Abstract
While widely conceived as distinct conditions, higher functioning autism spectrum disorder (ASD) and schizoid personality disorder (schizoid PD) share similar clinical symptomatology. This study explored the relationship between the two disorders by collecting extensively validated measures of autistic trait burden (SRS-2) and schizoid PD affectation (DIGS) from clinically ascertained verbal males with and without autism ages 12-25 (n=72) via parent, teacher, and self-report. Although only a small minority of ASD adolescents met full diagnostic criteria for schizoid PD, ASD participants endorsed a continuous distribution of schizoid PD traits that reflected a pronounced pathological shift in comparison to those in the control group, with one half of ASD males experiencing 3 or more DSM-IV schizoid PD criterion items “often” or “almost always.” Results suggest significant amplification of schizoid PD trait burden in adolescents with ASD. ASD-specific interventions should be considered for schizoid PD patients with premorbid histories of ASD.
Keywords: autism spectrum disorder, schizoid personality disorder, social impairment, differential diagnosis, adolescence
Introduction
Social isolation and emotional detachment are the core symptoms which constitute a diagnosis of schizoid personality disorder. Originally derived from the diagnostic criteria for schizophrenia (Michels et al., 1989), schizoid PD encompasses a constellation of features of social indifference and constriction of affect that affects approximately 3-5% of the population nationally (American Psychiatric Association, 2013). Although common in adulthood, schizoid PD is rarely diagnosed in childhood.
Autism Spectrum Disorder (ASD) exhibits a nearly opposite pattern of prevalence over the life course, in which diagnosis has become increasingly prevalent in childhood (Zablotsky et al., 2015), but traditionally rare in adulthood, especially for cohorts of adults who were born before the exponential rise in prevalence of the 1990s and early 2000s. Increased recognition of a constellation of childhood symptoms that characterize “higher-functioning” ASD syndromes has raised the possibility that what are now appreciable as childhood autistic traits may precede all or most cases of schizoid PD, even if this has been historically under-appreciated in prior studies of adults with the diagnosis.
Furthermore, a vast body of developmental research has revealed that the characteristic signs and symptoms of autism are continuously distributed in nature (see Constantino and Charman, 2016), and that at any level of severity with which an individual is affected, these traits are highly stable over the life course (Wagner et al., 2019). These traits, including proneness to isolation and limitations in range of affect, have historically been more readily attributed to autistic psychopathy in children (Asperger, 1944), despite their resemblance to schizoid PD (Wolff & Barlow, 1979; Wolff & McGuire, 1991). Although small previous studies have suggested associations between ASD and Schizoid PD that survive chance correction, all have relied upon ascertainment of comorbidity for categorical diagnosis of each respective condition (Larsen & Mouridsen, 1997; Nilsson et al., 1999), the most notable of which found 14 out of 54 adults with Asperger’s Syndrome qualifying for a diagnosis of schizoid PD (Lugnegard, Hallerbäck, & Gillberg, 2012).
Overlap in the respective symptom presentations for schizoid PD and ASD in adulthood has long been recognized, and the DSM-5 explicitly cites complexities of differential diagnosis between the two (American Psychiatric Association, 2013). Although patterns of social and communicative impairment in both disorders support a shared phenotype when subjected to factor analysis (Ford & Crewther, 2014), older clinical reports have suggested that patients with schizoid PD are more affected by deficits in social motivation, whereas those with ASD are more affected by deficits in social skills or capacity. More recent analysis of autistic traits in large populations has shown, however, that these constructs are highly correlated in nature, not only in clinically-affected patients, but in the entire general population (Frazier et al., 2014). Thus, the substantial overlap in the presentation of symptoms and their impact on social adaptation raises the question of whether they represent the same liability over the course of development. In this prospective longitudinal study, we examined schizoid PD traits in adolescent males with and without ASD whose autistic traits were serially quantitatively characterized throughout their transition to adulthood. To our knowledge, ours is the first study to employ quantitative methods or to explicitly examine the relationship between a childhood diagnosis of ASD and an adolescent diagnosis of schizoid PD in a prospective longitudinal context.
Methods
Participants
Participants included 72 high-functioning verbal males, recruited by their physicians from either the Washington University Child and Adolescent clinics or outpatient child psychiatry practices in the greater St. Louis metropolitan area. Participants were categorized as either having an ASD diagnosis (n = 50) or not (n = 22); a stipulation that all participants be verbal was incorporated into the study design to allow for self-report data to be collected and to help minimize potential effects of severe cognitive disability to influence the results. Those in the ASD group had received diagnoses established by expert clinician examination and as a further check of accuracy, a subset (one out of every four) ASD affected participants in the parent study underwent diagnostic confirmation by the Autism Diagnostic Observation Schedule and/or the Autism Diagnostic Interview; the rate of confirmation was over 95% (Wagner et al., 2019). Those in the non-ASD psychiatric control group carried expert clinician diagnoses of other psychiatric disorders including ADHD, unspecified neurodevelopmental disorder, anxiety disorder (separation anxiety, unspecified anxiety disorder), mood disorder (major depressive disorder, bipolar I disorder, unspecified episodic mood disorder), or trauma related disorder (adjustment disorder). For a breakdown of diagnosis prevalence amongst both groups, please see Table 1. All data were collected in the course of a longitudinal study and included a baseline SRS assessment at entry (ranging 1997-2010) and the latest of available follow up SRS assessments acquired in the longitudinal study (ranging 2002-2013, on average 7 years after baseline) coupled with acquisition of the DIGS at age 12 years or older (average age 14 years, range 12-19) (Supplemental Figure 1.3). For a full depiction of participant age at baseline and follow up, please see Supplemental Figures 1.1 and 1.2.
