Abstract
Meditation is commonly assumed to be associated with enhanced interoceptive accuracy. We previously found that experienced meditators did not exhibit a greater ability than nonmeditators to detect heartbeat sensations at rest, despite the meditators’ reported subjective ratings of higher accuracy and lower difficulty. Here, attempting to overcome previous methodological limitations, we assessed interoceptive awareness of heartbeat and breathing sensations across physiological arousal levels using infusions of isoproterenol, a beta-adrenergic agonist similar to adrenaline. We hypothesized that meditators would display greater interoceptive awareness than nonmeditators, as evidenced by higher interoceptive detection rates, increased interoceptive accuracy, and differences in localization of heartbeat sensations. We studied 15 meditators and 15 nonmeditators, individually matched on age-, gender-, and body mass index, using randomized, double-blinded, and placebo-controlled bolus infusions of isoproterenol. Participants reported their experience of heartbeat and breathing sensations using a dial during infusions, and the location of heartbeat sensations on a two-dimensional manikin afterwards. There was no evidence of higher detection rates or increased accuracy across any dose, although meditators showed a tendency to report cardiorespiratory sensation changes sooner at higher doses. Relative to nonmeditators, meditators exhibited prominent geographical differences in heartbeat localization, disproportionally reporting sensations throughout central regions of the chest, abdomen, neck, back, and head. To further assess indications of potential differences in cardiac interoceptive accuracy between meditators and nonmeditators, we conducted a meta-analysis including 724 participants, and found little evidence for such differences. We conclude that the practice of meditation is not associated with improved cardiac interoceptive awareness.
Keywords: Meditation, Interoception, Heartbeat detection, Mindfulness, Respiration
1. Introduction
The therapeutic application of meditation as a complementary approach in health management has increased considerably in recent years, with one in seven adults now reporting use within the previous year (Arias, Steinberg, Banga, & Trestman, 2006; Barnes, Bloom, & Nahin, 2008; Clarke, Barnes, Black, Stussman, & Nahin, 2018). While practiced typically in the context of spiritual traditions, the efficacy of meditation training in directly influencing the course of a variety of physical and mental disorders is also under increasing investigation (Goyal et al., 2014; Strauss, Cavanagh, Oliver, & Pettman, 2014). Recent large scale surveys of the available literature have concluded that there is some evidence for the efficacy of meditation as an adjuvant (i.e., superior to no treatment or treatment-as-usual) in certain healthcare settings, although the clinical effects often do not separate from other active treatments (Goldberg et al., 2018; Hedman-Lagerlof, Hedman-Lagerlof, & Ost, 2018; Wielgosz, Goldberg, Kral, Dunne, & Davidson, 2018). The low rates of adverse outcomes of this inexpensive, non-pharmacological approach have also led to increasing acceptance as a behavioral medicine tool by medical associations (Levine et al., 2017), but there is consensus that studies of meditation would benefit from the use of unbiased or standardized methods, and utilization of paradigms capable of making causal inferences (Levine et al., 2017; Ospina et al., 2007; Van Dam et al., 2018).
Most meditation traditions incorporate attention to internal body sensations as a component of the practice, especially in the beginning stages of instruction, possibly because the availability of these sensations from moment to moment makes them a convenient object to focus on. The most commonly attended body sensations include the breath, the position of the joints (i.e., proprioception), the degree of muscle tension, and the heartbeat (Kabat-Zinn, 1990; Kornfield, 1996; Nairn, 2000; Selby, 1992). Although attention to internal body sensations is most commonly practiced under conditions of rest, subjective experiences of these body sensations are also routinely modulated through manipulations of the breath and musculoskeletal posture, particularly during the practice of yoga exercises (Arambula, Peper, Kawakami, & Gibney, 2001; Bhajan & Khalsa, 2000; Lawrence et al., 2017; Peng et al., 2004; Youkhana, Dean, Wolff, Sherrington, & Tiedemann, 2016). Many traditions state that the repeated practice of attending to internal body signals results in enhanced awareness of them, and further assert that the meditation practice results in enhanced awareness of a variety of other internal events, such as the ongoing experience of thoughts and emotions (Hart, 1987; Kabat-Zinn, 1990; Kornfield, 1996; Nairn, 2000). From a clinical perspective, many questions remain about the mechanisms that underlie the changes commonly attributed to meditation. A natural question is whether meditation objectively enhances interoceptive awareness, defined as the act of consciously sensing, interpreting, and integrating information about the state of inner body systems (Khalsa, Adolphs, et al., 2018). Since abnormal interoception is a common feature of several medical conditions and especially certain psychiatric disorders (Khalsa, Adolphs, et al., 2018), knowing whether and how meditation might impact it could inform the potential development and delivery of meditation-based approaches as a health/clinical intervention.
In a previous study, we examined whether experienced Kundalini and Tibetan Buddhist meditators demonstrated evidence of increased interoceptive accuracy, using a rigorous heartbeat detection task (Khalsa et al., 2008). Contrary to expectations we did not find evidence to support this hypothesis. Meditators in both groups exhibited similar levels of interoceptive accuracy, and instead, demonstrated metacognitive differences in interoceptive insight (i.e., differential perceptions of their task performance and difficulty; see (Khalsa, Adolphs, et al., 2018) and Supplement for consensus definitions of interoceptive insight, accuracy, and other features of interoceptive awareness). While these negative results were surprising, they are corroborated by numerous studies using 1) the same task (Nielsen & Kaszniak, 2006), and 2) other measures of heartbeat perception (Fischer, Messner, & Pollatos, 2017; Melloni et al., 2013; Otten et al., 2015; Parkin et al., 2013). Studies of interoceptive accuracy in the respiratory domain have also been largely negative (Daubenmier, Sze, Kerr, Kemeny, & Mehling, 2013). Despite these numerous null findings, one important consideration is that these assessments were largely conducted under physiologically quiescent resting conditions, a period of homeostasis that is widely recognized to have lower significance for the subjective experience of interoceptive sensations (Khalsa, Adolphs, et al., 2018). Accordingly, less than 50% of the individuals in each study group demonstrated accurate rates of heartbeat detection, which is consistent with the low detection rates commonly seen in other studies (see (Khalsa & Lapidus, 2016) for a review). We have demonstrated previously that increases in interoceptive awareness for heartbeat and respiratory sensations can be induced by modulating physiological arousal (i.e., increasing the signal to noise ratio of sensory signals) (Khalsa, Rudrauf, Sandesara, Olshansky, & Tranel, 2009), providing an effective means for assessing group differences in interoceptive awareness across all participants within a sample (Khalsa et al., 2015).
In the current study, we evaluated whether there was evidence for increased interoceptive awareness in meditators under conditions of increased physiological arousal that were above the resting threshold for detection of cardiac signals. To increase interoceptive awareness for heartbeat as well as respiratory signals we used intravenous infusions of isoproterenol, a peripherally acting beta-adrenergic agonist similar to adrenaline, combined with a continuous measurement of subjective (rating dial) and objective (heart rate) signals. We predicted that meditators would display greater interoceptive awareness than nonmeditators across the spectrum of arousal as evidenced by 1) higher interoceptive detection rates, and 2) increased interoceptive accuracy scores. We also examined whether meditators differed with respect to the perceptual localization of heartbeat sensations in the body, using a recently developed method for quantitatively assessing cardiac body maps (Khalsa, Hassanpour, et al., 2018). We have shown that isoproterenol induces heartbeat sensations which are predominantly localized to the left anterior chest, head, neck, abdomen and arms (Khalsa, Rudrauf, Sandesara, et al., 2009), and that the processing of these sensations appears to rely on both somatosensory afferents from the skin and body sensitive brain regions including the insula, somatosensory cortices, and amygdala (Hassanpour et al., 2018; Hassanpour et al., 2016; Khalsa et al., 2016; Khalsa, Rudrauf, Feinstein, & Tranel, 2009). Given the difficulty in detecting this weak signal under resting conditions, and the theoretical argument that repeatedly attending to internal body signals during the meditation practice results in enhanced awareness of them, we hypothesized that meditators would exhibit a greater spatial extent of heartbeat sensations in the body although we did not have specific hypotheses regarding their location.
2. Method
2.1. Participants
15 nonmeditators and 15 meditators participated in the study. Each nonmeditator was individually matched to a corresponding meditator based on three criteria: age, sex, and body mass index (Table 1). 11 of the meditators were Vipassana practitioners, and the other four meditators were Kundalini practitioners.1 Meditators were considered eligible for participation if they reported a continuous (daily or near daily) meditation practice during the previous two years, and if they had also attended one or more weeklong meditation retreats within the previous year. Nonmeditators were considered eligible for participation if they had never received formal meditation training in meditation or yoga and did not practice self-taught meditation. All participants were screened for the presence of any neurological, psychiatric, cardiac or respiratory disease during a detailed phone interview, and were excluded if they reported a history of illness in any of these categories. None of the study participants were smokers, and none of the women took oral contraceptives or were pregnant, as assessed via urine pregnancy test. Each participant demonstrated a normal 12-lead electrocardiogram (EKG), as assessed by a board-certified cardiologist or neurologist. This study was approved by the General Clinical Research Center (GCRC) Advisory Committee and the Institutional Review Board of the University of Iowa. All participants provided written informed consent and received compensation.
