Abstract
Prunus mume is the only fragrant flowering species of Prunus. According to the previous studies, benzyl acetate and eugenol dominate its floral scent. However, the diversity of its floral scents remains to be elucidated. In this work, the floral volatiles emitted from eight intraspecific cultivars of P. mume with white, pink and red flowers, were collected and analyzed using headspace solid-phase microextraction combined with gas chromatograms-mass spectrometry (HS-SPME-GC-MS). In total, 31 volatile compounds were identified, in which phenylpropanoids/benzenoids accounted for over 95% of the total emission amounts. Surprisingly, except for benzyl acetate and eugenol, several novel components, such as benzyl alcohol, cinnamyl acohol, cinnamy acetate, and benzyl benzoate were found in some cultivars. The composition of floral volatiles in cultivars with white flowers was similar, in which benzyl acetate was dominant, while within pink flowers, there were differences of floral volatile compositions. Principal component analysis (PCA) showed that the emissions of benzyl alcohol, cinnamyl alcohol, benzyl acetate, eugenol, cinnamyl acetate, and benzyl benzoate could make these intraspecific cultivars distinguishable from each other. Further, hierarchical cluster analysis indicated that cultivars with similar a category and amount of floral compounds were grouped together. Our findings lay a theoretical basis for fragrant plant breeding in P. mume.
Keywords: floral scent, Prunus mume, intraspecific cultivars, volatile compounds, phenylpropanoids/benzenoids, HS-SPME-GC-MS
1. Introduction
For ornamental plants, floral fragrance is an attractive and significant character, playing a crucial role in plant–animal communication, including attracting pollinators and defense against pathogens [1]. Also, floral scent can increase aesthetic value and attract visitors [2]. The trait of floral scent is complex and composed of a series of low molecular weight volatile organic compounds (VOCs) [3]. The composition and number of VOCs vary among different species or even in interspecific and intraspecific cultivars [4,5]. In recent years, more and more studies focusing on floral fragrance profiles have been performed in many fragrant plants, including Lilium [6,7], Gelsemium sempervirens [8], Chimonanthus praecox [9], Camellia [4], Polianthes tuberosa [5], and bearded irises [10], etc. VOCs released from these flowers are basically divided into four classes by the biosynthesis origin: terpenoids, phenylpropanoids/benzenoids, fatty acid derivatives, and compounds containing nitrogen/sulfur [11].
P. mume (mei) from Rosaceae, is a famous ornamental and fruit tree blooming in early spring within January and February, and it is mainly cultivated in Yangtze valley that locates in the subtropical monsoon climate zone [12]. It emits recognizable and pleasant scents, making it distinguishable from other species in Prunus, such as P. sibirica, P. armeniaca, and P. persica [13]. It has been reported that the aromatic benzenoid, benzyl acetate, is the main component in its floral volatiles [14]. However, very little is known about the diversity of the floral scents in its cultivars with different coloring corollas, which includes two groups: one in white and light yellow without anthocyanin, the other one in light pink, pink and fuchsia with anthocyanin [15]. As reported, there is a close connection between metabolic biosynthesis of floral volatiles and flower coloration, because aromatic benzenoids and anthocyanin are derived from their common precursor, aromatic amino acid phenylalanine, through phenylpropanoids biosynthesis pathway [16]. There are some researches focusing on the relation between floral scent and flower colour, which usually mentioned the regulation of transcription factors (TF). A TF from MYB family, production of anthocyanin pigment 1 (pap1) in Arabidopsis thaliana can activate phenylpropanoids pathway to enhance the emission of phenylpropanoid/benzenoid compounds and accumulate anthocyanin pigments [17,18]. In Petunia hybrids, flower pigment and scent emission are also co-regulated by PH4 [16]. Whether the floral fragrance of P. mume intraspecific cultivars with different flower colours varies or not awaits further investigation. P. mume has been recognized to be an essential material to investigate the floral metabolic characteristics.
The present study aims to explore the diversity of floral scents in P. mume intraspecific cultivars with different corolla colours. It reports the floral scents through characterizing the emitted VOCs with HS-SPME-GC-MS. First, the specific floral volatile compounds from the investigated cultivars were identified. We analyzed the emission composition referring to their percentages and absolute amounts. Then, PCA was performed to categorize the intraspecific cultivars and clarify the major contributing component. Further, the eight cultivars were classified into two groups in terms of the absolute amount of main floral components through hierarchical cluster analysis. This study will provide a better understanding of the floral scent chemistry in P. mume cultivars and help breeders to develop new fragrant cultivars.
