Abstract
OBJECTIVE
Traditionally, diffusion MRI (dMRI) has been performed in parallel with high-resolution conventional MRI, which requires long scan times and may require sedation or general anesthesia in infants and young children. Conversely, fast brain MRI permits image acquisition without the need for sedation, although its short pulse sequences, susceptibility to motion artifact, and contrast resolution have limited its use to assessing ventricular size or major structural variations. Here, the authors demonstrate the feasibility of leveraging a 3-direction fast brain MRI protocol to obtain reliable dMRI measures.
METHODS
Fast brain MRI with 3-direction dMRI was performed in infants and children before and after hydrocephalus treatment. Regions of interest in the posterior limbs of the internal capsules (PLICs) and the genu of the corpus callosum (gCC) were drawn on diffusion-weighted images, and mean diffusivity (MD) data were extracted. Ventricular size was determined by the frontal occipital horn ratio (FOHR). Differences between and within groups pre- and posttreatment, and FOHR-MD correlations were assessed.
RESULTS
Of 40 patients who met inclusion criteria (median age 27.5 months), 15 (37.5%), 17 (42.5%), and 8 (20.0%) had posthemorrhagic hydrocephalus (PHH), congenital hydrocephalus (CH), or no intracranial abnormality (controls), respectively. A hydrocephalus group included both PHH and CH patients. Prior to treatment, the FOHR (p < 0.001) and PLIC MD (p = 0.027) were greater in the hydrocephalus group than in the controls. While the mean gCC MD in the hydrocephalus group (1.10 × 10−3 mm2/sec) was higher than that of the control group (0.98), the difference was not significant (p = 0.135). Following a median follow-up duration of 14 months, decreases in FOHR, PLIC MD, and gCC MD were observed in the hydrocephalus group and were similar to those in the control group (p = 0.107, p = 0.702, and p = 0.169, respectively). There were no correlations identified between FOHR and MDs at either time point.
CONCLUSIONS
The utility of fast brain MRI can be extended beyond anatomical assessments to obtain dMRI measures. A reduction in PLIC and gCC MD to levels similar to those of controls was observed within 14 months following shunt surgery for hydrocephalus in PHH and CH infants. Further studies are required to assess the role of fast brain dMRI for assessing clinical outcomes in pediatric hydrocephalus patients.
Keywords: hydrocephalus, fast brain MRI, diffusion MRI, diffusion tensor imaging
HYDROCEPHALUS is a common neurological disorder in which imbalances between CSF production and absorption typically result in ventricular enlargement and increased intracranial pressure.38 While the pathophysiology of hydrocephalus is not well understood, pathological changes in periventricular white matter (PVWM) have been associated with poor cognitive and neuromotor outcomes.33,54 Diffusion MRI (dMRI) has been used to delineate PVWM microstructural integrity and assess the effect of hydrocephalus on PVWM integrity and neurodevelopment.4,33,54 Traditionally, dMRI is performed in parallel with conventional high-resolution multidirectional MRI. In the clinical setting, this often requires sedation or general anesthesia in infants and young children, which carry risks of respiratory distress or pulmonary disease, hemodynamic instability, emotional distress, and potential long-term neurocognitive complications.32,49 Conversely, fast brain MRI permits image acquisition without the need for sedation,10,36,39,48 although its short pulse sequences, susceptibility to motion artifact, and contrast resolution have generally limited its use to assessing ventricular size or major structural variations.39,48 We present findings from a population of pediatric patients who underwent fast brain MRI using a dMRI protocol that allowed measurement of mean diffusivity (MD). Our hypothesis was that fast brain MRI can demonstrate dMRI indices before and after intervention for hydrocephalus.
Methods
Patients
Institutional review board approval was obtained at Washington University School of Medicine and St. Louis Children’s Hospital in St. Louis, Missouri. Our inclusion criteria for the hydrocephalus group were as follows: 1) age less than 18 years and 2) hydrocephalus as the indication for undergoing fast brain MRI. The exclusion criterion was having undergone conventional dMRI under sedation or general anesthesia. Overall, the records of all patients who underwent fast brain MRI at our institution between October 2009 and July 2018 were assessed (n = 191), and 40 patients met inclusion criteria. There were no biases introduced in excluding the 151 patients. Sixty-six of those patients were excluded a priori because their fast brain MRI scans were obtained for pathological but non–hydrocephalus-related indications such as trauma, tumor, subdural hematoma, arachnoid cyst, and Chiari malformation. Thirty-six patients did not have diffusion-weighted images available for analysis. An additional 49 patients were excluded because they lacked either a preoperative or a postoperative fast brain MRI scan for comparison. The indications and timing for fast brain MRI in the hydrocephalus patients were driven by standard clinical practice, where dMRI scans were obtained before and within a few days after shunt placement, and then also during their approximately 5-month and 14-month postoperative clinic visits. In the non-hydrocephalus controls, the indication for dMRI was suspicion of an intracranial pathology, which was found to be negative on MRI, both at the time of presentation and during their follow-up clinic visit. Each control patient had one clinically indicated follow-up MR image at approximately 14 months to ensure an intracranial pathology had not been previously missed. As such, as shown in Table 1, follow-up dMRI images that matched the controls to the hydrocephalus patients were only available at 14 months and not at 5 months. To account for expected age-related dMRI changes, the controls were age matched to the hydrocephalus group.19,33,54
TABLE 1.
