Abstract
Innate lymphoid cells (ILCs) are enriched at barriers surfaces of the mammalian body, rapidly respond to host- or microbial-derived stimuli, and become dysregulated in multiple human diseases. Over the past decade, substantial advances have been made in identifying the heterogeneity and functional diversity of ILC subsets, much of which has revealed striking similarities to T cell subsets. However, emerging evidence indicates that ILCs also have a complex role in directly influencing the adaptive immune response in the context of development, homeostasis, infection or inflammation. Adaptive immunity also reciprocally regulates ILCs, indicating that these interactions are a crucial determinant of immune responses within tissues. Here, we summarize our current understanding of functional interactions between ILCs and the adaptive immune system, discuss limitations and future areas of investigation, and consider the potential for these interactions to be therapeutically harnessed to benefit human health.
Introduction
The past decade has seen an explosion of research into an emerging arm of the innate immune system, collectively termed the innate lymphoid cell (ILC) family1,2. These studies have defined ILCs as important regulators of immunity, inflammation and barrier homeostasis through their rapid production of effector cytokines in response to tissue-derived signals, alarmins [G], environmental cues or neuronal mediators1,2. ILCs are broadly grouped into subsets based on their transcription factor expression and cytokine production (Box 1 and reviewed extensively elsewhere1,2). These ILC subsets have unique developmental, phenotypic and functional characteristics (Box 1).
Box 1 ∣. The innate lymphoid cell family.
Group 1 ILCs
Group 1 innate lymphoid cells (ILC1s) include both classical natural killer (NK) cells and ILC1s that express the transcription factor T-bet and produce the cytokines IFNγ and TNF to mediate immunity against intracellular pathogens. NK cells are distinguished by co-expression of eomesodermin (Eomes). Dysregulated ILC1 responses have been implicated in the pathogenesis of inflammatory bowel disease (IBD) and rheumatoid arthritis.
Group 2 ILCs
Group 2 ILCs (ILC2s) express high levels of GATA3 and produce the cytokines IL-4, IL-5, IL-9, IL-13 and amphiregulin in response to large multicellular helminth pathogens or protozoa. These include both inflammatory and natural ILC2 subgroups that exhibit some phenotype heterogeneity. Dysregulated ILC2 responses can drive allergic disease in the context of asthma and atopic dermatitis.
Group 3 ILCs
Group 3 ILCs (ILC3s) express RORγt and produce IL-17A and IL-22 in response to extracellular microorganisms, both commensal and pathogenic. ILC3 are heterogeneous and include T-bet+ ILC3 that express natural cytotoxicity receptors, CCR6+ ILC3 that are also known as lymphoid tissue inducer (LTi)-like cells, and ex-ILC3 that have lost RORγt expression and resemble ILC1. As with other ILC family members, inappropriate ILC3 responses have been implicated in chronic inflammatory disorders, including IBD and multiple sclerosis.
ILC subsets closely mirror the transcriptional and functional biology of both cytotoxic CD8+ T cells and CD4+ T helper (TH) cell subsets. However, unlike cells of the adaptive immune system, ILCs can colonize lymphoid and barrier tissue sites during fetal development, do not undergo somatic recombination, and lack antigen-specific receptors. Furthermore, ILCs are transcriptionally, epigenetically and functionally poised to rapidly mediate specialized functions in response to subset-specific danger signals1,2. In order to distinguish and dissect the contributions of ILC-derived cytokines from that of T helper cell subsets, many initial studies necessarily employed mice deficient in adaptive immunity, such as Rag1−/− mice. Although these experiments were a crucial step forward in our understanding of fundamental ILC biology, the approaches came with substantial caveats, including the inability to study ILCs in the context of adaptive immunity or appreciate the potential interplay between these innate and adaptive lymphocytes.
Analyses of defined patient populations and the development of new mouse models to more selectively target ILCs have indicated a degree of functional redundancy between ILC responses and adaptive immunity during infection3-6. For example, although ILCs represent an important source of cytokines during the initial stages of an infection, this function can be rendered dispensable following the establishment of an adaptive immune response, where Tcell subsets that can outnumber ILCs and produce identical cytokines in the tissue3-6. This redundancy has led to substantial questions regarding the shared biology and evolutionary relationships between these cell types. Indeed, ILCs are conserved among humans, rodents and zebrafish2,7,8, and associated cytokines or core transcriptional machinery are found in distant ancestors such as bony fish and lampreys7. This suggests that retaining ILC-like cells has conferred a distinct evolutionary advantage even in the presence of an adaptive immune system. Therefore, it is tempting to speculate that ILCs have additional and/or unique non-redundant functions beyond those shared with T cells. In support of this, recent studies have highlighted that ILCs express multiple factors that functionally modulate adaptive immunity, which together shape the magnitude and quality of an immune response. In this Review, we focus on substantial evidence that implicates ILC family members as previously unappreciated regulators of the adaptive immune system in the context of health and disease.
Anatomical distribution of ILCs
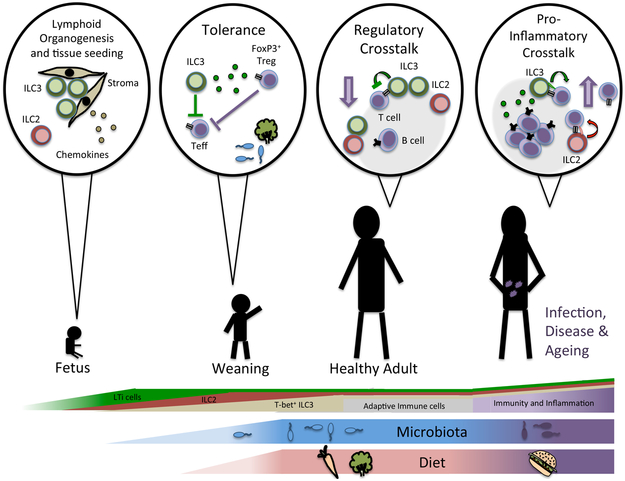
There are unique spatial and temporal interactions that occur between ILCs and adaptive immune populations throughout a lifetime. These include: (i) fetal periods where ILCs promote secondary lymphoid organogenesis and seed unique tissue niches, (ii) post-natal periods where ILCs undergo expansion and interact with adaptive immunity during initial microbial exposure, and (iii) stages of health and disease where communication between ILCs and adaptive immunity may critically control the outcome of immune homeostasis, infection, inflammation or aging (Figure 1). Defining the anatomical distribution of ILCs throughout these stages has allowed for a better understanding of their functional interactions with adaptive immune cells.
Figure 1 ∣. A dialogue between innate and adaptive lymphocytes throughout a lifetime.
LTi cells promote secondary lymphoid organogenesis prior to birth, and along with ILC2, seed multiple lymphoid or barrier tissues during fetal development and neonatal periods. Following birth, ILC2 expand within tissues and T-bet+ ILC3s expand in the intestine as a result of colonization with microbiota and exposure to other environmental stimuli. It is during this period and throughout stages of homeostasis that ILCs interact with adaptive immunity to promote tissue homeostasis. This occurs primarily through bi-directional interactions, and includes direct or indirect modulation of multiple facets of innate and adaptive immunity. Conversely, in the context of aging, infection, inflammation or immunodeficiency, ILCs and adaptive immunity engage in a pro-inflammatory Interaction that is a crucial determinant of the immune response.
Lymphoid tissues.
One of the earliest identified ILC subsets was characterized as a CD4+CD3− hematopoietic cell that seeded sites of secondary lymphoid tissue organogenesis during fetal development9,10. These populations express integrin α4β7 and lymphotoxin (LT), and are now defined as bona fide lymphoid tissue-inducer cells [G] (LTi cells) owing to their essential role in promoting secondary lymphoid tissue organogenesis11,12. The development of LTi cells requires the transcription factor RORγt13, resulting in their assignment to the ILC3 subset. Furthermore, LTi cells persist after birth and promote tertiary lymphoid structures [G] in the gut, such as cryptopatches and isolated lymphoid follicles (ILFs), which mature in response to microbiota colonization14-16. In general, LTi cells found in adult mice are termed LTi-like, express high levels of CCR6, and are heterogeneous in their expression of CD4. However, fundamental questions remain regarding the longevity, lineage relationships and differential functions of LTi-like cells in adult mammals, which are hampered by a lack of specific genetic tools.
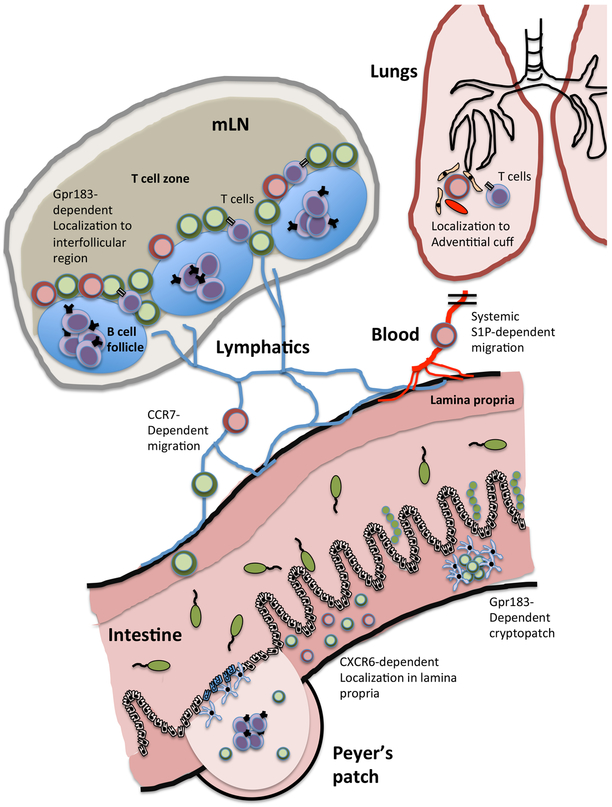
LTi-like cells are found following birth predominantly within organized lymphoid structures including draining lymph nodes, Peyer’s patches and tertiary lymphoid structures17-20. ILC2s are found in these tissues and fat-associated lymphoid clusters21,22. A majority of ILC2 in these sites and others discussed below are seeded during fetal development or neonatal periods, and acquire tissue-specific transcriptional signatures. There is variable replacement of these ILC2 across tissues with age, and rapid in situ expansion upon infectious or inflammatory challenge23 (Figure 1). Within lymph nodes, both LTi-like ILC3s and ILC2s selectively localize at inter-follicular regions (Figure 2)19. These sites surround B cell follicles at the key entry points for the afferent lymphatics, and are also the primary location where interactions between T cells and B cells are initiated. Thus, this localization pattern suggests that ILCs directly encounter recently migrated lymphocytes from the tissues, and influence T cell–B cell interactions or the initiation of humoral immune responses.
