Abstract
Ivermectin, a dihydro derivative of avermectin (AVM), was introduced into the veterinary, agricultural and aquaculture markets for animal health in 1981. Ivermectin was soon adopted in 1987 as a human medicine that was originally used for the treatment of onchocerciasis, a parasitic infection. Since then, ivermectin has also been used to control other human diseases and has exerted a significant effect on human health and welfare. In the past decade, many published studies have attempted to determine the role of ivermectin in cancer. In this review, we summarize the published studies to define the current progress in the characterization of ivermectin. Ivermectin causes cell death in cancer cell lines by inducing PAK1-mediated cytostatic autophagy, caspase-dependent apoptosis and immunogenic cell death (ICD) through the modulation of some pathways, including the WNT-T cell factor (TCF), Hippo and Akt/mTOR pathways. Ivermectin can affect the growth and proliferation of cancer cells and plays several different roles, such as its functions as an RNA helicase, a small-molecule mimetic of the surface-induced dissociation (SID) peptide, an activator of chloride channel receptors, and an inducer of mitochondrial dysfunction and oxidative stress. In addition, ivermectin induces the multidrug resistance protein (MDR), has potent anti-mitotic activity, targets angiogenesis and inhibits cancer stem-like cells (CSCs). Many studies have proven that ivermectin exerts antitumour effects and might thus benefit patients with cancer after sufficient clinical trials.
Keywords: ivermectin, cancer, molecular mechanisms, antitumour effects, drug therapy
Introduction
Satoshi Omura at the Kitasato Institute discovered ivermectin in 1979 and was awarded a Nobel Prize in Physiology or Medicine for this discovery in 2015. Ivermectin was first introduced as a veterinary medicine. In the 1980s, because ivermectin is safer and more effective than other avermectins (AVMs), scientists found that this compound could be administered to humans as an anti-parasitic drug. Ivermectin protects humans and domestic animals from parasitic infection by killing different types of parasites, such as the parasite that causes onchocerciasis (also known as river blindness), Enterobius vermicularis, Ascaris lumbricoides, the parasite that causes lymphatic filariasis (also known as elephantiasis), Strongyloides stercoralis, Ancylostoma duodenale and Trichuris trichiura, in livestock.1–3 Initial studies aiming to determine the molecular mechanisms of ivermectin revealed that it enhances the activity of glutamate-gated chloride channel (GluCl), a member of the Cys-loop receptor family that blocks nerve cell signals and subsequently the muscle cells of parasites, and thereby kills parasites, and these findings provide an adequate scientific basis for the sensitivity of drugs targeting mammalian γ-aminobutyric acid (GABA) receptors.4 Due to an improved understanding of the molecular mechanisms of ivermectin, scientists discovered that it also influences several ligand-gated ion channels and receptors, including 1) type 35 hydroxytryptamine receptor (5-HT3R); 2) an excitatory cation-permeable receptor to enhance the Ach-induced current (nAChR);5 3) P2X4 receptors, the upper transmembrane domain (TM) regions of which are near the extracellular surface of the plasma membrane, to induce mutations of all amino acids located at TM1 and TM2 to Ala or Trp; and 4) farnesoid X receptor (FXR), a member of the nuclear hormone receptor superfamily in the cytoplasm.6 Recently, I-Shan Chen and Yoshihiro Kubo also demonstrated that ivermectin directly activates GIRK2, a type of G-protein-gated that inwardly rectifies the K+ (GIRK) channel by phosphorylating phosphatidylinositol-4,5-biphosphate (PIP2) in a Gβγ-independent manner.6,7
Intriguingly, due to its wide range of effects, the results from in vivo and in vitro studies in many different medical fields resulted in the development of ivermectin for the control of many diseases in addition to those caused by parasites. For example, ivermectin has been shown to decrease the serum cholesterol and glucose levels and improve the insulin sensitivity of diabetic mice, reduce the viral replication of some flaviviruses, decrease the survival rate of the major insect vectors of trypanosomiasis and malaria and exert anti-inflammatory effects on T cell-induced skin disease.8
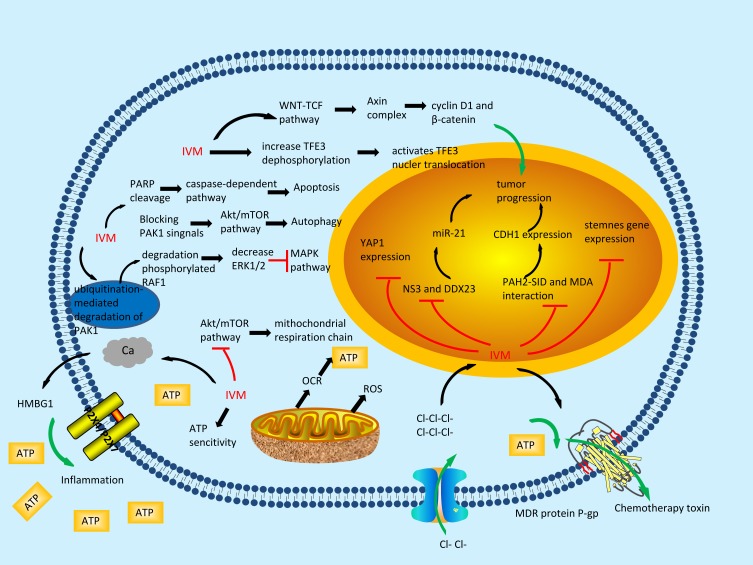
Ivermectin has long been known as a well-documented anti-parasitic drug. Over the past few years, increasing numbers of studies have indicated that ivermectin might have extensive uses as an anticancer agent for the treatment of different types of cancers, such as glioblastoma, breast cancer,9 ovarian cancer,10 leukaemia11 and neurofibromatosis type 2 (NF2) tumours,12 and thus might have strong potential as an anti-carcinogen. However, the anticancer activity of ivermectin at the molecular level remains to be clarified (Figure 1), which is important for determining the specific types of cancer that are susceptible to this drug. In this review, a great quantity of data showing that ivermectin exerts antitumour effects against a wide range of cancers were collected. Because ivermectin has already been registered for human use by the Federal Drug Administration (FDA), it will not be long before it is adopted as an anticancer drug.2
Figure 1.
The molecular mechanisms of the anti-tumour effects of ivermectin.