dMRI measures of 40 patients demonstrating reduction in ventricular size and MD measures following treatment for hydrocephalus
| Measure | Scan Time | Hydro | PHH | CH | Control | Pairwise Comparisons | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Hydro-Control | PHH-Control | CH-Control | PHH-CH | ||
| FOHR | Pre-Tx | 0.54 | 0.09 | 0.51 | 0.07 | 0.57 | 0.10 | 0.34 | 0.06 | <0.001* | <0.001* | <0.001* | 0.062 |
| 5 mos | 0.51 | 0.10 | 0.49 | 0.10 | 0.53 | 0.11 | — | — | — | — | — | 0.293 | |
| 14 mos | 0.44 | 0.10 | 0.44 | 0.11 | 0.44 | 0.09 | 0.38 | 0.04 | 0.107 | 0.154 | 0.087 | 1.000 | |
| PLIC MD × 10−3 mm2/sec | Pre-Tx | 1.00 | 0.18 | 1.09 | 0.17 | 0.92 | 0.16 | 0.85 | 0.06 | 0.027* | 0.001* | 0.247 | 0.007* |
| 5 mos | 0.94 | 0.11 | 0.98 | 0.12 | 0.90 | 0.08 | — | — | — | — | — | 0.032 | |
| 14 mos | 0.85 | 0.07 | 0.85 | 0.06 | 0.84 | 0.08 | 0.84 | 0.04 | 0.702 | 0.678 | 1.000 | 0.695 | |
| gCC MD × 10−3 mm2/sec | Pre-Tx | 1.10 | 0.21 | 1.22 | 0.17 | 0.97 | 0.17 | 0.98 | 0.14 | 0.135 | 0.003* | 0.886 | <0.001* |
| 5 mos | 1.10 | 0.18 | 1.16 | 0.19 | 1.03 | 0.15 | — | — | — | — | — | 0.039* | |
| 14 mos | 0.94 | 0.11 | 0.94 | 0.13 | 0.94 | 0.09 | 1.00 | 0.10 | 0.169 | 0.269 | 0.147 | 1.000 | |
Hydro = hydrocephalus; Tx = treatment; — = between-group comparison not performed as follow-up measures were not available for the control group at 5 months. The hydrocephalus group combines patients with CH and patients with PHH. dMRI studies were obtained prior to hydrocephalus treatment (Pre-Tx) and at median follow-up times of 5 and 14 months.
Benjamini-Hochberg corrected p values with p < 0.05 set as significant.
Imaging Acquisition
All images were acquired preoperatively and at a median follow-up of 5 or 14 months on either a Siemens 3-T Trio or a 1.5-T Aera/Avanto imager (Siemens Medical Solutions) using standardized fast brain MRI acquisition protocols: 2D-FLASH (2D spoiled gradient echo) T1-weighted images obtained from the skull base to the vertex with an acquisition time of 2 seconds per slice (TR 189 msec, TE 4.76 msec, flip angle 90°); and HASTE (singleshot turbo spin echo) T2-weighted images obtained with an acquisition time of 800 msec/slice (TR 800 msec, TE 80 msec, flip angle 150°). Both T1- and T2-weighted sequences were set for a slice thickness of 5 mm with spacing of 6.5 mm and an acquisition matrix of 256 × 256 mm, and a total time of scanning of 104 seconds. Echo planar imaging dMRI was performed using the following parameters: field of view 240 × 240 mm, matrix 96 × 96, voxel resolution 2.5 mm cubic, number of slices 76, TR/TE 9400/93.2 msec; 3 directions, 1 non–diffusion-weighted image; b = 1000 sec/mm2; IPAT factor 2. Total 3-plane (axial, sagittal, and coronal) acquisition time for the entire brain averaged 6 minutes, which approximates acquisition times reported with protocols of other institutions.10,36
Image Postprocessing, Interpretation, and Analysis
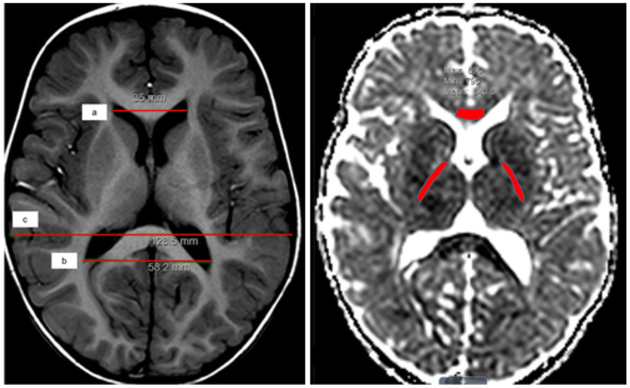
MD was calculated on the scanner from the trace of the diffusion tensor computed by averaging the diffusion measurement in the 3 axial directions derived from the conservation of the trace of the diffusion tensor under rotational transforms. Fractional anisotropy, axial diffusivity, and radial diffusivity were not evaluated as they require 6 or more directions of diffusion measurement. All images were reviewed independently by two pediatric neuroradiologists (J.S.S. and R.C.M.) and a neurosurgeon (D.D.L.), and all measures were performed by three independent reviewers (A.M.I., A.H., and M.C.). As demonstrated in Fig. 1 left, standard measurements described by O’Hayon et al.35 were used to calculate frontal occipital horn ratios (FOHRs) by all reviewers. When dysmorphic ventricular anatomy was encountered, the lead reviewer (A.M.I.) determined and demonstrated the margins of the ventricles to the other reviewers prior to all reviewers taking independent FOHR measurements. Hydrocephalus was defined as having the clinical phenotype and an FOHR that was greater than the upper limit of normal (0.44).35 As shown in Fig. 1 right, regions of interest (ROIs) in the genu of the corpus callosum (gCC) and the right and left posterior limbs of the internal capsules (PLICs) were delineated on the diffusion-weighted images in Imageweb imaging viewer, and their respective MD values were extracted. When white matter fibers appeared distorted, T2-weighted images were aligned to guide ROI placement. The left and right PLIC measures were averaged as no significant differences were detected between hemispheres.
