Abstract
Sex differences among neurons in the ventrolateral region of the ventromedial hypothalamic nucleus (VMHvl) allow for the display of a diversity of sex-typical behaviors and physiological responses, ranging from mating behavior to metabolism. Here we review recent studies that interrogate the relationship between sex-typical responses and changes in cellular phenotypes. We discuss technologies that increase the resolution of molecular profiling or targeting of cell populations, including single cell transcriptional profiling and conditional viral genetic approaches to manipulate neuron survival or activity. Overall, emerging studies indicate that sex-typical functions of the VMH may be mediated by phenotypically distinct and sexually differentiated neuron populations within the VMHvl. Future studies in this and other brain regions could exploit cell-type-specific tools to reveal the cell populations and molecular mediators that modulate sex-typical responses. Further, cell-type-specific analyses of the effects of sexually differentiating factors, including sex hormones, can test the hypothesis that distinct cell types within a single brain region vary with respect to sexual differentiation.
INTRODUCTION
Sex differences in the brain include molecular, cytoarchitectural, or connectivity features of cells and brain regions that are nonneutral between males and females. These sex differences are embedded within neural circuits and could provide the functional basis for sex-typical behaviors and physiological responses (Figure 1)1,2. The origins of these sex differences are not fully understood, but can be the result of genetic, hormonal, or environmental factors acting in adulthood or during development (reviewed in 3). Some of the best studied sex differences in the brain appear to arise from the effects of sex hormones, mainly testosterone or its metabolite estradiol. Activational effects of sex hormones arise in adulthood and are reversible. In contrast, organizational effects of sex hormones are programmed during a short perinatal time window, or critical period, and are permanent. For example, perinatal estradiol can alter cellular survival and lead to sex differences in neuron number in the sexually dimorphic nucleus of the preoptic area of the hypothalamus (SDN-POA) and the anteroventral periventricular nucleus (AVPV) in rodents4–9. However, other hypothalamic regions exhibit sex differences in gene expression or morphology rather than cell number. In the VMHvl, perinatal estradiol establishes permanent sex differences in gene expression4,5. This sexual differentiation of cellular phenotype extends to glial cells, including astrocytes10 and microglia11, and opens the door to more subtle differences between male and female brains.
Figure 1.
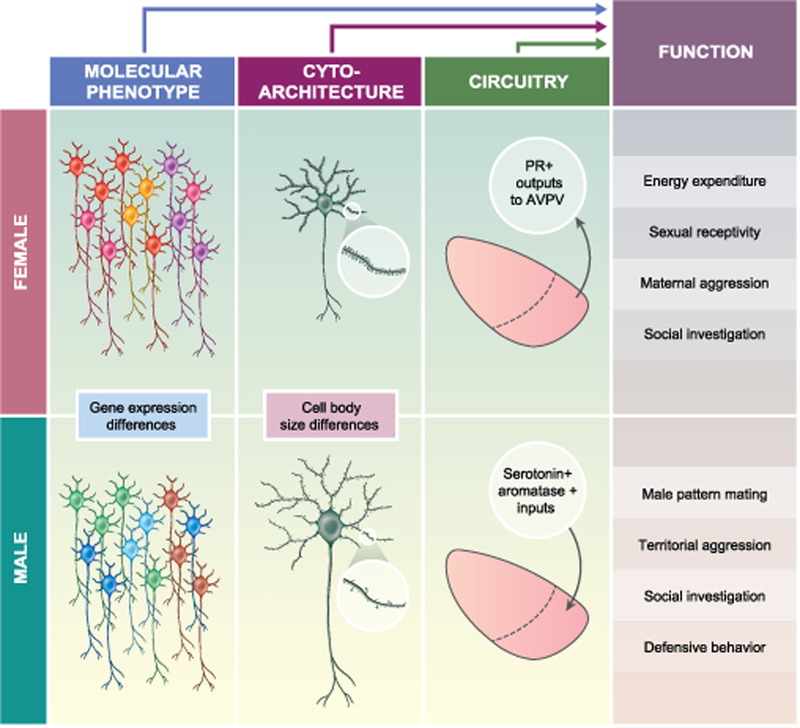
Schematic depiction of three types of sex differences in the VMH. Sex differences in molecular phenotype (gene expression), cytoarchitecture (cell body size, synaptic density in response to estradiol), and circuit connectivity (afferent and efferent projections) are thought to contribute to sex-typical functions, including behavioral and physiological outputs.
Earlier reviews have highlighted some of the challenges in characterizing sex differences in the brain: (1) sex differences rarely present as binary sexual dimorphisms but rather as quantitative differences2, and (2) while some cells and brain regions tend to be more strongly differentiated within each sex, the actual profile of masculinization and feminization depends on the hormonal and non-hormonal conditions of an individual12–14. These findings suggest that traditional methods of profiling sex differences in the brain, such as bulk tissue analysis, are unlikely to discriminate patterns of sexual differentiation specific to individual cells, and instead capture the average patterns of sexual differentiation within a brain region. Furthermore, bulk tissue-level analysis may be biased towards the characteristics of one or a few dominant cell populations and can miss those characteristics that are discordant among cell types and cell subpopulations15.