Table 1.
Demographic characteristics of groups
| ASD | Non-ASD Psychiatric Control |
|
|---|---|---|
| Mean age at baseline SRS parent report ascertainment (SD) | 9.7 (4.0) | 12.1 (2.6) |
| Mean age at follow up SRS parent report ascertainment (SD) | 16.7 (3.8) | 18.7 (3.1) |
| Mean age at baseline SRS teacher report ascertainment (SD) | 9.5 (3.9) | 12.6 (2.6) |
| Mean age at follow up SRS teacher report ascertainment (SD) | 14.5 (2.7) | 15.6 (1.8) |
| Mean age at DIGS ascertainment (SD) | 14.3 (2.1) | 14.8 (1.9) |
| Psychiatric diagnoses | ||
| Autism spectrum disorder | 50/50 100% | |
| ADHD | 2/22 9% | |
| Other neurodevelopmental disorder | 11/22 50% | |
| Anxiety disorder | 3/22 14% | |
| Mood disorder | 6/22 27% | |
| Trauma related disorder | 1/22 5% |
ASD: Schizoid PD Trait Index Parent Report n=49, Self Report n=50; SRS Baseline and Follow Up Parent Report n=50, Baseline and Follow Up Teacher Report n=46
Control: Schizoid PD Trait Index Parent and Self Report n = 22; SRS Baseline Parent Report n=21, Follow Up Parent report n=22, Teacher Baseline and Follow Up reports n=21
Measures
DIGS.
The Diagnostic Interview for Genetic Studies (DIGS) is a clinical interview-based diagnostic instrument for major psychiatric disorders. Parent and self-reports were collected from the schizoid PD section of the interview (questions 1-7, each of which corresponded to the DSM-IV criteria for schizoid PD). Each DSM-IV criterion is rated on a 4-point Likert scale (0 – Rarely/Never True, 1 – Somewhat/Sometimes True, 2 – Often True, 3 – Almost Always True.) To formally achieve the diagnostic threshold of schizoid PD on the DIGS, four or more items must be endorsed at the maximum severity rating of 3 “Almost Always True.”
SRS.
The SRS is a 65-item quantitative instrument designed to measure the severity of autism-related impairment in reciprocal social behavior through ratings of a child’s behavior in his or her natural social setting. Using a 4 point Likert scale to assess both parent and teacher reports of a child’s behavior at home and in school, SRS scores have been validated and normed to quantify diagnostic features of autism spectrum disorders in children and to distinguish these from traits of other psychiatric conditions (Constantino et al., 2000; Constantino & Gruber, 2005). In addition, SRS scores have demonstrated a high degree of heritability (Constantino & Todd, 2000, 2003, 2005) as well as a continuous distribution in the general population (Constantino & Todd, 2003).
For the subjects in this study, parent and teacher SRS reports had been collected serially prior to the ascertainment of schizoid PD symptoms; as described above, the stability of SRS measurements between the first rating at baseline and the rating closest to the time of schizoid PD assessment (on average 7 years apart) was ICC=0.62, indicating trait-like ASD-related characteristics of the subjects according to the standards of both Landis & Koch for observer agreement in multivariate categorical data (1977) as well as Cicchetti & Sparrow for interrater reliability (1981). In this sample, SRS scores overwhelmingly discriminated ASD patients from psychiatric controls (t(69) = 6.15, p < .001 at baseline and t(70) = 5.79, p < .001 at follow up by parent report; t(65) = 5.2, p < .001 at baseline and t(65) = 5.49, p < .001 at follow up by teacher report) in keeping with prior reports from larger samples (Wagner et al., 2019). For analysis, SRS raw (non-transformed) scores were used; the higher the SRS score, the higher the severity of socially-impairing autistic traits.
Data Analysis
First, we conducted an analysis of the distribution in DIGS-acquired schizoid PD traits comparing ASD and control participants using analysis of variance methods. We analyzed independent samples t-tests between each measure across the sample to determine statistically significant differences between ASD and non-ASD participant reports, as well as conducted Mann-Whitney U and Kolmogorov-Smirnov tests to confirm the observed between-groups differences in a more conservative context that did not assume normality of the respective underlying trait distributions.
