Abstract
Background
This study aimed to evaluate the efficacy of a porous polyoxymethylene thermoplastic regulator combined with a three-dimensional (3D) printed template to guide pedicle needle insertion in patients undergoing percutaneous pedicle screw fixation (PPSF) for thoracolumbar fracture.
Material/Methods
Forty patients were randomly divided into group A, treated using a porous polyoxymethylene thermoplastic regulator combined with a 3D printed template, and group B, who underwent conventional PPSF. Data recorded included the number of pedicle screws successfully inserted on the first attempt, the number of attempts, the time to successful needle insertion, the total time of fluoroscopy, and the duration of surgery. The Visual Analogue Scale (VAS) and the Oswestry Disability Index (ODI) scores one day before surgery, and at day 1, day 7, month 1, and month 3 after surgery were recorded. The postoperative vertebral posterior kyphotic angle (KA) and the rate of change of KA were recorded.
Results
Group A had a significantly increased total number of successful first insertions compared with group BV (P<0.05). Postoperative VAS and ODI scores of patients in both groups were significantly lower than before surgery (P<0.05), with no significant difference between the two groups at postoperative month 1 and month 3 (P>0.05). The postoperative vertebral posterior KA decreased significantly in both groups after surgery, with no significant difference between the two groups (P>0.05).
Conclusions
The use of a porous polyoxymethylene thermoplastic regulator combined with a 3D printed template may improve the success of pedicle insertion in patients undergoing PPSF.
MeSH Keywords: Bioprinting; Bone Screws; Fractures, Compression
Background
Percutaneous pedicle screw fixation (PPSF), using a posterior approach and internal screws, is a common treatment for fractures of the thoracolumbar and lumbar spine. The clinical outcome of PPSF depends on the accuracy of pedicle screw insertion. Internal pedicle screw fixation (PSF) is the first choice for thoracolumbar spinal fractures. Traditional PSF surgery often involves a large and extensive surgical incision in the paravertebral fascia, muscles, and other soft tissues, which results in prolonged postoperative recovery and postoperative symptoms that include low back pain [1]. Recently, with the development of minimally invasive technology, PPSF has become a more effective single-stage surgical method for the management of thoracolumbar compression fractures and has the advantages of short operation time and reduced soft tissue injury [2–4].
During PPSF, pedicle insertion is a crucial step. The accuracy of the insertion point and the insertion angle determines whether the pedicle screw can be accurately placed. The success rate of the initial insertion for conventional PPSF surgery is not high, and the insertion cannot be accurately adjusted. This problem is partly due to the experience of the surgeon, but most importantly, it is caused by the mobility of soft tissues and skin and is usually performed without navigation and guidance. If the screw is not placed accurately, the pedicle screw may be immobilized, injure the spinal cord, spinal nerves, and major blood vessels, resulting in paralysis or even death of the patient [5–7]. In conventional PPSF, repeat fluoroscopy with C-arm fluoroscopy may be needed to position and adjust the insertion point and insertion angle, which will be associated with increased radiation and hazards to the operator and patient [8]. Also, the prolonged prone position time is not good for patients with cardiopulmonary insufficiency.
However, the development and application of computer navigation technology, virtual reality technology, and the use of personalized three-dimensional (3D) printed templates to guide surgery provide a new approach for precise positioning of percutaneous pedicle insertion. Computed tomography (CT)-navigated surgery may improve the success rate of percutaneous pedicle insertion. Compared with conventional PPSF surgery [9,10], the use of computer navigation technology can guide the operator to perform pedicle insertion and pedicle screw placement in real-time during the operation, the equipment is expensive, and the duration of the procedure may be increased [11–14].
Computer-aided design (CAD) simulation software can include mixed reality technology based on preoperative CT data of the surgical site. During the operation, the virtual insertion vertebra can be matched with the real vertebra of the patient through imaging, and the pedicle insertion can be performed visually [15]. However, the real-time image is not stable during the actual operation process, which may not represent an accurate fit with the real vertebral body, and the image may also be lost. These limitations indicate that CAD system software still needs to be optimized. However, the use of customized instruments can improve the accuracy of positioning, but there are still some errors in actual operation due to the anatomic differences of each patient [12,16]. With the development of 3D printed technology, preoperative CT data of patients may be input for CAD-simulated pedicle insertion, and a personalized percutaneous guide template can be designed and printed for the positioning of the pedicle insertion point [17]. Because of the low flexibility of skin and muscle, the insertion needle might migrate during insertion, and once misplaced, the needle may not be modified easily from the single guide template. Then surgeons may then still need to use fluoroscopy several times to adjust the positioning, leading to longer operation times and increased radiation doses to patients.
