Abstract
Mild Traumatic Brain Injury (TBI) is an important public health problem generated by closed head injury. This study is focused on the impact of blast-induced mild TBI on auditory trace and delay fear conditioning, models of declarative and non-declarative memory, respectively, and the correlation of conditioned freezing and fractional anisotropy, a measure of axonal state. A supersonic helium pressure wave was generated by a shock tube to blast 8 week old male mice on Day 1 for 1.4 msec with an incident pressure of 16 psi, corresponding to a reflected pressure of 56.9 psi at the mouse head. On Day 3, the mice were subjected to auditory trace- or delay- fear conditioning. On Day 4, contextual freezing in the trained context, and pre-cue and cued freezing in a novel context were determined. After cardiac perfusion on Day 5, ex vivo images were obtained with diffusion tensor imaging at 14.1 Tesla. We observed that delay fear conditioning prevented or reversed the decrease in fractional anisotropy in both the medial and lateral corpus callosum suggesting axonal stabilization of potentially behavioral therapeutic significance. Moderately strong and statistically significant Pearson correlations were found between fractional anisotropy and contextual freezing in the medial and lateral corpus callosum of blasted and sham-blasted delay- or trace- fear conditioned mice. Thus, contextual freezing is a neurobehavioral biomarker for axonal injury in mild TBI and is a reliable and high-throughput behavioral assay for the evaluation of potential therapeutics to treat mild TBI.
Keywords: Delay fear conditioning, trace fear conditioning, declarative memory, non-declarative memory, post-concussion syndrome (PCS), posttraumatic stress disorder (PTSD)
Introduction
Traumatic brain injury (TBI) is an important global public health problem. In the United States, approximately 1.5–2 million TBIs and 52,000 associated deaths occur each year (1). Approximately 80% of hospital-reported TBIs are categorized as mild TBI (2). A similar prevalence applies to the military sector (3). Clinical severity of mild TBI is correlated with the duration of unconsciousness (4). The leading causes are falls (5), motor vehicle crashes (6), and impact sport events (7). Mild TBI has also been recognized as the “signature injury” in the recent war zones of Afghanistan and Iraq (8,9) mainly due to detonation of improvised explosive devices (IEDs) that generate high pressure gas waves (10).
Mild TBI has been recognized to a large extent as a white matter disease. Diffuse axonal injury, identified with diffusion tensor imaging (DTI), has been proposed as an essential feature of mild TBI in humans (2) and experimental animals (11). Besides evidence for the involvement of axonal injury in mild TBI, chronic traumatic encephalopathy (CTE), a pathological tau protein state, has been proposed to contribute to mild TBI (12). It is unclear at this time how axonal injury and CTE interact. Therefore, the discovery of an alternative biomarker of mild TBI may be helpful, especially given the fact that mild TBI is a risk factor for several stress-related and degenerative diseases such as post-concussive syndrome (13), post-traumatic stress disorder (8,14), major depression (14), post-traumatic epilepsy (15), Alzheimer’s Disease (16), and Parkinson’s Disease (17).
We selected the mouse as our model system to study TBI because numerous data from anatomical, physiological and behavioral experiments, and an abundance of transgenic animals to target specific genes are available for research, and the risk assessment strategies of mice are similar to those in humans (18). Those strategies are likely involved during training and testing of fear conditioning which we administered after applying a Friedlander pressure wave (19) from a shock tube to the head of isoflurane-anesthetized mice. The Friedlander wave is similar to what is produced by detonations of IEDs.
In the current study, we investigated if conditioned fear could serve as a neurobehavioral biomarker for mild TBI. Conditioned fear was generated by trace or delay fear conditioning to target declarative or non-declarative memory, respectively (20,21), and fractional anisotropy of the corpus callosum was used to assay the neurobiological effects of mild TBI (22) based upon the assumption that axonal injury is an important manifestation of the injury.
Materials and Methods
Animal preparation
Male C57BL/6N mice, 6–8 weeks old, were purchased from Harlan Laboratories and kept single-housed in the animal facility of Northwestern University for at least one week. Animal care was in accordance with institutional guidelines of Northwestern University. All experimental procedures were approved by the Northwestern University Animal Care and Use Committee. On Day 1 of the experiment, each mouse was individually anesthetized with 4% isoflurane in a plastic transparent induction box (22.9 × 12 × 11.5 cm). Then, its body was protected except for the head with a ballistic nylon coat tightened with Velcro tape. The animals’ hearing was protected with 1.5 × 1.5 mm foam plugs (Pura-fit, Moldex-Metric INC, Culver City, California) placed into their ears. Isoflurane anesthesia (2%; 0.5 l / min) was maintained through flexible tubing to the nose of the mouse after the animal was transferred to the shock tube apparatus.
Shock tube, blast procedure and post-blast sequence
A shock tube was used to deliver pressure waves, similar to those seen in free field blasts, to the head of a mouse. The components of the shock tube are described in Figure 1A. Helium was selected as the driver gas, because the pressure wave travels faster in Helium than in air, and it rises faster and becomes more similar to the pressure waves from IEDs in war zones (23). When the bursting pressure of the Mylar diaphragm was reached, a supersonic pressure wave traveled down the tube and hit the head of the mouse whose lungs and ears were protected (see preceding paragraph for details). The mouse was attached to a vertical board in the Mouse Chamber (Figure1A) with the top of the head facing the shock tube and positioned one inch from the end of the shock tube. A pad was placed behind the mouse head to allow a maximum of 45 degrees of head rotation in order to facilitate axonal injury. The pressure at the tube exit was uniform across the entire exit plane as determined with a pressure gauge. Incident pressures of 12, 16, and 19 psi measured at the end of the tube were applied. Exposure to 16 psi was linked to a mortality rate of less than 10%, whereas exposure to an incident pressure of 19 psi was not survived by any C57BL/6N mouse. The helium-driven pressure wave which exhibited a constant incident overpressure of 16.02 +/− 0.60 psi for 1.4 msec, was equivalent to a reflected pressure of 56.9 psi at the site of the mouse head (Figure 1B). Full recovery after blast was indicated by the presence of a grasp reflex (24) and a coordinated walk using all four legs on the top lid of their cages. On Day 3, all mice were subjected to training for auditory trace or delay fear conditioning. On Day 4, all mice were tested for conditioned fear to the training context and then to the auditory cue in a novel context (see Fear Conditioning Materials and Methods for details). On Day 5, all mice were subjected to cardiac perfusion. Subsequently, the isolated brains were analyzed with DTI in a 14.1 TESLA MR imager.