Recent technologies, such as single cell RNA sequencing (scRNA-seq) and viral genetic tools, have been used delineate which sexually differentiated cell populations contribute to the display of sex-typical responses in rodents. scRNA-seq tools isolate transcriptomes from single cells and examine transcriptional signatures among cell types (Figure 2A). Viral genetic tools include adeno-associated viruses that express genetically engineered proteins in a manner that is dependent on Cre recombinase (Figure 2B). This conditional gene expression allows for selective expression of genetic tools when delivered to mice that express Cre under the control of cell-type-specific gene markers. Here we discuss studies that induce cell-autonomous cell death using virally delivered Caspase-3 or bidirectionally manipulate neural activity using chemogenetic or optogenetic approaches. Briefly, chemogenetics refers to the use of synthetic G-protein coupled receptors that modulate neuronal excitability in response to an exogenous small molecule ligand, clozapine-N-oxide. Similarly, optogenetics refers to light-sensitive ion channels and implanted optical fibers to deliver light. Together, these studies reveal cell-type-specific effects on behavior that can differ between the sexes. Because these sex differences in function are restricted within and unique to distinct cell subpopulations, we suggest that sexual differentiation can have differential effects on cell populations. We propose the term “selective sexual differentiation” to describe heterogeneous responses to sexual differentiation factors, such as sex hormones, and suggest that these could underlie the sex-specificity and heterogeneity of functions mediated by the VMH. Finally, we discuss the potential and limitations of cell-type-specific analyses to advance our understanding of sex differences in the brain.
Figure 2.
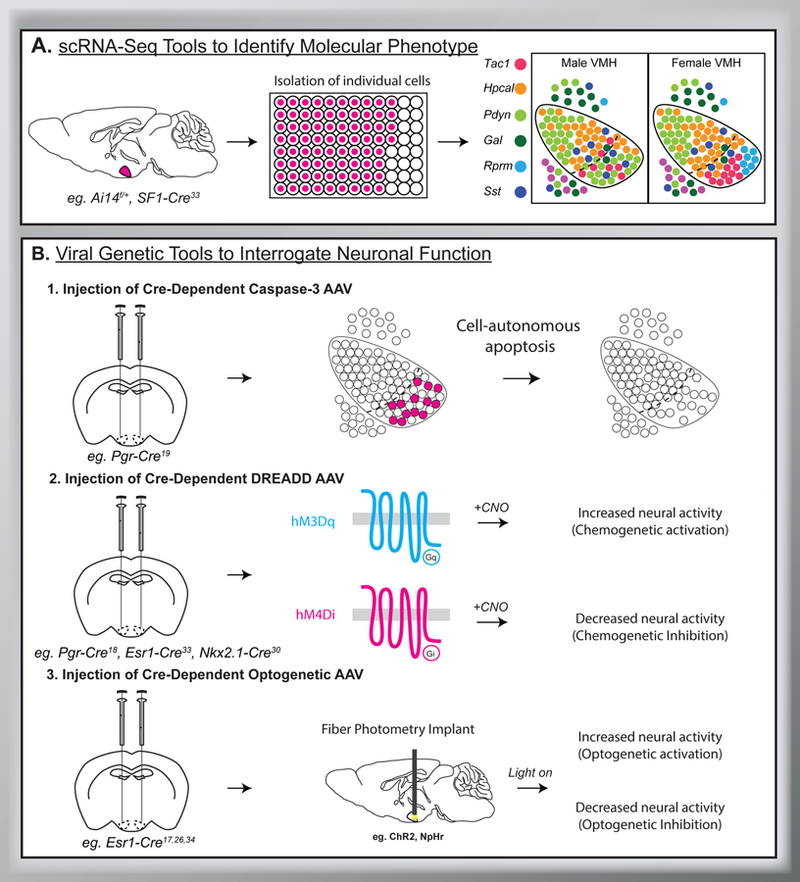
Single cell RNA sequencing (scRNA-Seq) and viral genetic tools can help identify the molecular phenotype of sexually differentiated neuronal populations within the VMH and determine their contribution to VMH-mediated functions. A. Pipeline for scRNA-Seq of VMH neurons as reported in33. Briefly, fluorescently labeled VMH neurons are isolated. A single-cell suspension allows for the transcriptome of individual cells to be analyzed. This approach revealed 6 molecularly distinct populations of neurons in the VMH. Figure adapted from33. B. Viral genetic tools that manipulate neuronal survival or activity patterns can be used to determine cellular function. Top row, injection of Cre-dependent Caspase-3 AAV virus allows for cell-autonomous apoptosis (cell death) of specific neurons within the VMH. Middle row, injection of Cre-dependent DREADD AAV (eg. hM3Dq, hM4Di) allows for chemogenetic activation or inhibition of neuronal activity within VMH neurons following delivery of clozapine-N-oxide (CNO). Bottom row, injection of Cre-dependent optogenetic AAV (eg. ChR2, NpHr) allows for optogenetic activation or inhibition of VMH neurons via fiber photometry.
SEX-SPECIFIC FUNCTIONS REGULATED BY THE VMH
The VMH is a sexually differentiated structure that regulates a wide range of physiological processes, from sexual receptivity and mating behaviors, to physical activity and metabolic processes. Manipulations of molecularly defined neuron populations in the VMHvl have revealed sex-specific regulation of mounting behavior16–19, sexual receptivity19–23, territorial aggression16–18,24,25, maternal behavior26–29, physical activity30,31, and temperature32,33. The VMHvl also regulates defensive social behaviors34 but an effect of sex has not been established. Similarly, SF-1 neurons in the central and dorsomedial regions of the VMH (VMHc and VMHdm, respectively) control escape and defensive behaviors in both males and females35–37. Additionally, VMH neurons are sensitive to metabolic signals, including leptin and glucose, similarly in both sexes38–41. These studies suggest that many, but not all, functions mediated by the VMH occur in a sex-biased manner.
