lack of a control group and should therefore be interpreted cautiously. Benralizumab, an eosinophil depleting antibody that induces antibody-dependent cellular toxicity (through IL5 receptor alpha subunit) has been recently granted orphan drug designation for the treatment of EoE. While no data from randomized-controlled trials in EoE are available so far, in a small trial in patients with hypereosinophilic syndrome, a subset had gastrointestinal involvement with eosinophilia, which normalized after benralizumab treatment.(26)
Anti-IL13
Interleukin-13 secreted by Th2 cells is a key mediator in EoE pathogenesis. Studies have revealed markedly elevated IL-13 mRNA levels in esophageal biopsies from patients with active EoE compared to healthy controls. Of note, in vitro treatment of esophageal epithelial cells with IL-13 results in a transcriptomic profile largely overlapping the EoE-specific transcriptome.(5) IL-13 activates eosinophils and promotes eosinophil chemotaxis through increasing levels of eotaxin-3 and periostin.(5, 27) Two monoclonal antibodies directly targeting IL-13 have been studied in active EoE, QAX576 and RPC4046. QAX576 was evaluated in a randomized-controlled trial including 25 adult patients.(28) While the trial did not meet the primary endpoint (>75% reduction in peak eosinophil counts), patients in the verum group showed a significant decrease in terms of esophageal eosinophilia (−60%) compared to patients randomized to the placebo group (+23%).(28) Furthermore, improvement in the EoE-transcriptome was demonstrated with QAX576. In terms of symptomatic improvement some trends were seen (decrease in frequency and severity of dysphagia), but overall there was no significant effect of QAX576 on clinical disease activity (though this outcome was not primary).(28) In contrast to QAX576, a randomized-controlled trial (n=99) with the anti-IL-13 antibody RPC4046 (180 or 360mg sc) resulted in a significant reduction of esophageal eosinophilia and endoscopic disease activity; there was also a strong trend towards reduction of dysphagia symptoms (p=0.07) in.(29) It is notable that in this study, half of enrolled patients were previously refractory to topical steroids but that had the same promising response rate to the biologic as the non-refractory patients. Indeed, the validated EEsAI PRO score significantly improved in patients with previous steroid failure.(29) RPC4046 was also used in a 52 week-long open label extension where response was maintained for the treatment period and the medication was generally well tolerated over this timeframe.(30)
Anti-IL13 and IL4 through inhibition of the shared IL-4 receptor alpha
Similarly to IL-13, IL-4 has been shown to be a key driver in Th2-mediated diseases. Through JAK-STAT signaling pathways, IL-4 leads to Th2 cell differentiation and IgE class switching in B cells.(31) In EoE, IL-4 is significantly upregulated in the esophageal mucosa and is among 8 key cytokines elevated in blood samples of EoE patients that unambiguously differentiate EoE from healthy controls.(32) Dupilumab is a monoclonal antibody that antagonizes both IL-4 and IL-13 as its target, the IL-4 receptor alpha, is shared by both the IL-13 and IL-4 receptor. It has been recently evaluated in a randomized-controlled phase II trial including 47 adult patients.(33) 12-week treatment with dupilumab resulted in a significant symptomatic, endoscopic and histological improvement.(31)
Anti-IgE and anti-TNF
Several findings encouraged the use of anti-IgE antibodies and anti-TNF as treatment for active EoE: 1) EoE is frequently associated with IgE-mediated allergic diseases; 2) increased levels of IgE positive cells are detected on biopsies from patients with active EoE; and 3) TNF is highly upregulated in esophageal epithelial cells of patients with active EoE.(31) Consistently, a first open label trial in 15 patients with the anti-IgE antibody omalizumab showed promising results with a clinico-histological remission in 33%.(34) However, a subsequent randomized-controlled trial in 30 patients did not reveal any significant benefit with regards to symptoms and histological disease activity after a 16-week treatment course.(35) Similarly, an open-label study including 3 male patients failed to show any beneficial effect of the anti-TNF antibody infliximab.(36) Based on these small trials, the use of omalizumab and infliximab for EoE is not supported.
