Abstract
Background:
Low 25-hydroxyvitamin D [25(OH)D] levels in serum are associated with breast cancer risk. This study was conducted to determine the impact of 25(OH)D deficiency on survival of breast cancer patients.
Methods:
In a retrospective cohort study of 303 patients diagnosed with breast cancer during 2011-2012 at the National Cancer Institute Thailand, all cases were followed up for 7 years. The 25(OH)D was measured by high-performance liquid chromatography (HPLC). Clinical and pathological data were collected. The Chi-square test, Kaplan-Meier and Cox regression model were used to assess the association between 25(OH)D levels and risk of death.
Results:
Of the 303 cases aged between 24 and 78 years 51 (16.8%) died during follow-up from any cause. The mean 25(OH)D levels was 25.1±7.54 ng/ml (8.2 – 61.0 ng/ml). Thirty-three patients (10.9%) were stratified as inadequate or deficient group (<16 ng/ml) with mean survival time of 60.65 months compared to 76.24 months in insufficient or sufficient group (≥16 ng/ml). Multivariate analysis adjusted for age, body mass index, stage, lymph node metastases, and immunohistochemical (IHC) findings (ER, PgR, HER-2, Ki-67 and P53) showed that patients with low 25(OH)D levels (<16 ng/ml) at diagnosis had a significantly higher risk of death (hazard ratio = 2.5-2.9) than the group with high 25(OH)D levels (≥16 ng/ml).
Conclusion:
A concentration of 25(OH)D below 16 ng/ml was found to be independently associated with poor survival in breast cancer patients, regardless of age, lymph node status, stage or breast cancer subtype. An investigation of potential benefit of 25(OH)D supplements appears warranted.
Key Words: Vitamin D deficiency, 25(OH)D, breast cancer, survival rate, prognosis
Introduction
Breast cancer is the leading female cancer worldwide in both incidence and mortality, with an estimated 1.7 million new cases and over half a million deaths in 2012 (Hu et al., 2018). A great deal of effort has been invested in countering this public health problem with implementation of screening mammography, improvement of access to cancer care and development of anti-cancer protocols. However, problems with poor outcome still remain in many countries.
25(OH)D, a fat-soluble vitamin generated by sunlight exposure or obtained through the diet, plays roles in calcium homeostasis, neuromuscular and immune functions and control of inflammation. Associations of low serum 25(OH)D with cancer incidence and mortality are in line with its anti-cancer properties (Lim et al., 2015), with contributions to regulation of cell growth and apoptosis (Kermani et al., 2011). Epidemiologic studies have generally indicated an inverse association between the 25(OH)D levels and risk of cancers including breast cancers (Goodwin et al., 2009; Gorham et al., 2007; Crew et al., 2009; Bao et al., 2010; Shui and Giovannucci, 2014). Furthermore, breast cancers in patients with low 25(OH)D levels (below 30 or 32 ng/ml) usually have more aggressive clinico-pathological characteristics that result in a poorer prognosis (Palmieri et al., 2006; Goodwin et al., 2009; Thanasitthichai et al., 2015). However, an optimal 25(OH)D cut-off level for prediction of survival outcome under given clinico-pathological conditions has yet to be established. Therefore, we conducted the present retrospective cohort study of 25(OH)D levels in breast cancer patients at the National Cancer Institute, Thailand.
Materials and Methods
Study participants
In this study, 303 patients newly diagnosed with breast cancer between July 2011 and June 2012 were enrolled. All patients were examined for their baseline 25(OH)D levels both before and after treatment. Analyses were carried out with age, BMI, clinical stage, lymph node metastasis, ER, PgR, HER-2, P53, Ki-67 and 25(OH)D levels as variables. The study was approved by the Institutional Review Board and Ethics Committee of NCI, Thailand. Written informed consent was obtained from all patients.
Serum collection and 25(OH)D levels assessment
Approximately 1.5 ml aliquots of serum were collected from 7 ml whole blood samples of each patient and stored at -20ºC until 25(OH)D concentrations were determined by high-performance liquid chromatography (HPLC) with UV detection at the Department of Immunology at NCI, Thailand ((after (Neyestani et al., 2007)).
