Abstract
The effects of plant inoculation with plant growth-promoting rhizobacteria (PGPR) and those resulting from the exogenous application of salicylic acid (SA) or methyl jasmonte (MeJA) on total phenolic content (TPC) and monoterpenes in Mentha x piperita plants were investigated. Although the PGPR inoculation response has been studied for many plant species, the combination of PGPR and exogenous phytohormones has not been investigated in aromatic plant species. The exogenous application of SA produced an increase in TPC that, in general, was of a similar level when applied alone as when combined with PGPR. This increase in TPC was correlated with an increase in the activity of the enzyme phenylalanine ammonia lyase (PAL). Also, the application of MeJA at different concentrations in combination with inoculation with PGPR produced an increase in TPC, which was more relevant at 4 mM, with a synergism effect being observed. With respect to the main monoterpene concentrations present in peppermint essential oil (EO), it was observed that SA or MeJA application produced a significant increase similar to that of the combination with rhizobacteria. However, when plants were exposed to 2 mM MeJA and inoculated, an important increase was produced in the concentration on menthol, pulegone, linalool, limonene, and menthone concentrations. Rhizobacteria inoculation, the treatment with SA and MeJA, and the combination of both were found to affect the amount of the main monoterpenes present in the EO of M. piperita. For this reason, the expressions of genes related to the biosynthesis of monoterpene were evaluated, with this expression being positively affected by MeJA application and PGPR inoculation, but was not modified by SA application. Our results demonstrate that MeJA or SA application combined with inoculation with PGPR constitutes an advantageous management practice for improving the production of secondary metabolites from M. piperita.
Keywords: salicylic acid, jasmonic acid, rhizobacteria, plant growth-promoting rhizobacteria (PGPR), mint, total phenolic content, monoterpene, menthol, pulegone
1. Introduction
Modern agriculture does not restrict itself to traditional food, forage, and fiber crops; rather, there is an increasing interest in crops that include species with secondary metabolites. These are present in all plant tissues at variable concentrations. They are low molecular weight compounds that are very important in plant ecology because they are responsible for the processes of adaptation of plants to their environment. Given their diverse biological and physio-chemical properties, secondary metabolites are of great interest as unique sources for pharmaceuticals, food additives, oils, waxes, perfumes, flavoring agents, dyes, and many other commercially important materials [1,2,3].
Peppermint (Mentha x piperita L.; family Labiatae) is an important, commonly-used flavoring agent world-wide. M. piperita plants contain ~3% volatile oils, consisting of >50 different compounds. The major EO components, which make up ~60% of its total oil volume, are limonene, linalool, menthone, menthol, and pulegone. Peppermint leaves are used for teas and flavoring foods and beverages, and its EOs are also used in chewing gum, candy, toothpaste, mouthwash, aromatherapy, pharmaceuticals, antimicrobial agents, and eco-friendly pesticides [4]. Not less important components of peppermint leaves are the phenolic compounds, including caffeic acid, rosmarinic acid, eriocitrin, and luteolin-7-O-glucoside [5,6], which represent about 20% of the dry weight. Seventy-five percent of these compounds can be extracted in an infusion [7,8,9,10]. The infusion of peppermint leaves is a common beverage with a refreshing flavor and a particular fragrance.
Many natural compounds extracted from plants have shown biological activities. There is a growing consumption of natural products with potential health benefits, and novel bioactive compounds are continually being discovered. Among others, plants are regarded as major sources of bioactive compounds with anticancer, antioxidant, antimicrobial, and anti-inflammatory effects [11,12]. Phytobioactive compounds commonly play important physiological roles in plants as secondary metabolites, which can be modified quantitatively and qualitatively through environmental changes as well as exposure to biotic and abiotic stress [13].
As knowledge has grown of the biological functionalities of secondary metabolites, so too has the search for new biotechnological alternatives to improve the production of economically important secondary metabolite compounds [2,3,14,15,16] with bioactive properties which can be used in agriculture as herbicides and pesticides, or as medicinal agents. Several strategies have been investigated to enhance the production of secondary metabolites from medicinal plants, including high-yielding cell line screening, media modification, elicitation, precursor feeding, large-scale cultivation systems, plant cell immobilization, hairy root culture, biotransformation, and others [17,18].
Jasmonic acid (JA), methyl jasmonate (MeJA), and salicylic acid (SA) are known as potent elicitors and plant defense hormones, which play significant roles in regulating plant defense responses against biotic and abiotic stress [19]. JA and its related compounds have long been known as transducers of elicitor signals for the production of plant secondary metabolites [20]. Exogenous application of JA signaling compounds stimulates the biosynthesis of secondary metabolites including a wide variety of plant secondary products such as terpenoids, flavonoids, alkaloids, and phenylpropanoids [21,22], while MeJA application has been reported to be both safe and inexpensive [23,24]. The JA signaling pathway is generally regarded as an integral signal for biosynthesis of many plant secondary products. Also, as many elicitors stimulate endogenous JA biosynthesis in plants, the JA signaling pathway is regarded as a transducer or mediator for elicitor signaling [25].
Salicylic acid (SA) is a well-known inducer of plant systematic acquired resistance (SAR) in plant–pathogen interaction, but it is not a universal inducer for the production of plant defensive metabolites. SA quickly accumulates at the infection site during pathogen attack and plant hypersensitive reaction, and it spreads to other parts of the plant to induce a wide range of defense responses, leading to the accumulation of plant secondary metabolites [26,27,28].
It is well known that a group of bacteria colonize the root systems of plants and can modulate plant growth, with beneficial effects on plant growth and crop yield and quality. Such bacteria, collectively termed “plant growth-promoting rhizobacteria” (PGPR), promote plant growth by enhancing the availability of nutrients, inducing metabolic activities by phytohormones and analogs, by shifting the phytohormonal balance, by inducing systemic resistance defense (ISR), or by reducing phytotoxic microbial communities [29,30,31]. Depending on the PGPR species, two or more of these growth-promoting mechanisms may be present [31,32,33,34,35].
Our previous studies showed that PGPR inoculation increased secondary metabolite production in various aromatic plant species [36,37,38]. The same beneficial bacteria inoculated in different plant species increased total EO yield (relative to controls), but not necessarily at the same level [37,38,39]. We observed that the effects of rhizobacteria on these secondary metabolites varied depending on the strain, suggesting that the rhizobacteria are recognized by the host plant in a strain-specific manner.
Thus, the elicitation of secondary metabolites appears to be a promising and innovative alternative. There are combinations of both biotic and abiotic forms of elicitors that stimulate metabolism but, while there are many studies of their potential use in improving secondary metabolism biosynthesis individually, few reports were found with the combination of both. Knowing that exogenous MeJA and SA applications, such as inoculation with PGPR, increase secondary metabolites, we inoculated the plants with PGPR and simultaneously applied phytohormones directly to learn how the combination of treatments affects total phenolic content (TPC) and the main monoterpenes of Mentha x piperita. New less aggressive biotechnological methods are needed to enhance the production of secondary metabolites from medicinal plant crops based on the use of beneficial microorganisms applied as biofertilizers.
2. Results
2.1. Total Phenolic Content (TPC)
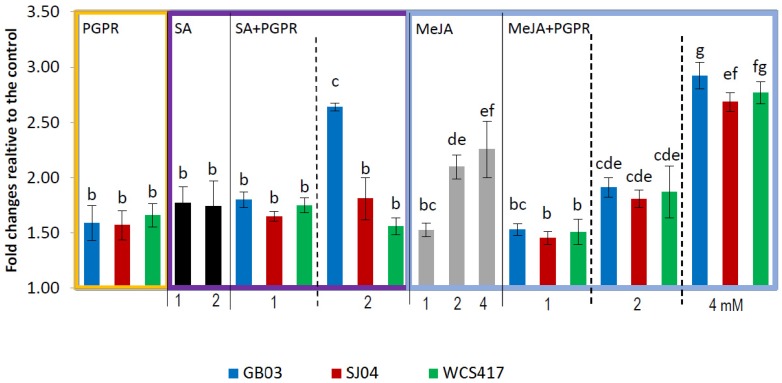
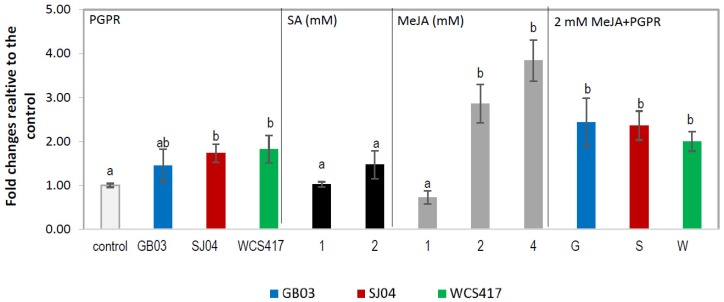
Phenolic compounds are plant secondary metabolites that can be released under the influence of multiple biotic and abiotic stresses; thus, we sprayed inoculated plants with SA or JA at different concentrations in order to determine the effects of the combination of treatments in TPC. An increased accumulation of total phenols was observed in inoculated mint plants compared to untreated controls (p < 0.05) (Figure 1). TPC in leaves of WCS417r inoculated plants showed further increased accumulation of total phenolic compounds (347.98 mg/mg fresh weight); this phenolic accumulation was similar for the three strains evaluated. The TPC in plants sprayed with SA at 1 or 2 mM concentrations showed similar phenolic content to plants only inoculated (~400 mg/mg fw) (Figure 1, Supplementary Table S1). The main effect was observed when plants were treated with 2 mM in combination with GB03 increasing up to 60% compared to individual treatments (2 mM SA or PGPR; p < 0.05).The response of plants exposed to MeJA depended on the concentration applied (Figure 1); as the concentration increased, the effect was also greater, almost 2.5 times with 4 mM in relation to control plants. When plants were inoculated and treated with 1 or 2 mM MeJA, the TPC was comparable to that of plants treated only with MeJA regardless of the strain inoculated; when plants were treated with 4 mM+GB03 or WCS417, it increased up to 3 times (613.11–563.22 mg/mg fw; Supplementary Tables S1 and S2) compared to control, and was higher than MeJA alone or inoculated plants.
Figure 1.
Inoculation with plant growth-promoting rhizobacteria (PGPR) and hormone treatments increase total phenolic compounds content in shoots of M. × piperita plants. Values are fold changes relative to the control. Different letters above bars for PGPR/methyl jasmonte (MeJA) + PGPR/salicylic acid (SA) + PGPR groups indicate significant differences according to the Tukey test (p < 0.05). The letter “a” indicates similar to the control. Three different ANOVA analyses were performed (i) PGPR (yellow box), (ii) SA–SA + PGPR (violet box), (iii) MeJA–MeJA + PGPR (blue box). TPC in control plants: for PGPR treatments = 209.84 ± 13.20 µg/g fresh weight, for SA treatments = 254.03 ± 35.26 µg/g fresh weight, for MeJA treatments = 192.43 ± 16.52 µg/g fresh weight. Native values are given in Supplementary Tables S1 and S2.