Table 1.
Demographic data for all participants.
| Meditators (M) | Nonmeditators (NM) | |
|---|---|---|
| Sex | 10 Men, 5 Women | 10 Men, 5 Women |
| Age (years) | 44.7 +/− 13.2 | 44.0 +/− 13.7 |
| Body Mass Index | 24.5 +/− 4.6 | 25.5 +/− 4.0 |
| Race | 15 Caucasian | 14 Caucasian, 1 Asian |
| Education (years) | 17.3 +/− 2.2 | 15.9 +/− 2.3 |
| Beck Anxiety Inventory score | 5.1 +/− 3.4 | 3.5 +/− 2.9 |
| Beck Depression Inventory score | 4.3 +/− 4.6 | 3.7 +/− 5.3 |
| Meditation practice (years) | 10.8 +/− 10.8 | 0 +/− 0 |
| Cumulative meditation practice (hours) | 4947 +/− 6251 | 0 +/− 0 |
| Retreat experience (days) | 19 +/− 14 | 0 +/− 0 |
| CD25 (micrograms) | 4.48 +/− 1.5 | 4.72 +/− 2.2 |
Chronotropic Dose 25 (CD25): dose of isoproterenol that would be required to increase the heart rate by 25 beats per minute. Means +/− standard deviation.
2.2. Interoceptive awareness task
Each participant rated the experience of internal body sensations both during and immediately after receiving bolus infusions of isoproterenol (0.1, 0.25, 0.5, 0.75, 1 and 2 micrograms (mcg)) and saline. Infusion administration was double blinded. Participants were told beforehand they would be receiving both isoproterenol and saline infusions, and were informed what the isoproterenol sensations might feel like (e.g., “you may notice your heart beating faster/stronger, and/or may feel an increase in your breathing sensations”). They were not informed when they would be receiving each agent, but were verbally notified of the beginning of each infusion (e.g., “infusion starting”). Each infusion period lasted approximately 2 minutes. During this period participants were instructed to pay attention to their heartbeat and breathing sensations, and to turn a dial to indicate their ongoing experience of the overall intensity of these body sensations. The dial could range from 0 (“normal, i.e., no change in intensity”) to 10 (“most ever”). The dial was always set to zero at the beginning of each infusion, and participants were specifically instructed to keep the dial at zero if they felt they did not notice any increase in the intensity of heartbeat and breathing sensations above baseline.
To assess interoceptive accuracy, cross correlations for each dose were calculated from mean-centered dial ratings and mean-centered instantaneous heart rate changes occurring during each two-minute infusion interval, at a sample rate of 200 Hz. This signal processing metric compares the degree of similarity between two different waveforms occurring within a specific time period. The measure is influenced by both the presence of peaks and troughs in each time series as well as the temporal relationship (i.e. degrees of overlap) between them. The associated product, the cross correlation, has a range between −1 and 1. The closer the value is to 1, the more closely associated the waveforms are, and the greater the level of interoceptive accuracy. Three measures are typically generated: the cross correlation at zero lag, the maximum cross correlation, and the lag time. The zero cross correlation is the cross correlation between the two time series as the signals have occurred naturally in time. The maximum cross correlation is the maximum possible correlation between the two time series; it is obtained by systematically shifting one waveform across the entire time series and recalculating the cross correlation at each point. The highest value obtained is the maximum cross correlation. The lag time is a variable associated with the maximum cross correlation, and reflects the amount of distance (in this case, time) that one waveform was shifted in order to obtain the maximum cross correlation. Thus in the current study, higher cross correlation values in one group would be expected to provide evidence of greater interoceptive accuracy.
After each infusion, participants provided retrospective ratings of the intensity of heartbeat and breathing sensations. Participants were also instructed to rate the intensity of physical anxiety, mental anxiety, and distress experienced during each infusion using the same 0 to 10 rating scale. Meditators were asked not to meditate during the task, and were instructed to keep their eyes open throughout the rating period. Heart rate was continuously measured throughout each infusion using a lead II electrocardiogram (Biopac Systems, Inc.). This approach to measuring interoceptive awareness was identical to our previously published pharmacological protocols for assessing interoceptive awareness (Khalsa, Rudrauf, Sandesara, et al., 2009; Khalsa et al., 2015; Khalsa et al., 2016). See Supplement for additional details.
2.3. Body map assessment
Immediately after each infusion participants were asked to “draw the areas where you felt your heartbeat (be as accurate as possible)” on a two-dimensional paper manikin. This approach was identical to our previously published studies involving cardiac body sensation maps (Khalsa, Rudrauf, Feinstein, et al., 2009; Khalsa, Rudrauf, Sandesara, et al., 2009)). To facilitate a statistical body map analysis, these outlines were traced manually onto a digital manikin by an expert tracer blinded to the participant grouping. Before moving further a reliability check across 90 randomly selected maps, traced onto the digital manikin by a second tracer, yielded an inter-tracer Dice (Dice, 1945) similarity coefficient ≥ 0.77, suggesting quantitatively reliable agreement comparable to other published manual tracing approaches (Theiss, Ridgewell, McHugo, Heckers, & Blackford, 2017). We then calculated proportional body maps from the binary tracings for each group and condition, selecting only doses for which all 15 participants generated tracings (2 mcg and saline). Thus, each pixel value in the proportional map was equal to the total number of participants reporting sensation in that pixel (maximum of 15) divided by total number of participants (maximum of 15). We then applied spatial smoothing using a Gaussian kernel with a full width half maximum of 6 pixels. This smoothing size was determined from the inter-tracer reliability analysis, representing half of the average non-overlapping areas observed between the two tracers.
2.4. Statistical analysis of body maps
To evaluate between-group differences in the body maps, for each pixel, we calculated the test statistic using the z-formula for proportion (Jekel, 2007)
where pM is the proportion of participants in the meditator group who reported having sensation on that pixel and pNM is the same for the nonmeditator group, pTot is the proportion of participants having sensation when both groups are combined, and NM and NNM are the number of participants in the meditator and nonmeditator groups. To estimate the p-value for the calculated z-value, we performed a permutation analysis in Matlab (Mathworks, Inc.). In this analysis, we assumed that under the null hypothesis, the group labeling of participants (M or NM) is arbitrary, and one can estimate the probability distribution of the test statistic under the null hypothesis by relabeling participants many times and computing the test statistic. When participants are independent from one another, the total number of possible relabelings would be (NM + NNM)!/(NM! NNM!). We used 5000 relabelings, randomly selected from all the possible relabelings, which resulted in an acceptable precision error for the p-value estimation (< .0001) via a random permutation or Monte-Carlo permutation test procedure (modeled after (Michael, Naveteur, Dupuy, & Jacquot, 2015)). The p-value for each pixel was subsequently calculated by computing the number of occurrences of z-value in the resampled conditions equal or larger than the z-value of the actual sample. Pixels with p-values ≤ .05 were considered to have significantly more hits (i.e., reported to have sensation) by the meditator group compared to nonmeditator group (Nichols & Holmes, 2002). This approach was identical to our recently described method for statistical body map analysis (Khalsa, Hassanpour, et al., 2018).
2.5. Statistical analysis of infusion data
2 x 6 repeated measures ANOVAs were performed on each univariate dependent variable of interest, with group (meditators, nonmeditators) as the between subjects factor and with dose of isoproterenol as the within subjects factor. All univariate repeated measures ANOVA tests were assessed for violations of the sphericity assumption, and when violated, were corrected with the Huynh-Feldt method. In these instances the corrected p values are reported, along with the Huynh-Feldt epsilon (ε) correction.
3. Results
3.1. Participants
Meditators reported significantly more years of meditation practice t(28) = 3.90, p = .002, hours of cumulative meditation practice t(28) = 3.07, p = .008, and days of retreat experience t(28) = 2.31, p = .037, than nonmeditators. The groups did not differ with respect to age t(28) = .15, p = .88, body mass index t(28) = −.63, p = .53, or education t(28) = 1.64, p = .11. The groups also did not differ with respect to baseline levels of anxiety (assessed via the Beck Anxiety Inventory) t(28) = 1.39, p = .18, or depression (assessed via the Beck Depression Inventory) t(28) = .33, p = .74.
3.2. Heart rate responses
A 2 x 6 repeated measures ANOVA revealed a main effect of dose F(1, 5) = 50.10, p < .0001, ηp2 = .64, ε = .807, indicating that increasing doses of isoproterenol elicited increases in mean heart rate response. There was no effect of group F(1, 28) = 1.27, p =.27, ηp2 = .04, and there was no group by dose interaction F(1, 5) = .04, p = .998, ηp2 = .00, indicating that isoproterenol infusions elicited equivalent increases in heart rate in both groups. An independent samples t test of CD25 values also did not reveal any group differences in heart rate reactivity to isoproterenol: t(28) = −.35, p = .73. Finally, there were no group differences in average heart rate during the saline infusions: t(28) = .25, p = .80.
