Abstract
Aim:
CAPOX treatment in CRC patients was reported to cause several dose-limiting toxicities, and are found responsible for treatment interruption or even discontinuation. Therefore there is a critical need for identifying the predictive biomarkers for such toxicities to prevent them. The aim of our present study is to find the influence of DPYD*9A, DPYD*6 and GSTP1 ile105val gene polymorphisms on CAPOX treatment-associated toxicities in south Indian patients with CRC.
Patients and Methods:
We have recruited 145 newly diagnosed and treatment naive CRC patients in the study. Each Patient received a standard treatment schedule of oxaliplatin 130 mg/m2 infusion over 2 hours on day 1 and oral capecitabine 1000mg/m2 in divided doses twice daily for the next 14 days of a 21-day cycle. 5 ml of the venous blood was collected from each patient and genomic DNA extraction and genotyping. The genotyping analysis of the selected genetic polymorphisms was carried out by real-time PCR using TaqMan SNP genotyping assays obtained from applied biosystems.
Results:
The major dose-limiting toxicities observed with CAPOX treatment were thrombocytopenia, HFS and PN. DPYD*9A carries were found to be at higher risk for HFS, diarrhoea and thrombocytopenia when compared to patients with wild allele. No significant association was found between DPYD*6, GSTP1 ile105val polymorphisms and CAPOX related toxicities except for thrombocytopenia.
Conclusion:
A significant association was observed between DPYD*9A polymorphism and CAPOX induced dose-limiting toxicities strengthening its role as a predictive biomarker.
Key Words: CAPOX, Toxicities, Predictive markers, DPYD*9A, DPYD*6, GSTP1 ile105va, Colon cancer
Introduction
5-fluorouracil and its oral prodrug capecitabine are the widely used anticancer agents and indeed their combination regimens with other anticancer drugs are the backbone in the treatment of various cancers like colon, rectum, breast, stomach, oesophagal and pancreatic cancer etc (Malet-Martino and Martino, 2002). Capecitabine and oxaliplatin combination regimen (CAPOX) is the standard chemotherapeutic care for the treatment of colorectal cancer (CRC) in the last decade. CAPOX treatment was proven to be equivalent or non-inferior to standard regimens like FOLFOX and FOLFRI in the treatment of advanced and metastatic CRC (Pectasides et al., 2015; Guo et al., 2016; Sobrero et al., 2018) and preferred over its counterparts due to convenience in administration and easy management. (Wehler et al., 2012) However, CAPOX treatment was associated with several dose-limiting haematological and non-haematological toxicities (Mullally et al., 2018; Baird et al., 2011) and patients show high inter-individual variation to its toxicity profile. (Haller et al., 2008) These toxicities may limit treatment effectiveness as they impose treatment interruption or even discontinuation and often require hospitalization which in turn increases health care costs. Therefore there is a critical need for identifying the predictive biomarkers for CAPOX related toxicities.
Dihydropyrimidine dehydrogenase (DPYD) is a rate-limiting enzyme and found to metabolize 80% of the administered capecitabine. (Caudle et al., 2013) DPYD gene was found to be genetically polymorphic and its deficiency was reported to cause severe toxicities with capecitabine treatment. The US food and drug administration (FDA) and European medical agency (EMA) approved drug label of capecitabine warns for the unexpected, severe toxicities like stomatitis, hand-foot syndrome (HFS), diarrhoea, mucosal inflammation, neutropenia in the deficiency of DPYD enzyme and states that no dose has been proven safe in patients with complete absence of DPYD enzyme. (Xeloda-Epar-Product-Information.; XELODA (Capecitabine) Tablets, for Oral Use,”) DPYD*2A polymorphism (1905+1 G>A splice donor variant) is a classic genetic variant and individuals who express this polymorphism were reported not to metabolize capecitabine at a normal rate and were found to be at a higher risk of developing severe life-threatening toxicities. (Toffoli et al., 2015; Deenen et al., 2016) However, the major limitation of DPYD*2A of being used as a predictive marker for toxicity is its lower minor allele frequency varying from 0.1 % to 1% in different ethnic groups. (Henricks et al., 2017) A recent study states that only a 50 % patients with DPYD*2A carriers actually develop toxicity with 5-FU treatment and reported that novel DPYD variants, DPYD*9 and DPYD*6 carry 2 fold higher risk for toxicities with 5 –FU treatment when compared to DPYD*2A polymorphism alone (Gentile et al., 2016). This emphasizes the search for novel genetic toxicity predictive markers for capecitabine treatment.
