Abstract
Background
Nuclear receptor subfamily 4 group A member 1 (Nr4a1) has been increasingly investigated in association with type 2 diabetes mellitus (T2DM). This study aimed to explore its efficacy with liver kinase B1 (LKB1) and potential signaling pathways in T2DM.
Material/Methods
A T2DM model in rats was established by high-fat diet and injection of 30 mg/kg streptozotocin. The ectopic expression of Nr4a1 or in combination with LKB1 was performed in T2DM rats to probe their effects on T2DM. Then, the weight and indicators of blood lipid and blood glucose in normal rats and T2DM rats were measured. The volume change of adipocytes and the size of lipid droplets in white adipose tissue (WAT) were observed by hematoxylin-eosin staining and oil red O staining, respectively. We also measured levels of Nr4a1, LKB1, and adenosine monophosphate-activated protein kinase (AMPK)/sirtuin 1 (SIRT1)/Nuclear factor-kappa B (NF-κB) axis-related proteins.
Result
In T2DM rats, Nr4a1 was highly expressed, and body weight, blood lipid and blood glucose were increased, and the volume of adipocytes and the size of lipid droplets in WAT were increased, which were all reversed by low expression of Nr4a1. After treatment with Nr4a1 and LKB1 together, T2DM rats showed decreased levels of blood lipid, blood glucose, and reduced volume of adipocytes and lipid droplet size in WAT, with activated AMPK/SIRT1 signaling pathway and inhibited NF-κB.
Conclusions
Our results highlight that interaction of Nr4a1 and LKB1 can mitigate T2DM by activating the AMPK/SIRT1 signaling pathway and inhibiting NF-κB activation. This may offer new insight for T2DM treatment.
MeSH Keywords: Adipose Tissue, White; Diabetes Mellitus, Type 2; Nuclear Receptor Subfamily 4, Group A, Member 1
Background
Diabetes mellitus (DM) is a metabolic disease characterized by hyperglycemia and deficient secretion or action of endogenous insulin, accompanied with enhanced free radical production or dysfunctional antioxidant defense [1]. The number of DM patients all over the world is projected to reach 366 million in 2030 among adults aged ≥20 years [2], and the number of people with type 2 diabetes mellitus (T2DM) has had a surprisingly large increase in the past 20 years [3]. T2DM is characterized by peripheral insulin resistance, pancreatic β-cell dysfunction, and aberrant hepatic gluconeogenesis [4], often accompanied with complications and senile syndrome [5]. Patients with T2DM are faced with a higher risk of cardiovascular events, such as coronary, cerebrovascular, and peripheral arterial ischemia [6]. Former observations point out that high consumption of fruit and vegetables is associated with a decreased risk of T2DM [7]. Diabetes managements include treatment of blood glucose, improvement in lifestyle, and pharmacological intervention [8,9]. White adipose tissue (WAT) is the most important organ involved in metabolic inflexibility [10]. Thus, this study investigated the treatment outcome of potential genes and signaling pathways in T2DM in relation to blood glucose in WAT.
Nuclear receptor subfamily 4 group A member 1 (Nr4a1), also known as Nur77, nerve growth factor I-B (NGFI-B), and TR3, is a member of the Nr4a nuclear receptor superfamily, which regulates inflammatory response [11,12]. Nr4a1 inhibits adipocyte differentiation and regulates the expression of glucose metabolism-related genes in skeletal muscle [4]. It is noteworthy that Nr4a1 is an attractive target for improving insulin resistance, as well as for preventing and treating T2DM and metabolic diseases [13]. Liver kinase B1 (LKB1) is a tumor suppressor and an upstream kinase of adenosine monophosphate-activated protein kinase (AMPK), and plays pivotal roles in monitoring energy metabolism and cell growth in adipose tissues [14,15]. Yamada E et al. found that the LKB1/AMPK axis is one of most promising targets for T2DM treatment [16]. AMPK is a vital energy sensor in cells and regulates metabolism, and in mammals it also regulates metabolism and helps maintain energy homeostasis at the whole-body level [17]. Ruderman et al. found that AMPK and sirtuin 1 (SIRT1) are mutually regulated and share several common targets, and their dysregulation may lead to T2DM and atherosclerotic cardiovascular diseases [18]. Additionally, previous research provided evidence of activated nuclear factor-kappa B (NF-κB) pathway in T2DM skeletal muscle after inflammation, which may contribute to insulin resistance [19]. Interestingly, Nr4a1 depletion can contribute to increased NF-κB activity in monocytes and peritoneal macrophages, probably owing to a reduction in inhibitor kappa B-α (IκBα) expression [11]. In light of these previous findings, we hypothesized that there may be interactions among Nr4a1, LKB1, AMPK, SIRT1, and NF-κB in T2DM. Therefore, we conducted a series of experiments to test our hypothesis.
Material and Methods
Ethics statement
This study was approved and supervised by the Animal Ethics Committee of Weifang People’s Hospital. All efforts were made to minimize the number of animals used and their suffering. All procedures were conducted strictly in accordance with the code of ethics.