2.2. Phenylalanine Ammonia Lyase Activity (PAL)
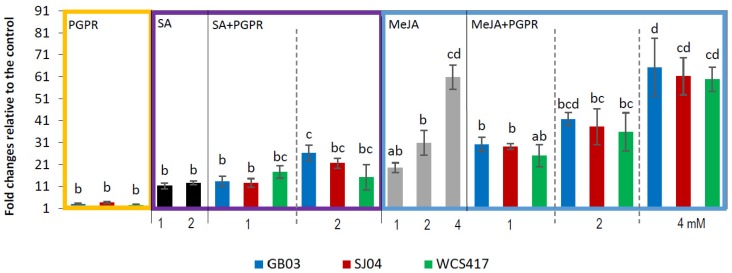
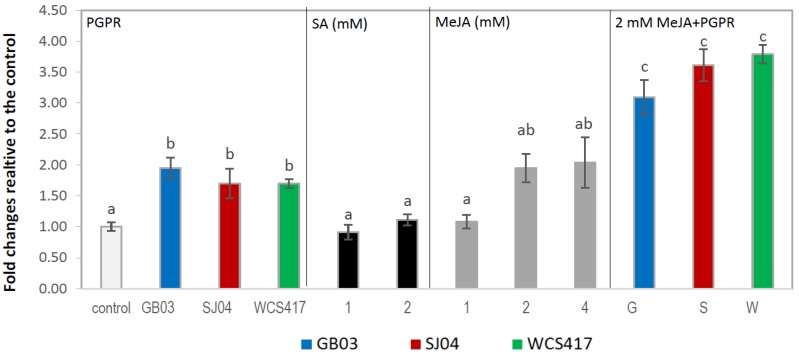
Phenylalanine ammonia lyase activity was affected by inoculation and/or exogenous phytohormone application. Significantly higher PAL activity was observed in inoculated plants compared to untreated controls (p < 0.05; Figure 2). SA applications led to 10-fold increased activity regardless of the concentration applied (p < 0.05; Figure 2). However, when PGPR and 1 mM SA spraying were combined, the rise in PAL activity was similar to the increase seen in plants treated with SA alone (p < 0.05), while in plants inoculated with GB03 and treated with 2 mM, the activity was almost double that in 2 mM treated plants. Spraying with MeJA also produced an increase in PAL activity, which was higher at each higher concentration applied, being ~20-, ~30- and ~60-fold higher in plants treated with 2 and 4 mM, respectively, compared to control plants. When PGPR and MeJA spraying were combined, the effect on PAL activity was statistically similar to that of MeJA alone for the different concentrations evaluated, while with 1 mM were slightly higher.
Figure 2.
Inoculation with PGPR and hormone treatments modify phenylalanine ammonia lyase (PAL) activity in shoots of M. × piperita plants. Values are fold changes relative to the control. Different letters above bars for PGPR, MeJA + PGPR, SA + PGPR groups indicate significant differences according to the Tukey test (p < 0.05). The letter “a” indicates similar to the control. Three different ANOVA analyses were performed (i) PGPR (yellow box), (ii) SA–SA + PGPR (violet box), (iii) MeJA–MeJA + PGPR (blue box). PAL activity in control plants: for PGPR treatments = 4.62 ± 0.32 µg trans-cinnamic acid min−1 mg−1protein, for SA treatments = 4.63 ± 0.30 µg trans-cinnamic acid/min × mg protein, for MeJA treatments = 4.60 ± 0.33 µg trans-cinnamic acid/min × mg protein. Native values are given in Supplementary Tables S1 and S2.
2.3. Quantification of Main EO Components
Since we showed in previous studies that PGPR and the exogenous application of SA and MeJA combined with inoculation increased EO content [40], we analyzed the response of the main EO components in plants treated with PGPR in addition to SA or MeJA. GC analysis of the yields of the major EO components ((+)-pulegone, (−)-menthone, (−)-menthol, limonene, and linalool) revealed great differences among plants treated with the phytohormone and control groups.
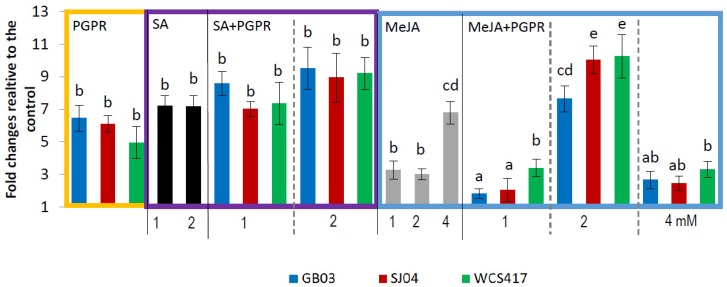
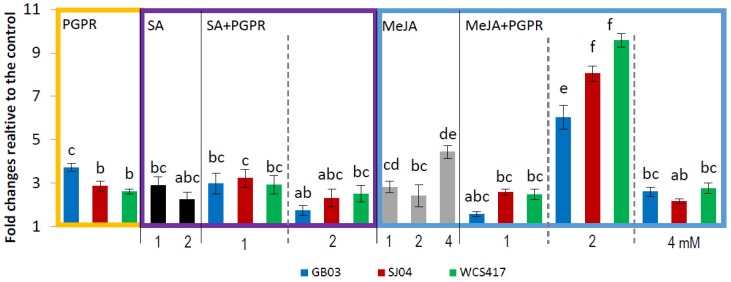
Plants only inoculated with the different strains produced an increase in all the major monoterpenes evaluated. The total amount of menthol increased (Figure 3) to 1.29 μg/g fresh weight in plants inoculated with GB03 in comparison with 0.20 μg/g fw in the control group (p < 0.05). The same trend was observed for pulegone (Figure 4), linalool, limonene, and menthone, where inoculation with GB03 generally produced the greater effect (Table 2).
Figure 3.
Inoculation with PGPR and hormone treatments increase menthol concentration in shoots of M. × piperita plants. Values are fold changes relative to the control. Different letters above bars for PGPR, MeJA + PGPR, SA + PGPR groups indicate significant differences according to the Tukey test (p < 0.05). The letter “a” indicates similar to the control. Three different ANOVA analyses were performed (i) PGPR (yellow box), (ii) SA–SA + PGPR (violet box), (iii) MeJA–MeJA + PGPR (blue box). Menthol concentrations in control plants: for PGPR treatments = 0.20 ± 0.02 μg/g fw, for SA treatments = 0.19 ± 0.02 μg/g fw, for MeJA treatments = 0.23 ± 0.07 μg/g fw.
Figure 4.
Inoculation with PGPR and hormone treatments increases pulegone concentration in shoots of M. × piperita plants. Values are fold changes relative to the control. Different letters above bars for PGPR, MeJA + PGPR, SA + PGPR groups indicate significant differences according to the Tukey test (p < 0.05). The letter “a” indicates similar to the control. Three different ANOVA analyses were performed (i) PGPR (yellow box), (ii) SA–SA + PGPR (violet box), (iii) MeJA–MeJA + PGPR (blue box). Pulegone concentrations in control plants: for PGPR treatments = 3.74 ± 0.16 μg/g fw, for SA treatments = 4.27 ± 0.27 μg/g fw, for MeJA treatments = 3.99 ± 0.28 μg/g fw.
The external application of SA modified the yield of the main monoterpene compounds of M. piperita (Table 1, Figure 3 and Figure 4), but, in general, there was no difference between the application of 1 and 2 mM (p > 0.05). The combination of SA with PGPR did not show any significant difference with the application of SA alone for all the compounds evaluated.
Table 1.
Effects of inoculation with PGPR strains and external application of SA on concentration of main shoot essential oil components of M. × piperita plants (mean ± SE). Means followed by the same letter in a given column are not significantly different according to the Tukey test (p < 0.05).
| Treatments | Linalool (µg/g fw) | (−)-Limonene (µg/g fw) | (−)-Menthone (µg/g fw) |
|---|---|---|---|
| Control | 0.15 ± 0.01 a | 0.20 ± 0.02 a | 0.50 ± 0.03 a |
| G | 0.61 ± 0.07 b | 0.76 ± 0.06 b | 1.15± 0.05 bc |
| S | 0.48 ± 0.08 b | 0.73 ± 0.08 b | 1.34 ± 0.15 c |
| W | 0.52 ± 0.05 b | 0.65 ± 0.07 b | 0.99 ± 0.09 b |
| Control for SA | 0.14 ± 0.02 a | 0.19 ± 0.04 a | 0.43 ± 0.09 a |
| 1 mM SA | 0.54 ± 0.07 b | 1.09 ± 0.18 b | 1.30 ± 0.09 b |
| 2 mM SA | 0.40 ± 0.07 ab | 1.01 ± 0.20 b | 1.40 ± 0.09 b |
| 1 mM SA + G | 0.41 ± 0.03 cd | 0.91 ± 0.06 b | 1.34 ± 0.07 b |
| 1 mM SA + S | 0.32 ± 0.03 ab | 0.99 ± 0.18 b | 1.71 ± 0.35 c |
| 1 mM SA + W | 0.40 ± 0.05 ab | 0.66 ± 0.17 ab | 1.45 ± 0.05 b |
| 2 mM SA + G | 0.34 ± 0.09 ab | 1.14 ± 0.03 b | 1.50 ± 0.22 b |
| 2 mM SA + S | 0.41 ± 0.05 ab | 1.18 ± 0.08 b | 1.53 ± 0.06 b |
| 2 mM SA + W | 0.22 ± 0.06 a | 1.06 ± 0.17 b | 1.31 ± 0.05 b |
G—GB03; S—SJ04; W— WCS417; SA—Salicylic Acid.
Menthol content in plants sprayed with SA increased 7 times in relation to uninoculated plants (Figure 3), similar values to those in inoculated plants alone (Supplementary Table S1). The same effect was observed for pulegone: SA application increased pulegone approximately three-fold in comparison to the control (Figure 4), similar to the increase in PGPR-inoculated plants (Supplementary Table S1).
Linalool content (Table 1) increased significantly only with 1 mM SA application (p < 0.05).
Limonene content in plants treated with 1 or 2 mM showed the same effect, an increase of 4–5 times compared to controls, a similar effect to inoculated plants (Table 1).
Menthone yield showed the same trend as limonene. Plants treated with SA showed an increase compared to control plants (p < 0.05) (Table 1), but when the treatments were combined, menthone content was similar in plants treated only with SA and in those also inoculated (p > 0.05), with the exception of 1mM SA + S, which resulted in a yield increase of 30% with respect to 1 mM SA.
