Abstract
Background and Aim
Acne is a common skin disease resulting from a complex interaction of various pathogenetic factors. The aim of this study was to find out lipid profile abnormalities in acne vulgaris patients.
Material and Methods
This descriptive analytic cross-sectional study was conducted on 45 acne patients and 45 age- and sex-matched healthy controls to assess plasma total cholesterol (TC), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and triglyceride (TG) levels.
Results
We showed a higher cholesterol level in acne patients (P=0.025), particularly in men (P=0.04). Other plasma lipids including TG, LDL, and HDL in male and female patients were higher compared to controls, but this is not statistically significant.
Discussion and Conclusion
In conclusion, lipid profile was altered in our patients, with high cholesterol level as the commonest derangement, particularly in men. Therefore, screening for lipid profile abnormalities could be considered in the treatment of acne patients.
Keywords: acne vulgaris, lipid profile, severity
Introduction
Acne vulgaris is the most common skin condition affecting late adolescents worldwide.1 The prevalence of acne is said to be 85% in adults aged 12 to 25 years.2 Almost all teenagers between 15 and 17 years report having some degree of acne.3 Estimates of the lifetime occurrence of acne in the literature range from 0.1%4 to as high as 85.1%5 in the general population, depending on the age range, region, and method of sampling. For instance, in a study the overall pooled prevalence rates of acne were 39.2%, the prevalence rates in different age groups were 10.2% overall, 50.2% for primary and secondary students and 44.5% for undergraduates.6 Acne is no longer considered a health problem strictly of adolescence, as its prevalence in adults has significantly increased.7 Overall, acne is more common in women,8,9 but in some literature the prevalence is approximately equal in men and women.10,11
Acne vulgaris is a multifactorial disorder of the pilosebaceous unit, resulting from sebum overproduction, follicular hyperkeratinization, inflammation, and bacterial colonization of hair follicles by Propionibacterium acnes. The sebaceous gland is controlled primarily by hormonal stimulation. In this way, the hormonal effect on sebum secretion is a key to the pathogenesis of acne.12–16 During puberty, alteration of the sebum component, called dysseborrhea, stress, irritation, cosmetics, and potential dietary factors lead to the formation of acne lesions.16 Changes in sebum secretion are considered to be an important factor of acne. In this way, increased sebum secretion can induce acne occurrence.17
Increasing evidence, in essence, indicates that changes in lipid profile are strongly related to acne occurrence. Accordingly, further information and awareness campaigns are needed to emphasize the role of balanced lipid metabolism to control acne. Although a correlation between the incidence of acne and lipid profile levels has been observed before, it must be documented in each population with etiological characteristics. Minimal reports are available on the relationship between plasma lipids and acne in our country. Hence, we aim to establish this study to estimate the presence of lipid alteration in acne patients.
Materials and Methods
The present study was carried out as a descriptive analytic cross-sectional work. The Ethics Committee of Hamadan University of Medical Sciences approved the research (Approval ID: IR.UMSHA.REC.1395.491). This study was conducted in accordance with the Declaration of Helsinki. The study population comprised all untreated patients with acne referred to Sina Hospital, Hamadan in 2017. Criteria for removing a patient from the study were as follows: reluctance to take part in the study, pregnancy or lactation, intake of oral contraception or hormone therapy, history of taking any medications that affect lipid metabolism or oral isotretinoin, and systemic diseases that affect the metabolism of lipids, such as uncontrolled diabetes, hypothyroidism, nephrotic syndrome, end-stage renal failure, HIV, familial hypercholesterolemia, and metabolic syndrome. The controls were age- and sex-matched healthy volunteers, not taking any medicine, and having no personal or family history of acne.
After the study had been fully described to the subjects who met the participation criteria, written informed consent was obtained from them. For participants under the age of 18 years, a parent or legal guardian provided written informed consent. They were examined accurately, and clinical and demographical information was recorded in a checklist. A checklist was used to collect demographical, clinical, and patients’ condition data. The recorded information included age, gender, and severity of involvement of the patients. The patients were then be categorized into 4 groups according to acne severity by Global Acne Grading System (GAGS), which was devised by Doshi and colleagues in 1997. The GAGS is a quantitative scoring system to assess acne severity [Global score: 0 = none; 1–18 = mild; 19–30 = moderate; 31–38 = severe; >39 = very severe].18 Thereafter, venous blood specimens were drawn from patients and controls who had been fasting for 12 h. The blood samples were taken to measure lipid profile levels as triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL), and high-density lipoprotein cholesterol (HDL) values. Serum TG, TC, and HDL were detected by Pars Azmun Kit and serum LDL was estimated using Friedewald's formula. Because there are some differences in lipid profile between males and females, in this study we decided to analyze data obtained as two groups, female and male acne patients, and these results were compared with those of healthy controls. Statistical analysis of the data was performed using the statistical package for the social sciences (SPSS version 16) program using descriptive statistics as well as analytic ones (independent sample t-test and logistic regression tests). The differences were considered significant if the obtained p-value was less than or equal to 0.05. The differences in TG, total cholesterol, HDL, and LDL levels between the patients and controls were statistically analyzed.
