Abstract
Both environmental and genetic factors are involved in the initiation and development of gastrointestinal cancer. Covalent closed circular RNAs (circRNAs) are produced by a mechanism called “back-splicing” from mRNAs. They are highly stable and show cell and tissue specific expression patterns. Although some functions such as “microRNA sponge” and “RNA binding protein sponge” have been reported for a small number of circRNAs, the function of thousands of other circRNAs is still unknown. Dysregulation of circRNAs has been reported in many GI cancers and are involved in metastasis and invasion. CircRNAs have been reported to be useful as prognostic markers and targets for developing new treatments. We first describe the properties and biogenesis of circRNAs. We then summarize recent reports about circRNA functions, expression status, and their potential to be used as biomarkers in GI cancers including, gastric cancer, colorectal cancer, esophageal cancer, hepatocellular carcinoma, gallbladder cancer and pancreatic cancer.
Keywords: Circular RNAs, gastrointestinal cancers, microRNA sponge, prognostic indicator, biomarkers, signaling pathways
1. Introduction
Gastrointestinal (GI) cancers can originate from the stomach, esophagus, pancreas, hepatobiliary system, large intestine (colorectal and anal regions) and together comprise a major cause of cancer-related mortality worldwide (Siegel et al., 2019). Smoking, obesity, Helicobacter pylori, hepatitis B virus (HBV) and hepatitis C virus (HCV) are known environmental risk factors for GI cancer development (Pourhoseingholi et al., 2015). Genetic factors such as, APC mutation predisposing to familial adenomatous polyposis (Leoz et al., 2015; Nallamilli and Hegde, 2017), and mutations in E-cadherin leading to hereditary diffuse-type gastric cancer (Brooks-Wilson et al., 2004; Liu and Chu, 2014) are known risk factors for GI development, but the exact molecular mechanisms underlying the progression and malignancy of other GI cancers remain largely unknown. Despite the success of chemotherapy in the treatment of some GI cancers, many patients continue to have a bad outcome and the 5-year survival rate in gastric cancer is less than 10% (Orditura et al., 2014; Sitarz et al., 2018). It is important to identify predictive and prognostic biomarkers to improve current treatment strategies and extend the 5-year survival rate.
CircRNAs are a group of single-stranded RNAs molecules that form a covalently closed loop structure as a result of joining the 3’ and 5’ ends. The existence of circRNAs has been known for more than 20 years (Nigro et al., 1991), but they have generally been considered to be artifacts of aberrant splicing (Cocquerelle et al., 1993). The first circRNA was serendipitously identified in a study that aimed to understand how viroids function as plant pathogens (Sanger et al., 1976). It was found by electron microscopy that viroid RNA is a single-stranded circular RNA which does not code for any proteins(Gross et al., 1978). Hepatitis delta was the second virus which was identified as a circular RNA, and in this case the circRNA encoded an open reading frame (ORF) which was translated to a protein (Kos et al., 1986). The first endogenously produced circRNA detected in human cells was a transcript of the DCC gene (deleted in colorectal cancer), which was identified in the early 1990s. The authors identified a transcript with the exons not in the expected order (Nigro et al., 1991). In the next two decades shuffled exons in transcripts of other genes such as SRY (sex-determining region Y) (Capel et al., 1993) and cytochrome P450 2C24 (CYPIIC24) (Zaphiropoulos, 1993, 1996) were also identified. However, recent work has revealed a large number of circRNAs in mammalian cells, and most of them are stable (Guo, J.U. et al., 2014; Rybak-Wolf, A. et al., 2015; Salzman et al., 2012). Most circRNAs in humans arise from coding genes (Wilusz, 2017). These transcripts are regulated independently from the linear transcripts of the underlying gene, and their transcription levels vary in a cell-specific manner (Salzman, J. et al., 2013; Salzman et al., 2012).
Studies have shown that circRNAs have some common properties. Exonic circRNAs are very stable and most of them have half-lives longer than 48 hours inside cells, in comparison to mRNAs that show average half-lives about 10 hours (Jeck, W. R. et al., 2013; Schwanhausser et al., 2011). However, circRNAs are not stable in serum, which could be due to the presence of circulating RNA endonucleases (Haupenthal et al., 2006). Their stability inside cells is because of their resistance to exonucleases. This resistance may explain why some circRNAs are more abundant than the linear RNA products of their respective genes (Salzman et al., 2012). CircRNAs do not contain the 2′−5′ linkage which is present in RNA lariats, and are therefore resistant to RNA debranching enzymes. Using different methods, some studies reported that exonic circRNAs localize in the cytoplasm, and they were susceptible to the siRNA-mediated decay system, which could be useful in clarifying the functional roles of circRNAs (Hansen et al., 2011). The human homologs of the Drosophila melanogaster Hel25E (helicase at 25E) similarly regulate circRNA localization, and their export from the nucleus to cytoplasm is size dependent (Azmi, 2018; Huang, C. et al., 2018). Exonic circRNAs share some sequence-dependent features. The circRNAs that have been described to date all involve a GT-AG pair of canonical splice sites, but this could be unreliable because of the detection methods employed (Jeck, W. R. et al., 2013). Moreover, some flanking introns are smaller than the average size, but most are usually longer than introns on average(Salzman, J. et al., 2013). Lastly, the length of the exons influences the circularization, and in circRNAs comprising a single exon, the given exon was found to be three fold longer compared to all expressed exons (Jeck, W. R. and Sharpless, N. E., 2014).
The lack of the free 3′ end in the circRNA molecule, which is needed for polyadenylation to take place, prevents researchers from using many molecular techniques that work by adding a poly-A tail to the RNA molecule. This makes it difficult to detect circRNAs and to explore their function. Moreover, because of the backsplicing process, the arrangements of exons are not in the expected order, and circRNAs are usually filtered out in sequencing algorithms. These problems in circRNA detection have been overcome with new bioinformatics tools such as, exonuclease-based enrichment approaches and sequencing of ribosomal RNA (rRNA)-depleted RNA libraries (instead of polyA-enriched libraries) (Jeck, W. R. and Sharpless, N. E., 2014).
Although they have been thought to be a class of non-coding RNAs, some studies have shown that these circRNAs could also act as mRNAs and be translated to produce functional proteins. Moreover this process could be tissue-dependent (Meganck et al., 2018). CircRNAs have been reported to be associated with ribosomes, and it has been reported that circRNAs from the muscleblind locus could encode a protein (Pamudurti et al., 2017). More recently, several studies have reported that dysfunction or dysregulation of circRNAs could be associated with the development of human diseases including Alzheimer’s(Akhter, 2018) and cancer (He et al., 2017). CircRNAs could act either as tumor suppressor genes or as oncogenes in cancer initiation and development. In this review we focus on new findings concerning circRNA function in gastrointestinal (GI) cancers and their potential as biomarkers and prognostic factors.
2. Circular RNA biogenesis
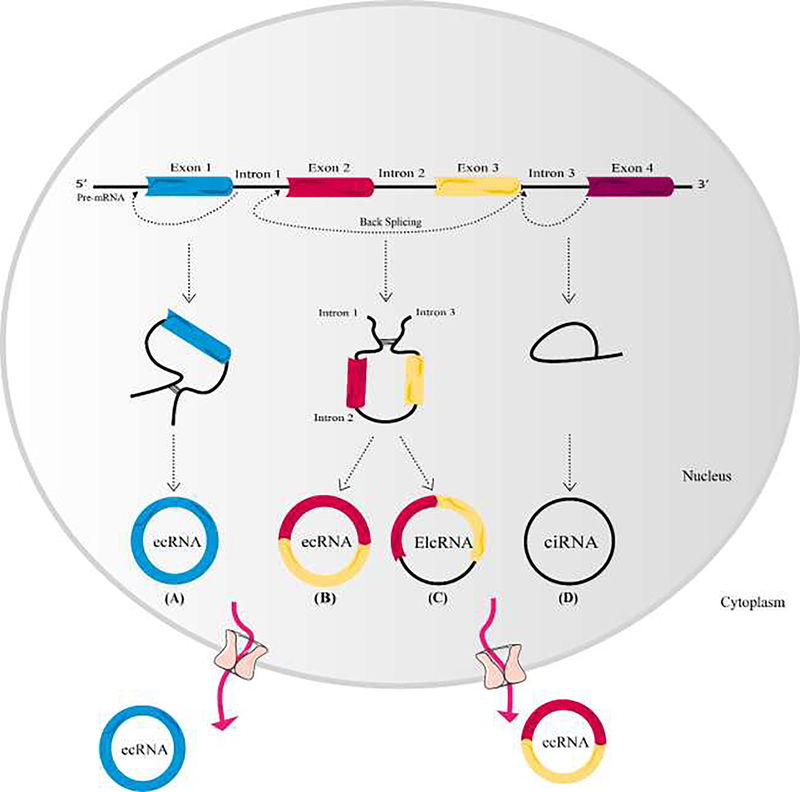
CircRNAs are produced during a unique type of splicing -called back-splicing- which is catalyzed by canonical spliceosomal machinery (Vicens and Westhof, 2014). They are formed whenthe 5’ and 3’ ends of transcribed exons and/or introns are joined covalently. During this process, down-stream splice site of donor exon/intron joins to the up-stream splice site of acceptor exon/intron in a pre-mRNA molecule (Figure 1) (Ragan et al., 2019). Several splice variants of circRNA transcripts could be generated from a single gene depending on which exons are selected by alternative back-splice site selection during the back-splicing process (Zhang et al., 2016). There are two types of alternative back-splicing; (a) 5’ back-splicing and (b) 3’ back-splicing. In the 5’ alternative back-splicing, two or more downstream splice sites are alternatively linked to the up-stream 3’ splice site. On the other hand, in the 3’ alternative back-splicing, two or more up-stream 3’ splice sites are alternatively linked to the down-stream 5’ splice site (Zhang et al., 2016). The expression level of circRNAs is regulated at three different stages, including transcription, back-splicing regulation, and circRNA turnover (Li, X. et al., 2018).
Figure 1. CircRNAs biogenesis.
Exons are shown as rectangles with different colors, and introns are depicted as black lines. Exon-derived circRNA (ecRNA) contains only exons (A and B), while circular intronic RNA (ciRNA) comprises only introns (D). In exon–intron circRNA (EIciRNA), an intron is introduced between two exons (C). The pathway by which mature ecRNAs are exported into the cytoplasm is still unclear. Some circRNAs are assumed to pass through the nuclear membrane via a nuclear pore complex.
Regulation of circRNA generation depends on cis-regulatory elements and trans-acting factors (Li, X. et al., 2018). Cis-regulatory elements are the same in all tissues and cells, and the tissue-specific pattern of circRNA expression suggests that trans-factors must be involved in regulation of circRNA expression (Zhang et al., 2016). Splicing machinery, RNA binding proteins, and repeated sequences, such as the Alu transposable element which is inverted (oriented in opposite direction) in the RNA can modulate the biogenesis of circRNA (Zhang et al., 2014). Back-splicing and linear splicing compete with each other in newly-synthesized RNA in a cell-specific manner (Ashwal-Fluss et al., 2014). CircRNAs biogenesis is not yet fully understood, but three main pathways have been identified. Exonic circRNA arise from exons and can be subdivided into two groups: single exon circRNAand multiple exon circRNA. The second group of circRNAs are exon-intron circRNAs (EIciRNA) which contain both intron and exon sequences. Intronic circRNAs originate from the introns of the underlying gene. Analysis of the splice sites in circRNAs has revealed that most exonic circRNAs contain canonical GT/AG splicing sites(Shen, T. et al., 2015). However the mechanism of splice-site selection in circularization by spliceosomes is poorly understood.
3. CircRNAs function
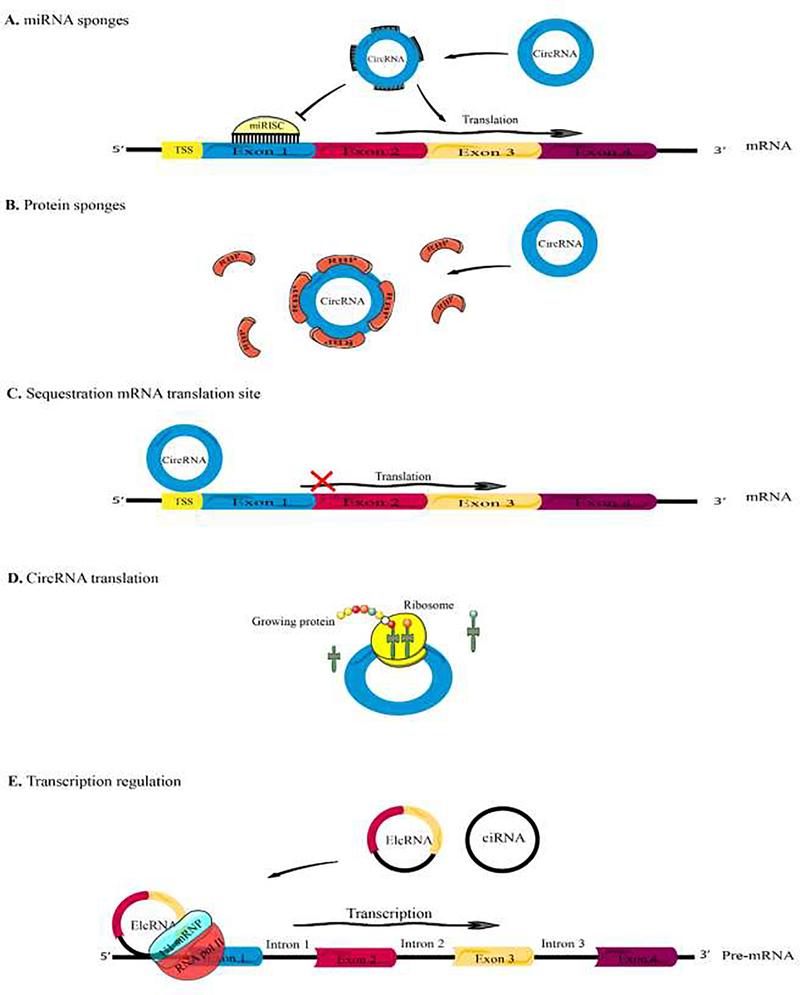
All the functions of circRNAs have not been fully elucidated, but some biological functions (especially in gene regulation) have been proposed (Figure 2). Their diversity, abundance and conservation suggest that they may play key roles in cell physiology (Lasda and Parker, 2014). Several functions such as the microRNA sponge and transcription regulators have been proposed for circRNAs (Holdt et al., 2018)
Figure 2. Schematic illustration of circRNA functions.
(A) CircRNAs might function as miRNA sponges by competing for binding of miRNA sequences, lessening the impact of miRNA-mediated regulationon gene expression. (B) CircRNAs might function as protein sponges, by binding to other RNA-binding proteins (RBPs). (C) Some circRNAs might control the expression of proteins by sequestering mRNA translation start sites. (D) CircRNAs might be translated to create functional proteins. (E) CircRNAs (e.g., EIciRNAs and ciRNAs) might interact with transcription complexes and increase the expression of their parental genes.
Based on competitive endogenous RNA (ceRNA) hypothesis, some transcripts with shared microRNA binding site compete for microRNA and post-transcriptional control (Thomson and Dinger, 2016). It was proposed that circRNAs could act as a decoy for binding to microRNAs, hence increasing the level of the microRNA target genes (Figure 3). The first example of a circRNA acting as a microRNA sponge, was circRNACDR1as which could be viewed as an extreme example as itcontained >70 microRNA binding sites for mir-7. Decreasing the level of CDR1as led to a decreased level of CDR1 mRNA (Hansen et al., 2011). This regulatory pathway was proposed to play a role in tumorigenesis and in the mammalian brain (Kleaveland et al., 2018; Xu et al., 2018). Likewise, the circular SRY contained 16 binding sites for miR-138, and it co-precipitated with Argonaute-2 (Ago-2) which is a key protein in the microRNA regulatory pathway (Hansen et al., 2013). These two are extreme examples of the microRNA sponging activity of circRNAs, and most other circRNAs contain a much smaller number of microRNA binding sites.
Some studies have reported that circRNAs can regulate gene transcription. Nuclear localized circRNAs can interact with the complex between RNA polymerase II and U1 snRNP (small nuclear ribonucleoprotein) that acts as a regulator of transcription and splicing machinery (Maiti et al., 2017). Some circRNAs regulate splicing and promote circularization thereby decreasing linear mRNA levels (Bose and Ain, 2018). CircRNAs could also regulate gene expression (and even its own expression) at a post-transcriptional level by acting as a RNA binding protein sponge. CircRNAs have some properties, which suggest they might act as scaffolds for RNA binding proteins (Dudekula et al., 2016; Zang et al., 2018). They may have roles as sequence targeting elements, which could simultaneously bind to RNA binding proteins and also to complementary sequences in target RNA or DNA molecules. However, the function of thousands of other circRNAs has not yet been elucidated and awaits further study.
4. Circular RNAs as Biomarkers
Hulka et al. have defined biomarkers as “cellular, biochemical or molecular alterations, which can be measured in biological media, including human tissues, cells, or fluids”. However, recently researchers have extended the above definition to cover biological processes, which may be objectively measured and assessed as indices of normal or pathogenic biological parameters, or responses to therapeutic interventions (Mayeux, 2004; Naylor, 2003).
Based on the National Cancer Institute, a biomarker is “a biological molecule found in blood, other body fluids, or tissues, which could be a marker of a normal or abnormal process, or of a condition or disease” such as cancer (Henry and Hayes, 2012). In general, biomarkers could differentiate an affected individual from a healthy individual (Henry and Hayes, 2012). Studies have identified a diverse range of biomarkers, such as proteins (enzymes or receptors) (Álvarez-Chaver et al., 2007; Watabe-Rudolph et al., 2012), nucleic acids (microRNAs or other non-coding RNAs) (Witwer, 2015; Zhou et al., 2015), antibodies (Schwarz et al., 2006), and peptides (Belogurov et al., 2008). Moreover, a biomarker may also consist of a set of modifications, including metabolomic and proteomic signatures, and gene expression profiles. It should be noted that many biomarkers are capable of being detected in the circulation: (serum, plasma, or whole blood); or in excretions or secretions (urine, stool, nipple discharge, or sputum). For this reason, they can be readily evaluated in a non-invasive serial manner. Other biomarkers may be only detectable in whole tissue, in which case, they would need special imaging or biopsies to be evaluated (Henry and Hayes, 2012).
Contemporary studies have shown that many features of circRNAs enable them to serve as biomarkers for some human diseases (Conn et al., 2015). One of these features is their stability. As a result of the covalently closed-loop structure with no free 5′ and 3′ ends, circRNA molecules show high resistance to the exonuclease RNase R compared to linear RNAs (Enuka et al., 2015; Memczak et al., 2013). Other studies have found that the average half-life of circRNAs in plasma exceeds 48 hours, which is much longer than the average half life of mRNAs (10 hours) (Jeck, William R and Sharpless, Norman E, 2014; Zhong, Y. et al., 2018). Another feature is universality. According to some researchers, circRNAs are among the most universal molecules found in human cells (Salzman et al., 2012). circRNAs can be more abundant than their linear isoforms under some conditions (Glažar et al., 2014). Moreover, circRNA is more abundant in the brain compared to other organs (Rybak-Wolf, Agnieszka et al., 2015). The relatively high concentration of circRNAs in the blood could be ascribed to their high stability (Li, M. et al., 2018; Memczak et al., 2015). Another advantageous feature is specificity; that is, the circRNAs are expressed in a tissue-specific and developmental stage-specific manner. This characteristic makes circRNAs useful biomarkers for particular diseases. Several studies have found that circRNAs are differentially expressed between cancerous and noncancerous tissues (Memczak et al., 2013) and that there identity and abundance are also distinctive of cancer cells (Floris et al., 2017; Salzman, Julia et al., 2013). Finally, circRNAs are strongly conserved across various species, meaning that a number of circRNA biomarkers that have been detected in murine models are also candidates for translation to clinical applications in humans (Jeck, William R et al., 2013).
