Abstract
Purpose
Neutrophils and platelets have been described as tumor-promoting factors, but lymphocytes have been described as tumor-inhibiting factors. The prognostic values of the neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) have been explored in human epidermal growth factor receptor (HER2)-positive breast cancer, however, the value of the systemic immune- inflammation index (SII) has not been studied in this molecular subtype. Our study aimed to compare the prognostic values of these inflammation-based indexes in Chinese HER2-positive breast cancer patients who received adjuvant trastuzumab.
Methods
A total of 147 HER2-positive breast cancer patients were retrospectively analyzed. The association between clinicopathological factors and inflammation-based indexes was investigated. The Kaplan-Meier method was used to evaluate overall survival (OS) and disease-free survival (DFS); the Log rank test was performed to comparatively evaluate the survivals between the high-value and low-value groups. Multivariate Cox regression analysis was used to identify independent prognostic factors.
Results
The SII value correlated significantly with histological grade (HG)(p=0.016). The cut-off values determined by ROC analysis for the NLR, PLR and SII were 1.69, 110 and 442, and the corresponding areas under the curves (AUCs) were 0.621, 0.639 and 0.674, respectively. The 5-year DFS was significantly lower in the NLR-high than in the NLR-low group (75.8% vs. 90.7%, p<0.01), in the PLR-high than in the PLR-low group (76.7% vs. 90.6%, p<0.01) and in the SII-high than in the SII-low group (66.8% vs. 90.7%, p<0.01). The 5-year OS was significantly lower in the PLR-high than in the PLR-low group (83.2% vs. 100%, p=0.035) and in the SII-high than in the SII-low group (77.3% vs. 96.4%, p=0.012). A multivariate regression model revealed that tumor size, lymph node involvement, HG, hormone receptor status, PLR and SII were independently correlated with DFS; lymph node involvement and SII were independently correlated with OS.
Conclusion
Our study suggests that SII is an independent prognostic factor for DFS and OS in HER2-positive breast cancer, and in terms of prognostic reliability, the SII is superior to other inflammation-based indexes.
Keywords: breast cancer, HER2, NLR, PLR, SII, prognosis
Introduction
Breast cancer has become the leading malignancy among women worldwide.1 According to the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2), breast cancer can be classified into several molecular subtypes: luminal like, HER2-positive and triple-negative breast cancer (TNBC). It has been reported that HER2 protein over-expression is present in approximately 20–25%of breast cancers and is generally linked to poor outcomes. Evidence from four randomized control trials, including the Herceptin Adjuvant (HERA), North Central Cancer Treatment Group (NCCTG) N9831, National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 and Breast Cancer International Research Group (BCIRG) 006 trials showed a significant improvement in disease-free survival (DFS) when trastuzumab was added to conventional chemotherapy.2–5 The updated data from the BCIRG006 trial demonstrated that there was a recurrence rate of 25% for HER2-positive breast cancer within 10 years after its initial diagnosis.
Several inflammation-based indexes, such as the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), have been evaluated as prognostic indicators in various malignant tumors.6–11 In breast cancer patients, preoperatively high value of NLR or PLR predicted a poor prognosis.12–14 In addition, several investigators evaluated the prognostic values of NLR and PLR in HER2-positive breast cancer, however, the conclusions were inconsistent.15–20 Moreover, these conclusions were drawn based on subgroup analysis. A meta-analysis of the HER2-positive subgroup including the above studies showed that neither NLR nor PLR had prognostic value for overall survival (OS), however, NLR had a prognostic value for DFS, while PLR did not.21
The systemic immune-inflammation index (SII), which is a parameter integrates neutrophils, platelets and lymphocytes, has recently been assessed in various solid cancers: colorectal cancer, nasopharyngeal cancer, hepatocellular cancer, pancreatic cancer, gastric cancer and prostate cancer.22–29 A recent study evaluated the prognostic significance of SII in hormone receptor (HR)-negative, HER2-positive breast cancer, and showed that an increased SII independently predicted poor survival for HER2-positive breast cancer patients.30 In this study, the median DFS rates of 15.1 and 31.5 months in high and low SII patients, respectively, indicated that the included patients were at an extremely high risk, as in the BCIRG006 trial, the 10-year DFS rate was as high as 75% in the general population. Hence, the prognostic value of the SII in common HER2-positive breast cancer patients treated with adjuvant trastuzumab should be further explored. Meanwhile, the optimal inflammation-based index for predicting the outcome of HER2- positive breast cancer has not been established. Therefore, our study aimed to investigate and compare the prognostic values of the inflammation-based indexes NLR, PLR and SII in HER2-positive breast cancer patients who were treated with adjuvant trastuzumab and chemotherapy.
