Abstract
In this review article, the occurrence of nor-lignans and their biological activities are explored and described. Nor-lignans have proven to be present in several different families also belonging to chemosystematically distant orders as well as to have many different beneficial pharmacological activities. This review article represents the first one on this argument and is thought to give a first overview on these compounds with the hope that their study may continue and increase, after this.
Keywords: nor-lignans, occurrence, biological activities
1. Introduction
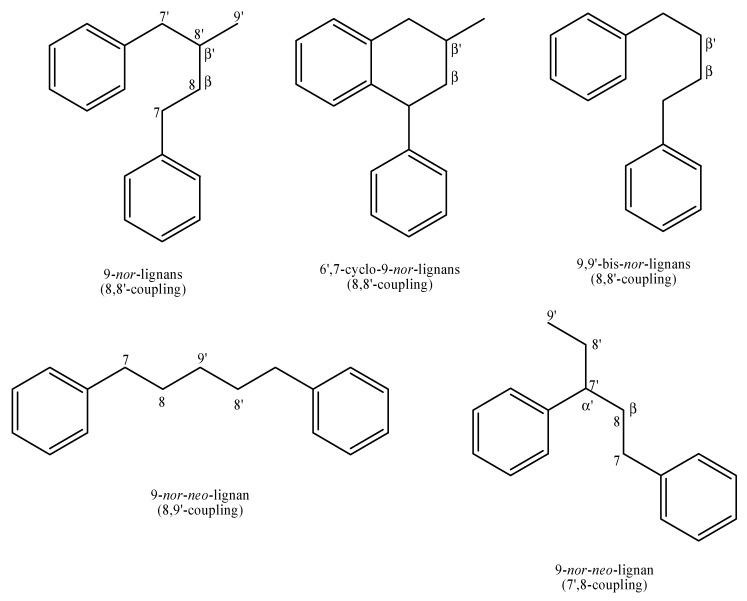
A large part of secondary plant phenolic natural products derives from the aromatic amino acids couple tyrosine/phenylalanine. The de-amination of these compounds gives raise, through the shikimic acid pathway, to the intermediate metabolites C(6)–C(3), i.e., propenyl-phenols and allyl-phenols, generally named as phenylpropanoids. These are the starting points of the biosynthesis of several classes of active constituents related to the stability of the cell wall and to the defense of plants against herbivorous animals and pathogens. The first line of defense in terrestrial plants is a mechanical one, related to a polymerization process which leads to the formation of lignin, the main component of wood. Lignin is a very strong and stable macromolecule. Its introduction inside the plant cell wall instead of the carbohydrate polymer cellulose, confers force and resistance, allowing the formation of giant tree’s structure and making the digestions of the adult parts of the plant from herbivorous animals very difficult. Yet, the lignin defense line has resulted to be quite insufficient in many cases and the incoming predominance of herbal species determined the shift towards another form of defense, i.e., the chemical one. Actually, the phenylpropanoids pathway has never been dismissed, but rather it has turned towards the synthesis of smaller products having more precise targets. Among these molecules, the dimerization process of C(6)–C(3) precursors gives rise to three important classes of natural secondary metabolites: lignans, neo-lignans and nor-lignans. These classes present similar features due to their common biosynthetic origin. Their general structure is characterized by the presence of two terminal phenyl groups, which are more or less functionalized with hydroxyl groups and connected by a central chain of six carbon atoms, differently arranged and oxidized. The main difference among lignans, neo-lignans and nor-lignans is due to the different type of junction between the two C(6)C(3) (=PhC3) units. In particular, in lignans, this junction is through a β-β (8-8′) bond and in neo-lignans the junction is not a β-β type. Therefore, lignans and neo-lignans, and their several different derived subclasses, can be identified depending upon the carbon skeletons which they possess. For what concerns nor-lignans, the structure is more complicated. In fact, nor-lignans own a peculiar characteristic, with respect to lignans and neo-lignans, which is the cut of one carbon from the central chain. This loss forces this chain to be differently arranged from lignans and neo-lignans, such as in a linear sequence or in a C(3)C(2) arrangement meaning 8,9′-coupling and 7′,8-coupling or alternatively in the bis-nor-lignan and cyclo-nor-lignan skeletons (8,8′) where chirality plays a central role. From this description, it is quite easy to understand the other definition of the structure of nor-lignans: natural compounds based on diphenyl-pentanes, derived by the union of two phenylpropanoid units in the positions α, β′ or β, γ′ and characterized with the loss of the terminal carbon of the chain [1,2,3].
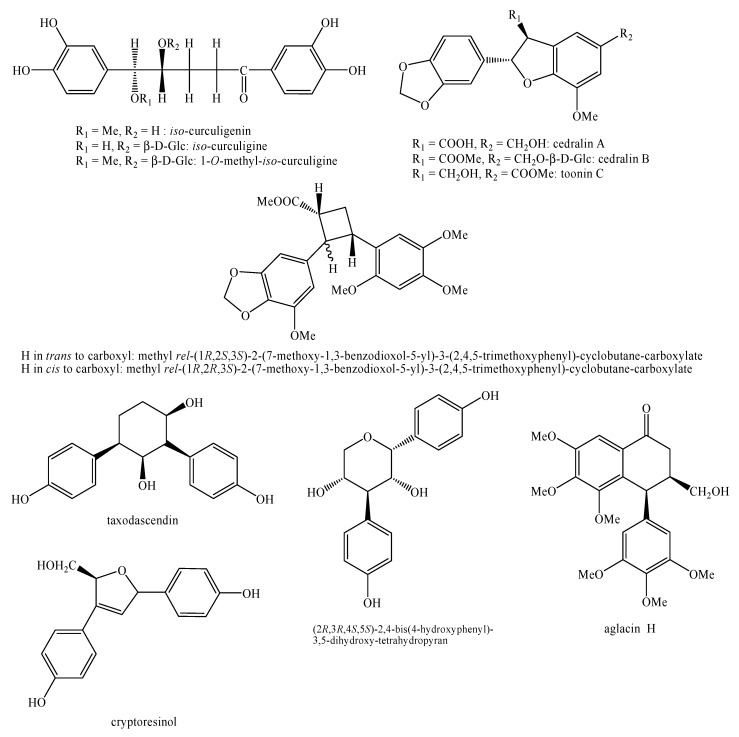
Figure 1 shows the possible different arrangements for nor-lignans.
Figure 1.
general nor-lignan basic structures.
2. Occurrence of Nor-Lignans in the Plant Kingdom
From the environmental and taxonomical points of view, lignans are mainly biosynthesized in woody plants, since main occurrences are related to Gymnospermae and Angiospermae. In particular, they can be found in the trees’ members of ancient forests like the Amazonian one, but, probably because of their simple biosynthetic pathway, they can be present also in herbal plants like those of monocotyledons.