5. GI cancers and circRNAs
The aberrant expression of circRNAs has been linked to many human diseases including cancer (He et al., 2017). CircRNAs are becoming an interesting subject in cancer research due to their abundance, stability and regulatory function. To date some circRNAs have been implicated with several hallmarks of cancer, such as cell death and survival, invasion, metastasis and angiogenesis (Kristensen et al., 2018). These circRNAs can be detected in body fluids such as saliva and blood, and also in exosomes, suggesting that they could be used as non-invasive biomarkers in cancer detection (Bahn et al., 2015; Memczak et al., 2015; Qian et al., 2018; Wang et al., 2016). The role of circRNAs in GI cancers remains unknown, but some studies have shown that they are differentially expressed, and could be linked with prognosis, stage determination, and 5-year survival. The results have similarities and differences between studies, which could be due to different sample size, expression analysis methods, and subject status or race. Here we briefly review the current knowledge of circRNAs in different GI cancers.
5.1. Role of circRNAs in gastric cancer
Gastric cancer (GC) is an aggressive disease and despite declining in prevalence in recent decades, due to improved nutrition, better food preservation, reduction of H. pylori infection, and earlier diagnosis, it still remains the fourth most frequent malignancy worldwide, with a poor prognosis (Ferro et al., 2014). GC is the result of a combination of environmental factors and an accumulation of genetic alterations (Carcas, 2014; Sitarz et al., 2018). This cancer is usually diagnosed at advanced stages, and unfortunately the treatment of advanced and metastatic cancers has made little progress with a median survival only around 1 year (Cervantes et al., 2013).
CircRNAs have been proposed to have a key role in GC carcinogenesis through functioning as a sponge for microRNA and interacting with RNA binding proteins. To date there have been 13 reported circRNAs which can act as microRNA sponges in gastric cancer, some of them can act as tumor suppressors, while others could act as oncogenes in GC progression (Table 1) (Wang and Dong, 2019). The microarray expression level analysis of circRNAs in 5 samples of GC in comparison with adjacent non-tumor tissues showed different expression patterns of 713 circRNAs in GC. In GC tissues, 522 circRNAs were down-regulated whereas 191 were up-regulated, and among them,hsa_circ_0076305, hsa_circ_0035431, and hsa_circ_0076304showed the greatest alteration levels. Pathway analysis of differentially expressed circRNAs revealed that they were mostly related to carcinogenesis (Dang et al., 2017). Shen et al. showed 603 down-regulated and 347 up-regulated circRNAs in GC tissue in comparison to healthy gastric tissue. Further analysis showed that these circRNAs expression levels did not relate to their host gene linear mRNA levels, suggesting a different mechanism of regulation in the circularization process (Shen et al., 2018). CircRNA expression analysis using a circRNA chip on 8 GC and normal paired tissue samples identified 1285 differentially expressed circRNAs, 594 were down-regulated and 691 were up-regulated. Functional analysis of these circRNAs showed that 69 circRNAs could potentially sponge microRNAs and therefore regulate the target mRNAs. They also suggested that cancer-related genes such as CD44, CXXC5, MYH9 and MALAT1 could be regulated in GC development through the interaction of circRNA-miRNA-mRNA pathways (Sui et al., 2017). Another study revealed 16 up-regulated and 84 down-regulated circRNAs in GC. Prediction of interactions between circRNAs and miRNAs targeting specific genes revealed that hsa-circ-0026 probably regulates gene silencing, gene expression, RNA metabolism, RNA transcription and other biological activities relevant to GC. They also suggested that hsa_circ-0026 could be a potential biomarker for diagnosing GC as well as for its targeted therapy (Chen, J. et al., 2017). In this report, uni-variate and multi-variate Cox proportional hazard models were used to assess whether circPVT1 levels were able to predict survival independently from other pathological and clinical parameters in GC patients. In addition, if the expression levels of circPVT1 and TNM phase were combined, they could provide a better prognostic indicator compared to the TNM phase alone. This study also examined the clinical importance of linear PVT1 expression. Differently from circPVT1, patients showing lower levels of PVT1 exhibited better DFS and OS in comparison to the patients having higher levels of PVT1. When the combined levels of circPVT1 and PVT1 were examined, patients with lower levels of circPVT1 and higher levels of PVT1 showed a considerably shorter DFS and OS compared to patients having higher levels of circPVT1 and lower levels of PVT1 (Chen, J. et al., 2017).
Table 1.
CircRNAs which act as microRNA sponges in gastric cancer
| CircRNA | Target | Model (In vitro, in vivo, Human) | Type of cell line | Ref |
|---|---|---|---|---|
| Hsa_circ_0000993 | miR-214–5p | Human | - | (Chen, H. et al., 2018) |
| Has_circ_0001461 | miR-548g, RUNX1 in the cytoplasm; YBX1 in the nucleus | Human, In vitro | GSE-1, SGC-7901, BGC-823, MKN-28, AGS, MGC-803, MKN-45 | (Fang et al., 2019) |
| Hsa_circ_0027599 | miR-101, PHLDA1 | Human, In vitro | SGC-7901, MGC-803, HGC-27, MKN-45, MKN-28 | (Wang, L. et al., 2018) |
| CircRNA_001569 | miR-145, NR4A2 | Human, In vitro | ... | (Shen et al., 2019) |
| CiRS-7 | miR-7, PTEN/PI3K/AKT pathway | In vitro | MGC-803, HGC-27, GES-1 | (Pan et al., 2018) |
| CircRNA_101057 | miR-424, LATS1 | Human, In vitro | SGC-7901, MKN-45, MKN-28, HGC-27, MGC-803, AGS, BGC-823, GES-1 | (Zhang, J. et al., 2017) |
| CircRNA_100269 | miR-630 | In vitro | AGS, MKN28, MKN45, BGC823, MGC803, SGC7901, GES1 | (Zhang, Y. et al., 2017) |
| CircZFR | miR-130a/miR-107, PTEN | In vitro | AGS, AZ521, HGC-27, GES-1 | (Liu, T. et al., 2018) |
| Has_circ_0002320 | miR-367–5p, p27 | In vitro | HGC-27, GES-1, MKN-45, | (Liu, H. et al., 2018) |
| Hsa_circ_0017639 | miR-182–5p, CREB1 | In vitro | MKN-45, BGC-823, MGC-803, SGC-7901 AGS | (Sun, H. et al., 2018b) |
| CircPDSS1 | miR-186–5p, NEK2 | Human, In vitro | MGC-803, HGC-27, BGC-823, GES-1 | (Ouyang et al., 2019) |
| CircRNA_0000284 | miR-124 and miR-29b, COL1A1, COL4A1 and CDK6 | In vitro | XGC-1, XGC-2, MGC-803, BGC-823 | (Cheng et al., 2018) |
| CircNF1 | miR-16, MAP7 and AKT3 | ... | (Wang, Z. et al., 2018) | |
| Hsa_circ_0058092 | PODXL | Human | - | (Bu et al., 2019) |
| circ_0001546 | miR-421, ATM/checkpoint kinase 2 (Chk2)/p53-dependent signaling pathway | In vitro | HGC-27, L-OPH | (Wu, Q. et al., 2019) |
| RNA circNHSL1 | miR-1306–3p/SIX1/vimentin axis | Human, In vitro | MKN-28, AGS, MKN-45, BGC-823, MGC-803, HGC-27, SGC-7901, GES-1 | (Zhu, Z. et al., 2019) |
| Hsa_circ_101882 | EMT signaling pathway | Human, In vitro | MGC-803, HGC-27, SGC-7901, MNK-45, GES | (Yin et al., 2019) |
| circHECTD1 | miR-1256 and activating β-catenin/c-Myc signaling | In vivo | BGC823, MKN45, HGC27, AGS, MGC803, SGC7901, GES-1 | (Cai, Juan et al., 2019) |
| Circ_SPECC1 | miR-526b on downstream KDM4A/YAP1 pathway | In vitro | AGS, BGC-823, HGC-27, MGC-803, MKN-45, SGC-7901, GES-1 | (Chen, L.-h. et al., 2019) |
| hsa_circ_0067582, hsa_circ_0005758 | CEA level and stages | Human | - | (Lu, R. et al., 2019) |
| miRNA-340 | tumorigenesis and invasion | Human, In vitro | HFE145, BGC-823 | (Wang, J. et al., 2019) |
| circ-NOTCH1 | sponge of miR-637, expression of Apelin | Human, In vitro | GC | (Guan et al., 2019) |
| circ-DCAF6 | sponging miR-1231 and miR-1256 | Human | - | (Wu, L. et al., 2019) |
736 unique annotated circRNAs were detected by RNA sequencing in several types of gastric tissues. Further analysis found that five microRNAs could be regulated by 5 differentially expressed circRNAs out of the 736 totals (Vidal et al., 2017). All of these circRNAs were previously shown to play roles in GC (Chen, J. et al., 2017; Shao et al., 2017; Tian et al., 2018).
In GC cells, hsa_circ_0000993 inhibits invasion as well as proliferation through sponging miR-214–5p(Zhong, S. et al., 2018). Hsa_circ_000146 is negatively associated with survival in GC patients, and could sponge miR-548g, which is a tumor suppressor microRNA (Hu et al., 2014). miR-548 directly targets mRNA, and could reverse the effects of hsa_circ_000146 overexpression, such as invasion, migration, and proliferation of GC cells.miR-548 directly targets runt-related transcription factor 1 (RUNX1),which controls genes expression implicated in the regulation ofcell cycle, the p53 and transforming growth factor β (TGF-β)signaling pathways(Cai et al., 2015; Chuang et al., 2013; Sood et al., 2017). Therefore, hsa_circ_000146 was revealed to act as a tumor suppressor viamodulatingthemiR-548/RUNX1 axis in GC cells (Fang et al., 2019). It has been also demonstrated that hsa_circ_0027599could sponge miR-101–3p, and its overexpression inhibited proliferation and metastasis in GC cells. Pleckstrin homology-like domain family A member 1 (PHLDA1) overexpression, a direct target of miR-101, decreased the growth and migration of GC cells(Wang, L. et al., 2018). Some studies reported pro-apoptotic and anti-proliferative roles ofPHLDA1, which is involved in drug resistance in cancer (Fearon et al., 2018; Nagai, 2016). The result of another study was in contradiction with the above results, since it reported that miR-101 overexpression inhibited invasion and proliferation in the AGS gastric cancer cell line(Wu et al., 2017). It seems that clarification of the role of miR-101 in GC needs more investigation, and it is worth distinguishing between the modulatory and regulatory functions of circRNAs. Knockdown of hsa-circ_001569, which sponges miR-145(a known tumor suppressor gene in human cancer) decreased cell viability and promoted apoptosis, while it led to increased miR-145 levels(Shen et al., 2019).
CircRNAs could sponge RNA binding proteins and decrease their availability within the cell. A down-regulated circRNAin GC cells called hsa_circ_104916 could suppress proliferation, invasion and migration by decreasing the expression of Slug, a transcription factor suppressing the expression of E-cadherin, and hence modulates the epithelial–mesenchymal transition (EMT)(Li, J. et al., 2017). Hsa_circ_104916 overexpression up-regulated E-cadherin, which could be due to downreglation of Slug (Li, J. et al., 2017).
Recent work has revealed the different expression of circRNAs in GC, and some studies have suggested specific circRNAs which could be used as biomarkers in GC diagnosis (Table 2). Hsa_circ_0000190 expression level was evaluated in 104 GC and adjacent non-tumor samples, and also in plasma samples from 104GC patients and normal subjects. The results showed down-regulation of this circRNA in GC, which could differentiate patients from normal samples and subjects with specificity and sensitivity values of 0.750 and 0.712 respectively (Chen, S. et al., 2017). Hsa_circ_002059 expression level was proposed as a biomarker for GC progression, and it was down-regulated in plasma samples from 36 post-operative patients in comparison to pre-operative plasma samples. According to the findings, Hsa_circ_002059 was down-regulated in gastric cancer tissues in comparison to the adjacent non-cancerous tissue. Moreover, it was found that the levels of hsa_circ_002059 in plasma collected from post-operative gastric cancer patients were significantly different from the levels obtained from pre-operative gastric cancer patients. Lower levels of expression showed a significant correlation with distant metastasis, TNM stage, and patient age (Li, P. et al., 2015). Hsa_circ_0003159 expression was evaluated in 108 paired GC samples, and it was suggested that its down regulation could be a biomarker in GC(Tian et al., 2018). Hsa_circ_0000467 was reported to promote cancer progression and was upregulated in GC cell lines, tissues, and plasma samples from GC patients. It was proposed as a promising non-invasive marker for GC prognosis as well as diagnosis. Findings also showed significant upregulation of Hsa_circ_0000467 in GC tissues in comparison to adjacent non-tumor tissues. The same results were observed in the MGC-803, HGC-27, NUGC-3, AGS, and GES-1 cell lines. and in samples of plasma from GC patients. Results showed that the area under the ROC curve of hsa_circ_0000467 was 0.790, which was higher than widely utilized biomarkers such as CA-724 and CEA. In addition, the levels of hsa_circ_0000467 expression were significantly reduced after surgical operation. Furthermore, a close relationship was found between the expression levels of hsa_circ_0000467 and the TNM phase. Cox multivariate analysis also suggested hsa_circ_0000467 could be a new independent prognostic marker. In-vitro experiments showed that proliferation, migration, and invasion of GC cells were considerably suppressed by knockdown of hsa_circ_0000467. In addition, hsa_circ_0000467 silencing resulted in increased tumor cell apoptosis in-vitro. Therefore, it was suggested that Hsa_circ_0000467 could function as a novel non-invasive biomarker in GC, and could also be a therapeutic target for GC (Lu, J. et al., 2019). Hsa_circ_KIAA1244 was down-regulated in GC patient plasma, tissues and exosomes, and was proposed as a diagnostic marker for GC. Its expression status was related to TNM stage, metastasis and shorter survival rates (Tang et al., 2018).
Table 2.
CircRNAs as biomarkers in gastric cancer
| CircRNA | Expression status | Target | Model (In vitro, in vivo, Human) | Type of cell line | Ref |
|---|---|---|---|---|---|
| Hsa_circ_0003159 | Down | - | Human | - | (Tian et al., 2018) |
| Hsa_circ_0001895 | Down | t-CEA | Human, In vitro | SGC-7901, AGS, HGC-27, MGC-803,, BGC-823, | (Shao et al., 2017) |
| Hsa-circ-104916 | Down | EMT | Human, In vitro | AGS, MKN-28, NCI-N87, MKN-45, GES-1 | (Li, J. et al., 2017) |
| Hsa_circ_0000190 | Down | - | Human | - | (Zhang, J. et al., 2017) |
| Hsa_circ_002059 | Down | - | Human | - | (Li, P. et al., 2015) |
| Hsa_circ_0000467 | Up | TNM stage | Human, In vitro | HGC-27, MGC-803, AGS, NUGC-3, GES-1 | (Lu, J. et al., 2019) |
| CircSMARCA5 | Down | Human, In vitro | MGC803, MKN45, MKN74, AGS, BGC823, SGC7901, GES-1 | (Cai, J. et al., 2019) | |
| Hsa_circ_0000181 | Down | Carbohydrate antigen, CEA | Human | - | (Zhao et al., 2018) |
| Hsa_circ_0000520 | Down | Human, In vitro | MKN-45, BGC-823, MGC-803, AGS | (Sun, H. et al., 2018a) | |
| Hsa_circ_0000745 | Down | CEA | Human | - | (Huang, M. et al., 2017) |
| Hsa_circ_00001649 | Down-regulated | PCHNTs | Human | - | (Li, W.H. et al., 2017) |
| Hsa_circ_0006633 | Down | CEA | Human, In vitro | HGC-27, SGC-7901, MGC-803, AGS, GES-1 | (Lu et al., 2017) |
| Hsa_circ_0066444 | Up | - | Human, In vitro | MKN-45, BGC-823, MGC-803, AGS, GES-1 | (Rong et al., 2018) |
| Hsa_circ_0074362 | Down | - | Human, In vitro | GES-1, AGS, BGC-823, HGC-27, MGC-803, SGC-7901 | (Xie et al., 2018) |
| Hsa_circ_0001017, Hsa_circ_0061276 | Down | - | Human, In vitro | BGC-823, MGC-803, SGC7901, GES-1 | (Li, T. et al., 2018) |
| Hsa-circ_0000096 | Down | CDK6, MMP-2, MMP-9, Ki67, VEGF | Human, In vitro, In vivo | AGS, BGC-823, HGC-27, SGC-7901, MGC-803 GES-1, And mouse model | (Li, P. et al., 2017) |
| circPVRL3 | Down | Human, In vitro | MKN-45, MGC-803, SGC-7901, AGS, GES-1 | (Sun, H.D. et al., 2018) | |
| hsa_circ_0103398, hsa_circ_0127859 hsa_circ_0103398, hsa_circ_0127859 | Up | - | Human | - | (Wang, F. et al., 2019) |
| hsa_circ_0005654 | Down | - | Human | - | (Wang, Y. et al., 2019) |
| hsa_circ_0000144 | Up | ErbB | Human, In vitro | MGC-803, line GES-1 | (Wei et al., 2019) |
| hsa_circ_0006848 | Down | CEA | Human | - | (Lu, Jun et al., 2019) |
| Hsa_circ_0065149 | Down | Human | - | (Shao et al., 2019) | |
| hsa_circ_006100 | Up | miR-195/GPRC5A signaling | In vivo | KN-45, MGC-803, AGS and SGC-7901, GES-1-T | (Liang et al., 2019) |
| hsa_circ_0001821 | Down | Human, In vitro | SGC-7901, HGC-27, BGC-823, AGS, MKN-1, GES-1 | (Kong et al., 2019) |
5.2. Role of circRNAs in colorectal cancer
Colorectal cancer (CRC) accounts for 10% of cancer-related deaths, and is the second and third most frequent malignancy among women and men, respectively(Marley and Nan, 2016). CRC prevalence has been increasing in recent decades mainly because of changes in life style, obesity, low physical activity, smoking, and dietary habits (Kuipers et al., 2015; Marmol et al., 2017). Most of the hereditary forms of CRC are caused by mutations in genes, including EPCAM,PMS2, MSH6, MSH2or MLH involved in DNA mismatch-repair system(Tiwari et al., 2016), and the adenomatous polyposis coli (APC) gene which handles the Wnt signaling pathway)(Vasen et al., 2015). Of note, polyposis is related to mutations in the mutY DNA glycosylase (MUTYH) gene (Kashfi et al., 2013; Markkanen et al., 2013).
The association between CRC and circRNAs was reported for the first time, with the identification of a circular transcript of the Deleted in CRC (DCC)gene (Nigro et al., 1991). As its name suggests the deletion of the DCC gene in CRC had been known for more than two decades (Fearon and Pierceall, 1995). Bachmayr-Heyda and colleagues (Bachmayr-Heyda et al., 2015)indicated the global decrease of the expression of circRNAs in tumor tissues and CRC cell lines in comparison with normal colonic mucosa. They found 39 circRNAs differentially expressed between CRC samples and normal mucosa. Of these, 28 circRNAs were down-regulatedand11 circRNAs were up-regulated. Interestingly, this data agreed with a recent study that also reported the global decrease of circRNAs in CRC tissues and cell lines (Taborda et al., 2017). It was hypothesized that circularization is less functional in tumors than in normal tissues, and this could be due to onco-micoRNAs which are up-regulated in CRC (Ragusa et al., 2015). CircRNA sequencing data in two CRC cell lines,SW620(a metastasis-derived cell line)and SW480 (a primary tumor cell line), and abnormal colorectal cell, NCM460,showed an overall circRNA abundance decreasein CRC cell lines in comparison to normal colonic epithelial cells. 2,919 circRNAs that were differentially expressed between CRC cells and normal cells were identified, and it was also found that circRNAs in CRC cells were usually shorter than those in normal cells (Jiang, W. et al., 2018).