Statistical analysis
The average of serum 25(OH)D levels was included in analysis, which homogeneity of variance by Cochran and Bartlett’s test were not different. Adopting cut-off points from the literature (Ross et al., 2011) serum 25(OH)D levels were classified as inadequate or deficient when <20 ng/ml (inadequate 12-20 ng/ml, deficient <12 ng/ml), insufficient with 20-30 ng/ml, and sufficient with >30 ng/ml. The impact of 25(OH)D deficiency on breast cancer survival was investigated using the 25(OH)D cut point of 16 ng/ml (calculated from median of IOM definitions for inadequate 25(OH)D, 12-20 ng/ml), above and below which patients were respectively considered to have sufficient and deficient levels. Descriptive statistics were applied with the Chi-square test used to evaluate the significance of differences in parameters between groups. Overall survival was calculated from the date of diagnosis to the date of last follow-up or death from any cause. To compare survival times, the Kaplan-Meier method and log-rank test were employed. Univariate and multivariate statistics were used to estimate hazard ratios (HRs) and 95% confidence intervals (95%CIs) with the Cox’s proportional hazard model. Significance was concluded with p-values < 0.05.
Results
The mean age for the 303 patients was 50.8±10.5 years (range: 24 – 78 years). Their mean 25(OH)D concentration was 25.1±7.54 ng/ml (8.2-61.0 ng/ml). Thirty-three cases constituted the deficient group (<16 ng/ml) with a mean serum 25(OH)D concentration of 13.3±1.9 ng/ml (8.2-15.6 ng/ml). The remaining 270 (86.0%) patients had a mean value of 26.6±6.7 ng/ml (16.0-61.0 ng/ml). Cases with high 25(OH)D levels (≥16 ng/ml) were mainly early stage or with HER-2 negative status (p = 0.042 and 0.046, respectively) as detailed in Table 1.
Table 1.
Patient Characteristics and 25(OH)D Levels
| Characteristics | Total n (%) |
Deficient (<16 ng/ml) n (%) |
Sufficient (≥16 ng/ml) n (%) |
p-value |
|---|---|---|---|---|
| Age group (years) | 0.332 | |||
| <50 | 143 (47.4) | 13 (39.4) | 130 (48.3) | |
| ≥50 | 159 (52.6) | 20 (60.6) | 139 (51.7) | |
| BMI (kg/m2) | 0.424 | |||
| <23 | 128 (43.4) | 16 (50.0) | 112 (42.6) | |
| ≥23 | 167 (56.6) | 16 (50.0) | 151 (57.4) | |
| Clinical Stage | 0.042 | |||
| Stages I-II | 189 (69.5) | 16 (53.3) | 173 (71.5) | |
| Stages III-IV | 83 (30.5) | 14 (46.7) | 69 (28.5) | |
| LN metastasis | 0.49 | |||
| Positive | 117 (51.3) | 15 (57.7) | 102 (50.5) | |
| Negative | 111 (48.7) | 11 (42.3) | 100 (49.5) | |
| ER | 0.94 | |||
| Positive | 170 (64.9) | 19 (65.5) | 151 (64.8) | |
| Negative | 92 (35.1) | 10 (34.5) | 82 (35.2) | |
| PR | 0.776 | |||
| Positive | 129 (49.2) | 15 (51.7) | 114 (48.9) | |
| Negative | 133 (50.8) | 14 (48.3) | 119 (51.1) | |
| HER-2 | 0.046 | |||
| Positive | 49 (19.2) | 6 (22.2) | 43 (18.9) | |
| Equivocal | 52 (20.4) | 10 (37.0) | 42 (18.4) | |
| Negative | 154 (60.4) | 11 (40.7) | 143 (62.7) | |
| P53 | 0.299 | |||
| Positive | 206 (78.0) | 24 (85.7) | 182 (77.1) | |
| Negative | 58 (22.0) | 4 (14.3) | 54 (22.9) | |
| Ki-67 | 0.225 | |||
| Positive | 238 (93.3) | 22 (88.0) | 216 (93.9) | |
| Negative | 17 (6.7) | 3 (12.0) | 14 (6.1) | |
BMI, body mass Index; LN, lymph node; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epithelial growth factor receptor-2.