Materials and Methods
Patient Selection
After approval from the Institutional Ethics Committee of Ningbo Medical Center Lihuili Hospital, we retrospectively analyzed the data of HER2-positive breast cancer patients seen in our hospital between April 2011 and September 2015. All patients were pathologically diagnosed and a radical operation (breast-conserving surgery or mastectomy, and sentinel lymph node biopsy or axillary lymph node dissection) was performed to every patient. Patients who met any of the following criteria were excluded from this study: (1) patients with metastatic breast carcinoma; (2) patients with non-invasive breast carcinoma including ductal carcinoma in situ (DCIS), lobular carcinoma in situ (LCIS) and Paget’s disease of the breast; (3) patients with inflammatory breast carcinoma (IBC); (4) patients with hematological, immune, infectious or inflammatory diseases; (5) patients who did not undergo trastuzumab therapy; (6) patients with incomplete data of hematological indexes or follow-up data. Written informed consent was obtained from every patient.
Data Collection
Data for age, histological pathology, tumor size, lymph node metastasis, tumor stage, histological grade (HG), ER, PR, HER2 and Ki-67label index were collected from the medical records of every patient. Differential blood counts were collected within the 3 days preceding surgery, and the counts of platelets, neutrophils, and lymphocytes were extracted from blood counts, and used to calculate the corresponding values of NLR (the ratio of neutrophil/lymphocyte), PLR (the ratio of platelet/lymphocyte), and SII (neutrophil x platelet/lymphocyte).
Positivity of HER2 was defined as a 3+ score with immunohistochemistry (IHC) or a 1+ and 2+ score in IHC but a state of amplification with fluorescence in situ hybridization (FISH). Adjuvant trastuzumab therapy was given to every patient and the overall duration was twelve months. Adjuvant chemotherapy, radiotherapy and endocrine therapy were administered in appropriate patients according to current available clinical practice guidelines.
Follow-Up
Regular follow-ups were scheduled in 3-month intervals within the first two years after surgery; biannually until the end of the fifth year and annually thereafter. Follow-up included assessing disease progression, and confirming patient’s death, or loss to follow-up. The status of the disease was evaluated using ultrasound imaging (US), mammography (MM), computed tomography (CT) and magnetic resonance imaging (MRI). If clinically feasible, any suspicious disease was biopsied to confirm or exclude the disease of breast cancer. In our study, we set January 2019 as the deadline for follow-up. DFS was calculated as the time from diagnosis to the time of the first disease recurrence. OS was calculated from the time of the diagnosis to the time of death for any reason. Once the recurrence or metastasis was confirmed, anti-HER2 therapy concurrently with chemotherapy was performed according to current available clinical practice guidelines.
Statistical Analysis
Analyses were performed using SPSS v. 20.0 Software (SPSS, Chicago, IL, http://www.spss.com). Chi-square or Fisher’s exact tests were employed for categorical variables. Multivariate logistic regression analysis, including all variables from the univariate analysis that were associated with survival or the widely accepted variables, was performed to test for factors’ independence. The Kaplan-Meier method was used to evaluate the DFS and OS, and the Log rank test was used to evaluate the survival differences between patients divided into two groups according to the optimal cut-off points of NLR, PLR or SII, which were obtained from the area under the curve (AUC). A p value of <0.05 was considered to have statistical significance and statistical tests were two-sided. The hazard ratio (HR)and 95% confidence intervals (CIs) were also calculated.