The response of plants treated with MeJA depended on the concentration applied. Plants treated with 1 or 2 mM MeJA increased menthol and pulegone content approximately three-fold in relation to the control, with the greater effect observed in plants treated with 4 mM MeJA (Figure 3 and Figure 4), in which the amounts of menthol and pulegone increased 7 and 5 times, respectively (Figure 3, Supplementary Table S2). The effect of spraying with MeJA and inoculation increased menthol concentration only when 2 mM was applied, reaching 10-fold compared to control plants and 3 times compared to 2 mM-sprayed mint plants, while the combination of 1 or 4 mM and inoculation showed no yield effect compared to sprayed plants alone. A similar effect was observed for pulegone content (Figure 4), where the combination of inoculation with any of the strains evaluated and the application of 1 and 4 mM MeJA showed no significant difference with 1 and 4 mM sprayed plants (p > 0.05), while the combination with 2 mM increased pulegone content approximately 6-, 8- and 10-fold in relation to control plants in the inoculated strains, GB03, SJ04 and WCS417, respectively, with higher yields than those obtained with individual treatments (Figure 4; Supplementary Table S2).
Linalool, limonene and menthone content in plants treated with 1 and 2 mM MeJA was not modified (p > 0.05) in comparison to control plants, while 4 mM significantly increased the amount of linalool, limonene and menthone, 6-, 3-, and 4-fold, respectively (Table 2). When the inoculation was combined with MeJA, significant differences were observed only at 2 mM, increasing the content of linalool, limonene, and menthone approximately 5-fold in relation to 2 mM-treated plants (Table 2).
Table 2.
Effects of direct inoculation with PGPR strains and external application of MeJA on concentration of main shoot essential oil components of M. x piperita plants (mean ± SE). Means followed by the same letter in a given column are not significantly different according to Fisher’s LSD test (p < 0.05).
| Treatments | Linalool (µg/g fw) | (−)-Limonene (µg/g fw) | (−)-Menthone (µg/g fw) |
|---|---|---|---|
| Control MeJA | 0.15 ± 0.02 a | 0.25 ± 0.02 a | 0.35 ± 0.03 a |
| 1 mM + MeJA | 0.16 ± 0.02 a | 0.25 ± 0.05 a | 0.43 ± 0.08 a |
| 2 mM + MeJA | 0.22 ± 0.04 a | 0.21 ± 0.04 a | 0.47 ± 0.13 a |
| 4 mM + MeJA | 0.97 ± 0.12 c | 0.83 ± 0.07 b | 1.42 ± 0.14 b |
| 1 mM MeJA + G | 0.23 ± 0.04 a | 0.40 ± 0.05 a | 0.23 ± 0.03 a |
| 1 mM MeJA + S | 0.32 ± 0.07 a | 0.25 ± 0.03 a | 0.51 ± 0.13 a |
| 1 mM MeJA + W | 0.35 ± 0.07 ab | 0.37 ± 0.04 a | 0.44 ± 0.13 a |
| 2 mM MeJA + G | 0.79 ± 0.15 bc | 1.09 ± 0.07 bc | 1.75 ± 0.17 bc |
| 2 mM MeJA + S | 1.12 ± 0.17 c | 1.23 ± 0.07 c | 1.95 ± 0.17 bc |
| 2 mM MeJA + W | 0.84 ± 0.21 c | 1.11 ± 0.10 bc | 2.54 ± 0.45 c |
| 4 mM MeJA + G | 0.21 ± 0.08 a | 0.22 ± 0.04 a | 0.27 ± 0.07 a |
| 4 mM MeJA + S | 0.20 ± 0.02 a | 0.23 ± 0.01a | 0.20 ± 0.04 a |
| 4 mM MeJA + W | 0.23 ± 0.02 a | 0.27 ± 0.09 a | 0.42 ± 0.12 a |
G—GB03; S—SJ04; W—WCS417; MeJA—Methyl Jasmonate.
2.4. PGPR Inoculation and External Phytohormones Application Induces Terpenoid Gene Expression in M. piperita
Both rhizobacteria inoculation and SA and MeJA treatment were found to affect the amount of the main monoterpenes present in the EO of M. piperita. We, therefore, evaluated qPCR gene expression of two enzymes involved in the biosynthetic pathway leading to the bioactive monoterpenes. Previous studies have established the biochemical pathway in mints that leads to the production of the most important monoterpenes [41]. We considered the early gene, limonene synthase (Ls), involved in the formation of limonene, which is responsible for the first dedicated step of monoterpene biosynthesis in mint species. Limonene synthase catalyzes the cyclization of geranyl diphosphate, the universal C10 precursor of the monoterpenes, to (−)-4-S-limonene [41] and one of the end genes, Pr, coding for the enzyme pulegone reductase (Pr), which produced menthone and isomenthone at an average ratio of 2.5:1 [42].
Considering that not all the treatments in the present study produced an increase in the main EO compounds, we determined the gene expression of the most important treatments. Ls, coding for the enzyme that catalyzes the conversion of geranyl diphosphate to limonene, one of the simplest of all terpenoid cyclization reactions, was upregulated almost 2-fold in plants inoculated with SJ04 and WCS417(p < 0.05). External application of 1 and 2 mM SA had no effect on the expression of Ls (p > 0.05; Figure 5). In contrast, the application of 2 and 4 mM MeJA upregulated Ls expression 3- and 4-fold, respectively (p < 0.05). When plants were inoculated and sprayed with 2 mM MeJA, they showed the same effect as with the application of 2 mM alone (Figure 5).
Figure 5.
Limonene synthase gene expression of M. x piperita plants inoculated with PGPR and hormone treatments with respect to control leaves. Bars indicate the standard error over the mean of at least three biological replicates. Means followed by the same letter in a given column are not significantly different according to the Tukey test (p < 0.05).
Pr, which codes for pulegone reductase, the enzyme responsible for NADPH-dependent reduction of the conjugated double bond of terpenone to yield (−)-menthone and lesser amounts of (+)-isomenthone [42], was upregulated almost 2-fold by PGPR inoculation (p < 0.05; Figure 6). SA did not affect Pr expression (p > 0.05; Figure 6). MeJA external application produced the same effect as in Ls gene expression, which upregulated Pr by over 2-fold when it was applied at 2 and 4 mM, but these two values were not statistically different (p > 0.05). Moreover, this effect increased (to approximately 3.5-fold) when plants were sprayed with 2 mM MeJA and inoculated.
Figure 6.
Inoculation with PGPR and hormone treatments increases pulegone reductase expression in shoots of M. x piperita plants. Values are fold changes relative to the control. Means followed by the same letter in a given column are not significantly different according to the Tukey test (p < 0.05). The letter “a” indicates similar to the control.
2.5. Principal Component Analysis
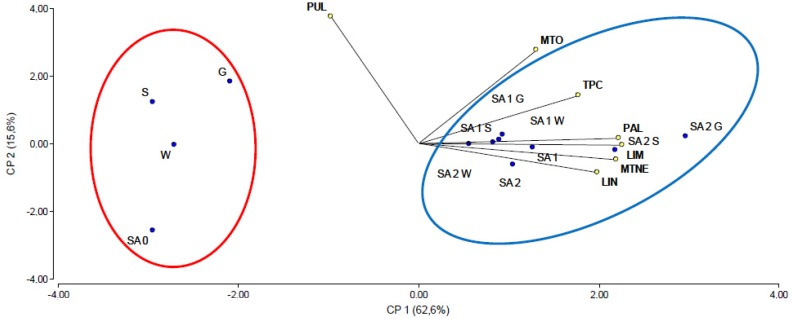
The PCA was performed to correlate the effects of inoculation with the three PGPR strains and spraying with SA or MeJA on the production of the main monoterpenes, TPC and PAL activity. This type of analysis provides a graph that facilitates the visualization and interpretation of the data set and the variables. Figure 7 shows the PCA correlating the effects of inoculation with the three PGPR strains combined with exogenous SA application. The variation in the data (78.2%) was explained by the first two principal components and gave a cophenetic correlation coefficient of 0.970. This plot shows that the strains, whichever was combined with 1 or 2 mM SA, were located in proximity to all the variables evaluated (blue circle): limonene content (LIM), linalool content (LIN), menthone (MTNE), pulegone (PUL), menthol content (MTO), TPC, and PAL. The control treatments SA and the strains alone are far from the variable evaluated (red circle), showing a low effect in relation to the other treatments. We observed a strong positive correlation (acute angle) between all monoterpene content and TPC content with the exception of PUL content (Figure 7).
Figure 7.
Principal component analysis illustrating relationships among PGR inoculation and SA external application on M. x piperita for TPC, PAL and major essential oil components: LIM (limonene content), LIN (linalool content), MTNE (menthone content), PUL (pulegone content), MTO (menthol content).
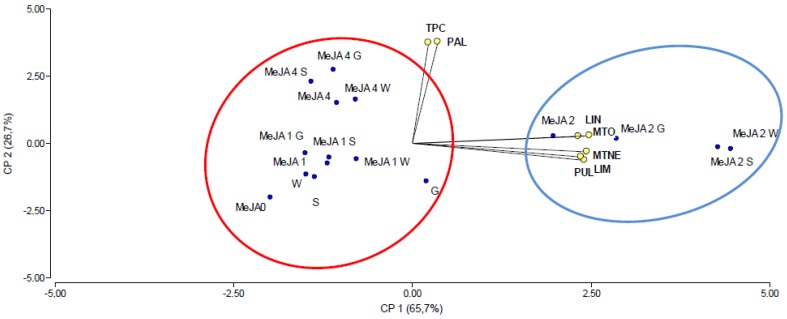
In relation to the PCA that correlates effects of inoculation with MeJA treatment, a plot defined by the first two principal components was sufficient for our purpose because it explained most (92.4%) of the variation in the data and gave a cophenetic correlation coefficient of 0.994 (Figure 8). In the two-dimensional coordinate system based on the first two principal components, it was possible to differentiate the strains combined with different MeJA concentrations. The inoculation and spraying with 1 and 4 mM MeJA were all together, far from the variable evaluated (red circle), while the three strains combined with 2 mM MeJA (blue circle) were located in proximity to the variables LIM, LIN, MTNE, PUL, and MTO. No one treatment was closer to TPC and PAL. In regard to associations among variables, there was a strong positive correlation (acute angle in Figure 8) between the limonene, menthone, menthol and pulegone content, as expected. Surprisingly, no associations (i.e., right angles in Figure 8) were observed between TPC and the major essential oil components.
Figure 8.
Principal component analysis illustrating relationships among strain inoculation and MeJA external application on M. x piperita for TPC, PAL, and major essential oil components: LIM (limonene content), LIN (linalool content), MTNE (menthone content), PUL (pulegone content), MTO (menthol content).
3. Discussion
Plants have been called chemical factories as they have the ability to fabricate important phytochemicals. Changes in secondary metabolites and the enhanced growth of host plants in interaction with different beneficial microbes have recently been studied [30,35].
