Abstract
Mutations in the mitochondrial alanyl-tRNA synthetase gene, AARS2, have been reported to cause leukoencephalopathy associated with early ovarian failure, a clinical presentation described as “ovarioleukodystrophy”. We present a sibling pair: one with cerebellar ataxia and one with vision loss and cognitive impairment in addition to ataxia. Neither shows evidence of leukoencephalopathy on MRI imaging. Exome sequencing revealed that both siblings are compound heterozygous for AARS2 variants (p.Phe131del and p.Ile328Met) Yeast complementation assays indicate that p.Phe131del AARS2 dramatically impairs gene function and that p.Ile328Met AARS2 is a hypomorphic allele. This work expands the phenotypic spectrum of AARS2-associated disease to include ataxia without leukoencephalopathy.
Keywords: AARS2, cerebellar ataxia, ovarioleukodystrophy, leukoencephalopathy, recessive ataxia
INTRODUCTION
Aminoacyl-tRNA synthetases (ARSs) are essential enzymes responsible for charging tRNA with cognate amino acids—this is a crucial step in translating genetic information into proteins [1]. There are 37 ARS genes encoded in the nuclear genome: 17 encode enzymes that function in the cytoplasm; 17 encode enzymes that function in the mitochondria; and three encode enzymes that function in both cellular compartments [1]. Bi-allelic mutations in all 17 genes encoding mitochondrial ARS enzymes have been implicated in recessive human disease phenotypes, and these mutations often affect the central nervous system [2]. Mutations in alanyl-tRNA synthetase 2 (AARS2; MIM: 612035), which encodes the mitochondrial enzyme that charges tRNAAla molecules with alanine, have been implicated in two phenotypes: infantile cardiomyopathy and childhood- or adulthood-onset leukoencephalopathy often with cerebellar atrophy, ataxia, and ovarian failure in female patients [3,4]. Additionally, a patient was recently reported with optic atrophy and retinopathy in addition to leukoencephalopathy [5]. We present two siblings from a single family who are both compound heterozygous for two AARS2 variants and who present with ataxia in the absence of leukoencephalopathy.
PATIENTS AND METHODS
Patients
Patients were seen through the University of Michigan Ataxia Clinic. The Institutional Review Board at the University of Michigan determined (HUM00164887) that the description of these cases does not fit the definition of human subjects research requiring IRB approval. Authorization was obtained from the patients in order to publish the description of their cases.
Magnetic resonance imaging
The proband had a brain MRI on a 1.5T GE scanner. MR sequences included axial, coronal and sagittal T1, with and without gadolinium contrast, axial T2, axial T2 FLAIR, Axial gradient echo, and diffusion weighted imaging (DWI/ADC).
The proband’s sibling underwent a brain MRI on a 3T Siemens scanner. MR sequences included axial and sagittal T2 FLAIR, Axial T1 without gadolinium, diffusion weighted imaging and axial, coronal and sagittal T1 sequences with intravenous gadolinium contrast.
Exome Sequencing
Commercial exome sequencing was performed through the University of Chicago as described previously [6]. Briefly, exome sequencing was performed using the Agilent SureSelect Clinical Research Exome kit (Agilent Technologies, Santa Clara, CA, USA), and sequencing was performed using Illumina NextSeq technology with 150-bp paired-end reads (Illumina, San Diego, CA, USA). Variants with a global population frequency of ≥1% in ExAC were excluded. Variants were interpreted by a team of board-certified geneticists, genetic counselors, and neurologists. The variants in AARS2 described here were confirmed by Sanger sequencing. Sequencing of the unaffected parents was used to confirm that the described variants were on two separate alleles in trans. Both parents were examined, and had a normal neurological examination.
Multiple-Species Alignment
AARS2 protein orthologs from multiple species were obtained using the following GenBank accession numbers: human (Homo sapiens, NP_065796.2), chimpanzee (Pan troglodytes, PNI77549.1), horse (Equus caballus, XP_023480797.1), cow (Box taurus, NP_001178140.1), rat (Rattus norvegicus, NP_001100361.1), mouse (Mus musculus, NP_001344929.1), chicken (Gallus gallus, NP_001026227.1), zebrafish (Danio rerio, XP_021329187.1), and yeast (Saccharomyces cerevisiae, NP_014980.3). Protein sequences were aligned using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/).