Figure 2 ∣. Anatomical distribution of ILCs and their interface with adaptive immunity.
The ability of ILCs to interact with adaptive immune cells and modulate their responses is highly dependent upon co-localization of ILCs within tissues and lymphoid structures. This is best characterized for ILC2s (red) and ILC3s (green), which are constitutively found within both mucosal barrier tissues and associated lymphoid tissues. Within the intestinal tissue, ILCs are largely found either localized within lymphoid structures such as the Peyer’s patches or cryptopatches of the small intestine or within the lamina propria. Localization of LTi-like ILC3s to cryptopatches is controlled through sensing of stromal derived cues via receptors such as GPR183, whereas T-bet+ ILC3s localization within the intestinal lamina propria is mediated via CXCR6-dependent homing. Increasing evidence suggests that subsets of ILCs (specifically, inflammatory ILC2s) can migrate out of tissues via blood vessels in an S1P-dependent manner in the context of infection and inflammation and establish residence in peripheral organs, including the lungs. Within such peripheral tissues, ILC2s are further found localized within “adventitial cuffs”, tissue niches formed around airways and blood vessels that are further characterized by stromal populations expressing ILC2-activating cytokines in multiple tissues. Additionally, ILCs can migrate in the lymphatics to draining lymph nodes, such as the intestinal-associated mesenteric lymph node (MLN) chain, in a CCR7-dependent manner and co-localize at the inter-follicular border between the B cell follicle and T-cell zone. ILCs interact with many types of T cells, inlcuding TFH cells, Treg cells, TH1 cells, TH2 cells and TH17 cells, which is described in greater detail in Figures 3-5.
A detailed understanding of the molecular machinery required for ILCs to localize to this lymphoid niche is still lacking. LTi cells and adult LTi-like ILC3s express CXCR5, which is required for the generation of Peyer’s patches and ILFs, but does not influence the localization of LTi-like ILC3s at the inter-follicular border in established lymph nodes24,25. In contrast, GPR183 (also known as EBI2, a receptor for oxysterols) is required for the localization of LTi-like ILC3s both within intestinal lymphoid structures in the large intestine and at the inter-follicular border of the mesenteric lymph node (MLN)17,18,25. Notably, the localization of LTi-like ILC3s in cryptopatches in the large intestine is mediated through gradients of the cholesterol ligand 7α,25-OHC, and maintained through spatial segregation of stromal cell populations expressing either the 7α,25-OHC-generating enzyme Ch25h, or the degrading enzyme Cyp7b118. This lymphoid niche for ILC3 may also be established prior to birth, as fetal stromal organizer cells have been found to give rise to adult marginal reticular cells and persist throughout life in close association with ILC326. In addition, LTi-like ILC3s are commonly characterized by expression of CCR6, and interactions between CCR6 and CCL20 are important for the formation of tertiary lymphoid structures in the small intestine, but not the colon14,27.
Barrier tissues.
Beyond lymphoid structures, ILCs are enriched in barrier tissues of the mammalian body, such as the gastrointestinal tract, airways and skin (Figure 2). T-bet+ ILC3s are dominantly found within the lamina propria of the intestine following birth and expand following colonization with the microbiota (Figure 1). This localization pattern is controlled in part through expression of CXCR628. ILC2 and ILC3 subsets are found throughout the different layers of the skin, with some LTi-like ILC3s localizing to hair follicles in a CCR6-dependent manner29. ILC2 populations in the lungs expand after birth, are found at branch points in the airway, and co-localize with regulatory T (Treg) cells, dendritic cells (DCs), and specialized stromal cells producing activating cytokines (such as IL-33 or TSLP)30. This is a conserved localization pattern for ILC2s, which also includes proximity to blood or lymphatic vessels in multiple tissues, including the skin, adipose tissue, liver, kidney, pancreas and intestine31-34.
During homeostasis, most ILCs in mice are tissue resident, absent from the circulation, and variably replaced from bone marrow hematopoietic progenitors35,36. However, several recent studies suggest that ILCs can migrate through lymphatics during homeostasis or enter the circulation during infection and inflammation (Figure 2). For example, both ILC2s and LTi-like ILC3s migrate from the intestine to the draining lymph node under steady-state conditions, as shown in photo-convertible mouse studies, and the LTi-like ILC3 migration is dependent upon expression of CCR719. Conversely, a switch from lymph node to gut homing receptors on ILCs can occur in the mucosal draining lymph nodes, mediated in part by retinoic acid (RA) and DCs37. Furthermore, following nematode infection a unique set of IL-25-elicited inflammatory ILC2s enter the circulation and migrate from the intestine to the airways in an S1P-dependent manner38,39. It is important to note that substantial differences may exist between humans and mice, as circulating ILC progenitors are constitutively present in human blood and are thought to differentiate into mature ILCs within tissues40.
Regulation of adaptive immunity by ILCs
While extensive research over the last decade has focused on the role of ILCs as rapid innate sources of effector cytokines during infection or inflammation, it is increasingly appreciated that ILCs also play important roles in bridging innate and adaptive immune responses. Occurring through a variety of indirect and direct interactions, ILCs functionally impact the quality and magnitude of adaptive immune responses within complex tissue microenvironments.
Indirect regulation through bystander cells.
ILCs markedly influence adaptive immunity through several discrete pathways. As highlighted above, LTi cells promote lymphoid tissue organogenesis through their production of LT. Following birth, this axis maintains homeostatic responses against the microbiota at mucosal barrier surfaces by supporting IgA production in gut-associated lymphoid tissues. This occurs through the provision of surface LTα1β2 heterotrimers by ILC3s, which activates DCs located in the subepithelial dome of Peyer’s patches or the lamina propria to express inducible nitric oxide synthase, subsequently support T cell-independent class production of IgA41,42. In addition, ILC3s produce soluble LTα3 that activates stromal cells to support T cell homing to the intestine and subsequent CD40L-dependent IgA responses41,43. In the spleen, production of LT and GM-CSF by ILC3s indirectly promotes marginal zone B cell responses by controlling stromal cell and neutrophil homeostasis, respectively44. Finally, following the destruction of stromal cell populations infected with lymphocytic choriomeningitis virus, ILC3 expression of LTα1β2 supports the restoration of secondary lymphoid tissues, and this is crucial to enable adaptive immune responses to secondary infections45.
Beyond LT, other canonical ILC-derived factors indirectly influence adaptive immune cells (Figure 3a). For example, in the context of allergen-induced airway inflammation, ILC2-derived IL-13 promotes the migration of lung-associated DCs to the draining lymph node to subsequently facilitate priming of naïve CD4+ T cells into T helper 2 (TH2) cells46. This is not limited to initial allergen exposure, as ILC2-derived IL-13 also promotes airway DCs to produce CCL17, which drives memory T cell migration to the airways during secondary challenges with allergens47. A similar paradigm may exist for NK cells or ILC1s, which provide a rapid source of IFNγ following exposure to intracellular pathogens48,49, although the mechanisms of action are less well characterized and it remains unclear whether IFNγ acts directly on CD4+ T cells or indirectly on associated myeloid cells (Figure 3b). In the context of autoimmunity, a T-bet+ ILC population orchestrates T cell entry into the central nervous system through the production of pro-inflammatory cytokines, chemokines and matrix metalloproteinases, collectively facilitating neuro-inflammation50. However it remains unclear whether this ILC population represents T-bet+ NK cells, ILC1s or ILC3s.
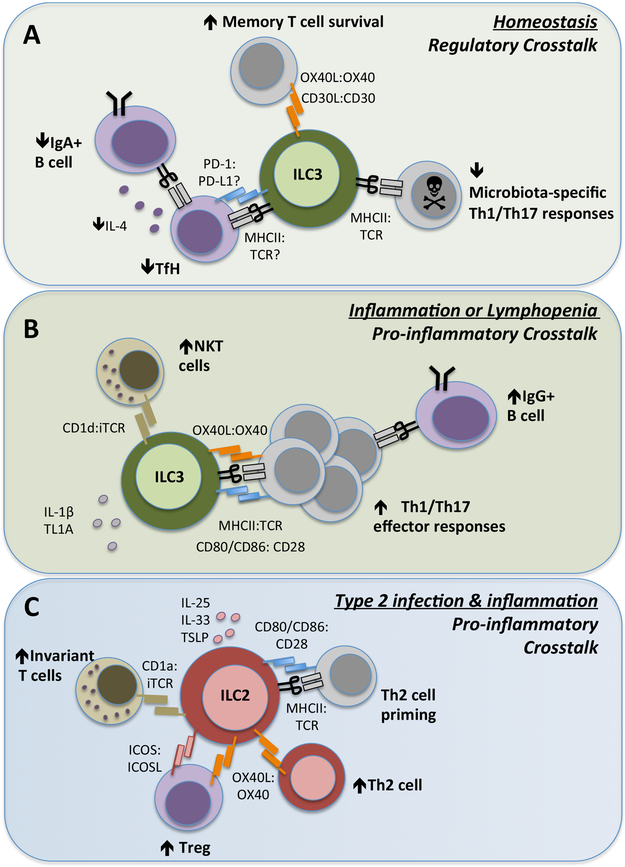
Figure 3 ∣. ILCs modulate adaptive immunity by producing soluble mediators with direct or indirect effects.
