Abstract
The seeds of Millettia ferruginea are used in fishing, pesticides, and folk medicine in Ethiopia. Here, the anti-cancer effects of isoflavones isolated from M. ferruginea were evaluated in human ovarian cancer cells. We found that isoflavone ferrugone and 6,7-dimethoxy-3’,4’-methylenedioxy-8-(3,3-dimethylallyl)isoflavone (DMI) had potent cytotoxic effects on human ovarian cancer cell A2780 and SKOV3. Ferrugone and DMI treatment increased the sub-G1 cell population in a dose-dependent manner in A2780 cells. The cytotoxic activity of ferrugone and DMI was associated with the induction of apoptosis, as shown by an increase in annexin V-positive cells. Z-VAD-fmk, a broad-spectrum caspase inhibitor, and z-DEVD-fmk, a caspase-3 inhibitor, significantly reversed both the ferrugone and DMI-induced apoptosis, suggesting that cell death stimulated by the isoflavones is mediated by caspase-3-dependent apoptosis. Additionally, ferrugone-induced apoptosis was found to be caspase-8-dependent, while DMI-induced apoptosis was caspase-9-dependent. Notably, DMI, but not ferrugone, increased the intracellular levels of reactive oxygen species (ROS), and antioxidant N-acetyl-L-cysteine (NAC) attenuated the pro-apoptotic activity of DMI. These data suggest that DMI induced apoptotic cell death through the intrinsic pathway via ROS production, while ferrugone stimulated the extrinsic pathway in human ovarian cancer cells.
Keywords: Millettia ferruginea; 6,7-dimethoxy-3’,4’-methylenedioxy-8-(3,3-dimethylallyl) isoflavone; ferrugone; apoptosis; caspase; ovarian cancer
1. Introduction
Ovarian cancer is the most lethal gynecological disease in the world. Approximately 225,000 women are diagnosed and about 140,200 women die each year from ovarian cancer [1]. Due to the non-specific symptoms, a majority of the patients are diagnosed when the disease is already advanced with poor prognosis [2]. The five-year survival rate of advanced ovarian cancer is only about 25%. Platinum-taxane chemotherapy following cytoreductive surgery is the standard treatment for ovarian cancer. Although primary therapy may be of therapeutic benefit in about 80% of ovarian cancer patients, around 70% of the patients eventually relapse [3]. Therefore, it is urgent to seek for new agents for the treatment of ovarian cancer. Natural products from plants have drawn great attention in this regard. Extensive studies have shown that plant-derived flavonoids have anti-cancer properties through the induction of apoptosis, which is usually abnormal in the cancer cells [4].
More than 200 species of the Millettia genus (Leguminosae) are distributed in the tropical and subtropical regions of Asia, Australia, and Africa [5]. In Africa, these plants are commonly used as insecticides and fish poison [6]. In addition, these plants have long been used in traditional medicine. For example, Millettia pachycarpa was used as an anthelminthic to evacuate the parasitic intestinal worms [7]. M. thonningii is known to treat dysentery, constipation, and diarrhea while M. carulea has anti-inflammatory effects [8,9]. M. macrophylla is also used to relieve menopausal symptoms [10].
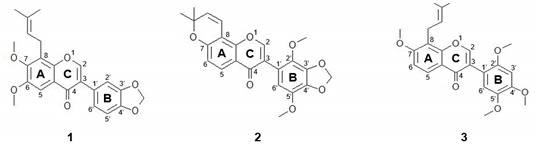
Phytochemical studies with Millettia species revealed that these species have a rich source of flavonoids, including isoflavonoids, rotenoids, chalcones, and pterocarpans [11,12]. M. ferruginea (Hochst) Baker, is an endemic of Ethiopia and has two representative species; ssp. ferruginea and darassana. Previously, from the seeds of M. ferruginea ssp. ferruginea, we isolated several isoflavones, including calopogonium isoflavone A, durmillone, barbigerone, ferrugone, prebarbigerone, and a novel prenylated isoflavone 6,7-dimethoxy-3’,4’-methylenedioxy-8-(3,3-dimethylallyl)isoflavone (DMI) [12]. Calopogonium isoflavone A and durmillone showed antimicrobial and DNA fragmentation effects against gram-negative bacteria and the promastigotes of Leishmania donovani [13], while barbigerone exhibited inhibitory effects on the growth and metastasis of tumors in melanoma [14]. However, to the best of our knowledge, the biological activities of ferrugone, prebarbigerone, and DMI have never been reported. Here, we investigated the anti-cancer effects of the isoflavones using human ovarian cancer cells.
2. Results
2.1. Ferrugone and DMI Isolated from M. ferruginea Had Potent Cytotoxic Activities against Human Ovarian Cancer Cells
We investigated the effects of the three isoflavones isolated from M. ferruginea, ferrugone, prebarbigerone, and DMI on the viability of two human ovarian cancer cells A2780 and SKOV3. Ferrugone and DMI had potent cytotoxic activities in A2780 cells (Table 1).
Table 1.
