Abstract
Phytoremediation soil polluted by cadmium has drawn worldwide attention. However, how to improve the efficiency of plant remediation of cadmium contaminated soil remains unknown. Previous studies showed that nitrogen (N) significantly enhances cadmium uptake and accumulation in poplar plants. In order to explore the important role of nitrogen in plants’ responses to cadmium stress, this study investigates the poplar proteome and phosphoproteome difference between Cd stress and Cd + N treatment. In total, 6573 proteins were identified, and 5838 of them were quantified. With a fold-change threshold of > 1.3, and a p-value < 0.05, 375 and 108 proteins were up- and down-regulated by Cd stress when compared to the control, respectively. Compared to the Cd stress group, 42 and 89 proteins were up- and down-regulated by Cd + N treatment, respectively. Moreover, 522 and 127 proteins were up- and down-regulated by Cd + N treatment compared to the CK group. In addition, 1471 phosphosites in 721 proteins were identified. Based on a fold-change threshold of > 1.2, and a p-value < 0.05, the Cd stress up-regulated eight proteins containing eight phosphosites, and down-regulated 58 proteins containing 69 phosphosites, whereas N + Cd treatment up-regulated 86 proteins containing 95 phosphosites, and down-regulated 17 proteins containing 17 phosphosites, when compared to Cd stress alone. N + Cd treatment up-regulated 60 proteins containing 74 phosphosites and down-regulated 37 proteins containing 42 phosphosites, when compared to the control. Several putative responses to stress proteins, as well as transcriptional and translational regulation factors, were up-regulated by the addition of exogenous nitrogen following Cd stress. Especially, heat shock protein 70 (HSP70), 14-3-3 protein, peroxidase (POD), zinc finger protein (ZFP), ABC transporter protein, eukaryotic translation initiation factor (elF) and splicing factor 3 B subunit 1-like (SF3BI) were up-regulated by Cd + N treatment at both the proteome and the phosphoproteome levels. Combing the proteomic data and phosphoproteomics data, the mechanism by which exogenous nitrogen can alleviate cadmium toxicity in poplar plants was explained at the molecular level. The results of this study will establish the solid molecular foundation of the phytoremediation method to improve cadmium-contaminated soil.
Keywords: poplar, cadmium stress, nitrogen, proteomics, phosphoproteome
1. Introduction
Heavy metal (HM) contamination has become a serious threat to the environment and human health [1]. Among heavy metals, cadmium (Cd), a widespread non-essential trace metal, is of great concern due to its widespread occurrence and high toxicity. Recently, Cd pollution has accelerated environmental deterioration. Notably, Cd contamination has widely degraded farmlands into the core polluted regions of the world [2,3]. Cd is easily taken up by plants through their root systems and can be accumulated through the food chain, which is a danger to animal and human health [4,5]. Cd has a negative effect on photosynthetic and nitrogen (N) metabolism in plants, leading to poor growth and a low biomass [6,7]. Additionally, Cd can stimulate the accumulation of reactive oxygen species (ROS), including the superoxide anion (O2−), hydroxyl radicals (OH) and hydrogen peroxide (H2O2), to disturb the redox balance in plants [8,9]. Therefore, the ability for ROS scavenging is vital for plant detoxification against Cd stress. According to previous research, the detoxification strategies of plants to Cd stress may include Cd sequestration, constraint, degradation, exclusion and inactivation by the exudation of organic ligands [10]. Sequestering Cd using phytochelatins (PCs), metallothioneins, organic acids, etc., and transporting Cd to the vacuole is confirmed to be an important pathway to detoxifying Cd [11].
These environmental and human health problems caused by heavy metals may be mitigated by many strategies. At present, many chemical, physical and biological techniques have been used to extract heavy metals from soils. However, among these techniques, phytoremediation has been proven to be effective and economical. Phytoremediation is a set of processes that uses plants to clean contamination in the environment [12,13,14]. Compared with traditional remediation technology, phytoremediation has been confirmed to be an optimal technique because it is a low-cost, sustainable and ecological method [15,16]. A number of different plants have an inherent ability to metabolize a variety of environmental pollutants [13,17]. Woody species, such as poplar and willow, have been used successfully for phytoremediation because of their characters of rapid growth, high-biomass, extensive root systems and amenability to transformation [18,19,20]. Additionally, using tall poplar and willow to remediate the contaminated environment is a functional approach that not only remediates the contaminated environment but also creates a landscape.
However, there are also several limitations for phytoremediation. Firstly, phytoremediation plants are often unique ecotypes and their specific habitations are limited [21,22]. Secondly, the ability to accumulate Cd varies significantly between species [23]. Thirdly, there is a lack of high-biomass Cd hyperaccumulators [24]. Fourth, phytoremediation is limited to shallow soils, streams and ground water. Fifth, high concentrations of hazardous materials can be toxic to plants. Therefore, how to improve the efficiency of phytoremediation is worthy of further study. Some documents have reported that nitrogen (N) can enhance the absorption of plants to Cd [25,26]. Li et al. [27] revealed that the addition of appropriate nitrogen to the nutrient solution could promote root system development and enhance the accumulation of Cd in Sedum alfredii Hance. Cd toxicity was mitigated by N deposition [11]. In leaves of Pentas lanceolate, N fertilization can promote the recovery of chlorophyll to alleviate the damages caused by Cd stress [28]. This study showed that excessive nitrates can enhance a plant’s uptake of Cd by up-regulating OsIRT1 expression in rice [29]. Our previous study revealed that addition N can weaken the damage caused by Cd stress [30,31]. In a word, the existing documents suggest that nitrogen application can effectively alleviate the harm of cadmium to plants when N supply is optimal. However, the mechanisms behind heavy metal detoxification in plants remain unclear.
Proteins are very important molecules in cells and are involved in virtually all cell functions, such as resistance to biological and abiotic stresses. Differential proteomic analysis is achieving great success as a reliable and reproducible high-through put approach to study the molecular mechanisms of plant responses to heavy metals [32,33]. Additionally, protein phosphorylation is one of the most widespread post-translational modifications, as well as the key factor in controlling signal transduction, and phosphorylation may regulate heavy metal stress responses [34]. However, at present, there is no study on the quantitative changes of the proteomics and protein phosphorylation induced by exogenous nitrogen in plants under cadmium stress. Therefore, the main objective in the study was to investigate the effects of nitrogen on protein expression patterns in poplar plants under Cd stress. Thus, we performed comparative proteomic and protein phosphorylation analyses. The results further elucidate the important role of N in detoxifying plants with Cd in poplar species.
2. Results
2.1. Exogenous Nitrogen Reduces Cadmium Toxicity in Poplar Leaf Photosynthesis and Promotes Growth
No obvious morphological differences were seen at the beginning of the treatment period. However, as the treatment time was prolonged, the growth of Cd-treated plants was significantly inhibited, and their leaves’ colors changed from green to yellow green, and even leaf etiolation was obvious in Cd-treated plants by the end of the experiment. The level of chlorophyll is an important index that reflects the growth of a plant and the tolerance of that plant to Cd stress. Here, we identified that Cd treatment decreased the chlorophyll a (Chl a), chlorophyll b (Chl b) and total chlorophyll (Chl) content by 30.7%, 53.3% and 36.5%, respectively, when compared with the control plants (Table 1). Additionally, Cd stress obviously reduced the plants’ photosynthetic capabilities and Chl fluorescence. Cd treatment decreased the net photosynthetic rate (Pnmax), transpiration rate (R), maximum quantum efficiency (AQE), stomatal conductance (gs) and maximum quantum efficiency of PS II (Fv/Fm) by 51.3%, 53.6%, 56.5%, 46.6% and 35.5%, respectively, when compared to the control plants (Table 2). The above data reveal that Cd stress severely inhibits growth.
Table 1.
Interactive effects of Cd and N treatment on the chlorophyll content in poplar leaves (unit: mg/g FW).
| Treatments | Chl a | Chl b | Total Chl |
|---|---|---|---|
| CK | 1.653 ± 0.021 A | 0.571 ± 0.013 A | 2.224 ± 0.017 A |
| Cd | 1.146 ± 0.014 C | 0.267 ± 0.007 B | 1.413 ± 0.011 B |
| N + Cd | 1.521 ± 0.039 B | 0.629 ± 0.026 A | 2.150 ± 0.033 A |
CK is the control, Cd indicates cadmium added and N + Cd indicates both nitrogen and cadmium added. Each value is the mean ± SD of three replicates, and the terms A, B, C and D within each column indicate significant differences between the means (p ≤ 0.05). The same is true below.
Table 2.
Effects of cadmium by supplementing nitrogen on photosynthesis and Chl fluorescence in poplar plants.
| Treatments | Pnmax (μmolCO2·m−2s−1) | R (μmolCO2·m−2s−1) | AQE (molCO2·m−1 photon) | gs (mmol·m−2s−1) | Fv/Fm |
|---|---|---|---|---|---|
| CK | 14.73 ± 0.25 A | 5.21 ± 0.19 A | 0.62 ± 0.002 A | 0.58 ± 0.001 A | 0.823 ± 0.012 A |
| Cd | 7.17 ± 0.34 C | 3.39 ± 0.23 B | 0.27 ± 0.004 C | 0.31 ± 0.002 B | 0.531 ± 0.007 C |
| N + Cd | 11.16 ± 0.51 B | 5.15 ± 0.32 A | 0.45 ± 0.002 B | 0.49 ± 0.003 A | 0.709 ± 0.004 B |
Pnmax, the net rate of photosynthesis; R, transpiration rate; AQE, maximum quantum efficiency; gs, stomatal conductance; Fv and Fm, maximum variable fluorescence in dark- and light-adapted leaves, respectively; Fv/Fm, maximum quantum efficiency of PS II.
Conversely, the N + Cd treated plants grew well; their leaves maintained their green color and even turned dark green as the treatments progressed. There was no significant difference in the Chl a, Chl b and total Chl between the N + Cd treated plants and the control plants (Table 1). On the other hand, Pnmax, R, AQE, gs and the ratio of Fv/Fm increased 55.6%, 51.9%, 66.7%, 58.1% and 33.5%, respectively, under N + Cd treatment compared to the sole Cd treatment. The above data reveal that Cd stress damaged the poplar plants’ viability. The addition of nitrogen to Cd treated plants may alleviate the suppression of Cd in poplar plants and promote growth.
2.2. Exogenous Nitrogen Can Enhance the Plants Absorption and Transportation of Cd
The Cd content in soil and in plants can effectively reflect the absorption ability of plant to Cd. As shown in Table 3, the Cd concentrations in soil, roots and leaves during Cd treatment or N + Cd treatment were observably higher than those of the control. Moreover, Cd content in roots was obviously higher than that in leaves, which indicates that Cd was mainly trapped in the root regions. In addition, Cd concentrations in the roots and in leaves under N + Cd treatment increased by 30.3% and 59.5%, respectively, while the Cd in soil decreased by 31.2% when compared to the Cd-stressed plants. The results reveal that the addition of N significantly enhanced the absorption and transportation of plants to Cd.
Table 3.
The application of nitrogen under Cd stress may significantly increase cadmium absorption by poplar plants from soil (unit: mg·kg−1).
| Treatments | Cd in Soil | Cd in Root | Cd in Leaves |
|---|---|---|---|
| CK | 0.68 ± 0.002 C | 2.77 ± 0.05 C | 1.41 ± 0.02 C |
| Cd | 221.19 ± 3.42 A | 128.56 ± 5.21 B | 78.09 ± 2.58 B |
| N + Cd | 152.13 ± 2.45 B | 167.50 ± 5.65 A | 124.52 ± 3.52 A |
2.3. Exogenous Nitrogen Reduces Cadmium-Induced H2O2 and MDA Generation and Enhances Glutathione (GSH) and Phytochelatin (PCs) Accumulation
Cd exerts its toxic effects on plants mainly through the production of large amounts of reactive oxygen species (ROS) [35]. Exogenous nitrogen can mitigate the damaging effects of ROS [26]. We examined whether exogenous nitrogen could alleviate cadmium toxicity in poplar plants by maintaining the redox balance in cells. H2O2 reflects levels of cellular oxidation, and MDA is an end-product of lipid peroxidation. On the other hand, GSH and PCs reflects scavenging Cd2+ ability of plants. Therefore, we detected the H2O2, MDA, GSH and PCs levels in poplar leaves following Cadmium or Cd +N treatment (Table 4). The data show that H2O2, MDA, GSH and PCs significantly increased when plants were faced with Cd stress, while the addition of Cd +N decreased cadmium-induced H2O2 and MDA accumulation and obviously increased GSH and PCs accumulation.
Table 4.
