Abstract
Introduction:
Females have a reduced risk of Parkinson’s disease (PD). However, it is unclear if sex is a prognostic factor. We aimed to examine differences in presentation, physician- and patient-reported PD outcomes, and progression by sex in a large clinical cohort.
Methods:
This study was a secondary analysis of a cohort of PD patients seen at a tertiary care center. Sociodemographic and clinical characteristics, treatment, care timing, and outcomes were examined by sex. Sex differences in progression of impairment, disability, and health-related quality of life (HRQoL) were tested with five-year piecewise linear mixed-effects models. A mediation analysis assessed drivers of sex differences.
Results:
The study included 914 males and 549 females. Females had significantly less social support, more psychological distress, and worse self-reported (but not physician-reported) disability and HRQoL at initial PD care visits, compared to males. Addressing anxiety symptoms may attenuate this difference. PD progression sex differences were minimal.
Conclusion:
PD progression does not differ by sex, yet patient-reported measures of disease severity are worse in females than males. To attenuate this sex difference in disease experience, psychological distress screening and management, particularly targeting females, should be implemented as part of PD clinical care.
Keywords: Parkinson’s Disease, Sex Differences, Activities of Daily Living, Quality of Life, Patient Reported Outcome Measures
INTRODUCTION
Males have 1.4 to 3.7 times the risk of developing Parkinson’s disease (PD), compared to females [1]. The etiology behind sex differences is likely multifactorial [2]. The neuroprotective effect of estrogen, genetic factors, differences in brain development/function, and differences in environmental exposures/lifestyle factors are potential explanations for this difference [1,2]. Risk factors for the development of PD are not necessarily prognostic factors, or factors that influence the disease course [3].
Sex may influence PD progression; however, findings are mixed on whether either or neither sex fares worse [4,5]. Sex differences in underlying biology [1], disease presentation and symptoms [1,6,7], referral barriers [8], treatment response [9], and disease management [9] may impact disease progression. Previous studies of PD progression often extrapolate longitudinal progression from cross-sectional data [10], assume progression is constant and linear over time [10], or have limited patient follow-up [4].
The impact of sex on progression may be domain specific. Previous studies have emphasized motor symptoms, or impairments; however, other outcomes may be more important to patients and their care teams [3]. Other outcomes include disability, health-related quality of life (HRQoL), nonmotor symptoms, and mortality [3,11]. Physician-reported disease severity based on the Unified Parkinson’s Disease Rating Scale (UPDRS) [12] may show discordance with patient-reported severity, due to differences in clinician versus patient perception [9,11,13].
To inform patient prognostic counseling and clinical management, this study aims to 1) examine whether male and female patients at a tertiary care center present or progress differently in multiple outcome domains and 2) examine underlying drivers of any found differences.
We hypothesized that females will have worse disease at presentation due to care delays but will have more mild disease progression.
METHODS
Study Setting and Sample
This study was a secondary analysis of data from the University of Maryland PD and Movement Disorders Center Health Outcomes Measurement (HOME) Study. The HOME study is a naturalistic cohort study with over 15 years of longitudinal data from patient visits. Some patients with a prevalent PD diagnosis are referred to the Center while others are initially diagnosed at the Center. As part of routine clinical care, patient and physician-reported scales are administered at each visit and pertinent medical information is recorded. Per the University of Maryland Institutional Review Board approved protocol, adult patients may consent to have their data entered into the HOME study database for research purposes.
For the current study, final analysis was restricted to patients with a PD diagnosis confirmed by one of the Center’s movement disorder specialists. Data was restricted to PD patient visits between April 1, 2002 and December 31, 2016. Eligible patients had at least one observation for all study outcome measures and information about at least one key date (diagnosis, symptom onset, or levodopa start year).
Variables
The independent variable was sex. The time scale used for progression models was years since diagnosis. For individuals with missing diagnosis dates, symptom and levodopa start dates, if available, were leveraged to impute missing diagnosis dates. Imputation algorithms were based on median intervals between the three dates. Intervals were calculated by sex and, due to potential treatment differences, by age [14]. Diagnosis occurred one year after symptom onset, regardless of age. Diagnosis occurred the same year that levodopa was started for those age ≥60 years and one year after diagnosis for those age <60 years. Intervals did not differ by sex.
Two other key date variables included first visit and initial PD visit dates. The first visit refers to the first time the patient was seen at the Center, irrespective of PD diagnosis. The initial PD visit is the first time the patient was seen at the Center with a PD diagnosis. For example, a patient could have been seen at the Center in 2009 for neurologic symptoms (first visit) but not diagnosed with PD until 2011 (initial PD visit).
Outcomes (Disease Severity)
Impairment
Impairment was captured with the physician-reported UPDRS motor sub-scale score (range=0–108, higher=worse) [12].