Cytotoxic activity of isoflavones isolated from Millettia ferruginea spp. ferruginea in human ovarian cancer cells.
| Compound No | Name | IC50 a | |
|---|---|---|---|
| A2780 | SKOV3 | ||
| 1 | 6,7-dimethoxy-3′,4′-methylenedioxy-8- (3,3-dimethylallyl)isoflavone (DMI) |
0.45 ± 0.06 μM (0.18 ± 0.02 μg/mL) |
> 100 μM (> 39.41 μg/mL) |
| 2 | Ferrugone | 0.51 ± 0.06 μM (0.21 ± 0.02 μg/mL) |
36.78 ± 5.26 μM (15.01 ± 2.15 μg/mL) |
| 3 | Prebarbigerone | 32.06 ± 8.31 μM (13.16 ± 3.41 μg/mL) |
> 100 μM (> 41.05 μg/mL) |
| - | Cisplatin b | 14.14 ± 0.59 μM (4.24 ± 0.18 μg/mL) |
36.64 ± 0.96 μM (10.99 ± 0.29 μg/mL) |
a IC50 values are defined as the concentration that results in a 50% decrease in the number of cells compared with that of the control cultures. b Cisplatin was used as a positive control.
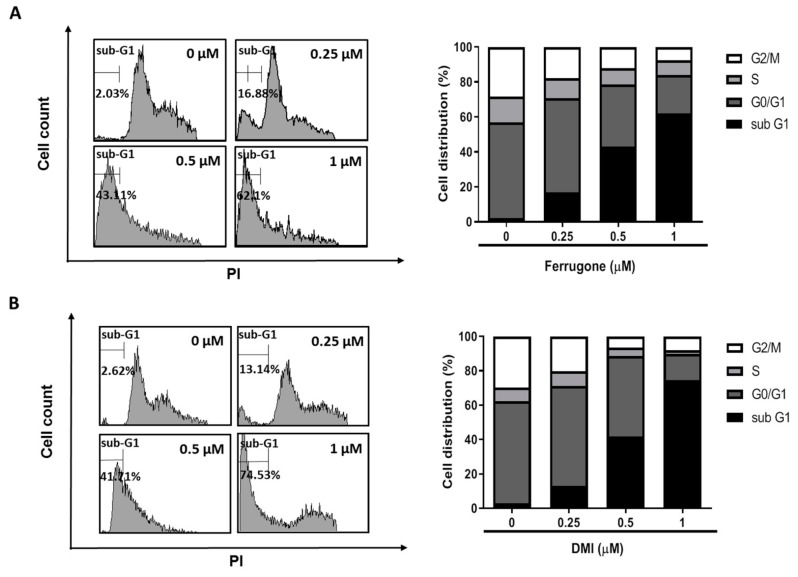
To examine whether the cytotoxic activities of ferrugone and DMI are related to cell cycle arrest, the distribution of the cells in different phases of cell cycle progression was analyzed in A2780 cells. As shown in Figure 1, treatment with ferrugone or DMI increased sub G1 phase in the ovarian cancer cells. After treatment with 0, 0.25, 0.5, and 1 µM of ferrugone, the proportions of the sub G1 phase cells were 2.03%, 16.88%, 43.11%, and 62.1% (Figure 1A). Following DMI treatment under the same condition, the proportions of the sub G1 cells were 2.62%, 13.14%, 41.71%, and 74.53% (Figure 1B). These data suggest that the cytotoxicities of ferrugone and DMI are mediated by the induction of cell death rather than cell cycle arrest.
Figure 1.
Effect of ferrugone and DMI on cell cycle distribution in human ovarian cancer cells. A2780 cells were treated with ferrugone (A) or DMI (B) (0.25, 0.5, and 1 µM) for 48 h and then stained with propidium iodide (PI). The cell-cycle distribution profiles of the cells were determined by flow cytometry. The graph indicates the percentages of cells in the sub-G1, G0/G1, S, and G2/M phases of the cell cycle. The data are representative of three independent experiments.
2.2. Ferrugone and DMI Isolated from M. ferruginea Induced Apoptotic Cell Death in the Ovarian Cancer Cells
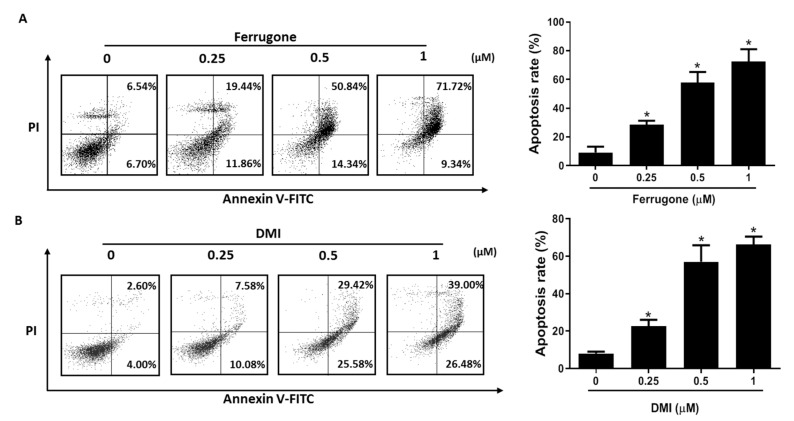
We further investigated whether the cytotoxic effects of ferrugone and DMI were related to apoptotic cell death using annexin V-FITC (fluorescein isothiocyanate) staining assay. As shown in Figure 2, both ferrugone and DMI increased annexin V-positive apoptotic cells in the right quadrants of the flow cytometry graphs. Treatment with 0.25, 0.5, and 1 μM of ferrugone induced a dose-dependent increase in the apoptotic cell population from 13.24% to 31.3%, 65.18%, and 81.06%, respectively (Figure 2A). Treatment with 0.25, 0.5, and 1 μM of DMI showed 17.66%, 55%, and 65.48% apoptotic cell death, respectively (Figure 2B). These results suggest that ferrugone and DMI dose-dependently induced apoptotic cell death in human ovarian cancer cells.