DPYD*9A (rs id 1801265) is a novel missense single nucleotide variant (A>G) located on chromosome 1, at position 97883329. The A>G replacement induces an amino acid change of cysteine to arginine in the coding region and found to alter the DPYD enzyme activity. DPYD*6 (rs id 1801160) is another missense single nucleotide variant (C>T) located in the chromosome 1, at position 97305364. The C>T replacement induces amino acid change valine to isoleucine. Both these polymorphisms were reported to alter the catalytic activity of DPYD enzyme and linked with capecitabine associated toxicities. (Offer et al., 2014; Gross et al., 2003; Baskin et al., 2015)
Glutathione-S-transferase P1 (GSTP1) is a rate-limiting enzyme involved in detoxification of oxaliplatin. It mediates oxaliplatin-glutathione conjugation (GSH) reaction for the easy elimination of oxaliplatin from the body through kidneys. GSTP*1 Ile105val (rs1695, A>G) is a missense single-nucleotide on chromosome 11 at position 67585218 and was found to lower the expression of GSTP1 enzyme. Oxaliplatin-related cumulative neuropathy and neutropenia were reported to be more frequent and severe in patients with heterozygous (AG) and homozygous (GG) genotype when compared to wild allele (AA) patients (Lecomte et al., 2006; Zhong et al., 2006) The aim of our study was to find the association between DPYD and GSTP1 gene polymorphisms and toxicities with CAPOX treatment in south Indian patients with colorectal cancer.
Materials and Methods
The study was approved by the JIPMER scientific advisory committee (JSAC Reg.No.JSAC 34/6/2016) and JIPMER ethics committee (JEC Reg.No: 25-5-2016). In a prospective cohort study, we recruited 145 newly diagnosed and treatment-naive CRC patients from January 2016 to December 2018. Patients with age ≥ 18 years of either gender, who were scheduled to receive CAPOX as their standard treatment care were included in the study. Previously treated, pregnant, lactating women and patients with abnormal liver function (serum transaminases ≥ 2 times the normal value) or renal function (creatinine >1.5 g/dl) parameters were excluded from the study. Patients received regimens other than CAPOX were excluded from the study. The demographic details and patients characteristics like age, sex, cancer stage, treatment setting, comorbidities, smoking and drinking habits were collected at baseline. Apart from the above data, baseline haematological values, renal and liver function parameters, starting dose of CAPOX, any dose reduction or treatment delay or drug discontinuation and treatment-related deaths were recorded during each follow-up. Informed consent was obtained from all the patients.
Each patient received a standard treatment schedule of oral capecitabine 1,000 mg/m2 in divided doses twice daily for 14 days and oxaliplatin 150 mg/m2 infusion over 2 hours on day 1 of a 21-day cycle. The median number of CAPOX cycles administered was 12. During each cycle, the treatment-related toxicities were noted and analyzed. The toxicities were divided into haematological and non-haematological toxicities. All the toxicities are graded for severity by using common terminology criteria for adverse events (CTCAE). (“Common Terminology Criteria for Adverse Events” 2017)
DNA extraction and genotyping
5 ml of the venous blood was collected from each patient and subjected to centrifugation for 5 min at 2,500 g for plasma separation. Plasma was discarded and the pellets containing red blood cells (RBC) with the buffy coat of white blood cells (WBC) were stored at −20°C until DNA extraction. Genomic DNA was extracted from the WBC by the phenol-chloroform method. (“Shared Protocol-Extracting-DNA-Using-Phenol-Chloroform.Pdf” n.d.) The extracted DNA was analyzed qualitatively and quantitatively using biophotometer plus (Eppendorf AG 22331, Hamburg, Germany). Each DNA sample was diluted to an optimal concentration of 50 ng/μL suitable for further downstream analysis and stored in aliquots at 4°C. The genotyping analysis for the selected single nucleotide polymorphism (DPYD*9A, DPYD*6 and Ile105val A>G was carried out by real-time PCR (7300 Applied Biosystems; Life Technologies Corporation, Carlsbad, CA, USA) using TaqMan SNP genotyping assays (rs id 1801265, rs id 1801160, rs 1695) purchased from applied biosystems. Version 1.4 of 7300 sequence detection software (SDS) was used for absolute quantification and allelic discrimination (Kodidela et al., 2015)
Diagram1.
