Abstract
Retinal remodeling is a progressive series of negative plasticity revisions that arise from retinal degeneration, and are seen in retinitis pigmentosa, age-related macular degeneration and other forms of retinal disease. These processes occur regardless of the precipitating event leading to degeneration. Retinal remodeling then culminates in a late-stage neurodegeneration that is indistinguishable from progressive central nervous system (CNS) proteinopathies. Following long-term deafferentation from photoreceptor cell death in humans, and long-lived animal models of retinal degeneration, most retinal neurons reprogram, then die. Glial cells reprogram into multiple anomalous metabolic phenotypes. At the same time, survivor neurons display degenerative inclusions that appear identical to progressive CNS neurodegenerative disease, and contain aberrant α-synuclein (α-syn) and phosphorylated α-syn. In addition, ultrastructural analysis indicates a novel potential mechanism for misfolded protein transfer that may explain how proteinopathies spread. While neurodegeneration poses a barrier to prospective retinal interventions that target primary photoreceptor loss, understanding the progression and time-course of retinal remodeling will be essential for the establishment of windows of therapeutic intervention and appropriate tuning and design of interventions. Finally, the development of protein aggregates and widespread neurodegeneration in numerous retinal degenerative diseases positions the retina as a ideal platform for the study of proteinopathies, and mechanisms of neurodegeneration that drive devastating CNS diseases.
Keywords: Retinal remodeling, Neurodegeneration, Ultrastructure, Alpha-synuclein, Proteinopathy, Transcellular debris removal
1. Introduction
Negative plasticity as a consequence of photoreceptor degeneration has been chronicled in the literature for nearly a century (Kolb and Gouras, 1974; Lewis et al., 1998; Wolff, 1935), with substantial additions to the literature in the 1980s through the early 2000s (Coblentz et al., 2003; Erickson et al., 1983, 1987; Fariss et al., 2000; Fisher and Lewis, 2003; Lewis et al., 1989, 1998; Li et al., 1995; Marc et al., 1998a; Santos et al., 1997; Strettoi and Pignatelli, 2000; Strettoi et al., 2002). In 2003, all of these associated processes of plasticity were consolidated into a single term: retinal remodeling (Jones et al., 2003; Marc and Jones, 2003). It has since become widely recognized as an integral component of pathology in retinal degeneration, regardless of the precipitating insult (Fisher et al., 2005). Retinal remodeling is a complex process that involves multiple mechanisms. Glial hypertrophy and metabolic dysregulation (Jones et al., 2011; Pfeiffer et al., 2016) are fundamental components of retinal remodeling. In addition, existing neurons extend new, synaptically active, aberrant processes that alter the circuitry of the retina in a process termed “rewiring” (Jones et al., 2003, 2012; Lewis et al., 1998). Phenotypic switching of bipolar cell classes via alterations of glutamate receptors on existing cells results in “reprogramming” of neural circuits (Marc et al., 2007). Translocation of neuronal somas alters the lamination of the retina (Jones et al., 2003), and the eventual cell death of many neurons of all types within the inner retina modifies the signal processing network, likely corrupting it.
Understanding the process of retinal remodeling and its progression will guide the vision research community towards windows of opportunity for vision rescue therapies following photoreceptor loss (e.g. in retinal degenerative diseases such as age-related macular degeneration (AMD) and retinitis pigmentosa (RP)) (Marc et al., 2014). Furthermore, evaluation of retinal remodeling, its components, and their origins provides insight into neural plasticity.
Early evaluation of human retinas with photoreceptor degeneration demonstrated neuronal loss in the inner retina and gross changes of retinal lamination (Kolb and Gouras, 1974; Wolff, 1935). Later work (Lewis and Fisher, 2005; Santos et al., 1997; Sethi et al., 2005) and our more recent results in human tissue demonstrate not only inner retinal neuronal loss, but also plasticity in the adult retina (Jones et al., 2016a, 2016c). Retinal remodeling as a consequence of photoreceptor degeneration has been well described in animal models as well (Jones et al., 2003, 2011). After prolonged retinal remodeling, we speculated that the associated plasticity would enter a plateau phase, perhaps in a predictable and pseudo-stable state. We were incorrect. Retinas engaged in remodeling continue to progress to a new phase of inner retina neurodegeneration with features consistent with proteinopathies. For brevity, neurodegeneration in the retina is referring specifically to inner retinal neurodegeneration, degeneration of the photoreceptors will be specified as photoreceptor degeneration. This review reassesses our understanding of retinal remodeling, including impacts on the outer and inner retina from a histological and ultrastructural perspective, as well as presents evidence for wide-spread retinal neurodegeneration, and discusses the role of proteinopathies in retinal degeneration.
It is our conclusion that neurodegeneration is an additional component of retinal remodeling, with a distinct etiology from reprogramming, rewiring, or currently known metabolic alterations, and is under active study. We will discuss these processes as a product of photoreceptor degeneration, will address the implications for therapeutics aimed at restoring vision, and close by discussing opportunities and advantages of using the retina to explore neurodegeneration and proteinopathies.
2. Retinal anatomy
The functional neuroanatomy of the retina has been studied since the earliest days of neuroscience through the work of Ramόn y Cajal, who illustrated the basic anatomy and principles of information flow through the retina, from photoreceptors to ganglion cells. The retina is a thin, organized, multilaminar strip of nervous tissue along the posterior surface of the eye, which developmentally originates from the frontal cortex. It is subdivided into six major histological layers: the photoreceptor layer (which may be further subdivided into the outer segment, inner segment, and outer nuclear layer), the outer plexiform layer (OPL), the inner nuclear layer (INL), the inner plexiform layer (IPL), the ganglion cell layer (GCL), and the nerve fiber layer (NFL) (Fig. 1A). It is composed of five major neuronal cell types: photo-receptors, horizontal cells, bipolar cells, amacrine cells, and ganglion cells (Marc, 1999). These cells working together play important roles in processing color, motion, intensity, and directionality from visual stimulus (Masland, 2012). The retinal circuitry is, in its simplest terms, divided into two major pathways: vertical and horizontal. The vertical pathway is the path from the photoreceptors, bipolar cells, and ganglion cells, which ultimately project axons into the brain (Inner retina pathways illustrated in Fig. 1B). It is primarily thought of as an amplification pathway, though the presence of ON and OFF cells permits the first level of light-detection processing, and ON-OFF motifs are preserved from bipolar cells through processing in the brain. The horizontal pathway tunes signals from the vertical pathway from being extremely broad regions of light detection to more defined regions of higher light intensity with less light intense regions surrounding, and consists of amacrine and horizontal cells (Fig. 1C). The higher intensity regions of focus are termed centers while the surrounding regions are aptly named the surround. Together, the vertical and horizontal pathways provide initial processing of visual information, prior to action potentials relaying signals to the brain. For a more comprehensive review of visual functioning in the retina see Marc, (2009) (Marc, 2009).
Fig. 1. Phases of remodeling.
(A) Combined phases of remodeling including all cell types. (B) Phases of remodeling in ganglion and bipolar cells. Arrows indicate neurite sprouting, circle highlights cell loss. (C) Phases of remodeling in amacrine and horizontal cells. Arrows indicate neurite sprouting and microneuroma formation. (D) Phases of remodeling in glia. Arrows indicate metabolic variation. The oval indicates a region of muller cell entanglement.
3. Retinal degeneration
Photoreceptor degeneration can be caused by numerous mechanisms including hereditary diseases directly affecting the photoreceptors such as retinitis pigmentosa (RP) and rod-cone dystrophies, diseases affecting the RPE such as Lebers Congenital Amaurosis (LCA) and age-related macular degeneration (AMD), or physical trauma leading to retinal detachment. Despite the variable mechanism of photoreceptor loss between cases, the ultimate outcome is the same: extensive loss of photoreceptors, leaving a deafferented neural retina followed by extensive remodeling, and eventually, neurodegeneration. The following subsections briefly describe the most common means of photoreceptor degeneration to introduce the reader to these concepts, but are not intended to be a comprehensive list of all photoreceptor degenerations.
3.1. Retinitis pigmentosa
Retinitis pigmentosa (RP) refers to a range of hereditary disorders, which onset with degeneration of the photoreceptors, commonly rod photoreceptors (Hamel, 2006). The name retinitis pigmentosa originates from the ophthalmoscopic observation of exposed pigmentation in the neural retina, arising from a presumed migration of pigmented RPE cells into the neural retina (Jones and Marc, 2005). The prevalence of RP is approximately 1 in 4000, with varying modes of inheritance where 50–60% of cases are inherited autosomal-recessive, 30–40% autosomal-dominant, and 5–15% X-linked. RP is known to arise from approximately 100 gene defects,1 which are subclassified by their genetic component, mode of inheritance, and whether the defect is constrained to the eye or is syndromic and affects nonocular tissues (Dias et al., 2018). Examples of associated syndromic diseases are Bardet-Biedl syndrome and Usher syndrome, which are both autosomal-recessive. Bardet-Biedl syndrome, in addition to photoreceptor loss, is associated with polydactyly, truncal obesity, renal dysfunction, and learning difficulties (Suspitsin and Imyanitov, 2016) while Usher syndrome is associated with hearing-loss in addition to RP (Mathur and Yang, 2015).
The speed of vision loss and the mechanism causing the photoreceptor degeneration varies widely across RP diseases. Some individuals will experience vision loss early in life, while others will remain relatively asymptomatic until adulthood. Despite the many modes of inheritance and genes involved with RP, rod photoreceptors degenerate in a non-uniform fashion across the periphery of the retina, followed by cone loss. In most cases, RP patients are legally blind by their mid-forties, and photoreceptor degeneration continues until the retina is completely devoid of photoreceptors, leaving patients completely unable to perceive light for years to decades of life (Hartong et al., 2006).
3.2. Age-related macular degeneration
Age-Related Macular Degeneration (AMD) predominately affects people over the age of 50, and is the leading cause of vision loss in elderly individuals. The risk of AMD increases with age.2 Though 52 genetic variants have been linked to AMD, environmental factors such as smoking and diet can greatly increase an individual’s risk, with smoking being the single largest risk factor, aside from age (Al-Zamil and Yassin, 2017; Fritsche et al., 2014; Lim et al., 2012).
AMD is associated with loss of proper function of the retinal pigment epithelium (RPE), which leads to photoreceptor death. The RPE separates the retina from the choroid and is a critical component of the retinoid cycle and photoreceptor maintenance. The RPE is essential for photoreceptor metabolism and phagocytosis of outer photoreceptor segments shed from photoreceptors in a circadian fashion. Histologically, dry AMD commonly presents with photoreceptor outer segment shortening and loss, drusen (lipid deposits above and below the RPE) separating the photoreceptors from RPE or from the choroid, and metabolic alterations of RPE cells that may predict progression of disease (Jones et al., 2016b). Regardless, loss of proper RPE function and/or death leads to the death of photoreceptors, principally with the loss of central cone vision and often followed by the loss of peripheral rod vision. The wet form of AMD arises from new blood vessel formation (neovascularization) in the choroid, neural retina, subretina and vitreous, leading to subsequent leakage of blood and serum which is damaging to the neural retina (Wong et al., 2008). Currently, there are only treatments for wet forms of AMD to prevent further neovascularization through anti-VEGF treatments. However, the AREDS study suggests a prophylactic effect of supplements in warding off dry forms of AMD (Age-Related Eye Disease Study Research, 2001a, b). In wet and dry forms, affected individuals often complain about blurriness in their central vision, which progresses to a loss of central vision and advances outward towards the periphery.
3.3. Injury-induced retinal degeneration
Both retinal detachment and light-induced retinal degeneration (LIRD) induce photoreceptor degeneration with subsequent retinal remodeling in the absence of genetic involvement in humans and animal models. Clinically, retinal detachment has been described for nearly a century (Jeremy, 1922). Following detachment, the patient commonly experiences photopsias and progressive loss of sight in the affected eye, though the exact presentation varies with the cause of the detachment. Experimentally, retinal detachment and LIRD are excellent models to explore the responses of the outer and inner retina to the specific insult of photoreceptor degeneration. Retinal detachment studies observed that the loss of photoreceptors leads to a number of deleterious changes within the inner retina, that were subsequentially found also be present in genetic models (Erickson et al., 1987; Fisher et al., 1995; Lewis et al., 1989, 1994, 1998; Marc et al., 1998b). LIRD was documented to involve ultrastructural alterations to the RPE (Grignolo et al., 1969) alterations in photoreceptor outer segment lipids and proteins (Organisciak et al., 1989b) and induces photoreceptor degeneration (Organisciak et al., 1989a), and later was found to progress into remodeling (Jones et al., 2006). Following detachment or light-damage, photoreceptor outersegments degenerate, while vacuolization of the soma and mitochondrial stress become evident by day 3 (Erickson et al., 1983). Glial involvement is evident via cell division, nuclear translocation, and protein changes in Muller cells of retinas detached for a prolonged period (Erickson et al., 1987; Lewis et al., 2010). Changes in metabolic signatures are also evident in both retinal detachment (Marc et al., 1998a) and LIRD (Marc et al., 2008). Finally, rewiring is evident in the inner retina of both models following photoreceptor degeneration (Jones et al., 2006; Lewis et al., 1998). The fact that these degenerations provoke features of remodeling consistent with genetic disease models indicates the loss of photoreceptors is the primary insult leading to remodeling.
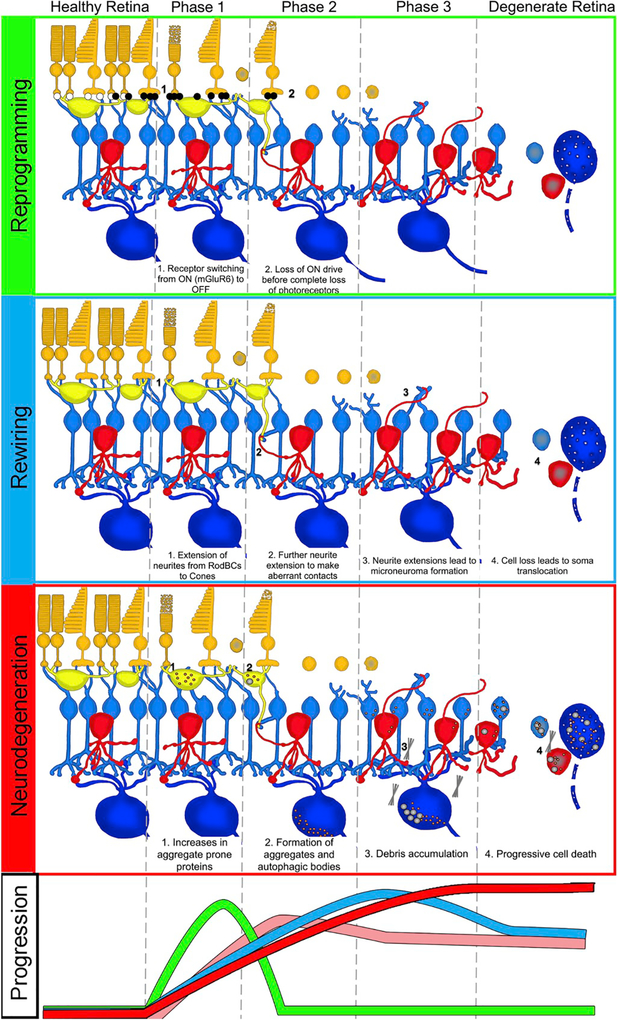
4. Retinal remodeling
Retinal remodeling is a phenomenon consequent to photoreceptor degeneration consisting of a series of alterations in retinal metabolism, receptor expression, neuronal network topologies, and eventual cell death. Retinal remodeling has been described as phases, characterized by the progression from photoreceptor stress through photoreceptor degeneration and neural retinal involvement (Fig. 1). Phase 0 is the healthy retina, wherein no photoreceptor stress or degeneration is observed. Phase 1 is characterized in by initial stress and degeneration of photoreceptors along with the emergence of neural reprogramming and glial responses. Phase 2 of remodeling is defined by the progressive loss of remaining photoreceptors, particularly cones. Phase 2 is maintained as long as remnant cones persist in the retina, widespread rewiring and cell death of phase 3 is held at bay. Phase 3 follows the complete loss of photoreceptors. At this point in degeneration, deafferentation of the retina is complete and leads to neurite outgrowth by all cell classes, followed by widespread cell death.
In the following sections we will refer to phases of remodeling, describing the discrete etiologies of the main processes of remodeling: reprogramming, rewiring, glial responses, and formally introduce the next phase of retinal remodeling in section 4: neurodegeneration. In short, regardless of the primary insult, the neural retina undergoes a series of increasingly deleterious changes in response to loss of photoreceptors. These processes will likely have an impact on any therapeutic scheme introduced to restore/preserve vision once remodeling is initiated, and more comprehensive understanding of precipitating factors will provide future targets. Understanding these components will be essential to recommending the best potential therapeutic for different stages of remodeling (Marc et al., 2014).