2. Results
2.1. Floral Volatiles in the Eight P. mume Cultivars
A total of 31 different floral volatiles were identified in the eight investigated cultivars with different corolla colours (Figure 1). The colour parameter and number of detected compounds in different cultivars were summarized in Table 1.
Figure 1.
The flowers of eight intraspecific cultivars with different colours.
Table 1.
Summary of compounds identified in the eight P. mume cultivars.
| Cultivars | ‘Fub’ | ‘Zah’ | ‘Sub’ | ‘Zao’ | ‘Fenp’ | ‘Jia’ | ‘Fenh’ | ‘Wuy’ |
|---|---|---|---|---|---|---|---|---|
| RHSCC | NN155B | NN155B | 155C | NN155C | 62B | 63B | 68B | 60B |
| Number of compounds | 20 | 20 | 21 | 18 | 23 | 20 | 22 | 19 |
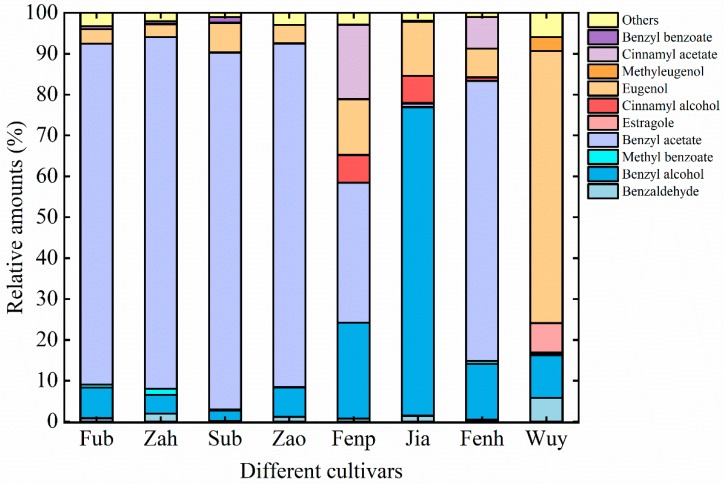
The composition of VOCs varied obviously among these cultivars. VOCs were classified according to their chemical categories, and their relative amounts are listed in Supplementary Table S1. The detected floral VOCs included 17 phenylpropanoids/benzenoids, 10 fatty acid derivatives, 4 terpenoids. Phenylpropanoids/benzenoids, as the most prior VOCs, accounted for as high as above 95% of total emissions. Ten compounds represented more than 1% of total emission in any cultivar (Figure 2), among which benzyl acetate, benzyl alcohol, eugenol, cinnamyl acetate, and cinnamyl alcohol accounted for a large percentage of total amounts. Except for in ‘Jia’ and ‘Wuy’, benzyl acetate accounted for the highest percentage in the other six cultivars and varied from 34.23% to 87.26%. Benzyl alcohol was emitted from all cultivars, while its relative amount ranged from 2.53% to 75.43%. Similarly, eugenol was detected in all samples, and its relative abundance reached as the highest as 70.92% in ‘Wuy’. Cinnamyl acetate and cinnamyl alcohol only presented in three of them. Their relative amounts ranged from 0.08% to 18.14% and from 0.88% to 6.81%, respectively.
Figure 2.
Major components (>1%) of floral volatiles emitted from P. mume cultivars.
The fragrance was suggested to be related to the absolute number of compounds, which was not always consistent with their relative amounts for the different adsorption coefficient of VOCs. As shown in Table 2, except for ‘Fenp’, the emission amount of benzyl acetate was also the most in five of the six cultivars mentioned above, which ranged from 239.2 to 1333.0 μg·g−1 fresh weight·h−1, indicating that benzyl acetate may play a major role in their floral fragrance. Flowers of ‘Fenp’ emitted the highest amount of cinnamyl acetate at 810.3 μg·g−1 fresh weight·h−1, which was much larger than other chemicals. It indicated cinnamyl acetate contributed the most to floral fragrance of ‘Fenp’. The emission of benzyl alcohol varied from 38.2 μg·g−1 fresh weight·h−1 in ‘Sub’ to 1094.0 μg·g−1 fresh weight·h−1 in ‘Jia’, whose fragrance might be dominated by benzyl alcohol. In addition, eugenol was emitted differently from 36.39 to 406.33 μg·g−1 fresh weight·h−1. Though accounting for the largest proportion of total floral emission amounts, the concentration of eugenol in ‘Wuy’ was lower than in ‘Zao’ while approximated it in ‘Jia’. Cinnamyl alcohol emission amounts were close between in ‘Fenp’ and ‘Jia’, which were more than that in ‘Fenh’. Benzyl benzoate was only emitted from ‘Fub’, ‘Zah’ and ‘Sub’, which amounts changed from 95.00 to 157.47 μg·g−1 fresh weight·h−1. In total, these results suggested that benzyl acetate, benzyl alcohol, cinnamyl acetate, and eugenol were the main constituents of P. mume floral scents.
