Abstract
Sleep deprivation (SD) is associated with a broad spectrum of cognitive and behavioral complications, including emotional lability, enhanced stress reactivity, as well as deficits in executive functions, decision making, and impulse control. These impairments, which have profound negative consequences on the health and productivity of many individuals, reflect alterations of the prefrontal cortex (PFC) and its connectivity with subcortical regions. However, the molecular underpinnings of these alterations remain elusive. Our group and others have begun examining how the neurobehavioral outcomes of SD may be influenced by neuroactive steroids, a family of molecules deeply implicated in sleep regulation and stress response. These studies have revealed that, similar to other stressors, acute SD leads to increased synthesis of the neurosteroid allopregnanolone (AP) in the PFC. Whereas this upregulation is likely aimed at counterbalancing the detrimental impact of oxidative stress induced by SD, the increase in prefrontal AP levels contributes to deficits in sensorimotor gating and impulse control, signaling a functional impairment of PFC. This scenario suggests that the synthesis of neuroactive steroids during acute SD may be enacted as a neuroprotective response in the PFC; however, such compensation may in turn set off neurobehavioral complications by interfering with the corticolimbic connections responsible for executive functions and emotional regulation.
Keywords: sleep deprivation, neurosteroids, AP, prefrontal cortex
Introduction
Sleep is an essential function for energy conservation as well as the homeostasis of multiple physiological and behavioral processes. Although most mechanisms of sleep remain partially elusive, the overwhelming consensus of the scientific and medical community indicates that healthy sleep habits are critical for the integrity of cognitive, metabolic, and immune functions. While the American Academy of Sleep Medicine and the National Sleep Foundation recommend that adults sleep at least 7 h per day [1,2], the increasing demand for long work shifts and “around-the-clock” work availability has led to a marked reduction of the average sleep duration in high-income countries [3,4]. Recent surveys have shown that approximately 20–30% of Americans experience occasional episodes of sleep restriction or fragmentation due to occupational demands, lifestyle choices, and behavioral disturbances [5–9]. The issue of sleep loss has become so pervasive in society that the Center for Disease Control (CDC) has recently elevated it to public health epidemic status [8]. In addition, sleep loss is a rampant problem among adolescents and college students, and often signals the excessive use of stimulants in this population [10–12]. The impact of sleep restriction is particularly harmful for high-responsibility professionals forced to lose sleep due to prolonged work shifts or emergency situations, e.g., health caregivers [13–17], military personnel [18–20], firefighters [21,22], and airline pilots [23–25]. The economic repercussions of sleep deficits are staggering [26], due to their negative influence on public safety and work productivity [27–30]. These devastating effects are known to be contributed by alterations of executive functions, a class of processes that enable the enactment of purposive, goal-directed tasks and attune cognitive and emotional responses to the environment. These effects reflect the adverse consequences of sleep loss on the prefrontal cortex (PFC) [31], a brain region that plays a key role in the orchestration of executive functions [32–35]. One of the best experimental models to study the neurochemical underpinnings of cognitive and neurobehavioral deficits induced by sleep loss is total sleep deprivation (SD), a condition of forced wakefulness lasting 24–72 h imposed to either volunteers or laboratory animals [36,37] Conversely, partial sleep deprivation entails a restriction of sleep duration to 2–6 h per night, over several nights [38, 39]. In animals, both conditions are typically achieved by exposing them to manipulations that interfere with sleep initiation, which can be inherently stressful [40]. For this and other reasons, neither procedure is fully appropriate to capture the complexity of sleep loss in the clinical setting; nevertheless, these manipulations have proven extremely useful to gain insight into the negative consequences of hyposomnia, as well as their neurobiological mechanisms. In this article, we will first discuss how SD studies in both volunteers and animal models have helped understand the nature of the neurobehavioral deficits associated with sleep loss. Then, we will overview recent preclinical evidence suggesting that neuroactive steroids - an important class of mediators that regulate sleep and stress response - may be critical in mediating and shaping the executive function deficits associated with SD. Finally, we will discuss how the implication of these steroids may offer a novel perspective to understand some of the pathophysiological links between hyposomnia and different psychopathological states, including schizophrenia and depression.
Neurobehavioral complications of sleep loss: clinical evidence
In this section, we briefly review the main cognitive and behavioral effects of sleep restriction in humans, as well as their neurological and endocrine underpinnings. A comprehensive description of the effects of sleep loss on brain and behavioral processes is beyond the scope of this article; the interested reader is referred to the references [41–45].
Cognitive consequences of SD.
Although the effects of sleep restriction are characterized by marked intra- and inter-subject variability, neuroimaging studies have shown that the PFC and its functional connections with subcortical regions (such as amygdala and hippocampus) are highly vulnerable to the harmful impact of this condition [46]. Specifically, most individuals experience a progressive intrusion of microsleep episodes, during which they lose the ability to respond to sensory stimulation for 5–30 s [47]. This situation likely translates into a gradual worsening of PFC activity and executive functions, including information processing and gating, attention, working memory, inhibitory control, cognitive flexibility, and problem solving [43, 48–52]. The escalation of wake-state instability results in prolonged reaction time, errors of omission and commission, poor short-term recall, perseveration of ineffective responses, reduced divergent thinking and poor insight into one’s own actions [53–56]. Under these conditions, the execution of multitasking and flexible cognitive performances entail the recruitment of compensatory adaptive mechanisms - such as the activation of the parietal cortex and thalamus [57–63]. Although sleep is critical for learning and memory [64,65], the available evidence on the effects of SD on these functions is somewhat inconsistent. For example, different studies have shown contradictory results with respect to the impact of SD on verbal recall and reasoning ability [66–70], possibly due to differences in study design and methodology. The cognitive effects of chronic partial SD are less well-understood, but recent data support that they are akin to those induced by total SD [43, 71–75].
Affective consequences of SD.
Depressive symptoms are one of the best-documented consequences of several conditions associated with poor sleep quality and alterations in sleep architecture, including fragmented sleep, decreased slow wave sleep, shortened REM latency, and increased REM density [76, 77, 78]. Conversely, a wide body of literature has established that acute SD has mood-enhancing properties, which may reflect the involvement of the PFC (as well as its connections with the amygdala) in socio-affective reactivity [79]. Indeed, sleep-deprived individuals are hyperresponsive to both positive and negative stimuli, due to the increased activation of reward-related circuits and the amygdala [80–82]. Furthermore, they display poor self-monitoring, behavioral disinhibition, attenuated response to losses and higher propensity to engage in impulsive actions, as a result of the combination of PFC deficits and hyperactivation of the nucleus accumbens [82–84]. The negative effects of SD on mood are likely to be contributed by the loss of REM sleep. This stage modulates emotional responses to facial cues [85]; in particular, the threat stimulation theory posits that the purpose of dreams in REM sleep is to rehearse real-life challenges and prepare for appropriate responses when similar scenarios occur [86]. The activation of the mesolimbic dopaminergic system during REM sleep leads to the acquisition and consolidation of emotionally and motivationally salient information [87].
Neuroendocrine consequences of SD.
Sleep is critical for circadian rhythmicity as well as homeostatic hormonal and metabolic balances. Accordingly, SD is associated with a broad range of alterations of endocrine factors, including cortisol, growth hormone (GH), prolactin, thyroid-stimulating hormone (TSH), gonadotropin-releasing hormone (GnRH), testosterone, leptin, ghrelin, and insulin [88–93]. In keeping with these effects, SD has been shown to result in increased appetite for highly caloric food, resulting in a higher risk of obesity and diabetes [94, 95]. In addition to these changes, SD has a complex effect on the endocrine reactivity to stress. Given that stress is defined as a condition of threatened homeostasis [79], SD inherently heightens stress sensitivity; furthermore, several studies have shown that poor sleep quality is associated with increased allostatic load [97, 98], defined as the cumulative damage of allostatic responses due to chronic stress [99, 100]. Accordingly, sleep loss is associated with the increased release of all major hormones of the hypothalamic-pituitary-adrenocortical (HPA) axis, namely corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH) and cortisol [101, 102]. The relationship between sleep and HPA axis regulation is complicated by the fact that, while NREM sleep is associated with a suppression of the HPA axis [103, 104], the mentation of stressful dreams during REM sleep leads to increased cortisol concentrations [105]. From this perspective, it is worth noting that CRH promotes both REM sleep [106] and wakefulness [103], and can therefore exacerbate some of the behavioral complications of SD. Several studies suggest that the 24-h urinary cortisol levels are positively correlated with the amount of REM sleep [107, 108], signifying a critical role of REM in stress response. In addition to the effects of SD on stress reactivity, several lines of evidence point to negative effects on sex hormones. Indeed, total SD has been shown to reduce the levels of progesterone in both sexes and testosterone in men [109]. Although no significant change in estradiol concentrations were found in sleep-deprived women and men [109], research in female rats has shown that REM SD during diestrus reduces the content of estrogens and increases corticosterone levels [110]. Furthermore, ovarian hormones promote recovery from SD in rats [111]. Overall, these studies suggest that SD is conducive to a wide array of endocrine disruptions, which may participate in the neurobehavioral complications of this manipulation. A critical tool to examine this hypothesis is afforded by rodent models [37, 112]. In the next section, we will outline how studies in laboratory animals have been instrumental for our current understanding of the neurobiological underpinnings of the negative impact of SD.
