Abstract
Objective
To determine if there is a benefit to adjuvant corneal crosslinking (CXL) and to compare natamycin versus amphotericin B for filamentous fungal keratitis.
Design
Outcome-masked, 2 × 2 factorial design, randomized controlled clinical trial
Study Participants
Consecutive patients presenting with moderate vision loss from a smear-positive fungal ulcer at Aravind Eye Hospital, Madurai, India.
Intervention
Study eyes were randomized to one of four treatment combinations using an adaptive randomization protocol. The treatment arms included: 1. Topical natamycin 5% alone, 2. Topical natamycin 5% plus CXL, 3. Topical amphotericin B 0.15% alone, and 4. Topical amphotericin 0.15% plus CXL.
Main Outcome Measures
The primary outcome of the trial was microbiological cure at 24 hours on repeat culture. Secondary outcomes included best spectacle corrected visual acuity (BSCVA) at 3 weeks and 3 months, % of study participants with epithelial healing at 3 days, 3 weeks and 3 months, infiltrate and/or scar size at 3 weeks and 3 months, 3-day smear and culture, and adverse events.
Results
Those randomized to CXL regardless of medication (topical natamycin or amphotericin) had 1.32-fold increased odds of 24-hour culture positivity although this was not statistically significant (95% CI 0.57 to 3.06; P=0.51). We were also unable to find a difference in 24-hour culture positivity between those randomized to amphotericin compared with natamycin when evaluating as a group regardless of whether or not they received CXL (coef 1.10, 95% CI 0.47 to 2.54; P=0.84). BSCVA was approximately 0.22 logMAR (2.2 Snellen lines) worse on average at 3 weeks among those receiving CXL regardless of medication (95% CI −0.04 to 0.40, P=0.04) and 0.32-logMAR (3.2 Snellen lines) worse visual acuity at 3 months after controlling for baseline visual acuity (95% CI 0.03 to 0.54; P=0.02). There was no difference in infiltrate and/or scar size, % epithelialized or adverse events when comparing CXL versus no CXL or the two topical medications.
Conclusions
There appears to be no benefit of adjuvant CXL in the primary treatment of moderate filamentous fungal ulcers and it may result in decreased visual acuity.
Trial Registration
Précis
Topical amphotericin has similar outcomes to natamycin in the treatment of filamentous fungal ulcers. However, there is no benefit to adjuvant corneal crosslinking.
Introduction
There has been recent interest in corneal crosslinking (CXL) as an adjuvant therapy for infectious keratitis.1 There are at least three potential mechanisms by which CXL may benefit patients with infectious corneal ulcers: antimicrobial and anti-inflammatory effects, as well as increased resistance of corneal tissue to enzymatic degradation. In vitro efficacy of UV-A + riboflavin against the most common bacterial ocular pathogens, such as Pseudomonas aeruginosa and Streptococcus pneumonia has been demonstrated.2 Unfortunately, in vitro CXL alone has failed to demonstrate fungal pathogen inactivation.2,3 However, one study showed improved inhibition of fungus in vitro with amphotericin plus riboflavin + UV-A compared with amphotericin alone.2 The authors suggest that CXL may improve diffusion of antifungal medications or inhibit fungal proliferation through more complex mechanisms. In addition to these data, multiple case reports have suggested potential benefits of CXL for treatment of bacterial and fungal keratitis, such as symptomatic improvement, resolution of resistant infection, and halting of progressive melting.4–7
Filamentous fungal corneal ulcers continue to have a poor prognosis with no new treatment since the 1960s. Although the evidence for CXL as a treatment for fungal keratitis is less robust, clinicians have already begun using UV-A + riboflavin in conjunction with antifungals in hopes that there will be some benefit in this challenging disease. Thus, scientific investigation into this treatment approach is necessary.
Methods
Trial Design
Cross-Linking Assisted Infection Reduction (CLAIR) was an institutionally-funded randomized, outcome-masked, clinical trial with 2×2 factorial design. Patients with moderate smear-positive filamentous fungal corneal ulcers were allocated to one of four treatment combinations using an adaptive randomization protocol. The treatment arms included: 1. natamycin alone, 2. natamycin plus CXL, 3. amphotericin alone, and 4. amphotericin plus CXL. All study participants received medical therapy with topical natamycin, 5%, eye drops (Ynaat, Optix) or topical amphotericin B 0.15% (United Biotech) every hour while awake, and homatropine 2% (Aurohom Aurolab) 3 times daily.
Ethical approval was obtained from the University of California, San Francisco, Committee on Human Research (IRB #14–14918) and the Aravind Eye Care System Institutional Review Board, Madurai, India. Written informed consent was obtained from all participants, and the trial conformed to the Declaration of Helsinki.8 The Data Safety and Monitoring Committee recommended one interim analysis to review safety, data quality and trial conduct.
Outcomes
The primary outcome of the trial was microbiological cure, which we defined as a negative culture, at 24 hours. Secondary outcomes included best spectacle corrected visual acuity (BSCVA) at 3 weeks and 3 months, percent of study participants with epithelial healing at 3 days, 3 weeks and 3 months, infiltrate and/or scar size at 3 weeks and 3 months, 24-hour smear result, 3-day smear and culture result, and adverse events including corneal perforation or the need for therapeutic penetrating keratoplasty (TPK).