FIG. 1.
Representative axial T1-weighted (left) and apparent diffusion coefficient (right) MR images demonstrating measurement of FOHR [(a+b)/2c] (left), and ROIs drawn to measure MDs in the gCC and right and left PLIC (right). Figure is available in color online only.
Statistical Analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute), and graphs were obtained from Prism 7.0e (GraphPad Software). FOHR and MD measures were obtained from the MR images of each patient acquired before the intervention for hydrocephalus, and median measurement occasions at 5 and 14 months following treatment. As FOHR and MD measures were continuous variables obtained by three independent observers, intraclass correlation coefficients were calculated to assess interrater reliability for all time points. Normality of data was determined with the Shapiro-Wilk test, and data are reported as the mean ± SD. An a priori power analysis was not performed, as all patients who met inclusion criteria were included in the analyses. Standard descriptive statistics of patient characteristics, including age and etiology of hydrocephalus, were obtained. Repeated measures ANOVA was used to perform between-group comparisons for the three time points (pretreatment and then median 5-month and 14-month posttreatment), first between the hydrocephalus and control groups, and then among posthemorrhagic hydrocephalus (PHH), congenital hydrocephalus (CH), and controls. All results of significance were Benjamini-Hochberg corrected. Given the reported variability in dMRI measures between different magnetic field strength MRI studies,21 the type of MRI used for data acquisition (Siemens 3-T Trio vs 1.5-T Aera/Avanto) was set as a covariate to adjust for the effect of MRI model/strength. Pearson’s correlations assessed relationships between FOHR and MD measures. The significance level was set at p < 0.05.
Results
Patients
Among the 40 patients who met inclusion criteria (median pretreatment age 27.5 months), 32 (80.0%) had hydrocephalus and were assigned to the hydrocephalus group, and the remaining 8 (20.0%) patients who had no intracranial abnormality were assigned to the control group. There was no significant difference between the mean age of the control patients and patients in the hydrocephalus group (p = 0.430). The hydrocephalus group comprised 15 (37.5%) and 17 (42.5%) patients who had PHH or CH, respectively.
Although the type of hydrocephalus treatment was not an exclusion criterion, all patients included in the study cohort had been treated with ventriculoperitoneal shunts; none were treated with endoscopic third ventriculostomy. There were no cases of hydranencephaly. Seventeen study subjects were female (42.5%). While 5 patients’ imaging studies had notable motion artifacts, their PLIC and gCC ROIs were easily identifiable and demarcated. No patients were excluded due to poor image quality.
Ventricular Size
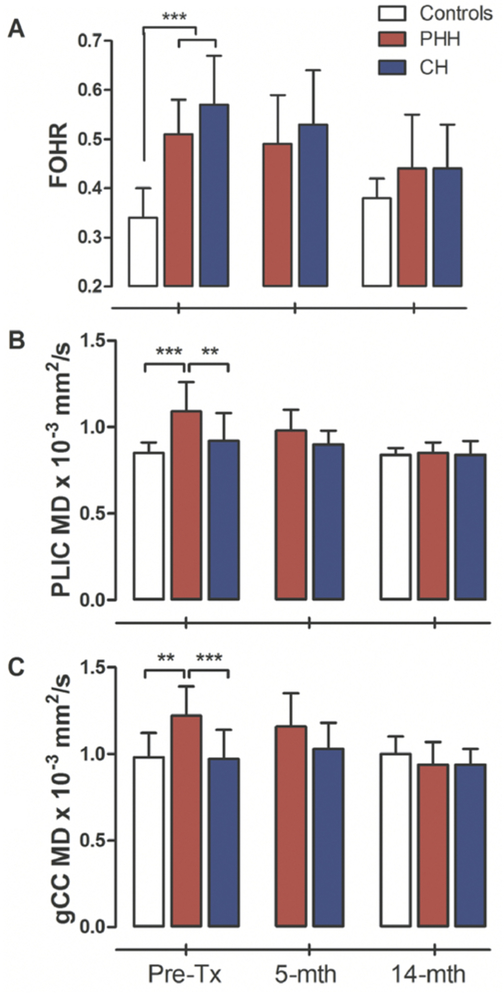
Prior to treatment, the mean FOHR in the hydrocephalus group was 0.54 ± 0.09, which was 58.82% larger than that of the controls (0.34 ± 0.06) (p < 0.001). In the hydrocephalus group, there was no significant difference between the FOHRs of the PHH and CH groups (p = 0.062); however, at the median 5- and 14-month follow-up visits, FOHRs had decreased by 5.88% and 22.72%, respectively, in the hydrocephalus group, relative to their respective preoperative ratios, which were no longer statistically different from the control (p = 0.107). Among the hydrocephalus patients, there was a 15.90% and 29.54% reduction in FOHRs in the PHH and CH groups, respectively, at the median 14-month follow-up, similar to levels seen in the control group (Fig. 2A). Table 1 shows the mean FOHRs and the PLIC and gCC MDs between the groups, prior to treatment for hydrocephalus and at the median 5-month and 14-month follow-up visits. Single measures intraclass coefficients among the three independent observers were 0.95 (95% CI 0.92–0.97, p < 0.001), 0.87 (95% CI 0.78–0.93, p < 0.001), and 0.91 (95% CI 0.85–0.95, p < 0.001) for the pretreatment, 5-month, and 14-month FOHR measurements, respectively.