Results
Patient Characteristics
Between April 2011 and September 2015, a total of 147 Chinese patients with HER2-positive breast cancer were included in this study. The baseline characteristics of the patients are shown in Table 1.
Table 1.
Clinicopathologic Characteristics of 147 HER2-Positive Breast Cancer Patients
| Variables | Number | % |
|---|---|---|
| Age (years) | ||
| ≤ 35 | 7 | 4.8 |
| > 35 | 140 | 95.2 |
| Tumor stage | ||
| T1 | 41 | 27.9 |
| T2 | 90 | 61.2 |
| T3 | 13 | 8.9 |
| T4 | 3 | 2.0 |
| Tumor differentiation | ||
| G1 | 3 | 2.0 |
| G2 | 76 | 51.7 |
| G3 | 68 | 46.3 |
| Lymph node involvement | ||
| N0 | 68 | 46.3 |
| N1 | 49 | 33.3 |
| N2 | 15 | 10.2 |
| N3 | 15 | 10.2 |
| AJCC stage | ||
| I | 33 | 22.4 |
| II | 84 | 57.2 |
| III | 30 | 20.4 |
| HR status | ||
| Positive | 62 | 42.2 |
| Negative | 85 | 57.8 |
| Ki67 | ||
| Low (≤30%) | 63 | 42.9 |
| High (>30%) | 84 | 57.1 |
| Surgery | ||
| Breast conserving surgery | 18 | 12.2 |
| Mastectomy | 129 | 87.8 |
| Radiotherapy | ||
| Yes | 87 | 59.2 |
| No | 60 | 40.8 |
| Endocrine therapy | ||
| Yes | 61 | 41.5 |
| No | 86 | 58.5 |
| NLR | ||
| ≤1.69 | 60 | 40.8 |
| >1.69 | 87 | 59.2 |
| PLR | ||
| ≤110 | 49 | 33.3 |
| >110 | 98 | 66.7 |
| SII | ||
| ≤442 | 87 | 59.2 |
| >442 | 60 | 40.8 |
Abbreviations: HER2, human epidermal growth factor receptor 2; T, tumor; N, lymph-node; AJCC, American Joint Committee on Cancer; HR, hormone receptor; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
The optimal cut-off values that were determined by the ROC for the NLR, PLR and SII are summarized in Table 2. The corresponding AUCs for the NLR, PLR and SII were 0.621, 0.639 and 0.674, respectively. The SII had a higher AUC than the NLR and PLR, however, the difference was not significant. According to these cut-off points, the patients were then separated into two groups (low-value group vs. high-value group) in each category, and 87 (59.2%), 98 (66.7%) and 60 (40.8%) of patients had a high NLR, PLR and SII values, respectively.
Table 2.
Receiver Operating Characteristics Analyses of Inflammation-Base Parameters in Patients with HER2-Positive Breast Cancer
| Variables | Cut-off Value | AUC (95% CI) | Sensitivity | Specificity |
|---|---|---|---|---|
| NLR | 1.69 | 0.621 (0.515–0.727) | 0.793 | 0.458 |
| PLR | 110 | 0.639 (0.539–0.740) | 0.897 | 0.390 |
| SII | 442 | 0.674 (0.582–0.767) | 0.759 | 0.678 |
Abbreviations: HER2, human epidermal growth factor receptor 2; AUC, area under curve; CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
The relationship between clinical characteristics and each inflammation-based index is shown in Table 3. The SII correlated significantly with HG (p<0.05).
Table 3.