Model establishment and grouping
Fifty-four male Sprague-Dawley (SD) rats (8 weeks old, 170±20 g) purchased from Laboratory Animal Center of Anhui Medical University (Hefei, Anhui, China) were adaptively fed for 1 week and then were randomize into a control group (n=6) and a T2DM group (n=48). Rats in the control group were fed with standard feed, while rats in the model group were fed with high-fat diet for 8 weeks and intraperitoneally injected with streptozotocin (30 mg/kg, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for model establishment. Afterwards, the model rats were sub-assigned into the T2DM group, the T2DM+Nr4a1-negative control (NC) group (T2DM rats injected with Nr4a1 mimic-NC vector via the tail vein), the T2DM+Nr4a1 group (T2DM rats injected with Nr4a1 mimic vector via the tail vein), the T2DM+Nr4a1-inhibitor-NC group (T2DM rats injected with si-Nr4a1-NC vector via the tail vein), the T2DM+Nr4a1-inhibitor group (T2DM rats injected with si-Nr4a1 vector via the tail vein), and the T2DM+Nr4a1+LKB1 group (T2DM rats injected with Nr4a1+LKB1), with 6 rats in each group. After feeding for 8 weeks, T2DM rats were fasted for 12 hours, and then euthanized after their blood samples and WATs were obtained. All rats were tested for blood lipid and blood glucose. Three rats in each group were used for RT-qPCR and Western blot analysis, and 3 for hematoxylin and eosin (HE) staining and oil red O staining.
Detection of weight and blood glucose
The weight of rats in each group was measured and recorded every 2 weeks after the experiment by an electronic balance. The blood glucose of rats was measured 6 hours after lunch on the day before the end of the experiment, and recorded as postprandial blood glucose (PBG). The blood glucose was measured on the second day after fasting 12 hours, and recorded as fasting blood glucose (FBG).
After 6 weeks of grouping, half of the rats in each group were tested for intraperitoneal insulin tolerance (IPIT) and the other half of the rats in each group were tested for intraperitoneal glucose tolerance (IPGT). After 12 hours of fasting, the blood glucose in tail vein was measured and recorded at 0, 30, 60, 90, and 120 minutes after intraperitoneal injection of insulin (0.5 U/Kg) and 50% glucose (2 g/Kg), respectively.
Detection of serum metabolic indicators
After fasting for 12 hours, all rats were anesthetized with sodium pentobarbital. Blood was taken from the abdominal aorta and serum was separated (centrifugation at 3000 rpm for 15 minutes). Levels of triglyceride (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), alanine transaminase (ALT), and aspartate transaminase (AST) were detected using a blood lipid kit (Sichuan Marker Biotechnology Co., Chengdu, Sichuan, China).
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA from WATs was obtained using the Trizol (Invitrogen, Carlsbad, CA, USA) to determine its concentration and purity, and then reversely transcribed into cDNA. The mRNA expression was quantified by SYBR PCR Master Mix kit (Applied Biosystems, Inc., Carlsbad, CA, USA) with β-actin as an internal reference. Data were analyzed with 2−ΔΔCt, indicating the ratio of target gene expression between the T2DM group and the control group. The formula was as follows: ΔΔCT=(Ct target gene–Ct β-actin) in the T2DM group–(Ct target gene–Ct β-actin) in the control group. The primers used in the experiment were designed by Primer 3Plus website and synthesized by Suzhou Genewiz Bioengineering Co. (Suzhou, Jiangsu, China). The primers are shown in Table 1.
Table 1.
Primer sequence for RT-qPCR.
| Primer | Sequence (5′→3′) |
|---|---|
| Nr4a1 | F: GTGTTGATGTTCCCGCCTTT |
| R: GGAGCCCGTGTCGATCAGT | |
| LKB1 | F: GTCACTCTGCTCTTCTTTCTCG |
| R: CTCTCTGTGGTGTTCTTCGTTG | |
| AMPK | F: CAAGGTGTACGGAAGGCAA |
| R: CACGCAAATAATAGGGGTT | |
| NF-κB | F: GGCCGGAAGACCTATCCTACT |
| R: CTACAGACACAGCGCACACT | |
| β-actin | F: GTCATTCCAAATATGAGAGATGCGT |
| R: GCTATCACCTCCCCTGTGTG |
RT-qPCR – reverse transcription quantitative polymerase chain reaction; Nr4a1 – nuclear receptor subfamily 4 group A member 1; LKB1 – liver kinase B1; AMPK – adenosine monophosphate-activated protein kinase; NF-κB – nuclear factor-kappa B; F – forward; R – reverse.
Western blot analysis
WATs of rats in each group were extracted and homogenized with radio-immunoprecipitation assay lysate. The tissues were centrifuged at 4°C and 12 000 rpm for 15 minutes. Then, the supernatant was obtained to extract and quantify the total protein. The protein levels were measured using Western blot analysis with β-actin as an internal reference. The Chemi Scope series (Bio-Rad Laboratories, Inc. Hercules, CA, USA) gel imaging system was applied to analyze the results. The antibodies used are shown in Table 2. All antibodies were purchased from Abcam, Inc. (Cambridge, MA, USA).
Table 2.
