Abstract
The technology of drug delivery systems (DDS) has expanded into many applications, such as for treating neurological disorders. Nanoparticle DDS offer a unique strategy for targeted transport and improved outcomes of therapeutics. Stroke is likely to benefit from the emergence of this technology though clinical breakthroughs are yet to manifest. This review explores the recent advances in this field and provides insight on the trends, prospects and challenges of translating this technology to clinical application. Carriers of diverse material compositions are presented, with special focus on the surface properties and emphasis on the similarities and inconsistencies among in vivo experimental paradigms. Research attention is scattered among various nanoparticle DDS and various routes of drug administration, which expresses the lack of consistency among studies. Analysis of current literature reveals lipid- and polymer-based DDS as forerunners of DDS for stroke; however, cell membrane-derived vesicles (CMVs) possess the competitive edge due to their innate biocompatibility and superior efficacy. Conversely, inorganic and carbon-based DDS offer different functionalities as well as varied capacity for loading but suffer mainly from poor safety and general lack of investigation in this area. This review supports the existing literature by systematizing presently available data and accounting for the differences in drugs of choice, carrier types, animal models, intervention strategies and outcome parameters.
Keywords: nanoparticle, drug delivery system, stroke, animal model, nano medicine, therapeutics
Introduction
Stroke remains among the major causes of mortality and the leading cause of impaired daily living.1 Progresses in promoting recovery have been achieved through elucidating the complex pathways and discovering potential drug solutions, but clinical translation of these prospective interventions has been slow and is affected by a multitude of factors, including the therapeutic time window of drugs, the heterogeneity of patient cases, the use of unrepresentative animal models and issues of drug safety and pharmacokinetics.2,3 Stroke is characterized by brain cell death and neurological deficits attributable to a lack of blood supply to the brain, due to cerebral blood vessel occlusion or hemorrhage. It is determined by clinical presentation and imaging to observe signs of an infarcted core or hematoma, and to eliminate possible non-vascular causes such as a brain tumor, traumatic injury, metabolic disorder and infection. It is categorized by the location of injury, type of abnormality and time-based progression from onset.4 Approximately 80% of stroke cases are ischemic in origin, while the remaining are due to hemorrhage in the brain parenchyma or ventricular space, or between the arachnoid membrane and pia mater of the brain, known as the subarachnoid space.1
Vessel occlusion by a thrombus or embolus causes ischemic stroke while hemorrhagic stroke may instead develop from a vascular deformity or aneurysm that ruptures, leading to increased pressure against surrounding cells and vasculature due to fluid build-up, while blood loss and vasoconstriction cause hypoperfusion.5,6 Resultant oxygen and glucose deprivation from cerebral hypoperfusion impair cellular energy production and cause ion dysregulation, leading to lactate acidosis, excitotoxicity, cytotoxic edema, loss of membrane integrity, oxidative stress, activation of degradative enzymes, microvascular injury and recruitment of resident microglia and migrating neutrophils and macrophages; eventually resulting in cell death and compromise of blood-brain barrier (BBB) permeability.3 Thus, stroke injury can be subdivided into phases: the initial ischemic cascade; followed by ischemia/reperfusion (I/R) injury which refers to the secondary damage upon restoring blood flow as a result of the spread and increased production of reactive oxygen species (ROS) and inflammatory cytokines, as well as the activation of the complement cascade and the recruitment of immune cells; and consequently, the post-ischemic inflammation.7
A multitude of molecular pathways may be involved in the onset and progression of stroke, thus an equally diverse arsenal of intervention strategies is needed. To date, the gold standard for intravenous (IV) intervention of stroke is by using the recombinant protein, tissue plasminogen activator (tPA), a thrombolytic agent that dissolves clots to restore blood flow. Unfortunately, tPA achieves meaningful clinical efficacy only when used within 3 to 4.5 hrs from stroke onset, is applicable only for thromboembolic phenotypes, and interacts with the BBB in multiple signalling pathways that may enhance permeability leading to hemorrhage.8 A number of strategies have been developed to circumvent these issues, such as to extend the therapeutic time window of tPA through developing novel thrombolytics and to pair IV tPA with an intra-arterial (IA) injection, but these did not alleviate the side effects considerably. Combining tPA with other drugs have been able to mitigate side effects relating to the BBB but a more effective method was to restrict the interactions and prolong the circulation of tPA.8 Targeting tPA to erythrocytes has achieved this without disrupting hemostatic clots.9 However, thrombolysis and anti-thrombotic approaches do not directly address I/R injury and post-ischemic inflammation.
Other approaches that deal with I/R injury include enhancing regeneration, reducing inflammation and conferring protection from excitotoxicity and oxidative stress. The basis of these approaches is to reverse or counter the effects of pathological molecular, cellular and systemic processes.10,11 However, translation of these approaches has been hampered by low clinical efficacy, possibly due to complex patient conditions that differ in responses due to age, gender and comorbidities. From this alone, given any one drug candidate, more than one model is necessary to substantiate its efficacy and provide insight to develop patient selection criteria.11 Also, by default, each drug will inevitably pose a unique set of pharmacological limitations, while preclinical studies that do account for patient differences may not be able to determine other clinically relevant issues like the therapeutic time window, the optimal duration for drug administration and suitable outcome measures.12 Regardless, recent years have seen the rise of nanoparticle drug delivery systems (DDS) as a solution to overcome these limits. In this review, preclinical studies on nanoparticle DDS for treating stroke are discussed. Comparable disease models that have different endpoints from stroke, such as traumatic brain injury, non-cerebral vascular disease and systemic inflammation, are not of interest for this review and will not be discussed.
Nanocarriers
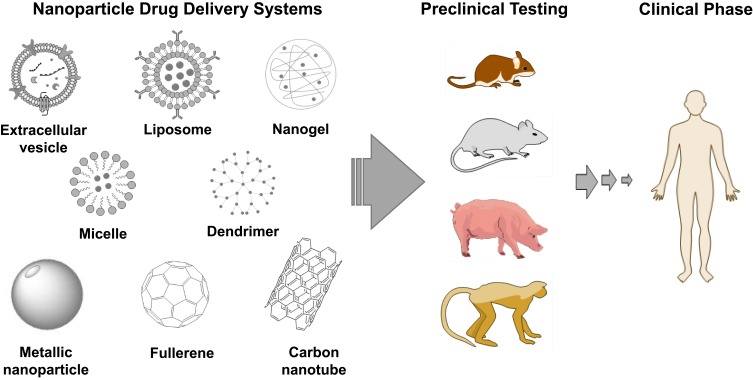
Nanoparticle DDS improve drug pharmacokinetics, pharmacodynamics and safety by protecting drugs from early deactivation and removal, by providing access to target sites and by preventing off-site interactions. This means that circulation time is prolonged, drugs accumulate at desired regions and dosage can be reduced; toxicity is thus mitigated. An ideal DDS should not induce a biological response that may lower drug efficacy or be toxic or cause immune clearance, nor be unstable and fail to enhance the drug delivery altogether. Simultaneously, the DDS should be biodegradable, easy to produce and scale up, and be economically feasible.13 As observed in Figure 1, nanoparticle DDS are distinct from pure targeting systems by virtue of the submicron colloidal or vesicular systems that fulfil drug delivery through carrier traits. These include surface binding or entrapment and ferrying of the active ingredient, confining its activity and distribution, hindering its loss during circulation, blocking adsorption of proteins which reduces the risk of opsonization and recognition by immune system, and controlling its release to be in burst, sustained or periodic patterns.13 This last feature may be expressed as diffusion or dissociation over time, activation by enzymatic degradation, or disassembly by a stimulus such as pH and temperature. Overall, these features justify the research and development of nanoparticle carriers for diagnostics, therapeutics and other applications as well, such as for food, agriculture, sanitation and cosmetics.14–16
Figure 1.
Nanoparticle DDS in the pipeline for stroke therapy.
In the context of neurological disease and targeting brain parenchyma via injection into systemic circulation, the ability to cross the BBB is an essential requirement. The BBB comprises all of the brain vasculature that is reinforced by tight junctions between endothelial cells, as well as by a luminal glycocalyx layer, the extravascular basal lamina and surrounding pericyte membrane, and astrocyte endfeet. All these together contribute to BBB resistance against large molecules. The threshold size for nonpolar compounds is 400 Da via endothelial cell membrane or 4 nm via junctions.17,18 Crossing may still be possible via several mechanisms, such as efflux transporters, adsorptive- and receptor-mediated transcytosis, and diffusion between endothelial cells in the midst of transient permeabilization by injury or disease. This is applicable to stroke because the activation of immune cells and the production of ROS compromise BBB integrity and potentiates vasogenic edema and leakage of molecules in time- and hypoxia-dependent phases.18
The exact behavior of a nanoparticle carrier will depend on several key aspects. The first is size because it influences biodistribution, cell internalization and clearance by immune cells and the reticuloendothelial system (RES). Specifically, sizes below 200 nm in diameter are ideal though as carriers become smaller, they are more prone to low encapsulation efficiency and rapid drug release. The second aspect is shape as it determines the transport properties, cellular uptake and stability, as well as the surface area available for functionalization and interaction with the surrounding environment. The third aspect is rigidity as flexible or shrinkable carriers were shown to have improved uptake and tissue penetration. The fourth and fifth aspects are hydrophobicity and surface charge which affect carrier toxicity, tendency to aggregate, interaction with cell membrane and adsorption by serum proteins. The sixth and seventh aspects are the mechanism of particle degradation and drug release, which is the system to unload encapsulated cargo while remaining stable at the site of action.19
The decisive elements affecting all the aforementioned aspects may be narrowed down to the material composition and the functionalization, which may involve the incorporation of certain molecules on the surface of the carrier by integration, encapsulation, conjugation, coating or adsorption. A timeless example is the functionalizing of surfaces with polyethylene glycol (PEG), also called PEGylation. When applied to liposomes, they become “stealth” liposomes because PEG masks them from recognition and removal by the immune system and the RES. Another classic example is the functionalizing of liposomal surface with covalently attached ligands that can bind to membrane-bound receptors on cell surfaces for the purpose of targeting specific cells and promoting uptake of encapsulated drugs.20 The optimization for this latter application may depend more on the receptor target characteristics, such as receptor location, expression and signalling system or internalization mechanism. Regardless, as mentioned previously, passive targeting may be sufficient to accumulate nanoparticles at the diseased site in stroke because of oxidative damage, vascular injury and immune cell activation, which cause increased blood flow and BBB permeability, leading to the escape of molecules across the BBB. Paired with mounting evidence that lymphatic drainage is affected negatively during stroke, this pathophysiological pathway appears similar to the enhanced permeability and retention (EPR) effect, although the lymphatic system is yet to be studied in the context of testing nanoparticle DDS.21–23
Stroke is primarily defined by a vascular abnormality that leads to infarction in the brain but this does not exclude a plethora of animal models that are insufficiently demonstrative of the disease progression. Guidelines from committees like the Stroke Therapy Academic Industry Roundtable (STAIR) and the Stem Cell as an Emerging Paradigm in Stroke (STEPS) can be summed up in the recommendations for study replications across different strains and in different species, and by alternative methods of inducing stroke. They also suggested including comorbidities, such as concomitant diseases, and applying functional outcome measures.24 Although models with comorbidities may imitate clinical cases well, they entail experimental paradigms that account for the roles of the added variables. These require a separate review and will not be discussed here. Instead, the animal models used in the selected articles for this review simply fulfilled part of the following elements: 1) the cause of stroke had a vascular origin; 2) the vascular abnormality in the brain was ischemic or hemorrhagic; 3) when ischemic, the vessel was occluded, constricted or damaged; 4) when occluded or constricted, the blood flow disruption could be either transient or permanent. Furthermore, articles were also selected based on the use of a nanoparticle DDS for a therapeutic purpose, before being grouped according to material composition.
