Abstract
Objective
The cost-effectiveness of exercise interventions in lung cancer survivors is unknown. We performed a model-based cost-effectiveness analysis of an exercise intervention in lung cancer survivors.
Design
We used Markov modeling to simulate the impact of the Lifestyle Interventions and Independence for Elders (LIFE) exercise intervention compared to usual care for stage I-IIIA lung cancer survivors following curative-intent treatment. We calculated and considered incremental cost-effectiveness ratios (ICERs) <$100,000/quality-adjusted life-year (QALY) as cost-effective and assessed model uncertainty using sensitivity analyses.
Results
The base-case model showed that the LIFE exercise program would increase overall cost by $4,740 and effectiveness by 0.06 QALYs compared to usual care and have an ICER of $79,504/QALY. The model was most sensitive to the cost of the exercise program, probability of increasing exercise, and utility benefit related to exercise. At a willingness-to-pay threshold of $100,000/QALY, LIFE had a 71% probability of being cost-effective compared to 27% for usual care. When we included opportunity costs, LIFE had an ICER of $179,774/QALY, exceeding the cost-effectiveness threshold.
Conclusions
A simulation of the LIFE exercise intervention in lung cancer survivors demonstrates cost-effectiveness from an organization but not societal perspective. A similar exercise program for lung cancer survivors may be cost-effective.
MESH: Cost-effectiveness analyses, exercise, lung neoplasms, survivorship
Introduction
Up to 50% of patients with non-small cell lung cancer (NSCLC) are diagnosed with stage I-IIIA disease,1 the treatment of which typical aims to cure through a combination of lung cancer resection surgery, definitive radiation, or concurrent chemoradiation. Following curative-intent treatment, lung cancer survivors are at risk for health impairments resulting from the effects and/or treatment of lung cancer and comorbidities. Perioperative pulmonary and cardiopulmonary complications (e.g. prolonged respiratory failure, bacterial pneumonia, atrial arrythmia) occur in 15%2 and 35%,3, 4 respectively of patients undergoing surgical resection and can lead to negative health consequences well beyond the perioperative period. Approximately 20% of patients experience prolonged (> 7 days) hospital stay following lung cancer resection surgery.5, 6 Additionally, patients typically lose 10–15% of lung function following lobectomy which can negatively affect symptom burden (e.g. dyspnea), exercise and functional capacity, and quality of life.7 Chemotherapy and radiotherapy, often part of the treatment for stage IB-IIIA NSCLC, can also lead to long-term health impairments, including cardiovascular disease and neurocognitive deficits.8 In time, partly due to the long-term effects of lung cancer treatment and comorbidities, patients experience disabling symptoms and a downward spiral of health. As such, clinical guidelines recommend health monitoring and counseling for wellness/health promotion for cancer including lung cancer survivorship care.10
Physical activity and exercise improve physical function and reduce symptom burden in cancer survivors.11 As such, the American College of Sports Medicine recommends exercise training as an adjunct cancer therapy12 and the American Cancer Society recommends cancer survivors to participate in physical activity/exercise regularly.13 However, the cost-effectiveness of exercise in cancer survivors, including lung cancer, is unknown. Model-based cost-effectiveness analyses (CEAs) of exercise interventions to improve lung cancer outcomes may stimulate future clinical trials to test the effectiveness and trial-based CEAs of exercise interventions in lung cancer survivors. In this study, we simulated an exercise program in lung cancer survivors following curative-intent treatment to evaluate its cost-effectiveness. We hypothesize that this exercise program will be cost-effective.
Methods
Study Overview
We developed a simulation model to investigate the cost-effectiveness of exercise interventions compared to usual care (UC) in stage I-IIIA lung cancer survivors following curative-intent treatment. We chose the interventions described in the Lifestyle Interventions and Independence for Elders (LIFE) study,14 partly due to a higher median age (70 years15) at diagnosis for lung cancer patients compared to other cancers and the availability of a previous CEA.16 In LIFE, 1,635 participants aged 70–89 years were randomized to a structured moderate-intensity exercise program (done in eight different centers and at home) or a health education program; participants in the exercise program had an approximately 20% lower risk of major mobility disability compared to health education after a mean of 2.6 years of follow-up.14 We created three different intervention conditions: participation in the LIFE exercise program, health education, or UC (Figure 1). Our primary outcome was the incremental cost-effectiveness ratio (ICER), expressed in US dollars per quality-adjusted life-years ($/QALYs). Institutional review board approval was not obtained for this study as the research did not involve human subjects, or include any interaction or intervention with human subjects, or involve access to identifiable private information.
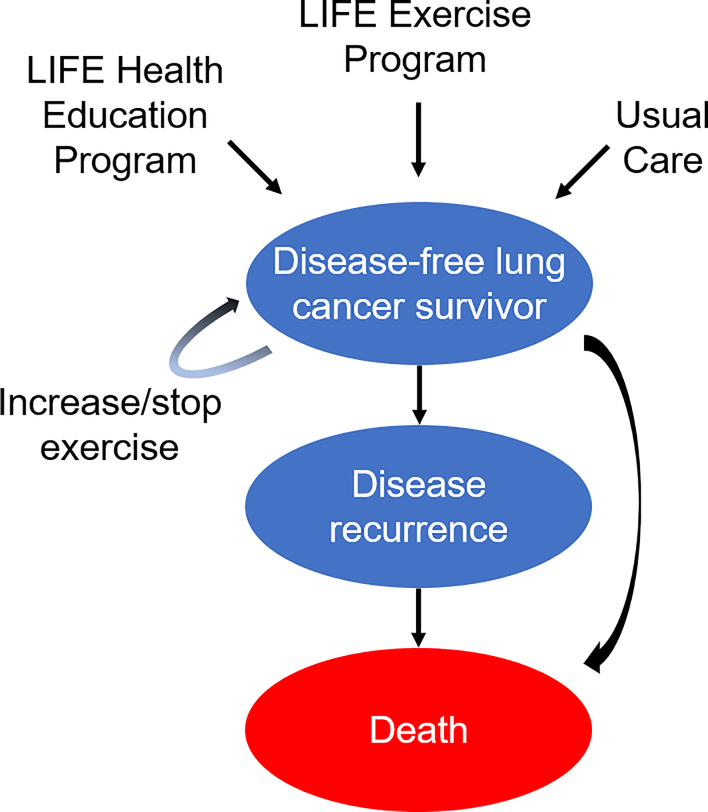
Figure 1:
State Transition Diagram
Caption: The health states are represented by ovals and include “disease-free, increase exercise,” “disease-free, no change in exercise,” “disease recurrence,” and “death.” Arrows represent possible transitions from one health state to another. Patients in the “disease-free” health state may experience a health utility benefit related to exercise if they increase their physical activity/exercise. Patients in the “disease recurrence” health state are assumed to not exercise. Patients who increase their exercise are subjected to a chance of stopping exercise after each year.