Effects of cadmium supplementation with nitrogen on H2O2, MDA, GSH and PCs.
| Treatments | H2O2 (nmol·g−1·FW) | MDA (μmol·g−1·FW) | GSH (nmol·g−1·FW) | PCs (nmol·g−1·FW) |
|---|---|---|---|---|
| CK | 52.9 ± 3.4 C | 9.24 ± 1.2 C | 24.6 ± 1.27 C | 121.3 ± 5.45 C |
| Cd | 452.7 ± 5.9 A | 22.99 ± 1.4 A | 52.7 ± 2.41 B | 325.9 ± 6.42 B |
| N + Cd | 132.1 ± 3.6 B | 13.21 ± 1.5 B | 106.4 ± 3.45 A | 543.4 ± 4.76 A |
2.4. Impacts of Cd or Cd + N on the Global Proteome of Poplar Plants
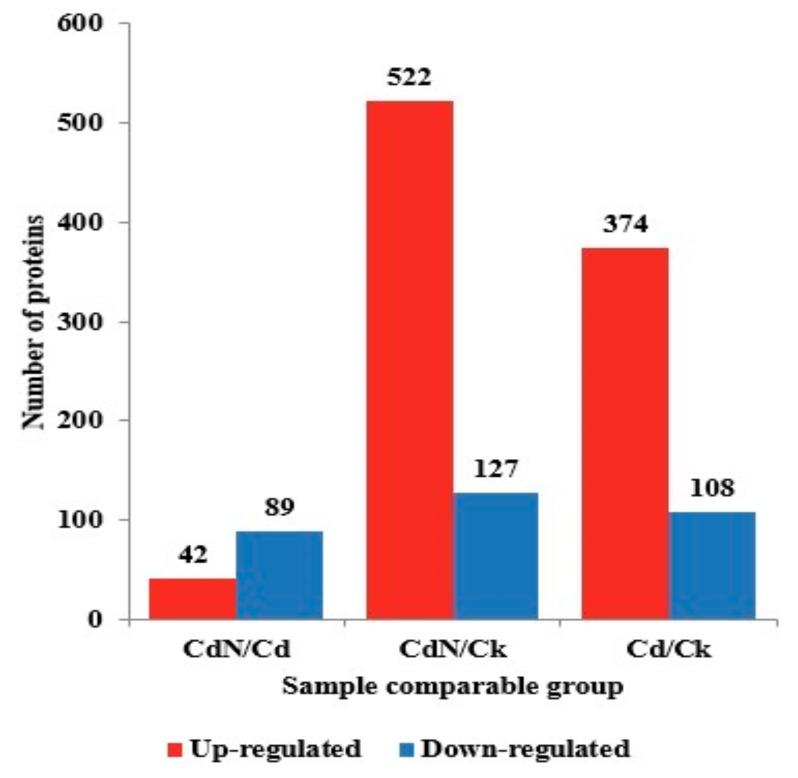
In order to elucidate the important role of N in plants against Cd toxicity in poplar species, we measured the protein abundance for the following three groups: (1) Cd/CK, (2) Cd + N/Cd and (3) Cd + N/CK. The primary proteome data and phosphoproteome data were deposited in the PRIDE Archive (No. PXD013360). In this study, 6573 proteins were identified, and 5838 of them were quantified. With a fold-change threshold of > 1.3 and a p-value < 0.05, 375 and 108 proteins were up- and down-regulated, respectively, in the Cd/CK group; 42 and 89 proteins were up- and down-regulated, respectively, in the Cd + N/Cd group; and 522 and 127 proteins were up- and down-regulated, respectively, in the Cd + N/CK group (Figure 1).
Figure 1.
The differentially expressed proteins in the Cd/CK, Cd + N/Cd and Cd + N/CK groups.
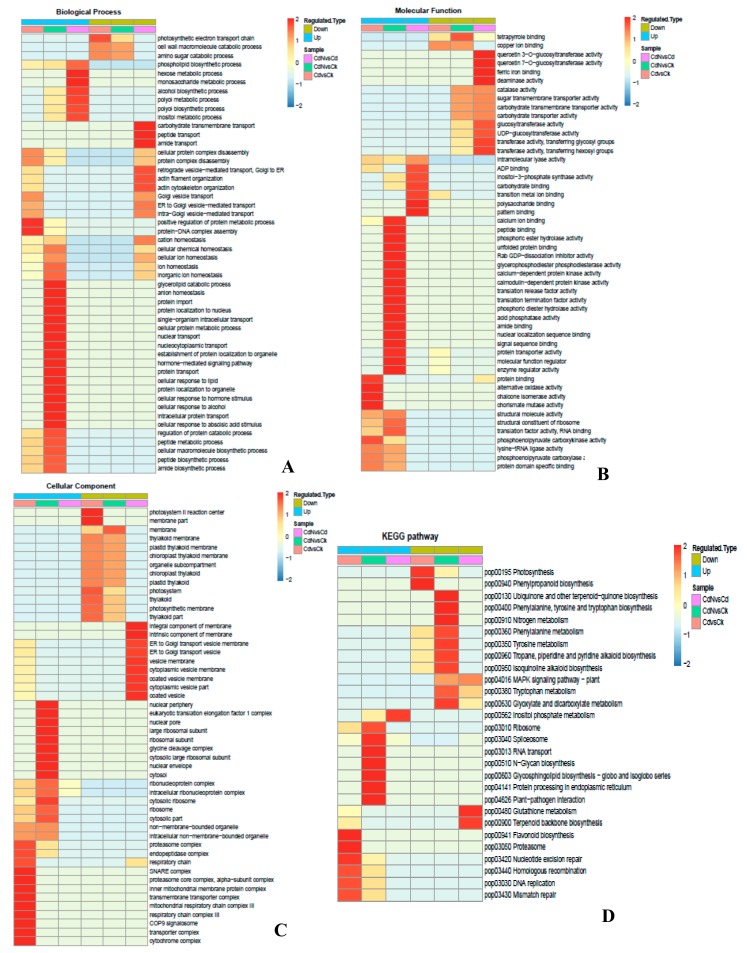
To characterize the functions of the differentially expressed proteins in the Cd/CK or Cd + N/Cd groups, gene ontology (GO) enrichment-based clustering analyses were performed (Figure 2). When comparing the differentially expressed proteins between the Cd treatment and CK, we found that, in the biological process category (Figure 2A), positive regulation of the protein metabolic process, protein-DNA complex assembly, cellular protein complex disassembly, protein complex disassembly and Golgi vesicle transport were highly enriched among the up-regulated proteins; down-expressed proteins were observed in the regulation of the photosynthetic electron transport chain, cell wall macromolecule and amino sugar catabolic processes. In the molecular function category (Figure 2B), the up-expressed proteins were mainly enriched in their protein binding, alternative oxidase activity, chalcone isomerase activity, chorismate mutase activity and phosphoenolpyruvate carboxykinase activity, while the down-expressed proteins mainly included copper ion binding and tetrapyrrole binding. In the cellular component category (Figure 2C), the up-expressed proteins were mainly enriched in 12 processes, including the cytochrome complex, transporter complex, COP9 signalosome, respiratory chain complex III, mitochondrial respiratory chain complex III, transmembrane transporter complex and others. Down-regulated proteins were primarily located in the photosystem II reaction center, membrane part, photosystem, thylakoid, photosynthetic membrane, thylakoid part, chloroplast thylakoid membrane and others.
Figure 2.
A comprehensive heatmap for cluster analysis of the enrichment patterns of differentially expressed proteins up-regulated and down-regulated based on their Z-score (–log10 (p-value); from –2 to 2). Cluster membership were visualized by a heat map using the heatmap.2” function from the “gplots” R-package. (A) A biological process category. (B) A molecular function category. (C) A cell component category. (D) A Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis for all differentially expressed proteins.
However, when comparing the functions of the differentially expressed proteins between Cd + N and Cd treatment, we found that in the biological process category (Figure 2A), the inositol metabolic process, polyol biosynthetic process, polyol metabolic process, alcohol biosynthetic process, monosaccharide metabolic process, hexose metabolic process and phospholipid biosynthetic process were up-regulated in the Cd + N treatment compared to sole Cd stress, while carbohydrate transmembrane transport, peptide transport, amide transport, retrograde vesicle-mediated transport, actin filament organization, actin cytoskeleton organization and Golgi vesicle transport were down-regulated by exogenous nitrogen addition under Cd stress. In the molecular function category (Figure 2B), the up-expressed proteins were mainly involved in pattern binding, polysaccharide binding, transition metal ion binding, carbohydrate binding, inositol-3-phosphate synthase activity, ADP binding and intramolecular lyase activity, while the down-regulated proteins were mainly involved in quercetin glucosyltransferase activity, ferric iron binding, deaminase activity, transferase activity, transferase activity and glucosyltransferase activity. For the cellular component category, Figure 2C showed that the down-expressed proteins in plant cells with N + Cd treatment primarily consisted of the integral component of membrane, intrinsic components of the membrane, ER to Golgi transport vesicle membrane, vesicle membrane, cytoplasmic vesicle membrane, coated vesicle membrane, cytoplasmic vesicle part and coated vesicle.
The cellular pathways of the different proteins regulated by Cd stress or Cd + N treatments were profiled using gene ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (Figure 2D). The flavonoid biosynthesis, proteasome, nucleotide excision repair, homologous recombination, DNA replication and mismatch repair pathways were up-regulated by Cd stress, while the photosynthesis and phenylpropanoid biosynthesis pathways were down-regulated by Cd stress. In addition, the inositol phosphate metabolism pathway was up-expressed in Cd + N treatment plants, while the glutathione metabolism and terpenoid backbone biosynthesis pathways were down-regulated by Cd + N treatment in comparison with sole Cd stress.
2.5. Impacts of Cd and Cd + N on the Protein Phosphorylation Levels in Poplar Plants
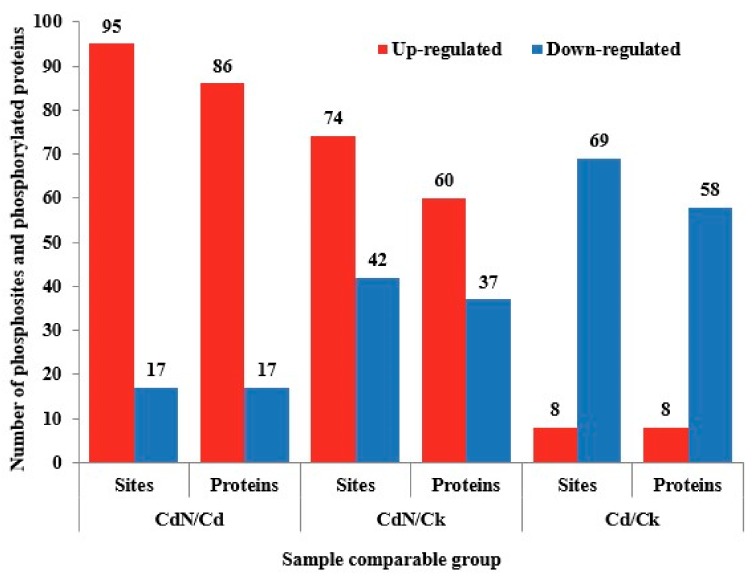
Protein phosphorylation is important in signal transduction systems, as it affects the activity of its target. In this study, the enrichment efficiency of phosphorylated peptides was 94.1% and the mean precursor ion fraction (PIF) value of phosphorylated peptides was 0.77. In addition, 7605 phosphosites in 3479 proteins were identified, and 5094 phosphosites in 2848 proteins were quantified based on a localization probability of > 0.75, according to the LC–MS/MS analysis. The phosphoproteome data were obtained after the proteomic difference was normalized. The results quantified 1471 phosphosites in 721 proteins. The differences in phosphosites and phosphorylated proteins upon Cd or Cd + N treatment were compared with the control and are shown in Figure 3. According to a fold-change threshold of > 1.2, and a p-value < 0.05, the Cd stress up-regulated eight phosphosites in eight proteins and down-regulated 69 phosphosites in 58 proteins in the Cd/CK group, whereas N + Cd treatment up-regulated 95 phosphosites in 86 proteins and down-regulated 17 phosphosites in 17 proteins when compared to sole Cd stress. In addition, N + Cd treatment up-regulated 74 phosphosites in 60 proteins and down-regulated 42 phosphosites in 37 proteins when compared to the control.
Figure 3.
The differentially expressed phosphosites and phosphorylated proteins (after being normalized) in the Cd/CK, Cd + N/Cd and Cd + N/CK groups.