Table 2.
Emission amount of main floral scent compounds in the eight P. mume cultivars.
| Compounds | RI 1 | Emission Amount [mean ± s.d. (μg·g−1 fresh weight·h−1)] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ‘Fub’ | ‘Zah’ | ‘Sub’ | ‘Zao’ | ‘Fenp’ | ‘Jia’ | ‘Fenh’ | ‘Wuy’ | ||
| Benzaldehyde | 982 | 10.1 ± 0.7 | 19.3 ± 14.3 | 1.5 ± 0.1 | 34.1 ± 22.0 | 6.0 ± 3.8 | 17.6 ± 2.4 | 6.0 ± 1.6 | 13.4 ± 3.6 |
| Benzyl alcohol | 1036 | 108.3 ± 21.2 | 149.2 ± 46.9 | 38.2 ± 7.6 | 221.6 ± 112.3 | 279.3 ± 8.3 | 1094.0 ± 0.8 | 227.0 ± 54.2 | 50.6 ± 9.9 |
| Methyl benzoate | 1060 | 6.3 ± 4.0 | 24.8 ± 11.3 | 3.6 ± 2.1 | 2.2 ± 0.7 | tr 2 | 0.97 ± 0.46 | 6.3 ± 1.3 | 0.8 ± 0.3 |
| Benzyl acetate | 1160 | 709.1 ± 152.7 | 1333.1 ± 360.5 | 778.2 ± 131.4 | 1691.0 ± 292.8 | 239.2 ± 1.9 | 7.10 ± 1.85 | 620.1 ± 104.6 | 0.6 ± tr |
| Estragole | 1172 | - 3 | - | 1.5 ± 1.5 | 1.4 ± 0.3 | 0.4 ± 0.1 | 1.0 ± tr | 0.3 ± tr | 9.6 ± 3.2 |
| Cinnamyl alcohol | 1243 | - | - | - | - | 116.1 ± 13.5 | 138.5 ± 34.1 | 17.8 ± 5.6 | - |
| Eugenol | 1392 | 88.1 ± 35.6 | 145.0 ± 85.6 | 166.6 ± 58.5 | 406.3 ± 16.3 | 274.3 ± 4.6 | 325.9 ± 46.9 | 168.0 ± 46.1 | 336.2 ± 54.1 |
| Methyleugenol | 1361 | 3.2 ± 1.0 | 6.1 ± 2.9 | 8.4 ± 1.9 | 5.1 ± 0.9 | 3.4 ± 0.7 | 7.2 ± 2.7 | tr | 32.3 ± 8.9 |
| Cinnamyl acetate | 1367 | - | - | - | - | 810.3 ± 177.1 | 4.4 ± 1.5 | 423.5 ± 139.6 | - |
| Benzyl benzoate | 1733 | 95.0 ± 42.4 | 119.87 ± 38.9 | 157.5 ± 82.5 | - | - | - | - | - |
1 retention index. 2 trace amount, less than 0.1. 3 not detected.
2.2. Principal Component Analysis of Floral Scent Emission
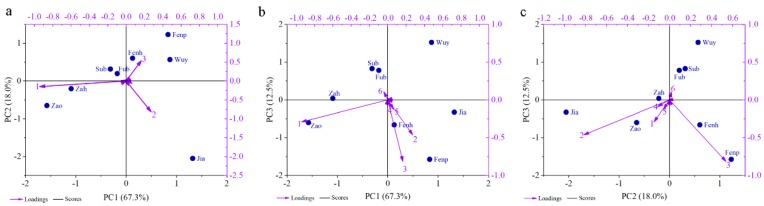
To categorize the intraspecific cultivars, PCA was performed based on the emission amount of floral volatiles, helping to highlight the similarity and difference in the dataset [5]. As shown in Figure 3, the variances of PC1, PC2, and PC3 were 67.3%, 18.0%, and 12.5%, respectively. The six highest loading values in each PC were selected as main factors, including benzyl acetate, benzyl alcohol, cinnamyl acetate, eugenol, cinnamyl alcohol and benzyl benzoate, which contributed to separate the eight cultivars. In Figure 3a,b, the emission amount of benzyl acetate was a main factor to separate ‘Zao’ and ‘Zah’ from other cultivars. Benzyl alcohol amount was one principal component to distinguish ‘Jia’ from others. In Figure 3a,c, cinnamyl acetate helped to separate ‘Fenp’ and ‘Fenh’ from other cultivars. Benzyl benzoate was the main component to separate ‘Sub’ and ‘Fub’ from others. In Figure 3b,c, ‘Wuy’ was obviously separated from others because of its low emission of VOCs.
