Abstract
In oral biology, tissue engineering aims at regenerating functional tissues through a series of key events that occur during alveolar/periodontal tissue formation and growth, by means of scaffolds that deliver signaling molecules and cells. Due to their excellent physicochemical properties and biomimetic features, nanomaterials are attractive alternatives offering many advantages for stimulating cell growth and promoting tissue regeneration through tissue engineering. The main aim of this article was to review the currently available literature to provide an overview of the different nano-scale scaffolds as key factors of tissue engineering for alveolar bone regeneration procedures. In this narrative review, PubMed, Medline, Scopus and Cochrane electronic databases were searched using key words like “tissue engineering”, “regenerative medicine”, “alveolar bone defects”, “alveolar bone regeneration”, “nanomaterials”, “scaffolds”, “nanospheres” and “nanofibrous scaffolds”. No limitation regarding language, publication date and study design was set. Hand-searching of the reference list of identified articles was also undertaken. The aim of this article was to give a brief introduction to review the role of different nanoscaffolds for bone regeneration and the main focus was set to underline their role for alveolar bone regeneration procedures.
Keywords: scaffolds, nanomaterials, tissue engineering, regenerative medicine, alveolar bone regeneration
1. Introduction
The reconstruction and augmentation of the alveolar bone is a complex and challenging field for the maxillofacial and periodontal surgeon. The crucial aim of the therapies in this field is mainly increasing the bone mass in patients who have lost this tissue as a result of a consequence of several conditions such as periodontal disease, aging, osteoporosis, trauma, neoplastic pathology and reconstructive surgery or as a result of congenital defects [1].
At present auto-transplantation from a patient’s extra-oral or intra-oral donor site is accepted as the gold standard and is the most frequently used method [2]. The critical limitations of this conventional approach are donor site morbidity, inadequate blood supply of graft tissue, associated pain and limited supply. For these reasons, autologous grafting is usually reserved for a restricted number of cases [2,3].
Alternative sources for bone grafts include allografts (grafts originating from another individual of the same species) and xenografts (grafts originating from different species such as bovine or porcine). However, these substitutes also have some certain disadvantages such as the possibility of immune rejection and pathogen transmission from donor to host [2,3].
Another strategy for bone grafts is the use of synthetic alloplasts made from polymers, ceramics or metals. Alloplasts represent some disadvantages including non-optimal integration with the native tissue at the site of the defect and the potential failure due to fatigue or infection caused during implantation. In addition to these, their applications are limited at locations of high stress or mechanical load [2,3].
Owing to the drawbacks and limitations of bone grafts, over the last few decades, several novel approaches involving tissue engineering and regenerative medicine (TE/RM) have emerged as alternatives to conventional treatments. The fundamental concept underlying TE/RM was to use scaffolds alone or in combination with growth factor, cell and/or gene delivery to form a so-called “tissue engineering construct,” that stimulates tissue repair and/or regeneration [2,3,4,5].
In brief, the four crucial factors that must be considered with TE/RM are:
Cells, which represent the fundamental structural unit of any tissue,
A matrix such as “scaffolds”, as framework material supporting the growth of cells to form a fully organized tissue,
Biological factors like growth factors (GF) and bone morphogenetic proteins (BMPs) to guide cellular activity and tissue formation,
Vascularization to provide oxygen and nutrients for the cell metabolism, and to remove catabolic waste products [6,7].
Current limitations in bone TE/RM strategies are the impaired cellular differentiation, inadequate synthesis of extrinsic factors essential for an effective osteogenesis and, most importantly, insufficient mechanical strength and physicochemical properties of the conventional scaffolds [7]. Conventional scaffolds combined with growth factors and cells do not always achieve successful bone regeneration in clinical settings, generally due to the limited capability of controlling framework degradation as well as delivery of biological factors and drugs [6,8,9].
The focus of this review was to give a brief introduction to the nanoscaffolds in TE/RM and to underline the role of different scaffolds in successful tissue formation for bone regeneration. The main aim of this literature-based article is to provide an overview of the different nanoscale scaffolds as key factors of the tissue-engineering paradigm, used for alveolar bone regeneration.
In order to find articles pertinent to this narrative review, PubMed/Medline, Scopus and Cochrane electronic databases were searched using key terms such as “tissue engineering”, “regenerative medicine”, “alveolar bone defects”, “alveolar bone regeneration”, “nanomaterials”, “scaffolds”, “nanospheres” and “nanofibrous scaffolds”. No limitation regarding study design, publication date and language was set. A hand search through the references of the identified articles and previous reviews was also undertaken. The last electronic search was performed on 3 December 2019.
1.1. Importance of Scaffolds in TE/RM
Scaffold material plays a key role in tissue regeneration, as it provides a micro-environment suitable for cell adhesion, proliferation and differentiation. In general, an ideal scaffold material should be biocompatible, have a controllable degradation and appropriate physico-chemical features in order to simulate the extracellular matrix (ECM) structure of the original tissues [7]. It should be able to balance various combinations of materials with specific functions by means of engineered surface, cell encapsulation and controlled release of chemicals [6,9]. Additionally, it should be able to endorse and control peculiar events occurring at the cellular and tissue level [10].
In scaffold based tissue engineering, the scaffold is expected to perform several functions. It should provide adequate mechanical strength and stiffness to replace the mechanical properties of the missing or the injured tissue [6,11]. Most importantly, a successful scaffold should stimulate not only the early ingrowth of new tissue, but also the progressive maturation and remodeling by providing adequate support and morphology [12]. Its design should also take into account degradation kinetics and physico-chemical features [6,11].
1.2. Nanotechnology
One thousand nm is equal to 1 micrometer (µm) and nanoparticles are smaller than 100 nanometers (nm). Nanomaterials compared with bulk materials possess features like quantum size, macroscopic quantum tunneling, as well as small size effects, resulting in altered physiochemical properties [7].
Current areas of research in nanotechnology for tissue regeneration are as follows:
Nanoparticle-based techniques for delivering bioactive molecules,
Nanoparticle-mediated cells tagging and targeting,
Nanoparticle-based scaffold manufacturing [7].
Additionally, the half-life and distribution of nanoparticles can be affected by their size. Their surface properties can determine their stability and their localization in the tissues [7]. A decreased size in nanomaterial particles is beneficial in terms of stiffness, effective surface area and area-to-volume ratio [7]. The charge of the nanoparticles is also another important factor affecting the internalization of nanosized particles into different target cells [7].
In oral biology, nanotechnology applications are mainly focused on augmentation procedures for bone tissue regeneration and enhancement of osseointegration of oral implants [7]. Natural bone itself is constituted by a highly organized extracellular matrix (ECM) in a nanometric scale, and the application of nanoscaffolds can represent an intrinsic advantage for tissue engineering regeneration procedures of the musculoskeletal apparatus [8,13]. Nanomaterials can overcome the main problems encountered with the current scaffolds used for bone regeneration such as: Inadequate mechanical strength, instability of growth factors released and impaired cellular differentiation [7,10].
1.3. Rationale of Nanotechnology Scaffolds in Tissue Engineering
Advanced materials play a crucial role in promoting the bench-to-bedside translation of tissue engineering technologies [14,15]. Direct application of therapeutic substances may be affected by some limitations of the conventional scaffold materials including non-specific targeting, insufficient physiological stability and low cell membrane permeability. Usually, supra-physiological doses are needed to compensate for the poor pharmacokinetics of such agents, which in return increase the potential risk of adverse effects [7]. Currently, nanotechnology has allowed the production of structures having the same size as the naturally occurring tissues and has opened a new era for TE/RM [8,16].
Nanoscaffolds can be produced so as to be extremely similar to tissue-specific ECM. The reduced size of the nanoparticles permits a fast response to external stimuli coming from the environment, like ultrasounds, magnetic fields, pH and iX-ray exposure.
Nanoscaffold materials may be used to deliver drugs, genetic material or biological factors in a controlled way, both systemically and locally [8]. Nanoscaffolds can stabilize the bioactive agents by means of encapsulation or surface attachment, promoting molecule internalization, targeting their delivery from cells and allowing to control biological factor release at the intended target [7]. Controlled and sustained delivery by nanoparticles mainly depends on their reduced size and related high specific surface area [17]. Thus they may represent stimulus-sensitive delivery vehicles for chemically or biologically active substances, which will provide a triggered delivery as a response to an external stimulus [7,16,17].
Nanoscaffolds have a considerable drug loading capability, high mobility of drug loaded particles, and efficient in vivo reactivity toward nearby tissues [17]. They can be used for labeling cells, in order to enable continuous cell tracking and monitoring [8,16]. Additionally, nanoparticles can provide enhanced osseointegration, osteoconduction and osteoinduction [16].
1.4. Nanofibrous Scaffold Systems
Fibrous nanoscaffolds are extremely-thin uninterrupted fibers with a short diffusional path, a considerable surface area to unit mass ratio and high porosity. The porous structure of the nanofibrous drug delivery systems is highly interconnected and represents an adequate substrate for cell adhesion and transport of nutrients. One of the limitations of these systems is the initial burst release phenomenon, which depends on the large surface area and the short diffusional path.
Fibrous nanoscaffolds, besides their role as drug carriers, provide mechanical strength. There had been worries that drug incorporation into the nanofibers might impair the mechanical features of the same nanofibers [8]. As an example for a solution to this problem, Ionescu et al. developed a microsphere-laden fibrous nanoscaffold structure in which the microspheres were to deliver drugs, while the nanofibers only worked as an engineered scaffold [18].