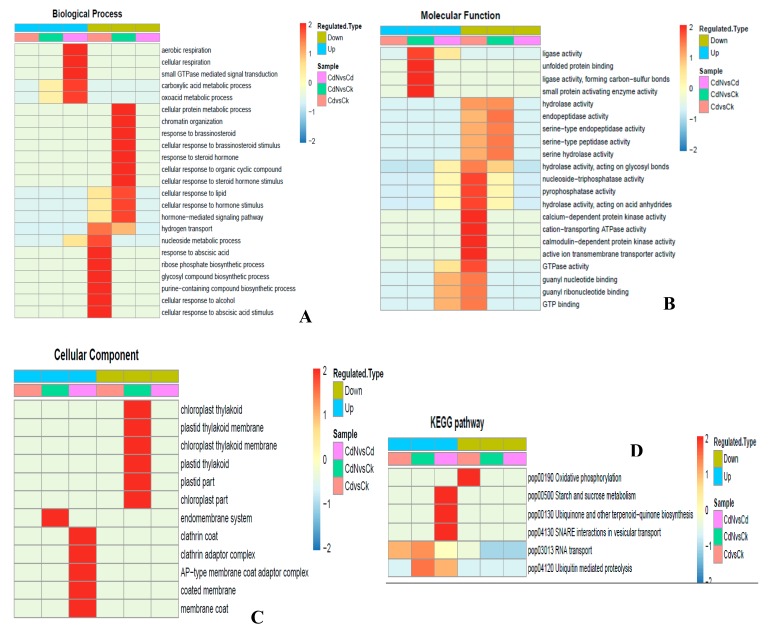
A GO enrichment method was performed to analyze the functions of the different phosphosites and the different phosphoproteins between Cd and Cd + N treatment. As shown in Figure 4A, in the biological process category, the process of the cellular response to abscisic acid stimulus, cellular response to alcohol, purine-containing compound biosynthesis, glycosyl compound biosynthesis, ribose phosphate biosynthesis, response to abscisic acid, nucleoside metabolic and hydrogen transport were down-regulated under Cd stress. For the molecular function cluster (Figure 4B), 17 processes including active ion transmembrane transporter activity, calmodulin protein kinase activity, cation transporting ATPase activity, calcium-dependent protein kinase activity, hydrolase activity, acting on acid anhydrides, pyrophosphatase activity and others, were down-regulated by Cd stress. However, there were no differentially expressed proteins enriched in the cellular component category (Figure 4C).
Figure 4.
Gene ontology (GO) enrichment-based clustering analysis of the quantified phosphoproteome based on Zscore (–log10 (p-value); from –2 to 2). (A) biological process category. (B) The molecular function category. (C) The cell component category. (D) KEGG pathway analysis.
However, when characterizing the functions of the differentially phosphorylated proteins between Cd + N and Cd treatment, we found that aerobic respiration, cellular respiration, small GTPase mediated signal transduction, the carboxylic acid metabolic process and the oxoacid metabolic process were enriched in the biological process category and were up-regulated by exogenous nitrogen addition following Cd stress (Figure 4A). For the molecular function cluster (Figure 4B), GTP binding, guanyl ribonucleotide binding, guanyl nucleotide binding and GTPase activity were up-regulated in the Cd + N treatment compared to Cd stress alone. In addition, the differentially expressed proteins in response to Cd + N treatments were chiefly located in the membrane coat, coated membrane, clathrin adaptor complex, AP-type membrane coat adaptor complex and clathrin coat (Figure 4C).
The alterations in signaling pathways induced by the Cd or Cd + N treatments were analyzed using the KEGG clustering method (Figure 4D). The oxidative phosphorylation pathway was down-regulated by Cd stress. Interestingly, some signaling pathways, including starch and sucrose metabolism, ubiquinone and other terpenoid−quinone biosynthesis and SNARE interactions in vesicular transport were up-regulated by exogenous nitrogen addition following Cd stress, suggesting that the addition of exogenous nitrogen under Cd stress is beneficial to enhance the photosynthetic ability of plants and promote the poplar to absorb and transport cadmium through different pathways. The above results are consistent with those of previous analyses (Table 2 and Table 3).
3. Discussion
3.1. Cd Stress Induced Oxidative Injuries and Inhibited Photosynthesis
The photosynthetic capacity of plants is closely related to their growth. Cd stress seriously destroys the photosystem through the formation of ROS, which decreases the photosynthetic capacity and inhibits growth [27,28]. In the present study, Cd induced an accumulation of H2O2 and MDA (Table 4). Moreover, Cd ions inhibited the activity of RuBisCo and interfered with the reactions in photosystem I and photosystem II. For example, some proteins (A0A2K2BNL0 and B9MYU1) related to the photosystem I reaction pathway and one protein (A0A2K1Z195) associated with the photosystem II reaction pathway were down-regulated by Cd stress. In addition, one RuBisCo protein (A9PJ06), one ATP synthase CF0 A subunit protein (A0A2K1WNK6) and one thioredoxin protein (B9H8W5) showed a downward in expression with longer-term (60 d) of Cd stress (Table 5).
Table 5.
List of partial proteins differentially regulated by Cd and Cd + N in poplar plants determined by LC–MS/MS analysis.
| Accession Number | Proteins Name | Cd/CK Ration | Regulated Type | Cd/Ck p Value |
| Photosynthesis and energy metabolite | ||||
| A0A2K1Z195 | photosystem II CP43 reaction center protein-like | 0.639 | down | 0.014 |
| A0A2K1WNK6 | ATP synthase CF0 A subunit (chloroplast) | 0.71 | down | 0.026 |
| A9PJ06 | ribulose bisphosphate carboxylase/oxygenase activase, chloroplastic isoform X1 | 0.747 | down | 0.037 |
| B9H8W5 | thioredoxin-like 2, chloroplastic | 0.634 | down | 0.015 |
| A0A2K2BNL0 | “Photosystem I reaction center subunit XI family protein | 0.631 | down | 0.033 |
| B9MYU1 | photosystem I subunit O-like | 0.627 | down | 0.034 |
| A0A2K1X1I0 | outer envelope pore protein 37, chloroplastic-like | 0.723 | down | 0.007 |
| A9PF53 | chaperone protein ClpB3, chloroplastic-like | 0.739 | down | 0.0173 |
| Response to stress | ||||
| A0A2K2BZL0 | heat shock protein 70 | 2.171 | up | 0.023 |
| A0A2K2BGF4 | 14-3-3 protein | 1.372 | up | 0.032 |
| A9P8Q7 | 14-3-3-like family protein | 2.068 | up | 0.028 |
| A9PCV6 | 14-3-3-like family protein | 1.806 | up | 0.018 |
| T2AUM9 | HSP90 | 1.331 | up | 0.0201 |
| Q6ZXH8 | Putative pathogenesis-related protein | 2.287 | up | 0.0225 |
| A0A2K1XHW1 | TMV resistance protein N | 1.324 | up | 0.016 |
| A0A2K1YCK6 | putative disease resistance protein RGA4 isoform X4 | 1.71 | up | 0.029 |
| A0A2K2C6K6 | NBS-like putative resistance family protein | 1.415 | up | 0.021 |
| A0A2K1XHW1 | TMV resistance protein | 1.324 | up | 0.016 |
| A0A1L6K4D3 | Cinnamyl alcohol dehydrogenase (CAD) | 3.77 | up | 0.010 |
| DNA and ion binding | ||||
| A0A2K1WMZ6 | DNA-binding family protein | 2.176 | up | 0.025 |
| A0A2K1XEE1 | nucleotide-binding protein | 1.629 | up | 0.001 |
| A0A2K1XN19 | oxidoreductase/transition metal ion-binding protein | 1.393 | up | 0.048 |
| A0A2K1Y9H8 | DNA-binding protein | 1.619 | up | 0.026 |
| A9P929 | DNA-binding family protein | 1.365 | up | 0.014 |
| A9PCK0 | DNA-binding family protein | 1.842 | up | 0.021 |
| B9I6G6 | calcium-binding EF hand family protein | 1.487 | up | 0.003 |
| U5GT53 | DNA-binding family protein | 1.34 | up | 0.011 |
| A0A2K1XU09 | zinc finger family protein | 1.577 | up | 0.004 |
| A9PEW2 | zinc finger CCCH domain-containing protein | 1.342 | up | 0.014 |
| Transporters related to cadmium transport | ||||
| A0A2K1Z3W9 | probable cadmium/zinc-transporting ATPase HMA1 | 1.321 | up | 0.014 |
| A0A2K2ADM8 | ABC transporter family protein | 1.513 | up | 0.016 |
| A9P875 | copper transport protein CCH | 1.352 | up | 0.001 |
| A9P8F9 | Copper-transporting ATPase RAN1 family protein | 1.342 | up | 0.049 |
| B9GJX7 | ABC transporter family protein | 1.442 | up | 0.013 |
| B9GTB1 | sugar transporter family protein | 1.575 | up | 0.031 |
| B9HIU2 | sugar transporter family protein | 1.751 | up | 0.028 |
| Antioxidant activity | ||||
| A0A2K1Z5Z6 | oxidoreductase family protein | 1.324 | up | 0.014 |
| A0A2K1XV17 | peroxisome biogenesis protein 6 | 1.381 | up | 0.0461 |
| B9ICD9 | “superoxide dismutase [Fe], chloroplastic isoform X2 | 1.338 | up | 0.0282 |
| Accession Number | Proteins Name | Cd + N/Cd Ration | Regulated Type | Cd + N/Cd p Value |
| Photosynthesis and energy metabolite | ||||
| A0A2K2BLH9 | probable glutamyl endopeptidase, chloroplastic | 1.336 | up | 0.039 |
| U7E2H1 | probable starch synthase 4, chloroplastic/amyloplastic isoform X2 | 1.634 | up | 0.004 |
| B9HQD5 | rubisco subunit binding-protein alpha subunit | 1.359 | up | 0.029 |
| Q3LUR8 | Glyceraldehyde-3-phosphate dehydrogenase | 1.484 | up | 0.002 |
| B9GHJ1 | thioredoxin family protein | 1.859 | up | 0.013 |
| Response to stress | ||||
| A0A2K1XHW1 | TMV resistance protein N | 1.353 | up | 0.015 |
| A0A2K1Y4T9 | signal recognition particle 14 kDa family protein | 1.307 | up | 0.008 |
| A0A2K1YPP0 | disulfide isomerase family protein | 2.338 | up | 0.002 |
| A0A2K2BTY0 | vacuolar-sorting receptor 6-like | 1.837 | up | 0.012 |
| A0A2K2CBE6 | probable disease resistance protein At4g27220 | 1.342 | up | 0.032 |
| B9GVR1 | stress inducible family protein | 1.431 | up | 0.015 |
| B9HKA3 | 6a-hydroxymaackiain methyltransferase family protein | 1.301 | up | 0.021 |
| B9HSN8 | UDP-N-acetylglucosamine pyrophosphorylase family protein | 1.954 | up | 0.048 |
| A0A2K2AYX4 | ATP-dependent RNA helicase family protein | 1.509 | up | 0.043 |
| A0A2K2C8D8 | DEAD-box ATP-dependent RNA helicase 46 | 1.405 | up | 0.047 |
| B9HX26 | huntingtin-interacting protein K-like | 2.393 | up | 0.013 |
| Transporters related to Cadmium transport | ||||
| A0A2K1ZUT7 | oligopeptide transporter family protein | 1.358 | up | 0.003 |
| B9HXP4 | vesical transport v-SNARE 12 family protein | 2.084 | up | 0.025 |
| A9PJD4 | transmembrane protein | 2.243 | up | 0.032 |
| Antioxidant activity | ||||
| K9MCB1 | Catalase | 1.647 | up | 0.018 |
| A0A2K1ZES8 | Peroxiredoxin family protein (Prx) | 3.867 | up | 0.004 |
| A0A193KWX3 | Glutathione S-transferase | 1.731 | up | 0.036 |
| A0A193KWY1 | Glutathione S-transferase | 1.642 | up | 0.014 |
| Q5CCP3 | Glutathione S-transferase | 1.481 | up | 0.006 |
| Regulation | ||||
| A0A2K2C504 | transcription initiation factor TFIID subunit 15b-like | 1.359 | up | 0.024 |
| A0A2K2B8L4 | zinc finger protein At1g67325-like isoform X1 (ZFPs) | 1.634 | up | 0.032 |
| A0A2K1YFZ8 | dof zinc finger protein DOF1.4-like (ZFPs) | 1.784 | up | 0.002 |
| Accession Number | Proteins Name | Cd + N/CK Ration | Regulated Type | Cd + N/CK p Value |
| Photosynthesis and energy metabolite | ||||
| Q3LUR8 | Glyceraldehyde-3-phosphate dehydrogenase | 1.42 | up | 0.002 |
| A9PFQ2 | ribulose bisphosphate carboxylase/oxygenase activase, chloroplastic-like isoform X1 | 1.476 | up | 0.014 |
| A9PJF4 | Ribulose bisphosphate carboxylase/oxygenase activase family protein | 1.371 | up | 0.032 |
| B9HQD5 | rubisco subunit binding-protein alpha subunit | 1.392 | up | 0.029 |
| B9I5M2 | rubisco accumulation factor 1, chloroplastic | 2.213 | up | 0.007 |
| A0A2K2C7R0 | Photosystem I reaction center subunit XI family protein | 2.657 | up | 0.001 |
| A9PEL0 | photosystem II 11 kDa family protein | 1.526 | up | 0.014 |
| A9PFW0 | photosynthetic NDH subunit of subcomplex B 4 | 2.815 | up | 0.004 |
| U5GXD4 | phosphoenolpyruvate carboxylase family protein | 1.351 | up | 0.012 |
| A0A0U1XA51 | Phosphoenolpyruvate carboxylase | 1.648 | up | 0.002 |