The circRNAs expression profile from three paired CRC samples and adjacent non-tumor tissues revealed 136 significantly over-expressed circRNAs and 243 down-regulated circRNAs in CRC tissues. Circ-BANP was up-regulated in 35 CRC samples and its knockdown attenuated the proliferation of CRC cells (Zhu, M. et al., 2017). CircRNA-seq analysis in 40 samples of CRC and CRC with liver metastasis (CRC-m) revealed 113 differentially expressed circRNAs, of which 92 circRNAs were up-regulated and 21 circRNAs were down-regulated.Hsa_circRNA_0001178 and hsa_circRNA_0000826wereconsiderably up-regulated in CRC-m tissues. In order to assess the utility of circRNAs as diagnostic biomarkers, the ROC curves of circRNA_0000826 and circRNA_0001178 were analyzed in CRC-m patients with liver metastasis. The results showed increased expression levels of circRNA_0000826 and circRNA_0001178 in patients with CRC-m. The ROC curves were analyzed for assessing the diagnostic value of both circRNAs in CRC-m patients. The analysis showed that AUC was 0.816 for circRNA_0000826 and 0.945 for circRNA_0001178. The mentioned circRNAs could be promising markers for the diagnosis of liver metastasis from CRC (Xu, H. et al., 2019).
Expression pattern analysis has shown significantly higher hsa_circ_001569 expression in CRC, which was related to aggressive features such as metastasis (Taborda et al., 2017). Over-expression of hsa_circ_001569 increased invasion as well as proliferation in the CRC cell lines, while its suppression showed a remarkable decrease in invasiveness and proliferation rate. It has also been shown that hsa_circ_001569 sponges miR-145 leading to increased protein levels of formin like 2 (FMNL2), “B-cell lymphoma 2 associated athanogene 4” (BAG4), and E2F5 (Taborda et al., 2017). E2F5 is a transcription factor regulating the expression of genes implicated in the cell cycle (Jiang et al., 2011). BAG4 and FMNL2 are implicated in invasion and growth as well as metastasis (Annunziata et al., 2007; Li et al., 2010; Liang et al., 2013). MiR-145 has been reported to be correlated with survival in patients with CRC (Li et al., 2012), and also inhibits invasion and migration in CRC cell lines (Sheng et al., 2017). Hsa_circ_001988 down-regulation was reported in CRC tissues compared with adjacent normal mucosa in 62 patients, and its expression level was related to differentiation and invasion (Wang, X. et al., 2015). Hsa_circ_0000069 was significantly up-regulated in 30 paired CRC samples and non-tumor adjacent tissues (Guo, J.N. et al., 2016). The knockdown of hsa_circ_0000069 can inhibit invasion, migration, and proliferation of tumor cells. CircCCDC66 expression was elevated in CRC and was associated with poor prognosis. CircCCDC66 could function as an oncogene and its knockdown significantly reduced cell migration and metastasis (Hsiao, K.Y. et al., 2017). Evaluation of ciRS-7-A in 44 matched healthy mucosal samples and 153 primary CRC tissues revealed that this biomarker was remarkably up-regulated in cancer tissues(Weng et al., 2017). CiRS-7 over-expression led to miR-7 suppression, and the RAF1 and EGFR oncogene activation, and was also correlated with poor patient survival. Cir-ITCH was found to be down-regulated in 45 CRC tissues in comparison to adjacent non-tumor tissues (Huang, G. et al., 2015). Functional analysis showed that cir-ITCH increases ITCH levels implicated in the Wnt/β-catenin pathway suppression. Therefore, cir-ITCH may play a key role in CRC through modulating the Wnt/β-catenin pathway.
A summary of the circRNAs that have been reported to be involved with CRC is shown in Table 3, and 4.
Table 3.
CircRNAs related to colorectal cancer
| Circular RNA | Expression in CRC | Target | miRNA sponge | Model/Source | Type of cell line | Ref |
|---|---|---|---|---|---|---|
| has_circ_103809 | Down | FOXO4 | miR-532–3P | In vitro, Human/CRC tissues (n=30) | SW620, HCT116, COCA-2, HT29 | (Bian et al., 2018) |
| circITCH | Down | Wnt/β-catenin signaling pathway | miR-214 | In vitro, Human/CRC tissues (n=45) | HCT116, SW480 | (Huang, Guanli et al., 2015) |
| hsa_circ_0000523 | Down | Dkk1 | miR-31 | In vitro | COLO205, COLO320HSR, DLD-1, HCT-15, HCT-8, SW480, SW620, SW1116, HT-29, LoVo, NCM460, FHC, LS 174T, Caco-2 | (Jin et al., 2018) |
| circDDX17 (hsa_circ_0002211) | Down | - | - | Human/CRC tissues (n=60) | - | (Li, X.N. et al., 2018) |
| hsa_circ_0001649 | Down | - | - | In vitro, Human/CRC tissues (n=64), Serum (n=18) | HCT116 | (Ji, W. et al., 2018) |
| hsa_circ_0000711 | Down | - | - | In vitro, Human/CRC tissues (n=101) | HCT116, H-T29, COLO205 | (Li, Jinyun et al., 2018) |
| hsa_circ_0000567 | Down | - | - | Human/ In vitro/CRC tissues (n=102) | SW480, SW620, RKO, HCT116, CACO2 | (Wang, J. et al., 2018) |
| hsa_circ_0026344 | Down | - | miR-31, miR-21 | In vitro, Human/CRC tissues (n=32) | SW620, HCT116, HT29, SW480 | (Yuan, Yuan et al., 2018) |
| hsa_circ_104700 | Down | - | - | Human/CRC tissues (n=170) | - | (Zhang, Peili et al., 2017) |
| circITGA7 | Down | NF1 | miR-370–3p | Human/CRC tissues (n=69) | - | (Li, Xiaomin et al., 2018) |
| hsa_circ_0014717 | Down | p16 | - | In vitro, Human/CRC tissues (n=46) | HCT116, SW480, HT29 | (Wang, Feng et al., 2018) |
| hsa_circ_001988 | Down | - | - | Human/CRC tissues (n=62) | - | (Wang, Xuning et al., 2015) |
| hsa_circ_0003906 | Down | - | - | In vitro, Human/CRC tissues (n=122) | HCT8, SW480, LoVo, HCT116, HT29, SW620 | (Zhuo, Fan et al., 2017) |
| circACAP2 | Up | Tiam1 | miR-21–5p | In vitro, Human/CRC tissues (n=21) | SW480 | (He et al., 2018) |
| hsa_circ_001569 | Up | E2F5, FMNL2, BAG4 | miR-145 | In vitro, Human/CRC tissues (n=30) | SW480, HCT116 | (Shen, H. et al., 2015) |
| hsa_circ_0020397 | Up | TERT PD-L1 | miR-138 | In vitro | SW480, LoVo, SW620, HCT116 | (Zhang, X.l. et al., 2017) |
| hsa_circ_0071589 | Up | EZH2 | miR-600 | In vitro, Human/CRC tissues (n=40) | HCT116 | (Yong et al., 2018) |
| hsa_circ_0000069 | Up | - | - | In vitro, Human/CRC tissues (n=30) | HCT-116, HT-29, SW480, LoVo | (Guo, J.-n. et al., 2016) |
| hsa_circ_0007534 | Up | - | - | Human/CRC tissues (n=33) | - | (Zhang, R. et al., 2018) |
| hsa_circ_000984 | Up | CDK6 | miR-106b | In vitro, Human/CRC tissues (n=32) | HT29, W480, SW620 | (Xu, X.W. et al., 2017) |
| hsa_circ_0055625 | Up | ITGB8 | miR-106b-5p | Human/CRC tissues | - | (Zhang, J. et al., 2019) |
| circBANP | Up | p-Akt | - | In vitro, Human/CRC tissues (n=35) | HT29, HCT116 | (Zhu, Mingchen et al., 2017) |
| ciRS-7 | Up | EGFR/RAF1/MAPK pathway | miR-7 | In vitro, Human/CRC tissues (n=44) | HT29, HCT116 | (Tang et al., 2017) |
| circRNA_100290 | Up | FZD4 | miR-516b | In vitro, Human/CRC tissues (n=24) | HT29, HCT116, SW620, SW480 | (Fang et al., 2018) |
| hsa_circ_0136666 | Up | SH2B1 | miR-136 | In vitro | Cell line | (Jin et al., 2019) |
| circHIPK3 | Up | FAK, YY1, EGFR, IGF1R | miR-7 | In vitro, Human/CRC tissues (n=178) | HCT116, SW620, HT29, DLD1, SW480 | (Zeng, K. et al., 2018) |
| circCCDC66 | Up | - | - | In vitro, Human/CRC tissues (n=48) | HCT116, HT-29 | (Hsiao, K.-Y. et al., 2017) |
Table 4.
CircRNAs as biomarkers in colorectal cancer
| circRNAs | Dysregulation | miRNA sponge | Related molecules or pathways | Model | Type of cell line | Ref |
|---|---|---|---|---|---|---|
| hsa_circRNA_104700 | Down | - | - | Human | - | (Zhang, P. et al., 2017) |
| circRNA0003906 | Down | - | - | Human In vitro | SW480, HCT8, SW620, LoVo, HT29, HCT116 | (Zhuo, F. et al., 2017) |
| circ_0026344 | Down | miR-31, miR-21 | - | Human | - | (Yuan, Y. et al., 2018) |
| hsa_circ_001988 | Down | - | - | Human | - | (Wang, Xuning et al., 2015) |
| hsa_circ_0000567 | Down | - | - | Human In vitro | SW480, SW620, RKO, HCT116, CACO2 | (Wang, J. et al., 2018) |
| Hsa_circ_0014717 | Down | - | p16 | Human In vitro | HCT116, SW480, HT29 | (Wang, F. et al., 2018) |
| hsa_circ_0000711 | Down | - | - | Human In vitro | HCT116, HT29, COLO205 | (Li, Jinyun et al., 2018) |
| circITGA7 | Down | miR-307–3p | ITGA7 | Human In vitro | SW480, Caco-2, RKO, SW620, HCT116, LoVo, DLD1 | (Li, Xiaomin et al., 2018) |
| hsa_circ_0001649 | Down | - | - | Human In vitro | H116 | (Ji, Wenxin et al., 2018) |
| hsa_circ_0136666 | Up | miR-136 | SH2B1 | Human In vitro | LoVo, DLD1, SW480, SW620, HCT116, HCT8, HT29 | (Jin et al., 2019) |
| circCCDC66 | Up | - | - | Human In vitro | - | (Hsiao, K.-Y. et al., 2017) |
| circRNA_100290 | Up | miR-516b | FZD4 | Human In vitro | HCT116, HT29, SW480, SW620 | (Fang et al., 2018) |
| circHIPK3 | Up | miR-7 | FAK, YY1, IGF1R, EGFR | Human In vitro | HT29, HCT116 | (Zeng, Kaixuan et al., 2018) |
| ciRS-7 | Up | miR-7 | EGFR/RAF1 | Human In vitro | HCT116, HT29 | (Weng et al., 2017) |
5.3. Role of circRNAs in esophageal cancer
Esophageal cancer (EC) has an overall five-year survival ranging from 15% to 20%, and is the eighth most frequent malignancy in the world, and the sixth most prevalent cause of cancer-related mortality. The two histological subtypes of EC are squamous cell carcinoma and adenocarcinoma, that each has varying geographical and racial distribution (Abbas and Krasna, 2017; Sohda and Kuwano, 2017). The risk factors for EC are abuse of tobacco and alcohol, obesity, diet, race and gender (Domper Arnal et al., 2015). EC continues to be a generally fatal disease with only a modest improvement in survival, and surgery still plays the main role in EC treatment (D’Journo and Thomas, 2014). The poor prognosis in EC is mainly because of late diagnosis, and more than 50% of patients already had metastatic tumor at the time of diagnosis (D’Journo and Thomas, 2014). Identifying a biomarker for the detection of EC at an early stage is crucial to improve disease management.
Accumulating evidence suggests that circRNAs have a crucial role in EC (Table 5). Microarray analysis of circRNAs in esophageal squamous cell carcinoma (ESCC) and adjacent-cancer tissues in three patients revealed 3,288 differentially expressed circRNAs of which 2,139 were up-regulated, and 1,149 were down-regulated. Further network analysis of circRNAs-mRNA-microRNA interactions showed that 32 differentially expressed circRNAs and 98 differentially expressed mRNAs were linked to 64 miRNAs in ESCC (Jiang, C. et al., 2019). The expression pattern of the circRNAs revealed that 1045 were up-regulated and 1032 were down-regulated in 3 pairs of frozen tumor and non-tumor ESCC tissues. Three circRNAs including hsa_circ_0043603, hsa_circ_0001946, andhsa_circ_0062459 were detected in plasma, and the combination of hsa_circ_0043603 and hsa_circ_0001946 could be utilized as a diagnostic biomarker with sensitivity of 0.928 and specificity of 98%.The findingsdemonstratethat these two circRNAs could be utilized as diagnostic biomarkers.
Table 5.
circRNAs and esophageal cancer
| CircRNAs | Dysregulation | Target | Model (In vitro, in vivo, Human) | Type of cell line | Ref |
|---|---|---|---|---|---|
| circPVT1 | Up | Paxs, PPARs | Human, In vitro | EC109, CaES-17, TE-1, TE-10,HEEC, HepG2, MKN45, SW60, A549 | (Zhong et al., 2019) |
| hsa_circ_0006168, miR-100 | Down | Human, In vitro | TE13, ECA109, Kyse150, Kyse450, Kyse510, Het-1A | (Shi et al., 2019) | |
| Hsa_circ_0004370 | Up | miR-1294/LASP1 pathway | Human, In vitro | Eca-109,TE-1, KYSE-150, Het-1A | (Zhang, Z. et al., 2019) |
| CircRNA_000543 | Up | miR-9 | Human, In vitro | NPC, CNE1, 5e8F | (Chen, L. et al., 2019) |
| has_circ_0067934 | Up | Human, In vitro | TE-13, KYSE-410, ECA-109, TE-1, HEEC | (Xia et al., 2016) | |
| circ-SMAD7 | Up | Human, In vitro | KYSE30, KYSE 70, KYSE 140, KYSE 150, KYSE 180, TE10, TE12, HET-1-A | (Zhang, Y. et al., 2019) | |
| ITCH | - | Wnt/β-catenin pathway | Human, In vitro | Eca-109, TE-1 | (Li, F. et al., 2015) |
| Circ-TTC17 | Up | Human, In vitro | HET-1A, KYSE30, KYSE450 | (Wang, Q. et al., 2019) |
A circular RNA micro-array was employed to analyze three pairs of ESCC frozen tumor and non-tumor tissue in order to detect ESCC-associated circRNAs. A total of 1045 up-regulated and 1032 down-regulated circRNAs were observed, among which six circRNAs (hsa_circ_0062459, hsa_circ_0076535, hsa_circ_0072215, hsa_circ_0042261, hsa_circ_0001946, and hsa_circ_0043603) were confirmed by qRT-PCR analysis. Because blood is the most widely utilized sample in the laboratory, these six circRNAs were also investigated in blood as diagnostic biomarkers. The results indicated there were no detectable levels of hsa_circ_0042261, hsa_circ_0072215, or hsa_circ_0076535 in the plasma or serum of either patients or healthy individuals. ROC curve analysis was used to assess the specificity and sensitivity of the other three circRNAs. The AUCs of hsa_circ_0001946 and hsa_circ_0062459 respectively were 0.894 (sensitivity=92%, specificity=80%) and 0.836 (sensitivity=64%, specificity=92%) respectively. A logistic regression analysis was run to establish the whether a combination of the expression levels of both circRNAs in plasma was useful. The formula was established as 3.272-(0.465*level of hsa_circ_0001946) – (1.706* level of hsa_circ_0062459). Logistic regression analysis showed better diagnostic precision with the combination AUC, specificity of 98% and sensitivity of 0.928. Moreover, hsa_circ_0001946 over-expression decreased invasion, migration, and proliferation in cell lines (Fan et al., 2019).
Another study reported that 267 circRNAs revealed considerably different levels of expression in ESCC tissues from five patients and adjacent non-tumor tissues (Jiang, C. et al., 2019). Of these circRNAs, 92 were up-regulated and 175 were down-regulated. Hsa_circRNA_406826and hsa_circRNA_101125 were proposed as novel potential markers for ESCC treatment and diagnosis. Another study identified 813 significantly up-regulated and 445 down-regulated circRNAs in normal epithelial and malignant esophageal cell lines(Sun et al., 2017). The five most up-regulated circRNAs were ciRNA11, circRNA904, circRNA3594, ciRNA101, circRNA1241, and the top five most down-regulated circRNAs were circRNA9864, circRNA9650, circRNA9865, circRNA9671 and circRNA9930. Pathway analysis showed that differentially expressed circRNAs were linked to cancer-related pathways including metabolism, cell apoptosis, proliferation and migration.
Evaluation of circRNA expression in ESCC using microarray technology revealed that 469 circRNAs were up-regulated, and 275 were down-regulated in ESCC in comparison with non-tumor adjacent tissue. Of these, the most up-regulated (increased 20.3-fold) circRNA was hsa_circRNA_103670, while the most down-regulated (decreased 12.1-fold) was hsa_circRNA_030162 (Shi et al., 2018).
In addition to profiling circRNAs in EC, there have been some reports, which have explored the expression of specific circRNAs in EC, and clarified their roles in vitro. Hsa_circ_0067934 was significantly up-regulated in 51 paired ESCC samples in comparison to the adjacent normal tissues. SiRNA inhibition of hsa_circ_0067934 blocked migration and proliferation, and suppressed the progression of cell cycle in ESCC cells (Xia et al., 2016). Cir-ITCH expression status was analyzed in a total of 684 ESCC and paired adjacent non-tumor tissue samples and its overall down-regulation was established in ESCC samples (Li, F. et al., 2015). Cir-ITCH sponges miR-7, −17, and −214. In addition, Cir-ITCH probably exerts its suppressive effects on ESCC through modulating the Wnt/β-catenin signaling pathway. The circ-TTC17 expression levels were observed to be greater in ESCC plasma and tissue from ESCC patients in comparison to healthy subjects. The ROC curves were utilized for investigating the diagnostic value of circ-TTC17 in differentiating ESCC samples from normal plasma. As shown by the analysis, the cutoff value of circ-TTC17 was –2.548, and the area under the ROC curve (AUC) was 0.8200. Moreover, this showed a sensitivity of 73.33% and a specificity of 88.00%. In addition, a positive relationship was observed between the expression of circ-TTC17 and the TNM phase and the presence of lymphatic metastasis. Kaplan–Meier survival analysis and log-rank statistics were used to compare patient postoperative survival and the expression levels of circ-TTC17 for the prognosis of ESCC patients. The ESCC patients with higher levels of circ-TTC17 expression demonstrated a considerably shorter survival time in comparison to patients with lower levels of circ-TTC17 expression. Univariate analysis revealed that the TNM phase, relative circ-TTC17 expression level, and lymphatic metastasis were all prognostic indices for OS rates of ESCC patients. Nevertheless, multi-variate analyses of the variance showed that only the size of the tumor was an independent marker to assess the prognosis of the ESCC patients. Hence, circ-TTC17 has potential to be utilized as a prognostic and diagnostic marker for ESCC. Circ-TTC17 can act as an oncogene, and promote ESCC cell proliferation and migration. Bioinformatics analysis suggested that circ-TTC17 could play a role in ESCC by sponging microRNAs such as miR-153, −217, −224, and −370 (Wang, Q. et al., 2019).