M. piperita leaves contain high levels of polyphenolic compounds [5,6]. In the present study, we confirm the strong effect of rhizobacteria inoculation on TPC observed previously in M. piperita leaves [43]. The exogenous application of SA produces an approximately 1.5-fold increase in TPC compared to controls, whatever the concentration (1 or 2 mM) applied. In plants inoculated with GB03 and then sprayed with 2 mM SA, TPC increased to a greater extent than with the application of the phytohormone alone (60% higher). These results suggest that the combination produces a synergistic effect on the biosynthesis of phenolic compounds. A similar result was observed for MeJA treatments, where 2 and 4 mM produced an increase of TPC greater than in inoculated plants. Further, when plants were inoculated with GB03 or SJ04 and sprayed with 4 mM, the TPC increased 30% in relation to the individual treatment (MeJA or PGPR), suggesting also a synergism between 4 mM and PGPR inoculation, as observed for 2 mM SA.
The observed results match those of a previous study in which exogenous applications of JA and SA significantly induced the accumulation of phenolic compounds in a wide variety of plant species [44]. M. piperita suspension cultures exposed to JA and MeJA showed increased accumulation of rosmarinic acid (one of the main TPC of peppermint) [45]. The increase in TPC was also observed in suspension cultures supplemented with JA or SA of Panax ginseng root [46], of Thevetia peruviana [47], Cucumis melo [48], in plants of Romaine lettuce [49], in buckwheat [50], radish sprouts [51] and Agastache rugosa treated only with JA [52]. Similarly, Figueroa Perez et al. [9] reported an increase in TPC of 65%, 35%, and 31% in peppermint treated with SA at 0.05, 0.10, and 0.50 mM, respectively. Similar results were reported in other aromatic plants, such as Thymus vulgaris treated with 1 and 2 mM SA [53], Rosmarinus officinalis [54], and Achillea millefolium [55].
The induction of phenolic accumulation by JA and SA is related with the stimulation of the phenylpropanoid pathway, increasing PAL activity [19,47] in agreement with our observation that PAL activity increases as the concentration of MeJA treatments increases and, for SA treatments, PAL activity increases whatever the concentration applied. Particularly with the combination of 2 mM SA and GB03, the increase of PAL activity was greater than the individual treatments, as observed in TPC content. However, with 4 mM MeJA and inoculation with GB03 or WCS417, PAL activity was similar to that observed for 4 mM treated plants. No synergism in the combination of treatments (4 mM + bacteria) was observed as had been seen in TPC production.
Kim et al. [49] observed an increase in transcript levels of phenylpropanoid biosynthetic genes after treatment with methyl jasmonate in cell cultures of Agastache rugosa. Liu et al. [56] found that 10 µmol JA treatment on pea leaves (Pisum sativum) led to a significant increase in the activities of plasma membrane NADPH oxidase and PAL. Jasmonate elicitation was also found to increase the production of phenylpropanoids and naphtodianthrones in Hypericum perforatum cell suspensions [57].
Rhizobacteria inoculation was also found to increase TPC and PAL activity in peppermint [43], as was also reported in Piper betle inoculated with Serratia marcescens and Tagetes minuta inoculated with WCS417r and Azospirillum brasilense Sp7 [38,58]. The inoculation of chickpea seeds with P. fluorescens and P. aeruginosa, singly or in combination, induced the synthesis of specific phenolic acids (gallic, ferulic, and chlorogenic) and increased total phenolic content at various stages of plant growth [59]. Salla et al. [60] showed that the inoculation of eucalyptus with Streptomyces increased total phenolic content in leaves. In addition, Panka et al. [61] reported that the presence of the endophyte fungus, Neotyphodium lolii, increased the content of phenolic compounds in the aerial part of three different genotypes of the perennial grass, Lolium perenne. Increased PAL activity was recorded in P. fluorescens-pretreated tomato plants challenged with pathogen compared to untreated control [62] and other plant species [63].
Regarding the concentrations of the main monterpene compounds, EO levels and composition in plants play several key roles in plant–environment interactions and plant–plant communication. Terpenoids are crucial components in plant defensive responses to abiotic and biotic stresses [64,65]. Our previous studies showed that PGPR inoculation increased pulegone, menthone, menthol, and linalool production in M. piperita [66] and in other aromatic plant species: A. brasilense inoculation increased the levels of ocimenone and tagetone by 71% and 66%, respectively, in Tagetes minuta [38]; in Origanum majorana, the main compounds, terpinen-4-ol, cis-sabinene hydrate, trans-sabinene hydrate, and a-terpineol were also increased by inoculation with P. fluorescens [67]; and greater amounts of terpineol and eugenol were reported for sweet basil inoculated plants [36].
In a previous study, we reported that M. piperita exposed to SA and MeJA increased the total EO yield. Particularly, external application of 1 and 2 mM SA increased the EO yield approximately two-fold, while the combination with inoculation led to a 3-fold increase compared to control plants [40]. Curiously, the main monoterpene in plants exposed to SA increased in greater proportions; particularly, menthol rose approximately 7-fold, and pulegone, linalool, limonene and menthone 3-fold in comparison with control plants. Moreover, in plants exposed to combined treatments (PGPR + SA), the levels of the main monoterpene were similar to those of exposure to SA alone. The genes involved in isoprenoid biosynthesis have been shown to be transcriptionally upregulated by SA in Salvia miltiorhiza and Michelia chapensis [68,69]. The expression of three prenyltransferases from the core terpenoid biosynthetic pathway has been shown to be upregulated by SA in different species as well [70,71,72]. Similarly, the levels of many isoprenoids, have been shown to be upregulated during drought or salt stress in parallel to increases in SA levels (see, for instance, [73,74]). In addition, holm oaks fumigated with SA showed higher monoterpene levels in leaves and enhanced volatile monoterpene emission [75]. Zhang et al. [76] reported that treatment of Glycine max with 1 mM SA induced transcription of a newly identified gene encoding a monoterpene synthase. Treatment of Cistus creticus subsp. Creticus with 5 mM SA increased the expression of two genes which encode for enzymes that catalyze the first reactions of methyl-erythritol-phosphate and mevalonate [77]. Xu et al. [78] reported that 8 days after exposure to SA, the medicinal herb, Houttuynia cordata, increased the accumulation of α-thujene, α-pinene, α-terpinene, β-pinene, β-myrcene, limonene, and β-ocimene. Although the complete mechanism of SA-mediated plant defense is still not completely understood, the central role of SA in plant defense is universally accepted [79]. Further research is needed to determine the effects of SA on the biosynthesis of particular terpenoids (especially in the committed steps leading to these particular terpenoids), and to establish the possible relationship between the biosynthetic pathways leading to different individual terpenoids.
In relation to the effects of MeJA on the total EO yield of peppermint, it was previously reported that it produced an increase depending on the concentration applied: 3-fold for 1 and 2 mM and 5-fold for 4 mM. The strongest effect was observed when plants were treated with 2 mM and inoculated with rhizobacteria, increasing approximately 8-fold in comparison with controls or inoculated plants, indicating that there is a synergism between PGPR and MeJA [40]. Regarding the amount of the main monoterpene evaluated, not all responded in the same way. Pulegone and menthol showed the same trend as total EOs reported, while limonene, linalool, and menthone did not differ significantly from control plants for exposure to 1 and 2 mM and approximately 4 times for 4 mM. In relation to the combination of treatments, in the present study, we observed a 10-fold increase in the amount of pulegone and menthone when combining 2 mM with inoculation with WCS417. This increase was 2-fold greater as shown in the total EO yield reported [40]. Wang and Wu [80] stated that MeJA is an effective inducer of the terpenoid in Taxus spp. and also induced rapid activation of PAL.
JA and its derivatives and precursors are involved in the induction of plant defense responses [81,82,83,84,85]. These hormones play a central role in the regulation of the biosynthesis of several secondary metabolites, such as alkaloids, terpenoids, phytoalexins, coumarins, anthocyanins, among others, by gene regulation, promoting an increase in the number of transcripts of the enzymes linked to the metabolic pathway of those compounds [86,87,88]. Similarly to the results obtained in the present study, Schmidt et al. [89] reported that the exogenous application of JA induced accumulation of monoterpenes and diterpenes in Picea abies stems. However, in this study, treatment with JA did not produce a significant increase in production of terpenoids in eucalyptus leaves, which contained abundant secretory cavities [90]. Likewise, both the emission of linalool and the content of this monoterpene in glandular trichomes of tomato plants increased after the application of JA 1 mM. Treatment with JA also increased the transcription levels of the LeMTS1 gene, which codes for a linalool synthase located in trichomes of tomato plants [91].
Few studies have been reported on the effects of exogenous application of JAs on the production of secondary metabolites in aromatic and medicinal plants. Złotek et al. [92] reported that treatment with JA significantly increased the content of monoterpenes linalool, eugenol, and limonene in Ocimum basilicum. The results obtained in this work indicate that the biosynthesis of terpenoids in aromatic plants and other plant species can be induced by treatment with JA or its methylated derivative, MeJA.
The increase observed in monoterpene accumulation is correlated with an increase in the density of glandular trichomes [93,94]. Biochemical studies with isolated peltate glandular trichomes of peppermint have revealed that the secretory cells are responsible for the secretion of monoterpenes in the oil-storage space [95,96]. A previous study showed that exposure to SA and MeJA produced a 2-fold increase in the density of peltate trichomes in peppermint [40]; this explains the increase in content of the main monoterpenes observed in the present study. Moreover, peltate trichomes also serve as the site of monoterpene biosynthesis [96]. Thus, upregulation of limonene synthase Ls and Pr gene expression was observed. The biosynthesis of monoterpenes from primary metabolism requires a series of enzymatic steps. First, using geranyl diphosphate as substrate, Ls generates limonene and minor amounts of myrcene, alpha-pinene, and beta-pinene [96]. In the present study, an upregulation of Ls was observed that correlates with the increase observed in limonene content in plants exposed to 2 and 4 mM MeJA alone and 2 mM MeJA + PGPR. In contrast, no effect on Ls expression was observed in plants treated with SA, despite the 5-fold increase in limonene observed in those plants. A similar response was observed for pulegone reductase Pr gene expression; menthone and isomenthone (in a 2:1 to 10:1 ratio) were formed by the action of the NADPH-dependent Pr using pulegone as substrate [96]. The enzyme responsible for the synthesis of menthone was upregulated only in plants exposed to MeJA (4 mM) and in the combined treatments 2 mM MeJA + PGPR, while SA treatment did not affect Pr expression, despite the accumulation of menthone in SA-treated plants being increased in relation to control plants. The poor or the total lack of correlation between gene expression of Pr and Ls with the respective monoterpene content was probably because at the time that the qPCR were performed (7 days after phytohormone application) the increase in the biosynthesis of monoterpene may have already occurred, probably 48–72 h after phytormone application [97], and it is well known that the monoterpenes are stored in the glandular trichomes. On the other hand, protein stability may be increased due to post-translational modifications such as phosphorylation, acetylatylation, glycosylation, and also it could be that protein may be long lived and accumulates over time whereas mRNA turnover is quick [98].