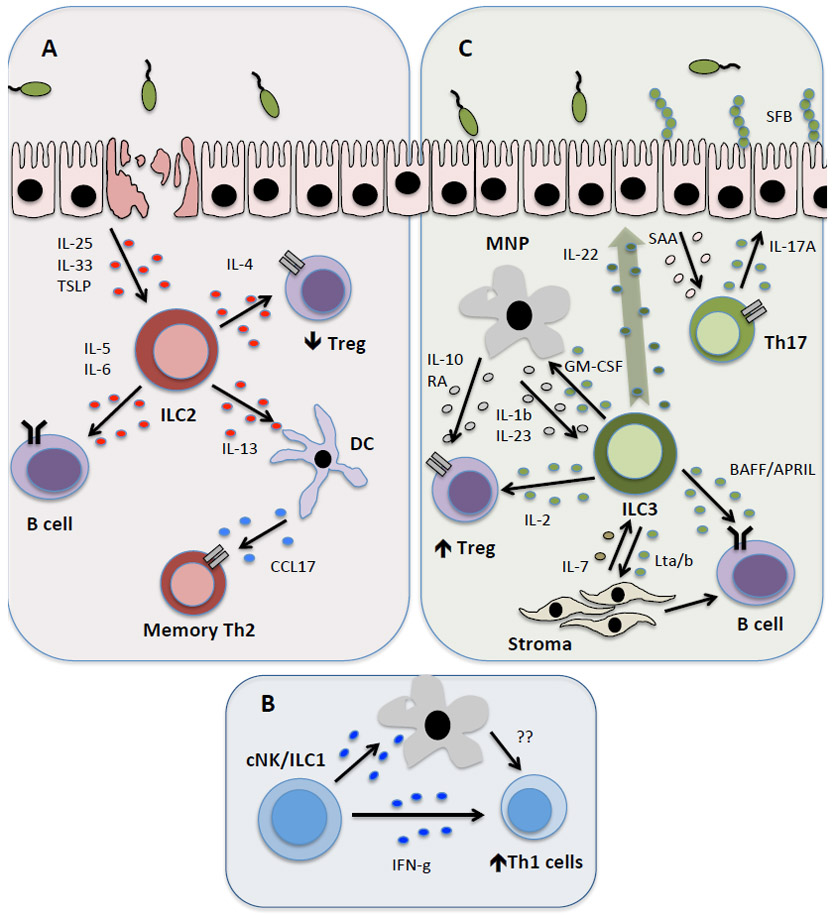
ILCs modulate adaptive immune responses through the production of soluble effector molecules that exert their effects either directly or through the activation of bystander cells such as epithelial cells and myeloid cells (dendritic cells (DCs) and mononuclear phagocytes (MNPs)) in the tissue. a) ILC2s are predominantly activated within tissues following production of cytokines, including IL-25, IL-33 and TSLP. Subsequently, ILC2 provide direct help for B cells within the tissue through production of IL-5 and IL-6. Conversely, IL-13 produced by ILC2s induces DC migration to the draining lymph node and production of the chemokine CCL17, which subsequently functions to rapidly recruit memory TH2 cells to the airways in the context of airway inflammation or infection. b) NK cells and ILC1 may also have roles in modulating T cells responses, in particular Th1 responses, through production of IFN-γ. This may occur directly or indirectly via myeloid cell populations. c) Similarly, ILC3s in the intestinal tract and lymphoid tissues produce lymphotoxin to support B cell class switching and IgA production. ILC3 in the spleen express the B cell-survival factors (BAFF/APRIL), but this has not yet been reported at mucosal sites. Additionally, multiple reports implicate a complex communication between ILC3s, MNPs and Treg cells in the intestine, which together reinforces tolerance and tissue homostasis. This is achieved either directly through ILC3-derived IL-2 secretion, which provides crucial survival signals to Treg cells, or indirectly via GM-CSF. The latter pathway subsequently results in a feedback loop whereby MNPs respond by producing IL-10 and RA to further reinforce Treg cell conversion and maintenance. ILC3 production of IL-22 has also been linked to induction of intestinal epithelial cell-derived SAA, which supports Th17 cell responses. Th17 cell production of IL-17A induces antimicrobial responses from intestinal epithelial cells and controls the composition of the microbiota, including limiting colonization with segmented filamentous bacteria (SFB).
ILC3-derived cytokines also augment adaptive immunity through several distinct mechanisms (Figure 3c). Production of IL-22 can have wide-ranging tissue-protective, anti-microbial and metabolic outcomes51. This pathway is engaged early following extracellular pathogen encounter and is necessary to promote intestinal immunity prior to the initiation of an adaptive immune response51-53. ILC3-derived IL-22 is also induced following exposure to the commensal microbiota and is necessary to modulate intestinal barrier function, control the composition of the microbiota and influence systemic metabolism1,2,51,54-58. The effects of this pathway on adaptive immunity are indirect and diverse, for example ILC3-derived IL-22 promotes acute phase responses from intestinal epithelial cells to facilitate serum amyloid A (SAA)-dependent induction of microbiota-specific TH17 cells in the small intestine (Figure 3c)59. Conversely other studies suggest that ILC3-derived IL-22 limits immune activation or tissue damage following encounter with pathogenic or commensal microorganisms, subsequently preventing activation of local T cells51,57,60. It remains possible that these diverse outcomes are context dependent and may need to be revisited as more selective approaches emerge to target ILC3s in the presence of adaptive immunity.
Another important aspect of intestinal barrier homeostasis is the ability to induce and maintain tolerance to the microbiota or dietary antigens. This is achieved in part by the induction and maintenance of peripheral Treg cell populations, which promote an immunosuppressive environment in the gut. An increasing body of evidence suggests that tissue-resident ILC3s have crucial roles in orchestrating direct and indirect communication with Treg cells (Figure 3c). For example, it was shown that microbiota-sensing macrophages produce IL-1β and induce GM-CSF expression by ILC3s61. ILC3-derived GM-CSF subsequently promotes IL-10 and RA production by intestinal macrophages to support local Treg cell conversion and maintain a tolerogenic environment in the large intestine61. Of note, GM-CSF produced by cells resembling ILC2s has also been implicated in the amplification of TH17 cell-mediated joint inflammation, implicating a role for GM-CSF production by multiple ILC subsets62. Collectively, these results highlight how ILCs orchestrate multiple other innate, epithelial and stromal cell compartments to shape adaptive immune responses throughout the mammalian body.
Direct regulation through soluble mediators.
In addition to indirect interactions mediated through bystander cells, ILCs produce multiple soluble factors that directly influence adaptive immunity in the context of health and disease (Figure 3c). For example, we recently defined that ILC3s are a dominant source of IL-2 that is induced by macrophage-derived IL-1β following MYD88- and NOD2-dependent sensing of the microbiota63. ILC3-derived IL-2 supports the population size of peripherally induced Treg cells in the small intestine and is essential to maintain tolerance to dietary antigens63. ILC2s also produce several soluble factors that can directly modulate CD4+ T cell responses (Figure 3a). Limited evidence suggests that ILC2-derived IL-4 is important for promoting TH2 cell differentiation or inhibiting Treg cell responses in the context of intestinal parasite infections or food allergy, respectively64,65. Furthermore, ILC2s can also produce IL-2 during homeostasis or following allergen challenge, and IL-2 from both innate and adaptive sources is a crucial co-factor in the activation of ILC2s20,66. ILCs may also directly modulate humoral responses via cytokine-mediated effects on B cells. For example, ILC2s also produce IL-5 and IL-6, which may support the self-renewal of B1 cells or enhance production of IgM and IgA21,22. Similarly, ILC3 production of the human B cell survival factor BAFF supports marginal zone B cell responses in the spleen44. Together, these findings suggest that in addition to their primary roles in producing effector cytokines during infection or inflammation, ILCs also directly modulate adaptive immunity through the release of soluble factors in the local tissue milieu.
Contact-dependent interactions
A key conceptual advance in ILC biology was the discovery that these populations have the potential to function as antigen-presenting cells and directly modulate adaptive immune responses (Figure 4). Early studies identified that fetal LTi cells express MHC class II molecules9. Renewed interest in the ILC family over the past decade led to further investigation of the potential antigen-presenting functions of ILC subsets.
Figure 4 ∣. ILCs control adaptive immunity through direct cellular interactions.
ILC subsets, in particular ILC2s and ILC3s, express a range of surface molecules that facilitate direct cell–cell interactions and modulation of T cell responses in health and disease. a) ILC3s constitutively express MHC class II molecules in intestinal-draining lymphoid tissues and the colon at homeostasis. In this context, ILC3s lack surface co-stimulatory molecules and present antigens to microbiota-specific CD4+ T cells, resulting in the induction of cell death. Similarly, interactions with TFH cells within the mesenteric lymph node limits TFH cell-derived IL-4 and subsequent class-switching of mucosal B cells to produce IgA. ILC3 also express PD-L1, which may interact with PD-1 on TFH cells, but this has yet to be extensively explored. ILC3 expression of non-canonical co-stimulatory molecules such as OX40L and CD30L can support memory T cell responses, although it is unknown whether this also requires MHC class II-dependent antigen presentation. b) By contrast, in the context of a maladapted immune system, such as in lymphopenia or chronic inflammation, the antigen-presenting function of ILC3s can change markedly. In response to pro-inflammatory cytokines such as IL-1β and TL1A, ILC3s upregulate expression of co-stimulatory molecules including CD80, CD86 and OX40L and promote inflammatory T cell effector responses. c) Upon activation by IL-25 and/or IL-33 (derived from epithelial cells, stromal cells and myeloid cells), ILC2s upregulate expression of MHC class II and co-stimulatory molecules such as CD80 and CD86, which facilitate direct interactions with naïve T cells and promote priming of TH2 cells in the context of allergic airway inflammation or infection. In addition, ILC2 expression of OX40L promotes the proliferation and effector responses of TH2 cells and further supports expansion of OX40+ Treg cells. ILC2s further enhance Treg cell responses through provision of surface ICOS, which binds ICOSL expressed on Treg cells.
Antigen presentation by ILCs.
At steady state, LTi-like ILC3s in the MLN and large intestine constitutively express MHC class II molecules at levels comparable with other professional antigen-presenting cells, and can acquire, process and present antigens67. However, in contrast to DCs, MHC class II+ ILC3s in the healthy intestine lack classical co-stimulatory molecules such as CD40, CD80 and CD86 and are unable to induce naïve T cell proliferation67. Rather, through a series of in vitro and in vivo studies, LTi-like ILC3s were found to suppress effector CD4+ T cell responses in the gut (Figure 4a). Consistent with this, mice with a deletion of ILC3-specific MHC class II resulted in dysregulated TH1 cell and TH17 cell responses against the commensal microbiota and spontaneous inflammation in the large intestine67-69. These studies were a fundamental advance in defining LTi-like ILC3s as a novel antigen-presenting cell type, and defined a novel selection pathway that regulates microbiota-specific CD4+ T cells in the intestine67,68. In addition, these results provoked many fundamental questions regarding the mechanisms by which ILCs acquire antigens, whether ILCs influence other types of T cell responses (such as Treg cells, TH2 cells or TH1 cells) through antigen-presentation, and the role of antigen-presenting ILCs during infection or inflammation. Furthermore, one recent study described a MHC class II+ ILC3-like population in peripheral lymph nodes that expresses the autoimmune regulator gene (AIRE)70, although it remains unclear whether this will permit ILC3-mediated regulation of self-specific CD4+ T cells.