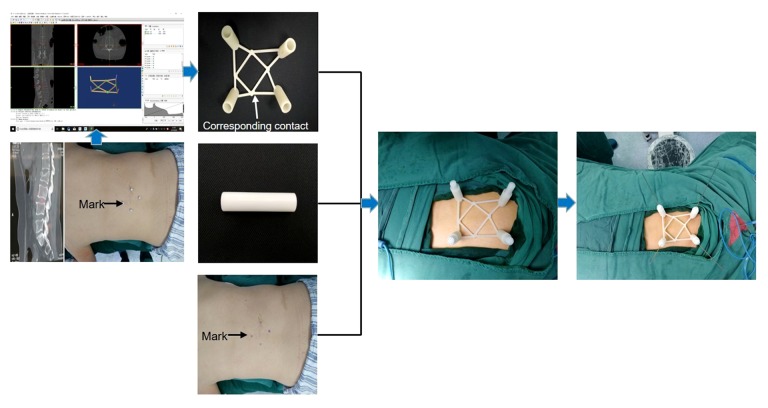
Therefore, this study aimed to evaluate the efficacy of a porous polyoxymethylene thermoplastic regulator (‘lotus-shaped’) combined with a three-dimensional (3D) printed template to guide pedicle needle insertion in patients undergoing percutaneous pedicle screw fixation (PPSF) for thoracolumbar fracture (Figure 1).
Figure 1.
Principles and procedures of percutaneous guide plate combined with lotus-style regulator assist puncture positioning.
Material and Methods
Patients
Between January 2018 to August 2019, there were 40 patients enrolled in the study, who underwent percutaneous pedicle screw fixation (PPSF), including 12 men and 28 women, with a mean age of 59.6 years, in the Xuzhou Medical University Affiliated to the Second Peoples’ Hospital of Huai’an. All patients signed an informed consent before surgery. This study was approved by the Ethics Committee of the Xuzhou Medical University Affiliated to the Second Peoples’ Hospital of Huai’an.
Design of the porous polyoxymethylene thermoplastic regulator and guide template
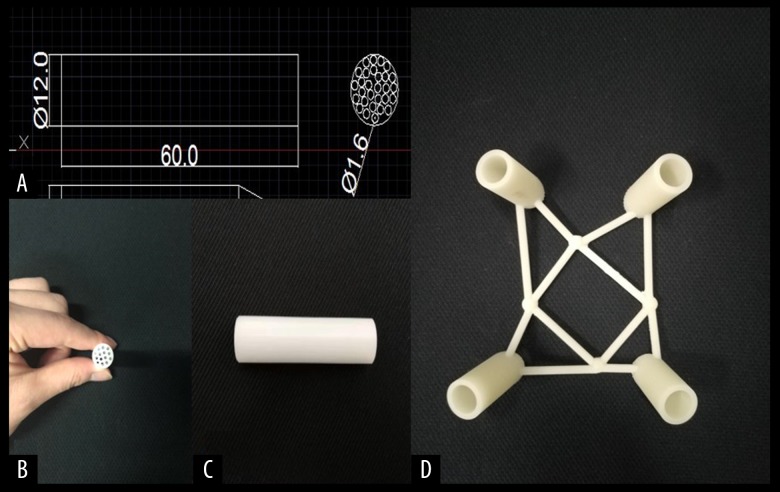
The porous polyoxymethylene thermoplastic regulator (a ‘lotus-root’ design) was designed and manufactured (Figure 2A–2C). The three-dimensional (3D) printed guide template was designed according to the corresponding computed tomography (CT) data of each patient, using the Materialise Mimics version 19.0 software and Magics version 21.0 software (Materialise, Leuven, Belgium). The guide template was printed using a 3D printer (Pulisheng Electromechanical Technology Co., Ltd., Shanghai, China) in the Key Laboratory of Medical 3D Printing in Huai’an city (Figure 2D). The parameters of the porous polyoxymethylene thermoplastic regulator and the guide template are shown in Table 1.
Figure 2.
(A) Lotus-type regulator parameter dimensions. (B, C): Top view and side view of the lotus-type regulator. (D) 3D printed percutaneous guide plate.
Table 1.
Characteristics of the porous polyoxymethylene thermoplastic regulator and the percutaneous guide template.
| Material | Porous polyoxymethylene thermoplastic regulator | Percutaneous guide template |
|---|---|---|
| Material composition | Polyoxymethylene | Photosensitive resin |
|
| ||
| Material parameter | Diameter of the main lumen: 12 mm | Outer diameter: 15 mm |
| Diameter of the sub-lumen: 1.6 mm | Inner diameter: 13 mm | |
| Length: 60 mm | ||
|
| ||
| Physicochemical properties | Solid, hard texture | Solid, hard texture |
|
| ||
| Biocompatibility | Poor | Poor |
|
| ||
| Adverse reactions | No potential cytotoxicity or allergic reaction | No potential cytotoxicity or allergic reaction |
The use of the personalized 3D printed guide template
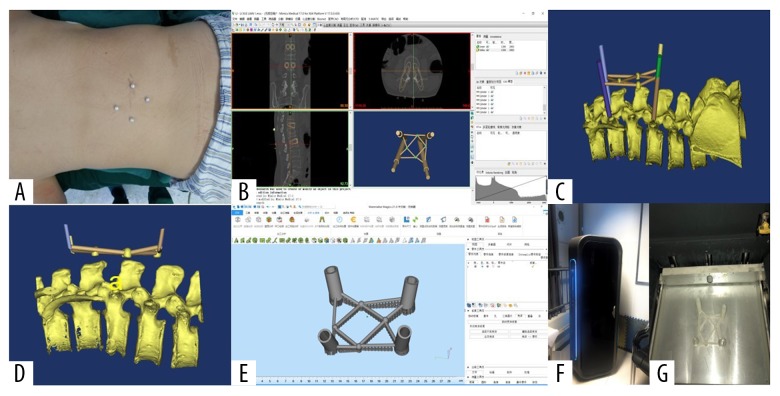
Briefly, patients received a CT scan before surgery. The area of the fractured vertebral body was marked using four hemispherical pearls (diameter of 1 cm) at four points, 4 cm away from the center of the vertebral body (Figure 3A). The Digital Imaging and Communications in Medicine (DICOM) file of the scanned CT data was imported into Materialise Mimics version 19.0 software (Materialise, Leuven, Belgium), and computer-aided design (CAD) was performed. The threshold segmentation function was used to reconstruct the 3D model of four markers and the surrounded area.