It was observed that hsa_circ_0004370 was up-regulated in both EC tissues and cell lines. Interestingly, loss of hsa_circ_0004370 function suppressed invasion as well as proliferation of EC cell lines and increased their apoptosis rate. Hsa_circ_0004370 can sponge miR-1294 and indirectly increase the level of “LIM and SH3 domain protein 1” (LASP1) suggesting that hsa_circ_0004370 can function as an oncogene affecting proliferation, apoptosis, and invasion via the miR-1294/LASP1 axis (Zhang, Z. et al., 2019). LASP1 enhances tumor invasion as well as proliferation in various cancers and could act as an essential EMT mediator by mediating the mitogen-activated protein kinase (MAPK) phosphorylation, triggering Smad and PI3K/AKT pathways (Wang et al., 2014; Zhang, X. et al., 2017). Circ-SMAD7 derived from the gene SMAD7 intron, is remarkably downmodulated in plasma samples from ESCC patients in comparison with healthy subjects, and showed a negative correlation with the TNM stage. In vitro circ-SMAD7 overexpression could suppress migration and proliferation in ESCC cells, while circ-SMAD7 knockdown showed the opposite effects. Taken together these finding suggested a tumor suppressor role for circ-SMAD7 in ESCC(Zhang, Y. et al., 2019). Functional analysis of circ0043898 shown to be downmodulated in ESCC tissue samples, indicating its inhibitory effects on invasion, migration, and proliferation of tumor cells, and could induce apoptosis in ECA-109 and Kyse-520 ESCC cells. In vivo experiments showed that circ0043898 was associated with inhibition of oncogenesis (Guo, S. et al., 2018). Besides playing a role in carcinogenesis, circRNAs could be involved in other aspects of cancer, such as the development of drug and radiation resistance. Evaluation of circRNAs found significant up-regulation of 57 circRNAs and down-regulation of 17 circRNAs in KYSE-150R, a radioresistant EC cell line, compared with the radiosensitive KYSE-150(Su et al., 2016).
5.4. Role of circRNAs in hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is increasing in incidence worldwide with an average survival rate between 6–20 months(Waller et al., 2015). HCC is more common among males, with a male: female ratio of 2.4 worldwide, and the most common age at presentation is between the third and fifth decades of life (Kulik and El-Serag, 2019). Chronic liver diseases caused by hepatitis B and C viruses, and excessive consumption of alcohol contributes most to the HCC subjects (Ghouri et al., 2017).HCC pathogenesis involves different genetic aberrations, and alterations in several signaling pathways, which lead to a heterogeneity in the disease at a molecular level(Bertino et al., 2014). An improved knowledge of the molecular mechanisms of hepatocarcinogenesis could help to improve diagnostic methods, and enable possible therapeutic strategies to inhibit cancer-driving signaling pathways.
Mounting data suggests that circRNAs are related to HCC development (Tables 6, 7), but their mechanism of action in the development of HCC remains unclear. Shang et al analyzed circRNA expression in 3 paired HCC and non-tumor tissues, and identified 61 differentially expressed circRNAs. Among them, 26 circRNAs were up-regulated and 35 were downregulated in tumor cells. Additionally, they reported that hsa_circ_0005075 levels were considerably up-regulated in HCC, was associated with HCC tumor size, and could be used as potential biomarker with sensitivity of 83.3%and specificity of 90.0%. Bioinformatics analysis of the circRNA-miRNA-mRNA interaction network predicted that a total of 4 miRNAs and 121 mRNAs could interact with hsa_circ_0005075 (Shang et al., 2016). Micro-array analysis detected twenty specific circRNAs with differential expression levels between tumor and non-tumor tissues. qRT-PCR was employed to verify the expression levels of the above 20 circRNAs. Moreover, the micro-array test results showed that hsa_circ_0091579 mRNA and hsa_circ_16245–1 expression was consistent with the respective levels. However, because the levels of hsa_circ_16245–1 and hsa_circ_0091579 were significantly enhanced in the HCC tissues, it was decided to evaluate their potential diagnostic value. ROC curves were obtained for hsa_circ_0091579 and hsa_circ_16245–1 levels in HCC. The areas under the ROC curve were 0.720 for hsa_circ_16245–1 and 0.656 for hsa_circ_0091579. For hsa_circ_0091579, the sensitivity and specificity were 0.4 and 0.97 respectively. The specificity and sensitivity of hsa_circ_16245–1 were 0.63 0.83 respectively. 75 pairs of HCC specimens were tested to validate the prognostic value of these circRNAs. Hsa_circ_0091579 expression was remarkably increased in HCC tumor tissue in comparison to the levels in adjacent non-tumor tissues. They could not detect hsa_circ_16245–1 in a number of the HCC samples. Thus, hsa_circ_0091579 up-regulation serves as a potential prognostic marker for individuals with HCC, and is related to overall survival (Zhang, C. et al., 2018). Hsa_circ_0001649 expression was considerably down-modulated and was correlated with tumor size in 89 HCC tissues in comparison to adjacent non-tumor tissues.(Qin, M. et al., 2016).Microarray analysis of circRNAs revealed that the expression of hsa_circ_0004018 was lower in HCC than non-tumor tissues. Hsa_circ_0004018 expression was also associated with TNM stage, differentiation, and tumor diameter (Fu, L. et al., 2017).
Table 6.
Role of circRNAs in hepatocellular carcinoma
| circRNAs | Dysregulation | Target | Model | Type of cell line | Ref |
|---|---|---|---|---|---|
| Hsa_circ_0091579 | Up | Human | - | (Zhang, C. et al., 2018) | |
| Hsa_circ-101368 | Up | HMGB1/RAGE signaling | Human, In vitro | HCCLM3 | (Li, S. et al., 2018) |
| CircSETD3 (hsa_circRNA_0000567/hsa_circRNA_101436) | Down | Human, In vitro | (WHO, 2019) | ||
| Hsa_circRNA-104718 | Up | miR-218–5p/TXNDC5 signaling pathway | Human, In vivo | SMMC-7721, HepG2, MHCC-LM3, and SK-Hep1, HEK293T | (Yu et al., 2019) |
| Hsa_circ_0003570 | Down | alpha-fetoprotein | Human, In vitro | HepG2, SMMC-7721, MHCC97L, MHCC97H, and HCCLM3, L02 | (Fu et al., 2018) |
| Hsa_circ_0068669 | Down | Human, In vitro | HCC | (Yao et al., 2018) | |
| Hsa_circ_0078602 | Down | Human | (Kou et al., 2019) | ||
| Circ5379–6 | Down | In vitro, In vivo | hepG2, 293T, Huh-7, SK-hep-1 | (Zhang, N. et al., 2018) | |
| CSMARCA5 (hsa_circ_0001445) | Down | TIMP3, Sponging miR-17–3p and miR-181b-5p | Human, In vitro, In vivo | (Yu, J. et al., 2018) | |
| CircPTGR1 | Up | miR449a-MET pathway | In vitro | HepG2, L-O2, SMCC-7721, HEP3B, HUH7, MHCC97-L (97 L), MHCC 97H (97H), HCC-LM3 (LM3) | (Wang, G. et al., 2019) |
| Hsa_circ_0079929 | Down | PI3K/AKT/mTOR signaling pathway | Human, In vitro, In vivo | SK-Hep-1, SMMC-7721, 7702, LM-3, PRL-RF5 | (Zheng et al., 2019) |
| CircHIPK3 | Up | miR-124, AQP3 | Human, In vitro | Huh7, MHCC-LM3, HepG2, SMMC-7721, PLC, L02, HEK293T | (Chen, G. et al., 2018) |
| Hsa_circ_0070269 | Up | miR-182/NPTX1 axis | Human, In vitro | (Hep-3B, SMMC-7721, HepG2, PLC, Huh-7, LO2 | (Su et al., 2019) |
| Hsa_circ_0028502, hsa_circ_0076251 | Down | Human | - | (Jiang, Z. et al., 2019) | |
| circular RNA PVT1 | Up | miR-203/HOXD3 pathway | Human, In vitro | Huh7, Sk-hep1, SMMC-7721, HepG2, L-02 | (Zhu, Y. et al., 2019) |
| Circ_0015756 | Up | miR-7 | Human, In vitro | WRL-68, MHCC97H, Bel-7402, Huh-7, Bel100 | (Liu et al., 2019b) |
| hsa_circ_0085616 | Up | β-catenin, p-ERK,p-AKT, | Human, In vitro | HepG2, MHCC-97L, MHCC-97H, Huh7, LM3 | (Li et al., 2019) |
| Hsa_circ_0003998 | Up | AFP, | Human, In vitro | HepG-2, HuH7, MHCC97H, PLC/PRF/5, L02 | (Qiao et al., 2019) |
Table 7.
CircRNAs as biomarkers in hepatocellular carcinoma
| Circular RNA | Expression in HCC | miRNA sponge | Model /Source | Type of cell line | Ref |
|---|---|---|---|---|---|
| hsa_circ_0005075 | Up | - | Human/HCC tissues (n=63) | - | (Zeng, K. et al., 2018) |
| circRNA_100338 | Up | miR-141–3p | In vitro, Human/HCC tissues (n=4) | BEL7402, Hep3B, HCCLM6, MHCC97H | (Huang, X.-Y. et al., 2017) |
| circ_0067934 | Up | miR-1324 | In vitro, Human HCC tissues (n=49) | BEL7402, HuH7, MHCC97-L, Hep3B | (Zhu, Q. et al., 2018) |
| ciRS-7 (Cdr1as) | Down | In vitro, Human HCC tissues (n=108) | (Xu, L. et al., 2017; Xu, Liangliang et al., 2017) | ||
| hsa_circ_0005986 | Down | miR-129–5p | In vitro, Human HCC tissues (n=81) | HepG2, Huh7, SMMC7721, HCCLM3, MHCC97L, MHCC97H | (Fu, Liyun et al., 2017a) |
| hsa_circ_0004018 | Down | - | In vitro, Human HCC tissues (n=102) | Huh7, HepG2, HCCLM3, MHCC97H, SMMC7721 | (Fu, Liyun et al., 2017b) |
| circMTO1 | Down | miR-9 | In vitro, Human HCC tissues (n=289) | QGY-7701, HepG2, SK-Hep1, SMMC-7721 | (Han, Dan et al., 2017) |
| cSMARCA5 | Down | miR-181b-5p, miR-17–3p | In vitro, Human HCC tissues (n=208) | HCCLM3 | (Yu, Jian et al., 2018) |
| circRNA_0046367 | Down | miR-34a | Human HCC tissues (n=5) | - | (Guo, X.-Y. et al., 2017) |
| hsa_circ_0001649 | Down | - | HCC tissues (n=89) | - | (Qin, Meilin et al., 2016) |
| circC3P1 | Down | miR-4641 | Human/HCC tissues | Cell line (???) | (Zhong, L. et al., 2018) |
| circ-ITCH | Down | - | Human/HCC tissues (n=1600) | - | (Guo, W. et al., 2017) |
| circZKSCAN1 | Down | In vitro, Human/HCC tissues (n=102) | BEL-7402, Huh7, SMMC-772, Hep3B, HepG2 | (Yao et al., 2017) |
The microRNA sponging function of circRNAs is involved in HCC development, and various pathways have been reported to be regulated by this mechanism. circMTO1 (mitochondrial translation optimization1 homologue) and hsa_circRNA_0007874/hsa_circRNA_104135 were detected to be remarkably down-modulate in HCC tissues, and were linked to shortened survival. MiR-9 was found to bind tocircMTO1, and circMTO1 silencing in HCC cells could down-modulate p21, the oncogenic miR-9 target, leading to HCC invasion and proliferation promotion. Furthermore, an inhibitor of miR-9 blocked the tumor-promoting impact of circMTO1 silencing (Han, D. et al., 2017). The expression of CircTRIM33–12 in HCC tissues was investigated, and it was revealed that circTRIM33–12 is down-regulated in HCC cell lines and tissues. CircTRIM33–12 can sponge miR-191, and its inhibition increased immune evasion, tumor invasion, migration, and proliferation (Zhang, P.F. et al., 2019). Compared to the adjacent non-tumor tissues, Cdr1as expression is up-regulated in HCC tissues, and its suppression inhibits miR-7 expression and increased tumor cells invasion and proliferation. Mentioned findings propose that Cdr1as acts as an oncogene in HCC, partly by targeting miR-7 (Yu et al., 2016). Expression analysis of circRNA-101368 showed that this circRNA is considerably up-modulated in HCC tissue samples, and the higher expression of hsa-circ-101368 was related to poor prognosis in patients with HCC. Hsa-circ-101368 was confirmed that directly binds to miR-200a and both could negatively modulate each other. The expression of miR-200a was negatively related to hsa-circ-101368 expression in tissue samples. Hsa-circ-101368 inhibition blocked migration, which could be partially attenuated via miR-200a suppression (Li, S. et al., 2018).
CircSETD3 (hsa_circRNA_101436/hsa_circRNA_0000567) was remarkably down-regulated in HCC cell lines and tissues, and was correlated with larger tumor size and poor HCC differentiation in affected subjects. CircSETD3 could suppress the proliferation of HCC cells and induced G1/S cell cycle arrest. Furthermore, it was shown that circSETD3 could act as a sponge for miR-421, which targets MAPK14 signaling (Xu, L. et al., 2019). MAPK14 may facilitate tumor cells proliferation and survival, and contribute to the progression of some tumor types (Igea and Nebreda, 2015).Yu et al reported that hsa-circ-104718 was physically associated and co-expressed with microRNA-218–5p in HCC. They found that exogenously expressed hsa-circ-104718 accelerated cell proliferation, migration, invasion, and inhibited apoptosis. Hsa-circ-104718 over-expression could increase tumor size and metastasis, while its silencing had the opposite effect. Conversely, miR-218–5p over-expression could decrease the proliferation, migration, invasion, and increase apoptosis (Yu et al., 2019).
5.5. Role of circRNAs in gallbladder cancer
Gallbladder cancer (GBC), the most prevalent biliary tract cancer, accounts for more than 80% of biliary tract malignancies (Lai and Lau, 2008). GBC has only a 6 month mean overall survival, while the 5-year survival rate remains low at about 5%. A genetic predisposition, congenital biliary tract anomalies, female sex, and age are considered to be the main risk factors for GBC development. Based on the geographical and ethnical considerations, the mentioned factors are different among populations (Hundal and Shaffer, 2014). This malignancy is most often diagnosed at late stages, frequently proving fatal. Although surgical resection is the only treatment option, only 10% of affected subjects are candidates for surgery with curative intent at initial presentation (Kanthan et al., 2015). The development of diagnostic markers is essential for improved GBC management, and could lead to the development of screening programs for individuals at risk.
Some evidence has shown that circRNAs are involved in GBC development. However, much remains unknown about the role of circRNAs in GBC, and further investigation is needed to clarify possible circRNA regulatory pathways in the initiation and development ofGBC. CircHIPK3 was found to be up-regulated in human GBC cells. Silencing of circHIPK3 using siRNA, induced apoptosis and suppressed proliferation and survival of both primary and established human GBC cells. The opposite effects occurred with circHIPK3 over-expression (Xu et al., 2013). Further investigation showed that circHIPK3 could sponge tumor-suppressive microRNA-124 resulting in enhanced miR-124 gene targets expression, such as CDK6 (rho-associated protein kinase) and ROCK1 (rho-associated protein kinase 1) (Pierson et al., 2008). They concluded that, through sponging miR-124, circHIPK3 could promote GBC cell growth (Kai et al., 2018). CircHIPK3 was also observed to sponge miR-379, and miR-379 up-regulation rescued the phenotype induced by over-expression of circHIPK3 (Tian et al., 2017).
5.6. Role of circRNAs in pancreatic cancer
Pancreatic cancer is the fourth most frequent cause of malignancy-associated mortality in USA. Although the approaches for management and detection of this cancer have been developed, 5-year survival remains at only 4%. The lethality is mainly caused by its propensity to rapidly metastasize to the lymphatic system and distant organs (Iovanna et al., 2012). The main risk factors for developing pancreatic cancer are high-fat diet, African-American ethnic origin, obesity, diabetes mellitus, male sex (Vincent et al., 2011), smoking, age, and family history of chronic pancreatitis (Klein et al., 2004). Up to now, surgical resection is the only therapeutic approach for pancreatic cancer, and it responds poorly to chemotherapeutic agents (McGuigan et al., 2018). Understanding the underlying mechanism contributing to pancreatic cancer is essential to develop new treatment strategies.
Studies have suggested that circRNAs could serve as diagnostic or predictive biomarkers for pancreatic cancer, and could provide new insights especially in pancreatic ductal adenocarcinoma (PDAC) (Table 8, 9) (Qu et al., 2015). Microarray analysis of circRNAs in 6 PDAC and paired non-tumor tissues revealed that 351 circRNAs were differentially expressed between PDAC and the healthy tissue, of which 142 circRNAs were downregulated and 209 circRNAs were up-regulated in tumor samples. Some differentially expressed circRNAs were evaluated by qRT-PCR in 20 paired tissues. Bioinformatics analysis predicted that hsa_circ_0005785 (one of the differentially expressed circRNAs) was potentially able to bind miR-181a and miR-181b (Li, H. et al., 2016). MiR-181a has a crucial role in modulating the migration as well as growth of pancreatic cancer cells (Zhang et al., 2015). MiR-181b is related to pancreatic cancer cell resistance to gemcitabine chemotherapy (Takiuchi et al., 2013). Gue et al. investigated the circRNA profile in 20 pancreatic cancer tissues and corresponding adjacent tissues. They reported that 128 circRNAs were up-regulated and 161 circRNAs were down-regulated in cancer tissues (Guo, S. et al., 2018). Moreover, these authors predicted circRNA-miRNA interactions, and identified eight circRNAs that bound to miR-15a.Three of these were up-regulated (hsa_circ_100435, hsa_circ_103076, hsa_circ_103309) and five were down-regulated (hsa_circ_000780, hsa_circ_101252, hsa_circ_102374, hsa_circ_104433, hsa_circ_104882).Moreover, four up-regulated circRNAs bound to miR-506 (hsa_circ_101717, hsa_circ_104084, hsa_circ_100646, hsa_circ_102213). It has been reported that miR-15a could inhibit the proliferation of pancreatic cancer cells and the EMT, and that miR-506 could suppress progression and chemoresistance (Guo, S. et al., 2014; Li, J. et al., 2016). Gue et al speculated that circRNAs could regulate gene expression via the miRNA sponging effect and may have a role in the progression of pancreatic cancer (Guo, S. et al., 2018).
Table 8.