Study Participants
All study participants were enrolled at Aravind Eye Hospital in Madurai, India. Consecutive patients who presented with smear-positive fungal corneal ulcers were screened for inclusion. Inclusion criteria were 1) presence of corneal ulcer 2) smear positive for filamentous fungus and 3) presenting visual acuity of 20/70 (logMAR 0.54) or worse in the affected eye. Exclusion criteria included involvement of the posterior 1/3 of the stroma, central pachymetry less than 350μm, evidence of concomitant infection with herpes or bacteria, impending or frank perforation or limbal involvement, no light perception vision in the affected eye or visual acuity worse than 20/200 in the unaffected eye, age less than 18 or greater than 70, and patients that were cognitively impaired or unable to complete follow up (eTable 1).
Randomization
Each study eye was randomly assigned to the treatment group through an adaptive randomization allocation using block randomization by the coordinating site (Statistical package R; Version 3.2 or above; R Foundation for Statistical Computing, Vienna, Austria). A drop-the-loser adaptive trial design used 24-hour culture result (coded as positive or negative) to perform interim response-adaptive randomization.9 Real time electronic data collection allowed for continuous updating of randomization allocation based on trial results. After eligibility was confirmed and written informed consent was obtained, the study participant was assigned a study participant ID and randomized to a treatment group. Once randomized, they were included in the intent to treat analysis.
Interventions
Microbiological methods used for this study were adapted from a protocol used in the Mycotic Ulcer Treatment Trial I which have been previously published in detail.10 Corneal scraping was performed at enrollment, and 24 hours and 3 days after enrollment by a masked microbiologist. A Kimura spatula using aseptic techniques was used to obtain a scrape from the leading edge and base of the corneal ulcer. Two scrapings were smeared directly on to two separate glass microbiology slides for Gram stain and for Potassium Hydroxide (KOH) wet mount while three additional scrapings were taken and directly inoculated on to sheep’s blood agar, chocolate agar, potato dextrose agar or Sabouraud’s agar for bacterial and fungal culture. A positive fungal smear was defined as fungal elements seen under low-power magnification and reduced light. Positive fungal cultures were defined as light growth on any 2 media or moderate to heavy growth on 1 medium.
For those randomized to CXL, the procedure was scheduled in the operating room within 24 hours of enrollment in the study. Using aseptic techniques, the patient was given a 30-minute loading dose of 0.1% topical riboflavin and 20% dextran T500 drops administered every 2 minutes. The cornea was then exposed to UV-A light at a wavelength of 365 nm with an irradiance of 3 mW/cm2. Throughout the UV-A treatment the study participant continued to receive topical riboflavin at 5-minute intervals. All patients were hospitalized for the first 3 days so that all medications were directly observed and recorded by a health technician.
Study participants were examined by a masked study physician at baseline, 3 days, 3 weeks and 3 months. A calibrated slit lamp biomicroscope was used to assess the epithelial defect size, infiltrate and/or scar dimensions and depth according to a protocol adapted from the Herpetic Eye Disease Study.11 The presence of corneal perforation, hypopyon, or other ocular adverse event was also recorded. Participants were asked about serious and non-serious systemic adverse events. All study ophthalmologists were certified to ensure adherence to the study protocol.
Using a protocol adapted from the Age-Related Eye Disease Study using Early Treatment Diabetic Retinopathy Study (ETDRS) tumbling E charts (charts 2305 and 2305A; Precision Vision), best spectacle-corrected visual acuity (BSCVA) was recorded at 4 meters at enrollment, 3 weeks, and 3 months by a certified masked refractionist.12 Low vision testing was also performed at a distance of 0.5m.
Masking
Study participants were not masked to their CXL intervention status but were asked not to share this information with any of the study personnel. While the surgeon performing CXL was not masked as to CXL intervention status, the microbiologist performing repeat scraping and culture analysis, treating physician involved in outcome assessment, and refractionist performing BSCVA were all masked to treatment arm. All participants, physicians, refractionists, and the microbiologist were masked to medication assignment.
Sample Size Calculation
The sample size was determined based on the primary end point of microbiological cure at 24 hours in the CXL versus no CXL group (regardless of study medication). Given the 4 arms of the trial, we have estimated a sample size based on a significance level of 0.0125. Assuming that 85% of study participants that are smear positive are subsequently culture positive and that there is an approximately 7.5% reduction is culture positivity on average per day of treatment as there was in Mycotic Ulcer Treatment Trial II (MUTT II)13, and if we consider initial scraping to have a similar effect as one day of treatment, we can estimate that there would be an approximately 8% reduction in culture positivity due to repeat scraping in our trial at 24 hours. In order to detect a 30% difference in culture positivity (approximately 80% in controls versus 50% in the crosslinking group), we would need to enroll 110 study participants. We anticipate similar power to detect a difference for the natamycin versus amphotericin comparison. This assumes no loss to follow-up, since the primary outcome occurs while the patient is still hospitalized one day after enrollment. In reality, we should have more statistical power since we will also take into account the results of the baseline culture, which we expect to be highly correlated with the follow-up culture.