4.1. Reprogramming
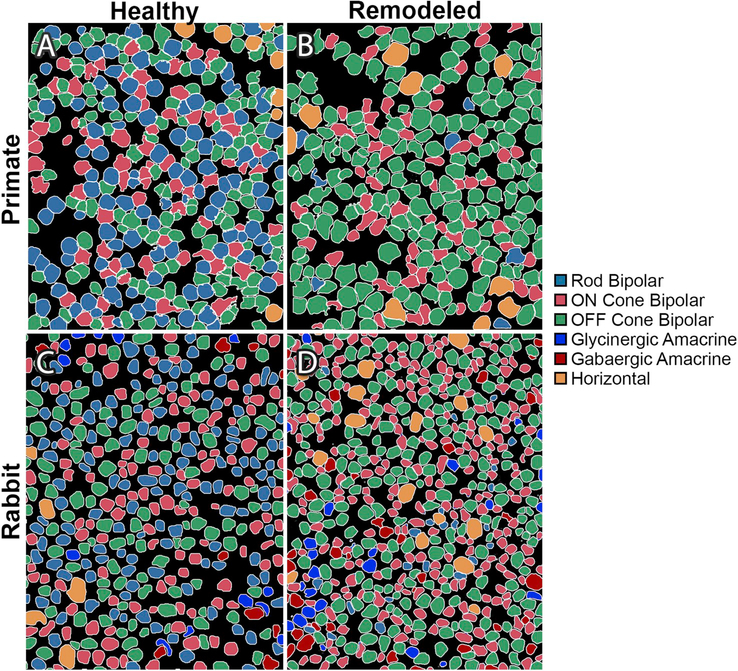
Photoreceptor input to bipolar cells is conveyed via graded glutamate release, dependent on light levels, which acts upon receptors of differing kinetics. In bipolar cells which depolarize in response to light (ON-BCs), the responsible receptor is metabotropic glutamate receptor 6 (mGluR6). In bipolar cells which depolarize in response to a decrease in light (OFF-BCs), the responsible receptors are ionotropic kainite and AMPA sensitive glutamate receptors (iGluRs). During phase 1 remodeling, ON-BCs change expression of their receptor phenotypes and become pharmacologically consistent with OFF-BCs. In healthy primate, the ratio of OFF-BC: ON-BC: RodBC is 40:30:30. In an RP affected retina, the ratio shifts to 70:20:05 (Fig. 2)(Jones et al., 2016a; Marc et al., 2007). These results were confirmed in rabbit retina with a shift of OFF-BCs from approximately 30–40% of the retina to over 56% of identifiable bipolar cells in a rabbit model of RP (Fig. 2) (Jones et al., 2011, 2012). In phase 2 remodeling, the shift of mGluR to iGluR expression in bipolar cells continues, in addition to mislocalization of mGluR receptors. As dendrites degenerate and disappear, mislocalized mGluR6 expression occurs in axons of RodBC (Strettoi and Pignatelli, 2000; Strettoi et al., 2002). In phase 3 remodeling, both mGluRs and iGluRs are functionally absent in all bipolar cells, though iGluRs persist in amacrine and ganglion cells (Marc et al., 2007). This end phenotype of remodeling might be due to prolonged lack of afferent input to bipolar cells by photoreceptors, but the precise mechanism of down-regulation is unknown. It is worth noting however, that iGluR functional expression persists in retinal regions containing remnant cones, granted it is unclear what signaling remnant cones are doing. The persistence of iGluRs downstream of bipolar cells in phase 3 remodeling suggests that some sort of bipolar input to amacrine and ganglion cells is still present, yet it is currently unknown what initiates that signaling. We believe that answer lies in the rewiring component of remodeling.
Fig. 2. Reprogramming.
Adapted with permission from Marc et al., (2007) and Jones et al., (2011) Theme maps of horizontal sections through the inner nuclear layer generated by cluster analysis of Kainic Acid driven (25 μM) signaling in normal and degenerate retinas. (A) Normal baboon retina, (B) Human RP retina, (C) Healthy rabbit retina, (D) Degenerate rabbit retina from a 10-Month Tg P347L rabbit.
4.2. Rewiring
Precise synaptic organization between neurons is central to proper neural network function. Corruption of neural wiring from disease or trauma alters how information is processed and complicates therapies designed to replace neurons lost through disease. Following insult, the central nervous system (CNS) is known to undergo rewiring events in epilepsy (Morimoto et al., 2004), Alzheimer’s disease (Agosta et al., 2010), Parkinson’s disease (Guo et al., 2015), and others. The retina is no different. The normal topology of retinal circuitry is disrupted during and following photoreceptor insult. In phase 1 remodeling, rod photoreceptors extend axonal processes beyond the OPL into the inner retina, in some cases extending to the inner limiting membrane (Fariss et al., 2000; Li et al., 1995; Sethi et al., 2005),. Simultaneously, RodBCs and rod-contacting horizontal cells retract their dendrites from rod spherules and a subset extend neurites towards cone pedicles (Linberg et al., 2006; Peng et al., 2000), making peripheral contacts (Fig. 1 B + C). In phase 2 remodeling, degeneration continues with photoreceptor cell death progression, with the end of phase 2 being marked by the absence of all photoreceptors. Bipolar cells continue to remodel their dendrites as their normal inputs disappear, before becoming completely deafferented once photoreceptors are gone (Strettoi and Pignatelli, 2000; Strettoi et al., 2003). Horizontal cells sprout neurites that extend into the IPL (Strettoi and Pignatelli, 2000; Strettoi et al., 2002, 2003), though their contacts, if any, are unknown. Amacrine cells and ganglion cells also initiate sprouting, creating new synaptic contacts in regions of novel neuropil (Jones et al., 2003). In phase 3 remodeling, the complete loss of afferent input leads to widespread neurite sprouting of all remaining retinal neurons (Jones et al., 2003; Marc and Jones, 2003; Marc et al., 2003). Extended neurites may coalesce together forming microneuromas, which are ultrastructurally found to contain ribbon synapses, vesicle clouds, and post-synaptic densities consistent with synaptic activity (Jones et al., 2003). Other neurites, and an allocation of entire neurons, have been found to escape the neural retina and extend within the choroid (Jones et al., 2003), or infiltrate epiretinal membranes caused by Muller cell extensions that break through the inner limiting membrane into the vitreous (Lewis et al., 2007). Mechanistically, neurite sprouting has been associated with retinoic acid (Lin et al., 2012a) and growth associated protein 43 (GAP43)(Coblentz et al., 2003). Due to the extensive neurite sprouting, the precise topology of novel circuits formed during remodeling are still under active investigation in our lab (Pfeiffer et al., 2018).
4.3. Glial responses
In addition to neurons, the retina also has numerous glial cells: astrocytes, microglia, and Mϋller cells, each providing critical functions in the retina (Fig. 1D). Each of these glia serves specific roles in the maintenance of the retina and proper neural signaling and become reactive early in remodeling. These roles and reactive states caused by remodeling are expounded below.
4.3.1. Astrocytes
Astrocytes are a class of glial cell found throughout the CNS, with well characterized roles in neuronal metabolism, formation and maintenance of the blood-brain-barrier, and stress response. In the brain, they anatomically tile with one another forming distinct spatial domains (Bushong et al., 2002; Ogata and Kosaka, 2002). In vascularized retinas, they are less prevalent and their localization is generally associated with the optic nerve head and blood vessels, with a minimal overlap tiling observed (Jammalamadaka et al., 2015). Retinal astrocytes have a distinct morphology of small processes which make broad flat contacts with blood vessels, as well as smaller contacts with ganglion cells (particularly their axons), and other astrocytes (Luna et al., 2016). Astrocyte responses to photoreceptor degeneration has been a difficult phenomenon to study. In the retina, Mϋller cells are the most common macroglia, which in the healthy retina can be quickly differentiated from astrocytes based on glial fibrillary acidic protein (GFAP) expression. As we will discuss below, Müller cells express low levels of GFAP under conditions of low stress, while astrocytes express high GFAP. In retinal degeneration however, Müller cells begin to increase their GFAP expression making astrocyte responses difficult to differentiate. Luna and colleagues (Luna et al., 2016) evaluated astrocyte response to retinal detachment in multiple species using dye filling strategies in mouse (where Müller glial up-regulation of GFAP is less pronounced), finding distinct morphologies associated with detachment. Following detachment, and the associated photoreceptor degeneration, astrocytes become more diffuse in their GFAP labelling combined with greater branching and dispersed contacts with blood vessels. Following prolonged detachment, astrocytes are found to extend processes into the inner retina. In human AMD, the regular appearance of astrocytic processes bordering ganglion cell axons also becomes irregular (Luna et al., 2016). The exact progression of astrocytic response to photoreceptor degeneration in remodeling remains to be clarified. Nevertheless, it is clear that astrocytes alter their normal morphology and clear contacts with blood vessels in retinal detachment and human AMD.
4.3.2. Microglia
Microglia are the resident macrophage cells of the CNS, including the retina. They play a central role in CNS maintenance, development, and plasticity through remodeling of synapses, the clearance of cellular debris, pathogens, and apoptotic cells (Sominsky et al., 2018). In the retina, microglia cell bodies are found in the plexiform layers (Karlstetter et al., 2015) from where they continuously extend and retract processes as they surveil their surroundings in healthy retina (Nimmerjahn et al., 2005). In phase 1 remodeling in RP, as rods begin to become stressed, microglia ramify and extent processes towards the photoreceptor layer where they phagocytize apoptotic rods (Gupta et al., 2003). In AMD, microglia are attracted to drusen (Killingsworth et al., 1990), and once photoreceptors begin to degenerate, microglia are involved in their clearance. In retinal detachment, microglia also rapidly migrate from the IPL to the ONL in response to photoreceptor degeneration (Lewis et al., 2005). Microglia are not restricted to phagocytosis of apoptotic cells and have been implicated in the degeneration of neighboring, otherwise healthy photoreceptors (Gupta et al., 2003). This, in addition to the secretion of cytokines, leads to bystander killing by microglia, likely advancing photoreceptor cell death and potentially impacting other cell classes in retina (Langmann, 2007). In phase 3 remodeling microglia are found to become less ramified and settle into an in between morphology suggesting a chronic para-inflammatory state (Karlstetter et al., 2015).
4.3.3. Müller Cells
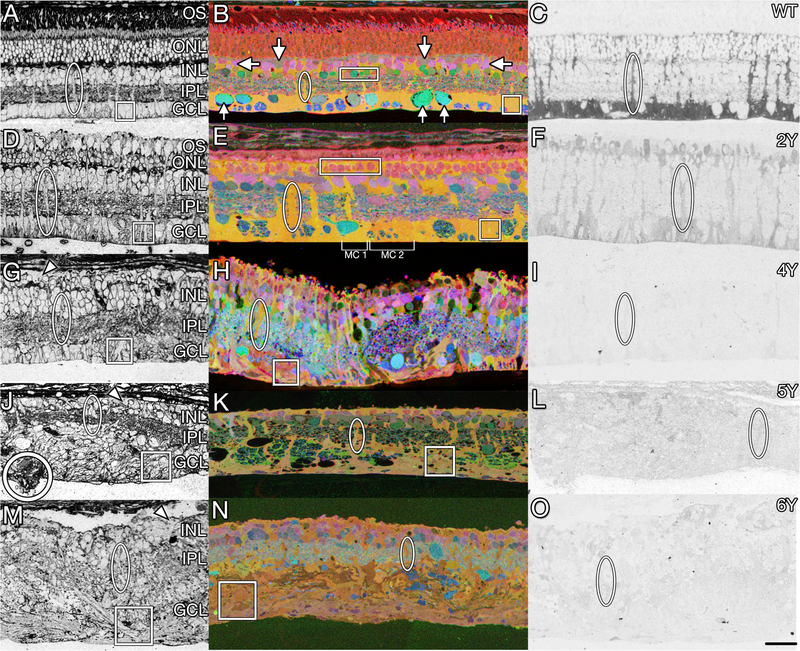
Müller cells are the primary macroglia of the retina that function in amino acid and ion homeostasis, water regulation, provide trophic and structural support, and play a central role in glutamate metabolism. In retinal degeneration, Müller cells are among the first to respond to stress. In phase 1, as the photoreceptors begin to become stressed and outer segments shorten and begin to degenerate, Müller cells begin to alter their metabolic signatures, then begin to hypertrophy and change their protein expression including upregulation of GFAP, (Erickson et al., 1987; Roesch et al., 2012), decrease in glutamine synthetase (GS) expression, and decreased cellular retinaldehyde-binding protein (CRALBP) expression (Lewis et al., 1994). The normal homogeneous signature unique to Müller cells: high glutamine and taurine (> 10 mM) and a low concentration of glutamate (< 0.1 mM) (Marc et al., 1995); become less stable and more heterogeneous. Müller cells begin to demonstrate global glutamine elevation (Jones and Marc, 2005), though local metabolic signatures begin to differ among neighboring Müller cells in regions of photoreceptor degeneration (Jones et al., 2011). Ultrastructurally, Müller cells demonstrate greater overlap and interdigitation of their endfeet while also demonstrating heterogeneity in their osmication affinity (Pfeiffer et al., In Press). In phase 2, Müller cells continue to hypertrophy and begin to form a glial seal between the inner retina and the RPE, as photoreceptors continue to degenerate (Jones and Marc, 2005). Metabolically, the increase in glutathione concentration in Müller cells along with variability in glutamate, glutamine, and taurine levels between individual cells suggests these cells are undergoing oxidative stress (Jones et al., 2016a, 2016b). In phase 3, Müller cells complete the glial seal, isolating the inner retina from the RPE and choroid. The glial seal is a combination of Muller cell apical processes and Muller cells which have delocalized their nuclei into the OPL and undergone division (Lewis et al., 2010). Müller cell protein expression becomes increasingly altered with high levels of GFAP expression and decreasing levels of glutamine synthetase (Jones et al., 2016a). These changes persist long term following the photoreceptor degeneration (Lewis et al., 1989). As glutamine synthetase expression decreases, the levels between individual Müller cells becomes increasingly chaotic until glutamine synthetase expression completely collapses (Jones et al., 2016a; Pfeiffer et al., 2016). Fig. 3 demonstrates features of long-term remodeling progression to neurodegeneration in the Tg P347L rabbit. Recently, we discovered that the extracellular matrix (ECM) can be visualized by tomato lectin (TL) immunoprobing to facilitate understanding of the topological morphology changes that are occurring in retinal degeneration and remodeling. An added benefit is that TL markers with this approach are also compatible with ultrastructural analysis, as evidenced by its clean staining of glutaraldehyde fixed tissues (Fig. 3, column 1). Computational Molecular Phenotyping (CMP) analyses of retinas from 2 to 6 year old Tg P347L rabbits show continued pathologic neurite and glial remodeling, progressive neurodegeneration, neuronal loss and anomalous, and chaotic metabolic reprogramming of Müller cells (MCs) (Fig. 3, column 2) (Pfeiffer et al., 2016); Of note, severe, progressive loss of MC GS occurs as previously noted, (Fig. 3, column 3), which is essential for retinal glutamate-glutamine recycling and consistent with that seen in humans (Jones et al., 2016a). Glial revision in phase 3 remodeling continues until MCs have no distinct metabolic signature and exhibit highly disorganized morphologies. MCs transform into thick, overlapping blocks of glia with processes invading debris-like assemblies in large areas of the remnant retina (Fig. 3, column1).
Fig. 3. Muller glial changes in remodeling.
Transition from normal (A–C) to remodeled (D–I) and neurodegenerative retina (J–O) in WT (A–C) versus 2yr (D–F), 4yr (G–I), 5yr (J–L), and 6yr (M–O) Tg P347L rabbits. Representative Müller cell columns and endfeet are highlighted with ovals and squares, respectively. Retinas probed for tomato lectin (left column) display extracellular matrix and cell patterning. White circle (J) marks Müller cell eruption through the basement membrane. Small molecule sCMP mapping (middle column) with τQE (taurine, glutamine, glutamate) > RGB differentiates major cell types as colored metabolite mixtures: Normal Müller cells, yellow-orange (ovals); ganglion cells, teal (up arrows); amacrine cells, pink or green (rectangles); bipolar cells, pink (down arrows), horizontal cells, dull green (side arrows. Metabolically anomalous Müller Cells are indicated by brackets (E). Protein CMP of glutamine synthetase (right column) reveals declining expression over disease duration. Scale bar 30 μm.
5. Phase 4: Progressive neurodegeneration in retinal degenerations
Retinal remodeling after photoreceptor loss is common to all retinal degenerations in humans (Jones et al., 2016a, 2016b), carnivores (Marc et al., 1998a), rodents (Jones et al., 2003; Strettoi et al., 2003) and lagomorphs (Jones et al., 2011), independent of the primary insult inducing photoreceptor degeneration. However, throughout much of the literature, short-lived rodent models have been used to characterize and quantify the nature, extent and mechanisms of remodeling (Chang et al., 2002). This creates a misinterpretation of the process of retinal degeneration and creates a disconnect between the biology described by the use of experimental rodent models of vision rescue, spanning less than a hundred days of degeneration, and the ultimate goal of restoring sight in humans who display severely remodeled retinas following decades of disease.
After continued monitoring of retinas and samples from human disease, we have identified a fourth phase of retinal remodeling: Progressive neurodegeneration that extends beyond simple cell loss to resembling proteinopathies observed in other forms of neurodegeneration, like Alzheimer’s, Parkinson’s, and others.
5.1. Neurodegeneration in humans
The hallmark of dry AMD and RP in humans is degeneration of photoreceptors, prompting numerous studies into the causes and progression of photoreceptor loss (Cuenca et al., 2014). Beyond photoreceptor loss, we know that retinal degeneration associated cell death is common in geographic atrophy, which follows late stage AMD and becomes progressively more severe with time. Geographic atrophy is characterized by a regional loss of RPE, photoreceptors, and the choriocapillaris following neovascularization of the retina. In addition to the loss of these outer retinal structures, neurons of the inner retina have also demonstrated signs of metabolic stress (Fig. 3)(Jones et al., 2016b) and cell death (Ramkumar et al., 2018). Retinal remodeling in humans and animal models of RP demonstrates the neural retina is not immune to cell death that occurs coincident and subsequent to photoreceptor stress and loss. Studies of post mortem RP eyes demonstrate loss of neurons in the inner retina (Jones et al., 2003, 2016a), including within the more preserved macula, with 70% loss of ganglion cells (Santos et al., 1997).