Figure 2.
Effect of ferrugone and DMI on apoptotic cell death in human ovarian cancer cells. A2780 cells were treated with ferrugone (A) and DMI (B) (0.25, 0.5, and 1 µM) for 48 h and co-stained with PI and Annexin V-FITC (fluorescein isothiocyanate). The translocation of phosphatidyl serine was detected by flow cytometry. The graph indicates the percentages of annexin V-positive apoptotic cells in the right quadrants of flow cytometry graphs. The data are representative of three independent experiments. Data were analyzed using one-way ANOVA followed by Dunnett’s multiple comparison test. * p < 0.05 as compared with untreated group.
2.3. Ferrugone and DMI Induced Caspase-Dependent Apoptotic Cell Death in the Ovarian Cancer Cells
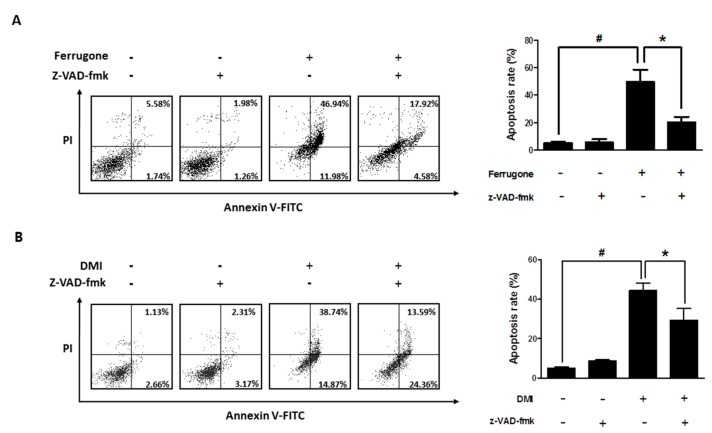
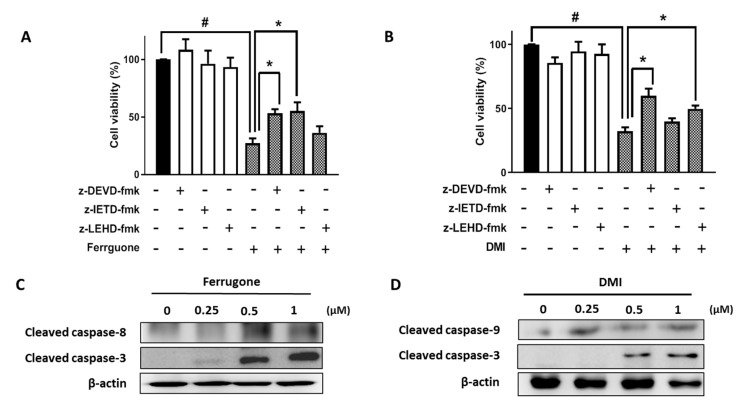
The involvement of caspases in apoptosis induced by ferrugone and DMI was further investigated considering the critical role of various caspases in apoptotic cell death. A broad caspase inhibitor, z-VAD-fmk, significantly suppressed the ferrugone- and DMI-induced apoptotic cell death in the A2780 cells, suggesting that both ferrugone and DMI induced caspase-dependent apoptosis in the human ovarian cancer cells (Figure 3A,B). To confirm which caspase was associated in particular, z-DEVD-fmk (caspase-3 inhibitor), z-IETD-fmk (caspase-8 inhibitor), and z-LEHD-fmk (caspase-9 inhibitor) were used (Figure 4). z-DEVD-fmk and z-IETD-fmk, but not z-LEHD, significantly decreased ferrugone-induced cell death (Figure 4A). On the other hand, DMI-induced cell death was significantly reversed by z-DEVD-fmk and z-LEHD-fmk, but not z-IETD-fmk (Figure 4B). Additionally, western blot analysis revealed that ferrugone activated caspase-3 and caspase-8 (Figure 4C) and DMI activated caspases-3 and caspase-9 (Figure 4D) in the A2780 cells resulting in increasing the density of their cleaved forms. These data suggest that ferrugone and DMI induced caspase-3-dependent apoptosis via the caspase-8 and caspase-9 activation, respectively, in the human ovarian cancer cells.
Figure 3.
Involvement of caspases in ferrugone and DMI-induced cell death in human ovarian cancer cells. A2780 cells were pretreated with broad caspase inhibitor z-VAD-fmk (50 µM) for 2 h, and then treated with ferrugone (A) and DMI (B) (0.5 µM) for 48 h. Annexin/PI V-FITC staining assay was performed to determine apoptosis. The graph indicates the percentages of annexin V-positive apoptotic cells in the right quadrants of flow cytometry graphs. The data are representative of three independent experiments. Student’s t-test (two-tailed) was applied to evaluate the significance. # p < 0.05 as compared with the untreated group. * p < 0.05 as compared with the ferrugone or DMI only-treated group.