1.5. Nanosphere Scaffold Systems
Nanospheres can deliver drugs, growth factors or genetic material [7]. The advantages of nanospheres over conventional monolithic bulk scaffolds can be listed as follows:
Mechanically weak scaffolds in load-bearing applications may be reinforced by the adjunct of nanospheres as cross-linking agents,
The porosity of the materials constituting traditional scaffolds may be significantly enhanced, by adding spheres like porogen. Increased porosity means allowing interior tissue infiltration into the scaffold,
Nanospheres can stimulate the creation of apatite crystals and the following mineralization of hydrogels through the release of the corresponding minerals, so that self-hardening biomaterials adapted to the regeneration of bone tissue can be produced.
Injectable and/or moldable materials can be developed thanks to the spherical nature of some nanomaterials, so that their application is possible by means of minimally invasive surgery [17].
1.6. Classification of Nanoparticle (NP) Materials
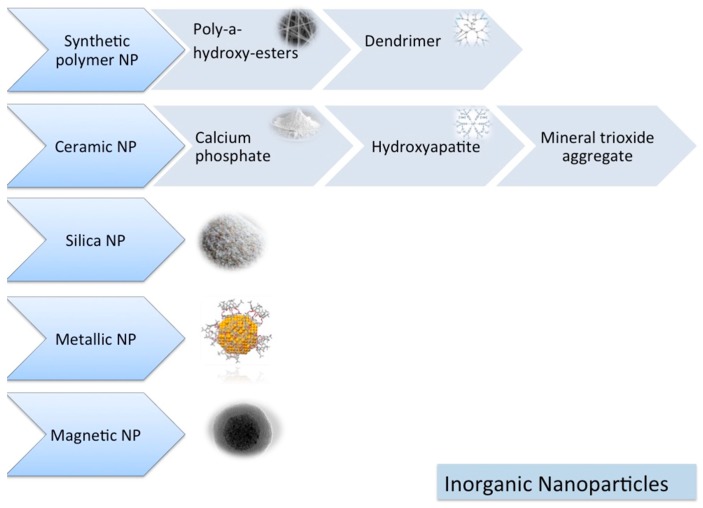
Nanoparticles can be classified as inorganic (Figure 1) and organic nanoparticles (Figure 2).
Figure 1.
Inorganic nanomaterials.
Figure 2.
Organic nanomaterials.
1.6.1. Inorganic NPs
Synthetic Polymers
Synthetic polymers have some advantages, such as sufficient supply, easy fabrication, easy adaptation, high safety profile and reasonable costs. Additionally, they have adjustable physiochemical and morphological features, which are valuable for wide-scale production and application [19,20].
The most common synthetic polymers for biomedical applications are poly-α-hydroxyesters (poly-glycolic acid (PGA), poly-lactic acid (PLA), poly-lactic-glycolic acid (PLGA) and poly-caprolactone (PCL)) [20]. However, there are also disadvantages of poly-α-hydroxyesters, which can be listed as follows: (i) Hydrophobicity, which causes failure of loading hydrophilic drugs or molecules and poor cell adhesion, (ii) degradation by autocatalysis, which causes unpredictable degradation behavior, (iii) an acidic degradation product, which leads to denaturation of bioactive proteins and inflammatory tissue response, and (iv) low capacity of loading therapeutic agents, which limits their penetration into the polymer network [20,21].
Various combinations of PLGA/PLLA/PEG/PCL were tested by several researchers with promising results for alveolar bone regeneration applications [1,22,23,24].
• Dendrimers
Dendrimers are synthetic polymers with branching treelike structures. They are biocompatible and biodegradable with uniform morphology. They have a high number of surface functional groups, which makes them suitable for several applications [16,25]. Dendrimers can cause an improvement on drug solubility [16,26] and are mostly used for targeted drug delivery reasons [16,27]. However, they have a few disadvantages, like cytotoxicity and possible poor drug retention inside the branches of the dendrimer [16].
Ceramic NPs
Ceramic materials are “synthethic crystalline, solid, inorganic non-metallic materials” [28]. Bioceramics include bioactive glass, bioactive glass–ceramic, calcium phosphate groups and alumina [16,29,30].
Bioceramics possess certain advantages like biocompatibility, nontoxicity and dimensional stability [29]. They are added to the scaffolds to improve their structural properties and delivery performance. Many ceramic materials such as calcium phosphate groups and mineral trioxide aggregate materials have been used and are currently in use for bone regeneration procedures [16].
Bioactive glass nanoscaffold was investigated and was reported to be beneficial for formation of new alveolar bone tissue [31].
• Calcium Phosphate (CP) Groups
Normal bone tissue is composed of 30% w/v organic collagen fibers and 70% inorganic matter, mainly CP crystals. The latter represents a model for mimicking natural bone material at the macro- and nanoscale level [7]. For this reason, CP nanoparticles (nano-tricalcium phosphate (nTCP) and nano-hydroxyapatite (nHA)) have been the most commonly applied materials for bone tissue regeneration [32].
CP has many favorable properties such as similarity to the inorganic portion of natural bone tissue, biocompatibility, biosafety, osteoconductivity, low cost and ease of manufacturing. The drawbacks are poor regulation of drug delivery and degradation rate [20,33]. However, when CP is used as a single component, it might represent some limitations due to its poor mechanical features and very low toughness. In order to overcome such drawbacks, researchers recently introduced composite scaffolds composed of nTCP/nHA plus further biological materials or synthetic polymeric materials [33].
Several applications of nanoHA [34,35,36,37,38,39] and nanoTCP [40,41] combinations were investigated in the literature and were reported as successful for alveolar bone tissue regeneration.
• Mineral Trioxide Aggregate (MTA)
MTA is prepared by bismuth oxide reaction, and is composed of tricalcium aluminate, tricalcium silicate, dicalcium silicate and calcium sulfate dehydrate [42,43]. During hydration, MTA releases Ca2+ and OH ions, which increases pH in order to neutralize the acidic metabolites produced by macrophages and osteoclasts. Nanoscale particle sized tricalcium aluminate may increase bone regeneration, inflammatory response and foreign body reactions [43].
Silica NPs
Silica, either itself or as a coating of other compounds, has been used for applications in the biomedical field, like imaging and drug delivery [16]. According to their application purposes, silica nanoparticles can be synthesized as bulk particles, core/shell silica NPs and mesoporous silica nanoparticles (MSNPs) [16]. In particular, MSNPs have attracted the attention of researchers for applications of controlled release. MSNPs can be synthesized in different ways to obtain particles with various dimensions and physical properties. MSNPs have unique pore structure, high surface area and large pore volume. Additionally, because of their honeycomb-like porous structure, they are able to encapsulate and absorb a number of biomolecules [16,44,45].
The typical advantages of silica nanoparticles are their uniform morphology, biocompatibility and chemical stability. Their disadvantage is their variable toxicity [16].
Metallic NPs
Metallic NPs are biocompatible materials with reduced cytotoxicity. Their functionalization is easy; however, they need long term cytotoxicity testing and their coating is advised [16]. In biomedical applications, gold NPs are considered as the safest and the most commonly utilized among other metallic NPs. Gold NPs can be synthesized as spheres, rods or cages and they can be applied in areas such as drug delivery, biosensing, bioimaging and photothermal therapy [16,46,47].
Combinations of gold and silver nanoparticles with chitosan for alveolar bone regeneration were reported for gold [48] and silver [22] with successful outcomes for enhanced mineralization and implant osseointegration.
Magnetic NPs
In biomedical applications, nickel, iron, cobalt and their oxides may be used as magnetic nanoparticles for drug delivery, three-dimensional cell organization, cell tracking, imaging procedures and as biosensors [16,49]. Some magnetic nanoparticles offer super paramagnetic properties with long-lasting and non-invasive tracking possibility. However, magnetic nanoparticles are cytotoxic and coating is required due to the safety concerns for overcoming their cytotoxicity and for producing biocompatible magnetic NPs [16].
1.6.2. Organic Nanoparticles
Liposomes
Liposomes are vesicles composed of bilayers of phospholipids [50]. Their properties are affected by their lipid size, surface charge, composition and preparation method [16]. Liposomes are biodegradable, stimuli responsive and can be incorporated with hydrophilic/hydrophobic drugs [16,51]. They are mainly used in drug delivery and imaging [52]. They are the most clinically tested nanosystems, with several commercialized formulations [51]. However, their rapid clearance from circulation and scale-up issues might represent some disadvantages [16,51].
Natural Polymeric NPs
Natural polymers are biocompatible and biodegradable materials with bulk physical properties. They are divided mainly as proteins and polysaccharides [53] and can be listed as collagen, alginate, fibrin, gelatin and chitosan [17]. Due to their intrinsic biocompatibility and biodegradability, natural polymers are very important for bone tissue engineering procedures [54]. The synthesis methods can be flexible and polymer chains can show a wide diversity of composition and properties [53]. Generally they have high drug loading capability, but the scale-up issues might represent some disadvantages [16,53].
• Collagen
Collagen is the most frequently tested natural polymer since the ECM of the natural bone is mainly composed of collagen. It has excellent biocompatibility, optimal biodegradability and negligible immunogenicity [17,55]. The limitations of the collagen can be listed as weak mechanical stability, possible denaturation on processing and the intrinsic risk of causing immune reactions and/or transmitting infectious agents. As an alternative solution, instead of collagen extracted from animals, recombinant collagen was proposed for tissue engineering procedures [17].