5.2. Animal models of RP
Extensive reorganization of the retina is associated with phase 3 retinal remodeling and has been described in numerous animal models as well as in human (Figs. 4 and 5) (Han et al., 2013; Jones and Marc, 2005; Jones et al., 2003, 2016a; Kalloniatis et al., 2016). Although not absolute, many rodent models of RP eventually demonstrate inner retinal cell death, particularly after prolonged loss of cone photoreceptors (Fig. 4). This occurs in a discontinuous fashion across the surface of the retina and is consistent with the regional retinal degeneration and the associated neuronal loss observed in humans. The key element leading to observable neurodegeneration is time. The rhodopsin transgenic (Tg) P347L rabbit permits evaluation of retinal remodeling over many years, recapitulating retinal remodeling observed in humans (Kondo et al., 2009), and also serving as a model for progressive neurodegeneration and proteinopathy as we discuss below. At 10 months old, the Tg P347L rabbit retina is largely devoid of rod and cone outer segments with a reduced electroretinogram (80% scotopic a and b-wave reduction, 70% photopic a and b wave reduction) reflecting the loss of photoreceptor input (Kondo et al., 2009). Over the following 1–2 years the sensory retina loses photoreceptors in a patchy fashion, until cone photoreceptors are lost, leading to phase 3 remodeling of the neural retina (Fig. 3) and (Jones et al., 2011). Following the complete loss of photoreceptors, widespread neural degeneration and remodeling is progressive and affects neurons of all classes (Jones et al., 2003). Loss of neuronal tissue as a percent of total tissue in the P347L rabbits is substantial. By 2 years there is ~40% cell loss and by 4 years–55%. In our 6-year TgP347L rabbit, the best-preserved region suffered ~78% loss, while the more degenerate regions showed > 99% neuronal loss.
Fig. 4. Retinal remodeling in animal models and humans.
Reprinted with permission from Jones et al., 2003. Representative γGE → rgb mappings of retinal degeneration models. Downward arrows indicate inversions from the inner nuclear layer (INL) to the ganglion cell layer (GCL); upward arrows indicate eversions from the inner nuclear layer to the distal margin of the remnant retina; vertical arrowheads indicate glial columns; circles denote blood vessels. Abbreviations: M, inner nuclear layer microneuroma; S, stricture; P, neuropil patch; γ. GABA; G, glycine Scales, 20 μm. (A) Human RP retina, FFB accession #378, 76 year old female, 2 h post-mortem, RP diagnosed at age 33, no vision at death. This vertical image demonstrates massive cell loss typical of late stage RP, with complete loss of the inner nuclear and ganglion cell layers at the right margin. A column of apparent neuronal migration is bordered by glycinergic amacrine cells (GAC) in the ganglion cell layer and GABAergic amacrine cells (γAC) at the distal margin of the remnant. Strictures distort the remaining inner plexiform layer and a Müller cell (MC) fibrotic seal separates the retina from the choroid/retinal pigmented epithelium. (B) rd1 mouse, pnd 630. A large column of neurons bridges the depleted inner nuclear layer, displaying inverted amacrine and bipolar cells (BC) and everted amacrine cells. (C) RCS rat retina, pnd 900. This image shows three columns of neuronal migration (vertical arrowheads) where bipolar and amacrine cells are displaced from their proper locations. Distorted regions of the inner plexiform layer often pass through strictures as small as 5 μm. (D) S334ter rat, pnd 363. This image shows two glial columns (vertical arrowheads), with right column exhibiting Müller cell hypertrophy breaking up the normal tiling of the retina. Everted and inverted glycinergic amacrine cells are abundant. (E) P23H rat, pnd 372. A glial column (vertical arrowhead) traverses the inner plexiform layer accompanied by migrating glycinergic and GABAergic amacrine cells. (F) GHL mouse, pnd 746. A broad glial column (vertical arrowhead) serves as a pathway for migration for amacrine and bipolar cells into the ganglion cell layer. A microneuroma has formed distal to the heavily depleted inner nuclear layer. (G) TG9N mouse, pnd 160. An oblique column forms a neuron migration path with both inverted and everted cells. Strictures deform the inner plexiform layer and microneuromas form in the midst of the inner nuclear layer, but a mere 5 μm from the distal glial seal. (H) nr mouse, pnd 720. Two columns of neuronal migration are indicated, with inversion and eversion of amacrine cells. The inner nuclear layer is thinned to less than half its normal thickness. (I) chx10 mouse, pnd 365. Few neurons survive in this defect, and only in small clusters with tiny patches of apparent neuropil surrounded by massive fields of apparent Müller cells. (J) Chx10/P27Kip1−/− hypocellularity rescue mouse, pnd 60. This model initially displays roughly normal lamination, but with few bipolar cells and severely reduced photoreceptor numbers. However, even in the absence of clear glial columns or seals, microneuromas emerge and columns of neurons strung through the inner plexiform layer are common, as are misplaced amacrine cells in the ganglion cell layer. (K) RKO mouse, pnd 365. No neuron migration has emerged, and positions of amacrine cells, including presumed starburst amacrine cells (S AC), are normal. Microneuromas are forming next to the glial seal. (L) RhoΔCTA mouse, pnd 541. Migration across the inner plexiform layer has not begun, but microneuromas are emerging and the entire inner nuclear layer displays disordering of neurons, with everted amacrine cells and bipolar cells moving into the former amacrine cell layer.(M) pcd mouse, pnd 321. At this age, retinal lamination appears normal and no microneuromas have emerged and proper localization of ganglion cells (GC) is observed.(N) rd2 mouse, pnd 151. At this age, retinal lamination appears normal and no microneuromas have emerged. These image data will be available for public access at http://www.marclab.org.
Fig. 5. Theme maps of remodeling.
Reprinted with permission from Jones et al., 2003 Theme maps of (A) normal pnd 700 Sprague-Dawley (SD) rat, (B) pnd 900 RCS rat, (c) P372 P23H line 1 transgenic rat, and (d) human RP (Foundation Fighting Blindness accession #133-OD, 67 year-, female, advanced RP, simplex, fixed 2.5 h post mortem). In normal retina, cell layers are precisely defined. Remodeling clearly disrupts lamination via migration on Müller glia columns (C), yielding eversion (E) of GABAergic and glycinergic amacrine cells to the distal Müller glial seal (M) and inversion of amacrine and bipolar cells (I) to the ganglion cell layer. Glial hypertrophy and neuronal movement can be so extensive that the inner plexiform layer is segmented, distorted and forced through strictures (S) as small as 10 μm.
6. Ultrastructural characterization of photoreceptor degeneration, retinal remodeling, and neurodegeneration
Electron microscopy has played an important role in our current understanding of many components of nervous system structure and function including synaptic architecture, cellular organization (Palay and Palade, 1955), and recently through connectomics efforts, neural network architecture (Marc et al., 2013). In addition, ultrastructural analysis, particularly transmission electron microscopy (Eskelinen, 2014; Klionsky et al., 2008) is the gold standard in the identification of cellular pathologies such as autophagy and necrosis. Clear descriptions of ultrastructural phenomena observed throughout retinal degeneration highlights multiple avenues of investigation for not only the progress of retinal degeneration, but also reveals pathological indications typical of multiple neurodegenerative diseases.
6.1. Early retinal degeneration
Early retinal degeneration is characterized by phase 1 remodeling (onset of photoreceptor stress and the beginnings of outer segment shortening). Some early studies of retinal degeneration explored advanced retinal degeneration in human tissues (phase 3 + remodeling), but most studies have been carried out in transgenic animal models which permit the evaluation of disease progression in the earliest phases. RP has been widely studied thanks to the large number of genetic mutations that lead to rod degeneration in models of RP. Fewer models are available for the study of AMD for a variety of reasons from genetic complexity to rodents’ lack of anatomical foveas resembling human and primate foveas. Among the over 100 genetic mutations associated with RP, the most common is the P23H mutation in rhodopsin used in many animal models including zebrafish (Tam and Moritz, 2006), mouse (Naash et al., 1993), rat (LaVail et al., 2018), and pig (Ross et al., 2012). This mutation, and others including the P347L mutation, causes degeneration of the photoreceptors, initiating with the loss of rods, leading to night blindness, with progressive tunnel vision prior to complete vision loss. Most ultrastructural studies of animal RP models are focused on photoreceptor structural anomalies associated with photoreceptor degeneration. These studies, like much of the AMD literature describe photoreceptor outer segment disorganization and degeneration (Asakawa et al., 2015; Jones et al., 2011), mitochondrial disorganization, swelling of endoplasmic reticulum or golgi apparatus (Blanks et al., 1982), and autophagic compartments in the photoreceptors (Bogea et al., 2015).
6.2. Advanced retinal degeneration
Early electron microscopy studies were typically of patients with advanced RP that demonstrated extensive loss of photoreceptors, with loss or irregular organization of outer segments in the foveal cones and widespread loss of photoreceptors in the periphery (Kolb and Gouras, 1974; Mizuno and Nishida, 1967). The RPE also demonstrates disorganization with pigmented cells present throughout the inner retina (Jones et al., 2016a), characteristic and likely the cause of pigmented bone spicules associated with advanced RP. In human eyes affected by advanced AMD, cone outer segments are largely degenerated (where still present), with shortened inner segments and swollen mitochondria (Litts et al., 2015), commonly associated with cell stress. Light microscopic approaches through computational molecular phenotyping demonstrate conclusive cell stress in both Müller cell populations as well as neuronal and photoreceptor populations (Jones et al., 2011, 2016a; Pfeiffer et al., 2016).
6.3. Effects of retinal degeneration on the inner retina
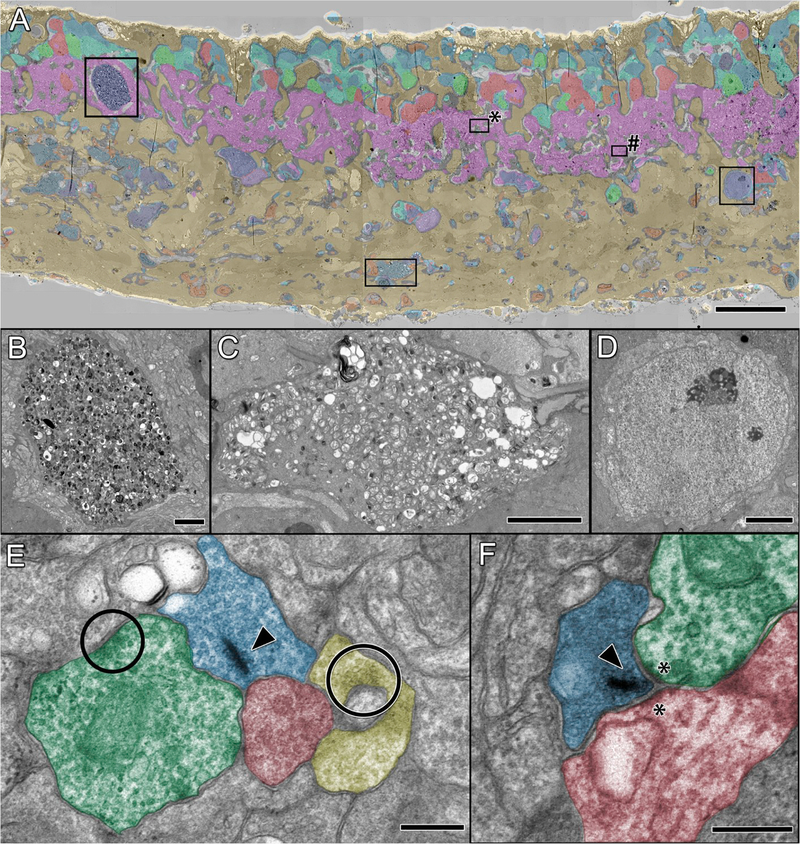
Over the past couple of decades, a few labs have worked to characterize the ultrastructural characteristics of the inner retina during retinal degeneration. A primary characteristic of remodeling in advanced retinal degeneration is the presence of microneuromas, where processes of numerous classes of neurons coalesce and run together, in some cases for many microns. These novel neuropil structures contain ultrastructural evidence of active synapses (Jones et al., 2003, 2012). Data show that Müller cells become and remain hypertrophic throughout the progression of remodeling, in addition to demonstrating evidence of variable hyper osmication both within cells and between Müller glia (Jones et al., 2011; Pfeiffer et al., In Press). In advanced aged models of retinal degeneration, many remnant ganglion cells somas and processes display severe cytopathologies (Asakawa et al., 2016), and extensive “internal whorl” structures, believed to be associated with ER or mitochondrial stress and autophagy (Fig. 6B and C), similar to pathologies found in CNS neurodegenerations (Hall et al., 1997; Park et al., 2014). Despite substantial neuronal loss following photoreceptor death, the retina continues to maintain synaptic structures (Fig. 6E and F).
Fig. 6. TEM of remodeling and neurodegeneration in P347L rabbit.
(A) Theme map overlay generated via k-means clustering of 7 yr Tg P347L rabbit retina using E, Q, J, D, G, γ, τ, and GS signals. Gold, MCs; magenta, IPL; salmon and green, amacrine cells; cyan and teal, bipolar cells; blue and purple, ganglion cells, scale 25 μm. (B) Large ganglion cell containing autophagic inclusions, scale 2.5 μm (C) Ganglion cell process filled with autophagic structures, scale 2.5 μm. (D) Ganglion cell without autophagic structures, scale 2.5 μm. (E) Conventional (circles) and ribbon (arrowhead) synapses in neurodegenerative retina (region * in A); scale, 300 nm. (F) Ribbon synapse (arrowhead) and typical post-synaptic densities (asterisks) persist (region # in A); scale, 300 nm.
6.4. Autophagy in retinal degenerations
The role of autophagy in retinal degeneration is a matter of ongoing discussion (Boya et al., 2016). Autophagy is the process of degrading and recycling cellular components intracellularly, by way of lysosomes (Dodson et al., 2013). In healthy tissue, this is an important part of cell maintenance, particularly among non-mitotic cells such as neurons. In diseased tissue, the role of autophagy becomes more complex. Autophagy is important for both the removal of damaged mitochondria (mitophagy) and the removal of other cellular debris including damaged proteins. Studies with genetically modified animals to inhibit autophagy found increased rod photoreceptor cell death, in addition to an accumulation of the protein transducin (a G-protein associated with phototransduction). These features combined, indicate a pro-survival or maintenance effect of autophagy in retinal degeneration (Yao et al., 2016; Zhou et al., 2015). It may also serve as a metabolic source when metabolic resources are scarce (Dodson et al., 2013).
Conversely, autophagy may initiate apoptosis in cells. In the retina, autophagy occurs in the developing retina as a component of programmed cell death. In the adult autophagy may be elicited through numerous mechanisms including genetic disorders, following trauma, or light damage of the photoreceptors. Autophagic compartments and oxidative stress are observed in photoreceptors following light damage in electron micrographs, in addition to an increase in LC3 expression (associated with autophagic activity (Kunchithapautham and Rohrer, 2007; Tanida et al., 2008)). Autophagic structures are also found in photoreceptors of transgenic animals preceding photoreceptor cell death (Bogea et al., 2015). Work in our lab using large-scale ultra-structural connectomics finds autophagic structures, multivesicular bodies, and endosomes are distributed throughout the inner retina in both human and animal retinal degenerations (Fig. 7). Some of these structures are likely associated with standard homeostasis, however the increase in prevalence of these structures and the subsequent decrease in neuronal numbers found in adult tissue reveals autophagy as a key component of neurodegeneration resulting from retinal degenerations.
Fig. 7. TEM of internal whorl structures associated with neurodegeneration.
(A) Human retina from 77yo male diagnosed with RP. (Ai and Aii) Regions of higher magnification from A containing whorl structures. (Ai* and Aii*) High resolution images of whorl structures. (B) Retina from a 2yo female P347L rabbit. (Bi and Bii) Regions of higher magnification from B containing whorl structures. (Bi* and Bii*) High resolution images of whorl structures. Scale bars 500 nm.
6.5. Possible contributions of debris exocytosis
A recent study by Meletijevic et al. (Melentijevic et al., 2017) described a novel mechanism by which neurons may respond to an increase in misfolded proteins. They find that adult neurons extrude misfolded proteins and pathologic mitochondria via novel structures, termed exophers. Exophers are large, complex, membrane enclosed structures associated with lysosomal autophagy. They are exported from within their origination neuron and may be taken up by a neighboring cell. This is proposed as a mechanism of misfolded protein transfer, that has been described in multiple neurodegenerative diseases (Lee et al., 2010). In addition, recent work by Fertig et al., has demonstrated extracellular vesicles extrusion of multivesicular bodies from teleocytes (Fertig et al., 2014). Our connectomics efforts with volumes of reconstructed serial section electron microscopy (Anderson et al., 2011a, 2011b) of pathological retina (unpublished), has revealed numerous instances of whorls and swollen multivesicular debris that are extruded from one cell and encapsulated by a neighboring cell, either neuron (Fig. 8A) or Müller cells (Fig. 8B). While these extrusions are inconsistent with apoptotic blebbing or typical autophagy, these whorls and endocyte-like structures are often found associated with swollen mitochondria. Although inconsistent with the assumption that cells degrade their own organelles, transcellular debris degradation is not without precedence, in fact the potential for neurons to retain a capacity for phagocytosis has been previously described (Bowen et al., 2007). In the optic nerve head, it has been shown that mitochondria originating from ganglion cell axons can be transferred to neighboring astrocytes and degraded (Davis et al., 2014). Based on this, we propose that these structures are associated with debris removal from stressed cells, potentially similar to the above described mechanisms or via an unknown mechanism of cell-to-cell transfer of debris. Discussed below, these structures may provide a mechanism for the spread of progressive neural degenerative disease. Further investigation is required to determine the origins, implications, molecular biology and functional consequences of these structures.
Fig. 8. Cell to cell transfer of debris-like structures in TEM serial sections.
(A) Serial sections of an amacrine cell (yellow) extruding a region of multiple large vesicles (indicated by arrow) into a bipolar cell (blue) near a potential lysosome. (B) Serial sections of apparent engulfment of a multivesicular structure (indicated by arrow) in a Muller cell (darker) by a bipolar cell (blue) near an apparent lysosome.