Yeast Complementation Assays
Yeast complementation assays were performed as previously described [7]. The open-reading frames for wild-type yeast ALA1 and wild-type human AARS and AARS2 were amplified, sequence-verified, and cloned into either pRS315 (ALA1) or pYY1 [8] (AARS and AARS2) using Gateway cloning technology (Invitrogen). Subsequently, each AARS2 variant was modeled only in yeast ALA1 and human AARS (primers available upon request). Mutagenesis was performed using the Quickchange II XL Site-Directed Mutagenesis Kit. Reactions were transformed into E. coli, and plasmid DNA was purified from individual colonies and sequenced to confirm the presence of the mutation and the absence of PCR-induced errors.
To assess the ability of ALA1, AARS, and AARS2 (wild-type and variants) to support cellular growth, a previously validated haploid strain with the endogenous ALA1 locus deleted and viability maintained via a pRS316 vector bearing wild-type ALA1 [9] was transformed with wild-type, mutant, or ‘empty’ (i.e., those not containing an ALA1, AARS, or AARS2 insert) expression constructs. Transformed yeast cells were selected for the presence of pYY1 or pRS315 by growth on solid medium lacking leucine (pYY1 and pRS315 harbor the LEU2 gene) and uracil (pRS316 harbors the URA3 gene). Colonies were grown to saturation in 2mL -leu-ura liquid medium at 30°C and 275 rpm for 48 hours. A 1mL aliquot from each 2mL culture was spun down at 10,000 rpm and re-suspended in 50uL UltraPure RNase/DNase-free water. Undiluted cultures and dilutions of 1:10 and 1:100 were spotted on 0.1% 5-FOA complete solid medium (Teknova, Hollister CA), which selects for cells that have spontaneously lost the URA3-bearing maintenance vector [10]. Yeast viability was assessed by visual inspection after 5 days of incubation at 30°C. Two colonies per transformation were assayed.
RESULTS
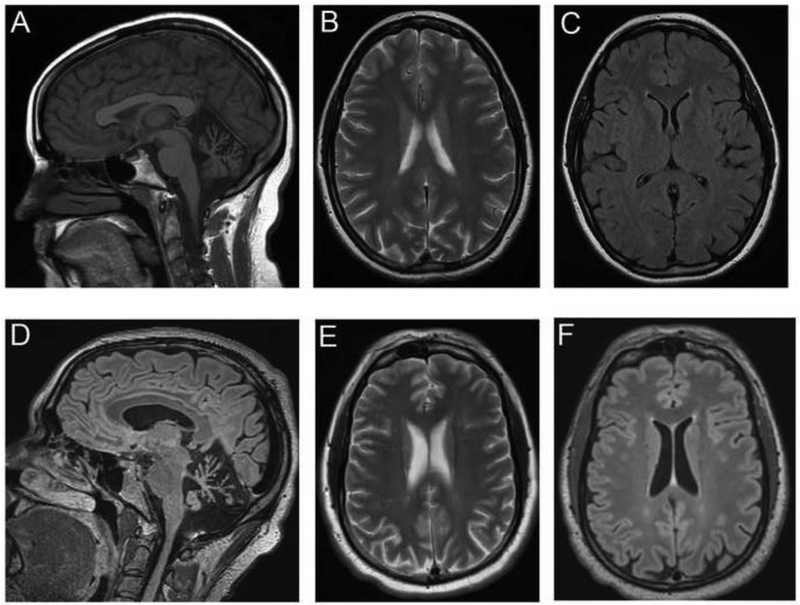
The 29-year-old proband was initially evaluated at age 26 with a prior two-year history of slurred speech. She sought a neurological evaluation due to progressive dysarthria followed by balance impairment. At age 26 she developed impaired hand dexterity and worsening handwriting. Her neurological examination revealed downbeating nystagmus in primary gaze and gaze-evoked nystagmus. Additionally, there was prominent ataxic dysarthria and moderate dysmetria on finger-nose-finger and heel-knee-shin testing. There was mild spasticity in the supine position in the lower extremities with otherwise normal deep tendon reflexes. She was able to ambulate with a wide-based staggering gait. She reported normal menstrual cycles and became pregnant at age 29 but had an early second trimester miscarriage. Brain MRI revealed mild cerebellar atrophy in sagittal T1-weight MRI images (Figure 1A) and no abnormal white matter signal on axial T1, T2-weighted images (Figure 1B) and fluid-attenuated inversion recovery images (Figure 1C). There were no lesions that either restricted diffusion or produced gadolinium enhancement.