SMALL MOLECULES
Several small molecules have been studied in the treatment of EoE. These therapies aim to target various inflammatory mechanisms such as Th2 cell function, mast cell degranulation, and eosinophil chemotaxis. Two small molecules were promising given their role in allergic disorders, the mast cell stabilizer cromolyn sodium and the leukotriene antagonist montelukast; though both have failed to show efficacy in randomized-controlled trials in EoE patients.(37, 38)
Chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) antagonist
Given the key involvement of Th2 cells in EoE pathogenesis and the high expression of CRTH2 on these cells, blocking CRTH2 appears to be a rational therapeutic approach. Straumann et al. evaluated the selective CRTH2 antagonist OC000459 in a randomized controlled trial including 26 patients.(39) After an 8-week treatment, esophageal eosinophilic infiltration was significantly reduced (by 36%) in the treatment but not placebo group.(39) In addition, global assessment of disease activity by treating physicians improved with OC000459, but not with placebo.(39) However, patient reported outcomes did not differ significantly between the two groups, and no effect on endoscopy was observed.(39) Since beneficial effects on eosinophilic infiltration are only modest and in light of more potent alternatives (steroids), this agent has yet to be evaluated any further in EoE management.
POTENTIAL MOLECULAR TARGETS
Based on in vitro findings and data emerging from studies in other Th2-mediated disorders, numerous potential therapeutic targets have been suggested for EoE (Figure 2). When considering these agents, it should be realized that positive effects on eosinophils have to be contextualized with symptom and endoscopic improvements, ideally using validated outcome measures. Ongoing or future clinical trials will answer the question whether or not targeting these proposed targets have positive effects in terms of clinical, endoscopic and histological disease activity.
FIGURE 2:
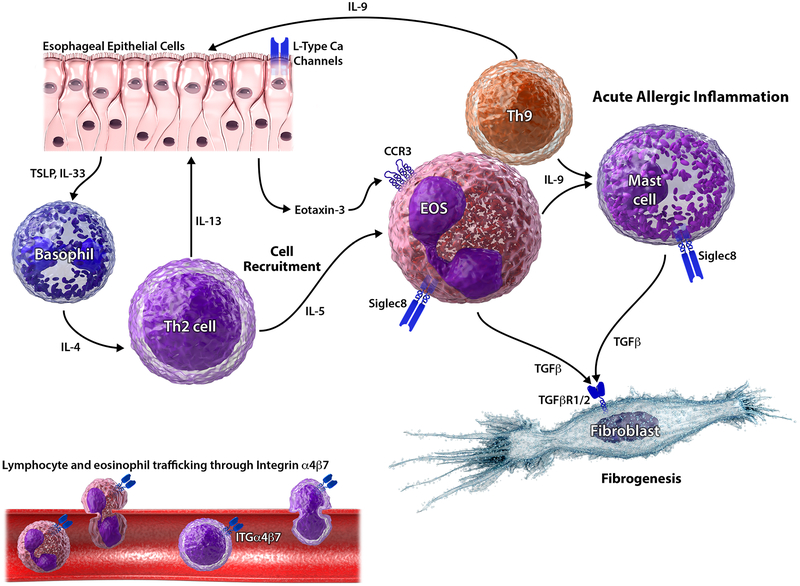
Potential therapeutic targets in EoE. In a simplified model, esophageal epithelial cell-released TSLP and IL-33 can trigger Th2 response through involvement of basophils and IL-4. Th2 cells secrete IL-5 which – together with calcium channel-mediated eotaxin release from epithelial cells – results in eosinophil recruitment. Integrin α4β7 is expressed on lymphocytes and eosinophils. By binding to its vascular counterpart MAdCAM1, it mediates cell trafficking to the esophageal mucosa. Secretion of IL-9 (from eosinophils and Th9 cells) further drives epithelial barrier disruption (together with IL-13 from Th2 cells) and mast cell recruitment. Release of TGFβ (secreted by eosinophils and mast cells) perpetuates eosinophilic infiltration and drives tissue remodeling through activation of fibroblasts. Siglec-8 is selectively expressed on eosinophils and mast cells with a key role in apoptosis.
Integrin α4β7
Integrin α4β7 is involved in lymphocyte trafficking to the site of inflammation by binding to its counterpart, the adhesion molecule MAdCAM1, which is selectively expressed on endothelial cells in the intestinal tract. Vedolizumab, an anti-integrin α4β7 antibody, blocks its interaction with MadCAM1 and binds with high affinity to CD4 positive T-cells and eosinophils. In addition, vedolizumab inhibits αE(CD103)/β7 integrin, a marker of intraepithelial T lymphocytes.(40) Lately, Wen and colleagues revealed that esophageal Th2 cells are also αE positive.(3) Thus αE(CD103)/β7 integrin might serve as therapeutic target in the future. Vedolizumab’s potential role in EoE management has been suggested by two case reports of patients with concomitant inflammatory bowel disease and EoE, where treatment resulted in clinical and histological response.(41, 42) Its anti-eosinophil effects have further been demonstrated in two case series on eosinophilic gastroenteritis.