7. Protein aggregation contributes to retinal neurodegeneration
The neurodegeneration found in retinal degenerations, including pronounced cell death, metabolic deviation, and debris accumulation, is reminiscent of CNS neurodegenerative diseases. Protein aggregates are a common pathology associated with neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, Pick’s disease, Huntington’s disease (Waelter et al., 2001), and multiple types of dementia. In retinal degeneration, protein aggregates have been attributed to components of cell death, predominately mechanisms of photoreceptor cell death (Illing et al., 2002; Saliba et al., 2002). Here, we make a case for protein aggregates playing a role in the widespread neuronal loss associated with late-stage retinal degeneration in a manner consistent with that seen in other CNS diseases. This is not meant to imply that all initiating factors are the same. Clearly the precipitating factors (genetics, trauma, environmental factors, or a combination thereof) are unique in the onset of these disorders, nonetheless the final outcome of neurodegeneration is the same. Here we simply describe common proteins whose aggregates appear to be involved in the final neurodegeneration processes of the retina and brain.
7.1. Protein aggregation in AMD
In AMD, amyloid-beta (Aβ), the protein commonly associated with Alzheimer’s disease, has been associated with AMD pathology (Ratnayaka et al., 2015). Aβ is a monomeric peptide produced by γ- and β-secretase cleavage of the amyloid precursor protein (APP) (Dawkins and Small, 2014). APP is a type I membrane glycoprotein with multiple spliced forms. Multiple factors including mutations in APP encoding genes and post-translational modifications of Aβ lead to greater self-association and the formation of amyloid fibrils and oligomers. These structures are associated with neurodegeneration (Lambert et al., 1998). APP is found in cells of the RPE, inner nuclear layer, and ganglion cells (Wang et al., 2011). Aβ has been identified in drusen deposits from eyes with AMD (Dentchev et al., 2003; Johnson et al., 2002). In addition to Aβ in drusen, Aβ depositions increase in the RPE with age, while levels of neprilysin (the enzyme responsible for clearing Aβ) are decreased, indicating a contributing mechanism of Aβ accumulation with age (Wang et al., 2012). These Aβ structures of varying sizes have been reported in drusen post-mortem in humans with a correlation of a greater number associated with more advanced AMD (Yoshida et al., 2005), however the identification appears to be highly dependent on the antibody used for detection (Anderson et al., 2004). These observations led to investigation of the contributions of Aβ to retinal stress and degeneration. In animal models, various forms of Aβ and Aβ oligomers were injected into the eye or exposed to cell culture leading to an increase in pro-inflammatory cytokines (Kurji et al., 2010; Liu et al., 2012) and oxidative stress responses (Bruban et al., 2009). Combined, these studies indicate a primary contribution of proteinopathy-associated oligomers contributing to AMD and a potential additional pathway to address the complex progression of AMD.
7.2. Protein aggregation in RP
Protein aggregation is not restricted to AMD as a mechanism of retinal degeneration progression. One common type of autosomal dominant RP is the P23H single-base substitution mutation of rhodopsin that leads to progressive rod death followed by secondary cone loss. In this mutation, the rhodopsin protein is misfolded, leading to ER stress and activation of the unfolded protein response (Gorbatyuk et al., 2010), and has been shown to aggregate (Saliba et al., 2002). These protein aggregates are found to be highly ubiquitinylated, and over time leads to an impairment of the ubiquitin proteasome system (Illing et al., 2002).
Members of the synuclein family are another proposed protein aggregate found in RP (Surguchov et al., 1999). The most well-known member of this family is alpha-synuclein (α-syn), the protein which, when mutated, is associated with Parkinson’s disease (Polymeropoulos et al., 1997). In the nervous system, α-syn is found enriched at synaptic terminals associated with the SNARE-complex (Burre et al., 2010). Mutant forms of α-syn, however, are prone to pathologic aggregations as oligomers, β-sheets, and Lewy Bodies, which are associated with neurodegeneration (Burre et al., 2015). α-Syn is found throughout the retina, with higher levels found in rod outer segments and the ONL (Martinez-Navarrete et al., 2007). Phosphorylation of the serine at position 129 of α-syn (Pα-syn) is a specific marker of α-syn lesions and is associated with an increased propensity to form aggregates (Fujiwara et al., 2002). We evaluated the distribution of α-syn in healthy retina and across aging timepoints in the degenerate retina of the P347L rabbit to better understand the potential contribution of protein aggregates in RP. In Tg retinas, the levels of both α-syn and Pα-syn vary with age and severity of retinal remodeling (Fig. 9). Although signals may vary depending on the level of remodeling, the basic pattern is as follows: Early in degeneration (3mo-1.5yrs), Pα-syn is increased in horizontal cells, along with a subtle uniform increase in the INL, IPL, and GCL (Fig. 9E). Higher levels of α-syn are found in the outer segments of degenerating rods and lower levels throughout neurons of the outer and inner retina (Fig. 9F). By ~2yrs the thinned ONL shows extracellular aggregation of Pα-syn around and within some ganglion cells (Fig. 9H). High levels of α-syn are observed, with increased concentrations in the inner retina as well. Of note, at this stage the α-syn in ganglion cells appears to no longer be uniformly distributed, but rather is aggregated along the outer cell membrane (Fig. 9I). At ~4yr degeneration the ONL is largely absent and Pα-syn levels are two-fold higher than α-syn throughout neurons of the inner retina. Pα-syn also begins to aggregate inside neurons, particularly ganglion cells (Fig. 9K). α-Syn is distributed in the neuronal nuclei of the inner retina, with some punctate staining distributed within the IPL, presumably at large synapses (Fig. 9L). At 5 yr, Pα-syn continues to aggregate within ganglion cells in displaced neurons found within the IPL, and is found at high concentrations in many INL neuronal soma (Fig. 9N). α-Syn is found highly expressed in all remaining neurons and is predominantly colocalized with Pα-syn, with an exception of punctate levels in the IPL, as IPL staining is unique to the full protein (Fig. 9O). By 6–7yrs the structure of the retina is severely decimated. Pα-syn is not only ubiquitous in the remaining neurons but is also found in aggregates in the cellular debris found distributed among the region of remodeled glial endfeet (Fig. 9Q). α-Syn is distributed widely throughout remnant neurons and processes of the retina (Fig. 9R). Combined, these studies suggest a role of the synuclein family, including α-syn and Pα-syn in retinal neurodegeneration, particularly during remodeling.
Fig. 9. Patterning of phosphorylated α-synuclein (Pα-syn), full α-synuclein (α-syn), and GFAP in normal WT (A–C) versus 3 mo, 2y, 4y, 5y, and 6y Tg rabbits (D–R).
Left column: pCMP mapping of Pα-syn, α-syn, GFAP > rgb. Middle column: grayscale density mapped Pα-syn channel. Right column, grayscale density mapped Pα-syn channel. (A–C) Normal retina has highest Pα-syn and α-syn levels in photoreceptors. (D–F) In early degeneration, Pα-syn but not total α-syn is transiently elevated in horizontal cells (arrows). (G–I) As retinal degeneration progresses, Pα-syn but not α-syn debris accumulates around ganglion cells (circle). (J–L) In advanced disease, circumscribed extracellular and intracellular Pα-syn aggregates become common in the ganglion cell layer. (M–R) In advanced neurodegenerative phases, both Pα-syn and total α-syn levels become elevated in virtually all survivor neurons. Scale, 30 μm.
7.3. Ubiquitin proteasome system
In addition to the autophagy-lysosome system, a common pathway involved in neurodegenerative disease is the ubiquitin-proteasome system (UPS) (Zheng et al., 2016). The UPS is responsible for approximately 80% of intracellular protein degradation in eukaryotes, where ubiquitin tagged proteins are targeted for degradation by the 26S proteasome in an ATP dependent manner (Glickman and Ciechanover, 2002). Proteasome end products are short peptides, which may be further degraded in lysosomes to single amino acids (Schreiber and Peter, 2014). However not all substrates degraded by lysosomes are first processed via the UPS. In healthy cells, the UPS system helps to maintain proteostasis by rapidly degrading redundant or mutated proteins. Subsequentially, the UPS can be impeded by protein aggregates (Bence et al., 2001). In neurodegenerative diseases, despite differing aggregate forming proteins, ubiquitin inclusions are a common feature (McKinnon and Tabrizi, 2014).
In the retina, the UPS is associated with retinal disease (Campello et al., 2013). An example of this in RP, is P23H rhodopsin aggregation associated with UPS impairment, where the impairment of degradation of the mutant rhodopsin leads to aggresome formation near the cellular centrosome (Illing et al., 2002). In the P347L rabbit, ubiquitin levels and localization as a measure of protein degradation and aggregation were mapped, revealing increased levels in neuronal somas soon after the onset of outer segment stress and degeneration. Focal increases were also found within ganglion cells underlying regions of photoreceptor stress (Fig. 10A–C). Later timepoints demonstrate increased levels of ubiquitin distributed throughout neuronal somas, with increases found throughout the retina at later time points (Fig. 10D and E). By 6 years, we see elevated ubiquitin levels in all remaining neurons, in addition to aggregation outside of somas (Fig. 10F). Combined, these data document protein aggregation and a disrupted UPS as components to retinal neurodegeneration in remodeling.
Fig. 10. Grayscale ubiquitin labelling in rabbit retinas.
(A) WT P347L littermate control demonstrating light ubiquitin immunoreactivity in INL and GCL. (B) 3 mo Tg P347L rabbit retina demonstrating slight accumulation in soma of INL and focal increases in GCL Somas. (C) 2 yr Tg P347L rabbit retina with continued focal increases in soma of INL and GCL. (D) 4 yr Tg P347L rabbit retina. Levels of ubiquitin have risen in some soma. (E) 5 yr Tg P347L rabbit retina, demonstrating variable level of ubiquitin across all cell types with punctate increase in some soma. (F) 6 yr Tg P347L rabbit retina. The remaining somas have varying levels of ubiquitin with extra cellular increases in unknown processes.
8. Implications for therapeutic interventions
RP is relatively rare, affecting 1 in 4000 people, however, AMD is the third leading cause of blindness worldwide and is expected to become more prevalent as the population over 65 increases. Because of this, a primary interest for current vision research is the advancement of new therapies for the treatment of photoreceptor-associated vision loss. Therapies including small electrode array implants to stimulate remaining neurons, optogenetic approaches involving the transfection of specialized opsins into remnant neurons, and a number of genetic and cellular based approaches are currently being investigated (Lamba et al., 2009; Petrs-Silva and Linden, 2014). All these interventions require a nominally intact retina with a stable retinal metabolic environment to either reactivate or repopulate neurons (Marc et al., 2014). The impact of proteinopathy and extensive neurodegeneration interferes with stable retinal environments conducive to proper function, and therefore needs to be addressed in any therapeutic intervention that hopes to have long-lasting function.
8.1. Optogenetics
The fundamental concept behind optogenetics as a treatment for retinal degenerative disorders is to engineer another cell class to genetically respond to light, restoring vision due to photoreceptor loss. Early studies demonstrated algal channelrhodopsin-2 (ChR2) when inserted into the membrane of mammalian cells, will induce a depolarization event in response to light (Nagel et al., 2003). Other studies have explored Archean halorhodopsin (NpHR) to induce hyperpolarization in response to light (Han and Boyden, 2007; Schobert and Lanyi, 1982; Zhang et al., 2007). Therefore, by genetically inserting these apoproteins into neurons, any neuron could be made light sensitive (Boyden et al., 2005). The idea that neurons of the retina could be given new light detecting capabilities following the loss of photoreceptors was proposed and successfully implemented by (Bi et al., 2006). However, which class of cell should be the appropriate recipient of the optogenetic therapy to induce, or restore, light-sensitivity? One early avenue was to restore light-sensitivity to cone somas, following the loss of outer segments with the goal of preserving native processing in the retina (Busskamp et al., 2010; Busskamp and Roska, 2011). As discussed above, in many forms of retinal degeneration, cone cell death occurs relatively early in the progression of the disease in humans, resulting in early alterations that may preclude or at least complicate potential treatments. Later work targeted the neurons of the inner retina to compensate for the complete loss of photoreceptors, either through a broadly delivered intravitreal injection approach, which predominantly targets ganglion cells (Greenberg et al., 2011; Tomita et al., 2009, 2010; Zhang et al., 2009), or through targeted delivery to specific cells of the inner retina (Doroudchi et al., 2011; Lagali et al., 2008). The discussion in the literature is still unresolved as to which cell is best for inducing light sensitivity, in what combination, and which promoters are best for cell-specific targeting. Another major problem with algal and protozoan opsins is their very low photosensitivity, requiring extremely bright light displays to generate responses (Marc et al., 2014). This hurdle is rapidly being overcome as more recent work has demonstrated opsins transduced into mouse bipolar cells offer improved performance (Ganjawala et al., 2019; Gaub et al., 2015). Others have successfully transduced melanopsin into retinas, but the time course of melanopsin signaling is so far, much too slow to provide functional vision (Lin et al., 2008). Recent work has also made advancements in targeting opsins in healthy retinas of humans and non-human primates, furthering the feasibility of this type of therapeutic intervention (Chaffiol et al., 2017; Khabou et al., 2018; Sengupta et al., 2016).
8.2. Chemical photoswitches
Another approach is temporarily inducing photosensitivity in neurons through the application of chemical photoswitches. These photosensitive small molecules regulate neuronal electrical activity by transiently blocking ion channels. An early study used the photochemical AAQ (acrylamide-azobenzene-quaternary ammonium), which in its trans form blocks K+ channels, thereby increasing excitability, and in its cis form unblocks K+ channels. AAQ photoisomerizes from its trans to cis conformation in the presence of short wavelength (~380 nm) light, which unblocks K+ channels and decreases excitability. Relaxation from cis to trans occurs slowly in darkness, or reisomerization can be rapidly induced using long wavelength (~540 nm) light (Polosukhina et al., 2012), allowing potentially rapidly gating, bi-directional optical control. Polosukhina et al. unexpectedly found that 380 nm light elicited action potentials, despite K+ channels becoming unblocked at that wavelength. This turned out to be the result of AAQ primarily effecting amacrine cells, causing ganglion cells to fire due to disinhibition. A distinct problem with AAQ is the necessity of high intensity UV light for isomerization which itself could be oxidatively damaging to retinal tissues if administered post cornea and lens. Another chemical photoswitch is DENAQ (diethylamino-azobenzene-quaternary ammonium) (Tochitsky et al., 2014). DENAQ is also a K+ channel photoswitch that blocks K+ channels in its trans confirmation and unblocks in cis. The main difference is that DENAQ is red-shifted and photoisomerizes trans to cis within the visible spectrum (450–550 nm) then rapidly relaxes back to the trans conformation in the dark (within ~600msec). Also, DENAQ has been shown to be responsive to lower intensity light than either AAQ or many optogenetic strategies. Recently investigators have begun exploring new photoswitches capable of acting in AMPA receptors, via the photoswitchable excitatory amino acid (ATA) (Stawski et al., 2012). ATA is active in the dark, and photoisomerizes from trans to cis in blue light (480 nm). The distribution of AMPA receptors causes a group of RGCs to respond to blue light, while another group fires in response to darkness (Laprell et al., 2016). A combination of specific photoswitches may facilitate restoring ON-OFF responses, though remodeling leading to altered glutamate receptor expression levels (Marc et al., 2007, Jones et al., 2012) will likely be of concern (Lin et al., 2012b) as it would complicate the normal programming that photoswitches would depend upon. Photoswitches also demonstrate a shorter lifespan than optogenetics, making them a potential diagnostic tool (Marc et al., 2014).
8.3. Cellular replacement strategies
Typically, in mammalian CNS, once a neuron dies it is not replaced. This is certainly the case in mammalian retina. The advent of stem cell techniques promises the ability to differentiate new cells in vitro, with the hope of replacement of cells or entire organs grown specifically for an individual. Because the death of photoreceptors is the precipitating feature of all retinal degenerative diseases, photoreceptor replacement strategies are being investigated as a potential treatment. Photoreceptor transplants have had early success with surviving, for at least a few weeks, and have been found to generate synapses in the neural retina (Barber et al., 2013; MacLaren et al., 2006; Pearson et al., 2012; Santos-Ferreira et al., 2016a). It has been shown following introduction of embryonic stem cell derived photoreceptors, that pupillary responses are increased in visually deficient mice (Singh et al., 2013), though it is unclear whether this is due to photoreceptor signaling or a change in the response of intrinsically-photosensitive retinal ganglion cells (ipRGCs). Research continues to determine the long-term viability of transplanted cells and their functionality in the context of the total retina (Pearson et al., 2014; Santos-Ferreira et al., 2016b). In addition, cellular replacement strategies involving RPE transplantation and implantation of full retinal sheets are also being explored. RPE transplant efforts have progressed to human trials in numerous RPE affecting disorders (Lu et al., 2017), following extensive validation in animal models (Khristov et al., 2018). These studies in humans have shown that RPE transplants are more effective in treating hemorrhagic AMD than choroidal neovascular membrane excision alone (Lu et al., 2017). Retinal transplants have been performed in mice (Arai et al., 2004; Radner et al., 2002), rats (Foik et al., 2018; Seiler and Aramant, 2012; Seiler et al., 2017), non-human primates (Tu et al., 2019), and current clinical trials are underway in humans. A study from earlier this year found that retinas derived from human induced pluripotent stem cells (iPSCs) are capable of surviving in light-damaged primate host retinas and have indications of restoring light responses (Tu et al., 2019). Further exploration and technological development into these transplants are necessary, as retinal detachments is a common side effect. However, we have also found that transplantation of fetal retina induces remodeling that occurs as fast as, or potentially faster than, that seen in degenerate retina without intervention (Seiler et al., 2012).
8.4. Bionic implants
Beyond the biologic interventions currently proposed, another methodology aims to intervene using principles similar to the widely successful cochlear implant; in this case, the retinal implant. For a more extensive review of retinal implants see: (Yue et al., 2016). There are multiple approaches to retinal implants: subretinal, epiretinal, and intrascleral (Bareket et al., 2017). A subretinal implant is surgically implanted under the retina between the photoreceptor layer (or former photoreceptor layer in the case of retinal degenerations) and the RPE, and uses microelectrodes to generate light responses to be processed through the remnant neural retina (Zrenner, 2002). An epiretinal implant is surgically placed along the vitreal surface, next to the ganglion cell layer of the retina, which does not require detachment of the retina from the RPE and generates action potentials in the associated ganglion cells, bypassing neural retina processing (Radner et al., 2002). Each approach has advantages and disadvantages.