Neurobehavioral complications of SD: preclinical evidence
Several rodent studies have indicated that SD induces profound consequences across an ample spectrum of behavioral paradigms. Such alterations reflect changes in diverse brain neurotransmitters, including dopamine, acetylcholine, serotonin, and GABA. In particular, the dopaminergic mesocortical pathway - which projects from the ventral tegmental area (VTA) to the prefrontal cortex (PFC) - plays an essential role in a number of cognitive and emotional processes and may be directly implicated in some key behavioral consequences of SD. In keeping with this idea, Tufik and colleagues (1981) reported that SD leads to a state of supersensitivity of post-synaptic dopamine receptors in the rat brain [113]. Accordingly, SD rats respond to the administration of low doses of dopaminergic agonists with paroxysmal increases in dopamine-dependent phenotypes [113]. These modifications are not related to changes in dopamine synthesis or release [114]. The two major classes of dopamine receptors, D1- and D2- like, are likely both implicated in the phenotypic complications of SD. Indeed, SD-subjected rats exhibit greater dopaminergic-mediated responses upon striatal upregulation of D2 receptors [115]; conversely, pre-treatment with the D2 receptor antagonist haloperidol rescues SD-mediated behavioral alterations [113]. At the same time, D1 receptors have been found to be implicated in SD-induced learning impairments in flies [116].
SD also affects serotonergic and noradrenergic signaling [117]. Unlike the effects on the dopaminergic system, binding studies showed an overall decrease of the serotonin receptors 5HT1A, 5HT2 and serotonin transporter (SERT) in rats subjected to SD [118]. Norepinephrine may indirectly modulate SD-mediated phenotypes by interacting with the dopamine system [119]. Furthermore, some reports suggest that these effects might be contributed by both α and β-adrenoceptors, as a reduction in β expression [120] as well as a down- and up-regulation of α1 and α2, has been found in the brain of SD rats [120].
Although these findings have been extremely useful to improve our understanding of the behavioral abnormalities associated with SD and their neurobiological basis, the translational value of most of these preclinical studies is often limited by a lack of clear correspondence between behavioral responses in animal models and human phenotypes. A novel opportunity to overcome this problem comes from the Research Domain Criteria (RDoC) initiative, a new research framework focused on dimensional and transdiagnostic approaches. The strategy outlined by the RDoC is aimed at the systematization of biological knowledge of risk factors (including information on genes, anatomical circuits and specific psychological responses) to gain insight into different domains of psychopathology [121]. The integration of cross-species tests focusing on these different dimensions enable the use of rodent models to test mechanistic hypotheses [122]. Building on this perspective, we observed that in rats, SD impairs prepulse inhibition (PPI) of the acoustic startle reflex [123]. This index, which can be studied in both rats and humans, measures how the startle response is reduced when the eliciting acoustic burst is immediately preceded by a weaker pre-stimulus. The importance of PPI in neurocognitive research comes from its value as an operational measure of sensorimotor gating, a key early-stage information-processing function that enables the formation of salience maps by filtering out irrelevant stimuli [124]. The importance of PPI in information processing is highlighted by its correlation with the efficiency and reaction time of preattentional and attentional performances, particularly in the context of complex tasks [125]; furthermore, higher PPI levels are associated with better and faster problem-solving [126]. A very exciting development of this research came in 2014, when our findings of SD-induced PPI deficits were replicated in humans [127]. Healthy volunteers subjected to 24-h SD exhibited PPI deficits in association with perceptual distortions and cognitive fragmentation. In striking analogy with our observations in rat models [123], these deficits were fully reversed after a night of normal sleep [127].
Other models of the negative consequences of SD on executive functions have been developed. For example, recent studies have shown that SD impairs the performance in the 5-choice continuous performance test (5C-CPT) in humans and rodents alike [128]. The 5C-CPT is the gold standard to assess deficits in sustained and selective attention, as well as vigilance, and has high cross-species validity [129, 130]. Another valuable example is afforded by the Iowa Gambling Task (IGT), a well-consolidated task for preference-based decision making [131]. In a rodent counterpart of this test [132], SD was recently shown to impair decision-making abilities [133].
Neurosteroids are involved in the neurobehavioral effects of SD.
Synthesis and molecular actions of neurosteroids.
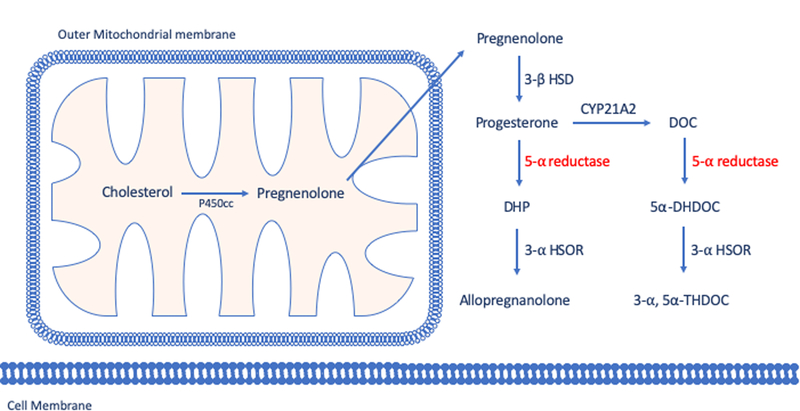
The key precursor of most neurosteroids, pregnenolone, derives from the cleavage of the side chain from cholesterol, and undergoes conversion to progesterone by 3β-hydroxysteroid dehydrogenase (3β-HSD). In turn, progesterone can be reconverted to testosterone via joint actions of cytochrome P450 17A1 (CYP17A1) and 17β-hydroxysteroid dehydrogenase (17β-HSD). An alternative metabolic pathway of progesterone entails its transformation into deoxycorticosterone (DOC) by 21α-hydroxylase. Progesterone and DOC are then converted by 5α-reductase (5αR) into their metabolites 5α-dihydroprogesterone (DHP) and 5α-dihydrodeoxycorticosterone (DHDOC) [134]. In turn, the enzyme 3α-hydroxysteroid oxidoreductase (3α-HSOR) converts these steroids to allopregnanolone (AP; 3α,5α−3-hydroxypregnan-20-one) and tetrahydrodeoxycorticosterone (THDOC; 3α,5α−3,21-dihydroxypregnan-20-one) (Fig.1). Generally, the acute effects of neurosteroids are not mediated by the classical genomic steroid hormone receptors. Several classes of neurosteroids modulate brain excitability primarily by interacting with neuronal membrane receptors and ion channels, principally GABA-A receptors [135, 136]. The effects of these neurosteroids occur rapidly (within minutes), whereas steroid hormone actions via intracellular steroid receptors are usually slow in onset and elicit long-lasting effects [136]. AP and THDOC are potent allosteric modulators of GABA-A receptors and can even open GABA-A channels directly in the absence of GABA at sufficiently high concentrations [137, 138]. Additionally, 3α-androstanediol potentiates GABA-A function, albeit with lower potency than AP and THDOC [139]. In contrast with these neurosteroids, many of their 3β-hydroxyl isomers act as non-competitive GABA-A receptor modulating steroid antagonists (GAMSAs) [140]. The positive modulation of GABA-A receptors by AP and THDOC is dependent on their specific subunit composition of GABA-A receptors. For example, extrasynaptic GABA-A receptors expressing δ subunits may primarily bind to GABA only in the presence of neurosteroids [141–143]. The importance of δ subunits is underscored by evidence indicating the key role of these proteins in the physiological effects of neurosteroids [144].
Figure 1. Simplified schematization of neurosteroids biosynthesis by 5-alpha reductase pathway.
For a complete steroid synthesis pathway including other neurosteroids, see Frau and Bortolato 2018.
Several preclinical and clinical studies suggest that the sex differences susceptibility reported in certain neuropsychiatric disorders result on dimorphic acute and chronic effects of brain and peripheral steroids. Sex differences in relation to steroid activity and imbalance have been described in several psychiatric conditions, such as depression and anxiety [145, 146], alcohol and cocaine addiction [147, 148] schizophrenia [149] and Tourette Syndrome [150], and others [151]. Of note, the majority of these sex-dependently psychiatric diseases are related to stress, GABA-A receptors and allopregnanolone [146, 148, 152–155], suggesting that, depending on sex, AP and other 5-alpha reduced neurosteroids differently modulate brain function in response to acute and chronic stress.
Role of neurosteroids in stress response regulation.
As mentioned above, neurosteroid synthesis is critical for the orchestration of stress response [156]. Indeed, neurosteroid synthesis is promoted by acute stress [157]. The mechanisms whereby stress promotes neurosteroidogenesis remain partially elusive; however, several lines of evidence suggest that a critical mechanism by which stress promotes the synthesis of AP is the upregulation of 5αR. The expression of this enzyme in the cortex is sensitive to acute stress [158] and reduced by chronic psychosocial stress [159]. Recent evidence suggests that, in the cortex, glutamatergic pyramidal neurons may be predominantly involved in the synthesis of GABAergic neurosteroids, possibly following the activation of NMDA glutamate receptors and neuronal nitric oxide synthase (n-NOS) [160–163]. Notably, our group showed that one of the two main 5αR isoenzymes (type 2) is strategically segregated in glutamatergic pyramidal neurons in the cortex [164], suggesting a connection between these mechanisms. To the best of our knowledge, however, the mechanisms whereby NMDA receptor activation may promote 5αR synthesis or activation remain unknown to date. Irrespective of these issues, the idea that AP and similar neurosteroids reduce excitability by modulating GABA-A receptors suggest that, in pyramidal cells, these compounds may serve an autocrine function aimed at preventing excessive activation and, possibly, excitotoxicity (a typical outcome of glutamatergic stimulation). The synthesis of AP and other neurosteroids is tightly linked with HPA axis regulation. For example, brain and plasma AP levels are enhanced by CRH and ACTH [165]; in turn, neurosteroids control the activity of HPA axis, and such action is contributed by a direct control of CRH synthesis and hypothalamic release [136, 166]. Of note, the positive modulation of GABA-A receptors by AP and THDOC during stress response is likely aimed at the reduction of some of the negative psychological outcomes of acute stress, such as anxiety. While GABAergic transmission is generally silenced during acute stress [167, 168], the enhanced neurosteroid synthesis is correlated with the restoration of GABAergic functions and reduced anxiety-like behaviors [169]. Again, this background suggests that the synthesis of neurosteroids like AP and THDOC is primarily aimed at compensating for some effects of stress response and re-establishing homeostatic balances in brain regions that play a critical role in emotional and affective regulation.