Recent advances suggest that MHC class II+ ILC3s co-localize and interact with T follicular helper (TFH) cells in the MLNs, and the absence of ILC3-intrinsic antigen presentation resulted in expansion of TFH cell populations, enhanced de novo germinal center reactions and IgA class switching25 (Figure 4A). The number of plasma cells in the colon secreting IgA was increased in mice lacking MHC class II+ ILC3s, and increased IgA production was preferentially found to target bacterial species residing within the colonic mucosa. This suggests that under steady-state conditions, ILC3-associated antigen presentation may further promote mutualism with the mucosal microbiota by limiting T cell-dependent IgA production25. Taken together, these studies suggest that antigen-presentation by MLN-resident ILC3s suppresses multiple aspects of inflammatory T cell responses towards the microbiota under immune homeostasis.
Conversely, antigen-presenting ILC3s have also been ascribed roles in promoting adaptive immune responses in the context of vaccination, chronic inflammation or lymphopenia (Figure 4b)71. Notably, IL-1β stimulation of in vitro-generated ILC3-like cells was shown to upregulate expression of MHC class II, IL-2 and co-stimulatory molecules, including CD40 and CD80, which was associated with the ability of these cells to promote antigen-specific CD4+ T cell responses71. This study may highlight tissue-specific roles for antigen-presenting ILC3s, as IL-1β did not promote co-stimulatory molecule expression on ILC3s directly isolated from the gastrointestinal tract, and further it was not required for constitutive high MHC class II expression67,68. Therefore, additional investigations are required to define what causes ILC3s to promote versus inhibit CD4+ T cell responses, and whether these represent the same ILC3 subset or unique subsets that arise only in inflammatory environments.
Whereas ILC3s seem to be the main antigen-presenting ILC subset under homeostatic conditions, MHC class II expression has also been reported on ILC2s, particularly in response to alarmins (Figure 4c). A subset of ILC2s elicited by either IL-25 or IL-33 has been reported to express genes associated with antigen presentation, including H2-Ab1 and Cd74, and MHC class II levels on ILC2s can be further augmented by trogocytosis [G] of antigen-containing MHC class II complexes from professional antigen-presenting cells20,72-74. MHC class II+ ILC2s from IL-33-treated mice further expressed CD80, and to a lesser extent CD86, and enhanced T cell responses in vitro and in vivo20,74. In addition, ILC subsets can express lipid antigen-presentation molecules of the CD1 family. Most notably, LTi-like ILC3s express CD1d and are capable of presenting lipid antigens to activate invariant NKT cells [G] in vitro and in vivo75, and human ILC2s express CD1a in the skin following stimulation with thymic stromal lymphopoietin (TSLP) and activate CD1a-responsive T cell populations during Staphylococcus aureus infection76. These advances define that both ILC3s and ILC2s have the potential to augment adaptive immunity through direct antigen presentation.
Auxiliary Surface Molecules on ILCs.
The consequences of ILC interactions with T cell subsets seem to differ depending upon the ILC subset, anatomical location and context of the encounter. This may, in part, be further regulated by the presence or absence of secondary signals, such as co-stimulatory or auxiliary molecules. Besides classical co-stimulatory molecules, ILC subsets express several auxiliary activating and inhibitory molecules with the capacity to modulate interactions with adaptive immune cells (Figure 4b), including a range of TNF receptor (TNFR)-family molecules. For example, LTi-like ILC3s express the TNFR-family molecule RANK while simultaneously expressing the cognate ligand (RANKL), resulting in regulation of cell clustering in intestinal lymphoid structures through homotypic interactions77. Furthermore, intestinal ILC3s recognize the TNFR ligand LIGHT (on a yet to be identified cell type) through expression of HVEM to modify cytokine production in the context of infection78. ILC1 and ILC2 also express GITR (TNFSF18), which supports their effector functions79-81. In contrast, ILC3 expression of the TNFR-family ligands OX40L and CD30L has a key role in modulating adaptive immune cell function through cognate interactions with OX40 and CD30, respectively, on T cell subsets82-85. LTi-like ILC3s may support T cell memory formation through direct OX40L and CD30L interactions, which suggests that this subset may have nuanced roles in modulating different stages of the adaptive immune response82,86. Furthermore, in an inflammatory and lymphopenic environment, ILC3-specific expression of OX40L is induced by TL1A (also known as TNFSF15) from CX3CR1+ mononuclear phagocytes, and subsequently promotes a TH1 cell response (Figure 4b)87. By contrast, ILC3 expression of OX40L may support the maintenance of OX40-expressing intestinal Treg cells within cryptopatches in the presence of lymphopenia but absence of inflammation88. In all of these studies, it remains unclear whether modulation of responses by CD30L or OX40L additionally requires direct antigen presentation by MHC class II+ ILC3 and whether it is a unique feature of specific ILC3 subsets or contexts.
ILC2s acquire OX40L expression upon stimulation to support OX40+ TH2 cell and Treg cell responses in the airways following helminth infection or allergic airway inflammation (Figure 4c)89. OX40L therefore seems to be a common mechanism through which ILCs interact with multiple subsets of CD4+ T cells. In addition, ILC2s are characterized by expression of surface ICOS, but also express ICOSL, which reinforces cell survival and effector function in ILC2s themselves through homotypic interactions. This pathway further supports Treg cell accumulation in response to IL-33 through engagement of ICOSL on the T cell (Figure 4c)30,90. Intriguingly, Treg cells in the gut and adipose tissue also respond to IL-33, suggesting there could be critical coordination or competition with ILC291,92.
As noted above, both ILC2s and ILC3s establish residence within the inter-follicular niche of lymph nodes where they may interact with multiple adaptive immune cell populations, including TFH cells, which themselves express ICOS, OX40 and PD-1. However whether ILCs further modulate TFH cells and subsequent B cell responses through these pathways remains poorly understood. Evidence suggests that ILC3s directly augment B cell responses through expression of CD40L and Notch ligands44,93. Multiple ILC subsets additionally express the inhibitory molecule PD-L1, and expression on ILC2s was surprisingly found to enhance rather than suppress interactions with TH2 cells following helminth infection94. ILC progenitors and mature cells in the periphery also express PD-1, thus suggesting that ILCs themselves may be sensitive to PD-L1-mediated inhibition95, and PD-1 has been reported to negatively regulate ILC2s in mice and humans96. Further studies are required to determine whether the expression of the cognate ligands for these pathways by ILC2s and ILC3s permit direct modulation of other specific TH cell subsets or B cells. Moreover, the expression of checkpoint molecules on ILC subsets raises the possibility that these cells may provide inhibitory signals in the context of cancer, where checkpoint blockade has shown unprecedented promise as a therapeutic approach97.
Reciprocal ILC–adaptive regulation
We have discussed the growing body of evidence supporting a role for ILCs in directly and indirectly modulating the adaptive immune system. Furthermore, emerging studies indicate that the adaptive immune system conversely modulates ILC function (Figure 5). Compelling recent evidence suggests this is indeed the case as lack of the adaptive immune system, specifically T cells, results in hyper-activation of ILC3s in the small intestine in response to microbial colonization55,98-100. These reciprocal interactions between ILCs and adaptive immunity may be crucially important in terms of establishing homeostasis during initial microbiota colonization of the intestine, when ILCs are the first lymphocytes to encounter these stimuli and are necessary to respond prior to the development of an adaptive immune response55. Furthermore, these findings and others have led to a hypothesis that ILCs are crucial pioneers that establish the lymphoid or tissue niches in early life development, which subsequently have long-term consequences for immune homeostasis101.
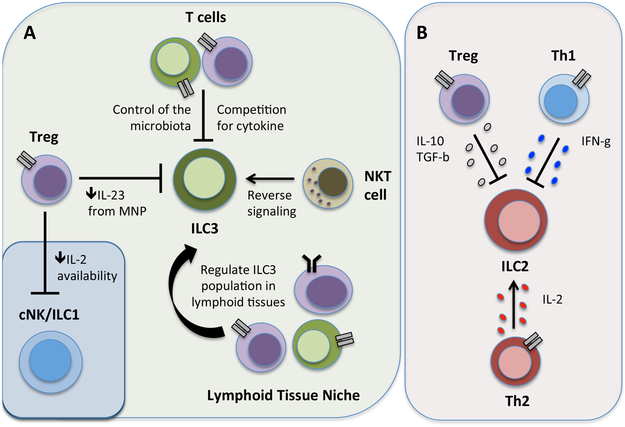
Figure 5 ∣. Counter regulation of ILCs by adaptive immunity.
Adaptive immunity can promote or inhibit ILC responses through a number of distinct mechanisms. a) ILC3 and ILC1 responses can be limited by Tregs through the direct sequestration of IL-2 or indirect regulation of MNP-derived IL-23. Furthermore, ILC3 responses are suppressed through the competition with adaptive immunity for pro-survival cytokines or by adaptive immune control of the microbiota. Conversely, ILC3 responses are promoted following antigen-dependent encounters with NKT cells, and the population of size of ILC3 is supported through undefined mechanisms by T and B cells in secondary lymphoid tissues. b) ILC2 responses are suppressed by Treg cell-derived IL-10 and TGF-β or TH1 cell-derived IFNγ, while ILC2 responses are conversely enhanced by TH2 cell-derived IL-2 in a context-dependent manner.
T cell-derived effector cytokines also cross-regulate ILC responses during an immune response. For example, IFNγ directly limits ILC2 expansion and type-2 immunopathology in the airway30,36,102,103, and TH17 cells indirectly limit intestinal ILC3 responses by controlling the intestinal microbiota60. Treg cells have a key role in regulating ILC3 responses, as transfer of FoxP3+ Treg cells prevents the hyper-activation of ILC3 responses in the small intestine of Rag1−/− mice. Mechanistically, LAG3 on Treg cells interacted with MHC class II on the surface of CX3CR1+ macrophages to inhibit production of IL-2355,98. Treg cells also suppress NK cell and ILC1 responses in multiple contexts by depriving them of IL-2104-106. This may reflect a general competition for pro-survival cytokines between innate and adaptive lymphocytes, that can have consequences on the population expansion of B cells or T cells and limit homeostatic proliferation in the context of lymphopenia68,107,108.