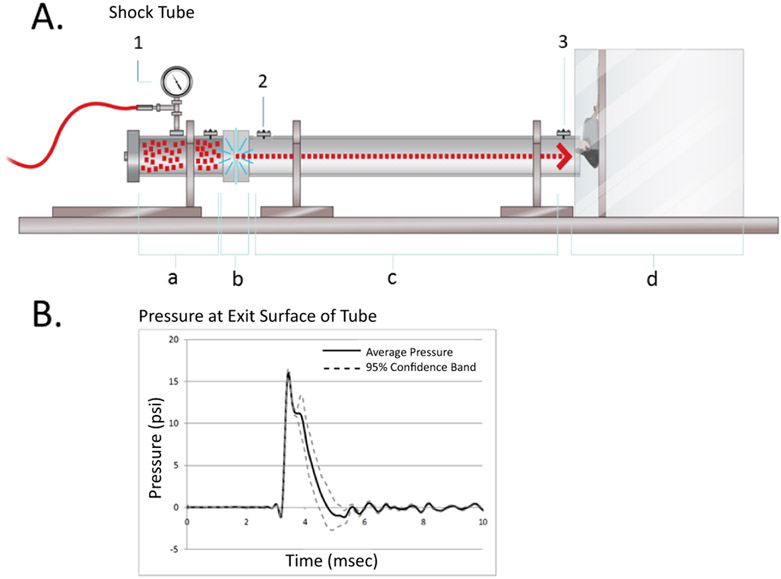
Fig. 1. Shock tube and pressure wave.
A. The Shock Tube. It was specifically designed by Paul Rigby (L3 Company) for blasting rodents. The length of the tube is 6 ft, the diameter 2 inches. It was made of aluminum. The tube consists of the Driver Section in the Compression Chamber (a) to be filled with helium gas, a Mylar Diaphragm (5 milli-inches thick in the presented experiments) (b), separating the Driver Section from the compression section (c). The diaphragm bursts at a defined pressure. A Mouse Chamber (d) is added to the end of the tube. It contains a movable acrylic board to which the anesthetized mouse is attached. An analog gauge (1) measures the helium gas pressure in the Compression Chamber. The pressure wave is monitored with digital incident pressure gauges at the beginning (2) and end (3) of the Expansion Section of the tube. B. Pressure at exit surface of tube. For each blast, the incident pressure is measured and recorded with a digital pressure gauge (3). The average incident pressure used in this study was 16.02 +/− 0.60 psi corresponding to a reflected pressure of 56.9 psi at the head of the mouse one inch away from the tube exit plane. The reflected pressure was determined in a separate experiment. The second spike approximately at 4 msec is most likely the reflected wave from the mouse head.
Experimental groups
Four groups of mice were used in the experiment. Two of the groups were blasted and then subjected to auditory trace fear conditioning (TFC) or auditory delay fear conditioning (DFC). Two additional groups served as sham-blasted controls. They were treated like the blasted mice, but without being exposed to the pressure wave. They were subsequently subjected to auditory trace or delay fear conditioning. Each group contained 9–10 mice.
Fear conditioning
Trace and delay fear conditioning were identical except that trace fear conditioning included a 15 s stimulus-free trace period between the end of the auditory conditioning stimulus (CS) and the start of the foot-shock unconditioned stimulus (US). The CS was a 30 s, 75 dB, broadband noise pulsed at 5 Hz with a 50% duty cycle and a 5 msec rise-fall time. The CS was presented 180 s after the mouse was placed onto a 25 × 29 cm grid floor in a 29 cm high conditioning chamber (Coulbourn) scented with Clidox (cleaner/disinfectant). The US was a 2 sec, 0.7 mA DC scrambled shock to the paws that was delivered at the end of the CS for delay fear conditioning, or at the end of the trace interval for trace fear conditioning. Mice were given only one conditioning trial of their assigned conditioning paradigm. They remained in the conditioning chamber for approximately 30 seconds and were then returned to their home-cages. Approximately 24 hours later, the mice were returned to the conditioning chamber for three minutes to test for contextual fear, i.e. freezing to the conditioning chamber that had been used for training. They were then returned to their home-cage for approximately one hour. The mice were subsequently placed in a novel chamber (34 × 28 × 18 cm) which was made of plastic, had a smooth floor, was scented with Windex cleaner, and illuminated with red, instead of white light. A single 60 s CS was delivered after a 180 s baseline period, and data were recorded for another 180 s period after the CS. For determination of preCue freezing, a period of 60 sec immediately before the start of the CS was monitored. The experiment was done inside an Industrial Acoustics Corporation acoustical cabinet and was controlled by Actimetrics’ FreezeFrame software and analyzed with Actimetrics’ FreezeView software.
Cardiac perfusion
Mice were anesthetized with a drug cocktail of ketamine (87 mg/kg) and xylazine (13 mg/kg) injected i.p. Depth of anesthesia was ascertained by absence of response to toe pinch. Anesthetized mice were pinned on a slanted board. The chest wall was opened by incision to expose the heart. A 25-gauge needle was inserted into the left ventricle, and an incision was made in the right atrium to drain blood. Heparinized saline was allowed to flow via the needle into the left ventricle. Perfusion of saline was continued for approximately 10–15 ml or until the return flow was colorless. Saline was then replaced with 4% paraformaldehyde, and perfusion was continued for another 10–15 ml. The skull was then opened, the brain removed and placed in 4% paraformaldehyde.
