Abstract
Two new carbazole alkaloids (1,2) and six known carbazole alkaloids (3–8) were isolated from Clausena anisum-olens. Their structures were elucidated based on extensive spectroscopic analysis. All isolated compounds (1–8) were evaluated for their anti-HIV effects on virus replication in MT-4 lymphocytes infected by HIV-1NL4-3 Nanoluc-sec virus, and new carbazole alkaloid 1 exhibited anti-HIV activity with an EC50 value of 2.4 μg/mL and SI of 7.1.
Keywords: Rutaceae, Clausena anisum-olens, carbazole alkaloids, anti-HIV
1. Introduction
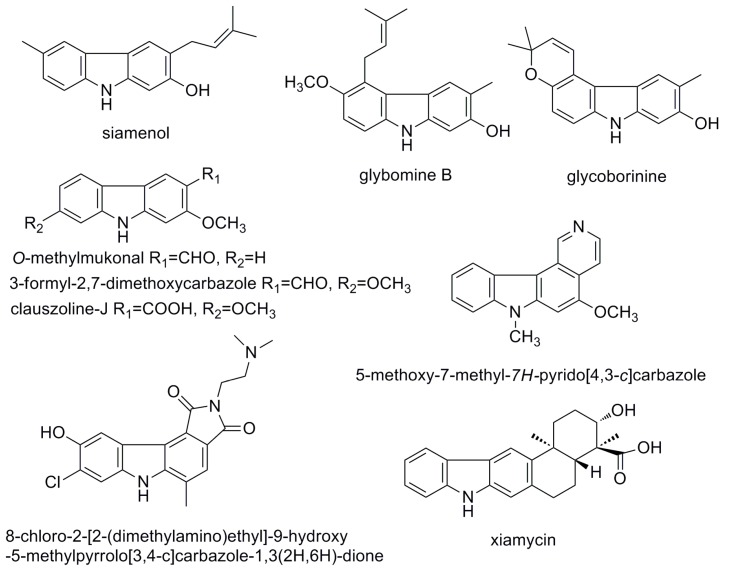
Carbazole alkaloids are characterized by a tricyclic aromatic basic skeleton with a central pyrrole ring fused between two benzene rings. The intriguing structural features and promising pharmacological activities of these natural products have led to enormous developments in the field of carbazole alkaloids [1,2]. Indeed, carbazoles have been widely investigated for their various biological properties, ranging from significant antimicrobial, antiprotozoal, and insecticidal effects to anti-inflammatory, antioxidative, antiplatelet aggregative, antiviral, and neuroprotective activities [1,2,3]. Natural and synthetic carbazole alkaloids have been reported to possess anti-HIV activity. Meragelman and co-workers found that an organic extract of Murraya siamensis from Thailand showed anti-HIV activity. Bioassay-guided fractionation of the extract led to the isolation of a C-6-prenylated carbazole alkaloid, siamenol, which exhibited HIV-inhibitory activity with an EC50 value of 2.6 µg/mL, reaching 50–60% maximum protection in the XTT-tetrazolium assay [4]. A crude extract from the underground parts of Clausena excavata inhibited HIV-1 activity in a syncytium assay [5]. Three carbazole alkaloids, O-methylmukonal, 3-formyl-2,7-dimethoxycarbazole, and clauszoline-J, displayed promising anti-HIV-1 effects with EC50 values of 2.7, 7.4, and 8.2 µg/mL, respectively, and potential therapeutic index (PTI) values of 56.7, 8.0, and 1.6, respectively [5]. The biological results supported the use of the C. excavata extracts for the treatment of AIDS infections [6]. In addition, the two C-5-prenylated carbazoles, glybomine B and glycoborinine, isolated from Glycosmis montana exhibited weak to moderate in vitro inhibitory activity against HIV replication in C8166 cells, with IC50 values of 9.73 and 4.47 µg/mL, respectively [7]. Hirata et al. prepared a series of N-alkyl-pyrido [4,3-c]carbazole derivatives starting from mukonal (2-hydroxy-9H-carbazole-3-carbaldehyde), a natural carbazole present in Rutaceous plants. Several compounds inhibited HIV replication in H9 lymphocytes. Among them, 5-methoxy-7-methyl-7H-pyrido[4,3-c]carbazole had the highest therapeutic index (TI = 503) in the series, with an EC50 value of 0.0054 µg/mL [3,8]. Yan et al. studied the antiviral activity of small molecular weight compounds with a carbazole structure using a single replication infectivity assay in HeLa 4.5/EGFP cells. 8-Chloro-2-[2-(dimethylamino)ethyl]-9-hydroxy-5-methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dionedemonstrated potent strand-transfer inhibitory activity and could be used as a lead compound to develop novel inhibitors [9]. Later, a pentacyclic indolocarbazole, xiamycin, from Streptomyces sp. GT2002/1503, was found to exhibit selective anti-HIV activity (Figure 1) [10]. Based on the above results, carbazole may be considered a “privileged scaffold” that can provide opportunities for the development of anti-HIV drugs; however, this approach is a relatively low-studied area of anti-HIV drug discovery. Therefore, more multidirectional approaches need to be continued in anti-HIV drug discovery, including finding new natural products, evaluating known natural products, modifying semi-synthetic anti-HIV natural products, etc. [3,11].