LIFE = Lifestyle Interventions and Independence for Elders study
LIFE exercise program
The LIFE study enrolled men and women aged 70 and older who could complete a walk test that covered 400 m within 15 min without sitting or using an assistive device.17 Patients with a history of New York Heart Association Class III or IV congestive heart failure, lung disease requiring oral corticosteroids or supplemental oxygen, cancer treatment in the past 3 years, and movement and/or mental health disorders were excluded.17 The LIFE exercise program involved three phases – adoption, transition, and maintenance – progressing from primarily center-based in the adoption phase to predominately home-based physical activity and exercise in the maintenance phase. It had a goal of walking 150 min/week and included strength, flexibility, and balance training. Exercise was prescribed by an exercise physiologist using the FITT (frequency, intensity, time, and type of exercise) principal for exercise prescription.14 On average, exercise participants attended two 60-min center-based visits per week and home-based activity 3–4 times per week. Individualized exercise sessions were led by exercise physiologists and progressed towards a goal of 30 min of walking daily at moderate intensity, 10 min of primarily lower extremity strength training using ankle weights, 10 min of balance training, and large muscle group flexibility exercises. Intensity was assessed by Borg ratings of perceived exertion (0 to 20 scale), recommended at 13 (somewhat hard) for walking and 15–16 (hard) for strength training. Moderate physical activity was assessed using accelerometry step-count, defined as > 760 counts/min. The intervention lasted the duration of the study follow-up period (mean 2.6 years).
LIFE health education program
Participants in the LIFE education program14 received health education focused on successful aging. They attended weekly workshops during the first 26 weeks of the program and monthly sessions thereafter. Topics covered in the workshops included how to navigate the health care system, travel safely, and information on preventive services and screening recommendations. Participants also had one 5- to 10-minute session of instructor-led upper extremity stretching or flexibility exercises, but no subsequent exercise sessions thereafter.
Usual care
We incorporated a UC arm into our model to more closely mimic real-life scenarios, as neither the LIFE exercise or health education program is available in routine care.
Utilities
We used baseline health utilities as assessed by the EuroQoL-5 Dimensions (EQ-5D) questionnaire and reported in the Cancer Care Outcomes Research and Surveillance Consortium for lung cancer survivors who completed treatment > 30 days previously:18 0.83 for stage I patients, 0.78 for stage II, 0.76 for stage III, calculated to an average (0.79) for all stage I-IIIA patients based on estimates of stage distribution by the American College of Chest Physicians. For the disease recurrence health state, we assumed a health utility of 0.76 as these patients are clinically similar to patients with advanced/metastatic disease (Table 1).18
Table 1:
Parameters for Cost-Effectiveness Model
| Variable | Base Case | Citation | Range Tested | Distribution‡ |
|---|---|---|---|---|
| Age | 70 | SEER12 | 55 – 85 | Binomial |
| Five-year disease-free survival | 0.61 | Dziedzic21 | 0.25 – 0.8 | Beta |
| Five-year overall survival | 0.55 | SEER12 | 0.3 – 0.6 | Beta |
| Health utilities | Beta | |||
| Disease-free | 0.79 | Tramontano15 | 0.76 – 0.83 | |
| Disease-recurrence | 0.76 | Tramontano15 | 0.70 – 0.80 | |
| Benefit related to exercise* | 0.05 | Groessl13 | 0.01 – 0.10 | |
| Costs ($/patient/year) | Gamma | |||
| Exercise interventions** | ||||
| LIFE physical activity | 1,361 (1,855) | Groessl13 | 500 – 2,500 | |
| LIFE health education | 413 (1,210) | Groessl13 | 250 – 1,000 | |
| Usual care | 0 (203) | N/A† | N/A | |
| Treatment of recurrent disease | 95,363 | Bradley19 | 80,000–120,000 | |
| Probability of increasing exercise | Beta | |||
| Physical activity/exercise | 0.60 | Gordon16 | 0.40 – 0.80 | |
| Health education | 0.10 | N/A† | 0.10 – 0.20 | |
| Usual care | 0 | N/A† | 0 – 0.10 | |
| Probability of dropout/lost to follow-up* | 0.10 | Speck17 | 0.10 – 0.20 | Beta |
Parameter values at one year.
Opportunity costs entered in parentheses.
Parameter assumed.
Per standard recommendations for cost-effectiveness analysis models 40.