The mean survival time was 74.8 months, with an overall survival (OS) of 81.5% at 60 months. Breast cancer patients in the deficient group (<16 ng/ml) had poorer outcomes for overall survival compared with patients with sufficient 25(OH)D (≥16 ng/ml) (p = 0.002). In the deficient patients with older age, BMI (≥23 kg/m2), advance stage, lymph node metastasis, negative for PgR, HER-2, P53, Ki-67, and positive for ER showed significantly poorer 5 year-survival rate compared to their sufficient counterparts (p = 0.003, 0.007, 0.024, 0.001, 0.001, 0.001, 0.001, 0.027, and 0.012, respectively) (Table 2). Figure 1 shows Kaplan-Meier plots. Patients with deficient serum 25(OH)D levels had a significantly poorer overall survival than the patients with sufficient serum 25(OH)D levels (p = 0.002).
Table 2.
Five Year Overall Survival Proportions with Patient Characteristics According to the Serum 25(OH)D cut-off
| Characteristics | n | 5-year survival (%) |
p-value | |
|---|---|---|---|---|
| <16ng/ml | ≥16ng/ml | |||
| Vitamin D | 303 | 64.3 | 83.5 | 0.002 |
| Age (years) | 0.003 | |||
| <50 | 143 | 80.8 | 89 | |
| ≥50 | 159 | 54 | 78.2 | |
| BMI 23 kg/m2 | 0.007 | |||
| <23 | 128 | 81.3 | 75.1 | |
| ≥23 | 167 | 51.9 | 91.9 | |
| Clinical stage | 0.024 | |||
| I-II | 189 | 81.8 | 92.6 | |
| III-IV | 83 | 42.9 | 61.3 | |
| LN metastasis | 0.001 | |||
| Positive | 117 | 45 | 72.2 | |
| Negative | 111 | 87.5 | 96.5 | |
| ER | 0.001 | |||
| Positive | 170 | 52.1 | 86.2 | |
| Negative | 92 | 70 | 77.7 | |
| PgR | 0.001 | |||
| Positive | 129 | 61.9 | 90.3 | |
| Negative | 133 | 57.1 | 76.2 | |
| HER-2 | 0.012 | |||
| Positive | 49 | 62.5 | 70.6 | |
| Equivocal | 52 | 75 | 76 | |
| Negative | 154 | 51.1 | 88.2 | |
| P53 | 0.001 | |||
| Positive | 206 | 65.5 | 85.7 | |
| Negative | 58 | 50 | 75.6 | |
| Ki-67 | 0.027 | |||
| Positive | 238 | 67.9 | 82.9 | |
| Negative | 17 | 66.7 | 85.7 | |
Figure 1.
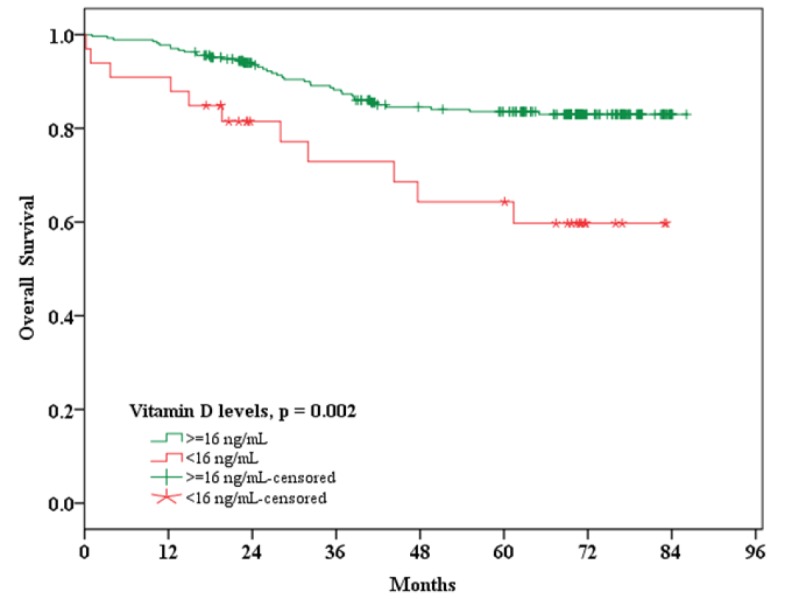
Kaplan-Meier plot for overall survival in breast cancer patients with serum 25(OH)D levels <16 ng/ml versus ≥16 ng/ml
We conducted univariate and multivariate analysis to determine risk factors affecting breast cancer survival. The multivariate findings after adjustment for age, body mass index, stage, lymph node metastases, IHC findings (ER, PgR, HER-2, Ki-67 and P53) showed that patients with 25(OH)D levels <16 ng/ml at diagnosis had a significantly higher risk of death (hazard ratio = 2.5-2.9) than the group with 25(OH)D levels ≥16 ng/ml (Table 3).