For these analyses, responses to each DIGS question were summed into one cumulative variable to create the Schizoid PD Trait Index; the greater its value, the greater the level of schizoid PD affectation. We note that use of this trait index was invoked as a research method to acquire multi-informant assessments of Schizoid PD trait burden in adolescents and was not fully validated as a diagnostic tool. Because many ASD participants who presented with considerable schizoid PD trait burden did not formally reach the threshold for case designation according to the DIGS (4 of 7 criterion items endorsed at a level of “almost always true”), yet still exhibited substantial schizoid PD trait burden, we conducted secondary analyses in which a relaxed threshold for clinically-significant schizoid PD symptom endorsement was considered (Supplement 2).
We then computed coefficients of cross trait correlation, using Intraclass or Pearson’s coefficient of correlation as appropriate.
Finally, we analyzed the frequency of individual criterion item endorsements on the DIGS to determine whether specific diagnostic criterion items disproportionately accounted for overlap or discrimination between groups.
Results
Relationship between Autistic Traits and Schizoid PD traits
The distribution of schizoid PD trait burden among ASD subjects exhibited a distinct pathological shift in comparison to their non-ASD control counterparts (see Figure 1). This pronounced shift held true for both self-report and parent report: ASD self-report (M = 6.7, SD = 3.7), control self-report (M = 3.5, SD = 1.9), ASD parent report (M = 8.3, SD = 4.1), and control parent report (M = 3.4, SD = 3.2) (see Table 2). Across informants, ASD participants consistently scored higher on the Schizoid PD Trait Index, meaning that they endorsed significantly more schizoid PD diagnostic criteria than the participants in the control sample, as shown in Table 2. Correlations between autistic traits and schizoid PD are presented in Table 3 and were substantial, consistent with an effect size (Cohen’s d) of 2.3, and generally statistically significant. A scatter plot depicting the association is provided in Figure 2.
Figure 1a.
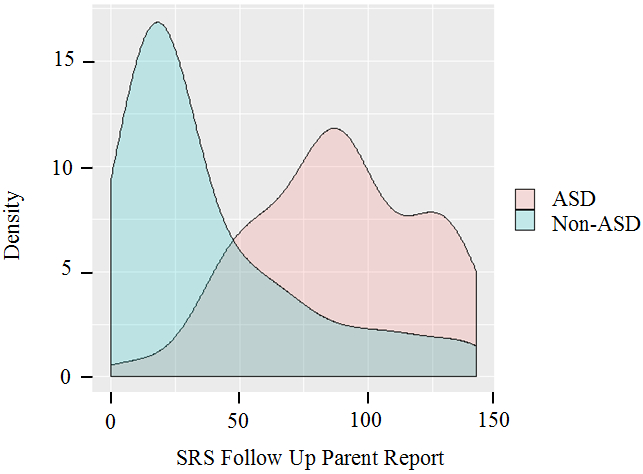
Distribution of SRS scores for ASD vs. control participants per follow up parent report (collected 2002-2013)
Table 2.
Descriptive statistics for all reports
| ASD | Control | t | p | Mann- Whitney U |
p | Kolmogorov- Smirnov |
p | |
|---|---|---|---|---|---|---|---|---|
| Mean Schizoid PD Trait Index Parent Report (SD) | 8.3 (4.1) | 3.4 (3.2) | 4.9 | <0.001 | 196 | <0.001 | 2.2 | <0.001 |
| Mean Schizoid PD Trait Index Self Report (SD) | 6.7 (3.7) | 3.5 (1.9) | 4.8 | <0.001 | 262.5 | <0.001 | 1.8 | .003 |
| Mean SRS Baseline Score Parent Report (SD) | 101.9 (25) | 54.7 (38.3) | 6.2 | <0.001 | 143.5 | <0.001 | 2.6 | <0.001 |
| Mean SRS Follow Up Score Parent Report (SD) | 88.4 (32) | 38.7 (37.1) | 5.8 | <0.001 | 169 | <0.001 | 2.5 | <0.001 |
| Mean SRS Baseline Score Teacher Report (SD) | 89.7 (29) | 49.8 (29.4) | 5.2 | <0.001 | 171 | <0.001 | 2.1 | <0.001 |
| Mean SRS Follow Up Score Teacher Report (SD) | 84.6 (32.5) | 41.2 (23.8) | 5.5 | <0.001 | 143 | <0.001 | 2.5 | <0.001 |
ASD: Schizoid PD Trait Index Parent Report n=49, Self Report n=50; SRS Baseline and Follow Up Parent Report n=50, Baseline and Follow Up Teacher Report n=46
Control: Schizoid PD Trait Index Parent and Self Report n = 22; SRS Baseline Parent Report n=21, Follow Up Parent report n=22, Teacher Baseline and Follow Up reports n=21
Table 3.