Ivermectin Induces Cell Death in Cancer Cells
Ivermectin Induces PAK1-Mediated Cytostatic Autophagy
Autophagy is an intracellular self-degradative process that plays a key role in regulating cell growth and metabolism.13 Unlike the ubiquitin-proteasome system, which is also involved in cell degradation, the substrate of autophagy is endogenous, and autophagy is the process responsible for cellular degeneration and the destruction of certain organelles or the local cytoplasm.14 PAK proteins are critical modulators of nuclear signal transduction and cytoskeletal reorganization; among the PAK proteins, p21 (RAC1)-activated kinase 1 (PAK1) modulates a wide range of signals involved in a large number of biological activities. The blockage of PAK1 signals contributes to tumour cell death.2 PAK1 is associated with the development of the great majority of all human cancers and acts as an Akt-binding protein that stimulates Akt phosphorylation and activation.15 The Akt/mTOR signalling pathway was previously shown to play a vital inhibitory role in autophagy.16 Some studies have indicated that ivermectin, a PAK1 inhibitor, inhibits the growth of breast cancer, ovarian cancer, glioblastoma and NF2 tumours by inducing cytostatic autophagy both in vitro and in vivo.2,12,15,17 Ivermectin inhibits the Akt/mTOR signalling pathway by increasing the ubiquitination-mediated degradation of PAK1, which results in increased autophagic flux.18 Some results also strongly suggest that the ivermectin-mediated inactivation of PAK1 decreases the levels of phosphorylated RAF1, extracellular signal-regulated kinase ½ (ERK1/2), and MAPK/ERK (MEK). All of these factors contribute to activation of the MAPK pathway and tumour growth and are involved in cell death in nasopharyngeal carcinoma (NPC) and melanoma.19,20 Furthermore, Qianhui Dou found that ivermectin can mediate the formation of autophagosomes by enhancing the interaction between Beclin 1 (an autophagy-related protein) and Atg14L or Vps34 (positive regulators) while attenuating the coimmunoprecipitation of Beclin 1 and Bcl-2 (negative regulators).17
Ivermectin Induces Caspase-Dependent Apoptosis
Apoptosis, which is the programmed and autonomous death of cells controlled by genes to maintain the stability of the internal environment in multicellular organisms, is generally unregulated in many types of cancers.21 Apoptosis mainly occurs through two pathways: the intracellular pathway (mitochondrial-mediated), the two principal features of which are collapse of the mitochondrial membrane potential (ΔΨm) and the release of cytochrome c, and the extracellular pathway (death receptor-mediated).22 AVM stimulates the release of cytochrome c into the cytosol in HeLa cells. Moreover, the effector caspase-3 and the initiating agent caspase-9 are both activated, and this activation induces the cleavage of poly ADP-ribose polymerase (PARP), which is a substrate of caspase-3 and a fundamental component of apoptosis. Both of these caspases associated with apoptosis are augmented by AVM, and the repair and healing of AVM-induced DNA damage are simultaneously blocked.22 Similarly, the treatment of breast cancer cells with ivermectin induces the cleavage of PARP, caspase-1 activity, which is characteristic of pyroptotic cell death, and the activation of caspase-3, which typically occurs in classic apoptosis.23 Studies have shown that Z-VAD-FMK, a pan-caspase inhibitor, eliminates the pro-apoptotic influence of ivermectin on K562, U87, NBM CD34, CML and T98G cells, which indicates that ivermectin induces apoptosis in glioblastoma and chronic myeloid leukaemia cells through a caspase-dependent pathway.24,25 OCI-AML2 leukaemia cells treated with ivermectin exhibit the same results.11 KPNB1, which encodes a nuclear transport factor, is a new therapeutic target gene. Kodama M recently reported that ivermectin initiates multiphase cell cycle arrest and apoptosis in epithelial ovarian cancer (EOC) cells, which is consistent with the alterations observed after KPNB1 suppression.10 In addition, another investigation demonstrated that ivermectin induces apoptosis in glioblastoma and HeLa cells by enhancing cytochrome c release, upregulating Bax and p53 expression, downregulating Bcl-2 expression and decreasing the levels of cyclin E, cyclin D1, CDK2, CDK6 and CDK4.26,27
Ivermectin Induces Immunogenic Cell Death (ICD)
ICD as a novel concept that has emerged in recent years. Damage-associated molecular patterns (DAMPs) induce the primary immunogenic features of ICD, including the release of high-mobility-group protein B1 (HMGB1), surface-exposed calreticulin (CRT) and secreted ATP.28,29 Through chimeric plasma membrane-targeted luciferase, a high extracellular ATP concentration at tumour sites was detected in vivo, which shows that extracellular ATP is essential for cancer.30 The release of extracellular ATP from cancer cells within the tumour microenvironment is strictly controlled and regulated, and ATP is degraded by tumour-associated extracellular ATPases such as CD73 and CD39. Through the inhibition of extracellular ATPases (CD39 and CD73) by PSB 069, ivermectin-induced cytotoxicity can be repressed in many types of cancer cell lines.23 Exogenous ATP, which controls the defence and repair processes in cellular tissue through the P2X, P2Y and P1 purinergic receptors and P2X7 signalling, has recently been connected with tumour growth and metastasis.31 Recent data support the capacity of ivermectin to kill mice and human triple-negative breast cancer (TNBC) cells through an inflammatory and immunogenic mechanism and demonstrate that tumour cells release ATP and suffer from ICD mediated by increased P2X4/P2X7-gated pannexin-1 channel opening, which causes cell death. This death is a mixture of necrotic and apoptotic cell death associated with caspase-1 activity that is consistent with pyroptosis.23 Ivermectin-induced killing is first strengthened by both blocking purinergic signalling using suramin (a non-selective inhibitor of P2 receptors) and decreasing the extracellular ATP levels with ATPases.23 Moreover, in P2X7-KO and WT mice, ivermectin combined with ATP enhances the maximal increase in [Ca2+] following formation of a hypothetical heterotrimer composed of P2X1 and P2X4 receptors or a homotrimer of P2X4 subunits.32 Ivermectin is indeed able to upregulate CRT on the surface of live murine (4T1.2, CT26) and MD Anderson-metastatic breast-231 (MDA-MB231) breast cancer cells, and these effects promote apoptosis and lead to the release of HMGB1, a key mediator of inflammation.23 In addition, ivermectin combined with a low concentration of ATP prolongs the activation of P2X4 receptors, thus ensuring sustained activation of the non-specific cation channel coupled with this receptor. Furthermore, pore formation, which can decrease the levels of K+ to sufficiently low levels to induce the release of IL-1β after caspase-1 activity, was also triggered.32