Sex hormone effects during development and puberty are the most robust mechanisms that drive sexual differentiation in the brain. Therefore, sex differences are most likely to manifest within populations of sex hormone-responsive neurons, and the VMH is rich in sex hormone receptor expression. In adult mice, neurons co-expressing estrogen receptor α (ERα) and progesterone receptor (PR) localize to VMHvl19,42 and are estimated to constitute 40–50% of cells in the VMHvl17,19. Furthermore, ERα is expressed at significantly higher levels in the VMHvl of females than males in rats43–45 and mice46. This sexually differentiated expression of ERα has been demonstrated in rats to arise during the early postnatal period (by postnatal day 10, P10) and persist into adulthood45. Neurons expressing the other ER subtype, estrogen receptor β (ERβ), are also localized to the VMHvl and largely overlap with ERα+ neurons, although ERβ expression is much lower than that of ERα in both mice47 and rats43. ERβ is also expressed at higher levels in females than males in neonatal (P5) rats; however this sex difference becomes marginal by adulthood and is not observed in mice43,47. Finally, androgen receptor (AR)-expressing neurons are localized throughout the VMH, with expression observed in mice in the early neonatal period (P1–P4). However, ARs do not show sex differences in expression in rats or mice48–50. Instead, AR neurons are thought to contribute to sex differences in the VMH through the regulation of aromatase expression and activity. In rats, testosterone treatment increases aromatase activity more in males than females, while mutations that render males unresponsive to androgens contribute to decreased aromatase activity and demasculinization of the VMH51,52.
Mating behavior (sexual receptivity and male-pattern mating behavior)
Subsets of VMHvl neurons contribute to the circuitry underlying female sexual receptivity and male pattern mating behavior. In females, the VMH is the final integration site for the hypothalamic and limbic circuits that underlie the expression of lordosis53. Female rodents are sexually receptive at specific times in their estrus cycle, starting with the evening of proestrus and ending with the morning of estrus, in a manner that is dependent on estradiol and progesterone53. RNAi-mediated silencing of ERα in the murine VMHvl impairs the display of receptive behavior, and instead triggers vigorous rejection of the male54. Unlike ERα, ERβ does not seem to play a major role in female sexual behavior, as ERβ-null mice show normal reproductive behavior55.
Gene targeting studies have identified several receptor and neuropeptide-encoding genes in the VMHvl that are necessary for female mating behavior. Cckar, the cholecystokinin A receptor, is expressed by the majority of ERα+/PR+ neurons in the VMHvl of female mice and is induced by estrogen signaling20,26. Cckar-null females56 show specific deficits in sexual receptivity, but show wild-type maternal behaviors and estrus cycles20. In contrast, Cckar is only very weakly expressed in males, and Cckar-null males show wild-type male-pattern mating behaviors19,20. The oxytocin receptor (Otr) is also necessary for estrogen-induced sexual receptivity behavior, as infusion of Otr antisense oligos into the VMH leads to reduced lordosis frequency and intensity without affecting locomotor activity following an estrogen priming paradigm in rats21. The gene encoding the precursor of the neuropeptide enkephalin (Penk) also contributes to lordosis behavior, as disruption of Penk function by intrahypothalamic antisense oligonucleotide injection decreases lordosis behavior without affecting locomotor behavior in rats22. Interestingly, although Penk is induced by estrogen signaling in adult guinea pigs, single cell RNA-seq and co-expression studies in mice find that only very few enkephalin-immunoreactive cells in the murine VMHvl co-express ERα or PR33,57. Lastly, subsets of neurons in the rat VMHvl encoding the neuropeptide substance P project to the dorsal midbrain central grey58, and injection of substance P to this target region facilitates lordosis behavior23. The gene encoding the substance P precursor (Tac1) is colocalizes with ERα in the rodent VMHvl33. However, a direct effect of Tac1 disruption on lordosis behavior remains to be shown. Together, these studies suggest that genes expressed by sex hormone-responsive cells, or induced by activational effects of sex hormones, have been linked to female-specific receptive behaviors.
In contrast to females, studies using genetic ablation, optogenetic or chemogenetic manipulation, and neural activity pattern recordings of VMHvl ERα+ or PR+ neurons have shown that these cells play a less robust role in male-pattern mating behavior16. Inducing death of PR+ neurons, using Cre-dependent Caspase-3 delivered to VMHvl of male mice expressing Cre under the Pgr promoter (Pgr-Cre), leads to reduced mounting, intromission, and reduced intromission length19. Optogenetic activation of neurons expressing Cre under the Esr1 promoter (Esr1-Cre) is not sufficient to increase mounting frequency towards females, although it does increase mounting duration in male mice17. Furthermore, unlike in females where mating-related VMHvl neurons increase in vivo spiking activity during and throughout a male encounter, VMHvl neurons in male mice show only a transient increase in in vivo spiking activity with a female encounter, and this is extinguished during progression of the behavior59. Similarly, optogenetic inhibition of Esr1-Cre neurons in male mice is insufficient to affect frequency, duration, or halt ongoing mounting of females17. Of note, residual neuronal activity in the optogenetic experiments may have impeded complete behavioral manipulation, accounting for some of these discrepancies17. Indeed, chemogenetic inhibition of VMHvl neurons in male Pgr-Cre mice reduces the quantity and duration of intromission events18. Nevertheless, optogenetic inhibition of Esr1-Cre neurons in the medial preoptic area (mPOA) is sufficient to inhibit mounting in both sexes, suggesting a nuanced role for the VMHvl in male-pattern mating behavior16,60.
Social behaviors (aggression and social investigation)
VMHvl neurons also contribute to aspects of various social behaviors, including defensive behavior, social investigation, and aggression. Optogenetic activation of Esr1-Cre neurons in the murine VMHvl can elicit either aggressive or defensive behaviors in males, while optogenetic inhibition of this same population compromises social defense34. Subsets of neurons in the murine VMHvl of males also show increased in vivo spiking activity during the initiation of an aggressive behavior towards a male intruder, and these neurons are normally inhibited during female encounters59. In contrast, optogenetic activation of these VMHvl neurons in males induces a rapid, time-bounded attack towards any intruders, including conspecific females59. Parallel findings from Esr1-Cre and Pgr-Cre mice show that these VMHvl neurons are necessary and sufficient to induce and maintain male intruder aggression17–19. Furthermore, intruder aggression can be elicited by chemogenetic activation of Pgr-Cre neurons in male mice even in the absence of testicular hormones18. In addition, the social behavior elicited by this population is scalable, such that a larger population of optogenetically or chemogenetically activated cells preferentially induces aggressive behavior, while a weaker activation of cells (via manipulation of the optogenetic or chemogenetic parameters) preferentially induces mounting and social investigative behavior towards both sexes17,18.