Statistical Analysis
Baseline characteristics between the two arms were compared using Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables. The pre-specified primary analysis used a logistic regression model to assess microbiological cure at 24 hours between groups (dichotomous culture positive or culture negative outcome) with a 4-level categorical variable for treatment (natamycin alone, natamycin plus CXL, amphotericin alone, amphotericin plus CXL) controlling for baseline culture status and accounting for the adaptive nature of the randomization. A Wald test was performed to assess the significance of this interaction. Similar logistic regression models were used to assess % with healed epithelium at 3 week and 3 months controlling for baseline epithelial defect size and status of smear (positive or negative) between groups although we did not include a co-variate for baseline smear since smear positivity was part of the inclusion criteria. Multiple linear regression was used to analyze BSCVA and infiltrate and/or scar size measured at 3 weeks and 3 months with baseline measurements as co-variates (adjusted semi-parametrically to improve model fit in the presence of non-linear relationships between baseline and follow up values). Cox proportional hazards regression models were used to estimate the hazard of perforation or need for TPK associated with CXL plus medical therapy versus medical therapy alone while correcting for baseline infiltrate and/or scar size as a fixed effect. Fischer exact test was used to compare adverse events between arms.
For missing data for BSCVA due to TPK, we used the last observation carried forward (LOCF) or logMAR of 1.7 (Approximate Snellen equivalent 20/1000) if there was no LOCF data available. If there were missing data for infiltrate and/or scar size or epithelial defect size resulting from a TPK, the LOCF prior to TPK was used. For all analyses, standard methods to compute the P value were adjusted for the adaptive nature of the randomization.14 All analyses were conducted using Stata, version 13 (StataCorp), and R including R package gam 1.14–4 were performed the week of March 8th 2019.
Results
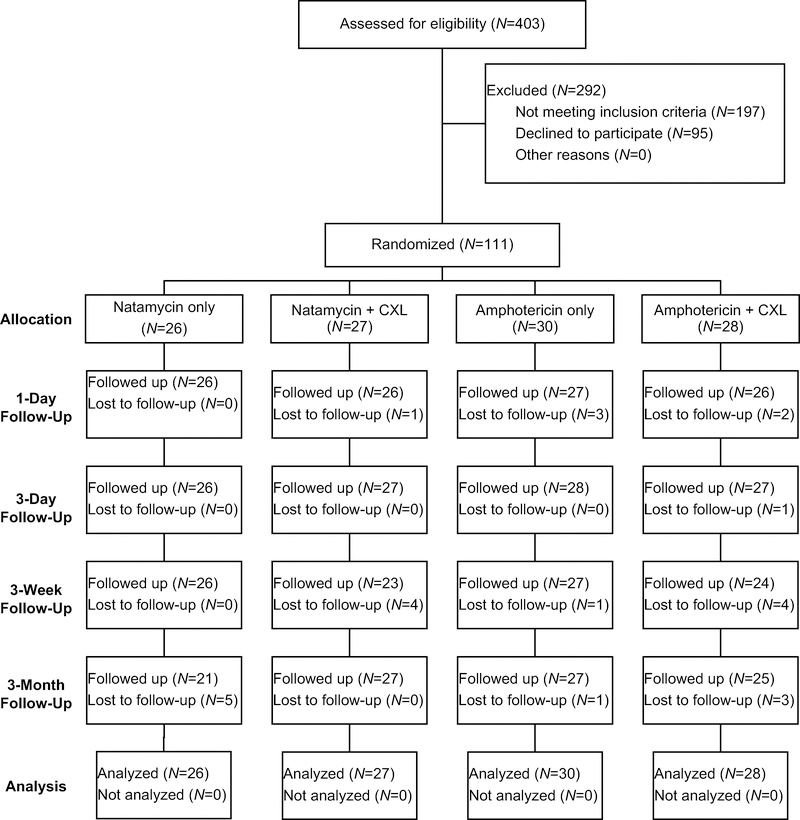
A total of 403 patients with smear positive ulcers were screened between January 4, 2016 and March 19, 2018 and 111 were randomized to either topical natamycin 5% or topical amphotericin 0.15% and CXL or no CXL, for a total of 4 groups: topical natamycin 5% alone, topical natamycin 5% plus CXL, topical amphotericin 0.15% alone, and topical amphotericin 0.15% plus CXL (eFigure1). All study participants were enrolled at the Aravind Eye Hospital, Madurai. Follow-up for the primary outcome of 24-hour repeat culture was available for 105/111 (95%) of study participants, and 3-month follow up was available for 100/111 (90%). Thirty-one (31%) of the 3-month visits were out of window. There was no evidence that loss to follow up was associated with baseline visual acuity or treatment arm. Baseline participant demographics and clinical characteristics are outlined in Table 1. No major baseline differences between groups were identified. The preliminary 24-hour culture result (i.e. not the final culture result) was used to guide adaptive randomization.
eFigure1.
CONSORT Flow Diagram
Table 1.
