Abstract
Anolis lizards have served as important research models in fields ranging from evolution and ecology to physiology and biomechanics. However, anoles are also emerging as important models for studies of embryo development and tissue regeneration. The increased use of anoles in the laboratory has produced a need to establish effective methods of anesthesia, both for routine veterinary procedures and for research procedures. Therefore, we tested the efficacy of different anesthetic treatments in adult female Anolis sagrei. Alfaxalone, dexmedetomidine, hydromorphone, ketamine, and tribromoethanol were administered subcutaneously (SC), either alone or combined at varying doses in a total of 64 female anoles. Drug induction time, duration, anesthesia level, and adverse effects were assessed.
Differences in anesthesia level were observed depending on injection site and drug combination. Alfaxalone/dexmedetomidine and tribromoethanol/dexmedetomidine were the most effective drug combinations for inducing a surgical plane of anesthesia in anoles. Brown anoles injected SC with alfaxalone (30 mg/kg) plus dexmedetomidine (0.1 mg/kg) or with tribromoethanol (400 mg/kg) plus dexmedetomidine (0.1 mg/kg) experienced mean durations of surgical anesthesia levels of 31.2±5.3 and 87.5±19.8 min with full recovery after another 10.9±2.9 and 46.2±41.8 min, respectively. Hydromorphone given with alfaxalone/dexmedetomidine resulted in deep anesthesia with respiratory depression, while ketamine/hydromorphone/dexmedetomidine produced only light to moderate sedation. We determined that alfaxalone/dexmedetomidine or tribromoethanol/dexmedetomidine combinations were sufficient to maintain a lizard under general anesthesia for coeliotomy. This study represents a significant step towards understanding the effects of anesthetic agents in anole lizards and will benefit both veterinary care and research on these animals.
Keywords: alfaxalone, tribromoethanol, dexmedetomidine, anesthesia, Anolis, lizard
The lizard genus Anolis includes approximately 400 described species, which are distributed throughout Central and South America and the islands of the Caribbean1. Anoles may be best known for the series of adaptive radiations that resulted in convergent evolution of similar sets of Anolis habitat specialists, or ecomorphs, on different islands of the Greater Antilles. They are, however, also emerging as an important new system to study reptile development. Among the Anolis species that are seeing increased use for developmental studies is the brown anole lizard, Anolis sagrei2–4. The brown anole is a small, Caribbean lizard native to Cuba, the Bahamas, and surrounding islands. This lizard is becoming more widely used for embryological research for several reasons, including ease of husbandry, high fecundity, and the ability to collect large numbers of lizards from invasive populations5. The popularity of anoles has produced a need for the establishment of appropriate methods for sedation and pain management of Anolis lizards, both for veterinary care of animals housed in research colonies as well as for surgical procedures performed during research studies.
An important consideration in establishing sedation methods in A. sagrei is the small size of this reptile. Mature females range in size from 2.5–3.5 g, while mature males typically range from 6–8 g. Therefore, intubation of these very small lizards may not be practical, and the use of inhaled anesthetic agents poses challenges in these reptiles6. Delivery of anesthetics intravenously (IV) or by intramuscular injection (IM) are also not ideal given the small size of the blood vessels and delicate musculature of these animals. While an intraperitoneal injection is an alternative method used in mice, it is not routinely performed in reptiles because of the hepatic-first pass effect where drugs are rapidly cleared by the hepatic portal and ventral abdominal veins and metabolized by the liver, inhibiting wide systemic circulation7–10. Thus, SC injections may be the most practical route to deliver anesthetics to brown anoles.
The use of multiple injectable anesthetic agents, either alone or in combination, has been reported in a variety of reptiles. Among these agents is alfaxalone, a synthetic neuro-active steroid that induces CNS depression through agonistic effects on GABAA receptors11. Alfaxalone is effective in inducing anesthesia in green iguanas12, leopard geckos9,10, and other reptiles9,10. Additional reptile studies have looked at the effects of ketamine, a dissociative agent that is known for its ability to inhibit NMDA receptors13,14. In reptiles, ketamine induces muscle relaxation and sedation and is routinely administered in combination with other drugs, like dexmedetomidine15, medetomidine16, or midazolam17 at doses ranging between 5–60 mg/kg. The sedation levels achieved when alfaxalone or ketamine are administered to reptiles varies significantly12,15–21. This variability is likely due to a combination of factors, such as differences in species sensitivity, drug combination, dose, and method of delivery.
Another anesthetic agent, 2,2,2-tribromoethanol (TBE), has frequently been used by researchers to sedate rodents22. TBE is easy to prepare, is a non-scheduled drug, and is relatively inexpensive. Thus, TBE continues to be used by the research community. Although its mechanism of action remains unknown, in mammals TBE causes CNS depression and can induce anesthesia levels sufficient for surgical procedures. Administration of TBE in certain species of lizards23, turtles24, and avians25,26 is also known to produce surgical levels of sedation. TBE use in mammals is somewhat controversial due to conflicting reports about inflammation at sites of injection when TBE is administered intraperitoneally27. Nonetheless, TBE is commonly used in rodents and adverse effects in reptiles are unknown.
To our knowledge, no systematic studies of anesthetic agents have been reported in Anolis lizards. In an effort to develop appropriate methods for sedation and pain management in brown anoles, we systemically tested the drugs alfaxalone, dexmedetomidine, hydromorphone, ketamine, and tribromoethanol alone or in combination in 64 adult female brown anoles.
Materials and Methods
Animals
Anolis sagrei were maintained at the University of Georgia following published guidelines5. Animals were wild-caught individuals from Orlando, FL (Note: No commercial breeders exist for this species. A. sagrei is an invasive species in Florida, and no state or federal permits are required to collect this species). As these animals were wild caught, the exact age and previous history of each animal was unknown. After capture, lizards were allowed to acclimate to the laboratory environment for a minimum of 3 weeks before any experiments were performed. Lizards were housed in a facility with a 12 hr light–dark cycle where room temperature was held between 80–85°F (27–29°C) with humidity maintained at or above 60%. Cages were equipped with appropriate UV lighting, perches, artificial plants that provided cover, and nest boxes containing moist vermiculite for egg lay. Cages were misted twice daily and live crickets coated with vitamin dust (Repashy Super Foods Calcium Plus Supplement) offered every other day. Colony health status was tracked by body condition scoring (BCS) for males and females and egg production for females. Our BCS scoring scale was as follows: emaciated, 1; thin or poor, 2; optimum/normal, 3; overweight/heavy, 4; and grossly obese, 5. Breeding cages housed up to 4 females and 1 male together. For this study, sixty-four mature female Anolis lizards were selected from cages that had a prior history of laying 3–4 eggs a week. A physical exam was performed on each animal prior to anesthesia. All female lizards had a BCS of 3 (normal) and exhibited normal reflexes. The reflexes scored were: righting response, jaw tone, and hindlimb withdrawal. Each reflex was scored as follows: absent, 0; reduced, 1; normal, 2; and excessive/exaggerated, 3. All experiments followed the National Research Council’s Guide for the Care and Use of Laboratory Animals and were performed with the approval and oversight of the University of Georgia Institutional Animal Care and Use Committee (A2016 09–008-Y2-A3).