FIG. 2.
FOHR and MD differences between hydrocephalus and control groups. At baseline, the CH and PHH groups had significantly larger ventricles than the controls, but this difference had resolved following treatment (A). Similar trends were observed in their PLIC (B) and gCC (C) measures at 5- and 14-month median follow-up. **p < 0.001 and ***p < 0.01, Benjamini-Hochberg corrected. Figure is available in color online only.
PLIC MD
As shown in Table 1, at presentation, the hydrocephalus group had a significantly higher mean PLIC MD (1.00 ± 0.18 × 10−3 mm2/sec) than the controls (0.85 ± 0.06) (p = 0.027). Within the hydrocephalus group, the PLIC MD of the PHH group (1.09 ± 0.17) was significantly higher than that of the CH group (0.92 ± 0.16) (p = 0.007). A between-groups comparison showed that the PLIC MD for the CH group was higher than that for controls but was not significantly different in this limited cohort (p = 0.247). Following treatment, the PLIC MD for the hydrocephalus group was reduced to 0.94 ± 0.11 at the median 5-month and 0.85 ± 0.07 at the median 14-month follow-ups, an overall 18.01% reduction, reaching levels similar to those seen in the controls (0.84 ± 0.08) (p = 0.702). Single measures intraclass coefficients among the three independent observers were 0.98 (95% CI 0.97–0.99, p < 0.001), 0.75 (95% CI 0.58–0.87, p < 0.001), and 0.75 (95% CI 0.60–0.86, p < 0.001) for the pretreatment, 5-month, and 14-month PLIC measurements, respectively.
gCC MD
Similar to the PLIC, the pretreatment gCC MD of the hydrocephalus group (1.10 ± 0.21 × 10−3 mm2/sec) was greater than that of the control group (0.98 ± 0.14), although the difference was not significant (p = 0.135). However, a between-groups comparison showed that the gCC MD for the PHH group (1.22 ± 0.17) was significantly greater than it was for both the CH patients (0.97 ± 0.17) (p < 0.001) and the controls (0.98 ± 0.14) (p = 0.003). At the 5-month follow-up, the gCC in the hydrocephalus group was largely unchanged (1.10 ± 0.18). However, at the 14-month follow-up, the gCC MD of the hydrocephalus group had decreased by 17% (0.94 ± 0.11), and the previous subgroup difference between PHH and CH had resolved (PHH 0.94 ± 0.13; CH 0.94 ± 0.09; p = 1.000) (Fig. 2C). Single measures intraclass coefficients among the three independent observers were 0.89 (95% CI 0.81–0.95, p < 0.001), 0.95 (95% CI 0.89–0.98, p < 0.001), and 0.89 (95% CI 0.80–0.94, p < 0.001) for the pretreatment, 5-month, and 14-month gCC measurements, respectively.
Ventricular Size and Mean Diffusion Correlations
No significant correlations were observed between FOHR and PLIC MD (r = 0.168, n = 40, p = 0.394) or gCC MD (r = 0.215, n = 40, p = 0.273) values. Furthermore, controlling for age at imaging did not yield any correlations between FOHR and PLIC or gCC MDs.
Discussion
Pediatric hydrocephalus patients commonly undergo serial neuroimaging for a wide range of indications, including postoperative confirmation of shunt placement, assessment of response to treatment, and workup for frequent treatment failures.17 MRI is the preferred neuroimaging modality in these patients because, unlike CT, it does not expose patients to ionizing radiation with its potential risks of developmental sequelae or radiationinduced cancers.6 However, standard MR images have lengthy acquisition times and require sedation or general anesthesia, especially in neonates and uncooperative children, which subjects them to risks of anesthesia-related complications.16,32 Furthermore, the United States Food and Drug Administration has recommended against repeated elective exposure to anesthesia in young children.49 Since 2002, fast brain MRI protocols have been implemented to circumvent the need for sedation during image acquisition in pediatric hydrocephalus patients. Fast brain MRI utilizes short pulse sequences to acquire MR images in 2–3 seconds per slice that are adequate for making prompt clinical decisions in most pediatric hydrocephalus patients.3,10 Nevertheless, due to concerns of poor image quality and susceptibility to motion artifacts, indications for fast brain MRI in hydrocephalus have been limited to gross anatomical/structural and ventricular catheter placement assessments.
