Abstract
We report a case of nivolumab‐induced delayed‐onset aseptic meningitis and a case of limbic encephalitis and peripheral nerve palsy with toxicity relapse 6 months after initial presentation. The atypical presentations contribute to our knowledge of these rare events and reinforce the necessity for vigilant monitoring and a multidisciplinary treatment approach.
Keywords: aseptic meningitis, checkpoint inhibitor, encephalitis, ipilimumab, neurotoxicity, nivolumab
We report a case of nivolumab‐induced delayed‐onset aseptic meningitis and a case of limbic encephalitis and peripheral nerve palsy with toxicity relapse 6 months after initial presentation. The atypical presentations contribute to our knowledge of these rare events and reinforce the necessity for vigilant monitoring and a multidisciplinary treatment approach.
1. INTRODUCTION
Neurologic immune‐related adverse events are uncommon but potentially life‐threatening complications associated with immune checkpoint inhibitors. Here, we review the literature and report two cases, a rare case of nivolumab‐induced delayed‐onset aseptic meningitis and a case of limbic encephalitis and peripheral nerve palsy with toxicity relapse 6 months after initial presentation.
Within the past decade, immune checkpoint inhibitors (ICIs) have demonstrated survival advantages in various solid tumors and are now a therapeutic pillar in oncology. The primary function of immune checkpoints is to maintain immune homeostasis by down‐regulating T‐cell activation.1 One mechanism by which tumor cells evade the immune system is through exploiting immune checkpoints, thereby suppressing T‐cell activity.2, 3, 4 T‐cell anergy may be induced when programmed death‐ligand 1 (PD‐L1), primarily expressed on the tumor cell, binds to its receptor on the T cell. Blockade of this ligand‐receptor interaction may reverse immune down‐regulation, allowing for a more robust T‐cell‐mediated response. Inhibiting these negative immune regulators of T‐cell function has proven to be a successful antitumor approach.
Since 2011, 7 ICIs have been approved by the US Food and Drug Administration: ipilimumab, an inhibitor of cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4); nivolumab, pembrolizumab, and cemiplimab‐rwlc, which inhibit programmed cell death protein 1 (PD‐1); and atezolizumab, avelumab, and durvalumab, which inhibit PD‐L1. Urothelial carcinoma (UC) is one of the many tumors that has demonstrated response to ICIs. Five PD‐1/PD‐L1 inhibitors have produced meaningful response rates in platinum‐refractory UC.5, 6, 7, 8, 9, 10 Furthermore, a significant improvement in median overall survival was demonstrated with pembrolizumab compared to chemotherapy in this population.10 Data also support the use of atezolizumab and pembrolizumab in chemotherapy‐naïve, cisplatin‐ineligible UC,11, 12 although the preferred first‐line treatment strategy remains controversial.13
Although robust data support the use of immunotherapies in many solid tumors, including UC, the efficacy of these agents in renal medullary carcinoma (RMC) is not well established. Published case reports provide a hint of activity in this rare disease.14, 15 Given the lack of standard treatment options with proven efficacy, an ICI in the setting of a clinical trial is a reasonable approach in this under‐researched population.
Severe immune‐related adverse events (irAEs), defined as grade ≥3, are estimated to occur in approximately 22%, 7.1%, and 6.3% of patients receiving a CTLA‐4, PD‐1, or PD‐L1 inhibitor, respectively.16 The skin, colon, endocrine organs, liver, lungs, and musculoskeletal systems are most commonly affected, although any organ system may be involved.17, 18 Despite some unique toxicities, PD‐1/PD‐L1 inhibitors have demonstrated a generally favorable toxicity profile compared to cytotoxic chemotherapy.19, 20 The incidence of any grade neurologic irAEs is estimated to be 3.8% with CTLA‐4 inhibitors, 6.1% with PD‐1 inhibitors, and 12% with the combination.21 Most irAEs are generally mild, with headache being predominantly reported; incidence of high‐grade events was <1%. Guillain‐Barré syndrome, myasthenia gravis, encephalopathies, and meningoradiculoneuritis are among the reported serious neurologic irAEs.21, 22 Corticosteroids remain the cornerstone of management of neurologic irAEs,17, 18, 23 but several cases have nevertheless proven fatal.24, 25 Select reports of autoimmune neurologic toxicities associated with ICIs are summarized in Table 1.