Figure 1.
Natural and synthetic carbazole alkaloids with anti-HIV activity.
The genus Clausena (Rutaceae) includes about 30 species, which are widely distributed in tropical and subtropical regions of the eastern hemisphere. About 10 species and 2 varieties are found in China, from the southwest to Taiwan [12]. Some species of this genus have been used in Asian folk medicine to treat various diseases for a long time [12]. Carbazole alkaloids are an important and characteristic chemotaxonomic feature typical of the genus Clausena. These compounds continue to attract high interest due to their structural diversity and promising pharmacological potential, i.e., antitumor, anti-inflammatory, antimicrobial, antifungal, anti-HIV, and neuroprotective activities [5,6,13,14,15,16,17,18,19,20,21,22,23]. Clausena anisum-olens Merr. is a perennial evergreen shrub or small tree mainly distributed in the Philippines, South China, and Southeast Asia. The aerial parts of C. anisum-olens, which grows in Yunnan Province, China, are used in folk medicine to treat dysentery and arthritis [12]. Our previous phytochemical investigations of this species led to the discovery of new monoterpenoid coumarins [12,24,25,26,27,28]. As part of our search for new natural products and bioactive compounds from medicinal plants of Clausena in Yunnan, a chemical reinvestigation of the ethanolic extract of the aerial parts of C. anisum-olens was then carried out. Two new carbazole alkaloids (1,2) together with six known carbazole alkaloids (3–8) were obtained. This paper describes the isolation, structural elucidation, and biological evaluation of the isolated carbazole alkaloids against HIV-1, using AZT as the standard in antiviral assays.
2. Results and Discussion
2.1. Structural Elucidation of the Compounds
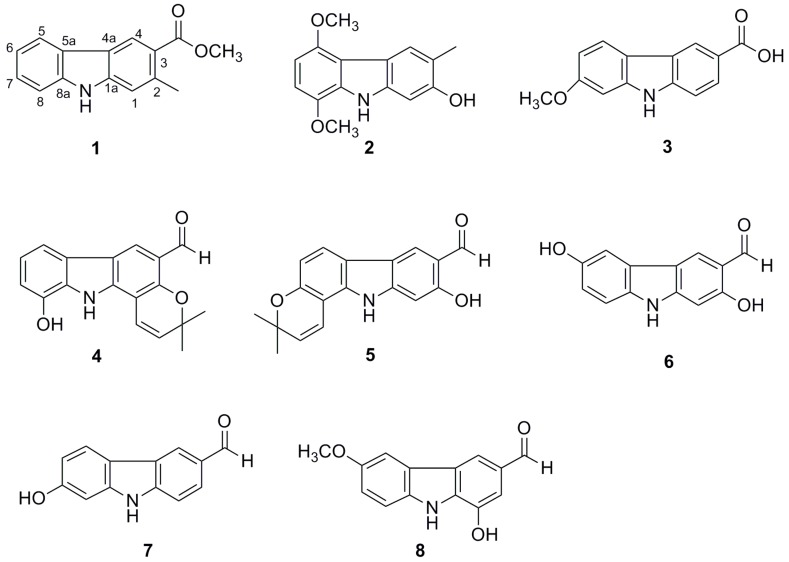
The 90% EtOH extract of the stems and leaves of C. anisum-olen Merr. was suspended in water and extracted successively with petroleum ether and ethyl acetate. The ethyl acetate fraction was repeatedly subjected to silica gel, Sephadex LH-20, RP-18 gel column chromatography to yield carbazole alkaloids 1–8, including two new compounds, as shown in Figure 2.
Figure 2.
Structures of compounds 1–8.