Ivermectin Modulates Several Pathways
Ivermectin Inhibits Proliferation by Inhibiting Yea-Associated Protein 1 (YAP1)
LATS1/2 potentially phosphorylates YAP1/TAZ in the presence of MOB1, which is the most important hub of the Hippo pathway, binds to the 14-3-3 protein into the cytoplasm, and thus protects it from activating TEA domain family member (TEAD)-mediated transcription, and the E3 ubiquitin ligase SCFβTRCP before being degraded.33 YAP1, a downstream target of the Hippo pathway that regulates organ size during growth, is a multi-functional protein that can activate gene expression by interacting with different transcription factors.34,35 Moreover, a large number of studies have associated increased accumulation of YAP1 in the nucleus with poor prognosis in patients with intrahepatic cholangiocellular carcinoma (ICC) and combined hepatocellular and cholangiocarcinoma (cHC-CC),33 colorectal cancer (CRC),36 ovarian cancer35,37,38 and gastric cancer (GC).34 Through in vitro proliferation assays and a xenograft model, Nambara S found that ivermectin exerts its antitumour effects and inhibits the proliferation of GC by decreasing the expression of the YAP1 protein in the nucleus and suggested that the nuclear expression of YAP1 is slightly associated with the YAP1 mRNA levels.39
Ivermectin Serves as a WNT-T Cell Factor (TCF) Pathway Response Blocker
WNTs and their downstream effectors are connected by a wide range of processes that play an important role in cancer progression, such as differentiation, metastasis, cell senescence, cell death, tumour initiation and tumour growth.40 In the absence of WNT, the cytoplasmic β-catenin protein, a key polyvalent protein that is phosphorylated at multiple sites, is constantly degraded by the action of the Axin complex, which is composed of the scaffolding protein Axin.40 This continual elimination of β-catenin prevents it from reaching the nucleus, and the target genes of WNT are thereby suppressed by the DNA-bound TCF/lymphoid enhancer factor (LEF) family of proteins.41 Studies have noted that ivermectin represses WNT-β-CATENIN/TCF transcriptional responses, which occur at a low level. This effect is rescued by the continuous and direct activation of TCF transcriptional activity and is related to repression of the levels of C-terminally phosphorylated β-catenin phosphoforms and cyclin D1, a vital target that appositively regulates the cell cycle and acts as an oncogene.42 Ivermectin suppressed the sensitivity of human colon cancer xenografts to TCF inhibition without discernible side effects.42,43 Ivermectin also inhibits lung cancer development in vivo, which supports the possibility of treating WNT-TCF-dependent diseases, such as intestine cancer, breast cancer, skin cancer, and lung cancer, with a blocker of the WNT-TCF pathway response.42 Moreover, after observing the anti-clonogenic effect of ivermectin through the analysis of spheroid formation, pretreatment with ivermectin in the examined cell lines eliminated floating spheroids by up to 73%, and this effect was accompanied by the suppression of cyclin D1, which demonstrates that ivermectin limits the formation of cancer stem cells.42
Ivermectin Increases TFE3 Activity
TFE3, a member of the MiT/TFE family, is a bHLH-leucine zipper transcription factor that is controlled through mTOR-induced phosphorylation. It contributes to not only lysosomal genes but also some newly characterized targets, including the oxidative stress response and oxidative metabolism.44 Ivermectin treatment increases TFE3(Ser321) dephosphorylation, activates TFE3 nuclear translocation and stimulates activation of the TFE3 reporter in human melanoma cells.45 Ivermectin treatment also clearly decreases phosphorylation of the mTORC1 substrate p-70S6K, which results in induction of mTORC1 deactivation.45 The inactivation of mTORC1 controls the intracellular localization of TFE3, which results in regulation of the dephosphorylation of Ser321 and thereby prevents the binding of TFE3 to 14-3-3 and leads to the accumulation of maximal TFE3 in the nucleus.44 In conclusion, ivermectin increases TFE3-dependent autophagy, and the repression of autophagy strengthens ivermectin-mediated apoptosis in human melanoma cells.45
Ivermectin Serves as an RNA Helicase Inhibitor
MicroRNAs (miRNAs) are a class of endogenous, 20-24-nucleotide-long small RNAs. By controlling protein expression via severing of the specific mRNA molecules of protein-coding genes or inhibiting their translation, miRNAs are involved in a series of fundamental processes, including early development, cell proliferation, apoptosis, cell death, fat metabolism and cell differentiation.46 These functions suggest a relationship between miRNAs and the development or maintenance of cancer. The upregulation of microRNA-21 (miR-21) is strongly associated with the development of glioma malignancy. Yin et al also demonstrated that DDX23 (a DEAD-box RNA helicase) promotes miR-21 production at the post-transcriptional level in glioma cells.47 Furthermore, it has also been reported that ivermectin, an RNA helicase inhibitor, potentially inhibits cell invasion and proliferation, thereby reducing the levels of precursor and mature miR-21 in U87MG glioma cells without affecting the expression of DDX23.47 Moreover, ivermectin significantly inhibits the cell proliferation of X01 and CSC2 cells, which are two glioma stem cell lines derived from patients, and A549, DU145, HeLa and PANC-1 cells, which are four carcinoma-derived cell lines.47
Ivermectin Serves as a Small-Molecule Mimetic of the SID Peptide