Table 1.
Case Reports and Management of Select Severe Neurologic irAEs
| Central Neurologic irAEs | ||||||
|---|---|---|---|---|---|---|
| Neurologic irAE | Grade | Checkpoint inhibitor | Approximate time to onset | Treatment of neurologic irAE | Outcome | Reference |
| Meningitis | ||||||
| Aseptic meningitis | 2 | Ipilimumab + nivolumab | 1‐2 wk | No treatment, ICI held then restarted | Complete resolution | Spain et al30 |
| Aseptic meningitis | 3 | Ipilimumab | 3‐5 wk | ICI stopped, no steroids due to spontaneous symptom improvement | Complete resolution | Spain et al30 |
| Aseptic meningitis | 3 | Ipilimumab | 3‐5 wk | Stop ICI, oral prednisolone | Complete resolution | Spain et al30 |
| Aseptic meningitis | N/A | Ipilimumab | 4 wk | Steroids administered | Resolved | Voskens et al31 |
| Aseptic meningitis | N/A | Ipilimumab (previous IL‐2) | 9‐11 wk | High‐dose dexamethasone | Compete resolution | Yang et al32 |
| Meningitis | N/A | Ipilimumab | 1‐3 wk | Dexamethasone 8 mg/day p.o. | Complete resolution | Bot et al24 |
| Meningitis | N/A | Atezolizumab | 1‐3 wk | No treatment; reinitiated w/o recurrence | Symptoms resolved | Genentech33 |
| Meningoencephalitis | ||||||
| Meningoencephalitis | N/A |
Ipilimumab + nivolumab |
19 wk |
ICI stopped; prednisone 100 mg/day tapered over 1 month | Full recovery | Fellner et al35 |
| Meningoencephalitis | N/A |
Ipilimumab + nivolumab |
12 wk |
ICI stopped then resumed 3 mo after symptom resolution; iv dexamethasone 10 mg twice daily for 8 d then tapered over 1 month | Full recovery | Fellner et al35 |
| Herpetic meningoencephalitis | N/A | Atezolizumab | 3 wk | No treatment | Patient died shortly thereafter from disease progression | Genentech33 |
| Encephalitis | ||||||
| Limbic encephalitis | N/A | Nivolumab | 5 d | iv dexamethasone 20 mg/day tapered over 12 d then oral prednisone 10 mg/day for 14 d followed by 5 mg/day | Full recovery | Fellner et al35 |
| Other | ||||||
| Cerebellar ataxia and dysarthria | N/A | Pembrolizumab | 29‐31 wk | ICI stopped; no treatment | Improved | Kao et al38 |
| Seizure | 2 |
Pembrolizumab (prior ipilimumab) |
7 wk | Levetiracetam 500 mg twice daily | Resolved; intracerebral bleeding 3 wk later | Zimmer et al39 |
| Seizure | 2 | Pembrolizumab | 20 wk | Lorazepam | Resolved | Zimmer et al39 |
| Recurring seizures; parkinsonoid/bradykinesia | 2 |
Pembrolizumab (prior ipilimumab) |
68 wk | ICI stopped; levetiracetam | Improved | Zimmer et al39 |
| Meningoradiculitis | 3 | Nivolumab | 9 wk | ICI stopped; dexamethasone 4 mg p.o. 4 times daily | Improved | Zimmer et al39 |
| Cranial polyneuropathy | N/A |
Ipilimumab + nivolumab |
8 wk | ICI stopped; prednisone 60 mg/day then tapered over 3 mo | Full recovery | Fellner et al35 |
| Phrenic nerve palsy with bulbar palsy | 4 | Nivolumab | 7 wk | ICI stopped; methylprednisolone 1 mg/kg; IVIG; pyridostigmine | Complete resolution | Spain et al30 |
| Peripheral Neurologic irAEs | ||||||
|---|---|---|---|---|---|---|
| Neurologic irAE | Grade | Checkpoint inhibitor | Approximate time to onset | Treatment of neurologic irAE | Outcome | Reference |