Disability
Patient-reported disability was assessed with the Older Americans Resource and Services Multidimensional Functional Assessment Questionnaire (OARS) Activities of Daily Living (ADLs) sub-section score and the OARS Instrumental Activities of Daily Living (IADLs) subsection score [15]. The questionnaire rates seven ADLs and seven IADLs, ranking the amount of difficulty performing each activity in the past week as 1 (no difficulty) through 5 (completely unable). Physician-reported disability was assessed with the Schwab & England ADL Scale (S&E) [16].
Health-Related Quality of Life
HRQoL was captured with the patient-reported 12 Item Short Form Health Survey (SF-12) [17]. Both mental health (SF-12 MH) and physical health (SF-12 PH) summary scores were used as outcomes. Responses were normalized to population T-scores (range=0–100; mean=50, SD=10; lower=worse).
Covariates
General
Covariates included sociodemographics (age at diagnosis, age at initial PD visit, race/ethnicity, education, marital status, living arrangement), clinical characteristics (Jankovic subtypes [tremor dominant, postural instability/gait difficulty, or indeterminate] [18], treatment complications, psychological distress, comorbidities, cognition, Hoehn & Yahr stage [19]), and treatment (deep brain stimulation, sex of clinician, carbidopa/levodopa use, dopaminergic drug use [levodopa, dopamine agonists, amantadine, monamine oxidase type B inhibitors, or catechol-O-methytransferase inhibitors]). Mortality was also examined.
The UPDRS Complications of Therapy section assessed treatment complications (dyskinesias, motor fluctuations, anorexia/nausea/vomiting, sleep disturbances, and orthostasis) [12]. Psychological distress was captured with the Brief Symptom Inventory-18 (BSI-18) [20], which separately assesses somatization, anxiety, and depression symptoms over the past week; scores are converted to normalized T-scores (higher=worse). Comorbidities were captured as the number of comorbidities endorsed on the Cumulative Illness Rating Scale-Geriatric (CIRS-G) (range=0–14) [21]. Cognition was assessed with the Mini Mental State Examination (MMSE) from 2002 to 2009 and the Montreal Cognitive Assessment (MoCA) from the end of 2009 onward. Although the MoCA is a preferred screening tool in PD [3], a PD-specific algorithm converted MoCA scores to MMSE scores [22].
Mediators
Mediators were chosen a priori and included treatment complications, cognition, comorbidities, psychological distress, and disease duration at first visit.
Statistical Analysis
Descriptive
All analysis was conducted in SAS v9.4 (Cary, NC). Differences between males and females for all covariates and outcome measures were examined at initial PD visits at the Center as well as at diagnosis.
Progression Models
To examine the rate of disease progression for each outcome by sex, linear mixed effects models were constructed (SAS procedure HPMIXED). To allow for non-uniform progression over time, progression was modeled with piecewise linear splines. Knots were placed at five-year intervals, the smallest interval that still afforded estimate stability, with a final knot placed at 20 years. Random intercept and slopes were added to the model to account for heterogeneity in disease severity at diagnosis and in annual progression, respectively. An unstructured correlation structure for the random effects was chosen. To capture the initial effect of specialty care, a term for the first year of clinical care at the Center was entered into the model as a fixed effect. To test for sex differences, sex was entered as a main effect, as a sex-time interaction term for each spline, and as a sex-first year of clinical care interaction. We also tested whether there were sex differences in the amount of inter-individual heterogeneity with likelihood ratio Chi-square testing of nested models. The final models were unadjusted as adjustment for any covariates, including medications, would constitute adjustment for factors on the causal pathway between sex and disease severity.
Elucidating Underlying Drivers of Sex Differences
To examine care delays, we calculated the differences in time between key dates (symptom onset, diagnosis, first visit, initial PD visit, levodopa initiation, and DBS treatment) by sex.
Based on study findings, a mediation analysis was conducted to assess if sex differences in patient-reported outcomes at initial PD visits could be attenuated. Linear regression models characterized the association between sex and all four patient-reported outcomes. A subsequent mediation analysis calculated controlled direct effects (CDE) [23]. A CDE is the effect of the independent variable if the population had the mediator set to, or controlled to, the same value for all individuals [23]. Mediators were examined individually with adjustment for mediator-outcome confounders.
To explore the effect of estrogen exposure proxies on PD progression, we conducted a subset analysis of post-menopausal females. We examined the identical sex progression models but replaced sex with individual reproductive health characteristics, including menopause type (natural versus surgical; natural indicating greater cumulative estrogen exposure [24]), parity (parous versus nulliparous; parous indicating greater exposure), and hormone replacement therapy (HRT) (current versus ever/never). We also adjusted for birth year and median income.