Figure 4.
Involvement of caspase-3, -8, and -9 in ferrugone and DMI-induced cell death in human ovarian cancer cells. (A,B) A2780 cells were pretreated with caspase-3 inhibitor z-DEVD-fmk (75 µM), caspase-8 inhibitor z-IETD-fmk (50 µM), and caspase-9 inhibitor z-LEHD-fmk (75 µM) for 2 h, and then treated with ferrugone (A) and DMI (B) (0.5 µM) for 24 h. MTT assay was performed to determine cell death. (C,D) A2780 cells were treated with ferrugone (C) and DMI (B) (0.25, 0.5, and 1 µM) for 48 h. Cleaved caspase-3 and caspase-8 levels were determined by Western blot assay. β-actin was used as an internal control. The data are representative of three independent experiments. Student’s t-test (two-tailed) was applied to evaluate the significance. # p < 0.05 as compared with the untreated group. * p < 0.05 as compared with the ferrugone or DMI only-treated group.
2.4. Apoptosis-Inducing Effect of DMI Is Associated with ROS Production
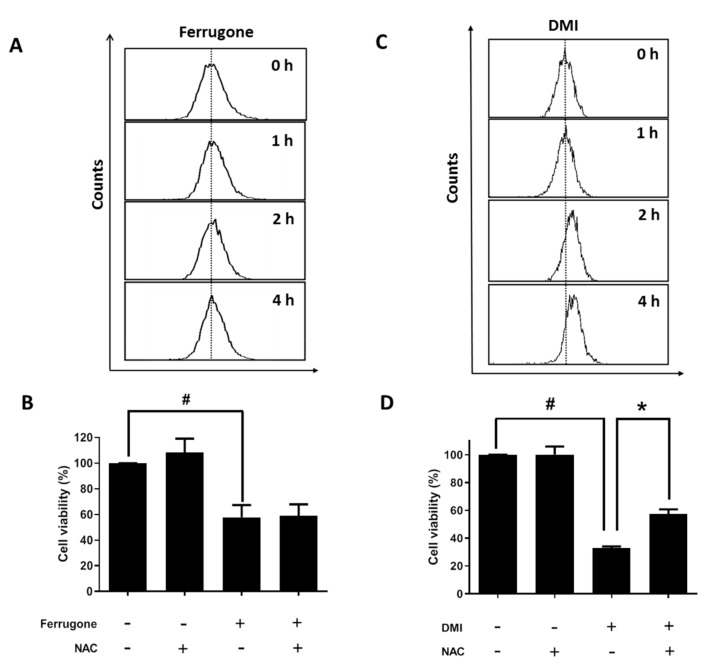
Reactive oxygen species (ROS) are known to stimulate apoptosis in various cancer cells [9]. Here, we investigated the involvement of ROS in ferrugone or DMI-induced cell death in human ovarian cancer. DCF-DA (2’,7’–dichlorofluorescin diacetate) staining assay showed that ferrugone did not induce any significant change in the intracellular ROS level (Figure 5A), and N-acetyl-L-cysteine (NAC) treatment did not affect ferrugone-induced cell death in A2780 cells (Figure 5B). On the other hand, DMI increased the intracellular ROS level in A2780 cells (Figure 5C). Additionally, DMI-induced cell death was significantly inhibited in the presence of N-acetyl-L-cysteine (NAC) (Figure 5D). These data reveal that DMI, but not ferrugone, induced apoptosis by ROS regulation in the ovarian cancer cells.
Figure 5.
Involvement of ROS (reactive oxygen species) in DMI-induced cell death in human ovarian cancer cells. (A) A2780 cells were treated with 0.5 µM of ferrugone for the indicated times (1, 2, and 4 h). The cells were stained with DCF-DA (2’,7’–dichlorofluorescin diacetate), and analyzed by flow cytometry. (B) A2780 cells were pretreated with N-acetyl-L-cysteine (NAC, 5 mM) for 30 min, then treated with ferrugone (0.5 µM) for 48 h. MTT assay was performed to examine the cell death. (C) A2780 cells were treated with 0.5 µM of DMI for the indicated times (1, 2, and 4 h). The cells were stained with DCF-DA and analyzed by flow cytometry. (D) A2780 cells were pretreated with NAC (5 mM) for 30 min, then treated with DMI (0.5 µM) for 48 h. MTT assay was performed to examine the cell death. Student’s t-test (two-tailed) was applied to evaluate the significance. # p < 0.05 as compared with the untreated group. * p < 0.05 as compared with the ferrugone or DMI only treated group.
3. Discussion
The genus Millettia appears in the African pharmacopeia. It has a wide range of biological activities as confirmed by pharmacological studies, such as pesticidal, antiviral, anti-inflammatory, antitumoral, and bactericidal activities, and therefore, this genus has attracted great attention in traditional medicine and research of new biologically active compounds [6]. In addition, the genus Millettia is rich in flavonoids, for example, isoflavones, showing various activities in women health. Isoflavones have been shown to have beneficial effects for women with menopausal complaints, postmenopausal syndromes, and breast and gynecologic cancers [15,16,17,18]. Similarly, griffonianone C, an isoflavone from the M. griffoniana, has been reported to have estrogenic activity in uterus and liver of ovariectomized rats [19]. In addition, isoflavones extracted from M. taiwaniana have shown cancer chemopreventive activities [20]. Durmillone isolated from M. pachyloba Drake induced apoptosis and autophagy in Hela and MCF-7 cells [21]. In this study, we demonstrated that two isoflavones, ferrugone and DMI, isolated from M. ferruginea, induced apoptotic cell death in human ovarian cancer cells.