There is ample evidence that ventricular dilatation in hydrocephalus, which is often associated with raised intracranial pressure, causes mechanical damage to adjacent PVWM fibers such as the corpus callosum and internal capsules, which have been associated with neuromotor and cognitive developmental deficits in children.12,13,20,24,52,53 More advanced dMRI sequences, such as diffusion tensor imaging (DTI), that utilize the directional diffusivity of water molecules within tissues to delineate white matter microstructural changes have been widely used to assess PVWM integrity in hydrocephalus.4,5,33,51–54 An important utility of dMRI measures is that they correlate with neurodevelopmental outcomes in children.33,54 For example, Mangano et al. (2016) showed that motor scores negatively correlated with PLIC MD scores, and, in general, patients with hydrocephalus had significantly lower conceptual, practical, social, and motor composite scores as measured using the Adaptive Behavior Assessment System (or ABAS-II) than healthy controls.33 Thompson et al. also showed that higher diffusivity values in the corpus callosum were associated with poor psychomotor development index scores at 2 years corrected age.47 Perhaps more importantly, dMRI measures tend to normalize following shunt surgery1,33 and, in some cases, well into adulthood. For example, Tan et al. demonstrated that higher fractional anisotropy measures correlated with better clinical outcomes assessed by the Headache Disability Inventory and the Hydrocephalus Outcome Questionnaire, among 21 adolescent and young adult patients who had been previously treated for hydrocephalus (at around age 2 years).46 As such, it is reasonable to suggest that serial conventional dMRI (and, as our data suggest, fast brain dMRI) may have both diagnostic and prognostic value in patient care.44 However, in spite of its utility, dMRI is usually performed as part of conventional high-resolution multisequence MR images and not typically as part of fast brain MRI protocols. Our goal was to demonstrate the feasibility of fast brain MRI for dMRI data acquisition. The PLIC and gCC were selected because their dMRI properties have been well characterized and documented as among the most important and vulnerable of white matter tissues that are affected by hydrocephalus2 and have also been associated with detrimental neurodevelopmental outcomes in hydrocephalic children.33
In our patient population, the ventricular size (FOHR) of the hydrocephalus patients was significantly larger than that of the controls, but by a median follow-up of 5 and 14 months following treatment, there was no significant difference between the groups. Similarly, the average MDs in the PLIC of the hydrocephalus group, which were significantly higher than those of the controls at initial presentation, had significantly decreased and normalized by 5 and 14 months posttreatment. While the initial gCC in the hydrocephalus group was higher than that of the controls, this difference was not statistically significant. However, within the hydrocephalus group, patients with PHH had significantly higher gCC MD values than both the controls and patients with CH. This difference may be attributed to certain factors that are unique to PHH. First, the vast majority of PHH patients were born prematurely, which is typically associated with high levels of inflammatory cytokines,11,22 delayed white matter maturation,27 and hypoxic-ischemic brain damage.40 In addition to the direct damage to the gCC tissue by the hemorrhage in PHH, there is also evidence that intraventricular hemorrhage induces secondary white matter injury through iron-mediated free radicals and hypoxanthine-derived oxidative damage.9 Decrements in gCC MDs were observed in the hydrocephalus group, after a median follow-up of 14 months, to levels almost similar to those of the controls.
Assessment of correlations between dMRI measures and ventricular size has yielded mixed results in the literature,2,8,29 and there is no consensus on whether ventricular size alone can be used as a surrogate marker for disease severity in hydrocephalus or as a predictor of neurological outcomes.7,29 While Akbari et al. found that corpus callosum fractional anisotropy, MD, and radial diffusivity correlated with FOHR in children with CH,2 Kulkarni et al. did not find any FOHR-dMRI associations in pediatric patients with treated, stable obstructive hydrocephalus.29 However, other MRI markers, such as cerebral blood flow, have been shown to correlate with ventricular size and have demonstrated some of the positive effects of ventricular decompression on cerebral hemodynamics in hydrocephalic infants.31,50 In the present study, while dMRI changes were noted in the hydrocephalus group, they did not correlate with ventricular size measures, supporting the notion that dMRI may be a sensitive measure of PVWM injury independent of ventricular size.54
Our findings of higher MD indices in the PVWM of patients with hydrocephalus are consistent with conventional dMRI reports in the literature that have found hydrocephalus to be associated with high MD (apparent diffusion coefficient) measures.2,4,33,54 Mangano et al. and Yuan et al. found that MD was significantly higher in the gCC of hydrocephalus children than healthy controls.33,54 Similarly, Assaf et al. assessed several white matter tracts, including the gCC and PLIC, in patients who presented with acute hydrocephalus and found that gCC MD was 29% higher in the hydrocephalus patients than in the 8 healthy controls.4 Increased MD in PVWM has been associated with impaired or delayed myelination or axonal damage,42,54 which may be hypothesized to be secondary to PVWM compression (e.g., from overt or occult ventricular pressure), infiltration by transependymal CSF,28 exposure to inflammatory or other factors in the CSF,22 or hydrocephalus-associated alterations in white matter development.14 Similar to previous clinical and experimental studies, our study showed that hydrocephalus-related PVWM injury, measured by dMRI indices, may be reversed by CSF-diversion surgery.4,15,33,41 Indeed, Mangano et al. reported improvements in dMRI measures that were observed in the PLIC of children with CH 12 months after CSF-diversion surgery.33 However, the rate of recovery may vary depending on a variety of factors.4,15,33,41 From a pathogenesis standpoint, white matter fibers that undergo impairment or delayed myelination may have a faster/better recovery following treatment than those that undergo axonal damage. In fact, several pathological studies have shown that, similar to neurodegenerative diseases,26,43,45 the corpus callosum in hydrocephalus undergoes neuronal degeneration,15,18 which may in part explain why recovery of the gCC MDs in our patient population was not as robust as that of the PLIC. Nevertheless, further mechanistic studies are required for substantiation.
