Abstract
Background: Vascular endothelial growth factor (VEGF) is upregulated by hypoxia and is a crucial stimulator for choroidal neovascularization (CNV) in age-related macular degeneration and pathologic myopia, as well as retinal neovascularization in proliferative diabetic retinopathy. Retinal and choroidal endothelial cells play key roles in the development of retinal and CNV, and subsequent fibrosis. At present, the effects of gold nanoparticles (AuNPs) on the VEGF-induced choroid-retina endothelial (RF/6A) cells are still unknown. In our study, we investigated the effects of AuNPs on RF/6A cell viabilities and cell adhesion to fibronectin, a major ECM protein of fibrovascular membrane. Furthermore, the inhibitory effects of AuNPs on RF/6A cell migration induced by VEGF and its signaling were studied. Methods: The cell viability assay was used to determine the viability of cells treated with AuNPs. The migration of RF/6A cells was assessed by the Transwell migration assay. The cell adhesion to fibronectin was examined by an adhesion assay. The VEGF-induced signaling pathways were determined by western blotting. Results: The 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) viability assay revealed no cytotoxicity of AuNPs on RF/6A cells. AuNPs inhibited VEGF-induced RF/6A cell migration in a concentration-dependent manner but showed no significant effects on RF/6A cell adhesion to fibronectin. Inhibitory effects of AuNPs on VEGF-induced Akt/eNOS were found. Conclusions: These results suggest that AuNPs are an effective inhibitor of VEGF-induced RF/6A cell migration through the Akt/eNOS pathways, but they have no effects on their cell viabilities and cell adhesion to fibronectin.
Keywords: gold nanoparticles (AuNPs), vascular endothelial growth factor (VEGF), cell migration, Akt, endothelial nitric oxide synthase (eNOS), choroidal and retinal neovascularization
1. Introduction
Angiogenesis is the physiological process involving the growth of new blood vessels from existing vasculature [1]. It plays a central role in cancer and various ischemic diseases [2,3]. Vascular endothelial growth factor (VEGF) is upregulated by hypoxia during ischemia [4] and is a major stimulatory factor for choroidal neovascularization (CNV) in age-related macular degeneration [5,6] and high myopia [7], as well as retinal neovascularization in diabetic retinopathy [6]. Retinal and choroidal endothelial cells play key roles in the development of retinal and choroidal neovascularization, and subsequent fibrosis.
The migration of endothelial cells (EC) is a notable and key step in angiogenesis [8], but the detailed signaling molecules responsible for this migration are still under investigation. Although numerous factors affect EC migration, the influence of VEGF has received considerable attention from researchers, as VEGF is widely used as a signaling molecule for the induction of EC migration [9,10].
Gold nanoparticles (AuNPs) have been shown to be the material of choice of many diagnostic platforms. Their unique electronic, biocompatible, and molecular-recognition properties of small-sized AuNPs broaden the potential of gold in several fields of application [11]. Gold nanoparticles displayed an anti-angiogenic effect. They induce nanostructural reorganization of VEGFR2 on the human umbilical vascular endothelial cells (HUVEC) to repress angiogenesis [12]. Moreover, AuNPs can significantly inhibit HepG2-conditioned medium (HepG2-CM) activated HUVEC proliferation and migration through the down-regulation of VEGF activity and disruption of cell morphology [13]. AuNPs were shown to inhibit VEGF-induced migration in HUVEC [14] and laser-induced CNV in mice [15]. Fabrication of resveratrol-coated gold nanoparticles can increase the retinal pigment epithelium-derived factor and decrease the VEGF-1 in streptozotocin-induced diabetic rats [16]. However, the effects of AuNPs on the choroid-retina endothelial (RF/6A) cells viability and VEGF-induced RF/6A cell migration are still unknown.
In the present study, we investigated the inhibitory effect of AuNPs on VEGF-induced RF/6A cell migration and the possible underlying mechanisms involved. These mechanisms include the influence of AuNPs on RF/6A cell viability, cell adhesion, and Akt/endothelial nitric oxide synthase (eNOS) pathway activation.
2. Results
2.1. AuNPs Showed No Cytotoxicity on RF/6A Cells
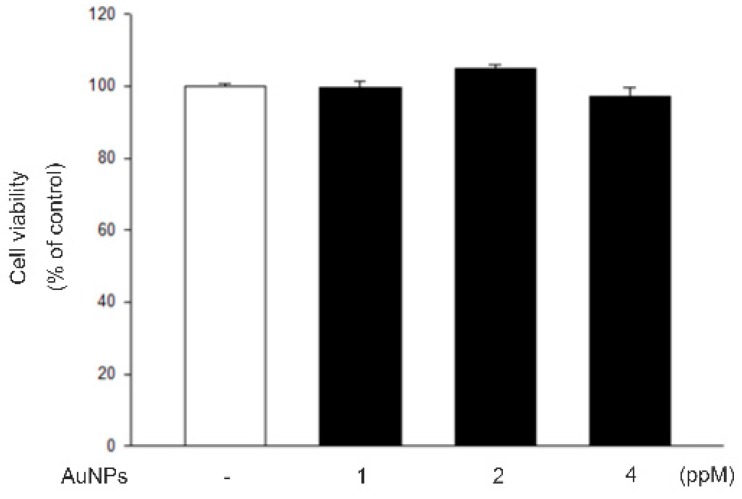
To eliminate the possibility that AuNPs have an effect on RF/6A cell migration through their effects on cell viability, cell viability was determined by MTT assays. As shown in Figure 1, the treatment of AuNPs (1, 2, and 4 ppm) did not change cell viability in MTT assays. These data show that AuNPs have no cytotoxicity toRF/6A cells and their effects on cell migration did not result from the reduction of cell viability.