Diffusion Tensor Imaging
Prior to imaging, brains were suspended in 0.2 M sodium phosphate buffer (pH 7.4) for 48 hours and then transferred to sample holders filled with perfluoro polyester (Fomblin). Ex vivo DTI of the mouse brains was acquired with a multi-slice spin-echo diffusion weighted imaging sequence on a 14.1 Tesla Bruker vertical bore Avance microimager. The following parameters were used: TR= 3000ms, TE= 16ms, time between diffusion gradient pulses Δ=7ms, duration of diffusion gradient δ=3 ms, slice thickness=500µm, field of view=15mm x 15mm, and matrix size= 256 × 256 yielding an in-plane voxel size of 59µm. Diffusion sensitizing gradients were applied along 30 directions with a single b value of 1000 s/mm2. DTI data were processed using Bruker Paravision software. The isotropic contribution of water mobility was determined as fractional anisotropy (FA). A high isotropic diffusion may result from increased radial diffusivity or decreased axial diffusivity and is suggestive for axonal damage, whereas a low isotropic diffusion leading to a FA value close to 1.0 is suggestive for axonal stabilization probably from re-myelination, dense axonal fiber packing or edema (25). FA values were determined for the medial and lateral corpus callosum from four slices spanning from near splenium to genu, as shown in Fig. 2.
Fig. 2. Images used for DTI.
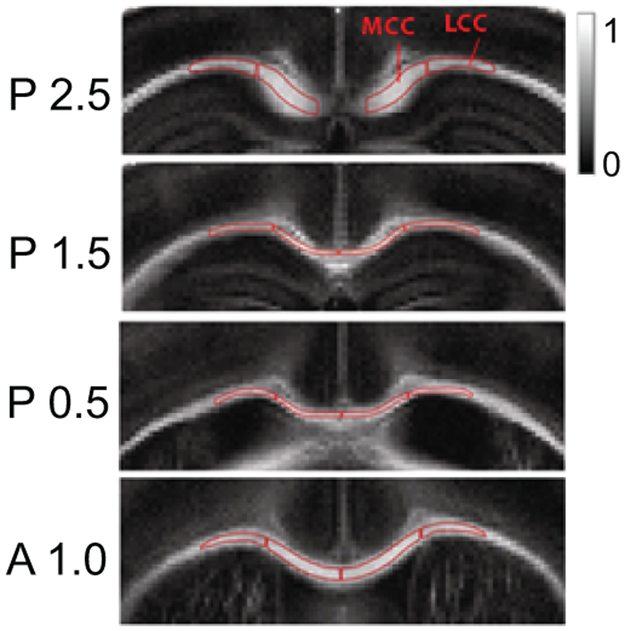
With a 14.1 T. microimager, four slices were analyzed per mouse, representing corpus callosum from genu/ anterior body (bregma A 1.0 mm) over posterior body (bregma P 0.5 – 1.5 mm) to splenium (bregma, P 2.5 mm). The mean FA values including left and right hemispheric medial and lateral corpus callosum were determined.
Data Analysis
Fractional anisotropy values of the four images were analyzed for each mouse with a 14.1 Tesla microimager (Fig. 2) and averaged across hemispheres. Statistical significance of the Pearson correlation between fractional anisotropy and contextual freezing behavior in the training context, as well as preCue and normalized Cue freezing in a novel context were determined using a Z test (StatView 5.0). All experimental groups were combined for this analysis to determine whether FA values were related to conditioned freezing, regardless of the cause of the FA alteration.
Video data from fear conditioning sessions were analyzed with Actimetrics’ FreezeView software. A threshold for movement was identified for each mouse based on a frequency distribution of the motion index (a measure of the number of video pixels that changed their state from “off” to “on”, or vice versa between adjacent video frames). A sharp inflection of the graph was typical and indicated the value that discriminated freezing from any movement except that required for respiration. A bout of at least 1 sec was required to be considered freezing (choosing shorter times would score respiration pauses as freezing).
Cue freezing was determined by comparing percent freezing during the 60 sec exposure to cue with the percent freezing during 60 sec immediately prior to cue onset. The data were also normalized to baseline levels of freezing so that cue-evoked freezing could be detected upon a background of increased freezing, i.e., if there was generalized fear to a novel context. Normalized freezing was calculated as: ((Cue freezing - preCue freezing)/preCue freezing) x100). An Excel file of percent freezing was generated for each batch of files, and StatView 5.0 was used to perform statistical analyses. FreezeView also generated a “fraster” (raster plot of freezing bouts for each mouse, Fig.3) to show the time course of freezing for each mouse in a group. A 2 × 2 factorial ANOVA (blast/sham-blast x trace/delay fear conditioning) was used for the primary analysis. Given that the differential effect of blast upon the trace and delay fear conditioning paradigms was of particular interest, individual post-hoc analyses of the effect were planned regardless of the significance of the interaction effect determined by ANOVA. Fisher’s PLSD tests or one-way ANOVAs were used for post-hoc analyses. The recovery times from blast were averaged for each group of mice and compared with a two-tailed t test.
Fig. 3. Graphical representation of freezing bouts by session and group for mice subjected to one trial of auditory delay or trace fear conditioning.
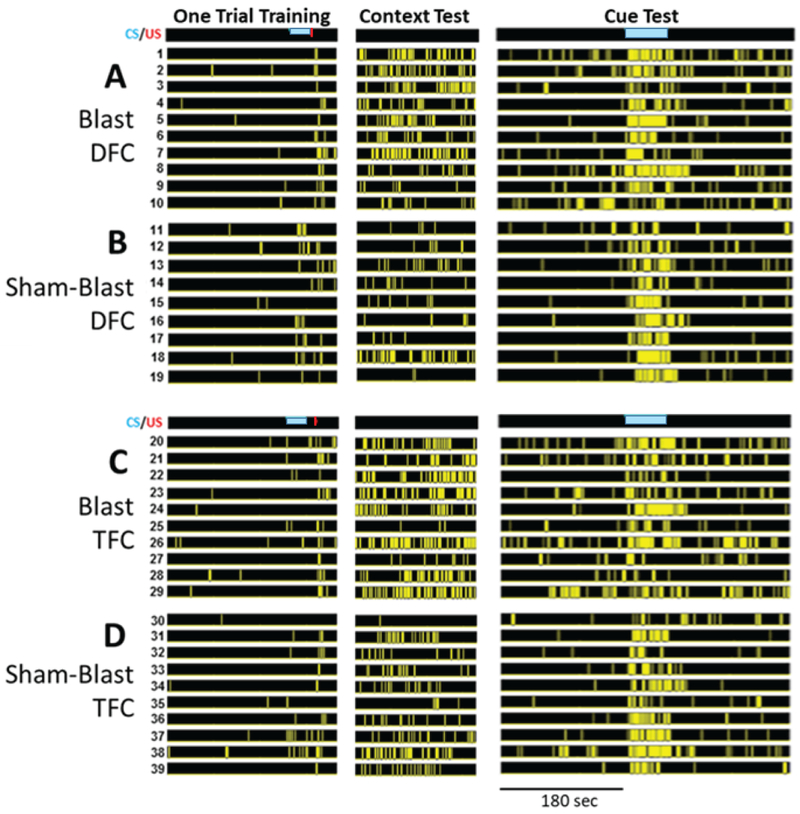
The timing of the conditioned stimulus (CS) (blue bar) is shown in the Training and Cue columns. The yellow bars in each subsequent row represent the timing of freezing bouts. Data are presented in a raster-type fashion such that each row represents the freezing behavior for each individual mouse. Columns represent the three phases of conditioning for each group, i.e., training, testing for contextual fear, and testing for cued fear including preCue freezing. A. Blast DFC: Blasted mice trained with delay fear conditioning (DFC). B. sham-Blast DFC: sham-blasted mice trained with DFC. C. Blast TFC: Blasted mice trained with trace fear conditioning (TFC). D. sham-Blast TFC: Sham-blasted mice trained with TFC. The 180 sec time scale applies to all three phases of the experiment.