Figure 3.
The main process of percutaneous guide plate design and 3D printing. (A) Preoperative localization of markers was performed by CT scan. (B–E) CAD of the main process of person alized percutaneous guide plate. (F, G) 3D printer is printing the real percutaneous guide plate.
The insertion point and insertion angle were accurately selected to ensure that the inner column was in the insertion vertebral body pedicle in the coronal plane, the sagittal plane, and the transverse plane. The inner diameter of the column was increased to 13 cm, as required by the design template. Four hollow navigation tubes were obtained by subtracting the outer column from the inner column by Boolean operation (Figure 3B–3D). Materialise Magics version 21.0 software (Materialise, Leuven, Belgium) was used to design the percutaneous guide template (Figure 3E), by using the functions of part cutting and Boolean operations. The stereolithography (STL) file of the designed percutaneous guide template was imported into the 3D printer (Pulisheng Electromechanical Technology Co., Ltd., Shanghai, China), and the guide template was printed with a photosensitive resin material (Figure 3F, 3G).
Surgical procedures
Forty patients were randomly divided into two groups. Patients who underwent PPSF surgery with the percutaneous guide template combined with the porous polyoxymethylene thermoplastic regulator were classified into group A (n=20), including five men and 15 women, with an average age of 61.5 years. Lesions included the segments of T11 (one case), T12 (two cases), L1 (three cases), L2 (eight cases), and L3 (six cases). Twenty patients in the control group, or group B, including seven men and 13 women, with an average age of 56.6 years. Lesions included the segments T11 (one case), T12 (one case), L1 (two cases), L2 (nine cases), and L3 (seven cases).
Surgery was performed by two experienced surgeons. The patients were in the prone position, with pads beneath the chest and pelvis to increase lordosis and obtain partial reduction. Twenty patients received the porous polyoxymethylene thermoplastic regulator and the 3D printed template that guided the PPSF procedure.
The use of the porous polyoxymethylene thermoplastic regulator and the 3D printed template guided PPSF (group A)
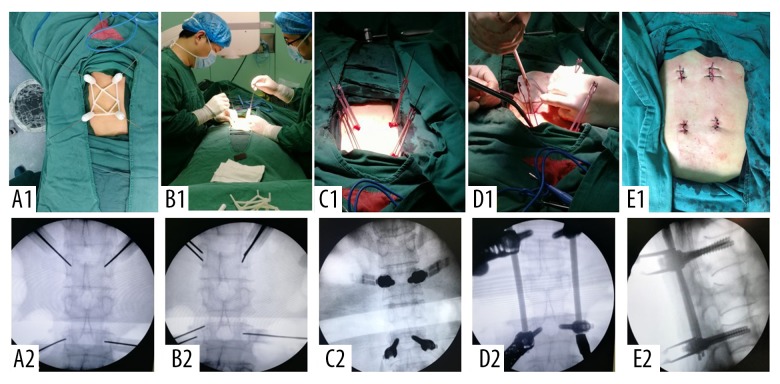
The percutaneous guide template was designed, and the four porous polyoxymethylene thermoplastic regulators were placed into the four navigation tubes. Four insertion needles with a diameter of 1.5 mm were inserted into the surface of the bone cortex along the center of the porous polyoxymethylene thermoplastic regulator, and then into the bone cortex. C-arm fluoroscopy was used to observe the position and the angle of the insertion needles (Figure 4A1, 4A2). If the first insertion ass successfully positioned, the insertion continued, and the needle reached the anterior one-third of the affected vertebra through the pedicle. If positioning was not ideal, the holes around the center hole of the first insertion were adjusted according to the offset position of the first insertion needle, which was used as a reference without moving the percutaneous guide template and the porous polyoxymethylene thermoplastic regulator (Figure 4B1, 4B2). When the insertion needle reached the ideal insertion point, insertion continued to the anterior one-third of the vertebral body. Four longitudinal incisions of about 2 cm in length were made through the insertion needles, and a sleeve was gradually placed to the bone surface to protect the surrounding soft tissues and to retain the outer expansion sleeve. The corresponding bone cortex flaring and tapping was performed using four percutaneous pedicle screws, which were placed and adjusted to ensure the same opening at the end of the lateral screw (Figure 4C1, 4C2).
Figure 4.
(A1–E1) The main steps of percutaneous guide combined with lotus root adjuster to assist PPSF. (A2–E2) The perspective image of the corresponding step.