Antibodies used in Western blot analysis.
| Antibody | Item No. | Dilution ratio |
|---|---|---|
| Nr4a1 | ab13851 | 1/500 |
| LKB1 | ab15095 | 1/100 |
| p-AMPK | ab23875 | 1/1000 |
| AMPK | ab32047 | 1/1000 |
| p-ACC | ab68191 | 1/5000 |
| ACC | ab72046 | 1/2000 |
| SIRT1 | ab110304 | 0.125 μg/mL |
| p-p65 | ab86299 | 1/2000 |
| p65 | ab16502 | 0.5 μg/mL |
| IκBα | ab32518 | 1/1000 |
| p-IκBα | ab133462 | 1/10000 |
| β-actin | ab179467 | 1/5000 |
Nr4a1 – nuclear receptor subfamily 4 group A member 1; LKB1 – liver kinase B1; AMPK – adenosine monophosphate-activated protein kinase; ACC – acetyl-CoA carboxylase; SIRT1 – sirtuin-1.
HE staining
WATs were fixed and embedded in paraffin, sliced at 5 μm, and dewaxed in xylene, washed in ethanol, and stained with hematoxylin, differentiated with hydrochloric acid and ethanol, and then stained with eosin after rinsing in running water. Afterwards, WATs were dehydrated regularly and sealed with neutral resin. Finally, WATs were observed under an optical microscope (Olympus, Tokyo, Japan).
Oil red O staining
WAT sections were stained in oil red staining (NanJing JianCheng Bioengineering Institute, Nanjing, Jiangsu, China) for 15 minutes and then washed in distilled water for 15 seconds. Next, WAT sections were counterstained for 3–5 minutes and washed for 60 seconds, followed by sealing, microscopic examination, and photographic analysis.
Statistical analysis
Statistical analysis was conducted using SPSS21.0 (IBM Corp. Armonk, NY, USA). The Kolmogorov-Smirnov test was used to assess whether the data were normally distributed. Measurement data are expressed as mean±standard deviation. The t test was used for comparisons between 2 groups, while one-way analysis of variance (ANOVA) was used for multiple groups, and Tukey’s multiple comparisons test for pair-wise comparisons was performed after ANOVA analyses. The p value was obtained by two-tailed test, and p<0.05 indicates statistically significant differences.
Results
Nr4a1 is upregulated in WATs of T2DM rats
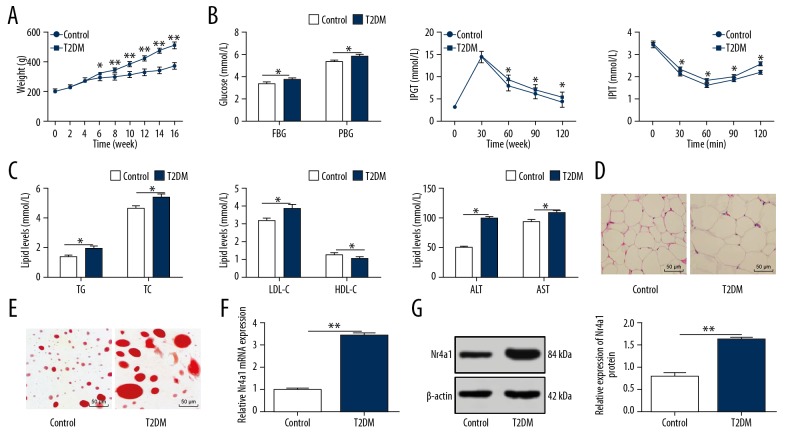
After the establishment of the T2DM model in rats, the relevant indicators and pathological changes were detected to verify whether the model was established successfully. The weight, FBG, PBG, IPGT, IPIT, TC, TG, LDL-C, ALT, and AST of T2DM rats were increased significantly, while HDL-C was decreased significantly (all p<0.05, Figure 1A–1C). The volume of adipocytes and the size of lipid droplets in WATs increased notably (both p<0.05, Figure 1D, 1E), inferring the successful establishment of the T2DM rat model. According to the literature, Nr4a1 is a newly-discovered malignant factor in diabetic renal damage [20]. Therefore, we speculated that Nr4a1 would have an effect on T2DM rats. We found that Nr4a1 levels in WATs of T2DM rats were noticeably increased (all p<0.05, Figure 1F, 1G).
Figure 1.
Nr4a1 is upregulated in WATs of T2DM rats. (A) Weight of normal rats and T2DM rats measured by an electronic balance, n=6; (B) Levels of blood glucose indicators in normal rats and T2DM rats measured by blood glucose meter, n=6; (C) Levels of blood lipid indicators in normal rats and T2DM rats measured by blood lipid kit, n=6; (D) Representative images of WAT volume in normal rats and T2DM rats detected by HE staining, n=3; (E) Representative images of WAT lipid droplets in normal rats and T2DM rats detected by oil red O staining, n=3; (F) Relative Nr4a1 mRNA expression in normal rats and T2DM rats measured by RT-qPCR, n=3; (G) Relative Nr4a1 protein level in normal rats and T2DM rats measured by western blot analysis, n=3. * Compared to the control group, p<0.05. Repetition=3. Data were analyzed by t test. Nr4a1 – nuclear receptor subfamily 4 group A member 1; WAT – white adipose tissue; T2DM – type 2 diabetes mellitus; HE – hematoxylin and eosin; RT-qPCR – reverse transcription quantitative polymerase chain reaction; AST – aspartate transaminase; ALT – alanine transaminase; FBG – fasting blood glucose; PBG – postprandial blood glucose; TC – total cholesterol; TG – triglyceride; HDL-C – high-density lipoprotein-cholesterol; LDL-C – low-density lipoprotein-cholesterol.