In contrast to males, activation of any-sized population of Pgr-Cre or Esr1-Cre VMHvl neurons in female mice generally fails to elicit aggressive behavior. Instead, activation of these neurons induces social investigation and occasional mounting17,18. However, the genetic background and reproductive state of the female can sometimes facilitate the expression of aggressive behavior following optogenetic activation of Esr1-Cre neurons, such as in the case of Swiss Webster mice or naturally aggressive lactating C57BL/6 mice26. In these females, the neurons that are active during the aggressive behavior are localized medially within the VMHvl and are functionally distinct from VMHvl neurons mediating mating behaviors26.
Finally, aggressive behavior can also be modulated by neurons projecting to the VMHvl. Optogenetic activation of lateral septum-VMHvl projections selectively inhibits an ongoing attack behavior in male mice, without affecting mounting behavior61. VMHvl neurons also receive synaptic inputs from GABAergic subparaventricular zone neurons, as well as neurons in the central VMH, as part of a larger circuitry that induces circadian phase-dependent regulation of VMH-mediated aggressive behaviors in mice62. Together, these studies suggest that social behaviors elicited by sex hormone-sensitive neurons in the VMHvl are modulated by both intrinsic factors and external circuitry.
Metabolism (energy expenditure and glucose sensing)
Neurons within the VMHvl also have important roles in regulating aspects of metabolism, including energy expenditure and glucose sensing, and many of these show sex-specific characteristics. Loss of ERα in the VMH by gene knockout or silencing impairs estrogen-dependent wheel running and ambulatory movement, metabolic rate, and brown adipose tissue (BAT) thermogenesis in female rats and mice30,31,63. The effects on movement in mice appear to be selectively mediated by a subset of ERα neurons that co-express Tac1. Indeed, developmental loss of ERα/Tac1 neurons leads to a reduction in movement without affecting BAT thermogenesis30. In rats, movement is regulated by Otr 44,64, but co-expression of Otr and Tac1 has not been analyzed.
We recently characterized a second population of ERα+ neurons in the murine VMHvl that is largely distinct from the Tac1 population and expresses the p53-induced gene reprimo (Rprm). Interestingly, siRNA-mediated knockdown of Rprm leads to a baseline increase in core body temperature in female mice33. This suggests that Rprm+/ERα+ neurons also contribute to sex-specific regulation of energy expenditure by modulating thermogenesis. While the roles of the Tac1+ and Rprm+ neurons have not been adequately interrogated in males, it is interesting to note that chemogenetic activation of ERα neurons in the VMHvl is sufficient to induce increased movement and energy expenditure in both males and females, suggesting that while the molecular pathways needed to elicit these aspects of metabolism may be enriched in females, synthetic activation of the circuitry can yield equivalent behavioral and physiological outputs in males and females (Kammel, van Veen, Correa unpublished).
Sex differences in glucose homeostasis are mediated in part by estrogen-sensitive glucose sensing neurons in the VMHvl. Glucose-excited (GE) and glucose-inhibited (GI) neurons, as well as a subclass of GI neurons that adapt to low extracellular glucose levels, are distributed throughout the murine VMH and work together to control glucose homeostasis40,41. The majority of glucose-sensing neurons within the VMHvl are GE, which increase their activity pattern with higher extracellular glucose levels as measured by patch-clamp electrophysiology40,65,66. While the percentage of GE neurons in the VMHvl is similar between male and female mice, males have a greater percentage of GI neurons that do not adapt to low extracellular glucose40. Furthermore, GI neurons in the male VMHvl show increased excitability in low levels of glucose, which suggests an inherent sex difference in glucose-elicited activity40. Finally, both types of GI neurons show activity blunting in response to estradiol40. Together, these findings suggest that female VMH neurons have a reduced ability to detect low glucose, and that this response can be further blunted by cyclic increases in estradiol40. One type of GI neuron was recently identified by expression of the pituitary adenylate cyclase-activating peptide (PACAP), and chemogenetic activation of this subset of VMH neurons was sufficient to inhibit insulin secretion and increase plasma glucose65. The sex difference in GI neuron activity could therefore underlie the clinical observations that women have weaker counterregulatory responses to hypoglycemia than men67.
Finally, developmental ablation of glucokinase (Gck), the major glucose-sensing enzyme, in murine VMH neurons leads to sex differences in fat mass, glucagon secretion, and autonomic nervous activity, with females showing increased gonadal, inguinal, and total fat mass, decreased hypoglycemia-induced glucagon secretion, and reduced parasympathetic and sympathetic nerve activity compared to males68. These findings further suggest that VMH Gck is required for hypoglycemia counter-regulation in females but not males68. Ultimately, sex differences in the VMH contribute to the differential recruitment of circuitry underlying metabolic homeostasis.