In this study, we focused on demonstrating the feasibility of fast brain MR images for dMRI data acquisition and estimated MD from the average of 3 axial direction measurements, which were mathematically derived from the conservation of the trace of the diffusion tensor under rotational transforms. While it has been shown that increasing the number of diffusion-encoding directions may improve the quality of dMRI data,25,37 there is a tradeoff between scan time and dMRI data.30 We would suggest that an ideal fast brain dMR protocol is one that utilizes a minimal number of diffusion-encoding directions, with only a marginal increase in scan time, yet yields reliable and clinically relevant dMRI data. In order to assess fractional anisotropy, axial diffusivity, and radial diffusivity, a minimum of 6 diffusion-encoding directions are needed and some studies have demonstrated 6 directions is capable of generating robust dMRI data.30,34 Therefore, to improve on our dMRI parameter estimates and measure fractional anisotropy, axial diffusivity, radial diffusivity, and MD, we have expanded our fast brain dMRI protocol to 6 directions in an ongoing prospective study. From our preliminary observations, we have found that the 6-direction dMR image acquisition is also feasible with fast brain MRI (< 6 minutes total scan time), which is similar to acquisition times reported for protocols at other institutions.10,36 At most, 6-direction dMRI adds 70 seconds to the scan time; with multiband MRI, dMRI sequences can be acquired in < 25 seconds. The issue of costs associated with dMR image acquisition23 has to be recognized when adding dMRI to fast brain MRI, as billing and reimbursement are highly variable across (and within) hospitals and regions. However, in many health systems, including ours, fast brain MR images are billed as standard brain MR images (without contrast), and there is no additional cost to add these sequences.
Despite the interesting findings of this study, one has to be cognizant of several limitations. First, while this study establishes the reliability of fast brain dMRI measures, validity was not directly assessed, as our patient cohort did not include infants who underwent conventional dMRI to permit a cross-sectional comparison of the dMRI measures between fast brain and conventional MR images. However, our MD indices were generally within range of the measures reported by Leliefeld et al.,31 Yuan et al.,54 Air et al.,1 Mangano et al.,33 Akbari et al.,2 and Assaf et al.4 Nevertheless, one must exercise caution when comparing discrete values of dMRI measures across studies as there is often significant variability in study designs and local factors that can affect reported dMRI indices. These factors include, but are not limited to, differences in patient characteristics such as age, sex, etiology of hydrocephalus, MRI equipment models and strengths, and dMRI acquisition parameters. Second, given that only one follow-up time point was available to compare the control and hydrocephalus groups, there may be background age-related dMRI changes in the observed MD changes.19,33,54 However, age matching the controls to the hydrocephalus group potentially helped to reduce this confounder. Third, while we took every precaution to draw our ROIs as accurately as possible, partial volume effects, especially in those patients in whom anatomy was distorted due to their pathology and shunt artifacts, are potential confounders. However, we controlled for this by using the average measures obtained by three independent reviewers. Last, the control group comprised patients who were initially referred for clinical evaluation and in whom no neurological abnormalities were found on MRI. The fact that a clinician suspected a neurological explanation for the patient’s presentation may also be a confounding factor. Although one would expect that FOHRs would be consistent across healthy controls, there was a small increase (0.05) in the control group, which was driven by 2 patients whose ventricular size had increased from 0.23 to 0.42 in one infant and from 0.32 to 0.42 in another; this effect would have likely been minimal had the sample size been larger.
Conclusions
This study demonstrates that with modern imaging processing algorithms, fast brain MRI can be utilized to obtain dMRI measures in pediatric patients. PVWM injury, assessed with dMRI MD measures, exhibits improvement following hydrocephalus treatment, in serially assessed patients. Specifically, MD measures in the PLIC and gCC decrease to levels similar to those seen in controls within 15 months of hydrocephalus treatment. With continued advances in technology, fast brain MRI protocols may be expanded to obtain other dMRI measures such as fractional anisotropy and axial and radial diffusivity.
Acknowledgments
Disclosures
Dr. Castaneyra-Ruiz reports receiving support for non-study-related clinical or research efforts that he oversees from Microbot Medical. Dr. McAllister reports receiving clinical or research support for the present study from the following: Rudi Schulte Research Institute, Hydrocephalus Association, National Institutes of Health, and Microbot Medical; he also reports owning stock in Aqueduct Neuroscience, Inc. Dr. Limbrick reports receiving support for non-study-related clinical or research efforts that he oversees from Microbot Medical.
ABBREVIATIONS
- CH
congenital hydrocephalus
- dMRI
diffusion MRI
- FOHR
frontal occipital horn ratio
- gCC
genu of the corpus callosum
- MD
mean diffusivity
- PHH
posthemorrhagic hydrocephalus
- PLIC
posterior limb of the internal capsule
- PVWM
periventricular white matter
- ROI
region of interest
Footnotes
Supplemental Information
Previous Presentations
Portions of this paper were presented in oral form at the 47th Annual Meeting of the AANS/CNS Section on Pediatric Neurological Surgery, Nashville, Tennessee, December 6–9, 2018.
