Abstract
A two-stage culture (with controlled sucrose concentrations and temperatures) of Sphingomonas paucimobilis for gellan gum production has been previously investigated. Herein, the mechanism of a two-stage culture favoring gellan gum overproduction was revealed by analysing the cell-membrane permeability and the proteomics for gellan gum biosynthesis. The two-stage culture, resulted in 79.8% increased content of unsaturated fatty acids, and 3.95% increased ratio of unsaturated to saturated fatty acids in the cell membrane. Moreover, cell membrane permeability increased and thus further enhanced gellan gum biosynthesis. Proteomic analysis results indicated that 13 identified protein spots were involved in energy generation, glycogen biosynthesis, and glycolysis. These findings revealed that two-stage culture impellel carbon flux flow toward gellan gum biosynthesis.
Keywords: Two-stage culture, Gellan gum, Sphingomonas paucimobilis, Proteomics
Introduction
Gellan gum, a linear polysaccharide, is composed of tetrasaccharide repeating units including d-glucose, l-rhamnose, and d-glucuronic acid at the ratio of 2:1:1 (Fialho et al. 2008). Due to its unique structure and excellent physical properties, gellan gum has been widely used as emulsifier, stabilizer, gelling agent, film former and thickening agent in the food industry (Alizadeh-Sani et al. 2019; Morris et al. 2012; Prajapati et al. 2013; Zia et al. 2018). In recent years, the demand for this unique biopolymer has been increasing.
Many microorganisms, such as Sphingomonas pseudosanguinis, Sphingomonas yabuuchiae and Sphingomonas paucimobilis NK 2000 produce gellan gum (Raghunandan et al. 2018; Jin et al. 2003). Among these microorganisms, S. paucimobilis is a typical strain producing gellan gum that is non-toxic and non-pathogenic (Dolan et al. 2016). Moreover, the yield of gellan gum could be improved by screening the strains with high performance and optimizing the fermentation conditions (Jin et al. 2003; West 2002; Zhang et al. 2015; Zhu et al. 2019). The concentration of carbon source and temperature are critical factors for high level production of gellan gum. Low sucrose concentration and high temperature are favorable for cell growth, but inhibits gellan gum biosynthesis. Therefore, two-stage culture strategy was developed to tackle the problem. The initial sucrose concentration was 10 g/L, to which 10 g/L of sucrose was added at 12 h and 24 h. The temperatures for the first-stage culture and the second-stage culture were 33 °C, and 28 °C, respectively. Gellan gum production was 35.71% higher than that of conventional batch culture. High gellan gum yield is related to high UDPG-pyrophosphorylase and glucosyltransferase activities (Zhu et al. 2013). However, the detailed mechanisms of improving the production by two-stage culture strategy remain still unclear.
In the present study, the permeability of cell membrane was determined by analyzing the fatty acid composition of S. paucimobilis cells. To discover proteins associated with different culture strategies, we performed proteomics to identify the proteins that were significantly up-regulated or down-regulated in the two-stage and conventional batch cultures. Our results could reveal the mechanism of two-stage culture strategy and supply a new perspective to understand the physiological response of gellan gum over-producing strains in the field of industrial fermentation.
Materials and methods
Microorganism and media
Sphingomonas paucimobilis ATCC 31461 was used in this study. The strain was maintained by monthly subculture on agar slants containing 3 g/L beef extract, 10 g/L peptone, 5 g/L NaCl, and 2 g/L agar at pH 7.4. The same medium without agar was used for seed cultivation. The seed was prepared in 250 mL flasks containing 50 mL of seed medium, and incubated in a rotary shaker at 200 rpm with a temperature of 30 °C for 24 h. The medium for shake flask culture contained 30 g/L sucrose, 1 g/L yeast extract, 2 g/L peptone, 3 g/L KH2PO4, 1 g/L K2SO4, 1 g/L K2HPO4, and 1 g/L MgSO4·7H2O at pH 7.0.
Shake flask culture conditions
Flask culture experiments were performed in 250 mL flasks each containing 50 mL medium after inoculating with 10% (v/v) of seed culture. The culture conditions of agitation rate, and growth period were fixed at 200 rpm and 72 h, respectively.