LIFE = Lifestyle Interventions and Independence for Elders study; SEER = Surveillance, Epidemiology, and End Results program
We assumed a health utility benefit related to exercise of 0.05 QALYs (as described in the LIFE study16) for patients in the disease-free and increase exercise health state. For patients in the LIFE exercise program, we conservatively estimated a probability of increasing exercise of 60% based on a pragmatic trial of breast cancer survivors19 and assumed probabilities of increasing exercise of 10% and 0% for those in the health education program and UC, respectively. We chose the pragmatic trial of breast cancer survivors due to previous evidence of effective exercise trials following treatment20 and the availability of a probability of increasing exercise.19 Patients who increased their exercise as the result of participating in the LIFE exercise or health education program were subjected to a yearly dropout rate of 10% based on a systematic review of controlled physical activity trials in cancer survivors.20
Costs
Following best practices for the conduct, methodological practices, and reporting of CEAs,21 we included costs from both organizational and societal perspectives. The organizational perspective included direct costs that would allow health care organizations to gauge approximately what it would cost to conduct similar programs in the future. The societal perspective incorporated all costs and additionally included costs related to patient-time and transportation to participating in exercise and unpaid lost productivity.21
For the organizational perspective, we used costs as previously reported from an organizational perspective for the LIFE interventions16 and assumed a cost of US$0 for UC. We used the treatment cost for advanced-stage NSCLC as the cost for recurrent disease which included costs for targeted therapy but not immunotherapy.22 For the societal perspective, we additionally included participant opportunity costs related to time spent in exercise or education as reported in LIFE (213 min/week and 173 min/week in the exercise and health education programs, respectively)14 and assumed 35 min/week of exercise for those in UC based on data from the National Health and Nutritional Examination Survey for participants aged > 70 years.23 We also included traveling costs for in-facility sessions using estimates of 40-min and 16-mile roundtrip for each session and a $2.42/gallon rate for gas cost and average light-duty vehicle efficiency of 23.9 miles/gallon. We measured the cost of time spent from lost work using the federal minimum wage ($7.25/hour as reported by the US Department of Labor) given that the median age at lung cancer diagnosis is 70 years,15 and therefore a substantial fraction of patients would be retired and not earning full wages. We adjusted all costs for inflation using the Consumer Price Index calculator to estimate costs in 2017 (Table 1).
Survival Probabilities
We obtained disease-free survival (DFS) data from the largest series to date24 involving 14,578 patients postresection for NSCLC and calculated a weighted 5-year DFS rate (61%) for patients aged 65–90 years, and 5-year overall survival (OS, 55%) from the Surveillance, Epidemiology, and End Results database15 for cases with segmentectomy or greater resection. We converted all 5-year to quarterly rates and calibrated them accordingly. We also incorporated the probability of dying of natural causes for a given age using data from the Social Security Actuarial Life Table.25
Model Structure
We used TreeAge® software to build a decision tree and capture outcomes including survival as described above, and Markov models to simulate health state progressions. We assumed that patients enter each intervention condition in equal probability and, thereafter, Markov models with four different health states: 1) disease-free and increased exercise; 2) disease-free with no change in exercise; 3) recurrent disease; or 4) death. Patients in the disease-free health state are subject to a chance of dying of natural causes, dropout/stopping exercise if they increased exercise, and disease recurrence. Patients with disease recurrence will either continue or be subjected to death from lung cancer recurrence (Figure 1).
Base case
Our base case assumed a 70 year-old lung cancer survivor with stage I-IIIA lung cancer who completed treatment > 30 days previously, referred to one of the three interventions, and experiencing probabilities of increasing exercise, health utility benefit related to exercise, and drop-out rates as described above. Since our simulated base case may differ from other lung cancer populations and published studies, we varied and tested ranges for the following lung cancer-related variables: age (55 to 80 years), stage (IA to IIIA), and disease-free (25 to 80%) and overall (30 to 60%) survival rates. We described these variations in detail in Table 1.
Statistical Analysis
We converted all yearly to quarterly (¼th) costs and utilities and used a cycle length of three months and time horizon five years with half-cycle corrections. We incorporated 3% annual discounts to costs and utilities,21 assumed a willingness-to-pay (WTP) threshold of $100,000 per QALY gained, and analyzed from health care organization and societal perspectives.21 We conducted sensitivity analyses on cost, probability of increasing exercise, and health utility benefit related to exercise to observe their effects on the ICER and probabilistic sensitivity analyses using Monte Carlo simulation with 100,000 iterations and parameters as reported in Table 1, with standard deviations (SD) 20% for all included variables.
Results
In LIFE, the mean (SD) age of participants was 78.9 (5.2) years. Sixty seven percent were females, 76% Caucasians, and 18% African Americans. Mean (SD) body-mass-index was 30.2 (6.0) kg/m2. The most common self-reported comorbidities were hypertension (71%), diabetes mellitus (26%), and history of cancer treated > 3 years previously (23%).14
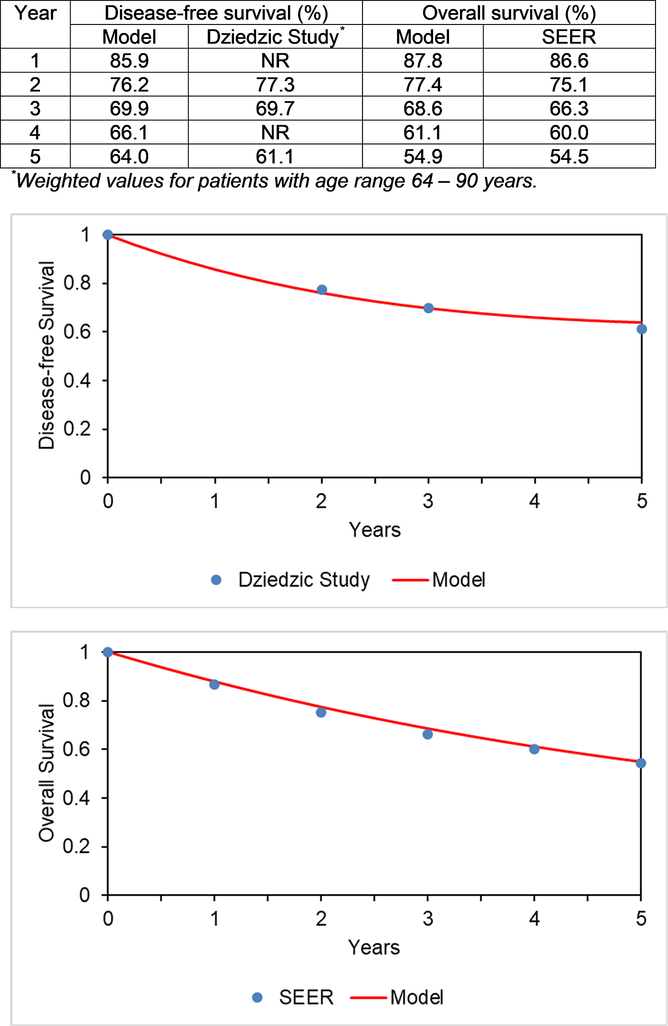
We calibrated our model using survival data as described above, the results are shown in Figure 2.