Circular RNAs in pancreatic cancer
| circRNAs | Dysregulation | miRNA sponge | Related molecules or pathways | Model | Type of cell line | Ref |
|---|---|---|---|---|---|---|
| circ-LDLRAD3 | Up | miR-137–3p | pleiotrophin (PTN) | In vitro | PANC-1, SW1990 | (Yao et al., 2019) |
| hsa_circRNA_0007334 | Up | hsa-miR-144–3p, hsa-miR-577 | - | Human | - | (Yang et al., 2019) |
| circ_0007534 | Up | miR-892b, miR-625 | - | In vitro Human | Capan-2, BxPC3, SW1990, AsPC-1, PANC1 | (Hao et al., 2019) |
| ciRS-7 | Up | miR-7 | EGFR/STAT3 signaling pathway | Human | - | (Liu et al., 2019a) |
| circ_0030235 | Up | miR-1294, miR-1253 | In vitro Human | AsPC-1, SW1990, Capan-2, PANC1, BxPC-3 | (Xu, Y. et al., 2019) | |
| circZMYM2 (hsa_circ_0099999) | Up | miR-335–5p | JMJD2C | In vitro Human | CFPAC-1, PANC-1 | (An et al., 2018) |
| circRHOT1 | Up | miR-330, miR-26b, miR-382, miR-125a | - | Human | PANC-1, Capan-1, Capan-2, AsPC-1, SW1990, BxPC-3 | (Qu et al., 2019) |
| hsa_circ_0000977 | Up | miR-874–3p | PLK1 | In vitro | - | (Huang, W.-J. et al., 2018) |
| circ-IRAS | Up | miR-122 | ZO-1, RhoA, F-actin | In vitro Human | AsPC-1, Hs 766 T | (Li, J. et al., 2018) |
| hsa_circ_0006215 | Up | - | Human | - | (Zhu, P. et al., 2018) | |
| circ-PDE8A | Up | miR-338 | MACC1/MET pathway | In vitro Human | BxPC-3, Capan-1, Hs 766T, Aspc-1, Hs 766T, HEK-293 | (Li, Z. et al., 2018) |
| CircRNA_100782 | Up | miR-124 | IL6-STAT3 pathway | Human | BxPC3, HPDE | (Chen, G. et al., 2017) |
Table 9.
Circular RNAs as biomarkers in pancreatic cancer
| circRNAs | Dysregulation | miRNA sponge | Model | Type of cell line | Ref |
|---|---|---|---|---|---|
| circRNA-ASH2L | Up | miR-34a | In vitro, Human | BxPC-3, AsPC-1, Capan-1, PANC-1, Hs 766 T, and SW1990 | (Jiang and Bu, 2019) |
| circ-LDLRAD3 | Up | - | In vitro, Human | HPDE6-C7, HPC-Y5 | (Yang et al., 2017) |
| circRNA_102213 | Up | miR-506 | Human | - | (Guo, Shixiang et al., 2018) |
| circRNA_100646 | Up | miR-506 | Human | - | (Guo, Shixiang et al., 2018) |
| circRNA_104084 | Up | miR-506 | Human | - | (Guo, Shixiang et al., 2018) |
| circRNA_101717 | Up | miR-506 | Human | - | (Guo, Shixiang et al., 2018) |
| hsa_circ_0001946 | Up | - | Human | - | (Li, Haimin et al., 2016) |
| hsa_circ_0005397 | Up | - | Human | - | (Li, Haimin et al., 2016) |
| circRNA_104882 | Down | miR-15a | Human | - | (Guo, Shixiang et al., 2018) |
| circRNA_104433 | Down | miR-15a | Human | - | (Guo, Shixiang et al., 2018) |
| circRNA_102374 | Down | miR-15a | Human | - | (Guo, Shixiang et al., 2018) |
| circRNA_101252 | Down | miR-15a | Human | - | (Guo, Shixiang et al., 2018) |
| circRNA_000780 | Down | miR-15a | Human | - | (Guo, Shixiang et al., 2018) |
| hsa_circ_0001649 | Down | - | In vitro, Human | PANC1, BxPC3 | (Jiang, Y. et al., 2018) |
| hsa_circ_0008719 | Down | - | Human | - | (Li, Haimin et al., 2016) |
| hsa_circ_0041150 | Down | - | Human | - | (Li, Haimin et al., 2016) |
| hsa_circ_0005785 | Down | - | Human | - | (Li, Haimin et al., 2016) |
| hsa_circ_0000257 | Down | - | Human | - | (Li, Haimin et al., 2016) |
| hsa_circ_0006913 | Down | - | Human | - | (Li, Haimin et al., 2016) |
Hsa_circ_0006215 was shown to be remarkably up-regulated in pancreatic cancer tissue. Based on bioinformatics analysis, it was proposed that hsa_circ_0006215 most likely regulated the expression of miR-378a-3p (Zhu, P. et al., 2018). Expression analysis of circ-IARS in human microvascular vein endothelial (HUVECs) cells, Hs 766 T, Hs 766 T-L2 pancreatic cancer cells, plasma exosomes, and PDAC tissues revealed that the expression of circ-IARS is up-modulated in plasma exosomes and in pancreatic cancer cells/tissues of metastatic disease patients (Zhang, C. et al., 2018). Also, the expression of circ-IARS is positively linked to TNM stage, vascular invasion and liver metastasis, and negatively associated with postoperative survival time. Circ-IARS sponges miR-122, and its overexpression considerably down-regulates miR-122. Taken together, it was proposed that circ-IARS promotes tumor metastasis and invasion (Zhang, C. et al., 2018). The expression of ciRS-7 and MiR-7 in 41 pairs of PDAC tumors and their adjacent tissues revealed that the expression of ciRS-7 is remarkably greater in PDAC tissue. However, the expression of miR-7 demonstrated the conflicting trend. Moreover, ciRS-7 suppression inhibits invasion and proliferation of PDAC cells. Using functional analysis they proposed an oncogenic role for ciRS-7 in PDAC, which could act partially through modulating the EGFR/STAT3 pathway as well as targeting miR-7 (Liu et al., 2019a). Qu and colleagues indicated that circRHOT1 was up-regulated in pancreatic cancer. CircRHOT1 could bind to miR-382, −330, −125a, and −26b, leading to modulate various tumor-related pathways. Moreover, circRHOT1 knockdown could inhibit pancreatic cancer cell proliferation, invasion and migration (Qu et al., 2019).
Gemcitabine (2’,2’-difluoro 2’-deoxycytidine, dFdC) (a cytidine analogue) is a cornerstone of PDAC therapy at all stages. Unfortunately, chemoresistance development within weeks of treatment initiation has limited its clinical use (Amrutkar and Gladhaug, 2017). Shao et al reported that the circRNA signature was different between the gemcitabine-resistant PANC1-GR cell line and the gemcitabine-sensitive PANC-1 counterpart (Shao et al., 2018). Differential analysis of gene expression between PANC-1 and PANC-1-GR cells showed that 126 circRNAs had significantly different expression between these two cell lines, with 68 up-regulated and 58 down-regulated in PANC-1-GR cells in comparison with PANC-1 cells. In another investigation, the expression levels of circ-LDLRAD3 were examined in 31 plasma samples from normal cases 31 plasma samples from pancreatic cancer patients, cell lines and 30 paired pancreatic cancer and adjacent non-tumor tissues (Yang et al., 2017). Their results showed that Ccirc-LDLRAD3 is up-regulated in plasma samples tissues and cell lines of pancreatic cancer. Besides, the expression of circ-LDLRAD3 is significantly associated with metastasis, as well as venous and lymphatic invasion.
6. Conclusions
CircRNAs, once thought to be merely random errors in transcription, have now been realized to be important players in cell biology and regulation of gene expression. The mechanism by which they regulate gene expression, and their overall function are not yet fully understood. Recent studies have clarified that these transcripts are dysregulated in many cancers, and play an essential role in cancer-related signaling pathways. Taken together, these findings highlight the important and diverse role of circRNAs in cancer initiation and progression. Accumulating evidence suggests that one important mechanisms of action for circRNAs is by acting as microRNA sponges, thus having the opposite effect on gene expression as manifested by the corresponding miRNAs involved. The present review has shown that circRNAs are involved in many different GI cancers, including esophagus, stomach, pancreas, liver, gallbladder and colorectal cancer. The fact that these cancers arise from several different cell types and have different risk factors for cancer development, underlines the likelihood that circRNAs may be involved in general signaling pathways in cancer, such as cell cycle regulation, cell death, proliferation, migration and metastasis. On the other hand, some circRNAs show tissue or malignancy-specific expression patterns, and may function in a single type of cancer or even a single-subtype. The fact that expression levels of many circRNAs can be detected in serum or plasma samples encourages the development of non-invasive screening or biomarker studies. High-throughput screening of thousands of circRNAs together with sophisticated computer algorithms may allow prognostic predictions, and monitoring of the development of treatment resistance. Inhibitors (small molecules or nucleic acid-based) of those circRNAs that function as oncogenes may be developed in the future. Clarifying the roles of circRNAs in GI cancers could open a new window in management and the development of new therapeutic strategies. Further investigation in the field of circRNAs and cancers is needed to fully characterize their role in carcinogenesis.
Acknowledgments
Funding and conflicts of interest
MRH was supported by US NIH Grants R01AI050875 and R21AI121700. MRH declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH; BeWell Global Inc, Wan Chai, Hong Kong; Hologenix Inc. Santa Monica, CA; LumiThera Inc, Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon Inc, Bee Cave, TX; Medical Coherence, Boston MA; NeuroThera, Newark DE; JOOVV Inc, Minneapolis-St. Paul MN; AIRx Medical, Pleasanton CA; FIR Industries, Inc. Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV Inc, Lansing MI; Illumiheal & Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp. Incline Village, NV; Niraxx Light Therapeutics, Inc, Boston, MA. Consulting; Lexington Int, Boca Raton, FL; USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V. Eindhoven, Netherlands; Johnson & Johnson Inc, Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholdings: Global Photon Inc, Bee Cave, TX; Mitonix, Newark, DE.
Abbreviation list
- GI
Gastro-Intestinal
- circRNAs
Covalently closed circular RNAs
- GC
gastric cancer
- CRC
colorectal cancer
- EC
esophageal cancer
- ESCC
esophageal squamous cell carcinoma
- HCC
Hepatocellular carcinoma
- GBC
Gallbladder cancer
- PC
pancreatic cancer
- PDAC
pancreatic ductal adenocarcinoma
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- APC
Adenomatous polyposis coli
- ORF
open reading frame
- DCC
Deleted in Colorectal cancer
- SRY
sex-determining region Y
- rRNA
ribosomal RNA
- EIciRNA
exon-intron circRNAs
- PHLDA1
pleckstrin homology-like domain family A member 1
- EMT
Epithelial–Mesenchymal transition
- BAG4
B-cell lymphoma 2 associated athanogene 4
- FMNL2
formin like 2
- LASP1
LIM and SH3 domain protein 1
- MAPK
mitogen-activated protein kinase
- ROCK1
rho-associated protein kinase 1
- qRT-PCR
the quantitative reverse transcription polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas G, Krasna M, 2017. Overview of esophageal cancer. Ann Cardiothorac Surg 6(2), 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter R, 2018. Circular RNA and Alzheimer’s Disease. Adv Exp Med Biol 1087, 239–243. [DOI] [PubMed] [Google Scholar]
- Álvarez-Chaver P, Rodríguez-Piñeiro AM, Rodríguez-Berrocal FJ, Martínez-Zorzano VS, de la Cadena MP, 2007. Identification of hydrophobic proteins as biomarker candidates for colorectal cancer. The international journal of biochemistry & cell biology 39(3), 529–540. [DOI] [PubMed] [Google Scholar]
- Amrutkar M, Gladhaug IP, 2017. Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers (Basel) 9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Y, Cai H, Zhang Y, Liu S, Duan Y, Sun D, Chen X, He X, 2018. circZMYM2 Competed Endogenously with miR-335–5p to Regulate JMJD2C in Pancreatic Cancer. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 51(5), 2224–2236. [DOI] [PubMed] [Google Scholar]
- Annunziata CM, Kleinberg L, Davidson B, Berner A, Gius D, Tchabo N, Steinberg SM, Kohn EC, 2007. BAG-4/SODD and associated antiapoptotic proteins are linked to aggressiveness of epithelial ovarian cancer. Clin Cancer Res 13(22 Pt 1), 6585–6592. [DOI] [PubMed] [Google Scholar]
- Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S, 2014. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 56(1), 55–66. [DOI] [PubMed] [Google Scholar]
- Azmi AS, 2018. Nuclear export mechanisms of circular RNAs: size does matter. Noncoding RNA Investig 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmayr-Heyda A, Reiner AT, Auer K, Sukhbaatar N, Aust S, Bachleitner-Hofmann T, Mesteri I, Grunt TW, Zeillinger R, Pils D, 2015. Correlation of circular RNA abundance with proliferation--exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Sci Rep 5, 8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn JH, Zhang Q, Li F, Chan TM, Lin X, Kim Y, Wong DT, Xiao X, 2015. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin Chem 61(1), 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belogurov AA, Kurkova IN, Friboulet A, Thomas D, Misikov VK, Zakharova MY, Suchkov SV, Kotov SV, Alehin AI, Avalle B, 2008. Recognition and degradation of myelin basic protein peptides by serum autoantibodies: novel biomarker for multiple sclerosis. The journal of Immunology 180(2), 1258–1267. [DOI] [PubMed] [Google Scholar]
- Bertino G, Demma S, Ardiri A, Proiti M, Gruttadauria S, Toro A, Malaguarnera G, Bertino N, Malaguarnera M, Malaguarnera M, Di Carlo I, 2014. Hepatocellular carcinoma: novel molecular targets in carcinogenesis for future therapies. Biomed Res Int 2014, 203693. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bian L, Zhi X, Ma L, Zhang J, Chen P, Sun S, Li J, Sun Y, Qin J, 2018. Hsa_circRNA_103809 regulated the cell proliferation and migration in colorectal cancer via miR-532–3p / FOXO4 axis. Biochem Biophys Res Commun 505(2), 346–352. [DOI] [PubMed] [Google Scholar]
- Bose R, Ain R, 2018. Regulation of Transcription by Circular RNAs. Adv Exp Med Biol 1087, 81–94. [DOI] [PubMed] [Google Scholar]
- Brooks-Wilson AR, Kaurah P, Suriano G, Leach S, Senz J, Grehan N, Butterfield YS, Jeyes J, Schinas J, Bacani J, Kelsey M, Ferreira P, MacGillivray B, MacLeod P, Micek M, Ford J, Foulkes W, Australie K, Greenberg C, LaPointe M, Gilpin C, Nikkel S, Gilchrist D, Hughes R, Jackson CE, Monaghan KG, Oliveira MJ, Seruca R, Gallinger S, Caldas C, Huntsman D, 2004. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet 41(7), 508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu X, Zhang X, Luan W, Zhang R, Zhang Y, Zhang A, Yan Y, 2019. Next-generation sequencing reveals hsa_circ_0058092 being a potential oncogene candidate involved in gastric cancer. Gene, 144176. [DOI] [PubMed] [Google Scholar]
- Cai J, Chen Z, Wang J, Wang J, Chen X, Liang L, Huang M, Zhang Z, Zuo X, 2019. circHECTD1 facilitates glutaminolysis to promote gastric cancer progression by targeting miR-1256 and activating β-catenin/c-Myc signaling. Cell death & disease 10(8), 576–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Chen Z, Zuo X, 2019. circSMARCA5 Functions as a Diagnostic and Prognostic Biomarker for Gastric Cancer. Dis Markers 2019, 2473652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Gao L, Teng L, Ge J, Oo ZM, Kumar AR, Gilliland DG, Mason PJ, Tan K, Speck NA, 2015. Runx1 Deficiency Decreases Ribosome Biogenesis and Confers Stress Resistance to Hematopoietic Stem and Progenitor Cells. Cell Stem Cell 17(2), 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, Goodfellow P, Lovell-Badge R, 1993. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 73(5), 1019–1030. [DOI] [PubMed] [Google Scholar]
- Carcas LP, 2014. Gastric cancer review. J Carcinog 13, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes A, Roda D, Tarazona N, Rosello S, Perez-Fidalgo JA, 2013. Current questions for the treatment of advanced gastric cancer. Cancer Treat Rev 39(1), 60–67. [DOI] [PubMed] [Google Scholar]
- Chen G, Shi Y, Liu M, Sun J, 2018. circHIPK3 regulates cell proliferation and migration by sponging miR-124 and regulating AQP3 expression in hepatocellular carcinoma. Cell death & disease 9(2), 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Shi Y, Zhang Y, Sun J, 2017. CircRNA_100782 regulates pancreatic carcinoma proliferation through the IL6-STAT3 pathway. Onco Targets Ther 10, 5783–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wang X, Yan X, Cheng X, He X, Zheng W, 2018. LncRNA MALAT1 regulates sepsis-induced cardiac inflammation and dysfunction via interaction with miR-125b and p38 MAPK/NFκB. International immunopharmacology 55, 69–76. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Zheng Q, Bao C, He J, Chen B, Lyu D, Zheng B, Xu Y, Long Z, Zhou Y, Zhu H, Wang Y, He X, Shi Y, Huang S, 2017. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer letters 388, 208–219. [DOI] [PubMed] [Google Scholar]
- Chen L.-h., Wang L.-p., Ma X.-q., 2019. Circ_SPECC1 enhances the inhibition of miR-526b on downstream KDM4A/YAP1 pathway to regulate the growth and invasion of gastric cancer cells. Biochemical and Biophysical Research Communications 517(2), 253–259. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhou H, Guan Z, 2019. CircRNA_000543 knockdown sensitizes nasopharyngeal carcinoma to irradiation by targeting miR-9/platelet-derived growth factor receptor B axis. Biochemical and biophysical research communications 512(4), 786–792. [DOI] [PubMed] [Google Scholar]
- Chen S, Li T, Zhao Q, Xiao B, Guo J, 2017. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin Chim Acta 466, 167–171. [DOI] [PubMed] [Google Scholar]
- Cheng J, Zhuo H, Xu M, Wang L, Xu H, Peng J, Hou J, Lin L, Cai J, 2018. Regulatory network of circRNA-miRNA-mRNA contributes to the histological classification and disease progression in gastric cancer. J Transl Med 16(1), 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang LS, Ito K, Ito Y, 2013. RUNX family: Regulation and diversification of roles through interacting proteins. Int J Cancer 132(6), 1260–1271. [DOI] [PubMed] [Google Scholar]
- Cocquerelle C, Mascrez B, Hetuin D, Bailleul B, 1993. Mis-splicing yields circular RNA molecules. FASEB J 7(1), 155–160. [DOI] [PubMed] [Google Scholar]
- Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ, 2015. The RNA binding protein quaking regulates formation of circRNAs. Cell 160(6), 1125–1134. [DOI] [PubMed] [Google Scholar]
- D’Journo XB, Thomas PA, 2014. Current management of esophageal cancer. J Thorac Dis 6 Suppl 2, S253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y, Ouyang X, Zhang F, Wang K, Lin Y, Sun B, Wang Y, Wang L, Huang Q, 2017. Circular RNAs expression profiles in human gastric cancer. Sci Rep 7(1), 9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A, 2015. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 21(26), 7933–7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudekula DB, Panda AC, Grammatikakis I, De S, Abdelmohsen K, Gorospe M, 2016. CircInteractome: A web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol 13(1), 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enuka Y, Lauriola M, Feldman ME, Sas-Chen A, Ulitsky I, Yarden Y, 2015. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic acids research 44(3), 1370–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Cao Q, Liu J, Zhang J, Li B, 2019. Circular RNA profiling and its potential for esophageal squamous cell cancer diagnosis and prognosis. Mol Cancer 18(1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G, Ye B-L, Hu B-R, Ruan X-J, Shi Y-X, 2018. CircRNA_100290 promotes colorectal cancer progression through miR-516b-induced downregulation of FZD4 expression and Wnt/β-catenin signaling. Biochemical and biophysical research communications 504(1), 184–189. [DOI] [PubMed] [Google Scholar]