Interactions between with adaptive immunity does not always limit subsequent ILC responses and may occur in a tissue-specific manner. For example, in contrast to the intestine, studies indicate that T cells promote the population size of ILC3s in the MLNs, and B cells support ILC3 numbers in the spleen19,109. Adaptive immunity also augments ILCs as a result of direct cellular contact. CD4+ T cells enhance ILC2 cytokine production following cognate interactions with MHC class II molecules and subsequent T cell provision of IL-220,74. Similarly, ICOS–ICOSL interactions between ILC2s and Treg cells not only regulate Treg cells, as discussed above, but also cross-regulate ILC2 cytokine production together with Treg cell-derived IL-10 and TGF-β30,110-112, which suggests that there is a bidirectional communication between these cell populations in response to IL-33. By contrast, MHC class II-dependent interactions between ILC3s and CD4+ T cells have no apparent effect on ILC3 production of IL-2267,68; however, presentation of lipid antigen by ILC3s in the context of CD1d was found to augment IL-22 production, which suggests that NKT cells support ILC3 function following activation75. Together these findings suggest that reciprocal interactions with T cells shape the magnitude and quality of the ILC responses in multiple tissues and contexts. Additional investigation is required to explore the nature of these interactions and the effector molecules that facilitate this dialogue in health and disease (Box 2).
Box 2 ∣. Perspectives and future challenges.
ILCs have been found to activate, regulate and inhibit adaptive immune responses. Although some reports may seem to be contradictory, the findings can be reconciled when considering the ILC subset involved, the anatomical location of the responses, and the contextual nature of the interaction.
As the field moves forward, there is an urgent need for new, specific genetic tools to target ILC subsets, and it will be important to carefully re-evaluate data side-by-side and taking into account the potential caveats of the approaches used. The interrogation of ILC1 and adaptive immune interactions is limited and should be explored further.
Lineage-specific targeting of ILCs is the most appropriate approach for evaluating functional interactions with adaptive immunity in vivo. However, this will be difficult to accomplish in the context of human samples.
It is important that studies carefully consider ILC subset heterogeneity, tissue location and the type of interacting B cell or T cell. In particular, the outcome of these interactions may differ within complex tissues such as the gastrointestinal tract, as the result of different properties imprinted by anatomical location, microbial colonization levels, barrier permeability, mucus organization or metabolite production.
The expression of co-stimulatory molecules may determine the outcome of a direct ILC–T cell interaction. However, relatively little is known about the signals that regulate expression of these molecules on ILCs, and whether the outcome also simultaneously requires antigen presentation.
It remains unclear how ILCs acquire antigens for presentation. Although studies are limited, ILCs do not seem to be major phagocytic populations. Co-localization of ILC-like cells with subcapsular sinus macrophages in lymphoid tissues has been observed, leading to the hypothesis that these cells transfer antigen-containing membrane blebs158,159. Alternatively, ILCs found within tertiary lymphoid structures may acquire antigens via microfold (M)-cells160 or from intestinal mononuclear phagocytes, as previously described for migratory DC populations161.
Human health and disease
ILCs have increasingly been implicated in the pathogenesis of a broad range of chronic inflammatory diseases in humans1,2. This is owing to the capacity of ILCs to produce pro-inflammatory cytokines in response to damage-associated or inflammatory cues, which exacerbate disease when ILCs are dysregulated in the context of a maladapted immune system, genetic predisposition and/or contributing environmental factors. However, in contrast to their increasingly appreciated roles as effector cells in the context of disease, the extent to which ILC interactions with adaptive immunity shape the pathogenesis of human diseases remains poorly defined.
Inflammatory bowel disease.
The contribution of ILCs to human disease is perhaps most extensively investigated in the context of inflammatory bowel disease (IBD), where many of the genetic polymorphisms linked to disease directly implicate dysregulated type 3 immune responses [G], such as those mediated by TH17 cells and ILC3s113,114. Early reports speculated a critical role for ILC3 in maintaining homeostasis in the healthy human intestine, with findings by Spits and colleagues that were among the first to define that human ILC3 express IL-2 and IL-22 in response to microbial stimulation115. Indeed, ILC3s are fundamentally altered in the inflamed human intestine. Several early studies described increased ILC3 numbers or effector responses in the intestine of patients with IBD116,117. By contrast, others have defined a substantial reduction in the number of ILC3s in the inflamed intestine of patients with IBD relative to multiple control samples, and have suggested that this is the result of an ILC3 to ILC1 conversion controlled by a balance of IL-12 and IL-23118,119, potentially reflecting similar plasticity that has been characterized for mouse ILCs120,121. The differences between these reports could result from the new appreciation of ILC1s or an inability to carefully examine for ILC3 heterogeneity in humans. We have defined that ILC3s isolated from the large intestine of pediatric patients with Crohn’s disease have significantly impaired expression of MHC class II molecules in comparison to controls without IBD, which correlated with increased frequencies of local inflammatory T cells and systemic antibody titers against the microbiota68. MHC class II expression was increased or unchanged on intestinal T cells or DCs, respectively, and these findings were subsequently independently verified in a cohort of adult patients with IBD122. Finally, we also defined a significant impairment of ILC3-derived IL-2 in the small intestine of patients with Crohn’s disease and a positive correlation of ILC3 and Treg cell frequencies63. As such, most studies so far implicate a protective role for ILC3s in orchestrating immune homeostasis in the intestine through multiple distinct mechanisms, as well as a substantial impairment of ILC3 responses in patients with IBD. Therefore, therapies aimed at restoring normal ILC3s may hold an important key to maintaining intestinal health in humans, in part by controlling pro-inflammatory T cell responses and homeostasis with the microbiota.
Other gastrointestinal pathologies.
Dysregulated communication between ILC3s and T cells may underlie other gastrointestinal pathologies that result from infection, dietary changes or clinical interventions. For example, ILC3s are significantly impaired in the intestine following HIV infection, or SIV infection in rhesus macaques123,124. This could subsequently lead to increases in CD4+ T cell activation via impairment of IL-22-mediated intestinal barrier function or loss of direct T cell regulation by antigen-presenting ILC3s or IL-2-producing ILC3s, collectively contributing to CD4+ T cell decline and progression to AIDS125. Similarly, circulating levels of ILC3-like cells correlate with reduced susceptibility to graft-versus-host-disease, potentially implicating similar mechanisms of ILC3-mediated regulation of T cells126,127, although the changes in intestinal ILCs have not been extensively investigated
Autoimmune disease.
Although ILC biology has been mainly studied at mucosal barrier sites, the interplay between ILCs and adaptive immunity could have important implications for disease pathology in systemic tissues. Indeed, ILCs have been implicated in the pathogenesis of a range of autoimmune diseases. Arthritic diseases are classically characterized by the presence of circulating autoantibodies and are often associated with the development of ectopic tertiary lymphoid follicles. Dysregulated communication between TFH cells, peripherally induced TFH-like cells and B cells has been implicated in the generation of autoantibodies in rheumatoid arthritis128,129. Intriguingly, it has been suggested that inflammatory TH17 cell and TFH cell responses generated within the intestinal tract, and driven by the microbiota, can exacerbate arthritic disease130,131. Furthermore, ILCs may also modulate adaptive immune responses that drive arthritic diseases, as they can promote tertiary lymphoid structures, augment TH cells, and control humoral immunity. In line with this, juvenile idiopathic arthritis has recently been associated with mutations in the NFIL3 gene132, a key transcriptional regulator of ILC development1,2. ILC2 are also substantially increased in the joints and circulation of patients with arthritis, and play a substantial role in limiting inflammatory responses in mouse models through production of IL-4, IL-9 and IL-13133,134, however it remains unclear whether there is also interactions between ILC2 and adaptive immunity in these contexts.
In the context of multiple sclerosis, RORγt-expressing ILC3-like cells have been reported in tertiary lymphoid aggregates within the brain of patients with progressive disease135. Increased numbers of circulating LTi-like ILC3s positively correlated with cerebrospinal fluid autoantibodies in multiple sclerosis patients, and were reduced in patients receiving monoclonal antibody therapies that targeted CD25136. These data implicate not only a role for ILCs in regulating adaptive immune responses in autoimmune disorders, but also the potential for novel therapeutic strategies in inflammatory disease.
Allergic disease.
ILC2s or associated responses are increased during multiple allergic human diseases, such as asthma, atopic dermatitis, nasal polyps, rhinosinusitis and chronic obstructive pulmonary disease137-146. However, a majority of these studies have yet to interrogate potential interactions between these altered ILC2 responses and an adaptive immune response, although these two pathways typically exhibit similar patterns of induction. One study revealed that human ILC2s express MHC class II molecules, as well as process and present allergen-derived antigens to CD4+ T cells, resulting in the differentiation and expansion of TH2 cell responses20. These studies suggest that targeting ILC2s in human disease may suppress multiple aspects of the overall type-2 immune response.
Cancer.
The investigation of ILCs in human cancer remains in very early stages. Initial reports have identified that ILCs are present in human colon, lung and breast tumors, are localized to lymphoid structures and express activation markers including MHC class II147-150. Furthermore, as highlighted above, ILCs express PD-1 and PD-L194-96, suggesting that these populations may be modified by checkpoint blockade immunotherapies. The expression of co-stimulatory and co-inhibitory molecules by ILCs opens up new avenues for intervention by co-opting these immune checkpoint pathways. In support of this, a human regulatory ILC3-like population was reported to limit tumor-associated T cells in an NKp46-dependent manner151. Additional studies are required to determine the contribution of ILC–adaptive immune cell interactions in cancer and the role of ILCs in checkpoint blockade immunotherapy, associated adverse events and long term outcomes.
Therapeutic modulation of ILCs.
In this regard, the emergence of next generation immune-therapeutics, such as monoclonal antibodies and small molecule antagonists, has the potential to revolutionize intervention strategies in chronic inflammatory diseases by potentially influence ILC-adaptive immune interactions. Advancing our understanding of the transcriptional and signal transduction pathways that mediate ILC functions may present additional treatment opportunities. Indeed, we previously showed that small molecule inhibitors of RORγt may have beneficial effects in mouse models of intestinal inflammation, as well as samples from patients with IBD, through the suppression of T cell-driven inflammatory responses, while unexpectedly leaving the regulatory functions of ILC3s intact152. In addition, targeting of inflammatory signaling pathways, such as JAK–STAT signaling, may prove beneficial in suppressing the pro-inflammatory roles of ILCs in a broad range of inflammatory diseases from arthritis to allergy153,154. However, to successfully harness ILC–adaptive immune interactions to benefit human health, a more complete understanding of these pathways in human diseases is required (Box 2).
Summary
ILCs are increasingly appreciated to play key roles in immune responses during both health and disease. While ILCs have many canonical roles via production of effector cytokines at barrier tissue sites, increasing evidence suggests ILCs also modulate the wider immune response through functional interactions with adaptive immune cells. These interactions begin early in life to facilitate organized lymphoid tissue formation and continue to play key roles following birth to promote tolerance to dietary antigens or the microbiota and facilitate tissue homeostasis. In adulthood, ILCs mediate multiple effects on the adaptive immune system either via complex communication with myeloid cells, provision of soluble factors to support regulatory populations, or through direct cell surface interactions including direct presentation of antigen to T cells.