Study design
Experiments were conducted with eight different anesthetic combinations, using a total of 64 female lizards. Each anesthesia trial comprised an anesthesia group (minimum of 4 lizards per group; groups consisted of females from the same cage) where drug treatment or dose varied (Fig. S1). A power calculation was performed to determine the minimum sample size needed to detect large treatment effects as previously outlined28,29. For a large effect size of 2.1, and using 20% as a value for beta (1-beta = 80% power) and 0.05 as a value for alpha, we estimated that the minimum sample size should be 4 lizards/group. Lizard groups were randomly assigned to a treatment. The initial objective was to qualitatively assess whether particular treatments were sufficient to induce a surgical depth of anesthesia. For those treatments that were sufficient to induce a surgical depth of anesthesia, statistical comparisons of treatment outcomes were performed using a nonparametric Mann-Whitney test (significance level p<0.05). Both the mean and one standard deviation from the mean were reported for each experimental condition. To investigate the longer term effects of anesthesia on lizard reproduction, egg production was monitored before and after anesthesia over a 2-month period. For these experiments the observer was not blinded.
Anesthetic/analgesic agents
Alfaxalone (A; Alfaxan, 10 mg/mL, Jurox); dexmedetomidine (D; Dexdomitor 5 mg/10 mL, Zoetis/Orion); hydromorphone (H; DILAUDID 1 mg/mL, Hospira), ketamine (K; Ketamine HCL, 100 mg/mL, Akorn); and tribromoethanol (T; 2,2,2-Tribromoethanol, 99%; ACROS organics) were administered either alone or in combination (Fig.S1). Additional analgesics, lidocaine 2.0 mg/kg (Lidocaine HCL, 2%, Hospira) and meloxicam 0.3 mg/kg (ELoxiject, 5 mg/mL, Henry Schein), were administered preoperatively to lizards undergoing surgical procedures. Lizards were randomly assigned to each treatment group. Due to the small volumes being administered, a 200–800 μL premix of the drug combinations (A/D, A/D/H, K/D/H, and T/D) were used. TBE is no longer available as a pharmaceutical grade compound and was compounded in the lab following the guidelines outlined by the University of Georgia Institutional Animal Care and Use Committee (Guidelines for Use of Tribromoethanol in Rodents, 12/21/2017 revision). A TBE stock solution (1.6 g/mL) was made as previously reported22 and stored in the dark at 4°C. TBE 2.5% solution was made fresh each morning of an experimental trial by diluting 15.6 μL of TBE stock solution with 984.4 μL Plasma-Lytes (final pH 7.2–7.4). TBE injection solutions were filter-sterilized by centrifuging through a 0.22 μm filtered tube (MILLIPORE ultrafree-MC, CAT No: UFC30GV25). The same TBE stock solution was used for all experimental conditions involving TBE reported in this paper and was disposed of after the conclusion of anesthesia trials (3 months).
Anesthesia induction
Prior to injection of anesthetizing agents, females were fasted for 24 hrs. Animals were assessed for overall condition, body weight, and normal reflexes (righting response, jaw tone and hindlimb withdrawal; see Animals section for details on scoring scale). All female lizards were in good physical condition and exhibited normal reflexes. Due to their small size (2.5–3.5 g), heart rate, blood pressure, doppler, ECG, SpO2, and body temperature of these small lizards could not be accurately measured and were not evaluated. A single drug or combination of drugs was administered via SC injection either dorsally into the thoracic epaxial region or laterally into the cervical area using 10 μL and 25 μL Hamilton syringes (RN Calibrated SYR Models 801, ref: CAL7642–01; and 802, ref: CAL7643–01) with 34 gauge hypodermic needles (Small Hub RN NDL, custom length: 10mm, point style 4, ref: 207434). Dispensed drug volume ranged from 5 to 60 μL, depending upon drug concentration and weight of the lizard. To avoid administering a single large volume of drug at one injection site, certain drug combinations were delivered by multiple smaller injections (2–3) on left and right sides of the body axis. Due to their small size, lizards were not supported with O2 gas or ventilated. In cases where respiratory depression occurred and breathing stopped, lizards were manually ventilated (minimum 4 breaths/min) by using delicate forceps (FST: Live Insect Handling Forceps, No: 26029–10) to expand the cranial coelom (i.e., grasping the cranial coelomic skin) and facilitate air flow and gas exchange. Lizards that underwent respiratory depression and received dexmedetomidine were reversed with atipamezole 1.0 mg/kg (Antisedan, 5 mg/mL, Zoetis) administered SC. Body temperature was maintained with a heat platform source (Fisher Scientific: model 77, serial # 802N0041CAT 12–594) with surgical towels providing a barrier between the heat source and the lizards. The contact temperature surface was held constant at 32°C. After recovery from anesthesia, females were housed together with their previous female mates and allowed to recover for 7 days prior to reintroducing the male.
Anesthesia assessment
Induction (time between injection and maximal effect), duration (plateau of anesthesia level), and recovery (restoration of muscle tone and all reflexes/responses) along with respiration rates were recorded for each treatment group. Anesthesia depth was evaluated every 5 minutes by scoring muscle tone (limb and jaw), loss of responses to increasing noxious stimuli (i.e. ranging from light—dorsal back/ hindlimb pinch, to moderate—pelvis pinch, to deep—cloacal/tail clamp) and reflexes (palpebral/corneal, limb withdrawal, and righting response). Level of anesthesia was scored (light, responsive; moderate, responsive/immobilized; surgical, non-responsive/immobilized; and deep, non-responsive/respiratory depression—less than 4 breaths/min) Data collected from individual lizards were recorded on anesthesia log sheets and responses––0, absent; 1, reduced; 2, normal, noted (Fig.S2). Over the next 5 weeks, egg production was recorded weekly and changes in egg yield were analyzed using the cage’s previous history to determine anesthesia effects on long-term health and reproduction (Fig.S3).