Lipid-Based Carriers
Liposomes
Liposomes are lipid bilayer membrane vesicles with a hollow aqueous core, measuring typically less than 200 nm in diameter, assembled by techniques like ethanol injection or thin-film hydration and extrusion.25,26 Liposomes have been applied in research for several decades and several applications are already in the market, such as for anti-cancer drugs, anti-fungal agents and vaccines, while others are in clinical phase trials.27 The timeline of liposome DDS development is reflected in the preclinical studies for stroke therapy, though inherent issues of inducing complement cascade and pseudoallergy are yet to be resolved.28
For stroke, liposomes were first used for the delivery of hemoglobin (Hb) with the aim of restoring oxygen to ischemic brain cells. In rat thrombotic stroke, liposomal Hb was administered via IV and penetrated the ischemic core but not healthy brain tissue. This resulted in higher oxygenation at the infarcted site and reduced infarct sizes in the treatment group, compared to control groups receiving free Hb, saline, empty vehicles and blood transfusion.29,30 However, the effects were observed only over 1 day. In rat transient middle cerebral artery occlusion (tMCAO) where I/R injury was studied, liposomal Hb was injected via the IA route after reperfusion. It was found that neutrophil infiltration and inflammatory markers were reduced.31 In a global transient ischemic model, behavioural data suggested that liposomal Hb protected from cognitive impairment.32 Other studies found that motor function deficits were improved and that water retention was reduced but depended on the dosage and time of intervention.33,34 Cross-validation studies of liposomal Hb were carried out on monkeys, which were subjected to craniotomy and vessel clipping before being treated. These showed that the infarct sizes were decreased while weight and muscle strength were regained in the treated group greater than in the controls.35,36
Besides Hb, other drugs had been studied with liposomal formulations. For example, FK506 and cyclosporine A (CsA) are neuroprotective agents that inhibit the activation of calcineurin when there is excess of calcium ions in cells, which would otherwise activate immune cell responses. However, these drugs were used in high doses or in conjunction with manipulating the BBB due to insufficient efficacy and thus, had undesirable side effects. Liposomal FK506 was given via IV to tMCAO rats and was found to reduce oxidative stress markers preferentially in the ischemic striatum as opposed to the healthy cortex region. When injected before reperfusion, it was able to suppress cell death during I/R injury better than vehicle and free drug controls in a dose-dependent manner.37 Similarly, liposomal CsA was compared to empty vehicles, an equivalent dose of free CsA and a much higher dose of free CsA in tMCAO rats. The experimental group demonstrated reductions in infarct volume and brain edema, as well as in inflammatory markers and behavioral deficits better than the control groups.38 These studies showed that liposome DDS can lower the minimum effective dose of drugs by prolonging drug circulation and accumulating drugs at the site of injury. Rho-kinase is an enzyme involved in cell homeostasis that is also a therapeutic target in stroke. Fasudil is a Rho-kinase inhibitor that failed clinical trials for neuroprotection in stroke due to low efficacy. A study on liposomal fasudil showed improved bioavailability and reduced infiltration of immune cells at the ischemic tissue but more importantly, it showed that passive accumulation was size-dependent, with formulations above 200 nm having distinctly less diffusibility into the injured brain parenchyma.39
Although accumulation was consistently greater in the ischemic core, it could still be detected in other tissues, which implies the feasibility but weak specificity of passive targeting. For example, the IV treatment of tMCAO rats with liposomal baicalin without any targeting or stealth device showed greater accumulation in brain tissue in the diseased group compared to healthy controls, with longer circulation times than free drugs. But simultaneously, there was high accumulation in the heart, lungs, liver and spleen; possibly the consequence of the RES response.40 One strategy to reduce undesired accumulation is to inject liposomal drugs in a combination therapy with thrombolytics. For instance, it was found that administering liposomal fasudil either after or at the same time as tPA improved the localization of fasudil at the site of injury and extended the therapeutic time window of tPA.41,42 Dexamethasone, a known anti-inflammatory agent, was also proposed to treat the inflammatory phases of stroke after thrombolysis. Free tPA and liposomal dexamethasone had been co-administered to a thrombotic rat model to mimic an embolic origin and downstream I/R injury. There were reductions in behavioral deficits and infarct volumes over a 1-week timeline compared to free drug and empty vehicle controls.43 Moreover, these studies together express the importance of time and inflammation as factors that affect observed efficacy.
Another way to improve efficacy while reducing undesired accumulation is to introduce liposomal drugs via alternative routes. Intragastric (IG) administration by oral gavage of liposomal lycopene into tMCAO rats showed improvements in levels of biomarkers for oxidative stress, inflammatory response and brain tissue damage compared to vehicles.44 Similarly, liposome surfaces were conjugated with oleoylethanolamine (OEA) and administered by oral gavage into tMCAO rats, which presented with reduced cerebral edema, infarct volume and inflammation markers, as well as improved behavioral outcomes.45 Alternatively, liposomal luteolin had been injected via the intraperitoneal (IP) route over 13 days and tested at 2 dosages. After 2 weeks, the higher dose was more effective with reduced infarct volumes and correspondingly reduced ROS production.46 However, all these studies did not precisely show presence of the drugs nor their carriers at the site of injury and required daily administration for 2 weeks. One study had improved on this by administering liposomal basic fibroblast growth factor (bFGF) via the intranasal (IN) route over 3 days and then showing its presence in the brain tissue. It was observed that infarct volumes were halved and persisted so over 3 weeks while functional outcomes were tracked for 3 days.47
To improve localization without co-administration with thrombolytics or multiple administrations, ligand conjugates were formulated to provide active targeting by binding to receptors unique to neurons undergoing ischemia. For example, asialo-erythropoietin (AEPO) had been conjugated to the surface of liposomes and had acted as a targeting device by binding to surface receptors on hypoxic neurons, as well as a therapeutic agent to promote cell survival. The liposomes protected AEPO from deactivation and removal from circulation for over a week compared to free AEPO and vehicle.48,49 In another case, penetration across the BBB endothelium was achieved for the novel neuroprotectant, ZL006, which had been encapsulated in liposomes functionalized with HAIYPRH (T7), a peptide that binds to transferrin receptors on BBB endothelial cells.50 In addition, when combined with T7 on liposome surfaces, stroke homing peptide (SHp) promoted specific uptake by targeting ischemic tissue.51
Besides targeting, surface charge and controlled release were also studied. One study had found that neutral and negatively charged liposomes loaded with simvastatin crossed the BBB better than positively charged counterparts due to lower rates of opsonisation.52 This runs counter to the view that due to the negative charge on BBB endothelium, cationic particles are more likely to cross. In a controlled-release study, ultrasound was used to trigger the release of xenon (Xe) gas from echogenic liposomes in stages from their 2-compartment structures, blocking early excitotoxicity and exerting lasting neuroprotection in rats with hemorrhagic stroke.53 The appeal of liposomes lies in the adaptable surface functionalization potential, the superior encapsulation efficiency and easy fabrication, as well as the predictable immunogenic properties.
Non-Liposomal Lipid Nanoparticles
Other lipid nanoparticles had also been studied though drawing far less attention than liposomes. PEGylated lipid nanoparticles (PLN) had been used as carriers in solid phase for the delivery of lipophilic drugs. Easy to produce and scale up, they are biocompatible and stable over time but have poor loading and release profiles.54 Conversely, nanostructured lipid carriers (NLC) have both solid and liquid phase lipids, and thus, have superior loading capacity and stability although this depends on the choice of lipids and surfactants.55 Most recently, nanoemulsions had been used for stroke, comparing injections via IN and IV. The formulation measured below 100 nm in diameter yet had very high drug content. Higher bioavailability was seen for IN delivery with the mucoadhesive function compared to IV control and non-functionalized nanoemulsions in both IN and IV treatments.56 Regardless, more studies are needed to develop their potential.
Polymer-Based Carriers
Micelles
Micelles comprise amphiphilic molecules that self-assemble around a hydrophobic core to form colloidal spheres below 100 nm in diameter. Micelles are typically monodisperse and stable in aqueous solution when above the critical micellar concentration (CMC). An individual molecular building block of a micelle may be monomeric, peptidic or copolymeric. Drugs may be conjugated to the surface of individual molecules or encapsulated in the core. The weaknesses of micelles include the tendency to dissociate when below the CMC, such as after injection into the blood, and the toxicity of the individual molecules. Selecting the suitable polymer, combining polymers, functionalizing the units or using surfactants have been applied to delay the dissociation or to design stimuli-directed degradation to improve drug delivery and reduce toxicity.61
The variability of polymers and associated properties have allowed experimentations with many drugs and strategies. Among the many anti-oxidative and anti-inflammatory compounds that had been studied with micelles to treat I/R injury and post-ischemic inflammation include antioxidants and anti-inflammatory agents such as superoxide dismutase, curcumin, luteolin, puerarin and resveratrol.62–68 An alternative strategy involves ligands to bind receptors and alter intracellular signalling, such as the channel blockers, MRZ2/576 and riluzole, as well as the neuropeptide, nerve growth factor (NGF).69–71 Another unique strategy is to deliver nucleic acids to modulate gene expression instead, such as miR-195, C3 siRNA and other plasmid vectors.72–74 Free radicals had also been used as ROS scavengers among different treatment approaches to reduce I/R injury. The nitric oxide radical, amino-TEMPO, had been conjugated to a copolymer which then formed micelles with the scavengers facing inward and with PEG serving as a stealth device against RES. Upon reaching the ischemic region which had low pH, the micelles had dissociated and exposed the radical scavenger. The IV injection resulted in decreased infarction volume and neurological deficits, while the IA injection reduced oxidative injury and BBB disruption.75,76 In a different ROS-mediated drug release strategy, copolymeric micelles were functionalized with phenylboronic motifs in order to dissociate and release cargo in response to high levels of ROS.77
Distinctly, several studies had addressed I/R injury by combining multiple therapeutic agents within a single micellar formulation. Dexamethasone was conjugated to polyethylenimine (PEI) that were then made to form micelles and were subsequently loaded with heme oxygenase-1 (HO-1) plasmid vectors. The corticosteroid was to counter inflammation and increase efficiency of cell and nuclear uptake by binding to glucocorticoid receptors in the cytoplasm, while the plasmid product was an antioxidant enzyme that would be activated in hypoxia.78 Although PEI had high efficiency, the monomer suffered from toxicity. In contrast, peptidic units are more biocompatible and readily degraded. R3V6 is an example of a peptidic unit that comprises three blocks of arginine and three blocks of valine. R3V6 micelles were loaded with HO-1 plasmid for delivery along with dexamethasone, which served to increase both core stability and transfection efficiency.79 The lesion size and inflammation markers were found to be reduced as compared to untreated controls. An alternative solution to reduce toxicity is to incorporate cleavable motifs into the individual monomers. One example is poly(oligo-arginine) polymers which incorporated reducible disulfide bonds (rPOA) within the individual units and had been used for gene delivery. Due to the rapid degradation, it showed high transfection efficiency and low cytotoxicity.80
In an application similar to liposomes, micelles were used in combination with thrombolysis. The micelles of poly-lactic-co-glycolic acid (PLGA) were loaded with the antioxidants, catalase and superoxide dismutase, and injected via the IA route after tPA. This had resulted in the migration of neural progenitor cells (NPC) from the subventricular zone and more neurogenesis than the untreated controls and the controls receiving tPA only.81 Drawing even further resemblances to liposomes, micelle drug delivery across the BBB could be enhanced by altering surface charge or conjugating ligands for specific interactions. One study developed drug-conjugated cationic bovine serum albumin (CBSA) micelles to augment crossing of the BBB, while another study had conjugated ligands onto micelle surfaces to bind to adenosine 2A receptors (A2AR) on the BBB lumen to improve uptake.82,83 One study had even combined the encapsulation of lexiscan and surface conjugation with chlorotoxin which together improved BBB uptake and delivery to the ischemic region.84 However, a strategy that is different from liposomes is the use of engineered degradation for intracellular release. Specifically, one study had incorporated a thrombin-cleavable peptide sequence into a copolymer, which then had a ligand conjugated to the surface for targeting to ischemic tissue. These micelles were loaded with glyburide and showed significant reduction of cerebral edema and infarct sizes, as well as improvement of neurological scores in tMCAO mice.85 Another distinct strategy that was not shown with liposomes is to coat micelles with chitosan or conjugate wheat germ agglutinin to their surfaces to enhance interaction with the nasal mucosa when delivered by IN injection.86,87
A unique development in the relationship between polymer- and lipid-based carriers is the use of lipid layers as coating for polymeric micelles to extend the half-life of micelles and improve their biocompatibility. PLGA nanoparticles had been loaded with panax notoginsenoside (PNS) and coated with a liposomal layer before being administered via the IG route by gavage. The outer liposomal layer prolonged drug circulation time while the inner micelle prevented drugs from leaking, resulting in superior encapsulation efficiency and drug potency compared to liposomal PNS and PLGA-PNS.88 Apart from liposomal layers, micelles were also coated with erythrocyte membrane functionalized with SHp to target ischemic tissue. The cell membrane coat protected the dextran micelles from early removal and improved their biocompatibility, while SHp provided specific targeting.89 A recent study had used membrane derived from neural cells that overexpressed a receptor to promote chemotaxis-driven migration of the coated PLGA micelles to the ischemic microenvironment. Glyburide was loaded into this carrier and IV injection into tMCAO mice resulted in reduced infarct volumes and improved neurological scores, as well as sustained release over 48 hrs.90 Taken together, these studies have shown the effectiveness of functionalized micelles through neurological scoring and immunostaining. Micelles are versatile and can solubilize lipophilic molecules well, but without functionalization, are either unstable or cytotoxic and have no target specificity.