The subretinal implant has the advantage of being a simpler more compact design. Some technologies are also including photodiodes with sensing and stimulating elements on one chip surface, to allow the wearer to preserve normal eye movements to shift visual attention (Stingl et al., 2013). The final advantage is that subretinal implants in theory have the capacity to utilize any circuitry remaining in the neural retina; however, as discussed in the remodeling section, it is likely that the circuitry of late retinal degenerations is corrupted.
Epiretinal implants, such as the Argus II are placed next to the ganglion cell layer in the vitreous, and stimulate nearby neurons to fire action potentials in a pattern controlled by a video camera headset. This approach has the potential to provide for coarse vision, allowing increased independence and mobility among individuals affected by retinal degenerations (Ahuja et al., 2011; da Cruz et al., 2013; Dorn et al., 2013). These results have been mostly maintained in long-term 5 year follow up studies, though 23% of individuals implanted had a serious side effect within the first 3 years (da Cruz et al., 2016).
All three methodologies are in clinical development. The epiretinal Argus II is approved for clinical use in Europe (2011), the United States (2013) and in Canada (2015). Another epiretinal stimulation device is the IRIS system (Pixium Vision), which is currently in clinical trials in Europe () and US (). For subretinal stimulation, the Alpha IMS and AMS systems (Retina Implant AG) have been leading the field with numerous clinical trials in the United States (NTC03629899), Europe (NTC02720640 and ) and Hong Kong ()(Kitiratschky et al., 2015; Stingl et al., 2015, 2017). For suprachoroidal implants a group out of Australia is leading this effort ( and )(Ayton et al., 2014; Petoe et al., 2017; Shivdasani et al., 2014, 2017; Slater et al., 2015)
These implants have all had early success in stimulating neurons and generating light mediated responses in patients with profound outer retinal degeneration. That said, complications are not uncommon. Epiretinal implants have a high rate of subsequent retinal detachment of 24%, while subretinal implants have a 22% rate of retinal breaks (Kitiratschky et al., 2015). In addition, a recent report from the Alpha AMS/IMS group demonstrated issues with changes in microchip positioning in clinical trials (Kuehlewein et al., 2019). Glial scarring is also a common issue with retinal implants, as this can reduce efficacy and lead to the implant being effectively “welded” to the scar. Further exploration into more biocompatible implants and further tuning of the stimulation is ongoing. This includes intrascleral (or suprachoroidal) implants which require greater stimulation to activate neurons within the retina, and these currents are not well defined for the number and types of retinal cells that may be activated.
8.5. Genetic approaches
Among therapeutic interventions for vision loss, genetic approaches are especially favored in cases where a specific gene defect is known to be the direct cause of vision loss. Leber’s Congenital Amaurosis (LCA) is the most notable example, in which one form is specifically caused by a loss of function mutation in the RPE specific gene RPE-65. LCA is an early onset retinal degeneration, where affected individuals typically experience severe vision loss in the first few years of life. Gene-replacement therapy delivered via adeno-associated viruses (AAVs) has been used to introduce functional RPE-65, and has shown promise in restoring proper RPE function in animal models, especially the Briard dog (Acland et al., 2001; Le Meur et al., 2007), and human patients (Cideciyan et al., 2009a, 2009b; Jacobson et al., 2015b; Sahel and Roska, 2013), gaining FDA approval in late 2017. Other gene therapies for replacing defective genes have been tested with x-linked mutations leading to RP with successful prevention of much of the early photoreceptor loss (Beltran et al., 2015).
A primary difficulty for the exclusive use of gene therapy to prevent or slow vision loss is the wide array of genetic defects that can lead to inherited retinal degenerations and the multiple environmental factors that contribute to the generation of AMD (Armstrong and Mousavi, 2015). Because of the diversity of potential defects, all gene therapies for retinal degenerative diseases, aside from perhaps AMD, will be orphan diseases. More importantly, intercession in most cases of recessive disease must occur perinatally, posing severe challenges for the medical ethics of early genetic manipulation. Also, many diseases are autosomal dominant in which a single defective gene can override the function of a normal gene and lead to photoreceptor death. Approaches to these disorders require suppression of the defective gene with methods such as shRNA targeting or new CRISPR/Cas9 gene editing methods, making a therapeutic path for human autosomal disease unclear. While autosomal diseases tend to progress more slowly, extending into adulthood (via unknown mechanisms), it is also not clear that the critical defect leading to widespread degeneration is operation of the defective gene or accumulation of toxic protein will not continue to complicate therapy, in which case late intervention will be of little use, and early intervention may be compromised. It is also important to note that despite the success of RPE65 gene therapy, the treated individuals still experience progressive cell death and slow rod kinetics (Jacobson et al., 2015a), illustrating that understanding the mechanisms behind retinal remodeling and plasticity requires more work before gene therapy can be deemed a full rescue.
8.6. Requirements for therapeutic interventions to be effective
There are many approaches to therapeutic interventions and treatment for photoreceptor-initiated vision loss. All are lofty and worthy goals. Unless you bypass the retina entirely, all therapeutic interventions involving the eye require a functional neural retina, or at least the preservation of ganglion cells and their axonal projections to LGN and brain.
Therapies that hope to utilize the neural processing afforded by the inner retina have a number of difficult problems to overcome. Cell-replacement therapies are unlikely to result in long-term vision restoration or preservation unless the initial insult leading to photoreceptor degeneration is also directly treated. It has not been shown that synapses made by newly introduced photoreceptors produce the same visual acuity as native photoreceptors (even within an order of magnitude), nor have photoreceptors been shown to persist. For most therapeutics targeted at stimulation of bipolar cells to be effective, regardless of whether via optogenetics, photoswitches, or subretinal implant, the primary circuitry of the retina must remain intact in addition to the metabolic support of surrounding glia. The basic idea that an epiretinal implant only stimulates ganglion cells, or that ganglion cells transduced by optogenetics or photoswitches could function independently is complicated by extensive coupling between ganglion cells and amacrine cells, as well as penetration of currents deep into retinal tissue. Previous data indicate that alterations in circuitry are present early in remodeling and it is unclear to what extent this affects the signals that are communicated to ganglion cells. We certainly know that retinal ganglion cells begin to display massive uncorrelated spiking in late phase 2 remodeling (Euler and Schubert, 2015; Margolis et al., 2008; Menzler and Zeck, 2011; Stasheff, 2008). In addition, other attributes of remodeling, such as, the metabolic variation of Müller cells during remodeling (Pfeiffer et al., 2016) and how it will affect the metabolic support provided by Müller cells (Bringmann et al., 2013; Reichenbach and Bringmann, 2010, 2013), remain to be explored in the context of therapeutic interventions.
The presence of neurodegeneration as a component of remodeling will present a difficult hurdle to overcome in implementing therapeutic interventions in especially advanced retinal degenerations. However, there are likely windows of opportunity during earlier stages of remodeling (Cuenca et al., 2014; Jones and Marc, 2005) and models of retinal degeneration are an ideal model system to evaluate the efficacy of neuroprotective compounds that could also be used for other neurodegenerative diseases.
9. Opportunities for retina as a model of CNS neurodegenerative disease
Retinal remodeling, specifically neurodegeneration and proteinopathy components, discussed above exhibit unmistakable correlates with CNS progressive neurodegenerative disease processes and illustrate the potential of the retina as a model for CNS neurodegenerative disease. The retina has been previously proposed for studying numerous brain disorders due to some features of those diseases manifesting in the retina during the course of the disease (London et al., 2013; Veys et al., 2019). While interesting and diagnostically useful, this underestimates the utility of specifically using the retina as a model for investigating mechanisms of neurodegeneration. Inner retinal neurodegeneration is a consequence of photoreceptor degeneration, and there is strong evidence for the role of protein aggregates being a causative factor in progressive retinal neurodegeneration. In addition, the retina has the advantage of being more accessible for evaluation of disease progression, and retina contains in a compact space, entire circuitries that are better understood in terms of organization and topology than most other regions of CNS(Marc et al., 2013).
Alzheimer’s Disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD) are all progressive neurodegenerative diseases that initially result in miswiring, followed by neuronal cell death and proteinopathy alterations that have correlates in retina. Following insult, the CNS is known to undergo rewiring events in epilepsy (Morimoto et al., 2004), AD (Agosta et al., 2010), PD (Guo et al., 2015), and others. The retina is no different.
9.1. Protein changes
In AD, the predominating causative hypothesis is the amyloid hypothesis, wherein amyloid (specifically amyloid-β) plaque accumulation is responsible for the progressive neurodegeneration observed in AD (Hardy and Higgins, 1992), granted that more recent studies indicate a potentially greater role of phosphorylated tau in the degree of cognitive decline (Hardy and Selkoe, 2002). In addition, Aβ oligomers have been shown to also be major contributors to neurodegeneration preceding much of the hallmark plaque and tangle formation (Jeong, 2017). In PD, second in neurodegenerative disease prevalence only to AD (Sarkar et al., 2016) the primary pathological characteristic is Lewy bodies, which are made up of tangles of α-synuclein, synphilinin-1, and ubiquitin. Of these proteins, α-synuclein (α-syn) has been specifically studied due to its role in the emergence of clinical symptoms when it is misfolded (α-synucleinopathies) (Burre et al., 2015). Other proteins have been associated with PD, including phosphorylated tau (Sarkar et al., 2016). HD is another progressive neurodegeneration associated with the aggregation of mutant protein (Ross and Tabrizi, 2011). Perhaps uniquely, Huntington’s has a single genetic cause (though diseases with similar symptoms have been identified (Govert and Schneider, 2013)). The huntingtin (HTT) gene, which is inherited in an autosomal dominant fashion and the length of the resultant polyglutamine stretch is predictive of disease onset (Li and Li, 2004).
AD, PD, and HD are by no means the only protein aggregation associated neurodegenerative diseases, as there are many more including those where protein aggregation is a secondary effect following the initial insult (i.e. traumatic brain injury, epilepsy, etc.). However, beyond protein aggregation, there are a number of other unifying characteristics observed in all of these disorders and in retinal neurodegeneration, which may ultimately reveal central mechanisms of neurodegeneration for broadly applicable therapeutics.
9.2. Receptor changes
As described in section 4.1, reprogramming is a key component of retinal remodeling. During this process, the loss of metabotropic-type glutamate receptors and a phenotypic switching to kainate and AMPA receptors provides a basis for beginning to untangle the changes in retinal physiology observed in degeneration. The simultaneous changes in NMDA receptors are interesting in different respects. First, the loss of NMDA receptors is a common thread between multiple neurodegenerations. In RD, a decrease in NMDA receptor expression has been demonstrated through both immunohistochemical (Grunder et al., 2001) and pharmacological approaches (Jones et al., 2011; Marc et al., 2007). In Parkinson’s Disease (PD), an increase in α-syn is associated with internalization of surface NMDA receptors (Chen et al., 2015) In Alzheimer’s disease (AD), expression of surface NMDA receptors are downregulated by Aβ oligomers leading to inhibition of long-term potentiation (Lacor et al., 2007). The loss of NMDA receptors appears in all cases to precede the gross cell death associated with each of these neurodegenerations, and understanding the mechanisms of this may help to provide treatments to delay memory loss in AD and PD, while preserving proper retinal signaling in RD. These results also conflict with the hypothesis of NMDA mediated neurotoxicity contributing to cell death in all of these degenerations.
9.3. Mitochondrial stress
Mitochondrial stress is a key feature found in numerous pathological CNS processes including neurodegeneration (Chaturvedi and Flint Beal, 2013; El-Hattab and Scaglia, 2016). Mitochondria play an important role in calcium buffering and cell metabolism, including energy production via oxidative phosphorylation and are central to the Krebs cycle. In addition to their roles in metabolism, they are found to be key regulators in cell death pathways, including apoptosis, autophagy, and necrosis. Reactive oxygen species (ROS) are a direct product of oxidative phosphorylation, and are normally found in tissues, but in large quantities can induce oxidative damage, which in turn activates autophagic pathways (Moreno et al., 2018). This phenomenon has been observed in RD (Moreno et al., 2018), AD (Hirai et al., 2001; Wang et al., 2014), PD (Ganguly et al., 2017; Liu et al., 2018), Huntington’s Disease (HD)(Jodeiri Farshbaf and Ghaedi, 2017), and other neurodegenerative disease (Vazquez et al., 2012), and is thought to be a mechanism of cell death in each of these diseases. In addition to ROS, mitochondrial dysfunction can lead to improper calcium handling, which can be especially damaging to neurons (Willems et al., 2008). In retinal degeneration, we observe mitochondrial swelling in addition to disorganized cristae, especially within cells that contain autophagic whorls. The increase in oxidative stress in RD is further supported by the observed increases in glutathione in RP and AMD retinas (Jones et al., 2016a, 2016b). The common thread of mitochondrial stress in neuronal degeneration has made treatments directed at mitochondria an attractive target for therapies and ongoing research in this may help to prevent neurodegeneration in a variety of diseases (Suliman and Piantadosi, 2016).
9.4. Autophagy
As described in section 6.4, autophagy is an important component of cell maintenance, but can also lead to cell death through mechanisms of autophagy mediated cell death and apoptosis (for a comprehensive review of autophagy see (Ravanan et al., 2017)). In neurodegeneration, autophagy is commonly implicated in the progression of neurons from healthy to stressed, and eventually to cell death. Autophagy is complex, and has many contributing factors, some of which are directly related to other components of neurodegeneration progression (e.g. mitochondrial stress, protein misfolding/aggregation, etc.). Although autophagy appears to be beneficial in many ways, impairment leading to degeneration (Ghavami et al., 2014) and increased autophagy leading to longer lifespan (Zhang et al., 2014), it has also been associated with production of extracellular Aβ plaques. A full description of all of the pathways leading to autophagy and its role in neurodegeneration diseases (including RD), is the subject of numerous reviews (Boya et al., 2016; Menzies et al., 2017; Moreno et al., 2018). Autophagy is a clear component of cell stress and progressive neurodegeneration, indicating the further evaluation of these pathways may uncover unified targets to prevent or delay neurodegeneration.
9.5. Ubiquitin-proteasome system
In addition to autophagy, the ubiquitin-proteasome system is important for regulating the catabolism of proteins when they are obsolete, damaged, or are misfolded (for a comprehensive review see (Glickman and Ciechanover, 2002)). This mechanism, as described in section 7.3 is affected by RD. In addition to its role in the neurodegeneration observed in RD (Campello et al., 2013), there is evidence for UPS disruption in other neurodegenerative diseases, especially involving those associated with protein aggregation (Bence et al., 2001; McKinnon and Tabrizi, 2014; Zheng et al., 2016). The proteins involved in aggregation vary depending upon the disease, but ubiquitin is a common component of the aggregates. This indicates the UPS may be a targetable pathway to reduce protein aggregation and prevent neurodegeneration in these diverse diseases (Harrigan et al., 2018; Opoku-Nsiah and Gestwicki, 2018).
10. Conclusions and future directions
Retinal remodeling as a clear consequence of retinal degenerative disease has been a growing field over the last two decades. Moreover, many of its features have been alluded to in the literature for almost a century. We have consolidated evidence for inner retinal neurodegeneration as a primary component of remodeling (likely arising from proteinopathy mechanisms), which occurs along with the other previously described features of remodeling: reprogramming, rewiring, and glial responses. All four of these processes occur as a result of deafferentation, or in the case of retina, photoreceptor degeneration, though their time-course and etiologies vary (Fig. 11). Ultimately, the end-state of remodeling is wide-spread neurodegeneration.
Fig. 11. Components of remodeling.
Progression panel at bottom represents relative timescale for the initiation and maximal contributions of each component of remodeling: Reprogramming (green), Rewiring (blue), Neurodegeneration (red), and Glial contributions (pink).
Beyond rewiring, reprogramming, and glial components of retinal remodeling; the presence of retinal neurodegeneration, and the evidence for a proteinopathy further complicates therapeutics for restoration of vision loss. All therapeutics directed at the retina require at a minimum, ganglion cells capable of transmitting signals to appropriate brain regions and metabolic support of these cells (effected by glial alterations), with others requiring specific intact neural network architectures (affected by rewiring), or predictable gene expression (affected by reprogramming). Therefore, it is reasonable to conclude the best time to intervene in retinal degenerations is earlier, prior to the more deleterious aspects of remodeling. This is not always possible meaning the path forward will require strategies to arrest, compensate for, or potentially reverse many aspects of retinal remodeling. This is not a small task. However, it is necessary for long-term effectiveness of each intervention. In addition, the lessons learned from attempting to restore vision will likely be instructive for future therapeutics in the rest of the CNS, especially in regards to neurodegenerative diseases.
Neurodegenerative diseases associated with protein aggregation are an active area of neuroscience research with widespread impact, particularly as a large portion of the population ages. Clearly, progressive neuronal cell death over a long period of time is a common thread, but underlying mechanisms provide cohesive targets for broad interventions. The relationship of protein aggregation and neurodegeneration associated with specific proteins such as amyloid-beta, alpha-synuclein, and tau were initially thought to be confined to specific diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and others. Further research finds protein aggregation to be a commonality between numerous neurodegenerations, with multiple modes of onset including trauma (Acosta et al., 2015), epilepsy (Yang et al., 2006), and retinal degeneration (Anderson et al., 2004). Of these neurodegenerations, retinal degeneration is an ideal system for studying the progression and mechanism of cell death in neurodegenerative proteinopathies. The retina is unrivaled by any other area of the CNS in its accessibility and our understanding of the associated metabolism and circuitry. These factors combined make the remodeled retina an attractive candidate tissue for the discovery of pathways leading to neurodegeneration and evaluating potential interventions.