Figure 3.
PCA of the emission amount of floral scent compounds separated the eight P. mume cultivars. (a) 2D type of component plot of PC1 and PC2. (b) 2D type of component plot of PC1 and PC3. (c) 2D type of component plot of PC2 and PC3. Violet axis represents the loading value of the compounds. Black axis represents the score of the cultivars. Numbers represent the major scent components: 1: benzyl acetate; 2: benzyl alcohol, 3: cinnamyl acetate, 4: eugenol, 5: cinnamyl alcohol, 6: benzyl benzoate.
2.3. Hierarchical Cluster Analysis of Floral Volatiles in the Eight P. mume Cultivars
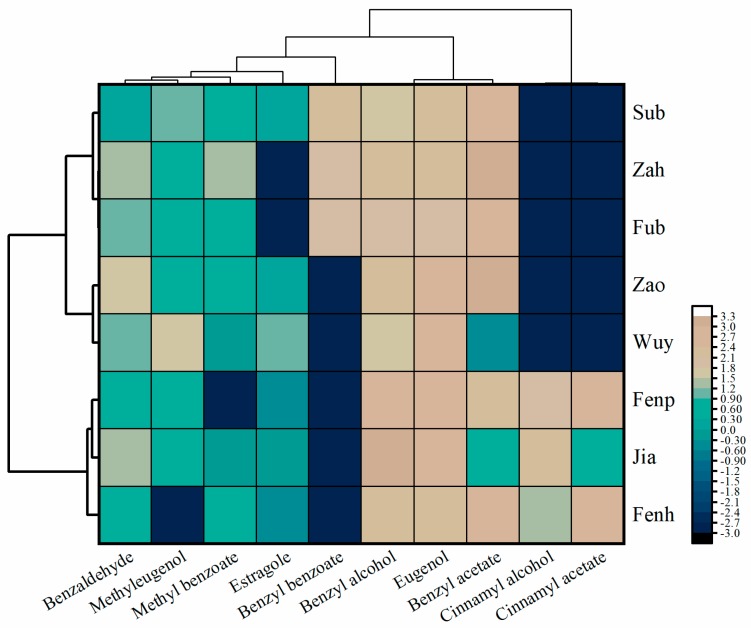
To explore the relationships among these cultivars, hierarchical cluster analysis was carried out according to the quantities of mainly detected chemicals. As shown in Figure 4, the cultivars clustered together emitting similar representative scent components. The eight cultivars were clustered into two groups. ‘Sub’, ‘Fub’ and ‘Zah’ were grouped into one-subgroup, in which ‘Fub’ and ‘Zah’ were closer, for the near contents of benzyl benzoate and benzyl acetate. ‘Zao’ and ‘Wuy’ were in the other sub-group. ‘Fenp’, ‘Jia’ and ‘Fenh’ were clustered in the other group albeit to the low level of benzyl acetate and cinnamyl acetate in ‘Jia’. ‘Fenp’ and ‘Jia’ were closer as the amounts of cinnamyl alcohol, benzyl alcohol and eugenol in them were significantly greater than in ‘Fenh’.
Figure 4.
Heatmap of core VOCs and hierarchical cluster analysis of eight P. mume cultivars. The colour of the heatmap ranges from dark blue (value, –3.0) to brown (value, 3.3) in the natural logarithmic scale. Data are presented with means of biological replicates. The emission values were normalized by log10 transformation.
3. Discussion
Floral scents always vary among different ornamental species or cultivars. Both the composition and the amount of the volatiles determine the specific flower fragrance [19]. The number of volatiles emitted from P. mume is less than other species, such as bearded iris (219) [10], Lithophragma (132) [20], G. sempervirens (81) [8], but approximate to Lilium [6], P. mume hybrids, and Cymbidium [21]. Comprehensive analysis of the floral scent profiles indicates that the fragrance may be not straightly connected with the number of compositions but their concentration. The number of components changes slightly from weak-scented ‘Wuy’ to heavy-scented ‘Zao’. Similar phenomenon occurs in Camellia that about 30 volatiles were identified in C. macrosepala (weak-scented) and C. buxifolia (moderate-scented) [4].
