Abstract
The fact that the number of people with Alzheimer’s disease is increasing, combined with the limited availability of drugs for its treatment, emphasize the need for the development of novel effective therapeutics for treating this brain disorder. Herein, we focus on generating 12 chalcone-donepezil hybrids, with the goal of simultaneously targeting amyloid-β (Aβ) peptides as well as cholinesterases (i.e., acetylcholinesterase (AChE) and butyrylcholinesterase (BChE)). We present the design, synthesis, and biochemical evaluation of these two series of novel 1,3-chalcone-donepezil (15a–15f) or 1,4-chalcone-donepezil (16a–16f) hybrids. We evaluate the relationship between their structures and their ability to inhibit AChE/BChE activity as well as their ability to bind Aβ peptides. We show that several of these novel chalcone-donepezil hybrids can successfully inhibit AChE/BChE as well as the assembly of N-biotinylated Aβ(1–42) oligomers. We also demonstrate that the Aβ binding site of these hybrids differs from that of Pittsburgh Compound B (PIB).
Keywords: Alzheimer’s disease, acetylcholinesterase, butyrylcholinesterase, 3H-PIB binding, Aβ assembly, Aβ dissociation
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that causes brain cells to waste away, resulting in deterioration of memory, cognitive, and executive functions [1,2,3,4,5,6]. Even though the estimates vary, there are as many as 5.5 million Americans age 60 and older with AD [7]. There are no medications available that cure AD or stop the disease progression in the brain. In the advanced stages, complications from the disease can cause dehydration, malnutrition, or infections that ultimately may lead to death [8,9,10,11,12,13]. Since increasing age is the most common risk factor for the development of AD, barring the implementation of an effective treatment or prevention of AD, the number of people with AD will increase significantly if the current population lifespan trends continue. The few medications that are approved for AD (donepezil, tacrine, rivastigmine, galantamine (acetylcholinesterase inhibitors (AChEIs)) and memantine (N-methyl-d-aspartate (NMDA) receptor antagonist) provide only limited symptomatic relief rather than affecting disease progression or providing a cure [14,15,16,17]. Since the number of AD-related drugs used in AD patients is limited, there is an urgent need for novel therapeutic candidates to treat this brain disorder.
Despite extensive research efforts, the causative factors of AD remain unclear [18]. However, various characteristics, such as low levels of acetylcholine (ACh), accumulation of amyloid-β (Aβ) deposits, tau (τ) protein aggregation, oxidative stress, inflammation, metal ion imbalance, and breakdown of homeostatic systems, play crucial roles in AD progression [19,20,21,22,23,24,25]. Currently, most of the primary therapeutic options for treating AD are based on AChEIs [26,27,28,29,30,31]. Low levels of acetylcholine (ACh) can lead to cognitive and memory deficits. The two types of cholinesterases present in the central nervous system, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), are capable of hydrolyzing ACh [32,33,34,35]. In healthy brains, BChE plays a secondary role to AChE, but as AD progresses the activity and expression of BChE in the brain increases [36]. Although developing inhibitors against these enzymes alone will not lead to a cure, combining their effect with another hallmark of AD progression, such as Aβ peptide accumulation, could potentially be beneficial. The accumulation of Aβ peptides in the cerebral cortex of the brain has been suggested as a part of AD pathogenesis [37,38]. Thus, additionally preventing the aggregation of Aβ and/or disrupting the existing Aβ plaques are potential therapeutic approaches for treating AD [22,39,40,41].
There are currently reports in the literature investigating new scaffolds with two or more pharmacophores connected to each other through a linker aimed at interacting with more than one biological target [42,43,44,45,46,47,48,49,50,51]. Previously, our group as well as others studied donepezil, the current drug of choice for treating AD with its AChE and BChE inhibiting capacity [52,53,54,55,56,57]. In addition, we explored the use of chalcone derivatives as AChE inhibitors as well as Aβ peptide and metal–Aβ complex-targeting compounds [58,59]. Here, we hypothesized that coupling various chalcones and donepezil with linkers of different lengths could result in chalcone-donepezil hybrids capable of inhibiting cholinesterases as well as targeting Aβ peptides. We report the design and synthesis of 12 such hybrids and evaluate their structure–activity relationship (SAR) for their ability to inhibit AChE and BChE as well as to prevent and/or disrupt Aβ peptide oligomerization. We also investigate the binding site of these hybrids by testing their ability to compete with Pittsburgh Compound B (PIB) for binding to natural and synthetic fibrils of Aβ.
2. Results and Discussion
2.1. Design and Synthesis of 1,3-Chalcone-Donepezil (15a–15f) and 1,4-Chalcone-Donepezil (16a–16f) Hybrids
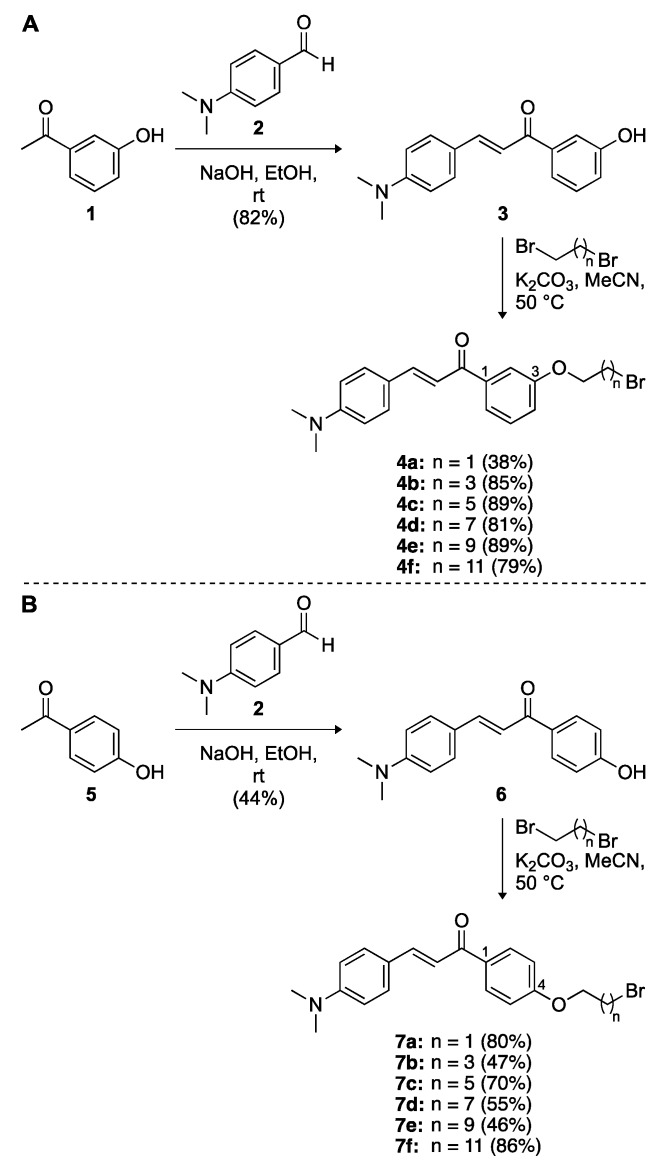
In order to develop multitarget-directed scaffolds for AD, we designed a series of 1,3- and 1,4-chalcone-donepezil hybrids in a way that the chalcone and donepezil scaffolds were attached to each other through linkers consisting of 2, 4, 6, 8, 10, and 12 carbons. Overall, an electrophilic center was introduced on the chalcone scaffold in the form of a brominated linker, which was then reacted with a donepezil nucleophile to generate the target molecules (Scheme 1). We first performed a two-step synthesis to add linkers of varied lengths to a series of 1,3- and 1,4-chalcones (Scheme 1). Briefly, the condensation of 4-(dimethylamino)benzaldehyde (2) with 3-hydroxyacetophenone (1) or 4-hydroxyacetophenone (5) in the presence of NaOH resulted in 1,3-chalcone 3 and 1,4-chalcone 6 in 82% and 44% yields, respectively. The free hydroxyl group of compounds 3 and 6 was reacted with n-alkyl dibromides with 2, 4, 6, 8, 10, and 12 carbons to generate compounds 4a–4f (38–89%) and 7a–7f (46–86%), respectively.
Scheme 1.
Synthetic schemes for the preparation of (A) 1,3-chalcones 4a–4f and (B) 1,4-chalcones 7a–7f.
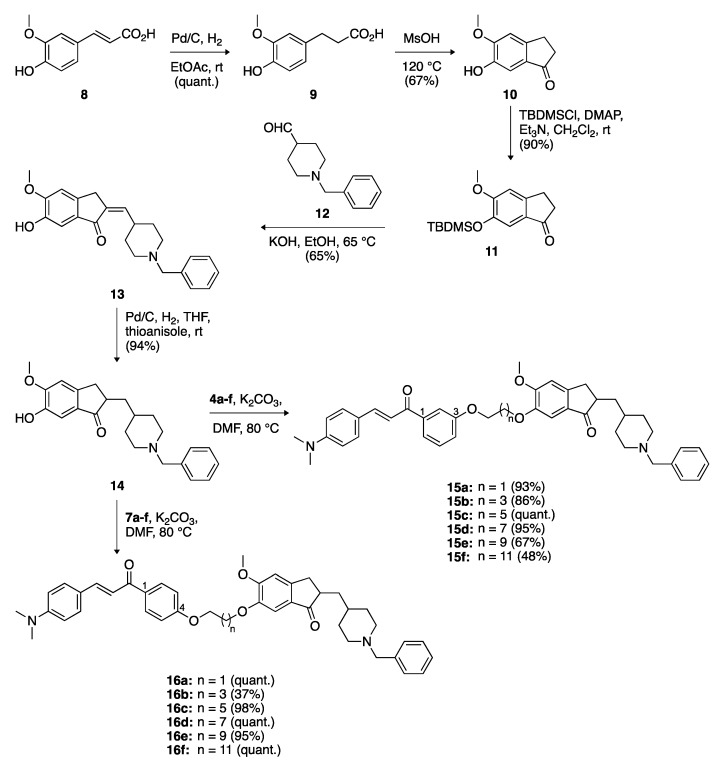
We next synthesized the donepezil nucleophile 14 in five steps (Scheme 2) consisting of the hydrogenation of ferulic acid 8 in the presence of Pd/C, followed by cyclization in the presence of methanesulfonic acid to result in formation of compound 10 in a 67% yield. Since the condensation of compound 10 with aldehyde 12 was found to be problematic, we had to protect the free hydroxyl group of compound 10 with a tert-butyldimethylsilyl (TBDMS) group to afford the protected compound 11 in 90% yield. Compound 11 was then successfully condensed with aldehyde 12 in the presence of KOH to give compound 13 in 65% yield. The controlled deactivation of the palladium catalyst with thioanisole was essential for selective reduction of the double bond in the presence of ketone and benzyl groups in compound 13 to provide the 6-O-desmethyl donepezil adduct 14 in 94% yield. With the donepezil nucleophile 14 in hand, we finally reacted its free hydroxyl group with two sets of electrophilic alkylated 1,3- and 1,4-chalcones, 4a–4f and 7a–7f, to afford the desired 1,3- and 1,4-chalcone-donepezil hybrids 15a–15f and 16a–16f in 48% to 100% and 37% to 100% yields, respectively.
Scheme 2.
Synthetic scheme for the preparation of 1,3-chalcone-donepezil hybrids 15a–15f and 1,4-chalcone-donepezil hybrids 16a–16f.
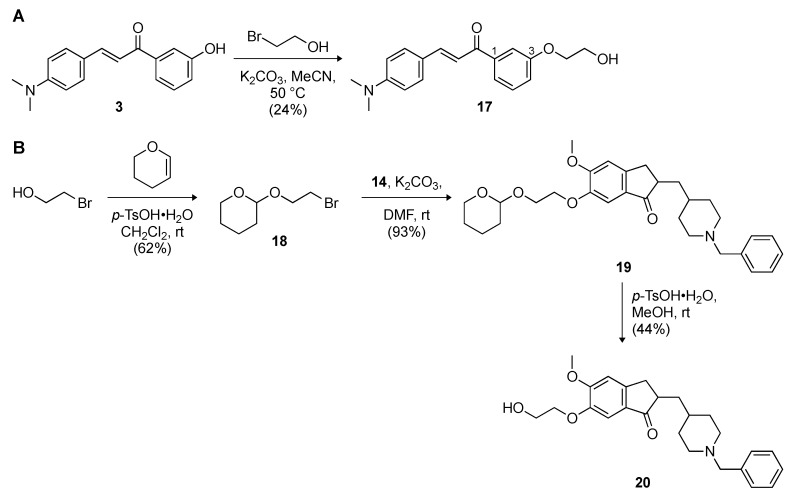
With the goal of investigating the importance of the covalent linkage between the chalcones and donepezil, we synthesized chalcone and donepezil fragments with a C2 linker (Scheme 3). The parent 1,3-chalcone 3 was reacted with bromoethanol in the presence of K2CO3 to give compound 17 in 24% yield. In the case of the donepezil fragment, the hydroxyl group of bromoethanol was protected with dihydropyran to give compound 18 in 62% yield. This molecule was subjected to a nucleophilic substitution reaction with the donepezil nucleophile 14 to yield 19, which was in turn subjected to the removal of the tetrahydropyran group to give compound 20 in 44% yield.
Scheme 3.
Synthetic schemes for the preparation of (A) 1,3-chalcone 17 used for combination studies with donepezil and comparison with 1,3-chalcone-donepezil hybrid 15a, and (B) donepezil derivative 20 used for combination studies with 1,3-chalcone 3 and comparison with 1,3-chalcone-donepezil hybrid 15a.
2.2. Cholinesterase Inhibitory Activity
To evaluate the potential cholinesterase inhibitory activity of the 12 newly synthesized 1,3- and 1,4-chalcone-donepezil hybrids, 15a–15f and 16a–16f, we determined their half maximal inhibitory concentration (IC50) values against EeAChE (AChE from Electrophorus electricus) (Table 1 and Figure S66) and EfBChE (BChE from Equus ferus) (Table 1 and Figure S67) using the Ellman’s method [60].
Table 1.
Inhibition (IC50 in μM) of EeAChE (from Electrophorus electricus) and EfBChE (from Equus ferus) by compounds 15a–15f and 16a–16f as well as the selectivity index (SI) for each inhibitor based on their IC50 values.
| Cpd | EeAChE | EfBChE | SI |
|---|---|---|---|
| Donepezil·HCl | 0.12 ± 0.01 | 2.0 ± 0.1 | 17 |
| 15a | 1.02 ± 0.33 | 0.020 ± 0.002 | 0.02 |
| 15b | 0.65 ± 0.14 | 0.03 ± 0.01 | 0.05 |
| 15c | 1.22 ± 0.18 | ≈4 | ≈3.3 |
| 15d | 1.22 ± 0.13 | ≈2 | ≈1.6 |
| 15e | 1.82 ± 0.45 | ≈10 | ≈5.5 |
| 15f | 1.67 ± 0.80 | >100 | >60 |
| 16a | 0.07 ± 0.01 | ≈0.1 | ≈1.4 |
| 16b | 0.14 ± 0.02 | ≈0.5 | ≈3.6 |
| 16c | ≈0.5 | ≈0.6 | ≈1.2 |
| 16d | 0.52 ± 0.06 | ≈1.0 | ≈1.9 |
| 16e | 1.38 ± 0.18 | ≈5.0 | ≈3.8 |
| 16f | >100 | >100 | 1 |
These experiments were performed in triplicate (n = 1 independent experiment). The errors represent standard deviations (SDEV).
2.2.1. AChE Inhibition
In general, from comparing 1,3- and 1,4-chalcone-donepezil hybrids (15 versus 16) pairwise (for example, 15a with 16a, etc.) we observed that in all cases, with the exception of 15f versus 16f, the 1,4-chalcone-donepezil hybrids were more effective at inhibiting EeAChE. In general, most of them displayed slightly higher IC50 values (1- to 15-fold) when compared to donepezil, with the exception of compounds 16a (0.07 ± 0.01 μM, 2 carbon linker) and 16b (0.14 ± 0.02 μM, 4 carbon linker), which showed equal or better inhibition than donepezil. Out of all the compounds, in the case of the 1,3-chalcone-donepezil series, the two best compounds were 15a (1.02 ± 0.33 μM, 2 carbon linker) and 15b (0.65 ± 0.14 μM, 4 carbon linker). Similarly, in the case of the 1,4-chalcone-donepezil series, 16a (0.07 ± 0.01 μM, 2 carbon linker) and 16b (0.14 ± 0.02 μM, 4 carbon linker) were the best at inhibiting EeAChE. It appears that increasing the linker length from 2 to 12 carbons gradually increased the IC50 values, as exemplified by the values shown by compounds 15a–15f (1.02 ± 0.33 μM, 0.65 ± 0.14 μM, 1.22 ± 0.18 μM, 1.22 ± 0.13 μM, 1.82 ± 0.45 μM, 1.67 ± 0.80 μM) and 16a–16f (0.07 ± 0.01 μM, 0.14 ± 0.02 μM, ≈0.5 μM, 0.52 ± 0.06 μM, 1.38 ± 0.18 μM, >100 μM). Interestingly, with an IC50 >100 μM, 16f (12 carbon linker) was unable to inhibit EeAChE, whereas 15f (12 carbon linker), with an IC50 value of 1.67 ± 0.80 μM, was much more potent. Since we observed better inhibition of HsAChE (from Homo sapiens) than EeAChE for donepezil derivatives in one of our previous studies [52], we postulated that the values observed here for EeAChE should translate to HsAChE. We therefore decided to only utilize EeAChE in the current study as it is much more easily accessible.
2.2.2. BChE Inhibition
When we tested the hybrids against EfBChE, we observed a similar trend to that seen against EeAChE. In general, 1,4-chalcone-donepezil hybrids (16) were better than 1,3-chalcone-donepezil hybrids (15) at inhibiting EfBChE, with the exception of compounds with 2 carbon (15a, 0.020 ± 0.002 μM) and 4 carbon linkers (15b, 0.03 ± 0.01 μM). In the case of 1,3-chalcone-donepezil hybrids, the compounds with 2 and 4 carbon linkers (15a and 15b) were much better than donepezil (2.0 ± 0.10 μM) at inhibiting EfBChE. In the 1,4-chalcone-donepezil series, the hybrids with 2 (16a, ≈0.1 μM), 4 (16b, ≈0.5 μM), 6 (16c, ≈0.6 μM), and 8 (16d, ≈1.0 μM) carbon linkers were better than donepezil (2.0 ± 0.10 μM) at inhibiting EfBChE. Against this enzyme, both compounds 15f (>100 μM, 12 carbon linker) and 16f (>100 μM, 12 carbon linker) were found to be inactive. The data gathered from the EeAChE and EfBChE inhibition studies point towards the benefit of having smaller linkers between the chalcones and donepezil.