Figure 1.
Viability of RF/6A cells was not influenced by AuNPs. The cells were treated with different concentrations of AuNPs for 24 h after being starved for 24 h. Cell viability was determined by the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The results are expressed as a percentage of control and represent the mean ± standard errors (SE) of four independent experiments.
2.2. AuNPs Suppressed VEGF-Induced RF/6A Cell Migration
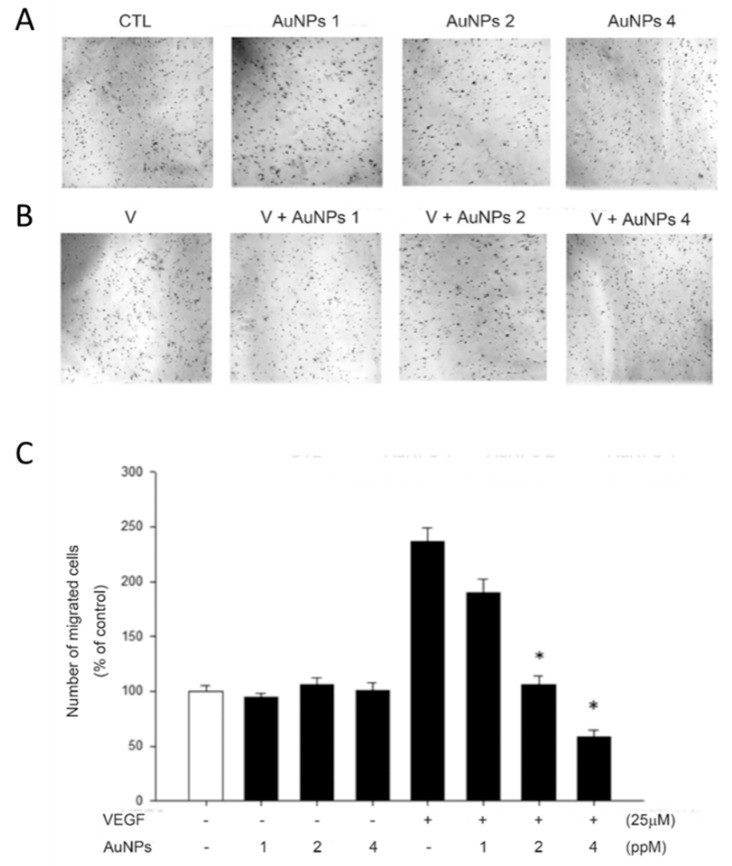
To decide the inhibitory activities of AuNPs on RF/6A cell migration, we carried out the Transwell migration assays. The data indicate that cell migration of RF/6A was increased by VEGF, and this effect was prominently inhibited by the preincubation of VEGF with AuNPs in a concentration-dependent manner. However, AuNPs had no effect on basal RF/6A cell migration without the treatment of VEGF (Figure 2).
Figure 2.
AuNPs suppress VEGF-induced cell migration in RF/6A cells by the Transwell migration assay. The Transwell inserts were coated with fibronectin (0.3 mg). RF/6A cells (5 × 104 in 200 μL) were seeded in the upper chamber in the absence or presence of AuNPs. The inserts were assembled in the lower chamber, which was filled with 600 μL serum-free medium without VEGF, (A) containing VEGF (25 ng/mL), (B) and preincubated with various concentrations of AuNPs for 30 min at 37 °C. After incubating for 5 h at 37 °C, fixation was performed. RF/6A cells that migrated to the underside of the filter membrane were photographed (A, B) and counted by phase-contrast light microscope under high power field (magnification, 100×); (C) All experiments were conducted in duplicates, and similar results were repeated four times. The results are expressed as a percentage of control and represent the mean ± standard errors (SE) of the eight experiments. * p < 0.05 significantly differs from VEGF-stimulated cells (the fifth bar).
2.3. AuNPs Had No Effect on RF/6A Cell Adhesion
To decide whether AuNPs suppressed RF/6A cell migration by interfering with their attachment to fibronectin, we conducted the effect of AuNPs on RF/6A cell adhesion with fibronectin-coated. As shown in Figure 3, the adhesion number was not affected by the treatment of AuNPs. These data suggest that the suppression of AuNPs on RF/6A cell migration was not produced by interference with the attachment of the cells to fibronectin.