Figure 2:
Calibration of Model Endpoints
Caption: Calibration of model endpoints: (top) disease-free survival and (bottom) overall survival. Red colored lines represent our model data and blue dots represent comparison study/data.
NR = not reported; SEER = Surveillance, Epidemiology, and End Results program
Base Case Analysis
Our base case CEA from an organizational perspective found total costs of $110,224, $106,923, $105,485 and effectiveness 2.78, 2.73, and 2.72 QALYs for the LIFE exercise program, health education, and UC, respectively. The ICER for the health education program was higher than that of the LIFE exercise program, suggesting lack of cost-effectiveness (in CEAs, this is referred to as extended dominance). The incremental cost for the LIFE exercise program was $4,740 and incremental effectiveness 0.06 QALYs more than UC, yielding an ICER of $79,504/QALY. The LIFE exercise program therefore would be cost-effective in lung cancer survivors from an organizational perspective at a WTP of $100,000/QALY. When we incorporated opportunity and traveling costs, the total cost for the LIFE exercise program was $116,685 and UC $105,967, yielding an incremental cost of $10,718 and ICER $179,774/QALY. The LIFE exercise program therefore would not be cost-effective from a societal perspective when costs related to time lost to exercise and traveling were included. When we excluded traveling costs ($620/patient/year) to simulate an equally effective home-based intervention, the ICER was $143,556/QALY and not cost-effective from a societal perspective.
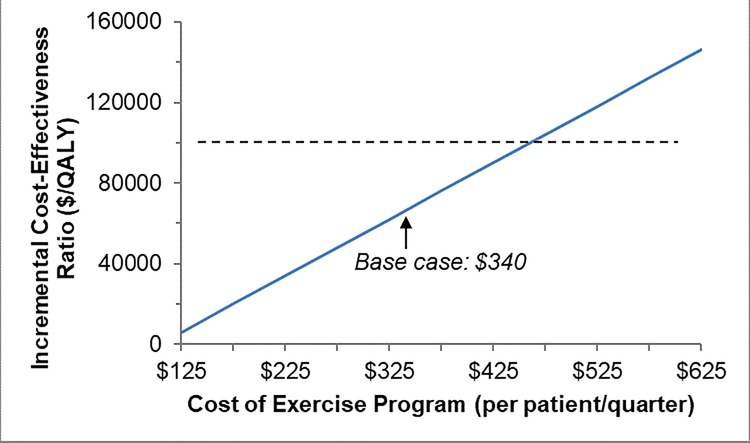
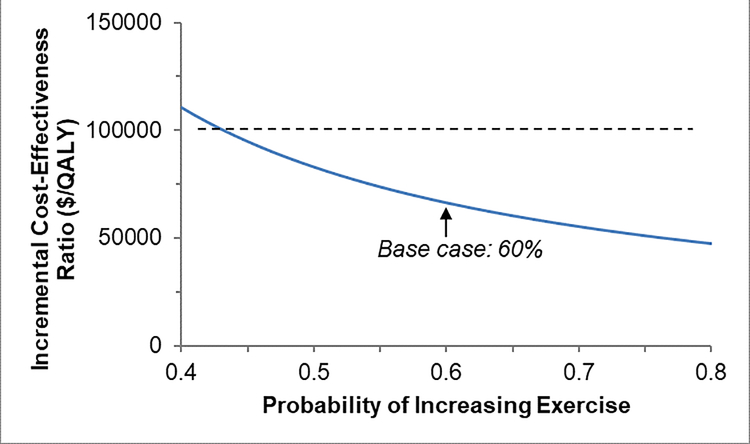
One-Way Sensitivity Analyses
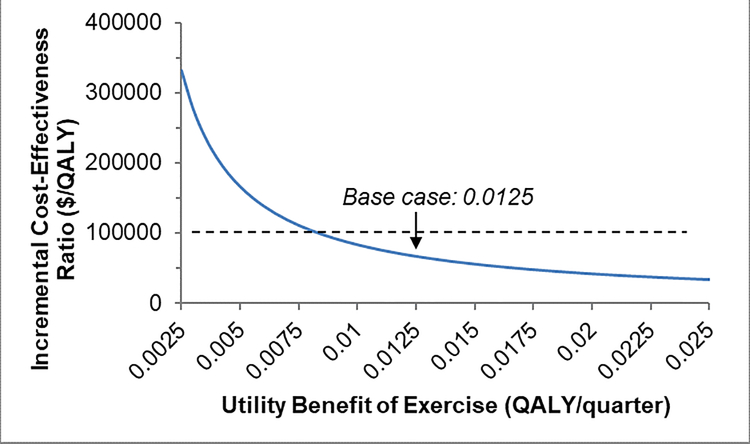
When we individually varied the parameters described in Table 1 to assess the effects of these changes on results, we found that our model was most sensitive to the costs of the exercise program, probability of increasing exercise, and health utility benefit related to exercise (Figure 3A–C). We found that at a WTP threshold of $100,000, the cost of the exercise program could be up to a maximum of $1,712/patient/year (approximately 26% increase from our base case) and still be considered cost-effective compared to UC. Also, at least 48% of lung cancer survivors referred would need to increase exercise and experience health utility benefits of 0.040 QALYs for the exercise program to be cost-effective.
Figure 3: One-way Sensitivity Analyses.