| Neuromuscular Junction Disorders | ||||||
| Myasthenia gravis/ paralysis (eyelids/hands) | 4 | Pembrolizumab (prior ipilimumab) | 10 wk | Methylprednisolone 1 gram iv for 3 d; pyridostigmine 30 mg p.o. for 3 d, plasmapheresis | Not resolved; death | Zimmer et al39 |
| Myasthenic crisis and polymyositis | N/A | Nivolumab | 2 wk | Steroids administered; immune absorption therapy; plasma exchange therapy; IVIG | N/A | Kimura et al40 |
| Demyelinating Disorders | ||||||
| Polyradiculitis | N/A |
Ipilimumab + nivolumab |
8 wk | ICI stopped; prednisone 80 mg/day tapered over 2 mo | Full recovery | Fellner et al35 |
| Polyradiculitis | N/A | Pembrolizumab | 18 wk | ICI stopped; iv methylprednisolone 1 gram/day tapered over 10 d then prednisone 80 mg/day tapered over 2 mo | Partial recovery | Fellner et al35 |
| Polyradiculitis | 3 | Pembrolizumab (prior ipilimumab) | 35 wk | ICI paused; prednisolone 1 gram iv then p.o. tapering | Improved | Zimmer et al39 |
| Polyneuropathy | 2 | Pembrolizumab | 4 wk | Pregabalin 75 mg p.o. twice daily | Not resolved | Zimmer et al39 |
| Polyneuropathy, worsening | 2 | Pembrolizumab (prior ipilimumab) | 6 wk | Magnesium | Not resolved | Zimmer et al39 |
| Sensorimotor neuropathy with bulbar palsy (Guillain‐Barré‐like syndrome) | 4 | Ipilimumab | 10 wk | ICI stopped; methylprednisolone 2 mg/kg; plasmapheresis | Partial improvement | Spain et al30 |
| Axonal Guillain‐Barré syndrome | 5 | Ipilimumab | 12 wk | IVIG 0.4 gram/kg for 5 d | Death secondary to respiratory failure 3 d after starting IVIG | Bot et al24 |
| Other | ||||||
| Tolosa‐Hunt syndrome | N/A | Ipilimumab | 18 wk | Methylprednisolone iv followed by dexamethasone p.o., local radiotherapy | Ongoing | Voskens et al31 |
| Paresis, neuritis (oculomotor nerve) | 2 | Pembrolizumab (prior ipilimumab) | 13 wk | ICI paused; prednisolone 100 mg/day | Resolved | Zimmer et al39 |
| Paresis (abducens nerve, facial nerve) | 3 | Nivolumab (prior ipilimumab) | 6 wk | ICI paused; methylprednisolone 1 mg/kg/day; IVIG | Resolved | Zimmer et al39 |
| Severe necrotizing myopathy | N/A | Pembrolizumab | 3‐5 wk | ICI stopped; prednisone 80 mg/day for 12 d; plasmapheresis | Death | Kao et al38 |
| Facial nerve paralysis | N/A | Ipilimumab | 7 wk | Steroids administered | Resolved | Voskens et al31 |
2. CASE PRESENTATIONS
2.1. Patient 1: Aseptic meningitis
A 58‐year‐old male with UC metastatic to the lung and lymph nodes who initially presented with 5 years of intermittent hematuria was found to have a left renal pelvis mass status postleft nephroureterectomy for a pT3Nx high‐grade UC followed by 4 cycles of adjuvant gemcitabine plus cisplatin with metastatic recurrence 13 months later. He was treated with 3 cycles of gemcitabine plus carboplatin without response. Relevant past medical history included obesity, noninsulin‐dependent diabetes mellitus, hypertension, hyperlipidemia, fatty liver disease, anxiety, depression, and obstructive sleep apnea. Following enrollment on a phase I clinical trial (NCT02496208), treatment was initiated with cabozantinib 40 mg p.o. once daily plus iv nivolumab 1 mg/kg every 2 weeks.