Results
Recovery from blast
The recovery time of blasted mice was three times longer than the recovery time of non-blasted mice, (7.27 vs 2.42 min) to recover in their own cages and show normal neurological reflex behavior, as demonstrated by their ability to perform a coordinated walk along the edges on top of their standard mouse home cage. The difference in recovery time was statistically significant (p<0.0001, df=13).
Overview of fear conditioning effects
The freezing behavior of all mice (9–10 mice/group) is presented in Fig. 3 and is separated by training paradigm and blast condition. The figure shows the time course of bouts of freezing for each animal in a rasterized fashion, i.e. each row represents data from a different mouse. These “frasters” (rasters of freezing data) provide a qualitative overview of the freezing behavior for each mouse in each group, and each column shows a different phase of training and testing. Qualitatively, blasted mice appear to exhibit a greater percentage of freezing than sham-blasted mice when placed back into the original training context; blasted mice also appear to exhibit more preCue freezing than sham-blasted mice when placed into a novel context to test for preCue and Cue freezing. Quantitative analyses support these observations (Fig. 4).
Fig. 4. Comparison of blasted and sham-blasted mice.
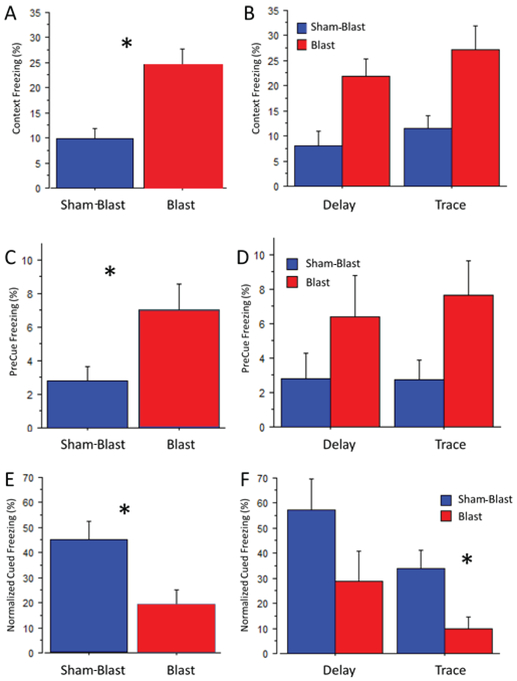
Percent freezing of all blasted or sham-blasted (control) mice to the original training context (A, B), to the novel context for the preCue period (C, D), and the cue period (E, F; normalized data). Significant differences are indicated by an asterisk and explained for panels A, C, E and F. A. Blast significantly increased contextual freezing relative to the behavior of sham-blasted mice (24.6±3.0 vs 9.9±1.9, F 1,34 =17.2, p=0.0002). C. Blast significantly increased preCue freezing relative to sham-blast (7.07±1.5 vs 2.79±0.9, F 1,34 =5.43, p=0.026). E. Blast significantly decreased normalized cue freezing (18.9+/−6.4 vs 45.0+/−7.3, F 1,34 =8.0, p=.008). F. Blast significantly decreased freezing relative to sham-blast for mice that underwent trace fear conditioning (9.9+4.5 vs 34.0+7.4, mean difference: 26.1, critical difference (Fisher’s test): 18.9, p=0.008), but not for mice that underwent delay fear conditioning.
Contextual fear
When the mice exposed to the training context on Day 3 were returned on Day 4 to the same context, a two-way ANOVA of group (Blast/ Sham-Blast) and paradigm (Trace/Delay fear conditioning) indicated a significantly greater percent of freezing for the blasted mice than for the sham-blasted control mice (Fig. 4A; 24.6+/−3.0 vs 9.9+/−1.9, F1,34=17.2, p=0.0002). There was no overall significant difference between trace and delay fear conditioning, and there was no significant interaction of blast and conditioning paradigm.
PreCue and Cue freezing
A two-way ANOVA of group and paradigm for the percent of preCue freezing revealed significantly more freezing by the blasted mice than by the sham-blasted control mice (Fig. 4C, 7.07+/−1.5 vs 2.79+/−0.9, F1,34=5.4, p=0.026). This blast-induced increase of preCue freezing is probably anxiety-related (13) caused by generalization. Again, there was no significant difference between mice that were treated with trace or delay fear conditioning, and there was no significant interaction between the two factors of blast and fear conditioning. These results suggest that fear was generalized to stimuli other than those comprising the training context.
Given that there was a difference in the amount of preCue baseline freezing for the two groups, a two-way ANOVA of group (Blast/Sham-Blast) and paradigm (trace/delay fear conditioning) was performed upon the percent of freezing to the cue after normalizing the data to the amount of preCue freezing [(Cue-preCue)/preCue) x100]. The results revealed significantly less normalized freezing for the blasted mice than for the sham-blasted mice during the cue period (Fig. 4E; F1,34=8.0, p=0.008). Although ANOVA indicated no significant interaction of the blast groups and training paradigms, the approach of a planned comparison of the blast effect for each training paradigm revealed significantly less normalized freezing for the trace fear conditioned mice than for the delay fear conditioned mice (Fig. 4 F; F1,34=5.2, p=0.030). This result suggests a greater cognitive impairment during trace conditioning than during delay conditioning. A similar planned comparison for results of contextual fear did not reveal a differential effect. Thus, cued auditory trace fear conditioning as a model for declarative memory was more impaired than auditory delay fear conditioning as a model for non-declarative memory. These results support our primary hypothesis.