Compound 1 was isolated as a white solid. The molecular formula, C15H13NO2, was established from13C NMR and HRESIMS data with 262.0839 [M + Na]+, calculated for 262.0838), suggesting 10 indices of hydrogen deficiency. The IR absorptions at 3354, 2946, 1692, 1634, 1488, 1452, 1381, and 1242 cm−1 showed the presence of a NH functionality from the carbazole alkaloid, methyl, ester carbonyl, and aromatic groups. The UV spectrum showed absorbances at 265 nm. Based on the IR and UV data, compound 1 was concluded to be a carbazole alkaloid [29,30].
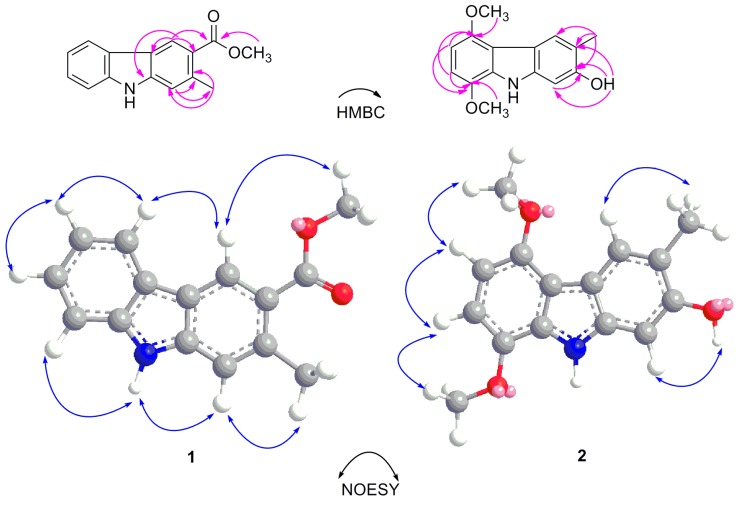
The 1H NMR spectrum of 1 (Table 1) indicated a set of ortho-disubstituted phenyl protons [δH 8.07 (1H, d, J = 7.8 Hz, H-5), 7.26 (1H, m, H-6), 7.42 (1H, m, overlap, H-7), 7.41 (1H, m, overlap, H-8); two lone aromatic protons [δH7.22 (1H, s, H-1), 8.74 (1H, s, H-4)] in the aromatic region; a broad NH singlet at δH 8.15; a singlet for one aromatic methyl group at δH 2.77; and a methoxy group at δH 3.95. The 13C NMR spectrum (Table 1), classified by the HSQC spectrum, revealed the presence of 15 carbon atoms, including 12 aromatic carbons (6 sp2 carbon atoms and 6 sp2 quaternary carbon atom), an ester carbonyl, a methyl, and a methoxy. The NMR data implied that compound 1 was a carbazole alkaloid [29,30]. In addition, the 12 sp2 carbon atoms and 10 indices of hydrogen deficiency were attributable to one carbazole ring group and an ester carbonyl group. The HMBC spectrum provided valuable information about the structure of 1 (Figure 2). Thus, while the two- and three-bond correlations of the methine proton singlets at δH 7.22 (H-1) and δH 8.74 (H-4), as well as the aromatic methyl proton signal at δH 3.95 with those of the neighboring carbons, clearly suggested the substitution at positions 2 and 3 in the molecule. The HMBC correlations from H-4 to the ester carbonyl carbon (δC 168.5) located the ester carbonyl at C-3 (δC 138.8). Additionally, correlations from the methyl proton to C-1 (δC 112.8), C-2 (δC 121.2), and C-3 (δC 138.8) supported that the location of the aromatic methyl group at C-2. The structure of 1 was finally confirmed by the clear NOE interactions observed in its NOESY spectrum, as depicted in Figure 3 (see Supplementary Materials). Hence, the complete structure of 1 (clauolenzole A (2-methyl-9H-carbazole-3-methyl carboxylate)) was established as shown.
Table 1.
600MHz and 13C NMR (150MHz) data of clauolenzoles A and B (1,2).