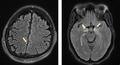
The first 12 cycles (48 weeks) of treatment were unremarkable. The patient achieved a complete response in the hilar and retrocrural lymph nodes and a partial response (PR) in the lung by cycle 11. Restaging evaluations at this time revealed a continuing PR to treatment, with a 51% reduction in the target right lower lobe mass and lymph node lesions. Soon thereafter, the patient presented with a fever of 39.3°C, chills, malaise, dry cough, headache, and bilateral eye pain. The patient described a dull frontal headache not relieved by analgesics and pain with ocular movement, but a neurologic examination was otherwise unremarkable. Concomitant medications were unchanged and included oxycodone, citalopram, loperamide, metoprolol succinate, opium tincture, rosuvastatin, aspirin, cholecalciferol, fexofenadine, levothyroxine, sitagliptin, and lisinopril. Treatment with nivolumab and cabozantinib was held. Three days later, the patient complained of worsening ocular pain with movement and intermittent shooting, lancinating right ear pain. MRI revealed normal orbits and a punctate focus of leptomeningeal enhancement (Figure 1). Ophthalmologic examination revealed normal vision, motility, and optic nerves. Lumbar puncture findings were consistent with aseptic meningitis (Table 2), prompting initiation of iv methylprednisolone 1 mg/kg (130 mg). Within 72 hours of starting corticosteroids, the patient experienced a rapid resolution of otic and ocular pain, fever, and headache. Methylprednisolone was transitioned to oral dexamethasone, which was then tapered over approximately 4‐5 weeks with no recurrence of symptoms. The patient was not rechallenged with nivolumab but continued cabozantinib for an additional year until disease progression in the contralateral retrocrural lymph nodes. He passed away 6 months after discontinuation of cabozantinib with extensive liver metastases. A brain autopsy showed no significant neuropathologic changes.
Figure 1.
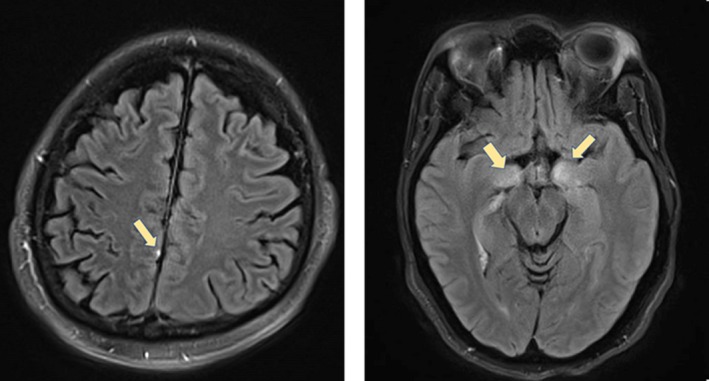
Imaging of patients 1 and 2. (Left) Meningitis, punctate focus of leptomeningeal enhancement of MRI‐Patient 1. Punctate focus of leptomeningeal enhancement on postcontrast FLAIR. (Right) Encephalitis signal abnormality in bilateral mesial temporal lobes on MRI‐Patient 2. Bilateral T2‐FLAIR hyperintense signal in the mesial temporal lobes bilaterally, with associated contrast enhancement
Table 2.
Case Summaries of Patients 1 and 2
| Patient 1 |
Patient 2 Initial presentation |
Patient 2 Relapse |
|
|---|---|---|---|
| Immunotherapy | Nivolumab × 24 doses | Ipilimumab/nivolumab x 4 doses then nivolumab x 4 doses | None since initial treatment |
| Neurologic autoimmune diagnosis | Aseptic meningitis (grade 3) | Limbic encephalitis (grade 3) | Relapsed limbic encephalitis (grade 3) |
| Presenting symptoms | Fever, chills, malaise, dry cough, headache, bilateral eye pain, and right ear pain | Blurry vision, headache, photophobia, and short‐term memory impairment | Confusion, paranoia, and short‐term memory impairment |
| Neurologic examination findings | Unremarkable |
Anterior bilateral uveitis (2 mo prior) (grade 1), ataxia, Impaired delayed recall, right 6th cranial nerve palsy |
Left 6th cranial nerve palsy, bilateral ptosisa, MoCA = 11/30 |
| Brain MRI results | Punctate focus of leptomeningeal enhancement | FLAIR hyperintensities in bilateral mesial temporal lobes | Bilateral lateral rectus atrophy |
| Lumbar puncture results |
Protein 64 mg/dL Glucose 56 mg/dL RBC 1/mm3 WBC 74/mm3 (91% lymphocytes) Opening pressure 21 mm H2O No organisms/growth Cytopathology negative |
Protein 34 mg/dL Glucose 53 mg/DL RBC 0/mm3 WBC 19/mm3 (99% lymphocytes) No organisms/growth Cytopathology negative Paraneoplastic panel negative AChR negative MuSK negative |
Protein 57 mg/dL Glucose 48 mg/dL RBC 0/mm3 WBC 4/mm3 NMDA receptor antibody negative bParaneoplastic, autoantibody panel negative Cytopathology negative No organisms/growth Pattern 4 oligoclonal bands |
| Additional findings/ assessments | EEG showed intermittent focal delta slowing in the bilateral frontal region | ||
| irAE treatment | Methylprednisolone 1 mg/kg iv x 1 then dexamethasone p.o. taper over 4‐5 wk | Methylprednisolone 1 gram iv daily x 5 then prednisone p.o. taper over 3 mo; mycophenolate unsuccessful | Methylprednisolone 1 gram iv daily x 5 then prednisone oral taper over 4 mo |
| irAE outcome | No sequelae | Partial improvement | Partial improvement |
Abbreviations: AChR, antiacetylcholine receptor antibody; EEG, electroencephalogram; FLAIR, fluid‐attenuated inversion recovery; iv, intravenous; irAE, immune‐related adverse event; LP, lumbar puncture; MoCA, Montreal Cognitive Assessment; MuSK, muscle‐specific receptor kinase; NMDA, N‐methyl‐D‐aspartate; RBC, red blood cell; and WBC, white blood cell.