RESULTS
The University of Maryland PD and Movement Disorders Center saw 2,696 PD patients during the study period. Of those, 72.3% (n=1,949) consented to participate in the HOME study; 486 were excluded due to missing outcome measures (n=413) and key date variables (n=73). Diagnosis date was imputed for 146 patients. Of 1,463 subjects, 914 males contributed 8,705 visits and 549 females contributed 5,115 visits. The median duration of follow-up and visits was 7.9 years and 7.0 visits, respectively
In total, 413 (28.3%) of patients were initially diagnosed at the Center and the median time from diagnosis to initial PD visits at the Center was 2.3 years (IQR=6.7). Table 1 displays the differences in sociodemographics, clinical characteristics, treatment, and outcomes between sexes at diagnosis as well as at initial PD visits. At diagnosis, females were more likely to be less educated, unmarried, residing alone, more anxious, seen by a female neurologist, taking a dopaminergic drug, and faced with greater IADL disability (p<0.05). At initial PD visits, the same differences were found (p<0.05), with the exception of anxiety and dopaminergic drug use. Additionally, females were older and more likely to have treatment complications, greater somatization, greater depressive symptoms, and a more advanced Hoehn & Yahr stage (p<0.05). At initial PD visits, females had worse ratings on all patient-reported health outcome measures.
Table 1.
Descriptive characteristics of sample at diagnosis (when diagnosed at the Center) and at initial Parkinson’s disease (PD) visit (irrespective of time since diagnosis), stratified by sex.
| Diagnosis | Initial PD Visit | |||||
|---|---|---|---|---|---|---|
| Males (n=255) |
Females (n=158) |
p-value | Males (n=914) |
Females (n=549) |
p-value | |
| Sociodemographics | ||||||
| Age, mean (SD) | 63.1 (10.19) | 63.4 (11.14) | 0.749* | 64.5 (10.37) | 65.7 (10.97) | 0.038* |
| Age at Diagnosis | N/A | N/A | N/A | 60.3 (11.08) | 61.1 (11.39) | 0.172* |
| Race/Ethnicity | 0.789‡ | 0.423‡ | ||||
| Non-Hispanic White | 196 (88.7) | 129 (89.6) | 761 (92.4) | 451 (91.1) | ||
| Other | 25 (11.3) | 15 (10.4) | 63 (7.7) | 44 (8.9) | ||
| Education, n (%) | 0.034‡ | <0.001‡ | ||||
| High School or Less | 51 (22.4) | 49 (34.3) | 176 (21.1) | 174 (35.0) | ||
| Trade/Some College/College | 96 (42.1) | 55 (38.5) | 325 (39.0) | 190 (38.2) | ||
| Advanced Degree | 81 (35.5) | 39 (27.3) | 332 (39.9) | 133 (26.8) | ||
| Marital Status, n (%) | <0.001‡ | <0.001‡ | ||||
| Married | 215 (85.0) | 109 (69.4) | 770 (84.7) | 355 (65.1) | ||
| Single | 14 (5.5) | 9 (5.7) | 44 (4.8) | 25 (4.6) | ||
| Divorced/Separated | 18 (7.1) | 20 (12.7) | 66 (7.3) | 63 (11.6) | ||
| Widowed | 6 (2.4) | 19 (12.1) | 29 (3.2) | 102 (18.7) | ||
| Living Arrangement, n (%) | <0.001§ | <0.001‡ | ||||
| Alone | 20 (8.8) | 25 (16.6) | 64 (8.2) | 103 (21.6) | ||
| Spouse or Partner | 201 (88.2) | 107 (70.9) | 682 (87.1) | 317 (66.3) | ||
| Other Family | 4 (1.8) | 14 (9.3) | 24 (3.1) | 44 (9.2) | ||
| Other | 3 (1.3) | 5 (3.3) | 13 (1.7) | 14 (2.9) | ||
| Clinical Characteristics | ||||||
| Jankovic Subtype, n (%) | 0.183‡ | 0.752‡ | ||||