Figure 1.
Brain MRI lacks changes associated with leukoencephalopathy. (A) The sagittal T1-weigted MRI from the proband at age 27 shows mild cerebellar atrophy. (B) The axial T2-weighted images show a lack of abnormal signal in the periventricular white matter. (C) The fluid-attenuated inversion recovery image shows no rarefaction of white matter areas. (D) The axial fluid-attenuated inversion recovery image from the sibling at age 36 shows marked cerebellar atrophy. (E) The axial T2-weighted images shows a lack of abnormal signal in the periventricular white matter. (F) The fluid-attenuated inversion recovery image shows no rarefaction of white matter in the centrum semiovale.
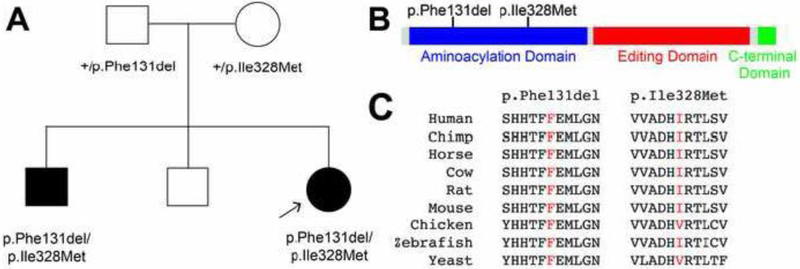
The proband’s 36-year old symptomatic male sibling was subsequently evaluated in the clinic. He had symptoms of vision problems starting at age five, first suspected by his kindergarten teacher, as he was touching the walls when he ambulated. Records of ophthalmological evaluation are only available from ages thirteen and sixteen. Visual acuity was noted to be 20/100 at age 13, with prominent downbeating nystagmus and mild optic atrophy. A second independent evaluation at age 16 reported a visual acuity of 20/200 with a normal funduscopic examination. The reduced visual acuity was attributed to prominent downbeating nystagmus. He developed gait changes later in childhood, but he was able to get a custodial position at age 18. Between the ages of 19 and 25, his balance got progressively worse, and he began to have impaired cognition. Over the subsequent 10 years he was no longer oriented to days and dates, and he developed more trouble with personal hygiene. At the time of evaluation, he had been determined to be legally blind. The Montreal Cognitive Assessment was administered to assess cognitive dysfunction. He scored 11 out of 30 at the time of evaluation. Impairments were identified primarily in the domains of visuospatial/executive attention and delayed recall. Orientation and registration were relatively preserved. No fluctuations in attention and concentration were identified, and there was no clinical concern for seizures or encephalopathy. Visual acuity was 20/400, and eye movements showed downbeating nystagmus. There was no ophthalmoparesis. Additionally, his speech was mildly dysarthric with hypophonia. Strength was intact in the upper and lower extremities, but he had a spastic-dystonic gait. Coordination was mildly impaired to finger-nose-finger and heel-knee-shin testing, with marked gait impairment requiring the assistance of another individual or a walker. Brain MRI revealed marked cerebellar atrophy in axial fluid-attenuated inversion recovery images (Figure 1D) and no abnormal white matter signal in axial T2-weighted images (Figure 1E) and axial or sagittal fluid-attenuated inversion recovery images (Figure 1F). There were no lesions that either restricted diffusion or produced gadolinium enhancement. No evidence of cortical atrophy was evident (Figure 1E,F). The proband was initially tested for repeat expansions associated with SCA1, 2, 3, 6, 7 and Friedreich ataxia. Since no expanded triplet repeats were detected, testing with commercial exome sequencing was obtained. Exome sequencing analysis revealed that the proband is compound heterozygous for two AARS2 variants (p.Phe131del and p.Ile328Met AARS2), and commercial genetic testing for AARS2 revealed that the proband’s symptomatic brother is also compound heterozygous for the same two AARS2 variants (Figure 2A). The maternally inherited variant (c.984C>G; p.Ile328Met) is not present in the gnomAD database [11], and the paternally inherited variant (c.390-392del; p.Phe131del) is present in gnomAD with a very low frequency (1 in 241,492 chromosomes) (Table 1).