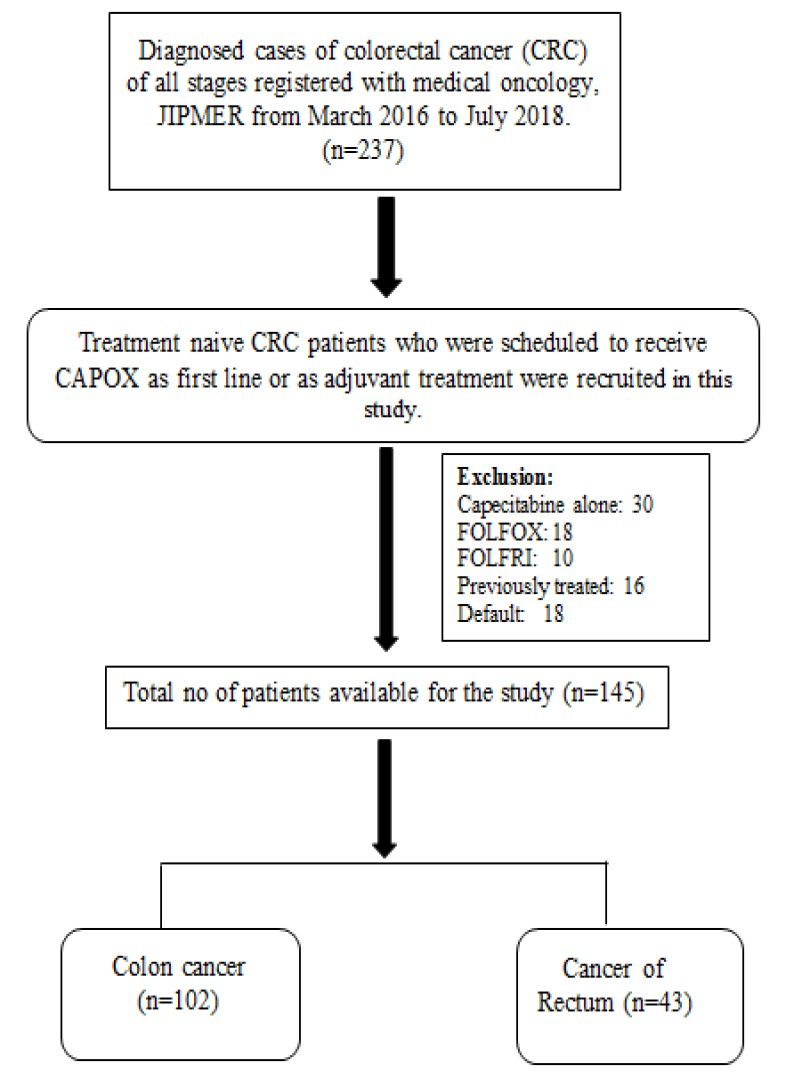
Flow- Diagram
Toxicity grading and Statistics
All the toxicities are graded according to common terminology criteria of adverse effects version 3.0 (CTCAE). Demographic parameters were expressed as mean ± Standard deviation. Adverse effects are represented in percentages and analyzed descriptively. Genotyping frequencies of the selected polymorphisms were analyzed for hardy Weinberg equilibrium. The association between genetic variants and CAPOX related toxicities were analyzed by chi-square association test using SPSS version 19.0 (IBM-SPSS). Multinomial regression analysis is done to find the influence of confounding factors on toxicities. A p-value of less than 0.05 was considered to be statically significant.
Results
The number of male patients was 90 with a mean age of 50 ± 13 and female patients were 55 with a mean age of 49 ± 12. The number of patients diagnosed with colon cancer were 102 and with the rectum cancer were 43. Most of the patients received CAPOX as an adjuvant treatment (48.2%) or palliative care (42%). The median number of CAPOX cycles administered were 12 and the median follow up time was 18 months. Other baseline characteristics of the patients included in the study are tabulated in Table 1.
Table 1.
Baseline Characteristics of the Patients
| S.no | No of Patients |
|||
|---|---|---|---|---|
| Characteristics | Colon | Rectum | Total (%) | |
| 1 | Gender | |||
| a Male | 62 | 28 | 90 (62) | |
| b. Female | 40 | 15 | 55 (38) | |
| 2 | Age in years – mean ± SD | 50±13 | 49±12 | -- |
| 3 | Ethnicity | |||
| a. Tamilian | 82 | 29 | 111 (76.5) | |
| b. Andhra | 11 | 9 | 11 (14.4) | |
| c. North Indians | 9 | 5 | 7 (9.6) | |
| 4 | Performance status | |||
| a. 0-1 | 76 | 30 | 106 (73.1) | |
| b. 2 | 18 | 9 | 27 (19) | |
| c. 3 | 8 | 4 | 12 (8) | |
| 5 | Tumour site | |||
| a. Right colon | 49 | -- | 49 (33.7) | |
| b. Left colon | 53 | -- | 53 (36.5) | |
| c. Rectum | -- | 43 | 43 (29.6) | |
| 6 | Cancer stage | |||
| a. II | 22 | 4 | 26 (18) | |
| b. III | 38 | 19 | 57 (39.3) | |
| c. IV | 42 | 20 | 62 (42.7) | |
| 7 | Chemotherapy setting | |||
| a. Neoadjuvant | 5 | 8 | 13 (9) | |
| b. Adjuvant | 55 | 15 | 70 (48.2) | |
| c. Palliative | 42 | 20 | 62 (42.7) | |
| 8 | Habits | |||
| a. Smoking | 15 | 7 | 22 (15.1) | |
| b. Alcoholic | 7 | 5 | 12 (8.2) | |
| c. Smoking+ Alcoholic | 10 | 8 | 18 (12.4) | |
| 9 | Comorbidities | |||
| a. Diabetes | 10 | 8 | 18 (12.4) | |
| b. Hypertension | 12 | 7 | 21 (14.4) | |
| c. Thyroid disorders | 2 | 0 | 2 (1.3) | |
The genotyping frequencies of DPYD*9, DPYD*6 and GSTP1 ile105val polymorphisms were in Hardy Weinberg Equilibrium. The most frequently observed haematological toxicities were anaemia, thrombocytopenia (TP) and neutropenia (NP), whereas vomiting, HFS and PN are the frequently observed non-haematological toxicities. The major dose-limiting toxicities were thrombocytopenia, HFS and PN. A total of 24% of the patients needed dose reduction, 14% of the patients needed treatment delay and 10% of the needed drug discontinuation due to toxicities. (Table 2 and 3).
Table 2.