In order to understand the utility of the hybrids, based on their IC50 values against EeAChE and EfBChE, we determined their selectivity index (SI) (Table 1). For all but two hybrids, 15a and 15b, EeAChE was inhibited equally to >60-fold better than EfBChE. In the case of 15a and 15b, the inhibition of EfBChE was 51-fold and 22-fold better than that of EeAChE, respectively.
2.3. 3H-PIB Binding Studies
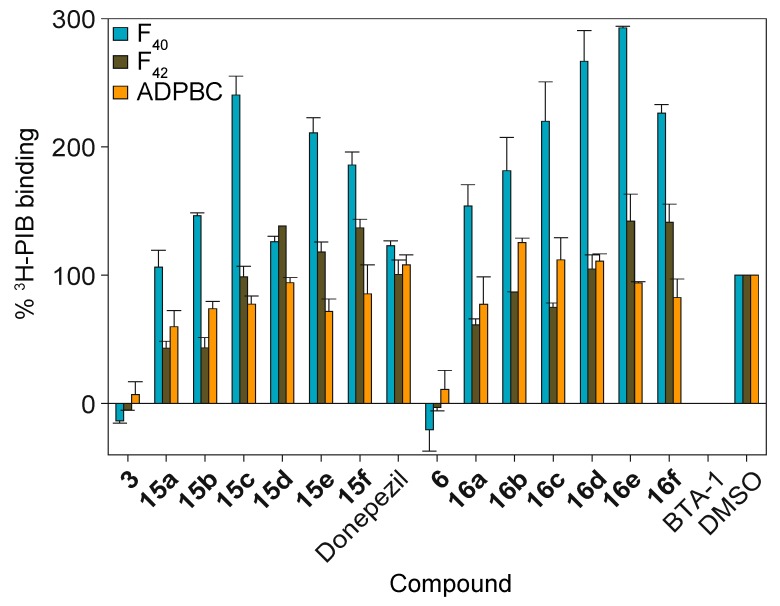
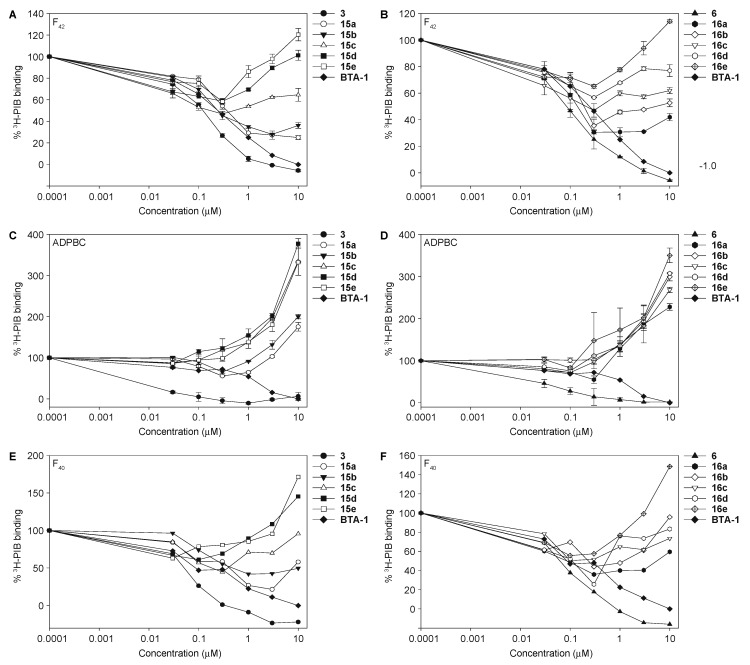
Previously, we demonstrated that various chalcones can displace 3H-PIB from the Alzheimer’s disease PIB binding complex (ADPBC) purified from AD brains, as well as from more widely available model fibrils assembled from synthetic Aβ(1–40) and Aβ(1–42) peptides (indicated herein as F40 and F42, respectively) [59]. When studied for their ability to displace 3H-PIB from F40 (Figure 1 turquoise bars), F42 (brown bars), and ADPBC (orange bars), we found that 1,3- and 1,4-chalcone-donepezil hybrids did not displace 3H-PIB as efficiently as chalcones 3 and 6. Even though there seemed to be no direct correlation between the linker length of the hybrids and their activity, hybrids with shorter linkers appeared to better displace 3H-PIB from F40 and F42 (i.e., compounds 15a and 15b displayed better binding to F40, F42, and ADPBC than 15c, 15d, 15e, and 15f; compound 16a displayed better affinity for F40 and F42 than 16b, 16c, 16d, 16e, and 16f). Interestingly, hybrids 16e and 16f displayed ADPBC affinity comparable to that of 16a. These results indicated that the chalcone-donepezil hybrids probably bind to the fibrils at a different location than 3H-PIB does in the AD brain. Interestingly, during the course of these experiments, we observed that hybrids 15a–15f and 16a–16f enhanced binding to F40. We speculate that when the hybrids bind to F40, which is known to be more malleable than F42, the conformation of the F40 peptide/fibril changes in a way that allow for additional PIB binding. Investigation of this phenomenon is beyond the scope of the current study.
Figure 1.
The measurement of competition of chalcone-donepezil hybrids 15a–15f and 16a–16f against 3H-PIB binding to Aβ fibrils and ADPBC at 10 μM. BTA-1 (2-(4′-methylaminophenyl)benzothiazole) was used as a positive control. F40 = Aβ(1–40) fibrils; F42 = Aβ(1–42) fibrils; ADPBC = PIB binding site located in the AD brain. Due to solubility-micellarization concerns that interferes with 3H-PIB binding measurements, compounds were not tested at concentrations greater than 10 μM. These experiments were performed in duplicate (n = 1 independent experiment). The error bars represent standard deviations (SDEV).
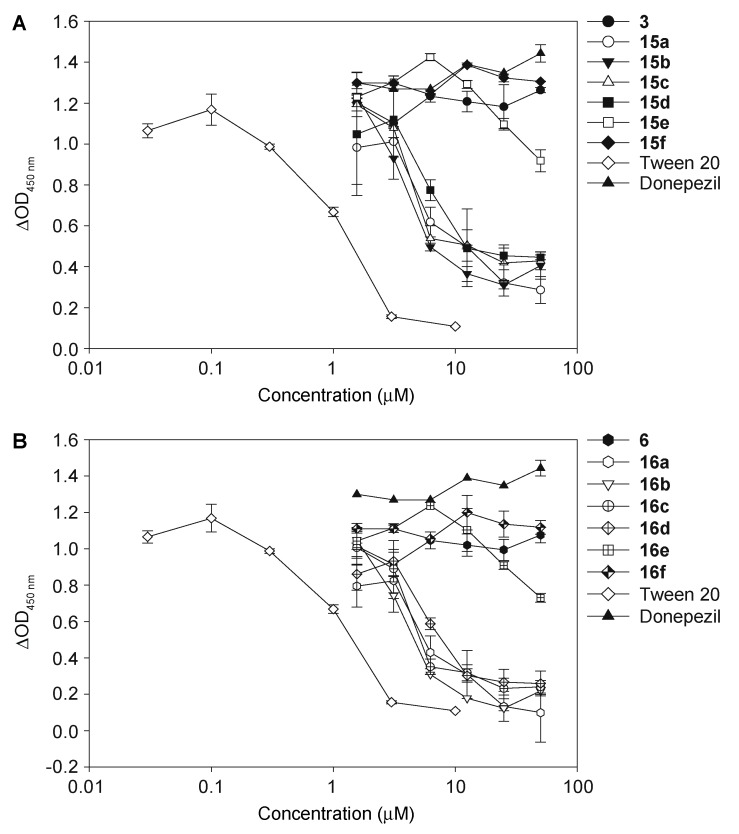
To confirm the results obtained from the 3H-PIB binding competition studies, we titrated the 1,3-and 1,4-chalcone-donepezil hybrids against 3H-PIB bound to F40, F42, and ADPBC fibrils. The experiments were performed in duplicate for F42 and ADPBC and as a single experiment for F40. Even though the single independent experiment may not be sufficient to determine the exact value of inhibition, we were able to observe a clear trend, which is sufficient at this stage of investigation. The data obtained by titration assays (Figure 2) were completely in accord with those displayed in Figure 1 for both the 1,3- and 1,4-chalcone-donepezil hybrids.
Figure 2.
The titration of chalcone-donepezil hybrids 15a–15e and 16a–16e against 3H-PIB binding to F42 (Aβ(1–42) fibrils) (A,B), ADPBC (PIB binding site located in the AD brain) (C,D), and F40 (Aβ(1–40) fibrils) (E,F). BTA-1 (2-(4′-methylaminophenyl)benzothiazole) was used as a positive control. The experiments with F42 and ADPBC were performed in duplicate (n = 1 independent experiment). The error bars represent standard deviations (SDEV).
2.4. The Effect of Chalcone-Donepezil Hybrids on Aβ Assembly and Dissociation
The progression of AD neurodegenerative disease is characterized by the accumulation of Aβ plaques followed by the loss of neurons and tau pathology accumulation leading to brain atrophy and dementia. Even though Aβ fibrils are responsible for plaque formation, recent research efforts have pointed out the role of soluble Aβ oligomers in synaptic neurotoxicity [61]. Genetic evidence and autopsy observations of human cases of AD suggest that the buildup and pathogenicity of Aβ is fundamental to the progression of the disease. Since AD progression is tightly connected to Aβ aggregation, it is imperative to evaluate the effect of the 1,3- and 1,4-chalcone-donepezil hybrids 15a–15f and 16a–16f on Aβ oligomer assembly and dissociation. Among Aβ peptide assemblies, such as Aβ(1–40) (F40) and Aβ(1–42) (F42) fibrils, the Aβ(1–42) peptide is the most abundant and neuro- and synaptotoxic [62,63,64,65].
We first examined the effect of the synthesized 1,3- and 1,4-chalcone-donepezil hybrids 15a–15f and 16a–16f on the assembly of N-biotinyl-Aβ(1–42) (bioAβ42) monomers into oligomers. We quantified the assembly of bioAβ42 into soluble oligomers by using an ELISA assay in which the soluble oligomers were captured on NeutrAvidinTM-coated ELISA plates and detected with streptavidin-horseradish peroxidase and colorimetric readout (Figure 3). Among the hybrids, those with shorter linkers (2–8 carbons), 15a–15d and 16a–16d, demonstrated better inhibition of bioAβ42 oligomer assembly than the parent chalcones 3 and 6 as well as the donepezil from which they were made, respectively. We found that hybrids with longer linkers (10 and 12 carbons), such as 15e, 15f, 16e, and 16f, did not prevent bioAβ42 oligomerization. From these data, we could infer that attaching donepezil to chalcones is highly beneficial as it increases their ability to prevent assembly of bioAβ42 oligomers.
Figure 3.
The measurement of biotinyl-Aβ(1–42) (bioAβ42) oligomer assembly inhibition of compounds 3 and 15a–15f (A), and compounds 6 and 16a–16f (B). The experiments were performed over a concentration range of 1.56 to 50 μM. Donepezil and Tween 20 (assembly inhibition) were used as controls. For Tween 20, the concentration range used was 0.03–10 μM. The absorbance of the enzymatic readout of oligomer content was determined at 450 nm with a BioTek HT Synergy plate reader. These experiments were performed in duplicate (n = 1 independent experiment). The error bars represent standard deviations (SDEV).
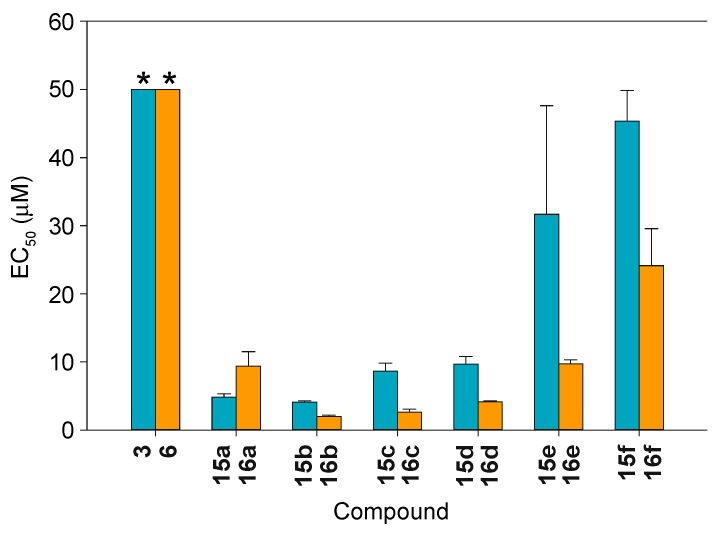
Next, we determined the half maximal effective concentration (EC50) values for all of the hybrids synthesized, 15a–15f and 16a–16f, against bioAβ42 oligomer assembly (Figure 4 and Table S1). In general, the 1,4-chalcone-donepezil hybrids (16b–16f) displayed lower EC50 values than their corresponding 1,3-chalcone-donepezil hybrid counterparts (15b–15f), with the exception of 16a, which had a slightly higher EC50 value than 15a. All compounds tested exhibited better EC50 values than chalcone alone. Compounds 15a (4.83 ± 0.47 μM), 15b (4.10 ± 0.17 μM), 15c (8.67 ± 0.15 μM), 15d (9.67 ± 1.15 μM), 16a (9.37 ± 2.10 μM), 16b (2.00 ± 0.20 μM), 16c (2.60 ± 0.46 μM), 16d (4.13 ± 0.15 μM), and 16e (9.73 ± 0.55 μM) displayed EC50 values <12 μM and a high affinity for bioAβ42, whereas 15e, 15f, and 16f had EC50 values >12 μM (up to 45.33 ± 4.51 μM). These data emphasized the importance of having a shorter linker between the chalcone and donepezil scaffolds. Overall, in the case of both 1,3- and 1,4-chalcone-donepzil hybrids, the less lipophilic compounds displayed better inhibition of bioAβ42 oligomerization.
Figure 4.
The measurement of half maximal effective concentration (EC50) values of chalcones 3 (turquoise bar) and 6 (orange bar) as well as 1,3- and 1,4-chalcone-donepzil hybrids 15a–15f (turquoise bars) and 16a–16f (orange bars) against biotinyl-Aβ(1–42) (bioAβ42) oligomer assembly inhibition. These experiments were performed in duplicate (n = 3 independent experiments). The error bars represent standard deviations (SDEV). The * on top of the bars for compounds 3 and 6 indicate that these values are in fact >50 μM.
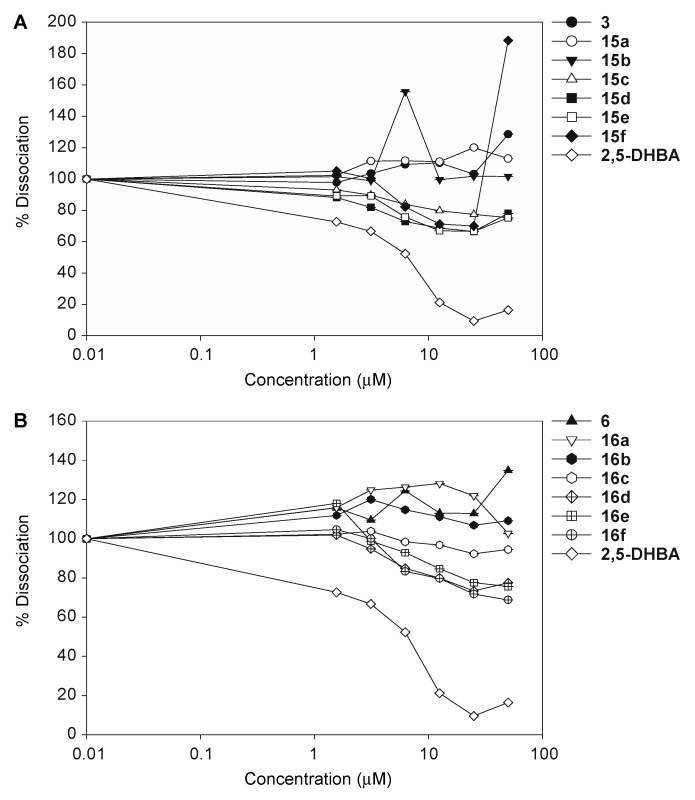
In addition to assess the effect of the synthesized 1,3- and 1,4-chalcone-donepezil hybrids 15a–15f and 16a–16f on the assembly of bioAβ42 monomers into oligomers, we also investigated their effect on the dissociation of preformed bioAβ42 oligomers. While some of these hybrids were able to prevent assembly, none of them could dissociate preformed bioAβ42 oligomers (Figure 5).
Figure 5.
The measurement of biotinyl-Aβ(1–42) (bioAβ42) oligomer dissociation of compounds 3 and 15a–15f (A), and compounds 6 and 16a–16f (B). The experiments were performed over a concentration range of 0.01 to 50 μM. 2,5-DHBA (2,5-dihydroxybenzoic acid) was used as a dissociator positive control. These experiments were performed in duplicate (n = 1 independent experiment). The error bars represent standard deviations (SDEV).
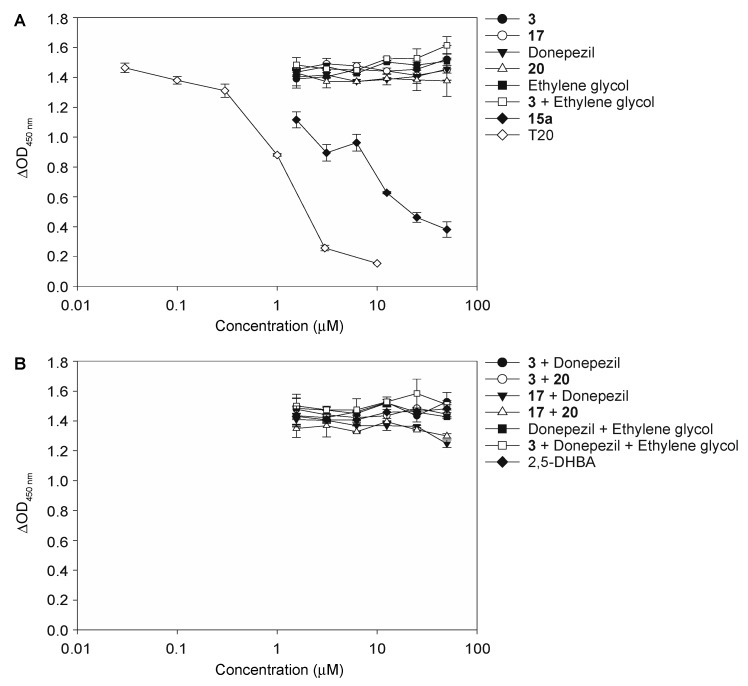
Having demonstrated the ability of 1,3- and 1,4-chalcone-donepezil hybrids 15a–15d and 16a–16d to inhibit bioAβ42 oligomer assembly (Figure 3), we wanted to investigate the importance of the covalent linkage of the chalcone and donepezil fragments on bioAβ42 assembly. To do so, we tested chalcone 3, chalcone 17, donepezil, and its analogue 20 alone for their ability to inhibit bioAβ42 oligomer assembly (Figure 6A). We found that chalcone 3, chalcone 17, donepezil, and its analogue 20 did not inhibit bioAβ42 assembly. Next, we explored 1:1 mixtures of chalcones 3 or 17 with donepezil or its analogue 20 against bioAβ42 oligomer assembly (Figure 6B). None of the above combinations prevented bioAβ42 oligomerization. Combining ethylene glycol linker to chalcone 3, donepezil, and chalcone 3 with donepezil in either 1:1 or 1:1:1 mixtures also did not result in inhibition of bioAβ42 oligomer assembly. In comparison, the 1,3-chalcone-donepezil hybrid 15a was successful in preventing the self-assembly of bioAβ42. These data confirm the importance of having an actual covalent linkage between the chalcones and donepezil for inhibiting bioAβ42 oligomer assembly.