The VOCs are also different among intraspecific or interspecific cultivars. In our work, 31 compounds were identified, predominant with aromatic benzenoids and phenylpropanoids, including benzyl alcohol, benzyl acetate, cinnamyl alcohol, cinnamyl acetate and eugenol. Compared to the preliminary study [14], benzyl acetate and eugenol are the major component in several cultivars indeed. Differently, emission amount of benzyl alcohol in the investigated ‘Jia’ is far more than that in other flowers of P. mume cultivars. In addition, cinnamyl alcohol and cinnamyl acetate are novel compounds released from P. mume. As shown in our analytical data, benzyl acetate, eugenol, benzyl alcohol and cinnamyl acetate contribute to the floral scents of P. mume.
Interaction of various VOCs forms the floral aroma that is characterized by a component with a higher odor value (content/olfactory threshold) [22]. Benzyl acetate is an important aromatic component of ripe fruits and perfumes in many ornamental plants, such as Myrtaceae fruit crops [23], Jasminum sambac [24], Zygopetalum maculatum [25]. Similarly, the floral scents in four of the eight cultivars, including strong-scented ‘Zao’ and ‘Zah’ and moderate-scented ‘Sub’ and ‘Fub’, are dominated by large amounts of benzyl acetate with fruit and jasmine aroma [26]. The cinnamon-fruity aroma of strong-scented ‘Fenp’ is decided by cinnamyl acetate. And floral odor with sweet-flowery-fruity of moderate-scented ‘Fenh’ is characterized by the interaction of benzyl acetate and cinnamyl acetate. Floral aroma of ‘Jia’ is provided by benzyl alcohol and eugenol. The light scented ‘Wuy’ is characterized by eugenol with a spicy clove odor. The high diversity of floral volatiles provides rich germplasm for developing new cultivars with diverse fragrance.
Through comprehensive analysis of the floral volatile compositions in the eight intraspecific cultivars, we conclude that the components of white flowers were similar but different in pink flowers. In white flowers, benzyl acetate and eugenol function as main components. While benzyl acetate, cinnamyl acetate and benzyl alcohol play important roles in the floral aroma of pink flowers. This result inspires us to further investigate about the complex molecular mechanism in our future study.
The synthesis of floral VOCs is mainly regulated by the expression of related genes, enzyme activity and substrate availability involved in the biosynthesis pathways. Our results indicate that benzenoids, especially aromatic esters, are the predominant components contributing to the unique aroma of P. mume cultivars. Similarly, the floral fragrance of many plants is dominated by aromatic esters, which are synthesized from the aromatic alcohol and acyl donor catalyzed with the acyltransferase. This has been reported in many studies, for example, acetyl CoA- benzyl alcohol acetyltransferase (CbBEAT) from Clarkia breweri [27], benzoyl-CoA: benzyl alcohol benzoyl transferase (PtBEBT) from Populus trichocarpa [28], and alcohol acetyltransferase 1 (RhAAT1) from rosa [29]. As several types of alcohol acyltransferases in different species of plants, CbBEAT and RhAAT1 can catalyze a broad range of acyl-CoAs and alcohols to form the relative aromatic esters [12]. Further, the esterification of alcohols, either benzyl or cinnamyl alcohol, results in the synthesis of a number of important fragrance and flavor compounds [26]. This suggests that formation of benzyl acetate and cinnamyl acetate may be catalyzed by one enzyme in P. mume, utilizing benzyl alcohol and cinnamyl alcohol as substrates, respectively. Though enzymes, including PmBEAT [12] and coniferyl alcohol acetyltransferase (PmCFAT) [30], with similar biochemical characteristics in P. mume have been reported, the process by which enzymes catalyze cinnamyl alcohol to produce cinnamyl acetate needs further investigation. Comparative transcriptome analysis can be an essential and effective strategy for fully understanding the scent biosynthesis in P. mume.
4. Materials and Methods
4.1. Plant Material
Eight P. mume cultivars with different flower colours grown in Jiufeng international registration park (Beijing, China) were selected, including P. mume ‘Fuban Lve’(Fub), P. mume ‘Zaohua Lve’(Zah), P. mume ‘Subai Taige’(Sub), P. mume ‘Zao Yudie’ (Zao), P. mume ‘Fenpi Gongfen’(Fenp), P. mume ‘Jiangsha Gongfen’ (Jia), P. mume ‘Fenhong Zhusha’(Fenh), and P. mume ‘Wuyuyu’(Wuy). They bloom during January to February every year. Names of these cultivars are referred to the book: Chinese Mei Flower Cultivars in Color (in chinese press). Flower colours were identified using the Royal Horticultural Society Color Chart (RHSCC).