Observed Genotype Frequency and Toxicity Frequency in CRC Patients (N=145)
| Toxicity frequency across genotypes –N (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| S.no | Gene & genotype | Freq | Anemia | TP | NP | Diarrhoea | Vomiting | HFS | PN |
| 64 (44%) | 50 (35%) | 31 (21%) | 33(22%) | 58 (40%) | 63(43%) | 46 (32%) | |||
| 1 | DPYD*9A | ||||||||
| AA | 100 | 45 | 28 | 20 | 14 | 30 | 26 | 26 | |
| AG | 35 | 12 | 17 | 6 | 12 | 22 | 29 | 17 | |
| GG | 10 | 7 | 5 | 5 | 7 | 6 | 8 | 3 | |
| 2 | DPYD*6 | ||||||||
| CC | 122 | 54 | 41 | 24 | 22 | 37 | 42 | 32 | |
| CT | 18 | 8 | 7 | 6 | 8 | 18 | 15 | 12 | |
| TT | 5 | 2 | 2 | 1 | 4 | 3 | 3 | 2 | |
| 3 | GSTP1 ile105val | ||||||||
| AA | 70 | 28 | 18 | 14 | 28 | 15 | 32 | 21 | |
| AG | 57 | 23 | 22 | 10 | 18 | 10 | 21 | 13 | |
| GG | 18 | 8 | 10 | 7 | 12 | 8 | 10 | 12 | |
TP, thrombocytopenia; NP, neutropenia; HFS, hand foot syndrome; PN, peripheral neuropathy.
Table 3.
No of Patients with Dose Reduction or Treatment Delay or Drug Discontinuation due to Toxicities (N=145)
| S.no | Toxicity | No of patients with dose reduction (%) | No of patients with treatment delay (%) | No of patients with drug discontinuation (%) |
|---|---|---|---|---|
| 1 | Anaemia | 6 (4.1) | 3 (2) | 0 (0) |
| 2 | TP | 9 (6.2) | 4 (2.7) | 4 (2.7) |
| 3 | HFS | 8 (5.5) | 3 (2) | 6 (4.1) |
| 4 | PN | 6 (4.1) | 5 (3.4) | 3 (2) |
| 5 | Diarrhoea | 5 (3.4) | 3 (2) | 0 (0) |
| 6 | Vomiting | 1 (0.6) | 3 (2) | 0 (0) |
| 7 | Infusional reaction | 0 (0) | 0 (0) | 2 (1.3) |
| Total | 35 (24%) | 21 (14%) | 15 (10.3%) |
In a dominant model of genotyping analysis, we found a significant association between DPYD*9A polymorphism and capecitabine related toxicities strengthening its role as a predictive biomarker. Patients with DPYD*9A polymorphism had a 2.4 (CI: 1.18-5.1) times higher risk for thrombocytopenia, 2.7 (CI: 1.8-4) times higher risk for diarrhoea and 2.3 (CI: 1.8-4.7) times higher risk for HFS when compared to wild type patients. No significant association was found between DPYD*6 polymorphism and capecitabine related toxicities. Similarly, we did not find any significant association between GSTP1 ile105val polymorphism and oxaliplatin-related toxicities except for thrombocytopenia. Patients with GSTP1 ile105val polymorphism had 2 (CI: 1.1-4.1) times higher risk for thrombocytopenia when compared to wild type (Table 4 and 5).
Table 4.
Association between Haematological Toxicities and Genetic Polymorphisms (DPYD*9A, DPYD*6 and GSTP1 ile105val)
| S.no | Model | Genotype | Freq | Observed toxicity across genotypes |
|||
|---|---|---|---|---|---|---|---|
| Anemia | Thrombocytopenia | Neutropenia | |||||
| 1 | DPYD*9A | AA | 100 | 45 | 28 | 20 | |
| AG | 35 | 12 | 17 | 6 | |||
| GG | 10 | 7 | 5 | 5 | |||
| Dominant model | AA (ref) AG+GG | 100 35+10 |
P-value Odds (CI) |
0.8 0.9 (0.4-1.8) |
0.01* 2.4 (1.18-5.1) |
0.5 1.24 (0.5-2.9) |
|
| 2 | DPYD*6 | CC | 122 | 54 | 41 | 24 | |
| CT | 18 | 8 | 7 | 6 | |||
| TT | 5 | 2 | 2 | 1 | |||
| Dominant model | CC(ref) CT+TT | 122 18+5 |
P-value Odds (CI) |
0.8 1.9 (0.4-2.6) |
0.6 1.2 (0.5-3.1) |
0.2 1.7 (0.6-1.7) |
|
| 3 | GSTP1 ile105val | AA | 70 | 28 | 18 | 14 | |
| AG | 57 | 23 | 22 | 10 | |||
| GG | 18 | 8 | 10 | 7 | |||
| Dominant model | AA (ref) AG+GG |
70 57+18 |
P-value Odds (CI) |
0.8 0.9 (0.4-1.8) |
0.04 2 (1.1-4.1) |
0.6 1.2 (0.5-2.6) |
|
ref, Reference; *, Significant; The frequency of wild type allele (high frequency allele) was taken as reference for calculating the odds ratio in dominant model of genotyping analysis.