SELECTIVE SEXUAL DIFFERENTIATION OF VMH NEURONS
Sexual differentiation of molecular phenotype
Selective sexual differentiation among sex hormone-sensitive neurons could allow for the diverse set of sex-specific physiological processes mediated by the VMHvl. Males and females share qualitatively similar pools of molecularly defined neurons2. Recently, we used single cell RNA sequencing to characterize the transcriptomes of VMH neurons from male and female P10 mice of the C57BL6 background on a cell-by-cell basis. We identified 6 clusters of glutamatergic neurons that were found in both males and females33. The top most differentially expressed gene within each of the clusters was Tac1, somatostatin (Sst), Rprm, prodynorphin (Pdyn), hippocalcin-like protein 1 (Hpcal1), and galanin (Gal), respectively. To determine if some of these genes identified subsets of estrogen-sensitive neurons, we co-localized ERα with each cluster marker. In the female VMHvl, the majority of ERα immunoreactivity was restricted to the two subpopulations identified by Tac1 and Rprm, and both Tac1 and Rprm were expressed at higher levels in the VMHvl of female compared to male mice. In the male VMHvl, we detected ERα immunoreactivity in Pdyn+ neurons, along with male-biased expression of Pdyn. Together, these findings suggest that the molecular signature of estrogen-responsive neurons in the VMHvl is heterogeneous and quantitatively different between males and females.
Non-hormonal mechanisms, such as the sex chromosome complement, can also contribute to neuronal sexual differentiation, either by supplementing or opposing hormonal sex differences69. In particular, X chromosome genes regulate aspects of metabolism, including food intake and adiposity70. However, we did not find evidence that the sex chromosome complement was contributing to the sex differences in Tac1, Rprm, or Pdyn expression in the murine VMHvl33. Rather, gonadal sex was critical for determining the sex difference in Tac1 and Rprm expression, suggesting that these patterns are established during development and maintained into adulthood. Finally, testicular hormones were required in adulthood for maintenance of the sex difference in Pdyn expression, as this difference was eliminated by castration. It was previously shown that half of the genes that exhibit sex differences in the VMHvl are regulated by adult effects of sex hormones in mice20. Therefore, while we cannot rule out the possibility that sex chromosome-linked genes may regulate other aspects of VMH neurons, the combined organizational and activational effects of gonadal hormones are likely to account for the majority of the sex differences in the molecular signature among estrogen-responsive neurons in the VMHvl.
Sexual differentiation of VMH architecture
Sex hormones and estrus cycle phase can induce sex differences in cell morphology and cytoarchitecture within the VMH. In rats, the volume of the VMH is greater in males than females across all subregions, suggesting organizational effects of sex hormones on aspects of VMH architecture71. However, due to a gain in VMH volume from diestrus to proestrus, this sex difference is smaller when comparing males to proestrus females than to diestrus females, indicating additional activational effects71. Male rats that are unresponsive to androgens also show significantly smaller VMHvl volumes compared to wild-type males51. Differences in VMH volume are primarily accounted for by the larger neuronal soma sizes and neuropil volume in males than females rather than reflecting relative differences in neuronal numbers between males and females within VMH subdivisions51,71. In particular, the male rat VMHvl receives more axodendritic contacts than the female VMHvl, and this sex difference in the synaptic pattern is reversible with neonatal castration in males or testosterone treatment in females72. Greater numbers of both serotonergic and aromatase-positive projections to the VMH in male rats and mice contribute to this sex difference in VMH innervation73,74. Finally, spine density is differentially regulated by sex hormones in males and females, with estradiol decreasing spine density in the male VMH but increasing spine density in the female VMH, though the mechanism underlying this dichotomy is unknown (reviewed in 75).
Sex differences in efferent projection patterns from hormone sensitive neurons in the VMH could also contribute to the display of sexually differentiated behaviors, in particular because subpopulations of ERα+ neurons along the anterior-posterior axis of the VMHvl differ in their projection targets. One study mapped efferent projections from murine Esr1-Cre neurons in the VMHvl and surrounding regions and found that caudally positioned Esr1-Cre neurons project rostrally to the amygdala and other areas of the hypothalamus, while rostrally positioned Esr1-Cre neurons project caudally to premotor brain areas76. However, no consistent differences between the sexes were detected76. In contrast, another study mapping efferent projections specifically from the VMHvl found major projections from murine Pgr-Cre neurons in the VMHvl to the AVPV in females that were largely absent in males19. Additional studies are required to determine if efferents are sexually dimorphic overall or differentially partitioned among subpopulations. Additionally, the behavioral or physiological significance of sex-specific projection patters will require functional circuit tracing studies in both sexes.
POTENTIAL AND LIMITATIONS OF UNICELLULAR ANALYSIS
Classically, sex differences are thought to initiate with the presence or absence of the Y-linked Sry gene, which leads to differentiation of the gonads through the activation of the testis or ovary differentiation pathways. Subsequent sex differences in gonadal hormones then lead to masculinization or feminization of other tissues. However, a large body of evidence has revealed cell autonomous sex differences in tissues all over the body3. The advent of single cell profiling technology provides the exciting opportunity to dissect sex differences at the level of single cells or cell populations in any tissue, including specific regions of the brain.
Transcriptional profiling studies with single cell resolution often include samples from both male and female mice. However, few studies are designed to detect sex differences or distinguish between sex differences in gene expression or cell composition within a tissue. A recent study examined sex differences in the various cell lineages of the developing mouse gonad77. Even during the differentiation of testes or ovaries, the cells of the gonad upregulate hundreds of genes equally in both sexes and the majority of sex differences are restricted to the lineage that expresses Sry. Thus, it is not surprising that scRNA-seq within the brain also finds limited sex differences in gene expression. In the medial amygdala, GABAergic neurons exhibit sex differences in gene expression, whereas gene expression in glutamatergic neurons is neutral with respect to sex78. Of the 40–60 genes that are differentially expressed in GABAergic neurons, at least two were confirmed by histological analysis; however, it is unclear how many are due to sexual differentiation of this neuron population compared to activational effects of hormone signaling. We analyzed VMH neurons from P10 male and female mice by scRNA-seq, before activational effects of sex steroids are induced. Many transcripts were enriched in males or females but these were expressed throughout the VMH and were not robust when analyzed by histology33. Instead, spatial analyses revealed robust sex differences in a few transcripts within the VMHvl sub-region. Importantly, manipulation of single transcripts, e.g. Tac1 or Rprm, seems to alter the function of the VMHvl30,33, suggesting that wholesale differences in molecular signature are not required for sex differences in cell, neuron, or circuit function.