Hearing after blast
An explanation for less cued freezing by the mice exposed to blast could be that they suffered from a blast-induced impairment of hearing which may not have been prevented by the plugs we placed into their ears prior to the blast. Our experiments show that mice of all groups responded to the auditory cue (Fig. 5). The raw freezing values are presented here (instead of normalized values) to emphasize that the mice responded to the auditory cue relative to the preCS background (44.7± 2.8% vs 4.9±0.9%), rather than to emphasize the degree of overall freezing; this difference was statistically significant according to an ANOVA of group x paradigm with the preCue and Cue periods as two levels of the repeated measure (F1,34=200.7, p<0.0001). No other effects were statistically significant. On the basis of these data, we conclude that there was no significant hearing loss associated with the blast. Minor hearing deficits not affecting fear conditioning could not be excluded.
Fig. 5. Effect of blast on hearing.
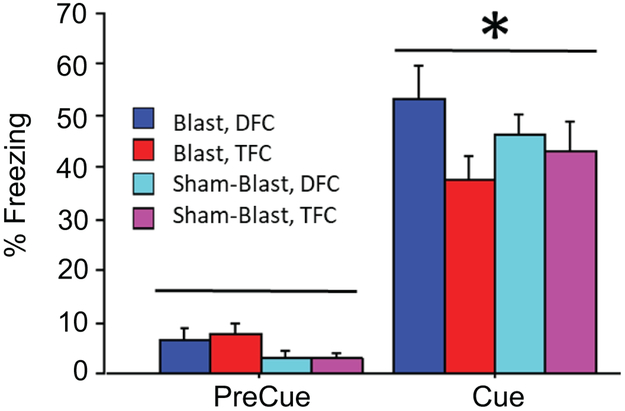
The freezing response of the mice of all groups to the cue stimulus was recorded. Raw values are presented here to emphasize that the mice respond to the auditory cue relative to the preCS background. The overall mean freezing to the cue was 44.7 +2.8% vs 4.9+0.9% freezing prior to the cue. The difference was statistically significant according to an ANOVA of group x paradigm with the preCue and Cue periods as a repeated measure (F1,34=200.7, p<0.0001). No other effects were statistically significant.
Change of fractional anisotropy by fear conditioning and blast in the medial and lateral corpus callosum
We compared the action of fear conditioning - in absence of blast or after blast - in isoflurane-treated mice on fractional anisotropy values in the medial and lateral corpus callosum using a two- way ANOVA for each region.
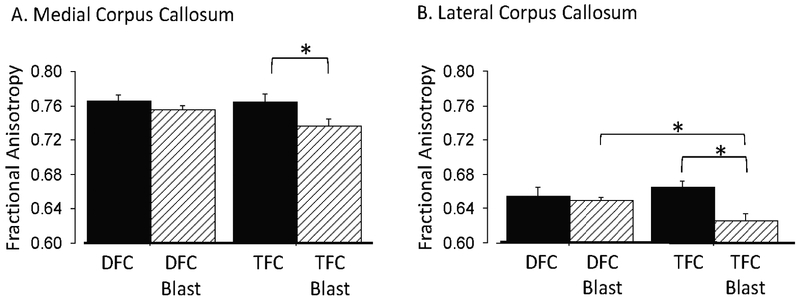
In the medial corpus callosum we found a significant effect of blast group on the fractional anisotropy values (F1.25=6.21, p=0.020). Although the interaction of blast group and training paradigm was not statistically significant, a planned comparison on the effect of the training paradigm revealed that delay fear conditioning prevented or reversed any possible blast-induced decrease in fractional anisotropy values (Fig. 6A). In contrast, the sequence of blast followed by trace fear conditioning resulted in a statistically significant reduction of fractional anisotropy (0.766±0.007, Blast, vs 0.756±0.004, Sham-Blast, F1.12=4.66, p=0.05).
Fig. 6. Impact of delay- and trace- fear conditioning (DFC/TFC) on fractional anisotropy in the corpus callosum after blast. A. Medial corpus callosum.
Delay, but not trace fear conditioning prevented a reduction in fractional anisotropy (F1,12 =4.66, p=0.05) due to the effects of the blast. Means: 0.764±0.009 (TFC), n=6 vs 0.737±0.009 (TFC+Blast), n=8. B. Lateral corpus callosum. Delay fear conditioning also prevented the lateral corpus callosum from a reduction in fractional anisotropy due to blast (F1,12 =11.98, p=0.0047). Means: 0.665±0.008, n=6 (TFC) vs 0.627±0.008, n=8 (TFC+Blast).
In the lateral corpus callosum, a significant effect of blast group (F1.25=8.2, p=0.008) and a significant interaction of blast group and training paradigm (F1.25=5.13, p=0.032) were found. When mice were blasted before trace fear conditioning, the resulting fractional anisotropy values were significantly reduced relative to sham-blasted mice that were administered trace fear conditioning (0.627+/−0.008, Blast, vs 0.665+/−0.008, Sham-Blast; Fig.6B). In contrast, when the blast was followed by delay fear conditioning, a statistically significant decrease in fractional anisotropy was not observed (0.650+/−0.003, Blast, vs 0.655+/−0.010, Sham-Blast).
Furthermore, no statistically significant differences were found between fractional anisotropy values for the left and right sides of the lateral corpus callosum according to an ANOVA with laterality used as a repeated measure (left: 0.648+/−0.007; right: 0.652+/−0.005). Interestingly, a comparison of the medial corpus callosum by ANOVA did reveal significantly lower fractional anisotropy values for the right side in each of the groups (F3,25=29.12, p<0.001). The mean anisotropy values for the left and right sides were 0.760+/−0.004 and 0.748+/−0.004, respectively.