In the two-stage culture strategy experiment, a pulse type fed-batch culture was used to 10 g/L sucrose added for 12 and 24 h. The batch culture had an initial sucrose concentration of 10 g/L. The first-stage control strategy was performed to realize fast cell growth within 24 h. The batch culture of S. paucimobilis ATCC 31,461 was initiated in the second stage. The temperature for the first-stage and second-stage culture was 33 °C, and 28 °C, respectively.
Lipid analysis
Fatty acids methyl esters were analyzed by gas chromatography as reported by Ahn et al. (2012).
Protein sample preparation for 2-D PAGE
Harvested cells were centrifuged at 15,000g for 20 min at 4 °C. The cell pellets were washed with ultrapure water three times and stored at − 80 °C.
Cells were suspended in acetone including 0.07% (w/v) dithiothreitol and10% (w/v) trichloroacetic acid and incubated at − 20 °C overnight. Proteins were extracted for 1 h at room temperature by vortex mixing. After centrifugation at 15,000g for 1 h at 4 °C, insoluble material was discarded, and soluble fraction was used for two-dimensional gel electrophoresis. The concentration of protein was determined using the Bradford method (Bradford 1976).
2D PAGE
For 2-DE, 24 cm IPG strip (immobiline drystrip) with linear gradient pH range (pH 3–11) was selected. The protein extracts (250 μg/gel) were loaded. 2D PAGE was performed as described by Sheng et al. (2014).
Image analysis
Protein spots were performed using the PDQuest (version 7.0, BioRad) software according to the manufacturers’ protocols. The quantity of each spot was normalized based on total valid spot intensity. Protein spots were identified as a significant expression variation if their expression level deviated over two folds compared with control or normal sample. Silver-stained gels were scanned using an Image Scanner (Amersham Biosciences). Data analysis was carried using the Image Master 2D Platinum 5.0 software (Amersham Biosciences). For each treatment, three gels from three independent treatments were studied. Gel spots were automatically tested, manually edited and deleted when necessary. Evidently spots were marked by calibrating and matching the gel. Next, the three replicate gels were compared to one another and merged into a master gel, containing the spots distributed on the three gels for every independent experiment. To quantify and contradistinguish the protein spots, we standardized the volume ratio. Significantly changed spots were chosen with at least a 0.2-fold decrease and 5.0-fold increase in the normalized volume ratio.
In-gel digestion
The differentially expressed protein spots were excised for digestion. The gel spot was washed, and the destaining solution was removed. Exactly 50% acetonitrile (ACN) and 100% CAN were successively added for 5 min. The samples were rehydrated in 2 − 4 μL of mass spectroscopy grade gold trypsin solution (Promega, Madison, WI, USA, 20 μg/mL in 25 mM NH4HCO3) for 30 min. Then, 20 μL of cover solution (25 mM NH4HCO3, 10% ACN) was added for digestion for 16 h at 37 °C. The supernatant was shifted to another tube, and the gel was extracted with 50 μL of extraction buffer containing 67% ACN and 5% trifluoroacetic acid (TFA). The supernatant of the gel spot and the peptide extracts was combined and freeze-dried for mass spectrometry.
Mass spectrometry analysis
The freeze-dried powder was redissolved in 5 μL of 0.1%TFA, and mixed with a saturated solution of α-cyano-4-hydroxycinnamic acid containing 50% ACN and 1% TFA at a ratio of 1:1. Exactly 1 μL of the sample was obtained for mass spectrometry point-of-target identification the positive ion mode in which data were automatically acquired. Mass spectrometry data were analyzed using GPS Explorer V3.6 (Applied Biosystems) and Mascot 2.3 (Matrix Science), and protein was identified using primary and secondary mass spectral data integration. The search parameters are as follows: the database was NCBInr-Funji, the enzyme was trypsin, the maximum missing cleavage site was 1, the fixed modification was carbamidomethyl (C), the variable modification was acetyl (ProteinN-term), deamidated (NQ), dioxidation (W) and oxidation (M), MS tolerance was 100 ppm and MS/MS tolerance was 0.5 Da. A protein score of C.I. % greater than 95% was successfully identified.