Sialic acid-binding immunoglobulin-like (Siglec) 8
Siglec-8 is selectively expressed on eosinophils, mast cells and basophils with a key role in apoptosis.(31) Preclinical data indicate anti-eosinophil properties of anti-Siglec-8 antibodies. Two different anti-Siglec-8 antibodies were able to induce eosinophil cell death in vitro, particularly in the presence of IL-5 stimulation.(43) In mouse models, treatment with anti-Siglec-8 resulted in depletion of IL-5 induced eosinophilia.(43) Moreover, anti-Siglec-8 antibody was shown to reduce both eosinophil and mast cell infiltration in the stomach and small intestine in a mouse model of eosinophilic gastroenteritis.(44) Very recently, a phase II trial evaluated the potential of anti-Siglec-8 (AK002) in eosinophilic gastritis and enteritis demonstrating depletion of gastrointestinal tissue eosinophilia and improvement of symptoms.(45) Data on AK002’s efficacy in EoE are currently not available.
Thymic Stromal Lymphopoietin (TSLP)
TSLP is an epithelial-derived cytokine, which is upregulated in active EoE.(2) It functions as a Th2 switch and thereby promotes a Th2-weighted inflammatory response.(31) Moreover, the chief EoE genetic susceptibility locus has been identified within the TSLP gene suggesting a crucial role for TSLP in EoE pathogenesis.(46, 47) Anti-TSLP has been demonstrated to block esophageal eosinophilia and food impactions in murine models for EoE.(48) The TSLP antibody tezepelumab has been successfully studied in severe uncontrolled asthma, but has yet to be studied in EoE.
Transforming growth factor beta (TGFβ)
TGFβ is known as a key regulator of fibrogenesis and has been shown to be involved in tissue remodeling and eosinophil recruitment in EoE.(49) It therefore represents an interesting target to treat both inflammatory and fibrostenotic aspects of EoE. Losartan, an angiotensin II receptor subtype 1 antagonist, can block TGFβ signaling and is currently studied in an open-label trial for EoE and eosinophilic gastroenteritis.(50)
Calcium channels
Very recently, the calcium channel blocker verapamil has been shown to attenuate IL-4 induced eotaxin expression in esophageal epithelial cells (in vitro).(51) Thus, calcium channels are a potential target to treat eotaxin-mediated eosinophil recruitment. No clinical data are available so far.
Eotaxin receptor anti CCR3
Eotaxin is a key driver of eosinophil recruitment to the esophageal mucosa. In mouse models, CCR3 antibody has been demonstrated to inhibit eosinophil inflammation and mucosal injury in eosinophilic gastroenteritis.(52) However, at least in asthma, a proof-of-mechanism study failed to show efficacy of the CCR3 antibody AXP1275.(53)
Interleukins 9, 15 and 33
Several interleukins besides IL-4, −5 and −13 have been shown to be involved in EoE pathogenesis and are thus considered potential therapeutic targets: 1) IL-9 is a major driver of epithelial barrier disruption and mast cell recruitment, key events in EoE;(54) 2) IL-15 expression is increased in human EoE and mediates EoE pathogenesis in in-vivo models;(55) and 3) elevated IL-33 expression is associated with pediatric EoE, while exogenous IL-33 promotes EoE development in mice.(2) Anti-IL-9 has been studied in asthma, where it does not appear to be of significant effect. The anti-IL-15 antibody CALY-002 has been granted orphan drug designation for the treatment of EoE by the European Medical Agency EMA. However, clinical data for its efficacy in EoE treatment have not been available yet. Similarly, there are no clinical data on the use of anti-IL-33 agents in EoE.
NEW DIETARY APPROACHES
Since EoE is considered a food-triggered disorder, dietary restriction has been extensively studied as an EoE treatment modality. Despite proven effectiveness of restrictive dietary approaches such as the 6-food elimination diet, several issues remain: 1) introduction and maintenance of dietary restrictions can be challenging; 2) quality of life of patients and their families might be affected; and 3) multiple endoscopies are required to finally identify one or multiple food triggers. IgE-based allergy tests have demonstrated very limited accuracy in the prediction of food triggers in EoE. Thus the clinical use of the empiric elimination diets is currently favored over allergy-testing directed diet therapy in EoE.