Correlation between fear conditioning and fractional anisotropy
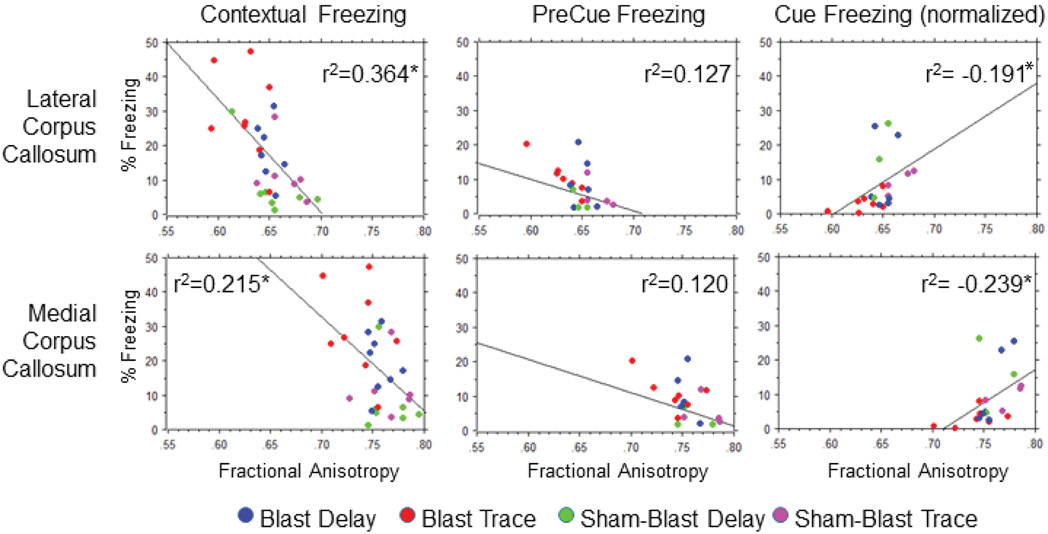
All four groups of mice (Blast/TFC, Blast/DFC, Sham-Blast/TFC, Sham-Blast/DFC) were combined to determine how fractional anisotropy was correlated with each measure of conditioned fear. Linear Pearson regressions of freezing behaviors and fractional anisotropy values are shown in Fig. 7 and include the r2 values to indicate the percent of variability accounted for by the regression function. The correlation between fractional anisotropy and contextual freezing was significant and of moderate strength in the lateral corpus callosum using a conventional threshold of r=0.5 (r= −0.603, r2=0.364, p=0.0004). The correlation with the medial corpus callosum was also statistically significant, but was of weak strength (r= −0.464, r2=0.215, p=0.010). The correlation of preCue freezing (in the novel context) was of low strength and not statistically significant in both the lateral (r= −0.356, r2= 0.127 p=0.058) and medial corpus callosum (r= −0.347, r2= 0.120 p=0.065). The correlation of normalized Cue freezing and fractional anisotropy was statistically significant (but of low correlation strength) in the medial corpus callosum (r=0.489, r2=0.239, p=0.028) and in the lateral corpus callosum (r=0.437, r2=0.191, p=0.05).
Fig. 7. Correlation analysis of fractional anisotropy values and several measures of freezing.
Linear regressions of fractional anisotropy values for the regions of interest shown in Fig. 2 were performed in relation to three assays of trace and delay fear conditioning performed 24 hours after the initial conditioning procedure: contextual freezing (to the training context), preCue freezing (in the novel context), and normalized freezing to cue presentation in the novel context ((Cue-preCue) / preCue). The r2 value indicating the relative variability (%) for each regression accounted for by the regression function is noted in each panel. A moderately strong correlation was found for contextual freezing and fractional anisotropy in the lateral corpus callosum (r=0.603, r2=0.364, p=0.0004). The correlation was statistically significant according to a Z test (p<0.05, N=29, power: 0.95). The relationship of contextual freezing and fractional anisotropy in the medial corpus callosum was statistically significant (p=0.01, N=29, power: 0.728), but of low strength (r= 0.464, r2=0.215). Fractional anisotropy and normalized cue freezing in the medial corpus callosum was also statistically significant (r=0.489, r2=0.239, p=0.028, power: 0.78, N=29), and of low strength.
Discussion
Selection of animal model and induction of axonal injury
Free location of explosive devices and exposure of mice or other animals at different distances from these devices can realistically mimic the exposure of humans in war zones (26). The advantage of using shock tubes is the flexibility to apply repetitive pressure wave exposures (blasts) with different speeds and shapes of pressure waves. The main parameters of the blast can be tailored to defined research aims and are reproducible.
In this study, we used a shock tube to induce axonal injuries. An important feature was the quick rise of high-pressure and its rapid return to baseline with a short negative phase and short exposure time. This feature was facilitated by the use of helium that transports the pressure wave faster than does air. The exposure times in small animal models should be in the range of 1 msec (which is realized here) in order to have a complete blast wave traverse the brain under the influence of the pressure wave.
Reduction of the reflective pressure to 35 psi under similar experimental conditions as described here resulted in the loss of axonal injury in the mouse model (Venkatasubramanian, P.N., Wyrwicz, A., Spiess, J., personal communication).
DTI measurements of changes in fractional anisotropy were performed ex vivo after cardiac perfusion. Direct comparison between in vivo and ex vivo rodent and primate brains have demonstrated the maintenance of diffusion anisotropy after tissue fixation (27, 28). One of the advantages of ex vivo DTI is that the scanning time can be extended to increase resolution without taking long-lasting immobilization effects of in vivo measurements into account.
Blast-induced mild TBI impact on fear conditioning
All three major behavioral expression modes of fear conditioning: contextual freezing in the training context, preCue and Cue freezing in a novel context, were affected. The blast-induced increase of contextual freezing was probably generated by anxiety caused by the blast procedure. The increase of preCue freezing showed generalization and a weakened ability to respond specifically to the contextual stimuli of the CS. The decreased normalized freezing in response to exposure to the Cue in a novel context indicated a cognitive dysfunction and memory deficit (Fig.4E).
The most significant reduction in fractional anisotropy, and thus the most significant axonal injury, was obtained by blast followed by auditory trace fear conditioning in the lateral corpus callosum (Fig.6B). This decrease in fractional anisotropy was occluded by the actions of delay fear conditioning and may be explained by the finding that delay fear conditioning results in more conditioned freezing than trace fear conditioning (Fig. 4F). This difference may reflect a greater activation of neuronal circuits mediating implicit memory acquisition (non-declarative memory) during delay fear conditioning as compared to explicit memory acquisition (declarative memory) during trace fear conditioning. Implicit memory is also impaired in neurodegenerative diseases such as Parkinson’s Disease (17).