Figure 2.
Pedigrees and conservation. (A) The simplex pedigree is shown for the affected family. Squares represent males and circles represent females. Genotypes are indicated under each symbol and filled shapes indicate affected individuals. An arrow indicates the proband. (B) AARS2 functional domains are indicated in blue (aminoacylation domain), red (editing domain), and green (C-terminal domain), and the positions of the variants are shown across the top. (C) The position of each variant is shown with flanking AARS2 amino-acid residues from multiple species. The position of the affected residue is shown in red for each species.
Table 1.
AARS2 variants identified
| Nucleotide changea | Amino acid changeb |
Detection in gnomADc |
Minor allele frequency |
|---|---|---|---|
| c.390-392del | p.Phe131del | 1 / 251,492 | 0.000004 |
| c.984C>G | p.Ile328Met | Not present | 0 |
Human nucleotide positions correspond to GenBank Accession number NM_020745.4
Human amino acid positions correspond to GenBank Accession number NP_065796.2
AARS2 is a nuclear-encoded enzyme responsible for charging tRNAAla molecules in the mitochondria of mammalian cells; AARS is responsible for charging tRNAAla molecules in the cytoplasm [1]. AARS2 contains an aminoacylation domain for catalyzing the ligation of alanine to tRNAAla molecules, an editing domain for deacylation of mischarged tRNAAla, and a C-terminal domain that promotes cooperative binding between the aminoacylation domain, the editing domain, and tRNAAla [12-14]. Both p.Phe131del and p.Ile328Met AARS2 map to the aminoacylation domain (Figure 2B). We assessed the evolutionary conservation of each affected AARS2 amino acid by aligning protein sequences of AARS2 orthologs from multiple species. The Phe131 residue is conserved among all analyzed species, and the Ile328 residue is conserved among the human, chimp, horse, cow, rat, mouse, and zebrafish AARS2 proteins (Figure 2C). In sum, the above data suggest that the two identified AARS2 variants affect protein function.
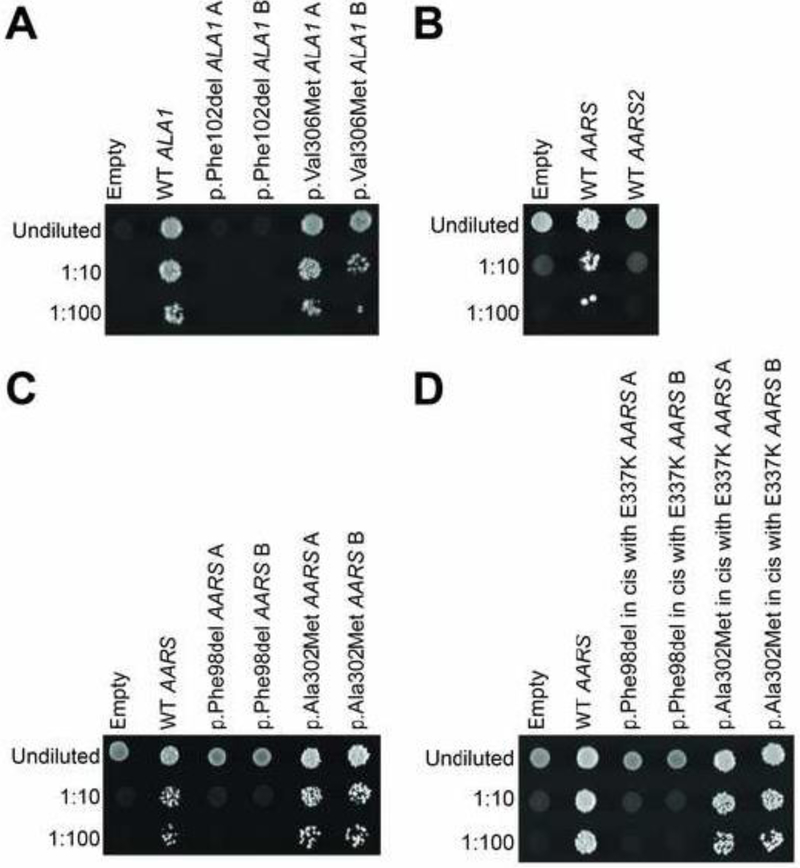
To assess the functional consequences of each AARS2 allele, we modeled each variant in ALA1, which encodes a bifunctional yeast enzyme that charges tRNA in both the cytoplasm and mitochondria [15]. We then compared the ability of mutant and wild-type ALA1 to complement the loss of ALA1 in yeast complementation assays. Briefly, we used a previously validated haploid yeast strain deleted for the endogenous ALA1 locus, with viability maintained due to the presence of ALA1 on a URA3-bearing vector [7, 9, 16]. Wild-type, p.Phe102del (corresponding to human p.Phe131del AARS2), or p.Val306Met (corresponding to human p.Ile328Met AARS2) ALA1, or a plasmid with no ALA1 insert (‘Empty’ in Figure 3A) was transformed into yeast, and growth was evaluated on 5-FOA medium, which selects for cells that have spontaneously lost the maintenance vector [10]. The plasmid with no ALA1 insert was unable to support yeast growth, consistent with ALA1 being an essential gene. The p.Phe102del ALA1 variant was unable to support yeast growth, indicating that p.Phe102del ALA1 represents a loss-of-function allele (Fig. 3A). The p.Val306Met ALA1 variant showed similar yeast cell growth compared to wild-type ALA1 (Figure 3A) indicating that this is not a loss-of-function allele in this assay. Similar results were observed for p.Phe102del and p.Val306Met ALA1 when yeast cell growth was evaluated on medium containing glycerol, a non-fermentable carbon source, instead of glucose (data not shown).