Step-up approach
Given the limitations in the use of empiric 6-food elimination diet, alternative strategies have been developed with the goal of less restrictive diets and greater efficiency in terms of food trigger identification. A 4-food elimination diet eliminating dairy, wheat, egg and soy/legumes has been shown to be effective in 54–64%.(56) Since milk frequently represents the culprit food in children with EoE, cow milk elimination attempts have been made. Recently, a randomized-controlled trial in 63 children has shown similar histological remission rates for milk elimination compared to a 4 food elimination.(57) Symptomatic improvement was seen in both groups, although to a greater extent in patients following the 4 food diet.(57) As it appears reasonable to assume that less restrictive dietary strategies are also less effective, step-up strategies have been developed. The 2-4-6 food elimination approach resulted in remission rates of 43% after elimination of 2 foods.(58) Step-up in non-responders to a 4-food and finally 6-food elimination diet resulted in similar remission rates than previously reported. Moreover, endoscopic procedures and diagnostic processing time were reduced by 20%. More recently, a computer-based simulation revealed that a 1–3 food (dairy followed by wheat, egg, dairy elimination) and a 1-4-8 food elimination step up approach (dairy followed by wheat, egg, dairy, soy, and then wheat, egg, dairy, soy, corn, chicken, beef and pork elimination) are most efficient in terms of culprit food identification.(59) Efficacy and efficiency of the latter two strategies have yet to be proven in clinical trials.
Targeted elimination
Since the IgE-based allergy tests have failed to predict response to elimination diets, novel strategies have been developed. Two of them show promising results. Based on evidence demonstrating an association of EoE with IgG4, food specific IgG4 from esophageal biopsies have been used to predict response to dietary restriction.(60) While there was high specificity for this strategy, the sensitivities, particularly for peanuts and soy, were low. Diagnostic accuracy could be increased by adding a second test, a lymphocyte proliferation assay performed on patient serum. A positive response on either test resulted in agreement between tests and elimination diet results of 53–75%, which is considerably higher than for classical allergy test modalities. A more invasive approach reported by the Amsterdam group is the esophageal skin prick test, where allergens are directly injected into the esophageal mucosa during upper endoscopy.(61) Reaction of the esophagus was seen in 5/8 (immediate) and 2/8 patients (delayed). Whether these reactions correlate with results from elimination diet strategies have yet to be determined.
FUTURE PERSPECTIVES
With increasing evidence for the efficacy of novel steroid formulations as well as of emerging biological agents, the question will arise where to position biological treatment. Looking at other inflammatory disorders such as asthma and inflammatory bowel diseases, where biological agents have been successfully introduced alongside topical steroid treatments, the following three positions within the treatment algorithm are conceivable for these agents: 1) treatment of steroid-refractory patients; 2) maintenance of steroid-induced remission; and 3) treatment of EoE patients with atopic comorbidities. At first glance and in light of histological response rates of up to 93%, the relevance of steroid-refractoriness appears to be limited with esophageal-specific formulations. However, significant heterogeneity in the shortterm response rates to steroids has been noted with histologic efficacy of around 50% in some studies. Furthermore, response rates to steroid treatment are considerably lower in long-term studies, and loss of response might be a particular concern in chronic management. In addition, need for daily intake can lead to medication nonadherence. Supervised and scheduled drug application every two to four weeks might result in better adherence and thus higher longterm remission rates. Moreover, biologic therapies might be an option for patients with several atopic comorbidities, where Th2-pathway directed strategies could target multiple diseases with a single agent. This would obviate the need for multiple formulations of topical steroids (i.e. swallowed for EoE, inhaled for asthma, cutaneous for eczema). Importantly, the optimal use of biologics in EoE will undoubtably be affected by the ongoing results of clinical trials regarding their long term safety, efficacy, and cost. Characterization of EoE endotypes in order to predict future disease course, need for aggressive treatment and response to therapies will further help to personalize EoE management. Molecular fingerprinting of EoE using the EoE diagnostic panel (EDP) has been demonstrated to identify three clusters of mRNA profiles associated with distinct phenotypes.(62) Such stratification might pave the road to individually tailored treatment strategies and precision medicine in EoE.