• Gelatin
Gelatin is a denatured form of collagen with similar beneficial biological properties and limitations. Gelatin degradation is controllable by tailoring the crosslinking procedures. The existing presence of functional groups additionally allow functionalization by chemical transformation [17].
• Chitosan
Chitosan has many advantageous biological and physicochemical properties. It is biocompatibile, antibacterial and its production is easy. Various studies have tested chitosan as a factor and/or drug delivery system for pharmaceutical and tissue engineering applications [5,17].
Chitosan nanomaterial was tested by researchers with different combinations of nanoparticles such as PLGA/bioactive glass [1], PCL/Sr-doped calcium phosphate [56], PLGA/silver [22] and gold [48] for alveolar bone regeneration procedures with successful results in favor of alveolar bone regeneration.
• Alginate
Alginates are biocompatible, hydrophilic, non-immunogenic and cost effective materials. Their intrinsic features can induce in vivo calcification without the use of any additives and they are suitable for a number of tissue engineering and drug delivery applications [17]. However their rather slow degradation when implanted in bone defects is poorly controllable and they do not have cell attachment sites for osteoblast anchorage [17].
Carbon
• Carbon Nanotubes
Carbon nanotubes (CNTs) are carbon allotropes with a long cylindrical structure. They might be single-walled or multi-walled graphitic hollow tubular structures. They have mechanical and chemical stability with optimum electrical properties. However, their good chemical stability might represent a drawback for the covalent functionalization process. Carbon materials are added to the composite scaffolds, in order to reinforce the structural properties [16].
Carbon nanotubes are effective on bone tissue regeneration as they promote cell proliferation and osteogenic differentiation [57]. Several carbon nanomaterials like CNTs, graphene oxide (GO), fullerenes, carbon dots (CDs), nano-diamonds and their derivatives were successfully tested as a scaffold for bone tissue engineering [57].
• Graphene and Its Derivates
Graphene is a film monolayer one-atom-thick, with a honeycomb-like structure made of carbon atoms, and arranged in a two-dimensional hexagonal structure [58]. Nanomaterials of the graphene family include a great variety of graphene derivatives such as, graphene oxide (GO), reduced graphene oxide (rGO) and graphene nanosheets [59,60]. Graphene and its derivatives represent advantageous mechanical, electrochemical and physical properties such as, small size, large surface area, thermal stability, electrical conductivity and mechanical strength. Additionally, graphene materials may be functionalized and combined with different biomaterials and biomolecules, such as small molecules, polymers or nanoparticles, by means of covalent or non-covalent interaction [50,51,52,53,54,55,56,57,58,59,60,61]. Graphene materials are favorable for cell differentiation, proliferation and osteogenic differentiation. Additionally they are biocompatible and represent antibacterial properties. However, the behavior of graphene is dependent on its size, surface functionalization and its coating [59,60,61]. Biomedical applications of graphene oxide can produce adverse effects mostly dependent on the dose and time. Currently, the cytotoxicity of the GO material represents a challenging situation for clinical applications. The quality of graphene plays a major role since the presence of impurities can cause undesired events [62]. Interactions between body and GO are still being investigated for in vivo applications and it is most likely that their primary clinical applications shall be the ones for topical use or short-time transient implantations [63].
GOs are able to enter into cytoplasm and nuclei. They can induce cell membrane damage and apoptosis. GO can induce lung diseases by causing severe toxicity. GOs also stay for a long time in kidneys since they are cleaned in the kidney by a very complex process [64]. Further studies are lacking in the literature to explore their toxicity and effect on cells/tissues.
Graphene oxide combined with silk fibroin [65,66], GO-coating of titanium implants [67] and GO-coating of collagen membranes [60,61] for guided bone regeneration were investigated by various researchers. The results showed that these applications can improve cell proliferation and osteogenic differentiation with success.
1.6.3. Composite Scaffolds
Inspired by the natural organic/inorganic composition of bone, different organic and/or inorganic particle combinations were tested as composite scaffolds that increase the advantages and decrease the drawbacks of each component [17]. In order to overcome the disadvantages of a single particle, strategies of different composite scaffold designs containing diverse materials have been developed by many researchers [68].
Combining nHA with polymers of high molecular weight was evaluated to overcome the disadvantages of a single material. PLA [19], PLGA [69], collagen [70], polyamide [71], coralline [72], chitosan and PCL [73] have been combined with nHA and were found to improve scaffold biocompatibility as well as mechanical strength. As an example for bone regeneration, several studies have shown that the combination of nHA and collagen scaffolds has favorable mechanical properties [19,70]. Collagen fiber provides an osteoinductive and absorbable scaffold for infiltration of osteoblast cells. However, the nonabsorbable nHA can reduce this effect. For this reason, the percentage of nHA and collagen in composite materials play a crucial role for tissue regeneration [19].
1.7. Manufacturing Methods to Fabricate Scaffolds
Strategies of scaffold designs utilizing particles can be listed as follows: (i) Particles as different single components embedded into matrices, solid polymers, hydrogels or calcium phosphate cements, (ii) particles combined into blocks for fabrication of scaffolds, (iii) haphazard packing, (iv) rapid prototyping and self-assembling scaffolds (assembly driven by electrostatic, magnetic interactions or hydrophobic interactions) [17].
Various methods have been developed to produce scaffolds with porous properties for bone regeneration, such as freeze-drying [74], gas foaming [75], salt leaching [76], phase transformation [77], sponge replication [78], electrospinning [79] and additive manufacturing methods such as 3D printing (selective laser sintering (SLS), stereolithography, laser assisted printing, inkjet printing and extrusion printing) [32,68,80].
The sol-gel phase, co-precipitation as well as chemo-mechanical techniques have been utilized for nHA production [81]. However, these procedures are complex with high cost and poor reproducibility. Additionally, the manufacturing variables, the low-yield end-products and high byproducts are difficult to control, and the produced nHA is different from the biological HA in terms of physicochemical properties [81].
1.8. Biphasic and Multiphasic Scaffolds
The nanoparticles have many limitations such as a high agglomeration rate and an uneven distribution pattern. Biphasic and multiphasic preparations might partially overcome the limitations of single-phase biomaterials by improving the bioactivity and biocompatibility [32] since they have the ability to combine different material compositions and physical structures [82,83,84].
The rationale of utilizing multiphasic/biphasic scaffolds in oral science mostly depends on the expectations from an ideal scaffold. An ideal scaffold should have various functions, such as the support for cell colonization, migration, differentiation and growth. The design should also take into account suitable physical and physicochemical properties together with degradation kinetics. Multiphasic scaffolds are needed for an appropriate control of the spatiotemporal events for periodontal tissue regeneration since they can allow compartmentalized tissue healing [82,83]. Manufacturing of multiphasic nanoscaffolds for periodontal tissue engineering can be divided into three approaches: (i) Bilayered occlusive membrane + bone compartment [84,85], (ii) compartmentalized biphasic scaffolds [86,87,88,89] and (iii) compartmentalized triphasic scaffold [90]. In vitro and in vivo investigations have reported promising results for biphasic/multiphasic scaffold designs. However, the methods still need to be refined for future clinical applications.
1.9. Three-Dimensional (3D) Porous Scaffolds
Three-dimensional porous scaffolds have been developed and tested in many tissues, including bone, cartilage, skin, muscle and vasculature [17]. The importance of 3D porous scaffolds comes from their ability to simulate extracellular matrices, which can sustain cellular activity. They can provide a pool of bioactive signaling molecules through a sustained release to the natural environment. In this way, they might regulate cell function and trigger tissue repair [17].
1.10. Three-Dimensional Bio-Printing
Three-dimensional bio-printing (3D additive manufacturing) is a precise layering of biomaterials, biochemical substances and living cells, with spatial control of the positioning of each component [91]. Tissue bio-printing strategies include inkjet, micro-extrusion and laser-assisted bio-printing. Three-dimensional printing is one of the hot topics in TE/RM and nanomaterials play a crucial role in this manufacturing method [92,93]. The major challenge in these technologies is to provide multiple cell types and micro-architecture of extracellular matrix (ECM) in an adequate resolution to reproduce biological function [92,93].
In periodontal regeneration, the main challenge is the scaffold design, which might mimic the periodontal tissue nature and organization. While 3D bio-printing may help to solve this challenge, the long-term success of these applications mostly depends on the properties of the current biomaterials [82,94].
1.11. Influence of Magnetic Fields on Biological Systems
Magnetism might play a significant role in the control of cell responses. A magnetic field can be applied to cells externally or internally; cells can be embedded in a scaffold material with magnetic properties [95]. Yun et al. investigated the additional effects of the outer static magnetic field (SMF) with magnetic polycaprolactone nanocomposite scaffold for bone regeneration. According to the results of this experiment, the combination of external and internal magnetism can be advantageous for new bone formation [95].
2. Nanoscaffold Applications for alveolar Bone Regeneration
In TE/RM, one of the major research topics is to create bioconstructs that can integrate with the in vivo tissues [10]. Current systems represent limitations such as restricted diffusion and uneven cell-matrix distribution. In order to overcome limitations of the current materials, during the last years different types of scaffolds and bioreactors have been designed and tested [10]. The regeneration of oral tissues is very challenging [96] and nanomaterials might represent a great potential for future TE/RM applications of various craniofacial and oral defects. The most frequently investigated nanomaterials for oral tissues can be listed as nanofibers, nanoparticles, nanosheets, nanotubesand nanospheres. Recently, the multi-layer nanoscaffolds for oral tissue regeneration were also tested by researchers [97,98].