Figure 3.
Cell adhesion of RF/6A cells was not influenced by AuNPs. BCECF-labeled cells were treated with DMSO or nanogold for 30 min. They were then seeded and allowed to adhere to plates with precoated fibronectin (fn) (15 µg/mL) at 37 °C for 1 h. Fluorescence was measured using excitation and an emission wavelength of 485 and 535 nm, respectively. The results are expressed as a percentage of control and represent the mean ± standard errors (SE) of three independent experiments.
2.4. AuNPs Suppressed VEGF-Induced Akt and eNOS Phosphorylation
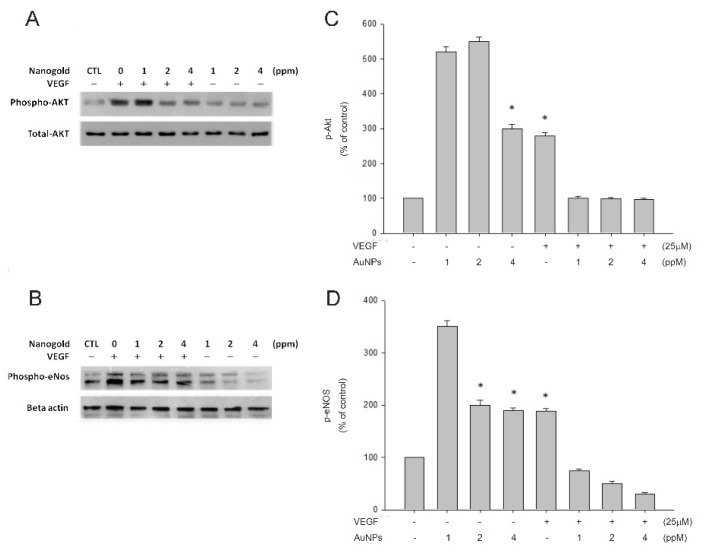
To decide whether VEGF-induced signaling pathways are influenced by AuNPs, the level of phosphorylation of Akt and eNOS was determined by Western blotting. Figure 4 indicates that Akt and eNOS phosphorylation were enhanced by the treatment of VEGF. Preincubation with AuNPs produced the decrease of VEGF-induced PI3K and Akt phosphorylation in a dose-dependent manner.
Figure 4.
VEGF-induced protein kinase B (Akt) and endothelial nitric oxide synthase (eNOS) phosphorylations were inhibited by AuNPs. RF/6A cells were preincubated with the indicated concentrations of AuNPs (1, 2, 4 ppm) and incubated with or without VEGF (25 ng/mL) at 37 °C for 30 min, the cells were collected, and their lysates were analyzed by Western blot analysis. The changes in phosphorylated Akt and eNOS expression were evaluated (A,C). The quantitative data of western blot are shown below the panels, which are expressed as a percentage of control and represent the mean ± standard errors (SE) of the three independent experiments (B,D). * p < 0.05 significantly differs from VEGF-stimulated cells (the second bar) (B,D).
3. Discussion
Angiogenesis is the physiological process of forming new blood vessels from preexisting vasculature. Physiological angiogenesis is highly regulated during wound repair [17,18]. Pathological choroidal and retinal angiogenesis are the leading causes of blindness, including age-related macular degeneration and diabetic retinopathy [19,20]. In angiogenesis, a serial process participated with several cells that include proteolytic degradation of the extracellular matrix, followed by migration and proliferation of capillary endothelial cells, pericyte recruitment, and assembly of the mature vessel [21]. The angiogenic process is regulated by a tight balance between pro-, and anti-angiogenic agents and vascular endothelial growth factor (VEGF) plays a critical regulatory role [22]. It is physiologically required for regulating proliferation and assembling endothelial cells during vasculogenesis, as well as for their maintenance and survival throughout the lifetime of blood vessels [23]. However, under subtle pathological alterations, abnormal angiogenesis with retinal and choroidal microvascular alterations have been observed during PDR, high myopia, and neovascular AMD [24,25,26].
AuNPs have been explored with a high expectation as they are effective and promising agents to improve the diagnosis and treatment of cancer [27,28,29]. Gold nanoparticles downregulate cellular cascades of interleukin-1β-induced pro-inflammatory response [30], and topical application of AuNPs decreases intraocular oxidative damage and inflammation [31]. Besides, AuNPs can effectively inhibit matrix metalloproteinase activity without causing cytotoxicity or inflammation [32]. AuNPs induce oxidative stress in mouse fibroblast, and the cells trigger the autophagic pathways as a survival mechanism to avoid cell death [33]. Gold nanoparticles can also inhibit retinal neovascularization through autophagy [34]. AuNPs can interrupt the crosstalk of tumor microenvironment and endothelial cells through the blockade of VEGF-VEGFR2 signaling during angiogenesis [35]. Under flow exposure conditions, anti-intercellular adhesion molecule-1 (ICAM-1) AuNPs can activate leukocyte adhesion receptors in tumor necrosis factor (TNF)-activated shear stress-adapted endothelial cells [36].