Surgical procedure
Following the anesthesia study, drug combinations that induced a surgical level of anesthesia were further evaluated by performing brief coeliotomies to access visceral organs. Coeliotomies were performed following the principles of good surgical technique30,31. Surgeries were performed in a dedicated area using aseptic procedures, including sterile gloves, sterile instruments, and aseptic techniques. In an effort to reduce pain, analgesics were used (meloxicam 0.3 mg/kg was administered SC prior to induction and lidocaine 2.0 mg/kg inverted L-blocks were injected at and around the surgical sites). After induction becoming non-responsive to any noxious stimuli, the lizard was placed into right lateral recumbency and the left flank was aseptically prepared by alternating disinfection with 70% ethanol and 7.5% povidone-iodine (Surgical Scrub Solution, 16 fl. oz. 473 mL, Dynarex) wipes for 5 minutes. Sterile iris scissors (FST: scissors, ref 15023–10) were used to make a 8–10mm vertical incision on the left side, in the mid-coelom region. The coelom was entered through an intercostal space and the organs—liver, ovary, and kidney were easily accessible by retracting intestines gently aside using sterile blunt forceps (FST: curved forceps, ref: 11051–10). Sterile drops of P-Lytes solution were applied to the coeliotomy opening as needed to prevent tissue dehydration. The muscle was not closed, but the skin was closed with a tissue adhesive (3M Vetbond, ref 1469SB). The lizard was re-positioned into left lateral recumbency and the procedure was repeated for a right coeliotomy. All surgeries were performed under a dissecting scope (Ziess Stemi SV11) with a top light (AmScope 80-LED illuminator) and each laparotomy was performed within 10–15 minutes.
Postoperative assessment and care
As noted above, the analgesic Meloxicam was administered immediately prior to the surgical procedure. Following surgery, triple antibiotic ointment (Bacitracin Zinc, Neomycin Sulfate, Polymyxin B Sulfate) was applied topically to the surgical wounds. Respiration was monitored every 15 minutes until the lizard was alert and responsive. For the first week post-surgery, lizards were visually monitored daily any signs of infection, pain, or inflammation. Indicators of pain or infection included: change in posture, decreased food intake, drop in body score, a slowness to escape when challenged, and/or a general inactivity of normal cage behavior (i.e. climbing and jumping on wall and roof surfaces). Any lizards exhibiting signs of pain would receive a second 0.3 mg/kg meloxicam dose SC and be monitored closely for signs of improvement. Euthanasia would be indicated for lizards that reached a BCS of 1 or exhibited a lack of flight response.
Results
Comparison of different SC injection sites
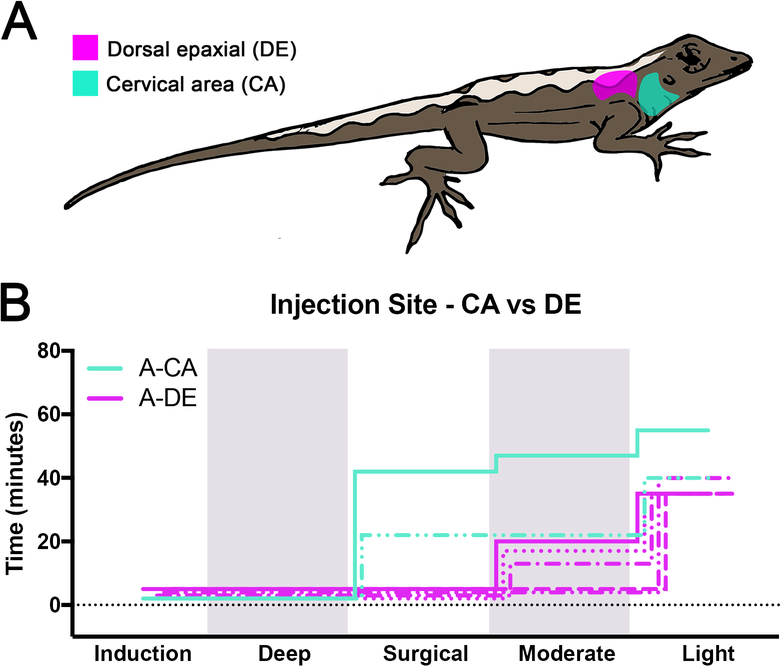
Before evaluating different anesthetics, we assessed two different subcutaneous injection sites with alfaxalone (Fig.1A). Female lizards received SC injections of alfaxalone at 30 mg/kg either laterally into the cervical area (treatment A-CA; n=2) or dorsally into the thoracic epaxial region (treatment A-DE; n=5). The two A-CA injected lizards were induced within 2 min, achieved a surgical plane of anesthesia lasting 20 and 40 min, and fully recovered within 13 and 18 min (Fig.1B, Fig.S4). Both lizards had normal respiration (mean RR ranged from 20–28 bpm) while under anesthesia. A second group of lizards (A-DE) was injected with alfaxalone SC in the dorsal epaxial region. Mean induction times in A-DE lizards was 3.8±1.3 min. None of the A-DE lizards reached a surgical plane of anesthesia. Three of the five lizards reached a moderate level of anesthesia lasting 13.3±2.9 min, and response to painful stimuli was present although reduced. 18.3±3.5 min of light anesthesia followed this period. The remaining 2 lizards only reached a light level of anesthesia, which was maintained for 35.5±0.7 min. All A-DE lizards fully recovered. For the remaining anesthesia trials, all drugs were administered SC into the cervical area.
Figure 1:
Location of subcutaneous drug injection sites and comparison of alfaxalone injected SC into the cervical area and dorsal epaxial region. (A) Dorsal epaxial (DE) and cervical area (CA) are highlighted in magenta and cyan, respectively. (B) Staggered stepwise graphs summing the total time individual lizards spent at each plane of anesthesia for A-DE (n=5) and A-CA (n=2) lizards. Each lizard is represented by a single line with different styles. Alfaxalone 30mg/kg was used in both A-DE and A-CA treatments. Shading delineates the boundaries of different anesthesia levels.