In general, nanomaterials have excellent physiochemical properties and biomimetic features for enhancing cell growth and tissue regeneration [96,97]. Nanomaterials, due to their size, can have an effective control of the release rate of the each agent upon matrix degradation. Furthermore, they can be used for delivery of therapeutic agents in alveolar bone and tooth regeneration. Nanofibers represent an ideal environment for cell migration due to their similarity to extracellular matrices and their high porosity. In addition, nanotubes and nanoparticles might enhance the chemical and mechanical features of the scaffold, increase cell migration and proliferation, and tissue regeneration [96]. While the application of nanomaterials in TE/RM is still in its infancy phase with several limitations to be addressed, the recent results of investigations indicate their potential role for future oral tissue engineering applications [93].
Three fabricating modes exist for TE/RM scaffolds: Growth factor-scaffold, cell-scaffold and cell growth factor-scaffold. In cell-scaffold, and cell growth factor-scaffold materials, cells are inserted on a biocompatible scaffold, in order to proliferate into the scaffold and to remodel the natural environment by secreting ECM. Biomaterials in periodontal tissue engineering are mainly growth factor-scaffold, used as the carrier of exogenous growth factors. The main challenge is the necessity to provide an adequate number of seeded cells integrated with the scaffold material in vitro and implanted into a defect position [96,99].
There is a huge number of experimental studies reported in the literature, searching for the best nanoscaffold material for various periodontal tissue regeneration applications. Ogawa et al. [41] tested nano-ß-TCP/collagen loaded with fibroblast growth factor-2 (FGF-2) on periodontal wound healing. In the study, nano-ß-TCP scaffold, nano-ß-TCP scaffold loaded with FGF-2 and non-coated collagen scaffold were implanted into one-wall infrabony defect models. nano-ß-TCP scaffold was fabricated by surface coating of collagen scaffold with nanosize ß-TCP dispersion. According to the four week post-surgery histological results, nano-ß-TCP scaffold loaded with FGF-2 displayed nearly five-times greater periodontal tissue repair when compared with the collagen scaffold [41].
Xue et al. [22] produced nanoparticles of chitosan, PLGA and silver (Ag) for investigating the optimal combination ratio for mineralization of periodontal membrane cells, and periodontal tissue regeneration. The antibacterial properties of each nanoparticle were also evaluated. Different combination ratios of nPLGA and chitosan were tested. According to the results, single nanoparticles did not show any cytotoxicity and were able to enhance cell mineralization. Additionally, chitosan and nAg showed antibacterial properties, while nAg limited cell proliferation. The 3:7 ratio of nPLGA/chitosan and 50 μg/mL nAg was the optimal proportion [22].
Hydrogels are soft materials with (3D) polymer structure, water-absorbability and adjustable physical and chemical properties [100]. Natural and synthetic hydrogels composed of micro-/nanostructures have been shown to imitate the chemical and physical properties of natural ECM for bone and periodontal tissue regeneration [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101]. Gelatin can be modified by methacryloyl (methacrylamide and methacrylate) side groups and the final product is GelMA [84]. Gelatin methacrylate (GelMA) is a biocompatible and photocrosslinkable hydrogel. Chen et al. [37] tested fabrication of GelMA/nanohydroxylapatite microgel arrays using a photocrosslinkable method. The group evaluated the regeneration and osteogenic proliferation of human periodontal ligament stem cells (hPDLSCs) encapsulated in microgels. According to the results GelMA/nHA microgels (10%/2% w/v) enhanced periodontal tissue regeneration. Since GelMA/nHA microgels enhanced hPDLSCs proliferation and osteogenic differentiation in vitro and supported bone regeneration in vivo [37].
In recent years, researchers proposed and tested graphene-based nanomaterials for oral tissue engineering because of the many advantageous properties and antibacterial capacities of the graphene [60,61,102].
Sowmya et al. [1] tested a scaffold system for the regeneration of oral tissues (periodontal ligament, cementum and alveolar bone) in a rabbit study [1]. The structure of the scaffold consisted of three layers. For bone tissue a layer of chitosan/PLGA/nano-sized bioactive glass layer enriched with PRP, for periodontal ligament a layer of chitosan/PLGA with the adjunct of fibroblast growth factor 2 (FGF-2), and finally for cementum a layer of chitosan/PLGA/nano-sized bioactive glass layer loaded with cementum protein 1 (CEMP1). According to the histological and tomographic evaluation results, new alveolar bone formation and complete periodontal healing was obtained in three months [1].
Zhang et al. [103] tested the incorporation of growth factors in nanomaterial-based silk fibroin scaffolds in a dog study for tissue regeneration. Nanomaterial-based silk fibroin scaffolds loaded with BMP-7 and/or platelet derived growth factor (PDGF)-ß adenovirus were used to test tissue regeneration. According to the results, the scaffolds loaded with BMP-7 mainly enhanced alveolar bone regeneration, while the scaffolds incorporated with PDGF-ß adenovirus produced a partial regeneration of the periodontal ligament. The combination of growth factors created a synergistic effect showing up to two times greater alveolar bone, periodontal ligament, and cementum formation, as compared with each factor alone [103].
Vaquette et al. [40] tested PCL and ß–TCP for periodontal ligament and alveolar bone regeneration. The scaffold design was composed of a flexible electrospun component for the periodontal ligament section and a fused deposition modeled component for the bone section. Cell sheet technology was utilized to manufacture biphasic scaffolds additively. While the results were promising with increased mechanical stability of the cell sheets and mineralization, the bone section did not provide sufficient ectopic bone proliferation [40].
Rasperini et al. [104] tested an individualized custom-made 3D-biomanufactured scaffold for regeneration of a periodontal osseous defect in a clinical study. For this purpose, a customized scaffold containing PCL powder and HA was produced by using selective laser sintering technology, according to the computed tomography scan of the patient’s defect. While the scaffold became exposed after 12 months, which led to failure, the results of this study are promising in terms of further research [104].
Nanomaterials seem to be promising for alveolar bone regeneration applications as they mostly provide favorable results, as listed in Table 1. However, the decreased size of nanomaterials can cause some potential problems. Nanomaterials possess sizes close to biological molecules, peptides, deoxyribonucleic acid and viruses. Thus, they can provoke adverse events by moving throughout the body, depositing in target organs (liver, lungs, kidney, heart and spleen), penetrating cell membranes and staying in the mitochondria [105,106,107,108]. Manganese oxide, titanium dioxide, aluminum oxide, zinc oxide and silver might accumulate and elicit harmful responses [109]. The brain is partly shielded by the blood–brain barrier, but the nanoscale size of these materials might cause them to cross this barrier or they might penetrate through the olfactory and sensory nerves. The biological toxicity of nanomaterials is thought to be induced by their oxidative properties [110,111] and further research should be conducted to evaluate and develop a science-based risk evaluation of the nanomaterials [109].
Table 1.
Nanoscaffold applications for alveolar bone regeneration.