Results
A total of 45 acne patients and 45 age- and sex-matched healthy controls were included in this study. In both groups, there were more women than men but the difference between patients and controls was not statistically significant (P=0.5). In the patient group, 28 (62.2%) were female and 17 (37.8%) were male, while in controls they were 31 (68.9%) and 14 (31.1%), respectively. The mean ages of acne patients and controls were 24.7±6.3 and 24.5±5.1, respectively, and there was no statistically significant difference (P=0.61). The age of participants was ranged from 15 to 44 years.
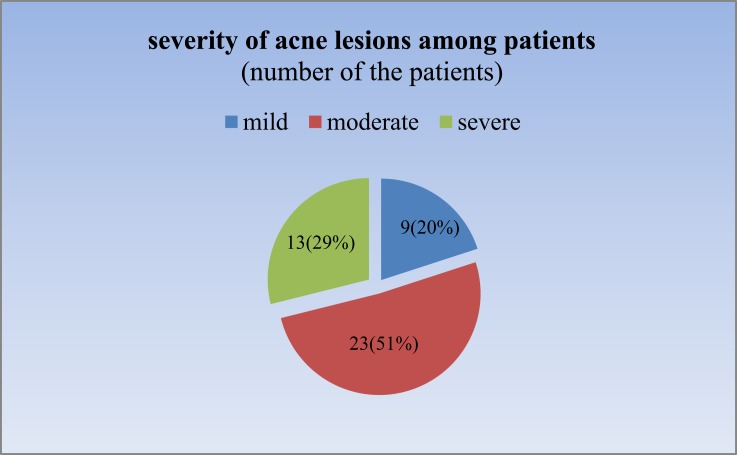
Table 1 shows the mean values for total cholesterol, HDL, LDL, and triglyceride in the two groups. According to this table, all of these means were in the normal range. All variables in male and female patients were higher than in the healthy control group. Statistical data analysis using an independent sample t-test showed there was no significant difference between TG, LDL, and HDL in acne vulgaris and control groups. Noticeably, TC was the only parameter that was significantly higher in patients than controls (P=0.025). Gender analysis showed the same results, which means that means of TG, HDL, and LDL were insignificantly higher in both male and female patients compared with healthy counterparts (all P-values>0.05), and only for cholesterol, a significant difference was seen between male patients and healthy counterparts (173.9±0.13 and 150.3±0.33 for patients and controls, respectively, p=0.04). In Table 2 the subjects were divided into two age groups as less than 25 and equal to or more than 25 years old. All lipid profiles were showed no significant difference in both age groups between acne patients and controls. In our study, the predominant clinical manifestations of acne were papules and pustules, described as moderate acne. About half of the patients were afflicted with moderate acne and none was very severe (Figure 1). As shown in Table 3, plasma lipid levels were elevated with an increase in severity of acne but this is not significant (P>0.05). The logistic regression model highlighted a positive correlation between serum cholesterol and acne (Table 4).
Table 1.
Comparison of Lipid Profile of Male and Female Subjects in Patient and Control Groups
| Parameters (mg/dL) | Male | Female | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Acne Patients (Mean±SD) | Controls (Mean±SD) | P (t-Test) | Acne Patients (Mean±SD) | Controls (Mean±SD) | P (t-Test) | Acne Patients (Mean±SD) | Controls (Mean±SD) | P (t-Test) | |
| TG | 108.1±69.5 | 99.6±43.4 | 0.936 | 88.5±40.7 | 78.9±29.8 | 0.536 | 94.6±51.4 | 86.7±36.6 | 0.725 |
| TC | 173.9±30.1 | 150.3±3 0.3 | 0.04 | 156.8±24.9 | 145.3±26.3 | 0.157 | 162.1±27.5 | 147.2±27.6 | 0.025 |
| LDL | 100.3±22.6 | 93.4±32.4 | 0.330 | 83±18.8 | 82.8±21.2 | 0.891 | 88.3±21.4 | 86.8±26.2 | 0.608 |
| HDL | 47.5±9.6 | 45.3±10.8 | 0.425 | 53.2±13 | 48.7±10.6 | 0.171 | 51.4±12.2 | 47.4±10.7 | 0.110 |
Abbreviations: TG, triglycerides; TC, total cholesterol; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; SD, standard deviation.