In this review article, the attention is focused on nor-lignans, their occurrence in the plant kingdom and their importance as bioactive molecules.
Table 1 reports on the nor-lignans identified in the plant kingdom differentiating them according to the species, genus and family. In addition, the organs from which these compounds have been isolated, and the techniques used for their isolation and identification were completely added.
Table 1.
of nor-lignans in the plant kingdom.
| Family | Species | Studied Organs | Compounds | Methods | References |
|---|---|---|---|---|---|
| Acanthaceae | Justicia patentiflora Hemsl. | Leaves and stems | justiflorinol | SE, CC, HPLC, [α]D, UV, IR, NMR, MS | [4] |
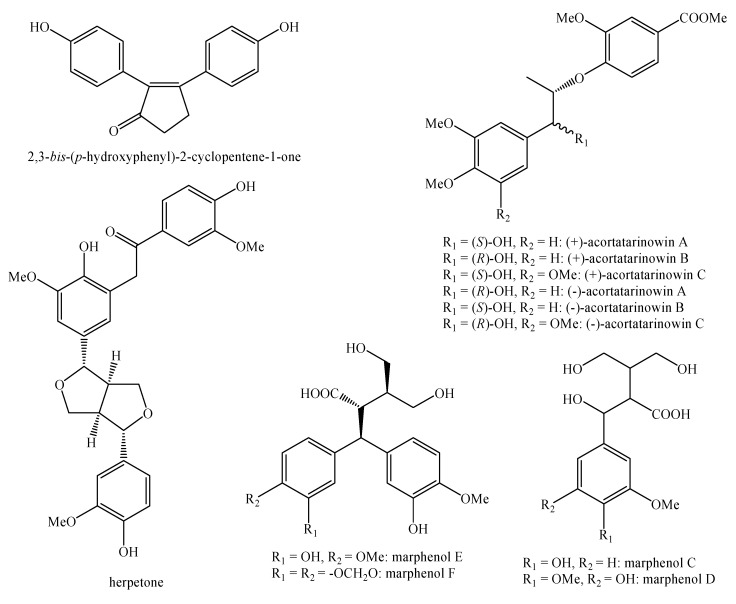
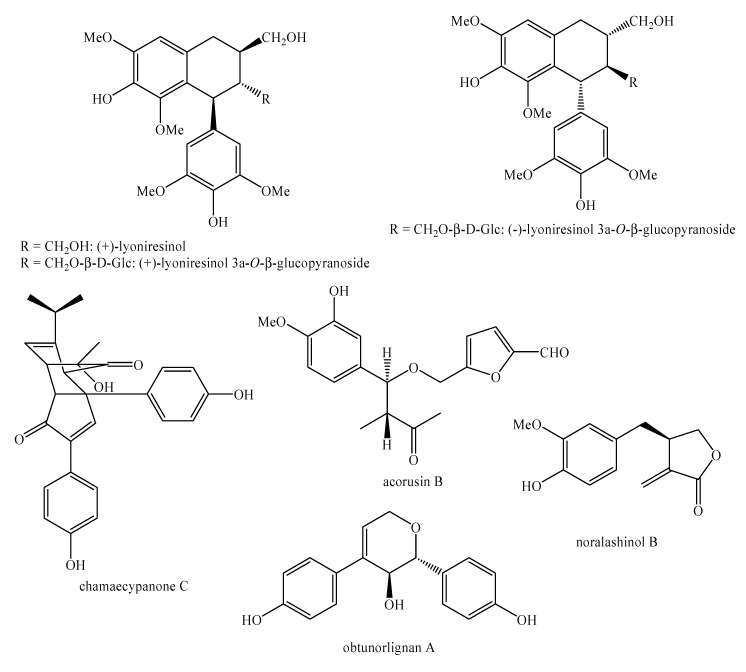
| Acoraceae | Acorus tatarinowii Schott | Rhizomes | (+)-acortatarinowin A, (+)-acortatarinowin B, (+)-acortatarinowin C, (−)-acortatarinowin A, (−)-acortatarinowin B, (−)-acortatarinowin C | SE, CC, HPLC, ECD, [α]D, UV, IR, NMR, MS | [5] |
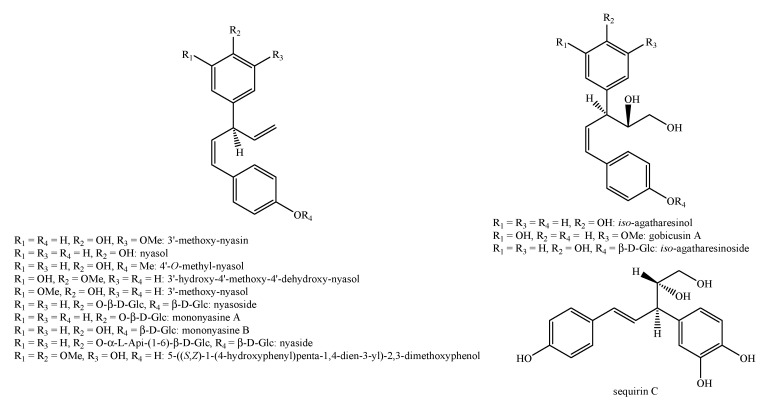
| acorusin B | SE, CC, [α]D, UV, IR, NMR, MS | [6] | |||
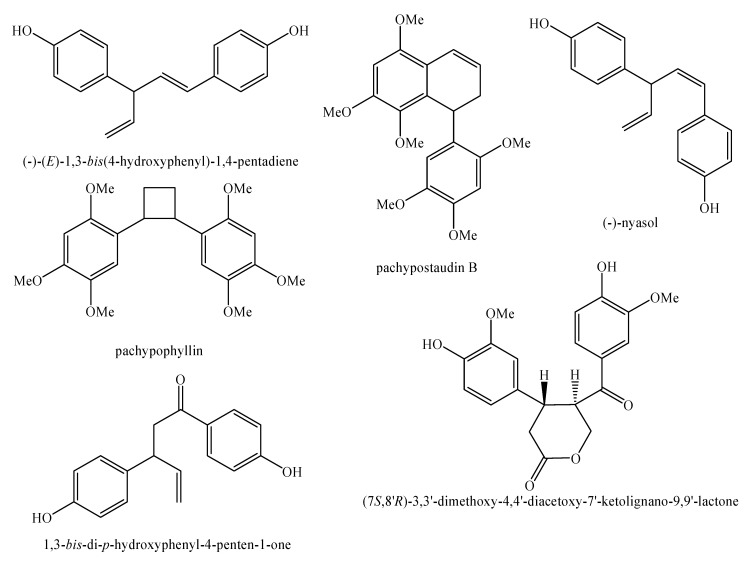
| Annonaceae | Duguetia confinis (Engl. and Diels) Chatrou | Bark | pachypostaudin A, pachypostaudin B | SE, CC, TLC, NMR, MS | [7] |
| Pachypodanthium staudlii (Engl. and Diels) Engl. and Diels | Bark | pachypostaudin A, pachypostaudin B, pachypophyllin | SE, MP, NMR, MS | [8] | |
| Araucariaceae | Araucaria angustifolia (Bertol.) Kuntze | Knot resin | 2,3-bis-(p-hydroxyphenyl)-2-cyclopentene-1-one, nyasol, cryptoresinol | SE, CC, HPLC, TLC, UV, IR, NMR, MS | [9] |
| Asparagaceae |
Anemarrhena asphodeloides Bunge |
Rhizomes | nyasol, 4′-O-methyl-nyasol, 1,3-di-p-hydroxyphenyl-4-penten-1-one | SE, CC, LC, [α]D, NMR, MS | [10,11] |
| nyasol, 4′-O-methyl-nyasol, 3″-methoxy-nyasol, 3″-hydroxy-4″-methoxy-4″-dehydroxy-nyasol | SE, CC, NMR, MS | [12] | |||
| nyasol | SE, CC, HPLC, UV, NMR, MS | [13] | |||
| Asparagus africanus Lam. | Roots | nyasol | CC, LC, HPLC, NMR, MS | [14] | |
| Asparagus cochinchinensis (Lour.) Merr. | Roots | iso-agatharesinoside, iso-agatharesinol | SE, CC, [α]D, UV, IR, NMR, MS | [15] | |
| Tubers | nyasol | SE, CC, [α]D, IR, NMR, MS | [16] | ||
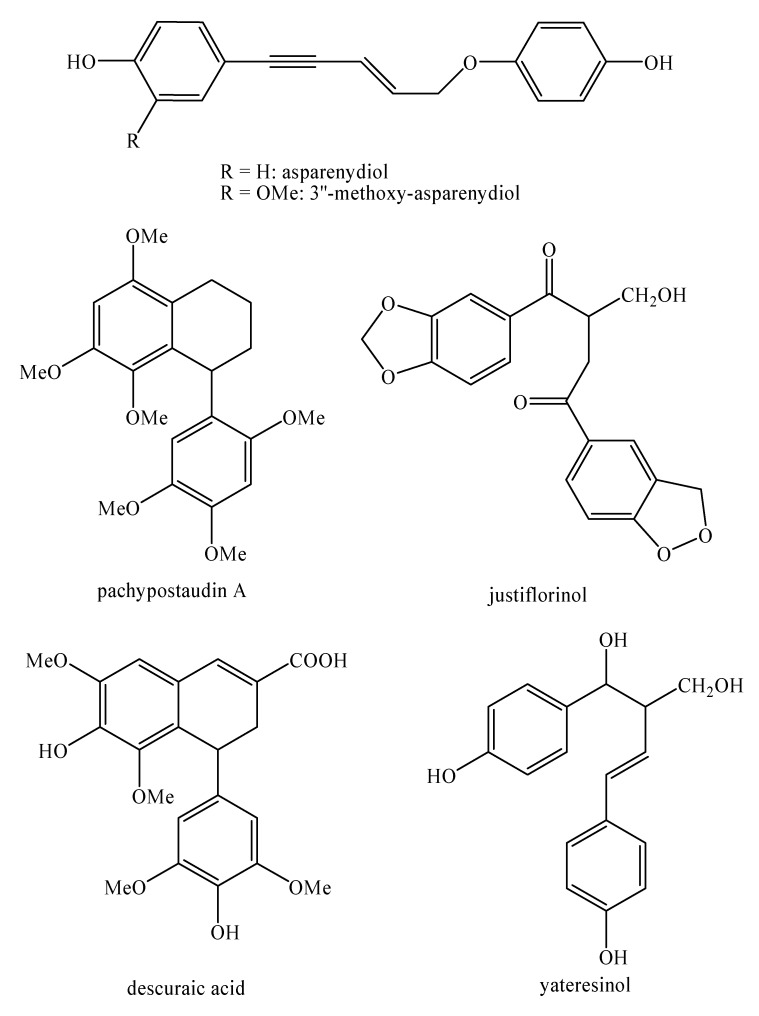
| Roots | 3′-hydroxy-4′-methoxy-4′-dehydroxy-nyasol, nyasol, 3′-methoxy-nyasol, 1,3-bis-di-p-hydroxyphenyl-4-penten-1-one, asparenydiol, 3′′-methoxy-asparenydiol | SE, CC, [α]D, UV, IR, NMR, MS | [17] | ||
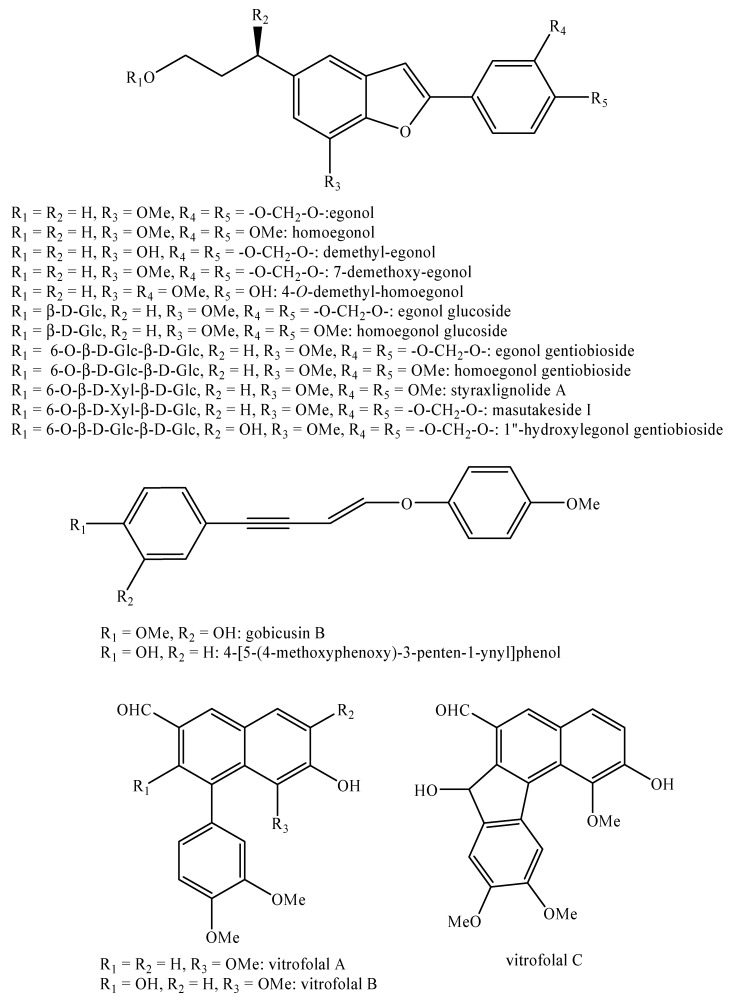
| Asparagus gobicus N.A.Ivanova ex Grubov | Roots | 3′-methoxy-nyasin, iso-agatharesinol, gobicusin A, gobicusin B, nyasol, 4-[5-(4-methoxyphenoxy)-3-penten-1-ynyl]phenol, sequirinC | SE, CC, [α]D, UV, IR, NMR, MS | [18] | |
| Asparagus racemosus Willd. | Whole plant | iso-agatharesinol, gobicusin A | SE, CC, NMR, MS | [19] | |
| Drimiopsis burkei Baker | Bulbs | (−)-nyasol | SE, CC, [α]D, NMR | [20,21] | |
| Drimiopsis maculata Lindl. and Paxton | Bulbs | (−)-(E)-1,3-bis(4-hydroxyphenyl)-1,4-pentadiene | SE, CC, [α]D, NMR | [20] | |
| Ledebouria ovatifolia (Baker) Jessop | Whole plant | 5-((S,Z)-1-(4-hydroxyphenyl)penta-1,4-dien-3-yl)-2,3-dimethoxyphenol | n.r. | [21] | |
| Rhodocodon campanulatus H. Perrier | Bulbs | (7S,8′R)-3,3′-dimethoxy-4,4′-diacetoxy-7′-ketolignano-9,9′-lactone | SE, CC, [α]D, ECD, IR, NMR, MS | [22] | |
| Berberidaceae | Dysosma versipellis (Hance) M.Cheng | Roots | dysosmanorlignan A, dysosmanorlignan B | SE, CC, HPLC, TLC, [α]D, IR, UV, NMR, MS | [23] |
| Brassicaceae | Descurainia sophia (L.) Webb ex Prantl | Roots | descuraic acid | SE, LC, CC, [α]D, NMR, MS | [24] |
| Compositae | Saussurea macrota Franch | Whole plant | egonol | SE, CC, TLC, [α]D, IR, NMR, MS | [25] |
| Cucurbitaceae | Herpetospermum pedunculosum (Ser.) C.B. Clarke | Whole plant | herpetone | SE, CC, HPLC, IR, UV, NMR, MS | [26] |
| Cupressaceae | Chamaecyparis formosensis Matsum. | Wood | yateresinol, nyasol | SE, CC, UV, IR, NMR | [27] |
| Chamaecyparis obtusa var. formosana (Hayata) Hayata | Heartwood | chamaecypanone C, obtunorlignan A | SE, CC, [α]D, UV, IR, NMR, MS | [28] | |
| Wood | trans-nyasol | SE, [α]D, MP, IR, MS | [29] | ||
| Cryptomeria japonica (Thunb. ex L.f.) D.Don | Whole plant | agatharesinol | IM | [30] | |
| n.a. | yateresinol | n.a. | [31] | ||