Many studies showed that AuNPs do not affect cell viabilities in several cell types [37], but some studies demonstrated their toxic effects [38,39]. The effect of AuNPs on choroid-retina endothelial cell viability is still unknown. Our study demonstrated that there are no toxic effects on RF/6A cell viabilities by AuNPs. Endothelial cell migration is essential to angiogenesis [40]. This motile and directional process is controlled by chemotactic, the directional migration toward a gradient of soluble chemoattractant; haptotaxis, the directional migration toward a gradient of immobilized ligands; and mechanotaxis, the directional migration generated by mechanical forces [41]. During angiogenesis, endothelial cell migration is the integrated as a result of the above three mechanisms. In this study, we found that AuNPs significantly inhibit VEGF-induced choroid-retinal endothelial RF/6A cell migration without any signs of cytotoxicity as well.
Fibronectin is the main structural component of internal/outer collagenous and the elastic layer of Bruch’s membrane [42]. It is also one of the serum autoantibody biomarkers for neovascular age-related macular degeneration [43]. In epiretinal membranes of vitreoproliferative retinopathy and proliferative diabetic retinopathy under immunohistochemical study, fibronectin is a major component in the extracellular matrix [44]. Under the retinal ischemic conditions, fibronectin is upregulated [45]. It is required for endothelial cell migration and tube morphogenesis and plays an important role during retinal and choroidal neovascularization [46]. However, our results indicate that AuNPs does not affect choroid-retinal endothelial RF/6A cell adhesion to fibronectin.
The PI3K/Akt pathway provides essential signaling for cell survival and proliferation. Signaling from different eNOS agonists, such as VEGF, insulin, and estrogen can affect eNOS activity through the PI3K/AKT pathway [47,48,49]. It has been reported that activations of the survival signal PI3K/Akt pathway and the endothelial-specific eNOS/NO pathway were closely associated with vascular remodeling and angiogenesis [50,51]. However, whether AuNPs affects the biological properties of choroid-retinal endothelial cells and the role of/Akt/eNOS signaling pathway in VEGF-induced migration, have remained poorly understood. In the present study, we determined the effects of AuNPs on the VEGF/Akt/eNOS signaling pathway on choroid-retinal endothelial RF/6A cell migration. The results showed that AuNPs suppressed VEGF-induced activation of the Akt/eNOS signaling pathway during these processes.
4. Material and Methods
4.1. Materials
Gold nanoparticles (AuNPs) were purchased from Gold NanoTech Inc. (Taipei, Taiwan) [52]. The synthesis methods of AuNPs have been described in our previous study [53]. The size of the AuNPs we used in this study was 3–5 nm (SA20130129b). Phenylmethylsulfonyl fluoride (PMSF), bovine serum albumin (BSA), leupeptin, aprotinin, sodium fluoride (NaF), and sodium orthovanadate were purchased from Sigma-Aldrich (St Louis, MO, USA). Antibodies (Ab) raised against phospho-eNOS and eNOS were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Abs raised against phospho-Akt and Akt were from Cell Signaling Technology, Inc. (Beverly, MA, USA).
4.2. Cell Cultures
The rhesus macaque choroid-retinal endothelial cell line RF/6A derived from the choroid-retina of a rhesus macaque fetus was purchased from Food Industry Research and Development Institute (Hsinchu, Taiwan). The cells were maintained in RPMI 1640 Medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin (Hyclone, UT, USA) at 37 °C, 5% CO2 and 95% humidified air. For most of the experiments, cells reaching a 90–95% of confluence were synchronized for 24 h by serum starvation before they were subjected to further analysis.
4.3. Nanogold Treatment and VEGF Incorporation
AuNPs were dissolved by distilled water in a series of dilutions. In Transmigration assays and the Western blot analysis, the 25 ng/mL of vascular endothelial growth factor (VEGF) were incorporated with different concentrations of AuNPs in the serum-free RPMI 1640 medium in culturing RF/6A cells at 37 °C for 30 min.
4.4. Transmigration Assays
By using a modified Boyden chamber model (Transwell apparatus, 8.0 mm pore size, Costar), the migration ability of RF/6A cell were demonstrated as previously described [54]. Briefly, the lower surface of the semi-permeable membrane was coated with 0.3 mg of fibronectin for 30 min in the laminar flow hood. Different concentrations of AuNPs were added to the lower chamber, which was filled with 0.6 mL of serum-free RPMI 1640 medium or 25 ng/mL VEGF-containing medium. 2.5 × 105/mL RF/6A cells were added to the upper chamber. Inserts were removed after 5 h, and the inner side was wiped with cotton swabs. Cells at the lower chamber were fixed and stained with 0.5% toluidine blue in 4% paraformaldehyde (PFA). The migrated cells were photographed and counted as the number of stained cells per ×100 field (high power field, HPF) under a phase-contrast microscope (Leica DMIL1) and photographed.