Alfaxalone in combination with dexmedetomidine
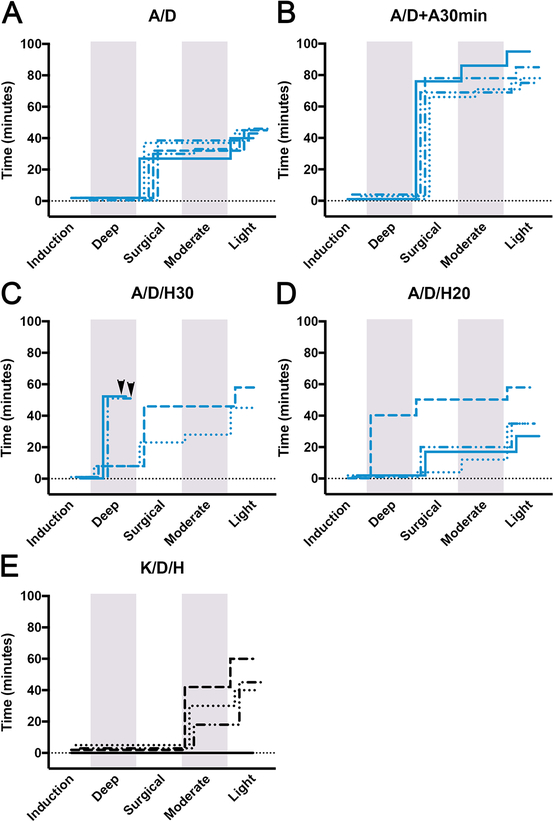
Dexmedetomidine has both anesthetic and analgesic properties in mammals, but its use in anoles has not been described. Therefore, dexmedetomidine 0.1 mg/kg was combined with alfaxalone 30 mg/kg and administered SC in the cervical area (treatment A/D; n=5). After a short induction period (1.7±0.7 min) lizards reached a surgical plane of anesthesia for 31.2±5.3 min (Fig.2A). All lizards remained under light anesthesia following surgical depth, except for one lizard that was at a moderate level for 3 min. Lizards fully recovered within 43.8±2.4 min of SC injection. Mean respiration rates ranged from 20–33 bpm. During the first 15–20 min of anesthesia, respiration depth was shallow and then returned to a more normal depth. To determine whether dexmedetomidine on its own produced any anesthetic effects, four lizards were injected with increasing amounts of dexmedetomidine (0.1, 0.5, 0.75, and 1.0 mg/kg). In all cases, anesthesia was not achieved. Instead, lizards retained all normal responses and behavior.
Figure 2:
Comparison of anesthesia duration in lizards injected SC with alfaxalone and ketamine combinations with hydromorphone/dexmedetomidine. (A-E) Staggered stepwise graphs summing the total time individual lizards spent at each plane of anesthesia. (A) A/D (n=5); alfaxalone 30mg/kg with dexmedetomidine 0.1mg/kg. (B) A/D/H30 (n = 4); alfaxalone 30mg/kg with dexmedetomidine 0.1mg/kg and hydromorphone 1mg/kg; lizard deaths represented as arrowheads. (C) A/D/H20 (n = 4); alfaxalone 20mg/kg with dexmedetomidine 0.67mg/kg and hydromorphone 0.67mg/kg. (D) A/D+A30min (n = 4); alfaxalone 30mg/kg with dexmedetomidine 0.1mg/kg followed by second injection of alfaxalone 30mg/kg at 30 min post induction. (E) K/D/H (n = 4); ketamine 30mg/kg with dexmedetomidine 0.1mg/kg and hydromorphone 1mg/kg. Shading delineates the boundaries of different anesthesia levels.
Alfaxalone and dexmedetomidine followed by second alfaxalone dose
In an effort to increase surgical window duration, alfaxalone was administered to one lizard at a higher dose (40 mg/kg) in combination with dexmedetomidine 0.1 mg/kg. This higher dose resulted in rapid induction (10 secs) followed by 50 min of deep anesthesia where the lizard stopped breathing and manual ventilation had to be applied. After this period respiration increased to 10 bpm and depth improved. The lizard was held at a surgical level for another 20 min followed by 14 min of light anesthesia and was fully recovered 85 min post induction. As an alternative method to increase surgical window time without inducing severe respiratory depression, alfaxalone 30 mg/kg and dexmedetomidine 0.1 mg/kg was administered followed by a second dose of alfaxalone 30 mg/kg that was injected 30 minutes post induction (treatment A/D + A30min; n=4). All A/D + A30min lizards were induced within 2.3±1.5 min and reached surgical planes lasting 70±7 min. Duration of moderate and light sedation levels were 3.8±4.8 min and 7.3±1.3 min, respectively (Fig.2B, Fig.S4). The total anesthesia time was 83.3±8.9 min. Respiration rates fluctuated between 24–33 bpm but had normal depth. All lizards became bright, alert, and responsive upon recovery.
Alfaxalone, dexmedetomidine, and hydromorphone
Hydromorphone 1 mg/kg was combined with alfaxalone 30 mg/kg and dexmedetomidine 0.1 mg/kg (treatment A/D/H30; n=4). Upon SC injection into the cervical area, all lizards were anesthetized within 15–60 secs. A/D/H30 lizards 1 and 2 in this group underwent deep anesthesia lasting for 7 min. During this interval, respiration rate dropped to 4 bpm and then increased to 12–16 bpm. Upon respiration increasing in rate and depth, lizards 1 and 2 were held at a surgical depth lasting 15 and 38 min. Lizard 1 underwent 5 min of moderate anesthesia and another 17 min of light anesthesia before totally recovering, while lizard 2 experienced an additional 12 min of light anesthesia before recovering. The two remaining A/D/H30 lizards immediately exhibited respiratory depression after being induced and did not recover despite manually ventilating over a 50 min period (Fig.2C, Fig.S4).
In an effort to avoid severe respiratory depression, the next group of lizards (treatment A/D/H20; n=4) received 2/3 of the original combined dose (alfaxalone 20 mg/kg, dexmedetomidine 0.067 mg/kg, and hydromorphone 0.67 mg/kg). All lizards were induced within 1–2 min with the exception of one lizard, which was rapidly induced within 15 secs. This lizard experienced a deep level of anesthesia lasting for 40 min. During this period, respiration ceased, and the lizard was manually ventilated. After 35 min, the lizard began breathing on its own and experienced a surgical level of anesthesia for 10 min with full recovery after an additional 8 min. The next two lizards achieved a surgical plane of anesthesia for 15 and 19 min followed immediately by a light level of anesthesia for 10 and 15 min, respectively. The last lizard within this group was at a surgical level of anesthesia for 2 min, moderate for 8 min, and light for 23 min (Fig.2D, Fig.S4). Respiration depth and mean rates in these three lizards were normal and ranged from 32–36 bpm. All four A/D/H20 lizards fully recovered within 27–58 min.