Role of neurosteroids in sleep regulation and SD.
Given that AP and THDOC are potent endogenous positive modulators of GABA-A receptors, it is not surprising that these steroids elicit hypnotic- and sedative-like effects. Compared with other GABA-A receptor agonists and positive modulators, however, the sleep-promoting properties of neurosteroids may be underlain by their peculiar affinity toward extrasynaptic GABA-A receptors. In rodents, AP elicits profound changes in sleep architecture, including shorter latencies of non-rapid eye movement sleep, prolonged REM sleep duration, longer NREM episodes and higher spindle activity within the NREM phase [170]. Similarly, THDOC dose-dependently shortens sleep latency, promotes the transition between NREM and REM sleep and prolongs NREM episodes [170]. The role of AP in the regulation of sleep architecture is also supported by the fact that elevations in its brain and/or plasma levels are time-locked with modifications in wake/sleep transitions induced by administration of its precursor progesterone [171, 172]; conversely, progesterone elicits hypnotic effects [173, 174], which are likely mediated by AP and other metabolites [175, 176]. Accordingly, the hypnotic properties of progesterone are dampened by 5α-reductase inhibitors, which block the conversion of progesterone to AP [176, 178]. However, it is worth noting that, at least in postmenopausal women, some of the sleep-inducing effects of intranasal progesterone may not reflect GABA-A receptor activation [179].
Another key neurosteroid that has been implicated in sleep regulation is pregnenolone sulfate. In rats, this steroid increases the amount of REM sleep without affecting NREM and modifies sleep–wake transitions [180–182]; conversely, in humans, it increases the amount of time spent in slow wave sleep and depresses EEG sigma power, via inverse agonistic GABA-A receptor modulation [183]. Moreover, infusions of this steroids in the pedunculopontine tegmentum - one of the main brain structures involved in REM sleep generation - promote REM sleep and the propensity to fall asleep during wakefulness [182]. Although this evidence supports an important role of neurosteroids in sleep regulation, it should be noted that a recent study challenged this idea by showing that finasteride, the prototypical 5αR inhibitor, does not alter sleep spindles in men [184].
Given the involvement of neurosteroids in the physiology of sleep and stress response, our group hypothesized their implication in the mechanisms of SD. To test this hypothesis, we recently measured the expression and activity of 5αR, as well as neuroactive steroid levels, in the PFC and other regions of sleep-deprived rats [185]. Our results showed that the PPI deficits induced by SD were underpinned by changes in corticolimbic expression of the two main 5αR isoenzymes. We detected that, after 72 h of SD, both 5αR1 and 5αR2 were upregulated in the PFC, but only the content of the latter was inversely correlated with PPI levels. In addition, we found a significant enhancement of AP in this region. To verify whether the behavioral complications induced by SD were underpinned by the enhancement in 5αR and AP levels, we tested the effects of the 5αR inhibitor finasteride on the behavioral changes induced by SD. The prototypical 5αR inhibitor finasteride countered both the PPI deficits and risk-taking behaviors induced by SD, while injections of AP exacerbated such defects [185]. Interestingly, we also found that low doses of progesterone had an effect similar to that of finasteride, possibly pointing to the importance of a balance between progesterone and its product AP within the PFC to maintain the integrity of executive functions [185]. Ongoing studies in our laboratories are evaluating how changes in AP may interfere with the function of the PFC. A potentially important molecular link to understand how AP and other neurosteroids may impair executive functions involves the dopaminergic system. Indeed, our group has documented that both pharmacological and genetic inactivation of 5αR type 1 interferes with the function of D1-like receptors on the regulation of sensorimotor gating [186– 189], in an AP-sensitive fashion [189]. Notably, preliminary data in our laboratories have shown that D1-like receptor antagonists reverse the gating deficits induced by SD in a fashion similar to the 5αR inhibitor finasteride. While the mechanisms whereby AP may contribute to the signaling of dopamine receptors remains unclear, several reports have pointed to the cross-talk of D1 and GABA-A receptors in the PFC [190–192]. Notably, D1 receptors are the most abundant class in the PFC [193,194], and their activation may cooperate with GABAergic functions to reduce glutamate release in this region [195]. This observation is particularly important given that the reduction of glutamatergic signaling and the reduction in NMDA receptor activation, is known to produce many of the impairments in executive functions observed after SD, including perceptual, attentional and working memory deficits [196–198]. From this perspective, it is also interesting to note that our previous research showed that D1 receptor activation potentiates the gating-disrupting effects of NMDA glutamate receptors [199].
Are neurosteroids involved in the link between sleep problems and gating disorders?
One of the major current limitations to establishing a dependable platform for translational neuropsychiatric research lies in the descriptive, qualitative nature of the DSM-5 classification criteria. Because of the exclusive reliance on symptoms in the diagnostic process, most psychiatric disorders are likely to encompass converging, yet diverse, pathophysiological conditions sharing some clinical phenotypes. For example, a recent genetic study has shown that the diagnostic classification of schizophrenia outlined in the DSM IV encompasses at least eight distinct clinical disorders, reflective of separate biological mechanisms [200]. The RDoC matrix offers a complementary phenomenological approach to demarcate the boundaries and define the overlaps across different psychopathological conditions, by integrating many information levels, such as genomics, biological underpinnings and behavioral components of normal and abnormal functioning [121].
A telling example of this strategy, which may apply to the outcomes of SD, is offered by PPI. This sensorimotor gating index has recently been integrated in the sensorimotor function RDoC domain, within the sub-construct Inhibition and Termination. The loss of PPI has been documented across multiple, diverse neuropsychiatric disorders, including schizophrenia, schizotypal personality disorder, bipolar disorders, obsessive compulsive disorder (OCD), Tourette’s Syndrome (TS), and post-traumatic stress disorder (PTSD) [201–203]. In keeping with the phenotypic perspective of RDoC, these otherwise highly heterogeneous conditions have been grouped under a common rubric of gating disorders [204]. This frame of reference may help gain novel insight into the mechanisms whereby SD triggers and/or exacerbates a wide array of distinct clinical entities. Indeed, all gating disorders have been shown to be accompanied by sleep disturbances and sensitive to their negative impact. For example, the risk of sleep disturbances in schizophrenia or bipolar disorder is almost twice as high as that reported in healthy controls, with insomnia as the most frequently reported sleep disturbance across groups [205]. Sleep disturbances are highly prevalent in bipolar disorder, and often consequent to a reduced need for sleep in mania [206, 207]. Sleep disruption is a triggering factor for manic/hypomanic episodes in some vulnerable patients [208, 209], or an exacerbating factor for depression in others [210, 211]. Sleep problems are also a hallmark of schizophrenia [212–215], with delays in falling asleep, difficulties in maintaining sleep, reduced total sleep time, several night time awakenings, nightmares, and non-restorative sleep [216]. Similar to bipolar patients, reduced need for sleep occurs particularly during episodes of psychotic exacerbation and before the first symptomatic signs of relapse as well as in patients with high risk for psychosis [217]. Further underscoring the relevance of sleep loss in psychosis and mania, recent research has shown that acute SD may serve as a proxy paradigm to study these conditions [218]. Indeed, acute SD results in psychotomimetic states, with perceptual distortions, anhedonia and cognitive disorganization [127, 218]; furthermore, SD leads to a rapid elevation of mood and exacerbation of manic symptoms [208]. Sleep loss is also associated with greater severity symptoms in TS [219, 220], OCD [221, 222], and PTSD [223]. Taken together, this evidence suggests that sleep problems may provide a relevant target for transdiagnostic treatment in patients affected by gating disorders.
As highlighted above, our findings suggest that neurosteroids may contribute to the link between SD and gating disorders. In line with this idea, we and others have pointed to the involvement of these molecules in the pathophysiology of schizophrenia [152, 186–188, 224–229], TS [230–232], and PTSD [233]. Future studies will be needed to fully evaluate the integration of neurosteroids with other pathogenic factors in these disorders.
The function of AP in SD: a double-edged sword?