Figure 6.
The measurement of biotinyl-Aβ(1–42) (bioAβ42) oligomer assembly inhibition of compounds 3, 17, donepezil, 20, ethylene glycol, 3 + ethylene glycol, and 15a (A) and compounds 3 + donepezil, 3 + 20, 17 + donepezil, 17 + 20, donepezil + ethylene glycol, and 3 + donepezil + ethylene glycol (B). Tween 20 (assembly inhibition) and 2,5-DHBA (2,5-dihydroxybenzoic acid) (dissociator) were used as controls. These experiments were performed in duplicate (n = 1 independent experiment). The error bars represent standard deviations (SDEV).
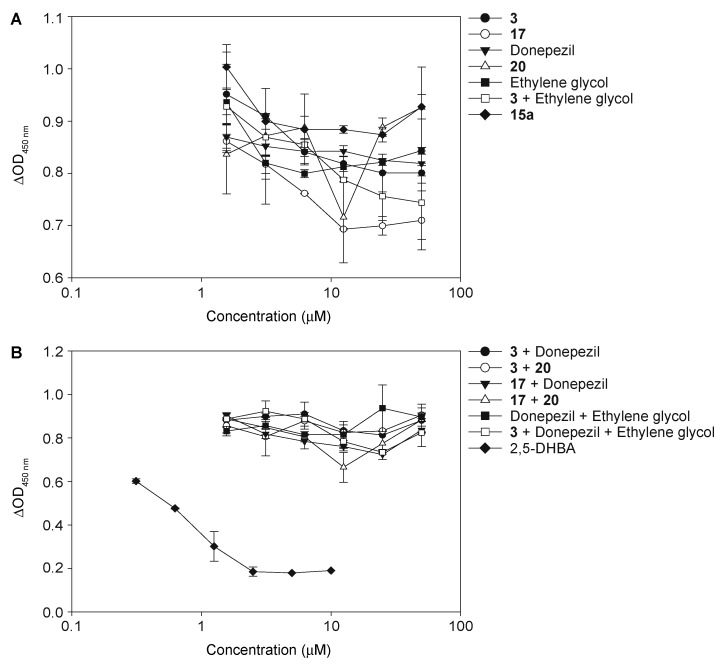
Since we had shown in a previous publication that some chalcones could dissociate preformed bioAβ42 oligomers [58], we wanted to investigate if the covalent attachment of the various chalcones to donepezil, as in hybrids 15a–15f and 16a–16f, was responsible for the lack of ability of these hybrids to dissociate the oligomers. We wanted to determine if chalcones 3 or 17, or donepezil, or its analogue 20 alone could dissociate the preformed bioAβ42 oligomers (Figure 7A). We also desired to see if combining chalcones 3 or 17 in a 1:1 mixture with donepezil or its analogue 20 would result in dissociation of bioAβ42 oligomers (Figure 7B). We found that chalcones 3 and 17 alone, as well as donepezil and its analogue 20, did not dissociate the preformed oligomers. Neither did the various combinations tested. We also explored the addition of the ethylene glycol linker to donepezil, chalcone 3, and donepezil with chalcone 3 in 1:1 or 1:1:1 mixtures. We found that these combinations also did not allow for dissociation of preformed bioAβ42 oligomers. These data highlight that these specific chalcones 3 and 17 with a hydroxyl moiety at position 3, unlike the previously published chalcone with an hydroxyl at position 2 [58], do not dissociate preformed bioAβ42 oligomers. This was not completely surprising, as not all chalcones in our previous study were able to do so.
Figure 7.
The measurement of biotinyl-Aβ(1–42) (bioAβ42) oligomer dissociation of compounds 3, 17, donepezil, 20, ethylene glycol, 3 + ethylene glycol, and 15a (A) and compounds 3 + donepezil, 3 + 20, 17 + donepezil, 17 + 20, donepezil + ethylene glycol, and 3 + donepezil + ethylene glycol (B). 2,5-DHBA (2,5-dihydroxybenzoic acid) was used as a positive dissociator control. These experiments were performed in duplicate (n = 1 independent experiment). The error bars represent standard deviations (SDEV).
2.5. Molecular Modeling
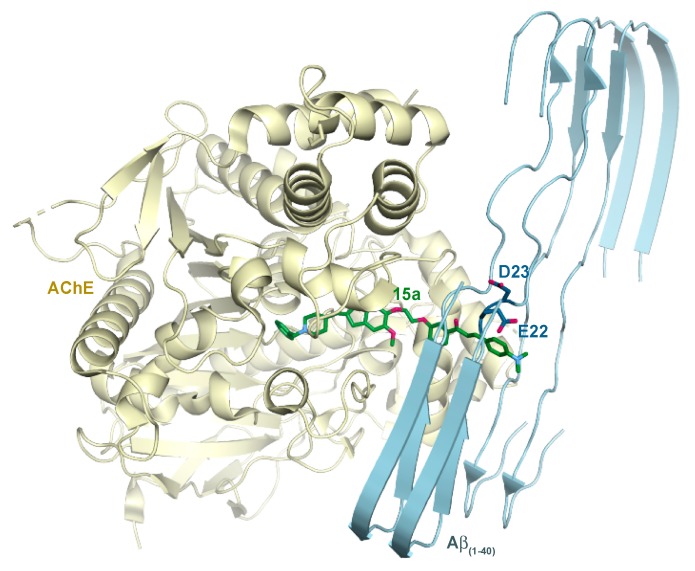
In order to understand how our chalcone-donepezil hybrid molecules may bind simultaneously the AChE enzyme and an Aβ fibril, we built a model of a HsAChE-15a-Aβ fibril complex using a crystal structure of the HsAChE-donepezil complex (PDB ID: 4EY7 [66]) and a cryo-EM structure of an Aβ (residues 1–40) fibril (PDB ID: 6SHS [67]) (Figure 8). The donepezil moiety of the hybrid was unambiguously defined by the bound donepezil in the former structure. In searching for a potential binding site for the chalcone-containing side of 15a in the Aβ fibril, we noticed that Glu22 and Asp23 formed a negatively-charged surface patch that could interact favorably with the terminal positively charged dimethyl amino group of 15a. The largely hydrophobic channel (lined with side chain of Phe19, Ala21, and Val24) would fit and favorably interact with the hydrophobic regions of the chalcone. The oxygen atom of the carbonyl group of chalcone may form hydrogen bonds with amide nitrogen atoms in this region of the Aβ, where β-sheets are highly distorted. Notably, the linker of 15a can span no more than two β-strand layers, as shown in Figure 8. In this model, the two β-strands are book-ended by the HsAChE on one side and the terminal dimethyl amino group on the other. These interactions would prevent propagation of the filament or even potentially cause a disassembly of a larger filament into at most two-strand-thick assemblies. It is obvious that in this model, a longer linker of the hybrid compound would allow a thicker filament to form; therefore, it would not be as potent in preventing filament propagation.
Figure 8.
A structural model of the HsAChE-15a-Aβ fibril complex. The HsAChE is shown in pale yellow, a two-layer Aβ (residues 1–40) fibril is in light blue, and 15a is in green. The acidic residues Glu22 and Asp23 of Aβ that may favorably interact with the dimethyl amino group of 15a are shown as dark blue sticks. The length of the linker of 15a can span no more than two β-strand layers, as shown in the figure. Note: Oxygen and nitrogen atoms are in red and blue, respectively.
3. Materials and Methods
3.1. Materials and Instrumentation
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), Alfa Aesar (Ward Hill, MA, USA), and AK scientific (Union City, CA, USA), and used without further purification. Chemical reactions were monitored by thin layer chromatography (TLC) using Merck Silica gel 60 F254 plates. Visualization was achieved using UV light and a ceric molybdate stain (5 g (NH4)2Ce(NO3)6, 120 g (NH4)6Mo7O24·4H2O, 80 mL H2SO4, and 720 mL H2O). 1H and 13C NMR spectra were recorded at 400 and 100 MHz (or 125 MHz), respectively, on a Varian 400 MHz spectrometer (MR400) (or a Varian 500 MHz spectrometer; VNMRS 500), using the indicated deuterated solvents. Chemical shifts (δ) are given in parts per million (ppm). Coupling constants (J) are given in Hertz (Hz), and conventional abbreviations used for signal shape are as follows: s = singlet; d = doublet; t = triplet; m = multiplet; dd = doublet of doublets; ddd = doublet of doublet of doublets; br s = broad singlet; dt = doublet of triplets. Liquid chromatography-mass spectrometry (LCMS) was carried out using an Agilent 1200 series Quaternary LC system equipped with a diode array detector, and Eclipse XDB-C18 column (250 mm × 4.6 mm, 5 μm). LCMS [M + H]+ signals were consistent with the expected molecular weights for all of the reported compounds. Tween 20 was purchased from Sigma-Aldrich (cat # P7949). N-biotinyl Aβ(1–42) (bioAβ42) was purchased from Anaspec (cat # AS-23523-05; Fremont, CA, USA). ELISA plates (Costar 9018), NeutrAvidinTM (Promega), adhesive film (NUNC), polypropylene 96-well plates (Costar 3365), and polypropylene Eppendorf tubes (Fisher 02-681-248) used for bioAβ42 oligomer assembly and dissociation assays were all purchased from Fisher Scientific (Pittsburgh, PA, USA). Plates were washed on a BioTek ELx50 plate washer (BioTek (Winooski, VT, USA)) and absorbance was read on a BioTek HT Synergy plate reader.
3.2. Synthesis of Compounds 3–20
(E)-3-(4-(Dimethylamino)Phenyl)-1-(3-Hydroxyphenyl)Prop-2-En-1-One (3) (SGT9). The known compound 3 was prepared as previously described [68]. A solution of compounds 1 (68 mg, 0.5 mmol) and 2 (75 mg, 0.5 mmol) in EtOH (1.5 mL) was treated with NaOH pellets (400 mg, 10.0 mmol). The reaction was stirred at room temperature (rt) overnight till completion. Most of the solvent was then removed and 1 N aqueous HCl was added. The precipitate was filtered to give the known compound 3 (110 mg, 82%) (Rf 0.26 in Hexanes:EtOAc/3:1) as an orange solid: 1H NMR (400 MHz, CDCl3, Figure S1, which matches the lit. [68]) δ 7.78 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.56–7.50 (m, 4H, aromatic), 7.35 (t, J = 8.0 Hz, 1H, aromatic), 7.29 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.03 (dd, J1 = 8.0 Hz, J2 = 2.0 Hz, 1H, aromatic), 6.69 (d, J = 9.2 Hz, 2H, aromatic), 5.30 (s, 1H, OH), 3.04 (s, 6H, N(CH3)2).
(E)-1-(3-(2-Bromoethoxy)Phenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-En-1-One (4a) (SGT653). A solution of compound 3 (80 mg, 0.30 mmol) and K2CO3 (165 mg, 1.20 mmol) in anhydrous MeCN (5 mL) was treated with 1,2-dibromoethane (0.10 mL, 1.20 mmol) and the resulting mixture was refluxed overnight. The solvent was then removed and the obtained crude product was purified by column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/4:1; Rf 0.38 in Hexanes:EtOAc/3:1) to yield compound 4a (43 mg, 38%) as a red solid: 1H NMR (400 MHz, CDCl3, Figure S2) δ 7.78 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.61 (d, J = 8.0 Hz, 1H, aromatic), 7.55–7.52 (m, 3H, aromatic), 7.39 (t, J = 8.0 Hz, 1H, aromatic), 7.29 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.10 (dd, J1 = 8.0 Hz, J2 = 2.8 Hz, 1H, aromatic), 6.70 (d, J = 8.8 Hz, 2H, aromatic), 4.37 (t, J = 6.0 Hz, 2H, BrCH2CH2OPh), 3.66 (t, J = 6.0 Hz, 2H, BrCH2CH2OPh), 3.04 (s, 6H, N(CH3)2); 13C NMR (100 MHz, CDCl3, Figure S3) δ 190.1, 158.3, 152.1, 146.0, 140.6, 130.5 (2 carbons), 129.5, 122.5, 121.5, 119.3, 116.7, 113.6, 111.8 (2 carbons), 68.0, 40.1 (2 carbons), 29.1; m/z calcd for C19H20BrNO2 373.1; found 374.0 [M + H]+.
(E)-1-(3-(4-Bromobutoxy)Phenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-En-1-One (4b) (SGT47). A solution of compound 3 (50 mg, 0.19 mmol) and K2CO3 (52 mg, 0.37 mmol) in anhydrous MeCN (5 mL) was treated with 1,4-dibromobutane (0.04 mL, 0.37 mmol) and the resulting mixture was stirred at 50 °C for 4 h. The solvent was then removed and the obtained crude product was purified by column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/4:1; Rf 0.60 in Hexanes:EtOAc/3:1) to yield compound 4b (64 mg, 85%) as a red solid: 1H NMR (400 MHz, CDCl3, Figure S4) δ 7.77 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.57-7.50 (m, 4H, aromatic), 7.36 (t, J = 8.4 Hz, 1H, aromatic), 7.29 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.06 (d, J = 8.4 Hz, 1H, aromatic), 6.69 (d, J = 8.4 Hz, 2H, aromatic), 4.06 (t, J = 6.0 Hz, 2H, BrCH2CH2CH2CH2OPh), 3.49 (t, J = 6.4 Hz, 2H, BrCH2CH2CH2CH2OPh), 3.03 (s, 6H, N(CH3)2), 2.08 (p, J = 7.2 Hz, 2H, BrCH2CH2CH2CH2OPh), 1.96 (p, J = 7.2 Hz, 2H, BrCH2CH2CH2CH2OPh); 13C NMR (100 MHz, CDCl3, Figure S5) δ 190.3, 159.0, 152.0, 145.8, 140.5, 130.4 (2 carbons), 129.4, 122.7, 120.9, 119.0, 117.0, 113.3, 111.9 (2 carbons), 67.0, 40.2 (2 carbons), 33.4, 29.4, 27.8; m/z calcd for C21H24BrNO2 401.1; found 402.0 [M + H]+.
(E)-1-(3-((6-Bromohexyl)Oxy)Phenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-En-1-One (4c) (SGT684). A solution of compound 3 (100 mg, 0.37 mmol) and K2CO3 (207 mg, 1.50 mmol) in anhydrous MeCN (5 mL) was treated with 1,6-dibromohexane (0.23 mL, 1.50 mmol) and the resulting mixture was stirred at 50 °C overnight. The solvent was then removed and the obtained crude product was purified by column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/4:1; Rf 0.60 in Hexanes:EtOAc/3:1) to yield compound 4c (143 mg, 89%) as an orange solid: 1H NMR (400 MHz, CDCl3, Figure S6) δ 7.78 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.57-7.50 (m, 4H, aromatic), 7.36 (t, J = 8.0 Hz, 1H, aromatic), 7.30 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.07 (d, J = 8.0 Hz, 1H, aromatic), 6.68 (d, J = 8.4 Hz, 2H, aromatic), 4.02 (t, J = 6.4 Hz, 2H, BrCH2CH2(CH2)2CH2CH2OPh), 3.42 (t, J = 6.8 Hz, 2H, BrCH2CH2(CH2)2CH2CH2OPh), 3.03 (s, 6H, N(CH3)2), 1.89 (br p, J = 6.4 Hz, 2H, BrCH2CH2(CH2)2CH2CH2OPh), 1.81 (br p, J = 5.6 Hz, 2H, BrCH2CH2(CH2)2CH2CH2OPh), 1.51 (m, 4H, BrCH2CH2(CH2)2CH2CH2OPh); 13C NMR (100 MHz, CDCl3, Figure S7) δ 190.4, 159.2, 152.0, 145.8, 140.4, 130.4 (2 carbons), 129.4, 122.6, 120.7, 119.0, 116.9, 113.4, 111.8 (2 carbons), 67.9, 40.2 (2 carbons), 33.9, 32.7, 29.0, 27.9, 25.3; m/z calcd for C23H28BrNO2 429.1; found 430.1 [M + H]+.
(E)-1-(3-((8-Bromooctyl)Oxy)Phenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-En-1-One (4d) (SGT685). A solution of compound 3 (100 mg, 0.37 mmol) and K2CO3 (207 mg, 1.50 mmol) in anhydrous MeCN (5 mL) was treated with 1,8-dibromooctane (0.28 mL, 1.50 mmol) and the resulting mixture was stirred at 50 °C overnight. The solvent was then removed and the obtained crude product was purified by column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/4:1; Rf 0.60 in Hexanes:EtOAc/3:1) to yield compound 4d (139 mg, 81%) as a yellow solid: 1H NMR (400 MHz, CDCl3, Figure S8) δ 7.78 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.57-7.50 (m, 4H, aromatic), 7.36 (t, J = 8.0 Hz, 1H, aromatic), 7.30 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.07 (d, J = 8.0 Hz, 1H, aromatic), 6.68 (d, J = 8.4 Hz, 2H, aromatic), 4.01 (t, J = 6.4 Hz, 2H, BrCH2CH2(CH2)4CH2CH2OPh), 3.40 (t, J = 6.4 Hz, 2H, BrCH2CH2(CH2)4CH2CH2OPh), 3.03 (s, 6H, N(CH3)2), 1.85 (p, J = 7.6 Hz, 2H, BrCH2CH2(CH2)4CH2CH2OPh), 1.79 (p, J = 7.6 Hz, 2H, BrCH2CH2(CH2)4CH2CH2OPh), 1.40–1.50 (m, 4H, BrCH2CH2(CH2)4CH2CH2OPh), 1.36 (m, 4H, BrCH2CH2(CH2)4CH2CH2OPh); 13C NMR (100 MHz, CDCl3, Figure S9) δ 190.4, 159.2, 152.0, 145.8, 140.4, 130.4 (2 carbons), 129.3, 122.6, 120.6, 119.0, 116.9, 113.4, 111.8 (2 carbons), 68.1, 40.1 (2 carbons), 34.1, 32.8, 29.2 (2 carbons), 28.7, 28.1, 25.9; m/z calcd for C25H32BrNO2 457.2; found 458.0 [M + H]+.