| Tremor Dominant | 97 (51.9) | 75 (61.5) | 253 (37.6) | 148 (36.1) | ||
| Postural Instability/Gait Dominant | 69 (36.9) | 33 (27.1) | 364 (54.1) | 223 (54.4) | ||
| Indeterminate | 21 (11.2) | 14 (11.5) | 56 (8.3) | 39 (9.5) | ||
| Treatment Complications, mean (SD) | 0.4 (0.77) | 0.5 (0.98) | 0.1611* | 1.3 (2.08) | 1.7 (2.49) | 0.009* |
| Treatment Complications, median (IQR) | 0 (1.00) | 0 (1.00) | 0.238† | 0 (2.00) | 1.0 (2.00) | <0.001† |
| BSI Somatization T-Score, mean (SD) | 53.2 (7.46) | 53.7 (9.00) | 0.592* | 53.6 (8.04) | 54.9 (8.95) | 0.023* |
| BSI Depression T-Score, mean (SD) | 49.4 (9.20) | 48.7 (9.04) | 0.513* | 51.1 (9.72) | 53.2 (9.38) | 0.001* |
| BSI Anxiety T-Score, mean (SD) | 50.3 (9.37) | 52.7 (9.53) | 0.028* | 50.4 (9.63) | 50.0 (9.65) | 0.571* |
| CIRS Comorbidity Count, mean (SD) | 2.9 (2.10) | 3.1 (2.27) | 0.355* | 3.1 (1.95) | 3.3 (2.12) | 0.165* |
| MMSE, mean (SD) | 29.0 (1.39) | 28.9 (1.38) | 0.639* | 28.5 (1.90) | 28.4 (2.40) | 0.444* |
| Hoehn & Yahr Stage, mean (SD) | 1.9 (0.54) | 1.9 (0.65) | 0.532* | 2.3 (0.83) | 2.4 (0.96) | 0.024* |
| Treatment | ||||||
| DBS at Current Visit, n (%) | 0 (0.0) | 1 (0.63) | 0.383‡ | 20 (2.2) | 14 (2.6) | 0.660‡ |
| Clinician Sex, n (%) | 0.015§ | <0.001‡ | ||||
| Male | 133 (86.4) | 72 (74.2) | 544 (86.5) | 275 (73.5) | ||
| Female | 21 (13.6) | 25 (25.8) | 85 (13.5) | 99 (26.5) | ||
| Carbo/Levodopa at Current Visit, n (%) | 37 (24.7) | 31 (36.1) | 0.063‡ | 406 (70.4) | 245 (72.7) | 0.452‡ |
| Dopaminergic at Current Visit, n (%) | 62 (35.6) | 50 (47.6) | 0.048‡ | 523 (75.6) | 307 (77.0) | 0.611‡ |
| Outcomes | ||||||
| UPDRS Motor, mean (SD) | 22.7 (10.24) | 22.1 (11.13) | 0.666* | 26.4 (12.37) | 26.3 (13.76) | 0.974* |
| OARS ADLs, mean (SD) | 8.7 (2.42) | 9.0 (2.87) | 0.302* | 10.2 (9.91) | 10.8 (10.37) | 0.021* |
| OARS IADLs, mean (SD) | 8.3 (2.80) | 9.2 (3.40) | 0.012* | 10.2 (5.19) | 11.4 (5.79) | 0.001* |
| S&E, mean (SD) | 88.8 (8.64) | 89.2 (9.90) | 0.730* | 81.1 (15.13) | 80.0 (17.19) | 0.273* |
| SF-12 MH, mean (SD) | 49.9 (10.47) | 48.6 (11.01) | 0.280* | 49.5 (10.65) | 47.7 (11.24) | 0.006* |
| SF-12 PH, mean (SD) | 46.3 (11.11) | 44.9 (11.72) | 0.273* | 42.9 (11.34) | 40.6 (12.63) | 0.002* |
Abbreviations: SD, Standard Deviation; IQR, Interquartile Range, N/A, Not Applicable
T-test with Satterthwaite Approximation
Wilcoxon Rank-Sum Test
Chi-Square Test
Fisher’s Exact Test
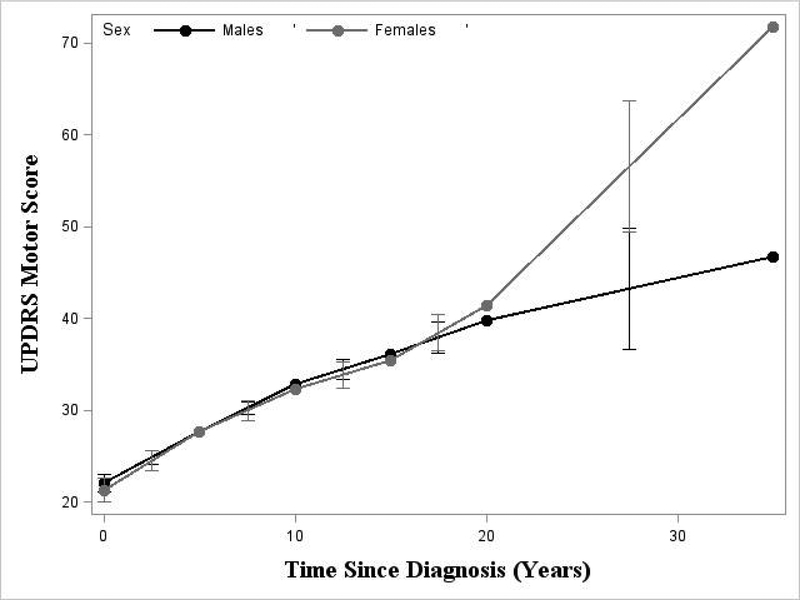
Males and females had similar rates of progression (Table 2, Figure 1, esuppTables 1–5, esuppFigures 1–5). For impairment (Table 2), the mean UPDRS score at diagnosis was similar for males and females (22.09 and 21.31, respectively). Also, the variation in patient-specific mean score at diagnosis was similar between males and females (SD=7.75 and 8.45, respectively). There were no significant differences between sexes in the rate of impairment progression until 20 years post-diagnosis (p=0.02); females, on average, after 20 years, had a significantly faster rate of progression. For mental HRQoL (esuppTable 4, esuppFigure 4), males progressed at a faster rate between 5 and 10 years since diagnosis (p=0.03).