However, it is critical to carefully consider the context in which ILCs and adaptive immune cells interact to fully understand the seemingly differential or contradictory roles ascribed to ILCs. For example, ILC3 suppress adaptive immune responses in the intestine and draining lymph nodes during homeostasis67-69, yet may promote peripheral T and B cell responses in the spleen following vaccination44,71. Additionally, ILC3 promote IgA responses via secretion of LT and acting on stromal cells or DCs41,43, yet conversely suppress IgA via antigen presentation to TFH cells25. Finally, ILC3-derived IL-2 is required to support regulatory T cells in the small intestine, but not the large intestine63, while antigen-presentation by ILC3 conversely regulates IgA responses in the large intestine, but not the small intestine25. Many of these differences represent the tissue- and context-specific nature of ILC interactions with adaptive immunity. The reasons for this compartmentalization of ILC3 communication remain poorly understood, but are likely to benefit from an increased understanding of the tissue microenvironments in which different ILC populations interact with adaptive immune cells and the signals or cellular heterogeneity present within each niche to instruct the function of ILCs.
Together these findings suggest a broader and more nuanced role for ILCs throughout the lifetime in orchestrating tissue immune responses. While the unifying feature of ILC communication with adaptive immunity in the context of health appears to promote tissue homeostasis, the outcomes of ILC interactions with adaptive immune cells may alter substantially in the context of ageing, infection or inflammation. Collectively, this may allow for an unprecedented opportunity to therapeutically target or harness ILC interactions with adaptive immunity to treat human disease.
Future challenges
The past decade of research on ILCs has moved at an extraordinary pace and yielded many exciting advances; however, the study of ILCs in modulating adaptive immunity is still in early stages of development. Reports from multiple groups have many functional interactions, but the precise nature of these signals and the consequences of this cross-talk are highly context-dependent. There are many challenges ahead for advancing our understanding of ILC–adaptive immune interactions, and additional considerations should be made for both existing and future studies (Box 2). The emerging concept that there is fundamental cross-regulation between ILCs and adaptive immunity may hold important keys to our understanding of the immune system in health and disease. Many early approaches to interrogate these interactions will need to be revisited as technologies and the appreciation of cellular heterogeneity advance. Our current approaches to target ILCs in the presence of adaptive immunity remain limited, and there is an urgent need to develop novel tools and strategies in mouse models. Furthermore, many limitations currently exist and need to be considered when re-evaluating published analyses of human ILCs. For example, recent research identified that many studies of ILCs in human blood may have examined ILC-like progenitors rather than mature populations40, and recent comprehensive investigations of ILC populations across human tissues have revealed important differences in terms of ILC distribution, phenotype and function155, which indicates the need to more carefully study ILCs in the appropriate anatomical location. We also currently do not appreciate how to accurately quantify human ILC heterogeneity, such as the identification of analogous LTi-like subsets versus T-bet+ ILC3s. Indeed, the expression of natural cytotoxicity receptors is potentially more reflective of the activation status of human ILCs, as opposed to being a defining marker of an ILC3 subset in mice156. Recent pioneering work on single-cell sequencing of these populations is paving the way towards better approaches to carrying out these studies157. Notwithstanding these limitations, it is clear from the promise of these early studies that ILCs are more than simply an innate counterpart to adaptive immunity, and rather may have evolved as master regulators of adaptive immunity and tissue homeostasis.
Acknowledgements
We thank members of the Hepworth and Sonnenberg Laboratories for discussions and critical reading of the manuscript. The Hepworth Laboratory is supported by a Royal Society and Wellcome Trust Sir Henry Dale Fellowship (Grant Number 105644/Z/14/Z) and a Lister Institute of Preventative Medicine Prize. The Sonnenberg Laboratory is supported by the National Institutes of Health (R01AI143842, R01AI123368, R01AI145989, R21DK110262 and U01AI095608), the NIAID Mucosal Immunology Studies Team (MIST), the Crohn’s and Colitis Foundation, the Searle Scholars Program, the American Asthma Foundation Scholar Award, Pilot Project Funding from the Center for Advanced Digestive Care (CADC), an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund, a Wade F.B. Thompson/Cancer Research Institute CLIP Investigator grant, the Meyer Cancer Center Collaborative Research Initiative, and the Roberts Institute for Research in IBD.
Glossary
- alarmins
Immune activating proteins or peptides that are released in response to tissue damage, infection or immune activation.
- lymphoid tissue-inducer cells
A member of the group 3 innate lymphoid cell family that is present during fetal development and initiates the generation of secondary lymphoid structures.
- tertiary lymphoid structures
Organized lymphoid aggregates that develop after birth in response to microbiota colonization or chronic inflammation.
- Trogocytosis
A process whereby cellular interactions results in the transfer of a surface protein from a donor cell and continued expression of that protein on the surface of a recipient cell.
- invariant NKT cells
A population of T cells that express surface molecules shared with NK cells and a restricted alpha beta T cell receptor repertoire.
- type 3 immune responses
A form of immune responses that develops in response to microbial exposure or chronic inflammation and is characterized by cells expressing the transcription factor RORγt and the cytokines IL-17A, IL-17F or IL-22 (such as TH17 cells and ILC3).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Spits H et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol 13, 145–149 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Vivier E et al. Innate Lymphoid Cells: 10 Years On. Cell 174, 1054–1066, doi: 10.1016/j.cell.2018.07.017 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Basu R et al. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37, 1061–1075 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rankin LC et al. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat Immunol 17, 179–186, doi: 10.1038/ni.3332 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song C et al. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J Exp Med 212, 1869–1882, doi: 10.1084/jem.20151403 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vely F et al. Evidence of innate lymphoid cell redundancy in humans. Nat Immunol 17, 1291–1299, doi: 10.1038/ni.3553 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivier E, van de Pavert SA, Cooper MD & Belz GT The evolution of innate lymphoid cells. Nat Immunol 17, 790–794, doi: 10.1038/ni.3459 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez PP et al. Single-cell transcriptional analysis reveals ILC-like cells in zebrafish. Sci Immunol 3, doi: 10.1126/sciimmunol.aau5265 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mebius RE, Rennert P & Weissman IL Developing lymph nodes collect CD4+CD3− LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997) [DOI] [PubMed] [Google Scholar]
- 10.Adachi S, Yoshida H, Kataoka H & Nishikawa S Three distinctive steps in Peyer’s patch formation of murine embryo. Int Immunol 9, 507–514, doi: 10.1093/intimm/9.4.507 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Meier D et al. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity 26, 643–654, doi: 10.1016/j.immuni.2007.04.009 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Mebius RE Organogenesis of lymphoid tissues. Nat Rev Immunol 3, 292–303, doi: 10.1038/nri1054 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Eberl G et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol 5, 64–73 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Bouskra D et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Saito H et al. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science 280, 275–278 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Kanamori Y et al. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med 184, 1449–1459, doi: 10.1084/jem.184.4.1449 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu C et al. Anti-microbial Functions of Group 3 Innate Lymphoid Cells in Gut-Associated Lymphoid Tissues Are Regulated by G-Protein-Coupled Receptor 183. Cell Rep 23, 3750–3758, doi: 10.1016/j.celrep.2018.05.099 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emgard J et al. Oxysterol Sensing through the Receptor GPR183 Promotes the Lymphoid-Tissue-Inducing Function of Innate Lymphoid Cells and Colonic Inflammation. Immunity 48, 120–132 e128, doi: 10.1016/j.immuni.2017.11.020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *19.Mackley EC et al. CCR7-dependent trafficking of RORgamma(+) ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat Commun 6, 5862 (2015).* This study highlighted the ability of ILCs to migrate from the intestinal tissue and to establish residence at inter-follicular sites within the draining lymph node.
- *20.Oliphant CJ et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 41, 283–295, doi: 10.1016/j.immuni.2014.06.016 (2014).* One of the first studies to show expression of MHC class II and co-stimulatory molecules on ILC2s, and to implicate a role for promoting TH2 cell responses during infection.
- *21.Moro K et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463, 540–544 (2010).* A seminal study that demonstrated a role for ILC2s in promoting B cell responses through production of IL-5.
- 22.Jackson-Jones LH et al. Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat Commun 7, 12651, doi: 10.1038/ncomms12651 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider C et al. Tissue-Resident Group 2 Innate Lymphoid Cells Differentiate by Layered Ontogeny andIn Situ Perinatal Priming. Immunity In Press, Corrected Proof, doi: 10.1016/j.immuni.2019.04.019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmutz S et al. Cutting edge: IL-7 regulates the peripheral pool of adult ROR gamma+ lymphoid tissue inducer cells. J Immunol 183, 2217–2221 (2009). [DOI] [PubMed] [Google Scholar]
- *25.Melo-Gonzalez F et al. Antigen-presenting ILC3 regulate T cell–dependent IgA responses to colonic mucosal bacteria. The Journal of Experimental Medicine, jem.20180871, doi: 10.1084/jem.20180871 (2019).* This study shows that antigen-presenting ILC3s modulate TFH cells and germinal centre formation in the MLN to limit IgA responses directed against mucosal microbiota.