In this study, to investigate the effects of isoflavones on the viability of human ovarian cancer cells, we have used two most commonly used human ovarian epithelial cancer cell lines A2780 and SKOV3 cells. Both DMI and ferrugone exhibited more potent cytotoxicity on A2780 cells with an observed IC50 value of 0.44 ± 17.24 and 0.51 ± 0.06 μM, respectively, than cisplatin (IC50 value of 14.14 ± 0.59 μM). However, in SKOV3 cells, ferrugone showed only mild cytotoxic effect (IC50 value of 36.78 ± 5.26 μM) and DMI was not active (IC50 value >100 uM). These data indicate that DMI and ferrugone have potent cytotoxicity only in A2780 cells, but not in SKOV3 cells. Notably, A2780 cells express wild-type p53 gene while SKOV3 cells are p53 null, suggesting that the isoflavone-induced cell death might be p53 dependent. It remains to be further demonstrated whether p53 mediate DMI- and ferrugone-induced apoptosis in human ovarian cancer cells.
Apoptosis is a natural mechanism of programmed cell death, which plays a critical role in the development and homeostasis of mammals that live long [22]. The prevention of apoptosis is an eminent hallmark of cancer, which may not only promote tumorigenesis but also render the cancer cells resistant to treatment [23,24]. Thus, apoptosis is a promising target for cancer therapy. The protease enzymes caspases (cysteinyl aspartate-specific protease) play critical roles in programmed cell death, as well as inflammation. Based on their function, mammalian caspase-2, -3, -7, -8, -9, and -10 are apoptotic caspases, whereas caspase-1, -4, -5, -11, and -12 are involved in inflammation [25]. The upstream signaling events induce dimerization and activation of initiator caspases-8 and -9, and then, the effector caspases-3, -6, and -7 are cleaved by initiator caspases resulting in apoptosis [26]. There are two common pathways that can be activated by caspase activation. In the extrinsic pathway, caspase-8 activation by death ligands binds to a death receptor in response to extracellular signals, while the intracellular stimulants activate caspase-9 in the intrinsic pathway [27]. The intrinsic and extrinsic pathways converge to caspase-3, which cleaves the inhibitor of the caspase-activated deoxyribonuclease, leading to apoptosis [28]. In this study, we found that both ferrugone and DMI induced the activation of an effector caspase-3 and caspases-dependent apoptosis in human ovarian cancer cells. Notably, ferrugone induced the activation of caspase-8, but not caspase-9, while DMI stimulated the activation of caspase-9, but not caspase-8. These data suggested that ferrugone and DMI differently induced apoptosis via a caspase-8-dependent extrinsic pathway and caspase-9-dependent intrinsic pathway, respectively.
The accumulation of reactive oxygen species (ROS) is involved in multiple diseases, including atherosclerosis, neurodegeneration, diabetes, aging, and cancer [29]. In cancer therapy, treatment with drugs that induce ROS generation has been suggested as an effective strategy to selectively push cancer cells over the ROS threshold and into cell death [30]. Intracellular ROS are known to activate the intrinsic apoptosis pathway through the reduction of mitochondria membrane potential, release of death-promoting factors, such as cytochrome c from the mitochondria, and the activation of caspase-9 [31,32]. Additionally, it has been suggested that oxidative modification of caspase-9 by ROS induces the interaction with apoptotic protease-activating factor-1 (Apaf-1), resulting in the stimulation of its auto-cleavage and activation [33]. In this study, we demonstrated that DMI increased the intracellular production of ROS. More importantly, caspase-9-dependent apoptotic cell death by DMI was significantly reversed by antioxidant NAC treatment. These data suggest that DMI-induced ROS production may stimulate caspase-9 activation, regulating apoptotic cell death. Although many polyphenols including isoflavones have antioxidant activity and show cytoprotective effect in some cells by reducing the ROS levels, some polyphenols have been reported to act both as an antioxidant and as a pro-oxidant. For instance, several polyphenols, including isoflavones and phlorotannins, increase intracellular levels of ROS to induce apoptosis and cell death in cancer cells [34,35]. That is consistent with our finding showing the isoflavone DMI induced ROS production and apoptosis in human ovarian cancer cells. In addition, increased levels of ROS have been reported to stimulate apoptotic cell death in cancer cells by regulating the stress kinase pathways, including PI3K/Akt and MAPK, as well as caspase pathway. Thus, whether those stress kinase pathways are involved in the DMI-induced ROS production and apoptosis should be further investigated. On the other hand, it is of note that the activation of the extrinsic apoptosis pathway by another isoflavone, ferrugone, was not associated with ROS production in human ovarian cancer cells. Further research is required to illuminate the upstream molecular mechanisms underlying ferrugone-induced apoptotic cell death.