Figure 3.
AARS2 variants display loss-of-function effects in yeast complementation assays. Yeast lacking endogenous ALA1 were transformed with a vector with no ALA1 or AARS insert (‘Empty’), or with vectors containing wild-type ALA1 or mutant ALA1 (A), wild-type AARS or AARS2 (B), wild-type AARS or mutant AARS (C), or E337K AARS or mutations in cis with E337K AARS (D). The vector used in each experiment is indicated across the top. Cultures were plated undiluted or diluted (1:10 or 1:100) on media containing 5-FOA and grown at 30°C. Two independently generated mutant constructs (‘A’ and ‘B’) were tested.
We also tested the ability of each AARS2 variant modeled in AARS to complement the loss of ALA1 since wild-type AARS has been shown to complement the loss of ALA1 [7], and since we found that wild-type AARS2 does not complement the loss of ALA1 (Figure 3B). Wild-type, p.Phe98del (corresponding to p.Phe131del AARS2), or p.Ala302Met (corresponding to p.Ile328Met AARS2) AARS, or a plasmid with no AARS insert were transformed into yeast, and growth was evaluated on 5-FOA medium as above. The p.Phe98del AARS variant was unable to support yeast growth, consistent with a loss-of-function allele (Figure 3C). The p.Ala302Met AARS variant showed similar yeast cell growth compared to wild-type AARS (Figure 3C). To improve the sensitivity of our yeast complementation experiments, each variant was also tested in cis with p.Glu337Lys AARS, a hypermorphic variant that increases growth of yeast expressing human AARS [7]. The p.Phe98del AARS variant in cis with p.Glu337Lys was unable to support yeast cell growth (Figure 3D). The p.Ala302Met in cis with p.Glu337Lys AARS variant supported reduced yeast growth compared to p.Glu337Lys only (Figure 3D) suggesting that this is a hypomorphic allele. Yeast cell growth was also evaluated on medium containing glycerol; both WT AARS and the hypermorphic variant, p.Glu337Lys AARS, were unable to support yeast growth on glycerol (data not shown), which is consistent with AARS functioning only in the cytoplasm. In summary, our yeast complementation assays indicate that p.Phe131del AARS2 dramatically impairs gene function, consistent with pathogenicity in the recessive phenotype described here. While our data suggest that p.Ile328Met AARS2 is a hypomorphic allele, additional studies will be required to verify this finding.
DISCUSSION
Our results indicate an expansion of the phenotypic spectrum of AARS2-related disorders to include a mitochondrial phenotype with ataxia even in the absence of leukoencephalopathy. We present two related patients: one with cerebellar ataxia and one with vision loss and cognitive impairment in addition to ataxia. Each patient is compound heterozygous for the same two AARS2 variants, which both affect highly-conserved amino acids in the aminoacylation domain. Our yeast complementation assays support a complete loss-of-function effect for p.Phe131del and a partial loss-of-function effect for p.Ile328Met AARS2; these data are consistent with both the recessive phenotype and the viability of the patients. Currently, the differences in clinical presentations of the siblings is not understood; however, genetic and environmental factors may contribute to phenotypic differences. Further studies, in larger patient cohorts, are needed to identify potential genetic modifiers that may explain the variability in presentation.