Ketamine, dexmedetomidine, and hydromorphone
Ketamine 30 mg/kg was administered SC in combination with dexmedetomidine 0.1 mg/kg and hydromorphone 1 mg/kg in four lizards (treatment K/D/H; n=4). Only three of the four lizards became anesthetized. Mean induction time for the anesthetized lizards was 3.3±1.5 min. Moderate levels of anesthesia were maintained for 26.7±12.6 min and light levels for 18.3±8.5 min (Fig.2E, Fig.S4). Mean respiration rates of these 3 lizards ranged from 22–40 bpm. All 3 lizards recovered within 48.3±10.4 min. The remaining lizard was not successfully anesthetized after receiving drugs and exhibited typical lizard behavior, remaining bright, alert, and responsive.
Tribromoethanol
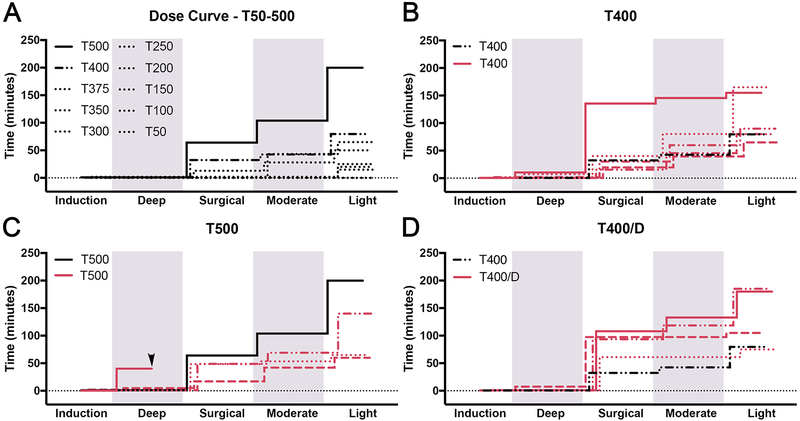
As we were unaware of any previous studies involving tribromoethanol in anoles, a dosing curve was established to determine the dose range needed to achieve a surgical plane of anesthesia. 11 lizards received TBE starting at 50 mg/kg and increasing to 500 mg/kg with 50–100 mg/kg increments. Induction occurred within 30–60 seconds at doses > 350 mg/kg, within 2 min at 200–250 mg/kg, and not at all at doses < 200 mg/kg. Lizards dosed with 375, 400 and 500 mg/kg reached surgical planes lasting 12, 32, and 63 min, respectively (Fig.3A, Fig.S4). Because TBE administered at 250–350 mg/kg only achieved light to moderate anesthesia levels, 400 mg/kg and 500 mg/kg doses were evaluated further.
Figure 3:
Anesthesia effects of TBE in lizards. (A-D) Staggered stepwise graphs summing the total time individual lizards spent at each plane of anesthesia. (A) Dose curve of TBE (n = 10); dose ranges from 50mg/kg (T50) to 500mg/kg (T500). Graphs (B-D) display T400 (n = 5), T500 (n = 4), and T400/D (n = 4). Arrowhead in (B) represents a lizard death. Shading delineates the boundaries of different anesthesia levels.
TBE was administered at 400 mg/kg (treatment T400; n=5). Within this group, the duration of anesthesia varied—a surgical depth of anesthesia was achieved for 44.0±45.8 min (surgical levels lasted 15, 19, 28, 33, and 125 min) (Fig.3B, Fig.S4). This was followed by an additional 26.0±11.4 min of moderate and 36.9±28.4 min of light anesthesia. Total duration of anesthesia was 111.8±45.7 min. All animals had normal respiration depth and a steady respiration rate consistently between 20–29 bpm throughout the procedure. Upon recovery, lizards were responsive but were slow to respond to stimulation/handling compared to A/D treated animals. We also noted that the pigmentation of T400 lizards darkened across the whole body. This dark coloration faded over the course of a few hours and was never observed in A/D treated animals.
The next four lizards were dosed with TBE 500 mg/kg (treatment T500; n=4). Induction occurred within 1.1±0.6 min in three of the lizards. These three lizards reached surgical levels lasting 36±20.8 min followed by 16.7±10.4 min of moderate and 33.5±32.6 min of light anesthesia (Fig.3C, Fig.S4). Respiration depth was normal and mean rates ranged between 20–35 bpm. Similar to the T400 lizards, T500 lizards were quiet but responsive upon recovery and displayed a dark color change. Recovery times varied and were 88.3±44.8 min. The remaining T500 lizard had an adverse response to the 500 mg/kg TBE dose, was rapidly induced within in 15 secs, and immediately underwent respiratory depression. This animal failed to recover despite assisted ventilation over a 40 min period.
Tribromoethanol and dexmedetomidine
To test the performance of TBE 400 mg/kg administered in combination with dexmedetomidine 0.1 mg/kg, four lizards were anesthetized with this combination (treatment T400/D; n=4). In T400/D lizards, induction occurred within 30–60 secs. After induction, one lizard was briefly at a deep level of anesthesia for 7 min before entering a surgical depth of anesthesia. The remaining three lizards directly entered a surgical depth of anesthesia. The four lizards experienced anesthesia at a surgical level for 87.5±19.8 min, a moderate level for 12.5±14.4 min, and a light level for 33.8±27.9 min. Total anesthesia time was 136.3±54.8 min (Fig.3D, Fig.S4). Respiration depth was normal and mean rates varied between 24–32 bpm. T400/D lizards retained normal coloration unlike the lizards in the T400 and T500 treatment groups.
Surgeries performed under Alfaxalone and TBE combinations with dexmedetomidine
To evaluate anesthetic performance under surgical conditions, two separate groups of lizards were anesthetized with either a combination of alfaxalone 30 mg/kg and dexmedetomidine 0.1 mg/kg followed by a second injection of alfaxalone 30 mg/kg 30 min post induction (n=4) or TBE 400 mg/kg and dexmedetomidine 0.1 mg/kg (n=4). Coeliotomies were then performed6. In all cases, anesthesia levels and times for the two surgical treatment groups were similar to our initial A/D + A30min and T400/D anesthesia treatment groups (total anesthesia time 89±23 min and 206±39 min, respectively). Lizards remained completely unresponsive to surgical manipulation and maintained steady respiration rates that ranged between 24–27 bpm (alfaxalone) and 31–35 bpm (TBE) with normal respiration depths. After 4 to 6hrs post recovery, lizard behavior and movement were assessed to determine pain levels. Lizards displayed typical escape behavior with no impact on movement when challenged. Appetite after surgery varied among lizards with the majority eating by the next day. Lizards continued to be monitored every 24 hrs for the following week after surgery. All lizards recovered from surgery with no physical/behavioral indications of infection, pain, or inflammation. No lizards in this study required supplemental pain-relief post surgery. All lizards exhibited normal behavior within two days of surgery, including eating, and all lizards exhibited normal egg production following surgery.