- 26.Hoorweg K et al. A Stromal Cell Niche for Human and Mouse Type 3 Innate Lymphoid Cells. J Immunol 195, 4257–4263 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baptista AP et al. Colonic patch and colonic SILT development are independent and differentially regulated events. Mucosal immunology 6, 511–521, doi: 10.1038/mi.2012.90 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satoh-Takayama N et al. The chemokine receptor CXCR6 controls the functional topography of interleukin-22 producing intestinal innate lymphoid cells. Immunity 41, 776–788, doi: 10.1016/j.immuni.2014.10.007S1074-7613(14)00383-5 [pii] (2014). [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi T et al. Homeostatic Control of Sebaceous Glands by Innate Lymphoid Cells Regulates Commensal Bacteria Equilibrium. Cell 176, 982–997 e916, doi: 10.1016/j.cell.2018.12.031 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molofsky AB et al. Interleukin-33 and Interferon-gamma Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity 43, 161–174, doi: 10.1016/j.immuni.2015.05.019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahlgren MW et al. Adventitial Stromal Cells Define Group 2 Innate Lymphoid Cell Tissue Niches. Immunity, doi: 10.1016/j.immuni.2019.02.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang SK et al. Stromal cell cadherin-11 regulates adipose tissue inflammation and diabetes. J Clin Invest 127, 3300–3312, doi: 10.1172/jci86881 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koga S et al. Peripheral PDGFRalpha(+)gp38(+) mesenchymal cells support the differentiation of fetal liver-derived ILC2. J Exp Med 215, 1609–1626, doi: 10.1084/jem.20172310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahlakoiv T et al. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci Immunol 4, doi: 10.1126/sciimmunol.aax0416 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gasteiger G, Fan X, Dikiy S, Lee SY & Rudensky AY Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–985, doi: 10.1126/science.aac9593 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moro K et al. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol 17, 76–86, doi: 10.1038/ni.3309 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Kim MH, Taparowsky EJ & Kim CH Retinoic Acid Differentially Regulates the Migration of Innate Lymphoid Cell Subsets to the Gut. Immunity 43, 107–119, doi: 10.1016/j.immuni.2015.06.009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Y et al. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol 16, 161–169, doi: 10.1038/ni.3078 [pii] (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Y et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359, 114–119, doi: 10.1126/science.aam5809 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim AI et al. Systemic Human ILC Precursors Provide a Substrate for Tissue ILC Differentiation. Cell 168, 1086–1100 e1010 (2017). [DOI] [PubMed] [Google Scholar]
- *41.Kruglov AA et al. Nonredundant function of soluble LTalpha3 produced by innate lymphoid cells in intestinal homeostasis. Science 342, 1243–1246 (2013).*This study demonstrated distinct roles for ILC3-derived LTα3 and LTα1β2 in regulating T cell-dependent and T cell-independent IgA responses within the small instestine, respectively.
- 42.Reboldi A et al. IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science 352, aaf4822, doi: 10.1126/science.aaf4822 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuji M et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity 29, 261–271 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Magri G et al. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol 15, 354–364 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scandella E et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol 9, 667–675 (2008). [DOI] [PubMed] [Google Scholar]
- *46.Halim TY et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40, 425–435, doi: 10.1016/j.immuni.2014.01.011 (2014).* This paper identified a critical role for ILC2s in orchestrating optimal TH2 cell responses during papain-induced airway inflammation.
- 47.Halim TY et al. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol 17, 57–64, doi: 10.1038/ni.3294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldszmid RS et al. TAP-1 indirectly regulates CD4+ T cell priming in Toxoplasma gondii infection by controlling NK cell IFN-gamma production. J Exp Med 204, 2591–2602, doi: 10.1084/jem.20070634 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weizman OE et al. ILC1 Confer Early Host Protection at Initial Sites of Viral Infection. Cell 171, 795–808 e712, doi: 10.1016/j.cell.2017.09.052 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwong B et al. T-bet-dependent NKp46+ innate lymphoid cells regulate the onset of TH17-induced neuroinflammation. Nat Immunol (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonnenberg GF, Fouser LA & Artis D Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol 12, 383–390 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Cella M et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satoh-Takayama N et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Wang X et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 514, 237–241, doi: 10.1038/nature13564 (2014). [DOI] [PubMed] [Google Scholar]
- *55.Mao K et al. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature, doi: 10.1038/nature25437 (2018).*This paper defined a critical window of time where ILC3 are regulated by adaptive immunity in the intestine.
- 56.Shih VF et al. Homeostatic IL-23 receptor signaling limits Th17 response through IL-22-mediated containment of commensal microbiota. Proc Natl Acad Sci U S A 111, 13942–13947, doi: 10.1073/pnas.1323852111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sonnenberg GF et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336, 1321–1325 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zenewicz LA et al. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J Immunol 190, 5306–5312, doi: 10.4049/jimmunol.1300016 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sano T et al. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell 163, 381–393, doi: 10.1016/j.cell.2015.08.061 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu J et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity 39, 386–399 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *61.Mortha A et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343, 1249288, doi: 10.1126/science.1249288 [pii] (2014).* This study describes complex interactions between tissue-resident ILC3s, macrophages and Treg cells in order to orchestrate intestinal tolerance.
- 62.Hirota K et al. Autoimmune Th17 Cells Induced Synovial Stromal and Innate Lymphoid Cell Secretion of the Cytokine GM-CSF to Initiate and Augment Autoimmune Arthritis. Immunity 48, 1220–1232 e1225, doi: 10.1016/j.immuni.2018.04.009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.Zhou L et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature 568, 405–409, doi: 10.1038/s41586-019-1082-x (2019).*This study demonstrates that ILC3 are a previously unappreciated cellular source of IL-2 that critically supports Treg cell homeostasis and oral tolerance in the small intestine.
- 64.Noval Rivas M, Burton OT, Oettgen HC & Chatila T IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. The Journal of allergy and clinical immunology 138, 801–811 e809, doi: 10.1016/j.jaci.2016.02.030 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pelly VS et al. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal immunology 9, 1407–1417, doi: 10.1038/mi.2016.4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roediger B et al. IL-2 is a critical regulator of group 2 innate lymphoid cell function during pulmonary inflammation. The Journal of allergy and clinical immunology 136, 1653–1663 e1657, doi: 10.1016/j.jaci.2015.03.043 (2015). [DOI] [PubMed] [Google Scholar]
- *67.Hepworth MR et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498, 113–117 (2013).* This was the first report that ILC3s are antigen presenting cells that suppresses microbiota-specific T cell responses to prevent intestinal inflammation.
- 68.Hepworth MR et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria specific CD4(+) T cells. Science 348, 1031–1035, doi: 10.1126/science.aaa4812 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goto Y et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 40, 594–607 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamano T et al. Aire-expressing ILC3-like cells in the lymph node display potent APC features. J Exp Med 216, 1027–1037, doi: 10.1084/jem.20181430 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.von Burg N et al. Activated group 3 innate lymphoid cells promote T-cell-mediated immune responses. Proc Natl Acad Sci U S A 111, 12835–12840 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wallrapp A et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549, 351–356, doi: 10.1038/nature24029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Angkasekwinai P et al. ILC2s activated by IL-25 promote antigen-specific Th2 and Th9 functions that contribute to the control of Trichinella spiralis infection. PloS one 12, e0184684, doi: 10.1371/journal.pone.0184684 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mirchandani AS et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol 192, 2442–2448, doi: 10.4049/jimmunol.1300974 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Saez de Guinoa J et al. CD1d-mediated activation of group 3 innate lymphoid cells drives IL-22 production. EMBO Rep, doi: 10.15252/embr.201642412 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hardman CS et al. CD1a presentation of endogenous antigens by group 2 innate lymphoid cells. Sci Immunol 2, doi: 10.1126/sciimmunol.aan5918 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bando JK et al. The Tumor Necrosis Factor Superfamily Member RANKL Suppresses Effector Cytokine Production in Group 3 Innate Lymphoid Cells. Immunity 48, 1208–1219 e1204, doi: 10.1016/j.immuni.2018.04.012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seo GY et al. LIGHT-HVEM Signaling in Innate Lymphoid Cell Subsets Protects Against Enteric Bacterial Infection. Cell Host Microbe 24, 249–260 e244, doi: 10.1016/j.chom.2018.07.008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vashist N et al. Influenza-Activated ILC1s Contribute to Antiviral Immunity Partially Influenced by Differential GITR Expression. Front Immunol 9, 505, doi: 10.3389/fimmu.2018.00505 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagashima H et al. GITR cosignal in ILC2s controls allergic lung inflammation. The Journal of allergy and clinical immunology 141, 1939–1943.e1938, doi: 10.1016/j.jaci.2018.01.028 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Galle-Treger L et al. Costimulation of type-2 innate lymphoid cells by GITR promotes effector function and ameliorates type 2 diabetes. Nat Commun 10, 713, doi: 10.1038/s41467-019-08449-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Withers DR et al. OX40 and CD30 signals in CD4(+) T-cell effector and memory function: a distinct role for lymphoid tissue inducer cells in maintaining CD4(+) T-cell memory but not effector function. Immunol Rev 244, 134–148 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Withers DR et al. Cutting edge: lymphoid tissue inducer cells maintain memory CD4 T cells within secondary lymphoid tissue. J Immunol 189, 2094–2098 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gaspal FM et al. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J Immunol 174, 3891–3896 (2005). [DOI] [PubMed] [Google Scholar]
- 85.Kim MY et al. CD4(+)CD3(−) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity 18, 643–654 (2003). [DOI] [PubMed] [Google Scholar]
- 86.Withers DR et al. The survival of memory CD4+ T cells within the gut lamina propria requires OX40 and CD30 signals. J Immunol 183, 5079–5084 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Castellanos JG et al. Microbiota-Induced TNF-like Ligand 1A Drives Group 3 Innate Lymphoid Cell-Mediated Barrier Protection and Intestinal T Cell Activation during Colitis. Immunity 49, 1077–1089 e1075, doi: 10.1016/j.immuni.2018.10.014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deng T et al. ILC3-derived OX40L is essential for homeostasis of intestinal Tregs in immunodeficient mice. Cell Mol Immunol, doi: 10.1038/s41423-019-0200-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Halim TYF et al. Tissue-Restricted Adaptive Type 2 Immunity Is Orchestrated by Expression of the Costimulatory Molecule OX40L on Group 2 Innate Lymphoid Cells. Immunity 48, 1195–1207 e1196, doi: 10.1016/j.immuni.2018.05.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maazi H et al. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity 42, 538–551, doi: 10.1016/j.immuni.2015.02.007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schiering C et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568, doi: 10.1038/nature13577 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vasanthakumar A et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol 16, 276–285, doi: 10.1038/ni.3085 (2015). [DOI] [PubMed] [Google Scholar]