3.3. Interoceptive accuracy ratings
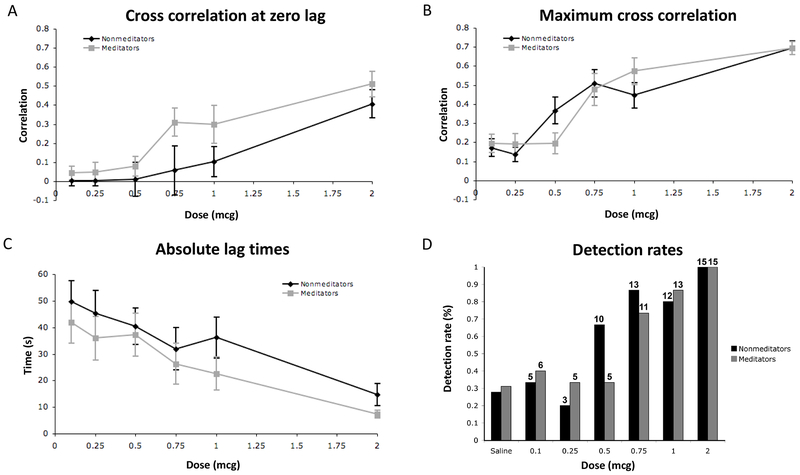
A 2 x 6 repeated measures ANOVA revealed a significant effect of dose on the zero order cross correlation F(1, 5) = 15.37, p < .0001, ηp2 = .35, ε = .857, indicating that participants generated greater zero lag cross correlations at increasing doses of isoproterenol. However, there was no effect of group F(1, 28) = 2.99, p = .10, ηp2 = .10, and there was no group by dose interaction F(1, 5) = 1.06, p = .38, ηp2 = .04, indicating that there were no group differences in the online tracking of interoceptive sensations (Figure 1A).
Figure 1.
Interoceptive accuracy ratings: A) Cross correlations at zero lag between objective heart rate and subjective dial rating for meditators and nonmeditators. B) Maximum cross correlations. C) Absolute lag times. D) Interoceptive detection rates derived from participant dial ratings. Numbers above each column indicate the number of individuals endorsing detection.
A 2 x 6 repeated measures ANOVA also revealed a significant effect of dose on the maximum cross correlation F(1, 5) = 39.92, p < .0001, ηp2 = .59, indicating that participants generated greater maximum cross correlations at increasing doses of isoproterenol. There was again no effect of group F(1, 28) = 0.00, p = .99, ηp2 = .00, and no group by dose interaction F(1, 5) = 2.23, p = .06, ηp2 = .07, indicating that there were no significant group differences in the online tracking of interoceptive sensations (Figure 1B).
A 2 x 6 repeated measures ANOVA revealed a significant effect of dose on the absolute lag times F(1, 5) = 11.81, p < .0001, ηp2 = .30, ε = .756, indicating that participants generated lower absolute lag times at increasing doses of isoproterenol. There was no effect of group F(1, 28) = 1.04, p = .32, ηp2 = .04, and there was no group by dose interaction F(1, 5) = .24, p = .91, ηp2 = .01, indicating that there were no group differences in the online tracking of interoceptive sensations (Figure 1C). (See Figures S1 and S2 in Supplement for depictions of the observed mean heart rate and corresponding mean dial ratings produced by all participants during each infusion.)
Finally, we did not find any significant correlations between life time practice measures in the meditators (cumulative years, cumulative hours, and retreat days) and interoceptive accuracy measures (zero cross correlation, maximum cross correlation, and absolute lag time).
3.4. Interoceptive detection rates
Examination of the individual online dial ratings revealed that for both meditators and nonmeditators, increasing numbers of participants detected increases in heartbeat and breathing sensations at increasing doses (Figure 1D). The lowest increases in sensation were reported during the saline infusions for both groups (28% for the nonmeditators versus 31% for the meditators). A minority of participants in both groups detected increased interoceptive sensations at two lowest doses (0.1, and 0.25 mcg) whereas a majority of participants in both groups detected increased interoceptive sensations at the three highest doses (0.75, 1 and 2 mcg). At the 0.5 mcg dose nonmeditators exhibited higher detection rates than meditators (66% vs 33% respectively). Every single participant (15 out of 15) in both groups perceived increases in sensation at the highest dose (2 mcg).
3.5. Interoceptive intensity ratings
A 2 x 6 repeated measures ANOVA revealed a significant effect of dose on retrospective ratings of heartbeat sensations F(1, 5) = 47.35, p < .0001, ηp2 = .63, ε = .446, indicating that increasing doses of isoproterenol elicited greater increases in the intensity of heartbeat sensations than saline. However, there was no effect of group F(1, 28) = .49, p = .49, ηp2 = .02, and there was no group by dose interaction F(1, 5) = 1.23, p = .30, ηp2 = .04, indicating that there were no group differences in the perceived change in intensity of heartbeat sensations (Supplementary Figure S3A). A 2 x 6 repeated measures ANOVA also revealed a significant effect of dose on retrospective ratings of breathing sensations F(1, 5) = 22.08, p < .0001, ηp2 = .44, ε = .597, indicating that increasing doses of isoproterenol elicited greater increases in the intensity of breathing sensations than saline. However, there was again no effect of group F(1, 28) = .74, p = .40, ηp2 = .03, and there was no group by dose interaction F(1, 5) = 1.29, p = .28, ηp2 = .04, indicating that there were no group differences the perceived change in intensity of breathing sensations (Supplementary Figure S3B).2
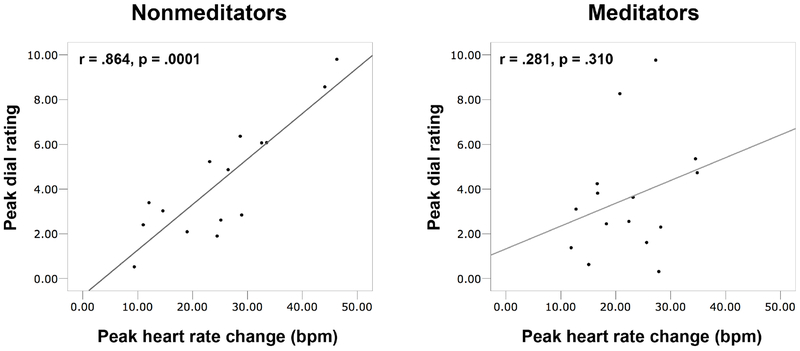
Since all participants reported an increase in sensations at the highest dose (2 mcg), peak heart rate changes and peak interoceptive ratings were examined for group differences. For the nonmeditators, the peak sensation ratings at this dose were highly correlated with the observed peak heart rate changes (r = .864, p = .0001). In contrast, for the meditators, the peak sensation ratings at the highest dose were uncorrelated (r = .281, p = .310) (Figure 2). A test for differences among these correlations was significant (Fisher r-to-z = 2.5, p = .012). There were no group differences in peak heart rate change t(28) = .84, p = .41, or peak dial rating t(28) = .81, p = .43.
Figure 2.
Correlations between peak subjective dial ratings and peak objective heart rate responses at 2 mcg for nonmeditators and meditators.
3.6. Cardiac body maps
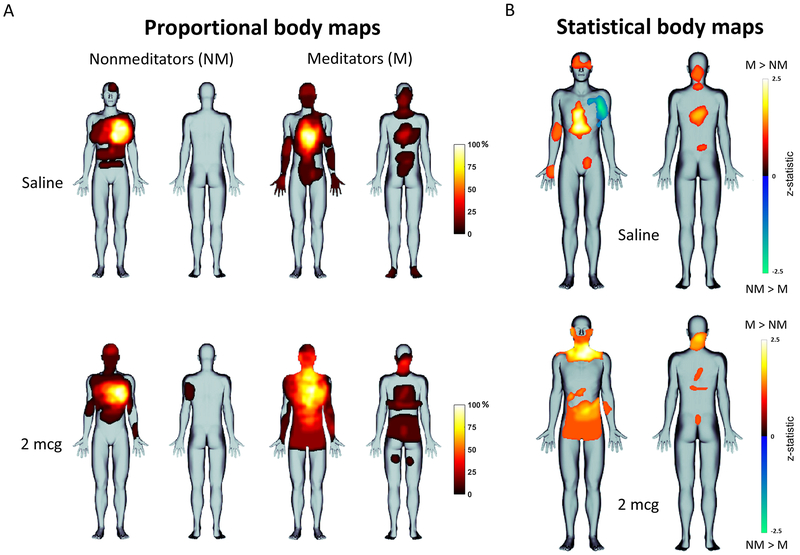
Proportional maps of heartbeat sensation location revealed that nonmeditators focally perceived heartbeat sensations in the same regions of the body at rest and during stimulation with isoproterenol, in the upper left region of the anterior chest. Meditators also perceived heartbeat sensations in the chest, but in the central region of the anterior and posterior chest across a broader area, and also in other regions of the body (Figure 3A). The statistical body map analysis showed that, during saline infusions, meditators felt heartbeat sensations prominently in the midline chest, upper face, neck, arms, lower abdomen, and central back. During the 2mcg infusion, meditators reported a substantial expansion of heartbeat sensation throughout the body, with statistically significant increases in the entire neck, lower face, lower abdomen, and central back relative to nonmeditators (Figure 3B).