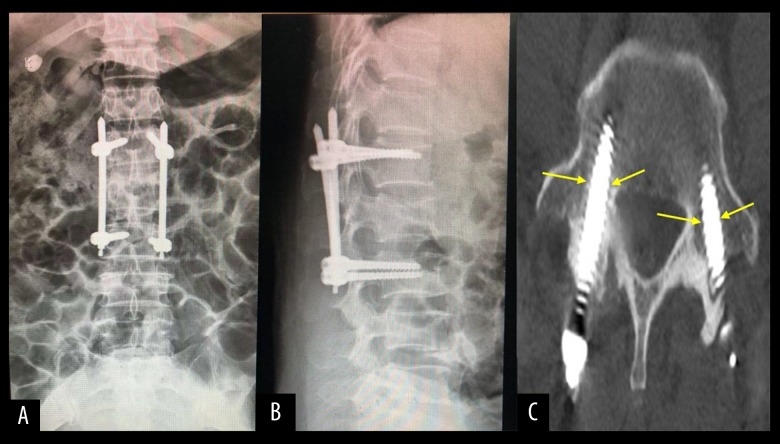
The preformed titanium connecting rod was installed by inserting the tip into the groove at the end of the screw tail with the rod holder, which was tightened. The other end of the titanium rod was placed into the groove at the end of the corresponding screw tail. The nut at the end was tightened, the spreader was tightened to restore the height of the affected vertebra, and the nuts at both ends of the fixation rod were tightened (Figure 4D1, 5D2). After the fluoroscopy nail rod was positioned satisfactorily, the surgical site was closed in layers (Figure 4E1, 4E2). The X-ray and CT images of the surgical site were re-examined within one week after surgery, and the position of the nail rod, height recovery of the affected vertebra (Figure 5A, 5B), and the position of the screw in the pedicle were observed (Figure 5C).
Figure 5.
(A, B): X-ray of group A 1 day after operation, and the heigh of the affected vertebra recovered well after operation. (C) CT of group A 1 day after operation, and the pedicle screw is in the pedicle.
Conventional PPSF (group B)
The target vertebra was determined by C-arm fluoroscopy, and the insertion point of the affected vertebra pedicle was initially located and marked. Screw insertion was performed according to the marked points. The offset position of the insertion needle through the C-arm perspective was observed, the orientation and the angle of the insertion needle was adjusted according to experience of the surgeon. A constant perspective was obtained through the C-arm, and the ideal insertion point and insertion angle were determined. The needle passed through the pedicle and entered the first third of the affected vertebra. The remaining steps were the same as for group A.
Patient clinical and surgical parameters
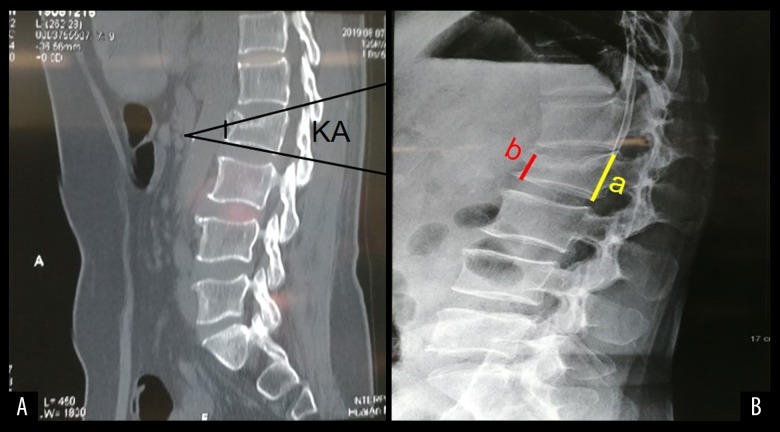
The number of successful pedicle insertions, the insertion times before the insertion needle reached the ideal position, the total fluoroscopy times, and the total volumes of intraoperative bleeding were recorded. The Visual Analogue Scale (VAS) and the Oswestry Disability Index (ODI) scores at one day before surgery, at day 1, day 7, month 1, and month 3 after surgery were recorded. The vertebral pedicle insertion number and the rate of change in the kyphosis angle (KA) were noted (Figure 6).
Figure 6.
(A) KA is the angle between the upper and lower endplate planes. (B) A Stands for normal vertebral height and b represents the height of the compressed vertebra; CR=(a–b)/a×100%.
Statistical analysis
Data were analyzed using SPSS version 19.0 software (IBM, Chicago, IL, USA). Data were expressed as the mean±standard deviation (SD). A paired t-test was used to compare the measurement data within the groups, and a t-test was used to compare data between the two groups. The chi-squared (χ2) test was used to compare quantitative data. The rank-sum test was used to compare qualitative data. A P-value <0.05 was considered to be statistically significant.
Results
Patient clinicopathological data
Forty patients who underwent percutaneous pedicle screw fixation (PPSF), including 12 men and 28 women, were randomly divided into two groups with 20 patients in each group. There were no significant differences between the two groups in gender, age, weight, degree of vertebral body compression, kyphotic angle (KA), preoperative Visual Analogue Scale (VAS) and the Oswestry Disability Index (ODI) scores, and position level of the affected vertebrae (P>0.05) (Table 2).
Table 2.