Discussion
Our goal was to identify a drug or combination of drugs that would successfully induce a stable surgical plane of anesthesia in anoles. Although inhalational agents such as isoflurane or sevoflurane are extensively used in reptiles32–37, and have been used on Anolis lizards38–40, there are challenges when using these agents in surgical protocols. First, unlike mammals and birds, in reptiles the intraventricular septum is absent or incomplete allowing the mixing of oxygenated and deoxygenated blood32,34,36,41,42. This can result in sudden changes in inhalant anesthetic blood concentrations, which can lead to significant changes in the depth and duration of anesthesia achieved34,36. Second, most reptiles develop apnea, or marked bradypnea and hypoventilation, when anesthetized32–37. Therefore, anesthesia using isoflurane or sevoflurane requires endotracheal intubation and intermittent positive-pressure ventilation32,34,36, which can be practically achieved in larger lizards, but is difficult when applied to Anole lizards due to their small size. Lastly, it has been reported that isoflurane levels adequate for surgical anesthesia often require mechanical ventilation37. These and other considerations associated with the use of inhalational agents in lizards33,36,37 prompted us to explore the use of injectable drugs to induce and maintain a surgical plane of anesthesia.
An important consideration when anesthetizing reptiles is animal size and drug volume. Female brown anoles are small—typically weighing between 2.5–3.5 grams, and administering volumes >10 μL IM in the craniodorsal and forelimb region is not practical. By injecting drugs SC in the cervical region, we were able to circumvent this issue, allowing for greater volumes to be administered. We found that brown anoles experience a surgical plane of anesthesia when alfaxalone 30 mg/kg or TBE 400 mg/kg are administered SC into the cervical area, either alone or in combination with dexmedetomidine 0.1 mg/kg. We also observed fast induction times. Work in leopard geckos has also shown SC administration to be very effective18. Therefore, administering drugs SC in the cervical area should be considered as an alternative to other routes in brown anoles and other small reptiles.
When alfaxalone or alfaxalone/dexmedetomidine was injected, a surgical plane of anesthesia of ~30 minutes was induced. These results are consistent with a report in green iguanas where alfaxalone (30 mg/kg IM) induced a surgical depth lasting approximately 40 min12. Successful anesthesia at this dose was also reported in leopard geckos18. We found that anoles sedated with alfaxalone rapidly recover and were alert an average of 11 minutes after exiting a surgical depth of anesthesia. Because some surgical procedures may require longer periods of anesthesia, the ability to increase the surgical window time is important. When alfaxalone/dexmedetomidine injected lizards received a second dose of alfaxalone (30 mg/kg) 30 minutes post induction, the surgical window was doubled without impacting recovery time. In contrast, when hydromorphone (1.0 mg/kg) is added to alfaxalone/dexmedetomidine, anoles are likely to be induced to a deep level of anesthesia, resulting in respiratory depression that may lead to death. It is possible that reducing the hydromorphone dose in alfaxalone/dexmedetomidine treated anoles might prevent severe respiratory depression while maintaining adequate analgesia coverage and good anesthesia depth, but further study of this drug combination is required.
We found that administration of TBE 400 mg/kg (alone or with dexmedetomidine 0.1 mg/kg) was also sufficient to generate a surgical plane of anesthesia. Historically, TBE has been used as an anesthetic agent for research procedures in rodents, but TBE has also been documented to be effective in reptiles24. Of the drug combinations we tested in brown anoles, TBE induced the longest surgical window (T400/D and A/D produced surgical windows of 87.5±19.8 and 31.2±5.3 min, respectively; p-value = 0.016). However, TBE injection volumes are also significantly greater (40–60 μL vs ~10 μL) and may contribute to bruising at the injection site. We observed that this bruising persisted for several days but was completely resolved within 2 weeks. Animals with bruising at injection sites displayed no outward signs of pain, inflammation, or feeding/behavioral changes. We also noted that upon awakening TBE injected lizards were subdued with some lizards showing lethargy for an additional 1.5 hours after regaining their righting response. Moreover, we observed variation in the duration of anesthesia levels among lizards injected with TBE or TBE/dexmedetomidine. This variation was likely attributable to TBE sensitivity of individual lizards. Alternatively, lizards may have been sensitive to slight differences between TBE injection solutions; we think this less likely since the same TBE stock solution was used for all experiments reported here and the variation in lizard response did not correlate with time of use after initial compounding date. That said, given that TBE solutions are known to exhibit batch-to-batch variation in effectiveness and some batches can exhibit toxicity22, a dosing curve should be established for each TBE stock solution that is prepared.
In mammals, dexmedetomidine is known to have both anesthetic and analgesic properties43, making it an ideal candidate for inducing anesthesia in other vertebrates. Studies using α2-adrenoceptor agonists combined with other drugs have been described in several reptile species16,18,44–47. When administered alone at doses ranging from 0.1–1.0 mg/kg, we found that dexmedetomidine was insufficient to induce anesthesia in anoles. Instead, lizard behavior was consistent with that of non-injected animals. However, we did find evidence that dexmedetomidine can alter anesthesia response when combined with other drugs. When lizards were injected with TBE alone, we noted that the pigmentation of the injected lizards darkened noticeably. Combining dexmedetomidine with TBE prevented this darkening. Since pigmentation darkening in anoles is associated with stress responses48, we speculate that dexmedetomidine may reduce stress responses generated by TBE. While this merits further study, we suggest that in the absence of respiratory depression it may be better to refrain from reversing lizards with atipamezole following surgical procedures, since treatment with atipamezole will also negate potential analgesic effects that dexmedetomidine may provide.
The final drug combination that we tested was ketamine 30 mg/kg with dexmedetomidine 0.1 mg/ml and hydromorphone 1.0 mg/kg. This treatment results in only moderate to light sedation. As one lizard in this group did not show any signs of sedation, we suggest that K/D/H at these doses is not an effective strategy to induce surgical anesthesia but might be adequate for purposes of restraint and minor, non-invasive procedures. Our finding that ketamine given at low doses results in moderate to light sedation levels in brown anoles is similar to findings in other reptiles17,49.
In summary, of the treatments we tested the most effective drug combinations to induce a surgical plane of anesthesia in anoles are alfaxalone 30 mg/kg and tribromoethanol 400 mg/kg. When combined with dexmedetomidine 0.1 mg/kg, these drugs performed well in lizards actively undergoing invasive surgical procedures. These drugs also appeared to be well tolerated in lizards, as egg production was still maintained in females that underwent anesthesia trials or coelomic surgery. Lastly, since anesthesia was induced readily by injecting drugs SC in the cervical area, this region may be an ideal location to administer anesthetics in small reptiles.