Baseline demographic and clinical characteristics
| Study Group |
|||||
|---|---|---|---|---|---|
| Characteristic | Natamycin only (N = 26) | Natamycin + CXL (N = 27) | Amphotericin only (N = 30) | Amphotericin + CXL (N = 28) | P-Values |
| Sex, No. (%) | 0.53 | ||||
| Male | 17 (65) | 15 (56) | 14 (47) | 17 (61) | |
| Female | 9 (35) | 12 (44) | 16 (53) | 11 (39) | |
| Age, median (IQR), y | 50 (36, 55) | 55 (47, 65) | 45 (40, 58) | 56 (45, 65) | 0.03 |
| Occupation, No. (%) | 0.74 | ||||
| Agriculture | 16 (62) | 13 (48) | 18 (60) | 13 (46) | |
| Non-agriculture | 10 (38) | 14 (52) | 12 (40) | 15 (54) | |
| Medication use at enrollment, No. (%) a | 18 (69) | 21 (78) | 19 (66) | 24 (86) | 0.31 |
| Trauma, No. (%) a | 0.77 | ||||
| Stick | 0 | 6 (22) | 4 (14) | 3 (11) | |
| Leaf | 0 | 2 (7) | 2 (7) | 5 (18) | |
| Dust | 9 (35) | 6 (22) | 5 (17) | ^3 (11) | |
| Stone | 1 (4) | 1 (4) | 0 | 1 (4) | |
| Cow tail | 2 (8) | 1 (4) | 0 | 0 | |
| Insect | 0 | 1 (4) | 2 (7) | 1 (4) | |
| Other b | 4 (15) | 1 (4) | 3 (10) | 1 (4) | |
| Unknown object | 0 | 1 (4) | 3 (10) | 2 (7) | |
| None | 10 (39) | 8 (30) | 10 (37) | 12 (43) | |
| Affected eye, No. (%) | 0.53 | ||||
| Right | 15 (58) | 15 (56) | 12 (40) | 15 (54) | |
| Left | 11 (42) | 12 (44) | 18 (60) | 13 (46) | |
| Visual acuity, median (IQR) | 0.27 | ||||
| LogMAR | 1.05 (0.62, 1.12) | 1.10 (0.60, 1.70) | 1.05 (0.62, 1.70) | 1.22 (0.91, 1.70) | |
| Approx. Snellen | 20/250 | 20/250 | 20/250 | 20/320 | |
| Ulcer location, No. (%) | 0.84 | ||||
| Central | 21 (81) | 23 (85) | 26 (87) | 22 (79) | |
| Peripheral | 5 (19) | 4 (15) | 4 (13) | 6 (21) | |
| Infiltrate and/or scar, median (IQR), mmc | 3.0 (2.5, 3.5) | 3.9 (2.6, 4.5) | 3.5 (2.7, 4.5) | 3.1 (2.8, 5.0) | 0.22 |
| Hypopyon, No. (%) | 0.27 | ||||
| No | 19 (73) | 16 (60) | 15 (50) | 14 (50) | |
| <0.5 mm | 1 (4) | 5 (19) | 5 (17) | 1 (4) | |
| >=0.5 mm | 6 (15) | 6 (22) | 10 (33) | 13 (46) | |
| % Depth, No. (%) a | 0.34 | ||||
| >0–33% | 14 (56) | 13 (50) | 13 (43) | 9 (32) | |
| >33–67% | 11 (44) | 12 (46) | 17 (57) | 19 (68) | |
| >67–100% | 0 | 1 (4) | 0 | 0 | |
| Epithelial defect, median (IQR), mmc | 3.0 (2.4, 4.0) | 3.9 (2.6, 4.5) | 3.5 (2.2, 4.5) | 3.4 (3.0, 4.7) | 0.29 |
| Duration of symptoms, median (IQR), d | 5 (4, 10) | 7 (3, 7) | 5 (3, 10) | 4 (3, 9) | 0.81 |
| Systemic disease, No. (%) | |||||
| None | 25 (96) | 26 (96) | 27 (90) | 26 (93) | 0.73 |
| Diabetes | 0 | 0 | 3 (10) | 0 | 0.04 |
| Asthma/eczema | 0 | 0 | 1 (3) | 1 (4) | 0.60 |
| Hypertension | 1 (4) | 1 (4) | 0 | 1 (4) | 0.77 |
Missing data
Includes fall, mattress, metal wire, mud, nail, sand, wood
Geometric mean
P-values from chi-square tests for categorical variables and ANOVA for continuous variables
Abbreviations: CF = count fingers, d = days, HM = hand motion, IQR = interquartile range, LogMAR = logarithm of the minimum angle, LP = light perception, mm = millimeters, No. = number, y = years
Organisms isolated from baseline cultures are described in Table 2. Fusarium grew in 45 (42%), Aspergillus in 15 (14%), other filamentous fungi in 48 (44%). Baseline cultures were negative in 22 cases (20%) which included 8 in the natamycin alone group, 5 in the natamycin plus CXL group, 3 in the amphotericin alone group and 6 of the amphotericin plus CXL group.
Table 2.