Table 5.
Association between Non-Haematological Toxicities and Genetic Variants (DPYD*9A, DPYD*6 and GSTP1 ile105val)
| S.no | Model | Genotype | Freq | Observed toxicity across genotypes |
||||
|---|---|---|---|---|---|---|---|---|
| Vomiting | Diarrhoea | HFS | PN | |||||
| 1 | DPYD*9A | AA | 100 | 35 | 18 | 37 | 32 | |
| AG | 35 | 17 | 8 | 18 | 10 | |||
| GG | 10 | 6 | 7 | 8 | 4 | |||
| Dominant model | AA (ref) AG+GG |
100 35+10 |
P value Odds (CI) | 0.06 1 (0.9-4) |
0.04 * 2.7 (1.8-4) |
0.02* 2.3 (1.8-4( |
0.9 09 (0.4-2) |
|
| 2 | DPYD*6 | CC | 122 | 49 | 25 | 54 | 38 | |
| CT | 18 | 9 | 6 | 7 | 5 | |||
| TT | 5 | 0 | 2 | 1 | 3 | |||
| Dominant model | CC (ref) CT+TT |
122 18+5 |
P value Odds (CI) | 0.9 1 (0.4-2) |
0.1 1.8 (0.7-2) |
0.3 0.6 (0.2-1) |
0.7 1.1 (0.4-2) |
|
| 3 | GSTP1 ile105val | AA | 70 | 28 | 15 | 32 | 21 | |
| AG | 57 | 18 | 10 | 21 | 13 | |||
| GG | 18 | 12 | 8 | 10 | 12 | |||
| Dominant model | AA (ref) AG+GG | 70 57+18 |
P value Odds (CI) | 0.8 1 (0.4-2) |
0.9 1 (0.4-2) |
0.2 1 (0.3-1) |
0.7 1.2 (0.5-2) |
|
ref, Reference; *, Significant; The frequency of wild type allele (high frequency allele) was taken as reference for calculating the odds ratio in dominant model of genotyping analysis.
We also performed a multinomial logistic regression analysis to find the influence of covariates such as age, sex, ethnicity, patient’s performance, cancer type, treatment setting and cancer stage on CAPOX related toxicities. We dint find any significant association between the covariates and toxicities except for age and thrombocytopenia. Patients with age group <50 years had shown a significant association with thrombocytopenia when compared with an age group >50 (Table 6 and 7).
Table 6.
Multinominal Regression Analysis between Covariates and Hematological Toxicities
| S.no | Co-variate | Anemia |
Thrombocytopenia |
Neutropenia |
|---|---|---|---|---|
| P value - Odds (CI) | P value-Odds (CI) | P value-Odds (CI) | ||
| 1 | Age | 0.5- 1 (0.4-2) | 0.01 – 2.5 (0.4-5) | 0.08 – 2.3 (0.8-6) |
| a. <50 | Ref | Ref | Ref | |
| b. >50 | ||||
| 2 | Sex | |||
| a. Male | 0.9 –1 (0.5-2.2) | 0.9 – 1 (0.4-2.3) | 0.1 – 0.8 (0.1-2) | |
| b. Female | Ref | Ref | Ref | |
| 3 | Ethnicity | |||
| a. Tamilian | 0.4 - 0.6 (0.1-2.1) | 0.5 – 1.4 (0.4-5) | 0.7- 1.2 (0.2-6) | |
| b. Andhra | 0.5 - 0.6 (0.1-2.4) | 0.3 - 2 (0.4-9) | 0.5 – 0.8 (0.1-3.5) | |
| c. North Indians | Ref | Ref | Ref | |
| 4 | Performance | |||
| a. 0-1 | 0.07 -1.7 (0.1-3) | 0.2 – 1.2 (0.4-1.3) | 0.6 – 0.9 (0.1-4) | |
| b. 2 | 0.1 - 0.9 (0.3-1.8) | 0.1 - 1 (0.6-1.7) | 0.1 – 1 (0.6-3) | |
| c. 3 | Ref | Ref | Ref | |
| 5 | Setting | |||
| a. Adjuvant | 0.5 – 1.4 (0.3-5) | 0.9 – 1 (0.2-4) | 0.2 – 1 (0.5-1.8) | |
| b. Palliative | Ref | Ref | Ref | |
| 6 | Cancer | |||
| a. Colon | 0.2 – 1.5 (0.7-3) | 0.4 – 1.3 (0.6-2) | 0.5 – 1.3 (0.4-3) | |
| b. Rectum | Ref | Ref | Ref | |
| 7 | Cancer stage | |||
| a. II | 0.3 – 0.5 (0.1-1.7) | 0.6 – 1 (0.2-2) | 0.8 – 1 (0.2 -4) | |
| b. III | 0.9 – 0.9 (0.2-4) | 0.9 – 1 (0.2-4) | 0.2 – 1.5 (0.4- 5) | |
| c. IV | Ref | Ref | Ref |
Ref, Reference; *, Significant
Table 7.