From these studies, it seems that sex differences in gene expression are restricted to certain cell populations and may involve a limited number of key transcripts. Therefore, sex differences in a few genes may lead to sex-specific functions without sex differences in the overall molecular signature of cells. However, the ability to detect these key differences that underlie sex differences in physiology will largely depend on the sensitivity of our methods to interrogate gene expression within sub-regions or individual cell populations. We argue that single cell resolution studies coupled with validation and functional manipulations will be crucial in our efforts to pinpoint the biological underpinnings of sex differences in physiology and behavior.
CONCLUDING REMARKS
Single cell resolution studies have revealed surprising cellular heterogeneity in a wide variety of tissues. In the VMH, it appears that the cells that mediate these sex-specific functions may be specialized, sexually differentiated, and intermingled with cells that are neutral with respect to sex. We propose that sexual differentiation contributes to the cellular heterogeneity within a tissue or brain region and leads to sex differences in some, but not all, cell populations. Some of this heterogeneity can be attributed to sex hormone receptor status, allowing for selective effects on cells that express androgen or estrogen receptors during the critical period of sexual differentiation. However, the studies reviewed here point to additional heterogeneity within hormone-sensitive cell types. Specifically, neurons that express ERα within the VMHvl form distinct subpopulations as identified by differences in molecular phenotype and activity patterns during VMH-mediated behaviors. Further studies will be necessary to determine if this molecular and functional specialization is established prior to or as a result of organizational effects of sex hormones.
It is possible that sexual differentiation builds upon pre-existing heterogeneity within hormone-sensitive cell populations to induce phenotypic diversity among cell types and between the sexes. In this framework, masculinization or feminization of a brain region is not uniform for all cell types, but rather reflects a cell-type-specific response to sex hormones during the critical period of sexual differentiation. Accordingly, we propose the use of unicellular analysis tools to identify sex differences within individual cell populations comprising a sexually dimorphic brain region. This high-resolution understanding of sex differences in the brain can be combined with hormone manipulations early in life to understand cell-type specific sexual differentiation and cell-type-specific manipulations of gene function in adulthood to pinpoint the key molecular mediators of sex-specific behaviors and physiologies.
Acknowledgements
The authors wish to thank Ed van Veen, Megan Massa, and three anonymous reviewers for constructive comments and input on the manuscript. Alison Schroeer provided artwork for Figure 1. LGK was supported by a pre-doctoral NRSA (F31 AG051381), a Hyde Fellowship, and a UCLA Dissertation Year Fellowship. The authors also acknowledge research support by the UCLA Division of Life Sciences, UCLA CTSI (NIH UL1TR001881), Iris Cantor-UCLA Women’s Health Center, and UCSD/UCLA Diabetes Research Center (NIH P30 DK063491).
Footnotes
The authors of the manuscript have no conflicts of interest to declare
REFERENCES
- 1.Forger NG, Strahan JA & Castillo-Ruiz A Cellular and molecular mechanisms of sexual differentiation in the mammalian nervous system. Front Neuroendocrinol 40, 67–86, 10.1016/j.yfrne.2016.01.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang CF & Shah NM Representing sex in the brain, one module at a time. Neuron 82, 261–278, 10.1016/j.neuron.2014.03.029 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold AP A general theory of sexual differentiation. J Neurosci Res 95, 291–300, 10.1002/jnr.23884 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forger NG et al. Deletion of Bax eliminates sex differences in the mouse forebrain. Proceedings of the National Academy of Sciences of the United States of America 101, 13666–13671, 10.1073/pnas.0404644101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zup SL et al. Overexpression of bcl-2 reduces sex differences in neuron number in the brain and spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 2357–2362 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleier R, Byne W & Siggelkow I Cytoarchitectonic sexual dimorphisms of the medial preoptic and anterior hypothalamic areas in guinea pig, rat, hamster, and mouse. J Comp Neurol 212, 118–130, 10.1002/cne.902120203 (1982). [DOI] [PubMed] [Google Scholar]
- 7.Davis EC, Popper P & Gorski RA The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain research 734, 10–18 (1996). [PubMed] [Google Scholar]
- 8.Gorski RA, Gordon JH, Shryne JE & Southam AM Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain research 148, 333–346, 10.1016/0006-8993(78)90723-0 (1978). [DOI] [PubMed] [Google Scholar]
- 9.Gorski RA Sexual dimorphisms of the brain. J Anim Sci 61 Suppl 3, 38–61, 10.1093/ansci/61.supplement_3.38 (1985). [DOI] [PubMed] [Google Scholar]
- 10.McCarthy MM, Todd BJ & Amateau SK Estradiol modulation of astrocytes and the establishment of sex differences in the brain. Ann N Y Acad Sci 1007, 283–297 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Lenz KM & McCarthy MM A starring role for microglia in brain sex differences. Neuroscientist 21, 306–321, 10.1177/1073858414536468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy MM in The Scientist 1–20 (2015).