| Libocedrus yateensis Guillaumin | Heartwood | yateresinol, nyasol | SE, CC, [α]D, IR, NMR, MS | [32] | |
|
Metasequoia glyptostroboides Huand W.C.Cheng |
Stems and leaves | metasequirin D, metasequirin E, metasequirin F, sequosempervirin B, sequosempervirin D, sequosempervirin F, agatharesinol, agatharesinol acetonide, sequirin C, nyasol | SE, CC, LC, HPLC, [α]D, IR, NMR, MS | [33] | |
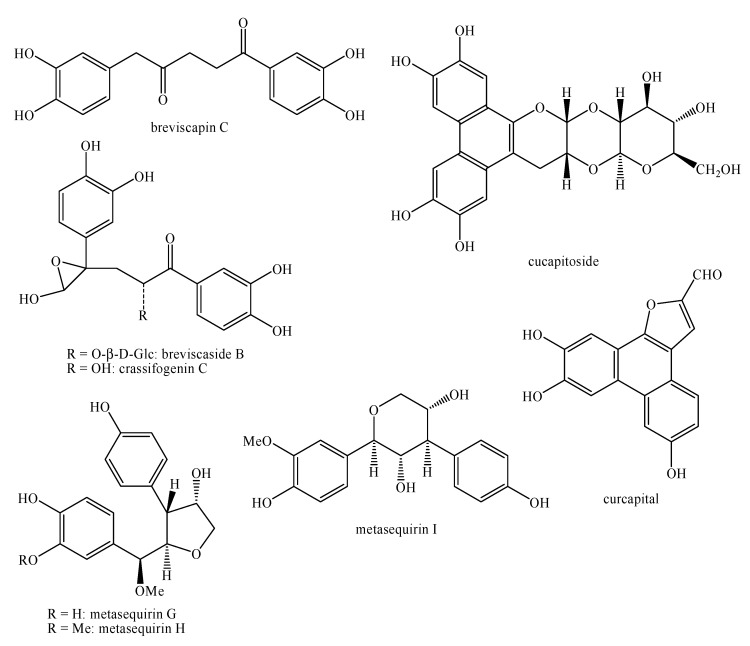
| Branches and stems | metasequirin G, metasequirin H, metasequirin I | SE, CC, LC, HPLC, [α]D, UV, IR, NMR, MS | [34] | ||
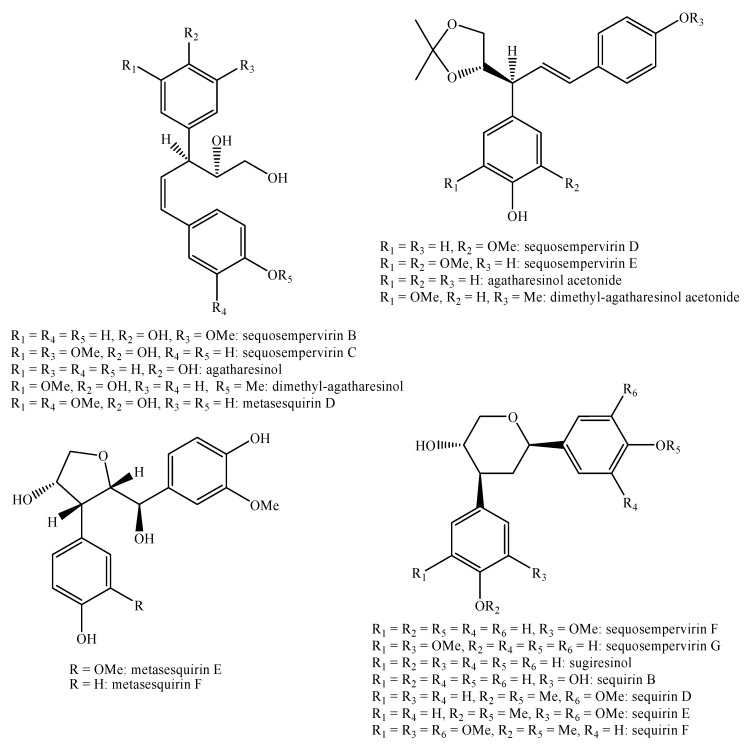
| Sequoia sempervirens (D.Don) Endl. | Branches and leaves | sequosempervirin B, sequosempervirin C, sequosempervirin D, sequosempervirin E, sequosempervirin F, sequosempervirin G, agatharesinol, agatharesinol acetonide, sugiresinol | SE, CC, [α]D, UV, NMR, MS | [35] | |
| Heartwood | sugiresinol, sequirin B, sequirin C, sequirin D, dimethyl-agatharesinol | SE, CC, LC, NMR, MS | [36] | ||
| Sequoiadendron giganteum (Lindl.) J.Buchholz | Heartwood | sequirin E, sequirin F, sequirin G, agatharesinol, dimethyl-agatharesinol, dimethyl-agatharesinol acetonide | SE, CC, TLC, NMR, MS | [36] | |
| Taxodium ascendens Brongn. | Leaves and branches | (2R,3R,4S,5S)-2,4-bis(4-hydroxyphenyl)-3,5-dihydroxy-tetrahydropyran, sequosemperverin B, agatharesinol, cryptoresinol | SE, CC, [α]D, IR, UV, NMR, MS | [37] | |
| Taxodium distichum var. imbricatum (Nutt.) Croom | Leaves and branches | taxodascendin, cryptoresinol, sequosempervirin B, agatharesinol | SE, CC, NMR, IR, UV, MS | [38] | |
| Hypericaceae | Hypericum chinense L. | Leaves | hyperione A, hyperione B | SE, CC, [α]D,IR, NMR, MS | [39] |
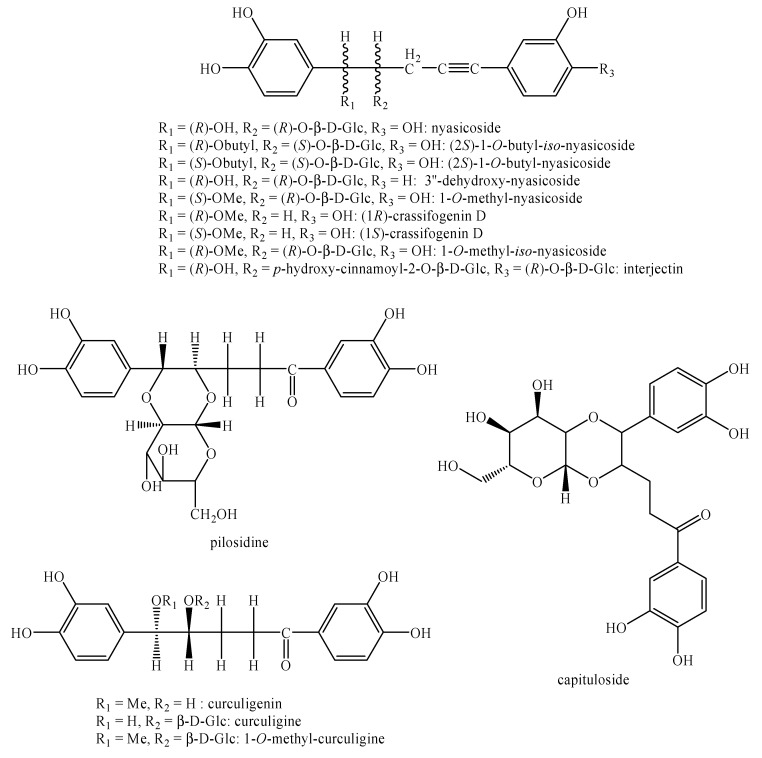
| Hypoxidaceae | Curculigo breviscapa S.C.Chen | Rhizomes | breviscapin C, breviscaside B, curcapital, capituloside, pilosidine, cucapitoside, crassifoside H, crassifoside F | SE, CC, LC, [α]D, IR, UV, NMR, MS | [40] |
| Curculigo capitulata (Lour.) Kuntze | Rhizomes | (2S)-1-O-butyl-iso-nyasicoside, (2S)-1-O-butyl-nyasicoside, nyasicoside, 3′′-dehydroxy-nyasicoside, 1-O-methyl-nyasicoside, curlignan | SE, CC, IR, UV, CD, NMR, MS | [41] | |
| capituloside, curculigenin, iso-curculigenin, curculigine, iso-curculigine, 1-O-methyl-curculigine, 1-O-methyl-iso-curculigine | SE, TLC, CC, IR, UV, NMR, MS | [42] | |||
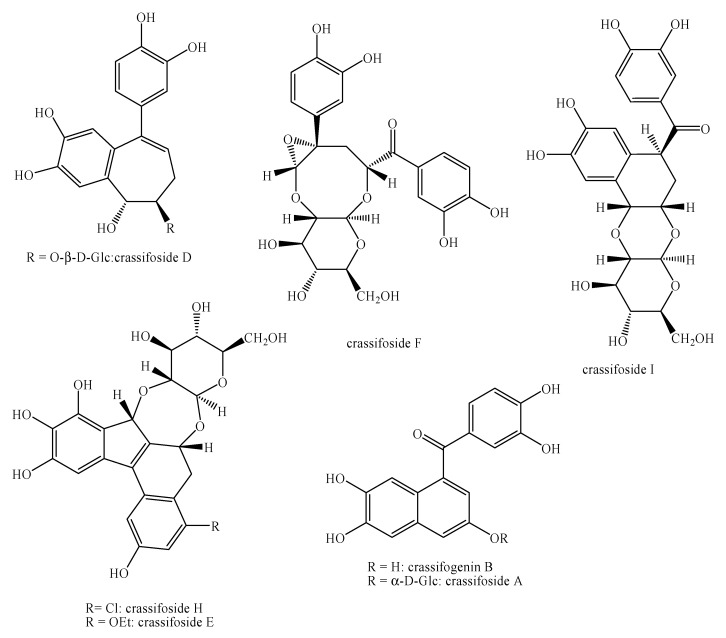
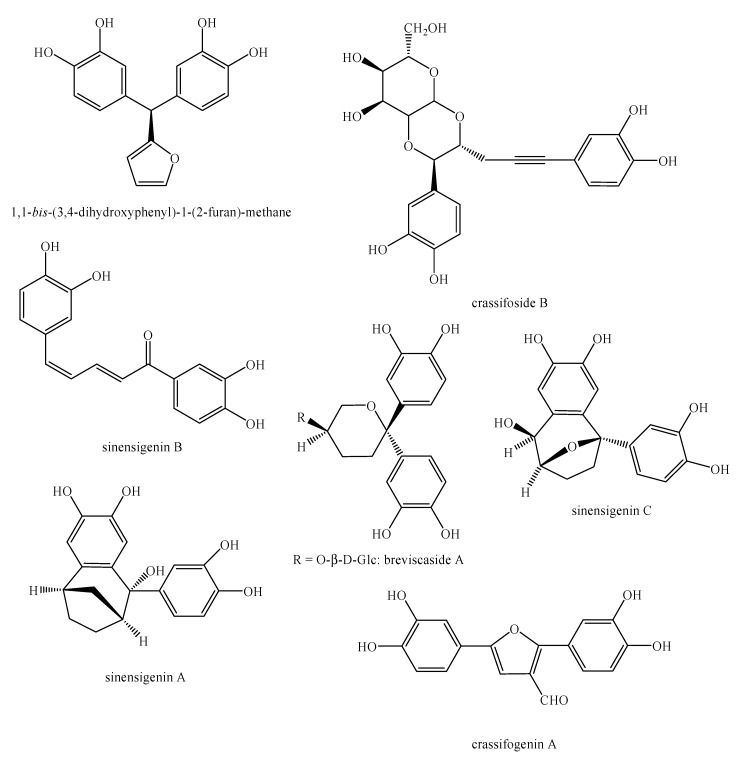
| crassifoside I, sinensigenin C, 1,1-bis-(3,4-dihydroxyphenyl)-1-(2-furan)-methane, crassifogenin B, crassifoside A, breviscaside A, crassifoside D, curcapital | SE, CC, [α]D, IR, UV, NMR, MS | [43] | |||
| Curculigo crassifolia (Baker) Hook.f. | Rhizomes | crassifogenin C, curcapital, crassifoside E, crassifoside F | SE, CC, IR, UV, NMR, MS | [44] | |
| 1-O-methyl-nyasicoside, 1-O-methyl-iso-nyasicoside, (1R)-crassifogenin D, (1S)-crassifogenin D | SE, CC, LC, [α]D, IR, UV, NMR, MS | [45] | |||
| crassifogenin A, crassifogenin B, crassifoside A, crassifoside B | SE, CC, [α]D, IR, UV, NMR, MS | [46] | |||
| Curculigo pilosa (Schumach. and Thonn.) Engl. | Rhizomes | nyasicoside, curculigine, pilosidine | SE, CC, [α]D, IR, UV, NMR, MS | [47,48] | |
| Curculigo recurvata W.T.Aiton | Rhizomes | curculigine, iso-curculigine, 1-O-methyl-curculigine, 1-O-methyl-iso-curculigine, nyasicoside | SE, CC, CE, CD, NMR, MS, | [49,50] | |
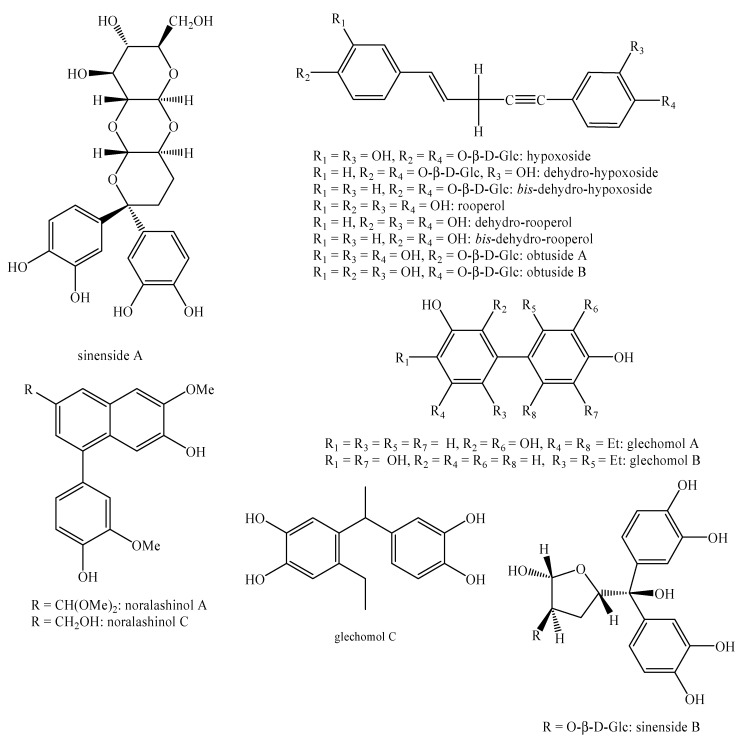
| Curculigo sinensis S.C.Chen | Rhizomes | sinensigenin A, sinensigenin B, crassifogenin B, cucapitoside, crassifoside B, crassifoside H, curculigine, iso-curculigine | SE, CC, LC, [α]D, IR, UV, NMR, MS | [51] | |
| sinenside A, sinenside B, crassifoside D, capituloside, 1-O-methyl-nyasicoside, 1-O-methyl-iso-nyasicoside, 1-O-methyl-curculigine, 1-O-methyl-iso-curculigine | SE, CC, [α]D, IR, UV, NMR, MS | [52] | |||
| Hypoxis angustifolia Lam. | Rhizomes | nyasol, hypoxoside, nyasosidenyaside, mononyasine A, mononyasine B | SE, CC, [α]D, IR, UV, NMR, MS | [53] | |
| Hypoxis hemerocallidea Fisch., C.A.Mey. and Avé-Lall. | Rhizomes | hypoxoside, dehydroxy-hypoxoside, bis-dehydroxy-hypoxoside, rooperol, dehydroxy-rooperol, bis-dehydroxy-rooperol | SE, HPLC, LC, UV, NMR, MS | [54] | |
| Hypoxis interjecta Nel | Rhizomes | interjectin | SE, CC, [α]D, IR, UV, NMR, MS | [55] | |
| Hypoxis multiceps Buchinger ex Baker | Rhizomes | interjectin | SE, CC, [α]D, IR, UV, NMR, MS | [55] | |