4.2. Floral Scent Collection and Quantitative Determination
The flower scents were collected through dynamic headspace collection method at 12:00–14:00 p.m. Branches with full bloom flowers were clipped in distilled water, and then instantly transported to the laboratory at 25 °C for volatile collection. In each of three experimental replicates, 0.2–0.3 g whole blooming flowers was collected and placed into 100 mL injection vials, which were held for 10 min before using extraction fiber to adsorb the volatiles for 30 min at 30 °C. SPME fiber coated with divinylbenzene/carboxen/polydimenthylsiloxane (50/30 μm DVB/CAR/PDMS) was selected to collect volatiles, as reported in previous studies [31]. The emitted volatiles were analyzed using GC-MS, carried out by Shimadzu QP2010 (Shimadzu, Kyoto, Japan) equipped with a DB-5MS capillary column (30 × 0.25 mm, 0.25 μm thickness, Shimadzu, Kyoto, Japan). The injection temperature was held at 250 °C. Helium was the carrier gas in the split mode with the split ratio at 20 and column flow at 27.0 mL min−1. The GC oven temperature started at 40 °C, maintained 2 min, and then increased to 250 °C by 5 °C min−1, holding for 6 min. The mass spectrometer interface temperature was 250 °C and the electron potential was set to 0.9 KV with mass scan range of 30 to 300 m/z units. The solvent cut time was 3.7 min.
The peak area of every scent compound was integrated to obtain the total ion current, with removing the peaks presented in control sample. Individual compound was tentatively identified by comparing the mass spectra with NIST11 library (the National Institute of Standards and Technology 2011, Shimadzu, Japan). Main compounds (relative amount more than 1%) were confirmed by comparing with authentic standard samples. 20 mg of standard compounds (benzaldehyde, benzyl alcohol, benzyl acetate, estragole, eugenol, methyl benzoate and propionic acid benzyl ester) were diluted using 1 mL of hexane. Then, 20 mg of standard compounds of cinnamyl alcohol, methyleugenol, cinnamyl acetate and benzyl benzoate were diluted using 1 mL of methanol. Further, 50 μL of each diluted solution was then co-added to a total volume of 1ml of hexane to make a mixed solution. Then, 5 μL of this mixed solution was placed into 100 mL capped vial for measurement. In addition, propionic acid benzyl ester as internal standard was added into capped vial in every sample. To improve the repeatability of the internal standard in every sample, 5 μL of propionic acid benzyl ester diluted solution was placed into the same position of the injection vial wall.
4.3. Data Analysis
Area normalization was used to calculate the relative content of components. The semi-quantification data was affirmed by standardizing the peak area of each component with the internal standard. PCA was performed with a covariance matrix to calculate the Eigenvalues of principal components. A heatmap of the emission amount of main chemicals and hierarchical cluster analysis of eight P. mume cultivars were conducted using Ward’s method, and squared Euclidean distance was chosen as the similarity metric. The cluster analysis and PCA were carried out by OriginPro 2018 (https://www.originlab.com). Microsoft Excel 2016 was used as the statistical tool.
5. Conclusions
In total, 31 VOCs were separated and identified from fully bloomed flowers of eight P. mume intraspecific cultivars by HS-SPME-GC-MS. Floral scent components varied within intraspecific cultivars. Besides benzyl acetate and eugenol, among the emitted volatiles, benzyl alcohol and cinnamyl acetate also contributed to the floral scent of P. mume. The composition of floral aroma from white-flower cultivars were similar, in which benzyl acetate dominated their floral scents. While there were differences in floral volatile compositions from pink flowers. Cinnamyl alcohol and cinnamyl acetate were synthesized only in pink flowers: ‘Fenp’, ‘Jia’ and ‘Fenh’. The high diversity of flower fragrance in P. mume provided a germplasm resource for developing new cultivars with a variety of fragrances. Comparative transcriptomic and metabolomic analyses could be an essential and effective strategy for fully understanding scent biosynthesis in P. mume.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/25/1/145/s1, Table S1: Relative amounts (>0.1%) of floral scent compounds in the eight P. mume cultivars.