Demographic and clinicopathological data of group A, treated using a porous polyoxymethylene thermoplastic regulator combined with a 3D printed template and group B, who underwent conventional percutaneous pedicle screw fixation (PPSF).
| Patient characteristics | Group A | Group B | Statistic | P-value |
|---|---|---|---|---|
| Age (years) | 56.57±5.50 | 57.33±4.63 | t=−0.227 | P=0.825 >0.05 |
| Gender (n) | χ2=0.476 | P=0.490 >0.05 | ||
| Men | 5 | 7 | ||
| Women | 15 | 13 | ||
| Weight (kg) | 67.50±5.75 | 66.67±6.47 | t=0.236 | P=0.818 >0.05 |
| Degree of vertebral compression (%) | 48.67±10.80 | 47.17±12.22 | t=0.225 | P=0.826 >0.05 |
| Preoperative kyphotic angle (KA) (°) | 26.67±5.64 | 25.33±4.32 | t=0.459 | P=0.656 >0.05 |
| Preoperative VAS scores | 6.50±1.05 | 6.17±1.17 | t=0.520 | P=0.615 >0.05 |
| Preoperative ODI scores | 81.17±1.47 | 82.17±1.60 | t=−1.126 | P=0.287 >0.05 |
| Level of injured vertebra (n) | z=1.894 | P=0.058 >0.05 | ||
| T11 | 1 | 1 | ||
| T12 | 2 | 1 | ||
| L1 | 3 | 2 | ||
| L2 | 8 | 9 | ||
| L3 | 6 | 7 |
VAS – Visual Analogue Scale; ODI – Oswestry Disability Index.
Intraoperative parameters
All surgical procedures for PPSF were successfully completed. The number of pedicles successfully pierced at first attempt in group A was greater than that in group B (P<0.05). The number of insertions, the operation time before the insertion needle reached the ideal position, the total number and time of intraoperative fluoroscopy sessions, and the intraoperative blood loss were all significantly lower in group A than in group B (P<0.05) (Table 3).
Table 3.
Comparison of the intraoperative parameters between group A, treated using a porous polyoxymethylene thermoplastic regulator combined with a 3D printed template and group B, who underwent conventional percutaneous pedicle screw fixation (PPSF).
| Patient characteristics | Group A | Group B | Statistic | P-value |
|---|---|---|---|---|
| Number of pedicles successful pierced at first attempt (n=80) | 10 | 3 | χ2=4.103 | P=0.043 <0.05 |
| Number of insertions before reaching the desired position (n) | 7.83±1.47 | 17.50±1.87 | t=−9.947 | P<0.01 |
| Radiation dosage before reaching the desired position (mSv) | 0.45±0.10 | 1.35±1.38 | t=−12.728 | P<0.01 |
| Operation time before reaching the desired position (min) | 15.17±2.64 | 29.50±2.43 | t=−9.788 | P<0.01 |
| Total radiation dosage of fluoroscopy (mSv) | 2.75±0.48 | 4.82±0.75 | t=−5.715 | P<0.01 |
| Total operation time (min) | 66.67±6.80 | 95.50±7.18 | t=−7.143 | P<0.01 |
| Intraoperative blood loss (ml) | 86.50±5.32 | 127.33±5.05 | t=−13.641 | P<0.01 |
Postoperative parameters
Postoperative computed tomography (CT) scans of the patients in the two groups showed that one pedicle screw insertion was required in the lateral cortex of the pedicle in group A and eight pedicle screws were required in group B, with no significant nerve and vascular damage and no symptoms of nerve injury (P<0.05). The postoperative rate of change in the kyphotic angle (KA) of group A was significantly higher than that of group B (P<0.05). All patients were followed up for 3 months after surgery. The average VAS and ODI scores of group A were significantly higher than those of group B on day 1 and day 7 after surgery (P<0.05). There was no significant difference in VAS and ODI scores between the two groups at month 1 and month 3 after surgery (P>0.05) (Table 4).
Table 4.
Comparison of the postoperative parameters between group A, treated using a porous polyoxymethylene thermoplastic regulator combined with a 3D printed template and group B, who underwent conventional percutaneous pedicle screw fixation (PPSF).
| Postoperative clinical parameters | Group A | Group B | Statistical analysis | P-value |
|---|---|---|---|---|
| Number of vertebral pedicles damaged | 1 | 8 | χ2=6.135 | P=0.029 <0.05 |
| Postoperative kyphotic angle (KA) (°) | 5.83±0.75 | 6.83±1.47 | t=−1.482 | P=0.169 >0.05 |
| Rate of change of KA (%) | 77.50±4.48 | 74.04±2.95 | t=1.582 | P=0.145 >0.05 |
| Visual Analogue Scale | ||||
| Day 1 | 3.17±0.75 | 6.50±0.55 | t=−8.771 | P<0.01 |
| Day 7 | 2.67±0.82 | 4.50±1.05 | t=−3.379 | P=0.041 <0.05 |
| Month 1 | 1.83±0.75 | 2.00±0.63 | t=−0.415 | P=0.687 >0.05 |
| Month 3 | 1.33±0.52 | 1.50±0.55 | t=−0.542 | P=0.60 >0.05 |
| Oswestry Disability Index | ||||
| Day 1 | 36.50±1.05 | 43.67±1.97 | t=−7.877 | P<0.01 |
| Day 7 | 27.17±1.17 | 35.50±1.05 | t=−12.997 | P<0.01 |
| Month 1 | 25.33±0.82 | 26.83±0.41 | t=−4.025 | P=0.652 >0.05 |
| Month 3 | 14.17±0.76 | 16.17±0.98 | t=−3.956 | P=0.627 >0.05 |
Discussion
Percutaneous pedicle screw fixation (PPSF) is widely used in spinal surgery and can achieve immediate spinal stability. The traditional pedicle screw fixation (PSF) procedure is characterized by tissue damage, extended operation time, and intraoperative bleeding, which increases the risk of postoperative complications. The emergence and development of the PPSF technique can effectively reduce soft tissue injury of the surgical site, reduce intraoperative bleeding, shorten the operation time, and improve the safety of the operation [18,19]. The correct choice of insertion point and insertion angle determines the success or failure of PPSF [20]. In conventional PPSF surgery, C-arm fluoroscopy is used for continuous imaging to select the insertion points and perform insertion. The success of the procedure relies on the experience of the surgeon, and the number of insertions and fluoroscopy sessions are significantly increased, the operation time is prolonged, and the surgical risk for the patient is increased.