| Position | 1 (CDCl3) | 2 (acetone-d6) | ||
|---|---|---|---|---|
| δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | |
| 1 | 7.22, s | 112.8, CH | 6.97, s | 96.2, CH |
| 2 | / | 121.2, C | / | 153.9, C |
| 3 | / | 138.8, C | / | 116.8, C |
| 4 | 8.74, s | 124.1, CH | 7.87, s | 123.8, CH |
| 5 | 8.07, d (7.8) | 120.4, CH | / | 149.8, C |
| 6 | 7.26, m | 120.2, CH | 6.46, d (5.6) | 98.8, CH |
| 7 | 7.42, m, overlap | 126.1, CH | 6.69, d (5.6) | 104.8, CH |
| 8 | 7.41, m, overlap | 110.8, CH | / | 140.4, C |
| 1a | / | 141.9, C | / | 139.2, C |
| 4a | / | 123.4, C | / | 116.0, C |
| 5a | / | 121.1, C | / | 113.8, C |
| 8a | / | 139.9, C | / | 130.8, C |
| 2-CH3 | 2.77, s | 23.0, CH3 | ||
| 3-CH3 | 2.29, s | 15.8, CH3 | ||
| 3-COOCH3 | / | 168.5, C | ||
| 3.95, s | 51.6, CH3 | |||
| 5-OCH3 | 3.93, s | 54.9, CH3 | ||
| 8-OCH3 | 3.86, s | 55.3, CH3 | ||
| 2-OH | 8.09, s | / | ||
| NH | 8.15, br s | / | 9.87, br s | / |
Figure 3.
Key HMBC and NOESY correlations of 1 and 2.
Compound 2 was obtained as a brown-yellow solid. The HRESIMS gave a molecular ion peak at m/z 256.0981 [M − H]− (calculated 256.0979) consistent with the molecular formula C15H15NO3, implying nine degrees of unsaturation. The UV spectrum showed absorbances at 234 nm. The IR spectrum displayed absorptions characteristic of hydroxy (3442 cm−1), aromatic ring (1600, 1521, 1452 cm−1), and methyl (2927, 2852, 1401, 1360 cm−1) groups. Thus, compound 2 was defined as having a carbazole alkaloid skeleton.
The 1H NMR spectrum of 2 showed the presence of three singlet protons, one for a phenolic hydroxyl group at δH 8.09 (1H, s, OH-2) and the other two for aromatic methine protons at δH 7.87 (1H, s, H-4) and 6.97 (1H, s, H-1), as well as an ortho-coupled aromatic proton [δH 6.69 (1H, d, J = 5.6 Hz, H-7) and 6.46 (1H, d, J = 5.6 Hz, H-6)] and a broad NH singlet at δH 9.87 (1H, brs, NH) in the aromatic proton region. The 1H NMR spectrum also displayed a single methyl peak at δH 2.29 (3H, s, 3-CH3) and two methoxy peaks at δH 3.93 (3H, s) and 3.86 (3H, s) at high field. These assignments are consistent with the 13C NMR data of 2 (Table 1), with resonances for 15 carbons: 12 aromatic carbons from two benzene rings, one methyl carbon, and two methoxy carbons. The long-range correlations in the HMBC experiment were observed between the methoxy groups (δH 3.93 and 3.86) and C-5 (δC 149.8 s) and C-8 (δC 140.4 s), respectively (Figure 3). Two methoxy (5-OCH3 and 8-OCH3) positions were further confirmed from the HMBC correlations between H-6 (δH 6.46) and C-5 (δC 149.8 s) and C-8 (δC 140.4 s), between H-7 (δH 6.69) and C-5 (δC 149.8 s) and C-8 (δC 140.4 s), as well as NOESY correlations between H-6 (δH 6.46) and 5-OCH3 (δH 3.93), between H-7 (δH 6.69) and 8-OCH3 (δH 3.86), and between H-6 (δH 6.46) and H-7 (δH 6.69). In addition, the HMBC data revealed three- and two-bond correlations between the phenolic hydroxyl group at δH 8.09 and C-1 (δC 96.2 d), C-2 (δC 153.9 s), and C-3 (δC 116.8 s) and between the aromatic methyl group at δH 2.29 and C-2 (δC 153.9 s), C-3 (δC 116.8 s), and C-4 (δC 123.8 d). The hydroxyl (2-OH) and methyl (3-CH3) positions were confirmed by the correlations between H-1 (δH 6.97) and OH-2 (δH 8.09) and between H-4 (δH 7.87) and CH3-3 (δH 2.29) in the NOESY spectrum of 2 (Figure 3). Ultimately, compound 2 was determined to be 5,8-dimethoxy-3-methyl-9H-carbazol-2-ol, named clauolenzole B.
The six known carbazole alkaloids were identified as clausine N (3) [31], heptazolicine (4) [32], clauszoline B (5) [33], clausine-O (6) [31], 7-dihydroxy-9H-carbazole-3-carboxaldehyde (7) [34], and clausine I (8) [35], based on their spectroscopic profiles and comparison with literature values.