si-Nr4a1 reduces glucose and lipid levels in T2DM rats
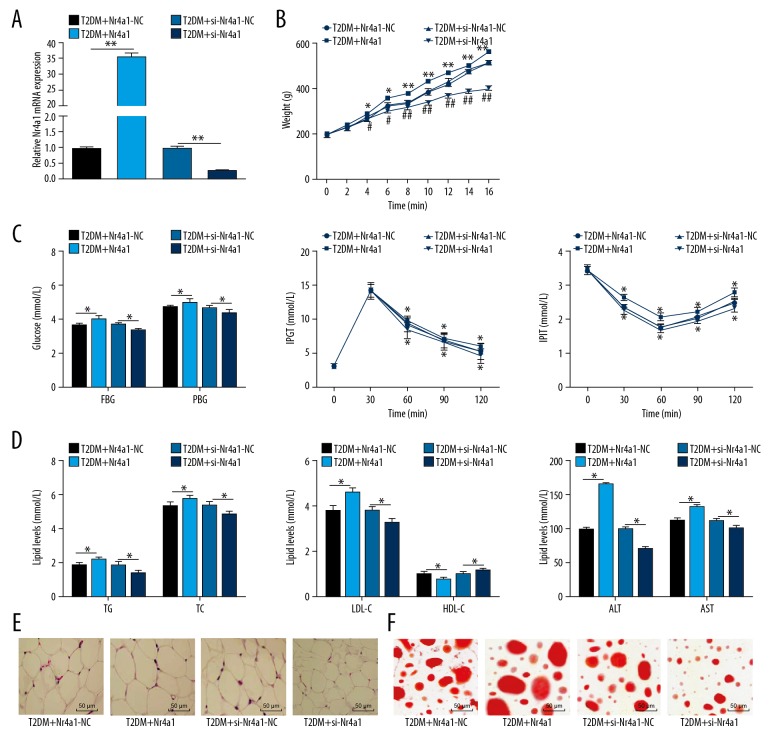
Nr4a1 expression in WATs of T2DM rats was increased obviously, so we speculated that silencing Nr4a1 is beneficial for T2DM rats. After silencing Nr4a1, the Nr4a1 mRNA expression in WATs of T2DM rats decreased significantly (p<0.05, Figure 2A.) Body weight, FBG, PBG, IPGT, IPIT, TC, TG, LDL-C, ALT, and AST were decreased notably, while HDL-C increased (all p<0.05, Figure 2B–2D). The volume of adipocytes and the size of lipid droplets in WATs were reduced notably (both p<0.05, Figure 2E, 2F). Rats with overexpressing Nr4a1 showed the opposite trends. Briefly, si-Nr4a1 reduces glucose and lipid levels in T2DM rats.
Figure 2.
Silencing Nr4a1 reduces glucose and lipid levels in T2DM rats. (A) Relative Nr4a1 mRNA expression in T2DM rats measured by RT-qPCR, n=3; (B) Weight of T2DM rats measured by an electronic balance, n=6; (C) Levels of blood glucose indicators in T2DM rats measured by blood glucose meter, n=6; (D) Levels of blood lipid indicators in T2DM rats measured by blood lipid kit, n=6; (E) Representative images of WAT volume in T2DM rats detected by HE staining, n=3; (F) Representative images of WAT lipid droplets in T2DM rats detected by oil red O staining, n=3. * For pair-wise comparison, p<0.05. Repetition=3. Data were analyzed by t test. Nr4a1 – nuclear receptor subfamily 4 group A member 1; WAT – white adipose tissue; T2DM – type 2 diabetes mellitus; HE – hematoxylin and eosin; RT-qPCR – reverse transcription quantitative polymerase chain reaction; AST – aspartate transaminase; ALT – alanine transaminase; FBG – fasting blood glucose; PBG – postprandial blood glucose; TC – total cholesterol; TG – triglyceride; HDL-C – high-density lipoprotein-cholesterol; LDL-C – low-density lipoprotein-cholesterol.