Here, we found that the two isoflavones, ferrugone and DMI, induced apoptosis through a different pathway in human ovarian cancer cells. The structural differences between two isoflavones are that ferrugone has two methoxy groups at 2, 5 positions of the B ring; and a methoxy group and an isoprene unit at 7, 8 positions of A ring constitutes a new ring and demethoxylation at 6 position of A ring. The difference in the mechanisms induced by ferrugone and DMI might be explicated by these structural differences, which potentially lead to the binding of different target molecules. Further research is required to clarify the detailed molecular mechanisms underlying the structure-activity relationship.
4. Materials and Methods
4.1. Sample Preparation
The mature seeds of Millettia ferruginea ssp. ferruginea were collected from Oromia region, Bacho Faleni district of rural lowland area, northern part of Ethiopia. The collected plant material was identified by one author (Deyou T), and a voucher specimen (TD-02/2016) was deposited at Jimma University Herbarium, Ethiopia. The phytochemical study of 6,7-dimethoxy-3’,4’-methylenedioxy-8-(3,3-dimethylallyl) isoflavone, ferrugone, and prebarbigerone (Table 1) used in the present study was described in our previous study [12]. Briefly, dry mature seeds of M. ferruginea ssp. ferruginea (300 g) were powdered and extracted with CH2Cl2/CH3OH (1:1, v/v). Resulting crude extract (160 g) was subjected to liquid–liquid extraction in CH3OH/n-hexane (7:3) to give 110 g of CH3OH extract. Successive chromatographic separation of CH3OH extract gave three prenylated isoflavone compounds, 6,7-dimethoxy-3’,4’-methylenedioxy-8-(3,3-dimethylallyl) isoflavone (34.6 mg), ferrugone (80.9 mg), and prebarbigerone (131.4 mg). The stock solution of each compound was prepared in DMSO (dimethyl sulfoxide) and serially diluted to set optimal concentration for bioassay. The final concentration of DMSO in the experiments was less than 0.1%.
4.2. Materials
Roswell Park Memorial Institute (RPMI) 1640, penicillin, fetal bovine serum (FBS), and streptomycin were purchased from Life Technologies Inc. (Grand Island, NY, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was obtained from Molecular Probes Inc. (Eugene, OR, USA). Propidium iodide (PI), N-acetyl-L-cysteine (NAC), and 2-mercaptoethanol were acquired from Sigma Chemical (St. Louis, MO, USA). Phenylmethylsulfonylfluoride (PMSF) and annexin V-fluorescein isothiocyanate (FITC) were purchased from BD Biosciences (San Jose, CA, USA). Dichlorofluorescein diacetate (DCFH-DA) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All inhibitors for caspases were purchased from Calbiochem (Bad Soden, Germany). Enhanced chemiluminescence (ECL) reagent was obtained from EMD Millipore (Billerica, MA, USA). Tris-buffered saline was obtained from Boster Biological Technology Ltd. (Wuhan, China). Caspase-3 and caspase-9 antibodies were procured from Cell Signaling Technology (Beverly, MA, USA). Caspase-8 and β-actin antibodies were procured from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Secondary antibodies were purchased from The Jackson Laboratory (West Grove, PA, USA).
4.3. Cell Culture
Ovarian cancer cells (A2780 and SKOV3) were cultured in RPMI 1640 supplemented with 5% FBS, penicillin (100 U/mL), and streptomycin sulfate (100 μg/mL). The cells were incubated in a 5% CO2 and 95% air humidified atmosphere at 37 °C.
4.4. MTT Assay
Cell viability was examined using the MTT assay. Human ovarian cancer cells (A2780 and SKOV3) were seeded in a 96-well plate containing 50 μL of RPMI medium at a density of 1 × 105 cells/mL in each well. After 24 h incubation, various concentrations of ferrugone and DMI dissolved in dimethyl sulfoxide were diluted with culture medium and were added into each well. After 48 h incubation, 50 μL of MTT (1 mg/mL stock solution) was added, and then the plates were incubated for an additional 4 h. Then the medium was discarded, and the formazan crystal that formed in the cells was dissolved in 50 μL of DMSO in each well. The optical density was measured at 540 nm by using a microplate spectrophotometer (SpectraMax; Molecular Devices, Sunnyvale, CA, USA).
4.5. Propidium Iodide (PI) Staining for Cell Cycle Analysis
A2780 cells were seeded in 60 mm culture dishes containing 3 mL of RPMI medium at a density of 1 × 105 cells/mL in each dish. After 24 h incubation, the cells were treated with ferrugone and DMI for 48 h and collected in ice-cold phosphate-buffered saline (PBS). After being washed twice with cold PBS, the cells were fixed with EtOH (70%) and stored at 4 °C for 2 h. Fixed cells were suspended in a PI staining solution (50 μg/mL) supplemented with RNase A (250 μg/mL). The suspended cells were incubated in the dark at room temperature for 20 min. The fluorescent intensity of the cells was measured using a Guava easyCyte flow cytometry system (EMD Milipore, Bilerica, MA, USA).