Associations Between Inflammation-Based Parameters and Clinicopathological Factors
| Variables | NLR | PLR | SII | ||||||
|---|---|---|---|---|---|---|---|---|---|
| H | L | P | H | L | P | H | L | P | |
| Age (years) | |||||||||
| ≤ 35 | 4 | 3 | 4 | 3 | 2 | 5 | |||
| > 35 | 83 | 57 | 0.910 | 94 | 46 | 0.584 | 58 | 82 | 0.499 |
| Tumor stage | |||||||||
| pT1 | 23 | 18 | 25 | 16 | 14 | 27 | |||
| pT2-4 | 64 | 42 | 0.636 | 73 | 33 | 0.363 | 46 | 60 | 0.306 |
| HG | |||||||||
| G1-2 | 41 | 28 | 42 | 27 | 21 | 48 | |||
| G3 | 46 | 32 | 0.956 | 56 | 22 | 0.161 | 39 | 39 | 0.016 |
| LN | |||||||||
| (-) | 39 | 27 | 47 | 19 | 24 | 42 | |||
| (+) | 48 | 33 | 0.984 | 51 | 30 | 0.291 | 36 | 45 | 0.321 |
| HR status | |||||||||
| Positive | 38 | 24 | 42 | 20 | 27 | 35 | |||
| Negative | 49 | 36 | 0.657 | 56 | 29 | 0.813 | 33 | 52 | 0.565 |
| Ki67 | |||||||||
| Low (≤30%) | 34 | 29 | 40 | 23 | 23 | 40 | |||
| High (>30%) | 53 | 31 | 0.265 | 58 | 26 | 0.480 | 37 | 47 | 0.357 |
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune- inflammation index; H, high; L, low; T, tumor; HG, histological grade; LN, lymph-node; HR, hormone receptor.
Survival
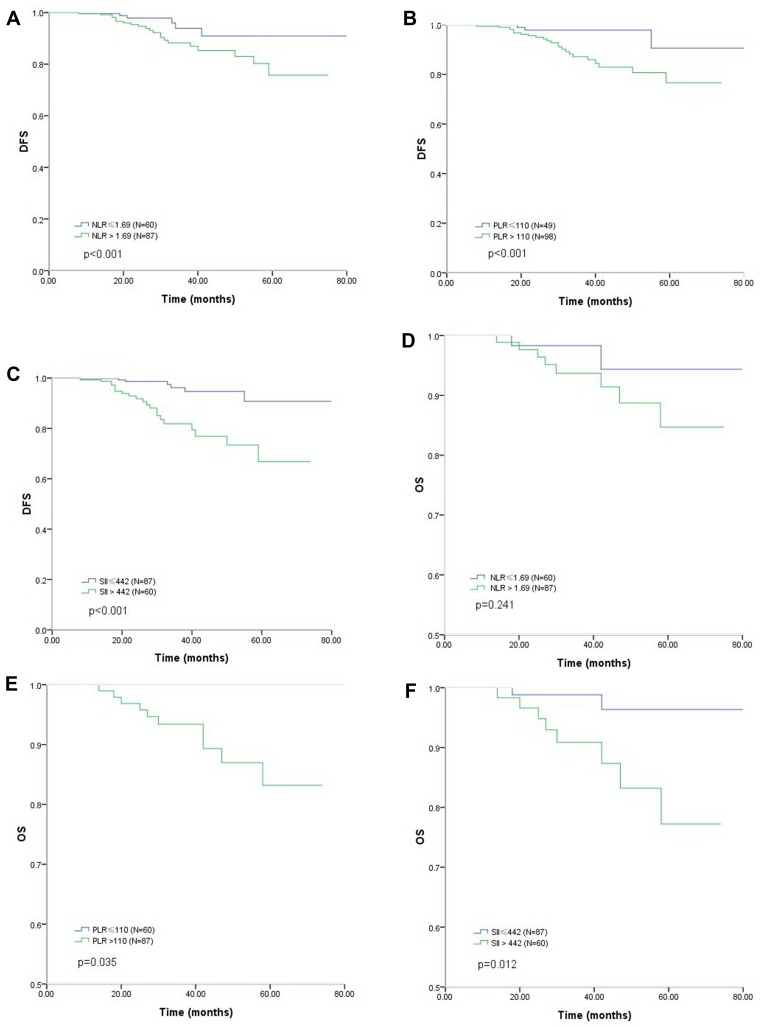
The median duration of follow-up was 42 months (range 15 to 78). During follow-up, 29 patients (19.7%) experienced recurrence, and 10 patients (6.8%) died from breast cancer, no patient died from other reasons. The Kaplan-Meier curves of DFS and OS according to the NLR, PLR and SII values are shown in Figure 1A–F. The DFS rate was significantly lower in the NLR>1.69 group than in the NLR≤1.69 group (estimated 5-year DFS: 75.8% vs. 90.7%, respectively; p <0.01), in the PLR >110 group than in the PLR≤110 group (estimated 5-year DFS: 76.7% vs. 90.6%, respectively; p <0.01) and in the SII>442 group than in the SII≤442 group (estimated 5-year DFS: 66.8% vs. 90.7%, respectively; p <0.01). The OS rate was significantly lower in the PLR >110 group than in the PLR≤110 group (estimated 5-year OS: 83.2% vs. 100%, respectively; p=0.035) as well as in the SII >442 group than in the SII≤442 group (estimated 5-year OS: 77.3% vs. 96.4%, respectively; p=0.012).