- Fang J, Hong H, Xue X, Zhu X, Jiang L, Qin M, Liang H, Gao L, 2019. A novel circular RNA, circFAT1(e2), inhibits gastric cancer progression by targeting miR-548g in the cytoplasm and interacting with YBX1 in the nucleus. Cancer Lett 442, 222–232. [DOI] [PubMed] [Google Scholar]
- Fearon AE, Carter EP, Clayton NS, Wilkes EH, Baker AM, Kapitonova E, Bakhouche BA, Tanner Y, Wang J, Gadaleta E, Chelala C, Moore KM, Marshall JF, Chupin J, Schmid P, Jones JL, Lockley M, Cutillas PR, Grose RP, 2018. PHLDA1 Mediates Drug Resistance in Receptor Tyrosine Kinase-Driven Cancer. Cell Rep 22(9), 2469–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon ER, Pierceall WE, 1995. The deleted in colorectal cancer (DCC) gene: a candidate tumour suppressor gene encoding a cell surface protein with similarity to neural cell adhesion molecules. Cancer Surv 24, 3–17. [PubMed] [Google Scholar]
- Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N, 2014. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer 50(7), 1330–1344. [DOI] [PubMed] [Google Scholar]
- Floris G, Zhang L, Follesa P, Sun T, 2017. Regulatory role of circular RNAs and neurological disorders. Molecular neurobiology 54(7), 5156–5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Chen Q, Yao T, Li T, Ying S, Hu Y, Guo J, 2017a. Hsa_circ_0005986 inhibits carcinogenesis by acting as a miR-129–5p sponge and is used as a novel biomarker for hepatocellular carcinoma. Oncotarget 8(27), 43878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Wu S, Yao T, Chen Q, Xie Y, Ying S, Chen Z, Xiao B, Hu Y, 2018. Decreased expression of hsa_circ_0003570 in hepatocellular carcinoma and its clinical significance. J Clin Lab Anal 32(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Yao T, Chen Q, Mo X, Hu Y, Guo J, 2017. Screening differential circular RNA expression profiles reveals hsa_circ_0004018 is associated with hepatocellular carcinoma. Oncotarget 8(35), 58405–58416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Yao T, Chen Q, Mo X, Hu Y, Guo J, 2017b. Screening differential circular RNA expression profiles reveals hsa_circ_0004018 is associated with hepatocellular carcinoma. Oncotarget 8(35), 58405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghouri YA, Mian I, Rowe JH, 2017. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog 16, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glažar P, Papavasileiou P, Rajewsky N, 2014. circBase: a database for circular RNAs. Rna 20(11), 1666–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross HJ, Domdey H, Lossow C, Jank P, Raba M, Alberty H, Sanger HL, 1978. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature 273(5659), 203–208. [DOI] [PubMed] [Google Scholar]
- Guan EC, Xu XG, Xue FX, 2019. circ-NOTCH1 acts as a sponge of miR-637 and affects the expression of its target gene Apelin to regulate gastric cancer cell growth. Biochemistry and cell biology = Biochimie et biologie cellulaire. [DOI] [PubMed] [Google Scholar]
- Guo J.-n., Li J, Zhu C.-l., Feng W.-t., Shao J.-x., Wan L, Huang M.-d., He J.-d., 2016. Comprehensive profile of differentially expressed circular RNAs reveals that hsa_circ_0000069 is upregulated and promotes cell proliferation, migration, and invasion in colorectal cancer. OncoTargets and therapy 9, 7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JN, Li J, Zhu CL, Feng WT, Shao JX, Wan L, Huang MD, He JD, 2016. Comprehensive profile of differentially expressed circular RNAs reveals that hsa_circ_0000069 is upregulated and promotes cell proliferation, migration, and invasion in colorectal cancer. Onco Targets Ther 9, 7451–7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Agarwal V, Guo H, Bartel DP, 2014. Expanded identification and characterization of mammalian circular RNAs. Genome Biol 15(7), 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Xu X, Ouyang Y, Wang Y, Yang J, Yin L, Ge J, Wang H, 2018. Microarray expression profile analysis of circular RNAs in pancreatic cancer. Molecular medicine reports 17(6), 7661–7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Xu X, Ouyang Y, Wang Y, Yang J, Yin L, Ge J, Wang H, 2018. Microarray expression profile analysis of circular RNAs in pancreatic cancer. Mol Med Rep 17(6), 7661–7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Xu X, Tang Y, Zhang C, Li J, Ouyang Y, Ju J, Bie P, Wang H, 2014. miR-15a inhibits cell proliferation and epithelial to mesenchymal transition in pancreatic ductal adenocarcinoma by down-regulating Bmi-1 expression. Cancer Lett 344(1), 40–46. [DOI] [PubMed] [Google Scholar]
- Guo W, Zhang J, Zhang D, Cao S, Li G, Zhang S, Wang Z, Wen P, Yang H, Shi X, 2017. Polymorphisms and expression pattern of circular RNA circ-ITCH contributes to the carcinogenesis of hepatocellular carcinoma. Oncotarget 8(29), 48169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X-Y, Chen J-N, Sun F, Wang Y-Q, Pan Q, Fan J-G, 2017. circRNA_0046367 prevents hepatoxicity of lipid peroxidation: an inhibitory role against hepatic steatosis. Oxidative medicine and cellular longevity 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Li J, Wang H, Su X, Hou J, Gu Y, Qian C, Lin Y, Liu X, Huang M, 2017. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology 66(4), 1151–1164. [DOI] [PubMed] [Google Scholar]
- Han D, Li J, Wang H, Su X, Hou J, Gu Y, Qian C, Lin Y, Liu X, Huang M, Li N, Zhou W, Yu Y, Cao X, 2017. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology (Baltimore, Md.) 66(4), 1151–1164. [DOI] [PubMed] [Google Scholar]
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J, 2013. Natural RNA circles function as efficient microRNA sponges. Nature 495(7441), 384–388. [DOI] [PubMed] [Google Scholar]
- Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, Kjems J, 2011. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J 30(21), 4414–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Rong W, Bai L, Cui H, Zhang S, Li Y, Chen D, Meng X, 2019. Upregulated circular RNA circ_0007534 indicates an unfavorable prognosis in pancreatic ductal adenocarcinoma and regulates cell proliferation, apoptosis, and invasion by sponging miR-625 and miR-892b. J Cell Biochem 120(3), 3780–3789. [DOI] [PubMed] [Google Scholar]
- Haupenthal J, Baehr C, Kiermayer S, Zeuzem S, Piiper A, 2006. Inhibition of RNAse A family enzymes prevents degradation and loss of silencing activity of siRNAs in serum. Biochem Pharmacol 71(5), 702–710. [DOI] [PubMed] [Google Scholar]
- He J-H, Li Y-G, Han Z-P, Zhou J-B, Chen W-M, Lv Y-B, He M-L, Zuo J-D, Zheng L, 2018. The CircRNA-ACAP2/Hsa-miR-21–5p/Tiam1 regulatory feedback circuit affects the proliferation, migration, and invasion of colon cancer SW480 cells. Cellular Physiology and Biochemistry 49(4), 1539–1550. [DOI] [PubMed] [Google Scholar]
- He J, Xie Q, Xu H, Li J, Li Y, 2017. Circular RNAs and cancer. Cancer Lett 396, 138–144. [DOI] [PubMed] [Google Scholar]
- Henry NL, Hayes DF, 2012. Cancer biomarkers. Molecular oncology 6(2), 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdt LM, Kohlmaier A, Teupser D, 2018. Molecular roles and function of circular RNAs in eukaryotic cells. Cell Mol Life Sci 75(6), 1071–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K-Y, Lin Y-C, Gupta SK, Chang N, Yen L, Sun HS, Tsai S-J, 2017. Noncoding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer research 77(9), 2339–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao KY, Lin YC, Gupta SK, Chang N, Yen L, Sun HS, Tsai SJ, 2017. Noncoding Effects of Circular RNA CCDC66 Promote Colon Cancer Growth and Metastasis. Cancer Res 77(9), 2339–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Ying X, Wang J, Piriyapongsa J, Jordan IK, Sheng J, Yu F, Zhao P, Li Y, Wang H, Ng WL, Hu S, Wang X, Wang C, Zheng X, Li W, Curran WJ, Wang Y, 2014. Identification of a tumor-suppressive human-specific microRNA within the FHIT tumor-suppressor gene. Cancer Res 74(8), 2283–2294. [DOI] [PubMed] [Google Scholar]
- Huang C, Liang D, Tatomer DC, Wilusz JE, 2018. A length-dependent evolutionarily conserved pathway controls nuclear export of circular RNAs. Genes Dev 32(9–10), 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Zhu H, Shi Y, Wu W, Cai H, Chen X, 2015. cir-ITCH plays an inhibitory role in colorectal cancer by regulating the Wnt/beta-catenin pathway. PLoS One 10(6), e0131225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Zhu H, Shi Y, Wu W, Cai H, Chen X, 2015. cir-ITCH plays an inhibitory role in colorectal cancer by regulating the Wnt/β-catenin pathway. PloS one 10(6), e0131225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, He YR, Liang LC, Huang Q, Zhu ZQ, 2017. Circular RNA hsa_circ_0000745 may serve as a diagnostic marker for gastric cancer. World J Gastroenterol 23(34), 6330–6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W-J, Wang Y, Liu S, Yang J, Guo S, Wang L, Wang H, Fan Y-F, 2018. Retraction notice to” Silencing circular RNA hsa_circ_0000977 suppresses pancreatic ductal adenocarcinoma progression by stimulating miR-874–3p and inhibiting PLK1 expression”[Cancer Letters 422C (2018) 70–80]. Cancer letters 438, 232. [DOI] [PubMed] [Google Scholar]
- Huang X-Y, Huang Z-L, Xu Y-H, Zheng Q, Chen Z, Song W, Zhou J, Tang Z-Y, Huang X-Y, 2017. Comprehensive circular RNA profiling reveals the regulatory role of the circRNA-100338/miR-141–3p pathway in hepatitis B-related hepatocellular carcinoma. Scientific reports 7(1), 5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal R, Shaffer EA, 2014. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 6, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igea A, Nebreda AR, 2015. The Stress Kinase p38alpha as a Target for Cancer Therapy. Cancer Res 75(19), 3997–4002. [DOI] [PubMed] [Google Scholar]
- Iovanna J, Mallmann MC, Goncalves A, Turrini O, Dagorn JC, 2012. Current knowledge on pancreatic cancer. Front Oncol 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeck WR, Sharpless NE, 2014. Detecting and characterizing circular RNAs. Nat Biotechnol 32(5), 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeck WR, Sharpless NE, 2014. Detecting and characterizing circular RNAs. Nature biotechnology 32(5), 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE, 2013. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19(2), 141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE, 2013. Circular RNAs are abundant, conserved, and associated with ALU repeats. Rna 19(2), 141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Qiu C, Wang M, Mao N, Wu S, Dai Y, 2018. Hsa_circ_0001649: A circular RNA and potential novel biomarker for colorectal cancer. Biochemical and biophysical research communications 497(1), 122–126. [DOI] [PubMed] [Google Scholar]
- Ji W, Qiu C, Wang M, Mao N, Wu S, Dai Y, 2018. Hsa_circ_0001649: A circular RNA and potential novel biomarker for colorectal cancer. Biochem Biophys Res Commun 497(1), 122–126. [DOI] [PubMed] [Google Scholar]
- Jiang C, Xu D, You Z, Xu K, Tian W, 2019. Dysregulated circRNAs and ceRNA network in esophageal squamous cell carcinoma. Front Biosci (Landmark Ed) 24, 277–290. [DOI] [PubMed] [Google Scholar]
- Jiang PC, Bu SR, 2019. Clinical value of circular RNAs and autophagy-related miRNAs in the diagnosis and treatment of pancreatic cancer. Hepatobiliary & pancreatic diseases international : HBPD INT. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang X, Chu Q, Lu S, Zhou L, Lu X, Liu C, Mao L, Ye C, Timko MP, Fan L, Ju H, 2018. The Circular RNA Profiles of Colorectal Tumor Metastatic Cells. Front Genet 9, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wang T, Yan L, Qu L, 2018. A novel prognostic biomarker for pancreatic ductal adenocarcinoma: hsa_circ_0001649. Gene 675, 88–93. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Yim SH, Xu HD, Jung SH, Yang SY, Hu HJ, Jung CK, Chung YJ, 2011. A potential oncogenic role of the commonly observed E2F5 overexpression in hepatocellular carcinoma. World J Gastroenterol 17(4), 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Shen L, Wang S, Wu S, Hu Y, Guo J, Fu L, 2019. Hsa_circ_0028502 and hsa_circ_0076251 are potential novel biomarkers for hepatocellular carcinoma. Cancer Medicine 0(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Wang A, Liu L, Wang G, Li G, 2019. Hsa_circ_0136666 promotes the proliferation and invasion of colorectal cancer through miR-136/SH2B1 axis. Journal of cellular physiology 234(5), 7247–7256. [DOI] [PubMed] [Google Scholar]
- Jin Y, Yu L, Zhang B, Liu C, Chen Y, 2018. Circular RNA hsa_circ_0000523 regulates the proliferation and apoptosis of colorectal cancer cells as miRNA sponge. Brazilian Journal of Medical and Biological Research 51(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai D, Yannian L, Yitian C, Dinghao G, Xin Z, Wu J, 2018. Circular RNA HIPK3 promotes gallbladder cancer cell growth by sponging microRNA-124. Biochem Biophys Res Commun 503(2), 863–869. [DOI] [PubMed] [Google Scholar]
- Kanthan R, Senger JL, Ahmed S, Kanthan SC, 2015. Gallbladder Cancer in the 21st Century. J Oncol 2015, 967472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashfi SM, Golmohammadi M, Behboudi F, Nazemalhosseini-Mojarad E, Zali MR, 2013. MUTYH the base excision repair gene family member associated with colorectal cancer polyposis. Gastroenterol Hepatol Bed Bench 6(Suppl 1), S1–S10. [PMC free article] [PubMed] [Google Scholar]
- Kleaveland B, Shi CY, Stefano J, Bartel DP, 2018. A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell 174(2), 350–362 e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, Griffin C, Cameron JL, Yeo CJ, Kern S, Hruban RH, 2004. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res 64(7), 2634–2638. [DOI] [PubMed] [Google Scholar]
- Kong S, Yang Q, Tang C, Wang T, Shen X, Ju S, 2019. Identification of hsa_circ_0001821 as a Novel Diagnostic Biomarker in Gastric Cancer via Comprehensive Circular RNA Profiling. Frontiers in genetics 10, 878–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos A, Dijkema R, Arnberg AC, van der Meide PH, Schellekens H, 1986. The hepatitis delta (delta) virus possesses a circular RNA. Nature 323(6088), 558–560. [DOI] [PubMed] [Google Scholar]
- Kou P, Zhang C, Lin J, Wang H, 2019. Circular RNA hsa_circ_0078602 may have potential as a prognostic biomarker for patients with hepatocellular carcinoma. Oncol Lett 17(2), 2091–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen LS, Hansen TB, Veno MT, Kjems J, 2018. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene 37(5), 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T, 2015. Colorectal cancer. Nat Rev Dis Primers 1, 15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik L, El-Serag HB, 2019. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 156(2), 477–491 e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH, Lau WY, 2008. Gallbladder cancer--a comprehensive review. Surgeon 6(2), 101–110. [DOI] [PubMed] [Google Scholar]
- Lasda E, Parker R, 2014. Circular RNAs: diversity of form and function. RNA 20(12), 1829–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoz ML, Carballal S, Moreira L, Ocana T, Balaguer F, 2015. The genetic basis of familial adenomatous polyposis and its implications for clinical practice and risk management. Appl Clin Genet 8, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Zhang L, Li W, Deng J, Zheng J, An M, Lu J, Zhou Y, 2015. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/beta-catenin pathway. Oncotarget 6(8), 6001–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Hao X, Wang H, Liu Z, He Y, Pu M, Zhang H, Yu H, Duan J, Qu S, 2016. Circular RNA expression profile of pancreatic ductal adenocarcinoma revealed by microarray. Cellular Physiology and Biochemistry 40(6), 1334–1344. [DOI] [PubMed] [Google Scholar]
- Li H, Hao X, Wang H, Liu Z, He Y, Pu M, Zhang H, Yu H, Duan J, Qu S, 2016. Circular RNA Expression Profile of Pancreatic Ductal Adenocarcinoma Revealed by Microarray. Cell Physiol Biochem 40(6), 1334–1344. [DOI] [PubMed] [Google Scholar]
- Li J, Li Z, Jiang P, Peng M, Zhang X, Chen K, Liu H, Bi H, Liu X, Li X, 2018. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. Journal of experimental & clinical cancer research : CR 37(1), 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ni S, Zhou C, Ye M, 2018. The expression profile and clinical application potential of hsa_circ_0000711 in colorectal cancer. Cancer management and research 10, 2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wu H, Li W, Yin L, Guo S, Xu X, Ouyang Y, Zhao Z, Liu S, Tian Y, Tian Z, Ju J, Ni B, Wang H, 2016. Downregulated miR-506 expression facilitates pancreatic cancer progression and chemoresistance via SPHK1/Akt/NF-kappaB signaling. Oncogene 35(42), 5501–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhen L, Zhang Y, Zhao L, Liu H, Cai D, Chen H, Yu J, Qi X, Li G, 2017. Circ-104916 is downregulated in gastric cancer and suppresses migration and invasion of gastric cancer cells. Onco Targets Ther 10, 3521–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JM, Zhao RH, Li ST, Xie CX, Jiang HH, Ding WJ, Du P, Chen W, Yang M, Cui L, 2012. Down-regulation of fecal miR-143 and miR-145 as potential markers for colorectal cancer. Saudi Med J 33(1), 24–29. [PubMed] [Google Scholar]
- Li M, Ding W, Sun T, Tariq MA, Xu T, Li P, Wang J, 2018. Biogenesis of circular RNA s and their roles in cardiovascular development and pathology. The FEBS journal 285(2), 220–232. [DOI] [PubMed] [Google Scholar]
- Li P, Chen H, Chen S, Mo X, Li T, Xiao B, Yu R, Guo J, 2017. Circular RNA 0000096 affects cell growth and migration in gastric cancer. Br J Cancer 116(5), 626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Chen S, Chen H, Mo X, Li T, Shao Y, Xiao B, Guo J, 2015. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta 444, 132–136. [DOI] [PubMed] [Google Scholar]
- Li S, Gu H, Huang Y, Peng Q, Zhou R, Yi P, Chen R, Huang Z, Hu X, Huang Y, Tang D, 2018. Circular RNA 101368/miR-200a axis modulates the migration of hepatocellular carcinoma through HMGB1/RAGE signaling. Cell Cycle 17(19–20), 2349–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Shao Y, Fu L, Xie Y, Zhu L, Sun W, Yu R, Xiao B, Guo J, 2018. Plasma circular RNA profiling of patients with gastric cancer and their droplet digital RT-PCR detection. J Mol Med (Berl) 96(1), 85–96. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou X, Wu X, Wei J, Huang Z, 2019. The role of circular RNA hsa_circ_0085616 in proliferation and migration of hepatocellular carcinoma cells. Cancer management and research 11, 7369–7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WH, Song YC, Zhang H, Zhou ZJ, Xie X, Zeng QN, Guo K, Wang T, Xia P, Chang DM, 2017. Decreased Expression of Hsa_circ_00001649 in Gastric Cancer and Its Clinical Significance. Dis Markers 2017, 4587698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang J, Zhang C, Lin C, Zhang J, Zhang W, Zhang W, Lu Y, Zheng L, Li X, 2018. Circular RNA circITGA7 inhibits colorectal cancer growth and metastasis by modulating the Ras pathway and upregulating transcription of its host gene ITGA7. The Journal of pathology 246(2), 166–179. [DOI] [PubMed] [Google Scholar]