4.6. Annexin V and PI Double Staining for Apoptosis Analysis
A2780 cells were seeded in 60 mm culture dishes containing 3 mL of RPMI medium at a density of 1 × 105 cells/mL in each dish. After 24 h incubation, the cells were treated with ferrugone and DMI for 48 h. The cells were harvested and washed twice with cold PBS and suspended with 500 μL of binding buffer (10 mM HEPES (4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid)/NaOH, 140 mM NaCl, 2.5 mM CaCl2, PH 7.4). After staining with 1.25 μL of FITC-conjugated Annexin V for 15 min and 10 μL of PI (50 mg/mL) for 5 min in the dark place, the mixture was analyzed by Guava® easyCyte flow cytometry.
4.7. Western Blot Analysis
A2780 cells were seeded in 60mm culture dishes containing 3 mL of RPMI medium at a density of 1 × 105 cells/mL in each dish. After 24 h incubation, the cells were treated with ferrugone and DMI for 48 h. The cells were collected and washed twice with cold PBS. Total cellular proteins were extracted using protein lysis buffer (Intron Biotechnology, Seoul, Korea) following manufacturer’s instructions and protein concentrations were measured by the Bradford assay. The protein extracts were mixed with 5x SDS (sodium dodecyl sulfate) sample buffer and heated for 5 min at 95 °C. The mixture was loaded on a gel for SDS (sodium dodecyl sulfate)-PAGE (polyacrylamide gel electrophoresis). After electrophoretic separation, separated proteins were blotted to PVDF (polyvinylidene difluoride) membranes. After blocking with 5 % skim milk for 1 h, the membranes were incubated overnight at 4 °C with diluted primary antibodies against caspase-3, -8, 9, and β-actin in TBS-T (Tris-buffered saline containing Tween-20). After a subsequent washing three times with TBS-T, the membranes were incubated with an appropriate secondary antibody at room temperature for 2 h. Immunoreactive bands were visualized by the ECL kit and detected by ImageQuant Las-4000 (GE Healthcare Life Science, WI, USA).
4.8. Measurement of Reactive Oxygen Species
The intracellular levels of ROS were measured by using the fluorescent probe DCFH-DA that is commonly used to measure H2O2. A2780 cells were seeded in 60 mm culture dishes containing 3 mL of RPMI medium at a density of 1 × 105 cells/mL in each dish. After 24 h incubation, the cells were collected by centrifugation after treatment with ferrugone and DMI at the desired time intervals, resuspended in PBS, and loaded with 20 μM DCFH-DA. The fluorescent intensity was analyzed by flow cytometry.
4.9. Statistical Analysis
One-way ANOVA or student’s t-tests were performed to identify statistically significant differences. p values less than 0.05 were considered statistically significant.
5. Conclusions
These data suggest that DMI induced apoptotic cell death through the intrinsic pathway via ROS production, while ferrugone stimulated the extrinsic pathway in human ovarian cancer cells. This is the first study to demonstrate the biological activity of DMI and ferrugone.
Author Contributions
Y.-Y.W. and J.H.K. performed the experiments and data analysis and wrote the manuscript. T.D. and Y.P.J. prepared isoflavones. K.-T.L. was involved in project design. J.-H.C. was involved in project design and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants of Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korea government (NRF-2019R1A2C2011213).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. Ca. Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Doubeni C.A., Doubeni A.R., Myers A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician. 2016;93:937–944. [PubMed] [Google Scholar]
- 3.Christian J., Thomas H. Ovarian cancer chemotherapy. Cancer Treat. Rev. 2001;27:99–109. doi: 10.1053/ctrv.2001.0219. [DOI] [PubMed] [Google Scholar]
- 4.Ravishankar D., Rajora A.K., Greco F., Osborn H.M. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013;45:2821–2831. doi: 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Banzouzi J.T., Prost A., Rajemiariaho M., Ongoka P. Traditional uses of african millettia. Int. J. Bot. 2008;4:406–420. doi: 10.3923/ijb.2008.406.420. [DOI] [Google Scholar]
- 6.Mollel N., Adema F. Revision of millettia section truncaticalyces (leguminosae papilionoideae) Blumea. 2006;51:333–343. doi: 10.3767/000651906X622274. [DOI] [Google Scholar]
- 7.Singhal A.K., Sharma R.P., Baruah J.N., Serengolam V., Govindant S.V., Herzt W. Rotenoids from roots of millettia pachycarpa. Phytochemistry. 1982;21:949–951. doi: 10.1016/0031-9422(82)80103-9. [DOI] [Google Scholar]
- 8.Bueno Perez L., Pan L., Munoz Acuna U., Li J., Chai H.B., Gallucci J.C., Ninh T.N., Carcache de Blanco E.J., Soejarto D.D., Kinghorn A.D. Caeruleanone A, a rotenoid with a new arrangement of the d-ring from the fruits of millettia caerulea. Org. Lett. 2014;16:1462–1465. doi: 10.1021/ol500266z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison J.J.E.K., Dankyi E., Kingsford-Adaboh R., Ishida H. In search of new leads: A closer look at the therapeutic potential of the constituents of millettia thonningii, millettia pachycarpa and their structural analogues. Int. J. Pharm. Pharm. Sci. 2011;3:71–81. [Google Scholar]