Godzik et al. [21] reported the use of the Excelsius GPS™ robotic navigation system in PPSF in a study in which 116 pedicle screws were successfully implanted, with an average two-dimensional (2D) accuracy of 2.6±1.1 mm and an average angle deviation of 5.6±4.3%. A 2 mm 2D PPSF surgical procedure was successfully and safely performed, which demonstrated the accuracy and safety of the surgery [21]. As the computer navigation system can be expensive, it has not been widely promoted. Wang et al. [15] applied magnetic resonance imaging (MRI) for percutaneous kyphoplasty (PKP) in cases of thoracolumbar compression fracture and compared this method with the conventional PKP. The insertion accuracy was significantly improved using MRI, and the insertion number, length of fluoroscopy, and operation time were reduced [15]. However, real-time MRI signaling is not stable, which is incompatible with real-time imaging, and the software system requires further optimization [15].
Prokop et al. [22] used a sextant in minimally invasive techniques for the treatment of 36 cases of thoracolumbar compression fractures. In this previous study, percutaneous pedicle screws were accurately placed and corresponded to the thoracolumbar vertebral body, the average surgical time was 49 minutes, and blood loss was 10–20 ml [22]. However, because of the specificity of each segment of the vertebral body, customized instruments currently in use also exist certain errors. The 3D printed personalized guide, created by preoperative computer-aided design (CAD) and simulation can improve the precision of intraoperative pedicle insertion. The 3D printed plate in auxiliary pedicle insertion increases the accuracy and safety of pedicle screw implantation and has been widely used [23,24].With the development of minimally invasive techniques, the use of a percutaneous insertion guide template is gradually applied in pedicle insertion and screw placement. Li et al. [17] used preoperative CAD technology to print a personalized guide template for PKP, which improved the accuracy of pedicle insertion, effectively reduced the number of pedicle insertion procedures, fluoroscopy sessions, and the operation time, and improved the postoperative recovery of patients. However, because of the mobility of the skin, muscles, and other soft tissue during insertion, the ideal position of the insertion point is more difficult to determine, and still required the multiple use of C-arm fluoroscopy for ideal insertion angle positioning [17].
In the present study, to overcome the difficulties of accurate adjustment of the percutaneous guide template after the failure of the first insertion, a percutaneous guide template combined with the porous polyoxymethylene thermoplastic regulator for the pedicle insertion for PPSF was designed. Based on the porous structure of the coupling regulator, the insertion sites were adjusted without moving the auxiliary device to achieve accurate positioning of the insertion point and insertion angle. The findings from the present study that the number of successful pedicle insertions, sessions of fluoroscopy, and operation time, and intra-operative blood loss were significantly reduced, and were beneficial to the postoperative recovery of the patient, have been supported by previous studies [25,26].
This study included 40 patients who were divided into group A, treated using a porous polyoxymethylene thermoplastic regulator combined with a 3D printed template, and group B, who underwent conventional PPSF. A total of 80 percutaneous pedicle screws were used, with postoperative computed tomography (CT) imaging, confirming that the lateral pedicle screws showed no excess wear, there was no neural or vascular injury. Previous studies supported these findings that PPSF surgery could more accurately ensure the insertion point and orientation of the pedicle screw placement and was more safe and effective [27–29].
In the present study, the kyphotic angle (KA) in both study groups was significantly reduced after surgery compared with the KA before surgery, but there was no significant difference in the rate of change of KA between the two groups. Therefore, it was possible for both groups to effectively recover the height of the affected vertebrae, as previously reported [30,31]. The results showed that the Visual Analogue Scale (VAS) and the Oswestry Disability Index (ODI) scores of postoperative patients were significantly lower than those of preoperative patients. The VAS and the ODI scores of group A were significantly lower than those of group B on day 1 and day 7 after surgery, and on the month 1 and month 3 after surgery. These findings indicated that in group A, postoperative symptoms of pain were more likely to be reduced in the short-term postoperative period [32–34], These findings may be due to the decrease in the total number of insertion in group A, which reduced soft tissue and spinal bone injury, leading to rapid recovery of soft tissue and bone. Pain at the surgical site was similar in the two study groups.