- Li X, Yang L, Chen LL, 2018. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol Cell 71(3), 428–442. [DOI] [PubMed] [Google Scholar]
- Li XN, Wang ZJ, Ye CX, Zhao BC, Li ZL, Yang Y, 2018. RNA sequencing reveals the expression profiles of circRNA and indicates that circDDX17 acts as a tumor suppressor in colorectal cancer. Journal of experimental & clinical cancer research : CR 37(1), 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhu X, Zeng Y, Wang J, Zhang X, Ding YQ, Liang L, 2010. FMNL2 enhances invasion of colorectal carcinoma by inducing epithelial-mesenchymal transition. Mol Cancer Res 8(12), 1579–1590. [DOI] [PubMed] [Google Scholar]
- Li Z, Yanfang W, Li J, Jiang P, Peng T, Chen K, Zhao X, Zhang Y, Zhen P, Zhu J, Li X, 2018. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett 432, 237–250. [DOI] [PubMed] [Google Scholar]
- Liang L, Li X, Zhang X, Lv Z, He G, Zhao W, Ren X, Li Y, Bian X, Liao W, Liu W, Yang G, Ding Y, 2013. MicroRNA-137, an HMGA1 target, suppresses colorectal cancer cell invasion and metastasis in mice by directly targeting FMNL2. Gastroenterology 144(3), 624–635 e624. [DOI] [PubMed] [Google Scholar]
- Liang M, Huang G, Liu Z, Wang Q, Yu Z, Liu Z, Lin H, Li M, Zhou X, Zheng Y, 2019. Elevated levels of hsa_circ_006100 in gastric cancer promote cell growth and metastasis via miR-195/GPRC5A signalling. Cell Proliferation 52(5), e12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Liu Y, Bian Z, Zhang J, Zhang R, Chen X, Huang Y, Wang Y, Zhu J, 2018. Circular RNA YAP1 inhibits the proliferation and invasion of gastric cancer cells by regulating the miR-367–5p/p27 (Kip1) axis. Mol Cancer 17(1), 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Liu FB, Huang M, Xie K, Xie QS, Liu CH, Shen MJ, Huang Q, 2019a. Circular RNA ciRS-7 promotes the proliferation and metastasis of pancreatic cancer by regulating miR-7-mediated EGFR/STAT3 signaling pathway. Hepatobiliary & pancreatic diseases international : HBPD INT. [DOI] [PubMed] [Google Scholar]
- Liu L, Yang X, Li NF, Lin L, Luo H, 2019b. Circ_0015756 promotes proliferation, invasion and migration by microRNA-7-dependent inhibition of FAK in hepatocellular carcinoma. Cell Cycle 18(21), 2939–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Liu S, Xu Y, Shu R, Wang F, Chen C, Zeng Y, Luo H, 2018. Circular RNA-ZFR Inhibited Cell Proliferation and Promoted Apoptosis in Gastric Cancer by Sponging miR-130a/miR-107 and Modulating PTEN. Cancer Res Treat 50(4), 1396–1417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu X, Chu KM, 2014. E-cadherin and gastric cancer: cause, consequence, and applications. Biomed Res Int 2014, 637308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang P-Y, Xie J-W, Wang J-B, Lin J-X, Chen Q-Y, Cao L-L, Li P, Zheng C-H, Huang C-M, 2019. Circular RNA hsa_circ_0006848 Related to Ribosomal Protein L6 Acts as a Novel Biomarker for Early Gastric Cancer. Disease markers 2019, 3863458–3863458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang PY, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Huang CM, Li P, Zheng CH, 2019. Hsa_circ_0000467 promotes cancer progression and serves as a diagnostic and prognostic biomarker for gastric cancer. J Clin Lab Anal 33(3), e22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Shao Y, Tao X, Ye G, Xiao B, Guo J, 2019. Clinical significances of hsa_circ_0067582 and hsa_circ_0005758 in gastric cancer tissues. Journal of Clinical Laboratory Analysis 0(0), e22984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Shao Y, Ye G, Xiao B, Guo J, 2017. Low expression of hsa_circ_0006633 in human gastric cancer and its clinical significances. Tumour Biol 39(6), 1010428317704175. [DOI] [PubMed] [Google Scholar]
- Maiti P, Al-Gharaibeh A, Kolli N, Dunbar GL, 2017. Solid Lipid Curcumin Particles Induce More DNA Fragmentation and Cell Death in Cultured Human Glioblastoma Cells than Does Natural Curcumin. 2017, 9656719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markkanen E, Dorn J, Hubscher U, 2013. MUTYH DNA glycosylase: the rationale for removing undamaged bases from the DNA. Front Genet 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley AR, Nan H, 2016. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet 7(3), 105–114. [PMC free article] [PubMed] [Google Scholar]
- Marmol I, Sanchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ, 2017. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci 18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux R, 2004. Biomarkers: potential uses and limitations. NeuroRx 1(2), 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS, 2018. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 24(43), 4846–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meganck RM, Borchardt EK, Castellanos Rivera RM, Scalabrino ML, Wilusz JE, Marzluff WF, Asokan A, 2018. Tissue-Dependent Expression and Translation of Circular RNAs with Recombinant AAV Vectors In Vivo. Mol Ther Nucleic Acids 13, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, 2013. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495(7441), 333. [DOI] [PubMed] [Google Scholar]
- Memczak S, Papavasileiou P, Peters O, Rajewsky N, 2015. Identification and Characterization of Circular RNAs As a New Class of Putative Biomarkers in Human Blood. PLoS One 10(10), e0141214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai MA, 2016. Pleckstrin homology-like domain, family A, member 1 (PHLDA1) and cancer. Biomed Rep 4(3), 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallamilli BR, Hegde M, 2017. Detecting APC Gene Mutations in Familial Adenomatous Polyposis (FAP). Curr Protoc Hum Genet 92, 10 18 11–10 18 16. [DOI] [PubMed] [Google Scholar]
- Naylor S, 2003. Biomarkers: current perspectives and future prospects. Taylor & Francis. [DOI] [PubMed] [Google Scholar]
- Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, Kinzler KW, Vogelstein B, 1991. Scrambled exons. Cell 64(3), 607–613. [DOI] [PubMed] [Google Scholar]
- Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A, Lieto E, Ciardiello F, De Vita F, 2014. Treatment of gastric cancer. World J Gastroenterol 20(7), 1635–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Li Y, Huang Y, Li X, Zhu Y, Long Y, Wang Y, Guo X, Gong K, 2019. CircRNA circPDSS1 promotes the gastric cancer progression by sponging miR-186–5p and modulating NEK2. J Cell Physiol 234(7), 10458–10469. [DOI] [PubMed] [Google Scholar]
- Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E, Perez-Hernandez D, Ramberger E, Shenzis S, Samson M, Dittmar G, Landthaler M, Chekulaeva M, Rajewsky N, Kadener S, 2017. Translation of CircRNAs. Mol Cell 66(1), 9–21 e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Li T, Jiang Y, Pan C, Ding Y, Huang Z, Yu H, Kong D, 2018. Overexpression of Circular RNA ciRS-7 Abrogates the Tumor Suppressive Effect of miR-7 on Gastric Cancer via PTEN/PI3K/AKT Signaling Pathway. J Cell Biochem 119(1), 440–446. [DOI] [PubMed] [Google Scholar]
- Pierson J, Hostager B, Fan R, Vibhakar R, 2008. Regulation of cyclin dependent kinase 6 by microRNA 124 in medulloblastoma. J Neurooncol 90(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Pourhoseingholi MA, Vahedi M, Baghestani AR, 2015. Burden of gastrointestinal cancer in Asia; an overview. Gastroenterol Hepatol Bed Bench 8(1), 19–27. [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Liu H, Li M, Shi J, Li N, Zhang Y, Zhang X, Lv J, Xie X, Bai Y, Ge Q, Ko EA, Tang H, Wang T, Wang X, Wang Z, Zhou T, Gu W, 2018. Potential Diagnostic Power of Blood Circular RNA Expression in Active Pulmonary Tuberculosis. EBioMedicine 27, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao G-L, Chen L, Jiang W-H, Yang C, Yang C-M, Song L-N, Chen Y, Yan H-L, Ma L-J, 2019. Hsa_circ_0003998 may be used as a new biomarker for the diagnosis and prognosis of hepatocellular carcinoma. OncoTargets and therapy 12, 5849–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Liu G, Huo X, Tao X, Sun X, Ge Z, Yang J, Fan J, Liu L, Qin W, 2016. Hsa_circ_0001649: A circular RNA and potential novel biomarker for hepatocellular carcinoma. Cancer Biomarkers 16(1), 161–169. [DOI] [PubMed] [Google Scholar]
- Qin M, Liu G, Huo X, Tao X, Sun X, Ge Z, Yang J, Fan J, Liu L, Qin W, 2016. Hsa_circ_0001649: A circular RNA and potential novel biomarker for hepatocellular carcinoma. Cancer Biomark 16(1), 161–169. [DOI] [PubMed] [Google Scholar]
- Qu S, Hao X, Song W, Niu K, Yang X, Zhang X, Shang R, Wang Q, Li H, Liu Z, 2019. Circular RNA circRHOT1 is upregulated and promotes cell proliferation and invasion in pancreatic cancer. Epigenomics 11(1), 53–63. [DOI] [PubMed] [Google Scholar]
- Qu S, Song W, Yang X, Wang J, Zhang R, Zhang Z, Zhang H, Li H, 2015. Microarray expression profile of circular RNAs in human pancreatic ductal adenocarcinoma. Genom Data 5, 385–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan C, Goodall GJ, Shirokikh NE, Preiss T, 2019. Insights into the biogenesis and potential functions of exonic circular RNA. Sci Rep 9(1), 2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragusa M, Barbagallo C, Statello L, Condorelli AG, Battaglia R, Tamburello L, Barbagallo D, Di Pietro C, Purrello M, 2015. Non-coding landscapes of colorectal cancer. World J Gastroenterol 21(41), 11709–11739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong D, Dong C, Fu K, Wang H, Tang W, Cao H, 2018. Upregulation of circ_0066444 promotes the proliferation, invasion, and migration of gastric cancer cells. Onco Targets Ther 11, 2753–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, 2015. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Molecular cell 58(5), 870–885. [DOI] [PubMed] [Google Scholar]
- Rybak-Wolf A, Stottmeister C, Glazar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, Herzog M, Schreyer L, Papavasileiou P, Ivanov A, Ohman M, Refojo D, Kadener S, Rajewsky N, 2015. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol Cell 58(5), 870–885. [DOI] [PubMed] [Google Scholar]
- Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO, 2013. Cell-type specific features of circular RNA expression. PLoS Genet 9(9), e1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO, 2013. Cell-type specific features of circular RNA expression. PLoS genetics 9(9), e1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO, 2012. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One 7(2), e30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK, 1976. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A 73(11), 3852–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M, 2011. Global quantification of mammalian gene expression control. Nature 473(7347), 337–342. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Spector L, Gortler M, Weisshaus O, Glass-Marmor L, Karni A, Dotan N, Miller A, 2006. Serum anti-Glc (α1, 4) Glc (α) antibodies as a biomarker for relapsing–remitting multiple sclerosis. Journal of the neurological sciences 244(1–2), 59–68. [DOI] [PubMed] [Google Scholar]
- Shang X, Li G, Liu H, Li T, Liu J, Zhao Q, Wang C, 2016. Comprehensive Circular RNA Profiling Reveals That hsa_circ_0005075, a New Circular RNA Biomarker, Is Involved in Hepatocellular Crcinoma Development. Medicine (Baltimore) 95(22), e3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao F, Huang M, Meng F, Huang Q, 2018. Circular RNA Signature Predicts Gemcitabine Resistance of Pancreatic Ductal Adenocarcinoma. Front Pharmacol 9, 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Chen L, Lu R, Zhang X, Xiao B, Ye G, Guo J, 2017. Decreased expression of hsa_circ_0001895 in human gastric cancer and its clinical significances. Tumour Biol 39(4), 1010428317699125. [DOI] [PubMed] [Google Scholar]
- Shao Y, Tao X, Lu R, Zhang H, Ge J, Xiao B, Ye G, 2019. Hsa_circ_0065149 is an Indicator for Early Gastric Cancer Screening and Prognosis Prediction. [DOI] [PubMed]
- Shen F, Liu P, Xu Z, Li N, Yi Z, Tie X, Zhang Y, Gao L, 2019. CircRNA_001569 promotes cell proliferation through absorbing miR-145 in gastric cancer. J Biochem 165(1), 27–36. [DOI] [PubMed] [Google Scholar]
- Shen H, Shen J, Wang L, Shi Z, Wang M, Jiang B. h., Shu Y, 2015. Low miR-145 expression level is associated with poor pathological differentiation and poor prognosis in non-small cell lung cancer. Biomedicine & Pharmacotherapy 69, 301–305. [DOI] [PubMed] [Google Scholar]
- Shen T, Han M, Wei G, Ni T, 2015. An intriguing RNA species--perspectives of circularized RNA. Protein Cell 6(12), 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Zhang J, Fu Z, Zhang B, Chen M, Ling X, Zou X, 2018. Gene microarray analysis of the circular RNAs expression profile in human gastric cancer. Oncol Lett 15(6), 9965–9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng N, Tan G, You W, Chen H, Gong J, Chen D, Zhang H, Wang Z, 2017. MiR-145 inhibits human colorectal cancer cell migration and invasion via PAK4-dependent pathway. Cancer Med 6(6), 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Sun J, He B, Song H, Li Z, Kong W, Wang J, Wang J, Xue H, 2018. Profiles of differentially expressed circRNAs in esophageal and breast cancer. Cancer Manag Res 10, 2207–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Guo Z, Fang N, Jiang W, Fan Y, He Y, Ma Z, Chen Y, 2019. hsa_circ_0006168 sponges miR-100 and regulates mTOR to promote the proliferation, migration and invasion of esophageal squamous cell carcinoma. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 117, 109151. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A, 2019. Cancer statistics, 2019. CA Cancer J Clin 69(1), 7–34. [DOI] [PubMed] [Google Scholar]
- Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP, 2018. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 10, 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohda M, Kuwano H, 2017. Current Status and Future Prospects for Esophageal Cancer Treatment. Ann Thorac Cardiovasc Surg 23(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood R, Kamikubo Y, Liu P, 2017. Role of RUNX1 in hematological malignancies. Blood 129(15), 2070–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Lin F, Deng X, Shen L, Fang Y, Fei Z, Zhao L, Zhang X, Pan H, Xie D, Jin X, Xie C, 2016. Profiling and bioinformatics analyses reveal differential circular RNA expression in radioresistant esophageal cancer cells. J Transl Med 14(1), 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Su J, He H, Zhan Y, Liu H, 2019. Hsa_circ_0070269 inhibits hepatocellular carcinoma progression through modulating miR-182/NPTX1 axis. Biomedicine & Pharmacotherapy 120, 109497. [DOI] [PubMed] [Google Scholar]
- Sui W, Shi Z, Xue W, Ou M, Zhu Y, Chen J, Lin H, Liu F, Dai Y, 2017. Circular RNA and gene expression profiles in gastric cancer based on microarray chip technology. Oncol Rep 37(3), 1804–1814. [DOI] [PubMed] [Google Scholar]
- Sun H, Tang W, Rong D, Jin H, Fu K, Zhang W, Liu Z, Cao H, Cao X, 2018a. Hsa_circ_0000520, a potential new circular RNA biomarker, is involved in gastric carcinoma. Cancer Biomark 21(2), 299–306. [DOI] [PubMed] [Google Scholar]
- Sun H, Xi P, Sun Z, Wang Q, Zhu B, Zhou J, Jin H, Zheng W, Tang W, Cao H, Cao X, 2018b. Circ-SFMBT2 promotes the proliferation of gastric cancer cells through sponging miR-182–5p to enhance CREB1 expression. Cancer Manag Res 10, 5725–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HD, Xu ZP, Sun ZQ, Zhu B, Wang Q, Zhou J, Jin H, Zhao A, Tang WW, Cao XF, 2018. Down-regulation of circPVRL3 promotes the proliferation and migration of gastric cancer cells. Sci Rep 8(1), 10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Yuan X, Li X, Wang D, Shan T, Wang W, Wan Q, Wang X, Yan J, Gao S, 2017. Comparative transcriptome analysis of the global circular RNAs expression profiles between SHEE and SHEEC cell lines. Am J Transl Res 9(11), 5169–5179. [PMC free article] [PubMed] [Google Scholar]
- Taborda MI, Ramirez S, Bernal G, 2017. Circular RNAs in colorectal cancer: Possible roles in regulation of cancer cells. World J Gastrointest Oncol 9(2), 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiuchi D, Eguchi H, Nagano H, Iwagami Y, Tomimaru Y, Wada H, Kawamoto K, Kobayashi S, Marubashi S, Tanemura M, Mori M, Doki Y, 2013. Involvement of microRNA-181b in the gemcitabine resistance of pancreatic cancer cells. Pancreatology 13(5), 517–523. [DOI] [PubMed] [Google Scholar]
- Tang W, Fu K, Sun H, Rong D, Wang H, Cao H, 2018. CircRNA microarray profiling identifies a novel circulating biomarker for detection of gastric cancer. Mol Cancer 17(1), 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Ji M, He G, Yang L, Niu Z, Jian M, Wei Y, Ren L, Xu J, 2017. Silencing CDR1as inhibits colorectal cancer progression through regulating microRNA-7. OncoTargets and therapy 10, 2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson DW, Dinger ME, 2016. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet 17(5), 272–283. [DOI] [PubMed] [Google Scholar]
- Tian F, Wang Y, Xiao Z, Zhu X, 2017. [Circular RNA CircHIPK3 Promotes NCI-H1299 and NCI-H2170 Cell Proliferation through miR-379 and its Target IGF1]. Zhongguo Fei Ai Za Zhi 20(7), 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Chen R, Li T, Xiao B, 2018. Reduced expression of circRNA hsa_circ_0003159 in gastric cancer and its clinical significance. J Clin Lab Anal 32(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari AK, Roy HK, Lynch HT, 2016. Lynch syndrome in the 21st century: clinical perspectives. QJM 109(3), 151–158. [DOI] [PubMed] [Google Scholar]
- Vasen HF, Tomlinson I, Castells A, 2015. Clinical management of hereditary colorectal cancer syndromes. Nat Rev Gastroenterol Hepatol 12(2), 88–97. [DOI] [PubMed] [Google Scholar]
- Vicens Q, Westhof E, 2014. Biogenesis of Circular RNAs. Cell 159(1), 13–14. [DOI] [PubMed] [Google Scholar]
- Vidal AF, Ribeiro-Dos-Santos AM, Vinasco-Sandoval T, Magalhaes L, Pinto P, Anaissi AKM, Demachki S, de Assumpcao PP, Dos Santos SEB, Ribeiro-Dos-Santos A, 2017. The comprehensive expression analysis of circular RNAs in gastric cancer and its association with field cancerization. Sci Rep 7(1), 14551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A, Herman J, Schulick R, Hruban RH, Goggins M, 2011. Pancreatic cancer. Lancet 378(9791), 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller LP, Deshpande V, Pyrsopoulos N, 2015. Hepatocellular carcinoma: A comprehensive review. World J Hepatol 7(26), 2648–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Li X, Zhao X, Xue Y, 2019. Detection of a 5-circRNA signature to improve prognostic prediction in gastric cancer. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. [DOI] [PubMed] [Google Scholar]