Figure 3.
A) Proportional body maps showing group heartbeat sensation topography in nonmeditators and meditators. B) Statistical body maps (thresholded at p ≤ .05) showing quantitative group differences in heartbeat sensation localization for saline (top) and isoproterenol 2 mcg (bottom).
3.7. Affective measures
A 2 x 6 repeated measures ANOVA revealed a significant effect of dose on retrospective ratings of physical anxiety F(1, 5) = 12.16, p < .0001, ηp2 = .38, ε = .419, indicating that participants experienced an increase in physical anxiety at increasing doses of isoproterenol. There was no effect of group F(1, 28) = .13, p = .72, ηp2 = .01, and there was no group by dose interaction F(1, 5) = .55, p = .59, ηp2 = .03, indicating that the groups did not differ in their experience of physical anxiety (Supplementary Figure S4A).
A 2 x 6 repeated measures ANOVA revealed a significant effect of dose on retrospective ratings of mental anxiety F(1, 5) = 2.73, p = .04, ηp2 = .12, ε = .711, indicating that participants experienced a small increase in mental anxiety at increasing doses of isoproterenol. There was no effect of group F(1, 28) = .90, p = .35, ηp2 = .04, and there was no group by dose interaction F(1, 5) = 1.01, p = .40, ηp2 = .05, indicating that the groups were not different in their experience of mental anxiety (Supplementary Figure S4B).
A 2 x 6 repeated measures ANOVA did not reveal any effect of dose on retrospective ratings of distress F(1, 5) = 1.78, p = .16, ηp2 = .08, ε = .600, indicating that participants did not experience any increases in distress at increasing doses of isoproterenol. There was also no effect of group F(1, 28) = .26, p = .61, ηp2 = .01, and there was no group by dose interaction F(1, 5) = .31, p = .82, ηp2 = .02 (Supplementary Figure S4C).
3.8. Post hoc analysis
Given our directional hypothesis that meditators would show a significantly greater interoceptive accuracy, and in light of the absence of such a finding, we examined the data in further detail. Since the majority of individuals in both groups reported increases in sensation and changes in interoceptive accuracy at the three highest doses (0.75, 1, 2 mcg), we computed average cross correlations for these doses and compared them for differences.3 This time there was a significant difference between the mean zero order cross correlation for the three highest doses: one tailed t(28) = 1.83, p = .04, indicating that across these doses on average, meditators generated greater zero cross correlations (Supplementary Figure S5A). However, when accounting for the lag time there was no difference in the mean maximum cross correlation: one tailed t(28) = .46, p = .32 (Supplementary Figure S5B), and there was no significant difference in the mean lag time at these doses: one tailed t(28) = 1.34, p = .10 (Supplementary Figure S5C). Since the zero order cross correlation is sensitive to temporal shifts between the signals being compared, a final component of the analysis examined whether the group difference in zero order cross correlations at the higher doses was related to differences in the timing of dial ratings, in the form of peak-to-peak delay (in this case, the peak dial rating relative to the peak heart rate change) (Supplementary Figure S6A). There was no significant evidence for this possibility (one tailed t(28) = −1.40, p = .09) (Supplementary Figure S6B).
The current study used a modest sample size, and since there was a small numerical difference in the average maximum cross correlation in favor of the meditators, we conducted a power analysis based on the current effects to determine the sample size required to achieve a statistically meaningful result. We found that even if group sizes were increased 10-fold, there would be a definite difference in the mean zero cross correlation (one tailed t(298) = 5.99, p < .0001) and mean absolute lag time (one tailed t(298) = 4.36, p < .0001) for the highest doses, but there would still not be a significant difference in the mean maximum cross correlation for the three highest doses (one tailed t(298) = 1.50, p = .07) (see Table 2 for confidence intervals (CI) for differences between means).
Table 2.
Confidence intervals for differences between means on interoceptive accuracy scores.
| Number per group (N) | 95 % Confidence interval | 99% Confidence interval | |
|---|---|---|---|
| Cross correlation at zero lag | |||
| N = 15 | −.021 to .391 | −.093 to .463 | |
| N = 30 | .044 to .325* | −.002 to .372 | |
| N = 150 | .125 to .245* | .107 to .264* | |
| Maximum cross correlation | |||
| N = 15 | −.106 to .167 | −.154 to .215 | |
| N = 30 | −.062 to .123 | −.092 to .153 | |
| N = 150 | −.010 to .077 | −.023 to .084 | |
| Absolute lag times | |||
| N = 15 | −22.4 to 4.7 | −27.2 to 9.4 | |
| N = 30 | −18.1 to .34 | −21.1 to 3.4 | |
| N = 150 | −12.9 to −4.9* | −14.1 to −3.6* |
Note: Confidence intervals are for differences between the mean cross correlation of the three highest doses (meditators minus nonmeditators). Confidence intervals for the larger samples were derived by extrapolation from the mean differences observed in the current sample.
Represents a mean difference greater than or less than zero.
3.9. Meta-analysis
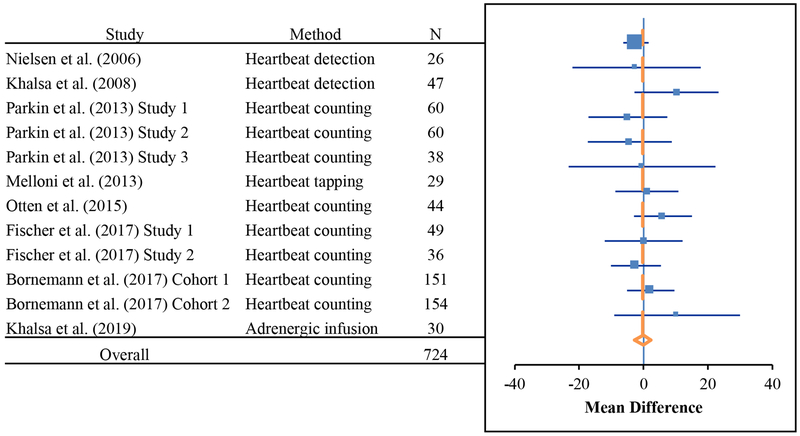
In order to support a more definitive conclusion with respect to the association between cardiac interoceptive accuracy and the practice of meditation, we subsequently applied a meta-analytic approach. We identified all English language articles (8 studies reporting a total of 12 experiments) reporting comparisons of cardiac interoceptive accuracy between meditators (N = 332) relative to nonmeditators (N = 392), and computed a grouped estimate of standardized differences between means using a random effects model. Some studies were cross sectional, others were longitudinal. Some included expert meditators, some included novices (e.g., longitudinal studies). The meditator groups in these studies spanned multiple traditions and techniques (e.g., Tibetan Buddhist, Kundalini, Vipassana, and Mindfulness). There were also a number of different tasks used to assess interoceptive accuracy (e.g., heartbeat detection, heartbeat counting, heartbeat tapping, and adrenergic infusion). In each case, we selected the mean that pertained to the contrast most likely to evaluate an association between meditation training and increased interoceptive accuracy (e.g., the post-training group means for a longitudinal intervention study). The result clearly indicated that there was no mean difference (MD) across all studies of cardiac interoceptive accuracy in meditators relative to nonmeditators (MD = −.30, 95% CI-: −9.12, CI+: 29.9) (Table 3, Figure 4). See Supplement for additional details including publication bias assessment.
Table 3.
Breakdown of individual studies included in meta-analysis, and their contribution to the overall estimated effect.