CONCLUSIONS
Newer formulations of swallowed topical corticosteroids have been shown to be highly efficacious in the short- and long-term management of EoE, which has led to the first approved EoE-specific treatment in Europe. In addition, several studies have proven high efficacy of dietary approaches, which appears to be of similar magnitude as seen with steroids. However, empirical food elimination results in a very restrictive diet and a high endoscopic burden until the culprit food is identified. New approaches such as the 2-4-6 food elimination (step up approach) are similarly efficacious but more efficient. Despite high efficacy of steroid formulations and dietary restrictions, a considerable proportion of patients do not achieve or maintain clinico-histological remission, particularly in the long-term. Biological agents might be an effective treatment alternative, anti-IL13 and anti-IL4r antibodies are most promising with phase 2 data to date. Several newer targets have been identified and are currently tested in clinical trials such as the AT1 receptor (losartan) or Siglec-8 (anti-Siglec-8).
Financial support:
This work was supported by a grant from the Swiss National Science Foundation to TG (grant no. P2ZHP3_168561), a young investigator award from the Swiss Society of Gastroenterology to TG, a research grant from the Novartis Foundation for medical-biological research to TG, and a training grant from the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) to TG. CEGIR (U54 AI117804) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED, CURED and EFC
Abbreviations
- AT1 receptor
angiotensin II receptor type 1
- BET
budesonide effervescent tablet
- b.i.d.
two times per day
- BOS
budesonide oral suspension
- BVS
budesonide viscous suspension
- CCR3
C-C chemokine receptor type 3
- CD4
cluster of differentiation 4
- CRTH2
Chemoattractant receptor-homologous molecule expressed on Th2 cells
- DSQ
dysphagia symptom questionnaire
- EDP
EoE diagnostic panel
- EEsAI PRO
EoE activity index patient reported outcome
- EG
eosinophilic gastritis
- EMA
European Medical Agency
- EoE
Eosinophilic esophagitis
- eos/hpf
number of eosinophils per high power field
- FDA
United States Food and Drug Agency
- Ig
Immunoglobulin
- IL
Interleukin
- MAdCAM1
mucosal vascular addressin cell adhesion molecule 1
- mRNA
messenger RNA
- Siglec 8
Sialic acid-binding immunoglobulin-like 8
- TGFb
transforming growth factor beta
- Th
T helper cell
- TNF
tumor necrosis factor
- TSLP
thymic stromal lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: TG has consulting contracts with Sanofi-Regeneron and Falk Pharma GmbH, received travel grants from Falk Pharma GmbH and Vifor, and an unrestricted research grant from Novartis. IH has received consulting fees from Receptos, Regeneron, Shire and Roche. ESD has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, Shire; consulting fees from Adare, Aimmune, Alivio, Allakos, AstraZeneca, Banner, Biorasi, Calypso, Celgene/Receptos, Enumeral, EsoCap, Gossamer Bio, GSK, Regeneron, Robarts, Salix, Shire; and educational grants from Allakos, Banner, Holoclara.
REFERENCES
- 1.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20.e6; quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 2.Travers J, Rochman M, Miracle CE, Cohen JP, Rothenberg ME. Linking impaired skin barrier function to esophageal allergic inflammation via IL-33. J Allergy Clin Immunol. 2016;138(5):1381–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen T, Aronow BJ, Rochman Y, Rochman M, Kc K, Dexheimer PJ, et al. Single-cell RNA sequencing identifies inflammatory tissue T cells in eosinophilic esophagitis. J Clin Invest. 2019;129(5):2014–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straumann A, Conus S, Degen L, Felder S, Kummer M, Engel H, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139(5):1526–37, 37.e1. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Lu TX, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120(6):1292–300. [DOI] [PubMed] [Google Scholar]