| Material | Reference | Outcome |
|---|---|---|
| 3 layer chitosan/PLGA/nano-sized bioactive glass | Sowmya et al. [1] | Complete periodontal healing and new alveolar bone deposition after three months |
| GO-coating of collagen membranes | Radunovic et al. [60] | Favourable on promoting osteoblastic differentiation process |
| GO-coating of collagen membranes | De Marco et al. [61] | Improved biocompatibility of collagen membranes on in vitro human primary gingival fibroblast model |
| PCL containing ß–TCP | Vaquette et al. [40] | Enhanced mechanical stability of the cell sheets, and mineralization. However, ectopic bone ingrowth was not sufficient |
| nano-ß- TCP/collagen scaffolds | Ogawa et al. [41] | nano-ß-TCP/collagen scaffolds loaded with fibroblast growth factor-2 (FGF-2) improved periodontal tissue wound healing results |
| Chitosan, PLGA, and silver (Ag) nanoparticles complex | Xue et al. [22] | Contributed to cell mineralization without cytotoxicity |
| GelMA/nHAmicrogels | Chen et al. [37] | Promoted in vivo osteogenesis of hPDLSCs encapsulated in microgels |
| nanomaterial-based silk fibroin scaffolds incorporating BMP-7 and/or PDGF-ß | Zhang et al. [103] | Promoted periodontal healing |
| PCLpowder containing HA | Rasperini et al. [104] | Clinical study with failure due exposure of the scaffold |
| Graphene | Xie et al. [112] | Favourable on osteogenic differentiation but not on osteoblastic differentiation |
| Graphene Oxide combined with silk fibroin | Rodríguez-Lozano et al. [65] | Favourable on mechanical resistance and hPDLSC proliferation and showed biocompatibility |
| Graphene Oxide combined with silk fibroin | Vera-Sánchez et al. [66] | PDLSC proliferation rate into osteo/cementoblast like cells improved with these combinations |
| GO-coating of titanium implants | Ren et al. [67] | Improved cell proliferation, osteogenic differentiation and biocompatibility of implants |
| Citric Acid-Based Nano Hydroxyapatite | Dayashankar et al. [39] | Significant bone regeneration |
| Nano-bioactive glass loaded with NELL1 gene | Zhang et al. [31] | Good osteoconductivity for promoting the formation of new alveolar bone tissue |
| Poly(l-lactic acid) (PLLA) nanofibrous spongy microspheres, PLLA/polyethylene glycol (PEG) co-functionalized mesoporous silica nanoparticles, and poly(lactic acid-co-glycolic acid) (PLGA) microspheres | Liu et al. [23] | In a mouse model of periodontitis, the injectable and biomolecule-delivering PLLA lead to Treg enrichment, expansion, and Treg-mediated immune therapy against bone loss |
| Nanofibrous yarn reinforced HA-gelatin | Manju et al. [34] | Promoted bone formation in critical sized alveolar defects in rabbit model |
| Silver nanoparticle-coated collagen membrane | Chen et al. [35] | Induced osteogenic differentiation of mesenchymal stem cells that guide bone regeneration. |
| Chitosan-gold nanoparticles mediated gene delivery | Takanche et al. [48] | Enchanced osseointegration of dental implant even in osteoporotic condition |
| Hydroxyapatite nanowires modified polylactic acid membrane | Han et al. [36] | Promoted bone regeneration in a rat mandible defect model |
| PCL/chitosan/Sr-doped calcium phosphate electrospun nanocomposite | Ye et al. [56] | Higher ALP activity level and a better matrix mineralization |
| Nano hydroxyapatite mineralized silk fibroin | Nie et al. [38] | Improved osteogenesis |
| PLGA/PCL Modification Including Silver Impregnation, Collagen Coating, and Electrospinning | Qian et al. [24] | Enhanced alveolar bone regeneration (31.8%) |
One of the limitations of the nanoscaffold systems is the incorporation of nanoscale material for reinforcement. In the regeneration of bone, utilizing micro- and nano-length scale materials can be beneficial for increasing the intrinsic fracture resistance of the bone by affecting bone strength and supporting plasticity. However, macro-length scale materials are also beneficial, influencing toughness and creating a resistance to fracture. In brief, nanoscale materials increase the capacity to withstand pressure, but toughness drops dramatically [6] and it is not realistic to expect both high strength and high toughness properties from one single material [113]. As an example, in ceramic based materials, toughness is provided majorly by their large scales. Ceramic based materials are able to shield crack by supporting uncracked material bridging the crack wake, which is impossible for micro/nano-scale materials [114,115]. Nanotechnology alone cannot be the optimum solution for improving the mechanical properties of the scaffolds. Incorporation of different scale materials with a hierarchical design exhibiting many scales seems to be beneficial and should be considered as a future perspective [6,116].
3. Conclusions
This review mainly focused on the basic information about the nanomaterials for bone regeneration to give researchers a general knowledge about their possible applications in the oral field. Currently their application in oral and maxillofacial clinics is very limited but interest is increasing for their possible applications in order to overcome the current problems associated with conventional materials.
As a conclusion, it can be underlined that nanomaterials have excellent physico-chemical properties and biomimetic features for promoting cell growth and stimulating tissue regeneration, and oral tissue engineering with nanomaterials seems to represent a great potential with vital importance as future treatment modalities.
However, nanomaterials should not be evaluated as optimum solutions for every current problem. It should be understood that there are also disadvantages of nanomaterials such as toughness, which can be solved with the incorporation of a hierarchical design encompassing many length-scales to generate stronger and tougher scaffold materials. They can also provoke adverse events by moving all over the body or by depositing in organs due to their nanosize, which is similar to biological molecules and viruses. Further research needs to be done in order to provide solutions for possible future applications.
Acknowledgments
None.
Abbreviations
| TE/RM | Tissue engineering and regenerative medicine |
| GF | Growth factors |
| BMP | Bone morphogenetic proteins |
| mRNA | Micro ribonucleic acid |
| TCP | Tricalcium phosphate |
| BMMSC | Bone marrow mesenchymal stem cells |
| MSC | Mesenchymal stem cells |
| PRP | Platelet rich plasma |
| ECM | Extracellular matrix |
| nm | Nanometers |
| µm | Micrometers |
| NP | Nanoparticles |
| PLA | Poly-lactic acid |
| PGA | Poly-glycolic acid, |
| PLGA | Poly-lactic-co-glycolic acid |
| PLLA | poly(l-lactic acid) |
| PEG | PLLA/polyethylene glycol |
| PCL | Poly-caprolactone |
| CP | Calcium Phosphate |
| HA | Hydroxyapatite |
| TCP | Tricalcium phosphate |
| nHA | Nano hydroxyapatite |
| nTCP | Nano tricalcium phosphate |
| MTA | Mineral trioxide aggregate |
| MSNP | Mesoporous silica nanoparticle |
| CNT | Carbon nanotube |
| GO | Graphene oxide |
| CD | Carbon dots |
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
| SLS | Selective laser sintering |
| 3D | 3 dimensional |
| SMF | Static magnetic field |
| FGF-2 | Fibroblast growth factor-2 |
| Ag | Silver |
| GelMA | Gelatin methacrylate |
| hPDLSC | Human periodontal ligament stem cells |
| CEMP1 | Cementum protein 1 |
| PDGF | Platelet derived growth factor |
| DNA | Deoxyribonucleic acid |
Author Contributions
Databases were searched and data was collected by G.F., M.D.F., E.G., G.A.B., S.T., S.P., D.G. All the authors contributed on analysis and interpretation of data for the work. G.F. drafted the work and wrote the manuscript with input from all authors. G.F. and M.D.F. revised the work critically for intellectual content. Integrity of the work was appropriately investigated and resolved by all authors. All authors contributed and approved equally to the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
All the authors have no conflict of interest to disclose.
References
- 1.Sowmya S., Mony U., Jayachandran P., Reshma S., Kumar R.A., Arzate H., Nair S.V., Jayakumar R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Healthc. Mater. 2017;6:1601251. doi: 10.1002/adhm.201601251. [DOI] [PubMed] [Google Scholar]
- 2.Pilipchuk S.P., Plonka A.B., Monje A., Tau A.D., Lanisdro A., Kang B., Giannobile W.V. Tissue Engineering for Bone Regeneration and Osseointegration in the Oral Cavity. Dent. Mater. 2015;31:317–338. doi: 10.1016/j.dental.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mudda J.A., Bajaj M. Stem cell therapy: A challenge to periodontist. Indian J. Dent. Res. 2011;22:132–139. doi: 10.4103/0970-9290.79978. [DOI] [PubMed] [Google Scholar]
- 4.Rios H.F., Lin Z., Oh B., Park C.H., Giannobile W.V. Cell- and Gene-Based Therapeutic Strategies for Periodontal Regenerative Medicine. J. Periodontol. 2011;82:1223–1237. doi: 10.1902/jop.2011.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goker F., Ersanlı S., Arısan V., Cevher E., Güzel E.E., İşsever H., Ömer B., Altun G.D., Morina D., Yılmaz T.E., et al. Combined effect of parathyroid hormone and strontiumranelate on bone healing in ovariectomized rats. Oral Dis. 2018;24:1255–1269. doi: 10.1111/odi.12895. [DOI] [PubMed] [Google Scholar]