| Hypoxis nyasica Baker | Rhizomes | nyasicoside, mononyasine A, mononyasine B, nyaside, hypoxoside, nyasoside | SE, CC, [α]D, IR, UV, NMR, MS | [56,57,58] | |
| Hypoxis obtusa Burch. ex Ker Gawl. | Rhizomes | hypoxoside | SE, CC, NMR, MS | [59] | |
| rooperol, obtuside A, obtuside B | SE, CC, [α]D, IR, UV, NMR, MS | [60] | |||
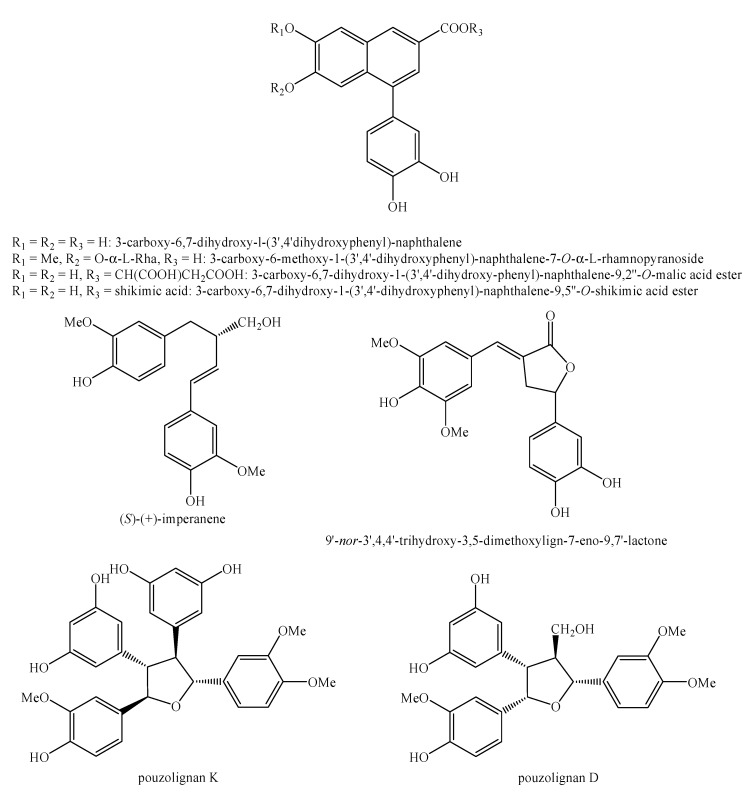
| Jungermanniaceae | Jungermannia exsertifolia Stephani | Whole plant | 3-carboxy-6,7-dihydroxy-l-(3′,4′dihydroxyphenyl)-naphthalene, 3-carboxy-6,7-dihydroxy-1-(3′,4′-dihydroxyphenyl)-naphthalene-9,5′′-O-shikimic acid ester | SE, CC, LC, HPLC, [α]D, NMR, MS | [61] |
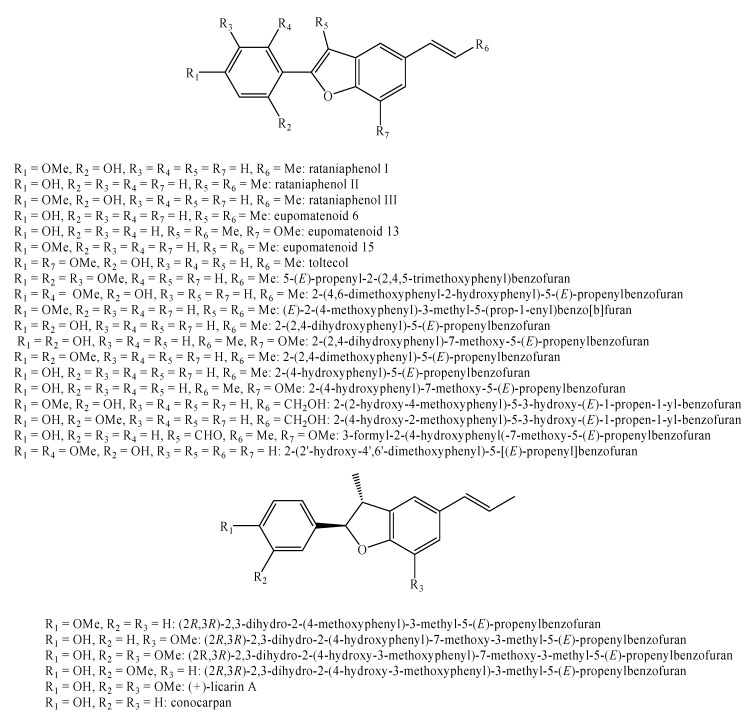
| Krameriaceae | Krameria cytisoides Cav. | Roots | (2R,3R)-2,3-dihydro-2-(4-hydroxy-3-methoxyphenyl)-3-methyl-5-(E)-propenylbenzofuran, (2R,3R)-2,3-dihydro-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-3-methyl-5-(E)-propenylbenzofuran, (2R,3R)-2,3-dihydro-2-(4-hydroxyphenyl)-7-methoxy-3-methyl-5-(E)-propenylbenzofuran, conocarpan, rataniaphenol II, eupomatenoid 13, 3-formyl-2-(4-hydroxyphenyl(-7-methoxy-5-(E)-propenylbenzofuran, 2-(2,4-dimethoxyphenyl)-5-(E)-propenylbenzofuran, rataniaphenol I, toltecol,2-(4-hydroxyphenyl)-7-methoxy-5-(E)-propenylbenzofuran, 2-(2,4-dihydroxyphenyl)-5-(E)-propenylbenzofuran, 2-(2,4-dihydroxyphenyl)-7-methoxy-5-(E)-propenylbenzofuran, olmecol,3,3′-didemethoxy-nectandrin B, 3′-demethoxy-nectandrin B | SE, CC, TLC, UV, IR, NMR, MS | [62] |
| Krameria grayi Rose and Painter | Roots | rataniaphenol I, eupomatenoid 6, 2-(2,4-dihydroxyphenyl)-5-(E)-propenylbenzofuran, (E)-2-(4-methoxyphenyl)-3-methyl-5-(prop-1-enyl)benzo[b]furan, rataniaphenol III, 2-(2,4-dimethoxyphenyl)-5-(E)-propenylbenzofuran, 2-(4-hydroxyphenyl)-5-(E)-propenylbenzofuran, 2-(4-hydroxy-2-methoxyphenyl)-5-3-hydroxy-(E)-1-propen-1-yl-benzofuran, 2-(2-hydroxy-4-methoxyphenyl)-5-3-hydroxy-(E)-1-propen-1-yl-benzofuran, (2R,3R)-2,3-dihydro-2-(4-methoxyphenyl)-3-methyl-5-(E)-propenylbenzofuran, (2R,3R)-2,3-dihydro-2-(4-hydroxyphenyl)-7-methoxy-3-methyl-5-(E)-propenylbenzofuran, (+)-licarin A, (2R,3R)-2,3-dihydro-2-(4-hydroxy-3-methoxyphenyl)-3-methyl-5-(E)-propenylbenzofuran, 4-(5-((R)-2-hydroxypropyl)-3-methylbenzofuran-2-yl)phenol | SE, CC, TLC, IR, UV, NMR, MS | [63] | |
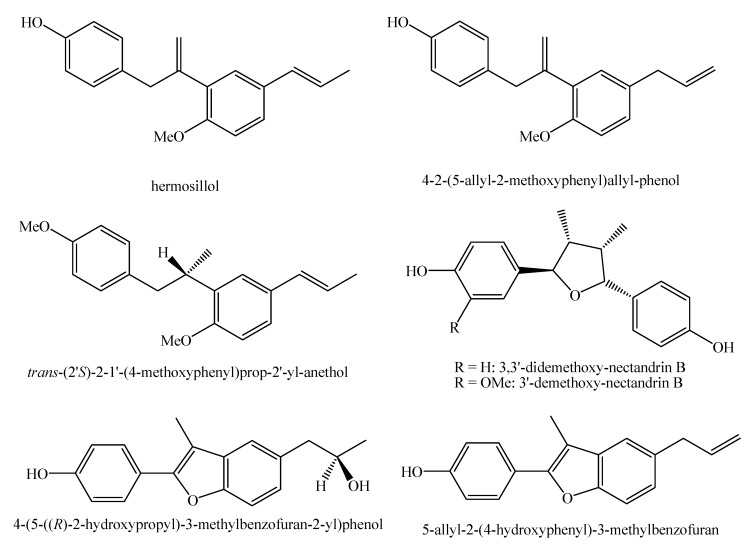
| Krameria ixine L. | Roots | conocarpan, ratanhiaphenol I, ratanhiaphenol II, 2-(4,6-dimethoxyphenyl-2-hydroxyphenyl)-5-(E)-propenylbenzofuran, 2-(4-hydroxyphenyl)-5-((E)-prop-2-en-1-yl)benzofuran, 2-(2,4-dihydroxyphenyl)-5-((E)-prop-2-en-1-yl)benzofuran, 5-(E)-propenyl-2-(2,4,5-trimethoxyphenyl)benzofuran, eupomatenoid15, 5-allyl-2-(4-hydroxyphenyl)-3-methylbenzofuran, hermosillol, 4-2-(5-allyl-2-methoxyphenyl)allyl-phenol, trans-(2′S)-2-1′-(4-methoxyphenyl)prop-2′-yl-anethol, 3,3′-didemethoxy-nectandrin B | SE, CC, TLC, [α]D, CD, UV, IR, NMR, MS | [64] | |
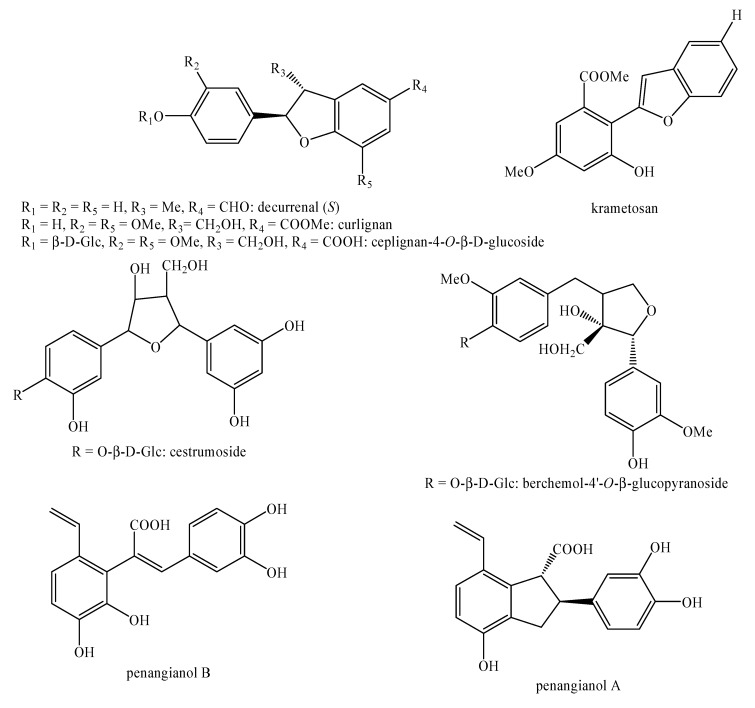
| Krameria tomentosa A. St.-Hil. | Roots | krametosan, ratanhiaphenol II,2-(2′-hydroxy-4′,6′-dimethoxyphenyl)-5-[(E)-propenyl]benzofuran, conocarpan, decurrenal (S) | SE, CC, [α]D, IR, NMR, MS | [65] | |
| Lamiaceae | Glechoma longituba (Nakai) Kuprian. | Whole plant | glechomol A, glechomol B, glechomol C | SE, CC, [α]D, IR, UV, NMR, MS | [66] |
| Tectona grandis L.f. | Leaves | balaphonin, tectonoelin A, tectonoelin B | SE, CC, HPLC, IR, NMR, MS | [67] | |
| Vitex negundo var. cannabifolia (Siebold and Zucc.) Hand.-Mazz. | Fruits | vitrofolal E, vitrofolal F | SE, CC, HPLC, NMR, MS | [68] | |
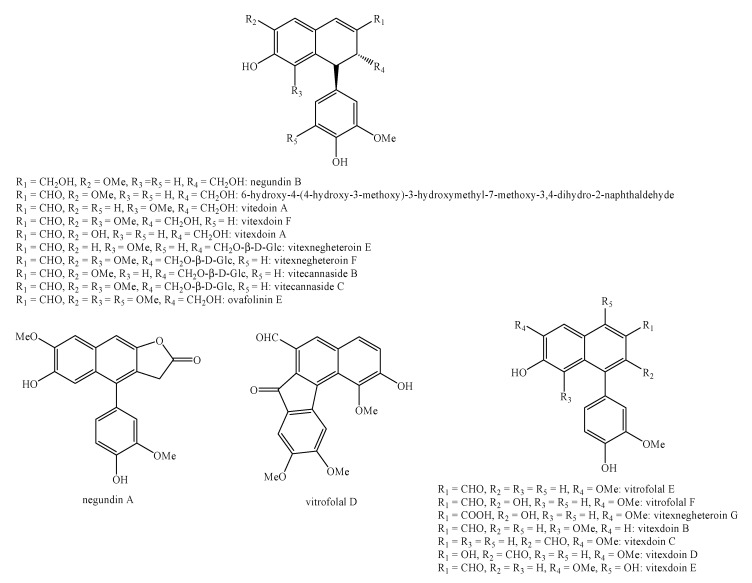
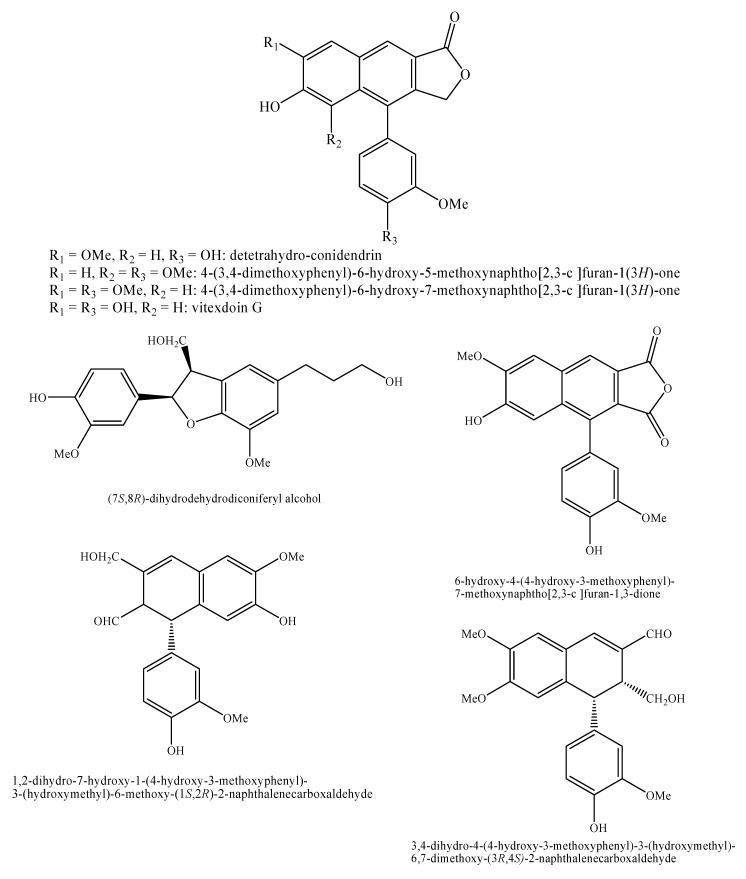
| Seeds | 6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde, vitexdoin A, vitexdoin E, vitexdoin C, vitexdoin D, vitexdoin B, vitexdoin F, vitrofolal A, vitrofolal B, vitrofolal E, vitrofolal F, negundin B, detetrahydro-conidendrin, vitedoin A, negundin B, 4-(3,4-dimethoxyphenyl)-6-hydroxy-5-methoxynaphtho[2,3-c ]furan-1(3H)-one, 4-(3,4-dimethoxyphenyl)-6-hydroxy-7-methoxynaphtho[2,3-c ]furan-1(3H)-one, 6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-7-methoxy-naphtho[2,3-c ]furan-1,3-dione, 1,2-dihydro-7-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-6-methoxy-(1S,2R)-2-naphthalenecarboxaldehyde,3,4-dihydro-4-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-6,7-dimethoxy-(3R,4S)-2-naphthalenecarboxaldehyde | SE, CC, LC, HPLC, [α]D, CD, NMR, MS | [69] | ||