- 10.Zingue S., Magne C.B., Clyne N.C., Njamen D. Elucidation of underlying mechanisms by which millettia macrophylla benth induces its estrogenic activity. Int. Sch. Res. 2014;2014:1–8. doi: 10.1155/2014/763781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deyou T., Gumula I., Pang F., Gruhonjic A., Mumo M., Holleran J., Duffy S., Fitzpatrick P.A., Heydenreich M., Landberg G., et al. Rotenoids, flavonoids, and chalcones from the root bark of millettia usaramensis. J. Nat. Prod. 2015;78:2932–2939. doi: 10.1021/acs.jnatprod.5b00581. [DOI] [PubMed] [Google Scholar]
- 12.Deyou T., Jang Y.P. A new prenylated isoflavone from the seeds of millettia ferruginea ssp ferruginea. S. Afr. J. Bot. 2018;117:155–157. doi: 10.1016/j.sajb.2018.05.004. [DOI] [Google Scholar]
- 13.Choudhury M.K., Shiferaw Y., Sur K.R., Dey D., Debnath S., Ghosh S., Ray R., Hazra B. Antimicrobial activity and DNA-fragmentation effect of isoflavonoids isolated from seeds of millettia ferruginea, and endemic legume tree in ethiopia. J. Coast. Life Med. 2016;4:556–563. [Google Scholar]
- 14.Yang J.H., Hu J., Wan L., Chen L.J. Barbigerone inhibits tumor angiogenesis, growth and metastasis in melanoma. Asian Pac. J. Cancer Prev. 2014;15:167–174. doi: 10.7314/APJCP.2014.15.1.167. [DOI] [PubMed] [Google Scholar]
- 15.Messina M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014;100:423S–430S. doi: 10.3945/ajcn.113.071464. [DOI] [PubMed] [Google Scholar]
- 16.Miadokova E. Isoflavonoids - an overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009;2:211–218. doi: 10.2478/v10102-009-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Franciscis P., Colacurci N., Riemma G., Conte A., Pittana E., Guida M., Schiattarella A. A nutraceutical approach to menopausal complaints. Medicina. 2019;55:544. doi: 10.3390/medicina55090544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill D.A., Crider M., Hill S.R. Hormone therapy and other treatments for symptoms of menopause. Am. Fam. Physician. 2016;94:884–889. [PubMed] [Google Scholar]
- 19.Wanda G.J., Starcke S., Zierau O., Njamen D., Richter T., Vollmer G. Estrogenic activity of griffonianone c, an isoflavone from the root bark of millettia griffoniana: Regulation of the expression of estrogen responsive genes in uterus and liver of ovariectomized rats. Planta Med. 2007;73:512–518. doi: 10.1055/s-2007-967186. [DOI] [PubMed] [Google Scholar]
- 20.Ito C., Itoigawa M., Kojima N., Tokuda H., Hirata T., Nishino H., Furukawa H. Chemical constituents of millettia taiwaniana: Structure elucidation of five new isoflavonoids and their cancer chemopreventive activity. J. Nat. Prod. 2004;67:1125–1130. doi: 10.1021/np030554q. [DOI] [PubMed] [Google Scholar]
- 21.Yan W., Yang J., Tang H., Xue L., Chen K., Wang L., Zhao M., Tang M., Peng A., Long C., et al. Flavonoids from the stems of millettia pachyloba drake mediate cytotoxic activity through apoptosis and autophagy in cancer cells. J. Adv. Res. 2019;20:117–127. doi: 10.1016/j.jare.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danial N.N., Korsmeyer S.J. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/S0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 24.Hassan M., Watari H., AbuAlmaaty A., Ohba Y., Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed. Res. Int. 2014;2014:150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Shalini S., Dorstyn L., Dawar S., Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22:526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIlwain D.R., Berger T., Mak T.W. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghobrial I.M., Witzig T.E., Adjei A.A. Targeting apoptosis pathways in cancer therapy. Ca. Cancer J. Clin. 2005;55:178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 29.Miller W.H., Jr., Schipper H.M., Lee J.S., Singer J., Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- 30.Parkinson EI H.P. Runaway ros as a selective anticancer strategy. Chem. Med. Chem. 2011;6:1957–1959. doi: 10.1002/cmdc.201100381. [DOI] [PubMed] [Google Scholar]
- 31.Wu C.C., Bratton S.B. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid. Redox Signal. 2013;19:546–558. doi: 10.1089/ars.2012.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadenbach B., Arnold S., Lee I., Huttemann M. The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases. Biochim. Biophys. Acta. 2004;1655:400–408. doi: 10.1016/j.bbabio.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Zuo Y., Xiang B., Yang J., Sun X., Wang Y., Cang H., Yi J. Oxidative modification of caspase-9 facilitates its activation via disulfide-mediated interaction with apaf-1. Cell Res. 2009;19:449–457. doi: 10.1038/cr.2009.19. [DOI] [PubMed] [Google Scholar]
- 34.Ahn J.H., Yang Y.I., Lee K.T., Choi J.H. Dieckol, isolated from the edible brown algae ecklonia cava, induces apoptosis of ovarian cancer cells and inhibits tumor xenograft growth. J. Cancer Res. Clin. Oncol. 2015;141:255–268. doi: 10.1007/s00432-014-1819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou K., Raffoul J.J. Potential anticancer properties of grape antioxidants. J. Oncol. 2012;2012:803294. doi: 10.1155/2012/803294. [DOI] [PMC free article] [PubMed] [Google Scholar]