References
- 1.Air EL, Yuan W, Holland SK, Jones BV, Bierbrauer K, Altaye M, et al. : Longitudinal comparison of pre- and postoperative diffusion tensor imaging parameters in young children with hydrocephalus. J Neurosurg Pediatr 5:385–391, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Akbari SH, Limbrick DD Jr, McKinstry RC, Altaye M, Ragan DK, Yuan W, et al. : Periventricular hyperintensity in children with hydrocephalus. Pediatr Radiol 45:1189–1197, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley WW Jr, McKinstry RC, Leonard JR, Smyth MD, Lee BC, Park TS: Use of rapid-sequence magnetic resonance imaging for evaluation of hydrocephalus in children. J Neurosurg 103 (2 Suppl):124–130, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Assaf Y, Ben-Sira L, Constantini S, Chang LC, Beni-Adani L: Diffusion tensor imaging in hydrocephalus: initial experience. AJNR Am J Neuroradiol 27:1717–1724, 2006 [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Sira L, Goder N, Bassan H, Lifshits S, Assaf Y, Constantini S: Clinical benefits of diffusion tensor imaging in hydrocephalus. J Neurosurg Pediatr 16:195–202, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Brenner DJ, Hall EJ: Computed tomography—an increasing source of radiation exposure. N Engl J Med 357:2277–2284, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Buckley RT, Yuan W, Mangano FT, Phillips JM, Powell S, McKinstry RC, et al. : Longitudinal comparison of diffusion tensor imaging parameters and neuropsychological measures following endoscopic third ventriculostomy for hydrocephalus. J Neurosurg Pediatr 9:630–635, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cauley KA, Cataltepe O: Axial diffusivity of the corona radiata correlated with ventricular size in adult hydrocephalus. AJR Am J Roentgenol 203:170–179, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Cherian S, Whitelaw A, Thoresen M, Love S: The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol 14:305–311, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christy A, Murchison C, Wilson JL: Quick brain magnetic resonance imaging with diffusion-weighted imaging as a first imaging modality in pediatric stroke. Pediatr Neurol 78:55–60, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Dammann O, Leviton A: Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res 75:376–380, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Bigio MR: Cellular damage and prevention in childhood hydrocephalus. Brain Pathol 14:317–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Bigio MR, da Silva MC, Drake JM, Tuor UI: Acute and chronic cerebral white matter damage in neonatal hydrocephalus. Can J Neurol Sci 21:299–305, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Del Bigio MR, Wilson MJ, Enno T: Chronic hydrocephalus in rats and humans: white matter loss and behavior changes. Ann Neurol 53:337–346, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Del Bigio MR, Zhang YW: Cell death, axonal damage, and cell birth in the immature rat brain following induction of hydrocephalus. Exp Neurol 154:157–169, 1998 [DOI] [PubMed] [Google Scholar]
- 16.DiMaggio C, Sun LS, Li G: Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg 113:1143–1151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinçer A, Özek MM: Radiologic evaluation of pediatric hydrocephalus. Childs Nerv Syst 27:1543–1562, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Ding Y, McAllister JP II, Yao B, Yan N, Canady AI: Axonal damage associated with enlargement of ventricles during hydrocephalus: a silver impregnation study. Neurol Res 23:581–587, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L: The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience 276:48–71, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Fletcher JM, Bohan TP, Brandt ME, Brookshire BL, Beaver SR, Francis DJ, et al. : Cerebral white matter and cognition in hydrocephalic children. Arch Neurol 49:818–824, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Fushimi Y, Miki Y, Okada T, Yamamoto A, Mori N, Hanakawa T, et al. : Fractional anisotropy and mean diffusivity: comparison between 3.0-T and 1.5-T diffusion tensor imaging with parallel imaging using histogram and region of interest analysis. NMR Biomed 20:743–748, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Habiyaremye G, Morales DM, Morgan CD, McAllister JP, CreveCoeur TS, Han RH, et al. : Chemokine and cytokine levels in the lumbar cerebrospinal fluid of preterm infants with post-hemorrhagic hydrocephalus. Fluids Barriers CNS 14:35, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock C, Bernal B, Medina C, Medina S: Cost analysis of diffusion tensor imaging and MR tractography of the brain. Open J Radiol 4:260–269, 2014 [Google Scholar]
- 24.Hannay HJ: Functioning of the corpus callosum in children with early hydrocephalus. J Int Neuropsychol Soc 6:351–361, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Hasan KM, Parker DL, Alexander AL: Comparison of gradient encoding schemes for diffusion-tensor MRI. J Magn Reson Imaging 13:769–780, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Kealey SM, Kim Y, Provenzale JM: Redefinition of multiple sclerosis plaque size using diffusion tensor MRI. AJR Am J Roentgenol 183:497–503, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Kelly CE, Cheong JL, Gabra Fam L, Leemans A, Seal ML, Doyle LW, et al. : Moderate and late preterm infants exhibit widespread brain white matter microstructure alterations at term-equivalent age relative to term-born controls. Brain Imaging Behav 10:41–49, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Kim H, Jeong EJ, Park DH, Czosnyka Z, Yoon BC, Kim K, et al. : Finite element analysis of periventricular lucency in hydrocephalus: extravasation