Baseline microbiologic culture results
| Study Group, No. (%) |
|||||
|---|---|---|---|---|---|
| Organism | Natamycin Only (N = 25) a | Natamycin + CXL (N = 27) | Amphotericin Only (N = 29) a | Amphotericin + CXL (N = 27) a | Total (N = 108) |
| Alternaria species | 1 (4) | -- | -- | -- | 1 (1) |
| Aspergillus species | |||||
| A. flavus | 2 (8) | 2 (7) | 6 (20) | 4 (15) | 14 (13) |
| A. terreus | -- | 1 (4) | -- | -- | 1 (1) |
| Bipolaris species | -- | 2 (7) | -- | 1 (4) | 3 (3) |
| Colletotrichum species | -- | -- | -- | 1 (4) | 1 (1) |
| Curvularias species | 2 (8) | 1 (4) | 2 (7) | 2 (7) | 7 (6) |
| Exserohilum species | 1 (4) | 1 (4) | 2 (2) | ||
| Fusarium species | 11 (44) | 10 (37) | 14 (47) | 10 (37) | 45 (42) |
| Scedosporium species | -- | 2 (7) | -- | -- | 2 (2) |
| Unidentified hyaline | 1 (4) | -- | 3 (10) | -- | 4 (4) |
| Unidentified dematiaceous | -- | 3 (11) | 1 (3) | 2 (7) | 6 (6) |
| Fungal culture negative | 8 (32) | 5 (19) | 3 (10) | 6 (22) | 22 (20) |
Missing data for one patient
Abbreviations: A. = Aspergillus, No. = number
At 24 hours, 17/26 (65%) of cultures were negative in the natamycin only group and 14/26 (54%) were negative in the natamycin plus CXL group, 15/27 (56%) of cultures were negative in amphotericin only group and 13/26 (50%) were negative in the amphotericin plus CXL group. Those randomized to CXL regardless of medication (topical natamycin or amphotericin) had 1.32-fold greater odds of 24-hour culture positivity (95% CI 0.57 to 3.06; P=0.51) and 1.08-fold odds of 3-day culture positivity (95% CI 0.44 to 2.66; P=0.87) however, this was not statistically significant. We were also unable to find a difference in 24-hour culture positivity between those randomized to amphotericin compared with natamycin when evaluating as a group regardless of whether or not they received CXL (coef 1.10, 95% CI 0.47 to 2.54; P=0.84). At 3-days, those randomized to amphotericin had 2.17-fold odds of culture positivity compared with natamycin after controlling for baseline culture status (95% CI 0.85 to 5.54; P=0.10). There was no evidence of an interaction between CXL and medication for the primary outcome of 24-hour culture positivity (P=0.89).
Outcomes of smear were similar, with those randomized to CXL regardless of the medication having 0.95-fold odds of smear positivity at 24 hours (95% CI 0.33 to 2.78; P=0.91) and 1.05-fold smear positivity at 3 days (95% CI 0.47 to 2.37; P=0.91). Those randomized to amphotericin were 1.35-fold more likely to be smear positive at 24 hours (95%CI 0.46 to 3.96; P=0.58) and 1.50-fold more likely to be smear positive at day 3 (95% CI 0.66 to 3.39, P=0.33). Smear and culture results at baseline, 1 and 3 days are outlined in Table 3.
Table 3.
Primary and secondary outcomes
| Study Group, No. (%) |
|||||
|---|---|---|---|---|---|
| Outcome | Natamycin only (N = 26) | Natamycin + CXL (N = 27) | Amphotericin only (N = 30) | Amphotericin + CXL (N = 28) | P-value a |
| Culture positive, N (%) | |||||
| Baseline | 17/25(68) | 22/27 (81) | 26/29 (90) | 21/27 (78) | |
| 24 hours | 9/26 (35) | 12/26 (46) | 12/27 (44) | 13/26 (50) | 0.51 |
| 3 days | 5/26 (19) | 4/27 (15) | 8/28 (29) | 10/26 (39) | 0.87 |
| Smear positive, N (%) | |||||
| Baseline | 25/26 (96) | 25/26 (96) | 29/29 (100) | 25/25 (100) | |
| 24 hours | 23/26 (88) | 19/26 (73) | 21/26 (19) | 24/26 (92) | 0.93 |
| 3 days | 16/26 (62) | 14/27 (52) | 18/28 (64) | 19/26 (73) | 0.90 |
| Visual acuity, median (IQR) | |||||
| Baseline | 1.05 (0.62, 1.12) | 1.10 (0.60, 1.70) | 1.05 (0.62, 1.70) | 1.22 (0.91, 1.70) | |
| 3 weeksb | 0.52 (0.22, 1.20) | 0.80 (0.32, 1.70) | 0.52 (0.50, 1.10) | 1.10 (0.57, 1.70) | 0.04 |
| 3 monthsb | 0.42 (0.10, 0.72) | 1.00 (0.30, 1.80) | 0.48 (0.20, 1.70) | 0.80 (0.50, 1.24) | 0.02 |