- 6.Saiz E., Zimmermann E.A., Lee J.S., Ulrike G.K., Wegst U.G.K., Tomsia A.P. Perspectives on the Role of Nanotechnology in Bone Tissue Engineering. Dent. Mater. 2013;29:103–115. doi: 10.1016/j.dental.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walmsley G.G., Mc Ardle A., Tevlin R., Momeni A., Atashroo D., Hu M.S., Feroze A.H., Wong V.W., Lorenz P.H., Longaker M.T., et al. Nanotechnology in bone tissue engineering. Nanomedicine. 2015;11:1253–1263. doi: 10.1016/j.nano.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbone E.J., Tao Jiang T., Nelson C., Henry N., Lo K.W.H. Small molecule delivery through nanofibrous scaffolds for musculoskeletal regenerative engineering. Nanomedicine. 2014;10:1681–1699. doi: 10.1016/j.nano.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosseinpour S., Ahsaie M.G., Rad M.R., Baghani M., Motamedian S.R., Khojasteh A. Application of selected scaffolds for bone tissue engineering: A systematic review. Oral Maxillofac. Surg. 2017;21:109–129. doi: 10.1007/s10006-017-0608-3. [DOI] [PubMed] [Google Scholar]
- 10.D’Amora U., Russo T., Gloria A., Rivieccio V., D’Ant V., Negri G., Ambrosio L., De Santis R. 3D additive-manufactured nanocomposite magnetic scaffolds: Effect of the application mode of a time-dependent magnetic field on hMSCs behavior. Bioact. Mater. 2017;2:138–145. doi: 10.1016/j.bioactmat.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferracane J.L., Giannobile W.V. Novel Biomaterials and Technologies for the Dental, Oral, and Craniofacial Structures. J. Dent. Res. 2014;93:1185–1186. doi: 10.1177/0022034514556537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Sun H., Song X., Gu X., Sun C. Biomaterials for periodontal tissue regeneration. Rev. Adv. Sci. 2015;40:209–214. [Google Scholar]
- 13.Deng M., James R., Laurencin C.T., Kumbar S.G. Nanostructured polymeric scaffolds for orthopaedic regenerative engineering. IEEE Trans. Nanobiosci. 2012;11:3–14. doi: 10.1109/TNB.2011.2179554. [DOI] [PubMed] [Google Scholar]
- 14.Laurencin C.T., Khan Y. Regenerative engineering. Sci. Transl. Med. 2012;14:160–169. doi: 10.1126/scitranslmed.3004467. [DOI] [PubMed] [Google Scholar]
- 15.Laurencin C.T., Ashe K.M., Henry N., Kan H.M., Lo K.W.H. Delivery of small molecules for bone regenerative engineering: Preclinical studies and potential clinical applications. Drug Discov. Today. 2014;19:794–800. doi: 10.1016/j.drudis.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vieira S., Vial S., Reis R.L., Oliveira J.M. Nanoparticles for Bone Tissue Engineering. Biotechnol. Prog. 2017;33:590–611. doi: 10.1002/btpr.2469. [DOI] [PubMed] [Google Scholar]
- 17.Wang H., Leeuwenburgh S.C.G., Li Y., Jansen J.A. The Use of Micro- and Nanospheres as Functional Components for Bone Tissue Regeneration. Tissue Eng. Part B. 2012;18:24–39. doi: 10.1089/ten.teb.2011.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ionescu L.C., Lee G.C., Sennett B.J., Burdick J.A., Mauck R.L. An anisotropic nanofiber/microsphere composite with controlled release of biomolecules for fibrous tissue engineering. Biomaterials. 2010;31:4113–4120. doi: 10.1016/j.biomaterials.2010.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X., Xing H., Zhang G., Wu X., Zou X., Feng L., Wang D., Li M., Zhao J., Du J., et al. Restoration of a critical mandibular bone defect using human alveolar bone-derived stem cells and porous nano-HA/Collagen/PLA Scaffold. Stem Cells Int. 2016:8741641. doi: 10.1155/2016/8741641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X., Li W. Biodegradable mesoporous bioactive glass nanospheres for drug delivery and bone tissue regeneration. Nanotechnology. 2016;27:225102. doi: 10.1088/0957-4484/27/22/225102. [DOI] [PubMed] [Google Scholar]
- 21.Mundargi R.C., Babu V.R., Rangaswamy V., Patel P., Aminabhavi T.M. Nano/micro technologies for delivering macromolecular therapeutics using poly(d,l-lactide-coglycolide) and its derivatives. J. Control. Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Xue Y., Hong X., Gao J., Shen R., Ye Z. Preparation and biological characterization of the mixture of poly(lactic-co-glycolic acid)/chitosan/Ag nanoparticles for periodontal tissue engineering. Int. J. Nanomed. 2019;14:483–498. doi: 10.2147/IJN.S184396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z., Chen X., Zhang Z., Zhang X., Saunders L., Zhou Y., Ma P.X. Nanofibrous Spongy Microspheres to Distinctly Release miRNA and Growth Factors To Enrich Regulatory T Cells and Rescue Periodontal Bone Loss. ACS Nano. 2018;12:9785–9799. doi: 10.1021/acsnano.7b08976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian Y., Zhou X., Zhang F., Diekwisch T.G.H., Luan X., Yang J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces. 2019;11:37381–37396. doi: 10.1021/acsami.9b07053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menjoge A.R., Kannan R.M., Tomalia D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today. 2010;15:171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Milhem O.M., Myles C., McKeown N.B., Attwood D., D’Emanuele A. Polyamidoamine Starburst dendrimers as solubility enhancers. Int. J. Pharm. 2000;197:239–241. doi: 10.1016/S0378-5173(99)00463-9. [DOI] [PubMed] [Google Scholar]
- 27.Gillies E.R., Frechet J.M.J. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 28.Yamamuro T. Bioceramics. In: Poitout D.G., editor. Biomechanics and Biomaterials in Orthopedics. Springer; London, UK: 2004. pp. 22–23. [DOI] [Google Scholar]
- 29.Raghavendra S.S., Jadhav G.R., Gathani K.M., Kotadia P. Bioceramics in endodontics–A review. J. Istanb. Univ. Fac. Dent. 2017;51:128–137. doi: 10.17096/jiufd.63659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pina S., Oliveira J.M., Reis R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015;27:1143–1169. doi: 10.1002/adma.201403354. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Chen Y., Xu J., Wang J., Li C., Wang L. Tissue engineering using 3D printed nano-bioactive glass loaded with NELL1 gene for repairing alveolar bone defects. Regen. Biomater. 2018;5:213–220. doi: 10.1093/rb/rby015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebrahimi M., Botelho M., Lu W., Monmaturapoj N. Synthesis and characterization of biomimetic bioceramic nanoparticleswith optimized physicochemical properties for bone tissue engineering. J. Biomed. Mater. Res. A. 2019;27 doi: 10.1002/jbm.a.36681. [DOI] [PubMed] [Google Scholar]
- 33.Liu L., Liu J., Wang M., Min S., Cai Y., Zhu L., Yao J. Preparation and characterization of nano-hydroxyapatite/silk fibroin porous scaffolds. J. Biomater. Sci. Polym. 2008;19:325–328. doi: 10.1163/156856208783721010. [DOI] [PubMed] [Google Scholar]
- 34.Manju V., Anitha A., Menon D., Iyer S., Nair S.V., Nair M.B. Nanofibrous yarn reinforced HA-gelatin composite scaffolds promote bone formation in critical sized alveolar defects in rabbit model. Biomed. Mater. 2018;13:065011. doi: 10.1088/1748-605X/aadf99. [DOI] [PubMed] [Google Scholar]
- 35.Chen P., Wu Z., Leung A., Chen X., Landao-Bassonga E., Gao J., Chen L., Zheng M., Yao F., Yang H., et al. Fabrication of a silver nanoparticle-coated collagen membrane with anti-bacterial and anti-inflammatory activities for guided bone regeneration. Biomed. Mater. 2018;13:065014. doi: 10.1088/1748-605X/aae15b. [DOI] [PubMed] [Google Scholar]
- 36.Han J., Ma B., Liu H., Wang T., Wang F., Xie C., Li M., Liu H., Ge S. Hydroxyapatite nanowires modified polylactic acid membrane plays barrier/osteoinduction dual roles and promotes bone regeneration in a rat mandible defect model. J. Biomed. Mater. Res. A. 2018;106:3099–3110. doi: 10.1002/jbm.a.36502. [DOI] [PubMed] [Google Scholar]
- 37.Chen X., Shi Z., Bai S., Li B., Huan Liu H., Guo F., Wu G., Liu S., Zhao Y. Fabrication of gelatin methacrylate/nanohydroxyapatite microgel arrays for periodontal tissue regeneration. Int. J. Nanomed. 2016;11:4707–4718. doi: 10.2147/IJN.S111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nie L., Zhang H., Ren A., Li Y., Fu G., Cannon R.D., Ji P., Wu X., Yang S. Nano-hydroxyapatite mineralized silk fibroin porous scaffold for tooth extraction site preservation. Dent. Mater. 2019;35:1397–1407. doi: 10.1016/j.dental.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Dayashankar C.P., Deepika P.C., Siddaramaiah B. Clinical and Radiographic Evaluation of Citric Acid-Based Nano Hydroxyapatite Composite Graft in the Regeneration of Intrabony Defects—A Randomized Controlled Trial. Contemp. Clin. Dent. 2017;8:380–386. doi: 10.4103/ccd.ccd_213_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaquette C., Fan W., Xiao Y., Hamlet S., Hutmacher D.W., Ivanovski S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials. 2012;33:5560–5573. doi: 10.1016/j.biomaterials.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa K., Miyaji H., Kato A., Kosen Y., Momose T., Yoshida T., Nishida E., Miyata S., Murakami S., Takita H., et al. Periodontal tissue engineering by nano beta-tricalcium phosphate scaffold and fibroblast growth factor-2 in one-wall infrabony defects of dogs. J. Periodontal. Res. 2016;51:758–767. doi: 10.1111/jre.12352. [DOI] [PubMed] [Google Scholar]
- 42.Camilleri J. Is mineral trioxide Aggregate a Bioceramic. ODOVTOS Int. J. Dent. Sci. 2016;18:13–17. doi: 10.15517/ijds.v18i1.23482. [DOI] [Google Scholar]