Funding
This work was supported by the National Institutes of Health [RO1 EY015128(BWJ), RO1 EY028927(BWJ) T32 EY024234 (RLP), P30 EY014800(Core)]; and an Unrestricted Research Grant from Research to Prevent Blindness, New York, NY to the Department of Ophthalmology & Visual Sciences, University of Utah.
Footnotes
Conflicts of interest
Drs. Rebecca L. Pfeiffer and Bryan William Jones have no competing or conflicting interests to declare.
Dr. Robert E. Marc is a principal of Signature Immunologics, the provider of some of the antibodies used in this manuscript.
https://sph.uth.edu/Retnet/(Accessed December 19, 2018).
References
- Ayton LN, Blamey PJ, Guymer RH, Luu CD, Nayagam DA, Sinclair NC,Shivdasani MN, Yeoh J, McCombe MF, Briggs RJ, Opie NL, Villalobos J, Dimitrov PN, Varsamidis M, Petoe MA, McCarthy CD, Walker JG, Barnes N, Burkitt AN, Williams CE, Shepherd RK, Allen PJ, 2014. Bionic vision Australia research, C. In: First-in-human Trial of a Novel Suprachoroidal Retinal Prosthesis. PLoS One 9, e115239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Ateh DD, Deinhardt K, Bird MM, Price KM, Baker CS, Robson JC,Swash M, Shamsuddin W, Kawar S, El-Tawil T, Roos J, Hoyle A, Nickols CD, Knowles CH, Pullen AH, Luthert PJ, Weller RO, Hafezparast M, Franklin RJ, Revesz T, King RH, Berninghausen O, Fisher EM, Schiavo G, Martin JE, 2007. The phagocytic capacity of neurones. Eur. J. Neurosci 25, 2947–2955. [DOI] [PubMed] [Google Scholar]
- Busskamp V, Duebel J, Balya D, Fradot M, Viney TJ, Siegert S, Groner AC,Cabuy E, Forster V, Seeliger M, Biel M, Humphries P, Paques M, Mohand-Said S, Trono D, Deisseroth K, Sahel JA, Picaud S, Roska B, 2010. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science 329, 413–417. [DOI] [PubMed] [Google Scholar]
- da Cruz L, Dorn JD, Humayun MS, Dagnelie G, Handa J, Barale PO, Sahel JA, Stanga PE, Hafezi F, Safran AB, Salzmann J, Santos A, Birch D, Spencer R, Cideciyan AV, de Juan E, Duncan JL, Eliott D, Fawzi A, Olmos de Koo LC, Ho AC, Brown G, Haller J, Regillo C, Del Priore LV, Arditi A, Greenberg RJ, Argus IISG, 2016. Five-year safety and performance results from the Argus II retinal prosthesis system clinical trial. Ophthalmology 123, 2248–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA Jr., Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovacs AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, Lopez-Otin C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Melendez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Munz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nurnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Talloczy Z, Tanaka K, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcategui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL, 2008. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4, 151–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl K, Schippert R, Bartz-Schmidt KU, Besch D, Cottriall CL, Edwards TL, Gekeler F, Greppmaier U, Kiel K, Koitschev A, Kuhlewein L, MacLaren RE, Ramsden JD, Roider J, Rothermel A, Sachs H, Schroder GS, Tode J, Troelenberg N, Zrenner E, 2017. Interim results of a multicenter trial with the new electronic subretinal implant alpha AMS in 15 patients blind from inherited retinal degenerations. Front. Neurosci 11, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J, 2001. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet 28, 92–95. [DOI] [PubMed] [Google Scholar]
- Acosta SA, Tajiri N, de la Pena I, Bastawrous M, Sanberg PR, Kaneko Y, Borlongan CV, 2015. Alpha-synuclein as a pathological link between chronic traumatic brain injury and Parkinson’s disease. J. Cell. Physiol 230, 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study Research, G., 2001a. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch. Ophthalmol 119, 1439–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study Research, G., 2001b. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol 119, 1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Rocca MA, Pagani E, Absinta M, Magnani G, Marcone A, Falautano M, Comi G, Gorno-Tempini ML, Filippi M, 2010. Sensorimotor network rewiring in mild cognitive impairment and Alzheimer’s disease. Hum. Brain Mapp 31, 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja AK, Dorn JD, Caspi A, McMahon MJ, Dagnelie G, Dacruz L, Stanga P,Humayun MS, Greenberg RJ, Argus IISG, 2011. Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. Br. J. Ophthalmol 95, 539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zamil WM, Yassin SA, 2017. Recent developments in age-related macular degeneration: a review. Clin. Interv. Aging 12, 1313–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV, 2004. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp. Eye Res 78, 243–256. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Jones BW, Watt CB, Shaw MV, Yang JH, Demill D, Lauritzen JS, Lin Y, Rapp KD, Mastronarde D, Koshevoy P, Grimm B, Tasdizen T, Whitaker R, Marc RE, 2011a. Exploring the retinal connectome. Mol. Vis 17, 355–379. [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, Mohammed S, Grimm B, Jones BW, Koshevoy P, Tasdizen T,Whitaker R, Marc RE, 2011b. The Viking viewer for connectomics: scalable multiuser annotation and summarization of large volume data sets. J. Microsc 241, 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S, Thomas BB, Seiler MJ, Aramant RB, Qiu G, Mui C, de Juan E, Sadda SR, 2004. Restoration of visual responses following transplantation of intact retinal sheets in rd mice. Exp. Eye Res 79, 331–341. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Mousavi M, 2015. Overview of risk factors for age-related macular degeneration (AMD). J. Stem Cells 10, 171–191. [PubMed] [Google Scholar]
- Asakawa K, Ishikawa H, Uga S, Mashimo K, Shimizu K, Kondo M, Terasaki H, 2015. Functional and morphological study of retinal photoreceptor cell degeneration in transgenic rabbits with a Pro347Leu rhodopsin mutation. Jpn. J. Ophthalmol 59, 353–363. [DOI] [PubMed] [Google Scholar]
- Asakawa K, Ishikawa H, Uga S, Mashimo K, Kondo M, Terasaki H, 2016Histopathological changes of inner retina, optic disc, and optic nerve in rabbit with advanced retinitis pigmentosa. Neuro Ophthalmol 40, 286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AC, Hippert C, Duran Y, West EL, Bainbridge JW, Warre-Cornish K,Luhmann UF, Lakowski J, Sowden JC, Ali RR, Pearson RA, 2013. Repair of the degenerate retina by photoreceptor transplantation. Proc. Natl. Acad. Sci. U. S. A 110, 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareket L, Barriga-Rivera A, Zapf MP, Lovell NH, Suaning GJ, 2017. Progress in artificial vision through suprachoroidal retinal implants. J. Neural Eng 14, 045002. [DOI] [PubMed] [Google Scholar]
- Beltran WA, Cideciyan AV, Iwabe S, Swider M, Kosyk MS, McDaid K, Martynyuk I, Ying GS, Shaffer J, Deng WT, Boye SL, Lewin AS, Hauswirth WW, Jacobson SG, Aguirre GD, 2015. Successful arrest of photoreceptor and vision loss expands the therapeutic window of retinal gene therapy to later stages of disease. Proc. Natl. Acad. Sci. U. S. A 112, 5844–5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bence NF, Sampat RM, Kopito RR, 2001. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555. [DOI] [PubMed] [Google Scholar]
- Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH, 2006. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 50, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanks JC, Mullen RJ, LaVail MM, 1982. Retinal degeneration in the pcd cerebellar mutant mouse. II. Electron microscopic analysis. J. Comp. Neurol 212, 231–246. [DOI] [PubMed] [Google Scholar]
- Bogea TH, Wen RH, Moritz OL, 2015. Light induces ultrastructural changes in rod outer and inner segments, including autophagy, in a transgenic Xenopus laevis P23H rhodopsin model of retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci 56, 7947–7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya P, Esteban-Martinez L, Serrano-Puebla A, Gomez-Sintes R, Villarejo-Zori B, 2016. Autophagy in the eye: development, degeneration, and aging. Prog. Retin. Eye Res 55, 206–245. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K, 2005. Millisecond-time-scale, genetically targeted optical control of neural activity. Nat. Neurosci 8, 1263–1268. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Grosche A, Pannicke T, Reichenbach A, 2013. GABA and glutamate uptake and metabolism in retinal glial (muller) cells. Front. Endocrinol 4, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruban J, Glotin AL, Dinet V, Chalour N, Sennlaub F, Jonet L, An N, Faussat AM, Mascarelli F, 2009. Amyloid-beta(1–42) alters structure and function of retinal pigmented epithelial cells. Aging Cell 8, 162–177. [DOI] [PubMed] [Google Scholar]
- Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC, 2010. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329, 1663–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burre J, Sharma M, Sudhof TC, 2015. Definition of a molecular pathway mediating alpha-synuclein neurotoxicity. J. Neurosci 35, 5221–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH, 2002. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci 22, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busskamp V, Roska B, 2011. Optogenetic approaches to restoring visual function in retinitis pigmentosa. Curr. Opin. Neurobiol 21, 942–946. [DOI] [PubMed] [Google Scholar]
- Campello L, Esteve-Rudd J, Cuenca N, Martin-Nieto J, 2013. The ubiquitin-protea-some system in retinal health and disease. Mol. Neurobiol 47, 790–810. [DOI] [PubMed] [Google Scholar]
- Chaffiol A, Caplette R, Jaillard C, Brazhnikova E, Desrosiers M, Dubus E,Duhamel L, Mace E, Marre O, Benoit P, Hantraye P, Bemelmans AP, Bamberg E, Duebel J, Sahel JA, Picaud S, Dalkara D, 2017. A new promoter allows optogenetic vision restoration with enhanced sensitivity in macaque retina. Mol. Ther 25, 2546–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR,2002. Retinal degeneration mutants in the mouse. Vis. Res 42, 517–525. [DOI] [PubMed] [Google Scholar]
- Chaturvedi RK, Flint Beal M, 2013. Mitochondrial diseases of the brain. Free Radic. Biol. Med 63, 1–29. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang W, Li X, Li X, Yang H, Xu Z, Yu S, 2015. alpha-Synuclein-induced internalization of NMDA receptors in hippocampal neurons is associated with reduced inward current and Ca(2+) influx upon NMDA stimulation. Neuroscience 300, 297–306. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Pang JJ, Roman AJ, Byrne BJ, Jacobson SG, 2009a. Human RPE65 gene therapy for leber congenital Amaurosis: persistence of early visual improvements and safety at 1 year. Hum. Gene Ther 20, 999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Roman AJ, Byrne BJ, Jacobson SG, 2009b. Vision 1 Year after gene therapy for leber’s congenital Amaurosis. N. Engl. J. Med 361, 725–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coblentz FE, Radeke MJ, Lewis GP, Fisher SK, 2003. Evidence that ganglion cells react to retinal detachment. Exp. Eye Res 76, 333–342. [DOI] [PubMed] [Google Scholar]
- Cuenca N, Fernandez-Sanchez L, Campello L, Maneu V, De la Villa P, Lax P, Pinilla I, 2014. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res 43, 17–75. [DOI] [PubMed] [Google Scholar]
- da Cruz L, Coley BF, Dorn J, Merlini F, Filley E, Christopher P, Chen FK, Wuyyuru V, Sahel J, Stanga P, Humayun M, Greenberg RJ, Dagnelie G, Argus IISG, 2013. The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. Br. J. Ophthalmol 97, 632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, Kinebuchi M, Phan S, Zhou Y, Bihlmeyer NA, Nguyen JV, Jin Y, Ellisman MH, Marsh-Armstrong N, 2014. Transcellular degradation of axonal mitochondria. Proc. Natl. Acad. Sci. U. S. A 111, 9633–9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins E, Small DH, 2014. Insights into the physiological function of the beta-amyloid precursor protein: beyond Alzheimer’s disease. J. Neurochem 129, 756–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentchev T, Milam AH, Lee VM, Trojanowski JQ, Dunaief JL, 2003. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol. Vis 9, 184–190. [PubMed] [Google Scholar]
- Dias MF, Joo K, Kemp JA, Fialho SL, da Silva Cunha A Jr., Woo SJ, Kwon YJ, 2018. Molecular genetics and emerging therapies for retinitis pigmentosa: basic research and clinical perspectives. Prog. Retin. Eye Res 63, 107–131. [DOI] [PubMed] [Google Scholar]
- Dodson M, Darley-Usmar V, Zhang J, 2013. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic. Biol. Med 63, 207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn JD, Ahuja AK, Caspi A, da Cruz L, Dagnelie G, Sahel JA, Greenberg RJ, McMahon MJ, gus IISG, 2013. The detection of motion by blind subjects with the epiretinal 60-electrode (Argus II) retinal prosthesis. JAMA Ophthalmol 131, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroudchi MM, Greenberg KP, Liu J, Silka KA, Boyden ES, Lockridge JA,Arman AC, Janani R, Boye SE, Boye SL, Gordon GM, Matteo BC, Sampath AP, Hauswirth WW, Horsager A, 2011. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol. Ther 19, 1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hattab AW, Scaglia F, 2016. Mitochondrial cytopathies. Cell Calcium 60, 199–206. [DOI] [PubMed] [Google Scholar]
- Erickson PA, Fisher SK, Anderson DH, Stern WH, Borgula GA, 1983. Retinal detachment in the cat: the outer nuclear and outer plexiform layers. Investig. Ophthalmol. Vis. Sci 24, 927–942. [PubMed] [Google Scholar]
- Erickson PA, Fisher SK, Guerin CJ, Anderson DH, Kaska DD, 1987. Glial fibrillary acidic protein increases in Muller cells after retinal detachment. Exp. Eye Res 44, 37–48. [DOI] [PubMed] [Google Scholar]
- Eskelinen E-L, 2014. To be or not to be? Examples of incorrect identification of autophagic compartments in conventional transmission electron microscopy of mammalian cells. Autophagy 4, 257–260. [DOI] [PubMed] [Google Scholar]
- Euler T, Schubert T, 2015. Multiple independent oscillatory networks in the degenerating retina. Front. Cell. Neurosci 9, 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariss RN, Li ZY, Milam AH, 2000. Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in human retinas with retinitis pigmentosa. Am. J. Ophthalmol 129, 215–223. [DOI] [PubMed] [Google Scholar]
- Fertig ET, Gherghiceanu M, Popescu LM, 2014. Extracellular vesicles release by cardiac telocytes: electron microscopy and electron tomography. J. Cell Mol. Med 18, 1938–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SK, Lewis GP, 2003. Müller cell and neuronal remodeling in retinal detachment and reattachment and their potential consequences for visual recovery: a review and reconsideration of recent data. Vis. Res 43, 887–897. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Lewis GP, Linberg KA, Barawid E, Verardo MR, 1995. Cellular remodeling in mammalian retina induced by retinal detachment. In: Kolb H, Fernandez E, Nelson R (Eds.), Webvision: the Organization of the Retina and Visual System, Salt Lake City (UT). [PubMed] [Google Scholar]
- Fisher SK, Lewis GP, Linberg KA, Verardo MR, 2005. Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment. Prog. Retin. Eye Res 24, 395–431. [DOI] [PubMed] [Google Scholar]
- Foik AT, Lean GA, Scholl LR, McLelland BT, Mathur A, Aramant RB, Seiler MJ, Lyon DC, 2018. Detailed visual cortical responses generated by retinal sheet transplants in rats with severe retinal degeneration. J. Neurosci 38, 10709–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A, 2014. Age-related macular degeneration: genetics and biology coming together. Annu. Rev. Genom. Hum. Genet 15, 151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T, 2002. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol 4, 160–164. [DOI] [PubMed] [Google Scholar]
- Ganguly G, Chakrabarti S, Chatterjee U, Saso L, 2017. Proteinopathy, oxidative stress and mitochondrial dysfunction: cross talk in Alzheimer’s disease and Parkinson’s disease. Drug Des. Dev. Ther 11, 797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganjawala TH, Lu Q, Fenner MD, Abrams GW, Pan ZH, 2019. Improved CoChR variants restore visual acuity and contrast sensitivity in a mouse model of blindness under ambient light conditions. Mol. Ther 27, 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaub BM, Berry MH, Holt AE, Isacoff EY, Flannery JG, 2015. Optogenetic vision restoration using rhodopsin for enhanced sensitivity. Mol. Ther 23, 1562–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Los MJ, 2014. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol 112, 24–49. [DOI] [PubMed] [Google Scholar]
- Glickman MH, Ciechanover A, 2002. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev 82, 373–428. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk M, Knox T, LaVail M, Gorbatyuk O, Noorwez S, Hauswirth W, Lin J, Muzyczka N, Lewin A, 2010. Restoration of visual funtion in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. PNAS 107 (13), 5961–5966. 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govert F, Schneider SA, 2013. Huntington’s disease and Huntington’s disease-like syndromes: an overview. Curr. Opin. Neurol 26, 420–427. [DOI] [PubMed] [Google Scholar]
- Greenberg KP, Pham A, Werblin FS, 2011. Differential targeting of optical neuro-modulators to ganglion cell soma and dendrites allows dynamic control of center-surround antagonism. Neuron 69, 713–720. [DOI] [PubMed] [Google Scholar]
- Grignolo A, Orzalesi N, Castellazzo R, Vittone P, 1969. Retinal damage by visible light in albino rats. An electron microscope study. Ophthalmologica 157, 43–59. [DOI] [PubMed] [Google Scholar]