Table 2.
Linear mixed effects model for Parkinson’s disease (PD) impairment progression by sex measured with the physician-reported, UPDRS Motor Scores
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| Effects | Mean Across Individuals | Between Individuals | Mean Across Individuals | Between Individuals | |||
| Parameters | β | 95% CI | SD | β | 95% CI | SD | p-value§ |
| Mean at Diagnosis* | 22.09 | 21.13, 23.05 | 7.75 | 21.31 | 20.02, 22.60 | 8.45 | 0.343 |
| Rates of Progression by Time | |||||||
| Since Diagnosis | |||||||
| 0 to <5 Years† | 1.12 | 0.83, 1.40 | 1.70 | 1.28 | 0.86, 1.70 | 2.05 | 0.531 |
| 5 to <10 Years† | 1.03 | 0.75, 1.30 | 1.69 | 0.90 | 0.45, 1.35 | 2.45 | 0.650 |
| 10 to <15 Years† | 0.65 | 0.22, 1.08 | 2.48 | 0.64 | 0.09, 1.20 | 2.55 | 0.991 |
| 15 to <20 Years† | 0.75 | 0.07, 1.43 | 2.46 | 1.19 | 0.41, 1.98 | 2.35 | 0.405 |
| ≥20 Years† | 0.46 | −0.42, 1.33 | 2.59 | 2.02 | 1.08, 2.97 | 1.85 | 0.017 |
| Rate of Progression Benefit from 1st Year in Clinic‡ | −1.66 | −2.43, −0.88 | N/A | −3.37 | −4.41, −2.33 | N/A | 0.010 |
Abbreviations: CI, Confidence Interval; SD, Standard Deviation
Intercept
β representing annual rate of change
One year rate of change
Comparison of sex differences
Figure 1.
Plot of average rate of Parkinson’s disease (PD) UPDRS Motor progression by sex
There was a high amount of inter-individual heterogeneity in the rate of progression and disease severity at diagnosis. For example, the SD of the rate of progression in UPDRS motor scores in the first 5 years for males was estimated to be 1.70 indicating that many males improved while others had a very fast annual decline (Table 2). Heterogeneity did not differ by sex.
Males and females experienced similar symptomatic improvement in their first year of care at the Center with the exception of UPDRS motor scores and OARS ADLs and IADLs. The average annual rate of UPDRS motor progression (Table 2) was attenuated in the first year of care at the Center by 3.37 points in females and only 1.66 points in males (p=0.10).
There was no evidence that males or females experience greater care delays (esuppTable 6).
Females reported worse disability and HRQoL, compared to males. The mediation analysis demonstrated that eliminating treatment complications, improving cognition, managing comorbidities, reducing psychological distress, or shortening the time from diagnosis to first specialty care visit could attenuate this sex disparity (Table 3). Reducing anxiety had the largest potential benefit. On crude analysis, females reported 1.13 units (95% CI: 0.48, 1.78) more IADL disability than males. Setting anxiety symptoms in the sample to a population normative T-score resulted in females reporting almost identical levels (difference=0.04 units) of IADL disability to males (95% CI: −0.59, 0.66).
Table 3.