Conclusions
This study aimed to evaluate the efficacy of a porous polyoxymethylene thermoplastic regulator combined with a three-dimensional (3D) printed template to guide pedicle needle insertion in patients undergoing percutaneous pedicle screw fixation (PPSF) for thoracolumbar fracture. The use of a porous polyoxymethylene thermoplastic regulator combined with a 3D printed template may improve the success of pedicle insertion in patients undergoing PPSF. In this small study at a single center when compared with conventional PPSF surgery, this new technique improved the safety of insertion and screw placement, significantly reduced the number of attempts at insertion, duration of fluoroscopy, shortened the operation time, reduced the amount of intraoperative bleeding, and resulted in reduced postoperative pain in the short term, resulting in more rapid postoperative recovery.
Footnotes
Source of support: This study was supported by the Key Research Program of Science, the Technology Support Program of Jiangsu Province (BK20171265), and the Research Program of Science and Technology Support Program of Huai’an (HAP201608)
Conflict of interest
None.
References
- 1.Lau D, Terman SW, Patel R, et al. Incidence of and risk factors for superior facet violation in minimally invasive versus open pedicle screw placement during transforaminal lumbar interbody fusion: A comparative analysis. J Neurosurg Spine. 2013;18(4):356–61. doi: 10.3171/2013.1.SPINE12882. [DOI] [PubMed] [Google Scholar]
- 2.Millimaggi DF, Norcia VD, Luzzi S, et al. Minimally invasive transforaminal lumbar interbody fusion with percutaneous bilateral pedicle screw fixation for lumbosacral spine degenerative diseases. A retrospective database of 40 consecutive cases and literature review. Turk Neurosurg. 2018;28(3):454–61. doi: 10.5137/1019-5149.JTN.19479-16.0. [DOI] [PubMed] [Google Scholar]
- 3.Alander DH, Cui S. Percutaneous pedicle screw stabilization: Surgical technique, fracture reduction, and review of current spine trauma applications. J Am Acad Orthop Surg. 2018;26(7):231–40. doi: 10.5435/JAAOS-D-15-00638. [DOI] [PubMed] [Google Scholar]
- 4.Mobbs RJ, Sivabalan P, Li J. Technique, challenges and indications for percutaneous pedicle screw fixation. J Clin Neurosci. 2011;18(6):741–49. doi: 10.1016/j.jocn.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Tinelli M, Matschke S, Adams M, et al. Correct positioning of pedicle screws with a percutaneous minimal invasive system in spine trauma. Orthop Traumatol Surg Res. 2014;100(4):389–93. doi: 10.1016/j.otsr.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima H, Sato K, Ando T, et al. Comparison of the percutaneous screw placement precision of isocentric C-arm 3-dimensional fluoroscopy-navigated pedicle screw implantation and conventional fluoroscopy method with minimally invasive surgery. J Spinal Disord Tech. 2009;22(7):468–72. doi: 10.1097/BSD.0b013e31819877c8. [DOI] [PubMed] [Google Scholar]
- 7.Patel K, Tajsic T, Budohoski KP, et al. Simultaneous navigated cervico-thoracic and thoraco-lumbar fixation. Eur Spine J. 2018;27(Suppl 3):318–22. doi: 10.1007/s00586-017-5233-1. [DOI] [PubMed] [Google Scholar]
- 8.Mariscalco MW, Yamashita T, Steinmetz MP, et al. Radiation exposure to the surgeon during open lumbar microdiscectomy and minimally invasive microdiscectomy: A prospective, controlled trial. Spine. 2011;36(3):255–60. doi: 10.1097/BRS.0b013e3181ceb976. [DOI] [PubMed] [Google Scholar]
- 9.Siasios ID, Pollina J, Khan A, et al. Percutaneous screw placement in the lumbar spine with a modified guidance technique based on 3D CT navigation system. J Spine Surg. 2017;3(4):657–65. doi: 10.21037/jss.2017.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang HY, Lee SH, Jeon SH, et al. Computed tomography-guided percutaneous facet screw fixation in the lumbar spine. Technical note. J Neurosurg Spine. 2007;7(1):95–98. doi: 10.3171/SPI-07/07/095. [DOI] [PubMed] [Google Scholar]
- 11.Prokop A, Löhlein F, Chmielnicki M, et al. [Minimally invasive percutaneous instrumentation for spine fractures]. Unfallchirurg. 2009;112(7):621–28. doi: 10.1007/s00113-008-1556-z. [in German] [DOI] [PubMed] [Google Scholar]
- 12.Garrido BJ, Wood KE. Navigated placement of iliac bolts: Description of a new technique. Spine J. 2011;11(4):331–35. doi: 10.1016/j.spinee.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Fraser JF, Von Jako R, Carrino JA, Härtl R. Electromagnetic navigation in minimally invasive spine surgery: Results of a cadaveric study to evaluate percutaneous pedicle screw insertion. SAS J. 2008;2:43–47. doi: 10.1016/SASJ-2007-0105-RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu JY, Yuan Q, Liu YJ, et al. Robot-assisted percutaneous transfacet screw fixation supplementing oblique lateral interbody fusion procedure: Accuracy and safety evaluation of this novel minimally invasive technique. Orthop Surg. 2019;11(1):25–33. doi: 10.1111/os.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefan P, Habert S, Winkler A, et al. A radiation-free mixed-reality training environment and assessment concept for C-arm-based surgery. Int J Comput Assist Radiol Surg. 2018;13(9):1335–44. doi: 10.1007/s11548-018-1807-6. [DOI] [PubMed] [Google Scholar]