each p-value given represents a logistic regression model comparing CXL versus no CXL for the stated outcome, controlling for the baseline value
missing data
Abbreviations: No. = number
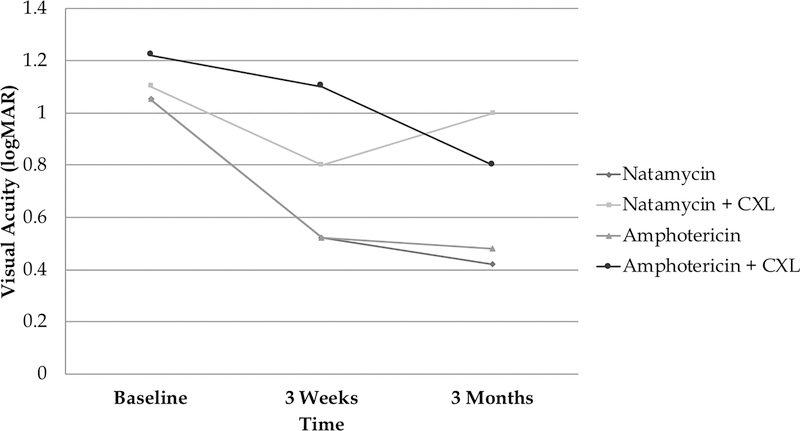
Median 3-week visual acuity was logMAR 0.52 (IQR 0.62, 1.12) in the natamycin only arm, logMAR 0.80 (IQR 0.32, 1.70) in the natamycin plus CXL arm, logMAR 0.52 (IQR 0.50, 1.10) in amphotericin only arm and logMAR 1.1 (IQR 0.57, 1.7) in the amphotericin plus CXL arm. BSCVA was approximately 0.22 logMAR (2.2 Snellen lines) worse on average at 3 weeks among those receiving CXL regardless of medication after controlling for baseline BSCVA and this was statistically significant (95% CI −0.04 to 0.40, P=0.04). Median 3-month visual acuity was 0.42 logMAR (IQR 0.10, 0.72) in the natamycin only arm, 1.00 logMAR(IQR 0.30, 1.80) in the natamycin plus CXL arm, 0.48 logMAR (IQR 0.20, 1.70) in the amphotericin only arm and 0.80 logMAR (IQR 0.5, 1.24) in the amphotericin plus CXL arm. Those randomized to CXL had 0.32-logMAR (3.2 Snellen lines) worse visual acuity at 3 months after controlling for baseline visual acuity (95% CI 0.03 to 0.54; P=0.02) (Figure 1).
Figure 1.
Visual Acuity by Treatment Arm
We were unable to detect a difference in visual acuity among those randomized to amphotericin versus natamycin, regardless of whether or not they had CXL, after controlling for baseline BSCVA at 3 weeks (coef 0.01, 95% CI −0.20 to 0.23; P=0.97) or 3 months (coef −0.09, 95% CI −0.34 to 0.16; P=0.48).
Ulcer healing was evaluated in two ways including % with healed epithelium and infiltrate and/or scar size at 3 weeks and 3 months. Those who were randomized to CXL, regardless of medication, had 1.57-fold higher odds of having an epithelial defect at 3 weeks (95% CI 0.70 to 3.52; P=0.27) and 1.39-fold higher odds at 3 months (95% CI 0.54 to 3.60; P=0.80) after controlling for baseline epithelial defect size. We were unable to find a difference in the ulcer infiltrate and/or scar size at 3 weeks (−0.02 mm, 95% CI −0.43 to 0.39; P=0.86) or at 3 months (0.27 mm, 95% CI −0.21 to 0.75; P=0.27) among those who received CXL compared to those who did not after controlling for baseline infiltrate and/or scar size. After controlling for baseline epithelial defect size, amphotericin-treated study participants had 1.10-fold higher odds of having an epithelial defect at 3 weeks (95% CI 0.54 to 3.60; P=0.48) and 1.93-fold higher odds at 3 months (95% CI 0.73 to 5.12; P=0.18) compared with natamycin although this was not statistically significant. There was no difference in ulcer infiltrate and/or scar size at 3 weeks (0.15 mm, 95% CI −0.27 to 0.57; P=0.71) or at 3 months (0.07 mm, 95% CI −0.42 to 0.55; P=0.80) among amphotericin treated patients compared with natamycin after controlling for baseline measurements.
Adverse events are outlined in Table 4. Overall there were 10 (9%) study participants who had full thickness corneal perforation, 3 (3%) in the CXL arms and 6 (5%) in the medication only arms. There was no evidence of an interaction between medication and CXL (P=0.67). Eighteen (16%) eventuated to TPK, including 8 (40%) in the CXL arms and 10 (9%) in the medication only groups. We were unable to find a difference in rate of perforation and/or TPK among those randomized to CXL, regardless of medication, after controlling for baseline infiltrate and/or scar depth (coef 0.90 95% CI 0.15 to 5.27; P=0.80). Those randomized to amphotericin, regardless of whether they had CXL, had 0.89-fold decreased hazard of TPK (95% CI 0.16 to 4.63; P=0.72). There was no evidence of an interaction between medication and CXL with regards to perforation and/or TPK (P=0.89). CXL conferred no increased hazard of experiencing any adverse event (coef 1.02, 95% CI 0.41 to2.53; P=0.94).
Table 4.