The four experimental groups (Blast/TFC, Blast/DFC, Sham-Blast/TFC, Sham-Blast/DFC) were all anesthetized with isoflurane. However, control mice with or without blast in the presence or absence of isoflurane, not exposed to delay or trace fear conditioning were not investigated. These controls would have provided insight into the effect of isoflurane on fractional anisotropy and the evolution of fractional anisotropy by the blast alone. We will perform these experiments in the future.
Axonal stabilization of fear conditioning
An increase in fractional anisotropy by delay fear conditioning was observed earlier for the cingulum bundle in a context not related to mild TBI, although data were not collected beyond a time point of one day (30). If auditory delay fear conditioning is preceded by the blast-inducing mild TBI, the resulting fractional anisotropy value is not significantly changed (at Day 5), whereas the combination of blast and auditory trace fear conditioning results in a significant reduction in fractional anisotropy (Fig.6A, B). This result suggests that delay fear conditioning either prevents or reverses axonal changes, as observed following blast and trace fear conditioning. The difference in fractional anisotropy values could be related to myelin synthesis and re-building of myelin organization (31), dense packing of axonal fibers, or edema (25). An increase, possibly signifying axonal repair, has also been observed under pathological conditions such as Parkinson’s Disease (32). There, significantly increased values were found in motor pathways involved in neuroplastic compensatory mechanisms (32)
The observation that delay fear conditioning preferentially prevented or reversed the blast-induced reduction in fractional anisotropy suggests that delay fear conditioning may be more efficient in axonal repair or protection than trace fear conditioning, in agreement with implicit tasks such as bicycling which has been applied for the treatment of Parkinson’s Disease (33). Immunological mechanisms may be involved additionally (34, 35).
Lastly, the finding of a significant hemispheric laterality of fractional anisotropy values in the medial corpus callosum independent of the presence of a blast action could not be explained by a technical bias of the blast procedure. This laterality may have resulted during ontogenetic development of the C57BL/6N mouse strain used in these experiments, and it is reminiscent of behavioral lateralization of paw preference of adult Swiss mice after mid-sagittal transection of the corpus callosum on the first post-natal day (36).
Correlation of conditioned freezing and fractional anisotropy
The regression analysis of the relationship between fractional anisotropy and conditioned fear responses revealed a significant inverse correlation between fractional anisotropy in the medial and lateral corpus callosum and contextual freezing to the training context (Fig.7). Fractional anisotropy also was significantly and directly correlated with Cue freezing after normalization of the freezing data from the medial corpus callosum. However, PreCue freezing, an indicator of anxiety, was not significantly correlated with fractional anisotropy.
Taking Pearson’s correlation strength analysis into account, the results presented here indicate that mainly conditioned contextual freezing can serve as a neurobehavioral biomarker of mild TBI, and that delay fear conditioning freezing can serve as a therapy to reverse reduced fractional anisotropy. These results are also in agreement with earlier preliminary data using a weight drop model that had revealed axonal injury in the corpus callosum and impairment of declarative memory without significant change of non-declarative memory (Joachim Spiess, personal observation). The three behavioral responses to blast and fear conditioning, increased contextual freezing, increased preCue freezing and reduced Cue freezing, are in agreement with post concussive syndrome- and post traumatic stress disorder-like behaviors (9,14,29).
Conclusions
We combined two approaches in the mouse model, blast-induced mild traumatic brain injury using a shock tube and trace and delay fear conditioning, models of declarative and non-declarative memory, respectively.
Two main results were found:
1. We demonstrated that conditioned fear can serve as a neurobehavioral biomarker. Specifically, we propose that contextual freezing with its statistically significant and moderately strong Pearson correlation with fractional anisotropy values (r=0.60), qualifies as a biomarker of axonal injury generated by mild TBI. This relationship is valid for auditory delay- and trace- fear conditioning. Therefore, contextual freezing can be used for pharmacological characterizations of drugs being tested for efficacy against mild TBI. In addition, contextual freezing is also an indicator for post-concussion syndrome and post-traumatic stress disorder. Thus, contextual fear can be used to screen potential therapeutics against mild TBI and possibly other related issues, e.g. post concussive syndrome and post-traumatic stress disorder.
2. We found evidence that delay fear conditioning repaired or prevented axonal injury indicated by reduced fractional anisotropy in the corpus callosum. The therapeutic potential of delay fear conditioning will be further investigated.
Acknowledgments:
We gratefully acknowledge support by Max Planck Society to J.S., the use of the Behavioral Phenotyping Core of Northwestern University, the imaging facilities of the Center for Basic MR Research of the Northshore University Health System, and the shock tube by L3 Applied Technologies. We also are thankful for many intensive discussions with Stuart Lipton (Scripps Research Institute), Juan Pina-Crespo (Sanford Burnham Medical Discovery Institute), Steven Schwulst (Northwestern University Feinberg School of Medicine), and Palamadai N. Venkatasubramanian (NorthShore Univ. Health System). We thank Sean Farley for his excellent technical help in our blast experiments and in the cardiac perfusion.
Support: NIH grants RO1NS059879 (CW), S10RR13880 (AMW), MH047340 (JFD), and Max Planck Society (JS).
References and Notes
- 1.Gardner AJ, Zafonte R: Neuroepidemiology of traumatic brain injury. Handb Clin Neurol 138:207–223, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, Lee H, Meeker M, Zimmerman RD, Manley GT, McCandliss BD: Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. Am J Neuroradiol 29:967–973, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DoD Numbers for Traumatic Brain Injury: Worldwide – Totals https://dvbic.dcoe.mil/files/tbi-numbers/worldwide-totals-2000-2018Q1-total_jun-21-2018_v1.0_2018-07-26_0.pdf [accessed 13 February 2019].