- 16.Acosta FL, Jr, Thompson TL, Campbell S, et al. Use of intraoperative isocentric C-arm 3D fluoroscopy for sextant percutaneous pedicle screw placement: case report and review of the literature. Spine J. 2005;5(3):339–43. doi: 10.1016/j.spinee.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Lin J, Yang Y, et al. 3-Dimensional printing guide template assisted percutaneous vertebroplasty: Technical note. J Clin Neurosci. 2018;52:159–64. doi: 10.1016/j.jocn.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Kim DY, Lee SH, Chung SK, Lee HY. Comparison of multifidus muscle atrophy and trunk extension muscle strength. Spine. 2005;30(1):123–29. [PubMed] [Google Scholar]
- 19.Ringel F, Stoffel M, Stüer C, Meyer B. Minimally invasive transmuscular pedicle screw fixation of the thoracic and lumbar spine. Neurosurgery. 2006;59(4 Suppl 2):ONS361–66. doi: 10.1227/01.NEU.0000223505.07815.74. [DOI] [PubMed] [Google Scholar]
- 20.Oh HS, Kim JS, Lee SH, et al. Comparison between the accuracy of percutaneous and open pedicle screw fixations in lumbosacral fusion. Spine J. 2013;13(12):1751–57. doi: 10.1016/j.spinee.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 21.Godzik J, Walker CT, Hartman C, et al. A quantitative assessment of the accuracy and reliability of robotically guided percutaneous pedicle screw placement: Technique and application accuracy. Oper Neurosurg. 2019;17(4):389–95. doi: 10.1093/ons/opy413. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji H, Takigawa T, Misawa H, et al. Minimally invasive percutaneous spinopelvic fixation for unstable pelvic ring fracture performed with the patient in a lateral position. Clin Spine Surg. 2019;32(5):191–97. doi: 10.1097/BSD.0000000000000798. [DOI] [PubMed] [Google Scholar]
- 23.Feng ZH, Li XB, Phan K, et al. Design of a 3D navigation template to guide the screw trajectory in spine: A step-by-step approach using Mimics and 3-Matic software. J Spine Surg. 2018;4(3):645–53. doi: 10.21037/jss.2018.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pu X, Luo C, Lu T, et al. Clinical application of atlantoaxial pedicle screw placement assisted by a modified 3D-printed navigation template. Clinics (Sao Paulo) 2018;73:e259. doi: 10.6061/clinics/2018/e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Wang Y, Yu B, et al. Comparison of cranial facet joint violation rate between percutaneous and open pedicle screw placement: A systematic review and meta-analysis. Medicine (Baltimore) 2015;94(5):e504. doi: 10.1097/MD.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Court C, Vincent C. Percutaneous fixation of thoracolumbar fractures: Current concepts. Orthop Traumatol Surg Res. 2012;98(8):900–9. doi: 10.1016/j.otsr.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Heintel TM, Berglehner A, Meffert R. Accuracy of percutaneous pedicle screws for thoracic and lumbar spine fractures: A prospective trial. Eur Spine J. 2013;22(3):495–502. doi: 10.1007/s00586-012-2476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kocis J, Kelbl M, Kocis T, Návrat T. Percutaneous versus open pedicle screw fixation for treatment of type A thoracolumbar fractures. Eur J Trauma Emerg Surg. :2018. doi: 10.1007/s00068-018-0998-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Tian F, Tu LY, Gu WF, et al. Percutaneous versus open pedicle screw instrumentation in treatment of thoracic and lumbar spine fractures: A systematic review and meta-analysis. Medicine (Baltimore) 2018;97(41):e12535. doi: 10.1097/MD.0000000000012535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan T, Rutges J, Marion T, et al. Anterior versus posterior approach in traumatic thoracolumbar burst fractures deemed for surgical management: Systematic review and meta-analysis. J Clin Neurosci. 2019;70:189–97. doi: 10.1016/j.jocn.2019.07.083. [DOI] [PubMed] [Google Scholar]
- 31.Cornelis FH, Joly Q, Nouri-Neuville M, et al. Innovative spine implants for improved augmentation and stability in neoplastic vertebral compression fracture. Medicina (Kaunas) 2019;55(8) doi: 10.3390/medicina55080426. pii: E426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang T, Guo N, Chen T, et al. Comparison of outcomes between cortical screws and traditional pedicle screws for lumbar interbody fusion: A systematic review and meta-analysis. J Orthop Surg Res. 2019;14(1):269. doi: 10.1186/s13018-019-1311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma SB, Lin GX, Jabri H, et al. Radiographic and clinical outcomes of huge lumbar disc herniations treated by transforaminal endoscopic discectomy. Clin Neurol Neurosurg. 2019;185:105485. doi: 10.1016/j.clineuro.2019.105485. [DOI] [PubMed] [Google Scholar]
- 34.Deng H, Li Y, Zhou J, et al. Therapeutic efficacy of transpedicular Intracorporeal cement augmentation with short segmental posterior instrumentation in treating osteonecrosis of the vertebral body: A retrospective case series with a minimum 5-year follow-up. BMC Musculoskelet Disord. 2019;20(1):305. doi: 10.1186/s12891-019-2671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]