Adverse events by treatment group
| Study Group, No. (%) |
||||||
|---|---|---|---|---|---|---|
| Adverse event | Natamycin only (N = 26) | Natamycin + CXL (N = 27) | Amphotericin only (N = 30) | Amphotericin + CXL (N = 28) | Total | P-value a |
| Endophthalmitis | -- | -- | 1 (3) | -- | 1 | |
| Glaucoma | -- | -- | -- | -- | -- | |
| Median tarsorrhaphy | -- | -- | -- | 1 (4) | 1 | |
| Medication reaction | -- | -- | -- | -- | -- | |
| Perforation | 2 (8) | 1 (4) | 4 (13) | 3 (11) | 10 | |
| Therapeutic penetrating keratoplasty | 5 (19) | 5 (19) | 5 (17) | 3 (1) | 18 | |
| Total | 7 | 6 | 11 | 7 | 31 | 0.99 |
Fisher’s exact comparing number of people with any adverse event in each arm
Abbreviations: No. = number
Discussion
We found no benefit of adjuvant CXL in the treatment of filamentous fungal ulcers in this randomized clinical trial. Specifically, we found no improvement in microbiological cure including culture and smear, no improvement in infiltrate and/or scar size, no increase in the % epithelialized at 3 week or 3 months, and no difference in adverse events including corneal perforation and need for TPK. Our results suggest that adjuvant CXL may have a negative effect on visual acuity. The reason for this is unclear. It is well known that CXL for ectatic conditions such as keratoconus result in topographic changes as well as corneal haze which decreases over time.15 It is possible that there is, therefore, increased irregular astigmatism or increased scar density that accounts for the worsened visual acuity. This may improve over time and we will continue to follow these patients up to 12 months postoperatively. Whether the patient was randomized to topical natamycin or amphotericin did not seem to have an effect on any of these outcomes.
Although multiple studies have suggested the efficacy of CXL for both in vitro and in vivo for bacterial keratitis, there has been less robust evidence for fungal keratitis. There have been two randomized clinical trials to date that have evaluated the efficacy of adjuvant CXL in fungal keratitis. One trial randomized patients with bacterial, fungal and Acanthameoba infections to CXL versus medical therapy. Although this trial did not identify a benefit of CXL, these results are difficult to interpret given inclusion of different types of keratitis and other study design limitations.16 Another small randomized clinical trial investigated CXL as adjuvant therapy for advanced, deep filamentous fungal ulcers and found an increased rate of perforation among those receiving CXL.17 Finally, one retrospective series found no benefit to adjuvant CXL in moderate mycotic keratitis.18
Limitations to this study include the fact that all patients enrolled in this study were from India, and the majority of infections were related to agricultural exposure and not contact lens wear, such as those seen in developed countries. Therefore, it is possible that organisms in this study exhibit different characteristics and response patterns to medications. Only a small number of each type of fungus was represented which may have made it difficult to detect a benefit of CXL for any particular organism. This study was powered to detect a difference in microbiological cure, a surrogate endpoint. Studies have suggested that in addition to providing an initial diagnosis, repeated culture can be used to assess response to treatment and is highly correlated with clinical outcomes such as visual acuity and outcomes such as these have become increasingly common in infectious disease trials.19–24 Although a negative culture implies a microbial load reduction on the ocular surface, it does not assess deeper infection. 24-hour repeat culture may have been to early a time point to detect a difference between arms. We chose this time point in order to isolate the effect of CXL on infectious keratitis as medical therapy decreases the culture positive rate over time. There may be anti-microbial effects of CXL that occur after the 3-day culture result which we may not have detected. However, due to the low numbers of culture positive ulcers at 3 days and the likelihood that this would be further reduced, it likely would have been difficult to detect a difference between arms. 3 day repeat culture and clinical outcomes such as visual acuity, scar size, rate of perforation and TPK in this study were supportive of the primary outcome. In addition, these clinical outcomes were designed to assess the other potential mechanisms by which CXL might be beneficial in infectious keratitis including biomechanical strength, and anti-inflammatory effect which would not be evaluated by our 24-hour microbiological outcome. This study had an adaptive randomization protocol that was based on the primary outcome of microbiological cure on culture. Because the protocol adapted based on the 24-hour culture result at day 1 rather than the final 7-day culture result, the adaptation did not completely reflect the results of the primary outcome. The result of this was that the adaptation did not change the randomization significantly from a traditional randomization protocol and is unlikely to have biased the outcomes. This study only evaluated CXL with the Dresden protocol, it is possible that alternative CXL duration or timing (i.e. not in the first 24 hours) may have had different outcomes. Additionally, new data suggest that UVC may be a more effect treatment for infectious keratitis than the UVA studied here.25
Conclusions
We found no benefit to adjuvant CXL in the primary treatment of filamentous fungal keratitis. Patients with mild to moderate filamentous fungal infections appear to have similar outcomes when treated with amphotericin compared with natamycin.
Supplementary Material
Acknowledgments
This work was supported by grants K23 EY025025 (Rose-Nussbaumer) from the National Eye Institute and Research to Prevent Blindness (UCSF). There are no conflicts of interest to report. Travis Porco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. NV Prajna contributed to the design and implementation of the study and editing of the manuscript. N Radhakrishnan contributed to the design and implementation of the study and editing of the manuscript. P Lalitha contributed to the design and implementation of the study and editing of the manuscript. Ariana Austin contributed to the design and implementation of the study, and writing of the manuscript. Tom Lietman contributed to design of the study and editing of the manuscript. Jennifer Rose-Nussbaumer contributed to the design and implementation of the study, and writing of the manuscript. Sumithra Rengasamy played a key role in study implementation as the principle study coordinator.