Table 3.
Univariate and Multivariate Analyses Evaluating the Baseline Risk Factors that Affect Survival
| Overall survival |
||||
|---|---|---|---|---|
| Unadjusted HRa (95%CI) | p-value | Adjusted HR (95%CI) | p-value | |
| 25(OH)D levels stratified by age b | ||||
| Insufficient/sufficient (≥16 ng/ml) | Reference | Reference | ||
| Inadequate (<16 ng/ml) | 2.626 (1.344-5.130) | 0.005 | 2.474 (1.084-5.644) | 0.031 |
| 25(OH)D levels stratified by BMI c | ||||
| Insufficient/sufficient (≥16 ng/ml) | Reference | Reference | ||
| Inadequate (<16 ng/ml) | 2.541 (1.261-5.122) | 0.009 | 2.699 (1.161-6.272) | 0.021 |
| 25(OH)D levels stratified by Stagec | ||||
| Insufficient/sufficient (≥16 ng/ml) | Reference | Reference | ||
| Inadequate (<16 ng/ml) | 2.138 (1.086-4.207) | 0.028 | 2.429 (1.148-5.139) | 0.02 |
| 25(OH)D levels stratified by HER-2c | ||||
| Insufficient/sufficient (≥16 ng/ml) | Reference | Reference | ||
| Inadequate (<16 ng/ml) | 2.448 (1.188-5.045) | 0.015 | 2.499 (1.095-5.704) | 0.03 |
| 25(OH)D levels stratified by LN d | ||||
| Insufficient/sufficient (≥16 ng/ml) | Reference | Reference | ||
| Inadequate (<16 ng/ml) | 3.190 (1.557-6.534) | 0.002 | 2.493 (1.090-5.704) | 0.03 |
| 25(OH)D levels stratified by PR e | ||||
| Insufficient/sufficient (≥16 ng/ml) | Reference | Reference | ||
| Inadequate (<16 ng/ml) | 2.942 (1.494-5.794) | 0.002 | 2.559 (1.113-5.884) | 0.027 |
| 25(OH)D levels stratified by P53 e | ||||
| Insufficient/sufficient (≥16 ng/ml) | Reference | Reference | ||
| Inadequate (<16 ng/ml) | 2.972 (1.469-6.014) | 0.002 | 2.518 (1.098-5.773) | 0.029 |
| 25(OH)D levels stratified by ER f | ||||
| Insufficient/sufficient (≥16 ng/ml) | Reference | Reference | ||
| Inadequate (<16 ng/ml) | 3.115 (1.581-6.141) | 0.001 | 2.966 (1.399-6.286) | 0.005 |
| 25(OH)D levels stratified by Ki-67 g | ||||
| Insufficient/sufficient (≥16 ng/ml) | Reference | Reference | ||
| Inadequate (<16 ng/ml) | 2.325 (1.075-5.031) | 0.032 | 2.462 (1.050-5.773) | 0.038 |
a, Hazard ratio; b, Adjusted for LN, ER, and HER-2; c, Adjusted for age, LN, and ER; d, Adjusted for age, ER, and HER-2; e, Adjusted for age, LN, ER, and HER-2; f, Adjusted for age, LN, and HER-2; g, Adjusted for LN and p53”
Discussion
From our analysis, we found that application of a cut-off value of 16 ng/ml for serum 25(OH)D levels is appropriate for division of patients regarding risk of mortality. Our findings are in line with other reports that low serum 25(OH)D has a negative effect on overall and disease-free survival (Ismail et al., 2018; Yao et al., 2017). Moreover, in an additional analysis, by dividing the patients into 3 groups: inadequate (<16 ng/ml), insufficient (16-30 ng/ml), and sufficient (>30 ng/ml) levels, we found that not only the patients with serum 25(OH)D >30 ng/ml had no survival benefit from higher 25(OH)D, but also they had worse survival trend compared to the intermediate level (data not shown). This might imply that the vitamin D supplement might have no additional benefit in patients who are not actually deficient.