Multinominal Regression Analysis between Covariates and Non-Hematological Toxicities
| S.no | Co-variate | HFS |
PN |
Diarrhoea |
Vomiting |
|---|---|---|---|---|---|
| P value-Odds (CI) | P value-odds (CI) | P value-odds (CI) | P value-odds (CI) | ||
| 1 | Age | ||||
| a. <50 | 0.851 -1.7 (0.5-2) | 0.3 - 1.3 (0.6-2.9) | 0.6 – 1.2 (0.5-2.7) | 0.7 – 1.1 (0.5-2.3) | |
| b. >50 | Ref | Ref | Ref | Ref | |
| 2 | Sex | ||||
| a. Male | 0.615 -1.2 (0.5-2) | 0.6 – 0.8 (0.3-1.8) | 0.3 – 1.4 (0.6-3.5) | 0.7 – 1.1 (0.5-2.4) | |
| b. Female | Ref | Ref | Ref | Ref | |
| 3 | Ethnicity | ||||
| a. Tamilian | 0.26 - 1.4 (0.6-2) | 0.8 – 1.1 (0.3-3.9) | 0.85 - 0.8 (0.2-3) | 0.9 – 1 (0.2-3.4) | |
| b. Andhra | 0.19 - 1.6 (0.6-2) | 0.4 – 1.8 (0.3-8.6) | 0.13 -0.29 (0.4-1) | 0.4 – 0.5 (0.1-2.4) | |
| c. North Indians | Ref | Ref | Ref | Ref | |
| 4 | Performance | ||||
| a. 0-1 | 0.09 - 0.5 (0.2-1) | 0.4 – 0.5 (0.7-3) | 0.4 - 0.9 (0.7-3) | 0.6 – 1.5 (0.2-3) | |
| b. 2 | 0.2 - 1 (0.3-2) | 0.9 – 1 (0.14-6) | 0.8 - 0.7 (0.1-4) | 0.1 - 1.2 (0.2-1.2) | |
| c. 3 | Ref | Ref | Ref | Ref | |
| 5 | Setting | ||||
| a. Adjuvant | 0.1 - 1.1 (0.7-1) | 0.8 – 1 (0.19-3) | 0.3 – 1.3 (0.4-2) | 0.4 – 1.4 (0.7-3.4) | |
| b. Palliative | Ref | Ref | Ref | Ref | |
| 6 | Cancer | ||||
| a. Colon | 0.8 - 1.3 (0.3-4) | 0.2 – 1.5 (0.7-3) | 0.9 – 1 (0.4-2) | 0.16 – 0.5 (0.2-1) | |
| b. Rectum | Ref | Ref | Ref | Ref | |
| 7 | Cancer stage | ||||
| a. II | 0.8 - 0.9 (0.2-2) | 0.7 – 0.8 (0.2-2.7) | 0.07 – 0.6 (0.1-1) | 0.2 – 0.9 (0.6-1.2) | |
| b. III | 0.7 - 1.2 (0.3-5) | 0.9 – 1 (0.2-5.1) | 0.9 – 1 (0.1-5) | 0.7 – 1.6 (0.8-2.4) | |
| c. IV | Ref | Ref | Ref | Ref |
Ref, Reference; *, significant; HFS, hand foot syndrome; PN, peripheral neuropathy
Discussion
The adverse drug effects (ADE) associated with cancer chemotherapy are a real concern for the patients and clinicians as they cause treatment interruption or even discontinuation. The current strategies of toxicity management with anticancer drugs either follow a holistic approach and nor addresses the long term complications. Looking for the inter-individual genetic makeup of an individual is a novel approach for predicting the toxicities associated with anticancer drugs. Studying genetic alterations, mainly the genes coding for the drug-metabolizing enzymes can serve as an important tool in identifying predictive biomarkers for drug-related toxicities. The prior screening and adjusting the dose in such patients can decrease the ADE rate.
In the present study, we looked for the adverse effects related to CAPOX treatment and their association with DPYD and GSTP1 gene polymorphisms. Thrombocytopenia, HFS and PN were the major dose-limiting toxicities observed with CAPOX treatment. HFS is a characteristic side effect with capecitabine with the symptoms ranging from mild blackish skin discolouration to severe skin changes like peeling, blisters, bleeding and pain mainly in the palm of the hands and sole of the feet. (Lassere and Hoff, 2004) PN is dose-limiting toxicity associated with oxaliplatin and occurs due to drug accumulation either in the sensory or motor neurons. The involvement of sensory neurons often results in disturbing sensations like numbness, burning and shooting pain in the affected areas. Motor involvement often causes muscle weakness and paralysis. (Saif and Reardon, 2005)
The implementation consortium guidelines (CPIC) of 2017 on DPYD genotyping states that DPYD*9A polymorphism reduces the enzyme activity however it doesn’t affect in a clinically relevant manner and limited its utility as a predictive toxicity biomarker. (Caudle et al., 2013) In the present study, we found a significant association between DPYD*9A polymorphism and capecitabine related toxicities strengthening its role as a predictive biomarker. The dominant model of genotyping analysis (AA vs AG+GG) has shown that heterozygous (AG) and homozygous (GG) carriers have a higher risk for HFS, diarrhoea and thrombocytopenia when compared to wild type (AA) carriers. Supporting to our study findings a recent study by Kushman et al. reported a significant association between DPYD*9A polymorphism and fluoropyrimidine induced toxicities in patients with gastrointestinal malignancies and recommend the oncologists to consider regular DPYD*9A screening along with other potential DPYD gene polymorphisms like DPYD*2A, 13 A and 9B (Khushman et al., 2018).