Footnotes
Disclosures: None of the authors have a proprietary/financial interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alio JL, Abbouda A, Valle DD, Del Castillo JM, Fernandez JA. Corneal cross linking and infectious keratitis: a systematic review with a meta-analysis of reported cases. Journal of ophthalmic inflammation and infection. 2013;3(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martins SA, Combs JC, Noguera G, et al. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Investigative ophthalmology & visual science. 2008;49(8):3402–3408. [DOI] [PubMed] [Google Scholar]
- 3.Sauer A, Letscher-Bru V, Speeg-Schatz C, et al. In vitro efficacy of antifungal treatment using riboflavin/UV-A (365 nm) combination and amphotericin B. Investigative ophthalmology & visual science. 2010;51(8):3950–3953. [DOI] [PubMed] [Google Scholar]
- 4.Panda A, Krishna SN, Kumar S. Photo-activated riboflavin therapy of refractory corneal ulcers. Cornea. 2012;31(10): 1210–1213. [DOI] [PubMed] [Google Scholar]
- 5.Iseli HP, Thiel MA, Hafezi F, Kampmeier J, Seiler T. Ultraviolet A/riboflavin corneal cross-linking for infectious keratitis associated with corneal melts. Cornea. 2008;27(5):590–594. [DOI] [PubMed] [Google Scholar]
- 6.Makdoumi K, Mortensen J, Crafoord S. Infectious keratitis treated with corneal crosslinking. Cornea. 2010;29(12):1353–1358. [DOI] [PubMed] [Google Scholar]
- 7.Shetty R, Nagaraja H, Jayadev C, Shivanna Y, Kugar T. Collagen crosslinking in the management of advanced non-resolving microbial keratitis. The British journal of ophthalmology. 2014;98(8):1033–1035. [DOI] [PubMed] [Google Scholar]
- 8.World Medical A World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013;310(20):2191–2194. [DOI] [PubMed] [Google Scholar]
- 9.Chow SC. Adaptive clinical trial design. Annu Rev Med. 2014;65:405–415. [DOI] [PubMed] [Google Scholar]
- 10.Prajna NV, Krishnan T, Mascarenhas J, et al. The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA ophthalmology. 2013;131(4):422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilhelmus KR, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology. 1994;101(12):1883–1895; discussion 1895–1886. [DOI] [PubMed] [Google Scholar]
- 12.Age-Related Eye Disease Study Research G. The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1. Controlled clinical trials. 1999;20(6):573–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prajna NV, Krishnan T, Rajaraman R, et al. Effect of Oral Voriconazole on Fungal Keratitis in the Mycotic Ulcer Treatment Trial II (MUTT II): A Randomized Clinical Trial. JAMA Ophthalmol. 2016;134(12):1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon R, Simon NR. Using Randomization Tests to Preserve Type I Error With Response-Adaptive and Covariate-Adaptive Randomization. Stat Probab Lett. 2011;81(7):767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37(1):149–160. [DOI] [PubMed] [Google Scholar]
- 16.Said DG, Elalfy MS, Gatzioufas Z, et al. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121(7):1377–1382. [DOI] [PubMed] [Google Scholar]
- 17.Uddaraju M, Mascarenhas J, Das MR, et al. Corneal Cross-linking as an Adjuvant Therapy in the Management of Recalcitrant Deep Stromal Fungal Keratitis: A Randomized Trial. Am J Ophthalmol. 2015;160(1):131–134 e135. [DOI] [PubMed] [Google Scholar]
- 18.Vajpayee RB, Shafi SN, Maharana PK, et al. Evaluation of corneal collagen cross-linking as an additional therapy in mycotic keratitis. Clin Exp Ophthalmol. 2015;43(2):103–107. [DOI] [PubMed] [Google Scholar]
- 19.Ray KJ, Prajna NV, Lalitha P, et al. The Significance of Repeat Cultures in the Treatment of Severe Fungal Keratitis. Am J Ophthalmol. 2018;189:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu JF, Chu SM, Huang YC, et al. Predictors of clinical and microbiological treatment failure in neonatal bloodstream infections. Clin Microbiol Infect. 2015;21(5):482 e489–417. [DOI] [PubMed] [Google Scholar]
- 21.Scangarella-Oman NE, Hossain M, Dixon PB, et al. Microbiological Analysis from a Phase 2 Randomized Study in Adults Evaluating Single Oral Doses of Gepotidacin in the Treatment of Uncomplicated Urogenital Gonorrhea Caused by Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2018;62(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timsit JF, de Kraker MEA, Sommer H, et al. Appropriate endpoints for evaluation of new antibiotic therapies for severe infections: a perspective from COMBACTE’s STAT-Net. Intensive Care Med. 2017;43(7):1002–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powers JH. Microbiologic surrogate end points in clinical trials of infectious diseases: example of acute otitis media trials. Pharmacotherapy. 2005;25(12 Pt 2):109S–123S. [DOI] [PubMed] [Google Scholar]
- 24.Ray KJ, Lalitha P, Prajna NV, et al. The Utility of Repeat Culture in Fungal Corneal Ulcer Management: A Secondary Analysis of the MUTT-I Randomized Clinical Trial. Am J Ophthalmol. 2017;178:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dean SJ, Petty A, Swift S, et al. Efficacy and safety assessment of a novel ultraviolet C device for treating corneal bacterial infections. Clin Exp Ophthalmol. 2011;39(2):156–163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.