Initially presented with left ptosis and progressed to bilateral ptosis.
VGKC‐complex Ab IPA, LGI1‐IgG CBA, CASPR2‐IgG CBA, GAD65 Ab Assay, GABA‐B‐R Ab CBA, AMPA‐R Ab CBA, ANNA‐1‐3, AGNA‐1, PCA‐1 and 2, PCA‐Tr, Amphiphysin Ab, CRMP‐5‐IgG
2.2. Patient 2: Limbic encephalitis
A 42‐year‐old female with newly diagnosed metastatic RMC to the bilateral lungs, bulky retroperitoneal lymph nodes, and multiple subcutaneous nodules presented for consultation and treatment initiation. Relevant past medical history included sickle cell trait; concomitant medications included oxycodone, acetaminophen, docusate/senna, and ondansetron. Following consent, treatment with immunotherapy and a targeted therapy were initiated based on the results of a published, ongoing clinical trial.26 Treatment consisted of cabozantinib 40 mg p.o. once daily and iv nivolumab 3 mg/kg plus iv ipilimumab 1 mg/kg every 3 weeks for 4 cycles, followed by cabozantinib 40 mg p.o. once daily and iv nivolumab 3 mg/kg every 2 weeks as maintenance therapy.
The patient experienced a PR following 7 cycles (approximately 4 months) of therapy. A biopsy of a residual subcutaneous nodule revealed necrotic tissue. Treatment was complicated with bilateral anterior uveitis that presented as blurry vision and resolved with topical agents. A short time later, the patient presented with a 5‐day history of diplopia, headache, photophobia, and difficulty with short‐term memory. Neurologic examination was significant for impaired delayed recall, right 6th cranial nerve palsy, and ataxia. MRI revealed a signal abnormality in bilateral mesial temporal lobes (Figure 1). Findings of a lumbar puncture and additional tests are included in Table 2. The patient experienced partial symptom improvement with methylprednisolone 1 gram iv daily for 5 days, followed by a prednisone taper. Approximately 2 weeks later, the patient developed acute bilateral intermittent ptosis during a prednisone dose reduction from 80 mg to 40 mg daily. Prednisone was re‐escalated, followed by a slower taper over approximately 3 months. A trial of mycophenolate also occurred during the taper period but without apparent symptomatic benefit.
The patient completed the steroid taper and was not rechallenged with immunotherapy. She remained neurologically stable until approximately 6 months after the initial presentation, at which time she presented with a 2‐week history of confusion, paranoia, and short‐term memory impairment. Neurologic examination revealed symptoms of contralateral left 6th cranial nerve palsy and a Montreal Cognitive Assessment of 11/30. MRI was notable for progression of atrophy but no alteration or enhancement to account for the new‐onset palsy. Cerebrospinal fluid (CSF) analysis is provided in Table 2. The recurrent symptom exacerbation was attributed to a relapse of autoimmune encephalitis, and the patient was treated with another round of methylprednisolone 1 gram iv daily for 5 days, followed by a slow (4‐month) prednisone taper. Similar to the first occurrence, the patient experienced a partial resolution of symptoms, but signs of neurocognitive slowing remained. The patient died 10 months later. At autopsy, gross examination of the brain showed no macroscopic abnormalities. Selected regions of the brain were examined with hematoxylin and eosin and various immunohistochemical stains (GFAP, NEUN, LFB‐PAS, NFTP, CD68, CD3, CD4, and CD8; Figure 2). Sections of the cerebellum show a marked loss of Purkinje cells, accompanied by microglial activation and Bergman gliosis. Sparse inflammatory cells mainly consist of scattered lymphocytes and macrophages. Additional changes of microglial activation and gliosis are seen in other regions, including the hippocampus.