Nr4a1 interacts with LKB1 to maintain glucose and lipid homeostasis in T2DM rats
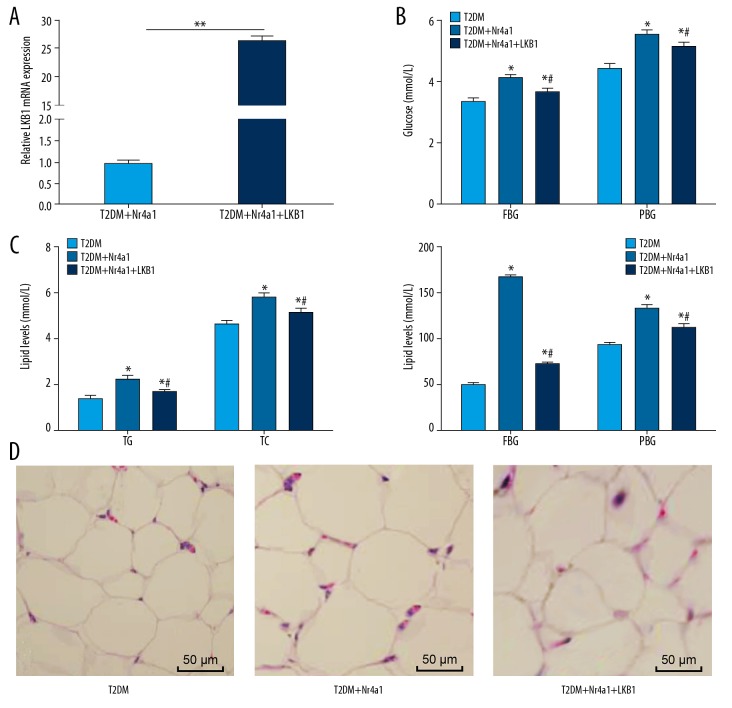
Adipose tissue, as an organ of energy metabolism and endocrine, is closely associated with metabolic diseases such as obesity, insulin resistance, and diabetes, and LKB1 plays crucial roles in regulating energy metabolism and cell growth in adipose tissue [15]. In this experiment, T2DM rats treated with overexpressing Nr4a1 and LKB1 together exhibited reduced levels of FBG, PBG, TC, TG, ALT, and AST (Figure 3A–3C), and decreased adipocytes in WAT (Figure 3D) (all p<0.05). In conclusion, overexpression of Nr4a1 and LKB1 together is beneficial for glucose and lipid homeostasis in T2DM rats.
Figure 3.
Nr4a1 interacts with LKB1 to maintain glucose and lipid homeostasis in T2DM rats. (A) Relative LKB1 mRNA expression in T2DM rats measured by RT-qPCR, n=3, data were analyzed by t test; (B) Levels of blood glucose indicators in T2DM rats measured by blood glucose meter, n=6; (C) Levels of blood lipid indicators in T2DM rats measured by blood lipid kit, n=6; (D) Representative images of WAT volume in T2DM rats detected by HE staining, n=3. Repetition=3. Data in panel B and C were analyzed by one-way ANOVA. * Compared with the T2DM group, p<0.05; # compared with the T2DM+Nr4a1 group, p<0.05. Nr4a1 – nuclear receptor subfamily 4 group A member 1; WAT – white adipose tissue; T2DM – type 2 diabetes mellitus; HE – hematoxylin and eosin; RT-qPCR – reverse transcription quantitative polymerase chain reaction; AST – aspartate transaminase; ALT – alanine transaminase; FBG – fasting blood glucose; PBG – postprandial blood glucose; TC – total cholesterol; TG – triglyceride; ANOVA – analysis of variance.
Interaction between Nr4a1 and LKB1 activates AMPK/SIRT1 signaling pathway and inhibits activation of NF-κB signaling pathway
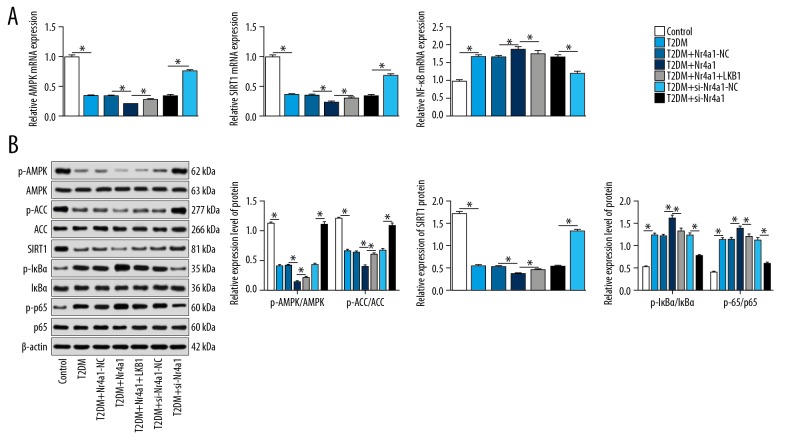
Metformin is reported to regulate energy balance by activating the LKB1/AMPK signaling pathway in T2DM [21]. Inflammation often occurs during the development of chronic DM. SIRT1 can reduce the levels of FBG, oxidative stress, and inflammatory injury-related proteins in T2DM rats through the antioxidant and anti-inflammatory effects of NF-κB and AMPK-dependent mechanisms [22]. In light of this, we overexpressed Nr4a1 and LKB1 in T2DM rats, and observed the changes in the AMPK/SIRT1/NF-κB axis. After overexpressing Nr4a1, the mRNA expression of AMPK and SIRT1 decreased, the mRNA expression of NF-κB increased, and protein levels of p-AMPK, p-ACC, SIRT1, p-IκBα, and p-p65 decreased significantly. The above trends were reversed when rats were treated with overexpression of Nr4a1 and LKB1 together (all p<0.05) (Figure 4A, 4B).
Figure 4.