| B9GHJ1 | thioredoxin family protein (TRX) | 2.181 | up | 0.023 |
| A0A2K2B424 | ferredoxin family protein (FRX) | 1.657 | up | 0.031 |
| A0A2K2B297 | cytochrome c oxidase family protein | 2.27 | up | 0.004 |
| A0A2K2BVX5 | PGR5-like protein 1A, chloroplastic | 5.151 | up | 0.006 |
| A0A2K2B5R5 | PGR5-like protein 1A | 2.849 | up | 0.014 |
| Response to stress | ||||
| A0A2K1WUP1 | HSP-interacting protein | 1.92 | up | 0.024 |
| B9HBT8 | hsp70 nucleotide exchange factor fes1-like | 4.264 | up | 0.017 |
| A0A2K1YTL5 | heat shock family protein | 2.386 | up | 0.019 |
| A0A2K2BZL0 | heat shock protein 70 | 2.46 | up | 0.038 |
| B9HMG7 | heat shock protein 70 cognate | 2.509 | up | 0.010 |
| B9HMG8 | heat shock protein 70 cognate | 2.533 | up | 0.012 |
| B9HTJ7 | heat shock protein 70 | 1.913 | up | 0.013 |
| B9HV59 | heat shock protein 70 | 1.421 | up | 0.046 |
| B9N9W5 | heat shock protein 70 cognate | 1.972 | up | 0.012 |
| B9NBF4 | heat shock protein 70 cognate | 2.374 | up | 0.007 |
| U5G4Y8 | heat shock cognate 70 kDa protein 2-like | 3.306 | up | 0.015 |
| B9HKN2 | DnaJ family protein | 5.113 | up | 0.005 |
| A0A2K2BGB8 | 14-3-3-like protein GF14 omicron | 3.609 | up | 0.021 |
| A9PBC6 | 14-3-3-like protein GF14 omicron | 3.217 | up | 0.048 |
| A9PCV6 | 14-3-3-like family protein | 1.579 | up | 0.035 |
| A0A2K1XHW1 | TMV resistance protein N | 1.791 | up | 0.003 |
| A0A2K2BWJ4 | Mitogen-activated protein kinase (MAPK) | 2.968 | up | 0.012 |
| A9PK38 | translationally controlled tumor-like family protein (TCTP) | 2.311 | up | 0.015 |
| B9NAI3 | translationally controlled tumor-like family protein (TCTP) | 2.239 | up | 0.014 |
| B9IGC6 | proliferation-associated protein 2G4-like (PA2G4) | 6.879 | up | 0.011 |
| A0A2K2C4E6 | proliferation-associated protein 2G4-like (PA2G4) | 2.238 | up | 0.035 |
| Transporters related to cadmium transport | ||||
| A0A2K1X4J9 | ABC transporter G family member 22 isoform X2 | 1.582 | up | 0.0211 |
| A0A2K2ADM8 | ABC transporter family protein | 1.658 | up | 0.010 |
| B9HGA2 | ABC transporter family protein | 1.481 | up | 0.002 |
| B9HQM5 | ABC transporter family protein | 1.511 | up | 0.042 |
| A0A2K2C2F2 | calcium-transporting ATPase 4, endoplasmic reticulum-type-like | 2.026 | up | 0.007 |
| A9P875 | copper transport protein CCH | 1.55 | up | 0.013 |
| A9P8F9 | Copper-transporting ATPase RAN1 family protein | 1.427 | up | 0.041 |
| Antioxidant activity | ||||
| A0A2K1XV17 | peroxisome biogenesis protein 6 (POD) | 1.534 | up | 0.004 |
| A0A2K1YAM2 | peroxisomal membrane protein PEX14-like isoform X2 | 1.94 | up | 0.011 |
| A0A2K1ZES8 | Peroxiredoxin family protein ((Prx) | 3.588 | up | 0.005 |
| B9NBW2 | glutathione transferase (GST) | 1.447 | up | 0.029 |
| D2WL67 | glutathione transferase GST | 1.373 | up | 0.002 |
| DNA and ion binding | ||||
| A0A2K1XEJ1 | oxidoreductase/transition metal ion-binding protein | 1.726 | up | 0.038 |
| A0A2K1XN19 | oxidoreductase/transition metal ion-binding protein | 1.449 | up | 0.037 |
| A0A2K1XB60 | GTP-binding protein TypA/BipA homolog | 1.388 | up | 0.025 |
| A0A2K2CA16 | calcium-binding EF-hand protein | 1.734 | up | 0.016 |
| A9P926 | GTP-binding protein beta chain | 1.533 | up | 0.013 |
| A9P929 | DNA-binding family protein | 1.446 | up | 0.002 |
| A9PCK0 | DNA-binding family protein | 1.626 | up | 0.008 |
| A9PCU6 | calcium-binding EF hand family protein | 2.142 | up | 0.007 |
| B9I2F7 | calcium binding family protein | 3.052 | up | 0.021 |
| B9I2G9 | GTP-binding protein beta chain | 1.448 | up | 0.026 |
| B9I6G6 | calcium-binding EF hand family protein | 1.43 | up | 0.029 |
| Storage protein | ||||
| A0A2K1Y5T5 | vacuolar protein sorting-associated protein 18 homolog (VPS) | 1.581 | up | 0.008 |
| A0A2K2BLH8 | vacuolar protein sorting-associated protein (VPS) | 1.625 | up | 0.039 |
| A0A2K2BTY0 | vacuolar-sorting receptor 6-like (VPS) | 2.753 | up | 0.0001 |
| U5GBE7 | Vacuolar protein sorting-associated protein 35 (VPS) | 2.391 | up | 0.019 |
| A9PGW6 | bark storage protein B-like (BSP) | 4.043 | up | 0.018 |
| Regulation | ||||
| A0A2K1XQU0 | transcription factor 4G-like (TF4G) | 2.062 | up | 0.003 |
| A0A2K1XWY2 | WRKY transcription factor 1 (WRKY1) | 1.34 | up | 0.001 |
| A0A2K1YGZ3 | eukaryotic translation initiation factor 4E family protein (elF4E) | 2.135 | up | 0.032 |
| A0A2K1Z7J5 | Translation initiation factor IF-3 (eIF3) | 1.751 | up | 0.042 |
| A0A2K2BI09 | transcription factor (TF) | 3.313 | up | 0.019 |
| A0A2K2CCU4 | translation initiation factor IF-2 family protein (eIF2A) | 3.319 | up | 0.023 |
| A9PC51 | eukaryotic translation initiation factor 4G isoform X1 (elF4G) | 1.64 | up | 0.029 |
| B9GP88 | eukaryotic translation initiation factor 4G isoform X1 (elF4G) | 1.518 | up | 0.005 |
| M9Z3T5 | Eukaryotic translation initiation factor 5A (eIF5A) | 1.816 | up | 0.002 |
| M9ZCJ4 | Eukaryotic translation initiation factor 5A (eIF5A) | 2.299 | up | 0.006 |
| A0A2K1XU09 | zinc finger family protein (ZFPs) | 1.671 | up | 0.007 |
| A9PCM2 | zinc finger protein GIS2-like (ZFPs) | 1.686 | up | 0.004 |
| U5FQR8 | zinc finger matrin-type protein 2 (ZFPs) | 1.485 | up | 0.026 |
3.2. Plants Actively Exert a Range of Approaches in the Defense against Cadmium Stress
Plants actively exert a range of strategies to strengthen their tolerance to heavy metal exposure. First, they up-regulate the expression of stress-responsive proteins; second, they stimulate the activities of antioxidant enzymes to scavenge the cellular ROS induced by heavy metal stress; third, they up-regulate the expression of cell wall proteins to constraint more heavy metal ions and fourth, they increase the activities of enzymes associated with glutathione (GSH) and phytochelation (PC) to sequestrate more heavy metal ions.
In this study, stress-responsive proteins including two heat shock proteins (HSPs; HSP70, A0A2K2BZL0; HSP90, T2AUM9), three 14-3-3 proteins (A0A2K2BGF4, A9P8Q7 and A9PCV6), and five disease resistance proteins (Q6ZXH8, A0A2K1XHW1, A0A2K1YCK6, A0A2K2C6K6 and A0A2K1XHW1) were up-regulated in poplar plants when exposed to Cd treatment. Heat shock proteins are stress-responsive proteins and play an important role in protecting cells from elevated environments. CeHSP17 was significantly up-regulated by cadmium and zinc in wild-type C. elegans [36]. In transgenic Arabidopsis, PfHSP17.2 showed a higher resistant ability under heat, cold and salt stresses [37]. HSP20, HSP70 and HSP90 were increased in poplar leaves under Cd stress [38]. In the Oxya chinensis, OcHsp40, OcHsp70 and OcHsp90 were significantly up-regulated by acute Cd stress [39]. In this study, HSP70 and HSP 90 were up-regulated by Cd stress in P. yunnanensis leaves.
In higher plants, 14-3-3 proteins always bind to the signaling molecules and play crucial roles in stress responses and signal transduction [40]. In rice, the genes encoding the 14-3-3 protein was obviously up-regulated by arsenic stress [41]. In aluminum (Al)-tolerant soybean roots, the expression of 14-3-3 proteins were significantly enhanced by Al stress [42]. In the present study, three 14-3-3 proteins were up-regulated by Cd stress.
Interestingly, five bioresistance proteins were up-regulated by cadmium stress in the study, which reveal that the resistance protein may have dual functions including bio-resistance and abio-resistance.
The cell wall is confirmed to be the first barrier to protect plants against environmental stresses [43]. Many documents have reported that the cell wall can effectively bio-sorb Cd ions to alleviate their toxicity to plants [44]. Meyer et al. (2015) revealed that metal-tolerant plants could accumulate abundant Cd in cell walls [45]. The proteomics results in this study showed that one cinnamyl alcohol dehydrogenase (CAD) protein (A0A1L6K4D3) had higher expression levels after Cd stress. CAD catalyzes key steps in the pathway of lignin monomer biosynthesis. Up-expressed CAD can promote the synthesis of plant cell walls to resist various environmental stresses [46].
Antioxidant enzymes maintain cellular redox balance by scavenging reactive oxygen species (ROS) [47]. Cd stress mainly stimulates the production of ROS to damage plants. In this study, our proteomics results showed that one oxidoreductase protein (A0A2K1Z5Z6), one peroxisome protein (A0A2K1XV17) and one superoxide dismutase protein (B9ICD9) were significantly up-regulated by Cd stress. Thus, our results reveal that Cd stress actively regulates the expression of antioxidant proteins to balance the cellular redox level and minimize the damage of Cd to poplar plants.
3.3. Exogenous Nitrogen Alleviated the Toxicity of Cadmium to Poplar Plants
In the present study, the results reveal that Cd + N observably promotes poplar plant growth, improves chlorophyll content, reduces the accumulation of H2O2 and MDA, and enhances the absorption and transportation of plants to Cd. The differentially expressed proteins in Table 5 show that the positive interaction effects between Cd and N could partially explain how exogenous nitrogen effectively protects plants against Cd stress.
The growth of plants is positively related to photosynthesis. In this study, Cd stress significantly inhibited the photosynthesis of poplar plants. Eight proteins involved in photosynthesis showed lower expression levels when compared to the control. However, 15 proteins correlated to photosynthesis and energy metabolite were obviously up-regulated by Cd + N treatment, relative to the control values. Similarly, five photosynthetic proteins showed higher expression levels in N + Cd treated plants when compared to acute Cd stress. The differentially expressed ratio of Cd + N/CK for the proton gradient regulation (PGR5) protein was as high as 5.515. The data revealed that the expression of PGR5 was markedly up-regulated by the interaction of Cd + N. Previous studies have shown that PGR5 is closely related to the cyclic electron flow in PS I [48]. Paredes and Quiles [49] revealed that when Hibiscus plants are subjected to cold stress, the amount of PGR5 polypeptide increases, suggesting that under abiotic stress, up-expressed PGR5 could protect photosystems and enhance the resistance of plants to environmental stress. Therefore, the PGR5 protein (A0A2K2BVX5) was screened out as a candidate protein for further research.