- 13.Bale TL Sex matters. Neuropsychopharmacology 44, 1–3, 10.1038/s41386-018-0239-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joel D & McCarthy MM Incorporating Sex As a Biological Variable in Neuropsychiatric Research: Where Are We Now and Where Should We Be? Neuropsychopharmacology 42, 379–385, 10.1038/npp.2016.79 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell JN et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nature neuroscience 20, 484–496, 10.1038/nn.4495 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashikawa K, Hashikawa Y, Falkner A & Lin D The neural circuits of mating and fighting in male mice. Curr Opin Neurobiol 38, 27–37, 10.1016/j.conb.2016.01.006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632, 10.1038/nature13169 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang T et al. Social Control of Hypothalamus-Mediated Male Aggression. Neuron 95, 955–970 e954, 10.1016/j.neuron.2017.06.046 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang CF et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909, 10.1016/j.cell.2013.04.017 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X et al. Modular genetic control of sexually dimorphic behaviors. Cell 148, 596–607, 10.1016/j.cell.2011.12.018 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy MM, Kleopoulos SP, Mobbs CV & Pfaff DW Infusion of antisense oligodeoxynucleotides to the oxytocin receptor in the ventromedial hypothalamus reduces estrogen-induced sexual receptivity and oxytocin receptor binding in the female rat. Neuroendocrinology 59, 432–440, 10.1159/000126689 (1994). [DOI] [PubMed] [Google Scholar]
- 22.Nicot A, Ogawa S, Berman Y, Carr KD & Pfaff DW Effects of an intrahypothalamic injection of antisense oligonucleotides for preproenkephalin mRNA in female rats: evidence for opioid involvement in lordosis reflex. Brain research 777, 60–68 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Dornan WA, Malsbury CW & Penney RB Facilitation of lordosis by injection of substance P into the midbrain central gray. Neuroendocrinology 45, 498–506, 10.1159/000124781 (1987). [DOI] [PubMed] [Google Scholar]
- 24.Falkner AL, Grosenick L, Davidson TJ, Deisseroth K & Lin D Hypothalamic control of male aggression-seeking behavior. Nature neuroscience 19, 596–604, 10.1038/nn.4264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remedios R et al. Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature 550, 388–392, 10.1038/nature23885 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashikawa K et al. Esr1(+) cells in the ventromedial hypothalamus control female aggression. Nature neuroscience 20, 1580–1590, 10.1038/nn.4644 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridges RS, Mann PE & Coppeta JS Hypothalamic involvement in the regulation of maternal behaviour in the rat: inhibitory roles for the ventromedial hypothalamus and the dorsal/anterior hypothalamic areas. J Neuroendocrinol 11, 259–266, 10.1046/j.1365-2826.1999.00322.x (1999). [DOI] [PubMed] [Google Scholar]
- 28.Mann PE & Babb JA Disinhibition of maternal behavior following neurotoxic lesions of the hypothalamus in primigravid rats. Brain research 1025, 51–58, 10.1016/j.brainres.2004.07.064 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Spanic T, Grgurevic N & Majdic G Haploinsufficiency for Steroidogenic Factor 1 Affects Maternal Behavior in Mice. Front Behav Neurosci 10, 131, 10.3389/fnbeh.2016.00131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correa SM et al. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep 10, 62–74, 10.1016/j.celrep.2014.12.011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musatov S et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America 104, 2501–2506, 10.1073/pnas.0610787104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez de Morentin PB et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab 20, 41–53, 10.1016/j.cmet.2014.03.031 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Veen Kammel JE et al. Single cell profiling of the VMH reveals a sexually dimorphic regulatory node of energy expenditure. bioRxiv 549725, 10.1101/549725 (2019). [DOI] [Google Scholar]
- 34.Wang L et al. Hypothalamic Control of Conspecific Self-Defense. Cell Rep 26, 1747–1758 e1745, 10.1016/j.celrep.2019.01.078 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Chen IZ & Lin D Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron 85, 1344–1358, 10.1016/j.neuron.2014.12.025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva BA et al. Independent hypothalamic circuits for social and predator fear. Nature neuroscience 16, 1731–1733, 10.1038/nn.3573 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva BA et al. The ventromedial hypothalamus mediates predator fear memory. Eur J Neurosci 43, 1431–1439, 10.1111/ejn.13239 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhillon H et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49, 191–203, 10.1016/j.neuron.2005.12.021 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Foster NN, Azam S & Watts AG Rapid-onset hypoglycemia suppresses Fos expression in discrete parts of the ventromedial nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol 310, R1177–1185, 10.1152/ajpregu.00042.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santiago AM, Clegg DJ & Routh VH Estrogens modulate ventrolateral ventromedial hypothalamic glucose-inhibited neurons. Mol Metab 5, 823–833, 10.1016/j.molmet.2016.08.002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song Z, Levin BE, McArdle JJ, Bakhos N & Routh VH Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 50, 2673–2681 (2001). [DOI] [PubMed] [Google Scholar]
- 42.McClellan KM, Parker KL & Tobet S Development of the ventromedial nucleus of the hypothalamus. Front Neuroendocrinol 27, 193–209, 10.1016/j.yfrne.2006.02.002 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Ikeda Y, Nagai A, Ikeda MA & Hayashi S Sexually dimorphic and estrogen-dependent expression of estrogen receptor beta in the ventromedial hypothalamus during rat postnatal development. Endocrinology 144, 5098–5104, 10.1210/en.2003-0267 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Devidze N, Mong JA, Jasnow AM, Kow LM & Pfaff DW Sex and estrogenic effects on coexpression of mRNAs in single ventromedial hypothalamic neurons. Proceedings of the National Academy of Sciences of the United States of America 102, 14446–14451, 10.1073/pnas.0507144102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokosuka M, Okamura H & Hayashi S Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol 389, 81–93, (1997). [DOI] [PubMed] [Google Scholar]