4.5. Viability Assays
The cell viability was detected by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, different concentrations of AuNPs were preincubated with RF/6A cells for 24 h in 96-well plates. After a brief wash with medium, 0.5 mg/mL MTT was added and incubated at 37 °C for 4 h to measure the amount of living and metabolically active cells. MTT was metabolized by mitochondrial dehydrogenases to a purple formazan dye, with the light absorbance at 570 nm. The absorbance was detected by a spectrophotometer and was proportional to cell viability.
4.6. Cell Adhesion Assays
The 50 µL of fibronectin (15 µg/mL in PBS, pH 7.4) was used to coat 96-well plates at 37 °C for 24 h. After gentle washed with PBS three times, the plates were blocked by 100 mg/mL bovine serum albumin (Sigma-Aldrich, St Louis, MO, USA) in PBS at room temperature for 1 h to avoid nonspecific binding to fibronectin. Afterward, RF/6A cells were labeled with 10 mg/mL BCECF/AM for 30 min at 37 °C in serum-free RPMI 1640 medium. After brief washed with the serum-free medium twice, the labeled cells were resuspended to a density of 1.0 × 105 and incubated with different concentrations of AuNPs in the serum-free RPMI 1640 medium for further 30 min at 37 °C. Then, the suspended cells were plated onto 96-well plates with 100 µL serum-free cell culture medium at 37 °C for 1 h. The nonadherent cells were removed from the plate by washing with PBS for three times and aspirated. The number of adhered cells in the 96-well plates was measured by the Wallac Victor 3 1420 multilabel counter (Perkin Elmer, Turku, Finland) at an excitation and emission wavelength of 485 and 535 nm.
4.7. Preparation of Cell Lysates for Western Blots
The RF/6A cells were cultured on 6 cm dishes until 90–95% confluent and changed into a serum-free RPMI 1640 medium for 24 h. Various concentrations of nanogold mixed with or without VEGF (25 ng/mL) at 37 °C for 30 min in serum-free medium were added to the cells. After 30 min of incubation, cell were washing with PBS twice and lysing with sonication and centrifugation at 14,000 g for 10 min at 4 °C in the radioimmunoprecipitation assay buffer. The supernatant was removed and the concentration of protein was quantified by a Pierce protein assay kit (Pierce, Rockford, IL, USA). Total protein was separated by electrophoresis on 10% SDS–polyacrylamide gels. The protein bands were then transferred onto polyvinylidene fluoride (PVDF) membrane and probed with the antibodies against the phosphorylation of Akt and eNOS. Signals were detected by enhanced chemiluminescence (Chemiluminescence Reagent Plus from NEN, Boston, MA, USA). The PVDF membrane was stripped at 60 °C for 30 min with a stripping buffer for staining internal control.
4.8. Statistical Analysis
All results were analyzed with SigmaPlot for Windows (Version 10.00, Chicago, IL, USA). Data are shown as mean ± standard error (SE) of four experiments. The t-test was performed to determine the difference between groups. A value of p < 0.05 was considered statistically significant.
5. Conclusions
As VEGF is a key mediator and upregulated in many ocular angiogenic diseases such as proliferative diabetic retinopathy and wet-type age-related macular degeneration, anti-VEGF therapy is the current main strategy of treatment. Our studies provide the mechanism of AuNPs on the inhibitory effects of the VEGF-induced RF/6A cell migration through the suppression of Akt/eNOS phosphorylation. However, AuNPs show no cytotoxic effects on the RF/6A cells. Moreover, AuNPs do not affect the normal physiological functions of RF/6A cell adhesion to fibronectin. These studies provide important insight into further research on the effects of AuNPs in RF/6A cells and the possible beneficial effect on the suppression of angiogenesis by AuNPs in ocular angiogenic diseases.