Results of mediation analysis demonstrating the controlled direct effect (CDE) of female sex on all patient-reported outcome measures at initial Parkinson’s disease (PD) visits
| OARS ADLs | OARS IADLS | SF-12 MH | SF-12 PH | |||||
|---|---|---|---|---|---|---|---|---|
| b | 95% CI | b | 95% CI | b | 95% CI | b | 95% CI | |
| Crude Sex Difference (Males Reference) | 0.62 | 0.12, 1.12 | 1.13 | 0.48, 1.78 | −1.82 | −3.10, −0.54 | −2.25 | −3.64, −0.85 |
| Mediators | CDE | 95% CI | CDE | 95% CI | CDE | 95% CI | CDE | 95% CI |
| Treatment Complications* | 0.22 | −0.51, 0.95 | 0.61 | −0.33, 1.54 | −0.58 | −2.48, 1.32 | −2.02 | −3.99, −0.04 |
| Cognition† | 0.32 | −0.27, 0.91 | 1.00 | 0.28, 1.73 | −1.82 | −3.57, −0.06 | −2.23 | −3.16, −0.28 |
| Comorbidities‡ | 0.37 | −0.31, 1.05 | 0.27 | −0.58, 1.13 | −2.27 | −4.26, −0.28 | −1.49 | −3.46, 0.48 |
| Psychological Distress§ | ||||||||
| Anxiety | −0.22 | −0.72, 0.28 | 0.04 | −0.59, 0.66 | 0.01 | −1.30, 1.33 | −0.27 | −1.82, 1.28 |
| Depression | 0.19 | −0.30, 0.67 | 0.55 | −0.05, 1.15 | −2.19 | −3.36, −1.03 | −0.59 | −2.09, 0.92 |
| Somatization | −0.17 | −0.70, 0.36 | 0.26 | −0.41, 0.93 | −0.91 | −2.49, 0.67 | −0.42 | −1.97, 1.13 |
| Disease Duration at First Visit to Center‖ | 0.41 | −0.13, 0.95 | 0.85 | 0.15, 1.54 | −1.51 | −3.01, −0.00 | −1.62 | −3.19, −0.06 |
Abbreviations: CDE, controlled direct effects
Adjusted for age, levodopa use, disease duration; mediator set to score = 0
Adjusted for age, disease duration, comorbidities, SES; mediator set to score = 30
Adjusted for age, disease duration, SES; mediator set to 1
Adjusted for age, drug side effects, disease duration, marital status, SES; mediator set to T-score = 50
Adjusted for SES; mediator set to 1 year
Proxy estrogen information was available for 463 post-menopausal females; 23.1% (n=94) had undergone surgical menopause, 53.2% (n=216) were ever HRT users, and 7.7% (n=30) were nulliparous. Significant differences in progression models were seen for all outcomes except mental HRQoL; however, differences did not follow a clear pattern.
DISCUSSION
This study showed that female PD patients present differently to specialty care but do not progress differently, compared to males. Differences were found among patient-reported outcomes; however, mediation analyses showed that these differences may be attenuated by various interventions
Consistent with previous studies [7], female PD patients had less social support and more psychological distress, compared to males. Female patients were more likely to see female specialists. As prior research suggests that the general population shows no sex preference for neurologists [25], further research is needed to understand this novel finding.
Females reported worse disability and HRQoL at their initial PD visits at the Center. Notably, this was not reflected in physician-reported impairment and disability. This lack of agreement could negatively impact shared decision making, particularly for female patients [26], and emphasizes the importance of patient perception and experience [9,11,13]. Some of the differences seen at initial PD visits, such as more advanced Hoehn & Yahr staging, could suggest that female patients may have experienced delays in specialist referral, which has been previously found [8]. In this study, there was no evidence that females experience greater care delays. As females are less likely to consult specialists [27], further studies are needed on female care-seeking patterns, including understanding referral patterns for those who never seek specialty care.
The mediation analysis demonstrated that managing patient psychological distress, particularly anxiety, to population norms, most drastically attenuated sex differences in patient-reported outcomes at initial PD visits. Managing psychological distress in PD is difficult and may be part of the disease etiology as well as a response to worsening disease [28]. Given that anxiety and depressive symptoms are often missed by neurologists [29], our study emphasizes the need to screen PD patients, especially females, for these symptoms.
With few exceptions, the average rates of PD progression were similar for males and females. Females had a faster rate of motor symptom (impairment) progression after 20 years. A prior study found that females had slower initial but faster later impairment progression; however, these patients were only followed for 10 years from baseline [30]. Given that more males died in our study (11.7% of males, 9.3% of females, p=0.15), the difference found late in the disease course could be the result of selective survival. To investigate this bias, additional mortality data is needed. However, there were only 717 observations available (out of 13,820) from ≥20 years after diagnosis; thus, differences late in progression may be the result of sparse data.
There was a large amount of heterogeneity in disease progression, although the amount of heterogeneity did not differ by sex. This heterogeneity in progression makes prognostic counseling difficult. Understanding underlying reasons for heterogeneity in PD progression is a priority research area [31].
Although we do not know if this was the first time patients saw a neurologist/movement disorders specialist or how patients would have progressed had they not sought out care at the Center, this study shows a significant initial benefit of specialist care for selected outcomes. PD patients seen by neurologists have improved outcomes, compared to those seen by primary care providers [27]. Willis et al. (2011) found that over a 48 month period, less than half (42%) of Medicare beneficiaries with PD saw a neurologist [27]. Further research is needed to improve patient access and utilization of specialty care.
The inconsistent impact of reproductive characteristics on progression seen in our analysis suggests that biological differences may not impact disease course. However, the characteristics examined in this study were crude proxies for estrogen exposure. Additional studies should include details such as age at menopause and menarche.