Mutations in proteins related to DNA repair and DNA replication epigenetic regulation suggest that runaway epigenetic regulation plays a key role in the development and progression of cancer. With respect to deregulated epigenetics, epithelial-mesenchymal transition (EMT) is an important biological process for the migration and invasion of malignant tumour cells derived from epithelial cells. EMT is also involved in angiogenesis, tumour growth and the generation of cancer stem-like cells (CSCs) that might influence tumour recurrence and resistance to conventional therapies.48,49 SIN3A and SIN3B, which are multidomain adaptor proteins, are important facilitators of epigenetic deregulation without intrinsic DNA-binding activity that act as molecular scaffolds that engender interactions between sequence-specific DNA-binding transcription factors and chromatin regulators.50 It was previously reported that the blockage of interactions between the PAH2 domain of the chromatin regulatory factor SIN3A and SIN3 interaction domain (SID)-containing proteins via the SID peptide leads to EMT reversal and the expression of silenced genes encoding proteins involved in cell differentiation and growth, including the RARβ, ERα and E-cadherin proteins, which maintain tight intercellular junctions.51 A new combination of treatment-related SID peptides has also been found to activate the RARα/β pathways, which are enhanced in combination with AM580 (RARα-specific agonist), to induce morphogenesis and inhibit tumour ball formation.51 As a small-molecule mimetic of the SID peptide, ivermectin inhibits the interaction between SIN3-PAH2 and MAD and thus induces the expression of CDH1, which encodes E-cadherin, and therefore, ivermectin plays an important role in EMT-mediated cancer progression. In addition, ivermectin restores the tamoxifen sensitivity of human MDA-MB-231 and mouse MMTV-Myc cells in vitro and TNBC cells.9 Ivermectin also increases the expression of ESR1, the ERα protein and progesterone receptor (PGR), a transcriptional target of ERα. In addition, the promotion of growth induced by 17β-oestradiol (E2) and the inhibition of growth induced by the ERα antagonist tamoxifen (Tam) in MDA-MB-231 and D3H2LN cells are reflected through the re-expression of ERα in response to ivermectin therapy.9
Ivermectin Inhibits Mitochondrial Respiration
Mitochondrial dysfunction is a major effect of reactive oxygen species (ROS), and superoxide, a precursor of many other forms of ROS, is formed mainly by mitochondrial electron leakage caused by mitochondrial dysfunction.25 Liu Yingying et al demonstrated the dose-dependent inhibitory effect of ivermectin on the basal oxygen consumption rate (OCR) and maximum OCR in U87, T98G and human microvascular endothelial cells (HBMEC) cells, which indicates that ivermectin inhibits mitochondrial respiration by decreasing the activity of respiratory complex I enzyme.24 These researchers also found that the exposure of these cells to ivermectin decreased the mitochondrial membrane potential, an electrochemical proton gradient generated by the mitochondrial respiratory chain.24 Consistently, obviously increased levels of ROS and mitochondrial superoxide as well as decreased ATP levels were also found in glioblastoma, HBMECs and chronic myeloid leukaemia (CML) cells treated with ivermectin.24,25 The anti-proliferative and apoptotic effects of ivermectin were abrogated through communication with the antioxidants α-tocopherol or mannitol, and the levels of phosphorylated Akt, mTOR and ribosomal S6 protein (rS6), which are downstream of mTOR, were reduced in U87, HBMEC, T98G and K562 cells exposed to ivermectin, reflecting the negative effect of ivermectin on mitochondrial function through its suppression of the Akt/mTOR pathway, which leads to oxidative stress.24,25 Similarly, acetyl-l-carnitine (ALCAR, a mitochondrial fuel) and N-acetyl-l-cysteine (NAC, an antioxidant) reversed the inhibitory effects of ivermectin in renal cell carcinoma (RCC) cells, which indicates that mitochondria are the target of ivermectin.52 Furthermore, consistent with ROS induction, ivermectin appears to dysregulate genes, including STAT1, which has been connected with increased ROS production, and IFIT3, OAS1, and TRIM22, which are downstream targets of STAT1.11 Some recent data demonstrate that ivermectin exhibits selective toxicity in inducing mitochondrial dysfunction and oxidative stress and enhances the role of BCR-ABL TKIs in CD34 CML cells.25 Studies have demonstrated that some cancers, including leukaemia, breast cancer and lymphoma, are more metabolically active and dependent on mitochondria than normal cells and therefore more responsive to ivermectin than their normal counterparts.52
Ivermectin Activates Mammalian Chloride Ion Channels
Ivermectin activates GluCls in invertebrates such as parasites and helminths, and this activation stimulates the opening of GABA receptors and Glu-Cl.2 At a higher concentration, ivermectin also stimulates chloride channels in mammals.11 Recent studies showed that the treatment of OCI-AML2 cells with ivermectin increased the concentration of intracellular chloride ions, leading to hyperpolarization of the plasma and mitochondrial membranes as well as the production of ROS. However, the mitochondrial membrane potential was not changed in a dose-dependent manner, and the changes in chloride flux were consistent with an increase in cell size, as measured by flow cytometry.11 The effect of ivermectin on calcium influx was detected in leukaemia cells because plasma membrane hyperpolarization could lead to calcium influx. Ivermectin increased the intracellular calcium, but the cytotoxicity of ivermectin was not related to this increase because the chelating of extracellular calcium with BAPTA-AM and EDTA did not block ivermectin-induced cell death.11
Other Novel Molecular Mechanisms of Ivermectin
Ivermectin Is an Angiogenesis Inhibitor
Angiogenesis (microvascular formation) is a cancer maker that provides substances necessary for tumour growth while removing waste and sediment from the tumour microenvironment.53 In a previous study, in vitro capillary network formation experiments using HBMECs revealed that 10 μM ivermectin completely eliminated the ability of HBMECs to form tubular structures, confirming the effect of ivermectin against angiogenesis.24 In conclusion, these findings indicate that ivermectin is a powerful angiogenesis inhibitor that also inhibits proliferation and induces apoptosis in HBMECs.24 Several miRNAs have been revealed to influence the expression of VEGF and its tyrosine kinase receptors downstream of hypoxia-inducible factor 1 (HIF-1) in tumour angiogenesis.53 Whether these miRNAs are targets of ivermectin remains to be confirmed, but the probable mechanisms have not been studied.