Figure 3A. Results of one-way sensitivity analyses for: cost of exercise program. QALY = quality-adjusted life-year
Figure 3B. Results of one-way sensitivity analyses for: probability of increasing exercise. QALY = quality-adjusted life-year
Figure 3C. Results of one-way sensitivity analyses for: utility benefit related to exercise. Dashed lines represent a willingness to pay threshold of $100,000, below which exercise would be considered cost-effective. QALY = quality-adjusted life-year
Probabilistic Sensitivity Analyses
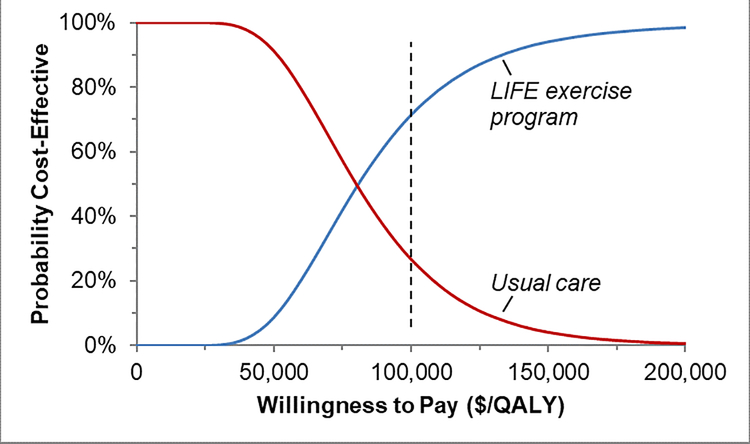
We conducted probabilistic sensitivity analyses by varying all costs, utilities, and probabilities simultaneously to assess their effects on findings. At a WTP of $100,000/QALY, we found that the LIFE exercise program was cost-effective 71% and UC 27% of the time (Figure 4), suggesting a high probability of cost-effectiveness for the LIFE exercise program compared to UC. If we increased the WTP to $150,000,26 these respective probabilities were 94% and 4%, strongly favoring the LIFE exercise program.
Figure 4:
Cost-Effectiveness Acceptability Curve
Caption: Results of probabilistic sensitivity analysis comparing the cost-effectiveness of the LIFE exercise program (blue line) with usual care (red line). Dashed line represents a willingness to pay threshold of $100,000/QALY, at which the probabilities of cost-effectiveness are 27% for usual care and 71% for the LIFE exercise program.
LIFE = Lifestyle Interventions and Independence for Elders study; QALY = quality-adjusted life-year
Discussion
In a model-based CEA, we found that the LIFE exercise program was cost-effective in lung cancer survivors following curative-intent treatment from an organizational but not societal perspective. While this program is currently not readily-available for lung cancer survivors in routine clinical care, these findings have implications in program design and implementation in future studies aimed at improving exercise to improve physical function and reduce symptom burden in these patients.
Following a cancer diagnosis, the Institute of Medicine recommends care planning and prevention and management of long-term and late treatment effects.27 While adult cancer survivors experience both physical and psychological impairments, evidence suggests that more cancer survivors have low health status as the result of physical than psychological impairments;28 these impairments often go undetected/untreated and may result in disability.28 As such, promoting physical activity and exercise is an important component of cancer survivorship and is recommended by the American Society of Clinical Oncology29–31 and American Cancer Society.30–33
Exercise interventions in lung cancer survivors commonly take place as part of comprehensive, multidisciplinary pulmonary rehabilitation programs, either in the preoperative or postoperative context.34 Recent systematic reviews suggest that preoperative rehabilitation that included aerobic training, resistance training, or respiratory muscle training improves cardiopulmonary fitness and may reduce the risk of surgical complications,35–38 while postoperative rehabilitation may improve functional and/or exercise capacity and physical health.37–39 One systematic review identified three randomized clinical trials (RCTs) involving a total of 178 participants who recently completed lung cancer resection surgery and found that exercise training resulted in functional exercise capacity improvements compared to UC, while global and physical health, and quadriceps muscle strength were not different.39 The mean age of the participants ranged from 58 to 65 years and 63% were males. Participants in the exercise training arm improved 50-m more in six-minute walk distance, suggested to be clinically meaningful in lung cancer.40 The authors concluded that while exercise training may potentially increase functional exercise capacity, the findings should be interpreted with caution due to disparities between studies, methodological limitations, and significant risks for bias and small sample sizes.39 Subsequent systematic reviews raised similar concerns.35, 38, 39 A more recent RCT that enrolled 112 advanced lung cancer survivors found that while physical activity/exercise did not reduce fatigue compared to UC, exploratory analyses of 38 (34%) participants who increased physical activity/exercise during the intervention period noted a significant reduction in fatigue symptoms.41
Moreover, cancer rehabilitation has been suggested to be effective in improving function and reducing symptom burden in cancer survivors and may be cost-effective in reducing health care costs.28 Exercise plays an important role in rehabilitation and may have role in cancer rehabilitation to decrease treatment-related morbidity, reduce costs, increase treatment options, and improve physical and psychological outcomes.28 A recent systematic review involving 350 participants suggested that four weeks of preoperative rehabilitation shortened length of hospital stay following lung cancer resection surgery and 12 weeks of postoperative rehabilitation improved functional exercise capacity and physical health and reduced dyspnea.37 Another systematic review suggested that preoperative rehabilitation also reduced postoperative complications following with lung cancer surgery.36 These results suggest that preoperative rehabilitation should be considered in clinical care due to the potential benefits discussed above. Such service may also assist lung cancer survivors overcome the physical and cardiopulmonary limitations resulting from chronic diseases such as COPD, heart failure, and diabetes. In addition, overcoming other psychological barriers (e.g. pain, dyspnea) may be important to achieve optimal effectiveness. Home-based interventions may also need to be incorporated to maintain exercise and/or facilitate program uptake for patients who have barriers for facility-based programs. Remote monitoring via accelerometry may help track physical activity/exercise levels and facilitate assessments of adherence to exercise prescriptions. Therefore, to effectively promote physical activity and exercise in lung cancer survivors, an integrated approach to evaluation and treatment is needed.42
Our CEA model is most similar to a previous analysis by van Waart and coworkers43 who performed a trial-based CEA of a home-based, low-intensity (Onco-Move) and a supervised, moderate-to-high intensity (OnTrack) program compared to UC. In 230 breast cancer patients enrolled from the initiation of adjuvant chemotherapy, Onco-Move was not cost-effective compared to OnTrack at approximately 9.5 months. Similarly, Kampshoff and coworkers44 evaluated the long-term cost-effectiveness of a high-intensity (HI) compared to low-moderate intensity (LMI) exercise program at 64 weeks, and found that in 277 cancer survivors randomized to a 12-week HI or LMI program, followed by booster sessions at 4, 10, and 18 weeks, the HI program gained 0.03 QALYs and had ICERs of -€72,859/QALY and -€87,831/QALY compared to LMI from the health care and societal perspectives, respectively. The cost saving was primarily due to significantly lower health care costs in secondary care and medications for participants in the HI compared to the LMI program.