Dendrimers
Unlike the lamellar arrangement of linear micellar polymers around a core of hydrophobic tails, dendrimers are made up of identical, branched polymers amassed around a central molecule, repeated in layers, called generations. The dendritic projections have functional groups at outer ends that serve as moieties for surface conjugation. Dendrimers are highly monodisperse and easy to fabricate in scale. Their size and shape depend on the number of generations but they are typically below 100 nm and globular. They may contain therapeutic agents as the core, between the branches or at the periphery. Dendrimers offer greater stability due to having bonds between generational layers and crosslinks between parallel units. They show high levels of cell uptake and vascular permeation but high cytotoxicity and hemolysis as well, resulting from their highly cationic surface charge which can be neutralized by conjugation of anionic molecules.91 In particular, it was shown that PEGylation improved the biocompatibility and reduced the clearance of polyamidoamine (PAMAM) dendrimers.92
Some studies had applied strategies comparable to micelles to improve cell uptake of dendrimers and consequent gene transfection. One study had conjugated dexamethasone as the targeting device, while another had incorporated reducible residues to dendritic polymers for increased degradability.93,94 These studies had applied intracerebral (IC) injection and could not report details regarding localization. Nevertheless, specific uptake by particular cell types was shown using core-shell PAMAM tecto-dendrimers that were taken up exclusively by microglia and astrocytes after IC injection.95 A later study using highly degradable dendrimers showed efficacy when delivered via IN but not via IV, suggesting an inability to cross the BBB when injected into the blood.96 However, one study had capitalized on the recruitment of immune cells to overcome the BBB and deliver antioxidants to the ischemic tissue. Proline-glycine-proline (PGP) peptides were conjugated to dendrimers loaded with catalase to target to neutrophils. In tMCAO mice, both the carriers and catalase were found co-localized in the immune cells and in the ischemic region, while the reduced ROS levels and infarct sizes implied anti-oxidative activity.97
Nanogels
In contrast to micelles and dendrimers, nanogels are nanoscale, three-dimensional, cross-linked polymers that act as reservoirs from which drugs are released. They offer the benefit of controlled release similar to other polymeric systems; however, their surface area may be greater due to the amorphous structure and high water content. Because of this, they are innately soft compared to other DDS and have a greater capacity of loading therapeutics, which may be suspended in the nanogel or form part of the structure.98 As yet, few animal studies are available of nanogels for stroke therapy. Interestingly, studies on nanogels had been for thrombolysis in rat pMCAO. One study had used a PEGylated thrombolytic agent, urokinase (UK), as the nanogel polymer that dissociated in low pH in the ischemic microenvironment.99 Another study had loaded UK onto chitosan nanogel that dissociated upon ultrasound stimulation.100 Although the outcome measured was not of recanalization but of effective drug release and protection of BBB integrity.
Inorganic Nanoparticles
Inorganic nanoparticles have been shown to have antioxidant properties and are known to have favorable characteristics for use as therapeutics and DDS. Cerium oxide (CeO2) nanoparticles were tested for their radical-scavenging activities when coated with PEGylated phospholipids. The CeO2 nanoparticles measured at 3 nm in diameter, while the coat measured at 30 nm. Rat tMCAO models were then treated with this formulation by IV administration. Results showed bioavailability and efficacy that were dose-dependent.101 More recently, conjugation of LWX7 to the surface of ceria nanoparticles improved localization to the ischemic penumbra in tMCAO rats due to interaction with integrin αvβ3.102 In a rat hemorrhagic stroke model, IV injection of CeO2 nanoparticles had reduced neuronal death, immune cell infiltration and cerebral edema.103 These do not however exemplify DDS technology. Therefore, in a recent study, CeO2 nanoparticles were loaded with edaravone and conjugated with angiopep-2 and PEG. The functionalization allowed targeting to the BBB and enhanced crossing while maintaining BBB integrity.104
Besides CeO2, platinum (Pt) nanoparticles were used in tMCAO rats and showed neuroprotective effects. When tPA was administered after reperfusion, it showed exacerbation of I/R injury. Using Pt nanoparticles improved motor function and reduced oxidative damage.105 Superparamagnetic iron oxide nanoparticles (SPIONs) have also been applied for controlled delivery of therapeutics in stroke using magnetic field guidance. SPIONs coated in silica were incubated with endothelial progenitor cells (EPCs) to be engulfed, before the EPCs were injected by IV into tMCAO mice, which resulted in improved behavioral outcomes and angiogenesis.106 Again, these studies do not show the true potential of inorganic nanoparticles as drug carriers. Similar to SPIONs but different in structure, iron oxide (Fe3O4) microrods are made of porous granular clusters, averaging 15 nm in size, that arrange into rods of 500 nm in diameter and 1.3 μm in length. Fe3O4 microrods had been loaded with tPA and were then guided by an external magnetic field to the clot in mice with thrombotic stroke. Results showed both successful delivery of tPA to the site as well as mechanical disruption of the clot by the microrods, on top of the chemical lysis by tPA. Additionally, despite the rod size, no damage to kidneys was observed. This might have been because Fe3O4 is biodegradable and could be recycled by immune cells for iron homeostasis.107
Carbon Allotropes
Carbon-based carriers are similar to inorganic nanoparticles, in that much of the studies of carbon nanoparticles are for use as the active agent after surface modification, instead of as a carrier. The spherical allotrope is fullerene. With the appropriate modification, fullerenes have free radical-scavenging potential like that of inorganic nanoparticles and have been tested using IV and IC injections, showing anti-oxidative and anti-inflammatory properties.108–112 Research on fullerenes has been limited, which may be due to inherently poor water solubility and evidence of toxic accumulation at RES organs.113 Structurally distinct from fullerenes but similar to microrods, carbon nanotubes (CNTs) are graphitic carbon arranged in tubular formation. One study showed neuroprotection in tMCAO rats that were treated beforehand with single-walled CNTs (SWCNTs) via injection into brain ventricles.114 Another study showed that after induced stroke, IC injection of hydrophobic multi-walled CNTs (MWCNTs) carrying NPCs promoted the integration and differentiation of NPCs within the lesion, resulting in improved behavioral outcomes, compared to hydrophilic MWCNTs and NPCs without carriers.115 Regardless, CNTs suffer the disadvantage of aberrant translocation to distal organs and toxic accumulation.116
Cell Membrane-Derived Vesicles
Cell Membrane-derived Vesicle (CMV) is a generic term encompassing all membrane vesicles that are produced by or manufactured from cells. They form an emergent class of DDS that has rapidly evolved and acquired massive research interest in the last decade. Extracellular vesicles (EVs) are heterogeneous nanoscale CMVs secreted by practically all cells. They carry a variety of molecules encapsulated and on the membrane surfaces, and have differentially expressed surface markers and tissues of origin, though their classification is based on size and different pathways of formation.118 Of particular interest are exosomes and microvesicles (MVs) because they serve as modes of communication between cells and have been shown to promote cardiovascular remodeling and angiogenesis after myocardial infarction.119 Exosomes are typically 30–150 nm in diameter and originate from the endosomal pathway, whereas MVs originate from outward blebbing of the plasma membrane and range in size up to 1000 nm in diameter. As a consequence of this diversity, EVs show tissue specificity at varying degrees and the mechanism of action that EVs exert on their target remains elusive; it is possible that binding alone may be sufficient to elicit a response in some cases, whereas in others, endocytosis or other forms of internalization may be necessary. EVs can thus influence stroke directly by affecting brain cells and indirectly by affecting immune cells.120 EVs are known to be involved in the pathogenesis of clinical cases as well, with potential for use as cell-free therapy and as biomarkers because they were found upregulated with miRNA contents that were implicated in diseases. Moreover, studies have revealed that EVs have low immunogenicity due to their endogenous components, and their uptake by endocytosis or macropinocytosis is affected by the tissue of origin and cell target.121 Due to these distinctive features, EVs have the potential to deliver therapeutic agents by modifying the parent cell to overexpress specific proteins and RNA, thus enriching EVs; or by directly loading EVs with drugs, RNA mimics, proteins and even plasmid vectors to transfect target cells.
Most studies had used EVs that originated from stem cells. This is because previous studies on stem cell-based therapeutics showed that despite promoting recovery, few of the transplanted cells actually incorporated into the brain parenchymal tissue of the animal models and thus, it was posited that instead of integration into and replacement of damaged tissue, the cells provided signals, growth factors and miRNAs via exosomes that enhanced tissue regeneration. This is plausible because mesenchymal stem cells (MSCs) are known to produce exosomes abundantly, especially upon exposure to conditioned medium.121 One group showed increased neuronal growth and development along with functional recovery when tMCAO rats were treated with unmodified exosomes from MSCs.122 Effect sizes were greater when the origin cells were made to overexpress miR-133b, which was observed in the exosomes having more miR-133b, implying the significance of the cargo.123 Along with neurons, astrocytes were affected by the enriched exosomes and contributed secondary exosomes that enhanced the outcomes.124
Studies have already shown that regeneration and functional outcomes were similarly enhanced whether treated with MSCs or EVs. However, EVs were specifically observed to have attenuated post-ischemic immunosuppression in the peripheral blood and separately, to have migrated into the infarcted brain more than stem cells have.125,126 Some studies explored the overexpression of diverse vectors and RNA mimics by transfecting cells using permeabilization methods, such as electroporation and Lipofectamine, and observing the effects of the enriched EVs. Exosomes enriched with miR-17-92 cluster enhanced neural plasticity and regeneration in tMCAO rats by modulating protein levels and downstream signalling, while exosomes enriched with miR-30d-5p conferred neuroprotection in rats with permanent middle cerebral artery occlusion (pMCAO) by promoting macrophage polarization to the anti-inflammatory phenotype.127,128
Other studies have functionalized EVs. One study had fused vectors for rabies virus glycoprotein to lysosome-associated membrane glycoprotein 2b (RVG-Lamp2b) and transfected them into MSCs to produce a recombinant surface protein on exosomes, before loading miR-124 mimics into these exosomes by electroporation. Exosomes with RVG-Lamp2b showed neuron-specific targeting, while their cargo promoted neurogenesis.129 Another study had directly conjugated peptides onto exosome surfaces before loading them with curcumin by way of sucrose gradient centrifugation. Specifically, the peptide c(RGDyK) can bind with high affinity to integrin αvβ3 which is overexpressed by ischemic cerebral vascular endothelium. The modified exosomes localized at the lesion and suppressed the inflammatory response.130 Other routes were also considered to improve targeting. One study had delivered exosomes enriched with pigment epithelium-derived factor (PEDF), via an IC injection, which resulted in improved autophagy and decreased apoptotic activity.131 Another study had loaded curcumin by rapid freeze-thawing into embryonic stem cell (ESC) exosomes and delivered them via the IN route, which resulted in reduced inflammation and restoration of the neurovascular unit.132
Regardless of the functionalization and loading, EVs have shown efficacy across stroke models. In a rat model using endothelin-1 (ET-1) to induce subcortical ischemic stroke, exosomes from rat adipose-derived stem cells (ADSCs) were administered via the IV route and showed reduction in infarct size, increase in axonal sprouting and glial cell development, and functional recovery over 28 days.133 In a mouse model of thrombotic stroke, EVs were generated from MSCs and neural stem cells (NSCs) that were both isogenically derived from H9 pluripotent stem cells to control for genetic confounders. The EVs produced by both cell lines had similar structure and protein expression profiles but MSC EVs had a size range up to 300 nm in diameter, whereas NSC EVs were mostly under 200 nm. Both EVs were efficacious but treatment with NSC EVs had superior results.134 However, whether the difference was due to EV size or cell source was not addressed. In a porcine model of ischemic stroke, NSC EVs that were injected via IV prevented hemorrhagic conversion in the experimental group compared to the control group which had seven incidences out of eight animals. Additionally, the treated group had significant decreases in lesion volume and brain edema, and improved behavioral outcomes, compared to untreated controls upon longitudinal observation.135 Similarly, in hemorrhagic stroke, exosome treatment increased white matter repair and improved functional outcomes progressively over 28 days.136,137
Selective studies on MVs for stroke therapy were particularly few compared to exosomes. In one study, human ADSCs were treated with either normal or stroke brain extract to produce different MVs that were injected via the IA route into pMCAO rats. Both showed similar efficacy in reducing infarct sizes and improving neurological deficits.138 In contrast, another study found that MVs from human brain microvascular endothelial cells (BMECs) exposed to normal culture conditions had promoted recovery, while MVs from BMECs exposed to oxygen and glucose deprivation had aggravated the condition.139 A novel subtype of CMVs is the bio-nanobubbles (BNBs), also known as exosome-mimetics or cell-derived nanovesicle, which are produced mechanically and had been previously used in cloaking strategies, hybrid carriers and vaccines.140–142 In stroke, BNBs were fabricated from human blood platelet by sonication and freeze-thawing, and had shown specific targeting as well as enhanced ultrasound imaging of the lesion in mouse thrombotic stroke, but no further studies had since been reported.143 The challenges facing the application of CMVs for stroke therapy are the difficulties in regulating surface interactions for specific localization and uptake, in studying factors and outcomes due to heterogeneity, and in sustaining production through scalability and stable long-term storage.