Exogenous nitrogen addition could help poplar plants actively respond to cadmium stress, which was validated by comparing the differentially expressed proteins between Cd + N treatment and the control or between Cd + N treatment and acute Cd treatment. Our proteomics results show that 21 and 11 differentially stress-responsive proteins were observably up-regulated by N + Cd treatments when contrasted with CK, and sole Cd, respectively. These 21 proteins mainly included eleven heat shock proteins (HSPs), three 14-3-3 proteins, one TMV resistance protein, one mitogen activated protein kinase protein (MAPK), two proliferation-associated proteins 2G4 (PA2G4), one DnaJ protein and two translationally controlled tumor proteins (TCTP). Notably, the differentially expressed ratio of Cd + N/CK about MAPK, TCTP, 14-3-3, HSP70, DnaJ, and PA2G4 was as high as 2.968, 2.311, 3.609, 4.264, 5.113 and 6.879, respectively. The functions of HSP and the 14-3-3 in stress-response were explained in the previous section. Research has shown that MAPK plays an important role in cell differentiation and stress response. The activity of MAPK was induced by heavy metals in alfalfa and rice [50]. Existing studies have shown that plant TCTP is involved in environment stress signaling and that overexpression of AtTCTP reinforce drought tolerance, depending on the abscisic acid pathway [51]. Wang et al. [52] revealed that OsTCTP could significantly reduce the accumulation of the Hg-induced reactive oxygen species (ROS), thereby enhancing the rice plants’ tolerance to Hg stress. In this study, two TCTPs were up-regulated by N + Cd treatment. The DnaJ protein was reported to be important in maintaining cellular homeostasis under stress conditions to enhance plants’ resistance. For example, a DnaJ protein located in the chloroplast maintained its photosystem II stability and improved the resistance of plants to chilling stress [53]. However, the mechanism by which the DnaJ protein facilitates plant resistance to Cd stress remains unclear.
The existing studies reported that the PA2G4 protein actively regulates the DNA replication in eukaryotic cells. At present, studies on the functions of PA2G4 proteins have mainly focused on humans and animals, with few studies on plants. In our study, the differentially expressed ratio of Cd + N/CK of PA2G4 ranged to 6.879, thus indicating that exogenous nitrogen addition helps to maintain normal DNA replication in plant cells and alleviate cadmium toxicity in poplar plants.
Antioxidant enzymes may be crucial for alleviating oxidative stress [54]. Our proteomics results show that peroxiredoxin (Prx), peroxisome (POD) and glutathione transferase (GST) are observably up-regulated by N + Cd treatments when contrasted with CK or sole Cd treatment, respectively. The differential expression ratio of Cd + N/CK or Cd + N/Cd for Prx was up to 3.588 and 3.867, respectively. Peroxiredoxins (Prxs) are a family of ubiquitous proteins in all kingdoms of life. Prxs are important for antioxidant defense and participate in redox signaling. The inactivation of Prx by phosphorylation causes H2O2 accumulation [55]. In the present study, Cd + N treatment activated the expressions of Prx and obviously decreased the accumulation of H2O2, which agrees with the results of past studies.
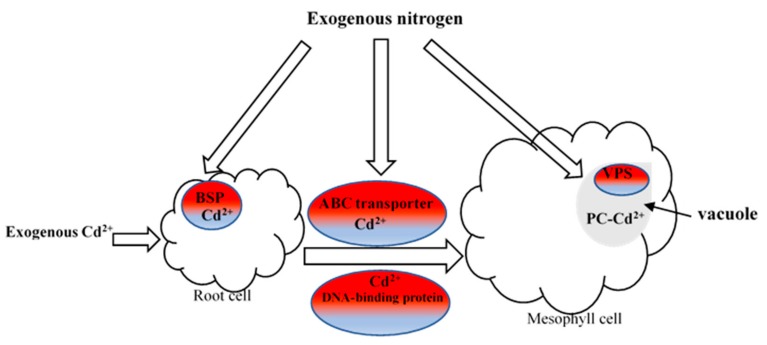
When cadmium ions are absorbed by plant roots, some of the ions are transferred aboveground from the plant and are then chelated with glutathione (GSH) and phytochelation (PC) to be sequestrated in the vacuoles. In this study, the addition of N significantly enhanced the transportation and absorption of Cd in plants. The data for the proteomics also revealed that eleven binding proteins, seven transporter proteins and five storage proteins associated with Cd2+ were up-regulated by N + Cd treatments. Of the eleven binding proteins, four were calcium-binding proteins, three were GTP-binding proteins, two were DNA-binding proteins and two were metal ion-binding proteins. The transporter proteins up-regulated by N + Cd were mainly ABC transporter proteins, which accounted for 57.1% of the total differential transporters. ABC transporter proteins are widely found in living organisms and belong to the superfamily of transporters. Evidence in the existing literature has shown that ABC transporters are important for the detoxification of heavy metals [56]. In Arabidopsis, AtSTAR1 (an annotated ABC transporter) is involved in the basic detoxification of aluminum stress [57]. AtMRP3, an ABC transporter gene, shows a higher expression levels in Cd-treated plants [58]. In the present study, we found much higher levels of ABC transporter proteins induced by Cd + N, which suggests that exogenous nitrogen addition could enhance the activities of ABC transporter proteins to carry and transport more Cd ions. Among the differentially expressed storage proteins, four were vacuolar proteins and one was a bark storage protein, which reveals that exogenous nitrogen addition can increase the accumulation of cadmium in poplar plants.
Existing studies have shown that transcriptional regulation and translational regulation are the conserved strategies for plant responses to biological and abiotic stress [59]. The responses of plants to Cd stress are often modulated by transcription factors (TFs) and translation initiation factors (IFs). These transcription factors usually include basic helix-loop-helix (bHLH), WRKY, ERF, MYB, NAC, MADS, Zinc-Finger transcription factor (ZFPs), etc. For example, overexpression of ThWRKY7 enhanced plant tolerance to cadmium stress [29]. ZAT6, belonging to the Zinc-Finger transcription factor family, positively regulates cadmium tolerance through the glutathione-dependent pathway in Arabidopsis [60]. In plants, the translation initiation factor (IFs) is the main eukaryotic translation initiation factor (eIF). Chou et al. [61] revealed that the expression of rice eIF5A genes, OseIF5A- and seIF5A-2 were remarkably up-regulated by salt and heavy metal stresses. In the present study, we found much higher levels of WRKY, ZFPs, TFs and eIFs in poplar plants under Cd + N treatment. These findings indicate that plant’s responses to Cd stress are positively regulated by exogenous nitrogen.
3.4. Exogenous Nitrogen Promoted Protein Phosphorylation to Enhance Poplar Plants’ Resistance to Cadmium Stress
Protein phosphorylation plays an important regulatory role in the immune system. Previous studies on the regulation of plant responses to stress by protein phosphorylation mainly focused on drought, cold, salinity and other biotic stress [62]. Moreover, the plant materials used were mainly model species, such as Arabidopsis thaliana, rice (Oryza sativa), etc. Heavy metal-responsive phosphoproteins may be involved in heavy metal (HM) uptake, HM transportation, HM sequestration and HM detoxification. However, there are few studies on the regulatory function of protein phosphorylation in woody plants responses to HM stress. Therefore, we are the first to investigate the protein phosphorylation changes in poplar plants under Cd stress or Cd + N treatment.
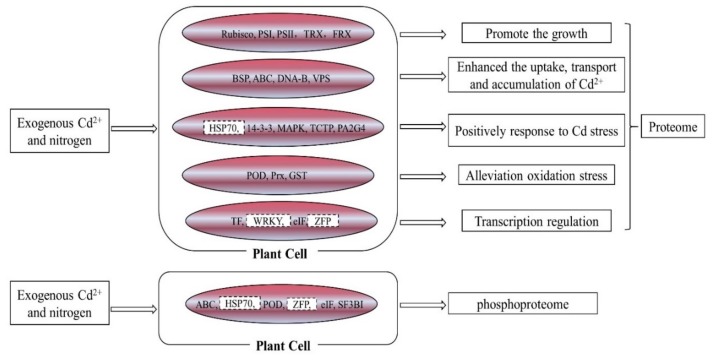
The differences in phosphorylated proteins under Cd or Cd + N treatment were compared with the control after the proteomic difference was normalized, and the results are shown in Figure 3. The changes in the trend for the phosphoproteome data were similar to those found in the proteomic data. Many proteins up-regulated by Cd + N treatment also had higher expression levels after phosphorylation (Table 6). In addition, both Cd stress and Cd + N treatment may affect oxidative phosphorylation. However, Cd + N treatment obviously lead to greater protein phosphorylation and more phosphorylated proteins being up-expressed, while sole Cd stress induced a greater number of phosphorylated proteins to be down-expressed. These proteins largely included zinc finger proteins (ZFPs), ABC transporter proteins (ABC), heat shock proteins (HSPs), translation initiation factors (IFs) and peroxidase (POD). Interestingly, eight sites in the splicing factor 3B subunit protein (SF3B1) (A0A2K2C6G4) were phosphorylated, and all of them were up-regulated under Cd + N treatment. In addition, three phosphosites in the HSP70 protein showed higher expression levels in Cd + N treated plants. SF3B1 is an mRNA splicing factor. Importantly, SF3B1 mutations have been detected in many cancers [63]. At present, studies on the important role of SF3B1 have mainly focused on humans and animals, while its role in plants has not been reported. Kim Guisbert et al. [64] revealed that SF3B1 regulates the activity of the heat shock transcription factor (HSF1) via mRNA splicing to enhance stress-sensitive abilities. In this study, eight phosphosites in the SF3B1 were up-expressed in Cd + N treated plants, which indicated that SF3B1 may modulate poplar plants’ resistant to Cd stress by mRNA splicing at the transcriptome. On the other hand, HSP70 is an important stress-responsive protein, and plays a vital role in stress responses against changes under extreme environments [65]. Assimon et al. [66] revealed that HSP70 autophosphorylation helps to bind its chaperone and further to realize its biological functions. Similarly, three phosphosites in HSP70 were all up-regulated by Cd + N treatment, which indicates the phosphorylated HSP70 may better exercise its function in protecting plant cells or tissues from Cd stress. However, in the study, the intriguing link between SF3B1 and Cd-response, as well as the variety of roles for both SF3B1 and HSP 70 in response to Cd stress, are still not clear, and deserve further study.
Table 6.
List of partial phosphorylated proteins differentially regulated by Cd + N in poplar plants.
| Protein Accession | Position | CdN/Ck Ratio | Regulated Type | Cd N/Ck p Value | Amino Acid | Protein Description |
|---|---|---|---|---|---|---|
| A0A2K1WUE2 | 493 | 1.28 | Up | 0.0064 | S | eukaryotic translation initiation factor isoform (eIF) |
| A0A2K1XQU0 | 1237 | 1.669 | Up | 0.0414 | S | eukaryotic translation initiation factor 4G-like (eIF) |
| B9GP88 | 987 | 1.704 | Up | 0.018 | T | eukaryotic translation initiation factor 4G isoform X1 (eIF) |
| B9GP88 | 988 | 1.899 | Up | 0.0271 | S | eukaryotic translation initiation factor 4G isoform X1 (eIF) |
| A0A2K1XBQ3 | 26 | 1.458 | Up | 0.0485 | S | ABC transporter G family member (ABC transporter protein) |
| A0A2K2ACX1 | 110 | 1.241 | Up | 0.00616 | S | ABC transporter G family member (ABC transporter protein) |
| A0A2K1XB30 | 251 | 1.671 | Up | 0.028 | S | zinc finger family protein (ZFP) |
| A0A2K1XB30 | 382 | 1.867 | Up | 0.0199 | S | zinc finger family protein (ZFP) |
| A0A2K2ATR0 | 296 | 1.991 | Up | 0.00726 | S | zinc-finger homeodomain protein 9-like (ZFP) |
| B9HN74 | 557 | 1.421 | Up | 0.0354 | S | heat shock protein 70 (HSP70) |
| B9HV59 | 10 | 1.345 | Up | 0.0299 | S | heat shock protein 70 (HSP70) |
| B9HV59 | 12 | 3.554 | Up | 0.00632 | S | heat shock protein 70 (HSP70) |
| B9HV59 | 14 | 1.493 | Up | 0.0000634 | T | heat shock protein 70 (HSP70) |
| U7E2Z8 | 604 | 2.388 | Up | 0.0185 | S | heat shock-related family protein (HSP70) |
| W8PVS7 | 250 | 2.7 | Up | 0.0191 | T | Peroxidase (POD) |
| A0A2K2C6G4 | 246 | 1.816 | Up | 0.0231 | T | splicing factor 3B subunit 1-like (SF3B1) |
| A0A2K2C6G4 | 185 | 2.175 | Up | 0.0149 | T | splicing factor 3B subunit 1-like (SF3B1) |
| A0A2K2C6G4 | 207 | 2.355 | Up | 0.0341 | T | splicing factor 3B subunit 1-like (SF3B1) |
| A0A2K2C6G4 | 209 | 2.086 | Up | 0.0351 | T | splicing factor 3B subunit 1-like (SF3B1) |
| A0A2K2C6G4 | 353 | 1.529 | Up | 0.0387 | T | splicing factor 3B subunit 1-like (SF3B1) |
| A0A2K2C6G4 | 244 | 1.92 | Up | 0.0127 | T | splicing factor 3B subunit 1-like (SF3B1) |
| A0A2K2C6G4 | 219 | 1.954 | Up | 0.0089 | T | splicing factor 3B subunit 1-like (SF3B1) |
| A0A2K2C6G4 | 125 | 1.484 | Up | 0.0462 | T | splicing factor 3B subunit 1-like (SF3B1) |
3.5. The Key Regulatory Proteins Play Their Important Roles in Plant Cellular Responses to Cd Stress
Plant cellular responses to heavy metal stress may involve complex interconnected networks of signaling pathways. Transcription factors (TFs) are proteins that have been identified their important roles in plant cellular responses to Cd stress. These TFs includes WRKY, MYB, NAC, HSF, ZIP, etc. [67]. In parallel with comparative proteomic and protein phosphorylation analyses, we performed transcriptome sequence using the same batch of materials. The transcriptomic data was not published, but can be checked in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) Sequence Database (accession number GSE140398). The data of transcriptome reveal that exogenous nitrogen addition under Cd stress induces more differentially expressed TFs, such as WRKY, HSF, LOB, NAC, MYB, ZAT and bHLH. Among these TFs, three WRKYs, five ZATs and one HSF showed significantly higher expression levels in poplar plants under Cd + N treatment compared to sole Cd stress. At the same time, proteomic data show that one WRKY, three ZFPs belonged to ZAT family, and one HSP70 belonged to HSF family, were significantly up-regulated by exogenous nitrogen applied to Cd stress. Therefore, combine transcriptomic data with proteomic data, WRKY, HSP70 and ZFP proteins can be considered as the key candidate proteins for further study.