In contrast, May and coworkers45 performed a CEA for an 18-week exercise program during adjuvant therapy, and found that at 9 months, supervised exercise had incremental effectiveness of 0.01 and 0.03 QALYs for breast and colon cancer, respectively, and societal costs of €2,912 and -€4,321 compared to UC. In their study, the 18-week exercise program was cost-effective only in colon cancer, partly due to cost saving in chemotherapy, hospitalization, medical contacts, and work absenteeism for the exercise group compared to UC.
Like these CEAs, our study suggests that the cost-effectiveness of exercise programs largely depends on the effectiveness (QALYs) gained. While our model is also sensitive to the costs of the program, these trial-based CEAs alternatively suggest that exercise may be associated with lower health care costs and therefore cost-effective. Unlike these CEAs, our study also incorporated a probability of increasing exercise which indirectly estimates the probability of exercise uptake and therefore may have implications for generalization/translation in health care delivery. In addition, we estimated the traveling costs for facility-based exercise programs which may be associated with more QALYs gained but higher participant costs compared to home-based interventions.11 Our model was somewhat sensitive to the 5-year DFS but not OS rate, likely due to the assumptions that patients with disease recurrence do not exercise and there is no survival benefit related to exercise.
Our study has limitations. First, we assumed a health utility improvement reported in LIFE which enrolled older adults without cancer and, therefore, may not translate to the lung cancer population. However, there are several advantages in choosing LIFE including: the advanced age of participants; multicenter and US setting; and the availability of cost-effectiveness data which maximize accuracy and real-life implications.16 In addition, recent systematic reviews suggest that exercise training in lung cancer patients may produce benefits in improving physical fitness37, 38 and, possibly, physical health.37 Furthermore, the physical benefits in LIFE extended across participants with and without comorbidity and among those with varying levels of fitness, suggesting that the LIFE exercise intervention could plausibly work among lung cancer patients. Second, the utility instrument used in LIFE (Quality of Well-being Scale) may have different psychometric properties including responsiveness to change compared to the EQ-5D. Nevertheless, similarities between these instruments include the use of preference-based scales (0–1) and the utility improvement in LIFE (0.05 QALYs over a median of 2.6 years) resembles the magnitude of EQ-5D utility improvement for COPD patients undergoing eight weeks of pulmonary rehabilitation.46 Third, we did not include possible negative health consequences related to exercise. However, in LIFE there was no difference in major adverse events related to exercise between the exercise and health education programs.16 In addition, the US Preventative Task Force suggests that potential harms are at most small and favors interventions to promote physical activity in individuals with and without cardiovascular disease risk factors.47 Fourth, we obtained DFS and OS data from patients undergoing lung cancer resection surgery and therefore may have limited generalizability to those undergoing definitive radiation or concurrent chemoradiation. However, the wide range of 5-year DFS and OS rates tested in our sensitivity analyses included stage IA patients undergoing lobectomy [respective rates ~80%24 and 60%48] and stage IIIA patients undergoing concurrent chemoradiation [respective rates ~25% and 30%49] and therefore facilitated inferences of cost-effectiveness for a broad range of treatment modalities. Moreover, we simultaneously varied and tested ranges of lung-cancer related variables (i.e. age, stage, DFS, and OS) in our probabilistic sensitivity analyses, further strengthening our findings and conclusions. Fifth, we did not incorporate possible benefits of exercise on functional changes associated with aging, chronic disease control, emotional well-being, independence, psychosocial functioning/social interactions, health care utilization including pulmonary rehabilitation, medical appointments, urgent care visits, hospitalizations, length of hospital stay, or survival; however, including these benefits would likely favor the cost-effectiveness of the LIFE exercise program since major mobility disability was reduced in LIFE with exercise compared to the health education program. Sixth, while this cost-effectiveness model strove to incorporate high-level evidence from a focused population of lung cancer patients, we lack a definitive RCT evaluating the LIFE exercise intervention in stage I-IIIA NSCLC patients. To identify model inputs for the CEA, we turned to research involving elderly non-cancer adults (for exercise program utility and cost), and breast cancer patients (for probability of exercise uptake). We measured the importance of these assumptions including a lower (40%) probability of increasing exercise and (0.01) QALY improvement related to exercise in our one-way sensitivity analyses, though the possibility of bias remains. Finally, our societal perspective analysis accounted for opportunity costs related to time lost to exercise which may overestimate costs since exercise can be performed as a leisure-time activity which may not lead to productivity loss.
Our model did not include immune checkpoint blockade (ICB) costs since they are not currently standard therapy for stage III or earlier NSCLC, although ICB therapy is moving rapidly to earlier-stage disease. A recent RCT comparing ICB with anti-programmed death (PD) ligand 1 antibody durvalumab after platinum-based chemoradiotherapy in patients with unresectable stage III NSCLC found longer progression-free survival with ICB compared to placebo.50 In a subgroup analysis, patients who started durvalumab < 14 days of completing chemoradiation had a progression-free survival hazard ratio (HR) of 0.39, compared to those patients who received ICB 14–56 days of completing chemoradiation who had an HR of 0.63. In addition to underlying comorbidities, these findings may support the hypothesis that ICB integrated more proximally, or potentially concurrently, with radiation may improve outcomes for patients with locally-advanced NSCLC treated with definitive chemoradiation. ANVIL is an ongoing NCI Cooperative Group RCT testing nivolumab (anti-PD-1 antibody) as adjuvant therapy after surgical resection in stage IB-IIIA (https://clinicaltrials.gov/ct2/show/NCT02595944), the results of which will further clarify the role of ICBs in patients with curable non-metastatic disease. While our model did not include the possible benefit of consolidation ICB therapy following or during traditional curative-intent treatment, including such benefit would likely further improve the cost-effectiveness of exercise in our model. Furthermore, future studies examining the utility of exercise in lung cancer may include not only improving function and reducing symptom burden, but also possible synergistic effects of exercise, radiation and ICB on lung cancer outcomes.