Pearson and Intraclass Correlation Coefficients across all measures for the entire sample (both ASD and control participants, n=72)
| Schizoid PD Trait Index Parent Report |
Schizoid PD Trait Index Self Report |
SRS Parent Baseline Report |
SRS Parent Follow Up Report |
SRS Teacher Baseline Report |
SRS Teacher Follow Up Report |
|
|---|---|---|---|---|---|---|
| Schizoid PD Trait Index Parent Report | 1 | |||||
| Schizoid PD Trait Index Self Report | 0.483** | 1 | ||||
| SRS Parent Baseline Report | 0.462** | 0.254* | 1 | |||
| SRS Parent Follow Up Report | 0.477** | 0.203 | 0.622** | 1 | ||
| SRS Teacher Baseline Report | 0.231 | 0.258* | 0.268** | 0.358** | 1 | |
| SRS Teacher Follow Up Report | 0.290* | 0.275* | 0.270** | 0.492** | 0.452** | 1 |
Intraclass Correlations denoted by italics; Pearson Correlations otherwise reported
Indicates significance at the 0.01 level (2-tailed)
Indicates significance at the 0.05 level (2-tailed)
Figure 2.
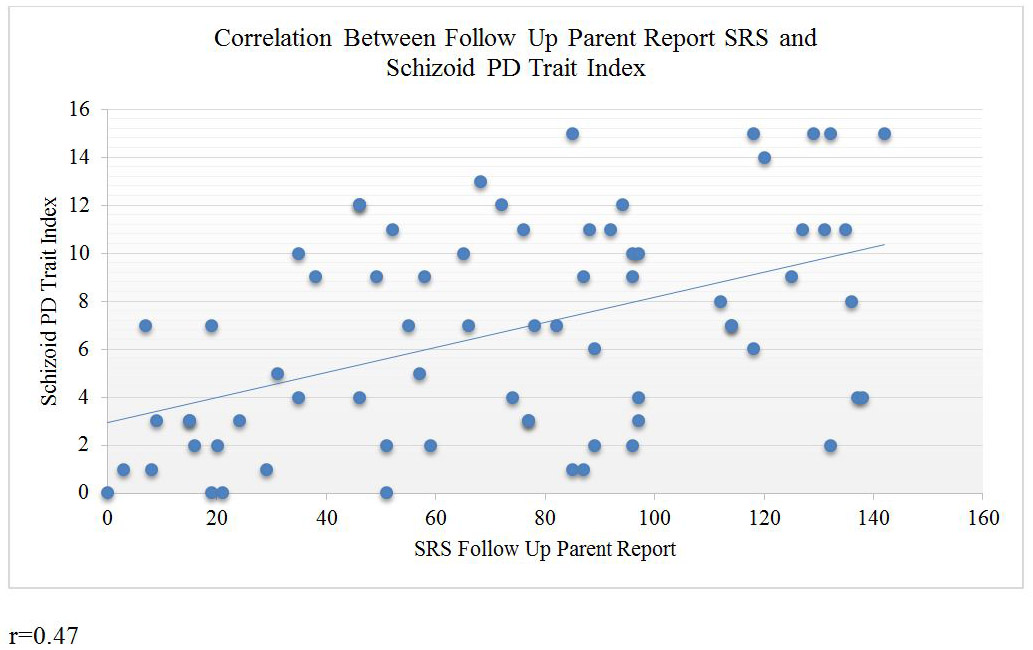
Correlation between follow up SRS scores and Schizoid PD Trait Index scores per parent report
As a confirmation of the homogeneity of association across age, we conducted an additional analysis in which SRS baseline scores, group, and age at time of schizoid PD assessment were entered into a linear regression model to predict Schizoid PD Trait Index. The results were as follows: F(3,66)=9.525, p < .001 with an R2 of .302. Only SRS and group (not age) were statistically significant; SRS (t = 2.003, p = .049), group (t = −2.8, p = .007), age (t = .91, p = .367).
Proportion of ASD participants meeting stringent diagnostic criteria for schizoid PD
When considering the DIGS standard for criterion endorsement meeting the threshold for schizoid PD diagnosis, (four or more items rated at a 3 “almost always true”), 3 out of 49 ASD participants and 0 out of 22 non-ASD control participants met full DSM-IV criteria for schizoid PD per parent report. Given our sample size, this did not reach statistical significance (Fischer’s exact p = .55)
Given the continuous nature of the marked pathological shift in the distribution of schizoid PD scores observed among ASD subjects, however, exclusive consideration of cases meeting strict criteria by this particular standard underrepresents discrepancies in clinically meaningful schizoid PD trait burden between adolescents with and without ASD. As an additional approach to explore such contrasts, we examined the profiles of symptoms between groups at the relaxed threshold of “often true.” Comparison of symptom profiles between ASD and controls at the respective nested thresholds of “often true” and “almost always true” are presented in the next section; in the supplemental materials, we provide an analysis indicating that endorsement of schizoid PD clinical trait burden at the lower threshold is five-fold more frequent among ASD subjects than controls (see Supplement 2).