2.2. Evaluation of In Vitro Anti-HIV Activity
All eight carbazole alkaloids isolated from C. anisum-olens were evaluated for anti-HIV activity by determining the inhibitory effects on virus replication in MT-4 lymphocytes infected by HIV-1NL4-3 Nanoluc-sec virus. Table 2 shows the anti-HIV results for the tested compounds with azidothymidine (AZT) as a positive control. New carbazole alkaloid 1 exhibited an anti-HIV EC50 value of 2.4 μg/mL and SI of 7.1. Thus, this compound might be a useful compound to further study carbazole alkaloids as potential anti-HIV agents. The remaining seven compounds did not show selective activity (CC50/EC50 < 5). The results prompted us to pursue whether synthetic modifications of the carbazole alkaloid structure could increase the anti-HIV-1 activity.
Table 2.
In vitro anti-HIV data of compounds 1–8 a.
| Compound | EC50 (μg/mL) NL4-3 b | CC50 (μg/mL) MT4 c | TI e |
|---|---|---|---|
| 1 | 2.4 ± 0.53 | 17 ± 2.5 | 7.1 |
| 2 | *- d 3.7 ± 1.9 | 15.2 ± 3.1 | |
| 3 | *- d | ||
| 4 | *- d | ||
| 5 | *- d | ||
| 6 | *- d | ||
| 7 | >25 | >25 | |
| 8 | *- d | ||
| AZT f | 0.0055 ± 0.0018 | >0.1 | 18.2 |
a The highest concentrations for the tested compounds and AZT were 25 μg/mL and 0.1 μg/mL, respectively. Testing was done with a series of 4-fold dilutions (six concentrations). b EC50: 50% HIV-inhibitory concentration (mean ± SD of 3 tests). c CC50: 50% cytotoxic concentration. d *-: no selective anti-HIV activity (CC50/EC50 < 5). e TI: CC50/EC50. f AZT: azidothymidine.
3. Materials and Methods
3.1. General Experimental Procedures
UV spectra were measured on a Shimadzu UV-2401PC spectrophotometer (Shimadzu Co. Ltd., Tokyo, Japan). IR spectra were obtained on a Bio-Rad FTS-135 (Bio-Rad, Richmond, Canada) infrared spectrophotometer. The 1D and 2D NMR spectra were obtained at 400 and 100, and 600 and 150 MHz for 1H and 13C, respectively, on Bruker DRX-400 and 600 spectrometers (Bruker, Bremerhaven, Germany) with TMS as an internal standard. MS data were recorded on a VG Autospec-3000 mass spectrometer (VG, Manchester, England). Commercially available silica gel (100−200 mesh or 200−300 mesh, Qingdao Makall Chemical Co. Ltd., Qingdao, China), Lobar LiChroprep RP-18 (40−63 μm, Merck, St. Louis, MO, USA), and Sephadex LH-20 (Pharmacia, Fine Chemical Co. Ltd., Sweden) were used for open-column chromatography. All solvents were distilled prior to use.
3.2. Plant Material
The leaves and twigs of Clausena anisum-olens Merr. were collected in the Hekou County of Yunnan Province, People’s Republic of China, in May 2015 and identified by Professor Yu Chen of the Kunming Institute of Botany. A voucher specimen (No. 02041705) was deposited in the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences.
3.3. Extraction and Isolation
The powdered leaves and twigs of Clausena anisum-olens Merr. (5.0 kg) were repeatedly extracted with 90% aqueous EtOH (3 × 10 L) at room temperature. The extract was concentrated under reduced pressure to give a brown syrup, which was partitioned into H2O and extracted successively with petroleum ether and ethyl acetate; the extracts were kept separately. The ethyl acetate extract (50.5 g) was subjected to silica gel column chromatography, eluting with petroleum ether-EtOAc (4:1, 2:1, 1:1, 2:3), EtOAc, EtOAc–MeOH (8:2, 7:3, 6:4, 1:1), and finally MeOH to afford eight fractions (I–VIII). Fraction II (12 g) was subjected again to silica gel column chromatography on Pharmadex LH-20 (MeOH) to give compound 1 (5 mg). Fraction III (6.2 g) was re-subjected to silica gel column chromatography on Pharmadex LH-20 (MeOH) to afford compounds 2 (3 mg) and 8 (3 mg). Fraction IV (14.2 g) was re-subjected to silica gel column chromatography on Pharmadex LH-20 (MeOH) and RP C18 to afford compounds 4 (3 mg) and 5 (6 mg). Fraction V (8 g) was subjected again to silica gel column chromatography on Pharmadex LH-20 (MeOH) to give compounds 3 (15 mg), 6 (6 mg), and 7 (8 mg).
Clauolenzole A (1).White solid. UV (MeOH) λmax 265 nm; IR (KBr) νmax 3354, 2946, 1692, 1634, 1488, 1452, 1381, 1242 cm−1; 1H and 13C NMR data see Table 1; HRESIMS [M + Na]+ z/z 262.0839 (calculated for C15H13NO2, 262.0838).