Figure 2.
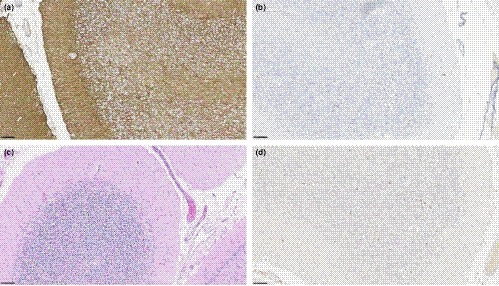
Pathologic findings in cerebellum on autopsy for patient 2. A, GFAP 20×, B, CD68 20×, C, HE 20×, and D, CD3 20×
3. DISCUSSIONS AND CONCLUSIONS
We present 2 cases of serious neurologic irAEs, aseptic meningitis and limbic encephalitis, both associated with the use of ICIs. Both patients had remarkable durable disease responses to immunotherapy. The patient with UC experienced a durable (approximately 2 years) PR on the nivolumab/cabozantinib regimen. Similarly, the patient with RMC experienced a PR on the nivolumab/ipilimumab/cabozantinib combination for approximately 5 months. Emerging data have begun to establish a correlation between irAEs and the efficacy of ICIs in patients with solid tumors27, 28; our cases provide additional evidence for this correlation.
Drug‐induced aseptic meningitis is a rare adverse event that has been reported with various pharmaceutical products, including nonsteroidal anti‐inflammatory drugs, antimicrobials, iv immunoglobulin (IVIG), vaccines,29 and most recently, ICIs.24, 30, 31, 32, 33 Patient 1 denied use of these agents, and there are no data supporting a link between cabozantinib and aseptic meningitis. Furthermore, viral, bacterial, and fungal causes of meningitis were ruled out. Presenting symptoms of fever and headache are classic signs of aseptic meningitis, but our patient also complained of ocular and otic pain. The reported median time to onset of neurologic irAEs is 6 weeks.21 Onset of meningitis‐associated symptoms in our patient was much later compared to previously published reports (24 doses vs 1‐2 doses, respectively).24, 30, 31, 32, 33 Drug‐induced aseptic meningitis may be self‐limited and resolve without treatment. Case reports of aseptic meningitis associated with ICIs have also demonstrated spontaneous symptom resolution.30, 33 Current guidelines recommend monitoring of steroids or considering steroids for moderate/severe symptoms or unwell patients.17, 23 Due to progressive symptoms during an initial work‐up period, we elected to initiate steroids in our patient.
Patient 2 met the diagnostic criteria for autoimmune limbic encephalitis34: subacute onset of memory deficits; fluid‐attenuated inversion recovery hyperintensities in bilateral medial temporal lobes; CSF pleocytosis; and a reasonable exclusion of alternative causes. One case of limbic encephalitis associated with nivolumab reported a symptom onset of 5 days followed by a full recovery with steroid treatment.35 To our knowledge, this is the first case of ICI‐associated limbic encephalitis with recurrent symptoms months after initial presentation. However, the patient did not fully recover to baseline cognition after the first neurologic irAE. Theoretically, the symptoms may have been suppressed by high‐dose steroids and a 3‐month taper and later progressed after steroid discontinuation. High‐level evidence for the treatment of immunotherapy‐associated encephalitis is lacking, and practice recommendations are based on case reports and/or expert opinion. Current guidelines recommend a trial of methylprednisolone 1‐2 mg/kg,17, 18, 23 and suggest methylprednisolone 1 gram iv daily for 3‐5 days plus IVIG for patients with severe or progressing symptoms or with oligoclonal bands.23 A further treatment option is plasmapheresis.23 Our patient was treated with pulse methylprednisolone 1 gram iv daily for 5 days for both the initial presentation and the recurrent episode, which improved but did not eradicate her cognitive symptoms. A trial with mycophenolate, a corticosteroid‐sparing agent, was unsuccessful.