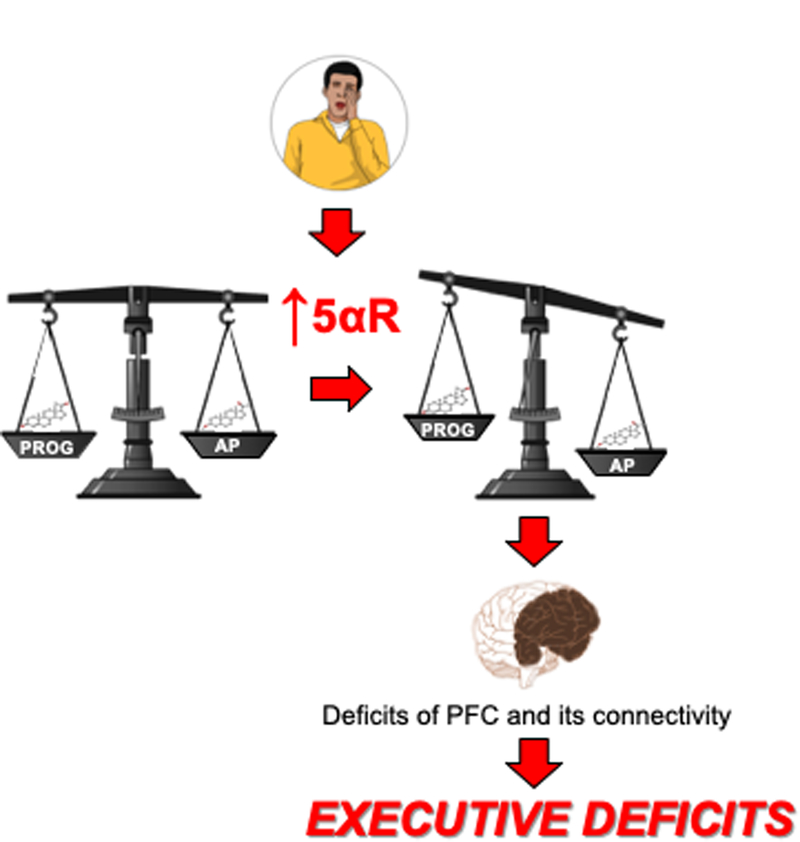
As outlined in the previous sections, our recent results have delineated a critical role of AP and progesterone in the executive complications of SD; some of these alterations may have important repercussions on the pathogenesis of gating disorders. The mechanisms that lead to the increased 5αR expression and AP synthesis in the PFC of SD-exposed subjects remain unknown. It should be noted that the negative role of AP on executive functions is in apparent contrast with the beneficial effects of this neurosteroid on anxiety-like behaviors and oxidative stress. Indeed, previous reports have documented that AP injections significantly improve anxiety-like behaviors and attenuate lipid peroxidation and nitrite levels, by activating GABA-A receptors [234]. AP has also been shown to reduce oxidative stress in cells [235–237]. In fact, positive modulation of GABA-A receptors by neurosteroids helps maintain neuronal redox homeostasis by increasing mitochondrial respiration and ATP generation [238]. These data collectively suggest that the upregulation of 5αR and AP levels may provide a compensatory response to SD; however, the price of this neuroprotective reaction may lead to subtle PFC deficits, possibly facilitating the intrusion of microsleep episodes and sleep attacks. Ultimately, the robust enhancement in AP synthesis may activate GABA-A receptors within the pyramidal cells of the PFC, opposing their activation, causing connectivity deficits and ultimately resulting in deficits of information processing and executive functions (Fig. 2).
Figure 2. Diagram of the hypothesized role of neurosteroids in the neurobehavioral complications induced by sleep deprivation.
Similar to other stressors, acute sleep deprivation leads to increased expression of 5-alpha reductase and synthesis of the neurosteroid allopregnanolone in the PFC. Whereas this upregulation is likely aimed at counterbalancing the detrimental impact of oxidative stress induced by sleep deprivation, such compensatory mechanism may in turn set off neurobehavioral complications by interfering with the corticolimbic connections responsible for executive functions and emotional regulation. Enzymes: CYP21A2: Steroid 21-hydroxylase; 3β-HSD; 3β-hydroxysteroid dehydrogenase; 3α-HSOR: 3α-hydroxysteroid oxidoreductase. Steroids: DOC, deoxycorticosterone; 5α-DHDOC, 5α-dihydro deoxycorticosterone; 3α,5α-THDOC, 3α,5α-tetrahydrodeoxycorticosterone, DHP, 5α-dihydroprogesterone. See text for further details.
This idea underscores that the negative cognitive consequences of SD may arise from the attempt of the brain to counteract the neurotoxic effect of sleep loss through compensatory mechanisms. In general, whereas neurosteroids promote neuronal adaptation to stress, they may also hinder the execution of multitasking performance and reduce cognitive flexibility. It should be noted that, while the upregulation of 5αR and AP in acute SD may represent an appropriate response to withstand the bioenergetic challenges of this condition, it is likely that chronic sleep restriction may elicit opposite outcomes. Accordingly, chronic stress has been shown to reduce both 5αR and AP in the PFC of rodents [239]. Future research will be needed to understand how neurosteroids support the pathophysiology and complications of sleep disturbances and stress coping, as well as their relations with gating disorders. This direction may pave the way for the identification of neurosteroid targets in the therapy of sleep problems and gating disorders as well as the identification of the role of 5αR in other executive tasks, such as 5C-CPT and IGT.
Acknowledgements
RF has been supported by a Research Grant from the Sardinia Region (Legge Regionale 7 25 agosto 2007, n. 7, Promozione della Ricerca Scientifica e dell’Innovazione 26 Tecnologica in Sardegna, and “Fondazione di Sardegna”. MB has been partially supported by the NINDS grant R21 NS108722–01.
References
- 1.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1:233–243. [DOI] [PubMed] [Google Scholar]
- 2.Watson NF, Badr MS, Belenky G, et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Sleep. 2015;38:1161–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. QuickStats: Percentage of Adults Who Reported an Average of ≤6 Hours of Sleep per 24-Hour Period, by Sex and Age Group—United States, 1985 and 2004. JAMA. 2005;294:2692–2692. [Google Scholar]
- 4.Ferrie JE, Kumari M, Salo P, et al. Sleep epidemiology—a rapidly growing field. Int J Epidemiol. 2011;40:1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown WD. Insomnia: Prevalence and Daytime Consequences In: Sleep: A Comprehensive Handbook. John Wiley & Sons, Ltd, 2005; pp. 91–98. [Google Scholar]
- 6.Institute of Medicine (US) Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington (DC): National Academies Press (US), 2006. [PubMed] [Google Scholar]
- 7.Lund HG, Reider BD, Whiting AB, et al. Sleep Patterns and Predictors of Disturbed Sleep in a Large Population of College Students. Journal of Adolescent Health. 2010;46:124–132. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC). Short sleep duration among workers—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012. April 27;61(16):281–5. [PubMed] [Google Scholar]
- 9.Liu Y, Wheaton AG, Chapman DP, et al. Prevalence of Healthy Sleep Duration among Adults--United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:137–141. [DOI] [PubMed] [Google Scholar]
- 10.Owens JA, Weiss MR. Insufficient sleep in adolescents: causes and consequences. Minerva Pediatr. 2017;69:326–336. [DOI] [PubMed] [Google Scholar]
- 11.Hershner SD, Chervin RD. Causes and consequences of sleepiness among college students. Nat Sci Sleep. 2014;6:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patrick Y, Lee A, Raha O, et al. Effects of sleep deprivation on cognitive and physical performance in university students. Sleep Biol Rhythms. 2017;15:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaba DM, Howard SK. Fatigue among Clinicians and the Safety of Patients. New England Journal of Medicine. 2002;347:1249–1255. [DOI] [PubMed] [Google Scholar]
- 14.Veasey S, Rosen R, Barzansky B, et al. Sleep Loss and Fatigue in Residency Training: A Reappraisal. JAMA. 2002;288:1116–1124. [DOI] [PubMed] [Google Scholar]
- 15.Papp KK, Stoller EP, Sage P, et al. The effects of sleep loss and fatigue on resident-physicians: a multi-institutional, mixed-method study. Acad Med. 2004;79:394–406. [DOI] [PubMed] [Google Scholar]
- 16.Philibert I Sleep loss and performance in residents and nonphysicians: a meta-analytic examination. Sleep. 2005;28:1392–1402. [DOI] [PubMed] [Google Scholar]
- 17.Mansukhani MP, Kolla BP, Surani S, et al. Sleep deprivation in resident physicians, work hour limitations, and related outcomes: a systematic review of the literature. Postgrad Med. 2012;124:241–249. [DOI] [PubMed] [Google Scholar]
- 18.Belenky G, Penetar DM, Thorne D, et al. The effects of sleep deprivation on performance during continuous combat operations In: Marriott BM, ed. Food Components to Enhance Performance. An Evaluation of Potential Performance-Enhancing Food Components for Operational Rations. Washington, DC: National Academy Press, 1994:127–35 [PubMed] [Google Scholar]
- 19.Peterson AL, Goodie JL, Satterfield WA, et al. Sleep disturbance during military deployment. Mil Med. 2008;173:230–235. [DOI] [PubMed] [Google Scholar]
- 20.Seelig AD, Jacobson IG, Smith B, et al. Sleep patterns before, during, and after deployment to Iraq and Afghanistan. Sleep. 2010;33:1615–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliot DL, Kuehl KS. The effects of sleep deprivation on fire fighters and EMS responders: final report [home page on internet]; 2007. Available from: https://www.iafc.org/docs/default-source/1safehealthshs/progssleep_sleepdeprivationreport.pdf?sfvrsn=f9e4da0d_2
- 22.Carey MG, Al-Zaiti SS, Dean GE, et al. Sleep problems, depression, substance use, social bonding, and quality of life in professional firefighters. J Occup Environ Med. 2011;53:928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price WJ, Holley DC. Shiftwork and safety in aviation. Occup Med. 1990;5:343–377. [PubMed] [Google Scholar]
- 24.Bourgeois-Bougrine S, Carbon P, Gounelle C, et al. Perceived fatigue for short- and long-haul flights: a survey of 739 airline pilots. Aviat Space Environ Med. 2003;74:1072–1077. [PubMed] [Google Scholar]
- 25.Caldwell JA. Crew Schedules, Sleep Deprivation, and Aviation Performance. Curr Dir Psychol Sci. 2012;21:85–89. [Google Scholar]
- 26.Hafner M, Stepanek M, Taylor J, et al. Why Sleep Matters-The Economic Costs of Insufficient Sleep: A Cross-Country Comparative Analysis. Rand Health Q. 2017;6:11. [PMC free article] [PubMed] [Google Scholar]
- 27.Rosekind MR. Underestimating the societal costs of impaired alertness: safety, health and productivity risks. Sleep Med. 2005;6 Suppl 1:S21–25. [DOI] [PubMed] [Google Scholar]
- 28.Swanson LM, Arnedt JT, Rosekind MR, et al. Sleep disorders and work performance: findings from the 2008 National Sleep Foundation Sleep in America poll. J Sleep Res. 2011;20:487–494. [DOI] [PubMed] [Google Scholar]
- 29.Williamson A, Lombardi DA, Folkard S, et al. The link between fatigue and safety. Accid Anal Prev. 2011;43:498–515. [DOI] [PubMed] [Google Scholar]