| Vitex negundo L. | Roots | negundin A, negundin B, 6-hydroxy-4-(4-hydroxy-3-methoxy)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde, (+)-lyoniresinol,(+)-lyoniresinol 3a-O-β-glucopyranoside, vitrofolal E, vitrofolal F | SE, CC, TLC, IR, UV, NMR, MS | [70,71] | |
| negundin A, negundin B, 6-hydroxy-4-(4-hydroxy-3-methoxy)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde, (+)-lyoniresinol,(+)-lyoniresinol 3a-O-β-glucopyranoside, vitrofolal E | SE, CC, [α]D, IR, UV, NMR, MS | [72] | |||
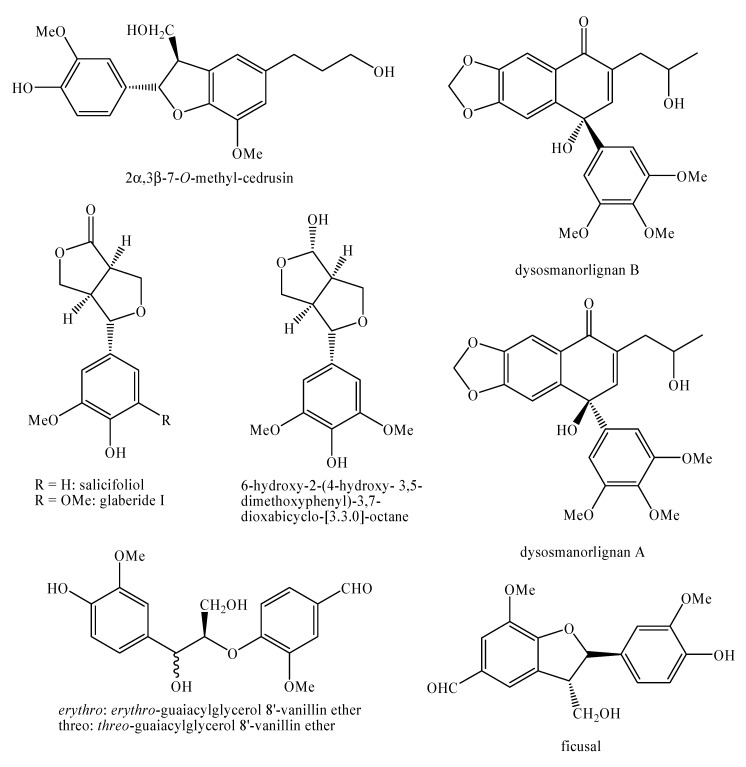
| Seeds | vitedoin A, 6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde, detetrahydro-conidendrin, vitrofolal E, vitrofolal F, 2α,3β-7-O-methyl-cedrusin | SE, CC, [α]D, NMR, MS | [73] | ||
| vitexnegheteroin E, vitexnegheteroin F, vitexnegheteroin G, vitecannaside B, 6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde, vitedoin A, vitexdoin A | SE, CC, [α]D, CD, UV, IR, NMR, MS | [74] | |||
| vitexdoin A, vitexdoin B, vitexdoin C, vitexdoin D, vitexdoin E, 6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde, vitrofolal E, vitrofolal F | SE, CC, LC, [α]D, CD, UV, IR, NMR, MS | [75] | |||
| Aerial parts | vitedoin A, 6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde, 2α,3β-7-O-methyl-cedrusin, vitexdoin F, vitexdoin A, (–)-lyoniresinol-3a-O-β-d-glucopyranoside, (+)-lyoniresinol-3a-O-β-d-glucopyranoside, vitecannaside B, ovafolinin E, (7S,8R)-dihydrodehydrodiconiferyl alcohol, vitecannaside C, vitexdoin G | SE, CC, LC, [α]D, UV, IR, NMR, MS | [76] | ||
| Vitex rotundifolia L.f. | Roots | vitrofolal A, vitrofolal B, vitrofolal C, vitrofolal D, vitrofolal E, vitrofolal F, detetrahydro-conidendrin, 4-(3,4-dimethoxyphenyl)-6-hydroxy-5-methoxynaphtho[2,3-c ]furan-1(3H)-one, 4-(3,4-dimethoxyphenyl)-6-hydroxy-7-methoxynaphtho[2,3-c ]furan-1(3H)-one | SE, CC, MP, UV, IR, NMR, MS | [77] | |
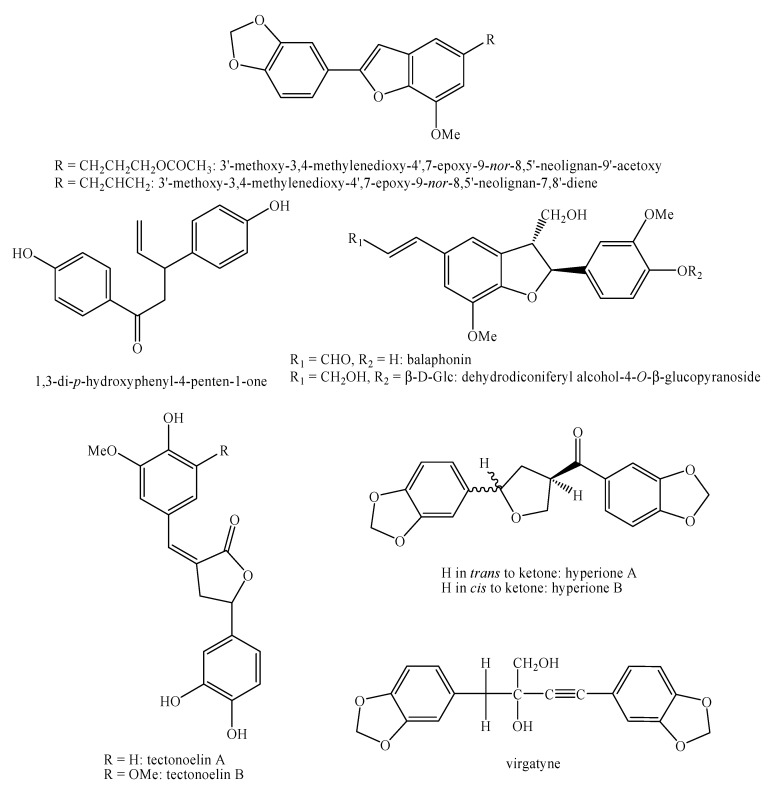
| Lauraceae | Nectandra lineata (Kunth) Rohwer | Young leaves | 3′-methoxy-3,4-methylenedioxy-4′,7-epoxy-9-nor-8,5′-neolignan-9′-acetoxy, 3′-methoxy-3,4-methylenedioxy-4′,7-epoxy-9-nor-8,5′-neolignan-7,8′-diene | SE, CC, IR, NMR, MS | [78] |
| Lepidoziaceae | Bazzania trilobata (L.) Gray | Whole plant | 3-carboxy-6,7-dihydroxy-l-(3′,4′dihydroxyphenyl)-naphthalene | SE, CC, HPLC, NMR, MS | [79] |
| Lepidozia incurvata Lindenb. | Whole plant | 3-carboxy-6,7-dihydroxy-l-(3′,4′dihydroxyphenyl)-naphthalene, 3-carboxy-6-methoxy-1-(3′,4′-dihydroxyphenyl)-naphthalene-7-O-α-L-rhamnopyranoside | SE, CC, LC, HPLC, [α]D, NMR, MS | [61] | |
| Lepidozia reptans (L.) Dumort. | n.a. | 3-carboxy-6,7-dihydroxy-l-(3′,4′dihydroxyphenyl)-naphthalene | n.a. | [80] | |
| Lophocoleaceae | Chiloscyphus polyanthos (L.) Corda | Whole plant | 3-carboxy-6,7-dihydroxy-l-(3′,4′dihydroxyphenyl)-naphthalene, 3-carboxy-6,7-dihydroxy-1-(3′,4′-dihydroxy-phenyl)-naphthalene-9,2′′-O-malic acid ester | SE, CC, LC, HPLC, [α]D, NMR, MS | [80] |
| Lythraceae | Sonneratia caseolaris (L.) Engl. | Fruits | nyasol, 4′-O-methyl-nyasol | SE, CC, TLC, NMR, MS | [81] |
| Sonneratia ovata Backer | Fruits | nyasol, 4′-O-methyl-nyasol | SE, CC, TLC, NMR, MS | [81] | |
| Trapa natans L. | Whole plant | nyasol | SE, CC, [α]D, IR, NMR, MS | [82] | |
| Magnoliaceae | Magnolia odora (Chun) Figlar and Noot. | Twigs | glaberide I, salicifoliol, 6-hydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-3,7-dioxabicyclo-[3.3.0]-octane, ficusal, erythro-guaiacylglycerol 8′-vanillin ether, threo-guaiacylglycerol 8′-vanillin ether | SE, CC, LC, HPLC, NMR, MS | [83] |
| Malvaceae | Urena lobata L. | Aerial parts | ceplignan-4-O-β-d-glucoside | SE, CC, [α]D, IR, UV, NMR, MS | [84] |
| Meliaceae | Aglaia cordata Hiern | Stem barks | aglacin H | SE, CC, HPLC, NMR, MS | [85] |
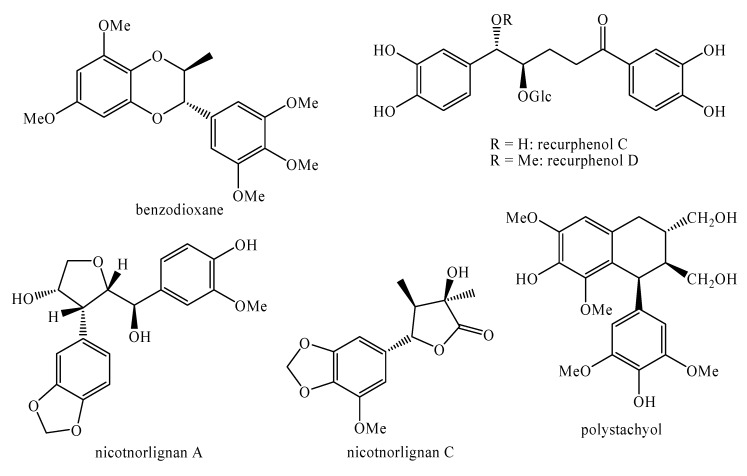
| Cedrela sinensis Juss. | Leaves | cedralin A, cedralin B | SE, IR, UV, NMR, MS | [86] | |
| Toona sinensis(Juss.) M.Roem. | Roots | toonin C | SE, CC, HPLC, [α]D, IR, NMR, MS | [87] | |
| Oleaceae | Syringa pinnatifolia Hemsl. | Stem barks | noralashinol A, vitrofolal E | SE, CC, UV, IR, NMR, MS | [88,89] |
| noralashinol B, noralashinol C | SE, CC, LC, [α]D, UV, IR, ECD, NMR, MS | [90] | |||
| Pelliaceae | Pellia epiphylla (L.) Corda | Gametophytes | 3-carboxy-6,7-dihydroxy-l-(3′,4′dihydroxyphenyl)-naphthalene | SE, CC, IR, NMR, MS | [91] |