- Grunder T, Kohler K, Guenther E, 2001. Alterations in NMDA receptor expression during retinal degeneration in the RCS rat. Vis. Neurosci 18, 781–787. [DOI] [PubMed] [Google Scholar]
- Guo L, Xiong H, Kim JI, Wu YW, Lalchandani RR, Cui Y, Shu Y, Xu T, Ding JB, 2015. Dynamic rewiring of neural circuits in the motor cortex in mouse models of Parkinson’s disease. Nat. Neurosci 18, 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Brown KE, Milam AH, 2003. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp. Eye Res 76, 463–471. [DOI] [PubMed] [Google Scholar]
- Hall DH, Gu G, Garcia-Anoveros J, Gong L, Chalfie M, Driscoll M, 1997Neuropathology of degenerative cell death in Caenorhabditis elegans. J. Neurosci 17, 1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel C, 2006. Retinitis pigmentosa. Orphanet J. Rare Dis 1, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Boyden ES, 2007. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS One 2, e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Dinculescu A, Dai X, Du W, Smith WC, Pang J, 2013. Review: the history and role of naturally occurring mouse models with Pde6b mutations. Mol. Vis 19, 2579–2589. [PMC free article] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA, 1992. Alzheimer’s disease: the amyloid cascade hypothesis.Science 256, 184–185. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ, 2002. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356. [DOI] [PubMed] [Google Scholar]
- Harrigan JA, Jacq X, Martin NM, Jackson SP, 2018. Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat. Rev. Drug Discov 17, 57–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP, 2006. Retinitis pigmentosa. The Lancet 368, 1795–1809. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA, 2001. Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci 21, 3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illing ME, Rajan RS, Bence NF, Kopito RR, 2002. A rhodopsin mutant linked to autosomal dominant retinitis pigmentosa is prone to aggregate and interacts with the ubiquitin proteasome system. J. Biol. Chem 277, 34150–34160. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Aguirre GD, Roman AJ, Sumaroka A, Hauswirth WW, Palczewski K, 2015a. Improvement in vision: a new goal for treatment of hereditary retinal degenerations. Expert Opin Orphan Drugs 3, 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Roman AJ, Sumaroka A, Schwartz SB, Heon E, Hauswirth WW, 2015b. Improvement and decline in vision with gene therapy in childhood blindness. N. Engl. J. Med 372, 1920–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammalamadaka A, Suwannatat P, Fisher SK, Manjunath BS, Hollerer T, Luna G, 2015. Characterizing spatial distributions of astrocytes in the mammalian retina. Bioinformatics 31, 2024–2031. [DOI] [PubMed] [Google Scholar]
- Jeong S, 2017. Molecular and cellular basis of neurodegeneration in alzheimer’s disease.Mol. Cells 40, 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremy HR, 1922. Retinal detachment at macula. Proc. R. Soc. Med 15, 34. [PMC free article] [PubMed] [Google Scholar]
- Jodeiri Farshbaf M, Ghaedi K, 2017. Huntington’s disease and mitochondria. Neurotox. Res 32, 518–529. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH, 2002. The Alzheimer’s A beta -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A 99, 11830–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Marc RE, 2005. Retinal remodeling during retinal degeneration. Exp. EyeRes 81, 123–137. [DOI] [PubMed] [Google Scholar]
- Jones BW, Watt CB, Frederick JM, Baehr W, Chen CK, Levine EM, Milam AH, Lavail MM, Marc RE, 2003. Retinal remodeling triggered by photoreceptor de-generations. J. Comp. Neurol 464, 1–16. [DOI] [PubMed] [Google Scholar]
- Jones BW, Marc RE, Watt CB, Vaughan DK, Organisciak DT, 2006. Neural plasticity revealed by light-induced photoreceptor lesions. Adv. Exp. Med. Biol 572, 405–410. [DOI] [PubMed] [Google Scholar]
- Jones BW, Kondo M, Terasaki H, Watt CB, Rapp K, Anderson J, Lin Y, Shaw MV, Yang JH, Marc RE, 2011. Retinal remodeling in the Tg P347L rabbit, a large-eye model of retinal degeneration. J. Comp. Neurol 519, 2713–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Kondo M, Terasaki H, Lin Y, McCall M, Marc RE, 2012. Retinal remodeling. Jpn. J. Ophthalmol 56, 289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Marmor M, Marc RE, 2016a. Retinal remodeling in human retinitis pigmentosa. Exp. Eye Res 150, 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Tucker J, Marc RE, 2016b. Retinal remodeling and metabolic alterations in human AMD. Front. Cell. Neurosci 10, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Tucker JF, Marc RE, 2016c. Retinal remodeling and metabolic alterations in human AMD. Front. Cell. Neurosci 10, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalloniatis M, Nivison-Smith L, Chua J, Acosta ML, Fletcher EL, 2016. Using the rd1 mouse to understand functional and anatomical retinal remodelling and treatment implications in retinitis pigmentosa: a review. Exp. Eye Res 150, 106–121. [DOI] [PubMed] [Google Scholar]
- Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T, 2015. Retinal microglia: just bystander or target for therapy? Prog. Retin. Eye Res 45, 30–57. [DOI] [PubMed] [Google Scholar]
- Khabou H, Garita-Hernandez M, Chaffiol A, Reichman S, Jaillard C, Brazhnikova E, Bertin S, Forster V, Desrosiers M, Winckler C, Goureau O, Picaud S, Duebel J, Sahel JA, Dalkara D, 2018. Noninvasive gene delivery to foveal cones for vision restoration. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khristov V, Maminishkis A, Amaral J, Rising A, Bharti K, Miller S, 2018Validation of iPS cell-derived RPE tissue in animal models. Adv. Exp. Med. Biol 1074, 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingsworth MC, Sarks JP, Sarks SH, 1990. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye (Lond) 4 (Pt 4), 613–621. [DOI] [PubMed] [Google Scholar]
- Kitiratschky VB, Stingl K, Wilhelm B, Peters T, Besch D, Sachs H, Gekeler F, Bartz-Schmidt KU, Zrenner E, 2015. Safety evaluation of “retina implant alpha IMS”–a prospective clinical trial. Graefes Arch. Clin. Exp. Ophthalmol 253, 381–387. [DOI] [PubMed] [Google Scholar]
- Kolb H, Gouras P, 1974. Electron microscopic observations of human retinitis pigmentosa, dominantly inherited. Investig. Ophthalmol 13, 487–498. [PubMed] [Google Scholar]
- Kondo M, Sakai T, Komeima K, Kurimoto Y, Ueno S, Nishizawa Y, Usukura J, Fujikado T, Tano Y, Terasaki H, 2009. Generation of a transgenic rabbit model of retinal degeneration. Investig. Ophthalmol. Vis. Sci 50, 1371–1377. [DOI] [PubMed] [Google Scholar]
- Kuehlewein L, Troelenberg N, Stingl K, Schleehauf S, Kusnyerik A, Jackson TL, MacLaren RE, Chee C, Roider J, Wilhelm B, Gekeler F, Bartz-Schmidt KU, Zrenner E, Stingl K, 2019. Changes in microchip position after implantation of a subretinal vision prosthesis in humans. Acta Ophthalmol epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kunchithapautham K, Rohrer B, 2007. Apoptosis and autophagy in photoreceptors exposed to oxidative stress. Autophagy 3, 433–441. [DOI] [PubMed] [Google Scholar]
- Kurji KH, Cui JZ, Lin T, Harriman D, Prasad SS, Kojic L, Matsubara JA, 2010. Microarray analysis identifies changes in inflammatory gene expression in response to amyloid-beta stimulation of cultured human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci 51, 1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL, 2007. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci 27, 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagali PS, Balya D, Awatramani GB, Munch TA, Kim DS, Busskamp V, Cepko CL, Roska B, 2008. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat. Neurosci 11, 667–675. [DOI] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Reh TA, 2009. Strategies for retinal repair: cell replacement and regeneration. Prog. Brain Res 175, 23–31. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL, 1998. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U. S. A 95, 6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmann T, 2007. Microglia activation in retinal degeneration. J. Leukoc. Biol 81,1345–1351. [DOI] [PubMed] [Google Scholar]
- Laprell L, Hull K, Stawski P, Schon C, Michalakis S, Biel M, Sumser MP, Trauner D, 2016. Restoring light sensitivity in blind retinae using a photochromic AMPA receptor agonist. ACS Chem. Neurosci 7, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM, Nishikawa S, Steinberg RH, Naash MI, Duncan JL, Trautmann N, Matthes MT, Yasumura D, Lau-Villacorta C, Chen J, Peterson WM, Yang H, Flannery JG, 2018. Phenotypic characterization of P23H and S334ter rhodopsin transgenic rat models of inherited retinal degeneration. Exp. Eye Res 167, 56–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, Mendes-Madeira A, Provost N, Pereon Y, Cherel Y, Ali RR, Hamel C, Moullier P, Rolling F, 2007. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 14, 292–303. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Desplats P, Sigurdson C, Tsigelny I, Masliah E, 2010. Cell-to-cell transmission of non-prion protein aggregates. Nat. Rev. Neurol 6, 702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GP, Fisher SK, 2005. Retinal plasticity and interactive cellular remodeling in retinal detachment and reattachment In: Pinaud R, Tremere LA, De Weerd P (Eds.), Plasticity in the Visual System: from Genes to Circuits. Springer, New York, NY, USA, pp. 55–78. [Google Scholar]
- Lewis GP, Erickson PA, Guerin CJ, Anderson DH, Fisher SK, 1989. Changes in the expression of specific Muller cell proteins during long-term retinal detachment. Exp. Eye Res 49, 93–111. [DOI] [PubMed] [Google Scholar]
- Lewis GP, Guerin CJ, Anderson DH, Matsumoto B, Fisher SK, 1994. Rapid changes in the expression of glial cell proteins caused by experimental retinal detachment. Am. J. Ophthalmol 118, 368–376. [DOI] [PubMed] [Google Scholar]
- Lewis GP, Linberg KA, Fisher SK, 1998. Neurite outgrowth from bipolar and horizontal cells after experimental retinal detachment. Investig. Ophthalmol. Vis. Sci 39, 424–434. [PubMed] [Google Scholar]
- Lewis GP, Sethi CS, Carter KM, Charteris DG, Fisher SK, 2005. Microglial cell activation following retinal detachment: a comparison between species. Mol. Vis 11, 491–500. [PubMed] [Google Scholar]
- Lewis GP, Betts KE, Sethi CS, Charteris DG, Lesnik-Oberstein SY, Avery RL, Fisher SK, 2007. Identification of ganglion cell neurites in human subretinal and epiretinal membranes. Br. J. Ophthalmol 91, 1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GP, Chapin EA, Luna G, Linberg KA, Fisher SK, 2010. The fate of Muller’s glia following experimental retinal detachment: nuclear migration, cell division, and subretinal glial scar formation. Mol. Vis 16, 1361–1372. [PMC free article] [PubMed] [Google Scholar]
- Li SH, Li XJ, 2004. Huntingtin-protein interactions and the pathogenesis ofHuntington’s disease. Trends Genet. 20, 146–154. [DOI] [PubMed] [Google Scholar]
- Li ZY, Kljavin IJ, Milam AH, 1995. Rod photoreceptor neurite sprouting in retinitis pigmentosa. J. Neurosci 15, 5429–5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY, 2012. Age-related macular degeneration. Lancet 379, 1728–1738. [DOI] [PubMed] [Google Scholar]
- Lin B, Koizumi A, Tanaka N, Panda S, Masland RH, 2008. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc. Natl. Acad. Sci. U. S. A 105, 16009–16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Jones BW, Liu A, Tucker JF, Rapp K, Luo L, Baehr W, Bernstein PS, Watt CB, Yang JH, Shaw MV, Marc RE, 2012a. Retinoid receptors trigger neuritogenesis in retinal degenerations. FASEB J. 26, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Jones BW, Liu A, Vazquez-Chona FR, Lauritzen JS, Ferrell WD, Marc RE, 2012b. Rapid glutamate receptor 2 trafficking during retinal degeneration. Mol. Neurodegener 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linberg KA, Lewis GP, Matsumoto B, Fisher SK, 2006. Immunocytochemical evidence that rod-connected horizontal cell axon terminals remodel in response to experimental retinal detachment in the cat. Mol. Vis 12, 1674–1686. [PubMed] [Google Scholar]
- Litts KM, Messinger JD, Freund KB, Zhang Y, Curcio CA, 2015. Inner segment remodeling and mitochondrial translocation in cone photoreceptors in age-related macular degeneration with outer retinal tubulation. Investig. Ophthalmol. Vis. Sci 56, 2243–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XC, Liu XF, Jian CX, Li CJ, He SZ, 2012. IL-33 is induced by amyloid-beta stimulation and regulates inflammatory cytokine production in retinal pigment epithelium cells. Inflammation 35, 776–784. [DOI] [PubMed] [Google Scholar]
- Liu XL, Wang YD, Yu XM, Li DW, Li GR, 2018. Mitochondria-mediated damage to dopaminergic neurons in Parkinson’s disease (Review). Int. J. Mol. Med 41, 615–623. [DOI] [PubMed] [Google Scholar]
- London A, Benhar I, Schwartz M, 2013. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol 9, 44–53. [DOI] [PubMed] [Google Scholar]
- Lu Y, Han L, Wang C, Dou H, Feng X, Hu Y, Feng K, Wang X, Ma Z, 2017. A comparison of autologous transplantation of retinal pigment epithelium (RPE) monolayer sheet graft with RPE-Bruch’s membrane complex graft in neovascular age-related macular degeneration. Acta Ophthalmol 95, e443–e452. [DOI] [PubMed] [Google Scholar]
- Luna G, Keeley PW, Reese BE, Linberg KA, Lewis GP, Fisher SK, 2016Astrocyte structural reactivity and plasticity in models of retinal detachment. Exp. Eye Res 150, 4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR, 2006. Retinal repair by transplantation of photoreceptor precursors. Nature 444, 203–207. [DOI] [PubMed] [Google Scholar]
- Marc RE, 1999. The structure of vertebrate retinas In: Toyoda (Ed.), The Retinal Basis of Vision. Elsevier, pp. 3–19. [Google Scholar]
- Marc R, 2009. Functional neuroanatomy of the retina In: Tasman W, Jaeger EA (Eds.), Duane’s Ophthalmology. Lippincott Williams and Wilkins, Philadelphia, United States. [Google Scholar]
- Marc RE, Jones BW, 2003. Retinal remodeling in inherited photoreceptor degenerations. Mol. Neurobiol 28, 139–147. [DOI] [PubMed] [Google Scholar]
- Marc RE, Murry RF, Basinger SF, 1995. Pattern recognition of amino acid signatures in retinal neurons. J. Neurosci 15, 5106–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Murry RF, Fisher SK, Linberg KA, Lewis GP, 1998a. Amino acid signatures in the detached cat retina. Investig. Ophthalmol. Vis. Sci 39, 1694–1702. [PubMed] [Google Scholar]
- Marc RE, Murry RF, Fisher SK, Linberg KA, Lewis GP, Kalloniatis M, 1998b. Amino acid signatures in the normal cat retina. Investig. Ophthalmol. Vis. Sci 39, 1685–1693. [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Strettoi E, 2003. Neural remodeling in retinal degeneration. Prog. Retin. Eye Res 22, 607–655. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Anderson JR, Kinard K, Marshak DW, Wilson JH, Wensel T, Lucas RJ, 2007. Neural reprogramming in retinal degeneration. Investig. Ophthalmol. Vis. Sci 48, 3364–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Vazquez-Chona F, Vaughan DK, Organisciak DT, 2008. Extreme retinal remodeling triggered by light damage: implications for age related macular degeneration. Mol. Vis 14, 782–806. [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Anderson JR, Sigulinsky C, Lauritzen S, 2013. Retinal connectomics: towards complete, accurate networks. Prog. Retin. Eye Res 37, 141–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc R, Pfeiffer R, Jones B, 2014. Retinal prosthetics, optogenetics, and chemical photoswitches. ACS Chem. Neurosci 5, 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DJ, Newkirk G, Euler T, Detwiler PB, 2008. Functional stability of retinal ganglion cells after degeneration-induced changes in synaptic input. J. Neurosci 28, 6526–6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Navarrete GC, Martin-Nieto J, Esteve-Rudd J, Angulo A, Cuenca N, 2007. Alpha synuclein gene expression profile in the retina of vertebrates. Mol. Vis 13, 949–961. [PMC free article] [PubMed] [Google Scholar]
- Masland RH, 2012. The neuronal organization of the retina. Neuron 76, 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, Yang J, 2015. Usher syndrome: hearing loss, retinal degeneration and associated abnormalities. Biochim. Biophys. Acta 1852, 406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon C, Tabrizi SJ, 2014. The ubiquitin-proteasome system in neurodegeneration. Antioxidants Redox Signal. 21, 2302–2321. [DOI] [PubMed] [Google Scholar]
- Melentijevic I, Toth ML, Arnold ML, Guasp RJ, Harinath G, Nguyen KC, Taub D, Parker JA, Neri C, Gabel CV, Hall DH, Driscoll M, 2017. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 542, 367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, Fullgrabe J, Jackson A, Jimenez Sanchez M, Karabiyik C, Licitra F, Lopez Ramirez A, Pavel M, Puri C, Renna M, Ricketts T, Schlotawa L, Vicinanza M, Won H, Zhu Y, Skidmore J, Rubinsztein DC, 2017. Autophagy and neurode-generation: pathogenic mechanisms and therapeutic opportunities. Neuron 93, 1015–1034. [DOI] [PubMed] [Google Scholar]
- Menzler J, Zeck G, 2011. Network oscillations in rod-degenerated mouse retinas. J.Neurosci 31, 2280–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Nishida S, 1967. Electron microscopic studies of human retinitis pigmentosa. I. Two cases of advanced retinitis pigmentosa. Am. J. Ophthalmol 63, 791–803. [DOI] [PubMed] [Google Scholar]