Figure 1.
Kaplan-Meier analysis of DFS and OS in patients with HER2- positive breast cancer. (A) DFS as derived by the NLR; (B) DFS as derived by the PLR; (C) DFS as derived by the SII; (D) OS as derived by the NLR; (E) OS as derived by the PLR; (F) OS as derived by the SII.
Abbreviations: DFS, disease free survival; OS, overall survival; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
Univariate analysis revealed a significant impact of tumor size, lymph node involvement, HG, hormone receptor (HR) status, Ki-67 index, NLR, PLR and SII on DFS. In the multivariate analysis, tumor size, lymph node involvement, HG, HR status, PLR and SII were independently correlated with poor DFS (Table 4).
Table 4.
Associations Between Clinicopathological Parameters and DFS in HER2-Positive Breast Cancer
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years) | ||||
| ≤ 35 | 1 | / | ||
| > 35 | 1.50 (0.17–12.97) | 0.711 | / | / |
| Tumor stage | ||||
| pT1 | 1 | 1 | ||
| pT2-4 | 6.67 (1.51–29.41) | 0.005 | 6.00 (1.57–22.82) | 0.009 |
| Tumor differentiation | ||||
| G1-2 | 1 | 1 | ||
| G3 | 3.24 (1.36–7.69) | 0.006 | 3.60 (1.33–9.73) | 0.012 |
| Lymph node involvement | ||||
| pN0 | 1 | 1 | ||
| pN1-3 | 3.14 (1.25–7.94) | 0.012 | 6.76 (2.56–17.84) | <0.001 |
| HR status | ||||
| Positive | 1 | 1 | ||
| Negative | 2.22 (0.91–5.41) | 0.076 | 3.74 (1.46–9.58) | 0.006 |
| Ki67 | ||||
| Low (≤30%) | 1 | 1 | ||
| High (>30%) | 2.30 (0.97–5.46) | 0.055 | 1.65 (0.65–4.20) | 0.295 |
| Surgery | ||||
| Mastectomy | 1 | / | ||
| Breast-conserving surgery | 1.19 (0.36–3.92) | 0.777 | / | / |
| Radiotherapy | ||||
| Yes | 1 | / | ||
| No | 1.16 (0.50–2.68) | 0.724 | / | / |
| Endocrine therapy | ||||
| Yes | 1 | / | ||
| No | 1.75 (0.74–4.17) | 0.202 | / | / |
| NLR | ||||
| ≤1.69 | 1 | 1 | ||
| >1.69 | 3.24 (1.23–8.55) | 0.014 | 0.84 (0.22–3.18) | 0.798 |
| PLR | ||||
| ≤110 | 1 | 1 | ||
| >110 | 5.52 (1.58–19.23) | 0.003 | 7.25 (1.98–26.54) | 0.003 |
| SII | ||||
| ≤442 | 1 | 1 | ||
| >442 | 6.62 (2.60–16.95) | <0.001 | 3.78 (1.10–13.08) | 0.035 |
Abbreviations: DFS, disease-free survival; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; CI, confidence interval; T, tumor; G, grade; N lymph-node; HR, hormone receptor; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
The univariate analysis showed that only SII had a significant influence on OS. In the multivariate analysis, lymph node involvement and SII were independently correlated with OS (Table 5).