In a previous study, we observed a significant increase in free jasmonic acid and the active form JA-Ile as well as SA in M. piperita plants inoculated with different rhizobacteria strains. The induction of SA and JA by rhizobacteria in the host plants suggests that plants may perceive these bacteria as a risk and thereby initiate a defensive response [40]. Elicitors such as SA and JA are considered as signal molecules that activate the signal-transduction cascade, leading to the activation and expression of genes related to the biosynthesis of secondary metabolites and playing a major role in the defense response [99]. It was reported that exogenous applications of MeJA result in the major reprogramming of genes, including genes that are known to be involved in plant stress responses [100,101], genes that induce defense by increasing activities of pathogenesis-related proteins, and genes that cause oxidative bursts, phytoalexin accumulation, lignification, and cell wall stiffening [102,103]. Furthermore, JA induces the expression of genes involved in biosynthesis, which leads to the accumulation of antimicrobial secondary metabolites, including alkaloids, terpenoids, flavonoids, anthraquinones, and glucosinolates in different plant species [104].
Total phenolic content has been related with stress tolerance, either through contributing with indirect photo protection or by participating directly as antioxidants [51,105]. The increase of phenolic compounds, which are considered the major antioxidant compounds in plants, resulted in a significant decrease in antioxidant capacity. Others organic compounds act as antioxidants like the monoterpenes, through the direct ROS scavenging pathway, and show a capacity for modulating the endogenous antioxidant system [106,107].
Interest in phenolic compounds has considerably increased in recent years because of their broad chemical spectrum and diverse biological properties [108]. In addition to their antioxidant properties, these compounds have been reported to be potential candidates in reducing cardiovascular diseases and anticarcinogenic activity, with antiallergenic, antiarthrogenic, antiinflammatory, antimicrobial, and antithrombotic effects [109]. Extracts of fruits, herbs, vegetables, cereals, and other plant materials rich in phenolics are increasingly of interest in the food industry, because they retard the oxidative degradation of lipids and thereby improve the quality and nutritional value of food [108].
Also, in view of the environmental, food-safety and health related issues associated with the application of chemical insecticides, growing emphasis is being laid on pest control through plant resources. In addition to the menthol and pulegone present in the essential oil of the Mentha species, which are the substances that give mints their characteristic aromas and flavors [4,110,111], they are also found to possess insecticidal, antiviral and fungicidal activities. Pulegone showed potent insecticidal activity against the mushroom scatopsid fly, Scatopse spp., in fumigant bioassay [112], and significant antibacterial activity is also observed for Mentha oil due to the presence of menthol [113]. These compounds form complexes with bacterial enzymes and protein and inhibit the growth of bacterial pathogens [114], causing the disruption of the plasma membrane, which increases its permeability and depolarizes its potential, finally leading to the death of the bacteria [115]. Menthol and limonene have also been reported as potential antifungal agents against plant pathogenic fungi [4].
4. Material and Methods
4.1. Plant Material, Bacterial Inoculation, and Treatments
Pseudomonas simiae WCS417r (formerly known as P. fluorescens WCS417r; [116]; P. putida SJ04) is a native fluorescent strain isolated from rhizospheric soil under a commercial crop of Mentha x piperita (San José) in Córdoba, Argentina, and demonstrated to have plant growth-promoting activity (GenBank KF312464.1) [117]; and Bacillus amyloliquefaciens GB03 (originally described as Bacillus subtilis GB03 [118]).
Bacteria were grown on LB medium [119] for routine use and maintained in nutrient broth with 15% glycerol at −80 °C for long-term storage.
Each bacterial culture was grown overnight at 30 °C with rotation at 120 rpm until reaching the exponential phase, washed twice in 0.9% NaCl with centrifugation (4300× g, 10 min, 4 °C), resuspended in sterile water, and adjusted to a final concentration of ~108 CFU/ mL for use as inoculum.
Plants were grown in plastic pots (diameter 12 cm, depth 22 cm) containing sterilized vermiculite. M. x piperita seedlings were planted (one per pot) in vermiculite and inoculated with 1000 µL bacterial suspension. Four experimental treatments were performed with the bacteria: sterile water (control), SJ04, WCS417r, and GB03.
4.2. Greenhouse Experiments
M. x piperita in vitro micropropagation was performed as described by Cappellari et al. [66]. On day 7 of culture, obtained by in vitro multiplication, they were transplanted directly into vermiculite in a greenhouse, and watered by a micro-irrigation system. All plants received Hoagland’s nutrient medium (20 mL/pot) twice per week [38]. Plants were grown in a growth chamber under controlled conditions of light (16/8-h light/dark cycle), temperature (22 ± 2 °C), and relative humidity (~70%). Bacterial suspensions as described above were applied to experimental seedlings, and sterile water was applied to control seedlings. After 7 days of inoculation, plants were sprayed until run-off with 1, 2, or 4 mM methyl jasmonate solution (MeJA) (Sigma–Aldrich, St. Louis, MO, USA, 1% methanol in water, v/v) or 1 or 2 mM SA solution (1% ethanol in water, v/v). The plants were left to dry for 30–60 min. For the phytohormone control treatments, a solution of the solvent used was applied. After phytohormone or control treatments, plants were transferred to a climate chamber with the phytohormone treatments spatially separated from other treatments because MeJA is very volatile.
After 14 days of applied phytohormone treatments, plants were removed from pots. Experiments were replicated 3 times (10 pots per treatment; 1 plant per pot) and were performed under non-sterile conditions.
4.3. Determination of Total Phenolic Content
Total phenols were determined using Folin–Ciocalteu reagent [120]. Each plant extract (0.5 mL) or gallic acid (standard phenolic compound) was mixed with Folin–Ciocalteu reagent (0.5 mL, diluted with 8 mL distilled water) and aqueous Na2CO3 (1 mL, 1 M). After 1 h, the level of total phenols was determined by colorimetry at a wavelength of 760 nm. Total phenol values were expressed in terms of µg gallic acid (a common reference compound) equivalent per g plant dry weight [38].
4.4. Determination of PAL Enzyme Activity
100 mg mint leaves were homogenized with liquid nitrogen using a mortar and pestle containing appropriate buffer solution (50 mM potassium phosphate and 1 mM EDTA, pH 7.8) and 1% PVP (polyvinylpyrrolidone) and then filtered through a 0.20 mm nylon filter into a centrifuge tube. The tissue extract was centrifuged at 12,000× g for 40 min at 4 °C. The supernatant to be used for enzyme activity determination was stored at 20 °C. Protein concentration was determined by the method described by Bradford [121].
PAL activity was assayed following the method described by Beaudoin–Eagan and Thorpe [122] by measuring the amount of trans-cinnamic acid formed at 290 nm. The reaction mixture consisted of 100 µL of enzyme extract, 900 µL 6 mM of l-phenylalanine, and 500 mM Tris HCl buffer solution (pH 8). The mixture was placed in a water bath at 37 °C for 70 min, and the reaction was stopped by the addition of 50 µL of 5 N HCl. Trans-cinnamic acid (1 mg/mL was used as standard and PAL activity was expressed as μg trans-cinnamic acid/min mg protein.
4.5. Extraction and Quantification of Main Monoterpene EO Components
Shoot samples were individually weighed and subjected to hydrodistillation in a Clevenger-like apparatus for 40 min. The volatile fraction was collected in dichloromethane, and β-pinene (1 μL in 50 μL ethanol) was added as an internal standard.
The major M. piperita EO components, which make up ~60% of total oil volume, are limonene, linalool, (−)-menthone, (−)-menthol, and (+)-pulegone. These compounds were quantified with respect to the standard added during the distillation procedure. The oil components were initially identified based on mass spectral and retention time data and confirmed by direct comparisons with commercial standards from Sigma–Aldrich Co. Flame ionization detector (FID) response factors for each compound generated essentially equivalent areas (differences <5%). Chemical analyses were performed as reported by Banchio et al. [36].
4.6. Total RNA Extraction and Quantitative Real-Time PCR
Total RNA from 50 mg lyophilized plant material was isolated using the Plant RNA Isolation Kit (Stratec, Berlin, BE, Germany), according to the manufacturer’s instructions but including an additional DNA digestion step (RNase Free DNase set (Qiagen, Valencia, CA, USA). Using identical amounts of total RNA, template cDNA for subsequent PCR reactions was generated using Superscript™ III (Invitrogen, Karlsruhe, BW, Germany) according to the manufacturer’s instructions. Quantitative real-time PCR was performed with SsoAdvanced Universal SYBR Green Supermix (BIO-RAD, Munich, Bavaria, Germany) and 10 pmol forward and 10 pmol reverse primer.
Relative RNA levels were calibrated and normalized with the level of housekeeping gene actin. Primer sequences for Actin (Act), Limonene synthase (Lim S), and Pulegone reductase (Pr) are shown in Table 3. PCR was performed using a CFX Connect Real-Time PCR system (BIO-RAD) according to the instruction manual. Transcript abundance was normalized to the transcript abundance of the actin.
Table 3.
Primer sequences for RT-PCR.
| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| Act | GCTCCAAGGGCTGTGTTCC | TCTTTCTGTCCCATGCCAAC |
| Ls | TTGTGGCGAATTCTCTCGCT | GGCTTCTGAGCTGGTCACTT |
| Pr | GCATGGAGATCCCAGATGGC | AGTAGAGCCAGGAAGGATGGA |
4.7. Statistical Analyses
Data were pooled and subjected to analysis of variance (ANOVA) followed by comparison of multiple treatment levels with controls using the Tukey test and principal component analysis (PCA). Control with solvent for MeJA and SA did not differ statistically with the control and is therefore not shown in the figures. For Figure 1, Figure 2, Figure 3 and Figure 4, the statistics were performed on the native data, but the figures were made using “fold changes” in order to facilitate the interpretation of the results. Differences between means were considered significant for p values <0.05. The Infostat software program, v. 2008 (Group Infostat, Universidad Nacional de Córdoba, Córdoba, Argentina), was used for all statistical analyses. In the Supplementary Tables S1 and S2, the native data is shown.