Clauolenzole B (2). Ochre solid. UV (MeOH) λmax 234 nm; IR (KBr) νmax 3442, 2927, 2852, 1600, 1521, 1452, 1401, 1360 cm−1; 1H and 13C NMR data see Table 1; HRESIMS [M − H]− z/z 256.0981 (calculated for C15H15NO3, 256.0979).
3.4. Bioassay Methods
MT4 cells (1 × 105 cells mL−1) in 96 well plates were infected by HIV-1 NL4-3 Nanoluc-sec at a dose of 50 TCID50/well in the presence of each test compound at various concentrations. On Day 3 post-infection, supernatants were collected and assayed for luciferase activity using the Nano-Glo Luciferase Assay System (Promega, Madison, WI, USA). The antiviral potency was defined as the compound concentration that reduces the luciferase activity by 50% (EC50) [36].
The cytotoxicity of the compound against MT4 cells was assessed using a CytoTox-Glo cytotoxicity assay (Promega, Madison, WI, USA). MT-4 cells were cultured in the presence of various concentrations of each test compound for 3 days, and the 50% cytotoxic concentration (CC50) causing a 50% reduction of cell viability was determined by following the manufacturer’s protocol [36].
4. Conclusions
In this work, eight carbazole alkaloids (1–8) were isolated and identified based on spectroscopic analyses and references from C. anisum-olens. Among them, compounds 1 and 2 were two new carbazole alkaloids, named clauolenzole A and B, respectively. Meanwhile, compounds 1–8 were evaluated for their in vitro anti-HIV activity on virus replication in MT-4 lymphocytes infected by HIV-1NL4-3 Nanoluc-sec virus with azidothymidine (AZT) as positive control. The new carbazole alkaloid 1 exhibited an anti-HIV EC50 value of 2.4 μg/mL and SI of 7.1, comparable or better than the values for similar carbazoles. Based on the current and prior literature results [4,5,6,7,8,9,10], carbazole alkaloids might be candidates for further study as potential anti-HIV agents. Structural analyses and synthetic modification of carbazole alkaloids and their derivatives are in progress to possibly increase the compounds’ anti-HIV-1 activity.
Supplementary Materials
The following are available online: 1H, 13C, 2D NMR, and HRMS spectra for 1–2.
Author Contributions
Y.-S.W. suggested the idea of the investigations; Y.-S.W. and K.-H.L. designed the experiments obtained; X.-Y.W. performed the isolation; J.-H.Y. performed the isolation and identification; J.-H.Y. and Y.-S.W. performed the elucidation of structures and prepared manuscript; R.L. and M.-H.Z. determined the spectroscopic data; C.-H.C. and Y.-P.Z. contributed to the evaluation of bioactivities; S.L.M.-N. revised the manuscript; Y.-Y.C. reviewed the manuscript; Y.-S.W. funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 81960629, 21662040, 21462048, 21162038, and 21262040) and the China Scholarship Council (CSC) Fund No. 201508535020, and Grant No. 2007PY01-23. Partial support was supplied by NIH grant AI033066 awarded to K.H. Lee.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Greger H. Phytocarbazoles: Alkaloids with great structural diversity and pronounced biological activities. Phytochem. Rev. 2017;16:1095–1153. doi: 10.1007/s11101-017-9521-5. [DOI] [Google Scholar]
- 2.Schmidt A.W., Reddy K.R., Knölker H.J. Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2012;112:3193–3328. doi: 10.1021/cr200447s. [DOI] [PubMed] [Google Scholar]
- 3.Caruso A., Ceramella J., Iacopetta D., Saturnino C., Mauro M.V., Bruno R., Aquaro S., Sinicropi M.S. Carbazole derivatives as antiviral agents: An overview. Molecules. 2019;24:1912. doi: 10.3390/molecules24101912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meragelman K.M., McKee T.C., Boyd M.R. Siamenol, a new carbazole alkaloid from Murraya siamensis. J. Nat. Prod. 2000;63:427–428. doi: 10.1021/np990570g. [DOI] [PubMed] [Google Scholar]