Symptom profiles for schizoid PD in ASD subjects vs. controls
Here (Table 4) we present the frequency with which schizoid PD traits were endorsed as a function of group, rater, and stringency of criterion (“often” vs. “almost always”). Notably, the items that most differentiated the ASD participants from the non-ASD control group per parent report (at either severity threshold) were “chooses solitary activities,” and “no close friends or confidants.” The most pronounced distinction was observed for “no close friends or confidants,” (Fisher’s exact p < .003), but this was by parent report only—no statistically significant difference was observed by self report at either threshold. While not a statistically significant difference between groups or raters, it is notable that the schizoid PD criterion item “Little, if any desire to have sexual experiences with another person,” was endorsed approximately twice as often by parents of ASD subjects than by the subjects themselves, and endorsed by ASD subjects roughly half as often as “No close friends or confidants.”
Table 4.
Frequency of itemized Schizoid PD Trait Index symptom endorsement in ASD vs. control participants per parent and self report
| Endorsement of “almost always true” | Endorsement of either “often true” or “almost always true” |
|||||||
|---|---|---|---|---|---|---|---|---|
| ASD | Control | ASD | Control | |||||
| Criterion | Parents (n=49) |
Self (n=50) |
Parents (n=22) |
Self (n=22) |
Parents (n=49) |
Self (n=50) |
Parents (n=22) |
Self (n=22) |
| Neither desires nor enjoys close relationships, including family | .08 | .04 | .05 | 0 | .22 | .18 | .09 | .05 |
| Almost always chooses solitary activities* | .18 | .18 | 0 | 0 | .49 | .44 | .18 | .09 |
| Rarely, if ever, claims or appears to experience strong emotions, anger/joy | .08 | .08 | .05 | .05 | .20 | .22 | .09 | .14 |
| Little, if any, desire to have sexual experiences with another person (age taken into account) | .35 | .18 | .14 | .09 | .39 | .26 | .18 | .18 |
| Indifferent to praise and criticism from others | .02 | .06 | 0 | .05 | .16 | .16 | 0 | .09 |
| No close friends or confidants, or only one, other than first-degree relatives ** | .53 | .34 | .14 | .18 | .76 | .46 | .23 | .23 |
| Constricted affect, aloof, cold, rarely reciprocated | .10 | .08 | 0 | 0 | .22 | .18 | 0 | .14 |
For between groups difference at the level of endorsement, “almost always true,” Fisher’s exact p = .049 for both parent and self report; for between groups differences at the level of “often” or “almost always true,” Fisher’s exact p = .018 for parent report and .006 for self
For between groups difference at the level of endorsement, “almost always true,” Fisher’s exact p = .002 for parent report and .261 for self report; for between groups differences at the level of “often” or “almost always true,” Fisher’s exact p = .0001 for parent report and .072 for self
Discussion
In this sample of ASD participants, the entire distribution of schizoid PD diagnostic symptoms was pathologically-shifted, strongly indicating that the condition of ASD is, at minimum, associated with a distinct increase in schizoid PD symptom burden in adolescence. Although the majority of participants did not meet strict diagnostic criteria for schizoid PD, the marked pathological shifts underlying the cases that fell at the extreme end of the continuous distribution implicates a substantial role of ASD symptomatology amplifying liability for schizoid PD. The stability of autistic traits in this sample fully reflected the marked stability of autistic traits that has been observed over time in prior research (Wagner et al., 2019). Thus, it is extremely unlikely that the endorsed schizoid PD traits represent the presentation of new behaviors. Rather, it is highly likely that the schizoid PD traits and symptoms endorsed in this adolescent sample reflect aspects of social dysfunction that had been present long before. Taken together, these results suggest causal overlap and/or developmental continuity between ASD trait burden in childhood and schizoid PD burden in adolescence: the higher the level of autistic impairment in childhood, the higher the level of schizoid PD symptomatology in adolescence. We observed no evidence that the small group of adolescents who met full diagnostic criteria for schizoid PD were discontinuous from the remainder of the high functioning ASD population (i.e. as a separate cluster).
Given that ASD symptoms are highly stable over the entire life course (Constantino & Todd, 2005; Wagner et al., 2019), the historic imbalance in prevalence between childhood and adulthood for these two disorders is indicative of as yet unresolved issues in diagnostic substitution over the course of development. The prevalence rate of schizoid PD affectation, while difficult to track, is estimated to occur in between 3-5% in the national population (American Psychiatric Association, 2013) and currently autism spectrum disorders are diagnosed in 1 in 59 children in the United States (Baio et al., 2014). Historically, adults have rarely carried diagnoses of autism spectrum conditions in the U.S. and now that the current generation of children born during the exponential rise in recognized prevalence of ASD are aging into adulthood, understanding continuities and discontinuities between these two conditions is a matter of increasing relevance.
While the two most distinguishing schizoid PD symptoms were a lack of close friends and a tendency to almost always choose solitary activities, substantially fewer ASD participants endorsed other established schizoid PD symptoms such as “Neither desires nor enjoys close relationships, including family,” “Indifferent to praise and criticism from others,” or “Constricted affect, aloof, cold, rarely reciprocated” (see Table 4). It is possible that ASD children lack close friendships not because they do not desire them, but rather because they struggle to form them (see Mendelson et al., 2013; Petrina et al., 2017), a distinction which may prove to be a discerning factor between schizoid PD and ASD. Absence of sexual interest, similarly, was only endorsed by a minority of ASD subjects and therefore another potential discerning feature between ASD and schizoid PD (see Cheak-Zamora et al., 2019), unless it was to be shown that such interest attenuates later in development in ASD (which we were unable to explore given the adolescent age range of our sample). Alternatively, the higher rates of endorsement in this symptom by parent report could be indicative that prior reports of its frequency in schizoid PD could be inflated if not obtained by self reports.