Discussion
Diversity and Complexity
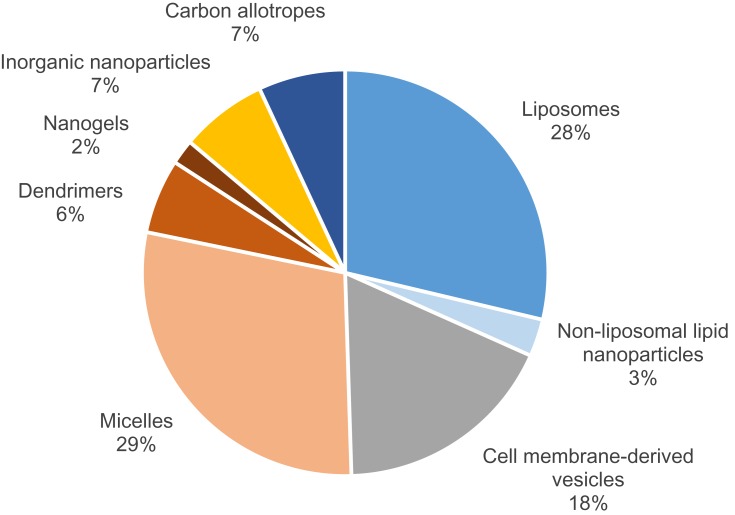
As seen in Figure 1, there are multiple categories of nanocarriers that differ in terms of material composition and structure. However, they all share either colloidal or vesicular mechanisms to carry and deliver therapeutics. Recent developments in each category have generally contributed to the growing field through preclinical testing although the potential is yet to be realized in clinical trials. Based on Figure 2, liposomes and micelles together constitute approximately two-thirds of animal studies in nanoparticle DDS for stroke therapy over the recent years. The improvements in liposomes and micelles have facilitated experimentation with other nanoparticle compositions, with some studies even combining them as hybrid, liposome-coated, micellar carriers. Liposomes are artificial systems and require surface functionalization for site-specificity and extended half-life. Else, the drug may need multiple dosing to achieve efficacy, which in some studies extended over 2 weeks of daily injections. Noticeably, functionalization of liposomes has been generic and restricted to mainly simple conjugation of targeting ligands or incorporation of charged molecules during fabrication (Table 1). More diverse functionalities had been achieved with their polymer-based counterparts because of the higher variability in the chemical composition and configuration of the monomeric units. This results in a wider range of interactions and emergent capacities, and explains the different direction taken for the development of polymer-based carriers (Table 2). Similarly, the functionalities of carbon-based and inorganic nanoparticles are unique but fundamentally chemical.
Figure 2.
Distribution of nanoparticles used in animal models of stroke based on their compositions.
Table 1.
Lipid-Based Carriers Used in Animal Models of Stroke, Categorized According to Surface Property and Therapeutic Cargo
| Carrier | Property | Surface Component | Therapeutic Agent | Animal: Disease Model (Route) | Source |
|---|---|---|---|---|---|
| LIPOSOME | Stealth | PEG | Hb, fasudil, Xe, luteolin, FK506, CsA, dexamethasone, acetate | Rat: tMCAO (IA, IP, IV), pMCAO (IV), thrombotic stroke (IV), hemorrhagic stroke (IV), global transient ischemia (IV) Monkey: tMCAO (IV) |
29–39,41–43,46,53,57 |
| Stealth, conjugated | PEG, AEPO, T7, SHp, anti-HSP72, anti-CD106, IgG-1, anti-PirB | AEPO, ZL006, citicoline, anti-PirB | Rat: tMCAO (IA, IV), pMCAO (IV) Mouse: thrombotic stroke (IV) |
48–51,58-60 | |
| Stealth, charged | PEG, CHOL(+), DOPA(-) | Simvastatin | Rat: tMCAO (IV) | 52 | |
| Non-stealth, conjugated | - | baicalin, lycopene, bFGF, OEA | Rat: tMCAO (IN, IV, IG), pMCAO (IV) | 40,44,45,47 | |
| PLN | Conjugated, loaded | PEG, anti-Fas | 3-n-Butylphthalide | Mouse: tMCAO (IV) | 54 |
| NLC | Loaded | - | Ferulic acid | Rat: Global transient ischemia (IV) | 55 |
| NANOEMULSION | Mucoadhesion | PEG, chitosan | Quercetin | Rat: tMCAO (IN, IV) | 56 |
Table 2.
Alternative Nanoparticles Studied in Animal Models of Stroke, Categorized According to Carrier Material Composition and Functionalization
| Carrier: Property | Functional Component | Therapeutic Agent | Animal: Disease Model (Route) | Source |
|---|---|---|---|---|
| MICELLES: Controlled degradability, coated, conjugated, mixed, loaded | Degradable motif, phospholipid coat, cell membrane coat, chitosan coat, tween80, polysorbate 80, amino-TEMPO, AMD3100, A2AR agonist, ApoE, anti-NR1, BHEM-Chol, CBSA, chlorotoxin, CREKA, HSAP, dexamethasone, lexiscan, WGA | catalase, curcumin, dexamethasone, edaravone, glyburide, glycyrrhizic acid, PNS, HSAP, luteolin, NGF, MRZ2/576, NEP1-40, NR2B9C, puerarin, rapamycin, amino-TEMPO, resveratrol, riluzole, superoxide dismutase, tanshinone IIA, C3 siRNA, HO-1 plasmid, miR-195 | Mouse: tMCAO (IA, IV), thrombotic stroke (IV) Rat: tMCAO (IA, IC, IN, IP, IV, IG), pMCAO (IV), thrombotic stroke (IA), hemorrhagic stroke (IV), global transient ischemia (IG) |
62–90 |
| DENDRIMERS: Controlled degradability, conjugated, loaded, tectonic | Degradable motif, dexamethasone, PGP | Catalase, HMGB-1 siRNA, HO-1 plasmid, dexamethasone | Mouse: tMCAO (IV), pMCAO (IV), unilateral cortical devascularization (IC) Rat: tMCAO (IC, IN, IV) |
92–97 |
| NANOGELS: Controlled release, loaded | Glycol chitosan, PEG-UK | UK, uPA | Rat: pMCAO (IV) | 99,100 |
| INORGANIC NANOPARTICLES: Conjugated, coated, loaded | LXW7, PEG-ANG coat, phospholipid coat, silica coat | CeO2, Pt, EPCs, edaravone, tPA | Mouse: tMCAO (IV), thrombotic stroke (IA) Rat: tMCAO (IV), hemorrhagic stroke (IV) |
101–107 |
| CARBON ALLOTROPES: Conjugated, coated, modified | Glucosamine, hydrocarbon layer, amine group, carboxyl group, polyhydroxyl group, sulfobutyl group | Carboxyfullerene, FC4S, fullerenol, glucosamine, C3 siRNA, NPCs | Rat: tMCAO (IC, IP, IV), ET-1 stroke (IC) | 108–112,114,115,117 |
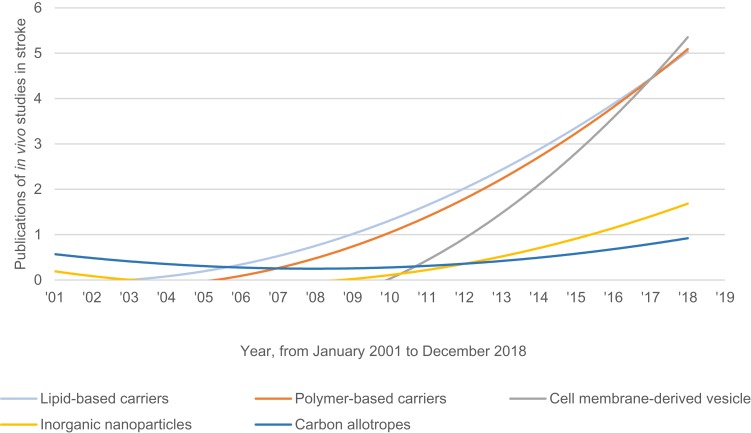
On the other hand, CMVs require entirely different strategies. One way is to load cargo or modify surface properties using cell engineering methods. This is achieved by exposing cells to specific conditions or tissue extracts, or by transduction to express proteins or miRNA (Table 3). Another approach is to load cargo or to modify the surface of the harvested CMVs using permeabilization methods or peptide conjugation, which is not unlike other nanoparticles. Regardless, a major distinction of CMVs is their efficacy despite having neither overexpressed nor encapsulated cargo (Table 3). Moreover, CMVs are innately biocompatible because of the endogenous origins and material composition that is analogous to cells of the body; chiefly, the phospholipid bilayer containing a mix of surface markers. This explains Figure 3 which depicts the publications of in vivo studies on nanoparticle DDS for stroke therapy. Lipid- and polymer-based carriers had been in the lead for two decades but have been overtaken recently by CMVs. As of 2019, only exosomes are in clinical trial for stroke according to the United States National Library of Medicine website.
Table 3.
Cell Membrane-Derived Vesicles Used in Animal Models of Stroke, Categorized According to Vesicle Type and Tissue of Origin; Exosomes Have Either Been Explicitly Described or Otherwise Inferred Based on Size and Protein Marker Detection
| Vesicle | Species (Tissue) | Modification | Therapeutic Agent | Animal: Disease Model (Route) | Source |
|---|---|---|---|---|---|
| EXOSOME | Rat (MSC, ADSC) | Cell transduction, cell transfection | miR-17-92 cluster vector, miR-137 vector, miR-184 vector, miR-210 vector, miR-30d-5p mimic, PEDF protein | Rat: tMCAO (IA, IC, IV), pMCAO (IV), ET-1 stroke (IV), hemorrhagic stroke (IV) | 122,124,125,127,128,131,133,136,137 |
| Mouse (MSC, ESC) | Cell transfection, exosome loading, exosome conjugation | RVG-Lamp2b vector, miR-124 mimic, curcumin, c[RGDyK] | Mouse: tMCAO (IN, IV), thrombotic stroke (IV) | 129,130,132 | |
| Human (MSC, NSC) | - | - | Mouse: tMCAO (IV), thrombotic stroke (IV) Pig: pMCAO (IV) |
126,134,135 | |
| MV | Human (ADSC, BMEC) | Cell exposure to tissue extract | - | Mouse: tMCAO (IV) Rat: pMCAO (IA) |
138,139 |
| BNB | Human (Platelet) | - | - | Mouse: thrombotic stroke (IV) | 143 |
Figure 3.