One of the variants reported here, p.Phe131del AARS2, was previously reported in a female patient who carried p.Phe131del on one allele and p.Arg199Cys and p.Val730Met on the other allele [17]. Her presentation differed from the patients reported here as she presented with leukoencephalopathy and ovarian failure [17]. Early ovarian failure, either primary amenorrhea or secondary amenorrhea in the 2nd decade of life is a well-described feature of AARS2-associated neurological disease [17], so much so that the disorder has been termed an ovarioleukodystrophy. Our findings indicate that the p.Phe131del AARS2 variant may not always be associated with leukoencephalopathy or with early ovarian failure.
To our knowledge, there has been only one other patient reported who had AARS2 variants and childhood to adulthood-onset disease without leukodystrophy; Srivastava et al. recently reported a 34-year-old man with a family history of tremor, imbalance, and Charcot-Marie-Tooth disease, and his presentation included tremor, polyneuropathy, ataxia, and cognitive and psychiatric decline [4]. He was compound heterozygous for a splice site variant, c.2598 +1G>T, and p.Arg199Cys AARS2, and he also carried a paternally inherited PMP22 duplication [4]. While the patients reported in both this study and Srivastava et al. presented with ataxia and no leukoencephalopathy, the patients reported here did not have any additional variants in other genes. Additionally, Srivastava et al. reported MRI signal abnormalities of the periventricular frontal white matter [4], which differs from the patients reported here who had no signal abnormalities. Our results further indicate that AARS2 variants can be associated with ataxia without leukoencephalopathy.
CONCLUSION
In conclusion, we report two siblings who are both compound heterozygous for AARS2 variants and who present with ataxia in the absence of leukoencephalopathy. The variants affect conserved residues in the aminoacylation domain, and yeast complementation assays indicate loss-of-function effects. This work expands the phenotypic spectrum of AARS2-associated disease to include ataxia without leukoencephalopathy.
ACKNOWLEDGEMENTS
M.E.K. is supported by the NIH Medical Scientist Training Program Training Grant (GM007863), the NIH Cellular and Molecular Biology Training Grant (GM007315), and an NIH National Research Service Award (F31) from the National Institute of Neurological Disorders and Stroke (NS113515). A.A. is supported by a grant from the National Institute of General Medical Sciences (GM118647). V.G.S is supported by a grant from the NINDS (NS085054).
Footnotes
Conflict of Interest: The authors declare that they have no conflicts of interest.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Antonellis A and Green ED. The role of aminoacyl-tRNA synthetases in genetic diseases. Annu Rev Genomics Hum Genet 2008: 9:87–107. Doi 10.1146/annurev.genom.9.081307.164204 [DOI] [PubMed] [Google Scholar]
- 2.Meyer-Schuman R and Antonellis A. Emerging mechanisms of aminoacyl-tRNA synthetase mutations in recessive and dominant human disease. Hum Mol Genet 2017: 26:R114–R27. doi 10.1093/hmg/ddx231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JY, Chen SF, Zhang HQ, Wang MY, Zhu JH and Zhang X. A homozygous mutation of alanyl-transfer RNA synthetase 2 in a patient of adult-onset leukodystrophy: A case report and literature review. Brain Behav 2019:e01313. doi 10.1002/brb3.1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava S, Butala A, Mahida S, Richter J, Mu W, Poretti A, Vernon H, VanGerpen J, Atwal PS, Middlebrooks EH, Zee DS and Naidu S. Expansion of the clinical spectrum associated with AARS2-related disorders. Am J Med Genet A 2019. doi 10.1002/ajmg.a.61188 [DOI] [PubMed] [Google Scholar]