(E)-1-(3-((10-Bromodecyl)Oxy)Phenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-En-1-One (4e) (SGT686). A solution of compound 3 (100 mg, 0.37 mmol) and K2CO3 (207 mg, 1.50 mmol) in anhydrous MeCN (5 mL) was treated with 1,10-dibromodecane (0.34 mL, 1.50 mmol) and the resulting mixture was stirred at 50 °C overnight. The solvent was then removed and the obtained crude product was purified by column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/4:1; Rf 0.60 in Hexanes:EtOAc/3:1) to yield compound 4e (160 mg, 89%) as a yellow solid: 1H NMR (400 MHz, CDCl3, Figure S10) δ 7.78 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.57–7.50 (m, 4H, aromatic), 7.36 (t, J = 8.0 Hz, 1H, aromatic), 7.30 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.07 (d, J = 8.4 Hz, 1H, aromatic), 6.68 (d, J = 8.4 Hz, 2H, aromatic), 4.01 (t, J = 6.8 Hz, 2H, BrCH2CH2(CH2)6CH2CH2OPh), 3.39 (t, J = 6.8 Hz, 2H, BrCH2CH2(CH2)6CH2CH2OPh), 3.03 (s, 6H, N(CH3)2), 1.81 (m, 4H), 1.44 (m, 4H), 1.30 (m, 8H); 13C NMR (100 MHz, CDCl3, Figure S11) δ 190.4, 159.3, 152.0, 145.8, 140.4, 130.4 (2 carbons), 129.3, 122.6, 120.6, 119.0, 116.9, 113.4, 111.8 (2 carbons), 68.1, 40.2 (2 carbons), 34.1, 32.8, 29.44, 29.35, 29.32, 29.2, 28.7, 28.1, 26.0; m/z calcd for C27H36BrNO2 485.2; found 486.0 [M + H]+.
(E)-1-(3-((12-Bromododecyl)Oxy)Phenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-En-1-One (4f) (SGT633). A solution of compound 3 (80 mg, 0.30 mmol) and K2CO3 (165 mg, 1.20 mmol) in anhydrous MeCN (5 mL) was treated with 1,12-dibromododecane (392 mg, 1.20 mmol) and the resulting mixture was refluxed overnight. The solvent was then removed and the obtained crude product was purified by column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/4:1; Rf 0.53 in Hexanes:EtOAc/3:1) to yield compound 4f (122 mg, 79%) as an orange solid: 1H NMR (400 MHz, CDCl3, Figure S12) δ 7.77 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.56–7.50 (m, 4H, aromatic), 7.36 (t, J = 8.0 Hz, 1H, aromatic), 7.30 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.07 (dd, J1 = 8.0 Hz, J2 = 2.0 Hz, 1H, aromatic), 6.71 (d, J = 8.4 Hz, 2H, aromatic), 4.02 (t, J = 6.8 Hz, 2H, BrCH2(CH2)10CH2OPh), 3.39 (t, J = 6.8 Hz, 2H, BrCH2(CH2)10CH2OPh), 3.04 (s, 6H, N(CH3)2), 1.87–1.75 (m, 4H), 1.47–1.27 (m, 16H); 13C NMR (100 MHz, CDCl3, Figure S13) δ 190.4, 159.3, 152.0, 145.8, 140.4, 130.4 (2 carbons), 129.3, 122.7, 120.6, 119.0, 117.0, 113.4, 111.8 (2 carbons), 68.2, 40.1 (2 carbons), 34.1, 32.8, 29.5 (3 carbons), 29.4, 29.3, 29.2, 28.7, 28.2, 26.0; m/z calcd for C29H40BrNO2 513.2; found 514.3 [M + H]+.
(E)-3-(4-(Dimethylamino)Phenyl)-1-(4-Hydroxyphenyl)Prop-2-En-1-One (6) (SGT649). The known compound 6 was prepared as previously described [69]. A solution of compounds 5 (0.50 g, 3.67 mmol) and 2 (0.55 g, 3.67 mmol) in EtOH (10 mL) was treated with NaOH pellets (2.94 g, 73.4 mmol). The reaction was stirred at rt overnight till completion. Most of the solvent was then removed and 1 N aqueous HCl was added. The precipitate was filtered to give the known compound 6 (0.43 g, 44%) (Rf 0.20 in Hexanes:EtOAc/2:1) as a red solid: 1H NMR (400 MHz, CDCl3, Figure S14, which matches the lit. [69]) δ 7.97 (d, J = 8.4 Hz, 2H, aromatic), 7.77 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.54 (d, J = 8.8 Hz, 2H, aromatic), 7.33 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 6.90 (d, J = 8.4 Hz, 2H, aromatic), 6.72 (d, J = 8.8 Hz, 2H, aromatic), 5.42 (s, 1H, OH), 3.04 (s, 6H, N(CH3)2).
(E)-1-(4-(2-Bromoethoxy)Phenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-En-1-One (7a) (SGT652). A solution of 6 (80 mg, 0.30 mmol) and K2CO3 (165 mg, 1.20 mmol) in anhydrous MeCN (5 mL) was treated with 1,2-dibromoethane (0.10 mL, 1.20 mmol) and the resulting mixture was refluxed overnight. The solvent was then removed and the obtained crude product was purified by column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/4:1; Rf 0.31 in Hexanes:EtOAc/3:1) to yield compound 7a (90 mg, 80%) as a yellow solid: 1H NMR (400 MHz, CDCl3, Figure S15) δ 8.01 (d, J = 8.8 Hz, 2H, aromatic), 7.77 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.55 (d, J = 8.8 Hz, 2H, aromatic), 7.34 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 6.97 (d, J = 8.8 Hz, 2H, aromatic), 6.75 (d, J = 8.8 Hz, 2H, aromatic), 4.36 (t, J = 6.0 Hz, 2H, BrCH2CH2OPh), 3.66 (t, J = 6.0 Hz, 2H, BrCH2CH2OPh), 3.04 (s, 6H, N(CH3)2); 13C NMR (100 MHz, CDCl3, Figure S16) δ 188.8, 161.3, 151.9, 145.1, 132.5, 130.6 (2 carbons), 130.3 (2 carbons), 122.8, 116.5, 114.2 (2 carbons), 111.8 (2 carbons), 67.8, 40.1 (2 carbons), 28.7; m/z calcd for C19H20BrNO2 373.1; found 374.0 [M + H]+.
(E)-1-(4-(4-Bromobutoxy)Phenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-En-1-One (7b) (SGT648). A solution of compound 6 (80 mg, 0.30 mmol) and K2CO3 (83 mg, 0.60 mmol) in anhydrous MeCN (5 mL) was treated with 1,4-dibromobutane (0.07 mL, 0.60 mmol) and the resulting mixture was refluxed overnight. The solvent was then removed and the obtained crude product was purified by column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/4:1; Rf 0.42 in Hexanes:EtOAc/3:1) to yield compound 7b (56 mg, 47%) as an orange solid: 1H NMR (400 MHz, CDCl3, Figure S17) δ 8.00 (d, J = 8.8 Hz, 2H, aromatic), 7.77 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.54 (d, J = 8.4 Hz, 2H, aromatic), 7.34 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 6.94 (d, J = 8.8 Hz, 2H, aromatic), 6.70 (d, J = 8.4 Hz, 2H, aromatic), 4.07 (t, J = 6.4 Hz, 2H, BrCH2CH2CH2CH2OPh), 3.49 (t, J = 6.4 Hz, 2H, BrCH2CH2CH2CH2OPh), 3.03 (s, 6H, N(CH3)2), 2.08 (p, J = 6.4 Hz, 2H, BrCH2CH2CH2CH2OPh), 1.97 (p, J = 6.4 Hz, 2H, BrCH2CH2CH2CH2OPh); 13C NMR (100 MHz, CDCl3, Figure S18) δ 188.8, 162.2, 151.8, 144.9, 131.8, 130.5 (2 carbons), 130.2 (2 carbons), 123.0, 116.7, 114.1 (2 carbons), 111.9 (2 carbons), 67.0, 40.2 (2 carbons), 33.3, 29.3, 27.8; m/z calcd for C21H24BrNO2 401.1; found 402.0 [M + H]+.
(E)-1-(4-((6-Bromohexyl)Oxy)Phenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-En-1-One (7c) (SGT691). A solution of compound 6 (50 mg, 0.19 mmol) and K2CO3 (103 mg, 0.75 mmol) in anhydrous MeCN (5 mL) was treated with 1,6-dibromohexane (0.12 mL, 0.75 mmol) and the resulting mixture was stirred at 50 °C overnight. The solvent was then removed and the obtained crude product was purified by column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/4:1; Rf 0.33 in Hexanes:EtOAc/4:1) to yield compound 7c (56 mg, 70%) as an orange solid: 1H NMR (400 MHz, CDCl3, Figure S19) δ 8.00 (d, J = 8.4 Hz, 2H, aromatic), 7.77 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.54 (d, J = 8.4 Hz, 2H, aromatic), 7.35 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 6.94 (d, J = 8.4 Hz, 2H, aromatic), 6.71 (d, J = 8.4 Hz, 2H, aromatic), 4.03 (t, J = 6.4 Hz, 2H, BrCH2CH2CH2CH2OPh), 3.42 (t, J = 6.4 Hz, 2H, BrCH2CH2CH2CH2OPh), 3.03 (s, 6H, N(CH3)2), 1.89 (br p, J = 6.4 Hz, 2H, BrCH2CH2(CH2)2CH2CH2OPh), 1.82 (br p, J = 6.4 Hz, 2H, BrCH2CH2(CH2)2CH2CH2OPh), 1.51 (m, 4H, BrCH2CH2(CH2)2CH2CH2OPh; 13C NMR (100 MHz, (CD3)2SO, Figure S20) δ 187.4, 162.6, 152.3, 144.7, 131.03 (2 carbons), 130.94 (2 carbons), 122.6, 116.5, 114.7 (2 carbons), 112.2 (2 carbons), 68.1, 65.0, 35.6 (2 carbons), 32.6, 28.8, 27.7, 25.0; m/z calcd for C23H28BrNO2 429.1; found 430.0 [M + H]+.
(E)-1-(4-((8-Bromooctyl)Oxy)Phenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-En-1-One (7d) (SGT692). A solution of compound 6 (50 mg, 0.19 mmol) and K2CO3 (103 mg, 0.75 mmol) in anhydrous MeCN (5 mL) was treated with 1,8-dibromooctane (0.14 mL, 0.75 mmol) and the resulting mixture was stirred at 50 °C overnight. The solvent was then removed and the obtained crude product was purified by column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/4:1; Rf 0.33 in Hexanes:EtOAc/4:1) to yield compound 7d (47 mg, 55%) as a yellow solid: 1H NMR (400 MHz, CDCl3, Figure S21) δ 8.00 (d, J = 8.8 Hz, 2H, aromatic), 7.77 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.54 (d, J = 8.8 Hz, 2H, aromatic), 7.35 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 6.94 (d, J = 8.8 Hz, 2H, aromatic), 6.70 (d, J = 8.4 Hz, 2H, aromatic), 4.02 (t, J = 6.4 Hz, 2H, BrCH2CH2(CH2)4CH2CH2OPh), 3.40 (t, J = 6.4 Hz, 2H, BrCH2CH2(CH2)4CH2CH2OPh), 3.03 (s, 6H, N(CH3)2), 1.85 (p, J = 6.8 Hz, 2H, BrCH2CH2(CH2)4CH2CH2OPh), 1.80 (p, J = 6.8 Hz, 2H, BrCH2CH2(CH2)4CH2CH2OPh), 1.40–1.50 (m, 4H), 1.36 (m, 4H); 13C NMR (100 MHz, CDCl3, Figure S22) δ 188.8, 162.5, 144.7, 131.6, 130.5 (2 carbons), 130.2 (2 carbons), 116.9, 114.1 (2 carbons), 112.1 (2 carbons), 68.1, 40.3 (2 carbons), 34.0, 32.7, 29.14, 29.07, 28.6, 28.1, 25.9; m/z calcd for C25H32BrNO2 457.2; found 458.4 [M + H]+.
(E)-1-(4-((10-Bromodecyl)Oxy)Phenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-En-1-One (7e) (SGT693). A solution of compound 6 (50 mg, 0.19 mmol) and K2CO3 (103 mg, 0.75 mmol) in anhydrous MeCN (5 mL) was treated with 1,10-dibromodecane (0.17 mL, 0.75 mmol) and the resulting mixture was stirred at 50 °C overnight. The solvent was then removed and the obtained crude product was purified by column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/4:1; Rf 0.41 in Hexanes:EtOAc/4:1) to yield compound 7e (41 mg, 46%) as a yellow solid: 1H NMR (400 MHz, CDCl3, Figure S23) δ 8.00 (d, J = 8.4 Hz, 2H, aromatic), 7.77 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.54 (d, J = 8.8 Hz, 2H, aromatic), 7.35 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 6.94 (d, J = 8.4 Hz, 2H, aromatic), 6.72 (d, J = 8.4 Hz, 2H, aromatic), 4.02 (t, J = 6.8 Hz, 2H, BrCH2CH2(CH2)6CH2CH2OPh), 3.40 (t, J = 6.8 Hz, 2H, BrCH2CH2(CH2)6CH2CH2OPh), 3.03 (s, 6H, N(CH3)2), 1.84 (p, J = 7.6 Hz, 2H, BrCH2CH2(CH2)6CH2CH2OPh), 1.79 (p, J = 6.8 Hz, 2H, BrCH2CH2(CH2)4CH2CH2OPh), 1.43 (m, 4H), 1.30 (m, 8H); 13C NMR (100 MHz, CDCl3, Figure S24) δ 188.8, 162.6, 144.7, 131.5, 130.5 (2 carbons), 130.2 (2 carbons), 116.9, 114.1 (2 carbons), 112.0 (2 carbons), 68.1, 40.3 (2 carbons), 34.0, 32.8, 29.4, 29.32, 29.28, 29.1, 28.7, 28.1, 25.9; m/z calcd for C27H36BrNO2 485.2; found 486.3 [M + H]+.
(E)-1-(4-((12-Bromododecyl)Oxy)Phenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-En-1-One (7f) (SGT654). A solution of compound 6 (80 mg, 0.30 mmol) and K2CO3 (165 mg, 1.20 mmol) in anhydrous MeCN (5 mL) was treated with 1,12-dibromododecane (392 mg, 1.20 mmol) and the resulting mixture was refluxed overnight. The solvent was then removed and the obtained crude product was purified by column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/4:1; Rf 0.47 in Hexanes:EtOAc/3:1) to yield compound 7f (132 mg, 86%) as an orange solid: 1H NMR (400 MHz, CDCl3, Figure S25) δ 8.00 (d, J = 8.8 Hz, 2H, aromatic), 7.77 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.54 (d, J = 8.4 Hz, 2H, aromatic), 7.35 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 6.94 (d, J = 8.8 Hz, 2H, aromatic), 6.73 (d, J = 8.4 Hz, 2H, aromatic), 4.02 (t, J = 6.8 Hz, 2H, BrCH2(CH2)10CH2OPh), 3.39 (t, J = 6.8 Hz, 2H, BrCH2(CH2)10CH2OPh), 3.03 (s, 6H, N(CH3)2), 1.87-1.75 (m, 4H), 1.47–1.27 (m, 16H); 13C NMR (100 MHz, CDCl3, Figure S26) δ 188.8, 162.6, 151.8, 144.8, 131.6, 130.5 (2 carbons), 130.2 (2 carbons), 123.0, 116.8, 114.1 (2 carbons), 111.9 (2 carbons), 68.2, 40.2 (2 carbons), 34.1, 32.8, 29.5 (3 carbons), 29.4, 29.3, 29.1, 28.7, 28.2, 26.0; m/z calcd for C29H40BrNO2 513.2; found 514.1 [M + H]+.
3-(4-Hydroxy-3-Methoxyphenyl)Propanoic Acid (9). To a solution of ferulic acid (6.0 g, 30.9 mmol) in degassed EtOAc (100 mL) was added a catalytic amount of 10% Pd/C (0.43 g). The reaction flask was then sealed with a rubber septum and freed of air. The reaction mixture was stirred at rt overnight under H2 atmosphere. Upon completion, the reaction mixture was filtered through a bed of celite, and concentrated to afford the known compound 9 [70] (6.1 g, quant.) as an off-white solid: 1H NMR (400 MHz, CDCl3, Figure S27, which matches the lit. [70]) δ 10.50 (very br s, 1H, CO2H), 6.82 (d, J = 7.6 Hz, 1H, aromatic), 6.69 (s, 1H, aromatic), 6.68 (d, J = 7.6 Hz, 1H, aromatic), 5.60 (very br s, 1H, OH), 3.85 (s, 3H, PhOCH3), 2.87 (t, J = 7.2 Hz, 2H, PhCH2CH2CO2H), 2.64 (t, J = 7.2 Hz, 2H, PhCH2CH2CO2H).
6-Hydroxy-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (10). A solution of 9 (6.3 g, 32.1 mmol) in methanesulfonic acid (50 mL) was refluxed at 120 °C for 1 h. After cooling to rt, the reaction mixture was poured into ice-water, stirred for 5 min, and filtered to afford a crude dark brown solid, which was recrystallized from EtOH to afford the known compound 10 [71] (3.8 g, 67%) as a yellow solid: 1H NMR (400 MHz, (CD3)2SO, Figure S28, which matches the lit. [71]) δ 9.38 (s, 1H, OH), 7.03 (s, 1H, aromatic), 6.89 (s, 1H, aromatic), 3.83 (s, 3H, OCH3), 2.92 (t, J = 5.6 Hz, 2H, CH2CH2C=O), 2.49 (t, J = 5.6 Hz, 2H, CH2CH2C=O).
6-((Tert-Butyldimethylsilyl)Oxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (11) (SGT640). TBDMSCl (3.2 g, 21.3 mmol) was added to a solution of 10 (1.9 g, 10.7 mmol), DMAP (0.5 g, 4.3 mmol), and Et3N (3.0 mL, 21.3 mmol) in freshly distilled CH2Cl2 (100 mL). The reaction mixture was stirred at rt overnight before being quenched with H2O (100 mL). The organic layer was separated, washed with H2O (2 × 100 mL) and brine (100 mL), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure to afford a crude dark brown solid, which was purified by flash column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/3:1, Rf 0.44 in Hexanes:EtOAc/3:1) to afford a brown solid, which was further triturated in Hexanes to give compound 11 (2.8 g, 90%) as a white solid: 1H NMR (400 MHz, CDCl3, Figure S29) δ 7.17 (s, 1H, aromatic), 6.84 (s, 1H, aromatic), 3.87 (s, 3H, PhOCH3), 3.02 (app. t, J = 5.6 Hz, 2H, CH2CH2C=O), 2.64 (app. t, J = 5.6 Hz, 2H, CH2CH2C=O), 0.98 (s, 9H, SiC(CH3)3), 0.14 (s, 6H, Si(CH3)2); 13C NMR (100 MHz, CDCl3, Figure S30) δ 205.7, 157.5, 150.9, 145.2, 130.0, 114.1, 107.8, 55.6, 36.6, 25.62 (3 carbons), 25.56, 18.4, −4.7 (2 carbons); m/z calcd for C16H24O3Si 292.2; found 293.2 [M + H]+.