| Phyllanthaceae | Phyllanthus virgatus G.Forst. | Whole plant | virgatyne | SE, CC, LC, [α]D, UV, NMR, MS | [92] |
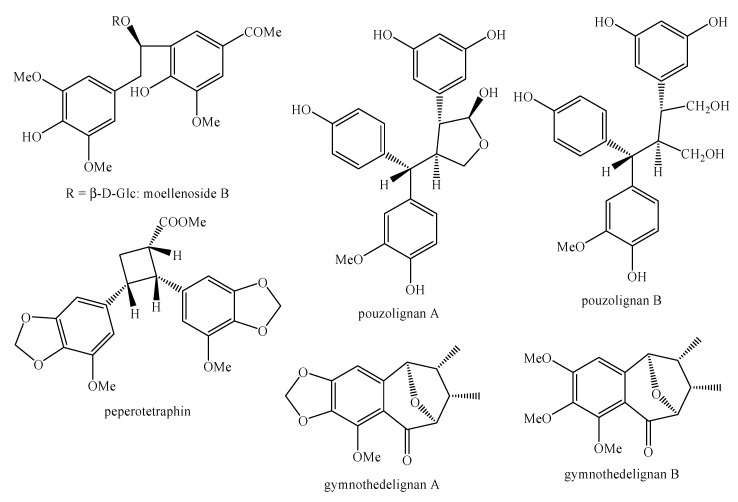
| Piperaceae | Peperomia tetraphylla (G.Forst.) Hook. and Arn. | Whole plant | methyl rel-(1R,2S,3S)-2-(7-methoxy-1,3-benzodioxol-5-yl)-3-(2,4,5-trimethoxyphenyl)-cyclobutane-carboxylate, methyl rel-(1R,2R,3S)-2-(7-methoxy-1,3-benzodioxol-5-yl)-3-(2,4,5-trimethoxyphenyl)-cyclobutane-carboxylate | SE, CC, LC, [α]D, UV, IR, CD, NMR, MS | [93] |
| peperotetraphin | SE, CC, LC, [α]D, UV, IR, NMR, MS | [94] | |||
| Piper obliquum Ruiz and Pav. | Leaves | justiflorinol | SE, CC, [α]D, UV, IR, NMR, MS | [95] | |
| Poaceae | Imperata cilindrica (L.) Raeusch. | Rhizomes | (S)-(+)-imperanene | SE, CC, [α]D, NMR, MS | [96] |
| Saururaceae | Gymnotheca chinensis Decne. | Whole plant | gymnothedelignan A, gymnothedelignan B | SE, CC, X-ray, NMR, MS | [97] |
| Selaginellaceae | Selaginella moellendorffii Hieron. | Whole plant | moellenoside B | SE, CC, LC, TLC, [α]D, CD, UV, IR, NMR, MS | [98] |
| Schisandraceae | Schisandra bicolor W.C.Cheng. | Fruits | marphenol C, marphenol D, marphenol E, marphenol F | SE, CC, LC, HPLC, [α]D, UV, IR, NMR, MS | [99] |
| Solanaceae | Cestrum diurnum L. | Leaves | cestrumoside, berchemol-4′-O-β-glucopyranoside, dehydrodiconiferyl alcohol-4-O-β-glucopyranoside, (+)-lyoniresinol 3a-O-β-glucopyranoside, (–)-lyoniresinol 3a-O-β-glucopyranoside | SE, CC, [α]D, UV, CD, IR, NMR, MS | [100] |
| Cestrum parqui (Lam.) L’Hér. | Leaves | 9′-nor-3′,4,4′-trihydroxy-3,5-dimethoxylign-7-eno-9,7′-lactone | SE, CC, [α]D, NMR, MS | [101] | |
| Nicotiana tabacum L. | Roots and stems | nicotnorlignan C, recurphenol C, recurphenol D, sequirin C, benzodioxane | n.r. | [102,103] | |
| Leaves | nicotnorlignan A, sequirin C, benzodioxane | n.r. | [102] | ||
| Solanum melongena L. | Roots | guaiacylglycerol 8′-vanillin ether, ficusal, polystachyol | SE, CC, HPLC, [α]D, NMR, MS | [104] | |
| Styracaceae | Styrax camporum Pohl | Whole plant | egonol, homoegonol | SE, pTLC, CC, HPLC-UV, NMR | [105] |
| Styrax ferrugineus Nees and Mart. | Leaves | egonol, homoegonol, egonol glucoside, homoegonol glucoside | SE, FCC, IR, NMR, MS | [106] | |
| Styrax japonica Sieb. et Zucc. | Stem bark | styraxlignolide A, egonol, masutakeside I | SE, CC, LC, [α]D, UV, NMR, MS | [107] | |
| Styrax obassis Sieboldi and Zucc. | Aerial parts | 1′′-hydroxylegonol gentiobioside, egonol glucoside | SE, CC, LC, NMR, MS | [108] | |
| Styrax officinalis L. | Fruits | egonol, dimethyl-egonol, homoegonol | SE, CC, NMR, MS | [109] | |
| Styrax pohlii A. DC. | Aerial parts | egonol, homoegonol, homoegonol gentiobioside, homoegonol glucoside, egonol gentiobioside | SE, CC, HPLC, NMR | [110] | |
| Styrax ramirezii Greenm. | Fruits | egonol, homoegonol, egonol glucoside, homoegonol glucoside, 7-demethoxy-egonol, 4-O-demethyl-homoegonol | SE, HPLC-DAD-MS | [111] | |
| Thelypteridaceae | Abacopteris penangiana (Hook.) Ching | Rhizomes | penangianol A, penangianol B | SE, CC, [α]D, UV, IR, NMR, MS | [112] |
| Urticaceae | Pouzolzia occidentalis (Liebm.) Wedd. | Aerial parts | pouzolignan A, pouzolignan B | SE, CC, LC, [α]D, UV, IR, NMR, MS | [113] |
| Pouzolzia zeylanica var. microphylla (Wedd.) Masam. | Aerial parts | pouzolignan D, pouzolignan K | n.a. | [114] |
Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18, Figure 19, Figure 20, Figure 21, Figure 22, Figure 23 and Figure 24 below show the structures of all the identified nor-lignans.
Figure 2.
The isolated nor-lignans in the plant kingdom—part 1.
Figure 3.
Isolated nor-lignans in the plant kingdom—part 2.
Figure 4.
Isolated nor-lignans in the plant kingdom—part 3.
Figure 5.
Isolated nor-lignans in the plant kingdom—part 4.
Figure 6.
Isolated nor-lignans in the plant kingdom—part 5.
Figure 7.
Isolated nor-lignans in the plant kingdom—part 6.
Figure 8.
Isolated nor-lignans in the plant kingdom—part 7.
Figure 9.
Isolated nor-lignans in the plant kingdom—part 8.
Figure 10.
Isolated nor-lignans in the plant kingdom—part 9.
Figure 11.
Isolated nor-lignans in the plant kingdom—part 10.
Figure 12.
Isolated nor-lignans in the plant kingdom—part 11.
Figure 13.
Isolated nor-lignans in the plant kingdom—part 12.
Figure 14.
Isolated nor-lignans in the plant kingdom—part 13.