Ivermectin Exerts Anti-Mitotic Activity
Microtubules, a type of polar cytoskeleton that is widely present in eukaryotic cells, are mainly composed of tubulins, which are crucial to mitosis. During mitosis, the duplicated chromosomes of one cell are divided into two identical groups before the cell is split into two daughter cells. Due to their importance in maintaining cell morphology, signal transduction, cell division, and material transport, microtubules have become a key target for the development of chemotherapeutics.54 A tubulin polymerization experiment performed by Shoaib Ashraf, who found an interaction between ivermectin and nematode tubulin, revealed that ivermectin improves the degree of tubulin polymerization in mammals.55 Furthermore, the in vitro treatment of HeLa cells with ivermectin at low micromolar concentrations prevented replication, stabilized mammalian tubulin, and aided the resistance to cold temperature depolymerization, but the inhibition of cell division was mitigated after the removal of ivermectin, which is similar to the effects of Taxol.55 Because ivermectin has already been used in humans, ivermectin might be adopted as an anti-mitotic agent for patients.56
Ivermectin Is a P-Glycoprotein (P-Gp) Inhibitor
Based on the phenomenon of multidrug resistance (MDR), cancer cells can develop resistance and cross-resistance to a variety of chemotherapeutic drugs that are not related to their function and structure after exposure to chemotherapeutic agents.57 Drug resistance is a new anticancer treatment strategy with important value. The development of the MDR phenotype in cancer cells is often attributable to the high expression of transmembrane proteins, particularly P-gp,58 which is encoded by the ABCB1 (also known as multiple drug resistance 1, MDR1) gene. P-gp is the most important member of the ATP-binding cassette (ABC) transporter family and part of the human blood-brain barrier, which limits drug intake into the brain.59 However, the genetic polymorphism of P-gp or co-treatment with ivermectin and a drug that blocks this efflux transport protein can alter the function or expression of P-gp, which, as expected, increases the concentration of ivermectin in the brain and induces serious neurotoxicity.60 Didier and Loor proved that ivermectin is not only an inhibitor but also a substrate of P-gp via a short-term assay, in which two types of P-gp probes were retained in the parent MDR cells and their recovery was measured.61 Therefore, the role of ivermectin in preventing the development of the MDR phenotype is not well understood.
Ivermectin Is an Inhibitor of CSCs
Embryonic stem (ES) cells are a type of cells isolated from the early embryo or primitive gonads that have unique characteristics, such as unlimited proliferation, self-renewal and multi-differentiation activities in vitro. ES cells can be induced to differentiate into almost all types of body cells both in vitro and in vivo. A few important ES cell-specific transcription factors, namely, octamer-binding protein 4 (Oct-4), the homeobox protein Nanog and SRY-box 2 (Sox-2), which are interconnected and interact with proteins from multiple inhibitory complexes such as the NuRD, Sin3A and Pml complexes, are vital transcription factors that control the self-renewal and pluripotency of ES cells.62 Ivermectin first suppresses CSCs and then downregulates the expression of “stemness” genes and decreases the activity and expression of CSC markers in breast cancer.63 Additionally, ivermectin at 0.5 µM reduced Nanog, Oct4 and Sox2 gene expression in D3H2LN cells by 50% to 80%, whereas the clonogenic, self-renewal-dependent tumoursphere growth was decreased by 90–100%.9
As part of the development of this antiparasitic agent, the pharmacology, safety and toxicity of ivermectin to humans and animals have been widely evaluated. For example, the LD50 of orally administered ivermectin in mice, rats and rabbits ranges from 10 to 50 mg/kg. In humans, the toxicity of ivermectin is very low, and no serious adverse reactions have been found in healthy volunteers even when the dose was increased to 120 mg (~2 mg/kg).64 Normal haematopoietic cells and AML cells were treated with ivermectin and compared. Ivermectin was found to be cytotoxic to AML patients at low micromolar concentrations. In contrast, at a concentration of up to 20 μm, ivermectin does not induce cell death in normal peripheral blood stem cells (PBSCs). However, when the CD34+ cells of a PBSC sample were gated, the IC50 of ivermectin-induced cell death was 10.5 ±0.6 μm, whereas that of ivermectin-induced cell death was approximately 5 μm. The researchers also evaluated the effects of ivermectin on primary normal haematopoietic and AML cell clone formation analysis. Ivermectin (6 μ m) decreased the clone formation ability of normal haematopoietic cells by 15%. In contrast, ivermectin decreased the clone formation ability of three of six primary AML samples by more than 40%.11 Importantly, two studies revealed that ivermectin exerts a preferential inhibitory effect on cancer cells, whereas its role in normal cells was significantly reduced or ineffective. The researchers found that 5, 10 μM and 15 μM ivermectin significantly inhibits the proliferation and induces apoptosis of all RCC cell lines in a dose-dependent manner. The range of IC50 values was 3–10 μM. In contrast, 15 μM ivermectin could inhibit the proliferation and induce apoptosis of HRPT (human proximal tubular), HRE (human renal epithelial) and HRCE (human renal cortical epithelial) cells, and the IC50 value was higher than 15 μM.52 Similarly, another study found that the same concentration of ivermectin did not induce apoptosis or increase apoptosis in normal bone marrow (NBM) CD34 cells compared with CML CD34 cells, which indicates that normal CD34 cells are not as sensitive to ivermectin as CML CD34 cells.25 The main reason for this finding is that ivermectin can significantly reduce the mitochondrial membrane potential and inhibit mitochondrial respiration. Many cancers exhibit higher metabolic activity and dependence on mitochondrial function than normal cells. Therefore, cancer cells are more sensitive to mitochondria-targeting drugs than normal cells, and these drugs might decrease the side effects of anticancer therapy.
Many in vitro and in vivo preclinical studies have shown that ivermectin is effective for the treatment of a variety of malignant diseases. The median concentration of ivermectin for in vitro treatment was found to be 5 μM (0.01–100 μM) in all the studies mentioned in Table 1. According to human pharmacokinetic data, this concentration can be achieved clinically.64 Various tumour cell lines were used in immunodeficient mice (Table 2) to evaluate the efficacy of ivermectin in vivo. The median dose used for the oral and intraperitoneal administration of ivermectin was 5 mg/kg (2.4 to 40 mg/kg), which corresponds to a dose of 0.40 mg/kg in humans65 and is lower than the highest dose (2 mg/kg) that is currently considered safe for use in human subjects.64 After 10 to 42 days, treatment with ivermectin can reduced the tumour volume by more than 50%.
Table 1.