Temporal timeline of research interest for stroke with nanoparticles, expressed via in vivo study publication.
Notwithstanding, the specific active ingredients in CMVs remain unclear still and the suggested protein and genetic elements have varied as much as the drugs that were applied in the studies of lipid- and polymer-based carriers. Interestingly, given the array of potential therapeutic cargo, most studies of CMVs did not investigate any potentially confounding biological factors beyond showing the sufficiency of the suspected miRNAs and the necessity of the CMVs in general to induce an effect. Also, there has yet to be a relevant comparative analysis of candidate miRNA sequences. Worth noting also is that there is a lack of clarity regarding the purpose of exposing cells to specific extracts; whether the aim was to produce more EVs or specific subtypes of EVs. Moreover, there are still issues of reproducibility, scalability and stable preservation that hamper the potential commercialization of EVs. However, these may be overcome by reconstituting cell membrane into artificial vesicles and showing their efficacy compared to their naturally produced counterparts. The fabrication methods may involve cell separation and reassembly by sonication, or cell extrusion through porous filters.141,144 These methods allow the amplification of yield by hundreds fold, with shorter turnover time and improved homogeneity.
Comparisons and Confounders
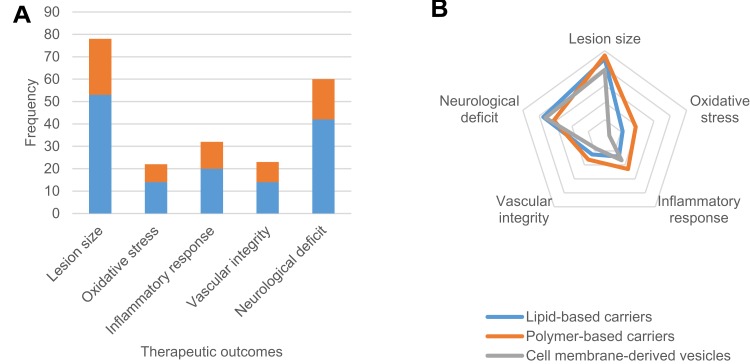
Another issue that is apparent across the research field is the general lack of studies comparing the efficacy between different nanoparticles and the lack of suitable controls within studies. One type of comparative analysis that was present is the comparison between exosomes of different cell origins; namely, between human cells and rat cells, as well as between mesenchymal stem cells and neural stem cells. Comparisons of free drugs against encapsulated drugs were present, as well as of empty carriers against loaded carriers. However, few studies actually applied both controls together as displayed in Figure 4A. The scarcity begs the question of whether there has been sufficient evidence of the therapeutic effect being due to the targeted delivery of the drug or agent as opposed to the nanoparticle exerting some effect on its own. This question, however, applies sparingly to inorganic nanoparticles and carbon allotropes because they tend to have therapeutic potential themselves, as well as to CMVs as their therapeutic mechanism remains to be established. These factors make the exercise of systematic meta-analysis more complicated but all the more essential to progress in the development of candidate nanoparticle DDS.
Figure 4.
Therapeutic outcome measures for lipid-based carriers, polymer-based carriers and cell membrane-derived carriers (CMVs). (A) Bar chart of the absolute total for each outcome measured. Studies using both empty vehicle and free drug controls (red) are fewer than those using only one or neither of the controls (blue). (B) Radar chart of the percentage-weighted differences in the types of outcome measures used to show efficacy in vivo.
Figure 4B lists the different therapeutic endpoints addressed in different studies. The data was corrected to display percentage from total of each group instead of absolute values to highlight the proportionate differences between the material-based classes. STAIR and STEPS guidelines do not recommend specific endpoints except for infarct size and behavioral deficits, which most studies have incorporated. In contrast, measures of edema and vascular integrity are consistently low across the three classes. Oxidative stress is particularly absent among studies of CMVs, with no such studies using it as a measure. These are important to show whether the intervention has a specific pathway of effect. An endpoint that is not listed is the detection of neurogenesis and angiogenesis, which were especially common in studies of CMVs. Since there are phases of stroke progression and multiple strategies of intervention, such as thrombolysis, neuroprotection and tissue regeneration, studies should be able to demonstrate principally the effects for each, which is why the guidelines stated the need for multiple stroke models and full disclosure when reporting results but could also recommend a list of relevant goals for research endeavors.
Apart from therapeutic outcomes, the time when drug is administered may be taken as another confounder. This is because one of the purposes of studying nanocarriers is to understand how they improve the current modes of intervention but most studies had not shown such a benefit in the therapeutic time window. The drug formulations were administered within 1 hr in half of all studies, or within 4.5 hrs in most of them to compare to tPA. Also, more than a fifth of studies had introduced the treatment prior to inducing stroke in the animal subjects, which does not seem useful except when studied as a preventative measure against recurrence. Some had been able to show differential effects at specific times of intervention, while others showed significant effects when administered at 24 hrs after stroke induction or later. This was a particular trend in the studies on CMVs but was scarce in others, implying their efficaciousness over a wider therapeutic window, which supports the notion of CMVs reaching clinical phase. Yet another confounder is that nearly a quarter of studies applied multiple dosing regimens for the formulations but few had shown the effective, toxic and therapeutic indices beyond optimising the dosage required to bring about an effect. Some of these regimens can last for weeks but the neurobehavioral assessments tend to not address longitudinal developments, missing the point of neuroprotection and tissue regeneration, which is to see the preservation and restoration of functions over time. Thus, in vivo evaluation protocols need to consider whether the results are sufficiently meaningful.
Translational Paradigms
Figure 5 shows that most studies had administered drugs via IV injection yet many studies had delivered via other routes as well. The problem with the IC route is its high invasiveness and the related complications, especially when piercing brain tissue. On the other hand, the IG route exposes the formulations to the harsh gastrointestinal environment, affecting bioavailability. Neither of these designs are clinically feasible. The more promising routes are IV and IA, followed by IN then IP based on the access to systemic circulation and brain tissue, and on the clearance mechanisms involved. The number of types of stroke models and their frequency used in animals almost epitomizes the clinical incidence rates; however, the problem is that none among these represent the same formulation except for liposomal Hb and EVs. This means most systems are tested only once and are not followed through in alternative stroke models or larger animal species. The drug and carrier combinations that had been used in different studies were either paired with different carriers or administered by different routes. This is unconstructive because each model and method has inherent limitations. Most studies had used rodents as they are small, easy to handle and relatively cheap to maintain. Mice offer the advantage of fast ageing and breeding, allowing genetic studies. As the smallest option, however, surgery and tissue analyses are cumbersome. Rats are larger and easier to monitor for physiological and functional outcomes.145 Monkeys and pigs are gyrencephalic, sharing analogous sensorimotor integration, but are too large and costly to maintain, and thus are used only in follow-up trials.146
Figure 5.
Distribution of routes of drug administration applied in animal models of stroke, for lipid-based carriers, polymer-based carriers and CMVs.
The main method of inducing stroke in animals is tMCAO, which offers reproducibility and control of reperfusion. The resultant lesion most closely mimics human stroke but its major side effect is the risk of hemorrhage from the surgery. This method is useful for studying neuroprotective agents and reperfusion injury but not thrombolytic therapy due to the artificial mechanism of recanalization. Thrombotic methods, on the other hand, involve relatively simpler procedures and low invasiveness. It involves light or chemical activation of an injected agent that either causes vascular constriction or clot formation. Alternatively, embolic clots can be formed without external stimulation by injecting thrombin or microspheres directly into the selected cerebral vasculature. All these methods allow the study of thrombolytic drugs through observing vascular responses and clot disintegration. However, they offer little control over the duration of blood flow depletion and size of injury, with embolic methods being at risk of spontaneous recanalization.147 To study hemorrhagic stroke, subarachnoid hemorrhage models may be induced by blood clot placement, vessel puncture or intracisternal blood injection. These methods can mimic intracranial aneurysm rupture to an extent but they lack fidelity in pathophysiology.148 By applying multiple models, the formulation can be shown to achieve efficacy convincingly through specified mechanisms and thus, can also be developed for specific clinical cases.
Conclusion
In summary, nanoparticle DDS for stroke therapy have evolved to match the evolving concepts in immunological and pathological mechanisms of stroke. Liposomes and micelles remain as the preferred candidates of carriers with high adaptability, reproducibility, stability and scalable production. In contrast, CMVs have superior biocompatibility and efficacy but are more difficult to produce and modify, are less chemically defined and are more heterogeneous. Also, CMVs require further improvements for long-term storage and scalability. There have been few studies conducted for inorganic nanoparticles and carbon-based allotropes in the context of stroke, although generally, poor safety profiles stand in the way of progress. Hybrid combinations of different materials may provide a novel approach to improve the design of nanocarriers, either using liposomes or cell membranes as coating. However, the inconsistent use of therapeutic outcome measures and unconstructive subsequent studies necessitate a revision of translational frameworks. A major challenge is to systematize the data within categories of carrier systems and drugs of choice, and subsequently account for intervention strategies, the animal models and the outcome measures, before comparisons can be made between DDS.
Abbreviations
A2AR, adenosine 2A receptor; ADSC, adipose-derived stem cell; AEPO, asialo-erythropoietin; BBB, blood-brain barrier; bFGF, basic fibroblast growth factor; BMEC, brain microvascular endothelial cell; BNB, bio-nanobubble; CBSA, cationic bovine serum albumin; CeO2, cerium oxide; CMC, critical micellar concentration; CMV, cell membrane-derived vesicle; CNT, carbon nanotube; CsA, cyclosporine A; DDS, drug delivery system; EPC, endothelial progenitor cell; EPR, enhanced permeability and retention; ESC, embryonic stem cell; ET-, endothelin-1; EV, extracellular vesicle; Fe3O4, iron(II,III) oxide; Hb, hemoglobin; HO-1, heme oxygenase-1; I/R, ischemia/reperfusion; IA, intra-arterial; IG, intragastric IN, intranasal; IP, intraperitoneal; IV, intravenous; MSC, mesenchymal stem cell; MV, microvesicle; MVB, multivesicular body; MWCNT, multi-walled carbon nanotube; NGF, nerve growth factor; NLC, nanostructured lipid carrier; NPC, neural progenitor cell; NSC, neural stem cells; OEA, oleoylethanolamine; PAMAM, polyamidoamine; PEDF, pigment epithelium-derived factor; PEG, polyethylene glycol; PEI, polyethylenimine; PGP, proline-glycine-proline; PLGA, poly-lactic-glycolic acid; PLN, PEGylated lipid nanoparticle; pMCAO, permanent middle cerebral artery occlusion; PNS, panax notoginsenoside; Pt, platinum; RES, reticuloendothelial system; ROS, reactive oxygen species; rPOA, reducible poly(oligo-arginine); RVG-Lamp2b, recombinant vector of rabies virus glycoprotein and lysosome-associated glycoprotein 2b; SHp, stroke homing peptide; SPION, superparamagnetic iron oxide nanoparticle; STAIR, Stroke Therapy Academy Industry Roundtable; STEPS, Stem Cell as an Emerging Paradigm in Stroke; SWCNT, single-walled carbon nanotube; T7, transferrin receptor-binding peptide; tMCAO, transient middle cerebral artery occlusion; tPA, tissue plasminogen activator; UK, urokinase; Xe, xenon gas.