Table 2.
Comparison of Lipid Profile of Patients and Controls in Two Age Groups
| Parameters (mg/dL) | <25 Years | ≥25 Years | ||||
|---|---|---|---|---|---|---|
| Acne Patients (Mean±SD) | Controls (Mean±SD) | P (t-Test) | Acne Patients (Mean±SD) | Controls (Mean±SD) | P (t-Test) | |
| TG | 95.8±58.1 | 92.8±41.9 | 0.93 | 93.3±44.7 | 81.4±31.1 | 0.45 |
| TC | 160.8±22.1 | 150.1±30.1 | 0.16 | 163.4±32.7 | 144.6±25.6 | 0.07 |
| LDL | 88.2±17.8 | 88.5±28.6 | 0.78 | 88.5±25.1 | 85.3±24.5 | 0.74 |
| HDL | 49.9±13.3 | 47.3±11.4 | 0.39 | 53.2±13 | 48.7±10.6 | 0.13 |
Figure 1.
Severity of acne among patients.
Table 3.
Lipid Profile Analysis in Different Severity of Acne
| Parameters (mg/dL) | Acne severity | P (t-Test) | ||
|---|---|---|---|---|
| Mild (Mean±SD) | Moderate (Mean±SD) | Severe (Mean±SD) | ||
| TG | 76.1±22.3 | 106.1±61.9 | 87±41.6 | 0.502 |
| TC | 153.5±19.2 | 163.7±26.6 | 165.2±33.9 | 0.530 |
| LDL | 82.5±21.7 | 88.6±20.8 | 91.8±23.1 | 0.836 |
| HDL | 54±18.2 | 49.9±10.9 | 52.3±9.9 | 0.903 |
Table 4.
Lipid Profile Analysis with Logistic Regression Model
| Variable | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | Exp(B) | Sig | 95% CI | OR | Exp(B) | Sig | 95% CI | |
| TG | 1.00 | 1.00 | 0.404 | 0.99–1.01 | 0.99 | 0.99 | 0.800 | 0.98–1.01 |
| TC | 1.02 | 0.05 | 0.016 | 1.00–1.03 | 1.05 | 1.04 | 0.007 | 1.01–1.09 |
| LDL | 1.00 | 0.70 | 0.756 | 0.98–1.02 | 0.95 | 0.96 | 0.020 | 0.92–0.99 |
| HDL | 1.03 | 0.15 | 0.108 | 0.99–1.07 | 1.00 | 1.01 | 0.805 | 0.95–1.06 |
| Gender (female) | 1.34 | 0.85 | 0.506 | 0.56–3.21 | 1.24 | 0.89 | 0.674 | 0.44–3.49 |
| Age | 1.00 | 0.723 | 0.838 | 0.93–1.08 | 0.99 | 1.00 | 0.834 | 0.91–1.07 |
Discussion
In our study, the male to female ratio was about 1:2, but this was not statistically significant (P=0.5). This result is in agreement with the findings of some studies that acne was more prevalent in women.8,9,19 Collier reported that adult women in different age categories have a significantly higher prevalence of acne than adult men.9 Meanwhile, a meta-analysis in mainland China found a higher prevalence rate of acne in males than females, due to differences in lifestyle, skincare routines, and androgen levels.6 In some other studies, no significant gender difference was noted and men and women are about equally affected.10,11
We found high cholesterol levels in acne patients (P=0.025), particularly in men (P=0.04). TG, LDL, and HDL in male and female patients were higher compared to controls, but this was not statistically significant. Some other results agree with these findings to some extent. In Jordan, El-Akawi et al reported that acne patients had significantly low HDL (P=0.000). Moreover, TG and LDL were significantly elevated in severe acne compared with those in healthy controls (P=0.004 and P=0.000 consequently).20 In addition, a case-control study conducted in China demonstrated higher TC and LDL in male and female patients with severe acne than in healthy controls. TG in male patients with severe and moderate acne, and also lipoprotein(a) in male and female patients with mild, moderate, and severe acne was higher than in controls.21 According to a case-control study in female acne patients, acne vulgaris is significantly associated with changes in lipid profile and body mass index (BMI). Bassi et al divided participants into three groups as follows: 50 obese female subjects with acne (Group-A), along with 50 non-obese female subjects with acne (Group-B) who were compared with 50 healthy females as controls (Group-C). BMI, TC, TG, and LDL in both Group-A and Group-B were significantly higher than Group-C (P<0.001), particularly in Group-A. A lower level of HDL was found in both Group-A and Group-B as compared to Group-C, and was more apparent in Group-A.22 Cunha et al conducted a retrospective transverse study in Brazil on 416 patients with a mean age of 32.23 years. Grade II acne as papules and pustules was seen in 156 patients (71%), which was similar to our study (51%). The patients had elevated TC and LDL regardless of their family history. Furthermore, he said there was no significant difference between the grade of acne and patients’ lipid profile, which was in agreement with our result.23