We conclude that in a simulation model, the LIFE exercise program is cost-effective compared to usual care in lung cancer survivors following curative-intent treatment from an organizational, but not societal perspective. A similar exercise program offered by a health care organization for lung cancer survivors may be effective and cost-effective.
Acknowledgments
b) Funding: This work was supported by the National Institutes of Health (L30CA208950 and 1T32HL134632-01); and the American Cancer Society (PF-17-020-01-CPPB).
c) Financial Benefits to Authors: none
Abbreviations
- CEA
cost-effectiveness analysis
- COPD
chronic obstructive pulmonary disease
- DFS
disease-free survival
- EQ-5D
EuroQoL-5 Dimensions
- HI
high intensity
- HR
hazard ratio
- ICB
immune checkpoint blockade
- ICER
incremental cost-effectiveness ratio
- LIFE
Lifestyle Interventions and Independence for Elders
- LMI
low-moderate intensity
- NSCLC
non-small cell lung cancer
- OS
overall survival
- PD
programmed death
- QALY
quality-adjusted life-years
- RCT
randomized clinical trial
- SD
standard deviation
- SEER
Surveillance, Epidemiology, and End Results
- UC
usual care
- US
United States
- WTP
willingness-to-pay
Footnotes
a) Conflict of Interest: All authors declare no conflict of interest exists
d)Previous manuscript: A pre-print version of this manuscript is available at https://www.biorxiv.org/content/10.1101/533281v1
References
- 1.Dinan MA, Curtis LH, Carpenter WR, et al. Stage migration, selection bias, and survival associated with the adoption of positron emission tomography among medicare beneficiaries with non-small-cell lung cancer, 1998–2003. J Clin Oncol. 2012;30( 22):2725–2730. [DOI] [PubMed] [Google Scholar]
- 2.Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: Are there any modifiable risk factors? Thorax. 2010;65( 9):815–818. [DOI] [PubMed] [Google Scholar]
- 3.Ha D, Choi H, Zell K, et al. Association of impaired heart rate recovery with cardiopulmonary complications after lung cancer resection surgery. J Thorac Cardiovasc Surg. 2015;149( 4):1168–73.e3. [DOI] [PubMed] [Google Scholar]
- 4.Hamada K, Irie M, Fujino Y, Hyodo M, Hanagiri T. Prognostic value of preoperative exercise capacity in patients undergoing thoracoscopic lobectomy for non-small cell lung cancer. Lung Cancer. 2019;128:47–52. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Mostofian F, Ivanovic J, et al. All grades of severity of postoperative adverse events are associated with prolonged length of stay after lung cancer resection. J Thorac Cardiovasc Surg. 2018;155( 2):798–807. [DOI] [PubMed] [Google Scholar]
- 6.Grigor EJM, Ivanovic J, Anstee C, et al. Impact of adverse events and length of stay on patient experience after lung cancer resection. Ann Thorac Surg. 2017;104( 2):382–388. [DOI] [PubMed] [Google Scholar]
- 7.Brunelli A, Xiume F, Refai M, et al. Evaluation of expiratory volume, diffusion capacity, and exercise tolerance following major lung resection: A prospective follow-up analysis. Chest. 2007;131( 1):141–147. [DOI] [PubMed] [Google Scholar]
- 8.Carver JR, Shapiro CL, Ng A, et al. American society of clinical oncology clinical evidence review on the ongoing care of adult cancer survivors: Cardiac and pulmonary late effects. J Clin Oncol. 2007;25( 25):3991–4008. [DOI] [PubMed] [Google Scholar]
- 9.Islam KM, Jiang X, Anggondowati T, Lin G, Ganti AK. Comorbidity and survival in lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2015;24( 7):1079–1085. [DOI] [PubMed] [Google Scholar]
- 10.Denlinger CS, Ligibel JA, Are M, et al. Survivorship: Healthy lifestyles, version 2.2014. J Natl Compr Canc Netw. 2014;12( 9):1222–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buffart LM, Kalter J, Sweegers MG, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz KH, Courneya KS, Matthews C, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42( 7):1409–1426. [DOI] [PubMed] [Google Scholar]
- 13.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62( 4):243–274. [DOI] [PubMed] [Google Scholar]
- 14.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The LIFE study randomized clinical trial. JAMA. 2014;311( 23):2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Cancer Stats Facts: Lung and Bronchus Cancer‥ https://seer.cancer.gov/statfacts/html/lungb.html. Accessed 10/16, 2017.
- 16.Groessl EJ, Kaplan RM, Castro Sweet CM, et al. Cost-effectiveness of the LIFE physical activity intervention for older adults at increased risk for mobility disability. J Gerontol A Biol Sci Med Sci. 2016;71( 5):656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rejeski WJ, Fielding RA, Blair SN, et al. The lifestyle interventions and independence for elders (LIFE) pilot study: Design and methods. Contemp Clin Trials. 2005;26( 2):141–154. [DOI] [PubMed] [Google Scholar]
- 18.Tramontano AC, Schrag DL, Malin JK, et al. Catalog and comparison of societal preferences (utilities) for lung cancer health states: Results from the cancer care outcomes research and surveillance (CanCORS) study. Med Decis Making. 2015;35( 3):371–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon LG, DiSipio T, Battistutta D, et al. Cost-effectiveness of a pragmatic exercise intervention for women with breast cancer: Results from a randomized controlled trial. Psychooncology. 2017;26( 5):649–655. [DOI] [PubMed] [Google Scholar]
- 20.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J Cancer Surviv. 2010;4( 2):87–100. [DOI] [PubMed] [Google Scholar]
- 21.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA. 2016;316( 10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 22.Bradley CJ, Yabroff KR, Mariotto AB, Zeruto C, Tran Q, Warren JL. Antineoplastic treatment of advanced-stage non-small-cell lung cancer: Treatment, survival, and spending (2000 to 2011). J Clin Oncol. 2017:JCO2016694166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the united states measured by accelerometer. Med Sci Sports Exerc. 2008;40( 1):181–188. [DOI] [PubMed] [Google Scholar]
- 24.Dziedzic DA, Rudzinski P, Langfort R, Orlowski T, Polish Lung Cancer Study Group (PLCSG). Risk factors for local and distant recurrence after surgical treatment in patients with non-small-cell lung cancer. Clin Lung Cancer. 2016;17( 5):e157–e167. [DOI] [PubMed] [Google Scholar]
- 25.Social Security Actuarial Life Table‥ https://www.ssa.gov/oact/STATS/table4c6.html. Accessed 10/16, 2017.