Results and discussion
Permeability of cell membrane
During gellan gum biosynthesis by S. paucimobilis, sugar-activated precursors are fistly synthesized in the cell, and then a tetrasaccharide repeat units is assembled, polymerized and transported out of the cell (Sá-Correia et al. 2002; Vartak et al. 1995). The permeability of the cell membrane affects the rate of gellan gum transport. The higher the permeability, the faster the transport rate is, and the higher the gellan gum production is (Santhiagu and Rathindra 2008). The permeability of the cell membrane is related to its lipid composition. The fatty acids in the cell membrane are mainly composed of palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), oleic acid (C18:1) and linoleic acid (C18:2) (Sheng et al. 2013). The permeability of cell membrane is highly dependent on the structure and relative content of unsaturated fatty acids. Plyunsaturated fatty acids play an important role in maintaining the structure and function and determining the fluidity of cell membranes (Yehuda et al. 2002; Mykytczuk et al. 2007).
Table 1 shows the fatty acid composition of the cells grown under two-stage culture during the late-logarithmic phase. The enhancement of gellan gum production was associated with changes in fatty acid composition in the cells. The major fatty acids contained in the cells were palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), oleic acid (C18:1) and linoleic acid (C18:2). The content of saturated fatty acids was 20.2%, while that of unsaturated fatty acids was 79.8% under two-stage culture. The ratio of unsaturated/saturated fatty acids increased from 2.57 to 3.95 under conventional batch and two-stage culture.
Table 1.
Fatty acid composition of S. paucimobilis cells grown under different cultures
| Fatty acids | % Total fatty acids | |
|---|---|---|
| Control | Two-stage culture | |
| C16:0 | 21.9 ± 1.2 | 17.6 ± 0.8 |
| C16:1 | 1.1 ± 0.9 | 5.2 ± 1.3 |
| C18:0 | 6.1 ± 0.7 | 2.6 ± 0.6 |
| C18:1 | 38.5 ± 0.4 | 45.4 ± 1.2 |
| C18:2 | 32.4 ± 1.1 | 29.2 ± 0.9 |
| Unsaturated/saturated | 2.57 | 3.95 |
The fatty acid composition of the cell membrane is also affected by temperature. When the temperature is low, the composition of unsaturated fatty acids in the cell increases, which elevates the fluidity of the membrane to adapt to the external environment (Shimiziu et al. 1988). High content of unsaturated fatty acids in plasma membrane, especially polyunsaturated fatty acids, decreases membrane viscosity and increases fluidity. Moreover, the polysaccharide is more secreted to outside of the cell. In the mechanism of improving the gellan gum production by the two-stage culture mass transfer is possibly affected either by changing the surface film resistance or the hydrodynamics.
Comparative proteomics
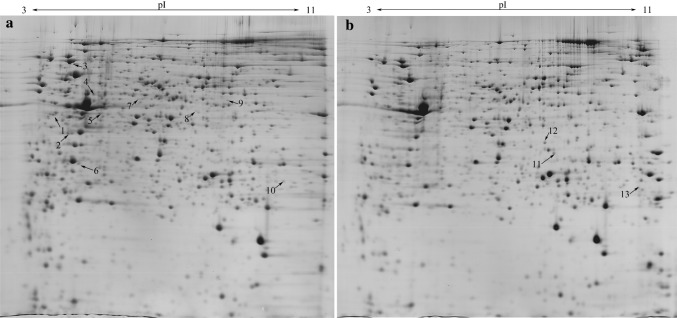
To further explore the metabolisms of S. paucimobilis by different culture, we analyzed the changes of expressed proteins. Two-stage and conventional batch cultures were performed in fermentation the medium, and proteins were extracted in the late-logarithmic phase. The protein extracts from two culture conditions were resolved on 2D-PAGE to visualize a considerable protein separation pattern. The representational gel maps of the proteins are shown in Fig. 1. As analyzed through Image Master, approximately 1000 visualized spots were found on the gels.
Fig. 1.
Comparative 2-DE gel analyses of cellular proteins of S. paucimobilis ATCC 31461cultivated in the conventional batch culture (a) and two-stage culture (b). Protein spots marked on the maps with arrows and numbers represent the 13 identified proteins analyzed with MALDI-TOF/TOF
In the MALDI-TOF/MASS analysis, the 21 most differential protein spots were matched with the proteins of other organisms, and 13 proteins were successfully identified (Fig. 1), and more detailed information about identified proteins is shown in Table 2.