Summary of the Studies in Tumors Using Ivermectin in vitro
| Cancer Type | Cell Lines | [µM] | IC50µM | Molecular Mechanisms | Reference |
|---|---|---|---|---|---|
| Triple-Negative Breast Cancer | 4T1 MMTV-Myc |
0.01 0.1 |
Selective Inhibition of SIN3 Corepressor and restoration of tamoxifen sensitivity. | 9 | |
| Mouse and human Triple-Negative Breast Cancer |
4T1.2 DDHer2 |
1 4 8 |
2 | A mixed apoptotic and necrotic mechanism | 23 |
| Human leukemia | OCI-AML2 HL60 U937 K61a TEX |
5 10 15 20 |
4.54 4.64 6.54 5.68 5.41 |
Induces chloride-dependent membrane hyperpolarization | 11 |
| Ovarian cancer | TYK-nu KOC7C SKOV3 RMUG-S HEI-193 |
0.1 1 10 100 |
5~20 10~20 10~20 5~20 5 |
Inactivates the kinase PAK1 and blocks the PAK1dependent growth | 12 |
| Breast cancer | MDAB-435 MDA-MB-231 MDA-MB-468 MDA-MB-361 MCF-7 HS578T |
5 10 15 20 |
20~40 10~20 |
Induces Cytostatic Autophagy by Blocking the PAK1/Akt Axis | 17 |
| Human glioblastoma | U87 T98G |
1 5 10 |
~5 ~5 |
Inducing mitochondrial dysfunction and oxidative stress | 24 |
| Cervical cancer | Hela | 2.5 5 10 15 20 |
7.87 5.78 |
Inducing DNA fragmentation and chromatin condensation | 27 |
| Human gastric cancer | MKN1 MKN7 MKN28 SH10TC |
2.5 5 10 |
10.2 31.9 25.4 21.2 |
Inhibition of Yes-associated protein 1 expression | 39 |
| Human melanoma | SKMe28 | 2.5 5 10 |
2.5 | Suppressing ROS-TFE3-dependent autophagy | 45 |
| Human colon cancer | CC14 CC36 Ls174T DLD1 |
0.1 1 5 5 |
2.3 1.7 1 0.8 |
Inhibit WNT-TCF pathway | 42 |
| Human breast cancer | MDA-MB-231 | 0.2 0.4 0.8 1 5 8 |
Preferentially inhibits cell viability and clonogenicity of the stem cell population | 63 |
Table 2.
Summary of the Studies in Tumors Using Ivermectin in vivo
| Cancer Type | Cell Line | Dose mg/kg | Results | Reference |
|---|---|---|---|---|
| Murine leukemia Human leukemia |
MDAY-D2 OCI-AML2 K562 |
3 5 6. |
Decreased tumor mass and volume in all 3 models by up to 70% | 11 |
| Breast cancer | MDA-MB-231-GFP | 2.4 | Tumor weight and size were reduced. | 17 |
| Human glioblastoma | U87 T98G |
40 | Decreases body weight of the mice were not observed but significantly inhibited growth of tumors. | 24 |
| Human gastric cancer | MKN1 | i.p | Tumor weight was reduced. | 39 |
| Human lung cancer | H358 | i.p. | Showing a ~ 50% repression of growth | 42 |
| Human colon cancer | DLD1 CC14 HT29 |
10 | Inhibited DLD1 tumor growth. Repressed HT29 tumor growth. Not affect the growth of CC14 tumors. | 42 |
| Human glioma | U87MG | 3 10 |
Reduces tumor size up to 50% at 3 mg/kg. | 47 |
Abbreviation: i.p., intraperitoneal.
Drug repositioning can provide another strategy to meet the economic cost and efficiency needs of finding new, effective and safe anticancer agents. The ultimate goal of drug repositioning in oncology is to promote patient access to innovative treatments. It is clear that innovative financial/regulatory solutions are needed to address the hurdles listed here. Although scientific and clinical work to demonstrate the efficacy of individual drug reuse is currently underway, it is now necessary to discuss the social and political framework for the management of drug approval and use, and sustainable solutions, such as adjustments to the current regulatory framework, should then be sought.66
Conclusion
The role of ivermectin in cancer remains to be discovered. Many targets share the same pathway. For example, the Akt/mTOR pathway is associated with mitochondrial dysfunction, oxidative stress and autophagy. Chloride-dependent membrane hyperpolarization is connected with anti-mitotic effects in the progression of cell death. The ICD and mTORC1 pathways are coupled with ROS, but these connections have not been clarified.
To manage patients with cancer in a more effective way, ivermectin can be adopted together with other medications. The combination of ivermectin with medications that are currently being used might result in more favourable prognoses for patients with certain types of cancer. For example, when combined with daunorubicin/cytarabine, tamoxifen, paclitaxel and anti-BRAF V600 inhibitors, ivermectin exhibits a more powerful anticancer effect against leukaemia, TNBC, EOC and melanoma, respectively. Oral ivermectin has been widely used at clinical doses for the treatment of human parasitic infections with no discernible side effects.
Obviously, the detailed mechanisms of this multi-functional drug remain to be understood. However, the proposed anticancer scheme and the application of new drugs are bringing us one step closer to better health, which is a goal that we should continue to pursue.
Funding Statement
The work was supported by the Department of Science and Technology, Jilin Province, China (CN) (20190101014JH, 20170622008JC).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Omura S. Ivermectin: 25 years and still going strong. Int J Antimicrob Agents. 2008;31(2):91–98. doi: 10.1016/j.ijantimicag.2007.08.023 [DOI] [PubMed] [Google Scholar]
- 2.Crump A. Ivermectin: enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations. J Antibiot (Tokyo). 2017;70(5):495–505. doi: 10.1038/ja.2017.11 [DOI] [PubMed] [Google Scholar]
- 3.Juarez M, Schcolnik-Cabrera A, Duenas-Gonzalez A. The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. Am J Cancer Res. 2018;8(2):317–331. [PMC free article] [PubMed] [Google Scholar]
- 4.Geary TG. Ivermectin 20 years on: maturation of a wonder drug. Trends Parasitol. 2005;21(11):530–532. doi: 10.1016/j.pt.2005.08.014 [DOI] [PubMed] [Google Scholar]