or transependymal CSF absorption? J Neurosurg 124:334–341, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni AV, Donnelly R, Mabbott DJ, Widjaja E: Relationship between ventricular size, white matter injury, and neurocognition in children with stable, treated hydrocephalus. J Neurosurg Pediatr 16:267–274, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Lebel C, Benner T, Beaulieu C: Six is enough? Comparison of diffusion parameters measured using six or more diffusion-encoding gradient directions with deterministic tractography. Magn Reson Med 68:474–483, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Leliefeld PH, Gooskens RHJM, Vincken KL, Ramos LMP, van der Grond J, Tulleken CAF, et al. : Magnetic resonance imaging for quantitative flow measurement in infants with hydrocephalus: a prospective study. J Neurosurg Pediatr 2:163–170, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Malviya S, Voepel-Lewis T, Eldevik OP, Rockwell DT, Wong JH, Tait AR: Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth 84:743–748, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Mangano FT, Altaye M, McKinstry RC, Shimony JS, Powell SK, Phillips JM, et al. : Diffusion tensor imaging study of pediatric patients with congenital hydrocephalus: 1-year postsurgical outcomes. J Neurosurg Pediatr 18:306–319, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni H, Kavcic V, Zhu T, Ekholm S, Zhong J: Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. AJNR Am J Neuroradiol 27:1776–1781, 2006 [PMC free article] [PubMed] [Google Scholar]
- 35.O’Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M: Frontal and occipital horn ratio: A linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg 29:245–249, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Patel DM, Tubbs RS, Pate G, Johnston JM Jr, Blount JP: Fastsequence MRI studies for surveillance imaging in pediatric hydrocephalus. J Neurosurg Pediatr 13:440–447, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Poonawalla AH, Zhou XJ: Analytical error propagation in diffusion anisotropy calculations. J Magn Reson Imaging 19:489–498, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Rekate HL: A contemporary definition and classification of hydrocephalus. Semin Pediatr Neurol 16:9–15, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Rozovsky K, Ventureyra EC, Miller E: Fast-brain MRI in children is quick, without sedation, and radiation-free, but beware of limitations. J Clin Neurosci 20:400–405, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Salmaso N, Jablonska B, Scafidi J, Vaccarino FM, Gallo V: Neurobiology of premature brain injury. Nat Neurosci 17:341–346, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheel M, Diekhoff T, Sprung C, Hoffmann KT: Diffusion tensor imaging in hydrocephalus—findings before and after shunt surgery. Acta Neurochir (Wien) 154:1699–1706, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH: Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17:1429–1436, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, et al. : Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26:132–140, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Sun M, Yuan W, Hertzler DA, Cancelliere A, Altaye M, Mangano FT: Diffusion tensor imaging findings in young children with benign external hydrocephalus differ from the normal population. Childs Nerv Syst 28:199–208, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Sundgren PC, Dong Q, Gómez-Hassan D, Mukherji SK, Maly P, Welsh R: Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology 46:339–350, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Tan K, Meiri A, Mowrey WB, Abbott R, Goodrich JT, Sandler AL, et al. : Diffusion tensor imaging and ventricle volume quantification in patients with chronic shunt-treated hydrocephalus: a matched case-control study. J Neurosurg 129:1611–1622, 2018 [DOI] [PubMed] [Google Scholar]
- 47.Thompson DK, Inder TE, Faggian N, Warfield SK, Anderson PJ, Doyle LW, et al. : Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage 59:3571–3581, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson EM, Baird LC, Selden NR: Results of a North American survey of rapid-sequence MRI utilization to evaluate cerebral ventricles in children. J Neurosurg Pediatr 13:636–640, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Food U.S. & Drug Administration: FDA Approves Label Changes for Use of General Anesthetic and Sedation Drugs in Young Children. FDA Drug Safety Communications: Safety Announcement, 2017. (https://www.fda.gov/media/104705/download) [Accessed May 29, 2019] [Google Scholar]
- 50.Yeom KW, Lober RM, Alexander A, Cheshier SH, Edwards MSB: Hydrocephalus decreases arterial spin-labeled cerebral perfusion. AJNR Am J Neuroradiol 35:1433–1439, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan W, Deren KE, McAllister JP II, Holland SK, Lindquist DM, Cancelliere A, et al. : Diffusion tensor imaging correlates with cytopathology in a rat model of neonatal hydrocephalus. Cerebrospinal Fluid Res 7:19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan W, McAllister JP II, Lindquist DM, Gill N, Holland SK, Henkel D, et al. : Diffusion tensor imaging of white matter injury in a rat model of infantile hydrocephalus. Childs Nerv Syst 28:47–54, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Yuan W, McAllister JPI, Mangano FT: Neuroimaging of white matter abnormalities in pediatric hydrocephalus. J Pediatr Neuroradiol 2:119–128, 2013 [Google Scholar]
- 54.Yuan W, McKinstry RC, Shimony JS, Altaye M, Powell SK, Phillips JM, et al. : Diffusion tensor imaging properties and neurobehavioral outcomes in children with hydrocephalus. AJNR Am J Neuroradiol 34:439–445, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]