| Experiment | Study | Type | Method | Meditators | Tradition | N | MD (95% CI) | z | p | Weight (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Nielsen et al. (2006) | Cross-sectional | Heartbeat detection | Experienced | Buddhist | 26 | −2.4 (−6.3, 1.5) | −1.20 | 0.23 | 41.7 |
| 2 | Khalsa et al. (2008) | Cross-sectional | Heartbeat detection | Experienced | Kundalini, Buddhist | 47 | −2.2 (−22.1, 17.7) | −0.22 | 0.83 | 1.6 |
| 3 | Parkin et al. (2013) Study 1 | Longitudinal | Heartbeat counting | Non experienced | Mindfulness | 60 | 10.2 (−2.9, 23.3) | 1.53 | 0.13 | 3.8 |
| 4 | Parkin et al. (2013) Study 2 | Longitudinal | Heartbeat counting | Non experienced | Mindfulness | 60 | −4.9 (−17.2, 7.4) | −0.78 | 0.43 | 4.3 |
| 5 | Parkin et al. (2013) Study 3 | Longitudinal | Heartbeat counting | Non experienced | Mindfulness | 38 | −4.3 (−17.3, 8.7) | −0.65 | 0.52 | 3.8 |
| 6 | Melloni et al. (2013) | Cross-sectional | Heartbeat tapping | Experienced & non experienced | Mindfulness | 29 | −0.5 (−23.3, 22.3) | −0.04 | 0.97 | 1.2 |
| 7 | Otten et al. (2015) | Cross-sectional | Heartbeat counting | Experienced | Mindfulness | 44 | 1 (−8.8, 10.8) | 0.20 | 0.84 | 6.7 |
| 8 | Fischer et al. (2017) Study 1 | Longitudinal | Heartbeat counting | Non experienced | Mindfulness | 49 | 6 (−3.0, 15.0) | 1.31 | 0.19 | 8.0 |
| 9 | Fischer et al. (2017) Study 2 | Longitudinal | Heartbeat counting | Non experienced | Mindfulness | 36 | 0 (−12.1, 12.1) | 0 | 1 | 4.4 |
| 10 | Bornemann et al. (2017) Cohort 1 | Longitudinal | Heartbeat counting | Non experienced | Mindfulness | 151 | −2.4 (−10.2, 5.4) | −0.61 | 0.54 | 10.7 |
| 11 | Bornemann et al. (2017) Cohort 2 | Longitudinal | Heartbeat counting | Non experienced | Mindfulness | 154 | 2.2 (−5.2, 9.6) | 0.59 | 0.56 | 12.0 |
| 12 | Khalsa et al. (2019) | Cross-sectional | Adrenergic infusion | Experienced | Vipassana, Kundalini | 30 | 10.4 (−9.1, 29.9) | 1.04 | 0.30 | 1.7 |
| Overall | 724 | −0.30 (−2.35, 2.24) | −0.23 | 0.81 | 100% | |||||
MD: mean difference. CI: confidence interval.
Figure 4.
Meta-analysis showing a negligible mean difference across 12 experiments evaluating interoceptive accuracy for heartbeat sensations in nonmeditators (N = 392) and meditators (N = 332).
4. Discussion
Our findings do not support the hypothesis that the practice of meditation is associated with increased interoceptive accuracy for the heartbeat. There were no group differences observed in any of the first pass interoceptive awareness measures employed including cross correlations of continuous dial ratings indexing interoceptive accuracy, dial deflections indexing interoceptive detection, and retrospective ratings assessing changes in the intensity of interoceptive sensations. A secondary analysis of the three highest doses combined, for which a majority of individuals in both groups reported increased interoceptive sensation, did reveal a group difference in the zero order cross correlations. However, group differences in this cross correlation measure were not maintained after accounting for the lag time, suggesting that the effect was related to a temporal shift in experience rather than a fundamental change in signal processing. Furthermore, a meta-analysis of all published studies examining the association between meditation training and cardiac interoceptive accuracy did not show any evidence of differences relative to nonmeditators. Overall, these results clearly demonstrate that meditation is not associated with increased interoceptive accuracy for 1) heartbeat sensations at rest, and 2) cardiorespiratory sensations during conditions of elevated physiological arousal.
Although our previous study of interoceptive accuracy (Khalsa et al., 2008) also did not find any differences, it was subject to the limitation that most individuals cannot accurately perceive their heartbeat under resting physiological conditions (Khalsa & Lapidus, 2016). This limitation was addressed by the current design using pharmacological modulation of heartbeat sensation, as evidenced by the fact that every individual in the study displayed increased interoceptive accuracy with increasing doses of isoproterenol. An additional feature of the current study was our selection of a group of predominantly Vipassana meditators, a tradition which heavily cultivates awareness of internal body sensations. These individuals were also in earlier phases of their training, when a greater emphasis is placed on practicing awareness of interoceptive sensations. Thus the current paradigm captured appropriate changes in interoceptive awareness in all subjects tested, incorporated a group of meditators from traditions that primarily practices awareness of interoceptive sensations, and extended the observation of a lack of differences in accuracy beyond heartbeat sensations to also include breathing sensations, a sensory capacity that is more commonly involved in the meditation practice. These results therefore provide the strongest evidence to date that practicing attention to internal body sensations, a core feature of meditation, is not associated with enhanced accuracy of awareness for the range of cardiorespiratory sensations that are commonly encountered throughout daily life.
These negative cross-sectional findings stand in prominent contrast with several recent reports concluding that the longitudinal practice of meditation improves interoceptive accuracy. Bornemann and colleagues found that individuals reported increases in self-perceived interoceptive awareness after engaging in a three-month contemplative training program (Bornemann, Herbert, Mehling, & Singer, 2015). The key finding was that individuals receiving ‘body scan’ and ‘breath meditation’ training reported greater increases in self-perceived interoceptive awareness after the training relative to a retest control group, on a German version of the Multidimensional Assessment of Interoceptive Awareness scale (Mehling et al., 2012) (differences were particularly prominent for the self-regulation, attention regulation, and body trusting subscales). While the use of a longitudinal design follows recent consensus recommendations, this finding relied on a self-report measure rather than an objective test of interoceptive accuracy. Furthermore, despite the fact that the test-retest control group did not differ on basic demographic variables, it is unclear whether both groups were equally masked from the study hypotheses. The authors make clear that individuals in the contemplative arm were informed that the daily practice of body scan and breath meditation activities were specifically aimed at improving attention and interoceptive awareness, whereas the comparison group was informed that the study was generally aimed at understanding ‘personality and emotion.’ In a follow up multi-cohort study, Bornemann & Singer (Bornemann & Singer, 2017) reported that participants in the same contemplative training program displayed increased interoceptive accuracy scores on the heartbeat counting test over the course of training. However, instead of an omnibus effect of training, they reported a significant group by training interaction. A closer inspection of the data revealed that the group by time interaction was actually non-significant immediately after the presence module training focusing on body awareness, but became significant after including the next training module (affect training in one cohort, perspective training in the other), suggesting a complex effect that was likely driven by multiple elements of contemplative training beyond the body-focused intervention. The advantages of this study were that it provided a longitudinal evaluation of the effects of contemplative training on interoceptive accuracy, in a large sample, using a task-based measure—namely, heartbeat counting accuracy scores. Another longitudinal study also reported a similar group by time interaction associated with increases in heartbeat counting accuracy following an 8 week body scan training intervention (Fischer et al., 2017). While the effect itself (i.e. the group by time interaction reported in both studies) is not in question, the interpretation of its meaning deserves careful scrutiny largely based on the utilization of a methodologically confounded measure. While we and others have repeatedly discussed the problems in interpreting heartbeat counting accuracy scores, including the lack of a statistical measure to evaluate individual performance, the possible influence of prior knowledge about the resting heart rate and the relative insensitivity of the task to actual changes in heart rate (Khalsa & Lapidus, 2016; Khalsa et al., 2008; Khalsa, Rudrauf, Sandesara, et al., 2009; Windmann, Schonecke, Frohlig, & Maldener, 1999), several recent studies have added empirical demonstrations casting doubt on its construct validity. Time estimation ability, IQ scores and knowledge of one’s resting heart rate substantially influence heartbeat counting accuracy (Murphy et al., 2018). Counting accuracy scores correlate poorly with measured heart rates (Zamariola, Maurage, Luminet, & Corneille, 2018), and are substantially influenced by cognitive strategies (Desmedt, Luminet, & Corneille, 2018). Moreover, counting accuracy scores show limited test-retest reliability (i.e., intraclass correlation coefficient (ICC) = 0.42 [95% CI: 0.27-0.58]) (Wittkamp, Bertsch, Vogele, & Schulz, 2018), and do not correlate with more rigorous measures of resting cardiac interoception (Ring & Brener, 2018). We have recently suggested an alternative interpretation of this measure within the framework of perceptual inference, whereby heartbeat counting accuracy scores predominantly reflect the accuracy of an individual’s prior beliefs about their heart rate rather than being an objective index of accuracy with respect to heart beat sensation signals (Paulus, Feinstein, & Khalsa, 2019). According to this concept, the repeated attentional focus on the internal state of the body during meditation leads to changes in the perceptual representation of the body—in the form of increased precision weighting of heart rate priors—leading to greater posterior probability estimates of heartbeat occurrence which are unrelated to the actual sensory evidence of cardiac data. Correspondingly, the observed changes in heartbeat counting accuracy observed by the meditators in Bornemann & Singer (2017) and Fischer et al. (2017) would be best interpreted as reflecting the modulation of an inferential belief system about the body. Such a phenomenon is consistent with the theoretical suggestion that meditation modifies interoceptive experiences by altering a simulation map, which is defined as ‘not identical to current sensation, but is rather an abstraction from recent sensory experience’ (Farb et al., 2015). Such a process does not have to be specific to interoception but could reflect a more general adaptation of inferential systems, as for example, participants in the Bornemann & Singer (2017) study also exhibited similar changes in self-reported levels of alexithymia. Overall, the results of our study and the meta-analysis of available literature lead us to the conclusion that it is highly unlikely that the practice of meditation results in improved interoceptive accuracy for heartbeat sensations.