- 43.Saghiri M.A., Orangi J., Tanideh N., Asatourian A., Janghorban K., Garcia-Godoy F., Sheibani N. Repair of bone defect by nano-modifed white mineral trioxide aggregates in rabbit: A histopathological study. Med. Oral. Patol. Oral Cir. Bucal. 2015;20:525–531. doi: 10.4317/medoral.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipski A.M., Pino C.J., Haselton F.R., Chen I.W., Shastri V.P. The effect of silica nanoparticle-modified surfaces on cell morphology, cytoskeletal organization and function. Biomaterials. 2008;29:3836–3846. doi: 10.1016/j.biomaterials.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moller K., Kobler J., Bein T. Colloidal suspensions of nanometer-sized mesoporous silica. Adv. Funct. Mater. 2007;17:605–612. doi: 10.1002/adfm.200600578. [DOI] [Google Scholar]
- 46.Yeh Y.C., Creran B., Rotello V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale. 2012;4:1871–1880. doi: 10.1039/C1NR11188D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vigderman L., Khanal B.P., Zubarev E.R. Functional gold nanorods: Synthesis, self-assembly, and sensing applications. Adv. Mater. 2012;24:4811–4841. doi: 10.1002/adma.201201690. [DOI] [PubMed] [Google Scholar]
- 48.Takanche J.S., Kim J.E., Kim J.S., Lee M.H., Jeon J.G., Park I.S., Yi H.K. Chitosan-gold nanoparticles mediated gene delivery of c-myb facilitates osseointegration of dental implants in ovariectomized rat. Artif. Cells Nanomed. Biotechnol. 2018;46:S807–S817. doi: 10.1080/21691401.2018.1513940. [DOI] [PubMed] [Google Scholar]
- 49.Colombo M., Carregal-Romero S., Casula M.F., Gutiérrez L., Morales M.P., Böhm I.B., Heverhagen J.T., Prosperi D., Parak W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012;41:4306–4334. doi: 10.1039/c2cs15337h. [DOI] [PubMed] [Google Scholar]
- 50.Bangham A.D. Surrogate cells or Trojan horses. The discovery of liposomes. Bioessays. 1995;17:1081–1088. doi: 10.1002/bies.950171213. [DOI] [PubMed] [Google Scholar]
- 51.Immordino M.L., Dosio F., Cattel L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 52.Allen T.M., Cullis P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 53.Nicolas J., Mura S., Brambilla D., Mackiewicz N., Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013;42:1147–1235. doi: 10.1039/C2CS35265F. [DOI] [PubMed] [Google Scholar]
- 54.Dhandayuthapani B., Yoshida Y., Maekawa T., Kumar D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011:290602. doi: 10.1155/2011/290602. [DOI] [Google Scholar]
- 55.Lutolf M.P., Weber F.E., Schmoekel H.G., Schense J.C., Kohler T., Müller R., Hubbell J.A. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat. Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 56.Ye H., Zhu J., Deng D., Jin S., Li J., Man Y. Enhanced osteogenesis and angiogenesis by PCL/chitosan/Sr-doped calcium phosphate electrospun nanocomposite membrane for guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2019;30:1505–1522. doi: 10.1080/09205063.2019.1646628. [DOI] [PubMed] [Google Scholar]
- 57.Eivazzadeh-Keihan R., Maleki A., de la Guardia M., Bani M.S., Chenab K.K., Pashazadeh-Panahi P., Baradaran B., Mokhtarzadeh A., Hamblin M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019;18:185–201. doi: 10.1016/j.jare.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.La W.G., Jin M., Park S., Yoon H.H., Jeong G.J., Bhang S.H., Park H., Char K., Kim B.S. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int. J. Nanomed. 2014;9:107–116. doi: 10.2147/IJN.S50742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Q., Yang P., Li X., Liu H., Ge S. Bioactivity of periodontal ligament stem cells on sodium titanate coated with graphene oxide. Sci. Rep. 2016;6:19343. doi: 10.1038/srep19343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radunovic M., De Colli M., De Marco P., Di Nisio C., Fontana A., Piattelli A., Cataldi A., Zara S. Graphene oxide enrichment of collagen membranes improves DPSCs differentiation and controls inflammation occurrence. J. Biomed. Mater. Res. A. 2017;105:2312–2320. doi: 10.1002/jbm.a.36085. [DOI] [PubMed] [Google Scholar]
- 61.De Marco P., Zara S., De Colli M., Radunovic M., Lazovi’c V., Ettorre V., Di Crescenzo A., Piattelli A., Cataldi A., Fontana A. Graphene oxide improves the biocompatibility of collagen membranes in an in vitromodel of human primary gingival fibroblasts. Biomed. Mater. 2017;12:055005. doi: 10.1088/1748-605X/aa7907. [DOI] [PubMed] [Google Scholar]
- 62.Liao C., Li Y., Tjong S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018;19:3564. doi: 10.3390/ijms19113564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bullock C.J., Bussy C. Biocompatibility Considerations in the Design of Graphene Biomedical Materials. Adv. Mater. Interfaces. 2019;6:1900229. doi: 10.1002/admi.201900229. [DOI] [Google Scholar]
- 64.Wang K., Ruan J., Song H., Zhang J., Wo Y., Guo S., Cui D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011;6:8. doi: 10.1007/s11671-010-9751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodríguez-Lozano F.J., García-Bernal D., Aznar-Cervantes S., Ros-Roca M.A., Algueró M.C., Atucha N.M., Lozano-García A.A., Moraleda J.M., Cenis J.L. Effects of composite films of silk fibroin and graphene oxide on the proliferation, cell viability and mesenchymal phenotype of periodontal ligament stem cells. J. Mater. Sci. Mater. Med. 2014;25:2731–2741. doi: 10.1007/s10856-014-5293-2. [DOI] [PubMed] [Google Scholar]
- 66.Vera-Sánchez M., Aznar-Cervantes S., Jover E., García-Bernal D., Oñate-Sánchez R.E., Hernández-Romero D., Moraleda J.M., Collado-González M., Rodríguez-Lozano F.J., Cenis J.L. Silk-Fibroin and Graphene Oxide Composites Promote Human Periodontal Ligament Stem Cell Spontaneous Differentiation into Osteo/Cementoblast-Like Cells. Stem Cells Dev. 2016;25:1742–1754. doi: 10.1089/scd.2016.0028. [DOI] [PubMed] [Google Scholar]
- 67.Ren N., Li J., Qiu J., Yan M., Liu H., Ji D., Huang J., Yu J., Liu H. Growth and accelerated differentiation of mesenchymal stem cells on graphene-oxide-coated titanate with dexamethasone on surface of titanium implants. Dent. Mater. 2017;33:525–535. doi: 10.1016/j.dental.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 68.Kinoshita Y., Maeda H. Recent developments of functional scaffolds for craniomaxillofacial bone tissue engineering applications. Sci. World J. 2013;21:863157. doi: 10.1155/2013/863157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang Y., Ren J., Ren T., Gu S., Tan Q., Zhang L., Lv K., Pan K., Jiang X. Bone marrow stromal cells cultured on poly (lactide-co-glycolide)/nanohydroxyapatite composites with chemical immobilization of Arg-Gly-Asp peptide and preliminary bone regeneration of mandibular defect thereof. J. Biomed. Mater. Res. A. 2010;95:993–1003. doi: 10.1002/jbm.a.32922. [DOI] [PubMed] [Google Scholar]
- 70.Han X., Liu H., Wang D., Su F., Zhang Y., Zhou W., Li S., Yang R. Alveolar bone regeneration around immediate implants using an injectable nHAC/CSH loaded with autogenic blood-acquired mesenchymal progenitor cells: An experimental study in the dog mandible. Clin. Implant Dent. Relat. Res. 2013;15:390–401. doi: 10.1111/j.1708-8208.2011.00373.x. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J.C., Lu H.Y., Lv G.Y., Mo A.C., Yan Y.G., Huang C. The repair of critical-size defects with porous hydroxyapatite/polyamide nanocomposite: An experimental study in rabbit mandibles. Int. J. Oral Maxillofac. Surg. 2010;39:469–477. doi: 10.1016/j.ijom.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 72.Du B., Liu W., Deng Y., Li S., Liu X., Gao Y., Zhou L. Angiogenesis and bone regeneration of porous nano-hydroxyapatite/coralline blocks coated with rhVegF165 in critical-size alveolar bone defects in vivo. Int. J. Nanomed. 2015;10:2555–2565. doi: 10.2147/IJN.S78331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su J., Xu H., Sun J., Gong X., Zhao H. Dual delivery of BMP-2 and bFGF from a new nano-composite scaffold, loaded with vascular stents for large size mandibular defect regeneration. Int. J. Mol. Sci. 2013;14:12714–12728. doi: 10.3390/ijms140612714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao F., Yin Y., Lu W.W., Leong J.C., Zhang W., Zhang J., Zhang M., Yao K. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials. 2002;23:3227–3234. doi: 10.1016/S0142-9612(02)00077-7. [DOI] [PubMed] [Google Scholar]
- 75.Xynos I., Hukkanen M., Batten J., Buttery L., Hench L., Polak J. Bioglass® 45S5 stimulates osteoblast turnover and enhances bone formation in vitro: Implications and applications for bone tissue engineering. Calcif. Tissue Int. 2000;67:321–329. doi: 10.1007/s002230001134. [DOI] [PubMed] [Google Scholar]
- 76.Kim H.D., Valentini R.F. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J. Biomed. Mater. Res. 2002;59:573–584. doi: 10.1002/jbm.10011. [DOI] [PubMed] [Google Scholar]
- 77.El-Ghannam A.R. Advanced bioceramic composite for bone tissue engineering: Design principles and structure—Bioactivity relationship. J. Biomed. Mater. Res. Part A. 2004;69:490–501. doi: 10.1002/jbm.a.30022. [DOI] [PubMed] [Google Scholar]