Understanding the difference between the motivation to pursue social connection and the capability of doing so may be critical in differentiating trajectories of ASD vs. schizoid PD and have important implications in treatment planning and intervention to increase adaptive functioning among affected individuals. We do note that despite a relatively lower frequency of endorsement of motivation-oriented schizoid PD symptoms, however, ASD participants still endorsed all schizoid PD criteria at a significantly higher rate than their non-ASD control counterparts.
It is conceivable that the array of educational and clinical interventions that have been afforded to children and adolescence with higher functioning ASD in the contemporary era have operated to offset risk for the full manifestation of schizoid PD in adulthood (i.e. cohort effects). Interventions that have consistently shown to be effective for adolescents with autism include social skills training (Laugeson et al., 2009; Lordo et al., 2016), positive behavior support plans (Carr et al., 2002), cognitive behavioral therapy (Sukhodolsky et al., 2013), and psychopharmacological approaches to mediate irritability and aggression (Parikh et al., 2008). These treatment advances represent potential therapeutic opportunities to address social impairment in schizoid PD by intervening when homologous autistic traits are identified in adolescence.
Limitations and Future Studies
Limitations of this study include sample size, age, and gender of participants; future research should target a larger epidemiologically representative group. However, our results show that even within a modest sample size, overlap in symptom burden between ASD and schizoid PD was prominent. Though the sample was relatively young, the stability of autistic traits over the lifetime suggests that future reports of these same participants would yield similar, if not exacerbated levels of schizoid PD affectation as they age. Although in one sense it could be construed as a limitation that our control group was not comprised of typical controls, our study provided a particularly stringent test of whether schizoid PD traits are disproportionately observed in individuals with ASD, by virtue of using a clinical control group of patients with non-ASD psychiatric disorders. If a control group more representative of the general population were used, we would expect an even more pronounced between-groups difference. Finally, our study did not control for potential effects of cognitive variation among the ASD subjects, which, if influential in the association between ASD and Schizoid PD symptomatology, might further refine estimation of their developmental overlap—this limitation was mitigated, however, by the fact that our entire sample was verbal, and that within the usual range of cognitive functioning manifested by verbal children with ASD, the correlations between measured IQ and quantitative autistic trait variation measured by the SRS are on the order of 0.20 or lower, thus accounting for less than 5 per cent of the variance (Constantino et al., 2007).
Future research to establish parameters of continuity between ASD and schizoid PD should examine caregiver reports of childhood histories of adults with schizoid PD using modern tools for ascertaining developmental history of ASD such as the Autism Diagnostic Interview Revised (ADI-R) or Social Communication Questionnaire (SCQ). Our data suggests that a substantial number of patients with ASD endorsed a very high level of schizoid PD symptomology, with some meeting full diagnostic criteria for schizoid PD; further data collection to delineate whether schizoid PD traits are continuously or bimodally distributed in adulthood will help to resolve the issue of whether ASD and schizoid PD symptoms lie along a single continuous severity gradient or whether they partially overlap but retain independent elements of causation. Further research will not only help to inform the etiological understanding of these disorders, but will promote increased social awareness, earlier clinical intervention, and if effective, higher quality of life for individuals at risk or affected by schizoid personality disorder.
Conclusions
The exponential rise in the prevalence of autism has been accompanied by data that confirms the enduring nature of the condition throughout life (Fountain et al., 2012; Wagner et al., 2019). Historically, the prevalence of ASD has been deemed much lower than that for schizoid PD but the cohort of children who have been diagnosed over the past twenty years—and who might not have been historically diagnosed in childhood—are now aging into adulthood. Many such individuals exhibit schizoid PD symptomatology and may account for cases of schizoid PD that historically would have been assumed to have had onset in adulthood. These data support overlap and possible developmental continuity between the two conditions and may help reconcile historic contrasts in their respective prevalence over the life course. Future research should be conducted to definitively clarify the distinction between the conditions to the extent that categorical distinctions in fact exist. Approaches to the support of individuals with higher functioning ASD that have proven successful in improving adaptation and quality of life should be considered for patients at risk or affected by Schizoid Personality Disorder.
Supplementary Material
Supplemental Figure 1.1 Individual Participant Age per Parent Report at Baseline and Follow-Up SRS Ascertainment
Supplemental Figure 1.2 Individual Participant Age per Teacher Report at Baseline and Follow-Up SRS Ascertainment
Supplemental Figure 1.3 Timeline of Ascertainment for All Measures
Supplemental Table 2.1 Proportion of Schizoid PD Trait Index scores meeting full vs. relaxed diagnostic criteria for Schizoid PD in ASD vs. control participants per parent and self report
Supplemental Figure 2 SRS Parent Reports and Schizoid PD Criteria Endorsement between Baseline and Follow Up
Figure 1b.