Disclosure
The authors declare no competing interest in this work. This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors. Figures of this review were created using Microsoft Office and ChemDraw software, using freely available templates.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics - 2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Reis C, Akyol O, Ho WM, et al. Phase I and Phase II therapies for acute ischemic stroke: an update on currently studied drugs in clinical research. Biomed Res Int. 2017;2017:4863079. doi: 10.1155/2017/4863079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shcharbina N, Shcharbin D, Bryszewska M. Nanomaterials in stroke treatment: perspectives. Stroke. 2013;44(8):2351–2355. doi: 10.1161/STROKEAHA.113.001298 [DOI] [PubMed] [Google Scholar]
- 4.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. doi: 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leclerc JL, Garcia JM, Diller MA, et al. A comparison of pathophysiology in humans and rodent models of subarachnoid hemorrhage. Front Mol Neurosci. 2018;11:71. doi: 10.3389/fnmol.2018.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlunk F, Greenberg SM. The pathophysiology of intracerebral hemorrhage formation and expansion. Transl Stroke Res. 2015;6(4):257–263. doi: 10.1007/s12975-015-0410-1 [DOI] [PubMed] [Google Scholar]
- 7.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstead WM, Ganguly K, Kiessling JW, et al. Signaling, delivery and age as emerging issues in the benefit/risk ratio outcome of tPA for treatment of CNS ischemic disorders. J Neurochem. 2010;113(2):303–312. doi: 10.1111/jnc.2010.113.issue-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danielyan K, Ganguly K, Ding BS, et al. Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation. 2008;118(14):1442–1449. doi: 10.1161/CIRCULATIONAHA.107.750257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel RAG, McMullen PW. Neuroprotection in the treatment of acute ischemic stroke. Prog Cardiovasc Dis. 2017;59(6):542–548. doi: 10.1016/j.pcad.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 11.Neuhaus AA, Couch Y, Hadley G, Buchan AM. Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain. 2017;140(8):2079–2092. doi: 10.1093/brain/awx126 [DOI] [PubMed] [Google Scholar]
- 12.Sas A, Horváth L, Oláh C, Valikovics A. A review of neuroinflammatory mechanisms in ischemic stroke: background and therapeutic approaches. Mech Neuroinflam. 2017:119. [Google Scholar]
- 13.Abed SN, Deb PK, Surchi HS, et al. Nanocarriers in different preclinical and clinical stages In Tekade RK, editor. Basic Fundamentals of Drug Delivery. Academic Press; 2019. 685–731. [Google Scholar]
- 14.Durán N, Marcato PD. Nanobiotechnology perspectives. Role of nanotechnology in the food industry: a review. Int J Food Sci Technol. 2013;48(6):1127–1134. doi: 10.1111/ijfs.2013.48.issue-6 [DOI] [Google Scholar]
- 15.Dasgupta N, Ranjan S, Ramalingam C. Applications of nanotechnology in agriculture and water quality management. Environ Chem Lett. 2017;15(4):591–605. doi: 10.1007/s10311-017-0648-9 [DOI] [Google Scholar]
- 16.Kaul S, Gulati N, Verma D, Mukherjee S, Nagaich U. Role of nanotechnology in cosmeceuticals: a review of recent advances. J Pharm (Cairo). 2018;2018:3420204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kutuzov N, Flyvbjerg H, Lauritzen M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier. Proc Natl Acad Sci U S A. 2018;115(40):E9429–E9438. doi: 10.1073/pnas.1802155115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrahari V, Burnouf PA, Burnouf T, Agrahari V. Nanoformulation properties, characterization, and behavior in complex biological matrices: challenges and opportunities for brain-targeted drug delivery applications and enhanced translational potential. Adv Drug Deliv Rev. 2019;148:146–180. doi: 10.1016/j.addr.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 20.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016–1024. doi: 10.1016/S1474-4422(18)30318-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nistal D, Mocco J. Central nervous system lymphatics and impact on neurologic disease. World Neurosurg. 2018;109:449–450. doi: 10.1016/j.wneu.2017.11.028 [DOI] [PubMed] [Google Scholar]
- 23.Nehoff H, Parayath NN, Domanovitch L, Taurin S, Greish K. Nanomedicine for drug targeting: strategies beyond the enhanced permeability and retention effect. Int J Nanomedicine. 2014;9:2539–2555. doi: 10.2147/IJN.S47129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl Stroke Res. 2013;4(3):279–285. doi: 10.1007/s12975-012-0209-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaafar-Maalej C, Diab R, Andrieu V, Elaissari A, Fessi H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J Liposome Res. 2010;20(3):228–243. doi: 10.3109/08982100903347923 [DOI] [PubMed] [Google Scholar]
- 26.Zhang H. Thin-film hydration followed by extrusion method for liposome preparation. Methods Mol Biol. 2017;1522:17–22. [DOI] [PubMed] [Google Scholar]
- 27.Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9(2):12. doi: 10.3390/pharmaceutics9020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szebeni J, Moghimi SM. Liposome triggering of innate immune responses: a perspective on benefits and adverse reactions. J Liposome Res. 2009;19(2):85–90. doi: 10.1080/08982100902792855 [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi AT, Fukumoto D, Haida M, Ogata Y, Yamano M, Tsukada H. Liposome-encapsulated hemoglobin reduces the size of cerebral infarction in the rat: evaluation with photochemically induced thrombosis of the middle cerebral artery. Stroke. 2007;38(5):1626–1632. doi: 10.1161/STROKEAHA.106.467290 [DOI] [PubMed] [Google Scholar]
- 30.Fukumoto D, Kawaguchi AT, Haida M, Yamano M, Ogata Y, Tsukada H. Liposome-encapsulated hemoglobin reduces the size of cerebral infarction in rats: effect of oxygen affinity. Artif Organs. 2009;33(2):159–163. doi: 10.1111/aor.2009.33.issue-2 [DOI] [PubMed] [Google Scholar]
- 31.Shimbo D, Abumiya T, Shichinohe H, Nakayama N, Kazumata K, Houkin K. Post-ischemic intra-arterial infusion of liposome-encapsulated hemoglobin can reduce ischemia reperfusion injury. Brain Res. 2014;1554:59–66. doi: 10.1016/j.brainres.2014.01.038 [DOI] [PubMed] [Google Scholar]
- 32.Hamadate N, Yamaguchi T, Sugawara A, et al. Liposome-encapsulated hemoglobin ameliorates impairment of fear memory and hippocampal dysfunction after cerebral ischemia in rats. J Pharmacol Sci. 2010;114(4):409–419. doi: 10.1254/jphs.10207FP [DOI] [PubMed] [Google Scholar]
- 33.Komatsu H, Furuya T, Sato N, et al. Effect of hemoglobin vesicle, a cellular-type artificial oxygen carrier, on middle cerebral artery occlusion- and arachidonic acid-induced stroke models in rats. Neurosci Lett. 2007;421(2):121–125. doi: 10.1016/j.neulet.2007.04.080 [DOI] [PubMed] [Google Scholar]
- 34.Kaneda S, Ishizuka T, Sekiguchi A, Morimoto K, Kasukawa H. Efficacy of liposome-encapsulated hemoglobin in a rat model of cerebral ischemia. Artif Organs. 2014;38(8):650–655. doi: 10.1111/aor.12358 [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi AT, Haida M, Yamano M, Fukumoto D, Ogata Y, Tsukada H. Liposome-encapsulated hemoglobin ameliorates ischemic stroke in nonhuman primates: an acute study. J Pharmacol Exp Ther. 2010;332(2):429–436. doi: 10.1124/jpet.109.160051 [DOI] [PubMed] [Google Scholar]
- 36.Kawaguchi AT, Haida M, Ohba H, Yamano M, Fukumoto D, Tsukada H. Liposome-encapsulated hemoglobin ameliorates ischemic stroke in nonhuman primates: longitudinal observation. Artif Organs. 2013;37(10):904–912. doi: 10.1111/aor.2013.37.issue-10 [DOI] [PubMed] [Google Scholar]
- 37.Fukuta T, Ishii T, Asai T, et al. Treatment of stroke with liposomal neuroprotective agents under cerebral ischemia conditions. Eur J Pharm Biopharm. 2015;97(Pt A):1–7. doi: 10.1016/j.ejpb.2015.09.020 [DOI] [PubMed] [Google Scholar]
- 38.Partoazar A, Nasoohi S, Rezayat SM, et al. Nanoliposome containing cyclosporine A reduced neuroinflammation responses and improved neurological activities in cerebral ischemia/reperfusion in rat. Fundam Clin Pharmacol. 2017;31(2):185–193. doi: 10.1111/fcp.2017.31.issue-2 [DOI] [PubMed] [Google Scholar]
- 39.Fukuta T, Asai T, Sato A, et al. Neuroprotection against cerebral ischemia/reperfusion injury by intravenous administration of liposomal fasudil. Int J Pharm. 2016;506(1–2):129–137. doi: 10.1016/j.ijpharm.2016.04.046 [DOI] [PubMed] [Google Scholar]
- 40.Li N, Feng L, Tan Y, Xiang Y, Zhang R, Yang M. Preparation, characterization, pharmacokinetics and biodistribution of baicalin-loaded liposome on cerebral ischemia-reperfusion after i.v. administration in rats. Molecules. 2018;23:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuta T, Asai T, Yanagida Y, et al. Combination therapy with liposomal neuroprotectants and tissue plasminogen activator for treatment of ischemic stroke. FASEB J. 2017;31(5):1879–1890. doi: 10.1096/fj.201601209R [DOI] [PubMed] [Google Scholar]
- 42.Fukuta T, Yanagida Y, Asai T, Oku N. Co-administration of liposomal fasudil and tissue plasminogen activator ameliorated ischemic brain damage in occlusion model rats prepared by photochemically induced thrombosis. Biochem Biophys Res Commun. 2018;495(1):873–877. doi: 10.1016/j.bbrc.2017.11.107 [DOI] [PubMed] [Google Scholar]
- 43.Tiebosch IA, Crielaard BJ, Bouts MJ, et al. Combined treatment with recombinant tissue plasminogen activator and dexamethasone phosphate-containing liposomes improves neurological outcome and restricts lesion progression after embolic stroke in rats. J Neurochem. 2012;123(Suppl 2):65–74. doi: 10.1111/j.1471-4159.2012.07945.x [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y, Xin Z, Li N, et al. Nano-liposomes of lycopene reduces ischemic brain damage in rodents by regulating iron metabolism. Free Radic Biol Med. 2018;124:1–11. doi: 10.1016/j.freeradbiomed.2018.05.082 [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Xu L, Zhou J, et al. Integration of phospholipid-complex nanocarrier assembly with endogenous N-oleoylethanolamine for efficient stroke therapy. J Nanobiotechnology. 2019;17(1):8. doi: 10.1186/s12951-019-0442-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao G, Zang SY, Jiang ZH, et al. Postischemic administration of liposome-encapsulated luteolin prevents against ischemia-reperfusion injury in a rat middle cerebral artery occlusion model. J Nutr Biochem. 2011;22(10):929–936. doi: 10.1016/j.jnutbio.2010.07.014 [DOI] [PubMed] [Google Scholar]
- 47.Zhao YZ, Lin M, Lin Q, et al. Intranasal delivery of bFGF with nanoliposomes enhances in vivo neuroprotection and neural injury recovery in a rodent stroke model. J Control Release. 2016;224:165–175. doi: 10.1016/j.jconrel.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 48.Ishii T, Asai T, Fukuta T, et al. A single injection of liposomal asialo-erythropoietin improves motor function deficit caused by cerebral ischemia/reperfusion. Int J Pharm. 2012;439(1–2):269–274. doi: 10.1016/j.ijpharm.2012.09.026 [DOI] [PubMed] [Google Scholar]
- 49.Ishii T, Asai T, Oyama D, et al. Amelioration of cerebral ischemia-reperfusion injury based on liposomal drug delivery system with asialo-erythropoietin. J Control Release. 2012;160(1):81–87. doi: 10.1016/j.jconrel.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 50.Wang Z, Zhao Y, Jiang Y, et al. Enhanced anti-ischemic stroke of ZL006 by T7-conjugated PEGylated liposomes drug delivery system. Sci Rep. 2015;5:12651. doi: 10.1038/srep12651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Y, Jiang Y, Lv W, et al. Dual targeted nanocarrier for brain ischemic stroke treatment. J Control Release. 2016;233:64–71. doi: 10.1016/j.jconrel.2016.04.038 [DOI] [PubMed] [Google Scholar]