- 5.Lynagh T, Lynch JW. Ivermectin binding sites in human and invertebrate Cys-loop receptors. Trends Pharmacol Sci. 2012;33(8):432–441. doi: 10.1016/j.tips.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 6.Chen IS, Kubo Y. Ivermectin and its target molecules: shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J Physiol. 2018;596(10):1833–1845. doi: 10.1113/JP275236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen IS, Tateyama M, Fukata Y, Uesugi M, Kubo Y. Ivermectin activates GIRK channels in a PIP2 -dependent, Gbetagamma -independent manner and an amino acid residue at the slide helix governs the activation. J Physiol. 2017;595(17):5895–5912. doi: 10.1113/JP274871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laing R, Gillan V, Devaney E. Ivermectin - old drug, new tricks? Trends Parasitol. 2017;33(6):463–472. doi: 10.1016/j.pt.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon YJ, Petrie K, Leibovitch BA, et al. Selective inhibition of SIN3 corepressor with avermectins as a novel therapeutic strategy in triple-negative breast cancer. Mol Cancer Ther. 2015;14(8):1824–1836. doi: 10.1158/1535-7163.MCT-14-0980-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodama M, Kodama T, Newberg JY, et al. In vivo loss-of-function screens identify KPNB1 as a new druggable oncogene in epithelial ovarian cancer. Proc Natl Acad Sci U S A. 2017;114(35):E7301–E7310. doi: 10.1073/pnas.1705441114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharmeen S, Skrtic M, Sukhai MA, et al. The antiparasitic agent ivermectin induces chloride-dependent membrane hyperpolarization and cell death in leukemia cells. Blood. 2010;116(18):3593–3603. doi: 10.1182/blood-2010-01-262675 [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto HMS, Sudo T, Maruta H. Ivermectin inactivates the kinase PAK1 and blocks the PAK1dependent growth of human ovarian cancer and NF2 tumor cell lines. Drug Discov Ther. 2009;3(6):3. [PubMed] [Google Scholar]
- 13.Desantis V, Saltarella I, Lamanuzzi A, et al. Autophagy: a new mechanism of prosurvival and drug resistance in multiple myeloma. Transl Oncol. 2018;11(6):1350–1357. doi: 10.1016/j.tranon.2018.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15(7):713–720. doi: 10.1038/ncb2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura1 HHTSHMR. The direct PAK1 inhibitor, TAT-PAK18, blocks preferentially the growth of human ovarian cancer cell lines in which PAK1 is abnormally activated by autophosphorylation at Thr 423. Drug Discov Ther. 2010;4(1):1–4. [PubMed] [Google Scholar]
- 16.Shinojima N, Yokoyama T, Kondo Y, Kondo S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy. 2014;3(6):635–637. doi: 10.4161/auto.4916 [DOI] [PubMed] [Google Scholar]
- 17.Dou Q, Chen HN, Wang K, et al. Ivermectin induces cytostatic autophagy by blocking the PAK1/Akt axis in breast cancer. Cancer Res. 2016;76(15):4457–4469. doi: 10.1158/0008-5472.CAN-15-2887 [DOI] [PubMed] [Google Scholar]
- 18.Wang K, Gao W, Dou Q, et al. Ivermectin induces PAK1-mediated cytostatic autophagy in breast cancer. Autophagy. 2016;12(12):2498–2499. doi: 10.1080/15548627.2016.1231494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallardo F, Mariame B, Gence R, Tilkin-Mariame AF. Macrocyclic lactones inhibit nasopharyngeal carcinoma cells proliferation through PAK1 inhibition and reduce in vivo tumor growth. Drug Des Devel Ther. 2018;12:2805–2814. doi: 10.2147/DDDT.S172538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallardo F, Teiti I, Rochaix P, et al. Macrocyclic lactones block melanoma growth, metastases development and potentiate activity of anti– BRAF V600 inhibitors. Clini Skin Cancer. 2016;1(1):4–14.e13. doi: 10.1016/j.clsc.2016.05.001 [DOI] [Google Scholar]
- 21.Bai L, Wang S. Targeting apoptosis pathways for new cancer therapeutics. Annu Rev Med. 2014;65:139–155. doi: 10.1146/annurev-med-010713-141310 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Luo M, Xu W, et al. Avermectin confers its cytotoxic effects by inducing DNA damage and mitochondria-associated apoptosis. J Agric Food Chem. 2016;64(36):6895–6902. doi: 10.1021/acs.jafc.6b02812 [DOI] [PubMed] [Google Scholar]
- 23.Draganov D, Gopalakrishna-Pillai S, Chen YR, et al. Modulation of P2X4/P2X7/Pannexin-1 sensitivity to extracellular ATP via Ivermectin induces a non-apoptotic and inflammatory form of cancer cell death. Sci Rep. 2015;5:16222. doi: 10.1038/srep16222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Fang S, Sun Q, Liu B. Anthelmintic drug ivermectin inhibits angiogenesis, growth and survival of glioblastoma through inducing mitochondrial dysfunction and oxidative stress. Biochem Biophys Res Commun. 2016;480(3):415–421. doi: 10.1016/j.bbrc.2016.10.064 [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Xu Y, Wan H, Hu J. Antibiotic ivermectin selectively induces apoptosis in chronic myeloid leukemia through inducing mitochondrial dysfunction and oxidative stress. Biochem Biophys Res Commun. 2018;497(1):241–247. doi: 10.1016/j.bbrc.2018.02.063 [DOI] [PubMed] [Google Scholar]
- 26.Song D, Liang H, Qu B, et al. Ivermectin inhibits the growth of glioma cells by inducing cell cycle arrest and apoptosis in vitro and in vivo. J Cell Biochem. 2019;120(1):622–633. doi: 10.1002/jcb.v120.1 [DOI] [PubMed] [Google Scholar]
- 27.Zhang P, Zhang Y, Liu K, et al. Ivermectin induces cell cycle arrest and apoptosis of HeLa cells via mitochondrial pathway. Cell Prolif. 2018;52(2):e12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–875. doi: 10.1038/nrc3380 [DOI] [PubMed] [Google Scholar]
- 29.Martins I, Wang Y, Michaud M, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014;21(1):79–91. doi: 10.1038/cdd.2013.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3(7):e2599. doi: 10.1371/journal.pone.0002599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27(4):211–217. doi: 10.1016/j.tips.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 32.Seil M, El Ouaaliti M, Fontanils U, et al. Ivermectin-dependent release of IL-1beta in response to ATP by peritoneal macrophages from P2X(7)-KO mice. Purinergic Signal. 2010;6(4):405–416. doi: 10.1007/s11302-010-9205-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishio M, Sugimachi K, Goto H, et al. Dysregulated YAP1/TAZ and TGF-beta signaling mediate hepatocarcinogenesis in Mob1a/1b-deficient mice. Proc Natl Acad Sci U S A. 2016;113(1):E71–E80. doi: 10.1073/pnas.1517188113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang W, Tong JH, Chan AW, et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17(8):2130–2139. doi: 10.1158/1078-0432.CCR-10-2467 [DOI] [PubMed] [Google Scholar]