Interaction between Nr4a1 and LKB1 activates AMPK/SIRT1 signaling pathway and inhibits activation of NF-κB signaling pathway. (A) Relative mRNA expression of AMPK, SIRT1 and NF-κB in T2DM rats measured by RT-qPCR; (B) Protein levels and phosphorylation levels of AMPK, ACC, SIRT1, IκBα and p-p65 measured by western blot analysis. Repetition=3. n=3, Data were analyzed by t test. * For pair-wise comparison, p<0.05. Nr4a1 – nuclear receptor subfamily 4 group A member 1; LKB1 – liver kinase B1; AMPK – adenosine monophosphate-activated protein kinase; ACC – acetyl-CoA carboxylase; SIRT1 – sirtuin-1; T2DM – type 2 diabetes mellitus; NF-κB – nuclear factor-kappa B; RT-qPCR – reverse transcription quantitative polymerase chain reaction.
Discussion
T2DM prevalence is estimated to increase with population aging and longer life expectancy, with complications of cognitive impairment, depression, and urinary incontinence in elderly patients caused by aging [23]. Thus, there is an urgent need to search for effective treatments for T2DM. In this study, we investigated potential genes and signaling pathways in T2DM from the aspects of blood glucose in WAT. Collectively, we highlighted that interaction between Nr4a1 and LKB1 mitigated T2DM by activating the AMPK/SIRT1 signaling pathway and inhibiting NF-κB activation.
Initially, we found T2DM rats exhibited increased weight and higher levels of FBG, PBG, IPGT, IPIT, TC, TG, LDL-C, ALT, and AST, as well as larger volume of adipocytes, larger lipid droplets, and reduced HDL-C. Elevated ALT is considered an alternative marker of nonalcoholic liver disease, which can predict the later development of DM and metabolic diseases [24]. Higher ALT and AST levels are commonly recognized as predictors of impaired glucose tolerance and DM incidence [25]. Research shows that low HDL-C level is an independent risk factor for T2DM development [26]. Compellingly, Adeneye et al. noted that diabetic dyslipidemia is usually characterized by elevated levels of TG, TC, and LDL-C, and decreased HDL-C, which are important risks of coronary heart disease in DM [27]. These studies further validated that the T2DM rat model in this study was constructed successfully. We also found Nr4a1 levels in WATs of T2DM rats were increased. In vivo, in response to glucagon and fasting, the cAMP axis induced Nr4a1 expression in the liver and in diabetic mice with hyperglycemia, and Nr4a1 overexpression stimulated glucose production and increased blood glucose level [28]. Additionally, Nr4a1 was also dramatically upregulated in obese patients and rodent models of diet-induced obesity [29]. In light of this, we injected si-Nr4a1 into T2DM rats to further evaluate its mechanism. We found that silencing Nr4a1 reduced blood lipid and glucose, adipocytes volume, and the size of lipid droplets. Consistently, the basal levels of Nr4a1, body weight, FBG, TG, TC, LDL-C, and HDL-C were higher in T2DM patients [30]. As recently reported, Nr4a1 was actually activated by chronic hyperglycemia, and higher Nr4a1 expression has strong links with glucose metabolism disorder, renal insufficiency, renal hypertrophy, and fibrosis, but Nr4a1 knockdown reduced diabetic renal damage [20]. Therefore, our results support that Nr4a1 depletion is beneficial for T2DM treatment.
Furthermore, our results revealed that T2DM rats with overexpressing Nr4a1 and LKB1 together exhibited reduced blood lipid and glucose and decreased adipocytes in WAT. LKB1 is a key regulator of energy metabolism and is necessary for WAT growth and differentiation, and LKB1 depletion led to severely decreased WAT mass, blood glucose levels, and adipocyte size [31]. Chikusetsu saponin IVa protected brain ischemia/reperfusion in diabetes through AMPK-mediated phosphorylation of glycogen synthase kinase 3β downstream of the adiponectin-LKB1 pathway [32]. The results suggested overexpression of Nr4a1 and LKB1 together is beneficial for glucose and lipid homeostasis in T2DM rats. Moreover, we noted that interaction of Nr4a1 and LKB1 activated AMPK/SIRT1 axis and inhibited NF-κB activation. Hyperglycemia decreased AMPK activation, resulting in impaired autophagy and matrix accumulation, and reduced AMPK activation was observed in human diabetic nephropathy [33]. AMPK is an underlying target for glucose and lipid homeostasis in T2DM [34] and AMPK activation itself increases insulin sensitivity in T2DM [35]. Yamada et al. revealed that the function of AMPK in regulating glucose transport, fatty acid oxidation, and mitochondria generation in the skeletal muscle and preventing hepatic glucose output made it a potential target for T2DM treatment [16]. Nr4a1 activated sestrin 2, which in turn activated the AMPK pathway to induce glucose metabolism genes and glucose uptake in C2C12 cells [36]. In addition, SIRT1 agonist SRT1720 treatment can reduce FBG, inflammation, and oxidative stress in T2DM rats by inhibiting NF-κB and upregulating AMPK [22]. The insulin sensitivity and glucose homeostasis of adipose tissue were improved after SIRT1 activation, suggesting that SIRT1 activation is a promising new treatment for T2DM [37]. Interestingly, Khare et al. reported NF-κB inhibitor rescued behavioral and neurochemical deficits in T2DM rats [38]. Nr4a1 is an important transcriptional factor in controlling IκBα expression in vascular endothelial cells, and protects endothelial cells from TNF-α-induced endothelial activation, as evidenced by attenuated NF-κB activation [39]. In monocytes and macrophages, Nr4a1 expression was induced by various inflammatory stimuli through NFκB, and Nr4a1 directly associated with p65 to prevent its binding to the κB element [40].