5. Conclusions
Due to the multiple properties of secondary metabolites from M. piperita, monoterpene and phenolic compounds arouse the interest of the pharmaceutical and food industry as well as cosmetics producers. Elicitation of secondary metabolites appears to be a promising and innovative alternative; there are combinations of both biotic (PGPR) and abiotic (phytohormone) forms of elicitors that stimulate metabolism, and there have been many studies of their potential use individually for improving secondary metabolism biosynthesis, but no reports were found with the combination of both. This study revealed that peppermint plants treated with elicitors SA or JA and simultaneously inoculated with PGPR could enhance the production of phenolic compounds and monoterpenes. Considering the different concentrations of SA and MeJA evaluated, we suggest using a concentration of MeJA 2 mM for the external application on M. piperita 7 days before harvest. This is a cost-effective concentration, which increased the main secondary metabolite content, and taking into account the fact that a concentration of MeJA 4 mM is more expensive, not necessarily any more effective, and did not increase the main monoterpene by as much as the 2mM concentration. This is the first report demonstrating that inoculation with PGPR in combination with an external phytohormone increases phytochemical production in relation to each treatment alone. Results from this study will help improve secondary metabolite production for this crop.
Acknowledgments
This study was supported by grants from the Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto, the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), MinCyT Córdoba, and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) PICT 0636-14, Argentina. E.B. obtained financial support from a Georg Forster-Research Fellowship of the Alexander von Humboldt Foundation. E.B. is Career Members of CONICET. L.d.R.C. received fellowships from CONICET- MinCyT. The authors are grateful to Joss Heywood, native speaker, for editorial assistance.
Abbreviations
| EO | essential oil |
| PGPR | plant growth-promoting rhizobacteria |
| JA | jasmonic acid |
| MeJA | methyl jasmonate |
| SA | salicylic acid |
| TPC | total phenolic content |
| PAL | phenylalanine ammonia lyase |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/1/50/s1.
Author Contributions
L.d.R.C. and M.V.S. performed the experiments; E.B. designed the research and analyzed the data. E.B., A.S. and J.G. were involved in data interpretation. E.B., A.S. and J.G. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto, the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), MinCyT Córdoba, the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT 0636-14), financial support to E.B. from the Georg Forster Research Fellowship of the Alexander von Humboldt Foundation, and to M.V.S., A.S., and J.G. from the Max Planck Society. E.B. is a Career Member of CONICET. L.d.R.C. received fellowship from CONICET.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Akula R., Ravishankar G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011;6:1720–1731. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerriero G., Berni R., Muñoz-Sanchez J.A., Apone F., Abdel-Salam E.M., Qahtan A.A., Alatar A.A., Cantini C., Cai G., Hausman J.F., et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes. 2018;9:309. doi: 10.3390/genes9060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonçalves S., Romano A. Production of Plant Secondary Metabolites by Using Biotechnological Tools. In: Vijayakumar R., Raja S.S.S., editors. Secondary Metabolites—Sources and Applications. IntechOpen; London, UK: 2018. pp. 81–99. [DOI] [Google Scholar]
- 4.Sing P., Pandey A.K. Prospective of Essential Oils of the Genus Mentha as Biopesticides: A Review. Front. Plant Sci. 2018 doi: 10.3389/fpls.2018.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorman H.J., Koşar M., Başer K.H., Hiltunen R. Phenolic profile and antioxidant evaluation of Mentha × piperita L. (peppermint) extracts. Nat. Prod. Comun. 2009;4:535–542. doi: 10.1177/1934578X0900400419. [DOI] [PubMed] [Google Scholar]
- 6.Farnad N., Heidari R., Aslanipour B. Phenolic composition and comparison of antioxidant activity of alcoholic extracts of Peppermint (Mentha piperita) J. Food Meas. Charact. 2014;8:113–121. doi: 10.1007/s11694-014-9171-x. [DOI] [Google Scholar]
- 7.McKay D.L., Blumberg J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytother. Res. 2006;20:619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 8.Figueroa Pérez M.G., Rocha-Guzmán N.E., Mercado-Silva E., Loarca-Piña G., Reynoso Camacho R. Effect of chemical elicitors on peppermint (Mentha piperita) plants and their impact on the metabolite profile and antioxidant capacity of resulting infusions. Food Chem. 2014;156:273–278. doi: 10.1016/j.foodchem.2014.01.101. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa-Pérez M.G., Rocha-Guzmán N.E., Pérez-Ramírez I.F., Mercado-Silva E., Reynoso-Camacho R. Metabolite profile, antioxidant capacity, and inhibition of digestive enzymes in infusions of peppermint (Mentha piperita) grown under drought stress. J. Agric. Food Chem. 2014;62:12027–12033. doi: 10.1021/jf503628c. [DOI] [PubMed] [Google Scholar]
- 10.Riachi L.G., De Maria C.A.B. Peppermint antioxidants revisited. Food Chem. 2015;176:72–81. doi: 10.1016/j.foodchem.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Fridlender M., Kapulnik Y., Koltai H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015;6:799. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L., Yang C., Li C. Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants. Sci. Bull. 2016;61:3–17. doi: 10.1007/s11434-015-0929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma N., Shukla S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants. 2015;2:105–113. doi: 10.1016/j.jarmap.2015.09.002. [DOI] [Google Scholar]
- 14.Sangwan N.S., Farooqi A.H.A., Shabih F., Sangwan R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001;34:3–21. doi: 10.1023/A:1013386921596. [DOI] [Google Scholar]
- 15.Wheatley R.E. The consequences of volatile organic compound mediated bacterial and fungal interactions. Antonie Van Leeuwenhoek. 2002;81:357–364. doi: 10.1023/A:1020592802234. [DOI] [PubMed] [Google Scholar]
- 16.Kai M., Haustein M., Molina F., Petri A., Scholz B., Piechulla B. Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 2009;81:1001–1012. doi: 10.1007/s00253-008-1760-3. [DOI] [PubMed] [Google Scholar]
- 17.Rao S.M., Ravishankar G.A. Plant cell cultures: Chemical factories of secondary metabolities. Biotechnol. Adv. 2002;20:101–153. doi: 10.1016/S0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 18.Vanisree M., Lee C.-Y., Lo S.-F., Nalawade S.M., Lin C.Y., Tsay H.-S. Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot. Bull. Acad. Sin. 2004;45:1–22. [Google Scholar]
- 19.Thiruvengadam M., Rekha K., Chung I.M. Induction of hairy roots by Agrobacterium rhizogenes-mediated transformation of spine gourd (Momordica dioica Roxb. ex. willd) for the assessment of phenolic compounds and biological activities. Sci. Hort. 2016;198:132–141. doi: 10.1016/j.scienta.2015.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farmer E.E., Alméras E., Krishnamurthy V. Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr. Opin. Plant Biol. 2003;6:372–378. doi: 10.1016/S1369-5266(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 21.Koo A.J.K., Howe G.A. The wound hormone jasmonate. Phytochemistry. 2009;70:1571–1580. doi: 10.1016/j.phytochem.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasternack C., Strnad M. Jasmonates are signals in the biosynthesis of secondary metabolites—Pathways, transcription factors and applied aspects—A brief review. N. Biotechnol. 2019;25:1–11. doi: 10.1016/j.nbt.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Ku K.M., Jeffery E.H., Juvik J.A. Optimization of Methyl Jasmonate Application to Broccoli Florets to Enhance Health-promoting Phytochemical Content. J. Sci. Food Agric. 2014;94:2090–2096. doi: 10.1002/jsfa.6529. [DOI] [PubMed] [Google Scholar]
- 24.Scognamiglio J., Jones L., Letizia C.S., Api A.M. Fragrance material review on methyl jasmonate. Food Chem. Toxicol. 2012;50:S572–S576. doi: 10.1016/j.fct.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 25.Zhao J., Davis L.C., Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Taguchi G., Yazawa T., Hayashida N., Okazaki M. Molecular cloning and heterologous expression of novel glucosyltransferases from tobacco cultured cells that have broad substrate specificity and are induced by salicylic acid and auxin. Eur. J. Biochem. 2001;268:4086–4094. doi: 10.1046/j.1432-1327.2001.02325.x. [DOI] [PubMed] [Google Scholar]
- 27.Rivas-San Vicente M., Plasencia J. Salicylic acid beyond: Its role in plant growth and development. J. Exp. Bot. 2011;62:3321–3338. doi: 10.1093/jxb/err031. [DOI] [PubMed] [Google Scholar]
- 28.Khan N., Bano A., Rahman M.A., Guo J., Kang Z., Babar M.A. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019;14:2097. doi: 10.1038/s41598-019-38702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kloepper J.W. Plant-Growth-Promoting Rhizobacteria as Biological Control Agents. In: Metting F.B., editor. Soil Microbial Ecology: Applications in Agricultural and Environmental Management. Marcel Dekker Inc.; New York, NY, USA: 1993. pp. 255–273. [Google Scholar]
- 30.Niranjan R.S., Shetty H.S., Reddy M.S. Plant Growth Promoting Rhizobacteria: Potential Green Alternative for Plant Productivity. In: Siddiqui Z.A., editor. PGPR: Biocontrol and Biofertilization. Springer; Dordrecht, The Netherlands: 2006. pp. 197–216. [Google Scholar]
- 31.van Loon L.C. Plant response to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 2007;119:243–254. doi: 10.1007/s10658-007-9165-1. [DOI] [Google Scholar]
- 32.Pieterse C.M., Zamioudis C., Berendsen R.L., Weller D.M., van Wees S.C.M., Bakker P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014;52:347–375. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- 33.Vessey J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- 34.Gupta G., Parihar S.S., Ahirwar N.K., Snehi S.K., Singh V. Plant growth promoting rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015;7:1013–1020. doi: 10.4172/1948-5948.1000188. [DOI] [Google Scholar]