On brain autopsy, sections of patient 2's cerebellum showed a marked loss of Purkinje cells accompanied by microglial activation and Bergman gliosis. The cerebellum is a frequent target of paraneoplastic autoimmunity. With paraneoplastic cerebellar degeneration (PCD), pathologic examination shows marked degeneration of Purkinje cells with minimal involvement of the molecular or granular cell layers. Depending on disease stage, inflammation can be marked, sparse, or absent in the cerebellar cortex.36 Inflammation in other regions of the brain can also be seen and might indicate an overlap with paraneoplastic encephalomyelitis. Three antibodies, anti‐Yo, anti‐Tr, and anti‐mGluR1, are predominantly associated with cerebellar dysfunction. In addition to antibody‐mediated immune responses, cytotoxic T‐cell responses are involved in the pathogenesis of PCD.37 In this case, anti‐Purkinje antibody titers performed a year prior to death were negative, but in the absence of concurrent antibody titers, we cannot rule out the possibility of a paraneoplastic syndrome. Also, given the patient's prior treatment with ICIs, the possibility of irAEs should be considered.
Although reports of aseptic meningitis and limbic encephalitis after treatment with ICIs have previously been published, these cases help expand our knowledge of these rare but potentially serious neurologic toxicities. Patient 1 provides evidence of an unusual late‐onset aseptic meningitis following 24 doses of nivolumab. Patient 2 is a unique case of recurrent limbic encephalitis. Neurologic sequelae from these agents may occur, requiring prompt attention and a multidisciplinary approach to reduce morbidity and mortality.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
LMC, NND, and ABA: made major contributions to the writing of this manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the Center for Cancer Research, NCI, National Institutes of Health.
Cordes LM, Davarpanah NN, Reoma LB, et al. Neurotoxicities associated with checkpoint inhibitors: Two case reports and a review of the literature. Clin Case Rep. 2020;8:24–32. 10.1002/ccr3.2534
REFERENCES
- 1. Disis ML. Mechanism of action of immunotherapy. Semin Oncol. 2014;41(suppl 5):S3‐13. [DOI] [PubMed] [Google Scholar]
- 2. Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35:S185‐S198. [DOI] [PubMed] [Google Scholar]
- 3. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA‐4‐Mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813‐5820. [PubMed] [Google Scholar]
- 5. Sharma P, Retz M, Siefker‐Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single‐arm, phase 2 trial. Lancet Oncol. 2017;18:312‐322. [DOI] [PubMed] [Google Scholar]
- 6. Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti–programmed death‐ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35:2117‐2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenberg JE, Hoffman‐Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: a single‐arm, multicentre, phase 2 trial. Lancet. 2016;387:1909‐1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN solid tumor): pooled results from two expansion cohorts of an open‐label, phase 1 trial. Lancet Oncol. 2018;19:51‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti‐programmed cell death ligand‐1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119‐3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balar AV, Castellano D, O'Donnell PH, et al. First‐line pembrolizumab in cisplatin‐ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE‐052): a multicentre, single‐arm, phase 2 study. Lancet Oncol. 2017;18:1483‐1492. [DOI] [PubMed] [Google Scholar]
- 12. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first‐line treatment in cisplatin‐ineligible patients with locally advanced and metastatic urothelial carcinoma: a single‐arm, multicentre, phase 2 trial. Lancet. 2017;389:67‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keytruda (pembrolizumab) or Tecentriq (atezolizumab): FDA alerts health care professionals and investigators: FDA statement ‐ decreased survival in some patients in clinical trials associated with monotherapy. Available from: https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm608253.htm [accessed 14 March 2019].