- 35.Schlegelmilch K, Mohseni M, Kirak O, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144(5):782–795. doi: 10.1016/j.cell.2011.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Z, Zhang HS, Zhang ZG, et al. Loss of HACE1 promotes colorectal cancer cell migration via upregulation of YAP1. J Cell Physiol. 2018;234:9663–9672. [DOI] [PubMed] [Google Scholar]
- 37.Xia Y, Chang T, Wang Y, et al. YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS One. 2014;9(3):e91770. doi: 10.1371/journal.pone.0091770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi Y, Banno K, Kunitomi H, Tominaga E, Aoki D. Current state and outlook for drug repositioning anticipated in the field of ovarian cancer. J Gynecol Oncol. 2019;30(1):e10. doi: 10.3802/jgo.2019.30.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nambara S, Masuda T, Nishio M, et al. Antitumor effects of the antiparasitic agent ivermectin via inhibition of Yes-associated protein 1 expression in gastric cancer. Oncotarget. 2017;8(64):107666–107677. doi: 10.18632/oncotarget.v8i64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2012;13:11. doi: 10.1038/nrc3419 [DOI] [PubMed] [Google Scholar]
- 41.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melotti A, Mas C, Kuciak M, Lorente-Trigos A, Borges I, Ruiz I Altaba A. The river blindness drug Ivermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. EMBO Mol Med. 2014;6(10):1263–1278. doi: 10.15252/emmm.201404084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seth C, Mas C, Conod A, et al. Long-lasting WNT-TCF response blocking and epigenetic modifying activities of withanolide f in human cancer cells. PLoS One. 2016;11(12):e0168170. doi: 10.1371/journal.pone.0168170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slade L, Pulinilkunnil T. The MiTF/TFE family of transcription factors: master regulators of organelle signaling, metabolism, and stress adaptation. Mol Cancer Res. 2017;15(12):1637–1643. doi: 10.1158/1541-7786.MCR-17-0320 [DOI] [PubMed] [Google Scholar]
- 45.Deng F, Xu Q, Long J, Xie H. Suppressing ROS-TFE3-dependent autophagy enhances ivermectin-induced apoptosis in human melanoma cells. J Cell Biochem. 2018. doi: 10.1002/jcb.27490 [DOI] [PubMed] [Google Scholar]
- 46.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65(14):6029–6033. doi: 10.1158/0008-5472.CAN-05-0137 [DOI] [PubMed] [Google Scholar]
- 47.Yin J, Park G, Lee JE, et al. DEAD-box RNA helicase DDX23 modulates glioma malignancy via elevating miR-21 biogenesis. Brain. 2015;138(Pt 9):2553–2570. doi: 10.1093/brain/awv167 [DOI] [PubMed] [Google Scholar]
- 48.Lee JY, Kong G. Roles and epigenetic regulation of epithelial-mesenchymal transition and its transcription factors in cancer initiation and progression. Cell Mol Life Sci. 2016;73(24):4643–4660. doi: 10.1007/s00018-016-2313-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19(11):1438–1449. doi: 10.1038/nm.3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silverstein RA, Ekwall K. Sin3: a flexible regulator of global gene expression and genome stability. Curr Genet. 2005;47(1):1–17. doi: 10.1007/s00294-004-0541-5 [DOI] [PubMed] [Google Scholar]
- 51.Farias EF, Petrie K, Leibovitch B, et al. Interference with Sin3 function induces epigenetic reprogramming and differentiation in breast cancer cells. Proc Natl Acad Sci U S A. 2010;107(26):11811–11816. doi: 10.1073/pnas.1006737107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu M, Li Y, Zhou Z. Antibiotic ivermectin preferentially targets renal cancer through inducing mitochondrial dysfunction and oxidative damage. Biochem Biophys Res Commun. 2017;492(3):373–378. doi: 10.1016/j.bbrc.2017.08.097 [DOI] [PubMed] [Google Scholar]
- 53.Goradel NH, Mohammadi N, Haghi-Aminjan H, Farhood B, Negahdari B, Sahebkar A. Regulation of tumor angiogenesis by microRNAs: state of the art. J Cell Physiol. 2019;234(2):1099–1110. doi: 10.1002/jcp.27051 [DOI] [PubMed] [Google Scholar]
- 54.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–265. doi: 10.1038/nrc1317 [DOI] [PubMed] [Google Scholar]
- 55.Ashraf S, Prichard R. Ivermectin exhibits potent anti-mitotic activity. Vet Parasitol. 2016;226:1–4. doi: 10.1016/j.vetpar.2016.06.015 [DOI] [PubMed] [Google Scholar]
- 56.Wang TG, Ye M. Advances on the roles of m (6)A in tumorigenesis. Yi Chuan. 2018;40(12):1055–1065. doi: 10.16288/j.yczz.18-098 [DOI] [PubMed] [Google Scholar]
- 57.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–234. doi: 10.1038/nrd1984 [DOI] [PubMed] [Google Scholar]
- 58.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706 [DOI] [PubMed] [Google Scholar]
- 59.Montanari F, Ecker GF. Prediction of drug-ABC-transporter interaction–recent advances and future challenges. Adv Drug Deliv Rev. 2015;86:17–26. doi: 10.1016/j.addr.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edwards G. Ivermectin: does P-glycoprotein play a role in neurotoxicity? Filaria J. 2003;2(Suppl 1):S8. doi: 10.1186/1475-2883-2-S1-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DAaL F. The abamectin derivative ivermectin is a potent P-glycoprotein inhibitor. Anticancer Drugs. 1996;7:745–751. [DOI] [PubMed] [Google Scholar]
- 62.Liang J, Wan M, Zhang Y, et al. Nanog and Oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nat Cell Biol. 2008;10(6):731–739. doi: 10.1038/ncb1736 [DOI] [PubMed] [Google Scholar]
- 63.Dominguez-Gomez G, Chavez-Blanco A, Medina-Franco JL, et al. Ivermectin as an inhibitor of cancer stemlike cells. Mol Med Rep. 2018;17(2):3397–3403. doi: 10.3892/mmr.2017.8231 [DOI] [PubMed] [Google Scholar]
- 64.Guzzo CA, Furtek CI, Porras AG, et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol. 2002;42(10):1122–1133. doi: 10.1177/009127002401382731 [DOI] [PubMed] [Google Scholar]
- 65.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF [DOI] [PubMed] [Google Scholar]
- 66.Verbaanderd C, Meheus L, Huys I, Pantziarka P. Repurposing Drugs in Oncology: next Steps. Trends Cancer. 2017;3(8):543–546. doi: 10.1016/j.trecan.2017.06.007 [DOI] [PubMed] [Google Scholar]