Investigation of prognostic effects of serum 25(OH)D levels in a prospective cohort of 512 women with early breast cancer, revealed those with serum 25(OH)D levels <20 ng/ml had poorer overall survival (HR = 1.73; 95% CI, 1.05-2.86) compared to those with >28.8 ng/ml, on univariate analysis. However, no statistical significance was found after adjusting for age, tumor stage, nodal stage, estrogen receptor status, and histological grading on multivariate analysis (HR = 1.60; 95%CI, 0.96-2.64) (Goodwin et al., 2009). In the study by Lim et al. clinico-pathological data were collected for patients, including serum 25(OH)D levels at diagnosis and at annual follow-up until 2012. Patients with advanced stage disease or older age in the non-deficient group (≥20 ng/ml), showed a significantly better survival compared with the deficient group, suggesting that sustaining optimal serum 25(OH)D levels should be advised for breast cancer patients (Lim et al., 2015). In a Norwegian study, patients who were diagnosed with breast cancer with higher serum 25(OH)D levels (>35 ng/ml) had a significantly decreased risk of breast cancer-specific mortality compared to those with lower serum 25(OH)D levels (<20 ng/ml) (Tretli et al., 2012). In a randomized clinical trial patients with high levels of 25(OH)D prior to chemotherapy had significantly improved overall survival and progression-free survival (Ng et al., 2019).
Mohr et al. also found that high serum 25(OH)D status was associated with lower mortality in patients with breast cancer, recommending that a normal range (30-80 ng/ml) should be maintained with appropriate monitoring (Mohr et al., 2014). In addition, O’Brien et al., (2017) reported high serum 25(OH)D levels and regular 25(OH)D supplement use to be associated with lower rates of breast cancer, especially in postmenopausal women. Similar findings were recently published by Estébanez et al., (2018). From a meta-analysis Kim and Je (2014) concluded that high 25(OH)D status is weakly associated with low breast cancer risk, but strongly associated with better breast cancer survival. Low 25(OH)D levels may be more significantly associated with locally advanced or metastatic breast cancer compared with early stages (Palmieri et al., 2006). Overall, the results support the hypothesis that 25(OH)D supplementation is useful in breast cancer prevention.
However a cohort study of 585 breast cancer survivors higher serum 25(OH)D was associated with improved survival, but the result was not statistically significant and interpretation is difficult (Villaseñor et al., 2013). Furthermore, other results did not support recommendations for vitamin D supplementation to improve breast cancer outcome (Lohmann et al., 2015). In a very recent nationwide, randomized, placebo-controlled trial, with a two-by-two factorial design, 25(OH)D (cholecalciferol) at a dose of 2,000 IU per day did not result in a lower incidence of invasive cancer or cardiovascular event than placebo (Manson et al., 2019).
As a strength of this study, we used a longitudinal cohort. As a possible limitation, to should be mentioned that only a single measurement of serum 25(OH)D levels was made. However, serum 25(OH)D has been reported to remain relatively stable over time, so this may not cause significantly bias (McKibben et al., 2016; Hu et al., 2018). A previous study found some evidence that serum 25(OH)D varies during the calendar year (Fohner et al., 2016).