- 14. Sodji Q, Klein K, Sravan K, Parikh J. Predictive role of PD‐L1 expression in the response of renal medullary carcinoma to PD‐1 inhibition. J Immunother Cancer. 2017;5:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beckermann KE, Jolly PC, Kim JY, et al. Clinical and immunologic correlates of response to PD‐1 blockade in a patient with metastatic renal medullary carcinoma. J Immunother Cancer. 2017;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El Osta B, Hu F, Sadek R, Chintalapally R, Tang SC. Not all immune‐checkpoint inhibitors are created equal: meta‐analysis and systematic review of immune‐related adverse events in cancer trials. Crit Rev Oncol Hematol. 2017;119:1‐12. [DOI] [PubMed] [Google Scholar]
- 17. Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2017;28: iv119‐iv142. [DOI] [PubMed] [Google Scholar]
- 18. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and tolerability of PD‐1/PD‐L1 inhibitors compared with chemotherapy in patients with advanced cancer: a meta‐analysis. Oncologist. 2017;22:470‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brahmer JR, Rodriguez‐Abreu D, Robinson AG, et al. Health‐related quality‐of‐life results for pembrolizumab versus chemotherapy in advanced, PD‐L1‐positive NSCLC (KEYNOTE‐024): a multicentre, international, randomised, open‐label phase 3 trial. Lancet Oncol. 2017;18:1600‐1609. [DOI] [PubMed] [Google Scholar]
- 21. Cuzzubbo S, Javeri F, Tissier M, et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer. 2017;73:1‐8. [DOI] [PubMed] [Google Scholar]
- 22. Hottinger AF. Neurologic complications of immune checkpoint inhibitors. Curr Opin Neurol. 2016;29:806‐812. [DOI] [PubMed] [Google Scholar]
- 23. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36:1714‐1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bot I, Blank CU, Boogerd W, Brandsma D. Neurological immune‐related adverse events of ipilimumab. Pract Neurol. 2013;13:278‐280. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki S, Ishikawa N, Konoeda F, et al. Nivolumab‐related myasthenia gravis with myositis and myocarditis in Japan. Neurology. 2017;89:1127‐1134. [DOI] [PubMed] [Google Scholar]
- 26. Nadal R, Mortazavi A, Stein M, et al. Clinical efficacy of cabozantinib plus nivolumab (CaboNivo) and CaboNivo plus ipilimumab (CaboNivoIpi) in patients (pts) with chemotherapy‐refractory metastatic urothelial carcinoma (mUC) either naïve (n) or refractory (r) to checkpoint inhibitor (CPI) [abstract]. J Clin Oncol. 2018;36:4528. [Google Scholar]
- 27. Haratani K, Hayashi H, Chiba Y, et al. Association of immune‐related adverse events with nivolumab efficacy in non‐small‐cell lung cancer. JAMA Oncol. 2018;4:374‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riudavets M, Barba A, Maroto P, et al. Correlation between immune‐related adverse events (irAEs) and efficacy in patients with solid tumors treated with immune‐checkpoints inhibitors (ICIs) [abstract]. J Clin Oncol. 2018;36:3064.30188784 [Google Scholar]
- 29. Jolles S, Sewell WA, Leighton C. Drug‐induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22:215‐226. [DOI] [PubMed] [Google Scholar]
- 30. Spain L, Walls G, Julve M, et al. Neurotoxicity from immune‐checkpoint inhibition in the treatment of melanoma: a single centre experience and review of the literature. Ann Oncol. 2017;28:377‐385. [DOI] [PubMed] [Google Scholar]
- 31. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti‐CTLA‐4 therapy in metastatic melanoma from the ipilimumab network. PLoS ONE. 2013;8:e53745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti‐CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Medical Information from Genentech Medical Communications to Lisa M. Cordes, PharmD.
- 34. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fellner A, Makranz C, Lotem M, et al. Neurologic complications of immune checkpoint inhibitors. J Neurooncol. 2018;137:601‐609. [DOI] [PubMed] [Google Scholar]
- 36. Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7:327‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Albert ML, Austin LM, Darnell RB. Detection and treatment of activated T cells in the cerebrospinal fluid of patients with paraneoplastic cerebellar degeneration. Ann Neurol. 2000;47:9‐17. [PubMed] [Google Scholar]
- 38. Kao JC, Liao B, Markovic SN, et al. Neurological complications associated with anti‐programmed death 1 (PD‐1) antibodies. JAMA Neurol. 2017;74:1216‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side‐effects of anti‐PD‐1 therapy. Eur J Cancer. 2016;60:210‐225. [DOI] [PubMed] [Google Scholar]
- 40. Kimura T, Fukushima S, Miyashita A, et al. Myasthenic crisis and polymyositis induced by one dose of nivolumab. Cancer Sci. 2016;107:1055‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]