Table 2.
Identification of differentially expressed protein spots from S. paucimobilis cultivated in the conventional batch culture (a) and two-stage culture (b) by proteomic analysis with 2D-GE and MALDI-TOF/TOF MS
| Spot no |
Accession no | Protein name | Organism | Mascot score |
Coverage (%) |
Theor Mw | Theor pI |
|---|---|---|---|---|---|---|---|
| 1 | gil460259 | Enolase | Bacillus subtilis subsp. subtilis str. 168 | 293 | 24 | 46,609 | 4.7 |
| 2 | gil385150300 | Malate dehydrogenase | Bacillus sp. 5B6 | 675 | 30 | 34,756 | 5.52 |
| 3 | gil4982302257 | X-Pro aminopeptidase | Sphingomonas elodea | 869 | 26 | 63,869 | 5.1 |
| 4 | gil518199993 | ATP-dependent protease | Sphingomonas sp. ATCC 31,555 | 450 | 18 | 47,271 | 5.19 |
| 5 | gil489312559 | Glyceraldehyde-3-phosphate dehydrogenase | Bacillales | 255 | 32 | 35,924 | 5.2 |
| 6 | gil489246620 | d-aminopeptidase | Bacillus amyloliquefaciens | 175 | 12 | 30,467 | 5.21 |
| 7 | gil489314523 | Serine hydroxymethyltransferase | Bacillus subtilis | 485 | 23 | 45,490 | 5.49 |
| 8 | gil657921822 | RNA methyltransferase | Sphingomonas elodea | 529 | 27 | 42,254 | 6.05 |
| 9 | gil498230410 | Acyl-CoA dehydrogenase | Sphingomonas elodea | 531 | 23 | 47,278 | 6.32 |
| 10 | gil657921661 | Pseudouridine synthase | Sphingomonas elodea | 122 | 13 | 24,374 | 8.74 |
| 11 | gil736961193 | ATPase AAA | Erythrobacter longus | 334 | 19 | 32,867 | 5.24 |
| 12 | gil498230141 | Hypothetical protein | Sphingomonas elodea | 490 | 22 | 58,346 | 6.96 |
| 13 | gil498230041 | Xylose isomerase | Sphingomonas elodea | 761 | 46 | 30,924 | 9.17 |
Ten proteins were down-regulated, whereas three were up-regulated under two-stage culture (Table 2) among the protein sequences obtained. Down-regulated proteins contained enolase, malate dehydrogenase, X-Pro aminopeptidase, ATP-dependent protease, glyceraldehyde-3-phosphate dehydrogenase, d-aminopeptidase, serine hydroxymethyl ransferase, RNA methyltransferase, and acyl-CoA dehydrogenase pseudouridine synthase. These proteins might be associated with basic metabolic enzymes. By comparison, ATPase AAA, hypothetical protein, and xylose isomerase were up-regulatied.
Glyceraldehyde 3-phosphate dehydrogenase catalyzes the sixth step of glycolysis (Tarze et al. 2007). Enolase is an enzyme responsible for the catalysis of the conversion of 2-phosphoglycerate to phosphoenolpyruvate, the ninth and penultimate step of glycolysis (Zhang et al. 1997). Thus glyceraldehyde 3-phosphate dehydrogenase and enolase break down glucose for energy and carbon molecules. Our results indicated that the conventional batch culture up-regulated glyceraldehyde 3-phosphate dehydrogenase and enolase protein, leading to the carbon flux toward glycolytic pathway for the growth of cell.
By contrast, ATPase AAA, hypothetical protein, and xylose isomerase were up-regulated. Xylose isomerase catalyzes the interconversion of d-xylose and d-xylulose (Katz et al. 2006), which could provide raw materials for the synthesis of gellan gum. Hence, these enzymes were involved in gellan gum biosynthesis. The results showed that the metabolic flux from the glycolytic pathway was converted to the gellan gum biosynthesis pathway.