4. Conclusions
Combing the proteomic data and phosphoproteomics data, we could deduce the reasons why exogenous nitrogen can enhance poplar plants’ resistance to cadmium and improve the phytoremediation efficiency of cadmium-contaminated soil. Exogenous nitrogen alleviated the toxicity of cadmium to poplar plants through multiple channels. First, the addition of nitrogen to Cd treated plants up-regulated the bark storage protein (BSP), ABS transporter protein, DNA-binding protein and vacuolar protein sorting-associated protein (VPS) to promote the uptake, transportation and accumulation of cadmium in poplar plants (Figure 5). Second, the addition of nitrogen induced the accumulation of glutathione (GSH) and phytochelatin and increased the activities of antioxidant enzymes to weaken the cadmium-induced damage to poplar plants (Table 4 and Table 5 and Figure 6). Third, the addition of nitrogen to Cd treated plants up-regulated the expressions of some proteins, such as the responsive proteins (HSP70, 14-3-3, MAPK, etc.), some transcription factors (WRKY, TF, ZFP, etc.), and eukaryotic translation initiation factor (elF), to strengthen the resistance of poplar plants to Cd stress. Lastly, phosphorylated SF3B1 and HSP70 possibly co-effected poplar plants’ resistance to cadmium stress. Taken together, the proteome and phosphoproteome data show that nitrogen serves a protective role in plants against Cd stress.
Figure 5.
Exogenous nitrogen enhanced the uptake, transport and accumulation of cadmium in poplar plants by up-regulated the expressions of proteins. BSP, bark storage protein; VPS, vacuolar protein sorting-associated protein. Red, up-regulated protein.
Figure 6.
Exogenous nitrogen alleviated the toxicity of cadmium to poplar plants through multiple channels. Red, up-regulated protein; PS I, Photosystem I; PS II, photosystem II; TRX, thioredoxin family protein; FRX, ferredoxin family protein; ABC, ABC transporter protein; DNA-B, DNA-binding protein; HSP70, heat shock protein 70; 14-3-3, 14-3-3 protein; MAPK, Mitogen-activated protein kinase; TCTP, translationally controlled tumor-like family protein; PA2G4, proliferation-associated protein 2G4-like; POD, Peroxidase; Prx, Peroxiredoxin; GST, glutathione transferase; TF, transcription factor; elF, Eukaryotic translation initiation factor; ZFP, zinc finger protein; SF3BI, splicing factor 3B subunit 1-like. WRKY, ZFP and HSP70 were selected as the key regulation proteins.
5. Materials and Methods
5.1. Plant Material and Treatment
The cuttings from Populus yunnanensis plants were used as the experimental materials and NH4HCO3 and CdCl2 as the major reagents in the study. Seventy-two healthy cuttings about 15 cm long were obtained from 15 Yunnan poplars (Populus yunnanensis). The P. yunnanensis samples were obtained from their native habitats in Butuo county of Liangshan prefecture, Sichuan province, China. Since the wide Yunnan poplar plants were not privately owned, and they were not listed among the endangered species, specific permits were not required for this study. Moreover, the selected trees shared the same habitat. After sampling, these 72 cuttings were replanted into 24 plastic pots (three cuttings per pot), and each pot was filled with 15 kg of dry soil. The diameter and deep of the pot were 37 and 28 cm, respectively. The basic characteristics of the cultivation soil were as following: pH 5.8, total organic matter 14.23 g·kg−1, total nitrogen 0.58 g·kg−1, total phosphorus 0.42 g·kg−1, total potassium 2.35 g·kg−1 and total cadmium 0.785 mg·kg−1. The pots with Yunnan poplar cuttings were placed in a chamber with a 16 h photoperiod, a 25/20 °C temperature, and 70% air humidity. A polyethylene disc under each pot was used to avoid run-off. When the plants were 20 cm in height with an average of 10–15 leaves, the 24 pots were divided into three sets. One set acted as a control, and the second set was treated with Cd. Meanwhile, the third set was treated with Cd and N. The method of cadmium treatment was based on the phenomenon of the gradual infiltration of cadmium into soil in nature. A total of 1.22 g CdCl2·2.5H2O and 2.52 g NH4HCO3 (equal to 40 mg Cd2+ ·kg−1 and 30 mg N·kg−1 dry weight soil) was added to each pot every 5 days. The amount of Cd and N added each time was based on the results from the preliminary test and our published research [30]. After 60 d of treatment, the fourth, fifth and sixth leaves from the apex of each seedling were sampled and immediately kept frozen at −80 °C for the subsequent analysis.
5.2. Chlorophyll Content and Cd Assays
Chlorophyll content was quantified according to the method of Lichtenthaler [68]. The Cd concentrations in soil, root and leaf tissue were detected using an inductively coupled plasma-optical emission spectrometer (ICP-OES; OPTIMA 2000A, PerkinElmer Co., Massachusetts, USA) after wet-digesting in HNO3–H2SO4–HClO4 [69]. Three independent biological replicates for each treatment and three technical repeats for each biological replicate were arranged to ensure the reproducibility.
5.3. Gas-Exchange Parameters and Chl Fluorescence Measurements
The fully expanded fourth, fifth and sixth leaves (from the apex) of the treatment and the control were used to monitor the photosynthetic rate and Chl fluorescence parameters. Gas-exchange parameters were quantified using a portable photosynthesis system (LI-6400, LI-COR Co., Nebraska, USA) according to the methods described by Zhang et al. [30]. Chlorophyll fluorescence parameters, such as the minimal fluorescence yield (F0), the maximal fluorescence yield (Fm), the minimal fluorescence (F0’) and maximal fluorescence (Fm’), were obtained according to the methods previously described [11]. The gas-exchange and fluorescence measurements were conducted on the same parts of the leaves.
5.4. H2O2, MDA (Malondialdehyde), Total GSH (Glutathione) and PCs (Phytochelatin) Content Analysis
H2O2 levels and MDA were measured as described previously [70]. The contents of GSH and PCs were quantified using the method of Gupta et al. [71]. GSH content was evaluated based on its absorbance at 412 nm. PC production was evaluated according to the D-value between non-protein thiols (NPT) and GSH [72]. The NPT was estimated as described by Ellman [73]. Three biological repeats were used to assess the content of these indexes.
5.5. The Differentially Expressed Proteins and Phosphorylated Proteins Analysis
The tandem mass tag (TMT) technique was applied to quantify the differentially expressed proteins in poplar leaves. TMT and IMAC (immobilized metal affinity chromatography) were used to identify the differentially phosphorylated proteins. Three independent biological replicates for each treatment and three technical repeats for each biological replicate were performed for assessment the proteome and protein phosphorylation levels.
5.5.1. Protein Extraction, Trypsin Digestion, TMT Labeling and HPLC Fractionation
Protein extraction, trypsin digestion, TMT labeling and HPLC fractionation were performed using the procedure described by Yang et al. [74], with minor modifications. Leaf samples were grinded by liquid nitrogen into powder and then transferred to a centrifuge tube (5 mL). After that, four volumes of lysis buffer (8 M urea, 1% Triton-100, 10 mM dithiothreitol and 1% protease inhibitor cocktail) were added to the cell powder, followed by sonication three times on ice using a high intensity ultrasonic processor (Scientz). The remaining debris was removed by centrifugation at 20,000× g at 4 °C for 10 min. Finally, the protein was precipitated with cold 20% TCA for 2 h at –20 °C. After centrifugation at 12,000× g 4 °C for 10 min, the supernatant was discarded. The remaining precipitate was washed with cold acetone three times. The protein was then redissolved in 8 M urea and the protein concentration was determined with a BCA kit, according to the manufacturer’s instructions.
For digestion, the protein solution was reduced with 5 mM dithiothreitol for 30 min at 56 °C and alkylated with 11 mM iodoacetamide for 15 min at room temperature in darkness. The protein sample was then diluted by adding 100 mM TEAB to a urea concentration of less than 2 M. Finally, trypsin was added at a 1:50 trypsin-to-protein mass ratio for the first digestion overnight at 37 °C, and a 1:100 trypsin-to-protein mass ratio was used for a second 4 h-digestion to complete the digestion cycle.
After trypsin digestion, the peptide was desalted by a Strata XC 18 SPE column (Phenomenex) and vacuum-dried. The peptide was reconstituted in 0.5 M TEAB and processed according to the manufacture’s protocol for the TMT kit/iTRAO kit. Briefly, one unit of TMT kit/iTRAO reagent was thawed and reconstituted in in acetonitrile. The peptide mixtures were then incubated for 2 h at room temperature and pooled, desalted, and dried by vacuum centrifugation.
The dried peptides were fractionated by high pH reverse-phase HPLC using a Thermo Betasil C18 column (5 μm particles, 10 mm ID, 250 mm length) (Vigorous Co., USA). Briefly, peptides were first separated with a gradient of 8%–32% acetonitrile (pH 9.0) over 60 min into 60 fractions. Then, the peptides were combined into 18 fractions and dried by vacuum centrifugation.
5.5.2. IMAC Enrichment
The phosphopeptide was enriched by immobilized metal affinity chromatography (IMAC) as previously described by Chen et al. [60]. Peptide mixtures were first incubated with an IMAC microsphere suspension with vibrations in a loading buffer, including 50% acetonitrile (CAN) and 6% trifluoroacetic acid (TFA). The IMAC microspheres with enriched phosphopeptides were collected by centrifugation (4 °C, 5000× g for 10 min), and the supernatant was removed. To remove nonspecific adsorbed peptides, the IMAC microspheres were washed with 50% acetonitrile/6% trifluoroacetic acid and 30% acetonitrile/0.1% trifluoroacetic acid, sequentially. To elute the enriched phosphopeptides from the IMAC microspheres, an elution buffer containing 10% NH4OH was added and the enriched phosphopeptides were eluted with vibration. The supernatant containing phosphopeptides was collected and lyophilized for LC–MS/MS analysis.
5.5.3. LC–MS/MS Analysis
The tryptic peptides were analyzed using LC–MS/MS, as described by Yang et al. [74]. The enriched phosphopeptides were analyzed on a Q ExactiveTM Plus hybrid quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), as described by Chen et al. [60]. The tryptic peptides were dissolved in 0.1% formic acid (solvent A) and directly loaded onto a home-made reversed-phase analytical column (15-cm length, 75 μm i.d.). The gradient comprised an increase from 6% to 23% for solvent B (0.1% formic acid in 98% acetonitrile) over 26 min, 23%–35% in 8 min and climbing to 80% in 3 min before holding at 80% for the last 3 min, all at a constant flow rate of 400 nL/min on an EASY-nLC 1000 UPLC system.
The peptides were subjected to NSI source analysis followed by tandem mass spectrometry (MS/MS) in a Q ExactiveTM Plus (Thermo Fisher Scientific, Waltham, MA, USA) coupled online to the UPLC. The electrospray voltage applied was 2.0 kV. The m/z scan range was 350–1800 for the full scan, and intact peptides were detected in the Orbitrap at a resolution of 70,000. Peptides were then selected for MS/MS using an NCE setting of 28, and the fragments were detected in the Orbitrap at a resolution of 17,500. A data-dependent procedure that alternated between one MS scan followed by 20 MS/MS scans with 15.0 s dynamic exclusion. The automatic gain control (AGC) was set at 5E4. The fixed first mass was set as 100 m/z.