The available data on DPYD*6 polymorphism as a predictive biomarker is limited and conflicting. The DPYD implementation consortium guidelines (CPIC) of 2017 states that DPYD*6 presence may not always result in toxicity and its association with toxicities was not consistently replicated (Caudle et al., 2013) However, a recent study by Del Re et al., (2019) reported a 29% reduction in the DPYD enzyme activity in presence DPYD*6 polymorphism when compared to wild type and found a significant association with capecitabine related adverse effects. They also suggest for preemptive analysis for DPYD*6 polymorphism and 20% dose reduction in the homozygous variants and close monitoring of heterozygous variants. A study by Gentile et al., (2016) also reported that DPYD*6 and DPYD*9A are in strong haplotype association (hap 7) and their presence carries 2 fold higher risk of 5-FU toxicity compared to DPYD*2A polymorphism alone in the Italian population. However, in the present study, we observed no significant association between DPYD*6 polymorphism and capecitabine related toxicities. The lack of association may be due to observed low frequency of DPYD*6 hetero and homozygous mutants in our study cohort.
GSTP1 ile105val is one of the widely studied variants and has been highly linked for causing oxaliplatin-induced PN. Several independent studies reported a significant association between GSTP1 ile105val polymorphism and oxaliplatin-related PN (Lecomte et al., 2006; Chen et al., 2010; Kumamoto et al., 2013). However, a meta-analysis which is based on twelve prospective trials and two retrospective trials reported for no significant association between GSTP1 ile105val polymorphism and oxaliplatin-induced cumulative PN in allele dominant model and recessive model of analysis (Peng et al., 2013). Our study results are consistent with the meta-analysis data. We didn’t find any significant association between GSTP1 le105val polymorphism and oxaliplatin-induced PN in the dominant model of analysis.
In conclusion Thrombocytopenia, HFS and PN were the major dose-limiting toxicities with CAPOX regimen. A significant association was observed between DPYD*9A polymorphism and CAPOX induced toxicities like HFS, diarrhoea and thrombocytopenia strengthening its role as a predictive biomarker. No significant association was found between DPYD*6, GSTP1 ile105val polymorphisms and CAPOX induced toxicities.
References
- Baird R, Biondo A, Chhaya V, et al. Toxicity associated with capecitabine plus oxaliplatin in colorectal cancer before and after an institutional policy of capecitabine dose reduction. Br J Cancer. 2011;104:43–50. doi: 10.1038/sj.bjc.6605995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin Y, Arsalan A, Olcun UU, Gizem C, Ilhan O. Dihydropyrimidine dehydrogenase 85T>C mutation is associated with ocular toxicity of 5-fluorouracil: A case report. Am J Ther. 2015;22:36–9. doi: 10.1097/MJT.0b013e31829e8516. [DOI] [PubMed] [Google Scholar]
- Caudle KE, Thorn CF, Klein TE, et al. Clinical pharmacogenetics implementation consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin Pharmacol Ther. 2013;94:640–5. doi: 10.1038/clpt.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Cheng-Hwai T, Po-Min C, et al. Influence of GSTP1 I105V polymorphism on cumulative neuropathy and outcome of FOLFOX-4 treatment in Asian patients with colorectal carcinoma. Cancer Sci. 2010;101:530–5. doi: 10.1111/j.1349-7006.2009.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenen MJ, Didier M, Annemieke C, et al. Upfront genotyping of DPYD*2A to individualize fluoropyrimidine therapy: A safety and cost analysis. J Clin Oncol. 2016;34:227–34. doi: 10.1200/JCO.2015.63.1325. [DOI] [PubMed] [Google Scholar]
- Del Re M, Saverio C, Angela M, et al. DPYD*6 Plays an important role in fluoropyrimidine toxicity in addition to DPYD*2A and c 2846A>T: A comprehensive analysis in 1254 patients. Pharmacogenomics J. 2019 doi: 10.1038/s41397-019-0077-1. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile G, Botticelli A, Lionetto L, et al. Genotype-phenotype correlations in 5-fluorouracil metabolism: A candidate DPYD haplotype to improve toxicity prediction. Pharmacogenomics J. 2016;16:320–5. doi: 10.1038/tpj.2015.56. [DOI] [PubMed] [Google Scholar]
- Gross E, Tobias U, Katharina S, et al. Detailed analysis of five mutations in dihydropyrimidine dehydrogenase detected in cancer patients with 5-fluorouracil-related side effects. Hum Mutat. 2003;22:498–507. doi: 10.1002/humu.9201. [DOI] [PubMed] [Google Scholar]