Supplementary Material
Acknowledgments
Special thanks to Dr. Steve Harvey and the Animal Resources at the University of Georgia for generously supplying the reagents necessary to conduct these anesthesia trials.
Funding
This work was supported by grants from the National Science Foundation (IOS-1149453; IOS-1827647), the National Institutes of Health (T32GM007103), a Society for Developmental Biology Emerging Models Grant, and the Achievement Rewards for College Scientists (ARCS) Foundation.
Footnotes
Declaration of Conflicting of Interests
The authors declare that there is no conflict of interest.
References:
- 1.Poe S, Nieto-Montes de Oca A, Torres-Carvajal O, et al. A phylogenetic, biogeographic, and taxonomic study of all extant species of Anolis (Squamata; Iguanidae). Syst Biol. 2017;66(5):663–697. doi: 10.1093/sysbio/syx029. [DOI] [PubMed] [Google Scholar]
- 2.Sanger TJ, Losos JB, and Gibson-Brown JJ. A developmental staging series for the lizard genus Anolis: a new system for the integration of evolution, development, and ecology. J Morphol. 2008;269(2):129–137. doi: 10.1002/jmor.10563. [DOI] [PubMed] [Google Scholar]
- 3.Park S, Infante CR, Rivera-Davila LC, et al. Conserved regulation of hoxc11 by pitx1 in Anolis lizards. J Exp Zool B Mol Dev Evol. 2014;322(3):156–165. doi: 10.1002/jez.b.22554. [DOI] [PubMed] [Google Scholar]
- 4.Sanger TJ and Kircher BK. Model Clades Versus Model Species: Anolis lizards as an integrative model of anatomical evolution. Methods Mol Biol. 2017;1650 (Chapter 19):285–297. doi: 10.1007/978-1-4939-7216-6_19. [DOI] [PubMed] [Google Scholar]
- 5.Sanger TJ, Hime PM, Johnson MA, et al. Laboratory protocols for husbandry and embryo collection of Anolis lizards. Herpetol Rev. 2008;39(1):58–63. [Google Scholar]
- 6.Alworth LC, Hernandez SM, and Divers SJ. Laboratory reptile surgery: principles and techniques. J Am Assoc Lab Anim Sci. 2011;50(1):11–26. [PMC free article] [PubMed] [Google Scholar]
- 7.Scheelings TF. Use of intravenous and intramuscular alfaxalone in Macquarie River Turtles (Emydura Macquarii). J Herpetol Med Surg 2014;23:91–94. doi: 10.5818/1529-9651-23.3.91. [DOI] [Google Scholar]
- 8.Kummrow MS, Tseng F, Hesse L, et al. Pharmacokinetics of buprenorphine after single-dose subcutaneous administration in red-eared sliders (Trachemys scripta elegans). J Zoo Wildl Med. 2008;39(4):590–595. doi: 10.1638/2008-0033.1. [DOI] [PubMed] [Google Scholar]
- 9.Holz P, Barker IK, Burger JP, et al. The effect of the renal portal system on pharmacokinetic parameters in the red-eared slider (Trachemys scripta elegans). J Zoo Wildl Med. 1997;28(4):386–393. [PubMed] [Google Scholar]
- 10.Holz P, Barker IK, Crawshaw GJ, et al. The anatomy and perfusion of the renal portal system in the red-eared slider (Trachemys scripta elegans). J Zoo Wildl Med. 1997;28(4):378–385. doi: 10.2307/20095678. [DOI] [PubMed] [Google Scholar]
- 11.Harrison NL and Simmonds MA. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323(2):287–292. [DOI] [PubMed] [Google Scholar]
- 12.Bertelsen MF and Sauer CD. Alfaxalone anaesthesia in the green iguana (Iguana iguana). Vet Anaesth Analg. 2011;38(5):461–466. doi: 10.1111/j.1467-2995.2011.00640.x. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald JF, Bartlett MC, Mody I, et al. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J Physiol (Lond). 1991;432:483–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irifune M, Shimizu T, Nomoto M, et al. Ketamine-induced anesthesia involves the N-methyl-D-aspartate receptor-channel complex in mice. Brain Res. 1992;596(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 15.McGuire JL, Hernandez SM, Smith LL, et al. Safety and utility of an anesthetic protocol for the collection of biological samples from gopher tortoises. Wildl Soc Bull. 2013;38(1):43–50. doi: 10.1002/wsb.364. [DOI] [Google Scholar]
- 16.Greer LL, Jenne KJ, and Diggs HE. Medetomidine-ketamine anesthesia in red-eared slider turtles (Trachemys scripta elegans). Contemp Top Lab Anim Sci. 2001;40(3):9–11. [PubMed] [Google Scholar]
- 17.Bienzle D and Boyd C. Sedative effects of ketamine and midazolam in snapping turtles (Chelydra serpentina). J Zoo Wildl Med. 1992;23(2):201–204. doi: 10.2307/20095209. [DOI] [Google Scholar]
- 18.Doss GA, Fink DM, Sladky KK, et al. Comparison of subcutaneous dexmedetomidine-midazolam versus alfaxalone-midazolam sedation in leopard geckos (Eublepharis macularius). Vet Anaesth Analg. 2017;44(5):1175–1183. doi: 10.1016/j.vaa.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Kischinovsky M, Duse A, Wang T, et al. Intramuscular administration of alfaxalone in red-eared sliders (Trachemys scripta elegans)-effects of dose and body temperature. Vet Anaesth Analg. 2013;40(1):13–20. doi: 10.1111/j.1467-2995.2012.00745.x. [DOI] [PubMed] [Google Scholar]
- 20.Morici M, Di Giuseppe M, Spadola F, et al. Intravenous alfaxalone anaesthesia in leopard geckos (Eublepharis macularius). J Ex Pet Med. 2018;27(3):11–14. doi: 10.1053/j.jepm.2017.08.008. [DOI] [Google Scholar]
- 21.Kleinschmidt LM, Hanley CS, Sahrmann JM, et al. Randomized controlled trial comparing the effects of alfaxalone and ketamine hydrochloride in the Haitian giant galliwasp (Celestus warreni). J Zoo Wildl Med. 2018;49(2):283–290. doi: 10.1638/2017-0164.1. [DOI] [PubMed] [Google Scholar]
- 22.Lieggi CC, Fortman JD, Kleps RA, et al. An evaluation of preparation methods and storage conditions of tribromoethanol. Contemp Top Lab Anim Sci. 2005;44(1):11–16. [PubMed] [Google Scholar]