Author Contributions
Conceptualization, T.Z., F.B. and Q.Z.; Methodology, T.Z. and F.B.; Software, T.Z.; Validation, T.Z., Y.Y. and J.W.; Formal Analysis, T.Z. and A.D. (Anqi Ding); Investigation, T.Z., Y.Y. and A.D. (Aiqin Ding); Resources, Q.Z. and T.C.; Data Curation, T.Z.; Writing—Original Draft Preparation, T.Z.; Writing—Review & Editing, T.Z. L.H. and F.B.; Project Administration, Q.Z.; Funding Acquisition, Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities (2017ZY23) and Special Fund for Beijing Common Construction Project.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Schiestl F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010;13:643–656. doi: 10.1111/j.1461-0248.2010.01451.x. [DOI] [PubMed] [Google Scholar]
- 2.De Vega C., Herrera C.M., Dotterl S. Floral volatiles play a key role in specialized ant pollination. Perspect Plant. Ecol. 2014;16:32–42. doi: 10.1016/j.ppees.2013.11.002. [DOI] [Google Scholar]
- 3.Dudareva N., Klempien A., Muhlemann J.K., Kaplan I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013;198:16–32. doi: 10.1111/nph.12145. [DOI] [PubMed] [Google Scholar]
- 4.Fan Z.Q., Li J.Y., Li X.L., Yin H.F. Composition analysis of floral scent within genus Camellia uncovers substantial interspecific variations. Sci. Hortic-Amsterdam. 2019;250:207–213. doi: 10.1016/j.scienta.2019.02.050. [DOI] [Google Scholar]
- 5.Kutty N.N., Mitra A. Profiling of volatile and non-volatile metabolites in Polianthes tuberosa L. flowers reveals intraspecific variation among cultivars. Phytochemistry. 2019;162:10–20. doi: 10.1016/j.phytochem.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Du F., Wang T., Fan J.M., Liu Z.Z., Zong J.X., Fan W.X., Han Y.H., Grierson D. Volatile composition and classification of Lilium flower aroma types and identification, polymorphisms, and alternative splicing of their monoterpene synthase genes. Hortic. Res.-Engl. 2019;6 doi: 10.1038/s41438-019-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong Y., Sun M., Pan H.T., Zhang Q.X. Composition and Emission Rhythm of Floral Scent Volatiles from Eight Lily Cut Flowers. J. Am. Soc. Hortic Sci. 2012;137:376–382. doi: 10.21273/JASHS.137.6.376. [DOI] [Google Scholar]
- 8.Johnson B.O., Golonka A.M., Blackwell A., Vazquez I., Wolfram N. Floral Scent Variation in the Heterostylous Species Gelsemium sempervirens. Molecules. 2019;24:2818. doi: 10.3390/molecules24152818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z.G., Cao H., Lee M.R., Shen D.L. Analysis of Volatile Compounds Emitted from Chimonanthus praecox (L.) Link in Different Florescence and QSRR Study of GC Retention Indices. Chromatographia. 2009;70:1153–1162. doi: 10.1365/s10337-009-1289-3. [DOI] [Google Scholar]
- 10.Yuan Y., Sun Y., Zhao Y.C., Liu C.G., Chen X.L., Li F.T., Bao J.Z. Identification of Floral Scent Profiles in Bearded Irises. Molecules. 2019;24:1773. doi: 10.3390/molecules24091773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhlemann J.K., Klempien A., Dudareva N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014;37:1936–1949. doi: 10.1111/pce.12314. [DOI] [PubMed] [Google Scholar]
- 12.Bao F., Ding A.Q., Zhang T.X., Luo L., Wang J., Cheng T.R., Zhang Q.X. Expansion of PmBEAT genes in the Prunus mume genome induces characteristic floral scent production. Hortic. Res.-Engl. 2019;6 doi: 10.1038/s41438-018-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao R.J., Du D.L., Wang T., Yang W.R., Wang J., Zhang Q.X. A comparative analysis of characteristic floral scent compounds in Prunus mume and related species. Biosci. Biotech. Bioch. 2014;78:1640–1647. doi: 10.1080/09168451.2014.936346. [DOI] [PubMed] [Google Scholar]
- 14.Hao R.J., Zhang Q., Yang W.R., Wang J., Cheng T.R., Pan H.T., Zhang Q.X. Emitted and endogenous floral scent compounds of Prunus mume and hybrids. Biochem. Syst. Ecol. 2014;54:23–30. doi: 10.1016/j.bse.2013.12.007. [DOI] [Google Scholar]
- 15.Zhang Q., Hao R.J., Xu Z.D., Yang W.R., Wang J., Cheng T.R., Pan H.T., Zhang Q.X. Isolation and functional characterization of a R2R3-MYB regulator of Prunus mume anthocyanin biosynthetic pathway. Plant Cell Tiss. Org. 2017;131:417–429. doi: 10.1007/s11240-017-1294-4. [DOI] [Google Scholar]
- 16.Cna’ani A., Spitzer-Rimon B., Ravid J., Farhi M., Masci T., Aravena-Calvo J., Ovadis M., Vainstein A. Two showy traits, scent emission and pigmentation, are finely coregulated by the MYB transcription factor PH4 in petunia flowers. New Phytol. 2015;208:708–714. doi: 10.1111/nph.13534. [DOI] [PubMed] [Google Scholar]