- Guo Y, Bing-Hong X, Tao Z, Yong C, Li M. XELOX vs. FOLFOX in metastatic colorectal cancer: An updated meta-analysis. Cancer Invest. 2016;34:94–104. doi: 10.3109/07357907.2015.1104689. [DOI] [PubMed] [Google Scholar]
- Haller DG, Jim C, Stephen JC, et al. Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol. 2008;26:2118–23. doi: 10.1200/JCO.2007.15.2090. [DOI] [PubMed] [Google Scholar]
- Henricks LM, Opdam FL, Beijnen JH, et al. DPYD genotype-guided dose individualization to improve patient safety of fluoropyrimidine therapy: Call for a drug label update. Ann Oncol. 2017;28:2915–22. doi: 10.1093/annonc/mdx411. [DOI] [PubMed] [Google Scholar]
- Kodidela S, Pradhan SC, Dubashi.B , Basu D. Influence of dihydrofolate reductase gene polymorphisms rs408626 (-317A>G) and rs442767 (-680C>A) on the outcome of methotrexate-based maintenance therapy in South Indian patients with acute lymphoblastic leukaemia. Eur J Clin Pharmacol. 2015;11:1349–58. doi: 10.1007/s00228-015-1930-z. [DOI] [PubMed] [Google Scholar]
- Khushman Moh’d, Girijesh KP, Peter JH, et al. Germline pharmacogenomics of DPYD*9A (c85T>C) variant in patients with gastrointestinal malignancies treated with fluoropyrimidines. J Gastrointest Oncol. 2018;9:416–24. doi: 10.21037/jgo.2018.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto K, Keiichiro I, Norimichi O, et al. Polymorphisms of GSTP1, ERCC2 and TS-3’UTR are associated with the clinical outcome of MFOLFOX6 in colorectal cancer patients. Oncol Lett. 2013;6:648–54. doi: 10.3892/ol.2013.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassere Y, Paulo H. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda) Eur J Oncol Nurs. 2004;8:31–40. doi: 10.1016/j.ejon.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Lecomte T, Bruno L, Philippe B, et al. Glutathione S-transferase P1 polymorphism (Ile105Val) predicts cumulative neuropathy in patients receiving oxaliplatin-based chemotherapy. Clin Cancer Res. 2006;12:3050–6. doi: 10.1158/1078-0432.CCR-05-2076. [DOI] [PubMed] [Google Scholar]
- Malet-Martino M, Martino R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): A review. Oncologist. 2002;7:288–323. doi: 10.1634/theoncologist.7-4-288. [DOI] [PubMed] [Google Scholar]
- Mullally WJ, Awis N, Colum D, et al. Safety and tolerability of adjuvant FOLFOX vs CAPOX in colon cancer: A real-world experience. J Clin Oncol. 2018;36:e15689. [Google Scholar]
- Offer SM, Croix CF, Natalie JW, et al. Comparative functional analysis of DPYD variants of potential clinical relevance to dihydropyrimidine dehydrogenase activity. Cancer Res. 2014;74:2545–54. doi: 10.1158/0008-5472.CAN-13-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pectasides D, Vasilios K, George P, et al. Randomized phase III clinical trial comparing the combination of capecitabine and oxaliplatin (CAPOX) with the combination of 5-fluorouracil, leucovorin and oxaliplatin (Modified FOLFOX6) as adjuvant therapy in patients with operated high-risk stage II or stage III colorectal cancer. BMC Cancer. 2015;15:384–95. doi: 10.1186/s12885-015-1406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Qianqian W, Jing G, et al. Association between GSTP1 Ile105Val polymorphism and oxaliplatin-induced neuropathy: A systematic review and meta-analysis. Cancer Chemother Pharmacol. 2013;72:305–14. doi: 10.1007/s00280-013-2194-x. [DOI] [PubMed] [Google Scholar]
- Saif MW, John R. Management of oxaliplatin-induced peripheral neuropathy. Ther Clin Risk Manag. 2005;1:249–58. [PMC free article] [PubMed] [Google Scholar]
- Sobrero A, Sara L, Gerardo R, et al. FOLFOX or CAPOX in stage II to III colon cancer: Efficacy results of the Italian three or six colon adjuvant trial. J Clin Oncol. 2018;36:478–85. doi: 10.1200/JCO.2017.76.2187. [DOI] [PubMed] [Google Scholar]
- Toffoli G, Luciana G, Angela B, et al. Clinical validity of a DPYD-based pharmacogenetic test to predict severe toxicity to fluoropyrimidines. Int J Cancer. 2015;137:2971–80. doi: 10.1002/ijc.29654. [DOI] [PubMed] [Google Scholar]
- Wehler TC, Yang C, Peter RG, et al. Combination therapies with oxaliplatin and oral capecitabine or intravenous 5-FU show similar toxicity profiles in gastrointestinal carcinoma patients if hand-food syndrome prophylaxis is performed continuously. Oncol Lett. 2012;6:1191–4. doi: 10.3892/ol.2012.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Shu-Feng Z, Xiao C, et al. Relationship between genotype and enzyme activity of glutathione S-transferases M1 and P1 in Chinese. Eur J Pharm Sci. 2006;28:77–85. doi: 10.1016/j.ejps.2006.01.002. [DOI] [PubMed] [Google Scholar]