Limitations
One potential limitation is the misspecification of progression models. However, extensive exploratory analysis was undertaken to select the final models that balanced progression complexity with parsimony and interpretability. Although an extensive amount of patient information is available in the HOME database, information on medication dosage and some non-motor symptoms are unavailable, which precluded analysis of these factors as mediators. Some patients completed questionnaires with the assistance of a caregiver or a caregiver completed the questionnaire on behalf of the patient. In PD, proxies rate health systematically worse than patients [32]. Proxies completed questionnaires without the patient in 0.5% of visits; however, co-reporting could also have influenced estimates. Selection bias due to loss to follow-up could be a concern as the HOME study does not have information on why individuals no longer present to the Center for care. These individuals could have switched clinicians, been admitted to a nursing home, or died. Consequently, the progression data from late in the disease course reflects a limited sample. Data on advanced disease progression may be more reflective of individuals who were diagnosed at a younger age, who differ from those with a later age of onset [33]. Lastly, patients seeking care at a tertiary center may not represent the general PD population in the community or primary care clinics. The progression patterns in this study reflect those of patients in specialty care; thus, this study merits replication in less specialized care settings.
Strengths
This study used a unique database with over 15 years of longitudinal data on numerous PD patients with specialist confirmed diagnoses. The database includes extensive information on both physician- and patient-reported outcome measures. Only one other study has simultaneously examined impairment, disability, and HRQoL PD progression; however the study was limited to 5 years of follow-up and examined progression linearly [34]. Lastly, we were able to implement causal inference approaches, namely mediation analysis, to provide insight into the reasons for sex differences in PD.
Conclusions
Our study suggests that sex influences disease presentation but minimally impacts progression. Differences in disability and HRQoL at initial visits to specialty care were pronounced but could be attenuated with interventions—particularly those targeting anxiety. Emphasis is needed on screening and management of anxiety symptoms in PD, particularly among female patients. Lastly, to inform shared decision-making, it is important to consider patient-reported disease severity.
Supplementary Material
Highlights.
Females with PD have less social support and report worse psychological distress
Sex minimally influences PD progression
Females report worse disability and HRQoL at initial visits for PD specialty care
Improved anxiety management can attenuate sex differences in disability and HRQoL
Patient reported outcome measures are vital to understanding patients’ experiences
ACKNOWLEDGEMENTS
This research was supported by grants from the National Institute on Aging [T32 AG000262, K01 AG50723]. We also acknowledge support from the Rosalyn Newman Foundation and the contribution of patients and the clinical team at the University of Maryland Parkinson’s Disease and Movement Disorders Center.
The funding sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTEREST
DSA, ALG-B, and SET received grant funding from National Institute on Aging during the conduct of the study. DSA received financial support from the Parkinson’s Foundation. LSM, PFM, EB, KS, and LMS have nothing to disclose.
REFERENCES
- [1].Gillies GE, Pienaar IS, Vohra S, Qamhawi Z. Review: Sex differences in Parkinson’s disease. Front Neuroendocrinol. 2014;35:370–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Review: Risk factors for Parkinson’s disease may differ in men and women: an exploratory study. Horm Behav. 2013;63:308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Puschmann A, Brighina L, Markopoulou K, Aasly J, Chung SJ, Frigerio R, et al. Clinically meaningful parameters of progression and long-term outcome of Parkinson disease: An international consensus statement. Parkinsonism Relat Disord. 2015. May/01. [DOI] [PubMed] [Google Scholar]
- [4].Post B, Merkus MP, De Haan R, Speelman JD. Prognostic factors for the progression of Parkinson’s disease: A systematic review. Mov Disord. 2007;22(13):1839–51. [DOI] [PubMed] [Google Scholar]
- [5].Marras C, Rochon P, Lang AE. Predicting motor decline and disability in Parkinson disease: a systematic review. Arch Neurol. 2002. November;59(11):1724–8. [DOI] [PubMed] [Google Scholar]
- [6].Haaxma CA, Bloem BR, Borm GF, Oyen WJG, Leenders KL, Eshuis S, et al. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007. August;78(8):819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pavon JM, Whitson HE, Okun MS. Parkinson’s disease in women: A call for improved clinical studies and for comparative effectiveness research. Maturitas. 2010. April;65(4):352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Saunders-Pullman R, Wang CL, Stanley K, Bressman SB. Diagnosis and referral delay in women with parkinson’s disease. Gend Med. 2011;8(3):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shulman LM. Gender differences in Parkinson’s disease. Gend Med. 2007;4:8–18. [DOI] [PubMed] [Google Scholar]