- 17.Zuker A., Tzfira T., Ben-Meir H., Ovadis M., Shklarman E., Itzhaki H., Forkmann G., Martens S., Neta-Sharir I., Weiss D. Modification of flower color and fragrance by antisense suppression of the flavanone 3-hydroxylase gene. Mol. Breeding. 2002;9:33–41. doi: 10.1023/A:1019204531262. [DOI] [Google Scholar]
- 18.Ben Zvi M.M., Shklarman E., Masci T., Kalev H., Debener T., Shafir S., Ovadis M., Vainstein A. PAP1 transcription factor enhances production of phenylpropanoid and terpenoid scent compounds in rose flowers. New Phytol. 2012;195:335–345. doi: 10.1111/j.1469-8137.2012.04161.x. [DOI] [PubMed] [Google Scholar]
- 19.Pichersky E., Dudareva N. Scent engineering: Toward the goal of controlling how flowers smell. Trends Biotechnol. 2007;25:105–110. doi: 10.1016/j.tibtech.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Friberg M., Schwind C., Guimaraes P.R., Raguso R.A., Thompson J.N. Extreme diversification of floral volatiles within and among species of Lithophragma (Saxifragaceae) Proc. Natl. Acad. Sci. USA. 2019;116:4406–4415. doi: 10.1073/pnas.1809007116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baek Y.S., Ramya M., An H.R., Park P.M., Lee S.Y., Baek N.I., Park P.H. Volatiles Profile of the Floral Organs of a New Hybrid Cymbidium, ‘Sunny Bell’ Using Headspace Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry Analysis. Plants-Basel. 2019;8 doi: 10.3390/plants8080251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buttery R.G., Seifert R.M., Guadagni D.G., Ling L.C. Characterization of additional volatile components of tomato. J. Agric. Food Chem. 1971;19:524–529. doi: 10.1021/jf60175a011. [DOI] [Google Scholar]
- 23.Cordeiro G.D., dos Santos I.G.F., da Silva C.I., Schlindwein C., Alves-dos-Santos I., Dotterl S. Nocturnal floral scent profiles of Myrtaceae fruit crops. Phytochemistry. 2019;162:193–198. doi: 10.1016/j.phytochem.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Yu Y., Lyu S.H., Chen D., Lin Y., Chen J.J., Chen G.X., Ye N.X. Volatiles Emitted at Different Flowering Stages of Jasminum sambac and Expression of Genes Related to alpha-Farnesene Biosynthesis. Molecules. 2017;22:546. doi: 10.3390/molecules22040546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bera P., Chakrabarti S., Gaikwad N.K., Kutty N.N., Barman M., Mitra A. Developmental variation in floral volatiles composition of a fragrant orchid Zygopetalum maculatum (Kunth) Garay. Nat. Prod. Res. 2019;33:435–438. doi: 10.1080/14786419.2018.1451999. [DOI] [PubMed] [Google Scholar]
- 26.Surburg H., Panten J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses. 6th ed. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2016. [Google Scholar]
- 27.Dudareva N., D’Auria J.C., Nam K.H., Raguso R.A., Pichersky E. Acetyl-CoA:benzylalcohol acetyltransferase—An enzyme involved in floral scent production in Clarkia breweri. Plant. J. 1998;14:297–304. doi: 10.1046/j.1365-313X.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- 28.Chedgy R.J., Kollner T.G., Constabel C.P. Functional characterization of two acyltransferases from Populus trichocarpa capable of synthesizing benzyl benzoate and salicyl benzoate, potential intermediates in salicinoid phenolic glycoside biosynthesis. Phytochemistry. 2015;113:149–159. doi: 10.1016/j.phytochem.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Shalit M., Guterman I., Volpin H., Bar E., Tamari T., Menda N., Adam Z., Zamir D., Vainstein A., Weiss D., et al. Volatile ester formation in roses. Identification of an acetyl-coenzyme A. Geraniol/citronellol acetyltransferase in developing rose petals. Plant Physiol. 2003;131:1868–1876. doi: 10.1104/pp.102.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang T., Huo T., Ding A., Hao R., Wang J., Cheng T., Bao F., Zhang Q. Genome-wide identification, characterization, expression and enzyme activity analysis of coniferyl alcohol acetyltransferase genes involved in eugenol biosynthesis in Prunus mume. PLoS ONE. 2019;14:e0223974. doi: 10.1371/journal.pone.0223974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y.Q., Pan H.T., Zhang Q.X., Sun M., Pan B.C. Studies on the Volatile Constituent from Cultivars of Prunus mume. J. Trop. Subtrop. Bot. 2010;3:98–103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.