As mentioned above, many studies have revealed lipid profile changes in acne patients, but, on the other hand, a few other studies showed similar values for lipid profile parameters in patients and controls. Ekiz et al reported that there was no relationship between acne and thyroid function, TG, TC, and LDL. Meanwhile, cigarette smoking (P<0.001) was significantly associated with post-adolescent acne. A high HDL level can decrease the risk of acne.24 A cross-sectional study in Indonesia involving 30 acne vulgaris and 30 healthy subjects found no association between lipid profiles with acne.25
The obtained results concerning lipid profile abnormalities in our study were similar to those demonstrated in some other countries. These findings suggest the need for assessing serum lipid levels during acne treatments. However, this study had some potential limitations including small sample size and cross-sectional study design. More work needs to be done analyzing samples from a greater geographic area or be expanded to multi-center research.
Conclusions
Our study and some others highlighted that altered serum lipid levels are prevalent in acne, indicating that lipid profile tests are an important consideration in acne. It is noteworthy that the evidence generated from papers concerning the association between lipid profile and acne vulgaris may prove beneficial in terms of understanding the role of lipid profile abnormalities in the pathogenesis as well as in the treatment of acne. Hence, further investigations with more patients are needed to determine other pathogenetic factors.
Acknowledgments
The authors would like to thank the vice-chancellor for research and technology of Hamadan University of Medical Sciences for support during the research proposal writing as well as during the completion of the original article.
Disclosure
There is no conflict of interest with any of the authors with this work.
References
- 1.Lynn D, Umari T, Dunnick CA, Dellavalle RP. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13–25. doi: 10.2147/AHMT.S55832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Degitz K, Ochsendorf F. Pharmacotherapy of acne. Expert Opin Pharmacother. 2008;9(6):955–971. doi: 10.1517/14656566.9.6.955 [DOI] [PubMed] [Google Scholar]
- 3.Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474–485. doi: 10.1111/bjd.2013.168.issue-3 [DOI] [PubMed] [Google Scholar]
- 4.Gibbs S. Skin disease and socioeconomic conditions in rural africa: tanzania. Int J Dermatol. 1996;35:633–639. doi: 10.1111/ijd.1996.35.issue-9 [DOI] [PubMed] [Google Scholar]
- 5.Law MP, Chuh AA, Lee A, Molinari N. Acne prevalence and beyond: acne disability and its predictive factors among Chinese late adolescents in Hong Kong. Clin Exp Dermatol. 2010;35(1):16–21. doi: 10.1111/j.1365-2230.2009.03340.x [DOI] [PubMed] [Google Scholar]
- 6.Li D, Chen Q, Liu Y, Tang W, Li S. The prevalence of acne in Mainland China: a systematic review and meta-analysis. Br Med J Open. 2017;7:e015354. doi: 10.1136/bmjopen-2016-015354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veltri KT. Acne pharmacotherapy: a review. US Pharm. 2013;38(5):43–46. [Google Scholar]