- 52.Campos-Martorell M, Cano-Sarabia M, Simats A, et al. Charge effect of a liposomal delivery system encapsulating simvastatin to treat experimental ischemic stroke in rats. Int J Nanomedicine. 2016;11:3035–3048. doi: 10.2147/IJN.S107292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miao YF, Peng T, Moody MR, et al. Delivery of xenon-containing echogenic liposomes inhibits early brain injury following subarachnoid hemorrhage. Sci Rep. 2018;8(1):450. doi: 10.1038/s41598-017-18914-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu YM, Huang JY, Wang H, et al. Targeted therapy of brain ischaemia using Fas ligand antibody conjugated PEG-lipid nanoparticles. Biomaterials. 2014;35(1):530–537. doi: 10.1016/j.biomaterials.2013.09.093 [DOI] [PubMed] [Google Scholar]
- 55.Hassanzadeh P, Arbabi E, Atyabi F, Dinarvand R. Ferulic acid-loaded nanostructured lipid carriers: a promising nanoformulation against the ischemic neural injuries. Life Sci. 2018;193:64–76. doi: 10.1016/j.lfs.2017.11.046 [DOI] [PubMed] [Google Scholar]
- 56.Ahmad N, Ahmad R, Naqvi AA, et al. Intranasal delivery of quercetin-loaded mucoadhesive nanoemulsion for treatment of cerebral ischaemia. Artif Cells Nanomed Biotechnol. 2018;46(4):717–729. doi: 10.1080/21691401.2017.1337024 [DOI] [PubMed] [Google Scholar]
- 57.So PW, Ekonomou A, Galley K, et al. Intraperitoneal delivery of acetate-encapsulated liposomal nanoparticles for neuroprotection of the penumbra in a rat model of ischemic stroke. Int J Nanomedicine. 2019;14:1979–1991. doi: 10.2147/IJN.S193965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agulla J, Brea D, Campos F, et al. In vivo theranostics at the peri-infarct region in cerebral ischemia. Theranostics. 2013;4(1):90–105. doi: 10.7150/thno.7088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu H, Jablonska A, Li Y, et al. Label-free CEST MRI detection of citicoline-liposome drug delivery in ischemic stroke. Theranostics. 2016;6(10):1588–1600. doi: 10.7150/thno.15492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Zhang Y, Xia J, et al. Neuronal PirB upregulated in cerebral ischemia acts as an attractive theranostic target for ischemic stroke. J Am Heart Assoc. 2018;7(3):e007197. doi: 10.1161/JAHA.117.007197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basalious EB, Shamma RN. Novel self-assembled nano-tubular mixed micelles of Pluronics P123, Pluronic F127 and phosphatidylcholine for oral delivery of nimodipine: in vitro characterization, ex vivo transport and in vivo pharmacokinetic studies. Int J Pharm. 2015;493(1–2):347–356. doi: 10.1016/j.ijpharm.2015.07.075 [DOI] [PubMed] [Google Scholar]
- 62.Reddy MK, Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. FASEB J. 2009;23(5):1384–1395. doi: 10.1096/fj.08-116947 [DOI] [PubMed] [Google Scholar]
- 63.Yun X, Maximov VD, Yu J, Zhu H, Vertegel AA, Kindy MS. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J Cereb Blood Flow Metab. 2013;33(4):583–592. doi: 10.1038/jcbfm.2012.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Luo J, Li SY. Nano-curcumin simultaneously protects the blood-brain barrier and reduces M1 microglial activation during cerebral ischemia-reperfusion injury. ACS Appl Mater Interfaces. 2019;11(4):3763–3770. doi: 10.1021/acsami.8b20594 [DOI] [PubMed] [Google Scholar]
- 65.Mukherjee A, Sarkar S, Jana S, Swarnakar S, Das N. Neuro-protective role of nanocapsulated curcumin against cerebral ischemia-reperfusion induced oxidative injury. Brain Res. 2019;1704:164–173. doi: 10.1016/j.brainres.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 66.Tan L, Liang C, Wang Y, Jiang Y, Zeng S, Tan R. Pharmacodynamic effect of luteolin micelles on alleviating cerebral ischemia reperfusion injury. Pharmaceutics. 2018;10(4):248. doi: 10.3390/pharmaceutics10040248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao L-X, Liu A-C, Yu S-W, et al. The permeability of puerarin loaded poly(butylcyanoacrylate) nanoparticles coated with polysorbate 80 on the blood-brain barrier and its protective effect against cerebral ischemia/reperfusion injury. Biol Pharm Bull. 2013;36(8):1263–1270. doi: 10.1248/bpb.b12-00769 [DOI] [PubMed] [Google Scholar]
- 68.Xu H, Hua Y, Zhong J, et al. Resveratrol delivery by albumin nanoparticles improved neurological function and neuronal damage in transient middle cerebral artery occlusion rats. Front Pharmacol. 2018;9:1403. doi: 10.3389/fphar.2018.01403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sopala M, Schweizer S, Schäfer N, et al. Neuroprotective activity of a nanoparticulate formulation of the GlycineB site antagonist MRZ 2/576 in transient focal ischaemia in rats. Arzneimittelforschung. 2002;52(3):168–174. doi: 10.1055/s-0031-1299875 [DOI] [PubMed] [Google Scholar]
- 70.Verma SK, Arora I, Javed K, Akhtar M, Samim M. Enhancement in the neuroprotective power of riluzole against cerebral ischemia using a brain targeted drug delivery vehicle. ACS Appl Mater Interfaces. 2016;8(30):19716–19723. doi: 10.1021/acsami.6b01776 [DOI] [PubMed] [Google Scholar]
- 71.Feczko T, Piiper A, Ansar S, et al. Stimulating brain recovery after stroke using theranostic albumin nanocarriers loaded with nerve growth factor in combination therapy. J Control Release. 2019;293:63–72. doi: 10.1016/j.jconrel.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 72.Cheng HY, Wang YS, Hsu PY, Chen CY, Liao YC, Juo SH. miR-195 has a potential to treat ischemic and hemorrhagic stroke through neurovascular protection and neurogenesis. Mol Ther Methods Clin Dev. 2019;13:121–132. doi: 10.1016/j.omtm.2018.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Li SY, Shen S, Wang J. Protecting neurons from cerebral ischemia/reperfusion injury via nanoparticle-mediated delivery of an siRNA to inhibit microglial neurotoxicity. Biomaterials. 2018;161:95–105. doi: 10.1016/j.biomaterials.2018.01.039 [DOI] [PubMed] [Google Scholar]
- 74.Oh J, Lee J, Piao C, Jeong JH, Lee M. A self-assembled DNA-nanoparticle with a targeting peptide for hypoxia-inducible gene therapy of ischemic stroke. Biomater Sci. 2019;7(5):2174–2190. doi: 10.1039/C8BM01621F [DOI] [PubMed] [Google Scholar]
- 75.Marushima A, Suzuki K, Nagasaki Y, et al. Newly synthesized radical-containing nanoparticles enhance neuroprotection after cerebral ischemia-reperfusion injury. Neurosurgery. 2011;68(5):1416–1418. doi: 10.1227/NEU.0b013e31820c02d9 [DOI] [PubMed] [Google Scholar]
- 76.Hosoo H, Marushima A, Nagasaki Y, et al. Neurovascular unit protection from cerebral ischemia-reperfusion injury by radical-containing nanoparticles in mice. Stroke. 2017;48(8):2238–2247. doi: 10.1161/STROKEAHA.116.016356 [DOI] [PubMed] [Google Scholar]
- 77.Lu Y, Li C, Chen Q, et al. Microthrombus-targeting micelles for neurovascular remodeling and enhanced microcirculatory perfusion in acute ischemic stroke. Adv Mater. 2019;31(21):e1808361. doi: 10.1002/adma.v31.21 [DOI] [PubMed] [Google Scholar]
- 78.Hyun H, Lee J, Hwang DW, et al. Combinational therapy of ischemic brain stroke by delivery of heme oxygenase-1 gene and dexamethasone. Biomaterials. 2011;32(1):306–315. doi: 10.1016/j.biomaterials.2010.08.116 [DOI] [PubMed] [Google Scholar]
- 79.Lee J, Hyun H, Kim J, et al. Dexamethasone-loaded peptide micelles for delivery of the heme oxygenase-1 gene to ischemic brain. J Control Release. 2012;158(1):131–138. doi: 10.1016/j.jconrel.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 80.Hyun H, Won YW, Kim KM, Lee J, Lee M, Kim YH. Therapeutic effects of a reducible poly(oligo-D-arginine) carrier with the heme oxygenase-1 gene in the treatment of hypoxic-ischemic brain injury. Biomaterials. 2010;31(34):9128–9134. doi: 10.1016/j.biomaterials.2010.08.038 [DOI] [PubMed] [Google Scholar]
- 81.Petro M, Jaffer H, Yang J, Kabu S, Morris VB, Labhasetwar V. Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials. 2016;81:169–180. doi: 10.1016/j.biomaterials.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu X, An C, Jin P, Liu X, Wang L. Protective effects of cationic bovine serum albumin-conjugated PEGylated tanshinone IIA nanoparticles on cerebral ischemia. Biomaterials. 2013;34(3):817–830. doi: 10.1016/j.biomaterials.2012.10.017 [DOI] [PubMed] [Google Scholar]
- 83.Jin Q, Cai Y, Li S, et al. Edaravone-encapsulated agonistic micelles rescue ischemic brain tissue by tuning blood-brain barrier permeability. Theranostics. 2017;7(4):884–898. doi: 10.7150/thno.18219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han L, Cai Q, Tian D, et al. Targeted drug delivery to ischemic stroke via chlorotoxin-anchored, lexiscan-loaded nanoparticles. Nanomedicine. 2016;12(7):1833–1842. doi: 10.1016/j.nano.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo X, Deng G, Liu J, et al. Thrombin-responsive, brain-targeting nanoparticles for improved stroke therapy. ACS Nano. 2018;12(8):8723–8732. doi: 10.1021/acsnano.8b04787 [DOI] [PubMed] [Google Scholar]
- 86.Ahmad N, Al-Subaiec AM, Ahmad R, et al. Brain-targeted glycyrrhizic-acid-loaded surface decorated nanoparticles for treatment of cerebral ischaemia and its toxicity assessment. Artif Cells Nanomed Biotechnol. 2019;47(1):475–490. doi: 10.1080/21691401.2018.1561458 [DOI] [PubMed] [Google Scholar]
- 87.Li R, Huang Y, Chen L, et al. Targeted delivery of intranasally administered nanoparticles-mediated neuroprotective peptide NR2B9c to brain and neuron for treatment of ischemic stroke. Nanomedicine. 2019;18:380–390. doi: 10.1016/j.nano.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 88.Zhang J, Han X, Li X, et al. Core-shell hybrid liposomal vesicles loaded with panax notoginsenoside: preparation, characterization and protective effects on global cerebral ischemia/reperfusion injury and acute myocardial ischemia in rats. Int J Nanomedicine. 2012;7:4299–4310. doi: 10.2147/IJN.S32385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lv W, Xu J, Wang X, Li X, Xu Q, Xin H. Bioengineered boronic ester modified dextran polymer nanoparticles as reactive oxygen species responsive nanocarrier for ischemic stroke treatment. ACS Nano. 2018;12(6):5417–5426. doi: 10.1021/acsnano.8b00477 [DOI] [PubMed] [Google Scholar]
- 90.Ma J, Zhang S, Liu J, et al. Targeted drug delivery to stroke via chemotactic recruitment of nanoparticles coated with membrane of engineered neural stem cells. Small. 2019;15:e1902011. doi: 10.1002/smll.v15.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kesharwani P, Jain K, Jain NK. Dendrimer as nanocarrier for drug delivery. Prog Polym Sci. 2014;39(2):268–307. doi: 10.1016/j.progpolymsci.2013.07.005 [DOI] [Google Scholar]
- 92.Santos SD, Xavier M, Leite DM, et al. PAMAM dendrimers: blood-brain barrier transport and neuronal uptake after focal brain ischemia. J Control Release. 2018;291:65–79. doi: 10.1016/j.jconrel.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 93.Kim ID, Lim CM, Kim JB, et al. Neuroprotection by biodegradable PAMAM ester (e-PAM-R)-mediated HMGB1 siRNA delivery in primary cortical cultures and in the postischemic brain. J Control Release. 2010;142(3):422–430. doi: 10.1016/j.jconrel.2009.11.011 [DOI] [PubMed] [Google Scholar]
- 94.Jeon P, Choi M, Oh J, Lee M. Dexamethasone-conjugated polyamidoamine dendrimer for delivery of the heme oxygenase-1 gene into the ischemic brain. Macromol Biosci. 2015;15(7):1021–1028. doi: 10.1002/mabi.v15.7 [DOI] [PubMed] [Google Scholar]
- 95.Murta V, Schilrreff P, Rosciszewski G, Morilla MJ, Ramos AJ. G5G2.5 core-shell tecto-dendrimer specifically targets reactive glia in brain ischemia. J Neurochem. 2018;144(6):748–760. doi: 10.1111/jnc.2018.144.issue-6 [DOI] [PubMed] [Google Scholar]