For explanation of anticancer effects, 25(OH)D can regulate the whole process of tumorigenesis from initiation to metastasis and cell-microenvironment interactions. 1α,25-dihydroxyvitamin D (1α,25(OH)2D3, calcitriol) roles are mediated by the vitamin D receptor (VDR) (Hu et al., 2018). Regulation of apoptosis, autophagy, inhibits cell proliferation, differentiation, epithelial-mesenchymal transition (EMT), and cell-microenvironment interactions have been proposed (Jeon and Shin, 2018). Metastasis is a complex and multistep process, during which circulating tumor cell (CTC) spread from the primary tumor mass, in the reversible EMT form to the distant organs. Once distant organs are reached, these mesenchymal tumor cells reverse to an epithelial identity via mesenchymal-epithelial (MET) to regain the ability to proliferate (Thanasitthichai et al., 2015).
Furthermore, vitamin D also activates transcription factors of FoxO protein, which control cell proliferation and survival by inducing deacetylation and dephosphorylation, for example, in neuroblastoma cells (Jeon and Shin, 2018). FoxO is a tumor suppressor associated with longevity. There is evidence that 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3, calcitriol) and FoxO regulate common target genes. VDR is linked with FoxO protein and also regulates the sirtuin 1 (Sirt1) class III histone deacetylase (HDAC) and protein phosphatase 1, providing a molecular basis for cancer chemopreventive actions of 1α,25(OH)2D3 (An et al., 2010). Apoptosis can be induced by 25(OH)D compound (1α,25(OH)2D3, EB 1089, and CB 1093) inhibited by Bcl-2 in MCF-7 and T47D human breast cancer cells expressing wild-type and mutant p53, respectively. This finding may indicate potential for treatment of tumors that are resistant to therapeutic agents that are dependent on the activation of p53 and/or caspases (Mathiasen et al., 1999).
In conclusion, our findings suggest that low levels of serum 25(OH)D are significantly associated with poor survival of breast cancer patients. It remains unclear whether adding vitamin D supplement to traditional breast cancer therapy would be a safe way to improve overall survival of breast cancer patients. Further prospective cohort studies and clinical trials are needed for clarification.
Acknowledgments
We would like to thank you Dr. Malcolm Anthony Moore and Dr. Sunanta Chariyalertsak for being good advisors and preparing the manuscript. This study was supported by a grant from National Cancer Institute, Thailand.
Statement conflict of Interest
We declare that we have no conflict of interest.
References
- An BS, Tavera-Mendoza LE, Dimitrov V, et al. Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor. Mol Cell Biol. 2010;30:4890–4900. doi: 10.1128/MCB.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Ng K, Wolpin BM, et al. Predicted vitamin D status and pancreatic cancer risk in two prospective cohort studies. Br J Cancer. 2010;102:1422–7. doi: 10.1038/sj.bjc.6605658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew KD, Gammon MD, Steck SE, et al. Association between plasma 25-hydroxyvitamin D and breast cancer risk. Cancer Prev Res Phila Pa. 2009;2:598–604. doi: 10.1158/1940-6207.CAPR-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estébanez N, Gómez-Acebo I, Palazuelos C, et al. Vitamin D exposure and risk of breast cancer: a meta-analysis. Sci Rep. 2018;8:9039. doi: 10.1038/s41598-018-27297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fohner AE, Wang Z, Yracheta J, et al. Genetics, diet, and season are associated with serum 25-hydroxycholecalciferol concentration in a Yup’ik study population from Southwestern Alaska. J Nutr. 2016;146:318–25. doi: 10.3945/jn.115.223388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DM, Looker AC, Chang SC, et al. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99:1594–1602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, et al. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–63. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32:210–6. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Hu K, Callen DF, Li J, et al. Circulating vitamin D and overall survival in breast cancer patients: a dose-response meta-analysis of cohort studies. Integr Cancer Ther. 2018;17:217–25. doi: 10.1177/1534735417712007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail A, El-Awady R, Mohamed G, et al. Prognostic significance of serum vitamin D levels in Egyptian females with breast cancer. Asian Pac J Cancer Prev. 2018;19:571–6. doi: 10.22034/APJCP.2018.19.