(Z)-2-((1-Benzylpiperidin-4-Yl)Methylene)-6-Hydroxy-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (13) (SGT641). To a solution of compounds 11 (1.00 g, 3.42 mmol) and 12 (0.68 mL, 3.42 mmol) in EtOH (10 mL) was added KOH (0.5 g), and the mixture was refluxed at 65 °C. After 1 h, the reaction was analyzed by TLC (CH2Cl2:MeOH/19:1, Rf 0.30 in CH2Cl2:MeOH/19:1). The reaction mixture was concentrated under reduced pressure to give a crude yellow solid, which was re-dissolved in H2O (10 mL). Then, 1 N aqueous HCl was then slowly added until pH 5 to give a yellow precipitate, which was recrystallized in MeCN to afford compound 13 (0.81 g, 65%) as a yellow solid: 1H NMR (400 MHz, CDCl3, Figure S31) δ 7.32–7.24 (m, 6H, aromatic), 6.87 (s, 1H, aromatic), 6.63 (d, J = 10.0 Hz, 1H, C=CH), 5.70 (br s, 1H, OH), 3.98 (s, 3H, OCH3), 3.56 (s, 2H), 3.51 (s, 2H), 2.91 (d, J = 11.6 Hz, 2H), 2.30 (m, 1H), 2.04 (t, J = 11.6 Hz, 2H), 1.70-1.60 (m, 4H); 13C NMR (100 MHz, CDCl3, Figure S32) δ 192.5, 152.6, 145.8, 143.4, 139.8, 138.2, 135.5, 132.5, 129.2 (2 carbons), 128.2 (2 carbons), 127.0, 108.7, 106.8, 63.5, 56.2, 53.1 (2 carbons), 37.2, 31.2 (2 carbons), 29.5; m/z calcd for C23H25NO3 363.2; found 364.2 [M + H]+.
2-((1-Benzylpiperidin-4-Yl)Methyl)-6-Hydroxy-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (14) (SGT332). To a solution of 13 (101 mg, 0.28 mmol) in degassed THF (2.5 mL) was added 10% Pd/C (wet support, Sigma 520829-10G, 10 mg). The reaction flask was then sealed with a rubber septum and freed of air. Thioanisole (14.2 × 10−7 mL, obtained using 5 μL of a stock solution comprising 14.2 μL of thioanisole in 50 mL of anhydrous THF) was added and the reaction mixture was stirred at rt overnight under H2 atmosphere. Upon completion, the reaction mixture was filtered through a bed of celite, and concentrated to afford the known compound 14 (96 mg, 94%) as a yellow solid: 1H NMR (400 MHz, CDCl3, Figure S33) δ 7.30-7.20 (m, 6H, aromatic), 6.82 (s, 1H, aromatic), 3.96 (s, 3H, OCH3), 3.49 (s, 2H, NCH2Ph), 3.20 (dd, J1 = 18.0 Hz, J2 = 7.6 Hz, 1H), 2.87 (m, 2H), 2.66 (dt, J1 = 13.6 Hz, J2 = 3.6 Hz, 2H), 1.98–1.82 (m, 3H), 1.72–1.63 (m, 2H), 1.48 (m, 1H), 1.39–1.24 (m, 3H); 13C NMR (100 MHz, CDCl3, Figure S34) δ 207.8, 152.9, 147.5, 145.8, 138.3, 130.0, 129.3 (2 carbons), 128.1 (2 carbons), 126.9, 108.0, 106.9, 63.4, 56.2, 53.7 (2 carbons), 45.3, 38.7, 34.4, 33.4, 32.9, 31.7; m/z calcd for C23H27NO3 365.2; found 366.2 [M + H]+.
(E)-2-((1-Benzylpiperidin-4-Yl)Methyl)-6-(2-(3-(3-(4-(Dimethylamino)Phenyl)Acryloyl)Phenoxy)Ethoxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (15a) (SGT656). A solution of compound 14 (15 mg, 0.041 mmol), compound 4a (19 mg, 0.049 mmol), and K2CO3 (17 mg, 0.12 mmol) in anhydrous DMF (5 mL) was heated at 65 °C overnight. The reaction mixture was then diluted with H2O, and extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×) and brine (3×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.39 in CH2Cl2:MeOH/19:1) to yield compound 15a (25 mg, 93%) as an orange oil: 1H NMR (400 MHz, CDCl3, Figure S35) δ 7.77 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.59–7.56 (m, 2H, aromatic), 7.52 (d, J = 8.4 Hz, 2H, aromatic), 7.37 (t, J = 8.0 Hz, 1H, aromatic), 7.30–7.23 (m, 7H, aromatic), 7.13 (dd, J1 = 8.0 Hz, J2 = 2.4 Hz, 1H, aromatic), 6.83 (s, 1H, aromatic), 6.66 (d, J = 8.4 Hz, 2H, aromatic), 4.41 (m, 4H), 3.89 (s, 3H, OCH3), 3.52 (s, 2H, NCH2Ph), 3.20 (dd, J1 = 17.6 Hz, J2 = 8.0 Hz, 1H), 3.02 (s, 6H, N(CH3)2), 2.91 (m, 2H), 2.66 (dt, J1 = 14.0 Hz, J2 = 2.4 Hz, 2H), 1.99 (m, 2H), 1.89 (m, 1H), 1.65–1.55 (m, 2H), 1.55–1.40 (m, 1H), 1.40–1.25 (m, 3H); 13C NMR (100 MHz, CDCl3, Figure S36) δ 207.7, 190.1, 158.8, 155.9, 152.0, 149.2, 148.4 (2 carbons), 145.9, 140.4, 130.4 (2 carbons), 129.41, 129.37 (2 carbons), 129.1, 128.2 (2 carbons), 127.1, 122.6, 121.2, 119.2, 116.7, 113.8, 111.8 (2 carbons), 107.7, 106.3, 67.6, 66.5, 63.2, 56.1, 53.6, 53.5, 45.4, 40.1 (2 carbons), 38.6, 34.3, 33.4, 32.7, 31.5; m/z calcd for C42H46N2O5 658.3; found 659.3 [M + H]+.
(E)-2-((1-Benzylpiperidin-4-Yl)Methyl)-6-(4-(3-(3-(4-(Dimethylamino)Phenyl)Acryloyl)Phenoxy)Butoxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (15b) (SGT681). A solution of compound 14 (30 mg, 0.082 mmol), compound 4b (40 mg, 0.099 mmol), and K2CO3 (34 mg, 0.25 mmol) in anhydrous DMF (5 mL) was heated at 65 °C overnight. The reaction mixture was then diluted with H2O, and extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×) and brine (3×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.39 in CH2Cl2:MeOH/19:1) to yield compound 15b (48 mg, 86%) as an orange oil: 1H NMR (400 MHz, CDCl3, Figure S37) δ 7.77 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.58–7.46 (m, 4H, aromatic), 7.38–7.22 (m, 7H, aromatic), 7.14 (s, 1H, aromatic), 7.06 (dd, J1 = 8.4 Hz, J2 = 2.0 Hz, 1H, aromatic), 6.80 (s, 1H, aromatic), 6.67 (d, J = 8.8 Hz, 2H, aromatic), 4.11 (m, 4H), 3.89 (s, 3H, OCH3), 3.57 (s, 2H, NCH2Ph), 3.19 (dd, J1 = 17.6 Hz, J2 = 8.4 Hz, 1H), 3.02 (s, 6H, N(CH3)2), 2.96 (m, 2H), 2.65 (dt, J1 = 14.0 Hz, J2 = 4.4 Hz, 2H), 2.05-1.98 (m, 6H), 1.89–1.84 (m, 1H), 1.70 (m, 2H), 1.60–1.22 (m, 4H); 13C NMR (100 MHz, CDCl3, Figure S38) δ 207.7, 190.3, 159.1, 155.8, 152.0, 148.7 (3 carbons), 145.8, 140.4, 130.4 (2 carbons), 129.8 (2 carbons), 129.3, 129.0, 128.4 (2 carbons), 127.7, 122.5, 120.7, 119.0, 116.8, 113.4, 111.8 (2 carbons), 107.4, 105.4, 68.6, 67.6, 62.7, 56.2, 53.3 (2 carbons), 45.1, 40.1 (2 carbons), 38.4, 33.7, 33.4, 31.8, 31.0, 26.1, 25.6; m/z calcd for C44H50N2O5 686.4; found 687.3 [M + H]+.
(E)-2-((1-Benzylpiperidin-4-Yl)Methyl)-6-((6-(3-(3-(4-(Dimethylamino)Phenyl)Acryloyl)Phenoxy)Hexyl)Oxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (15c) (SGT687). A solution of compound 14 (30 mg, 0.082 mmol), compound 4c (42 mg, 0.099 mmol), and K2CO3 (34 mg, 0.24 mmol) in anhydrous DMF (5 mL) was heated at 80 °C overnight. The reaction mixture was then diluted with H2O, and extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×) and brine (3×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.39 in CH2Cl2:MeOH/19:1) to yield compound 15c (61 mg, quantitative yield) as an orange solid: 1H NMR (400 MHz, CDCl3, Figure S39) δ 7.77 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.58–7.48 (m, 4H, aromatic), 7.38–7.24 (m, 7H, aromatic), 7.13 (s, 1H, aromatic), 7.06 (d, J = 8.4 Hz, 1H, aromatic), 6.81 (s, 1H, aromatic), 6.67 (d, J = 8.4 Hz, 2H, aromatic), 4.02 (m, 4H), 3.91 (s, 3H, OCH3), 3.60 (s, 2H, NCH2Ph), 3.20 (dd, J1 = 17.2 Hz, J2 = 8.4 Hz, 1H), 3.03 (s, 6H, N(CH3)2), 2.97 (m, 2H), 2.65 (m, 2H), 2.06 (m, 2H), 1.92–1.78 (m, 5H), 1.71 (m, 2H), 1.54 (m, 4H), 1.45–1.23 (m, 4H); 13C NMR (100 MHz, CDCl3, Figure S40) δ 207.8, 190.4, 159.2, 155.8, 152.0, 148.8, 148.6 (2 carbons), 145.8, 140.4, 130.4 (2 carbons), 129.6, 129.3 (2 carbons), 129.1, 128.3 (2 carbons), 127.5, 122.6, 120.6, 119.0, 116.9, 113.4, 111.8 (2 carbons), 107.4, 105.4, 68.9, 67.9, 62.9, 56.2, 53.4 (2 carbons), 45.2, 40.1 (2 carbons), 38.5, 34.0, 33.4, 32.2, 31.2, 29.1, 28.8, 25.82, 25.75; m/z calcd for C46H54N2O5 714.4; found 715.2 [M + H]+.
(E)-2-((1-Benzylpiperidin-4-Yl)Methyl)-6-((8-(3-(3-(4-(Dimethylamino)Phenyl)Acryloyl)Phenoxy)Octyl)Oxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (15d) (SGT688). A solution of compound 14 (30 mg, 0.082 mmol), compound 4d (45 mg, 0.099 mmol), and K2CO3 (34 mg, 0.24 mmol) in anhydrous DMF (5 mL) was heated at 80 °C overnight. The reaction mixture was then diluted with H2O, and extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×) and brine (3×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.39 in CH2Cl2:MeOH/19:1) to yield compound 15d (58 mg, 95%) as an orange solid: 1H NMR (400 MHz, CDCl3, Figure S41) δ 7.77 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.56–7.48 (m, 4H, aromatic), 7.38–7.24 (m, 7H, aromatic), 7.13 (s, 1H, aromatic), 7.06 (d, J = 8.4 Hz, 1H, aromatic), 6.82 (s, 1H, aromatic), 6.67 (d, J = 8.4 Hz, 2H, aromatic), 4.01 (t, J = 6.4 Hz, 4H), 3.92 (s, 3H, OCH3), 3.57 (s, 2H, NCH2Ph), 3.20 (dd, J1 = 17.2 Hz, J2 = 8.0 Hz, 1H), 3.03 (s, 6H, N(CH3)2), 2.95 (m, 2H), 2.65 (m, 2H), 2.03 (m, 2H), 1.92–1.65 (m, 7H), 1.45 (m, 5H), 1.42–1.23 (m, 7H); 13C NMR (100 MHz, CDCl3, Figure S42) δ 207.8, 190.4, 159.3, 155.7, 152.0, 148.8, 148.5 (2 carbons), 145.8, 140.4, 130.4 (2 carbons), 129.5, 129.3 (2 carbons), 129.1, 128.2 (2 carbons), 127.3, 122.6, 120.6, 119.0, 116.9, 113.4, 111.8 (2 carbons), 107.4, 105.4, 69.0, 68.1, 63.1, 56.2, 53.5 (2 carbons), 45.3, 40.1 (2 carbons), 38.6, 34.2, 33.4, 32.4, 31.4, 29.3 (2 carbons), 29.2, 28.9, 25.95, 25.85; m/z calcd for C48H58N2O5 742.4; found 743.4 [M + H]+.
(E)-2-((1-Benzylpiperidin-4-Yl)Methyl)-6-((10-(3-(3-(4-(Dimethylamino)Phenyl)Acryloyl)Phenoxy)Decyl)Oxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (15e) (SGT689). A solution of compound 14 (30 mg, 0.082 mmol), compound 4e (48 mg, 0.099 mmol), and K2CO3 (34 mg, 0.24 mmol) in anhydrous DMF (5 mL) was heated at 80 °C overnight. The reaction mixture was then diluted with H2O, and extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×) and brine (3×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.39 in CH2Cl2:MeOH/19:1) to yield compound 15e (42 mg, 67%) as a yellow solid: 1H NMR (400 MHz, CDCl3, Figure S43) δ 7.77 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.56–7.48 (m, 4H, aromatic), 7.38–7.24 (m, 7H, aromatic), 7.13 (s, 1H, aromatic), 7.07 (d, J = 8.4 Hz, 1H, aromatic), 6.81 (s, 1H, aromatic), 6.67 (d, J = 8.4 Hz, 2H, aromatic), 4.01 (t, J = 6.8 Hz, 4H, 2×OCH2CH2), 3.91 (s, 3H, OCH3), 3.54 (s, 2H, NCH2Ph), 3.20 (dd, J1 = 17.2 Hz, J2 = 8.0 Hz, 1H), 3.03 (s, 6H, N(CH3)2), 2.92 (m, 2H), 2.65 (m, 2H), 2.01 (m, 2H), 1.92-1.65 (m, 7H), 1.43 (m, 5H), 1.31 (m, 11H); 13C NMR (100 MHz, CDCl3, Figure S44) δ 207.8, 190.4, 159.3, 155.7, 152.0, 148.8, 148.5 (2 carbons), 145.8, 140.4, 130.4 (2 carbons), 129.4, 129.3 (2 carbons), 129.2, 128.2 (2 carbons), 127.2, 122.6, 120.6, 119.0, 116.9, 113.4, 111.8 (2 carbons), 107.4, 105.4, 69.0, 68.2, 63.2, 56.2, 53.6 (2 carbons), 45.3, 40.1 (2 carbons), 38.6, 34.2, 33.3, 32.6, 31.5, 29.7 (2 carbons), 29.4, 29.3, 29.2, 28.9, 26.0, 25.9; m/z calcd for C50H62N2O5 770.5; found 771.3 [M + H]+.
(E)-2-((1-Benzylpiperidin-4-Yl)Methyl)-6-((12-(3-(3-(4-(Dimethylamino)Phenyl)Acryloyl)Phenoxy)Dodecyl)Oxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (15f) (SGT679). A solution of compound 14 (30 mg, 0.082 mmol), compound 4f (51 mg, 0.099 mmol), and K2CO3 (34 mg, 0.25 mmol) in anhydrous DMF (5 mL) was heated at 80 °C overnight. The reaction mixture was then diluted with H2O, and extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×) and brine (3×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.39 in CH2Cl2:MeOH/19:1) to yield compound 15f (32 mg, 48%) as an orange oil: 1H NMR (400 MHz, CDCl3, Figure S45) δ 7.77 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.56–7.50 (m, 4H, aromatic), 7.35 (t, J = 8.0 Hz, 1H, aromatic), 7.33–7.28 (m, 6H, aromatic), 7.12 (s, 1H, aromatic), 7.06 (dd, J1 = 8.0 Hz, J2 = 2.0 Hz, 1H, aromatic), 6.81 (s, 1H, aromatic), 6.67 (d, J = 8.8 Hz, 2H, aromatic), 4.00 (m, 4H), 3.91 (s, 3H, OCH3), 3.57 (s, 2H, NCH2Ph), 3.19 (dd, J1 = 17.6 Hz, J2 = 8.0 Hz, 1H), 3.02 (s, 6H, N(CH3)2), 2.95 (m, 2H), 2.65 (dt, J1 = 14.0 Hz, J2 = 4.4 Hz, 2H), 2.03 (m, 2H), 1.90–1.75 (m, 5H), 1.73–1.60 (m, 2H), 1.50–1.40 (m, 6H), 1.40–1.25 (m, 14H); 13C NMR (100 MHz, CDCl3, Figure S46) δ 207.8, 190.4, 159.3, 155.7, 152.0, 148.8, 148.5 (2 carbons), 145.8, 140.4, 130.4 (2 carbons), 129.5, 129.3 (2 carbons), 129.1, 128.3 (2 carbons), 127.3, 122.6, 120.6, 119.0, 116.9, 113.4, 111.8 (2 carbons), 107.4, 105.4, 69.1, 68.2, 62.9, 56.2, 53.4 (2 carbons), 45.3, 40.1 (2 carbons), 38.6, 34.1, 33.3, 32.3, 31.3, 29.5 (3 carbons), 29.5, 29.4, 29.3, 29.2, 28.9, 26.0, 25.9; m/z calcd for C52H66N2O5 798.5; found 799.3 [M + H]+.