- 35.Backer R., Rokem J.S., Ilangumaran G., Lamont J., Praslickova D., Ricci E., Subramanian S., Smith D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018;9:1473. doi: 10.3389/fpls.2018.01473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banchio E., Xie X., Zhang H., Paré P.W. Soil bacteria elevate essential oil accumulation and emissions in sweet basil. J. Agric. Food Chem. 2009;5:653–657. doi: 10.1021/jf8020305. [DOI] [PubMed] [Google Scholar]
- 37.Banchio E., Bogino P., Santoro M.V., Torres L., Zygadlo J., Giordano W. Systemic induction of monoterpene biosynthesis in Origanum x majoricum by soil bacteria. J. Agric. Food Chem. 2010;58:650–654. doi: 10.1021/jf9030629. [DOI] [PubMed] [Google Scholar]
- 38.Cappellari L., Santoro M.V., Nievas F., Giordano W., Banchio E. Increase of secondary metabolite content in marigold by inoculation with plant growth-promoting rhizobacteria. Appl. Soil Ecol. 2013;70:16–22. doi: 10.1016/j.apsoil.2013.04.001. [DOI] [Google Scholar]
- 39.Santoro M.V., Zygadlo J., Giordano W., Banchio E. Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita) Plant Physiol. Biochem. 2011;49:1177–1182. doi: 10.1016/j.plaphy.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Cappellari L., Santoro V.M., Schmidt A., Gershenzon J., Banchio E. Induction of essential oil production in Mentha x piperita by plant growth promoting bacteria was correlated with an increase in jasmonate and salicylate levels and a higher density of glandular trichomes. Plant Physiol. Biochem. 2019;141:142–153. doi: 10.1016/j.plaphy.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 41.McConkey M.E., Gershenzon J., Croteau R.B. Developmental regulation of monoterpene biosynthesis in the glandular trichomes of peppermint. Plant Physiol. 2000;122:215–224. doi: 10.1104/pp.122.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner G.W., Gershenzon J., Nielson E.E., Froehlich J.E., Croteau R.B. Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol. 1999;120:879–886. doi: 10.1104/pp.120.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cappellari L.R., Chiappero J., Santoro M., Giordano W., Banchio E. Inducing phenolic production and volatile organic compounds emission by inoculating Mentha piperita with plant growth-promoting rhizobacteria. Sci. Hort. 2017;220:193–198. doi: 10.1016/j.scienta.2017.04.002. [DOI] [Google Scholar]
- 44.Sudha G., Ravishankar G.A. Involvement and interaction of various signaling compounds on the plant metabolic events during defense response, resistance to stress factors, formation of secondary metabolites and their molecular aspects. Plant Cell Tissue Organ. Cult. 2002;71:181–212. doi: 10.1023/A:1020336626361. [DOI] [Google Scholar]
- 45.Krzyzanowska J., Czubacka A., Pecio L., Przybys M., Doroszewska T., Stochmal A., Oleszek W. The effects of jasmonic acid and methyl jasmonate on rosmarinic acid production in Mentha × piperita cell suspension cultures. Plant Cell Tiss. Organ Cult. 2012;108:73–81. doi: 10.1007/s11240-011-0014-8. [DOI] [Google Scholar]
- 46.Ali M.B., Hahn E.J., Paek K.Y. Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules. 2007;12:607–621. doi: 10.3390/12030607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mendoza D., Cuaspud O., Arias J.P., Ruiz O., Arias M. Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol. Rep. 2018;3:e00273. doi: 10.1016/j.btre.2018.e00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nafie E., Hathout T., Mokadem A.S.A. Jasmonic acid elicits oxidative defense and detoxification systems in Cucumis melo L. cells. Braz. J. Plant Physiol. 2011;23:161–174. doi: 10.1590/S1677-04202011000200008. [DOI] [Google Scholar]
- 49.Kim H.J., Fonseca J.M., Choi J.H., Kubota C. Effect of methyl jasmonate on phenolic compounds and carotenoids of romaine lettuce (Lactuca sativa L.) J. Agric. Food Chem. 2007;12:5510366. doi: 10.1021/jf071927m. [DOI] [PubMed] [Google Scholar]
- 50.Park C.H., Yeo H.J., Park Y.E., Chun S.W., Chung Y.S., Lee S.Y., Park S.U. Influence of chitosan, salicylic acid and jasmonic acid on phenylpropanoid accumulation in germinated buckwheat (Fagopyrum esculentum Moench) Foods. 2019;6:153. doi: 10.3390/foods8050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H., Seguin P., Archambault A., Constan L., Jabaji S. Gene expression and isoflavone concentrations in soybean sprouts treated with chitosan. Crop Sci. 2009;49:224–236. doi: 10.2135/cropsci2007.09.0536. [DOI] [Google Scholar]
- 52.Kim Y.B., Kim J.K., Uddin M.R., Xu H., Park W.T., Tuan P.A., Li X., Chung E., Lee J.-H., Park S.U. Metabolomics analysis and biosynthesis of rosmarinic acid in Agastache rugosa Kuntze treated with methyl jasmonate. PLoS ONE. 2013;8:e64199. doi: 10.1371/journal.pone.0064199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khalil N., Fekry M., Bishr M., El-Zalabani S., Salama O. Foliar spraying of salicylic acid induced accumulation of phenolics, increased radical scavenging activity and modified the composition of the essential oil of water stressed Thymus vulgaris L. Plant Physiol. Biochem. 2018;123:65–74. doi: 10.1016/j.plaphy.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 54.El-Esawi M.A., Elansary H.O., El-Shanhorey N.A., Abdel-Hamid A.M.E., Ali H.M., Elshikh M.S. Salicylic acid-regulated antioxidant mechanisms and gene expression enhance rosemary performance under saline conditions. Front. Physiol. 2017;8:716. doi: 10.3389/fphys.2017.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorni P.H., Pacheco A.C. Growth promotion and elicitor activity of salicylic acid in Achillea millefolium L. Afr. J. Biotechnol. 2016;15:657–665. doi: 10.5897/AJB2016.15320. [DOI] [Google Scholar]
- 56.Liu Y., Pan Q.-H., Yang H.-R., Liu Y.-Y., Huang W.-D. Relationship between H2O2 and jasmonic acid in pea leaf wounding response. Russ. J. Plant Physl. 2008;55:765. doi: 10.1134/S1021443708060058. [DOI] [Google Scholar]
- 57.Gadzovska S., Maury S., Delaunay A. Jasmonic acid elicitation of Hypericum perforatum L. cell suspensions and effects on the production of phenylpropanoids and naphtodianthrones. Plant Cell Tissue Organ. Cult. 2007;89:1–13. doi: 10.1007/s11240-007-9203-x. [DOI] [Google Scholar]
- 58.Lavania M., Chauhan P.S., Chauhan S.V.S., Singh H.B., Nautiyal C.H. Induction of plant defense enzymes and phenolics by treatment with plant growth promoting rhizobacteria Serratia marcescens NBRI1213. Curr. Microbiol. 2006;52:363–368. doi: 10.1007/s00284-005-5578-2. [DOI] [PubMed] [Google Scholar]
- 59.Singh U.P., Sarma B.K., Singh D.P. Effect of plant growth-promoting rhizobacteria and culture filtrate of Sclerotium rolfsii on phenolic and salicylic acid contents in Chickpea (Cicer arietinum) Curr. Microbiol. 2003;46:131–140. doi: 10.1007/s00284-002-3834-2. [DOI] [PubMed] [Google Scholar]
- 60.Salla T.D., Ramos T., Astarita L.V., Santarém E.R. Streptomyces rhizobacteria modulate the secondary metabolism of Eucalyptus plants. Plant Physiol. Biochem. 2014;85:14–20. doi: 10.1016/j.plaphy.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Panka D., Piesik D., Jeske M., Baturo-Ciesniewska A. Production of phenolics and the emission of volatile organic compounds by perennial ryegrass (Lolium perenne L.)/Neotyphodium lolii association as a response to infection by Fusarium poae. J. Plant Physiol. 2013;170:1010–1019. doi: 10.1016/j.jplph.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 62.Vanitha S.C., Umesha S. Pseudomonas fluorescens mediated systemic resistance in tomato is driven through an elevated synthesis of defense enzymes. Biol. Plant. 2011;55:317. doi: 10.1007/s10535-011-0045-3. [DOI] [Google Scholar]
- 63.Ramamoorthy V., Samiyappan R. Induction of defenserelatedgenes in Pseudomonas fluorescens treated chilli plants in response to infection by Colletotrichum capsici. J. Mycol. Plant Pathol. 2001;31:146–155. [Google Scholar]
- 64.Unsicker S.B., Kunert G., Gershenzon J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 2009;1:479–485. doi: 10.1016/j.pbi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Vickers C.E., Gershenzon J., Lardau M.T., Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009;5:283–291. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- 66.Cappellari L., Santoro M., Reinoso H., Travaglia C., Giordano W., Banchio E. Anatomical, morphological, and phytochemical effects of inoculation with plant growth promoting rhizobacteria on peppermint (Mentha piperita) J. Chem. Ecol. 2015;41:149–158. doi: 10.1007/s10886-015-0549-y. [DOI] [PubMed] [Google Scholar]
- 67.Banchio E., Bogino P., Zygadlo J., Giordano W. Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem. Syst. Ecol. 2008;36:766–771. doi: 10.1016/j.bse.2008.08.006. [DOI] [Google Scholar]
- 68.Yan X., Zhang L., Wang J. Molecular characterization and expression of 1-deoxy- d-xylulose 5-phosphate reductoisomerase (DXR) gene from Salvia miltiorrhiza. Acta Physiol. Plant. 2009;31:1015. doi: 10.1007/s11738-009-0320-5. [DOI] [Google Scholar]
- 69.Cao X.Y., Li C.G., Miao Q., Zheng Z.J., Jiang J.H. Molecular cloning and expression analysis of a leaf-specific expressing 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase gene from Michelia chapensis Dandy. J. Med. Plants Res. 2011;5:3868–3875. doi: 10.1007/s11033-015-3922-6. [DOI] [Google Scholar]
- 70.Cao X.Y., Yin T., Miao Q., Li C.G., Ju X.Y., Sun Y. Molecular characterization and expression analysis of a gene encoding for farnesyl diphosphate synthase from Euphorbia pekinensis Rupr. Mol. Biol. Rep. 2012;39:1487–1492. doi: 10.1007/s11033-011-0886-z. [DOI] [PubMed] [Google Scholar]
- 71.Shabani L., Ehsanpour A.A., Asghari G., Emami J. Glycyrrhizin production by in vitro cultured glycyrrhiza glabra elicited by methyl jasmonate and salicylic acid. Russ. J. Plant Physiol. 2009;56:621–626. doi: 10.1134/S1021443709050069. [DOI] [Google Scholar]
- 72.Kai M., Crespo E., Cristescu S.M., Harren F.J.M., Francke W., Piechulla B. Serratia odorifera: Analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Appl. Microbiol. Biotechnol. 2010;88:965–976. doi: 10.1007/s00253-010-2810-1. [DOI] [PubMed] [Google Scholar]
- 73.Munne-Bosch S., Peñuelas J. Photo- and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta. 2003;217:758–766. doi: 10.1007/s00425-003-1037-0. [DOI] [PubMed] [Google Scholar]
- 74.Eraslan F., Inal A., Gunes A., Alpaslan M. Impact of exogenous salicylic acid on the growth, antioxidant activity and physiology of carrot plants subjected to combined salinity and boron toxicity. Sci. Hort. 2007;113:120–128. doi: 10.1016/j.scienta.2007.03.012. [DOI] [Google Scholar]