Conclusions
Our study offers insights into the mechanism of Nr4a1, LKB1, AMPK/SIRT1, and NF-κB signaling pathways in T2DM, whereby interaction between Nr4a1 and LKB1 activated AMPK/SIRT1 signaling pathway and inhibited activation of the NF-κB signaling pathway, thus maintaining glucose and lipid homeostasis, and alleviating symptoms in T2DM rats. This study may provide new insights for further understanding the pathology of T2DM and finding new targets for molecular-targeted therapies. Further research should be conducted to determine the best possible approach for managing T2DM based on the results obtained from this study.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Asgary S, Rahimi P, Mahzouni P, Madani H. Antidiabetic effect of hydroalcoholic extract of Carthamus tinctorius L. in alloxan-induced diabetic rats. J Res Med Sci. 2012;17(4):386–92. [PMC free article] [PubMed] [Google Scholar]
- 2.Li MZ, Su L, Liang BY, et al. Trends in prevalence, awareness, treatment, and control of diabetes mellitus in mainland china from 1979 to 2012. Int J Endocrinol. 2013;2013 doi: 10.1155/2013/753150. 753150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter P, Gray LJ, Troughton J, et al. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ. 2010;341:c4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mussig K, Machicao F, Machann J, et al. No association between variation in the NR4A1 gene locus and metabolic traits in white subjects at increased risk for type 2 diabetes. BMC Med Genet. 2010;11:84. doi: 10.1186/1471-2350-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno G, Mangione CM. Management of cardiovascular disease risk factors in older adults with type 2 diabetes mellitus: 2002–2012 literature review. J Am Geriatr Soc. 2013;61(11):2027–37. doi: 10.1111/jgs.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Udell JA, Scirica BM, Braunwald E, et al. Statin and aspirin therapy for the prevention of cardiovascular events in patients with type 2 diabetes mellitus. Clin Cardiol. 2012;35(12):722–29. doi: 10.1002/clc.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mursu J, Virtanen JK, Tuomainen TP, et al. Intake of fruit, berries, and vegetables and risk of type 2 diabetes in Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2014;99(2):328–33. doi: 10.3945/ajcn.113.069641. [DOI] [PubMed] [Google Scholar]
- 8.Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174(8):1227–34. doi: 10.1001/jamainternmed.2014.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zinman B, Harris SB, Neuman J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): A double-blind randomised controlled study. Lancet. 2010;376(9735):103–11. doi: 10.1016/S0140-6736(10)60746-5. [DOI] [PubMed] [Google Scholar]
- 10.Grenier-Larouche T, Labbe SM, Noll C, et al. Metabolic inflexibility of white and brown adipose tissues in abnormal fatty acid partitioning of type 2 diabetes. Int J Obes Suppl. 2012;2(Suppl 2):S37–42. doi: 10.1038/ijosup.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamers AA, Hanna RN, Nowyhed H, et al. NR4A nuclear receptors in immunity and atherosclerosis. Curr Opin Lipidol. 2013;24(5):381–85. doi: 10.1097/MOL.0b013e3283643eac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMorrow JP, Murphy EP. Inflammation: A role for NR4A orphan nuclear receptors? Biochem Soc Trans. 2011;39(2):688–93. doi: 10.1042/BST0390688. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y, Luo L, Luo N, et al. NR4A orphan nuclear receptors modulate insulin action and the glucose transport system: potential role in insulin resistance. J Biol Chem. 2007;282(43):31525–33. doi: 10.1074/jbc.M701132200. [DOI] [PubMed] [Google Scholar]
- 14.Wang F, Yang X, Lu Y, et al. The natural product antroalbol H promotes phosphorylation of liver kinase B1 (LKB1) at threonine 189 and thereby enhances cellular glucose uptake. J Biol Chem. 2019;294(27):10415–27. doi: 10.1074/jbc.RA118.007231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z, You W, Wang F, et al. Elucidating the role of Lkb1 and mTOR in adipose tissue. Adipocyte. 2019;8(1):26–30. doi: 10.1080/21623945.2018.1535743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada E, Lee TW, Pessin JE, Bastie CC. Targeted therapies of the LKB1/AMPK pathway for the treatment of insulin resistance. Future Med Chem. 2010;2(12):1785–96. doi: 10.4155/fmc.10.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardie DG, Ross FA, Hawley SA. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruderman NB, Xu XJ, Nelson L, et al. AMPK and SIRT1: A long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298(4):E751–60. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreasen AS, Kelly M, Berg RM, et al. Type 2 diabetes is associated with altered NF-kappaB DNA binding activity, JNK phosphorylation, and AMPK phosphorylation in skeletal muscle after LPS. PLoS One. 2011;6(9):e23999. doi: 10.1371/journal.pone.0023999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheng J, Li H, Dai Q, et al. NR4A1 promotes diabetic nephropathy by activating Mff-mediated mitochondrial fission and suppressing parkin-mediated mitophagy. Cell Physiol Biochem. 2018;48(4):1675–93. doi: 10.1159/000492292. [DOI] [PubMed] [Google Scholar]