Author Contributions
Formal analysis, C.-F.H.; Funding acquisition, C.-M.C. and D.-C.C; Investigation, C.-F.H.; Methodology, C.-M.C., C.-Y.H. and J.-Y.F.; Resources, C.-Y.H.; Writing—original draft, C.-M.C.; Writing—review & editing, C.-F.H., H.-J.L. and D.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the research grants from the National Science Council and from Cardinal Tien Hospital, Taipei, Taiwan (CTH-102-1-2A30, CTH-104-1-2B05). The fourth author is partially supported by an United States National Science Foundation grant DMS-1408839 and a McDevitt Endowment Fund at Georgetown University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Okamoto T., Usuda H., Tanaka T., Wada K., Shimaoka M. The Functional Implications of Endothelial Gap Junctions and Cellular Mechanics in Vascular Angiogenesis. Cancers. 2019;11:237. doi: 10.3390/cancers11020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apte R.S., Chen D.S., Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176:1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuazo-Gaztelu I., Casanovas O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018;8:248. doi: 10.3389/fonc.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreira-Soares M., Coimbra R., Rebelo L., Carvalho J., Travasso R.D. Angiogenic Factors produced by Hypoxic Cells are a leading driver of Anastomoses in Sprouting Angiogenesis-a computational study. Sci. Rep. 2018;8:8726. doi: 10.1038/s41598-018-27034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrenberg M., Benny O. Evolving multidimensional pharmacological approaches to CNV therapy in AMD. Curr. Eye. Res. 2018;43:147–154. doi: 10.1080/02713683.2017.1385088. [DOI] [PubMed] [Google Scholar]
- 6.Campbell M., Doyle S.L. Current perspectives on established and novel therapies for pathological neovascularization in retinal disease. Biochem. Pharmacol. 2019;164:321–325. doi: 10.1016/j.bcp.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Teo K.Y., Ng W.Y., Lee S.Y., Cheung C.M. Management of Myopic Choroidal Neovascularization: Focus on Anti-VEGF Therapy. Drugs. 2016;76:1119–1133. doi: 10.1007/s40265-016-0605-0. [DOI] [PubMed] [Google Scholar]
- 8.Kick K., Nekolla K., Rehberg M., Vollmar A.M., Zahler S. New View on Endothelial Cell Migration: Switching Modes of Migration Based on Matrix Composition. Arterioscler. Thromb. Vasc. Biol. 2016;36:2346–2357. doi: 10.1161/ATVBAHA.116.307870. [DOI] [PubMed] [Google Scholar]
- 9.Tang Y., Zhou X. Antagonistic effects of exogenous Slit2 on VEGF-induced choroidal endothelial cell migration and tube formation. Exp. Ther. Med. 2019;17:2443–2450. doi: 10.3892/etm.2019.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu C.L., Shyu J.F., Wu C.C., Hung C.F., Liao M.T., Liu W.C., Zheng C.M., Hou Y.C., Lin Y.F., Lu K.C. Association of Anabolic Effect of Calcitriol with Osteoclast-Derived Wnt 10b Secretion. Nutrients. 2018;10:1164. doi: 10.3390/nu10091164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahangirian H., Kalantari K., Izadiyan Z., Rafiee-Moghaddam R., Shameli K., Webster T.J. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int. J. Nanomedicine. 2019;14:1633–1657. doi: 10.2147/IJN.S184723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan Y., Ding H., Qin L., Zhao X., Cai J., Du B. Gold nanoparticles induce nanostructural reorganization of VEGFR2 to repress angiogenesis. J. Biomed. Nanotechnol. 2013;9:1746–1756. doi: 10.1166/jbn.2013.1678. [DOI] [PubMed] [Google Scholar]
- 13.Pan Y., Wu Q., Liu R., Shao M., Pi J., Zhao X., Qin L. Inhibition effects of gold nanoparticles on proliferation and migration in hepatic carcinoma-conditioned HUVECs. Bioorg. Med. Chem. Lett. 2014;24:679–684. doi: 10.1016/j.bmcl.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 14.Pan Y., Wu Q., Qin L., Cai J., Du B. Gold nanoparticles inhibit VEGF165-induced migration and tube formation of endothelial cells via the Akt pathway. Biomed. Res. Int. 2014;2014:418624. doi: 10.1155/2014/418624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roh Y.J., Rho C.R., Cho W.K., Kang S. The Antiangiogenic Effects of Gold Nanoparticles on Experimental Choroidal Neovascularization in Mice. Invest. Ophthalmol. Vis. Sci. 2016;57:6561–6567. doi: 10.1167/iovs.16-19754. [DOI] [PubMed] [Google Scholar]