Table 5.
Associations Between Clinicopathologic Parameters and OS in HER2-Positive Breast Cancer
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years) | ||||
| ≤ 35 | 1 | / | ||
| > 35 | 0.42 (0.05–3.80) | 0.420 | / | / |
| Tumor stage | ||||
| pT1 | 1 | 1 | ||
| pT2-4 | 3.72 (0.46–30.30) | 0.191 | 1.93 (0.21–17.55) | 0.559 |
| Tumor differentiation | ||||
| G1-2 | 1 | / | ||
| G3 | 1.81 (0.49–6.71) | 0.367 | / | / |
| Lymph node involvement | ||||
| pN0 | 1 | 1 | ||
| pN1-3 | 3.72 (0.76–18.18) | 0.085 | 6.39 (1.28–31.90) | 0.038 |
| HR status | ||||
| Positive | 1 | / | ||
| Negative | 1.04 (0.28–3.86) | 0.950 | / | / |
| Ki67 | ||||
| Low (≤30%) | 1 | / | ||
| High (>30%) | 0.73 (0.20–2.65) | 0.636 | / | / |
| Surgery | ||||
| Mastectomy | 1 | / | ||
| Breast-conserving surgery | 0.78 (0.09–6.58) | 0.823 | / | / |
| Radiotherapy | ||||
| Yes | 1 | / | ||
| No | 0.60 (0.15–2.43) | 0.471 | / | / |
| Endocrine therapy | ||||
| Yes | 1 | / | ||
| No | 2.01 (0.50–8.06) | 0.318 | / | / |
| NLR | ||||
| ≤1.69 | 1 | 1 | ||
| >1.69 | 2.93 (0.60–14.29) | 0.165 | 3.10 (0.35–27.46) | 0.256 |
| PLR | ||||
| ≤110 | 1 | 1 | ||
| >110 | 4.84 (0.60–40.0) | 0.105 | 2.91 (0.25–33.28) | 0.390 |
| SII | ||||
| ≤442 | 1 | 1 | ||
| >442 | 6.54 (1.34–32.26) | 0.009 | 2.32 (1.25–4.31) | 0.01 |
Abbreviations: OS, overall survival; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; CI, confidence interval; T, tumor; G, grade; N lymph-node; HR, hormone receptor; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
Discussion
The NLR and PLR have been widely used to predict the prognosis in various cancers,6–11 and the SII has recently been determined to be an independent prognostic factor in many kinds of cancers.22–29 These inflammation-based indexes, however, have rarely been investigated simultaneously in HER2-positive breast cancer. To the best of our knowledge, the current study is the first to comprehensively investigate and compare the prognostic values of these inflammation-based indexes in Chinese population with HER2-positive breast cancer who received scheduled adjuvant trastuzumab. In our study, the AUCs derived from the NLR, PLR and SII were 0.621, 0.639 and 0.674, respectively. There was not a significant difference among these AUCs, however, a superior trend could be seen.
The prognostic significance of the NLR and PLR in HER2-positive breast cancer has been explored previously, however, the conclusions have been inconsistent.15–20 Moreover, the conclusions were based on subgroup analysis. A meta-analysis including 6 previous studies showed that neither the NLR nor the PLR had a prognostic value for OS, however, the NLR had a prognostic value for DFS, while PLR did not. In the current study, there was a significantly lower DFS rate in the high-NLR and hign-PLR groups than in the low-NLR and low-PLR groupsgroup, and a significantly lower OS rate in the high-PLR and the high-SII groups than in the low-PLR and the low-SII groups, while there was no significant difference between the high-NLR and the low-NLR group. The difference between our findings and those of the meta-analysis can be partly explained by the difference in patient race and the cut-off points set. In our study, the cut-off point was determined by the AUC; however, most previous studies used the median value as cut-off points. In addition, the cut-off points of every category (including the NLR, PLR and SII) in our study were lower than those in other studies.15–20 However, the prognostic values of the NLR and PLR are still uncertain, and the intrinsic associations should be further explored in large, sample size, prospective studies.