(E)-2-((1-Benzylpiperidin-4-Yl)Methyl)-6-(2-(4-(3-(4-(Dimethylamino)Phenyl)Acryloyl)Phenoxy)Ethoxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (16a) (SGT683). A solution of compound 14 (20 mg, 0.055 mmol), compound 7a (25 mg, 0.066 mmol), and K2CO3 (23 mg, 0.16 mmol) in anhydrous DMF (5 mL) was heated at 80 °C overnight. The reaction mixture was then diluted with H2O, and extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×) and brine (3×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.39 in CH2Cl2:MeOH/19:1) to yield compound 16a (48 mg, quantitative yield) as a yellow solid: 1H NMR (400 MHz, CDCl3, Figure S47) δ 8.00 (d, J = 8.8 Hz, 2H, aromatic), 7.76 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.53 (d, J = 8.8 Hz, 2H, aromatic), 7.33 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.32–7.24 (m, 5H, aromatic), 7.23 (s, 1H, aromatic), 7.00 (d, J = 8.8 Hz, 2H, aromatic), 6.84 (s, 1H, aromatic), 6.67 (d, J = 8.8 Hz, 2H, aromatic), 4.41 (m, 4H), 3.90 (s, 3H, OCH3), 3.55 (s, 2H, NCH2Ph), 3.21 (dd, J1 = 17.6 Hz, J2 = 8.0 Hz, 1H), 3.02 (s, 6H, N(CH3)2), 2.92 (m, 2H), 2.67 (dt, J1 = 14.4 Hz, J2 = 3.2 Hz, 2H), 2.02 (m, 2H), 1.89 (m, 1H), 1.70 (m, 2H), 1.50 (m, 1H), 1.40–1.25 (m, 3H); 13C NMR (100 MHz, CDCl3, Figure S48) δ 207.6, 188.9, 161.9, 155.9, 151.9, 149.3, 148.4 (2 carbons), 145.0, 132.2, 130.5 (2 carbons), 130.3 (2 carbons), 129.4 (2 carbons) 129.1, 128.2 (2 carbons), 127.2, 122.8, 116.6, 114.3 (2 carbons), 111.8 (2 carbons), 107.8, 106.3, 67.4, 66.4, 63.1, 56.2, 53.5 (2 carbons), 45.3, 40.1 (2 carbons), 38.6, 34.2, 33.4, 32.5, 31.5; m/z calcd for C42H46N2O5 658.3; found 659.3 [M + H]+.
(E)-2-((1-Benzylpiperidin-4-Yl)Methyl)-6-(4-(4-(3-(4-(Dimethylamino)Phenyl)Acryloyl)Phenoxy)Butoxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (16b) (SGT682). A solution of compound 14 (20 mg, 0.055 mmol), compound 7b (27 mg, 0.066 mmol), and K2CO3 (23 mg, 0.16 mmol) in anhydrous DMF (5 mL) was heated at 65 °C overnight. The reaction mixture was then diluted with H2O, and extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×) and brine (3×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.39 in CH2Cl2:MeOH/19:1) to yield compound 16b (14 mg, 37%) as an orange oil: 1H NMR (400 MHz, CDCl3, Figure S49) δ 7.98 (d, J = 8.8 Hz, 2H, aromatic), 7.76 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.53 (d, J = 8.8 Hz, 2H, aromatic), 7.33 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.33–7.26 (m, 5H, aromatic), 7.13 (s, 1H, aromatic), 6.93 (d, J = 8.8 Hz, 2H, aromatic), 6.81 (s, 1H, aromatic), 6.67 (d, J = 8.8 Hz, 2H, aromatic), 4.41 (q, J = 5.6 Hz, 4H), 3.89 (s, 3H, OCH3), 3.62 (s, 2H, NCH2Ph), 3.21 (dd, J1 = 17.6 Hz, J2 = 8.4 Hz, 1H), 3.02 (s, 6H, N(CH3)2), 2.92 (m, 2H), 2.67 (dt, J1 = 14.0 Hz, J2 = 4.0 Hz, 2H), 2.10 (m, 2H), 2.02 (m, 4H), 1.88 (m, 1H), 1.72 (m, 2H), 1.60–1.20 (m, 4H); 13C NMR (100 MHz, CDCl3, Figure S50) δ 207.7, 188.9, 162.4, 155.8, 151.9, 148.7 (3 carbons), 144.9, 132.1, 131.7, 131.0, 130.5 (2 carbons), 130.2 (2 carbons), 129.7, 129.1, 128.3 (2 carbons), 122.8, 116.6, 114.1 (2 carbons), 111.8 (2 carbons), 107.5, 105.5, 68.6, 67.6, 62.7, 56.1, 53.3, 45.2, 40.1 (2 carbons), 38.5, 33.9, 33.4, 31.9, 31.1, 29.7, 26.0, 25.5; m/z calcd for C44H50N2O5 686.4; found 687.4 [M + H]+.
(E)-2-((1-Benzylpiperidin-4-Yl)Methyl)-6-((6-(4-(3-(4-(Dimethylamino)Phenyl)Acryloyl)Phenoxy)Hexyl)Oxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (16c) (SGT694). A solution of compound 14 (25 mg, 0.068 mmol), compound 7c (35 mg, 0.082 mmol), and K2CO3 (28 mg, 0.21 mmol) in anhydrous DMF (5 mL) was heated at 80 °C overnight. The reaction mixture was then diluted with H2O, and extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×) and brine (3×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.39 in CH2Cl2:MeOH/19:1) to yield compound 16c (48 mg, 98%) as a yellow solid: 1H NMR (400 MHz, CDCl3, Figure S51) δ 7.99 (d, J = 8.8 Hz, 2H, aromatic), 7.76 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.53 (d, J = 8.4 Hz, 2H, aromatic), 7.36–7.24 (m, 6H, aromatic), 7.14 (s, 1H, aromatic), 6.93 (d, J = 8.4 Hz, 2H, aromatic), 6.82 (s, 1H, aromatic), 6.68 (d, J = 8.8 Hz, 2H, aromatic), 4.032 (t, J = 6.0 Hz, 2H), 4.025 (t, J = 6.0 Hz, 2H), 3.91 (s, 3H, OCH3), 3.54 (s, 2H, NCH2Ph), 3.20 (dd, J1 = 17.6 Hz, J2 = 8.4 Hz, 1H), 3.02 (s, 6H, N(CH3)2), 2.92 (m, 2H), 2.65 (m, 2H), 2.00 (m, 2H), 1.90–1.80 (m, 9H), 1.60–1.20 (m, 6H); 13C NMR (100 MHz, CDCl3, Figure S52) δ 207.7, 188.8, 162.5, 155.7, 151.8, 148.8, 148.5 (3 carbons), 144.8, 131.6, 130.5, 130.2, 129.5, 129.1 (2 carbons), 128.2 (2 carbons), 127.3, 122.8 (2 carbons), 116.6, 114.1, 111.8 (2 carbons), 107.4, 105.4, 68.8, 67.9, 63.0, 56.2, 56.1, 53.4, 45.3, 40.1, 38.5 (2 carbons), 33.9, 33.3, 29.0, 28.8, 25.71, 25.66, 25.58, 24.9; m/z calcd for C46H54N2O5 714.4; found 715.3 [M + H]+.
(E)-2-((1-Benzylpiperidin-4-Yl)Methyl)-6-((8-(4-(3-(4-(Dimethylamino)Phenyl)Acryloyl)Phenoxy)Octyl)Oxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (16d) (SGT695). A solution of compound 14 (25 mg, 0.068 mmol), compound 7d (38 mg, 0.082 mmol), and K2CO3 (28 mg, 0.21 mmol) in anhydrous DMF (5 mL) was heated at 80 °C overnight. The reaction mixture was then diluted with H2O, and extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×) and brine (3×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.40 in CH2Cl2:MeOH/19:1) to yield compound 16d (51 mg, quantitative yield) as a yellow solid: 1H NMR (400 MHz, CDCl3, Figure S53) δ 7.99 (d, J = 9.2 Hz, 2H, aromatic), 7.76 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.53 (d, J = 8.8 Hz, 2H, aromatic), 7.36–7.24 (m, 6H, aromatic), 7.13 (s, 1H, aromatic), 6.93 (d, J = 8.8 Hz, 2H, aromatic), 6.82 (s, 1H, aromatic), 6.68 (d, J = 8.4 Hz, 2H, aromatic), 4.01 (t, J = 6.4 Hz, 4H), 3.92 (s, 3H, OCH3), 3.54 (s, 2H, NCH2Ph), 3.20 (dd, J1 = 17.6 Hz, J2 = 8.4 Hz, 1H), 3.02 (s, 6H, N(CH3)2), 2.92 (m, 2H), 2.65 (m, 2H), 2.00 (m, 2H), 1.90–1.76 (m, 5H), 1.76–1.65 (m, 2H), 1.50–1.20 (m, 12H); 13C NMR (100 MHz, CDCl3, Figure S54) δ 207.8, 188.9, 162.5, 155.7, 151.8, 148.8, 148.5 (3 carbons), 144.8, 131.6, 130.5 (2 carbons), 130.2 (2 carbons), 129.4, 129.2, 128.2 (2 carbons), 127.2, 122.9, 116.7, 114.1 (2 carbons), 111.8 (2 carbons), 107.4, 105.4, 69.0, 68.1, 63.2, 56.2, 53.6 (2 carbons), 45.4, 40.1 (2 carbons), 38.6, 34.2, 33.3, 31.5, 29.7, 29.2 (2 carbons), 29.1, 28.8, 25.9, 25.8; m/z calcd for C48H58N2O5 742.4; found 743.3 [M + H]+.
(E)-2-((1-Benzylpiperidin-4-Yl)Methyl)-6-((10-(4-(3-(4-(Dimethylamino)Phenyl)Acryloyl)Phenoxy)Decyl)Oxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (16e) (SGT696). A solution of compound 16 (20 mg, 0.055 mmol), compound 7e (32 mg, 0.066 mmol), and K2CO3 (23 mg, 0.16 mmol) in anhydrous DMF (5 mL) was heated at 80 °C overnight. The reaction mixture was then diluted with H2O, and extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×) and brine (3×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.40 in CH2Cl2:MeOH/19:1) to yield compound 16e (40 mg, 95%) as a yellow solid: 1H NMR (400 MHz, CDCl3, Figure S55) δ 7.99 (d, J = 8.8 Hz, 2H, aromatic), 7.76 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.53 (d, J = 8.8 Hz, 2H, aromatic), 7.36–7.24 (m, 6H, aromatic), 7.13 (s, 1H, aromatic), 6.93 (d, J = 8.8 Hz, 2H, aromatic), 6.82 (s, 1H, aromatic), 6.68 (d, J = 8.0 Hz, 2H, aromatic), 4.01 (t, J = 6.8 Hz, 4H), 3.92 (s, 3H, OCH3), 3.55 (s, 2H, NCH2Ph), 3.20 (dd, J1 = 17.6 Hz, J2 = 8.4 Hz, 1H), 3.02 (s, 6H, N(CH3)2), 2.94 (m, 2H), 2.65 (m, 2H), 2.02 (m, 2H), 1.92–1.60 (m, 7H), 1.50–1.20 (m, 16H); 13C NMR (100 MHz, CDCl3, Figure S56) δ 207.8, 188.9, 162.6, 155.8, 151.8, 148.8, 148.5 (3 carbons), 144.8, 131.6, 130.5 (2 carbons), 130.2 (2 carbons), 129.4, 129.2, 128.2 (2 carbons), 127.2, 122.9, 116.7, 114.1 (2 carbons), 111.8 (2 carbons), 107.4, 105.4, 69.0, 68.2, 63.1, 56.2, 53.6 (2 carbons), 45.3, 40.1 (2 carbons), 38.6, 34.2, 33.4, 31.5, 29.7, 29.42, 29.40, 29.30, 29.28, 29.1, 28.9, 26.0, 25.9; m/z calcd for C50H62N2O5 770.5; found 771.4 [M + H]+.
(E)-2-((1-Benzylpiperidin-4-Yl)Methyl)-6-((12-(4-(3-(4-(Dimethylamino)Phenyl)Acryloyl)Phenoxy)Dodecyl)Oxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (16f) (SGT680). A solution of compound 14 (25 mg, 0.068 mmol), compound 7f (42 mg, 0.082 mmol), and K2CO3 (28 mg, 0.21 mmol) in anhydrous DMF (5 mL) was heated at 80 °C overnight. The reaction mixture was then diluted with H2O, and extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×) and brine (3×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.39 in CH2Cl2:MeOH/19:1) to yield compound 16f (56 mg, quantitative yield) as an orange solid: 1H NMR (400 MHz, CDCl3, Figure S57) δ 7.99 (d, J = 8.8 Hz, 2H, aromatic), 7.76 (d, J = 15.6 Hz, 1H, HC=CH-Ph), 7.53 (d, J = 8.8 Hz, 2H, aromatic), 7.38–7.26 (m, 6H, aromatic), 7.12 (s, 1H, aromatic), 6.93 (d, J = 8.8 Hz, 2H, aromatic), 6.81 (s, 1H, aromatic), 6.67 (d, J = 8.4 Hz, 2H, aromatic), 4.01 (t, J = 6.4 Hz, 2H), 4.00 (t, J = 6.4 Hz, 2H), 3.91 (s, 3H, OCH3), 3.67 (s, 2H, NCH2Ph), 3.21 (dd, J1 = 17.6 Hz, J2 = 8.4 Hz, 1H), 3.03 (m, 2H), 3.02 (s, 6H, N(CH3)2), 2.64 (m, 2H), 2.16 (m, 2H), 2.00 (m, 1H), 1.92–1.70 (m, 7H), 1.50–1.20 (m, 19H); 13C NMR (100 MHz, CDCl3, Figure S58) δ 207.6, 188.9, 162.6, 155.8, 151.8, 148.9, 148.5 (3 carbons), 144.8, 131.6, 130.5 (2 carbons), 130.2 (2 carbons), 129.8, 129.1, 128.4 (2 carbons), 127.8, 122.9, 116.7, 114.1 (2 carbons), 111.8 (2 carbons), 107.4, 105.4, 69.1, 68.2, 62.5, 56.2, 53.2 (2 carbons), 45.1, 40.1 (2 carbons), 38.4, 33.6, 33.4, 31.0, 29.7, 29.50 (3 carbons), 29.47, 29.33, 29.30, 29.1, 28.9, 26.0, 25.9; m/z calcd for C52H66N2O5 798.5; found 799.3 [M + H]+.
(E)-3-(4-(Dimethylamino)Phenyl)-1-(3-(2-Hydroxyethoxy)Phenyl)Prop-2-En-1-One (17) (SGT863). A solution of compound (50 mg, 0.19 mmol) and K2CO3 (52 mg, 0.37 mmol) in anhydrous MeCN (5 mL) was treated with 2-bromoethanol (30 μL, 0.37 mmol) and the resulting mixture was refluxed overnight. The solvent was then removed and the obtained crude product was purified by column chromatography (SiO2 gel, pure Hexanes to Hexanes:EtOAc/1:1; Rf 0.32 in Hexanes:EtOAc/1:1) to yield compound 17 (14 mg, 24%) as a red solid: 1H NMR (400 MHz, CDCl3, Figure S59) δ 7.78 (d, J = 15.2 Hz, 1H, HC=CH-Ph), 7.60–7.58 (m, 1H, aromatic), 7.55–7.51 (m, 3H, aromatic), 7.38 (t, J = 8.4 Hz, 1H, aromatic), 7.29 (d, J = 16.0 Hz, 1H, HC=CH-Ph), 7.10 (ddd, J1 = 8.4 Hz, J2 = 2.8 Hz, J3 = 0.8 Hz, 1H, aromatic), 6.68 (d, J = 8.8 Hz, 2H, aromatic), 4.15 (t, J = 4.4 Hz, 2H, HOCH2CH2OPh), 3.98 (q, J = 4.0 Hz, 2H, HOCH2CH2OPh), 3.03 (s, 6H, N(CH3)2); 13C NMR (125 MHz, CDCl3, Figure S60) δ 189.3, 157.9, 145.1, 139.6, 129.6 (3 carbons), 128.6, 120.3, 118.2 (2 carbons), 116.0, 112.7 (2 carbons), 111.1, 68.5, 60.5, 39.3 (2 carbons); m/z calcd for C19H21NO3 311.2; found 312.1 [M + H]+.
2-(2-Bromoethoxy)Tetrahydro-2H-Pyran (18) (SGT864). A mixture of 2-bromoethanol (353 mg, 2.8 mmol), 3,4-dihydro-2H-pyran (0.31 mL, 3.4 mmol), and p-TsOH monohydrate (11 mg, 0.06 mmol) in anhydrous CH2Cl2 (10 mL) was stirred at rt overnight. The reaction mixture was washed with NaHCO3, H2O, and brine, dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, Hexanes:EtOAc/19:1; Rf 0.52 in Hexanes:EtOAc/9:1) to yield the known compound 18 [72] (362 mg, 62%) as a colorless oil: 1H NMR (400 MHz, CDCl3, Figure S61, which matches the lit. [72]) δ 4.66 (t, J = 3.6 Hz, 1H), 3.99 (dt, J1 = 11.2 Hz, J2 = 6.4 Hz, 1H), 3.87 (ddd, J1 = 11.6 Hz, J2 = 8.0 Hz, J3 = 2.8 Hz, 1H), 3.75 (dt, J1 = 11.2 Hz, J2 = 6.8 Hz, 1H), 3.54–3.44 (m, 3H), 1.88–1.77 (m, 1H), 1.76–1.66 (m, 1H), 1.65–1.48 (m, 4H).
2-((1-Benzylpiperidin-4-Yl)Methyl)-5-Methoxy-6-(2-((Tetrahydro-2H-Pyran-2-Yl)Oxy)Ethoxy)-2,3-Dihydro-1H-Inden-1-One (19) (SGT1729). A solution of compound 14 (50 mg, 0.14 mmol) and K2CO3 (39 mg, 0.27 mmol) in anhydrous DMF (5 mL) was treated with a solution of compound 18 (57 mg, 0.27 mmol) in DMF (1 mL), and the resulting mixture was stirred at rt overnight. The reaction mixture was quenched with H2O and extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×), and brine (3×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.21 in CH2Cl2:MeOH/19:1) to yield compound 19 (63 mg, 93%) as a yellow oil: 1H NMR (400 MHz, CDCl3, Figure S62) δ 7.35–7.22 (m, 5H, aromatic), 7.21 (s, 1H, aromatic), 6.82 (s, 1H, aromatic), 4.70 (t, J = 3.6 Hz, 2H), 4.21 (t, J = 4.8 Hz, 2H), 4.05 (m, 1H), 3.91 (s, 3H, OCH3), 3.87–3.82 (m, 2H), 3.49 (m, 2H, NCH2Ph), 3.20 (dd, J1 = 17.6 Hz, J2 = 8.4 Hz, 1H), 2.88 (m, 2H), 2.66 (dt, J1 = 14.4 Hz, J2 = 3.6 Hz, 2H), 2.00–1.76 (m, 4H), 1.75–1.48 (m, 8H), 1.38–1.23 (m, 3H); 13C NMR (100 MHz, CDCl3, Figure S63) 207.6, 155.9, 148.8, 148.7, 129.5 (2 carbons), 129.1 (2 carbons), 128.2 (2 carbons), 127.3, 107.6, 106.3, 98.9, 68.5, 65.42, 65.41, 63.1, 62.1, 56.12, 56.10, 53.5, 45.3, 38.6, 34.1, 33.4, 31.4, 30.4, 25.4, 19.2; m/z calcd for C30H39NO5 493.3; found 494.2 [M + H]+.