- 46.Walker VR & Korach KS Estrogen receptor knockout mice as a model for endocrine research. ILAR J 45, 455–461, 10.1093/ilar.45.4.455 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Zuloaga DG, Zuloaga KL, Hinds LR, Carbone DL & Handa RJ Estrogen receptor beta expression in the mouse forebrain: age and sex differences. J Comp Neurol 522, 358–371, 10.1002/cne.23400 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simerly RB, Chang C, Muramatsu M & Swanson LW Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294, 76–95, 10.1002/cne.902940107 (1990). [DOI] [PubMed] [Google Scholar]
- 49.Juntti SA et al. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 66, 260–272, 10.1016/j.neuron.2010.03.024 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McAbee MD & DonCarlos LL Ontogeny of region-specific sex differences in androgen receptor messenger ribonucleic acid expression in the rat forebrain. Endocrinology 139, 1738–1745, 10.1210/endo.139.4.5940 (1998). [DOI] [PubMed] [Google Scholar]
- 51.Dugger BN, Morris JA, Jordan CL & Breedlove SM Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav 51, 195–201, 10.1016/j.yhbeh.2006.10.001 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roselli CE, Salisbury RL & Resko JA Genetic evidence for androgen-dependent and independent control of aromatase activity in the rat brain. Endocrinology 121, 2205–2210, 10.1210/endo-121-6-2205 (1987). [DOI] [PubMed] [Google Scholar]
- 53.Micevych PE & Meisel RL Integrating Neural Circuits Controlling Female Sexual Behavior. Front Syst Neurosci 11, 42, 10.3389/fnsys.2017.00042 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Musatov S, Chen W, Pfaff DW, Kaplitt MG & Ogawa S RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proceedings of the National Academy of Sciences of the United States of America 103, 10456–10460, 10.1073/pnas.0603045103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogawa S et al. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proceedings of the National Academy of Sciences of the United States of America 96, 12887–12892 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kopin AS et al. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J Clin Invest 103, 383–391, 10.1172/JCI4901 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olster DH & Blaustein JD Immunocytochemical colocalization of progestin receptors and beta-endorphin or enkephalin in the hypothalamus of female guinea pigs. J Neurobiol 21, 768–780, 10.1002/neu.480210510 (1990). [DOI] [PubMed] [Google Scholar]
- 58.Dornan WA, Akesson TR & Micevych PE A substance P projection from the VMH to the dorsal midbrain central gray: implication for lordosis. Brain Res Bull 25, 791–796 (1990). [DOI] [PubMed] [Google Scholar]
- 59.Lin D et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226, 10.1038/nature09736 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei YC et al. Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender. Nature communications 9, 279, 10.1038/s41467-017-02648-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong LC et al. Effective Modulation of Male Aggression through Lateral Septum to Medial Hypothalamus Projection. Curr Biol 26, 593–604, 10.1016/j.cub.2015.12.065 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Todd WD et al. A hypothalamic circuit for the circadian control of aggression. Nature neuroscience 21, 717–724, 10.1038/s41593-018-0126-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu Y et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14, 453–465, 10.1016/j.cmet.2011.08.009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Narita K, Murata T & Matsuoka S The ventromedial hypothalamus oxytocin induces locomotor behavior regulated by estrogen. Physiol Behav 164, 107–112, 10.1016/j.physbeh.2016.05.047 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Khodai T et al. PACAP Neurons in the Ventromedial Hypothalamic Nucleus Are Glucose Inhibited and Their Selective Activation Induces Hyperglycaemia. Front Endocrinol (Lausanne) 9, 632, 10.3389/fendo.2018.00632 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cotero VE & Routh VH Insulin blunts the response of glucose-excited neurons in the ventrolateral-ventromedial hypothalamic nucleus to decreased glucose. Am J Physiol Endocrinol Metab 296, E1101–1109, 10.1152/ajpendo.90932.2008 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cryer PE Are gender differences in the responses to hypoglycemia relevant to iatrogenic hypoglycemia in type 1 diabetes? J Clin Endocrinol Metab 85, 2145–2147, 10.1210/jcem.85.6.6659 (2000). [DOI] [PubMed] [Google Scholar]
- 68.Steinbusch LK et al. Sex-Specific Control of Fat Mass and Counterregulation by Hypothalamic Glucokinase. Diabetes 65, 2920–2931, 10.2337/db15-1514 (2016). [DOI] [PubMed] [Google Scholar]
- 69.De Vries GJ Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145, 1063–1068, 10.1210/en.2003-1504 (2004). [DOI] [PubMed] [Google Scholar]
- 70.Chen X et al. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet 8, e1002709, 10.1371/journal.pgen.1002709 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madeira MD, Ferreira-Silva L & Paula-Barbosa MM Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J Comp Neurol 432, 329–345 (2001). [DOI] [PubMed] [Google Scholar]
- 72.Matsumoto A & Arai Y Development of sexual dimorphism in synaptic organization in the ventromedial nucleus of the hypothalamus in rats. Neuroscience letters 68, 165–168 (1986). [DOI] [PubMed] [Google Scholar]
- 73.Wu MV et al. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell 139, 61–72, 10.1016/j.cell.2009.07.036 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patisaul HB, Fortino AE & Polston EK Sex differences in serotonergic but not gamma-aminobutyric acidergic (GABA) projections to the rat ventromedial nucleus of the hypothalamus. Endocrinology 149, 397–408, 10.1210/en.2007-0666 (2008). [DOI] [PubMed] [Google Scholar]
- 75.Flanagan-Cato LM Sex differences in the neural circuit that mediates female sexual receptivity. Front Neuroendocrinol 32, 124–136, 10.1016/j.yfrne.2011.02.008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lo L et al. Connectional architecture of a mouse hypothalamic circuit node controlling social behavior. Proceedings of the National Academy of Sciences of the United States of America, 10.1073/pnas.1817503116 (2019). [DOI] [PMC free article] [PubMed]
- 77.Stevant I et al. Dissecting Cell Lineage Specification and Sex Fate Determination in Gonadal Somatic Cells Using Single-Cell Transcriptomics. Cell Rep 26, 3272–3283 e3273, 10.1016/j.celrep.2019.02.069 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Chen PB et al. Sexually Dimorphic Control of Parenting Behavior by the Medial Amygdala. Cell 176, 1206–1221 e1218, 10.1016/j.cell.2019.01.024 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