Despite the lack of quantitative differences in interoceptive accuracy in the current study, meditators exhibited several important differences in the quality of heartbeat sensation experience. First, meditators exhibited an uncoupling between the peak magnitude of the heart rate response during the 2mcg infusion, as evidenced by a lack of correlation between peak subjective and objective measures. This finding is notable given the tight correspondence of such sensations observed in the nonmeditator group, although it could be considered preliminary given that correlation coefficients are typically magnified by small sample sizes and can be substantially influenced by outliers when samples are small (Schonbrodt & Perugini, 2013). Second, meditators displayed differences in the location of perceived heartbeat sensations within the body. Under resting conditions meditators reported preferentially feeling the heartbeat in midline regions of the body including the central chest, upper abdomen, upper face, and central back, whereas nonmeditators felt the heartbeat more in the upper left region of the chest. This pattern shifted markedly during cardiorespiratory arousal modulation, with meditators preferentially reporting perception of the heartbeat in the entire neck, lower face, and lower abdomen. The resting heartbeat sensation has been previously localized to many of these same regions in nonmeditators during resting conditions in heartbeat detection studies (Jones, 1994; Jones, Jones, Rouse, Scott, & Caldwell, 1987; Ring & Brener, 1992) as well as during our previous studies involving physiological arousal modulation (Khalsa, Rudrauf, Feinstein, et al., 2009; Khalsa, Rudrauf, Sandesara, et al., 2009; Hassanpour et al., 2018). To our knowledge, no studies have previously described group differences in the localization of these sensations in meditators, although the present study is not capable of precisely distinguishing the neural origin of the heartbeat sensation signal in the meditators and nonmeditators (e.g., baroreceptor afferents within the thoracic cavity vs. somatosensory afferents within the skin; see Supplement for a discussion of potential underlying factors).
There are also alternative explanations for the differences in localization of heartbeat sensations to consider beyond the mechanistic accounts offered here. The first relates to potential differences in the manner in which meditators conceptualize body processes. For example, in line with notions of diffuse and localized forces in the body such as “energy” or “Chi” that are articulated in certain meditation and alternative medicine traditions, meditators in the current study could have been thinking differently about their heartbeats in a manner distinct from the process of physiological manipulation employed by the current study (see (Ma-Kellams, 2014) for a discussion of cultural influences on conceptualization of interoceptive processes). Importantly however, our observation of a shift in heartbeat sensation location during the isoproterenol infusions suggest that such conceptualizations, if present, were actually modified by the induced changes occurring within the body. The second relates to the possibility that the meditators were somehow interpreting their heartbeat sensations differently than the nonmeditators. This could occur in the context of a disproportionate preoccupation with physical symptoms, as is often observed in individuals with anxiety, depression, or somatic symptom disorders. Although there is presently no evidence to support these notions (i.e., self-reported psychiatric and neurological disorders were exclusionary in the current study), nearly a third of individuals in the United States report using meditation to help with problems of anxiety, stress, or depression (Cramer et al., 2016), and negatively valenced experiences have sometimes been associated with meditation practice (Lindahl, Fisher, Cooper, Rosen, & Britton, 2017). Future studies could more carefully examine these possibilities, perhaps by conducting detailed psychological and psychiatric evaluations of meditators and nonmeditators (e.g., using standardized clinical interviews).
4.1. Limitations and study considerations
Although the current findings were derived using rigorous and unbiased methods, several limitations warrant deliberation. The current study employed a small sample size, which raises concerns that it may not have been sufficiently powered to detect the hypothesized effects. To address this concern, we conducted a power analysis to examine the potential impact increasing the sample size on the observed results. This analysis revealed that while increasing sample size by an order of magnitude (e.g., to 150 individuals per group) might be sufficient to detect an effect on interoceptive attention via greater zero order cross correlations using two-tailed tests, it was highly unlikely to impact the critical comparison, namely, the mean difference in maximum cross correlation for the highest doses. We also conducted a meta-analysis of all existing studies comparing interoceptive accuracy for heartbeat sensations in nonmeditators and meditators (N = 724 total), and found no evidence for any differences whatsoever. Considering this information, it seems highly unlikely that the present findings are spurious.
The current study also employed a cross sectional design, which limits the ability to make causal inferences about the impact of meditation training on interoceptive ability. Conducting longitudinal investigations into the effects of meditation on interoceptive awareness would help to overcome this limitation. Such studies should ideally incorporate randomized recruitment in order to remove the potential bias of expectation of effects. This is not an inconsequential issue, as we have previously showed that the practice of meditation is associated with differences in beliefs about interoceptive task performance and task difficulty (Khalsa et al., 2008). In the interest of practicality, such designs might focus on the effects of shorter periods of intensive training, as often occurs in the context of meditation retreats. These retreats provide a unique opportunity to identify causal effects that might occur over discrete time periods, as a result of standardized training. Inclusion of a highly rigorous measure of interoceptive awareness is an absolute requirement of such studies, as the absence of such measures critically impairs the interpretability of any subsequent finding.
There was likely to be some variation in the types of meditation training received by the meditators in the current study, even for individuals within the same meditation tradition. For example, many of the Kundalini and Vipassana practitioners in the study received training from different meditation teachers. Although this was not expected to have a great influence, we cannot guarantee that differences in instruction (or consequently, differences in daily meditation practice) might not have influenced the observed effects.
The methodology of the current study relied on several assumptions to induce and then measure changes in interoceptive awareness. 1) We assumed a strong relationship between induced cardiac changes and resulting cardiac intensity ratings, based on the fact that human heart rate responses to isoproterenol are highly correlated (r = 0.7) with induced myocardial contractility changes (Cleaveland, Rangno, & Shand, 1972; George, Conolly, Fenyvesi, Briant, & Dollery, 1972; Quinones, Gaasch, & Alexander, 1976; Quinones, Gaasch, Cole, & Alexander, 1975). The heart rate response is routinely used in human studies employing isoproterenol likely because it is easier to measure than other contractility approaches (e.g., impedance cardiography, which is technically more complicated and provides an indirect estimate). Following the lead of these studies, we elected to use heart rate responses as a proxy for isoproterenol’s effect on the body. Heart rate responses to isoproterenol are commonly used to calculate an index of peripheral beta-adrenergic receptor sensitivity called the Chronotropic Dose 25 (CD25), which refers to the isoproterenol dose required to increase the heart rate by 25 beats per minute. It is estimated by linear regression for each individual from the heart rate response to graded infusions of isoproterenol (Cleaveland et al., 1972; Dimsdale, Ziegler, & Graham, 1988; Mills, Dimsdale, Ancoli-Israel, Clausen, & Loredo, 1998). We calculated CD25 values in the current study and did not find any group differences. We have previously found (using heart rate as an index of isoproterenol response) strong evidence that heart rate responses are positively related with subjective intensity ratings at the 2 mcg dose in nonmeditators, both in terms of overall dose-dependent increases in intensity ratings at different doses of isoproterenol, and also in terms of a correlation between peak dial intensity ratings and peak heart rate responses (r = .745, p = .001, in Khalsa et al 2008; r = .864, p =.0001, in the current study, both N = 15). While these observations do not address etiology (i.e. why heart rate responses are related to subjective intensity ratings), they provide sufficient evidence that it is possible to measure both physiological responses to isoproterenol and infer the relationship between objective physiological and subjective perceptual responses in modest samples of human participants using a rate-based measure. 2) We instructed participants to use the dial to rate the overall intensity of perceived heartbeat/breathing sensations (i.e., rather than heartbeat alone) because a) the functions of the heart and lungs are integrally linked, b) isoproterenol impacts both organs simultaneously, and c) we wanted to maximize our ability to detect all perceptual changes induced by the drug. In order to evaluate whether a person was perceiving their heartbeat or breath during infusions, after each infusion we also collected separate retrospective ratings of perceived heartbeat and breathing sensations. This approach should have been capable of identifying if there was an isolated effect on changes in heartbeat perception, or separately, on breath perception. Neither of which was observed. The validity of this approach has been directly supported by our repeated observations of dose-dependent increases in a) retrospective heartbeat intensity, b) retrospective breathing intensity, and c) concurrent cardiorespiratory interoceptive accuracy (i.e. cross-correlations between dial ratings and heart rate) in previous studies, all of which used the same randomized, double-blinded, and placebo controlled approach (Khalsa et al., 2015; Khalsa et al., 2016; Khalsa, Hassanpour, et al., 2018; Khalsa, Rudrauf, Sandesara, et al., 2009). Future studies could consider incorporating a measure of respiratory change in computing the cross-correlation measure of interoceptive accuracy (as well as a cardiac contractility measure) to evaluate whether it would yield a more precise indication of the perceptual response to the drug. Based on these considerations, the methodological rigor in measuring interoceptive awareness provided by the isoproterenol approach, and the meta-analysis described earlier, we argue that the null findings are more likely to be explained by the lack of an association between increased interoceptive accuracy and meditation practice than by methodological limitations.
Finally, while we did not observe evidence of group differences in respiratory perception in this study, we did not focus primarily on this physiological sensation, which is more commonly involved in meditation practices. Therefore, we cannot as confidently rule out the possibility of an effect in this domain (e.g., see (Levinson, Stoll, Kindy, Merry, & Davidson, 2014; Wielgosz, Schuyler, Lutz, & Davidson, 2016)).