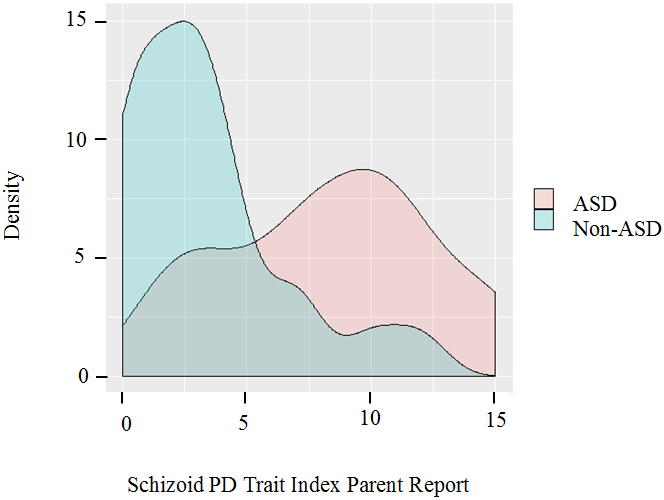
Distribution of Schizoid PD Trait Index scores for ASD vs. control participants per parent report (collected 2005-2013)
Acknowledgments:
This work was supported by grants HD042541 and HD087011 (the Intellectual and Developmental Disabilities Research Center at Washington University in St. Louis) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to Dr. John Constantino. The study protocol was approved by the Washington University School of Medicine Human Research Protection Office. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors sincerely and gratefully acknowledge Pearl Igwe for her assistance with an earlier version of this manuscript, Dr. Rob Fitzgerald and Rachael Wagner for their contribution to the data analysis, and the parents and families participating in the Washington University Social Developmental Studies program (sdslab.wustl.edu), for their contribution to this research effort.
Conflicts of Interest and Source of Funding:
Dr. Constantino receives royalties from Western Psychological Services for the commercial distribution of the Social Responsiveness Scale. No royalties were generated by the use of the instrument for this research study. For the remaining authors none were declared.
References
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Asperger H(1944). Die „Autistischen Psychopathen” im Kindesalte. Archiv für Psychiatrie und Nervenkrankheiten, 117, 76–136. [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson C, White T, Durkin MS, Imm P, Nikolaou L, Yeargin-Allsopp M, Lee L, Harrington R, Lopez M, Fitzgerald RT, Hewitt A, Pettygrove S, Constantino JN, Vehorn A, Shenouda MS, Hall-Lande J, Van K, & Dowling NF (2014). Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States. MMWR Surveillance Summaries 2018, 67(6), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr EG, Dunlap G, Horner RH, Koegel RL, Turnbull AP, Sailor W, Anderson JL, Albin RW, Koegel LK, & Fox L (2002). Positive behavior support: Evolution of an applied science. Journal of Positive Behavior Interventions, 4(1) 4–16. [Google Scholar]
- Cheak-Zamora NC, Teti M, Maurer-Batjer A, O’Connor KV, Randolph JK (2019). Sexual and relationship interest, knowledge, and experiences among adolescents and young adults with autism spectrum disorder. Ach Sex Behav, [April 22, Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV & Sparrow SA (1981). Developing criteria for establishing interrater reliability of specific items: Applications to assessment of adaptive behavior. American Journal of Mental Deficiency, 86(2), 127–37. [PubMed] [Google Scholar]
- Constantino JN, Abbacchi AM, LaVesser PD, Reed H, Givens L, Chiang L, Gray T, Gross M, Zhang Y, & Todd RD (2009). Developmental course of autistic social impairment in males. Developmental Psychopathology, 21(1), 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Charman T (2016). Diagnosis of autism spectrum disorder: reconciling the syndrome, its diverse origins, and variation in expression. The Lancet Neurology, 15(3), 279–291. [DOI] [PubMed] [Google Scholar]
- Constantino JN & Gruber CP (2005). Social Responsive Scale (SRS) Manual. Western Psychological Service, Los Angeles. [Google Scholar]
- Constantino JN, & Todd RD (2000). Genetic structure of reciprocal social behavior. American Journal of Psychiatry, 157(12), 2043–2045. [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Todd RD (2003). Autistic traits in the general population: A twin study, Archives of General Psychiatry, 60(5), 524–530. [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Todd RD (2005). Intergenerational transmission of subthreshold autistic traits in the general population. Biological Psychiatry, 57(6), 655–660. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Przybeck T, Friesen D, & Todd RD (2000). Reciprocal social behavior in children with and without pervasive developmental disorders. Journal of Developmental and Behavioral Pediatrics, 21(1), 2. [DOI] [PubMed] [Google Scholar]
- Dovgan KN, Mazurek MO (2019). Relations among activity participation, friendship, and internalizing problems in children with autism spectrum disorder. Autism, 23(3):750–758. [DOI] [PubMed] [Google Scholar]