- 5.Kongkathip B., Kongkathip N., Sunthitikawinsakul A., Napaswat C., Yoosook C. Anti-HIV-1 constituents from Clausena excavata: Part II. Carbazoles and a pyranocoumarin. Phytother. Res. 2005;19:728–731. doi: 10.1002/ptr.1738. [DOI] [PubMed] [Google Scholar]
- 6.Kongkathip N., Kongkathip B. Constituents and bioactivities of Clausena excavata. Heterocycles. 2009;79:121–144. doi: 10.3987/REV-08-SR(D)2. [DOI] [Google Scholar]
- 7.Wang J., Zheng Y., Efferth T., Wang R., Shen Y., Hao X. Indole and carbazole alkaloids from Glycosmis montana with weak anti-HIV and cytotoxic activities. Phytochemistry. 2005;66:697–701. doi: 10.1016/j.phytochem.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Hirata K., Ito C., Furukawa H., Itoigawa M., Cosentino L.M., Lee K.H. Substituted 7H-pyrido[4,3-c]carbazoles with potent anti-HIV activity. Bioorg. Med. Chem. Lett. 1999;9:119–122. doi: 10.1016/S0960-894X(98)00708-2. [DOI] [PubMed] [Google Scholar]
- 9.Yan H., Mizutani T.C., Nomura N., Takakura T., Kitamura T., Kitamura Y., Miura H., Nishizawa M., Tatsumi M., Yamamoto N., et al. A novel small molecular weight compound with a carbazole structure that demonstrates potent human immunodeficiency virus type-1 integrase inhibitory activity. Antivir. Chem. Chemother. 2005;16:363–373. doi: 10.1177/095632020501600603. [DOI] [PubMed] [Google Scholar]
- 10.Ding L., Munch J., Goerls H., Maier A., Fiebig H.H., Lin W.H., Hertweck C. Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorg. Med. Chem. Lett. 2010;20:6685–6687. doi: 10.1016/j.bmcl.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Singh I.P., Bodiwala H.S. Recent advances in anti-HIV natural products. Nat. Prod. Rep. 2010;27:1781–1800. doi: 10.1039/c0np00025f. [DOI] [PubMed] [Google Scholar]
- 12.Institutum Botanicum Kunmingenge Academiae Sinicae . In: Flora Yunnanica (Spermatophyta) Wu C.Y., editor. Science Press; Beijing, China: 2001. p. 767. [Google Scholar]
- 13.Wu T.S., Huang S.C., Lai J.S., Teng C.M., Ko F.N., Kuoh C.S. Chemical and antiplatelet aggregative investigation of the leaves of Clausena excavata. Phytochemistry. 1993;32:449–451. [Google Scholar]
- 14.Wu T.S., Huang S.H., Wu P.L. Pyrano and furocarbazole alkaloids from the root bark of Clausena excavata. Heterocycles. 1997;45:969–973. doi: 10.3987/COM-97-7786. [DOI] [Google Scholar]
- 15.Wu T.S., Huang S.C., Wu P.L. Carbazole alkaloids from bark of Clausena excavata. Phytochemistry. 1996;43:1427–1429. doi: 10.1016/0031-9422(96)00212-9. [DOI] [PubMed] [Google Scholar]
- 16.Ito C., Katsuni S., Ohta H., Omura M., Kajiura I., Furukawa H. Constituents of Clausena excavata. Isolation and structural elucidation of new carbazole alkaloids. Chem. Pharm. Bull. 1997;45:48–52. doi: 10.1248/cpb.45.48. [DOI] [Google Scholar]
- 17.Songsiang U., Thongthoom T., Boonyarat C., Yenjai C. Claurailas A-D, cytotoxic carbazole alkaloids from the roots of Clausena harmandiana. J. Nat. Prod. 2011;74:8–12. doi: 10.1021/np100654m. [DOI] [PubMed] [Google Scholar]
- 18.Maneerat W., Ritthiwigrom T., Cheenpracha S., Promgool T., Yossathera K., Deachathai S., Phakhodee W., Laphookhieo S. Bioactive carbazole alkaloids from Clausena wallichii roots. J. Nat. Prod. 2012;75:741–746. doi: 10.1021/np3000365. [DOI] [PubMed] [Google Scholar]