- Wang F, Nazarali AJ, Ji S, 2016. Circular RNAs as potential biomarkers for cancer diagnosis and therapy. Am J Cancer Res 6(6), 1167–1176. [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang J, Cao X, Xu L, Chen L, 2018. Hsa_circ_0014717 is downregulated in colorectal cancer and inhibits tumor growth by promoting p16 expression. Biomedicine & Pharmacotherapy 98, 775–782. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang J, Cao X, Xu L, Chen L, 2018. Hsa_circ_0014717 is downregulated in colorectal cancer and inhibits tumor growth by promoting p16 expression. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 98, 775–782. [DOI] [PubMed] [Google Scholar]
- Wang G, Liu W, Zou Y, Wang G, Deng Y, Luo J, Zhang Y, Li H, Zhang Q, Yang Y, Chen G, 2019. Three isoforms of exosomal circPTGR1 promote hepatocellular carcinoma metastasis via the miR449a-MET pathway. EBioMedicine 40, 432–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Shi J, Luo Y, Liao Q, Niu Y, Zhang F, Shao Z, Ding Y, Zhao L, 2014. LIM and SH3 protein 1 induces TGFbeta-mediated epithelial-mesenchymal transition in human colorectal cancer by regulating S100A4 expression. Clin Cancer Res 20(22), 5835–5847. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen W, Lin H, Zhang J, 2019. [Role of miRNA-340 in modulating gastric cancer cell proliferation and bioinformatic analysis]. Nan fang yi ke da xue xue bao = Journal of Southern Medical University 39(7), 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li X, Lu L, He L, Hu H, Xu Z, 2018. Circular RNA hsa_circ_0000567 can be used as a promising diagnostic biomarker for human colorectal cancer. J Clin Lab Anal 32(5), e22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KW, Dong M, 2019. Role of circular RNAs in gastric cancer: Recent advances and prospects. World J Gastrointest Oncol 11(6), 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shen J, Jiang Y, 2018. Circ_0027599/PHDLA1 suppresses gastric cancer progression by sponging miR-101–3p.1. Cell Biosci 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang Q, Sun H, Tang W, Yang L, Xu Z, Liu Z, Jin H, Cao X, 2019. Circ-TTC17 Promotes Proliferation and Migration of Esophageal Squamous Cell Carcinoma. Dig Dis Sci 64(3), 751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Huang L, Zhang J, Pan F, Li B, Yan Y, Jia B, Liu H, Li S, 2015. Decreased expression of hsa_circ_001988 in colorectal cancer and its clinical significances. International journal of clinical and experimental pathology 8(12), 16020. [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Huang L, Zhang J, Pan F, Li B, Yan Y, Jia B, Liu H, Li S, Zheng W, 2015. Decreased expression of hsa_circ_001988 in colorectal cancer and its clinical significances. Int J Clin Exp Pathol 8(12), 16020–16025. [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu S, Chen Y, Zheng X, Li T, Guo J, 2019. Identification of hsa_circ_0005654 as a new early biomarker of gastric cancer. Cancer Biomark. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma K, Pitts S, Cheng Y, Liu X, Ke X, Kovaka S, Ashktorab H, Smoot DT, Schatz M, Wang Z, Meltzer SJ, 2018. Novel circular RNA NF1 acts as a molecular sponge, promoting gastric cancer by absorbing miR-16. Endocr Relat Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Rudolph M, Song Z, Lausser L, Schnack C, Begus-Nahrmann Y, Scheithauer M-O, Rettinger G, Otto M, Tumani H, Thal D, 2012. Chitinase enzyme activity in CSF is a powerful biomarker of Alzheimer disease. Neurology 78(8), 569–577. [DOI] [PubMed] [Google Scholar]
- Wei J, Wang J, Gao X, Qi F, 2019. Identification of differentially expressed circRNAs and a novel hsa_circ_0000144 that promote tumor growth in gastric cancer. 19, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng W, Wei Q, Toden S, Yoshida K, Nagasaka T, Fujiwara T, Cai S, Qin H, Ma Y, Goel A, 2017. Circular RNA ciRS-7-A Promising Prognostic Biomarker and a Potential Therapeutic Target in Colorectal Cancer. Clin Cancer Res 23(14), 3918–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, W.H.O., 2019. Hepatitis C
- Wilusz JE, 2017. Circular RNAs: Unexpected outputs of many protein-coding genes. RNA Biol 14(8), 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer KW, 2015. Circulating microRNA biomarker studies: pitfalls and potential solutions. Clinical chemistry 61(1), 56–63. [DOI] [PubMed] [Google Scholar]
- Wu L, Liu D, Yang Y, 2019. Enhanced expression of circular RNA circ-DCAF6 predicts adverse prognosis and promotes cell progression via sponging miR-1231 and miR-1256 in gastric cancer. Experimental and molecular pathology 110, 104273. [DOI] [PubMed] [Google Scholar]
- Wu Q, Wang H, Liu L, Zhu K, Yu W, Guo J, 2019. Hsa_circ_0001546 acts as a miRNA-421 sponge to inhibit the chemoresistance of gastric cancer cells via ATM/Chk2/p53-dependent pathway. Biochem Biophys Res Commun. [DOI] [PubMed] [Google Scholar]
- Wu X, Zhou J, Wu Z, Chen C, Liu J, Wu G, Zhai J, Liu F, Li G, 2017. miR-101–3p Suppresses HOX Transcript Antisense RNA (HOTAIR)-Induced Proliferation and Invasion Through Directly Targeting SRF in Gastric Carcinoma Cells. Oncol Res 25(8), 1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Qiu M, Chen R, Wang S, Leng X, Wang J, Xu Y, Hu J, Dong G, Xu PL, Yin R, 2016. Circular RNA has_circ_0067934 is upregulated in esophageal squamous cell carcinoma and promoted proliferation. Sci Rep 6, 35576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Shao Y, Sun W, Ye G, Zhang X, Xiao B, Guo J, 2018. Downregulated expression of hsa_circ_0074362 in gastric cancer and its potential diagnostic values. Biomark Med 12(1), 11–20. [DOI] [PubMed] [Google Scholar]
- Xu B, Yang T, Wang Z, Zhang Y, Liu S, Shen M, 2018. CircRNA CDR1as/miR-7 signals promote tumor growth of osteosarcoma with a potential therapeutic and diagnostic value. Cancer Manag Res 10, 4871–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wang C, Song H, Xu Y, Ji G, 2019. RNA-Seq profiling of circular RNAs in human colorectal Cancer liver metastasis and the potential biomarkers. Mol Cancer 18(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Feng X, Hao X, Wang P, Zhang Y, Zheng X, Li L, Ren S, Zhang M, Xu M, 2019. CircSETD3 (Hsa_circ_0000567) acts as a sponge for microRNA-421 inhibiting hepatocellular carcinoma growth. J Exp Clin Cancer Res 38(1), 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zhang M, Zheng X, Yi P, Lan C, Xu M, 2017. The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of hepatic microvascular invasion in hepatocellular carcinoma. Journal of cancer research and clinical oncology 143(1), 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zhang M, Zheng X, Yi P, Lan C, Xu M, 2017. The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of hepatic microvascular invasion in hepatocellular carcinoma. Journal of cancer research and clinical oncology 143(1), 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Li S, Lin Y, Chen H, Hu Z, Mao Y, Xu X, Wu J, Zhu Y, Zheng X, Luo J, Xie L, 2013. MicroRNA-124–3p inhibits cell migration and invasion in bladder cancer cells by targeting ROCK1. J Transl Med 11, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XW, Zheng BA, Hu ZM, Qian ZY, Huang CJ, Liu XQ, Wu WD, 2017. Circular RNA hsa_circ_000984 promotes colon cancer growth and metastasis by sponging miR-106b. Oncotarget 8(53), 91674–91683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Yao Y, Gao P, Cui Y, 2019. Upregulated circular RNA circ_0030235 predicts unfavorable prognosis in pancreatic ductal adenocarcinoma and facilitates cell progression by sponging miR-1253 and miR-1294. Biochem Biophys Res Commun 509(1), 138–142. [DOI] [PubMed] [Google Scholar]
- Yang F, Liu DY, Guo JT, Ge N, Zhu P, Liu X, Wang S, Wang GX, Sun SY, 2017. Circular RNA circ-LDLRAD3 as a biomarker in diagnosis of pancreatic cancer. World J Gastroenterol 23(47), 8345–8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Cong X, Ren M, Sun H, Liu T, Chen G, 2019. Circular RNA hsa_circRNA_0007334 is Predicted to Promote MMP7 and COL1A1 Expression by Functioning as a miRNA Sponge in Pancreatic Ductal Adenocarcinoma. 2019, 7630894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Zhang C, Chen Y, Gao S, 2019. Downregulation of circular RNA circ-LDLRAD3 suppresses pancreatic cancer progression through miR-137–3p/PTN axis. Life sciences 239, 116871. [DOI] [PubMed] [Google Scholar]
- Yao T, Chen Q, Shao Z, Song Z, Fu L, Xiao B, 2018. Circular RNA 0068669 as a new biomarker for hepatocellular carcinoma metastasis. J Clin Lab Anal 32(8), e22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Luo J, Hu K, Lin J, Huang H, Wang Q, Zhang P, Xiong Z, He C, Huang Z, Liu B, Yang Y, 2017. ZKSCAN1 gene and its related circular RNA (circZKSCAN1) both inhibit hepatocellular carcinoma cell growth, migration, and invasion but through different signaling pathways. Mol Oncol 11(4), 422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G-H, Gao F-C, Tian J, Zhang W-B, 2019. Hsa_circ_101882 promotes migration and invasion of gastric cancer cells by regulating EMT. Journal of Clinical Laboratory Analysis 0(0), e23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong W, Zhuoqi X, Baocheng W, Dongsheng Z, Chuan Z, Yueming S, 2018. Hsa_circ_0071589 promotes carcinogenesis via the miR-600/EZH2 axis in colorectal cancer. Biomedicine & Pharmacotherapy 102, 1188–1194. [DOI] [PubMed] [Google Scholar]
- Yu J, Xu Q. g., Wang Z. g., Yang Y, Zhang L, Ma J. z., Sun S. h., Yang F, Zhou W. p., 2018. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. Journal of hepatology 68(6), 1214–1227. [DOI] [PubMed] [Google Scholar]
- Yu J, Xu QG, Wang ZG, Yang Y, Zhang L, Ma JZ, Sun SH, Yang F, Zhou WP, 2018. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J Hepatol 68(6), 1214–1227. [DOI] [PubMed] [Google Scholar]
- Yu J, Yang M, Zhou B, Luo J, Zhang Z, Zhang W, Yan Z, 2019. CircRNA-104718 acts as competing endogenous RNA and promotes hepatocellular carcinoma progression through microRNA-218–5p/TXNDC5 signaling pathway. Clin Sci (Lond) 133(13), 1487–1503. [DOI] [PubMed] [Google Scholar]
- Yu L, Gong X, Sun L, Zhou Q, Lu B, Zhu L, 2016. The Circular RNA Cdr1as Act as an Oncogene in Hepatocellular Carcinoma through Targeting miR-7 Expression. PLoS One 11(7), e0158347. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yuan Y, Liu W, Zhang Y, Zhang Y, Sun S, 2018. CircRNA circ_0026344 as a prognostic biomarker suppresses colorectal cancer progression via microRNA-21 and microRNA-31. Biochem Biophys Res Commun 503(2), 870–875. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Liu W, Zhang Y, Zhang Y, Sun S, 2018. CircRNA circ_0026344 as a prognostic biomarker suppresses colorectal cancer progression via microRNA-21 and microRNA-31. Biochemical and biophysical research communications 503(2), 870–875. [DOI] [PubMed] [Google Scholar]
- Zang J, Lu D, Xu A, 2018. The interaction of circRNAs and RNA binding proteins: An important part of circRNA maintenance and function. J Neurosci Res. [DOI] [PubMed] [Google Scholar]
- Zaphiropoulos PG, 1993. Differential expression of cytochrome P450 2C24 transcripts in rat kidney and prostate: evidence indicative of alternative and possibly trans splicing events. Biochem Biophys Res Commun 192(2), 778–786. [DOI] [PubMed] [Google Scholar]
- Zaphiropoulos PG, 1996. Circular RNAs from transcripts of the rat cytochrome P450 2C24 gene: correlation with exon skipping. Proc Natl Acad Sci U S A 93(13), 6536–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng K, Chen X, Xu M, Liu X, Hu X, Xu T, Sun H, Pan Y, He B, Wang S, 2018. CircHIPK3 promotes colorectal cancer growth and metastasis by sponging miR-7. Cell Death Dis 9(4), 417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zeng K, Chen X, Xu M, Liu X, Hu X, Xu T, Sun H, Pan Y, He B, Wang S, 2018. CircHIPK3 promotes colorectal cancer growth and metastasis by sponging miR-7. Cell death & disease 9(4), 417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang C, Zhang C, Lin J, Wang H, 2018. Circular RNA Hsa_Circ_0091579 Serves as a Diagnostic and Prognostic Marker for Hepatocellular Carcinoma. Cell Physiol Biochem 51(1), 290–300. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu H, Hou L, Wang G, Zhang R, Huang Y, Chen X, Zhu J, 2017. Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424–5p and regulating LATS1 expression. Mol Cancer 16(1), 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu H, Zhao P, Zhou H, Mao T, 2019. Has_circ_0055625 from circRNA profile increases colon cancer cell growth by sponging miR-106b-5p. Journal of cellular biochemistry 120(3), 3027–3037. [DOI] [PubMed] [Google Scholar]
- Zhang N, Li G, Li X, Xu L, Chen M, 2018. Circ5379–6, a circular form of tumor suppressor PPARalpha, participates in the inhibition of hepatocellular carcinoma tumorigenesis and metastasis. Am J Transl Res 10(11), 3493–3503. [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Guo Z, Hu R, He X, Jiao X, Zhu X, 2015. Interaction between microRNA-181a and TNFAIP1 regulates pancreatic cancer proliferation and migration. Tumour Biol 36(12), 9693–9701. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zuo Z, Shang W, Wu A, Bi R, Wu J, Li S, Sun X, Jiang L, 2017. Identification of differentially expressed circular RNAs in human colorectal cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 39(3), 1010428317694546. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zuo Z, Shang W, Wu A, Bi R, Wu J, Li S, Sun X, Jiang L, 2017. Identification of differentially expressed circular RNAs in human colorectal cancer. Tumor Biology 39(3), 1010428317694546. [DOI] [PubMed] [Google Scholar]
- Zhang PF, Wei CY, Huang XY, Peng R, Yang X, Lu JC, Zhang C, Gao C, Cai JB, Gao PT, Gao DM, Shi GM, Ke AW, Fan J, 2019. Circular RNA circTRIM33–12 acts as the sponge of MicroRNA-191 to suppress hepatocellular carcinoma progression. Mol Cancer 18(1), 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Xu J, Zhao J, Wang X, 2018. Silencing of hsa_circ_0007534 suppresses proliferation and induces apoptosis in colorectal cancer cells. Eur Rev Med Pharmacol Sci 22(1), 118–126. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu Y, Fan C, Wang L, Li A, Zhou H, Cai L, Miao Y, Li Q, Qiu X, Wang E, 2017. Lasp1 promotes malignant phenotype of non-small-cell lung cancer via inducing phosphorylation of FAK-AKT pathway. Oncotarget 8(43), 75102–75113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.l., Xu L.l., Wang F, 2017. Hsa_circ_0020397 regulates colorectal cancer cell viability, apoptosis and invasion by promoting the expression of the miR-138 targets TERT and PD-L1. Cell biology international 41(9), 1056–1064. [DOI] [PubMed] [Google Scholar]
- Zhang XO, Dong R, Zhang Y, Zhang JL, Luo Z, Zhang J, Chen LL, Yang L, 2016. Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res 26(9), 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L, 2014. Complementary sequence-mediated exon circularization. Cell 159(1), 134–147. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu H, Li W, Yu J, Li J, Shen Z, Ye G, Qi X, Li G, 2017. CircRNA_100269 is downregulated in gastric cancer and suppresses tumor cell growth by targeting miR-630. Aging (Albany NY) 9(6), 1585–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Q, Zhu D, Rong J, Shi W, Cao X, 2019. Up-regulation of circ-SMAD7 inhibits tumor proliferation and migration in esophageal squamous cell carcinoma. Biomed Pharmacother 111, 596–601. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lin W, Gao L, Chen K, Yang C, Zhuang L, Peng S, Kang M, Lin J, 2019. Hsa_circ_0004370 promotes esophageal cancer progression through miR-1294/LASP1 pathway. Biosci Rep 39(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Chen S, Li T, Xiao B, Zhang X, 2018. Clinical values of circular RNA 0000181 in the screening of gastric cancer. J Clin Lab Anal 32(4), e22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Chen T, Li C, Xu C, Ding C, Chen J, Ju S, Zhang Z, Liang Z, Cui Z, Zhao J, 2019. A circular RNA hsa_circ_0079929 inhibits tumor growth in hepatocellular carcinoma. Cancer Manag Res 11, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Wang Y, Cheng Y, Wang W, Lu B, Zhu L, Ma Y, 2018. Circular RNA circC3P1 suppresses hepatocellular carcinoma growth and metastasis through miR-4641/PCK1 pathway. Biochem Biophys Res Commun 499(4), 1044–1049. [DOI] [PubMed] [Google Scholar]
- Zhong R, Chen Z, Mo T, Li Z, Zhang P, 2019. Potential Role of circPVT1 as a proliferative factor and treatment target in esophageal carcinoma. 19, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Wang J, Hou J, Zhang Q, Xu H, Hu J, Zhao J, Feng J, 2018. Circular RNA hsa_circ_0000993 inhibits metastasis of gastric cancer cells. Epigenomics 10(10), 1301–1313. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Du Y, Yang X, Mo Y, Fan C, Xiong F, Ren D, Ye X, Li C, Wang Y, 2018. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Molecular cancer 17(1), 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Yin C, Dang Y, Ye F, Zhang G, 2015. Identification of the long non-coding RNA H19 in plasma as a novel biomarker for diagnosis of gastric cancer. Scientific reports 5, 11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Xu Y, Chen Y, Yan F, 2017. Circular BANP, an upregulated circular RNA that modulates cell proliferation in colorectal cancer. Biomedicine & Pharmacotherapy 88, 138–144. [DOI] [PubMed] [Google Scholar]
- Zhu M, Xu Y, Chen Y, Yan F, 2017. Circular BANP, an upregulated circular RNA that modulates cell proliferation in colorectal cancer. Biomed Pharmacother 88, 138–144. [DOI] [PubMed] [Google Scholar]
- Zhu P, Ge N, Liu D, Yang F, Zhang K, Guo J, Liu X, Wang S, Wang G, Sun S, 2018. Preliminary investigation of the function of hsa_circ_0006215 in pancreatic cancer. Oncol Lett 16(1), 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Lu G, Luo Z, Gui F, Wu J, Zhang D, Ni Y, 2018. CircRNA circ_0067934 promotes tumor growth and metastasis in hepatocellular carcinoma through regulation of miR-1324/FZD5/Wnt/β-catenin axis. Biochemical and biophysical research communications 497(2), 626–632. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Liu Y, Xiao B, Cai H, Liu M, Ma L, Yin H, Wang F, 2019. The circular RNA PVT1/miR-203/HOXD3 pathway promotes the progression of human hepatocellular carcinoma. Biol Open 8(9), bio043687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Rong Z, Luo Z, Yu Z, Zhang J, Qiu Z, Huang C, 2019. Circular RNA circNHSL1 promotes gastric cancer progression through the miR-1306–3p/SIX1/vimentin axis. Molecular cancer 18(1), 126–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo F, Lin H, Chen Z, Huang Z, Hu J, 2017. The expression profile and clinical significance of circRNA0003906 in colorectal cancer. OncoTargets and therapy 10, 5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo F, Lin H, Chen Z, Huang Z, Hu J, 2017. The expression profile and clinical significance of circRNA0003906 in colorectal cancer. Onco Targets Ther 10, 5187–5193. [DOI] [PMC free article] [PubMed] [Google Scholar]