- 75.Peñuelas J., Llusià J., Filella I. Methyl salicylate fumigation increases monoterpene emission rates. Biol. Plant. 2007;51:372–376. doi: 10.1007/s10535-007-0078-9. [DOI] [Google Scholar]
- 76.Zhang M., Liu J., Li K., Yu D. Identification and characterization of a novel monoterpene synthase from soybean restricted to neryldiphosphate precursor. PLoS ONE. 2013;4:e75972. doi: 10.1371/journal.pone.0075972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pateraki I., Kanellis A.K. Stress and developmental responses of terpenoid biosynthetic genes in Cistus creticus subsp. Creticus. Plant Cell Rep. 2010;29:629–641. doi: 10.1007/s00299-010-0849-1. [DOI] [PubMed] [Google Scholar]
- 78.Xu Y.W., Lv S.S., Zhao D., Chen J.W., Yang W.T., Wu W. Effects of salicylic acid on monoterpene production and antioxidant systems in Houttuynia cordata. Afr. J. Biotechnol. 2012;11:1364–1372. [Google Scholar]
- 79.Anand A., Uppalapati S.R., Ryu C.M., Allen S.N., Kang L., Tang Y., Mysore K.S. Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens. Plant Physiol. 2008;146:703–715. doi: 10.1104/pp.107.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang J.W., Wu J.Y. Nitric oxide is involved in methyl jasmonate-induced defense responses and secondary metabolism activities of Taxus cells. Plant Cell Physiol. 2005;46:923–930. doi: 10.1093/pcp/pci098. [DOI] [PubMed] [Google Scholar]
- 81.Karban R., Baldwin I.T. Induced Responses to Herbivory. University Chicago Press; Chicago, IL, USA: 1997. pp. 33–38. [Google Scholar]
- 82.Hu P., Zhou W., Cheng Z., Fan M., Wang L., Xie D. JAV1 controls jasmonate-regulated plant defense. Mol. Cell. 2013;50:504–515. doi: 10.1016/j.molcel.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 83.Kautz S., Trisel J.A., Ballhorn D.J. Jasmonic acid enhances plant cyanogenesis and resistance to herbivory in lima bean. J. Chem. Ecol. 2014;40:1186–1996. doi: 10.1007/s10886-014-0524-z. [DOI] [PubMed] [Google Scholar]
- 84.Tian D., Peiffer M., De Moraes C.M., Felton G.W. Roles of ethylene and jasmonic acid in systemic induced defense in tomato (Solanum lycopersicum) against Helicoverpa zea. Planta. 2014;239:577–589. doi: 10.1007/s00425-013-1997-7. [DOI] [PubMed] [Google Scholar]
- 85.Yan C., Xie D. Jasmonate in plant defence: Sentinel or double agent? Plant Biotech. J. 2015;13:1233–1240. doi: 10.1111/pbi.12417. [DOI] [PubMed] [Google Scholar]
- 86.Howe G.A. Cyclopentenone signals for plant defense: Remodeling the jasmonic acid response. Proc. Natl. Acad. Sci. USA. 2001;98:12317–12319. doi: 10.1073/pnas.231480898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maes L., Van Nieuwerburgh F.C., Zhang Y., Reed D.W., Pollier J., Vande Casteele S.R., Inzé D., Covello P.S., Deforce D.L., Goossens A. Dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytol. 2011;189:176–189. doi: 10.1111/j.1469-8137.2010.03466.x. [DOI] [PubMed] [Google Scholar]
- 88.Wasternack C., Song S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transciption. J. Exp. Bot. 2016;68:1303–1321. doi: 10.1093/jxb/erw443. [DOI] [PubMed] [Google Scholar]
- 89.Schmidt A., Nagel R., Krekling T., Christiansen E., Gershenzon J., Krokene P. Induction of isoprenyl diphosphate synthases, plant hormones and defense signalling genes correlates with traumatic resin duct formation in Norway spruce (Picea abies) Plant Mol. Biol. 2011;77:577–590. doi: 10.1007/s11103-011-9832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henery M.L., Wallis I.R., Stone C., Foley W.J. Methyl jasmonate does not induce changes in Eucalyptus grandis leaves that alter the effect of constitutive defense on larvae of a specialist herbivore. Oecologia. 2008;56:847–859. doi: 10.1007/s00442-008-1042-x. [DOI] [PubMed] [Google Scholar]
- 91.Rodríguez-Saona C., Crafts-Brandner S.J., Paré P.W., Henneberry T.J. Exogenous methyl jasmonate induces volatile emissions in cotton plants. J. Chem. Ecol. 2001;27:679–695. doi: 10.1023/A:1010393700918. [DOI] [PubMed] [Google Scholar]
- 92.Złotek U., Michalak-Majewska M., Szymanowska U. Effect of jasmonic acid elicitation on the yield, chemical composition, and antioxidant and anti-inflammatory properties of essential oil of lettuce leaf basil (Ocimum basilicum L.) Food Chem. 2016;213:1–7. doi: 10.1016/j.foodchem.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 93.Lange B.M., Mahmoud S.S., Wildung M.R., Turner G.W., Davis E.M., Lange I., Baker R.C., Boydston R.A., Croteau R.B. Improving peppermint essential oil yield and composition by metabolic engineering. Proc. Natl. Acad. Sci. USA. 2011;108:16944–16949. doi: 10.1073/pnas.1111558108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lange B.M., Turner G.W. Terpenoid biosynthesis in trichomes-current status and future opportunities. Plant Biotechnol. J. 2013;11:2–22. doi: 10.1111/j.1467-7652.2012.00737.x. [DOI] [PubMed] [Google Scholar]
- 95.Gershenzon J., McCaskill D., Rajaonarivony J.I.M., Mihaliak C., Karp F., Croteau R. Isolation of secretory cells from plant glandular trichomes and their use in biosynthetic studies of monoterpenes and other gland products. Anal. Biochem. 1992;200:130–138. doi: 10.1016/0003-2697(92)90288-I. [DOI] [PubMed] [Google Scholar]
- 96.McCaskill D., Gershenzon J., Croteau R. Morphology and monoterpene biosynthetic capabilities of secretory cell clusters isolated from glandular trichomes of peppermint (Mentha piperita L.) Planta. 1992;187:445–454. doi: 10.1007/BF00199962. [DOI] [PubMed] [Google Scholar]
- 97.Boughton A.J., Hoover K., Felton G.W. Methyl jasmonate application induces increased densities of glandular trichomes on tomate, Lycopersicon esculentum. J. Chem. Ecol. 2005;31:2211–2216. doi: 10.1007/s10886-005-6228-7. [DOI] [PubMed] [Google Scholar]
- 98.Greenbaum D., Colangelo C., Williams K. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4:117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van der Fits L., Memelink J. ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science. 2000;289:295–297. doi: 10.1126/science.289.5477.295. [DOI] [PubMed] [Google Scholar]
- 100.Schenk P.M., Kazan K., Wilson I., Anderson J.P., Richmond T., Somerville S.C., Manners J.M. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc. Natl. Acad. Sci. USA. 2000;97:11655–11660. doi: 10.1073/pnas.97.21.11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reymond P., Bodenhausen N., Van Poecke R.M.P., Krishnamurthy V., Dicke M., Farmer E.E. A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell. 2000;16:3132–3147. doi: 10.1105/tpc.104.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Durrant W.E., Dong X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 103.Guo X., Stotz H.U. Defense against Sclerotinia sclerotiorum in Arabidopsis is dependent on jasmonic acid, salicylic acid, and ethylene signaling. Mol. Plant Microbe Interact. 2007;20:1384–1395. doi: 10.1094/MPMI-20-11-1384. [DOI] [PubMed] [Google Scholar]
- 104.Memelink J., Verpoorte R., Kijne J.W. ORCAnization of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant Sci. 2001;6:212–219. doi: 10.1016/S1360-1385(01)01924-0. [DOI] [PubMed] [Google Scholar]
- 105.Agati G., Tattini M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010;186:786–793. doi: 10.1111/j.1469-8137.2010.03269.x. [DOI] [PubMed] [Google Scholar]
- 106.González-Burgos E., Gómez-Serranillos M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012;19:5319–5341. doi: 10.2174/092986712803833335. [DOI] [PubMed] [Google Scholar]
- 107.Janda T., Gondor O.K., Yordanova R., Szalai G., Pál M. Salicylic acid and photosynthesis: Signalling and effects. Acta Physiol. Plant. 2014;36:2537–2546. doi: 10.1007/s11738-014-1620-y. [DOI] [Google Scholar]
- 108.Lattanzio V. Phenolic Compounds: Introduction. In: Ramawat K.G., Me’rillon J.M., editors. Natural Products. Springer; Berlin/Heidelberg, Germany: 2013. [Google Scholar]
- 109.Huxley R.R., Neil H.A. The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2003;57:904–908. doi: 10.1038/sj.ejcn.1601624. [DOI] [PubMed] [Google Scholar]
- 110.Lubbe A., Verpoorte R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crop. Prod. 2011;34:785–801. doi: 10.1016/j.indcrop.2011.01.019. [DOI] [Google Scholar]
- 111.Salehi B., Stojanović-Radić Z., Matejić J., Sharopov F., Antolak H., Kręgiel D., Sen S., Sharifi-Rad M., Acharya K., Sharifi-Rad R., et al. Plants of Genus Mentha: From Farm to Food Factory. Plants. 2018;7:70. doi: 10.3390/plants7030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Basbagci G., Erler F. Evaluation of some essential oils and their major components against mushroom scatopsid flies as fumigants. Fresenius Environ. Bull. 2013;22:3173–3181. [Google Scholar]
- 113.Dorman H.J.D., Deans S.G. Antibacterial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 114.Rhouma A., Daoud H.B., Ghanmi S., Salah H.B., Romdhane M., Demak M. Antimicrobial activities of leaf extracts of Pistacia and Schinus species against some plant pathogenic fungi and bacteria. J. Plant Pathol. 2009;91:339–345. [Google Scholar]
- 115.Xu J., Zhou F., Ji B.P., Pei R.S., Xu N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008;47:174–179. doi: 10.1111/j.1472-765X.2008.02407.x. [DOI] [PubMed] [Google Scholar]
- 116.Berendsen R.L., van Verk M.C., Stringlis I.A. Unearthing the genomes of plant-beneficial Pseudomonas model strains WCS358, WCS374 and WCS417. BMC Genom. 2015;16:539. doi: 10.1186/s12864-015-1632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santoro V.M., Bogino P.C., Nocelli N., Cappellari L., Giordano W.F., Banchio E. Analysis of plant growth-promoting effects of fluorescent Pseudomonas strains isolated from Mentha piperita rhizosphere and effects of their volatile organic compounds on essential oil composition. Front. Microb. 2016;7:1085. doi: 10.3389/fmicb.2016.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Choi S.K., Jeong H., Kloepper J.W., Ryu C.M. Genome sequence of Bacillus amyloliquefaciens GB03, an active ingredient of the first commercial biological control product. Genome Announc. 2014;2:e01092-14. doi: 10.1128/genomeA.01092-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bertani G. Studies on lysogenesis. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 121.Bradford M.A. Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 122.Beaudoin-Eagan L.D., Thorpe T.A. Tyrosine and phenylalanine ammonia-lyase activities during shoot initiation in tobacco callus cultures. Plant Physiol. 1985;78:438–441. doi: 10.1104/pp.78.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.