- 5.Peragallo JH, Keller S, van der Knaap MS, Soares BP and Shankar SP. Retinopathy and optic atrophy: Expanding the phenotypic spectrum of pathogenic variants in the AARS2 gene. Ophthalmic Genet 2018: 39:99–102. doi 10.1080/13816810.2017.1350723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun M, Johnson AK, Nelakuditi V, Guidugli L, Fischer D, Arndt K, Ma L, Sandford E, Shakkottai V, Boycott K, Chardon JW, Li Z, Del Gaudio D, Burmeister M, Gomez CM, Waggoner DJ and Das S. Targeted exome analysis identifies the genetic basis of disease in over 50% of patients with a wide range of ataxia-related phenotypes. Genet Med 2019: 21:195–206. doi 10.1038/s41436-018-0007-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weterman MAJ, Kuo M, Kenter SB, Gordillo S, Karjosukarso DW, Takase R, Bronk M, Oprescu S, van Ruissen F, Witteveen RJW, Bienfait HME, Breuning M, Verhamme C, Hou YM, de Visser M, Antonellis A and Baas F. Hypermorphic and hypomorphic AARS alleles in patients with CMT2N expand clinical and molecular heterogeneities. Hum Mol Genet 2018: 27:4036–50. doi 10.1093/hmg/ddy290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien CI, Chen YW, Wu YH, Chang CY, Wang TL and Wang CC. Functional substitution of a eukaryotic glycyl-tRNA synthetase with an evolutionarily unrelated bacterial cognate enzyme. PLoS One 2014: 9:e94659. doi 10.1371/journal.pone.0094659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin HM, Sakaguchi R, Giblin W, Program NCS, Wilson TE, Biesecker L, Lupski JR, Talbot K, Vance JM, Zuchner S, Lee YC, Kennerson M, Hou YM, Nicholson G and Antonellis A. A recurrent loss-of-function alanyl-tRNA synthetase (AARS) mutation in patients with Charcot-Marie-Tooth disease type 2N (CMT2N). Hum Mutat 2012: 33:244–53. doi 10.1002/humu.21635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boeke JD, Trueheart J, Natsoulis G and Fink GR. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol 1987: 154:164–75. [DOI] [PubMed] [Google Scholar]
- 11.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O'Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG and Exome Aggregation C. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016: 536:285–91. doi 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotz A, Tyynismaa H, Euro L, Ellonen P, Hyotylainen T, Ojala T, Hamalainen RH, Tommiska J, Raivio T, Oresic M, Karikoski R, Tammela O, Simola KO, Paetau A, Tyni T and Suomalainen A. Exome sequencing identifies mitochondrial alanyl-tRNA synthetase mutations in infantile mitochondrial cardiomyopathy. Am J Hum Genet 2011: 88:635–42. doi 10.1016/j.ajhg.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swairjo MA, Otero FJ, Yang XL, Lovato MA, Skene RJ, McRee DE, Ribas de Pouplana L and Schimmel P. Alanyl-tRNA synthetase crystal structure and design for acceptor-stem recognition. Mol Cell 2004: 13:829–41. [DOI] [PubMed] [Google Scholar]
- 14.Guo M, Chong YE, Beebe K, Shapiro R, Yang XL and Schimmel P. The C-Ala domain brings together editing and aminoacylation functions on one tRNA. Science 2009: 325:744–7. doi 10.1126/science.1174343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang HL, Yeh LS, Chen NK, Ripmaster T, Schimmel P and Wang CC. Translation of a yeast mitochondrial tRNA synthetase initiated at redundant non-AUG codons. J Biol Chem 2004: 279:49656–63. doi 10.1074/jbc.M408081200 [DOI] [PubMed] [Google Scholar]
- 16.Motley WW, Griffin LB, Mademan I, Baets J, De Vriendt E, De Jonghe P, Antonellis A, Jordanova A and Scherer SS. A novel AARS mutation in a family with dominant myeloneuropathy. Neurology 2015: 84:2040–7. doi 10.1212/WNL.0000000000001583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dallabona C, Diodato D, Kevelam SH, Haack TB, Wong LJ, Salomons GS, Baruffini E, Melchionda L, Mariotti C, Strom TM, Meitinger T, Prokisch H, Chapman K, Colley A, Rocha H, Ounap K, Schiffmann R, Salsano E, Savoiardo M, Hamilton EM, Abbink TE, Wolf NI, Ferrero I, Lamperti C, Zeviani M, Vanderver A, Ghezzi D and van der Knaap MS. Novel (ovario) leukodystrophy related to AARS2 mutations. Neurology 2014: 82:2063–71. doi 10.1212/WNL.0000000000000497 [DOI] [PMC free article] [PubMed] [Google Scholar]