- 26.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371( 9):796–797. [DOI] [PubMed] [Google Scholar]
- 27.Institute of medicine, committee on quality of healthcare in america. crossing the quality chasm: A new health system for the 21st century. washington, DC: National Academy Press; 2001. [Google Scholar]
- 28.Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: An essential component of quality care and survivorship. CA Cancer J Clin. 2013;63( 5):295–317. [DOI] [PubMed] [Google Scholar]
- 29.Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American society of clinical oncology clinical practice guideline endorsement. J Clin Oncol. 2015;33( 9):1078–1085. [DOI] [PubMed] [Google Scholar]
- 30.Runowicz CD, Leach CR, Henry NL, et al. American cancer society/american society of clinical oncology breast cancer survivorship care guideline. J Clin Oncol. 2016;34( 6):611–635. [DOI] [PubMed] [Google Scholar]
- 31.Runowicz CD, Leach CR, Henry NL, et al. American cancer society/american society of clinical oncology breast cancer survivorship care guideline. CA Cancer J Clin. 2016;66( 1):43–73. [DOI] [PubMed] [Google Scholar]
- 32.Skolarus TA, Wolf AM, Erb NL, et al. American cancer society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64( 4):225–249. [DOI] [PubMed] [Google Scholar]
- 33.El-Shami K, Oeffinger KC, Erb NL, et al. American cancer society colorectal cancer survivorship care guidelines. CA Cancer J Clin. 2015;65( 6):428–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bade BC, Thomas DD, Scott JB, Silvestri GA. Increasing physical activity and exercise in lung cancer: Reviewing safety, benefits, and application. J Thorac Oncol. 2015;10( 6):861–871. [DOI] [PubMed] [Google Scholar]
- 35.Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev. 2017;6:CD012020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sebio Garcia R, Yanez Brage MI, Gimenez Moolhuyzen E, Granger CL, Denehy L. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: A systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2016;23( 3):486–497. [DOI] [PubMed] [Google Scholar]
- 37.Ni HJ, Pudasaini B, Yuan XT, Li HF, Shi L, Yuan P. Exercise training for patients pre- and postsurgically treated for non-small cell lung cancer: A systematic review and meta-analysis. Integr Cancer Ther. 2017;16( 1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Driessen EJ, Peeters ME, Bongers BC, et al. Effects of prehabilitation and rehabilitation including a home-based component on physical fitness, adherence, treatment tolerance, and recovery in patients with non-small cell lung cancer: A systematic review. Crit Rev Oncol Hematol. 2017;114:63–76. [DOI] [PubMed] [Google Scholar]
- 39.Cavalheri V, Tahirah F, Nonoyama M, Jenkins S, Hill K. Exercise training for people following lung resection for non-small cell lung cancer - a cochrane systematic review. Cancer Treat Rev. 2014;40( 4):585–594. [DOI] [PubMed] [Google Scholar]
- 40.Granger CL, Holland AE, Gordon IR, Denehy L. Minimal important difference of the 6-minute walk distance in lung cancer. Chron Respir Dis. 2015;12( 2):146–154. [DOI] [PubMed] [Google Scholar]
- 41.Dhillon HM, Bell ML, van der Ploeg HP, et al. Impact of physical activity on fatigue and quality of life in people with advanced lung cancer: A randomized controlled trial. Ann Oncol. 2017;28( 8):1889–1897. [DOI] [PubMed] [Google Scholar]
- 42.Granger CL, Parry SM, Denehy L, Remedios L. Evidence, education and multi-disciplinary integration are needed to embed exercise into lung cancer clinical care: A qualitative study involving physiotherapists. Physiother Theory Pract. 2018;34( 11):852–860. [DOI] [PubMed] [Google Scholar]
- 43.van Waart H, van Dongen JM, van Harten WH, et al. Cost-utility and cost-effectiveness of physical exercise during adjuvant chemotherapy. Eur J Health Econ. 2017. [DOI] [PubMed] [Google Scholar]
- 44.Kampshoff CS, van Dongen JM, van Mechelen W, et al. Long-term effectiveness and cost-effectiveness of high versus low-to-moderate intensity resistance and endurance exercise interventions among cancer survivors. J Cancer Surviv. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.May AM, Bosch MJ, Velthuis MJ, et al. Cost-effectiveness analysis of an 18-week exercise programme for patients with breast and colon cancer undergoing adjuvant chemotherapy: The randomised PACT study. BMJ Open. 2017;7( 3):e012187-2016-012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nolan CM, Longworth L, Lord J, et al. The EQ-5D-5L health status questionnaire in COPD: Validity, responsiveness and minimum important difference. Thorax. 2016;71( 6):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LeFevre ML U.S. Preventive Services Task Force. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. preventive services task force recommendation statement. Ann Intern Med. 2014;161( 8):587–593. [DOI] [PubMed] [Google Scholar]
- 48.van den Berg LL, Klinkenberg TJ, Groen HJ, Widder J. Patterns of recurrence and survival after surgery or stereotactic radiotherapy for early stage NSCLC. J Thorac Oncol. 2015;10( 5):826–831. [DOI] [PubMed] [Google Scholar]
- 49.Senan S, Brade A, Wang LH, et al. PROCLAIM: Randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2016;34( 9):953–962. [DOI] [PubMed] [Google Scholar]
- 50.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16( 13):e498–509. [DOI] [PubMed] [Google Scholar]