Our study found that there was a lower DFS and OS rate in the high-SII group than in the low-SII group, which was consistent with a previous study.30 However, there were several differences between these two studies. First, our study included HER2-positive breast cancer patients including HR-positive and HR-negative breast cancer, while the other study only included HR-negative breast cancer. Second, the survival in that study was much worse than that in our study, and this can be explained by the high lymph node involvement and TNM stage. Third, all the patients included in our study received a standard duration of trastuzumab therapy, which is essential in HER2-positive breast cancer, while in the other study as much as 47.7% of patients did not receive trastuzumab therapy. Overall, our study reflects the real world state of HER2-positive breast cancer patients, and the conclusions based on our study should be more reliable.
Our study also demonstrated that established prognostic factors such as tumor size, lymph node involvement and ER stats have no association with NLR, PLR and SII. Histological grade was associated with SII, not with NLR and PLR. The lacking association with established prognostic factors made our findings more valuable.
The prognostic value of the SII in solid malignancies may be explained by the function of its component factors: platelets, neutrophils, and lymphocytes. Platelets promote angiogenesis and metastases, in addition, they shield tumor cells from the anti-tumor immune response.31 Neutrophils play an important role in the proliferation and metastasis of tumors by releasing inflammatory mediators.32 In contrast, lymphocytes infiltrate tumors and their effects can prevent tumor growth and metastasis.33,34 Essentially, whether tumor development or progression mainly depends on the balance between tumor-promoting factors such as platelets and neutrophils and tumor-inhibiting factors such as lymphocytes. The more factors that are considered for the prognosis, and the higher the accuracy that can be achieved. As an integrated parameter, the SII includes three relative factors, while the NLR and the PLR only include two factors, this difference can partially explain the prognostic value of the SII, but not the NLR or the PLR identified in our study.
Despite the novelty and potential of this study, several limitations also should be acknowledged. First, it was a single-center study without a validation group. Second, its retrospective nature and small sample-size may lead to bias in data selection and analysis. Third, the study involved a relatively short follow-up period, therefore, the distant prognostic value could not be investigated in the current study. Nevertheless, our data indicated that an increased preoperative SII value may represent an independent prognostic factor in patients with HER2-positive breast cancer.
Conclusion
Our study is the first to comprehensively compare the prognostic value of peripheral inflammation-based indexes in HER2-positive breast cancer. Our findings suggest that the preoperative SII value correlates significantly with DFS and OS in HER2-positive breast cancer, and the SII value is not significantly superior to the NLR and PLR in terms of prognostic reliability. However, large and multicenter clinical trials are required to validate the findings in our study.
Acknowledgments
We would like to thank all subjects included in this study. This study was funded by Ningbo Science and Technology Foundation (2018A610325).