- 8.Dréno B. Treatment of adult female acne: a new challenge. J Eur Acad Dermatol Venereol. 2015;29(Suppl 5):14–19. doi: 10.1111/jdv.2015.29.issue-S5 [DOI] [PubMed] [Google Scholar]
- 9.Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56–59. doi: 10.1016/j.jaad.2007.06.045 [DOI] [PubMed] [Google Scholar]
- 10.Karciauskiene J, Valiukeviciene S, Gollnick H, et al. The prevalence and risk factors of adolescent acne among schoolchildren in Lithuania: a cross‐sectional study. J Eur Acad Dermatol Venereol. 2014;28:733–740. doi: 10.1111/jdv.2014.28.issue-6 [DOI] [PubMed] [Google Scholar]
- 11.Sharma RK, Dogra S, Singh A, Kanwar AJ. Epidemiological patterns of acne vulgaris among adolescents in North India: a cross-sectional study and brief review of literature. Indian J Peadiatr Dermatol. 2017;18(3):196–201. doi: 10.4103/ijpd.IJPD_82_16 [DOI] [Google Scholar]
- 12.Williams HC, Dellavalla RP, Garner S. Acne vulgaris. Lancet. 2012;379:361–372. doi: 10.1016/S0140-6736(11)60321-8 [DOI] [PubMed] [Google Scholar]
- 13.Degitz K, Ochsendorf F. Acne. J Dtsch Dermatol Ges. 2017;15(7):709–722. [DOI] [PubMed] [Google Scholar]
- 14.Gollnick HP. From new findings in acne pathogenesis to new approaches in treatment. J Eur Acad Dermatol Venereol. 2015;29(Suppl 5):1–7. [DOI] [PubMed] [Google Scholar]
- 15.Moradi Tuchayi S, Makrantonaki E, Ganceviciene R, Dessinioti C, Feldman SR, Zouboulis CC. Acne vulgaris. Nat Rev Dis Primers. 2015;1:15029. doi: 10.1038/nrdp.2015.29 [DOI] [PubMed] [Google Scholar]
- 16.Dréno B. What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol. 2017;31(5):8–12. doi: 10.1111/jdv.2017.31.issue-S5 [DOI] [PubMed] [Google Scholar]
- 17.Li X, He C, Chen Z, Zhou C, Gan Y, Jia Y. A review of the role of sebum in the mechanism of acne pathogenesis. J Cosmet Dermatol. 2017;16:168–173. doi: 10.1111/jocd.12345 [DOI] [PubMed] [Google Scholar]
- 18.Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36(6):416–418. doi: 10.1046/j.1365-4362.1997.00099.x [DOI] [PubMed] [Google Scholar]
- 19.El-Hamd MA, Nada EEA, Moustafa MA, Mahboob-Allah RA. Prevalence of acne vulgaris and its impact of the quality of life among secondary school-aged adolescents in Sohag Province, Upper Egypt. J Cosmet Dermatol. 2017;16(3):370–373. doi: 10.1111/jocd.12328 [DOI] [PubMed] [Google Scholar]
- 20.El-Akawi Z, Abdel-Latif N, Abdul-Razzak K, Al-Aboosi M. The relationship between blood lipids profile and acne. J Health Sci. 2007;53(5):596–599. doi: 10.1248/jhs.53.596 [DOI] [Google Scholar]
- 21.Jiang H, Li C, Zhou L, et al. Acne patients frequently associated with abnormal plasma lipid profile. J Dermatol. 2015;42:296–299. doi: 10.1111/jde.2015.42.issue-3 [DOI] [PubMed] [Google Scholar]
- 22.Bassi R, Sharma S, Kaur M, Sharma A. A study of changes in lipid profile in obese and non-obese females with acne vulgaris. Natl J Physiol Pharm Pharmacol. 2014;4:125–127. doi: 10.5455/njppp. [DOI] [Google Scholar]
- 23.Cunha MG, Batista AL, Macedo MS, Machado Filho CD, Fonseca FL. Study of lipid profile in adult women with acne. Clin Cosmet Investig Dermatol. 2015;8:449–454. doi: 10.2147/CCID.S83248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekiz O, Balta I, Unlu E, Bulbul Sen B, Rifaioğlu EN, Dogramaci AC. Assessment of thyroid function and lipid profile in patients with postadolescent acne in a mediterranean population from Turkey. Int J Dermatol. 2015;54:1376–1381. doi: 10.1111/ijd.12547 [DOI] [PubMed] [Google Scholar]
- 25.Nasution K, Putra I, Jusuf N. No association between lipid profiles and acne vulgaris. Mol Cell Biomed Sci. 2018;2(2):70–72. doi: 10.21705/mcbs.v2i2.33 [DOI] [Google Scholar]