- 30.Rosekind MR, Gregory KB, Mallis MM, et al. The cost of poor sleep: workplace productivity loss and associated costs. J Occup Environ Med. 2010;52:91–98. [DOI] [PubMed] [Google Scholar]
- 31.Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends Cogn Sci. 2002. November 1;6(11):475–481. [DOI] [PubMed] [Google Scholar]
- 32.Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnsten AFT, Li B-M. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. [DOI] [PubMed] [Google Scholar]
- 34.Stuss DT. Functions of the frontal lobes: relation to executive functions. J Int Neuropsychol Soc. 2011;17:759–65. [DOI] [PubMed] [Google Scholar]
- 35.Gyurak A, Goodkind MS, Kramer JH, et al. Executive functions and the down-regulation and up-regulation of emotion. Cogn Emot. 2012;26:103–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136:375–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toth LA, Bhargava P. Animal models of sleep disorders. Comp Med. 2013;63:91–104. [PMC free article] [PubMed] [Google Scholar]
- 38.Elmenhorst D, Elmenhorst EM, Luks N, et al. Performance impairment during four days partial sleep deprivation compared with the acute effects of alcohol and hypoxia. Sleep Med. 2009;1:189–97. [DOI] [PubMed] [Google Scholar]
- 39.Goulart LI, Pinto LR Jr, Perlis ML, et al. Effects of different sleep deprivation protocols on sleep perception in healthy volunteers. Sleep Med. 2014. October;15:1219–24. [DOI] [PubMed] [Google Scholar]
- 40.Revel FG, Gottowik J, Gatti S, et al. Rodent models of insomnia: a review of experimental procedures that induce sleep disturbances. Neurosci Biobehav Rev. 2009;33:874–99. [DOI] [PubMed] [Google Scholar]
- 41.Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. [DOI] [PubMed] [Google Scholar]
- 42.Alhola P, Polo-Kantola P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3:553–567. [PMC free article] [PubMed] [Google Scholar]
- 43.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129. [DOI] [PubMed] [Google Scholar]
- 44.Van Dongen HP, Maislin G, Mullington JM, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. [DOI] [PubMed] [Google Scholar]
- 45.Krause AJ, Simon EB, Mander BA, et al. The sleep-deprived human brain. Nat Rev Neurosci. 2017. July;18(7):404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoo SS, Hu PT, Gujar N, et al. A deficit in the ability to form new human memories without sleep. Nature Neuroscience. 2007;10:385–392. [DOI] [PubMed] [Google Scholar]
- 47.Harrison Y, Horne JA. Occurrence of “microsleeps’ during daytime sleep onset in normal subjects. Electroencephalogr Clin Neurophysiol. 1996;98:411–6. [DOI] [PubMed] [Google Scholar]
- 48.Wu JC, Gillin JC, Buchsbaum MS, et al. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31:2783–2792. [DOI] [PubMed] [Google Scholar]
- 49.Goel N, Rao H, Durmer JS, et al. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9:637–671. [DOI] [PubMed] [Google Scholar]
- 51.Petruo VA, Mückschel M, Beste C. On the role of the prefrontal cortex in fatigue effects on cognitive flexibility - a system neurophysiological approach. Sci Rep. 2018;8:6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drummond SP, Brown GG, Stricker JL, et al. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999;10:3745–3748. [DOI] [PubMed] [Google Scholar]
- 53.Kribbs NB, Dinges D. Vigilance decrement and sleepiness In: Sleep onset: Normal and abnormal processes. Washington, DC, US: American Psychological Association, 1994, pp. 113–125. [Google Scholar]
- 54.Horne JA. Sleep loss and ‘divergent’ thinking ability. Sleep. 1988;11:528–536. [DOI] [PubMed] [Google Scholar]
- 55.Wimmer F, Hoffmann R, Bonato R, et al. The effects of sleep deprivation on divergent thinking and attention processes. J Sleep Res. 1992;1:223–230. [DOI] [PubMed] [Google Scholar]
- 56.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236–249. [DOI] [PubMed] [Google Scholar]
- 57.Rogers NL, Kennaway DJ, Dawson D. Neurobehavioural performance effects of daytime melatonin and temazepam administration. J Sleep Res. 2003;12:207–212. [DOI] [PubMed] [Google Scholar]
- 58.Dorrian J, Dinges DF. Sleep Deprivation and Its Effects on Cognitive Performance In: Sleep: A Comprehensive Handbook. John Wiley & Sons, Ltd, pp. 137–144. [Google Scholar]
- 59.Chee MWL, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mu Q, Mishory A, Johnson KA, et al. Decreased brain activation during a working memory task at rested baseline is associated with vulnerability to sleep deprivation. Sleep. 2005;28:433–446. [DOI] [PubMed] [Google Scholar]
- 61.Drummond SP, Brown GG, Gillin JC, et al. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–657. [DOI] [PubMed] [Google Scholar]
- 62.Mu Q, Nahas Z, Johnson KA, Yamanaka K, Mishory A, Koola J, Hill S, Horner MD, Bohning DE, George MS. Decreased cortical response to verbal working memory following sleep deprivation. Sleep. 2005;28:55–67 [DOI] [PubMed] [Google Scholar]
- 63.Chengyang L, Daqing H, Jianlin Q, et al. Short-term memory deficits correlate with hippocampal-thalamic functional connectivity alterations following acute sleep restriction. Brain Imaging Behav. 2017;11:954–963. [DOI] [PubMed] [Google Scholar]
- 64.Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93:681–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ackermann S, Rasch B. Differential effects of non-REM and REM sleep on memory consolidation? Curr Neurol Neurosci Rep. 2014;14:430. [DOI] [PubMed] [Google Scholar]
- 66.Drummond SP, Brown GG, Salamat JS, et al. Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep; 2004;27:445–451. [PubMed] [Google Scholar]
- 67.Forest G, Godbout R. Effects of sleep deprivation on performance and EEG spectral analysis in young adults. Brain Cogn. 2000;43:195–200. [PubMed] [Google Scholar]
- 68.Harrison Y, Horne JA. One night of sleep loss impairs innovative thinking and flexible decision making. Organ Behav Hum Decis Process; 1999;78:128–45. [DOI] [PubMed] [Google Scholar]
- 69.Monk TH, Carrier J. Speed of mental processing in the middle of the night. Sleep. 1997;20:399–401. [DOI] [PubMed] [Google Scholar]
- 70.Quigley N, Green JF, Morgan D, et al. The effect of sleep deprivation on memory and psychomotor function in healthy volunteers. Hum Psychopharmacol. 2000;15:171–177. [DOI] [PubMed] [Google Scholar]
- 71.Drake CL, Roehrs TA, Burduvali E, et al. Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology. 2001;38:979–987. [DOI] [PubMed] [Google Scholar]
- 72.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- 73.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. [DOI] [PubMed] [Google Scholar]
- 74.Lo JC, Groeger JA, Santhi N, et al. Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS One. 2012;7:e45987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lo JC, Groeger JA, Cheng GH, et al. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med. 2016;17:87–98. [DOI] [PubMed] [Google Scholar]
- 76.Kahn-Greene ET, Killgore DB, Kamimori GH, et al. The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med. 2007;8:215–21. [DOI] [PubMed] [Google Scholar]
- 77.Riemann D, Spiegelhalder K, Nissen C, et al. REM sleep instability--a new pathway for insomnia? Pharmacopsychiatry. 2012;45:167–76. [DOI] [PubMed] [Google Scholar]
- 78.Wassing R, Benjamins JS, Dekker K, et al. Slow dissolving of emotional distress contributes to hyperarousal. Proc Natl Acad Sci U S A. 2016;113:2538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Helm E, Yao J, Dutt S, et al. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr Biol. 2011;21:2029–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoo S-S, Gujar N, Hu P, et al. The human emotional brain without sleep--a prefrontal amygdala disconnect. Curr Biol. 2007;17:877–878. [DOI] [PubMed] [Google Scholar]
- 81.Gujar N, Yoo S-S, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J Neurosci. 2011;31:4466–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Venkatraman V, Chuah YML, Huettel SA, et al. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–609. [DOI] [PubMed] [Google Scholar]
- 83.Wallis JD. Neuronal mechanisms in prefrontal cortex underlying adaptive choice behavior. Ann N Y Acad Sci. 2007;1121:447–460. [DOI] [PubMed] [Google Scholar]
- 84.Womack SD, Hook JN, Reyna SH, et al. Sleep loss and risk-taking behavior: a review of the literature. Behav Sleep Med. 2013;11:343–359. [DOI] [PubMed] [Google Scholar]
- 85.Gujar N, McDonald SA, Nishida M, Walker MP. A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cereb Cortex. 2011. January;21(1):115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valli K, Revonsuo A. The threat simulation theory in light of recent empirical evidence: a review. Am J Psychol. 2009;122:17–38. [PubMed] [Google Scholar]
- 87.Perogamvros L, Schwartz S. The roles of the reward system in sleep and dreaming. Neurosci Biobehav Rev. 2012;36:1934–1951. [DOI] [PubMed] [Google Scholar]
- 88.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. [DOI] [PubMed] [Google Scholar]
- 89.Kasper S, Sack DA, Wehr TA, et al. Nocturnal TSH and prolactin secretion during sleep deprivation and prediction of antidepressant response in patients with major depression. Biol Psychiatry. 1988;24:631–641. [DOI] [PubMed] [Google Scholar]
- 90.Luboshitzky R, Zabari Z, Shen-Orr Z, et al. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J Clin Endocrinol Metab. 2001;86:1134–1139. [DOI] [PubMed] [Google Scholar]