2-((1-Benzylpiperidin-4-Yl)Methyl)-6-(2-Hydroxyethoxy)-5-Methoxy-2,3-Dihydro-1H-Inden-1-One (20) (SGT1732). A solution of compound 19 (50 mg, 0.10 mmol) and p-TsOH monohydrate (19 mg, 0.10 mmol) in MeOH (5 mL) was stirred at rt overnight. The solvents were removed, and the crude material obtained was dissolved in EtOAc, washed with NaHCO3, H2O, and brine, dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude product obtained was purified by column chromatography (SiO2 gel, pure CH2Cl2 to CH2Cl2:MeOH/19:1; Rf 0.16 in CH2Cl2:MeOH/19:1) to yield compound 20 (18 mg, 44%) as a colorless oil: 1H NMR (400 MHz, CDCl3, Figure S64) δ 7.35–7.22 (m, 5H, aromatic), 7.18 (s, 1H, aromatic), 6.84 (s, 1H, aromatic), 4.12 (t, J = 4.4 Hz, 2H), 3.96 (t, J = 4.4 Hz, 2H), 3.92 (s, 3H, OCH3), 3.55 (br s, 2H, NCH2Ph), 3.22 (dd, J1 = 17.6 Hz, J2 = 8.0 Hz, 1H), 2.93 (m, 2H), 2.67 (dt, J1 = 14.0 Hz, J2 = 3.2 Hz, 2H), 2.10–1.94 (m, 2H), 1.93–1.84 (m, 1H), 1.80–1.30 (m, 6H); 13C NMR (100 MHz, CDCl3, Figure S65) 207.5, 155.9, 149.2, 148.4, 129.6 (2 carbons), 129.2 (2 carbons), 128.3 (2 carbons), 127.5, 107.6, 106.7, 70.7, 62.9, 61.0, 56.1, 53.41, 53.35, 45.2, 38.5, 33.9, 33.4 (2 carbons), 31.2; m/z calcd for C25H31NO4 409.23; found 410.2 [M + H]+.
3.3. In Vitro Inhibition of EeAChE and EfBChE
The ChE inhibition assays were performed as previously described [29]. The 1,3- and 1,4-chalcone-donepezil hybrids (0.1 nM to 100 μM) were dissolved in sodium phosphate buffer ((100 μL), 0.1 M, pH 8.0). They were diluted 5-fold and either EeAChE or EfBChE was added to the solution of the inhibitors (50 μL, containing 0.08 U/mL ChE (final concentration for both EeAChE and EfBChE). The enzymes and inhibitors were incubated for 10 min followed by the addition of DTNB (50 μL, 0.25 mM final concentration) and acylthiocholine (acetylthiocholine for EeAChE and butyrylthiocholine for EfBChE). The reactions were monitored at 412 nm by using a SpectraMax M5 plate reader (Molecular Devices, San Jose, CA) at 25 °C every 30 s for 10 min. Using the initial rates, the rate of no reaction was subtracted and normalized that value to the rate of no inhibitor. All assays were performed in triplicate. The data was plotted as a sigmoidal curve and IC50 values were calculated using SigmaPlot 14.0 (Systat Software, San Jose, CA, USA). The IC50 values for EeAChE and EfBChE inhibitions are presented in Table 1 and the corresponding graphs are presented in Figures S66 and S67, respectively.
3.4. Materials Used for 3H-PIB Binding Assays and the Assays Themselves
3.4.1. Aβ(1–40) and Aβ(1–42) Fibril Assembly
In total, 250 μg of lyophilized NH4OH-treated Aβ(1–40) (cat # A-1157-02) and Aβ(1–42) (cat # A-1167-02) purchased from rPeptide (Watkinsville, GA, USA) were each solubilized in their glass vials with 250 μL of a buffer solution comprised of 20 mM NaPi, 145 mM NaCl, 0.02% w/v NaN3 at pH 7.5 and incubated at 37 °C for 3 days, vortexing once per day. Prior to being transferred to screw-top polypropylene vials and being stored at −75 °C, these 1 mg/mL fibril suspensions were further diluted with an equal volume of the buffer solution. Their fibril content was determined in aliquots before and after centrifugation at 13,000× g by thioflavin fluorescence [73].
3.4.2. ADPBC Isolation
Human AD brain frontal cortical tissue (from the Sanders-Brown Center on Aging Alzheimer’s Disease Center Brain Bank at the University of Kentucky) was processed as follows. The insoluble Pittsburgh Compound B-binding fraction (ADPBC = AD PIB-binding complex) was prepared from the frontal cortex by sequential differential centrifugation of the tissue homogenate followed by sodium dodecyl sulfate extraction [74]. After detergent extraction, the pellet was washed with 20 mM NaPi buffer with 145 mM NaCl at pH 7.5 and collected by centrifugation at 100,000× g and aliquots stored in screw-top polypropylene vials at −75 °C.
3.4.3. 3H-PIB Binding Assays
Competition of compounds for the binding of 3H-PIB (VT 278, specific activity 70.2 Ci/mmol) purchased from Vitrax Radiochemicals (Placentia, CA) to Aβ(1–40) and Aβ(1–42) fibrils and AD brain extract (ADPBC) was determined by radioligand binding. 200 μL of 1.2 nM 3H-PIB in 20 mM NaPi buffer with 145 mM NaCl at pH 7.5, and 5% v/v EtOH-containing compounds and solvent controls were added to 200 ng of Aβ(1–40) or Aβ(1–42) fibrils in 20 μL of 20 mM NaPi buffer with 145 mM NaCl at pH 7.5 in individual wells of a polypropylene 96-well plate (Costar 3355). For the ADPBC, 25 μL of 20 mM NaPi buffer with 145 mM NaCl at pH 7.5 containing the equivalent of 133 μg wet weight of original tissue were incubated with compounds and solvent controls. The plates were sealed and incubated for 3 h at 22 °C, without shaking, and transferred to a 96-well Millipore Multiscreen HTS Hi Flow FB (GF/B) filter plate, and filtered with a multi-well plate vacuum manifold (Millipore Corporation, Bedford, MA, USA). The filters were rapidly washed 4× with 200 μL of 20 mM NaPi buffer with 145 mM NaCl at pH 7.5, and 5% v/v EtOH, dried, and transferred to scintillation vials and Budget-Solve (Fisher) scintillation fluid was added. Specific binding of 3H-PIB was determined by subtracting the non-specific binding determined in the presence of 1 μM BTA-1. These data are presented in Figure 1.
3.4.4. 3H-PIB Titration Assays
The titration of compounds with 3H-PIB bound to Aβ(1–40) and Aβ(1–42) fibrils and AD brain extract (ADPBC) were performed to further determine the competition of our compounds for the 3H-PIB binding site of the AD brain. The experiments were performed as described in the 3H-PIB binding assays of Section 3.4.3 with the use of a concentration range of 10 to 0.03 μM for the compounds tested. These data are presented in Figure 2.
3.5. Biotinyl-Aβ(1–42) (bioAβ42) Oligomer Assembly and Dissociation Assays
3.5.1. Preparation of ELISA Plates Coated with NeutrAvidinTM
Each well of ELISA plates (Costar 9018) were coated with 50 μL of 1 μg/mL NeutrAvidinTM in 10 mM NaPi buffer at pH 7.5 overnight at 4 °C. The next day, they were blocked with 200 μL of 20 mM NaPi buffer at pH 7.5 containing 0.145 M NaCl and 0.1% v/v Tween 20 for at least 2 h at rt. The plates were then stored in their blocked form at 4 °C prior to use for the oligomer assembly and dissociation assays.
3.5.2. Overview of bioAβ42 Oligomer Assembly
The assembly of bioAβ42 into soluble oligomers was quantified with an ELISA assay in which the soluble oligomers were captured with the NeutrAvidinTM coating the ELISA plates. The bound oligomers were detected with streptavidin-horseradish peroxidase (SA-HRP) [75]. The colorimetric or other signal produced was specific for oligomeric bioAβ42 species because the biotin of the NeutrAvidinTM-captured monomeric bioAβ(1–42) was complexed with the NeutrAvidinTM and was unable to react with the SA-HRP. Using biotinylated reagents and biotin-binding proteins for capture and detection avoids the interaction of small molecules with antibodies and this selectivity is useful for screening compound libraries [75,76]. Below, please find the details of the three steps (Steps 1–3) used for the oligomer assembly assay.
Step 1: Preparation of monomeric bioAβ42. For a low background, it was essential to rigorously pretreat the bioAβ42 peptide to convert (disaggregate) the multimeric species that form upon storage in the solid state, or even frozen in 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) to monomers. The required amount of stock bioAβ42 peptide (0.5 mg dissolved at 1 mg/mL in HFIP) was pipetted into a polypropylene microfuge tube, dried to a thin film under a gentle air or N2 stream, and then disaggregated for 10 min at rt in neat trifluoroacetic acid (TFA). After removal of the TFA by drying with an air or N2 stream, DMSO was added to produce a 2.3 μg/mL (50×) stock solution.
Step 2: Assembly of bioAβ42 oligomers. 2 μL of disaggregated monomeric 50× bioAβ42 peptide in DMSO (prepared in Step 1) was pipetted into the bottom of each well of a polypropylene 96-well plate. The assembly reaction was started by the addition of 100 μL of diluted compound containing up to 1% v/v DMSO in 20 mM NaPi buffer containing 0.145 M NaCl at pH 7.5. The 96-well plate was sealed and incubated at rt for 30 min without shaking. The bioAβ42 oligomer assembly was stopped by adding 50 μL of 0.3% Tween 20 v/v in distilled H2O.
Step 3: Measurement of bioAβ42 oligomers. To start measuring the bioAβ42 oligomers, the blocking solution for the NeutrAvidinTM-coated ELISA plate was removed and 50 μL of bioAβ42 oligomers (up to total of 20 nM bioAβ42 in 20 mM NaPi buffer with 0.145 M NaCl and 0.1% v/v Tween 20 at pH 7.5) was added to each well. The 96-well plate was then sealed and incubated for 2 h while shaking at 150 rpm at rt. The plate was then washed 3× with 200 μL of a solution comprised of 20 mM Tris-HCl, 34 mM NaCl, 0.1% v/v Tween 20 at pH 7.5, and then 50 μL of SA-HRP (1:20,000) in 20 mM NaPi buffer with 0.145 M NaCl and 0.1% v/v Tween 20 at pH 7.5 was added to each well. The plate was sealed and incubated for 1 h while shaking at 150 rpm at rt. The plate was washed as previously described, and 100 μL/well of HRP substrate (i.e., 1 mM:0.07 mM/tetramethylbenzidine:H2O2) in 0.2 M citrate buffer, pH 4.0 was added. The plate was incubated at rt for 5 to 10 min and the reaction was stopped by the addition of 100 μL/well of 1% v/v H2SO4. The absorbance of each well was read at 450 nm with a BioTek HT Synergy plate reader. These data are presented in Figure 3.
3.5.3. EC50 Determination against bioAβ42 Oligomer Assembly
The EC50 values of hybrids 15a–15f and 16a–16f against bioAβ42 oligomer assembly was determined as described in the overview of bioAβ42 oligomer assembly in Section 3.5.2. A concentration range of 50 to 1.56 μM for the compounds was used in this study. These data are presented in Figure 4 and Table S1.
3.5.4. Overview of bioAβ42 Oligomer Dissociation
Dissociation of preformed oligomers of bioAβ42 was measured by pretreating biotinylated oligomers with solvent controls or compounds for a period of time, capturing them with NeutrAvidinTM on ELISA plates, and detecting the remaining bound oligomers with SA-HRP [77] as described above. Below, please find the details of the two steps (Steps 1 and 2) used for the oligomer dissociation assay.
Step 1: Preformed bioAβ42 oligomers (sufficient for 400 wells). In total, 1 μg of bioAβ42 peptide (1 mg/mL dissolved in HFIP) was pipetted into 20 μL of HFIP in a polypropylene microfuge tube, and dried to a thin film under a gentle air or N2 stream. The film was dissolved in 250 μL of DMSO and vortexed. After 10 min at rt with intermittent vortexing, the DMSO-solubilized bioAβ42 was added rapidly to 12.5 mL of 20 mM NaPi buffer with 0.145 M NaCl at pH 7.5 in a 17 × 100 mm polypropylene tube, sealed, and vortexed. After incubation at rt for 1 h with intermittent vortexing, 0.375 mL of 100 mM Tween 20 (10% v/v) in distilled H2O was added to make the final Tween 20 concentration 0.3% v/v. The preformed oligomers were mixed by inversion and aliquoted for storage at −75 °C. Note: Multiple freeze-thaw cycles should be avoided.
Step 2: Oligomer dissociation assay. Preformed bioAβ42 oligomers (16.3 nM total bioAβ42) in Tween 20 were added to diluted compound in 20 mM NaPi buffer with 0.145 M NaCl at pH 7.5 (final concentration of bioAβ42 = 2.7 nM, ≤1% v/v DMSO) and incubated overnight (~18 h) with shaking. Then, 25 μL of the preformed bio42 oligomers adjusted to 0.6% v/v Tween 20 was pipetted into each well of a polypropylene 96-well plate. In total, 125 μL of diluted compound in 20 mM NaPi buffer with 0.145 M NaCl at pH 7.5 containing up to 1% v/v DMSO were added to the oligomers in the plates. The plates were sealed and shaken at 150 rpm overnight at rt. After 18 h of incubation, the plates were centrifuged at 1000× g for 10 min and a 100-μL aliquot was transferred from each well into a well of an NA-coated ELISA plate that had been blocked with Tween 20. The oligomer content of that aliquot was measured as described for the bioAβ42 oligomer assembly assay. These data are presented in Figure 5.
3.5.5. Determination of the Effects of Chalcone and Donepezil Fragments (Alone or in Combination) on Biotinyl-Aβ(1–42) (bioAβ42) Oligomer Assembly and Dissociation
The effects of various chalcones, donepezil, donepezil analogue, and 1,3-chalcone-donepezil hybrids were tested for their ability to inhibit the bioAβ42 oligomer assembly and dissociation either alone or in combination with each other. The experiments were performed as described in the biotinyl-Aβ(1–42) (bioAβ42) oligomer assembly and dissociation assays section. 1:1 or 1:1:1 ratios of the compounds were used, and these data are presented in Figure 6 and Figure 7.
3.6. Molecular Modeling
In the modeling of the HsAChE-15a-Aβ complex, we used the crystal structure of the HsAChE-donepezil complex (PDB ID: 4EY7 [66]) and the cryo-electron microscopy (cryo-EM) structure of an Aβ fibril (PDB ID: 6SHS [67]). The modeling was performed with Crystallographic Oriented Toolkit (Coot [78]) software. To generate the model, we superimposed the donepezil moiety of 15a with the bound donepezil in the HsAChE-donepezil crystal structure. The Aβ fibril was stripped down to two layers of Aβ and manually docked onto the chalcone side of 15a in an extended conformation while avoiding steric clashes and maximizing electrostatic and hydrophobic interactions between 15a and Aβ, assuming that the amino group of 15a would interact with the highly negatively-charged surface of Aβ defined by adjacent residues Glu22 and Asp23.
4. Conclusions
In summary, we synthesized 12 novel 1,3- and 1,4-chalcone-donepezil hybrids, 15a–15f and 16a–16f, and evaluated their ability to inhibit AD therapeutic targets. In general, most of these compounds were able to inhibit the actions of EeAChE and EfBChE with low to sub-micromolar IC50 values. In general, 1,4-chalcone-donepezil hybrids (16) were better at inhibiting EeAChE and EfBChE than 1,3-chalcone-donepezil hybrids (15), with the exception of 15f against EeAChE and 15a and 15b against EfBChE. The presence of shorter linkers between chalcones and donepezil seemed to have a positive impact on EeAChE and EfBChE inhibition. In the displacement of 3H-PIB from F40, F42, and ADPBC fibrils assays, we found that 1,3- and 1,4-chalcone-donepezil hybrids did not displace 3H-PIB as effectively as chalcones 3 and 6, which pointed towards a different binding site for these hybrids compared to PIB. Compared to donepezil and parent 1,3- and 1,4-chalcones 3 and 6, compounds 15a, 15b, 15c, 15d, 16a, 16b, 16c, and 16d exhibited better inhibition of bioAβ42 oligomer assembly. However, none of these 1,3- and 1,4-chalcone-donepezil hybrids were effective at dissociating preformed bioAβ42 oligomers. Finally, we performed a combination study that convincingly showed that a covalent linkage between chalcones and donepezil is essential for the prevention of bioAβ42 oligomerization. These studies demonstrate the promise of chalcone-donepezil hybrids as bifunctional molecules against two hallmarks of AD.
Acknowledgments
We thank the College of Pharmacy NMR Center for NMR support and Steven Van Lanen laboratory in the College of Pharmacy for LCMS support.
Abbreviations
| Aβ | amyloid-β |
| ACh | acetylcholine |
| AChEI | acetylcholinesterase inhibitor |
| AD | Alzheimer’s disease |
| ChE | cholinesterase |
| EC50 | half maximal effective concentration |
| EeAChE | acetylcholinesterase (from Electrophorus electricus) |
| EfBChE | butyrylcholinesterase (from Equus ferus) |
| HsAChE | acetylcholinesterase (from Homo sapiens) |
| IC50 | half maximal inhibitory concentration |
| KOH | potassium hydroxide |
| MsOH | methanesulfonic acid |
| NMDA | N-methyl-d-aspartate |
| PIB | Pittsburgh Compound B |
| ROS | reactive oxygen species |
| SAR | structure-activity relationship |
| SDEV | standard deviation |
| TBDMS | tert-butyldimethylsilyl |
Supplementary Materials
The following are available online, the Supplementary Materials include 1H and 13C NMR spectra for all the molecules synthesized (Figures S1–S65). The IC50 curves for the inhibition of EeAChE (Figure S66) and EfBChE (Figure S67) are also provided.
Author Contributions
N.T.C. collected NMR data, analyzed all the chemical and biological data, made all the figures, and wrote the manuscript and prepared all Supplementary Materials. M.Y.F. synthesized all the compounds and conducted the EeAChE and EfBChE inhibition assays. H.L.III performed the oligomer assembly and oligomer dissociation experiments and the 3H-PIB binding competition studies. O.V.T. performed the modeling experiments and wrote the corresponding sections. S.G.-T. analyzed all the data, helped with the preparation of all figures, and wrote the manuscript and Supplementary Materials with N.T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by startup funds (to S.G.-T.) from the College of Pharmacy at the University of Kentucky and by a BrightFocus Foundation grant A2014044S (to H.L.I.).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of all compounds are available from the authors.