- 16.Dong Y., Wan G., Yan P., Qian C., Li F., Peng G. Fabrication of resveratrol coated gold nanoparticles and investigation of their effect on diabetic retinopathy in streptozotocin induced diabetic rats. J. Photochem. Photobiol. B. 2019;195:51–57. doi: 10.1016/j.jphotobiol.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Lilly A.J., Mazan A., Scott D.A., Lacaud G., Kouskoff V. SOX7 expression is critically required in FLK1-expressing cells for vasculogenesis and angiogenesis during mouse embryonic development. Mech. Dev. 2017;146:31–41. doi: 10.1016/j.mod.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiPietro L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016;100:979–984. doi: 10.1189/jlb.4MR0316-102R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ved N., Hulse R.P., Bestall S.M., Donaldson L.F., Bainbridge J.W., Bates D.O. Vascular endothelial growth factor-A165b ameliorates outer-retinal barrier and vascular dysfunction in the diabetic retina. Clin. Sci. (Lond.) 2017;131:1225–1243. doi: 10.1042/CS20170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesquita J., Castro-de-Sousa J.P., Vaz-Pereira S., Neves A., Passarinha L.A., Tomaz C.T. Vascular endothelial growth factors and placenta growth factor in retinal vasculopathies: Current research and future perspectives. Cytokine Growth Factor Rev. 2018;39:102–115. doi: 10.1016/j.cytogfr.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Logsdon E.A., Finley S.D., Popel A.S., Mac Gabhann F. A systems biology view of blood vessel growth and remodelling. J. Cell. Mol. Med. 2014;18:1491–1508. doi: 10.1111/jcmm.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karaman S., Leppanen V.M., Alitalo K. Vascular endothelial growth factor signaling in development and disease. Development. 2018;145:dev151019. doi: 10.1242/dev.151019. [DOI] [PubMed] [Google Scholar]
- 23.Shibuya M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013;153:13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pakzad-Vaezi K., Mehta H., Mammo Z., Tufail A. Vascular endothelial growth factor inhibitor use and treatment approach for choroidal neovascularization secondary to pathologic myopia. Expert Opin. Biol. Ther. 2016;16:873–881. doi: 10.1517/14712598.2016.1167868. [DOI] [PubMed] [Google Scholar]
- 25.Gucciardo E., Loukovaara S., Salven P., Lehti K. Lymphatic Vascular Structures: A New Aspect in Proliferative Diabetic Retinopathy. Int. J. Mol. Sci. 2018;19:4034. doi: 10.3390/ijms19124034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L., Lee A.Y., Wigg J.P., Peshavariya H., Liu P., Zhang H. miRNA involvement in angiogenesis in age-related macular degeneration. J. Physiol. Biochem. 2016;72:583–592. doi: 10.1007/s13105-016-0496-2. [DOI] [PubMed] [Google Scholar]
- 27.Ning L., Zhu B., Gao T. Gold Nanoparticles: Promising Agent to Improve the Diagnosis and Therapy of Cancer. Curr. Drug Metab. 2017;18:1055–1067. doi: 10.2174/1389200218666170925122513. [DOI] [PubMed] [Google Scholar]
- 28.Singh P., Pandit S., Mokkapati V., Garg A., Ravikumar V., Mijakovic I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mo.l Sci. 2018;19:1979. doi: 10.3390/ijms19071979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng J., Liang X. Progress in research on gold nanoparticles in cancer management. Medicine. 2019;98:e15311. doi: 10.1097/MD.0000000000015311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumbayev V.V., Yasinska I.M., Garcia C.P., Gilliland D., Lall G.S., Gibbs B.F., Bonsall D.R., Varani L., Rossi F., Calzolai L. Gold nanoparticles downregulate interleukin-1beta-induced pro-inflammatory responses. Small. 2013;9:472–477. doi: 10.1002/smll.201201528. [DOI] [PubMed] [Google Scholar]
- 31.Pereira D.V., Petronilho F., Pereira H.R., Vuolo F., Mina F., Possato J.C., Vitto M.F., de Souza D.R., da Silva L., da Silva Paula M.M., et al. Effects of gold nanoparticles on endotoxin-induced uveitis in rats. Invest. Ophthalmol. Vis. Sci. 2012;53:8036–8041. doi: 10.1167/iovs.12-10743. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto M., Sasaki J.I., Yamaguchi S., Kawai K., Kawakami H., Iwasaki Y., Imazato S. Gold Nanoparticles Inhibit Matrix Metalloproteases without Cytotoxicity. J. Dent. Res. 2015;94:1085–1091. doi: 10.1177/0022034515589282. [DOI] [PubMed] [Google Scholar]
- 33.Gioria S., Chassaigne H., Carpi D., Parracino A., Meschini S., Barboro P., Rossi F. A proteomic approach to investigate AuNPs effects in Balb/3T3 cells. Toxicol. Lett. 2014;228:111–126. doi: 10.1016/j.toxlet.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Shen N., Zhang R., Zhang H.R., Luo H.Y., Shen W., Gao X., Guo D.Z., Shen J. Inhibition of retinal angiogenesis by gold nanoparticles via inducing autophagy. Int. J. Ophthalmol. 2018;11:1269–1276. doi: 10.18240/ijo.2018.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., Xiong X., Huai Y., Dey A., Hossen M.N., Roy R.V., Elechalawar C.K., Rao G., Bhattacharya R., Mukherjee P. Gold Nanoparticles Disrupt Tumor Microenvironment—Endothelial Cell Crosstalk to Inhibit Angiogenic Phenotypes in vitro. Bioconjug. Chem. 2019;30:1724–1733. doi: 10.1021/acs.bioconjchem.9b00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klingberg H., Loft S., Oddershede L.B., Moller P. The influence of flow, shear stress and adhesion molecule targeting on gold nanoparticle uptake in human endothelial cells. Nanoscale. 2015;7:11409–11419. doi: 10.1039/C5NR01467K. [DOI] [PubMed] [Google Scholar]