- 91.Taheri S, Lin L, Austin D, et al. Short Sleep Duration Is Associated with Reduced Leptin, Elevated Ghrelin, and Increased Body Mass Index. PLoS Med;. 2004; 1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Redwine L, Hauger RL, Gillin JC, et al. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597–3603. [DOI] [PubMed] [Google Scholar]
- 93.Spiegel K, Tasali E, Penev P, et al. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. [DOI] [PubMed] [Google Scholar]
- 94.Knutson KL. Impact of sleep and sleep loss on glucose homeostasis and appetite regulation. Sleep Med Clin. 2007;2:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.LeBlanc ES, Smith NX, Nichols GA, et al. Insomnia is associated with an increased risk of type 2 diabetes in the clinical setting. BMJ Open Diabetes Res Care. 2018;6:e000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goldstein DS, McEwen B. Allostasis, homeostats, and the nature of stress. Stress. 2002;5:55–58. [DOI] [PubMed] [Google Scholar]
- 97.Chen X, Redline S, Shields AE, et al. Associations of allostatic load with sleep apnea, insomnia, short sleep duration, and other sleep disturbances: findings from the National Health and Nutrition Examination Survey 2005 to 2008. Ann Epidemiol. 2014;24:612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clark AJ, Dich N, Lange T, et al. Impaired sleep and allostatic load: cross-sectional results from the Danish Copenhagen Aging and Midlife Biobank. Sleep Med. 2014;15:1571–8. [DOI] [PubMed] [Google Scholar]
- 99.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–101. [PubMed] [Google Scholar]
- 100.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8:367–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guyon A, Morselli LL, Balbo ML, et al. Effects of Insufficient Sleep on Pituitary-Adrenocortical Response to CRH Stimulation in Healthy Men. Sleep. 2017;40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–70. [PubMed] [Google Scholar]
- 103.Ehlers CL, Reed TK, Henriksen SJ. Effects of corticotropin-releasing factor and growth hormone-releasing factor on sleep and activity in rats. Neuroendocrinology. 1986;42:467–74. [DOI] [PubMed] [Google Scholar]
- 104.Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–3114. [DOI] [PubMed] [Google Scholar]
- 105.Payne JD, Nadel L. Sleep, dreams, and memory consolidation: The role of the stress hormone cortisol. Learn Mem. 2004;11:671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kimura M, Müller-Preuss P, Lu A, et al. Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep. Mol Psychiatry. 2010;15:154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vgontzas AN, Bixler EO, Papanicolaou DA, et al. Rapid Eye Movement Sleep Correlates with the Overall Activities of the Hypothalamic-Pituitary-Adrenal Axis and Sympathetic System in Healthy Humans. J Clin Endocrinol Metab. 1997;82:3278–3280. [DOI] [PubMed] [Google Scholar]
- 108.Somers VK, Dyken ME, Mark AL, et al. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. [DOI] [PubMed] [Google Scholar]
- 109.Carter JR, Durocher JJ, Larson RA, et al. Sympathetic neural responses to 24-hour sleep deprivation in humans: sex differences. Am J Physiol Heart Circ Physiol. 2012;302:H1991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Antunes IB, Andersen ML, Baracat EC, et al. The effects of paradoxical sleep deprivation on estrous cycles of the female rats. Horm Behav. 2006;49:433–40. [DOI] [PubMed] [Google Scholar]
- 111.Deurveilher S, Seary ME, Semba K. Ovarian hormones promote recovery from sleep deprivation by increasing sleep intensity in middle-aged ovariectomized rats. Horm Behav. 2013;63:566–76. [DOI] [PubMed] [Google Scholar]
- 112.Colavito V, Fabene PF, Grassi-Zucconi G, et al. Experimental sleep deprivation as a tool to test memory deficits in rodents. Front Syst Neurosci. 2013;7:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tufik S. Changes of response to dopaminergic drugs in rats submitted to REM-sleep deprivation. Psychopharmacology 1981a;72: 257–260 [DOI] [PubMed] [Google Scholar]
- 114.Tufik S. Increased responsiveness to apomorphine after REM sleep deprivation: supersensitivity of dopamine receptors or increase in dopamine turnover? J Pharm Pharmacol. 1981b;33:732–738. [DOI] [PubMed] [Google Scholar]
- 115.Nunes JR, Tufik S, Nobrega JN. Autoradiographic analysis of D1 and D2 dopaminergic receptors in rat brain after paradoxical sleep deprivation. Brain Res Bull 1994; 34:453–456. [DOI] [PubMed] [Google Scholar]
- 116.Seugnet L, Suzuki Y, Vine L, et al. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr Biol. 2008;18:1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Farooqui SM, Brock JW, Zhou J. Changes in monoamines and their metabolite concentrations in REM sleep-deprived rat forebrain nuclei. Pharmacol Biochem Behav 1996; 54: 385–391. [DOI] [PubMed] [Google Scholar]
- 118.Tufik S, Andersen ML, Bittencourt LR, Mello MT. Paradoxical sleep deprivation: neurochemical, hormonal and behavioral alterations. Evidence from 30 years of research. An Acad Bras Cienc. 2009;81:521–38. [DOI] [PubMed] [Google Scholar]
- 119.Troncone LRP, Tufik S. Effects of selective adrenoceptor agonists and antagonists on aggressive behavior elicited by apomorphine, DL-DOPA and fusaric acid in REM-sleep deprived rats. Physiol Behav. 1991; 50: 173–178. [DOI] [PubMed] [Google Scholar]
- 120.Hipòlide DC, Tufik S, Raymond R AND Nobrega JN. Heterogeneous effects of rapid eye movement sleep deprivation on binding to α-and β-adrenergic receptor subtypes in rat brain. Neuroscience 1998; 86: 977–987. [DOI] [PubMed] [Google Scholar]
- 121.Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cope ZA, Powell SB, Young JW. Modeling neurodevelopmental cognitive deficits in tasks with cross-species translational validity. Genes Brain Behav. 2016;15:27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Frau R, Orrù M, Puligheddu M, et al. Sleep deprivation disrupts prepulse inhibition of the startle reflex: reversal by antipsychotic drugs. Int J Neuropsychopharmacol. 2008;11:947–955. [DOI] [PubMed] [Google Scholar]
- 124.Giakoumaki SG, Bitsios P, Frangou S. The level of prepulse inhibition in healthy individuals may index cortical modulation of early information processing. Brain Res. 2006;1078:168–170. [DOI] [PubMed] [Google Scholar]
- 125.Bitsios P, Giakoumaki SG. Relationship of prepulse inhibition of the startle reflex to attentional and executive mechanisms in man. Int J Psychophysiol. 2005;55:229–241. [DOI] [PubMed] [Google Scholar]
- 126.Bitsios P, Giakoumaki SG, Theou K, et al. Increased prepulse inhibition of the acoustic startle response is associated with better strategy formation and execution times in healthy males. Neuropsychologia. 2006;44:2494–2499. [DOI] [PubMed] [Google Scholar]
- 127.Petrovsky N, Ettinger U, Hill A, et al. Sleep deprivation disrupts prepulse inhibition and induces psychosis-like symptoms in healthy humans. J Neurosci. 2014;34:9134–9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van Enkhuizen J, Acheson D, Risbrough V, et al. Sleep deprivation impairs performance in the 5-choice continuous performance test: similarities between humans and mice. Behav Brain Res. 2014;261:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Young JW, Light GA, Marston HM, et al. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One. 2009;4:e4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cope ZA, Young JW. The Five-Choice Continuous Performance Task (5C-CPT): A Cross-Species Relevant Paradigm for Assessment of Vigilance and Response Inhibition in Rodents. Curr Protoc Neurosci. 2017;78:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu L, Zhou R. Effect of 72 h of Sleep Deprivation on the Iowa Gambling Task. Noro Psikiyatr Ars. 2016;53:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–2343. [DOI] [PubMed] [Google Scholar]
- 133.Pittaras E, Callebert J, Dorey R, et al. Mouse Gambling Task reveals differential effects of acute sleep debt on decision-making and associated neurochemical changes. Sleep. 2018;41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Paba S, Frau R, Godar SC, et al. Steroid 5α-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders. Curr Pharm Des. 2011;17:151–67. [DOI] [PubMed] [Google Scholar]
- 135.Lambert JJ, Belelli D, Peden DR, et al. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. [DOI] [PubMed] [Google Scholar]
- 136.Gunn BG, Brown AR, Lambert JJ, et al. Neurosteroids and GABA(A) Receptor Interactions: A Focus on Stress. Front Neurosci. 2011;5:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Majewska MD, Harrison NL, Schwartz RD, et al. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. [DOI] [PubMed] [Google Scholar]
- 138.Shu HJ, Eisenman LN, Jinadasa D, et al. Slow actions of neuroactive steroids at GABAA receptors. J Neurosci. 2004;24:6667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reddy DS, Jian K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J Pharmacol Exp Ther. 2010;334:1031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang M, He Y, Eisenman LN, et al. 3beta -hydroxypregnane steroids are pregnenolone sulfate-like GABA(A) receptor antagonists. J Neurosci. 2002;22:3366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brown N, Kerby J, Bonnert TP, et al. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J.Neurosci. 2002;22:1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shu HJ, Bracamontes J, Taylor A, et al. Characteristics of concatemeric GABA(A) receptors containing α4/δ subunits expressed in Xenopus oocytes. Br J Pharmacol. 2012;165:2228–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maguire J, Mody I. Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology. 2009;34S:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bicíková M, Tallová J, Hill M, Krausová Z, Hampl R. Serum concentrations of some neuroactive steroids in women suffering from mixed anxiety-depressive disorder. Neurochem Res. 2000;25:1623–7. [DOI] [PubMed] [Google Scholar]