Conclusion
On basis of the lipid composition of the cell membrane, and the changes of expressed protein analysis by comparative proteomics, the mechanism of improving gellan gum production by two-stage culture was revealed. Proteins that were significantly up-regulated or down-regulated in different cultures were identified. Results indicated that two-stage culture increased permeability of cell membrane and impelled carbon flux flow toward gellan gum biosynthesis. The approaches presented in this study were helpful to understanding the mechanism of other polysaccharide overproduction by culture optimization and regulation.
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (31401657).
Authors' contributions
GZ is responsible for experiment design. XC is responsible for revise the manuscript. ZZ is responsible for data analysis. ZF is responsible for instrument operation. QT is responsible for providing overall ideas.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Ahn JW, Hwangbo K, Lee SY, Choi HG, Park YI, Liu JR, Jeong WJ. A new arcticchlorellaspecies for biodiesel production. Bioresource Technol. 2012;125:340–343. doi: 10.1016/j.biortech.2012.09.026. [DOI] [PubMed] [Google Scholar]
- Alizadeh-Sani Mahmood, Ehsani Ali, Moghaddas Kia Ehsan, Khezerlou Arezou. Microbial gums: introducing a novel functional component of edible coatings and packaging. Applied Microbiology and Biotechnology. 2019;103(17):6853–6866. doi: 10.1007/s00253-019-09966-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid sensitive method for the quantitation of microgram quantities utilizing the principle of protein-dye binding. Anal.Biochem. 1976;1976(72):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dolan LC, Matulka RA, Lebeau AL, Boulet JM. Two new nontoxic, non-pathogenic strains of sphingomonas elodea for gellan gum production. Regul Toxicol Pharm. 2016;78:37–44. doi: 10.1016/j.yrtph.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Fialho AM, Moreira LM, Granja AT, Popescu AO, Sá-Correia I. Occurrence, production, and applications of gellan: current state and perspectives. Appl Microbiol Biotechnol. 2008;79:889–900. doi: 10.1007/s00253-008-1496-0. [DOI] [PubMed] [Google Scholar]
- Jin Hyuck, Lee Nam-Kyu, Shin Myung-Kyo, Kim Sung-Koo, Kaplan David L, Lee Jin-Woo. Production of gellan gum by Sphingomonas paucimobilis NK2000 with soybean pomace. Biochemical Engineering Journal. 2003;16(3):357–360. doi: 10.1016/S1369-703X(03)00076-7. [DOI] [Google Scholar]
- Katz AK, Li X, Carrell HL, Hanson BL, Langan P, Coates L, Schoenborn BP, Glusker JP, Bunick GJ. Locating active-site hydrogen atoms in D-xylose isomerase: time-of-flight neutron diffraction. Proc Natl Acad Sci USA. 2006;103:8342–8347. doi: 10.1073/pnas.0602598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ER, Nishinari K, Rinaudo M. Gelation of gellan-a review. Food Hydrocolloid. 2012;28:373–411. doi: 10.1016/j.foodhyd.2012.01.004. [DOI] [Google Scholar]
- Mykytczuk NCS, Trevors JT, Leduc LG, Ferroni GD. Fluorescence polarization in studies of bacterial cytoplasmic membrane fluidity under environmental stress. Prog Biophys Mol Bio. 2007;95:60–82. doi: 10.1016/j.pbiomolbio.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Pan S, Yao D, Chen J, Wu S. Influence of controlled pH on the activity of UDPG-pyrophosphorylase in Aureobasidium pullulans. Carbohydr Polym. 2013;92:629–632. doi: 10.1016/j.carbpol.2012.08.099. [DOI] [PubMed] [Google Scholar]
- Prajapati VD, Jani GK, Zala BS, Khutliwala TA. An insight into the emerging exopolysaccharide gellan gum as a novel polymer. Carbohydr Polym. 2013;93:670–678. doi: 10.1016/j.carbpol.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Raghunandan K, Kumar A, Kumar S et al (2018) 3 Biotech 8:71. 10.1007/s13205-018-1096-3 [DOI] [PMC free article] [PubMed]