- 4.Hall RC, Hall RC, Chapman MJ: Definition, diagnosis, and forensic implications of postconcussional syndrome. Psychosomatics 46(3):195–202, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Almeida OP, Hankey GJ, Yeap BB, Golledge J, Flicker L: Prevalence, associated factors, mood and cognitive outcomes of traumatic brain injury in later life: the health in men study (HIMS). Int J Geriatr Psychiatry 30:1215–1223, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Shults RA, Jones BH, Kresnow MJ, Langlois JA, Guerrero JL: Disability among adults injured in motor vehicle crashes in the United States. J. Safety Research. 35:447–452, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Mihalik JP, Lynall RC, Teel EF, Carneiro KA: Concussion management in soccer. J Sport and Health Science 3:307–313, 2014. [Google Scholar]
- 8.Ling G, Bandak F, Armonda R, Grant G, Ecklund J: Explosive Blast Neurotrauma. Journal of Neurotrauma 26:815–825, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Ling G, Ecklund J: Traumatic brain injury in modern war. Curr Opin Anesthesiol 24:124–130, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Fievisohn E, Bailey Z, Guettler A, VandeFord P: Primary Blast Injury Mechanisms: Current Knowledge, Limitations, and Future Directions. J Biomech Eng 140(2):1–12, 2018. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL: Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury, Exper Neurol 205:116–131, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daneshvar DH, Goldstein LE, Kiernan PT, Stein TD, McKee AC: Post-traumatic neurodegeneration and chronic traumatic encephalopathy. Mol. Cell Neurosci 66:81–90, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Broshek DK, De Marco AP, Freeman JR: A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj. 29:228–37, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Cnossen MC, Scholten AC, Lingsma HF, Synnot A, Haagsma J, Steyerberg PEW, Polinder S: Predictors of major depression and posttraumatic stress disorder following traumatic brain injury: a systematic review and meta-analysis. J Neuropsychiatry Clin Neurosci 29:206–24, 2017. [DOI] [PubMed] [Google Scholar]
- 15.Xu T, Yu X, Ou S, Liu X, Yuan J, Huang H, Yang J, He I, Chen Y: Risk factors for posttraumatic epilepsy: a systematic review and meta-analysis. Epilepsy Behav 67:1–6, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Julien J, Joubert S, Ferland MC, Frenette LC, Boudreau-Duhaime MM, Malo-Veronneau L, de Guise E: Association of traumatic brain injury and Alzheimer disease onset: a systematic review. Ann Phys Rehabil Med 60:347–56, 2017. [DOI] [PubMed] [Google Scholar]
- 17.Wong JC, and Hazrati LN: Parkinson’s disease, parkinsonism, and traumatic brain injury. Crit Rev Clin Lab Sci 50:103–6, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Balci F, Freestone D, Gallistel CR: Risk assessment in man and mouse. Proc.Nat. Acad.Sci USA 106:2459–65, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tasissa AF, Hautefeuille M, Fitek JH, Radovitzky RA: On the formation of Friedlander waves in a compressed-gas-driven shock tube. Proc.R.Soc.A 472(2186):20150611, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Squire LR, Zola SM: Structure and function of declarative and nondeclarative memory systems. Proc. Natl. Acad. Sci. USA 93:13515–13522, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark RE, Manns JR, Squire LR: Classical conditioning, awareness, and brain systems. Trends Cogn Sci 6:524–531, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Cui J, Ng LJ, Volman V: Callosal dysfunction explains injury sequelai in a computational network model of axonal injury. J Neurophysiol 116:2892–2908, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundaramurthy A, Chandra N: A parametric approach to shape field-relevant blast wave profiles in compressed-gas-driven shock tube. Front Neurol 5:253, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox WM: Reflex-ontogeny and behavioural development of the mouse. Anim Behav 13:234–241, 1965. [DOI] [PubMed] [Google Scholar]
- 25.Alexander AL, Lee JE, Lazar M, Field AS: Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–29, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubovitch V, Ten-Bosch M, Zohar O, Harrison CR, Tempel-Brami C, Stein E, Hoffer BJ, Balaban CD, Schreiber S, Chiu WT, et al. : A mouse model of blast-induced mild traumatic brain injury. Exper Neurol 232:280–289, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun SW, Neil JJ, Liang HF, He YY, Schmidt R., Hsu CY, Song SK: Formalin fixation alters water diffusion coefficient magnitude but not anisotropy in infarcted brain. Mag Res Med 53:1447–1451, 2005. [DOI] [PubMed] [Google Scholar]
- 28.D’Arceuil HE, Westmoreland S, de Crespigny AJ: An approach to high resolution diffusion tensor imaging in fixed primate brain. Neuroimage 35:553–65, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Kaouane N, Porte Y, Vallee M, Brayda-Bruno L, Mons N, Calanndreau L, Marighetto A, Piazza PV, Desmedt A: Glucocorticoids can induce PTSD-like memory impairments in mice. Science 335:1510–1513, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Ding AY, Li Q, Zhou IY, Ma SJ, Tong G, McAlonan GM, Wu EX: MR diffusion tensor imaging detects rapid microstructural changes in amygdala and hippocampus following fear conditioning in mice. PLoS one 8:e51704, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zatorre RJ, Fields RD, Hohansen-Berg H: Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nature Neurosci 15:528–536, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mole JP, Subramanian L, Bracht T, Morris H, Metzler-Baddeley C, Linden DE: Increased fractional anisotropy in the motor tracts of Parkinson’s disease suggests compensatory neuroplasticity or selective neurodegeneration. Eur Radiol 26:3327–35, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hazamy AA, Altmann LJP, Stegemöller E, Bowers D, Lee HK, Wilson J, Okun MS, Hass CJ: Improved cognition while cycling in Parkinson’s disease patients and healthy adults. Brain Cogn 113:23–31, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang SH, Gangidine M, Pritts TA, Goodman MD, Lentsch AB: Interleukin 6 mediates neuroinflammation and motor coordination deficits after mild traumatic brain injury and brief hypoxia in mice. Shock 40(6):471–475, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trahanas DM, Cuda CM, Perlman H, Schwulst SJ: Differential Activation of Infiltrating Monocyte-Derived Cells After Mild and Severe Traumatic Brain Injury. Shock 43(3):255–60, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manhaes AC, Krahe TE, Caparelli-Daquer E, Ribeiro-Carvalho A, Schmidt SL, Filgueiras CC: Neonatal transection of the corpus callosum affects paw preference lateralization of adult Swiss mice. Neurosci Lett 348:69–72, 2003. [DOI] [PubMed] [Google Scholar]