- Ford TC & Crewther DP(2014). Factor analysis demonstrates a common schizoidal phenotype within autistic and schizotypal tendency: Implications for neuroscientific studies. Frontiers in Psychiatry, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain C, Winter AS, & Bearman PS(2012). Six developmental trajectories characterize children with autism. Pediatrics, 129(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier T, Thompson L, Youngstrom E, Law P, Hardan A, Eng C, & Morris N (2014). A twin study of heritable and shared environmental contributions to autism. Journal of Autism and Developmental Disorders, 44(8), 2013–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis JR & Koch GG (1977). The measurement of observer agreement for categorical data. Biometrics, 33(1), 159–174. [PubMed] [Google Scholar]
- Larsen FW & Mouridsen SE(1997). The outcome in children with childhood autism and Asperger syndrome originally diagnosed as psychotic. A 30-year follow-up study of subjects hospitalized as children. European Child & Adolescent Psychiatry, 6(4), 181–190. [DOI] [PubMed] [Google Scholar]
- Laugeson EA, Frankel F, Mogil C, Dillon AR (2009). Parent-assisted social skills training to improve friendships in teens with autism spectrum disorders. J Autism Dev Disord, 39(4):596–606. [DOI] [PubMed] [Google Scholar]
- Lordo DN, Bertolin M, Sudikoff EL, Keith C, Braddock B, & Kaufman DAS(2016). Parents perceive improvements in socio-emotional functioning in adolescents with ASD following social skills treatment. Journal of Autism and Developmental Disorders, 47(1), 203–214. [DOI] [PubMed] [Google Scholar]
- Lugnegard T, Hallberbäck MU, & Gillberg C (2012). Personality disorders and autism spectrum disorders: What are the connections? Comprehensive Psychiatry, 53(4), 333–340. [DOI] [PubMed] [Google Scholar]
- Mendelson JL, Gates JA, Lerner MD (2016). Friendship in school-age boys with autism spectrum disorder: A meta-analytic summary and developmental, process-based model. Psychol Bull, 142(6):601–622. [DOI] [PubMed] [Google Scholar]
- Michels R, Cooper AM, Guze SB, Judd LL, Klerman GL, & Solnit AJ (Eds.).(1989). Psychiatry (Vol 1). Philadelphia, PN: J.B. Lippincott Company and New York, NY: Basic Books, Inc., Publishing. [Google Scholar]
- Nilsson EW, Gillberg C, Gillberg IC, & Rastam M (1999). Ten-year follow-up of adolescent-onset anorexia nervosa: Personality disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 38(11), 1389–1395. [DOI] [PubMed] [Google Scholar]
- Parikh MS, Kolevzon A, & Hollander E (2008). Psychopharmacology of aggression in children and adolescents with autism: A critical review of efficacy and tolerability. Journal of Child and Adolescent Psychopharmacology, 18(2). [DOI] [PubMed] [Google Scholar]
- Petrina N, Carter M, Stephenson J, Sweller N (2017). Friendship satisfaction in children with autism spectrum disorder and nominated friends. J Autism Dev Disord, 47(2):384–392. [DOI] [PubMed] [Google Scholar]
- Sukhodolsky DG, Bloch MH, Panza KE, & Reichow B(2013). Cognitive-behavioral therapy for anxiety in children with high-functioning autism: A meta-analysis. Pediatrics, 123(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RE, Zhang Y, Gray T, Abbacchi A, Cormier D, Todorov A, and Constantino JN (2019). Autism-Related Variation in Reciprocal Social Behavior: A Longitudinal Study. Child Development, 90, 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S & Barlow A(1979). Schizoid personality in childhood: A comparative study of schizoid, autistic and normal children. Journal of Child Psychology and Psychiatry and Allied Disciplines, 20(1), 29–46. [DOI] [PubMed] [Google Scholar]
- Wolff S, Townshend R, Mcguire RJ, & Weeks DJ(1991). ‘Schizoid’ personality in childhood and adult life II: Adult adjustment and the continuity with schizotypal personality disorder. The British Journal of Psychiatry, 159(5), 620–629. [DOI] [PubMed] [Google Scholar]
- Zablotsky B, Black LI, Maenner MJ, Schieve LA, & Blumberg SJ (2015). Estimated prevalence of autism and other developmental disabilities following questionnaire changes in the 2014 National Health Interview Survey. National Statistics Reports, 87. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1.1 Individual Participant Age per Parent Report at Baseline and Follow-Up SRS Ascertainment
Supplemental Figure 1.2 Individual Participant Age per Teacher Report at Baseline and Follow-Up SRS Ascertainment
Supplemental Figure 1.3 Timeline of Ascertainment for All Measures
Supplemental Table 2.1 Proportion of Schizoid PD Trait Index scores meeting full vs. relaxed diagnostic criteria for Schizoid PD in ASD vs. control participants per parent and self report
Supplemental Figure 2 SRS Parent Reports and Schizoid PD Criteria Endorsement between Baseline and Follow Up