- Sá-Correia I, Fialho AM, Videira P, Moreira LM, Marques AR. Gellan gum biosynthesis in Sphingomonas paucimobilis ATCC 31461: genes, enzymes and exopolysaccharide production engineering. J Ind Microbiol Biotechnol. 2002;29:170–176. doi: 10.1038/sj.jim.7000266. [DOI] [PubMed] [Google Scholar]
- Santhiagu A, Rathindra MB. Optimization of gellan gumproduction by Sphingomonas paucimobilis ATCC 31461 with nonionic surfactants using central composite design. J Biosci Bioeng. 2008;105:204–210. doi: 10.1263/jbb.105.204. [DOI] [PubMed] [Google Scholar]
- Sheng L, Zhu G, Tong Q. Mechanism study of Tween 80 enhancing the pullulan production by Aureobasidium pullulans. Carbohydr Polym. 2013;97:121–123. doi: 10.1016/j.carbpol.2013.04.058. [DOI] [PubMed] [Google Scholar]
- Sheng Long, Zhu Guilan, Tong Qunyi. Comparative Proteomic Analysis of Aureobasidium pullulans in the Presence of High and Low Levels of Nitrogen Source. Journal of Agricultural and Food Chemistry. 2014;62(43):10529–10534. doi: 10.1021/jf503390f. [DOI] [PubMed] [Google Scholar]
- Shimiziu S, Kawashima H, Shinmen Y, Akimoto K, Yamada H. J Am Oil Chem Soc. 1988;65:1455–1459. doi: 10.1007/BF02898307. [DOI] [Google Scholar]
- Tarze A, Deniaud A, Le Bras M, Maillier E, Molle D, Larochette N, Zamzami N, Jan G, Kroemer G, Brenner C. GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization. Oncogene. 2007;26:2606–2620. doi: 10.1038/sj.onc.1210074. [DOI] [PubMed] [Google Scholar]
- Vartak NB, Lin CC, Cleary JM, Fagan MJ, Jr, SM Glucose metabolism in 'sphingomonas elodea': pathway engineering via construction of a glucose-6-phosphate dehydrogenase insertion mutant. Microbiology. 1995;141:2339–2350. doi: 10.1099/13500872-141-9-2339. [DOI] [PubMed] [Google Scholar]
- West T P. Isolation of a mutant strain of Pseudomonas sp. ATCC 31461 exhibiting elevated polysaccharide production. Journal of Industrial Microbiology and Biotechnology. 2002;29(4):185–188. doi: 10.1038/sj.jim.7000278. [DOI] [PubMed] [Google Scholar]
- Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging. 2002;23:843–853. doi: 10.1016/S0197-4580(02)00074-X. [DOI] [PubMed] [Google Scholar]
- Zhang E, Brewer JM, Minor W, Carreira LA, Lebioda L. Mechanism of enolase: the crystal structure of asymmetric dimer enolase-2-phospho-D-glycerate/enolase-phosphoenolpyruvate at 2.0 A resolution. Biochemistry. 1997;36:12526–12534. doi: 10.1021/bi9712450. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dong YC, Fan LL, Jiao ZH, Chen QH. Optimization of culture medium compositions for gellan gum production by a halobacterium sphingomonas paucimobilis. Carbohydr Polym. 2015;115:694–700. doi: 10.1016/j.carbpol.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Zhu G, Sheng L, Tong Q. A new strategy to enhance gellan production by two-stage culture in Sphingomonas paucimobilis. Carbohydr Polym. 2013;98:829–834. doi: 10.1016/j.carbpol.2013.06.060. [DOI] [PubMed] [Google Scholar]
- Zhu G, Guo N, Yong Y, et al. Effect of 2-deoxy-d-glucose on gellan gum biosynthesis by Sphingomonas paucimobilis. Bioprocess Biosyst Eng. 2019;42:897–900. doi: 10.1007/s00449-019-02078-w. [DOI] [PubMed] [Google Scholar]
- Zia Khalid Mahmood, Tabasum Shazia, Khan Muhammad Faris, Akram Nadia, Akhter Naheed, Noreen Aqdas, Zuber Mohammad. Recent trends on gellan gum blends with natural and synthetic polymers: A review. International Journal of Biological Macromolecules. 2018;109:1068–1087. doi: 10.1016/j.ijbiomac.2017.11.099. [DOI] [PubMed] [Google Scholar]