- 146.Zimmerberg B, Kajunski EW. Sexually dimorphic effects of postnatal allopregnanolone on the development of anxiety behavior after early deprivation. Pharmacol Biochem Behav. 2004;78:465–71. [DOI] [PubMed] [Google Scholar]
- 147.Finn DA, Beckley EH, Kaufman KR, et al. Manipulation of GABAergic steroids: Sex differences in the effects on alcohol drinking- and withdrawal-related behaviors. Horm Behav. 2010;57:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kohtz AS, Paris JJ, Frye CA. Low doses of cocaine decrease, and high doses increase, anxiety-like behavior and brain progestogen levels among intact rats. Horm Behav. 2010;57:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Godar SC, Bortolato M. Gene-sex interactions in schizophrenia: focus on dopamine neurotransmission. Front Behav Neurosci. 2014;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bortolato M, Frau R, Godar SC, et al. The implication of neuroactive steroids in Tourette’s syndrome pathogenesis: A role for 5α-reductase? J Neuroendocrinol. 2013;25:1196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Frau R, Abbiati F, Bini V, et al. Targeting neurosteroid synthesis as a therapy for schizophrenia-related alterations induced by early psychosocial stress. Schizophr Res. 2015;168:640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305:541–8. [DOI] [PubMed] [Google Scholar]
- 154.Quinones-Jenab V, Minerly AC, Niyomchia T, et al. Progesterone and allopregnanolone are induced by cocaine in serum and brain tissues of male and female rats. Pharmacol Biochem Behav. 2008;89:292–7. [DOI] [PubMed] [Google Scholar]
- 155.Ford MM, Yoneyama N, Strong MN, et al. Inhibition of 5alpha-reduced steroid biosynthesis impedes acquisition of ethanol drinking in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gunn BG, Cunningham L, Mitchell SG, et al. GABAA receptor-acting neurosteroids: a role in the development and regulation of the stress response. Front Neuroendocrinol. 2015;36:28–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Purdy RH, Morrow AL, Moore PH Jr, et al. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sánchez P, Torres JM, Gavete P, et al. Effects of swim stress on mRNA and protein levels of steroid 5alpha-reductase isozymes in prefrontal cortex of adult male rats. Neurochem Int. 2008;52:426–31. [DOI] [PubMed] [Google Scholar]
- 159.Bortolato M, Devoto P, Roncada P, et al. Isolation rearing-induced reduction of brain 5α-reductase expression: relevance to dopaminergic impairments. Neuropharmacology. 2011;60:1301–8. [DOI] [PubMed] [Google Scholar]
- 160.Saalmann YB, Kirkcaldie MT, Waldron S, et al. Cellular distribution of the GABAA receptor-modulating 3alpha-hydroxy, 5alpha-reduced pregnane steroids in the adult rat brain. J Neuroendocrinol. 2007;19:272–84. [DOI] [PubMed] [Google Scholar]
- 161.Suzuki T, Kodama S, Hoshino C, et al. A plateau potential mediated by the activation of extrasynaptic NMDA receptors in rat hippocampal CA1 pyramidal neurons. Eur J Neurosci. 2008;28:521–34. [DOI] [PubMed] [Google Scholar]
- 162.Chisari M, Eisenman LN, Covey DF, et al. The sticky issue of neurosteroids and GABA(A) receptors. Trends Neurosci. 2010;33:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J Neurosci. 2011;31:9905–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Castelli MP, Casti A, Casu A, et al. Regional distribution of 5α-reductase type 2 in the adult rat brain: an immunohistochemical analysis. Psychoneuroendocrinology. 2013;38:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Torres JM, Ruiz E, Ortega E. Effects of CRH and ACTH administration on plasma and brain neurosteroid levels. Neurochem Res. 2001;26:555–8. [DOI] [PubMed] [Google Scholar]
- 166.Sarkar J, Wakefield S, MacKenzie G, et al. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci. 2011;31:18198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sanna E, Cuccheddu T, Serra M, et al. Carbon dioxide inhalation reduces the function of GABAA receptors in the rat brain. Eur J Pharmacol. 1992;216:457–8. [DOI] [PubMed] [Google Scholar]
- 168.Barbaccia ML, Roscetti G, Bolacchi F, et al. Stress-induced increase in brain neuroactive steroids: antagonism by abecarnil. Pharmacol Biochem Behav. 1996;54:205–10. [DOI] [PubMed] [Google Scholar]
- 169.Barbaccia ML, Affricano D, Purdy RH, et al. Clozapine, but not haloperidol, increases brain concentrations of neuroactive steroids in the rat. Neuropsychopharmacology. 2001;25:489–97. [DOI] [PubMed] [Google Scholar]
- 170.Müller-Preuss P, Rupprecht R, Lancel M. The effects of the neuroactive steroid 3 alpha,5 alpha-THDOC on sleep in the rat. Neuroreport. 2002;13:487–90. [DOI] [PubMed] [Google Scholar]
- 171.Lancel M, Faulhaber J, Schiffelholz T, et al. AP affects sleep in a benzodiazepine-like fashion. J Pharmacol Exp Ther. 1997;282:1213–1218. [PubMed] [Google Scholar]
- 172.Friess E, Tagaya H, Trachsel L, Holsboer F, Rupprecht R. Progesterone-induced changes in sleep in male subjects. Am J Physiol. 1997;272:E885–91. [DOI] [PubMed] [Google Scholar]
- 173.Selye H. The antagonism between anesthetic steroid hormones and pentamethylenetetrazol (metrazol). J Lab Clin Med. 1942;27:1051–1053 [Google Scholar]
- 174.Reddy DS, Gangisetty O, Briyal S. Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Neuropharmacology. 2010;59:573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Arafat ES, Hargrove JT, Maxson WS, et al. Sedative and hypnotic effects of oral administration of micronized progesterone may be mediated through its metabolites. Am J Obstet Gynecol. 1988;159:1203–9. [DOI] [PubMed] [Google Scholar]
- 176.Söderpalm AH, Lindsey S, Purdy RH, et al. Administration of progesterone produces mild sedative-like effects in men and women. Psychoneuroendocrinology. 2004;29:339–54. [DOI] [PubMed] [Google Scholar]
- 177.Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7:171–7. [DOI] [PubMed] [Google Scholar]
- 178.Korneyev A, Costa E. Allopregnanolone (THP) mediates anesthetic effects of progesterone in rat brain. Horm Behav. 1996;30:37–43. [DOI] [PubMed] [Google Scholar]
- 179.Schüssler P, Kluge M, Adamczyk M, et al. Sleep after intranasal progesterone vs. zolpidem and placebo in postmenopausal women - A randomized, double-blind cross over study. Psychoneuroendocrinology. 2018;92:81–86. [DOI] [PubMed] [Google Scholar]
- 180.Darnaudéry M, Bouyer JJ, Pallarés M, et al. The promnesic neurosteroid pregnenolone sulfate increases paradoxical sleep in rats. Brain Res. 1999;818:492–8. [DOI] [PubMed] [Google Scholar]
- 181.Schiffelholz T, Holsboer F, Lancel M. High doses of systemic DHEA-sulfate do not affect sleep structure and elicit moderate changes in non-REM sleep EEG in rats. Physiol Behav. 2000;69:399–404. [DOI] [PubMed] [Google Scholar]
- 182.Darbra S, George O, Bouyer JJ, et al. Sleep-wake states and cortical synchronization control by pregnenolone sulfate into the pedunculopontine nucleus. J Neurosci Res. 2004;76:742–7. [DOI] [PubMed] [Google Scholar]
- 183.Steiger A, Trachsel L, Guldner J, et al. Neurosteroid pregnenolone induces sleep-EEG changes in man compatible with inverse agonistic GABAA-receptor modulation. Brain Res. 1993;615:267–74. [DOI] [PubMed] [Google Scholar]
- 184.Goldstein MR, Cook JD, Plante DT. The 5α-reductase inhibitor finasteride is not associated with alterations in sleep spindles in men referred for polysomnography. Hum Psychopharmacol. 2016;31:70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Frau R, Bini V, Soggiu A, et al. The Neurosteroidogenic Enzyme 5α-Reductase Mediates Psychotic-Like Complications of Sleep Deprivation. Neuropsychopharmacology. 2017;42:2196–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bortolato M, Frau R, Orrù M, et al. Antipsychotic-like properties of 5-alpha-reductase inhibitors. Neuropsychopharmacology. 2008;33:3146–3156. [DOI] [PubMed] [Google Scholar]
- 187.Frau R, Pillolla G, Bini V, et al. Inhibition of 5α-reductase attenuates behavioral effects of D1-, but not D2-like receptor agonists in C57BL/6 mice. Psychoneuroendocrinology. 2013;38:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Frau R, Mosher LJ, Bini V, et al. The neurosteroidogenic enzyme 5α-reductase modulates the role of D1 dopamine receptors in rat sensorimotor gating. Psychoneuroendocrinology. 2016;63:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mosher LJ, Cadeddu R, Yen S, et al. Allopregnanolone is required for prepulse inhibition deficits induced by D(1) dopamine receptor activation. Psychoneuroendocrinology. 2019;108:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu F, Wan Q, Pristupa ZB, et al. Direct protein-protein coupling enables cross-talk between dopamine D5 and gamma-aminobutyric acid A receptors. Nature. 2000;403:274–80. [DOI] [PubMed] [Google Scholar]
- 191.Shrivastava AN, Triller A, Sieghart W. GABA(A) Receptors: Post-Synaptic Co-Localization and Cross-Talk with Other Receptors. Front Cell Neurosci. 2011;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee FJ, Wang YT, Liu F. Direct receptor cross-talk can mediate the modulation of excitatory and inhibitory neurotransmission by dopamine. J Mol Neurosci. 2005;26:245–52. [DOI] [PubMed] [Google Scholar]