- Moreno ML, Merida S, Bosch-Morell F, Miranda M, Villar VM, 2018. Autophagy dysfunction and oxidative stress, two related mechanisms implicated in retinitis pigmentosa. Front. Physiol 9, 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ, 2004. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog. Neurobiol 73, 1–60. [DOI] [PubMed] [Google Scholar]
- Naash MI, Hollyfield JG, al-Ubaidi MR, Baehr W, 1993. Simulation of human autosomal dominant retinitis pigmentosa in transgenic mice expressing a mutated murine opsin gene. Proc. Natl. Acad. Sci. U. S. A 90, 5499–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E, 2003. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. U. S. A 100, 13940–13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F, 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Ogata K, Kosaka T, 2002. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience 113, 221–233. [DOI] [PubMed] [Google Scholar]
- Opoku-Nsiah KA, Gestwicki JE, 2018. Aim for the core: suitability of the ubiquitin-independent 20S proteasome as a drug target in neurodegeneration. Transl. Res 198, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisciak DT, Jiang YL, Wang HM, Pickford M, Blanks JC, 1989a. Retinal light damage in rats exposed to intermittent light. Comparison with continuous light exposure. Investig. Ophthalmol. Vis. Sci 30, 795–805. [PubMed] [Google Scholar]
- Organisciak DT, Wang HM, Xie A, Reeves DS, Donoso LA, 1989b. Intense-light mediated changes in rat rod outer segment lipids and proteins. Prog. Clin. Biol. Res 314, 493–512. [PubMed] [Google Scholar]
- Palay SL, Palade GE, 1955. The fine structure of neurons. J. Biophys. Biochem. Cytol 1, 69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Schertel A, Lee KE, Han SS, 2014. Ultra-structural analysis of the brain in a Drosophila model of Alzheimer’s disease using FIB/SEM microscopy. Microscopy (Oxf) 63, 3–13. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, Duran Y, Smith AJ, Chuang JZ, Azam SA, Luhmann UF, Benucci A, Sung CH, Bainbridge JW, Carandini M, Yau KW, Sowden JC, Ali RR, 2012. Restoration of vision after transplantation of photoreceptors. Nature 485, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Hippert C, Graca AB, Barber AC, 2014. Photoreceptor replacement therapy: challenges presented by the diseased recipient retinal environment. Vis. Neurosci 31, 333–344. [DOI] [PubMed] [Google Scholar]
- Peng YW, Hao Y, Petters RM, Wong F, 2000. Ectopic synaptogenesis in the mammalian retina caused by rod photoreceptor-specific mutations. Nat. Neurosci 3, 1121–1127. [DOI] [PubMed] [Google Scholar]
- Petoe MA, McCarthy CD, Shivdasani MN, Sinclair NC, Scott AF, Ayton LN, Barnes NM, Guymer RH, Allen PJ, Blamey PJ, Bionic Vision Australia C, 2017. Determining the contribution of retinotopic discrimination to localization performance with a suprachoroidal retinal prosthesis. Investig. Ophthalmol. Vis. Sci 58, 3231–3239. [DOI] [PubMed] [Google Scholar]
- Petrs-Silva H, Linden R, 2014. Advances in gene therapy technologies to treat retinitis pigmentosa. Clin. Ophthalmol 8, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer RL, Anderson JA, Emrich DP, Dahal J, Sigulinsky C, Morrison H, Yang JH, Watt CB, Rapp KD, Kondo M, Terasaki H, Garcia JC, Marc RE, Jones BW, (in press). Pathoconnectome analysis of muller cells in early retinal remodeling, in: Ash J, Anderson RE, Lavail MM, Bowes-Rickman C, Hollyfield JG, Grimm B (Eds.), Retinal Degenerative Diseases; Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer RL, Marc RE, Kondo M, Terasaki H, Jones BW, 2016. Muller cell metabolic chaos during retinal degeneration. Exp. Eye Res 150, 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer RL, Marc RE, Anderson JA, Emrich DP, Watt CB, Yang JH, Rapp KD, Dahal J, Kondo M, Terasaki H, Jones BW, 2018. A pathoconnectome of early retinal remodeling. IOVS (Investig. Ophthalmol. Vis. Sci.) 59. [Google Scholar]
- Polosukhina A, Litt J, Tochitsky I, Nemargut J, Sychev Y, De Kouchkovsky I,Huang T, Borges K, Trauner D, Van Gelder RN, Kramer RH, 2012. Photochemical restoration of visual responses in blind mice. Neuron 75, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL, 1997. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Radner W, Sadda SR, Humayun MS, Suzuki S, de Juan E Jr., 2002. Increased spontaneous retinal ganglion cell activity in rd mice after neural retinal transplantation. Investig. Ophthalmol. Vis. Sci 43, 3053–3058. [PubMed] [Google Scholar]
- Ramkumar HL, Nguyen B, Bartsch DU, Saunders LJ, Muftuoglu IK, You Q, Freeman WR, 2018. Reduced ganglion cell volume on optical coherence tomography in patients with geographic atrophy. Retina 38, 2159–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnayaka JA, Serpell LC, Lotery AJ, 2015. Dementia of the eye: the role of amyloid beta in retinal degeneration. Eye (Lond) 29, 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanan P, Srikumar IF, Talwar P, 2017. Autophagy: the spotlight for cellular stress responses. Life Sci. 188, 53–67. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A, 2010. Muller Cells in the Healthy Retina, Muller Cells in the Healthy and Diseased Retina. Springer Science+Business Meddia, LLC, New York, pp. 35–299. [Google Scholar]
- Reichenbach A, Bringmann A, 2013. New functions of Muller cells. Glia 61, 651–678. [DOI] [PubMed] [Google Scholar]
- Roesch K, Stadler MB, Cepko CL, 2012. Gene expression changes within Müller glial cells in retinitis pigmentosa. Mol. Vis 18, 1197–1214. [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Tabrizi SJ, 2011. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 10, 83–98. [DOI] [PubMed] [Google Scholar]
- Ross JW, Fernandez de Castro JP, Zhao J, Samuel M, Walters E, Rios C, Bray-Ward P, Jones BW, Marc RE, Wang W, Zhou L, Noel JM, McCall MA, DeMarco PJ, Prather RS, Kaplan HJ, 2012. Generation of an inbred miniature pig model of retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci 53, 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahel JA, Roska B, 2013. Gene therapy for blindness. Annu. Rev. Neurosci 36, 467–488. [DOI] [PubMed] [Google Scholar]
- Saliba RS, Munro PM, Luthert PJ, Cheetham ME, 2002. The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation. J. Cell Sci 115, 2907–2918. [DOI] [PubMed] [Google Scholar]
- Santos A, Humayun MS, de Juan E Jr., Greenburg RJ, Marsh MJ, Klock IB, Milam AH, 1997. Preservation of the inner retina in retinitis pigmentosa. A morphometric analysis. Arch. Ophthalmol 115, 511–515. [DOI] [PubMed] [Google Scholar]
- Santos-Ferreira T, Volkner M, Borsch O, Haas J, Cimalla P, Vasudevan P,Carmeliet P, Corbeil D, Michalakis S, Koch E, Karl MO, Ader M, 2016a. Stem cell-derived photoreceptor transplants differentially integrate into mouse models of cone-rod dystrophy. Investig. Ophthalmol. Vis. Sci 57, 3509–3520. [DOI] [PubMed] [Google Scholar]
- Santos-Ferreira TF, Borsch O, Ader M, 2016b. Rebuilding the missing part-A review on photoreceptor transplantation. Front. Syst. Neurosci 10, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Raymick J, Imam S, 2016. Neuroprotective and therapeutic strategies against Parkinson’s disease: recent perspectives. Int. J. Mol. Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobert B, Lanyi JK, 1982. Halorhodopsin is a light-driven chloride pump. J. Biol. Chem 257, 10306–10313. [PubMed] [Google Scholar]
- Schreiber A, Peter M, 2014. Substrate recognition in selective autophagy and the ubiquitin-proteasome system. Biochim. Biophys. Acta 1843, 163–181. [DOI] [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB, 2012. Cell replacement and visual restoration by retinal sheet transplants. Prog. Retin. Eye Res 31, 661–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Jones BW, Aramant RB, Yang PB, Keirstead HS, Marc RE, 2012. Computational molecular phenotyping of retinal sheet transplants to rats with retinal degeneration. Eur. J. Neurosci 35, 1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Lin RE, McLelland BT, Mathur A, Lin B, Sigman J, De Guzman AT, Kitzes LM, Aramant RB, Thomas BB, 2017. Vision recovery and connectivity by fetal retinal sheet transplantation in an immunodeficient retinal degenerate rat model. Investig. Ophthalmol. Vis. Sci 58, 614–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Chaffiol A, Mace E, Caplette R, Desrosiers M, Lampic M, Forster V, Marre O, Lin JY, Sahel JA, Picaud S, Dalkara D, Duebel J, 2016. Red-shifted channelrhodopsin stimulation restores light responses in blind mice, macaque retina, and human retina. EMBO Mol. Med 8, 1248–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi CS, Lewis GP, Fisher SK, Leitner WP, Mann DL, Luthert PJ, Charteris DG, 2005. Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci 46, 329–342. [DOI] [PubMed] [Google Scholar]
- Shivdasani MN, Sinclair NC, Dimitrov PN, Varsamidis M, Ayton LN, Luu CD, Perera T, McDermott HJ, Blamey PJ, Bionic Vision Australia C, 2014. Factors affecting perceptual thresholds in a suprachoroidal retinal prosthesis. Investig. Ophthalmol. Vis. Sci 55, 6467–6481. [DOI] [PubMed] [Google Scholar]
- Shivdasani MN, Sinclair NC, Gillespie LN, Petoe MA, Titchener SA, Fallon JB, Perera T, Pardinas-Diaz D, Barnes NM, Blamey PJ, Bionic Vision Australia C, 2017. Identification of characters and localization of images using direct multiple-electrode stimulation with a suprachoroidal retinal prosthesis. Investig. Ophthalmol. Vis. Sci 58, 3962–3974. [DOI] [PubMed] [Google Scholar]
- Singh MS, Charbel Issa P, Butler R, Martin C, Lipinski DM, Sekaran S, Barnard AR, MacLaren RE, 2013. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc. Natl. Acad. Sci. U. S. A 110, 1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater KD, Sinclair NC, Nelson TS, Blamey PJ, McDermott HJ, Bionic Vision Australia C, 2015. neuroBi: a highly configurable neurostimulator for a retinal prosthesis and other applications. IEEE J Transl Eng Health Med 3, 3800111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sominsky L, De Luca S, Spencer SJ, 2018. Microglia: key players in neurodevelopment and neuronal plasticity. Int. J. Biochem. Cell Biol 94, 56–60. [DOI] [PubMed] [Google Scholar]
- Stasheff SF, 2008. Emergence of sustained spontaneous hyperactivity and temporary preservation of OFF responses in ganglion cells of the retinal degeneration (rd1) mouse. J. Neurophysiol 99, 1408–1421. [DOI] [PubMed] [Google Scholar]
- Stawski P, Sumser M, Trauner D, 2012. A photochromic agonist of AMPA receptors.Angew Chem. Int. Ed. Engl 51, 5748–5751. [DOI] [PubMed] [Google Scholar]
- Stingl K, Bartz-Schmidt KU, Besch D, Braun A, Bruckmann A, Gekeler F,Greppmaier U, Hipp S, Hortdorfer G, Kernstock C, Koitschev A, Kusnyerik A, Sachs H, Schatz A, Stingl KT, Peters T, Wilhelm B, Zrenner E, 2013. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc Biol Sci 280, 20130077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl K, Bartz-Schmidt KU, Besch D, Chee CK, Cottriall CL, Gekeler F, Groppe M, Jackson TL, MacLaren RE, Koitschev A, Kusnyerik A, Neffendorf J, Nemeth J, Naeem MA, Peters T, Ramsden JD, Sachs H, Simpson A, Singh MS, Wilhelm B, Wong D, Zrenner E, 2015. Subretinal visual implant alpha IMS– clinical trial interim report. Vis. Res 111, 149–160. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Pignatelli V, 2000. Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc. Natl. Acad. Sci. U. S. A 97, 11020–11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Porciatti V, Falsini B, Pignatelli V, Rossi C, 2002. Morphological and functional abnormalities in the inner retina of the rd/rd mouse. J. Neurosci 22, 5492–5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Pignatelli V, Rossi C, Porciatti V, Falsini B, 2003. Remodeling of second-order neurons in the retina of rd/rd mutant mice. Vis. Res 43, 867–877. [DOI] [PubMed] [Google Scholar]
- Suliman HB, Piantadosi CA, 2016. Mitochondrial quality control as a therapeutic target. Pharmacol. Rev 68, 20–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguchov A, Surgucheva I, Solessio E, Baehr W, 1999. Synoretin–A new protein belonging to the synuclein family. Mol. Cell. Neurosci 13, 95–103. [DOI] [PubMed] [Google Scholar]
- Suspitsin EN, Imyanitov EN, 2016. Bardet-biedl syndrome. Mol Syndromol 7, 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, 2006. Characterization of rhodopsin P23H-induced retinal degeneration in a Xenopus laevis model of retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci 47, 3234–3241. [DOI] [PubMed] [Google Scholar]
- Tanida I, Ueno T, Kominami E, 2008. LC3 and autophagy. Methods Mol. Biol 2008/04/22, 77–88. [DOI] [PubMed] [Google Scholar]
- Tochitsky I, Polosukhina A, Degtyar VE, Gallerani N, Smith CM, Friedman A, Van Gelder RN, Trauner D, Kaufer D, Kramer RH, 2014. Restoring visual function to blind mice with a photoswitch that exploits electrophysiological remodeling of retinal ganglion cells. Neuron 81, 800–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Sugano E, Fukazawa Y, Isago H, Sugiyama Y, Hiroi T, Ishizuka T, Mushiake H, Kato M, Hirabayashi M, Shigemoto R, Yawo H, Tamai M, 2009. Visual properties of transgenic rats harboring the channelrhodopsin-2 gene regulated by the thy-1.2 promoter. PLoS One 4, e7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Sugano E, Isago H, Hiroi T, Wang Z, Ohta E, Tamai M, 2010Channelrhodopsin-2 gene transduced into retinal ganglion cells restores functional vision in genetically blind rats. Exp. Eye Res 90, 429–436. [DOI] [PubMed] [Google Scholar]
- Tu HY, Watanabe T, Shirai H, Yamasaki S, Kinoshita M, Matsushita K, Hashiguchi T, Onoe H, Matsuyama T, Kuwahara A, Kishino A, Kimura T, Eiraku M, Suzuma K, Kitaoka T, Takahashi M, Mandai M, 2019. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine 39, 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez MC, Balboa E, Alvarez AR, Zanlungo S, 2012. Oxidative stress: a pathogenic mechanism for Niemann-Pick type C disease. Oxid Med Cell Longev 2012, 205713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veys L, Vandenabeele M, Ortuno-Lizaran I, Baekelandt V, Cuenca N, Moons L, De Groef L, 2019. Retinal alpha-synuclein deposits in Parkinson’s disease patients and animal models. Acta Neuropathol. 137, 379–395. [DOI] [PubMed] [Google Scholar]
- Waelter S, Boeddrich A, Lurz R, Scherzinger E, Lueder G, Lehrach H, Wanker EE, 2001. Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol. Biol. Cell 12, 1393–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhu C, Xu Y, Liu B, Wang M, Wu K, 2011. Development and expression of amyloid-beta peptide 42 in retinal ganglion cells in rats. Anat. Rec 294, 1401–1405. [DOI] [PubMed] [Google Scholar]
- Wang J, Ohno-Matsui K, Morita I, 2012. Elevated amyloid beta production in senescent retinal pigment epithelium, a possible mechanism of subretinal deposition of amyloid beta in age-related macular degeneration. Biochem. Biophys. Res. Commun 423, 73–78. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X, 2014. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 1842, 1240–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems PH, Valsecchi F, Distelmaier F, Verkaart S, Visch HJ, Smeitink JA,Koopman WJ, 2008. Mitochondrial Ca2+ homeostasis in human NADH:ubiquinone oxidoreductase deficiency. Cell Calcium 44, 123–133. [DOI] [PubMed] [Google Scholar]
- Wolff E, 1935. A Pathology of the Eye, first ed. (Blakiston: ). [Google Scholar]
- Wong TY, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R, Fahrbach K, Probst C, Sledge I, 2008. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology 115, 116–126. [DOI] [PubMed] [Google Scholar]
- Yang JW, Czech T, Felizardo M, Baumgartner C, Lubec G, 2006. Aberrant expression of cytoskeleton proteins in hippocampus from patients with mesial temporal lobe epilepsy. Amino Acids 30, 477–493. [DOI] [PubMed] [Google Scholar]
- Yao J, Jia L, Feathers K, Lin C, Khan NW, Klionsky DJ, Ferguson TA, Zacks DN, 2016. Autophagy-mediated catabolism of visual transduction proteins prevents retinal degeneration. Autophagy 12, 2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Ohno-Matsui K, Ichinose S, Sato T, Iwata N, Saido TC, Hisatomi T, Mochizuki M, Morita I, 2005. The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J. Clin. Investig 115, 2793–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Weiland JD, Roska B, Humayun MS, 2016. Retinal stimulation strategies to restore vision: fundamentals and systems. Prog. Retin. Eye Res 53, 21–47. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG,Bamberg E, Nagel G, Gottschalk A, Deisseroth K, 2007. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ivanova E, Bi A, Pan ZH, 2009. Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. J. Neurosci 29, 9186–9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen S, Song L, Tang Y, Shen Y, Jia L, Le W, 2014. MTOR-independent, autophagic enhancer trehalose prolongs motor neuron survival and ameliorates the autophagic flux defect in a mouse model of amyotrophic lateral sclerosis. Autophagy 10, 588–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Huang T, Zhang L, Zhou Y, Luo H, Xu H, Wang X, 2016. Dysregulation of ubiquitin-proteasome system in neurodegenerative diseases. Front. Aging Neurosci 8, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Doggett TA, Sene A, Apte RS, Ferguson TA, 2015. Autophagy supports survival and phototransduction protein levels in rod photoreceptors. Cell Death Differ. 22, 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrenner E, 2002. The subretinal implant: can microphotodiode arrays replace degenerated retinal photoreceptors to restore vision? Ophthalmologica 216 (Suppl. 1), 8–20 discussion 52–23. [DOI] [PubMed] [Google Scholar]