5.5.4. MS/MS Data Search
The resulting MS/MS data were processed using MaxQuant (v.1.5.2.8) (Max Planck Institute of Biochemistry). Tandem mass spectra were searched against the Populus trichocarpa protein database concatenated with a reverse decoy database. Trypsin/P was specified as cleavage enzyme allowing up to four missing cleavages. The mass tolerance for precursor ions was set as 20 ppm in first search and 5 ppm in the main search, and the mass tolerance for fragment ions was set as 0.02 Da. Carbamidomethyl on Cys was specified as a fixed modification, and phosphorylation modification and oxidation on Met were specified as variable modifications. False discovery rate (FDR) thresholds were adjusted to < 1%, and the minimum score for modified peptides was set as > 40.
5.5.5. Relatively Quantified Phosphopeptides
The phosphopeptides were relatively quantified after the database search. For TMT quantification, the ratios of the TMT reporter ion intensities in MS/MS spectra (m/z 126–131) from raw data sets were used to calculate fold changes between samples. Only peptides unique for a given protein were considered for relative quantitation. For each sample, the quantification was normalized using the average ratio of all the unique peptide. Protein quantitation calculated from the median ratio of protein corresponding unique peptides when there were at least two unique peptides in a protein. To evaluate changes in differential abundance of protein, one-sample two-sided t-tests were performed with unique peptide ratio of protein corresponding. In general, a significance level of 0.05 was used for statistical testing, and we reported the p value or significance level any time a statistical test was performed. For overall group comparison, we regarded three replicate samples as one sample. In brief, the average reporter ion intensities of three replicate samples represented overall sample intensities. Then, we recalculated fold changes and statistics tests between samples.
5.5.6. Bioinformatics Analysis
Gene ontology (GO) annotation was applied to determine the protein categories that were enriched. If some identified proteins were not annotated by the UniProt-GOA database, then InterProScan soft (The Minnesota Supercomputing Institute) was used to annotate the protein’s GO function based on the protein sequence alignment method. Then, the proteins were classified by gene ontology annotation based on three categories: biological process, cellular component and molecular function. Identified proteins domain functional description were annotated by InterProScan (a sequence analysis application) based on the protein sequence alignment method, and the InterPro domain database (http://www.ebi.ac.uk/interpro/) was used.
The pathways of differentially-expressed proteins and phosphoproteins were determined using KEGG tools-first, by using the KEGG online service tool KAAS to annotated the protein’s KEGG database descriptions and then by mapping the annotation results on the KEGG pathway database using the KEGG online service tools KEGG mapper.
5.5.7. The Heat Map of Different Protein Functional Classification
The heat map of different protein functional classification was figured. We first collated all the categories obtained after enrichment along with their p values, and then filtered for those categories, which were at least enriched in one of the clusters with p value < 0.05. This filtered p value matrix was transformed by the function x = −log10 (p value). Finally, these x values were z-transformed for each functional category. These z scores were then clustered by one-way hierarchical clustering in Genesis. Cluster membership was visualized by a heat map using the “heatmap.2” function from the “gplots” R-package.
5.6. Statistical Analysis
Data were analyzed using SPSS statistical software, version 20.0 (IBM Co., Armonk, New York, USA). Results were evaluated by one-way ANOVA using a general model procedure followed by Tukey’s HSD post-hoc test. The results were presented as means ± SD, and a probability at p ≤ 0.05 was considered significant.
Abbreviations
| HM | Heavy metal |
| N | nitrogen |
| Cd | cadmium |
| HSP70 | heat shock protein 70 |
| POD | peroxidase |
| ROS | reactive oxygen species |
| O2− | superoxide radicals |
| OH | hydroxyl radicals |
| H2O2 | hydrogen peroxide |
| GSH | glutathione |
| PCs | phytochelatins |
| Chl a | chlorophyll a |
| Chl b | chlorophyll b |
| Pnmax | the net rate of photosynthesis |
| R | transpiration rate |
| AQE | maximum quantum efficiency |
| gs | stomatal conductance |
| Fv/Fm | maximum quantum efficiency of PS II |
| ZFP | zinc finger protein |
| elF | eukaryotic translation initiation factor |
| SF3BI | eukaryotic translation initiation factor |
| GO | Gene Ontology |
| MAPK | mitogen activated protein kinase protein |
| PA2G4 | proliferation-associated proteins 2G4 |
| TCTP | translationally controlled tumor proteins |
| Prx | peroxiredoxin |
| GST | glutathione transferase |
| ABC transporter | the ATP-binding cassette transporter |
| TFs | transcription factors |
| Bhlh | helix-loop-helix |
| VPS | vacuolar protein sorting-associated protein |
Author Contributions
J.H., writing original draft; J.H., X.W. (Xiaolu Wu), F.T., Q.C. and P.L., project administration; F.Z., supervision; X.W. (Xueqin Wan), design the study, funding acquisition and resources; Y.Z., resources; Q.L., software and data curation; T.L., writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Fund of China (No. 31870645). The funding body provided the funds for this study.
Conflicts of Interest
The authors declare no conflict of interest in the study. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Sarwar N., Ishaq W., Farid G., Shaheen M.R., Imran M., Geng M., Hussain S. Zincecadmium interactions: Impact on wheat physiology and mineral acquisition. Ecotox. Environ. Saf. 2015;122:528–536. doi: 10.1016/j.ecoenv.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Cuypers A., Plusquin M., Remans T., Jozefczak M., Keunen E., Gielen H., Opedenakker K., Nair A.R., Munters E., Artois T.J., et al. Cadmium stress: An oxidative challenge. Biometals. 2010;23:927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- 3.Brunetti G., Farrag K., Solerrovira P., Ferrara M., Nigro F., Senesi N. Heavy metals accumulation and distribution in durum wheat and barley grown in contaminated soils under Mediterranean field conditions. J. Plant. Interact. 2012;7:160–174. doi: 10.1080/17429145.2011.603438. [DOI] [Google Scholar]
- 4.Chaney R.L., Ryan J.A., Li Y.M., Brown S.L. Soil cadmium as a threat to human health. In: McLaughlin M.J., Singh B.R., editors. Cadmium in Soils and Plants. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1999. pp. 219–256. [Google Scholar]
- 5.McLaughlin M.J., Parker D.R., Clarke J.M. Metals and micronutrients-food safety issues. Field. Crop. Res. 1999;60:143–163. doi: 10.1016/S0378-4290(98)00137-3. [DOI] [Google Scholar]
- 6.Metwally A., Safronova V.I., Belimov A.A., Dietz K.J. Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J. Exp. Bot. 2005;56:167–178. doi: 10.1093/jxb/eri017. [DOI] [PubMed] [Google Scholar]
- 7.Küpper H., Kochian L.V. Transcriptional regulation of metal transport genes and mineral nutrition during acclimatization to cadmium and zinc in the Cd/Zn hyperaccumulator, Thlaspicaerulescens (Ganges population) New Phytol. 2010;185:114–129. doi: 10.1111/j.1469-8137.2009.03051.x. [DOI] [PubMed] [Google Scholar]
- 8.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 9.Romero-Puertas M.C., Corpas F.J., Sandalio L.M., Leterrier M., Rodrı’guez-Serrano M., del Rı’o L.A., Palma J.M. Glutathione reductase from pea leaves: Response to abiotic stress and characterization of the peroxisomal isozyme. New Phytol. 2006;170:43–52. doi: 10.1111/j.1469-8137.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 10.Saifullah G.C., Bolan N., Bibi S., Iqbal M., Rengel Z., Kunhikrishnan A., Ok Y.S. Cellular mechanisms in higher plants governing tolerance to cadmium toxicity. Crit. Rev. Plant Sci. 2014;33:374–391. [Google Scholar]
- 11.Chen L., Han Y., Jiang H., Korpelainen H., Li C. Nitrogen nutrient status induces sexual differences in responses to cadmium in Populus yunnanensis. J. Exp. Bot. 2011;62:5037–5050. doi: 10.1093/jxb/err203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strycharz S., Newman L. Use of native plants for remediation of trichloroethvlene: I. deciduous trees. Int. J. Phytorem. 2009;11:150–170. doi: 10.1080/15226510802378442. [DOI] [PubMed] [Google Scholar]
- 13.Fan K.C., His H.C., Chen C.W., Lee H.L., Hseu Z.Y. Cadmium accumulation and tolerance of mahogany (Swietenia macrophylla) seedlings for phytoextraction applications. J. Environ. Manag. 2011;92:2818–2822. doi: 10.1016/j.jenvman.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 14.Sarwar N., Imranm M., Shaheen M.R., Ishaque W., Kamran M.A., Matloob A., Rehim A., Hussain S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere. 2017;171:710–721. doi: 10.1016/j.chemosphere.2016.12.116. [DOI] [PubMed] [Google Scholar]
- 15.Li J.T., Liao B., Lan C.Y., He Z.H., Baker A.J.M., Shu W.S. Cadmium tolerance and accumulation in cultivars of a high biomass tropical tree (Averrhoa carambola) and its potential for phytoextraction. J. Environ. Qual. 2010;39:1262–1268. doi: 10.2134/jeq2009.0195. [DOI] [PubMed] [Google Scholar]
- 16.Wan X., Lei M., Chen T. Cost–benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total. Environ. 2016;563:796–802. doi: 10.1016/j.scitotenv.2015.12.080. [DOI] [PubMed] [Google Scholar]
- 17.Salam M.M.A., Kaipiainen E.K., Mohsin M., Villa A., Kuittinen S., Pulkkinen P., Pelkonen P., Mehtätalo L., Pappinen A. Effects of contaminated soil on the growth performance of young Salix (Salix schwerinii E. L. Wolf) and the potential for phytoremediation of heavy metals. J. Environ. Manag. 2016;183:467–477. doi: 10.1016/j.jenvman.2016.08.082. [DOI] [PubMed] [Google Scholar]
- 18.Komárek M., Tlustoš P., Száková J., Chrastný V., Ettler V. The use of maize and poplar in chelant-enhanced phytoextraction of lead from contaminated agricultural soils. Chemosphere. 2007;67:640–651. doi: 10.1016/j.chemosphere.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Wu F., Yang W., Zhang J., Zhou L. Cadmium accumulation and growth responses of a poplar (Populus deltoides×Populus nigra) in cadmium contaminated purple soil and alluvial soil. J. Hazard. Mater. 2010;177:268–273. doi: 10.1016/j.jhazmat.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 20.Lee K.Y., Strand S.E., Doty S.L. Phytoremediation of chlorpyrifos by populus and salix. Int. J. Phytoremediation. 2012;14:48–61. doi: 10.1080/15226514.2011.560213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali H., Khan E., Sajad M.A. Phytoremediation of heavy metals concepts and applications. Chemosphere. 2013;91:869–881. doi: 10.1016/j.chemosphere.2013.01.075. [DOI] [PubMed] [Google Scholar]
- 22.Pollard A.J., Reeves R.D., Baker A.J. Facultative hyperaccumulation of heavy metals and metalloids. Plant Sci. 2014;217:8–17. doi: 10.1016/j.plantsci.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Lone M.I., He Z.L., Stoffella P.J., Yang X. Phytoremediation of heavy metal polluted soils and water: Progresses and perspectives. J. Zhejiang Univ. Sci. B. 2008;9:210–220. doi: 10.1631/jzus.B0710633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaney R.L., Angle J.S., Broadhurst C.L., Peters C.A., Tappero R.V., Sparks D.L. Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. J. Environ. Qual. 2007;36:1429–1443. doi: 10.2134/jeq2006.0514. [DOI] [PubMed] [Google Scholar]
- 25.Chia M.A., Lombardi A.T., Melão M.D.G.G., Parrish C.C. Combined nitrogen limitation and cadmium stress stimulate total carbohydrates, lipids, protein and amino acid accumulation in chlorella vulgaris (Trebouxiophyceae) Aquat Toxicol. 2015;160:87–95. doi: 10.1016/j.aquatox.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Zouari M., Elloumi N., Ahmed C.B., Delmail D., Rouina B.B., Abdallah F.B., Labrousse P. Exogenous proline enhances growth, mineral uptake, antioxidant defense, and reduces cadmium-induced oxidative damage in young date palm (Phoenix dactylifera L.) Ecol. Eng. 2016;86:202–209. doi: 10.1016/j.ecoleng.2015.11.016. [DOI] [Google Scholar]