- 96.Kim ID, Shin JH, Kim SW, et al. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol Ther. 2012;20(4):829–839. doi: 10.1038/mt.2011.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang C, Ling CL, Pang L, et al. Direct macromolecular drug delivery to cerebral ischemia area using neutrophil-mediated nanoparticles. Theranostics. 2017;7(13):3260–3275. doi: 10.7150/thno.19979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cuggino JC, Blanco ERO, Gugliotta LM, Alvarez Igarzabal CI, Calderon M. Crossing biological barriers with nanogels to improve drug delivery performance. J Control Release. 2019;307:221–246. doi: 10.1016/j.jconrel.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 99.Cui W, Liu R, Jin H, et al. pH gradient difference around ischemic brain tissue can serve as a trigger for delivering polyethylene glycol-conjugated urokinase nanogels. J Control Release. 2016;225:53–63. doi: 10.1016/j.jconrel.2016.01.028 [DOI] [PubMed] [Google Scholar]
- 100.Teng Y, Jin H, Nan D, et al. In vivo evaluation of urokinase-loaded hollow nanogels for sonothrombolysis on suture embolization-induced acute ischemic stroke rat model. Bioact Mater. 2018;3(1):102–109. doi: 10.1016/j.bioactmat.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim CK, Kim T, Choi IY, et al. Ceria nanoparticles that can protect against ischemic stroke. Angew Chem Int Ed Engl. 2012;51(44):11039–11043. doi: 10.1002/anie.201203780 [DOI] [PubMed] [Google Scholar]
- 102.Zhang T, Li CY, Jia JJ, et al. Combination therapy with LXW7 and ceria nanoparticles protects against acute cerebral ischemia/reperfusion injury in rats. Curr Med Sci. 2018;38(1):144–152. doi: 10.1007/s11596-018-1858-5 [DOI] [PubMed] [Google Scholar]
- 103.Jeong H-G, Cha BG, Kang D-W, et al. Ceria nanoparticles synthesized with aminocaproic acid for the treatment of subarachnoid hemorrhage. Stroke. 2018;49(12):3030–3038. doi: 10.1161/STROKEAHA.118.022631 [DOI] [PubMed] [Google Scholar]
- 104.Bao Q, Hu P, Xu Y, et al. Simultaneous blood-brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano. 2018;12(7):6794–6805. doi: 10.1021/acsnano.8b01994 [DOI] [PubMed] [Google Scholar]
- 105.Takamiya M, Miyamoto Y, Yamashita T, Deguchi K, Ohta Y, Abe K. Strong neuroprotection with a novel platinum nanoparticle against ischemic stroke- and tissue plasminogen activator-related brain damages in mice. Neuroscience. 2012;221:47–55. doi: 10.1016/j.neuroscience.2012.06.060 [DOI] [PubMed] [Google Scholar]
- 106.Li Q, Tang G, Xue S, et al. Silica-coated superparamagnetic iron oxide nanoparticles targeting of EPCs in ischemic brain injury. Biomaterials. 2013;34(21):4982–4992. doi: 10.1016/j.biomaterials.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 107.Hu J, Huang S, Zhu L, et al. Tissue plasminogen activator-porous magnetic microrods for targeted thrombolytic therapy after ischemic stroke. ACS Appl Mater Interfaces. 2018;10(39):32988–32997. doi: 10.1021/acsami.8b09423 [DOI] [PubMed] [Google Scholar]
- 108.Huang SS, Tsai SK, Chih CL, et al. Neuroprotective effect of hexasulfobutylated C60 on rats subjected to focal cerebral ischemia. Free Radic Biol Med. 2001;30(6):643–649. doi: 10.1016/S0891-5849(00)00505-0 [DOI] [PubMed] [Google Scholar]
- 109.Lin AM-Y, Fang S-F, Lin S-Z, Chou C-K, Luh T-Y, Ho L-T. Local carboxyfullerene protects cortical infarction in rat brain. Neurosci Res. 2002;43(4):317–321. doi: 10.1016/S0168-0102(02)00056-1 [DOI] [PubMed] [Google Scholar]
- 110.Fluri F, Grunstein D, Cam E, et al. Fullerenols and glucosamine fullerenes reduce infarct volume and cerebral inflammation after ischemic stroke in normotensive and hypertensive rats. Exp Neurol. 2015;265:142–151. doi: 10.1016/j.expneurol.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 111.Vani JR, Mohammadi MT, Foroshani MS, Jafari M. Polyhydroxylated fullerene nanoparticles attenuate brain infarction and oxidative stress in rat model of ischemic stroke. EXCLI J. 2016;15:378–390. doi: 10.17179/excli2016-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sarami Foroshani M, Sobhani ZS, Mohammadi MT, Aryafar M. Fullerenol nanoparticles decrease blood-brain barrier interruption and brain edema during cerebral ischemia-reperfusion injury probably by reduction of interleukin-6 and matrix metalloproteinase-9 transcription. J Stroke Cerebrovasc Dis. 2018;27(11):3053–3065. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.042 [DOI] [PubMed] [Google Scholar]
- 113.Johnston HJ, Hutchison GR, Christensen FM, Aschberger K, Stone V. The biological mechanisms and physicochemical characteristics responsible for driving fullerene toxicity. Toxicol Sci. 2009;114(2):162–182. doi: 10.1093/toxsci/kfp265 [DOI] [PubMed] [Google Scholar]
- 114.Lee HJ, Park J, Yoon OJ, et al. Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat Nanotechnol. 2011;6(2):121–125. doi: 10.1038/nnano.2010.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moon SU, Kim J, Bokara KK, et al. Carbon nanotubes impregnated with subventricular zone neural progenitor cells promotes recovery from stroke. Int J Nanomedicine. 2012;7:2751–2765. doi: 10.2147/IJN.S30273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Czarny B, Georgin D, Berthon F, et al. Carbon nanotube translocation to distant organs after pulmonary exposure: insights from in situ 14C-radiolabeling and tissue radioimaging. ACS Nano. 2014;8(6):5715–5724. doi: 10.1021/nn500475u [DOI] [PubMed] [Google Scholar]
- 117.Al-Jamal KT, Gherardini L, Bardi G, et al. Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc Natl Acad Sci U S A. 2011;108(27):10952–10957. doi: 10.1073/pnas.1100930108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Antimisiaris S, Mourtas S, Marazioti A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics. 2018;10(4):218. doi: 10.3390/pharmaceutics10040218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851–858. doi: 10.1002/stem.2575 [DOI] [PubMed] [Google Scholar]
- 120.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- 121.Ramirez SH, Andrews AM, Paul D, Pachter JS. Extracellular vesicles: mediators and biomarkers of pathology along CNS barriers. Fluids Barriers CNS. 2018;15(1):19. doi: 10.1186/s12987-018-0104-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33(11):1711–1715. doi: 10.1038/jcbfm.2013.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xin H, Li Y, Liu Z, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31(12):2737–2746. doi: 10.1002/stem.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xin H, Wang F, Li Y, et al. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplant. 2017;26(2):243–257. doi: 10.3727/096368916X693031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moon GJ, Sung JH, Kim DH, et al. Application of mesenchymal stem cell-derived extracellular vesicles for stroke: biodistribution and microRNA study. Transl Stroke Res. 2019;10(5):509–521. doi: 10.1007/s12975-018-0668-1 [DOI] [PubMed] [Google Scholar]
- 126.Doeppner TR, Herz J, Gorgens A, et al. Extracellular vesicles improve post-stroke neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med. 2015;4(10):1131–1143. doi: 10.5966/sctm.2015-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xin H, Katakowski M, Wang F, et al. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48(3):747–753. doi: 10.1161/STROKEAHA.116.015204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang M, Wang H, Jin M, et al. Exosomes from miR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem. 2018;47(2):864–878. doi: 10.1159/000490078 [DOI] [PubMed] [Google Scholar]
- 129.Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tian T, Zhang HX, He CP, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 131.Huang X, Ding J, Li Y, et al. Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Exp Cell Res. 2018;371(1):269–277. doi: 10.1016/j.yexcr.2018.08.021 [DOI] [PubMed] [Google Scholar]
- 132.Kalani A, Chaturvedi P, Kamat PK, et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol. 2016;79:360–369. doi: 10.1016/j.biocel.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Otero-Ortega L, Laso-Garcia F, Gomez-de Frutos MD, et al. White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke. Sci Rep. 2017;7:44433. doi: 10.1038/srep44433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Webb RL, Kaiser EE, Scoville SL, et al. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl Stroke Res. 2018;9(5):530–539. doi: 10.1007/s12975-017-0599-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Webb RL, Kaiser EE, Jurgielewicz BJ, et al. Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke. 2018;49(5):1248–1256. doi: 10.1161/STROKEAHA.117.020353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Han Y, Seyfried D, Meng Y, et al. Multipotent mesenchymal stromal cell-derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J Neurosurg. 2018;1:1–11. [DOI] [PubMed] [Google Scholar]
- 137.Otero-Ortega L, Gomez de Frutos MC, Laso-Garcia Fet al,. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J Cereb Blood Flow Metab. 2018;38(5):767–779. doi: 10.1177/0271678X17708917 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Lee JY, Kim E, Choi SM, et al. Microvesicles from brain-extract-treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci Rep. 2016;6:33038. doi: 10.1038/srep33038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pan Q, He C, Liu H, et al. Microvascular endothelial cells-derived microvesicles imply in ischemic stroke by modulating astrocyte and blood brain barrier function and cerebral blood flow. Mol Brain. 2016;9(1):63. doi: 10.1186/s13041-016-0243-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yurkin ST, Wang Z. Cell membrane-derived nanoparticles: emerging clinical opportunities for targeted drug delivery. Nanomedicine. 2017;12(16):2007–2019. doi: 10.2217/nnm-2017-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Goh WJ, Zou S, Ong WY, et al. Bioinspired cell-derived nanovesicles versus exosomes as drug delivery systems: a cost-effective alternative. Sci Rep. 2017;7(1):14322. doi: 10.1038/s41598-017-14725-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goh WJ, Zou S, Lee CK, et al. EXOPLEXs: chimeric drug delivery platform from the fusion of cell-derived nanovesicles and liposomes. Biomacromolecules. 2018;19(1):22–30. doi: 10.1021/acs.biomac.7b01176 [DOI] [PubMed] [Google Scholar]
- 143.Li M, Liu Y, Chen J, et al. Platelet bio-nanobubbles as microvascular recanalization nanoformulation for acute ischemic stroke lesion theranostics. Theranostics. 2018;8(18):4870–4883. doi: 10.7150/thno.27466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jo W, Kim J, Yoon J, et al. Large-scale generation of cell-derived nanovesicles. Nanoscale. 2014;6(20):12056–12064. doi: 10.1039/C4NR02391A [DOI] [PubMed] [Google Scholar]
- 145.Tajiri N, Dailey T, Metcalf C, et al. In vivo animal stroke models: a rationale for rodent and non-human primate models. Transl Stroke Res. 2013;4(3):308–321. doi: 10.1007/s12975-012-0241-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87(1):179–197. doi: 10.1016/j.pbb.2007.04.015 [DOI] [PubMed] [Google Scholar]
- 147.Fluri F, Schuhmann MK, Kleinschnitz C. Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther. 2015;9:3445–3454. doi: 10.2147/DDDT.S56071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marbacher S, Gruter B, Schopf S, et al. Systematic review of in vivo animal models of subarachnoid hemorrhage: species, standard parameters, and outcomes. Transl Stroke Res. 2019;10(3):250–258. doi: 10.1007/s12975-018-0657-4 [DOI] [PubMed] [Google Scholar]