- 6.Greuter T, Alexander JA, Straumann A, Katzka DA. Diagnostic and Therapeutic Long-term Management of Eosinophilic Esophagitis-Current Concepts and Perspectives for Steroid Use. Clin Transl Gastroenterol. 2018;9(12):e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katzka DA, Tadi R, Smyrk TC, Katarya E, Sharma A, Geno DM, et al. Effects of topical steroids on tight junction proteins and spongiosis in esophageal epithelia of patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12(11):1824–9.e1. [DOI] [PubMed] [Google Scholar]
- 8.Dellon ES, Woosley JT, Arrington A, McGee SJ, Covington J, Moist SE, et al. Efficacy of Budesonide vs Fluticasone for Initial Treatment of Eosinophilic Esophagitis in a Randomized Controlled Trial. Gastroenterology. 2019;157(1):65–73.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellon ES, Sheikh A, Speck O, Woodward K, Whitlow AB, Hores JM, et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology. 2012;143(2):321–4.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greuter T, Safroneeva E, Bussmann C, Biedermann L, Vavricka SR, Katzka DA, et al. Maintenance Treatment Of Eosinophilic Esophagitis With Swallowed Topical Steroids Alters Disease Course Over A 5-Year Follow-up Period In Adult Patients. Clin Gastroenterol Hepatol. 2019;17(3):419–28.e6. [DOI] [PubMed] [Google Scholar]
- 11.Miehlke S, Hruz P, Vieth M, Bussmann C, von Arnim U, Bajbouj M, et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut. 2016;65(3):390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucendo AJ, Miehlke S, Schlag C, Vieth M, von Arnim U, Molina-Infante J, et al. Efficacy of Budesonide Orodispersible Tablets as Induction Therapy for Eosinophilic Esophagitis in a Randomized Placebo-Controlled Trial. Gastroenterology. 2019;157(1):74–86.e15. [DOI] [PubMed] [Google Scholar]
- 13.Lucendo A, Miehlke S, Vieth M, Schlag S, Biedermann L, Santander C, et al. Budesonide orodispersable tablets are highly effective to maintain clinico-histological remission in adult patients with eosinophilic esophagitis: Results from the 48-weeks, double-blind, placebo-controlled, pivotal EOS-2 trial. Gastroenterology. 2019; 156(6, Suppl. 1):S–1509. [Google Scholar]
- 14.Dellon ES, Katzka DA, Collins MH, Hamdani M, Gupta SK, Hirano I, et al. Budesonide Oral Suspension Improves Symptomatic, Endoscopic, and Histologic Parameters Compared With Placebo in Patients With Eosinophilic Esophagitis. Gastroenterology. 2017;152(4):776–86.e5. [DOI] [PubMed] [Google Scholar]
- 15.Dellon ES, Katzka DA, Collins MH, Gupta SK, Lan L, Williams J, et al. Safety and Efficacy of Budesonide Oral Suspension Maintenance Therapy in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2019;17(4):666–73.e8. [DOI] [PubMed] [Google Scholar]
- 16.Hirano I, Collins M, Katzka D, Mukkada V, Falk G, Williams J, et al. Efficacy of budesonide oral suspension for eosinophilic esophagitis in adolescents and adults: Results from a Phase 3 randomized, placebo-controlled trial Am J Gastroenterol. In press, 2019. Oral presentation, ACG meeting 2019. [Google Scholar]
- 17.Hirano I, Schoepfer A, Commer G, Safroneeva E, Meltzer B, Falk G. A Randomized, Double-Blind, Placebo-Controlled Trial of a Fluticasone Propionate Orally Disintegrating Tablet in Adult and Adolescent Patients with Eosinophilic Esophagitis: A Phase 1/2A Safety and Tolerability Study. Gastroenterology 2017;152 (Suppl 1):S195. [Google Scholar]
- 18.Greuter T, Bussmann C, Safroneeva E, Schoepfer AM, Biedermann L, Vavricka SR, et al. Long-Term Treatment of Eosinophilic Esophagitis With Swallowed Topical Corticosteroids: Development and Evaluation of a Therapeutic Concept. Am J Gastroenterol. 2017;112(10):1527–35. [DOI] [PubMed] [Google Scholar]
- 19.Dellon ES, Woosley JT, Arrington A, McGee SJ, Covington J, Moist SE, et al. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Doubleblind, Double-dummy Trial. Clin Gastroenterol Hepatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butz BK, Wen T, Gleich GJ, Furuta GT, Spergel J, King E, et al. Efficacy, dose reduction, and resistance to high-dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology. 2014;147(2):324–33.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, Buckmeier BK, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118(6):1312–9. [DOI] [PubMed] [Google Scholar]
- 22.Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59(1):21–30. [DOI] [PubMed] [Google Scholar]