- 21.Saraei P, Asadi I, Kakar MA, Moradi-Kor N. The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances. Cancer Manag Res. 2019;11:3295–313. doi: 10.2147/CMAR.S200059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Shang Y, Zhang R, et al. A SIRT1 agonist reduces cognitive decline in type 2 diabetic rats through antioxidative and antiinflammatory mechanisms. Mol Med Rep. 2019;19(2):1040–48. doi: 10.3892/mmr.2018.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yakaryilmaz FD, Ozturk ZA. Treatment of type 2 diabetes mellitus in the elderly. World J Diabetes. 2017;8(6):278–85. doi: 10.4239/wjd.v8.i6.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Bonito P, Sanguigno E, Di Fraia T, et al. Association of elevated serum alanine aminotransferase with metabolic factors in obese children: Sex-related analysis. Metabolism. 2009;58(3):368–72. doi: 10.1016/j.metabol.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Tan PC, Aziz AZ, Ismail IS, Omar SZ. Gamma-glutamyltransferase, alanine transaminase and aspartate transaminase levels and the diagnosis of gestational diabetes mellitus. Clin Biochem. 2012;45(15):1192–96. doi: 10.1016/j.clinbiochem.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 26.Abbasi A, Corpeleijn E, Gansevoort RT, et al. Role of HDL cholesterol and estimates of HDL particle composition in future development of type 2 diabetes in the general population: the PREVEND study. J Clin Endocrinol Metab. 2013;98(8):E1352–59. doi: 10.1210/jc.2013-1680. [DOI] [PubMed] [Google Scholar]
- 27.Adeneye AA. The leaf and seed aqueous extract of Phyllanthus amarus improves insulin resistance diabetes in experimental animal studies. J Ethnopharmacol. 2012;144(3):705–11. doi: 10.1016/j.jep.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Pei L, Waki H, Vaitheesvaran B, et al. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med. 2006;12(9):1048–55. doi: 10.1038/nm1471. [DOI] [PubMed] [Google Scholar]
- 29.Kasch J, Kanzleiter I, Saussenthaler S, et al. Insulin sensitivity linked skeletal muscle Nr4a1 DNA methylation is programmed by the maternal diet and modulated by voluntary exercise in mice. J Nutr Biochem. 2018;57:86–92. doi: 10.1016/j.jnutbio.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Huang Q, Xue J, Zou R, et al. NR4A1 is associated with chronic low-grade inflammation in patients with type 2 diabetes. Exp Ther Med. 2014;8(5):1648–54. doi: 10.3892/etm.2014.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Wang Q, Song P, Zou MH. Liver kinase b1 is required for white adipose tissue growth and differentiation. Diabetes. 2013;62(7):2347–58. doi: 10.2337/db12-1229. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Duan J, Yin Y, Cui J, et al. Chikusetsu Saponin IVa ameliorates cerebral ischemia reperfusion injury in diabetic mice via adiponectin-mediated AMPK/GSK-3beta pathway in vivo and in vitro. Mol Neurobiol. 2016;53(1):728–43. doi: 10.1007/s12035-014-9033-x. [DOI] [PubMed] [Google Scholar]
- 33.Kundu S, Pushpakumar S, Khundmiri SJ, Sen U. Hydrogen sulfide mitigates hyperglycemic remodeling via liver kinase B1-adenosine monophosphate-activated protein kinase signaling. Biochim Biophys Acta. 2014;1843(12):2816–26. doi: 10.1016/j.bbamcr.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang SJ, Dong H, Li JB, et al. Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats. World J Gastroenterol. 2015;21(25):7777–85. doi: 10.3748/wjg.v21.i25.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123(7):2764–72. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohankumar K, Lee J, Wu CS, et al. Bis-indole-derived NR4A1 ligands and metformin exhibit NR4A1-dependent glucose metabolism and uptake in C2C12 cells. Endocrinology. 2018;159(5):1950–63. doi: 10.1210/en.2017-03049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712–16. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khare P, Datusalia AK, Sharma SS. Parthenolide, an NF-kappaB inhibitor ameliorates diabetes-induced behavioural deficit, neurotransmitter imbalance and neuroinflammation in type 2 diabetes rat model. Neuromolecular Med. 2017;19(1):101–12. doi: 10.1007/s12017-016-8434-6. [DOI] [PubMed] [Google Scholar]
- 39.You B, Jiang YY, Chen S, et al. The orphan nuclear receptor Nur77 suppresses endothelial cell activation through induction of IkappaBalpha expression. Circ Res. 2009;104(6):742–49. doi: 10.1161/CIRCRESAHA.108.192286. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Liu Y, Chen HZ, et al. Impeding the interaction between Nur77 and p38 reduces LPS-induced inflammation. Nat Chem Biol. 2015;11(5):339–46. doi: 10.1038/nchembio.1788. [DOI] [PubMed] [Google Scholar]