Figure 15.
Isolated nor-lignans in the plant kingdom—part 14.
Figure 16.
Isolated nor-lignans in the plant kingdom—part 15.
Figure 17.
Isolated nor-lignans in the plant kingdom—part 16.
Figure 18.
Isolated nor-lignans in the plant kingdom—part 17.
Figure 19.
Isolated nor-lignans in the plant kingdom—part 18.
Figure 20.
Isolated nor-lignans in the plant kingdom—part 19.
Figure 21.
Isolated nor-lignans in the plant kingdom—part 20.
Figure 22.
Isolated nor-lignans in the plant kingdom—part 21.
Figure 23.
Isolated nor-lignans in the plant kingdom—part 22.
Figure 24.
Isolated nor-lignans in the plant kingdom—part 23.
3. Chemotaxonomy
As Table 1 clearly shows, nor-lignans have been recognized as phytochemical constituents of several families, even chemosystematically far away from each other.
This is in accordance with the easy phytochemical pathway connected with very common PhC3 intermediate metabolites. However, the rearrangements following the junction of the two originating moieties are another matter.
Therefore, some specific compounds can be evidenced as chemotaxonomic makers at every classification level.
In particular, (+)-acortatarinowins A-C, (−)-acortatarinowins A-C (Figure 8) and acorusin B (Figure 15) may be useful chemotaxonomic markers for the species Acorus tatarinowii Schott. since they have been isolated only from that species [5,6].
Pachypostaudins A-B and pachypophyllin (Figure 16 and Figure 17) may be chemotaxonomic markers for the entire Annonaceae family given their specific occurrence here [7,8].
Asparenydiol (Figure 17) and its derivatives are considered as some of the chemotaxonomic markers for the genus Asparagus L. [17].
Capituloside (Figure 4) and the crassifosides (Figure 10 and Figure 11) may be used as chemotaxonomic markers for the genus Curculigo Gaertn. given their occurrence limited to only it [40,43,44,46,51,52].
For the same reason, hypoxoside and related compounds (Figure 12) are a possible chemotaxonomic marker for the genera Hypoxis L. and Curculigo Gaertner [54,56,59] whereas rataniaphenols I-II (Figure 22) may serve as chemotaxonomic markers for the genus Krameria L. [62,63,64].
Within the Lamiaceae family, surely negundins A–B (Figure 20) are chemotaxonomic markers for the species Vitex negundo L given their occurrence in several exemplars of this species [69,70,71].
Indeed, egonol, homoegonol and their derivatives (Figure 3) can serve as chemotaxonomic markers for the Styrax L. genus since their occurrence is quite limited to it [105,106,107,108,109,110,111].
4. Biological Activities
Nor-lignans show several interesting biological activities, i.e., antioxidant, antifungal, antibacterial, antiallergic, antiasthma, analgesic, anticomplement, antiatherogenic, antiparasitic, vascular, antistress, anti-inflammatory, cytotoxic, phytotoxic, inhibitory of enzymes, proteins and platelet aggregation. In the following pages, these are characterized one by one.
4.1. Antioxidant
Egonol (Figure 3) highly inhibits the production of NO and highly reduces the release of ROS in a dose dependent manner. The same is valid for homoegonol but in a minor way [111].
Indeed, curcapital, crassifogenin C (Figure 9), crassifoside E and crassifoside F (Figure 10) show strong radical scavenging activity by the 1,1-diphenyl-2-picrylhydrazyl (DPPH•) assay with IC50 values equal to 7.76, 13.48, 15.54 and 17.07 μM, respectively, which are much higher than the control, L-ascorbic acid (IC50 = 27.59 μM) [44].
Moreover, hypoxoside and rooperol (Figure 12) show high effects towards the inhibition of lipid peroxidation withIC50 values equal to 12.6 and 2.6 μM, respectively [54].
Nyasol (Figure 7) exerts medium effects against ABTS•+ cation and superoxide anion radicals with IC50 values equal to 45.6 and 40.5 μM, respectively [82].
Vitexdoin F, vitedoin A, 6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde, vitexdoin A, negundin B, vitexdoin E, vitrofolal F, 1,2-dihydro-7-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-6-methoxy-(1S,2R)-2-naphthalenecarboxaldehyde, vitexdoin C, vitexdoin D, vitrofolal E, vitexdoin B, vitrofolal A and detetrahydro-conidendrin (Figure 20) showed stronger effects than ascorbic acid [69,73].
4.2. Antiradical
Vitrofolal E, vitrofolal F, viteodin A, 6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde (Figure 20), detetrahydro-conidendrin (Figure 23) and 2α,3β-7-O-methyl-cedrusin (Figure 21) exert high effects against the stable free radical, 1,1-diphenyl-2-picrylhydrazyl (DPPH•), more than L-cysteine and, in most cases, similar to α-tocopherol [73].
Vitexnegheteroin E, vitexnegheteroin F, vitexnegheteroin G, vitecannaside B and vitexdoin A (Figure 20) also exhibit strong effects in the ABTS•+ assay with IC50 values lower than 3.20 μM [74].
Vitexdoin A, vitexdoin B, vitexdoin C, vitexdoin D, vitexdoin E, vitrofolal E and vitrofolal F (Figure 20) are potent NO production inhibitors with IC50 values equal to 0.38 μM, 0.20 μM, 0.57 μM, 0.13 μM, 0.15 μM, 0.50 μM and 0.11 μM, respectively.
Instead, 6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-7-methoxy-3,4-dihydro- 2-naphthaldehyde (Figure 20) has a weaker effect with an IC50 value equal to 3.54 μM. Anyway, they were all more powerful than the positive control L-nitroarginine (IC50= 43.6 μM) [75].
4.3. Antifungal and Antibacterial
Homoegonol and egonol (Figure 3) exhibit strong effects against Candida albicans, Cladosporium sphaerospermum and Staphylococcus aureus with MIC values equal to 10, 5 and 10 μg/mL, respectively for the former compound, and 12, 10 and 10 μg/mL respectively for the latter compound. Indeed, egonol (Figure 3) and homoegonol (Figure 3) exhibit lower effects only against Candida albicans and Staphylococcus aureus with MIC values equal to 15 and 15 μg/mL, respectively for the former compound and 20 and 20 μg/mL, respectively for the latter compound [106].
Conversely, homoegonol (Figure 3) is totally inactive against Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenza, Pseudomonas aeruginosa and Klebsiella pneumonia showing MIC values higher than 400 μg/mL. Instead, egonol (Figure 3) is weakly active only against Streptococcus pneumonia showing a MIC value equal to 400 μg/mL [115].
Iso-agatharesinol and gobicusin A (Figure 7) are also able to exert these effects. In particular, gobicusin A is a better antibacterial compound against Escherichia coli and Staphylococcus aureus than iso-agatharesinol given its MIC values (0.12 and 0.05 mg/mL vs. 0.25 and 0.12 mg/mL, respectively) and its efficacy is extremely comparable to streptomycin especially against Staphylococcus aureus (MIC = 0.01 mg/mL) [19].
Nyasol (Figure 7) is able to inhibit the mycelial growth of Colletotrichum orbiculare, Phytophthora capsici, Pythium ultimum, Rhizoctonia solani and Cladosporium cucumerinum in a MIC range comprised between 1 and 50mg/mL [13]. Moreover, it potently inhibits the growth of Leishmania major with an IC50 value equal to 12 μM and moderately inhibits Plasmodium falciparum with an IC50 value equal to 49 μM [14].
Vitrofolal C (Figure 3), vitrofolal D, vitrofolal E (Figure 20) and detetrahydro-conidendrin (Figure 23) have good activity against methicillin-resistant Staphylococcus aureus with a MIC value below 64 μg/mL [77].
4.4. Antiviral
Nicotnorlignan A, benzodioxane (Figure 19) and sequirin C (Figure 7) showed high effects against HIV-1 with IC50 values equal to 3.15, 7.62 and 9.56 μM, respectively [103].
Moreover, nicotnorlignan C, recurphenol C, recurphenol D, benzodioxane (Figure 19) and sequirin C (Figure 7) and possess moderate activity against the anti-tobacco mosaic virus with inhibition rates equal to 14.7%, 22.5%, 23.4%, 21.4% and 17.6% respectively [103]. Nicotnorlignan A (Figure 19) also shows similar effects [103].
4.5. Anti-Allergic
Nyasol and 4′-O-methyl-nyasol (Figure 7) exert good effects with IC50 values equal to 2.06 and 1.89 μM, respectively. These values are extremely compatible with that of DSCG (IC50 = 1.78 μM), a very common antiallergic compound used in pharmacy [10].
4.6. Antiasthma
Homoegonol (Figure 3) is the only compound able to exert antiasthma effects by a complex mechanism of action composed by several paths [116]. The most important of these is that this compound is able to reduce the expression of the protease MMP-9 in the lung tissue and the presence of this protease greatly increases the asthmatic effect [117].
4.7. Analgesic
Hypoxoside (Figure 12) does not display any effect on the locomotor activity in mice but exerts a high analgesic effect even at low doses (5 mg/kg) probably via an anti-inflammatory mechanism [59].
4.8. Anticomplement
Styraxlignolide A, egonol and masutakeside I (Figure 3) show a strong effect with IC50 values equal to 123, 33 and 166 μM, respectively. This activity, in the case of egonol (Figure 3), is much higher than the control, i.e., rosmarinic acid, which shows an IC50 value equal to 182 μM [107].
4.9. Antiatherogenic
Nyasol (Figure 7) is able to act as inhibitor against LDL-oxidation with an IC50 value equal to 5.6 μM, which is very similar to that of probucol (IC50 = 2.0 μM), the typical compound uses for these purposes. Indeed, it exerts extremely weak inhibitory effects against hACAT1, hACAT2 (cholesterol acyltransferases) and Lp-PLA2 (lipoprotein-associated phospholipase A2) with IC50 values equal to 280.6, 398.9 and 284.7 μM, respectively [82].
4.10. Antiparasitic
3′-methoxy-3,4-methylenedioxy-4′,7-epoxy-9-nor-8,5′-neolignan-9′-acetoxy (Figure 5) has a medium effect against Trypanosoma cruzi with an IC50 value equal to 111 μM whereas 3′-methoxy-3,4-methylenedioxy-4′,7-epoxy-9-nor-8,5′-neolignan-7,8′-diene (Figure 5) is a good compound in this context with an IC50 value equal to 60 μM [78].
4.11. Vascular
Pilosidine, nyasicoside and curculigine (Figure 4), in low doses ranging from 1 to 30 mM, are able to induce a reversible facilitating effect on adrenaline evoked contractions [47]. Moreover, they all have a dose dependent vasoconstricting effect on rabbit aorta strips [48]. Their mechanism of action involves an interaction with the peripheral adrenergic system, in particular with α1 and β1 adrenoceptors [48].
(2S)-1-O-butyl-nyasicoside and nyasicoside (Figure 4) possess high effects against the ouabain-induced arrhythmia in the heart preparations of guinea pig at the doses of 3 μM, especially at the left atrium level.
(2S)-1-O-butyl-nyasicoside (Figure 4) has the same effect but in minor extent [41].
Lastly, 2-(2′-hydroxy-4′,6′-dimethoxyphenyl)-5-[(E)-propenyl]benzofuran (Figure 22) inhibits the vasodilatory effect produced by acetylcholine with an IC50 value equal to 31.2 μM. This effect is concentration-dependent. Moreover, the compound inhibits basal nitric oxide production [118].
4.12. Antistress
Negundin A (Figure 20) shows a very good effect in mice by greatly decreasing the number of writhes at the dose of 25 mg/kg. Moreover, it is able to reduce the blood glucose level and serum cholesterol level but at higher doses (50 and 100 mg/kg) [119].
4.13. Anti-Inflammatory
Egonol, homoegonol, homoegonol gentiobioside, homoegonol glucoside and egonol gentiobioside (Figure 3) were found to exert weak or medium effects against COX-1 and COX-2 with percentages of inhibition ranging from 1.3 for homoegonol gentiobioside against COX-1 to 35.7 of homoegonol glucoside against COX-1 at the concentration of 30 mM [110]. Yet, egonol (Figure 7) is able to reduce the mRNA expression levels of inducible nitric oxide synthase (iNOS), COX-2, interleukin-1β (IL-1β) and interleukin-6 (IL-6). The same effect was observed also for homoegonol (Figure 7) but in a minor extent [111].