- [10].Marinus J, van der Heeden JF, van Hilten JJ. Calculating clinical progression rates in Parkinson’s disease: Methods matter. Parkinsonism Relat Disord. 2014;20(11):1263–7. [DOI] [PubMed] [Google Scholar]
- [11].Poewe W. Clinical measures of progression in Parkinson’s disease. Mov Disord. 2009;24 Suppl 2:S671–6. [DOI] [PubMed] [Google Scholar]
- [12].Fahn S, Elton RL, Members of the UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent developments in Parkinson’s disease. Volume 2 ed. Florham Park, NJ: Macmillan Health Care Information; 1987. p. 153–164. [Google Scholar]
- [13].Shulman LM. Understanding disability in Parkinson’s disease. Mov Disord. 2010;25 Suppl 1:S131–5. [DOI] [PubMed] [Google Scholar]
- [14].Marras C, Lang A. Invited article: changing concepts in Parkinson disease: moving beyond the decade of the brain. Neurology. 2008. May/20;70(21):1996–2003. [DOI] [PubMed] [Google Scholar]
- [15].Fillenbaum GG. Multidimensional functional assessment of older adults: The Duke Older Americans Resources and Services procedures. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- [16].Schwab J, England A. Gillingham FJ, Donaldson IML, editors. Third Symposium on Parkinson’s Disease, held at the Royal College of Surgeons of Edinburgh on 20, 21 and 22 May 1968. Edinburgh, E & S Livingstone, 1969; 1969. p. 152–7. [Google Scholar]
- [17].Ware John E., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996(3):220. [DOI] [PubMed] [Google Scholar]
- [18].Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: Comparison with the unified Parkinson’s disease rating scale. Mov Disord. 2013;28(5):668–70. [DOI] [PubMed] [Google Scholar]
- [19].Hoehn MM, Yahr MD. Parkinsonism: Onset, progression, and mortality. Neurology. 1967. [PubMed] [Google Scholar]
- [20].Derogatis LR. BSI-18: Administration, Scoring and Procedures Manual. Minneapolis, MN: National Computer Systems; 2000. [Google Scholar]
- [21].Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968. January/01;16(5):622–6. [DOI] [PubMed] [Google Scholar]
- [22].van Steenoven I, Aarsland D, Hurtig H, Chen-Plotkin A, Duda JE, Rick J, et al. Conversion between mini-mental state examination, montreal cognitive assessment, and dementia rating scale-2 scores in Parkinson’s disease. Mov Disord. 2014. December;29(14):1809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Valeri L, VanderWeele TJ. Mediation analysis allowing for exposure–mediator interactions and causal interpretation: Theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013. June;18(2):137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gatto NM, Deapen D, Stoyanoff S, Pinder R, Narayan S, Bordelon Y, et al. Lifetime exposure to estrogens and Parkinson’s disease in California teachers. Parkinsonism Relat Disord. 2014;20:1149–56. [DOI] [PubMed] [Google Scholar]
- [25].Kerssens JJ, Bensing JM, Andela MG. Patient preference for genders of health professionals. Soc Sci Med. 1997. May;44(10):1531–40. [DOI] [PubMed] [Google Scholar]
- [26].Lindström Egholm C, Krogh NS, Pincus T, Dreyer L, Ellingsen T, Glintborg B, et al. Discordance of global assessments by patient and physician Is higher in female than in male patients regardless of the physician’s sex: Data on patients with rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis from the DANBIO Registry. J Rheumatol. 2015. October;42(10):1781–5. [DOI] [PubMed] [Google Scholar]
- [27].Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA.. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology. 2011. August/30;77(9):851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov Disord. 2001;16(3):507–10. [DOI] [PubMed] [Google Scholar]
- [29].Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2002;8:193–7. [DOI] [PubMed] [Google Scholar]
- [30].Reinoso G, Allen JC J., Au W, Seah S, Tay K, Tan LCS. Clinical evolution of Parkinson’s disease and prognostic factors affecting motor progression: 9-year follow-up study. Eur J Neurol. 2015. March;22(3):457–63. [DOI] [PubMed] [Google Scholar]
- [31].Sieber B, Landis S, Koroshetz W, Bateman R, Siderowf A, Galpern WR, et al. Prioritized research recommendations from the National Institute of Neurological Disorders and Stroke Parkinson’s Disease 2014 Conference. Ann Neurol. 2014. October;76(4):469–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fleming A, Cook KF, Nelson ND, Lai EC. Proxy reports in Parkinson’s disease: Caregiver and patient self-reports of quality of life and physical activity. Mov Disord. 2005;20(11):1462–8. [DOI] [PubMed] [Google Scholar]
- [33].Mehanna R, Moore S, Hou JG, Sarwar AI, Lai EC. Comparing clinical features of young onset, middle onset and late onset Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:530–4. [DOI] [PubMed] [Google Scholar]
- [34].Velseboer DC, Broeders M, Post B, van Geloven N, Speelman JD, Schmand B, et al. Prognostic factors of motor impairment, disability, and quality of life in newly diagnosed PD. Neurology. 2013. February/12;80(7):627–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.