- 19.Maneerat W., Phakhodee W., Ritthiwigrom T., Cheenpracha S., Promgool T., Yossathera K., Deachathai S., Laphookhieo S. Antibacterial carbazole alkaloids from Clausena harmandiana twigs. Fitoterapia. 2012;83:1110–1114. doi: 10.1016/j.fitote.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Du Y.Q., Liu H., Li C.J., Ma J., Zhang D., Li L., Sun H., Bao X.Q., Zhang D.M. Bioactive carbazole alkaloids from Clausena wallichii roots. Fitoterapia. 2015;103:122–128. doi: 10.1016/j.fitote.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y.L., Liu Y.P., Zhang C., Zhao W.H., Shi S., He D.N., Zhang P., Liu X.H., Han T.T., Fu Y.H. Carbazole alkaloids from Clausena hainanensis with their potential antiproliferative activities. Bioorg. Chem. 2018;76:359–364. doi: 10.1016/j.bioorg.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Huang L., Feng Z.L., Wang Y.T., Lin L.G. Anticancer carbazole alkaloids and coumarins from Clausena plants: A review. Chin. J. Nat. Med. 2017;15:881–888. doi: 10.1016/S1875-5364(18)30003-7. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y.P., Guo J.M., Liu Y.Y., Hu S., Yan G., Qiang L., Fu Y.H. Carbazole Alkaloids with Potential Neuroprotective Activities from the Fruits of Clausena lansium. J. Agric. Food Chem. 2019;67:5764–5771. doi: 10.1021/acs.jafc.9b00961. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y.S., He H.P., Yang J.H., Di Y.T., Hao X.J. New monoterpenoid coumarins from Clausena anisum-olens. Molecules. 2008;13:931–937. doi: 10.3390/molecules13040931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y.S., Huang R., Li L., Zhang H.B., Yang J.H. O-Terpenoidal coumarins from Clausena anisum-olens. Biochem. Syst. Ecol. 2008;36:801–803. doi: 10.1016/j.bse.2008.06.005. [DOI] [Google Scholar]
- 26.Wang Y.S., Xu H.Y., Lu H., Wang D.X., Yang J.H. A new O-terpenoidal coumarin from Clausena anisum-olens Merr. Molecules. 2009;14:771–776. doi: 10.3390/molecules14020771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y.S., Huang R., Li N.Z., Yang J.H. Coumarins from Clausena anisum-olens Merr. Biosci. Biotechnol. Biochem. 2010;74:1483–1484. doi: 10.1271/bbb.100143. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y.S., Li B.T., Liu S.X., Wen Z.Q., Yang J.H., Zhang H.B., Hao X.J. Anisucoumaramide, a bioactive coumarin from Clausena anisum-olens. J. Nat. Prod. 2017;80:798–804. doi: 10.1021/acs.jnatprod.6b00391. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y.S., He H.P., Hong X., Zhao Q., Hao X.J. A new binary carbazole alkaloid from Murraya koenigii. Chin. Chem. Lett. 2002;13:849–850. [Google Scholar]
- 30.Ma Q.G., Tian J., Yang J.B., Wang A.G., Ji T.F., Wang Y.G., Su Y.L. Bioactive carbazole alkaloids from Murraya koenigii (L.) Spreng. Fitoterapia. 2013;87:1–6. doi: 10.1016/j.fitote.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Wu T.S., Huang S.C., Wu P.L., Kuoh C.S. Alkaloidal and other constituents from the root bark of Clausena excavata. Phytochemistry. 1999;52:523–527. doi: 10.1016/S0031-9422(99)00220-4. [DOI] [Google Scholar]
- 32.Bhattacharyya P., Chakraborty A., Chowdhury B.K. Heptazolicine, a carbazole alkaloid from Clausena heptaphylla. Phytochemistry. 1984;23:2409–2410. doi: 10.1016/S0031-9422(00)80575-0. [DOI] [PubMed] [Google Scholar]
- 33.Ito C.H., Ohta H., Tan H.T.W., Furukawa H. Constituents of Clausena excavata. Isolation and structural elucidation of seven new carbazole alkaloids and a new coumarin. Chem. Pharm. Bull. 1996;44:2231–2235. doi: 10.1248/cpb.44.2231. [DOI] [Google Scholar]
- 34.Ito C., Okahana N., Wu T.S., Wang M.L., Lai J.S., Kuoh C.S., Furukawa H. New carbazole alkaloids from Murraya euchrestifolia. Chem. Pharm. Bull. 1992;40:230–232. doi: 10.1248/cpb.40.230. [DOI] [Google Scholar]
- 35.Wu T.S., Huang S.C., Wu P.L., Teng C.M. Carbazole alkaloids from Clausena excavate and their biological activity. Phytochemistry. 1996;43:133–140. doi: 10.1016/0031-9422(96)00212-9. [DOI] [PubMed] [Google Scholar]
- 36.Liu Q.B., Li W., Huang L., Asada Y., Morris-Natschke S.L., Chen C.H., Lee K.H., Koike K. Identification, structural modification, and dichotomous effects on human immunodeficiency virus type 1 (HIV-1) replication of ingenaneesters from Euphorbia kansui. Eur. J. Med. Chem. 2018;156:618–627. doi: 10.1016/j.ejmech.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.