References
- 1.Querfurth H.W., LaFerla F.M. Alzheimer’s disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Roberson E.D., Mucke L. 100 years and counting: Prospects for defeating Alzheimer’s disease. Science. 2006;314:781–784. doi: 10.1126/science.1132813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finder V.H. Alzheimer’s disease: A general introduction and pathomechanism. J. Alzheimers Dis. 2010;22(Suppl. 3):5–19. doi: 10.3233/JAD-2010-100975. [DOI] [PubMed] [Google Scholar]
- 4.Gitler A.D., Dhillon P., Shorter J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model Mech. 2017;10:499–502. doi: 10.1242/dmm.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canter R.G., Penney J., Tsai L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature. 2016;539:187–196. doi: 10.1038/nature20412. [DOI] [PubMed] [Google Scholar]
- 6.Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539:180–186. doi: 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzheimer’s Association 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019;15:321–387. doi: 10.1016/j.jalz.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Przedborski S., Vila M., Jackson-Lewis V. Neurodegeneration: What is it and where are we? J. Clin. Investig. 2003;111:3–10. doi: 10.1172/JCI200317522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pievani M., Filippini N., van den Heuvel M.P., Cappa S.F., Frisoni G.B. Brain connectivity in neurodegenerative diseases—From phenotype to proteinopathy. Nat. Rev. Neurol. 2014;10:620–633. doi: 10.1038/nrneurol.2014.178. [DOI] [PubMed] [Google Scholar]
- 10.Abeliovich A., Gitler A.D. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature. 2016;539:207–216. doi: 10.1038/nature20414. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs G.G. Molecular pathological classification of neurodegenerative diseases: Turning towards precision medicine. Int. J. Mol. Sci. 2016;17:189. doi: 10.3390/ijms17020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor J.P., Brown R.H., Jr., Cleveland D.W. Decoding als: From genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson P.T., Abner E.L., Schmitt F.A., Kryscio R.J., Jicha G.A., Smith C.D., Davis D.G., Poduska J.W., Patel E., Mendiondo M.S., et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rampa A., Bartolini M., Bisi A., Belluti F., Gobbi S., Andrisano V., Ligresti A., Di Marzo V. The first dual che/faah inhibitors: New perspectives for Alzheimer’s disease? ACS Med. Chem. Lett. 2012;3:182–186. doi: 10.1021/ml200313p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen R.C. Early diagnosis of Alzheimer’s disease: Is MCI too late? Curr. Alzheimer Res. 2009;6:324–330. doi: 10.2174/156720509788929237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molano J.R., Bratt R., Shatz R. Treatment and management of dementia due to Alzheimer’s disease. Curr. Treat. Options Neurol. 2015;17:363. doi: 10.1007/s11940-015-0363-4. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A., Singh A., Ekavali A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015;67:195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt C., Wolff M., Weitz M., Bartlau T., Korth C., Zerr I. Rapidly progressive Alzheimer disease. Arch. Neurol. 2011;68:1124–1130. doi: 10.1001/archneurol.2011.189. [DOI] [PubMed] [Google Scholar]
- 19.Marco-Contelles J., Unzeta M., Bolea I., Esteban G., Ramsay R.R., Romero A., Martinez-Murillo R., Carreiras M.C., Ismaili L. Ass234, as a new multi-target directed propargylamine for Alzheimer’s disease therapy. Front. Neurosci. 2016;10:294. doi: 10.3389/fnins.2016.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salama M., Shalash A., Magdy A., Makar M., Roushdy T., Elbalkimy M., Elrassas H., Elkafrawy P., Mohamed W., Abou Donia M.B. Tubulin and Tau: Possible targets for diagnosis of Parkinson’s and Alzheimer’s diseases. PLoS ONE. 2018;13:e0196436. doi: 10.1371/journal.pone.0196436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsden I.T., Minamide L.S., Bamburg J.R. Amyloid-beta-induced amyloid-beta secretion: A possible feed-forward mechanism in Alzheimer’s disease. J. Alzheimers Dis. 2011;24:681–691. doi: 10.3233/JAD-2011-101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 23.Buee L., Bussiere T., Buee-Scherrer V., Delacourte A., Hof P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 2000;33:95–130. doi: 10.1016/S0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 24.Pratico D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: Lights and shadows. Ann. N. Y. Acad. Sci. 2008;1147:70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- 25.Giacobini E. Cholinergic function and Alzheimer’s disease. Int. J. Geriatr. Psychiatry. 2003;18:S1–S5. doi: 10.1002/gps.935. [DOI] [PubMed] [Google Scholar]
- 26.Black S.E., Doody R., Li H., McRae T., Jambor K.M., Xu Y., Sun Y., Perdomo C.A., Richardson S. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology. 2007;69:459–469. doi: 10.1212/01.wnl.0000266627.96040.5a. [DOI] [PubMed] [Google Scholar]
- 27.Rountree S.D., Chan W., Pavlik V.N., Darby E.J., Siddiqui S., Doody R.S. Persistent treatment with cholinesterase inhibitors and/or memantine slows clinical progression of Alzheimer disease. Alzheimers Res. Ther. 2009;1:7. doi: 10.1186/alzrt7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran T.-D., Nguyen T.-C.-V., Nguyen N.-S., Nguyen D.-M., Nguyen T.-T.-H., Le M.-T., Thai K.-M. Synthesis of novel chalcones as acetylcholinesterase inhibitors. Appl. Sci. 2016;6:198. doi: 10.3390/app6070198. [DOI] [Google Scholar]
- 29.Eckroat T.J., Green K.D., Reed R.A., Bornstein J.J., Garneau-Tsodikova S. Investigation of the role of linker moieties in bifunctional tacrine hybrids. Bioorg. Med. Chem. 2013;21:3614–3623. doi: 10.1016/j.bmc.2013.02.047. [DOI] [PubMed] [Google Scholar]
- 30.Bornstein J.J., Eckroat T.J., Houghton J.L., Jones C.K., Green K.D., Garneau-Tsodikova S. Tacrine-mefenamic acid and hybrids for inhibition of acetylcholinesterase. Med. Chem. Commun. 2011;2:406–412. doi: 10.1039/c0md00256a. [DOI] [Google Scholar]
- 31.Kochi A., Eckroat T.J., Green K.D., Mayhoub A.S., Lim M.H., Garneau-Tsodikova S. A novel hybrid of 6-chlorotacrine and metal–amyloid-β modulator for inhibition of acetylcholinesterase and metal-induced amyloid-β aggregation. Chem. Sci. 2013;11:4137–4145. doi: 10.1039/c3sc51902c. [DOI] [Google Scholar]
- 32.Bartus R.T., Dean R.L., 3rd, Beer B., Lippa A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 33.Shah A.A., Dar T.A., Dar P.A., Ganie S.A., Kamal M.A. A current perspective on the inhibition of cholinesterase by natural and synthetic inhibitors. Curr. Drug Metab. 2017;18:96–111. doi: 10.2174/1389200218666161123122734. [DOI] [PubMed] [Google Scholar]
- 34.Perry E., Walker M., Grace J., Perry R. Acetylcholine in mind: A neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273–280. doi: 10.1016/S0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- 35.Bartus R.T. On neurodegenerative diseases, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 36.Unzeta M., Esteban G., Bolea I., Fogel W.A., Ramsay R.R., Youdim M.B., Tipton K.F., Marco-Contelles J. Multi-target directed donepezil-like ligands for Alzheimer’s disease. Front. Neurosci. 2016;10:205. doi: 10.3389/fnins.2016.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selkoe D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 38.Manchikalapudi A.L., Chilakala R.R., Kalia K., Sunkaria A. Evaluating the role of microglial cells in clearance of Abeta from Alzheimer’s brain. ACS Chem. Neurosci. 2019;10:1149–1156. doi: 10.1021/acschemneuro.8b00627. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y.J., Zhou H.D., Zhou X.F. Clearance of amyloid-beta in Alzheimer’s disease: Progress, problems and perspectives. Drug Discov. Today. 2006;11:931–938. doi: 10.1016/j.drudis.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Hamley I.W. The amyloid beta peptide: A chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem. Rev. 2012;112:5147–5192. doi: 10.1021/cr3000994. [DOI] [PubMed] [Google Scholar]
- 41.Pascoal T.A., Mathotaarachchi S., Mohades S., Benedet A.L., Chung C.O., Shin M., Wang S., Beaudry T., Kang M.S., Soucy J.P., et al. Amyloid-beta and hyperphosphorylated Tau synergy drives metabolic decline in preclinical Alzheimer’s disease. Mol. Psychiatry. 2017;22:306–311. doi: 10.1038/mp.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dias Viegas F.P., de Freitas Silva M., Divino da Rocha M., Castelli M.R., Riquiel M.M., Machado R.P., Vaz S.M., Simoes de Lima L.M., Mancini K.C., Marques de Oliveira P.C., et al. Design, synthesis and pharmacological evaluation of n-benzyl-piperidinyl-aryl-acylhydrazone derivatives as donepezil hybrids: Discovery of novel multi-target anti-Alzheimer prototype drug candidates. Eur. J. Med. Chem. 2018;147:48–65. doi: 10.1016/j.ejmech.2018.01.066. [DOI] [PubMed] [Google Scholar]
- 43.Lee S.Y., Chiu Y.J., Yang S.M., Chen C.M., Huang C.C., Lee-Chen G.J., Lin W., Chang K.H. Novel synthetic chalcone-coumarin hybrid for Abeta aggregation reduction, antioxidation, and neuroprotection. CNS Neurosci. Ther. 2018;24:1286–1298. doi: 10.1111/cns.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J., Qiu J., Wang M., Wang L., Su L., Gao J., Gu Q., Xu J., Huang S.L., Gu L.Q., et al. Synthesis and characterization of 1H-phenanthro [9,10-d]imidazole derivatives as multifunctional agents for treatment of Alzheimer’s disease. Biochim. Biophys. Acta. 2014;1840:2886–2903. doi: 10.1016/j.bbagen.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Li S.Y., Jiang N., Xie S.S., Wang K.D., Wang X.B., Kong L.Y. Design, synthesis and evaluation of novel tacrine-rhein hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Org. Biomol. Chem. 2014;12:801–814. doi: 10.1039/C3OB42010H. [DOI] [PubMed] [Google Scholar]
- 46.Jiang N., Huang Q., Liu J., Liang N., Li Q., Li Q., Xie S.S. Design, synthesis and biological evaluation of new coumarin-dithiocarbamate hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018;146:287–298. doi: 10.1016/j.ejmech.2018.01.055. [DOI] [PubMed] [Google Scholar]
- 47.Xie S.S., Lan J.S., Wang X., Wang Z.M., Jiang N., Li F., Wu J.J., Wang J., Kong L.Y. Design, synthesis and biological evaluation of novel donepezil-coumarin hybrids as multi-target agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2016;24:1528–1539. doi: 10.1016/j.bmc.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 48.Cao Z., Yang J., Xu R., Song Q., Zhang X., Liu H., Qiang X., Li Y., Tan Z., Deng Y. Design, synthesis and evaluation of 4’-OH-flurbiprofen-chalcone hybrids as potential multifunctional agents for Alzheimer’s disease treatment. Bioorg. Med. Chem. 2018;26:1102–1115. doi: 10.1016/j.bmc.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 49.Liu Q., Qiang X., Li Y., Sang Z., Li Y., Tan Z., Deng Y. Design, synthesis and evaluation of chromone-2-carboxamido-alkylbenzylamines as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2015;23:911–923. doi: 10.1016/j.bmc.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 50.Qiang X., Sang Z., Yuan W., Li Y., Liu Q., Bai P., Shi Y., Ang W., Tan Z., Deng Y. Design, synthesis and evaluation of genistein-o-alkylbenzylamines as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014;76:314–331. doi: 10.1016/j.ejmech.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y., Ye F., Xu J., Liao Q., Chen L., Zhang W., Sun H., Liu W., Feng F., Qu W. Design, synthesis and evaluation of novel bivalent beta-carboline derivatives as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2018;26:3812–3824. doi: 10.1016/j.bmc.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 52.Green K.D., Fosso M.Y., Garneau-Tsodikova S. Multifunctional donepezil analogues as cholinesterase and BACE1 inhibitors. Molecules. 2018;23:3252. doi: 10.3390/molecules23123252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costanzo P., Cariati L., Desiderio D., Sgammato R., Lamberti A., Arcone R., Salerno R., Nardi M., Masullo M., Oliverio M. Design, synthesis, and evaluation of donepezil-like compounds as AChE and BACE-1 inhibitors. ACS Med. Chem. Lett. 2016;7:470–475. doi: 10.1021/acsmedchemlett.5b00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mishra C.B., Kumari S., Manral A., Prakash A., Saini V., Lynn A.M., Tiwari M. Design, synthesis, in-silico and biological evaluation of novel donepezil derivatives as multi-target-directed ligands for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017;125:736–750. doi: 10.1016/j.ejmech.2016.09.057. [DOI] [PubMed] [Google Scholar]
- 55.Lan J.S., Zhang T., Liu Y., Yang J., Xie S.S., Liu J., Miao Z.Y., Ding Y. Design, synthesis and biological activity of novel donepezil derivatives bearing N-benzyl pyridinium moiety as potent and dual binding site acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2017;133:184–196. doi: 10.1016/j.ejmech.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 56.Li F., Wang Z.M., Wu J.J., Wang J., Xie S.S., Lan J.S., Xu W., Kong L.Y., Wang X.B. Synthesis and pharmacological evaluation of donepezil-based agents as new cholinesterase/monoamine oxidase inhibitors for the potential application against Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2016;31:41–53. doi: 10.1080/14756366.2016.1201814. [DOI] [PubMed] [Google Scholar]
- 57.Brewster J.T., 2nd, Dell’Acqua S., Thach D.Q., Sessler J.L. Classics in chemical neuroscience: Donepezil. ACS Chem. Neurosci. 2019;10:155–167. doi: 10.1021/acschemneuro.8b00517. [DOI] [PubMed] [Google Scholar]
- 58.Fosso M.Y., LeVine H., 3rd, Green K.D., Tsodikov O.V., Garneau-Tsodikova S. Effects of structural modifications on the metal binding, anti-amyloid activity, and cholinesterase inhibitory activity of chalcones. Org. Biomol. Chem. 2015;13:9418–9426. doi: 10.1039/C5OB01478F. [DOI] [PubMed] [Google Scholar]
- 59.Fosso M.Y., McCarty K., Head E., Garneau-Tsodikova S., LeVine H., 3rd Differential effects of structural modifications on the competition of chalcones for the PIB amyloid imaging ligand-binding site in Alzheimer’s disease brain and synthetic Abeta fibrils. ACS Chem. Neurosci. 2016;7:171–176. doi: 10.1021/acschemneuro.5b00266. [DOI] [PubMed] [Google Scholar]
- 60.Ellman G.L., Courtney K.D., Andres V., Jr., Feather-Stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 61.Walsh D.M., Selkoe D.J. Abeta oligomers—A decade of discovery. J. Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 62.Chauhan K., Datta A., Adhikari A., Chuttani K., Kumar Singh A., Mishra A.K. (68)Ga based probe for Alzheimer’s disease: Synthesis and preclinical evaluation of homodimeric chalcone in beta-amyloid imaging. Org. Biomol. Chem. 2014;12:7328–7337. doi: 10.1039/C4OB00941J. [DOI] [PubMed] [Google Scholar]
- 63.Citron M. Alzheimer’s disease: Strategies for disease modification. Nat. Rev. Drug Discov. 2010;9:387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- 64.Haass C., Selkoe D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 65.Hayne D.J., Lim S., Donnelly P.S. Metal complexes designed to bind to amyloid-beta for the diagnosis and treatment of Alzheimer’s disease. Chem. Soc. Rev. 2014;43:6701–6715. doi: 10.1039/C4CS00026A. [DOI] [PubMed] [Google Scholar]
- 66.Cheung J., Rudolph M.J., Burshteyn F., Cassidy M.S., Gary E.N., Love J., Franklin M.C., Height J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012;55:10282–10286. doi: 10.1021/jm300871x. [DOI] [PubMed] [Google Scholar]
- 67.Kollmer M., Close W., Funk L., Rasmussen J., Bsoul A., Schierhorn A., Schmidt M., Sigurdson C.J., Jucker M., Fandrich M. Cryo-EM structure and polymorphism of Abeta amyloid fibrils purified from Alzheimer’s brain tissue. Nat. Commun. 2019;10:4760. doi: 10.1038/s41467-019-12683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao P.L., Liu C.L., Huang W., Wang Y.Z., Yang G.F. Synthesis and fungicidal evaluation of novel chalcone-based strobilurin analogues. J. Agric. Food Chem. 2007;55:5697–5700. doi: 10.1021/jf071064x. [DOI] [PubMed] [Google Scholar]
- 69.Xie H., Wu S. Synthesis of chemical modified b-cyclodextrin and its inclusion behavior in alcohol/water mixed solvents. Supramolec. Chem. 2001;13:545–556. doi: 10.1080/10610270108028301. [DOI] [Google Scholar]
- 70.Allais F., Pla T.J.L., Ducrot P.-H. An access to chiral b-benzyl-g-butyrolactones and its application to the synthesis of enantiopure (+)-secoisolariciresinol, (−)-secoisolariciresinol, and (−)-enterolactone. Synthesis. 2011;9:1456–1464. doi: 10.1055/s-0030-1259982. [DOI] [Google Scholar]
- 71.Meng F.C., Mao F., Shan W.J., Qin F., Huang L., Li X.S. Design, synthesis, and evaluation of indanone derivatives as acetylcholinesterase inhibitors and metal-chelating agents. Bioorg. Med. Chem. Lett. 2012;22:4462–4466. doi: 10.1016/j.bmcl.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 72.O’Rourke N.F., Micalizio G.C. Cyclopropenes in metallacycle-mediated cross-coupling with alkynes: Convergent synthesis of highly substituted vinylcyclopropanes. Org. Lett. 2016;18:1250–1253. doi: 10.1021/acs.orglett.6b00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.LeVine H., 3rd Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- 74.Matveev S.V., Spielmann H.P., Metts B.M., Chen J., Onono F., Zhu H., Scheff S.W., Walker L.C., LeVine H., 3rd A distinct subfraction of Abeta is responsible for the high-affinity Pittsburgh Compound B-binding site in Alzheimer’s disease brain. J. Neurochem. 2014;131:356–368. doi: 10.1111/jnc.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.LeVine H., 3rd Biotin-avidin interaction-based screening assay for Alzheimer’s beta-peptide oligomer inhibitors. Anal. Biochem. 2006;356:265–272. doi: 10.1016/j.ab.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 76.LeVine H., 3rd, Ding Q., Walker J.A., Voss R.S., Augelli-Szafran C.E. Clioquinol and other hydroxyquinoline derivatives inhibit Abeta(1–42) oligomer assembly. Neurosci. Lett. 2009;465:99–103. doi: 10.1016/j.neulet.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.LeVine H., 3rd, Lampe L., Abdelmoti L., Augelli-Szafran C.E. Dihydroxybenzoic acid isomers differentially dissociate soluble biotinyl-Abeta(1–42) oligomers. Biochemistry. 2012;51:307–315. doi: 10.1021/bi201288x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Emsley P., Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.