- 93.Komlosi ZI et al. Human CD40 ligand-expressing type 3 innate lymphoid cells induce IL-10-producing immature transitional regulatory B cells. The Journal of allergy and clinical immunology 142, 178–194 e111, doi: 10.1016/j.jaci.2017.07.046 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Schwartz C et al. ILC2s regulate adaptive Th2 cell functions via PD-L1 checkpoint control. J Exp Med 214, 2507–2521, doi: 10.1084/jem.20170051 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu Y et al. Single-cell RNA-seq identifies a PD-1hi ILC progenitor and defines its development pathway. Nature 539, 102–106 (2016). [DOI] [PubMed] [Google Scholar]
- 96.Taylor S et al. PD-1 regulates KLRG1(+) group 2 innate lymphoid cells. J Exp Med 214, 1663–1678, doi: 10.1084/jem.20161653 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ribas A & Wolchok JD Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355, doi: 10.1126/science.aar4060 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bauche D et al. LAG3(+) Regulatory T Cells Restrain Interleukin-23-Producing CX3CR1(+) Gut-Resident Macrophages during Group 3 Innate Lymphoid Cell-Driven Colitis. Immunity 49, 342–352 e345, doi: 10.1016/j.immuni.2018.07.007 (2018). [DOI] [PubMed] [Google Scholar]
- 99.Korn LL et al. Conventional CD4+ T cells regulate IL-22-producing intestinal innate lymphoid cells. Mucosal immunology 7, 1045–1057, doi: 10.1038/mi.2013.121 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sawa S et al. RORgammat+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol 12, 320–326 (2011). [DOI] [PubMed] [Google Scholar]
- 101.Kotas ME & Locksley RM Why Innate Lymphoid Cells? Immunity 48, 1081–1090, doi: 10.1016/j.immuni.2018.06.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duerr CU et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol 17, 65–75, doi: 10.1038/ni.3308 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duster M et al. T cell-derived IFN-gamma downregulates protective group 2 innate lymphoid cells in murine lupus erythematosus. Eur J Immunol 48, 1364–1375, doi: 10.1002/eji.201747303 (2018). [DOI] [PubMed] [Google Scholar]
- 104.Gasteiger G et al. IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J Exp Med 210, 1167–1178, doi: 10.1084/jem.20122462 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gasteiger G, Hemmers S, Bos PD, Sun JC & Rudensky AY IL-2-dependent adaptive control of NK cell homeostasis. J Exp Med 210, 1179–1187, doi: 10.1084/jem.20122571 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sitrin J, Ring A, Garcia KC, Benoist C & Mathis D Regulatory T cells control NK cells in an insulitic lesion by depriving them of IL-2. J Exp Med 210, 1153–1165, doi: 10.1084/jem.20122248 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin CE et al. Interleukin-7 Availability Is Maintained by a Hematopoietic Cytokine Sink Comprising Innate Lymphoid Cells and T Cells. Immunity 47, 171–182 e174 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Bank U et al. Cutting Edge: Innate Lymphoid Cells Suppress Homeostatic T Cell Expansion in Neonatal Mice. J Immunol 196, 3532–3536, doi: 10.4049/jimmunol.1501643 (2016). [DOI] [PubMed] [Google Scholar]
- 109.Kim MY et al. Heterogeneity of lymphoid tissue inducer cell populations present in embryonic and adult mouse lymphoid tissues. Immunology 124, 166–174 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rigas D et al. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator-inducible T-cell costimulator ligand interaction. The Journal of allergy and clinical immunology 139, 1468–1477 e1462, doi: 10.1016/j.jaci.2016.08.034 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ogasawara N et al. IL-10, TGF-beta, and glucocorticoid prevent the production of type 2 cytokines in human group 2 innate lymphoid cells. The Journal of allergy and clinical immunology 141, 1147–1151 e1148, doi: 10.1016/j.jaci.2017.09.025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morita H et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity 43, 175–186, doi: 10.1016/j.immuni.2015.06.021 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jostins L et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124, doi: 10.1038/nature11582 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khor B, Gardet A & Xavier RJ Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Crellin NK et al. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity 33, 752–764 (2010). [DOI] [PubMed] [Google Scholar]
- 116.Geremia A et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med 208, 1127–1133, doi: 10.1084/jem.20101712 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Longman RS et al. CX(3)CR1(+) mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J Exp Med 211, 1571–1583, doi: 10.1084/jem.20140678 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bernink JH et al. Interleukin-12 and −23 Control Plasticity of CD127(+) Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity 43, 146–160, doi: 10.1016/j.immuni.2015.06.019 (2015). [DOI] [PubMed] [Google Scholar]
- 119.Bernink JH et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol 14, 221–229 (2013). [DOI] [PubMed] [Google Scholar]
- 120.Klose CS et al. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature 494, 261–265 (2013). [DOI] [PubMed] [Google Scholar]
- 121.Vonarbourg C et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity 33, 736–751, doi: 10.1016/j.immuni.2010.10.017S1074-7613(10)00403-6 [pii] (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li J et al. Enrichment of IL-17A(+) IFN-gamma(+) and IL-22(+) IFN-gamma(+) T cell subsets is associated with reduction of NKp44(+) ILC3s in the terminal ileum of Crohn’s disease patients. Clin Exp Immunol 190, 143–153, doi: 10.1111/cei.12996 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kloverpris HN et al. Innate Lymphoid Cells Are Depleted Irreversibly during Acute HIV-1 Infection in the Absence of Viral Suppression. Immunity 44, 391–405, doi: 10.1016/j.immuni.2016.01.006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mudd JC et al. Hallmarks of primate lentiviral immunodeficiency infection recapitulate loss of innate lymphoid cells. Nat Commun 9, 3967, doi: 10.1038/s41467-018-05528-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brenchley JM et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12, 1365–1371, doi: 10.1038/nm1511 (2006). [DOI] [PubMed] [Google Scholar]
- 126.Munneke JM et al. Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease. Blood 124, 812–821, doi: 10.1182/blood-2013-11-536888 (2014). [DOI] [PubMed] [Google Scholar]
- 127.Hanash AM et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37, 339–350, doi: 10.1016/j.immuni.2012.05.028 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Craft JE Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol 8, 337–347, doi: 10.1038/nrrheum.2012.58 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rao DA et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114, doi: 10.1038/nature20810 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Teng F et al. Gut Microbiota Drive Autoimmune Arthritis by Promoting Differentiation and Migration of Peyer’s Patch T Follicular Helper Cells. Immunity 44, 875–888, doi: 10.1016/j.immuni.2016.03.013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu HJ et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827, doi: 10.1016/j.immuni.2010.06.001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schlenner S et al. NFIL3 mutations alter immune homeostasis and sensitise for arthritis pathology. Ann Rheum Dis, doi: 10.1136/annrheumdis-2018-213764 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rauber S et al. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat Med 23, 938–944, doi: 10.1038/nm.4373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Omata Y et al. Group 2 Innate Lymphoid Cells Attenuate Inflammatory Arthritis and Protect from Bone Destruction in Mice. Cell Rep 24, 169–180, doi: 10.1016/j.celrep.2018.06.005 (2018). [DOI] [PubMed] [Google Scholar]
- 135.Serafini B et al. RORgammat Expression and Lymphoid Neogenesis in the Brain of Patients with Secondary Progressive Multiple Sclerosis. J Neuropathol Exp Neurol 75, 877–888, doi: 10.1093/jnen/nlw063 (2016). [DOI] [PubMed] [Google Scholar]
- 136.Perry JS et al. Inhibition of LTi cell development by CD25 blockade is associated with decreased intrathecal inflammation in multiple sclerosis. Sci Transl Med 4, 145ra106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mjosberg JM et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol 12, 1055–1062, doi: 10.1038/ni.2104 (2011). [DOI] [PubMed] [Google Scholar]
- 138.Monticelli LA et al. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat Immunol 17, 656–665 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Monticelli LA et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12, 1045–1054 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bal SM et al. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol 17, 636–645, doi: 10.1038/ni.3444 (2016). [DOI] [PubMed] [Google Scholar]
- 141.Barnig C et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med 5, 174ra126, doi: 10.1126/scitranslmed.3004812 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim BS et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med 5, 170ra116, doi: 10.1126/scitranslmed.3005374 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Salimi M et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med 210, 2939–2950, doi: 10.1084/jem.20130351 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Silver JS et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol 17, 626–635, doi: 10.1038/ni.3443 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu S et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: The role of thymic stromal lymphopoietin. The Journal of allergy and clinical immunology 141, 257–268.e256, doi: 10.1016/j.jaci.2017.03.032 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kabata H et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun 4, 2675, doi: 10.1038/ncomms3675 (2013). [DOI] [PubMed] [Google Scholar]
- 147.Carrega P et al. NCR(+)ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat Commun 6, 8280, doi: 10.1038/ncomms9280 (2015). [DOI] [PubMed] [Google Scholar]
- 148.Kirchberger S et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med 210, 917–931, doi: 10.1084/jem.20122308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Salimi M et al. Activated innate lymphoid cell populations accumulate in human tumour tissues. BMC Cancer 18, 341, doi: 10.1186/s12885-018-4262-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Simoni Y et al. Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency. Immunity 46, 148–161, doi: 10.1016/j.immuni.2016.11.005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *151.Crome SQ et al. A distinct innate lymphoid cell population regulates tumor-associated T cells. Nat Med 23, 368–375, doi: 10.1038/nm.4278 (2017).* This study implicates ILC3-like cells as crucial suppressors of tumor-infiltrating T cells in human patients with ovarian cancer and identifies ILC interactions with adaptive immunity as a potential predictor of cancer recurrence.
- 152.Withers DR et al. Transient inhibition of ROR-gammat therapeutically limits intestinal inflammation by reducing TH17 cells and preserving group 3 innate lymphoid cells. Nat Med 22, 319–323, doi: 10.1038/nm.4046 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Oetjen LK et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 171, 217–228 e213, doi: 10.1016/j.cell.2017.08.006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Robinette ML et al. Jak3 deficiency blocks innate lymphoid cell development. Mucosal immunology (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yudanin NA et al. Spatial and Temporal Mapping of Human Innate Lymphoid Cells Reveals Elements of Tissue Specificity. Immunity, doi: 10.1016/j.immuni.2019.01.012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bar-Ephraim YE et al. Cross-Tissue Transcriptomic Analysis of Human Secondary Lymphoid Organ-Residing ILC3s Reveals a Quiescent State in the Absence of Inflammation. Cell Rep 21, 823–833, doi: 10.1016/j.celrep.2017.09.070 (2017). [DOI] [PubMed] [Google Scholar]
- 157.Bjorklund AK et al. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol 17, 451–460, doi: 10.1038/ni.3368 (2016). [DOI] [PubMed] [Google Scholar]
- 158.Gray EE, Friend S, Suzuki K, Phan TG & Cyster JG Subcapsular sinus macrophage fragmentation and CD169+ bleb acquisition by closely associated IL-17-committed innate-like lymphocytes. PloS one 7, e38258 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang Y et al. Migratory and adhesive cues controlling innate-like lymphocyte surveillance of the pathogen-exposed surface of the lymph node. Elife 5, doi: 10.7554/eLife.18156 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mabbott NA, Donaldson DS, Ohno H, Williams IR & Mahajan A Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol 6, 666–677 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mazzini E, Massimiliano L, Penna G & Rescigno M Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity 40, 248–261, doi: 10.1016/j.immuni.2013.12.012 (2014). [DOI] [PubMed] [Google Scholar]