- 37.Aueviriyavit S., Phummiratch D., Maniratanachote R. Mechanistic study on the biological effects of silver and gold nanoparticles in Caco-2 cells--induction of the Nrf2/HO-1 pathway by high concentrations of silver nanoparticles. Toxicol. Lett. 2014;224:73–83. doi: 10.1016/j.toxlet.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 38.Sun H., Jia J., Jiang C., Zhai S. Gold Nanoparticle-Induced Cell Death and Potential Applications in Nanomedicine. Int. J. Mol. Sci. 2018;19:754. doi: 10.3390/ijms19030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitchaimani A., Nguyen T.D.T., Koirala M., Zhang Y., Aryal S. Impact of cell adhesion and migration on nanoparticle uptake and cellular toxicity. Toxicol. In Vitro. 2017;43:29–39. doi: 10.1016/j.tiv.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Senger D.R., Davis G.E. Angiogenesis. Cold Spring Harb. Perspect. Biol. 2011;3:a005090. doi: 10.1101/cshperspect.a005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamalice L., Le Boeuf F., Huot J. Endothelial cell migration during angiogenesis. Circ. Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 42.Nita M., Grzybowski A., Ascaso F.J., Huerva V. Age-related macular degeneration in the aspect of chronic low-grade inflammation (pathophysiological parainflammation) Mediators Inflamm. 2014;2014:930671. doi: 10.1155/2014/930671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morohoshi K., Patel N., Ohbayashi M., Chong V., Grossniklaus H.E., Bird A.C., Ono S.J. Serum autoantibody biomarkers for age-related macular degeneration and possible regulators of neovascularization. Exp. Mol. Pathol. 2012;92:64–73. doi: 10.1016/j.yexmp.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Ioachim E., Stefaniotou M., Gorezis S., Tsanou E., Psilas K., Agnantis N.J. Immunohistochemical study of extracellular matrix components in epiretinal membranes of vitreoproliferative retinopathy and proliferative diabetic retinopathy. Eur. J. Ophthalmol. 2005;15:384–391. doi: 10.1177/112067210501500312. [DOI] [PubMed] [Google Scholar]
- 45.Reinhard J., Renner M., Wiemann S., Shakoor D.A., Stute G., Dick H.B., Faissner A., Joachim S.C. Ischemic injury leads to extracellular matrix alterations in retina and optic nerve. Sci. Rep. 2017;7:43470. doi: 10.1038/srep43470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller C.G., Budoff G., Prenner J.L., Schwarzbauer J.E. Minireview: Fibronectin in retinal disease. Exp. Boil. Med. 2017;242:1–7. doi: 10.1177/1535370216675245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long Y., Xia J.Y., Chen S.W., Gao C.L., Liang G.N., He X.M., Wu J., Jiang C.X., Liu X., Huang W., et al. ATP2B1 gene Silencing Increases Insulin Sensitivity through Facilitating Akt Activation via the Ca(2+)/calmodulin Signaling Pathway and Ca(2+)-associated eNOS Activation in Endothelial Cells. Int. J. Biol. Sci. 2017;13:1203–1212. doi: 10.7150/ijbs.19666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hohmann N., Xia N., Steinkamp-Fenske K., Forstermann U., Li H. Estrogen Receptor Signaling and the PI3K/Akt Pathway Are Involved in Betulinic Acid-Induced eNOS Activation. Molecules. 2016;21:973. doi: 10.3390/molecules21080973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma S., Guru S.K., Manda S., Kumar A., Mintoo M.J., Prasad V.D., Sharma P.R., Mondhe D.M., Bharate S.B., Bhushan S. A marine sponge alkaloid derivative 4-chloro fascaplysin inhibits tumor growth and VEGF mediated angiogenesis by disrupting PI3K/Akt/mTOR signaling cascade. Chem. Biol. Interact. 2017;275:47–60. doi: 10.1016/j.cbi.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Namkoong S., Kim C.K., Cho Y.L., Kim J.H., Lee H., Ha K.S., Choe J., Kim P.H., Won M.H., Kwon Y.G., et al. Forskolin increases angiogenesis through the coordinated cross-talk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signaling. Cell. Signal. 2009;21:906–915. doi: 10.1016/j.cellsig.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 51.Xing Y., Lai J., Liu X., Zhang N., Ming J., Liu H., Zhang X. Netrin-1 restores cell injury and impaired angiogenesis in vascular endothelial cells upon high glucose by PI3K/AKT-eNOS. J. Mol. Endocrinol. 2017;58:167–177. doi: 10.1530/JME-16-0239. [DOI] [PubMed] [Google Scholar]
- 52.Yen H.J., Hsu S.H., Tsai C.L. Cytotoxicity and Immunological Response of Gold and Silver Nanoparticles of Different Sizes. Small. 2009;5:1553–1561. doi: 10.1002/smll.200900126. [DOI] [PubMed] [Google Scholar]
- 53.Lu P.H., Li H.J., Chang H.H., Wu N.L., Hung C.F. Gold nanoparticles induce cell death and suppress migration of melanoma cells. J. Nanopart. Res. 2017;19:342. doi: 10.1007/s11051-017-4036-y. [DOI] [Google Scholar]
- 54.Chan C.M., Chang H.H., Wang V.C., Huang C.L., Hung C.F. Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRbeta, PI3K/Akt and MAPK pathways. PLoS ONE. 2013;8:e56819. doi: 10.1371/journal.pone.0056819. [DOI] [PMC free article] [PubMed] [Google Scholar]