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SM, Shin EA. Exploring vitamin D metabolism and function in cancer. Exp Mol Med. 2018;50:20. doi: 10.1038/s12276-018-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermani IA, Kojidi HT, Gharamaleki JV, et al. Association of serum level of 25 hydroxy-vitamin D with prognostic factors for breast cancer. Asian Pac J Cancer Prev. 2011;12:1381–4. [PubMed] [Google Scholar]
- Kim Y, Je Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysis. Br J Cancer. 2014;110:2772–84. doi: 10.1038/bjc.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, Jeon YW, Suh YJ. Association between alterations in the serum 25-hydroxyvitamin D status during follow-up and breast cancer patient prognosis. Asian Pac J Cancer Prev. 2015;16:2507–13. doi: 10.7314/apjcp.2015.16.6.2507. [DOI] [PubMed] [Google Scholar]
- Lohmann AE, Chapman JAW, Burnell MJ, et al. Prognostic associations of 25 hydroxy vitamin D in NCIC CTG MA21, a phase III adjuvant randomized clinical trial of three chemotherapy regimens in high-risk breast cancer. Breast Cancer Res Treat. 2015;150:605–11. doi: 10.1007/s10549-015-3355-x. [DOI] [PubMed] [Google Scholar]
- Manson JE, Cook NR, Lee IM, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiasen IS, Lademann U, Jäättelä M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res. 1999;59:4848–56. [PubMed] [Google Scholar]
- McKibben RA, Zhao D, Lutsey PL, et al. Factors associated with change in 25-hydroxyvitamin D levels over longitudinal follow-Up in the ARIC Study. J Clin Endocrinol Metab. 2016;101:33–43. doi: 10.1210/jc.2015-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr SB, Gorham ED, Kim J, et al. Meta-analysis of vitamin D sufficiency for improving survival of patients with breast cancer. Anticancer Res. 2014;34:1163–6. [PubMed] [Google Scholar]
- Neyestani TR, Gharavi A, Kalayi A. Determination of serum 25-hydroxy cholecalciferol using high-performance liquid chromatography: a reliable tool for assessment of vitamin D status. Int J Vitam Nutr. 2007;77:341–6. doi: 10.1024/0300-9831.77.5.341. [DOI] [PubMed] [Google Scholar]
- Ng K, Nimeiri HS, McCleary NJ, et al. Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: The SUNSHINE Randomized Clinical Trial. JAMA. 2019;321:1370–9. doi: 10.1001/jama.2019.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KM, Sandler DP, Taylor JA, et al. Serum vitamin D and risk of breast cancer within five Years. Environ Health Perspect. 2017;125:077004. doi: 10.1289/EHP943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri C, MacGregor T, Girgis S, et al. Serum 25-hydroxyvitamin D levels in early and advanced breast cancer. J Clin Pathol. 2006;59:1334–6. doi: 10.1136/jcp.2006.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AC, Taylor CL, Yaktine AL, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui I, Giovannucci E. Vitamin D status and cancer incidence and mortality. Adv Exp Med Biol. 2014;810:33–51. doi: 10.1007/978-1-4939-0437-3_3. [DOI] [PubMed] [Google Scholar]
- Thanasitthichai S, Chaiwerawattana A, Prasitthipayong A. Association of vitamin D level with clinicopathological features in breast cancer. Asian Pac J Cancer Prev. 2015;16:4881–3. doi: 10.7314/apjcp.2015.16.12.4881. [DOI] [PubMed] [Google Scholar]
- Tretli S, Schwartz GG, Torjesen PA, et al. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: a population-based study. Cancer Causes Control. 2012;23:363–70. doi: 10.1007/s10552-011-9885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaseñor A, Ballard-Barbash R, Ambs A, et al. Associations of serum 25-hydroxyvitamin D with overall and breast cancer-specific mortality in a multiethnic cohort of breast cancer survivors. Cancer Causes Control. 2013;24:759–67. doi: 10.1007/s10552-013-0158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Kwan ML, Ergas IJ, et al. Association of serum level of vitamin D at diagnosis with breast cancer survival: a case-cohort analysis in the pathways study. JAMA Oncol. 2017;3:351–7. doi: 10.1001/jamaoncol.2016.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]