Disclosure
The authors declare no competing financial and non-financial interests in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2- positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306 [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122 [DOI] [PubMed] [Google Scholar]
- 4.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366–3373. doi: 10.1200/JCO.2011.35.0868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. doi: 10.1056/NEJMoa0910383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137(2):425–428. doi: 10.1016/j.jtcvs.2008.05.046 [DOI] [PubMed] [Google Scholar]
- 7.Urrejola GI, Bambs CE, Espinoza MA, et al. An elevated neutrophil/lymphocyte ratio is associated with poor prognosis in stage II resected colon cancer. Rev Med Chil. 2013;141(5):602–608. doi: 10.4067/S0034-98872013000500008 [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. doi: 10.1186/1471-2407-13-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai Q, Castro Santa E, Rico Juri JM, Pinheiro RS, Lerut J. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int. 2014;27(1):32–41. doi: 10.1111/tri.12191 [DOI] [PubMed] [Google Scholar]
- 10.Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109(2):416–421. doi: 10.1038/bjc.2013.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factoring patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:58. doi: 10.1186/1477-7819-12-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19(1):217–224. doi: 10.1245/s10434-011-1814-0 [DOI] [PubMed] [Google Scholar]
- 13.Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer. 2013;16(1):55–59. doi: 10.4048/jbc.2013.16.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azab B, Shah N, Radbel J, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. 2013;30(1):432. doi: 10.1007/s12032-012-0432-4 [DOI] [PubMed] [Google Scholar]
- 15.Ulas A, Avci N, Kos T, et al. Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio associated with prognosis in patients with HER2-positive early breast cancer receiving adjuvant trastuzumab? JBUON. 2015;20(3):714–722. [PubMed] [Google Scholar]
- 16.Liu C, Huang Z, Wang Q, et al. Usefulness of neutrophil-to-lymphocyte ratio and platelet-to- lymphocyte ratio in hormone-receptor-negative breast cancer. Onco Targets Ther. 2016;9:4653–4660. doi: 10.2147/OTT.S106017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao M, Liu Y, Jin H, et al. Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. Onco Targets Ther. 2014;7:1743–1752. doi: 10.2147/OTT.S69657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to- lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110:2524–2530. doi: 10.1038/bjc.2014.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho U, Park HS, Im SY, et al. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS One. 2018;13(7):e0200936. doi: 10.1371/journal.pone.0200936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gündüz S, Göksu SS, Arslan D, et al. Factors affecting disease-free survival in patients with human epidermal growth factor receptor 2-positive breast cancer who receive adjuvant trastuzumab. Mol Clin Oncol. 2015;3(5):1109–1112. doi: 10.3892/mco.2015.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo W, Lu X, Liu Q, et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: an updated meta-analysis of 17079 individuals. Cancer Med. 2019;8(9):4135–4148. doi: 10.1002/cam4.2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 23.Aziz MH, Sideras K, Aziz NA, et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels. Ann Surg. 2018:1. doi: 10.1097/SLA.0000000000002660 [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Yan Y, Zhu L, et al. Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manag Res. 2017;9:849–867. doi: 10.2147/CMAR.S151026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Guo X, Wang M, et al. Pre-treatment inflammatory indexes as predictors of survival and cetuximab efficacy in metastatic colorectal cancer patients with wild-type RAS. Sci Rep. 2017;7(1):17166. doi: 10.1038/s41598-017-17130-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan L, Wang R, Chi C, et al. Systemic immune-inflammation index predicts the combined clinical outcome after sequential therapy with abiraterone and docetaxel for metastatic castration-resistant prostate cancer patients. Prostate. 2018;78(4):250–256. doi: 10.1002/pros.23465 [DOI] [PubMed] [Google Scholar]
- 27.Lolli C, Basso U, Derosa L, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget. 2016;7(34):54564–54571. doi: 10.18632/oncotarget.10515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang W, Chen Y, Huang J, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with nasopharyngeal carcinoma: a propensity score-matched analysis. Oncotarget. 2017;8(39):66075–66086. doi: 10.18632/oncotarget.19796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong YS, Tan J, Zhou XL, Song YQ, Song YJ. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med. 2017;15(1):221. doi: 10.1186/s12967-017-1326-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, Li W, Li AJ, et al. Increased systemic immune-inflammation index independently predicts poor survival for hormone receptor-negative, HER2-positive breast cancer patients. Cancer Manag Res. 2019;11:3153–3162. doi: 10.2147/CMAR.S190335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi: 10.1182/blood-2014-08-531582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houghton AM, Rzymkiewicz DM, Ji H, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16(2):219–223. doi: 10.1038/nm.2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammed ZM, Going JJ, Edwards J, et al. The role of the tumour inflammatory cell infiltrate in predicting recurrence and survival in patients with primary operable breast cancer. Cancer Treat Rev. 2012;38(8):943–955. doi: 10.1016/j.ctrv.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 34.Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25(8):1536–1543. doi: 10.1093/annonc/mdu191 [DOI] [PubMed] [Google Scholar]