- 193.Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2009;19:849–60. [DOI] [PubMed] [Google Scholar]
- 194.Santana N, Artigas F. Laminar and Cellular Distribution of Monoamine Receptors in Rat Medial Prefrontal Cortex. Front Neuroanat. 2017;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Harte M, O’Connor WT. Evidence for a differential medial prefrontal dopamine D1 and D2 receptor regulation of local and ventral tegmental glutamate and GABA release: a dual probe microdialysis study in the awake rat. Brain Res. 2004;1017:120–9. [DOI] [PubMed] [Google Scholar]
- 196.Krystal JH, Bennett A, Abi-Saab D, et al. Dissociation of ketamine effects on rule acquisition and rule implementation: possible relevance to NMDA receptor contributions to executive cognitive functions. Biol Psychiatry. 2000;47:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Driesen NR, McCarthy G, Bhagwagar Z, et al. The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology. 2013;38:2613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chang JP, Lane HY, Tsai GE. Attention deficit hyperactivity disorder and N-methyl-D-aspartate (NMDA) dysregulation. Curr Pharm Des. 2014;20:5180–5. [DOI] [PubMed] [Google Scholar]
- 199.Bortolato M, Aru GN, Fà M, et al. Activation of D1, but not D2 receptors potentiates dizocilpine-mediated disruption of prepulse inhibition of the startle. Neuropsychopharmacology. 2005;30:561–74. [DOI] [PubMed] [Google Scholar]
- 200.Arnedo J, Svrakic DM, Del Val C, et al. Uncovering the hidden risk architecture of the schizophrenias: confirmation in three independent genome-wide association studies. Am J Psychiatry. 2015;172:139–53. [DOI] [PubMed] [Google Scholar]
- 201.Perry W, Minassian A, Feifel D, et al. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiatry. 2001;50:418–424. [DOI] [PubMed] [Google Scholar]
- 202.Parwani A, Duncan EJ, Bartlett E, et al. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry. 2000;47:662–669. [DOI] [PubMed] [Google Scholar]
- 203.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl). 2001;156:234–258. [DOI] [PubMed] [Google Scholar]
- 204.Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotox Res. 2006;10:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Laskemoen JF, Simonsen C, Büchmann C, et al. Sleep disturbances in schizophrenia spectrum and bipolar disorders - a transdiagnostic perspective. Compr Psychiatry. 2019;91:6–12. [DOI] [PubMed] [Google Scholar]
- 206.Robillard R, Naismith SL, Rogers NL, et al. Delayed sleep phase in young people with unipolar or bipolar affective disorders. J Affect Disord. 2013;145:260–263. [DOI] [PubMed] [Google Scholar]
- 207.Gold AK, Sylvia LG. The role of sleep in bipolar disorder. Nat Sci Sleep. 2016;8:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lewis KS, Gordon-Smith K, Forty L, et al. Sleep loss as a trigger of mood episodes in bipolar disorder: individual differences based on diagnostic subtype and gender. Br J Psychiatry. 2017;211:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wehr TA, Sack DA, Rosenthal NE. Sleep reduction as a final common pathway in the genesis of mania. Am J Psychiatry. 1987;144:201–204. [DOI] [PubMed] [Google Scholar]
- 210.Talbot LS, Stone S, Gruber J, Hairston IS, et al. A test of the bidirectional association between sleep and mood in bipolar disorder and insomnia. J Abnorm Psychol. 2012;121:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Perlman CA, Johnson SL, Mellman TA. The prospective impact of sleep duration on depression and mania. Bipolar Disorders. 2006;8:271–274. [DOI] [PubMed] [Google Scholar]
- 212.Cohrs S. Sleep disturbances in patients with schizophrenia : impact and effect of antipsychotics. CNS Drugs. 2008;22:939–962. [DOI] [PubMed] [Google Scholar]
- 213.Krysta K, Krzystanek M, Bratek A, et al. Sleep and inflammatory markers in different psychiatric disorders. J Neural Transm (Vienna). 2017;124:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Benson KL. Sleep in schizophrenia: impairments, correlates, and treatment. Psychiatr Clin North Am. 2006;29:1033–1045 [DOI] [PubMed] [Google Scholar]
- 215.Waters F. Multidisciplinary approaches to understanding auditory hallucinations in schizophrenia and nonschizophrenia populations: the International Consortium on Hallucination Research. Schizophr Bull. 2012;38:693–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Monti JM, Monti D. Sleep in schizophrenia patients and the effects of antipsychotic drugs. Sleep Med Rev. 2004;8:133–148. [DOI] [PubMed] [Google Scholar]
- 217.Ettinger U, Kumari V. Effects of sleep deprivation on inhibitory biomarkers of schizophrenia: implications for drug development. Lancet Psychiatry. 2015;2:1028–35. [DOI] [PubMed] [Google Scholar]
- 218.Meyhöfer I, Kumari V, Hill A, Petrovsky N, et al. Sleep deprivation as an experimental model system for psychosis: Effects on smooth pursuit, prosaccades, and antisaccades. J Psychopharmacol. 2017;31:418–433 [DOI] [PubMed] [Google Scholar]
- 219.Ghosh D, Rajan PV, Das D, et al. Sleep disorders in children with Tourette syndrome. Pediatr Neurol. 2014;51:31–5 [DOI] [PubMed] [Google Scholar]
- 220.Godar SC, Bortolato M. What makes you tic? Translational approaches to study the role of stress and contextual triggers in Tourette syndrome. Neurosci Biobehav Rev. 2017;76:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Paterson JL, Reynolds AC, Ferguson SA, et al. Sleep and obsessive-compulsive disorder (OCD). Sleep Med Rev. 2013;17:465–74. [DOI] [PubMed] [Google Scholar]
- 222.Timpano KR, Carbonella JY, Bernert RA, et al. Obsessive compulsive symptoms and sleep difficulties: exploring the unique relationship between insomnia and obsessions. J Psychiatr Res. 2014;57:101–7. [DOI] [PubMed] [Google Scholar]
- 223.Pace-Schott EF, Germain A, Milad MR. Sleep and REM sleep disturbance in the pathophysiology of PTSD: the role of extinction memory. Biol Mood Anxiety Disord. 2015;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Devoto P, Frau R, Bini V, et al. Inhibition of 5α-reductase in the nucleus accumbens counters sensorimotor gating deficits induced by dopaminergic activation. Psychoneuroendocrinology. 2012;37:1630–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Koethe D, Bortolato M, Piomelli D, et al. Improvement of general symptoms in a chronic psychotic patient treated with finasteride: case report. Pharmacopsychiatry. 2008;41:115–116. [DOI] [PubMed] [Google Scholar]
- 226.Marx CE, Stevens RD, Shampine LJ, et al. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006;31:1249–1263. [DOI] [PubMed] [Google Scholar]
- 227.Marx CE, Bradford DW, Hamer RM, et al. Pregnenolone as a novel therapeutic candidate in schizophrenia: emerging preclinical and clinical evidence. Neuroscience. 2011;191:78–90. [DOI] [PubMed] [Google Scholar]
- 228.Cai H, Cao T, Zhou X, et al. Neurosteroids in Schizophrenia: Pathogenic and Therapeutic Implications. Front Psychiatry. 2018;9:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ritsner MS. Pregnenolone, dehydroepiandrosterone, and schizophrenia: alterations and clinical trials. CNS Neurosci Ther. 2010;16:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Muroni A, Paba S, Puligheddu M, et al. A preliminary study of finasteride in Tourette syndrome. Mov Disord. 2011;26:2146–2147. [DOI] [PubMed] [Google Scholar]
- 231.Mosher LJ, Godar SC, Nelson M, et al. Allopregnanolone mediates the exacerbation of Tourette-like responses by acute stress in mouse models. Sci Rep. 2017;7:3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cadeddu R, Bäckström T, Floris G, et al. Isoallopregnanolone reduces tic-like behaviours in the D1CT-7 mouse model of Tourette syndrome. J Neuroendocrinol. 2019;e12754. [DOI] [PubMed] [Google Scholar]
- 233.Pineles SL, Nillni YI, Pinna G, et al. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology. 2018;93:133–141. [DOI] [PubMed] [Google Scholar]
- 234.Singh A, Kumar A. Possible GABAergic modulation in the protective effect of AP on sleep deprivation-induced anxiety-like behavior and oxidative damage in mice. Methods Find Exp Clin Pharmacol. 2008;30:681–689. [DOI] [PubMed] [Google Scholar]
- 235.Zampieri S, Mellon SH, Butters TD, et al. Oxidative stress in NPC1 deficient cells: protective effect of AP. J Cell Mol Med. 2009;13:3786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Qian X, Cao H, Ma Q, et al. AP attenuates Aβ25–35-induced neurotoxicity in PC12 cells by reducing oxidative stress. Int J Clin Exp Med. 2015;8:13610–13615. [PMC free article] [PubMed] [Google Scholar]
- 237.Lejri I, Grimm A, Miesch M, et al. AP and its analog BR 297 rescue neuronal cells from oxidative stress-induced death through bioenergetic improvement. Biochim Biophys Acta Mol Basis Dis. 2017;1863:631–642. [DOI] [PubMed] [Google Scholar]
- 238.Grimm A, Schmitt K, Lang UE, et al. Improvement of neuronal bioenergetics by neurosteroids: implications for age-related neurodegenerative disorders. Biochim Biophys Acta. 2014;1842:2427–38. [DOI] [PubMed] [Google Scholar]
- 239.Bortolato M, Devoto P, Roncada P, et al. Isolation rearing-induced reduction of brain 5α-reductase expression: relevance to dopaminergic impairments. Neuropharmacology. 2011;60:1301–8. [DOI] [PubMed] [Google Scholar]