- 78.Iviglia G., Saeid Kargozar S., Baino F. Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. J. Funct. Biomater. 2019;10:3. doi: 10.3390/jfb10010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li G., Zhang T., Li M., Fu N., Fu Y., Ba K., Deng S., Jiang Y., Hu J., Peng Q., et al. Electrospun fibers for dental and craniofacial applications. Curr. Stem Cell Res. 2014;9:187–195. doi: 10.2174/1574888X09666140213151717. [DOI] [PubMed] [Google Scholar]
- 80.Gmeiner R., Deisinger U., Schönherr J., Lechner B., Detsch R., Boccaccini A., Stampfl J. Additive manufacturing of bioactive glasses and silicate bioceramics. J. Ceram. Sci. Technol. 2015;6:75–86. [Google Scholar]
- 81.Kosachan N., Jaroenworaluck A., Jiemsirilers S., Jinawath S., Stevens R. Hydroxyapatite nanoparticles formed under a wet mechanochemical method. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017;105:679–688. doi: 10.1002/jbm.b.33590. [DOI] [PubMed] [Google Scholar]
- 82.Ivanovski S., Vaquette C., Gronthos S., Hutmacher D.W., Bartold P.M. Multiphasic Scaffolds for Periodontal Tissue Engineering. J. Dent. Res. 2014;93:1212–1221. doi: 10.1177/0022034514544301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carter S.D., Costa P.F., Vaquette C., ivanovski S., Hutmacher D.W., Malda J. Additive Biomanufacturing: An Advanced Approach for Periodontal Tissue Regeneration. Ann. Biomed. Eng. 2017;45:12–22. doi: 10.1007/s10439-016-1687-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carlo-Reis E.C., Borges A.P., Araújo M.V., Mendes V.C., Guan L., Davies J.E. Periodontal regeneration using a bilayered PLGA/calcium phosphate construct. Biomaterials. 2011;32:9244–9253. doi: 10.1016/j.biomaterials.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 85.Requicha J.F., Viegas C.A., Muñoz F., Azevedo J.M., Leonor I.B., Reis R.L., Gomes M.E. A tissue engineering approach for periodontal regeneration based on a biodegradable double-layer scaffold and adipose-derived stem cells. Tissue Eng. Part A. 2014;20:2483–2492. doi: 10.1089/ten.tea.2013.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park C.H., Rios H.F., Jin Q., Bland M.E., Flanagan C.L., Hollister S.J., Giannobile W.V. Biomimetic hybrid scaffolds for engineering human tooth ligament interfaces. Biomaterials. 2010;31:5945–5952. doi: 10.1016/j.biomaterials.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park C.H., Rios H.F., Jin Q., Sugai J.V., Padial-Molina M., Taut A.D., Flanagan C.L., Hollister S.J., Giannobile W.V. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials. 2012;33:137–145. doi: 10.1016/j.biomaterials.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vaquette C., Cooper-White J. A simple method for fabricating 3-D multilayered composite scaffolds. Acta Biomater. 2013;9:4599–4608. doi: 10.1016/j.actbio.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 89.Costa P.F., Vaquette C., Zhang Q., Reis R.L., Ivanovski S., Hutmacher D.W. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 2014;41:283–294. doi: 10.1111/jcpe.12214. [DOI] [PubMed] [Google Scholar]
- 90.Lee C.H., Hajibandeh J., Suzuki T., Fan A., Shang P., Mao J.J. Three dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng. Part A. 2014;20:1342–1351. doi: 10.1089/ten.tea.2013.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 92.Dababneh A.B., Ozbolat I.T. Bioprinting technology: A current state-of-the-art review. J. Manuf. Sci. Eng. 2014;136:061016. doi: 10.1115/1.4028512. [DOI] [Google Scholar]
- 93.Berthiaume F., Maguire T.J., Yarmush M.L. Tissue engineering and regenerative medicine: History, progress, and challenges. Annu. Rev. Chem. Biomol. Eng. 2011;2:403–430. doi: 10.1146/annurev-chembioeng-061010-114257. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Y.S., Yue K., Aleman J., Mollazadeh-Moghaddam K., Bakht S.M., Yang J., Weitao J.A., Dell’Erba V., Assawes P., Shin S.R., et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2017;45:148–163. doi: 10.1007/s10439-016-1612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yun H.M., Ahn S.J., Park K.R., Kim M.J., Kim J.J., Jin G.Z., Kim H.W., Ahn S.J., Kim H.W., Kim E.C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials. 2016;85:88–98. doi: 10.1016/j.biomaterials.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 96.Sculean A., Nikolidakis D., Nikou G., Ivanovic A., Chapple I.L., Stavropoulos A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontology 2000. 2015;68:182–216. doi: 10.1111/prd.12086. [DOI] [PubMed] [Google Scholar]
- 97.Kargozar S., Mozafari M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018;5:15492–15500. doi: 10.1016/j.matpr.2018.04.155. [DOI] [Google Scholar]
- 98.Neel E.A.A., Chrzanowski W., Salih V.M., Kim H.W., Knowles J.C. Review Tissue engineering in dentistry. J. Dent. 2014;42:915–928. doi: 10.1016/j.jdent.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y., Miron R.J., Li S., Shi B., Sculean A., Cheng X. Novel Meso Porous BioGlass/silk scaffold containing ad PDGF-B and ad BMP 7 for the repair of periodontal defects in beagle dogs. J. Clin. Periodontol. 2015;42:262–271. doi: 10.1111/jcpe.12364. [DOI] [PubMed] [Google Scholar]
- 100.Yue K., Trujillo-de Santiago G., Alvarez M.M., Tamayol A., Annabi N., Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen X., Liu Y., Miao L., Wang Y., Ren S., Yang X., Hu Y., Sun W. Controlled release of recombinant human cementum protein 1 from electrospun multiphasic scaffold for cementum regeneration. Int. J. Nanomed. 2016;11:3145–3158. doi: 10.2147/IJN.S104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guazzo R., Gardin C., Bellin G., Sbricoli L., Ferroni L., Ludovichetti F.S., Piattelli A., Antoniac I., Bressan E., Zavan B. Graphene-Based Nanomaterials for Tissue Engineering in the Dental Field. Nanomaterials. 2018;8:349. doi: 10.3390/nano8050349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Y., Song J., Shia B., Wanga Y., Chena X., Huanga C., Yanga X., Xua D., Cheng X., Chen X. Combination of scaffold and adenovirus vectors expressing bone morphogenetic protein-7 for alveolar bone regeneration at implant defects. Biomaterials. 2007;28:4635–4642. doi: 10.1016/j.biomaterials.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 104.Rasperini G., Pilipchuk S.P., Flanagan C.L., Park C.H., Pagni G., Hollister S.J., Giannobile W.V. 3D-printed bioresorbable scaffold for periodontal repair. J. Dent. Res. 2015;94:153–157. doi: 10.1177/0022034515588303. [DOI] [PubMed] [Google Scholar]
- 105.Feng X., Chen A., Zhang Y., Wang J., Shao L., Wei L. Application of dental nanomaterials: Potential toxicity to the central nervous system. Int. J. Nanomed. 2015;10:3547–3565. doi: 10.2147/IJN.S79892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee C.M., Jeong H.J., Yun K.N., Kim D.W., Sohn M.H., Lee J.K., Jeong J., Lim S.T. Optical imaging to trace near infrared fluorescent zinc oxide nanoparticles following oral exposure. Int. J. Nanomed. 2012;7:3203–3209. doi: 10.2147/IJN.S32828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y., Chen Z., Ba T., Pu J., Chen T., Song Y., Gu Y., Qian Q., Xu Y., Xiang K., et al. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small. 2013;9:1742–1752. doi: 10.1002/smll.201201185. [DOI] [PubMed] [Google Scholar]
- 108.Fernandez-Urrusuno R., Fattal E., Rodrigues J.M., Jr., Féger J., Bedossa P., Couvreur P. Effect of polymeric nanoparticle administration on the clearance activity of the mononuclear phagocyte system in mice. J. Biomed. Mater. Res. 1996;31:401–408. doi: 10.1002/(SICI)1097-4636(199607)31:3<401::AID-JBM15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 109.Karmakar A., Zhang Q., Zhang Y. Neurotoxicity of nanoscale materials. J. Food Drug Anal. 2014;22:147–160. doi: 10.1016/j.jfda.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma L., Liu J., Li N., Wang J., Duan Y., Yan J., Liu H., Wang H., Hong F. Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO2 delivered to the abdominal cavity. Biomaterials. 2010;31:99–105. doi: 10.1016/j.biomaterials.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 111.Adamcakova-Dodd A., Stebounova L.V., Kim J.S., Vorrink S.U., Ault A.P., O’Shaughnessy P.T., Grassian V.H., Thorne P.S. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibretoxicol. 2014;1:11–15. doi: 10.1186/1743-8977-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xie H., Chua M., Islam I., Bentini R., Cao T., Viana-Gomes J.C., Castro Neto A.H., Rosa V. CVD-grown monolayer graphene induces osteogenic but not odontoblastic differentiation of dental pulp stem cells. Dent. Mater. 2017;33:e13–e21. doi: 10.1016/j.dental.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 113.Launey M.E., Munch E., Alsem D.H., Barth H.B., Saiz E., Tomsia A.P., Ritchie R.O. Designing highly toughened hybrid composites through nature-inspired hierarchical complexity. Acta Mater. 2009;57:2919–2932. doi: 10.1016/j.actamat.2009.03.003. [DOI] [Google Scholar]
- 114.Dzenis Y. Materials science—Structural nanocomposites. Science. 2008;319:419–420. doi: 10.1126/science.1151434. [DOI] [PubMed] [Google Scholar]
- 115.Ritchie R.O. The quest for stronger, tougher materials. Science. 2008;320:448. doi: 10.1126/science.320.5875.448a. [DOI] [PubMed] [Google Scholar]
- 116.Evans A.G. Perspective on the development of high-toughness ceramics. J. Am. Ceram Soc. 1990;73:187–206. doi: 10.1111/j.1151-2916.1990.tb06493.x. [DOI] [Google Scholar]