- 23.Assa’ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141(5):1593–604. [DOI] [PubMed] [Google Scholar]
- 24.Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(2):456–63, 63. e1–3. [DOI] [PubMed] [Google Scholar]
- 25.Markowitz JE, Jobe L, Miller M, Frost C, Laney Z, Eke R. Safety and Efficacy of Reslizumab for Children and Adolescents With Eosinophilic Esophagitis Treated for 9 Years. J Pediatr Gastroenterol Nutr. 2018;66(6):893–7. [DOI] [PubMed] [Google Scholar]
- 26.Kuang FL, Legrand F, Makiya M, Ware J, Wetzler L, Brown T, et al. Benralizumab for. N Engl J Med. 2019;380(14):1336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1(4):289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;135(2):500–7. [DOI] [PubMed] [Google Scholar]
- 29.Hirano I, Collins MH, Assouline-Dayan Y, Evans L, Gupta S, Schoepfer AM, et al. RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis. Gastroenterology. 2019;156(3):592–603.e10. [DOI] [PubMed] [Google Scholar]
- 30.Dellon ES, Collins MH, Assouline-Dayan Y, Evans L, Gupta S, Schoepfer A, et al. Long-Term Efficacy and Safety of RPC4046, an Anti-Interleukin-13 Monoclonal Antibody, in Patients With Eosinophilic Esophagitis: Results From the Open-Label Extension of the HEROES Study. J Allergy Clin Immunol 2019; 143 (2, Suppl): AB208. [Google Scholar]
- 31.de Rooij WE, Dellon ES, Parker CE, Feagan BG, Jairath V, Ma C, et al. Pharmacotherapies for the Treatment of Eosinophilic Esophagitis: State of the Art Review. Drugs. 2019;79(13):1419–34. [DOI] [PubMed] [Google Scholar]
- 32.Blanchard C, Stucke EM, Rodriguez-Jimenez B, Burwinkel K, Collins MH, Ahrens A, et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127(1):208–17, 17.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirano I, Dellon E, Hamilton J, Collins M, Peterson K, Chehade M, et al. Efficacy of Dupilumab in a Phase 2 Randomized Trial of Adults With Active Eosinophilic Esophagitis.Gastroenterology. In press, 2019. [DOI] [PubMed] [Google Scholar]
- 34.Loizou D, Enav B, Komlodi-Pasztor E, Hider P, Kim-Chang J, Noonan L, et al. A pilot study of omalizumab in eosinophilic esophagitis. PLoS One. 2015;10(3):e0113483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147(3):602–9. [DOI] [PubMed] [Google Scholar]
- 36.Straumann A, Bussmann C, Conus S, Beglinger C, Simon HU. Anti-TNF-alpha (infliximab) therapy for severe adult eosinophilic esophagitis. J Allergy Clin Immunol. 2008;122(2):425–7. [DOI] [PubMed] [Google Scholar]
- 37.Lieberman JA, Zhang J, Whitworth J, Cavender C. A randomized, double-blinded, placebo-controlled study of the use of viscous oral cromolyn sodium for the treatment of eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2018;120(5):527–31. [DOI] [PubMed] [Google Scholar]
- 38.Alexander JA, Ravi K, Enders FT, Geno DM, Kryzer LA, Mara KC, et al. Montelukast Does not Maintain Symptom Remission After Topical Steroid Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2017;15(2):214–21.e2. [DOI] [PubMed] [Google Scholar]
- 39.Straumann A, Hoesli S, Bussmann C, Stuck M, Perkins M, Collins LP, et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68(3):375–85. [DOI] [PubMed] [Google Scholar]
- 40.Hardenberg JB, Braun A, Schön MP. A Yin and Yang in Epithelial Immunology: The Roles of the a. J Invest Dermatol. 2018;138(1):23–31. [DOI] [PubMed] [Google Scholar]
- 41.Taft TH, Mutlu EA. The Potential Role of Vedolizumab in Concomitant Eosinophilic Esophagitis and Crohn’s Disease. Clin Gastroenterol Hepatol. 2018;16(11):1840–1. [DOI] [PubMed] [Google Scholar]
- 42.Nhu QM, Chiao H, Moawad FJ, Bao F, Konijeti GG. The Anti-α4β7 Integrin Therapeutic Antibody for Inflammatory Bowel Disease, Vedolizumab, Ameliorates Eosinophilic Esophagitis: a Novel Clinical Observation. Am J Gastroenterol. 2018;113(8):1261–3. [DOI] [PubMed] [Google Scholar]