- 27.Li J.G., Jin S.L., Chen Y.Q., Lin G.L., Han X.R., Li T.Q., Yang X.E., Zhu E. Effects of nitrogen fertilizer on the root morphology and cadmium accumulation in low cadmium treatment Sedum alfredii Hance. Chin. Agric. Sci. Bull. 2007;23:260–265. [Google Scholar]
- 28.Chang Y.S., Chang Y.J., Lin C.T., Lee M.C., Wu C.W., Lai Y.H. Nitrogen fertilization promotes the phytoremediation of cadmium in Pentas lanceolate. Int. Biodeterior. Biodegrad. 2013;85:709–714. doi: 10.1016/j.ibiod.2013.05.021. [DOI] [Google Scholar]
- 29.Yang G., Wang C., Wang Y., Guo Y., Zhao Y., Yang C., Gao C. Overexpression of ThVHAc1 and its potential upstream regulator, ThWRKY7, improved plant tolerance of Cadmium stress. Sci. Rep. 2016;6:18752. doi: 10.1038/srep18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang F., Wan X.Q., Zhong Y. Nitrogen as an important detoxification factor to cadmium stress in poplar plants. J. Plant. Interact. 2014;9:249–258. doi: 10.1080/17429145.2013.819944. [DOI] [Google Scholar]
- 31.Zhang F., Li J.Q., Huang J.L., Lin L.H., Wan X.Q., Zhao J.L., Dong J.F., Sun L.X., Chen Q.B. Transcriptome Profling Reveals the Important Role of Exogenous Nitrogen in Alleviating Cadmium Toxicity in Poplar Plants. J. Plant. Growth. Regul. 2017;36:942–956. doi: 10.1007/s00344-017-9699-1. [DOI] [Google Scholar]
- 32.Brahim S., Joke D., Ann C., Jean-Paul N., Marjo T., Arja T., Sirpa K., Frank V.B., Karen S., Jaco V. Leaf proteome responses of Arabidopsis thaliana exposed to mild cadmium stress. J. Plant Physiol. 2010;167:247–254. doi: 10.1016/j.jplph.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Cvjetko P., Zovko M., Balen B. Proteomics of heavy metal toxicity in plants. Arh. Hig. Rada. Toksikol. 2014;65:1–18. doi: 10.2478/10004-1254-65-2014-2443. [DOI] [PubMed] [Google Scholar]
- 34.Ai-Momani S., Qi D., Ren Z., Jones A.R. Comparative qualitative phosphoproteomics analysis identifies shared phosphorylation motifs and associated biological processes in evolutionary divergent plants. J. Proteom. 2018;181:152–159. doi: 10.1016/j.jprot.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shahid M., Pourrut B., Dumat C., Nadeem M., Aslam M., Pinelli E. Heavy-metal-induced reactive eoxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014;232:1–44. doi: 10.1007/978-3-319-06746-9_1. [DOI] [PubMed] [Google Scholar]
- 36.Ezemaduka A.N., Wang Y., Li X. Expression of CeHSP17 protein in response to heat shock and heavy metal ions. J. Nematol. 2017;49:334–340. doi: 10.21307/jofnem-2017-081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L., Hu W., Gao Y., Pan H., Zhang Q. A cytosolic class II small heat shock protein, PfHSP17.2, confers resistance to heat, cold, and salt stresses in transgenic Arabidopsis. Genet. Mol. Biol. 2018;41:649–660. doi: 10.1590/1678-4685-gmb-2017-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y., Li X., Yang S., Zhou Y., Dong C., Ren J., Sun X., Yang Y. Comparative Physiological and Proteomic Analysis Reveals the Leaf Response to Cadmium-Induced Stress in Poplar (Populus yunnanensis) PLoS ONE. 2015 doi: 10.1371/journal.pone.0137396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., Liu Y., Zhang J., Guo Y., Ma E. Molecular Cloning and mRNA Expression of Heat Shock Protein Genes and Their Response to Cadmium Stress in the Grasshopper Oxya chinensis. PLoS ONE. 2015;10:e0131244. doi: 10.1371/journal.pone.0131244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Q., Zhang S., Liu B. 14-4-4 proteins: Macro-regulators with great potential for improving abiotic stress tolerance in plants. Biochem. Biophys. Res. Commun. 2016;477:9–13. doi: 10.1016/j.bbrc.2016.05.120. [DOI] [PubMed] [Google Scholar]
- 41.Pathare V., Srivastava S., Sonawane B.V., Suprasanna P. Arsenic stress affects the expression profile of genes of 14-3-3 proteins in the shoot of mycorrhiza colonized rice. Physiol. Mol. Bio. Plants. 2016;22:515–522. doi: 10.1007/s12298-016-0382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo C.L., Chen Q., Zhao X.L., Chen X.Q., Zhao Y., Wang L., Li K.Z., Yu Y.X., Chen L.M. Al-enhanced expression and interaction of 14-3-3 protein and plasma membrane H+-ATPase is related to Al-induced citrate secretion in an Al resistant black Soybean. Plant Mol. Biol. Rep. 2013;31:1012–1024. doi: 10.1007/s11105-013-0569-0. [DOI] [Google Scholar]
- 43.Parrotta L., Guerriero G., Sergeant K., Cai G., Hausman J.F. Target or barrier? The cell wall of early- and later-diverging plants vs cadmium toxicity: Differences in the response mechanisms. Front. Plant Sci. 2015;6:133. doi: 10.3389/fpls.2015.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lukačová Z., Švubová R., Kohanová J., Lux A. Silicon mitigates the Cd toxicity in maize in relation to cadmium translocation, cell distribution, antioxidant enzymes stimulation and enhanced endodermal apoplasmic barrier development. Plant Growth Regul. 2013;70:89–103. doi: 10.1007/s10725-012-9781-4. [DOI] [Google Scholar]
- 45.Meyer C.L., Juraniec M., Huguet S., Chaves-Rodriguez E., Salis P., Isaure M.P., Goormaghtigh E., Verbruggen N. Intraspecific variability of cadmium tolerance and accumulation, and cadmium-induced cell wall modifications in the metal hyperaccumulator Arabidopsis helleri. J. Exp. Bot. 2015;66:3215–3227. doi: 10.1093/jxb/erv144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim Y.H., Bae J.M., Huh G.H. Transcriptional regulation of the cinnamyl alcohol dehydrogenase gene from sweetpotato in response to plant developmental stage and environmental stress. Plant Cell Rep. 2010;29:779–791. doi: 10.1007/s00299-010-0864-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero-Puertas M.C., Rodríguez-serrano M., Corpas F.J., Gómez M., Delrío L.A., Sandalio L.M. Cadmium-induced subcellular accumulation of O2 and H2O2 in pea leaves. Plant Cell. Environ. 2004;27:1122–1134. doi: 10.1111/j.1365-3040.2004.01217.x. [DOI] [Google Scholar]
- 48.Tikkanen M., Rantala S., Aro E.M. Electron flow from PSII to PSI under high light is controlled by PGR5 but not by PSBS. Front Plant Sci. 2015;6:521. doi: 10.3389/fpls.2015.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paredes M., Quiles M.J. The Effects of Cold Stress on Photosynthesis in Hibiscus Plants. PLoS ONE. 2015;10:e0137472. doi: 10.1371/journal.pone.0137472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jonak C., Nakagami H., Hirt H. Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol. 2004;136:3276–3283. doi: 10.1104/pp.104.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim Y.M., Han Y.J., Hwang O.J., Lee S.S., Shin A.Y., Kim S.Y., Kim J.I. Overexpression of Arabidopsis translationally controlled tumor protein gene AtTCTP enhances drought tolerance with rapid ABA-induced stomatal closure. Mol. Cells. 2012;33:617–626. doi: 10.1007/s10059-012-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z.Q., Li G.Z., Gong Q.Q., Li G.X., Zheng S.J. OsTCTP, encoding a translationally controlled tumor protein, plays an important role in mercury tolerance in rice. BMC Plant Biol. 2015;15:123. doi: 10.1186/s12870-015-0500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong F., Deng Y., Zhou B., Wang G., Wang Y., Meng Q. A chloroplast-targeted DnaJ protein contributes to maintenance of photosystem II under chilling stress. J. Exp. Bot. 2014;65:143–158. doi: 10.1093/jxb/ert357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muradoglu F., Gundogdu M., Ercisli S., Encu T., Balta F., Jaafar H.Z.E., Zia-Ui-Haq M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015;48:11. doi: 10.1186/s40659-015-0001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woo H.A., Yim S.H., Shi D.H., Kang D., Yu D.Y., Rhee S.G. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Brunetti P., Zanella L., Paolis A.D., Litta D.D., Cecchetti V., Falasca G., Barbieri M., Altamura M.M., Costantino P., Cardarelli M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015;66:3815–3829. doi: 10.1093/jxb/erv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang C.F., Yamaji N., Ma J.F. Knockout of a bacterial-type ATP-binding cassette transporter gene, AtSTAR1, results in increased aluminum sensitivity in Arabidopsis. Plant Physiol. 2010;153:1669–1677. doi: 10.1104/pp.110.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bovet L., Feller U., Martinoia E. Possible involvement of plant ABC transporters in cadmium detoxification: A cDNA submicroarray approach. Environ. Int. 2005;31:263–267. doi: 10.1016/j.envint.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 59.Wang L., Xu C., Wang C., Wang Y. Characterization of a eukaryotic translation initiation factor 5A homolog from Tamarix androssowii involved in plant abiotic stress tolerance. BMC Plant Biol. 2012;12:118–135. doi: 10.1186/1471-2229-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J., Yang L., Yan X., Liu Y., Wang R., Fan T., Ren Y., Tang X., Xiao F., Liu Y., et al. Zinc-Finger transcription factor ZAT6 positively regulates cadmium tolerance through the glutathione-dependent pathway. Plant Physiol. 2016;171:707–719. doi: 10.1104/pp.15.01882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chou W.C., Huang Y.W., Tsay W.S., Chiang T.Y., Huang D.D., Huang H.J. Expression of genes encoding the rice translation initiation factor, eIF5A, is involved in developmental and environmental responses. Physiol. Plant. 2004;121:50–57. doi: 10.1111/j.0031-9317.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 62.Albataineh M.T., Kadosh D. Regulatory roles of phosphorylation in model and pathogenic fungi. Med. Mycol. 2016;54:333–352. doi: 10.1093/mmy/myv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonnal S., Vigevani L., Valcarcel J. The spliceosome as a target of novel antitumour drugs. Nat. Rev. Drug Discov. 2012;11:847–859. doi: 10.1038/nrd3823. [DOI] [PubMed] [Google Scholar]
- 64.Kim Guisbert K.S., Guisbert E. SF3B1 is a stress-sensitive splicing factor that regulates both HSF1 concentration and activity. PLoS ONE. 2017;12:e0176382. doi: 10.1371/journal.pone.0176382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiappori F., Merelli I., Milanesi L., Colombo G., Morra G. An atomistic view of Hsp70 allosteric crosstalk: From the nucleotide to the substrate binding domain and back. Sci. Rep. 2016;6:23474. doi: 10.1038/srep23474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Assimon V.A., Southworth D.R., Gestwicki J.E. Specific binding of tetratricopeptide repeat (TPR) proteins to heat shock protein 70 (Hsp70) and heat shock protein 90 (Hsp90) is regulated by affinity and phosphorylation. Biochemistry. 2015;54:7120–7131. doi: 10.1021/acs.biochem.5b00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ai T.N., Naing A.H., Yun B.W., Lim S.H., Kim C.K. Overexpression of RsMYB1 enhances anthocyanin accumulation and heavy metal stress tolerance in transgenic Petunia. Front Plant Sci. 2018;9:1330. doi: 10.3389/fpls.2018.01388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lichtenthaler H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzym. 1987;148:350–382. [Google Scholar]
- 69.Wu J.Z., Ge Y. Comparative studies on five pretreatment methods in the determination of elements in plant standard sample by ICP-AES. Spectrosc. Spectr. Anal. 1999;19:369–372. [PubMed] [Google Scholar]
- 70.Yang L., Tian D., Todd C.D., Luo Y., Hu X. Comparative proteome analyses reveal that nitric oxide is an important signal molecule in the response of rice to aluminum toxicity. J. Proteome Res. 2013;12:1316–1330. doi: 10.1021/pr300971n. [DOI] [PubMed] [Google Scholar]
- 71.Gupta S.C., Goldsbrough P.B. Phytochelatin accumulation and cadmium tolerance in selected tomato cell lines. Plant Physiol. 1999;97:306–312. doi: 10.1104/pp.97.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhargava P., Srivastava A.K., Urmil S., Rai L.C. Phytochelatin plays a role in UV-B tolerance in N2-fixing cyanobacterium Anabaena doliolum. J. Plant Physiol. 2005;162:1220–1225. doi: 10.1016/j.jplph.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 74.Yang F., Fan Y., Wu X., Cheng Y., Liu Q., Feng L., Chen J., Wang Z., Wang X., Yong T., et al. Auxin-to-gibberellin ration as a signal for light intensity and quality in regulating soybean growth and matter partitioning. Front Plant Sci. 2018;9:1–13. doi: 10.3389/fpls.2018.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]