- 23.Hamasaki DI and Dodt E. Light sensitivity of the lizard’s epiphysis cerebri. Pflugers Arch. 1969;313(1):19–29. [DOI] [PubMed] [Google Scholar]
- 24.Heisey SR. Cerebrospinal and extracellular fluid spaces in turtle brain. Am J Physiol. 1970;219(6):1564–1567. doi: 10.1152/ajplegacy.1970.219.6.1564. [DOI] [PubMed] [Google Scholar]
- 25.Evans RR, Goertz JW, and Williams CT. Capturing wild turkeys with tribromoethanol. J Wildl Manage. 1975;39(3):630. doi: 10.2307/3800410. [DOI] [Google Scholar]
- 26.Krapu GL. Experimental responses of mallards and Canada Geese to tribromoethanol. J Wildl Manage. 1976;40(1):180. doi: 10.2307/3800178. [DOI] [Google Scholar]
- 27.Thompson JS, Brown SA, Khurdayan V, et al. Early effects of tribromoethanol, ketamine/xylazine, pentobarbitol, and isoflurane anesthesia on hepatic and lymphoid tissue in ICR mice. Comp Med. 2002;52(1):63–67. [PubMed] [Google Scholar]
- 28.Lenth RV. Some practical guidelines for effective sample size determination. Am Stat. 2001;55(3):187–193. doi: 10.1198/000313001317098149. [DOI] [Google Scholar]
- 29.Festing MFW and Altman DG. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002;43(4):244–258. [DOI] [PubMed] [Google Scholar]
- 30.Brown MJ, Pearson PT, and Tomson FN. Guidelines for animal surgery in research and teaching. AVMA Panel on Animal Surgery in Research and Teaching, and the ASLAP (American Society of Laboratory Animal Practitioners). Am J Vet Res. 1993;54(9):1544–1559. [PubMed] [Google Scholar]
- 31.Guide for the care and use of laboratory animals (8th edn):. Lab Anim. 2012;46(3):267–268. doi: 10.1258/la.2012.150312. [DOI] [Google Scholar]
- 32.Heard DJ. Reptile anesthesia. Vet Clin North Am Exot Anim Pract. 2001;4(1):83–117. doi: 10.1016/S1094-9194(17)30053-1. [DOI] [PubMed] [Google Scholar]
- 33.Read MR. Evaluation of the use of anesthesia and analgesia in reptiles. J Am Vet Med Assoc. 2005;224(4):547–552. doi: 10.2460/javma.2004.224.547. [DOI] [PubMed] [Google Scholar]
- 34.Mosley CAE. Anesthesia and analgesia in reptiles. Semin Avian Exot Pet Med. 2005;14(4):243–262. doi: 10.1053/j.saep.2005.09.005. [DOI] [Google Scholar]
- 35.Bertelsen MF. Squamates (snakes and lizards) In: West G, Heard DJ, and Caulkett N (eds) Zoo Animal and Wildlife Immobilization and Anesthesia. Ames, IA: Blackwell, 2007:233–244. [Google Scholar]
- 36.Sladky KK and Mans C. Clinical analgesia in reptiles. J Ex Pet Med. 2012;21(2):158–167. doi: 10.1053/j.jepm.2012.02.012. [DOI] [Google Scholar]
- 37.Ferrell ST. Anesthesia and analgesia in reptiles NAVC Conference 2013 Small Animal. January 19–23, 2013, Orlando, FLORIDA. [Google Scholar]
- 38.Holmes MM and Wade J. Seasonal plasticity in the copulatory neuromuscular system of green anole lizards: A role for testosterone in muscle but not motoneuron morphology. J Neurobiol. 2004;60(1):1–11. doi: 10.1002/neu.10334. [DOI] [PubMed] [Google Scholar]
- 39.Christensen-Dalsgaard J and Manley GA. Acoustical coupling of lizard eardrums. JARO. 2008;9(4):407–416. doi: 10.1007/s10162-008-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brittan-Powell EF, Christensen-Dalsgaard J, Tang Y, et al. The auditory brainstem response in two lizard species. J Acoust Soc Am. 2010;128(2):787–794. doi: 10.1121/1.3458813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koshiba-Takeuchi K, Mori AD, Kaynak BL, et al. Reptilian heart development and the molecular basis of cardiac chamber evolution. Nature 2009 461:7260. 2009;461(7260):95–98. doi: 10.1038/nature08324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen B, Moorman AFM, and Wang T. Structure and function of the hearts of lizards and snakes. Biol Rev Camb Philos Soc. 2014;89(2):302–336. doi: 10.1111/brv.12056. [DOI] [PubMed] [Google Scholar]
- 43.Hunter JC, Fontana DJ, Hedley LR, et al. Assessment of the role of alpha2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br J Pharmacol. 1997;122(7):1339–1344. doi: 10.1038/sj.bjp.0701520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen LL and Bertelsen MF. Assessment of the effects of intramuscular administration of alfaxalone with and without medetomidine in Horsfield’s tortoises (Agrionemys horsfieldii). Vet Anaesth Analg. 2013;40(6):e68–e75. doi: 10.1111/vaa.12045. [DOI] [PubMed] [Google Scholar]
- 45.Dennis PM and Heard DJ. Cardiopulmonary effects of a medetomidine-ketamine combination administered intravenously in gopher tortoises. J Am Vet Med Assoc. 2002;220(10):1516–1519. doi: 10.2460/javma.2002.220.1516. [DOI] [PubMed] [Google Scholar]
- 46.Heaton-Jones TG, Ko JCH, and Heaton-Jones DL. Evaluation of medetomidine-ketamine anesthesia with atipamezole reversal in American alligators (Alligator mississippiensis). J Zoo Wildl Med. 2002;33(1):36–44. doi: 10.1638/1042-7260(2002)033[0036:EOMKAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 47.Olsson A and Phalen D. Preliminary studies of chemical immobilization of captive juvenile estuarine (Crocodylus porosus) and Australian freshwater (C. johnstoni) crocodiles with medetomidine and reversal with atipamezole. Vet Anaesth Analg. 2012;39(4):345–356. doi: 10.1111/j.1467-2995.2012.00721.x. [DOI] [PubMed] [Google Scholar]
- 48.Hadley ME and Goldman JM. Physiological color changes in reptiles. Am Zool. 1969;9(2):489–504. [DOI] [PubMed] [Google Scholar]
- 49.Holz P and Holz RM. Evaluation of ketamine, ketamine/xylazine, and ketamine/midazolam anesthesia in red-eared sliders (Trachemys scripta elegans). J Zoo Wildl Med. 1994;25(4):531–537. doi: 10.2307/20095413. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.