- 43.Legrand F, Cao Y, Wechsler JB, Zhu X, Zimmermann N, Rampertaap S, et al. Sialic acid-binding immunoglobulin-like lectin (Siglec) 8 in patients with eosinophilic disorders: Receptor expression and targeting using chimeric antibodies. J Allergy Clin Immunol. 2019;143(6):2227–37.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youngblood BA, Brock EC, Leung J, Falahati R, Bochner BS, Rasmussen HS, et al. Siglec-8 antibody reduces eosinophil and mast cell infiltration in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dellon E, Peterson K, Murray J, Falk G, Gonsalves N, Chehade M, et al. Efficacy and Safety of AK002 in Adult Patients with Active Eosinophilic Gastritis and Eosinophilic Enteritis: Primary Results from a Randomized, Double-Blind Placebo-Controlled Phase 2 Trial (ENIGMA Study) Am J Gastroenterol. In press, 2019. Oral presentation, ACG meeting 2019. [Google Scholar]
- 46.Sherrill JD, Gao PS, Stucke EM, Blanchard C, Collins MH, Putnam PE, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126(1):160–5.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42(4):289–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19(8):1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abonia JP, Franciosi JP, Rothenberg ME. TGF-β1: Mediator of a feedback loop in eosinophilic esophagitis--or should we really say mastocytic esophagitis? J Allergy Clin Immunol. 2010;126(6):1205–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng K, Gupta SK, Kantor S, Kuhl JT, Aceves SS, Bonis PA, et al. Creating a multi-center rare disease consortium - the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). Transl Sci Rare Dis. 2017;2(3–4):141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Odiase E, Zhang X, Chang Y, Huo X, Zhang Q, Pan Z, et al. L-type calcium channel inhibitors (verapamil and diltiazem) block Th2-cytokine-stimulated eotaxin-3 secretion in esophageal squamous cells from patients with eosinophilic esophagitis. Gastroenterology. 2019;156(6, Suppl1): S–39.31462210 [Google Scholar]
- 52.Song DJ, Shim MH, Lee N, Yoo Y, Choung JT. CCR3 Monoclonal Antibody Inhibits Eosinophilic Inflammation and Mucosal Injury in a Mouse Model of Eosinophilic Gastroenteritis. Allergy Asthma Immunol Res. 2017;9(4):360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gauvreau GM, FitzGerald JM, Boulet LP, Watson RM, Hui L, Villineuve H, et al. The effects of a CCR3 inhibitor, AXP1275, on allergen-induced airway responses in adults with mild-to-moderate atopic asthma. Clin Exp Allergy. 2018;48(4):445–51. [DOI] [PubMed] [Google Scholar]
- 54.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205(4):897–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu X, Wang M, Mavi P, Rayapudi M, Pandey AK, Kaul A, et al. Interleukin-15 expression is increased in human eosinophilic esophagitis and mediates pathogenesis in mice. Gastroenterology. 2010;139(1):182–93.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cotton CC, Durban R, Dellon ES. Illuminating Elimination Diets: Controversies Regarding Dietary Treatment of Eosinophilic Esophagitis. Dig Dis Sci. 2019;64(6):1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kliewer K, Aceves SS, Atkins D, Bonis PA, Chehade M, Collins MH, et al. fficacy of 1-food and 4-food elimination diets for pediatric eosinophilic esophagitis in a randomized multi-site study. Gastroenterology. 2019; 156(6, Suppl1): S–172. [Google Scholar]
- 58.Molina-Infante J, Arias Á, Alcedo J, Garcia-Romero R, Casabona-Frances S, Prieto-Garcia A, et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: The 2-4-6 study. J Allergy Clin Immunol. 2017. [DOI] [PubMed] [Google Scholar]
- 59.Zhan T, Ali A, Choi JG, Lee M, Leung J, Dellon ES, et al. Model to Determine the Optimal Dietary Elimination Strategy for Treatment of Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2018;16(11):1730–7.e2. [DOI] [PubMed] [Google Scholar]
- 60.Dellon E, Guo R, SJ M, al e. An allergen-specific immune signature identifies food triggers in Eosinophilic Esophagitis with high accuracy. Gastroenterology.2018; 154:S260. [Google Scholar]
- 61.Warners MJ, Terreehorst I, van den Wijngaard RM, Akkerdaas J, van Esch BCAM, van Ree R, et al. Abnormal Responses to Local Esophageal Food Allergen Injections in Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2018;154(1):57–60.e2. [DOI] [PubMed] [Google Scholar]
- 62.Shoda T, Wen T, Aceves SS, Abonia JP, Atkins D, Bonis PA, et al. Eosinophilic oesophagitis endotype classification by molecular, clinical, and histopathological analyses: a cross-sectional study. Lancet Gastroenterol Hepatol. 2018;3(7):477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]