4.2. Conclusion
Overall, the results of this study provide strong evidence against the notion that practicing attention to internal body sensations, a core feature of meditation, is associated with increased accuracy of awareness for the heartbeat sensation.
Supplementary Material
Acknowledgements
We thank Shinzen Young for assistance with participant recruitment, Brian Olshansky, Chirag Sandesara, and Erik St. Louis for assistance with electrocardiogram review, Sonia Schubert for help with administration of the isoproterenol protocol, Becky Triplett and the IV ads pharmacy staff for isoproterenol preparation, Eric Breese and Rachel Lapidus for assistance with body map ratings transfer, the University of Iowa General Clinical Research Center for institutional oversight. This project was supported by the National Center for Complementary & Integrative Health (NCCIH) NIH/NCCIH F31AT003061 (S.S.K.), the Mind and Life Institute (S.S.K.), the National Institute for Mental Health (NIMH) NIH/NIMH K23MH112949 (to S.S.K.), by NIGMS P20GM121312 (to S.S.K.), The William K. Warren Foundation (to S.S.K), Support for D.T. includes: NIH P50 MH0942581, NIH U01 NS103780, and Kiwanis International Neuroscience Research Foundation. The authors do not have any financial or non-financial conflicts of interest to report in the materials discussed in this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The inclusion of practitioners from multiple traditions was justified by our previous study in which we observed similar differences in interoceptive awareness in Tibetan Buddhist and Kundalini practitioners, as well as a theoretical motivation to discern the effects of meditation on interoception across contemplative traditions.
A secondary analysis not reported here examined the 11 Vipassana meditators and the corresponding 11 matched nonmeditators. The same pattern of results was found, namely, significant effects of isoproterenol dose on increasing detection rates, cross correlations, and retrospective intensity ratings, in the absence of any group effects or group by dose interactions whatsoever.
For this analysis, any values from participants who did not turn the dial above zero were excluded, as this would have artificially lowered the zero order cross correlations and increased the lag times for each group.
References
- Arambula P, Peper E, Kawakami M, & Gibney KH (2001). The physiological correlates of Kundalini Yoga meditation: a study of a yoga master. Appl Psychophysiol Biofeedback, 26(2), 147–153. 10.1023/A:1011343307783 [DOI] [PubMed] [Google Scholar]
- Arias AJ, Steinberg K, Banga A, & Trestman RL (2006). Systematic review of the efficacy of meditation techniques as treatments for medical illness. J Altern Complement Med, 12(8), 817–832. 10.1089/acm.2006.12.817 [DOI] [PubMed] [Google Scholar]
- Barnes PM, Bloom B, & Nahin RL (2008). Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report(12), 1–23. 10.1037/e623942009-001 [DOI] [PubMed] [Google Scholar]
- Bhajan Y, Khalsa GS (2000). Breathwalk: breathing your way to a revitalized body, mind and spirit. New York: Broadway Books. [Google Scholar]
- Bornemann B, Herbert BM, Mehling WE, & Singer T (2015). Differential changes in self-reported aspects of interoceptive awareness through 3 months of contemplative training. Front Psychol, 5, 1504 10.3389/fpsyg.2014.01504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornemann B, & Singer T (2017). Taking time to feel our body: Steady increases in heartbeat perception accuracy and decreases in alexithymia over 9 months of contemplative mental training. Psychophysiology, 54(3), 469–482. 10.1111/psyp.12790 [DOI] [PubMed] [Google Scholar]
- Clarke TC, Barnes PM, Black LI, Stussman BJ, & Nahin RL (2018). Use of Yoga, Meditation, and Chiropractors Among U.S. Adults Aged 18 and Over. NCHS Data Brief (325), 1–8. [PubMed] [Google Scholar]
- Cleaveland CR, Rangno RE, & Shand DG (1972). A standardized isoproterenol sensitivity test. The effects of sinus arrhythmia, atropine, and propranolol. Arch Intern Med, 130(1), 47–52. https://doi:10.1001/archinte.1972.03650010035007 [DOI] [PubMed] [Google Scholar]
- Cramer H, Hall H, Leach M, Frawley J, Zhang Y, Leung B, … Lauche R. (2016). Prevalence, patterns, and predictors of meditation use among US adults: A nationally representative survey. Sci Rep, 6, 36760 10.1038/srep36760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Sze J, Kerr CE, Kemeny ME, & Mehling W (2013). Follow your breath: respiratory interoceptive accuracy in experienced meditators. Psychophysiology, 50(8), 777–789. 10.1111/psyp.12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt O, Luminet O, & Corneille O (2018). The heartbeat counting task largely involves non-interoceptive processes: Evidence from both the original and an adapted counting task. Biol Psychol, 138, 185–188. 10.1016/j.biopsycho.2018.09.004 [DOI] [PubMed] [Google Scholar]
- Dice LR (1945). Measures of the amount of ecologic association between species. Ecology, 26, 297–302. 10.2307/1932409 [DOI] [Google Scholar]
- Dimsdale J, Ziegler M, & Graham R (1988). The effect of hypertension, sodium, and race on isoproterenol sensitivity. Clin Exp Hypertens A, 10(5), 747–756. 10.1080/07300077.1988.11878781 [DOI] [PubMed] [Google Scholar]
- Farb N, Daubenmier J, Price CJ, Gard T, Kerr C, Dunn BD, … Mehling WE (2015). Interoception, contemplative practice, and health. Front Psychol, 6, 763 10.3389/fpsyg.2015.00763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Messner M, & Pollatos O (2017). Improvement of Interoceptive Processes after an 8-Week Body Scan Intervention. Front Hum Neurosci, 11, 452 10.3389/fnhum.2017.00452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CF, Conolly ME, Fenyvesi T, Briant R, & Dollery CT (1972). Intravenously administered isoproterenol sulfate dose-response curves in man. Arch Intern Med, 130(3), 361–364. https://doi.org/0.1001/archinte.1972.03650030041010 [PubMed] [Google Scholar]
- Goldberg SB, Tucker RP, Greene PA, Davidson RJ, Wampold BE, Kearney DJ, & Simpson TL (2018). Mindfulness-based interventions for psychiatric disorders: A systematic review and meta-analysis. Clin Psychol Rev, 59, 52–60. 10.1016/j.cpr.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, … Haythornthwaite JA (2014). Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med, 174(3), 357–368. 10.1001/jamainternmed.2013.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart W (1987). The art of living: Vipassana meditation as taught by S.N. Goenka. New York: Harper Collins. [Google Scholar]
- Hassanpour MS, Simmons WK, Feinstein JS, Luo Q, Lapidus RC, Bodurka J, … Khalsa SS (2018). The insular cortex dynamically maps changes in cardiorespiratory interoception. Neuropsychopharmacology, 43(2), 426–434. 10.1038/npp.2017.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour MS, Yan L, Wang DJ, Lapidus RC, Arevian AC, Simmons WK, … Khalsa SS (2016). How the heart speaks to the brain: neural activity during cardiorespiratory interoceptive stimulation. Philos Trans R Soc Lond B Biol Sci, 371(1708). 10.1098/rstb.2016.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman-Lagerlof M, Hedman-Lagerlof E, & Ost LG (2018). The empirical support for mindfulness-based interventions for common psychiatric disorders: a systematic review and meta-analysis. Psychol Med, 48(13), 2116–2129. 10.1017/S0033291718000259 [DOI] [PubMed] [Google Scholar]
- Jekel JF (2007). Epidemiology, Biostatistics, and Preventive Medicine (3rd edition ed.). Philadelphia, PA: Elsevier Health Sciences-Medical. [Google Scholar]
- Jones GE (1994). Perception of visceral sensations: a review of recent findings, methodologies, and future directions. (Vol. 5). London: Jessica Kingsley Publishers. [Google Scholar]
- Jones GE, Jones KR, Rouse CH, Scott DM, & Caldwell JA (1987). The effect of body position on the perception of cardiac sensations: an experiment and theoretical implications. Psychophysiology, 24(3), 300–311. 10.1111/j.1469-8986.1987.tb00300.x [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J (1990). Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness: Delta Trade Paperbacks. [Google Scholar]
- Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, … Interoception Summit Participants. (2018). Interoception and Mental Health: A Roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging, 3(6), 501–513. 10.1016/j.bpsc.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Craske MG, Li W, Vangala S, Strober M, & Feusner JD (2015). Altered interoceptive awareness in anorexia nervosa: Effects of meal anticipation, consumption and bodily arousal. Int J Eat Disord, 48(7), 889–897. 10.1002/eat.22387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Feinstein JS, Li W, Feusner JD, Adolphs R, & Hurlemann R (2016). Panic anxiety in humans with bilateral amygdala lesions: Pharmacological induction via cardiorespiratory interoceptive pathways. J Neurosci, 36(12), 3559–3566. 10.1523/jneurosci.4109-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Hassanpour MS, Strober M, Craske MG, Arevian AC, & Feusner JD (2018). Interoceptive anxiety and body representation in anorexia nervosa. Front Psychiatry, 9, 444 10.3389/fpsyt.2018.00444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, & Lapidus RC (2016). Can interoception improve the pragmatic search for biomarkers in psychiatry? Front Psychiatry, 7, 121 10.3389/fpsyt.2016.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Damasio AR, Davidson RJ, Lutz A, & Tranel D (2008). Interoceptive awareness in experienced meditators. Psychophysiology, 45(4), 671–677. 10.1111/j.1469-8986.2008.00666.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, & Tranel D (2009). The pathways of interoceptive awareness. Nat Neurosci, 12(12), 1494–1496. 10.1038/nn.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Sandesara C, Olshansky B, & Tranel D (2009). Bolus isoproterenol infusions provide a reliable method for assessing interoceptive awareness. Int J Psychophysiol, 72(1), 34–45. 10.1016/j.ijpsycho.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfield J (1996). Living Dharma: Teachings of Twelve Buddisht Masters. Boston: Shambala Publications. [Google Scholar]
- Lawrence M, Celestino Junior FT, Matozinho HH, Govan L, Booth J, & Beecher J (2017). Yoga for stroke rehabilitation. Cochrane Database Syst Rev, 12, CD011483 10.1002/14651858.CD011483.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine GN, Lange RA, Bairey-Merz CN, Davidson RJ, Jamerson K, Mehta PK, … Council on Hypertension. (2017). Meditation and cardiovascular risk reduction: A scientific statement from the American Heart Association. J Am Heart Assoc, 6(10). 10.1161/JAHA.117.002218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DB, Stoll EL, Kindy SD, Merry HL, & Davidson RJ (2014). A mind you can count on: validating breath counting as a behavioral measure of mindfulness. Front Psychol, 5, 1202 10.3389/fpsyg.2014.01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl JR, Fisher NE, Cooper DJ, Rosen RK, & Britton WB (2017). The varieties of contemplative experience: A mixed-methods study of meditation-related challenges in Western Buddhists. PLoS ONE, 12(5), e0176239 10.1371/journal.pone.0176239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma-Kellams C (2014). Cross-cultural differences in somatic awareness and interoceptive accuracy: a review of the literature and directions for future research. Front Psychol, 5, 1379 10.3389/fpsyg.2014.01379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, & Stewart A (2012). The Multidimensional Assessment of Interoceptive Awareness (MAIA). PLoS ONE, 7(11), e48230 10.1371/journal.pone.0048230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni M, Sedeno L, Couto B, Reynoso M, Gelormini C, Favaloro R, … Ibanez A (2013). Preliminary evidence about the effects of meditation on interoceptive sensitivity and social cognition. Behav Brain Funct, 9, 47 10.1186/1744-9081-9-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GA, Naveteur J, Dupuy MA, & Jacquot L (2015). My heart is in my hands: the interoceptive nature of the spontaneous sensations felt on the hands. Physiol Behav, 143, 113–120. 10.1016/j.physbeh.2015.02.030 [DOI] [PubMed] [Google Scholar]
- Mills PJ, Dimsdale JE, Ancoli-Israel S, Clausen J, & Loredo JS (1998). The effects of hypoxia and sleep apnea on isoproterenol sensitivity. Sleep, 21(7), 731–735. 10.1093/sleep/21.7.731 [DOI] [PubMed] [Google Scholar]
- Murphy J, Millgate E, Geary H, Ichijo E, Coll MP, Brewer R, … Bird G (2018). Knowledge of resting heart rate mediates the relationship between intelligence and the heartbeat counting task. Biol Psychol, 133, 1–3. 10.1016/j.biopsycho.2018.01.012 [DOI] [PubMed] [Google Scholar]
- Nairn R (2000). What is meditation? Budhhism for everyone. Boston: Shambala Publications. [Google Scholar]
- Nichols TE, & Holmes AP (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp, 15(1), 1–25. 10.1002/hbm.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L, & Kaszniak AW (2006). Awareness of subtle emotional feelings: A comparison of long-term meditators and nonmeditators. Emotion, 6(3), 392–405. 10.1037/1528-3542.6.3.392 [DOI] [PubMed] [Google Scholar]
- Ospina MB, Bond K, Karkhaneh M, Tjosvold L, Vandermeer B, Liang Y, … Klassen TP. (2007). Meditation practices for health: state of the research. Evid Rep Technol Assess (Full Rep)(155), 1–263. [PMC free article] [PubMed] [Google Scholar]
- Otten S, Schotz E, Wittmann M, Kohls N, Schmidt S, & Meissner K (2015). Psychophysiology of duration estimation in experienced mindfulness meditators and matched controls. Front Psychol, 6, 1215 10.3389/fpsyg.2015.01215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin L, Morgan R, Rosselli A, Howard M, Sheppard A, Evans D, … Dunn B. (2013). Exploring the relationship between mindfulness and cardiac perception. Mindfulness. 10.1007/s12671-012-0181-7 [DOI] [Google Scholar]
- Paulus MP, Feinstein JS, & Khalsa SS (2019). An active inference approach to interoceptive psychopathology. Ann Rev Clin Psychol, 15, 97–122. 10.1146/annurev-clinpsy-050718-095617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CK, Henry IC, Mietus JE, Hausdorff JM, Khalsa G, Benson H, & Goldberger AL (2004). Heart rate dynamics during three forms of meditation. Int J Cardiol, 95(1), 19–27. 10.1016/j.ijcard.2003.02.006 [DOI] [PubMed] [Google Scholar]
- Quinones MA, Gaasch WH, & Alexander JK (1976). Influence of acute changes in preload, afterload, contractile state and heart rate on ejection and isovolumic indices of myocardial contractility in man. Circulation, 53(2), 293–302. 10.1161/01.cir.53.2.293 [DOI] [PubMed] [Google Scholar]
- Quinones MA, Gaasch WH, Cole JS, & Alexander JK (1975). Echocardiographic determination of left ventricular stress-velocity relations. Circulation, 51(4), 689–700. 10.1161/01.cir.51.4.689 [DOI] [PubMed] [Google Scholar]
- Ring C, & Brener J (1992). The temporal locations of heartbeat sensations. Psychophysiology, 29(5), 535–545. 10.1111/j.1469-8986.1992.tb02027.x [DOI] [PubMed] [Google Scholar]
- Ring C, & Brener J (2018). Heartbeat counting is unrelated to heartbeat detection: A comparison of methods to quantify interoception. Psychophysiology, 55(9), e13084 10.1111/psyp.13084 [DOI] [PubMed] [Google Scholar]
- Schonbrodt F, & Perugini M (2013). At what sample size do correlations stabilize? J. Res. Pers, 47, 609–612. 10.1016/j.jrp.2013.05.009 [DOI] [Google Scholar]
- Selby J (1992). Kundalini awakening: a gentle guide to chakra activation and spiritual growth. New York: Bantam Books. [Google Scholar]
- Strauss C, Cavanagh K, Oliver A, & Pettman D (2014). Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS ONE, 9(4), e96110 10.1371/journal.pone.0096110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss JD, Ridgewell C, McHugo M, Heckers S, & Blackford JU (2017). Manual segmentation of the human bed nucleus of the stria terminalis using 3T MRI. Neuroimage, 146, 288–292. 10.1016/j.neuroimage.2016.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam NT, van Vugt MK, Vago DR, Schmalzl L, Saron CD, Olendzki A, … Meyer DE. (2018). Mind the Hype: A Critical Evaluation and Prescriptive Agenda for Research on Mindfulness and Meditation. Perspect Psychol Sci, 13(1), 36–61. 10.1177/1745691617709589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielgosz J, Goldberg SB, Kral TRA, Dunne JD, & Davidson RJ (2018). Mindfulness meditation and psychopathology. Annu Rev Clin Psychol. 10.1146/annurev-clinpsy-021815-093423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielgosz J, Schuyler BS, Lutz A, & Davidson RJ (2016). Long-term mindfulness training is associated with reliable differences in resting respiration rate. Sci Rep, 6, 27533 10.1038/srep27533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmann S, Schonecke OW, Frohlig G, & Maldener G (1999). Dissociating beliefs about heart rates and actual heart rates in patients with cardiac pacemakers. Psychophysiology, 36(3), 339–342. 10.1017/S0048577299980381 [DOI] [PubMed] [Google Scholar]
- Wittkamp MF, Bertsch K, Vogele C, & Schulz A (2018). A latent state-trait analysis of interoceptive accuracy. Psychophysiology, 55(6), e13055 10.1111/psyp.13055 [DOI] [PubMed] [Google Scholar]
- Youkhana S, Dean CM, Wolff M, Sherrington C, & Tiedemann A (2016). Yoga-based exercise improves balance and mobility in people aged 60 and over: a systematic review and meta-analysis. Age Ageing, 45(1), 21–29. 10.1093/ageing/afv175 [DOI] [PubMed] [Google Scholar]
- Zamariola G, Maurage P, Luminet O, & Corneille O (2018). Interoceptive accuracy scores from the heartbeat counting task are problematic: Evidence from simple bivariate correlations. Biol Psychol, 137, 12–17. 10.1016/j.biopsycho.2018.06.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.