Lastly, nyasol and 5-((S,Z)-1-(4-hydroxyphenyl)penta-1,4-dien-3-yl)-2,3-dimethoxyphenol (Figure 7) show high effects. In particular, nyasol is able to inhibit microsomal cells by 100% as well as COX-1 while it inhibits COX-2 by 19%. Conversely, 5-((S,Z)-1-(4-hydroxyphenyl)penta-1,4-dien-3-yl)-2,3-dimethoxyphenol (Figure 7) inhibits microsomal cells by 72% and COX-2 by 23% [21].
4.14. Cytotoxic
3′-methoxy-nyasin and nyasol (Figure 7) possess moderate effects against HO-8910 (human ovarian carcinoma) and Bel-7402 (human hepatoma) cell lines. In particular, the former compound shows IC50 values equal to 84.0 and 26.2 μM, respectively whereas the latter compound shows IC50 values equal to 30.6 and 29.4 μM, respectively [18]. Nyasol and 4′-O-methyl-nyasol (Figure 7) exert moderate effects against the rat glioma C-6 cell line with IC50 values equal to 19.02 and 20.21 mg/mL, respectively [81]. Nyasol (Figure 7) is also able to inhibit the basic fibroblast growth factor (bFGF) and the vascular endothelial growth factor (VEGF)-induced endothelial cell proliferation [11]. The mechanism of action is related to its strong estrogen receptor binding ability [11]. In addition, nyasol (Figure 7) has medium effects against the human HL60 cancer cell line with IC50 value equal to 15.5 μM [27]. Moreover, nyasol, 4′-O-methyl-nyasol and 3′′-methoxy-nyasol (Figure 7) have a modest effect on the inhibition of β-hexosaminidase release in RBL-2H3 cells stimulated by DNP-BSA with IC50 values ranging from 18.08 μM for the latter to 52.67 μM for the second compound. These values are higher than the control compound ketotifen, which owns an IC50 value equal to 10.12 μM. Conversely, 3′′-hydroxy-4′′-methoxy-4′′-dehydroxy-nyasol (Figure 7) is more efficient than the control displaying an IC50 value equal to 2.85 μM [12].
Egonol and homoegonol (Figure 3) exhibit medium effects against B16F10 (murine melanoma), MCF-7 (human breast adenocarcinoma), HepG2 (human hepatocellular liver carcinoma), HeLa (human cervical adenocarcinoma) and MO59J (human glioblastoma) cell lines. These effects were observed to be higher with the passing of time reaching their peaks after 72 h. Anyway, they were not better than the controls doxorubicin, camptotechin and etoposide [105]. For what concerns egonol (Figure 3), the results for MCF-7 and HeLa were confirmed in another study and it was also observed that it is active against theHL-60 (human leukemia) cell line with an IC50 value equal to 47.8 μM [108].
Agatharesinol acetonide (Figure 1) exhibits strong effects on the A549 cell line (non-small-cell lung cancer) with an IC50 value equal to 27.1 μM, quite higher than taxol 33.72 μM [35].
Sequirin C (Figure 7) exerts good effects against the HL-60 cell line with an IC50 value of 5.5 μM, which is comparable to that of cisplatin (2.0 μM) [33].
Cedralin A (Figure 6) has weak activities against the HL-60 and K562 (myelogenous leukemia) cell lines with IC50 values equal to 26.2 and 22.4 mg/mL, respectively [86].
Methyl rel-(1R,2S,3S)-2-(7-methoxy-1,3-benzodioxol-5-yl)-3-(2,4,5-trimethoxyphenyl)-cyclobutane -carboxylate and methyl rel-(1R,2R,3S)-2-(7-methoxy-1,3-benzodioxol-5-yl)-3- (2,4,5-trimethoxyphenyl)-cyclobutane-carboxylate (Figure 6) exert modest effects against the HepG2, A549 and HeLa cell lines with IC50 values equal to 38.0, 56.4 and 64.9 μM for the former in corresponding order, and 42.4, 66.3 and 77.7 μM for the latter in corresponding order [52].
Noralashinol B (Figure 15) exhibits a weak activity against the HepG2 cancer cell line with an IC50 value equal to 31.7 μM, which is higher than the positive control, methotrexate showing an IC50 value equal to 15.8 μM [90]. Its mechanism of action is via apoptosis [90].
Metasequirin G, metasequirin H and metasequirin I (Figure 9) possess low cytotoxic effects against the A549 cell line with IC50 values close to 100 μM [34].
Chamaecypanone C (Figure 15) exerts potent effects against KB (human oral epidermoid carcinoma), HONE-1 (human nasopharyngeal carcinoma) and TSGH (human gastric carcinoma) cell lines with IC50 values equal to 0.19,0.24 and 0.52 μM, respectively [28].
Acorusin B (Figure 15) exert moderate effects against the CI-H1650 (non-small cell lung carcinoma), HepG2, BGC 823 (human stomach carcinoma), HCT-116 (human colon carcinoma) and MCF-7 cancer cell lines with IC50 values equal to 6.51, 4.80, 7.23, 8.81, 3.58 and 0.52 μM, respectively [6].
Yateresinol (Figure 17) is a decent cytotoxic compound against the human HL60 and Hepa G2 cancer cell lines with IC50 values higher than 20 μM [27].
Vitedoin A (Figure 20) exerts moderate effects against HCT116 cell lines with an IC50 value equal to 10.18 μM [74].
6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde (Figure 20) shows high effects against HepG2 cell lines with an IC50 value equal to 8.24 μM, which is comparable to doxorubicin (IC50 = 6.49 μM) [74].
4.15. Phytotoxic
9′-nor-3′,4,4′-trihydroxy-3,5-dimethoxylign-7-eno-9,7′-lactone (Figure 18) is a phytotoxic compound against Lactuca sativa L. (lettuce) and Lycopersicon esculentum Mill. (tomato) preventing their development [101]. Moreover, only in the case of L. esculentum, it inhibits the shoot length [101].
4.16. Inhibition on Enzymes, Proteins and Platelet Aggregation
Negundin A, negundin B, 6-hydroxy-4-(4-hydroxy-3-methoxy)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde, vitrofolal E (Figure 20) and (+)-lyoniresinol (Figure 15) showed medium effects against tyrosinase with IC50 values equal to 10.06, 6.72, 7.81, 9.76 and 3.21 μM which are, anyway, higher than kojic acid (IC50 = 16.67μM) [71].
Indeed, negundin B (Figure 20) has potent effect against lipoxygenase with an IC50 value equal to 6.25 μM [70].
Vitrofolal E (Figure 20) shows also moderate effects against butyryl-cholinesterase with an IC50 value equal to 35.0 μM [70].
6-hydroxy-4-(4-hydroxy-3-methoxy)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde and vitrofolal E (Figure 20) have modest α-chymotrypsin (serine protease) competitive inhibitory effects with Ki values equal to 31.75 and 47.11 μM, respectively [72].
Cestrumoside (Figure 14) is a strong protein kinase C inhibitor in an animal food additive [120].
Lastly, (S)-(+)-imperanene (Figure 18) strongly inhibits tyronase isolated from HMV-II cells with an IC50 value equal to 1.85 mM [121]. Its mechanism of action is essentially identical to that of arbutin [121]. Moreover it shows a high effect in rabbits giving a complete inhibition at the concentration of 6 × 10−4 M when the platelet aggregation is induced by thrombin [96].
5. Conclusions
Nor-lignans have proven to be quite present in the plant kingdom. Nevertheless, some of them can be even considered to be chemotaxonomic markers. In addition, they are endowed with a vast number of biological activities with a myriad of possible application in several medicinal and pharmacological fields. Yet, not all the nor-lignans have been studied and discovered at the present. This review article means to be a first step towards the understanding of how important nor-lignans are as well as to be an incentive to continue their research and study.
Abbreviations
[α]D: Optical Rotation Spectroscopy; ECD: Electronic Circular Dichroism Spectroscopy; CC: Column Chromatography; CD: Circular Dichroism Spectroscopy; FCC: Flash Column Chromatography; HPLC: High Performance Liquid Chromatography; IM: Immunohistochemistry Methods; IR: Infrared Spectroscopy; LC: Liquid Chromatography; MP: Melting Point; MS: Mass Spectrometry; NMR: Nuclear Magnetic Resonance Spectroscopy; n.a.: not accessible; n.r.: not reported; pTLC: Performance Thin Layer Chromatography; SE: solvent extraction; TLC: Thin Layer Chromatography; UV: Ultra-Violet Spectroscopy.
Author Contributions
M.N., conceptualization; M.G., M.N., M.S., A.B., supervision; all the authors, writing, reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ayres D.C., Loike J.D. Lignans: Chemical, Biological and Clinical Properties. 1st ed. Cambridge University Press; Cambridge, UK: 1990. [Google Scholar]
- 2.Ward R.S. Lignans Neolignans, and Related Compounds. Nat. Prod. Rep. 1993;10:1–28. doi: 10.1039/np9931000001. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki S., Umezawa T. Biosynthesis of lignans and norlignans. J. Wood Sci. 2007;53:273–284. doi: 10.1007/s10086-007-0892-x. [DOI] [Google Scholar]
- 4.Susplugas S., Van Hung N., Bignon J., Thoison O., Kruczynski A., Sévenet T., Guéritte F. Cytotoxic arylnaphthalene lignans from a Vietnamese Acanthaceae, Justicia patentiflora. J. Nat. Prod. 2005;68:734–738. doi: 10.1021/np050028u. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y., Xue Y., Liu J., Yao G., Li D., Sun B., Zhang J., Liu Y., Qi C., Xiang M., et al. (±)-Acortatarinowins A–F, Norlignan, Neolignan, and Lignan Enantiomers from Acorus tatarinowii. J. Nat. Prod. 2015;78:2205–2214. doi: 10.1021/acs.jnatprod.5b00328. [DOI] [PubMed] [Google Scholar]
- 6.Ni G., Shi G.-R., Zhang D., Fu N.-J., Yang H.-Z., Chen X.-G., Yu D.-Q. Cytotoxic Lignans and Sesquiterpenoids from the Rhizomes of Acorus tatarinowii. Planta Med. 2016;82:632–638. doi: 10.1055/s-0035-1568248. [DOI] [PubMed] [Google Scholar]
- 7.Mathouet H., Elomri A., Lameiras P., Daich A., Vérité P. An alkaloid, two Conjugate Sesquiterpenesand a Phenylpropanoid from Pachypodanthium confine Engl. and Diels. Phytochemistry. 2007;68:1813–1818. doi: 10.1016/j.phytochem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Ngadjui B.T., Ayafor J.F., Lontse D. Unusual Norlignans and Antiviral Agents from Pachypodanthium staudtii. Fitoterapia. 1987;57:340–341. [Google Scholar]
- 9.Ohashi H., Kawai S., Sakurai Y., Yasue M. Norlignan from the Knot Resin of Araucaria angustifolia. Phytochemistry. 1992;31:1371–1373. doi: 10.1016/0031-9422(92)80293-N. [DOI] [Google Scholar]
- 10.Jeong S.-J., Ahn N.-H., Kim Y.-C., Inagaki M., Miyamoto T., Higuchi R. Norlignans with Hyaluronidase Inhibitory Activity from Anemarrhena asphodeloides. Planta Med. 1999;65:367–368. doi: 10.1055/s-2006-960789. [DOI] [PubMed] [Google Scholar]
- 11.Jeong S.J., Higuchi R., Ono M., Kuwano M., Kim Y.C., Miyamoto T. Cis-Hinokiresinol, a Norlignan from Anemarrhena asphodeloides, Inhibits Angiogenic Response in Vitro and in Vivo. Biol. Pharm. Bull. 2003;26:1721–1724. doi: 10.1248/bpb.26.1721. [DOI] [PubMed] [Google Scholar]
- 12.Bak J.P., Cho Y.M., Kim I., Park D.W., Kwon J.E., Jeong Y.J., Kwak J.H., Kang S.C. Inhibitory Effects of Norlignans Isolated from Anemarrhena asphodeloides on Degranulation of Rat Basophilic Leukemia-2H3 cells. Biomed. Pharmacother. 2016;84:1061–1066. doi: 10.1016/j.biopha.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Park H.J., Lee J.Y., Moon S.S., Hwang B.K. Isolation and Anti-Oomycete Activity of Nyasol from Anemarrhena asphodeloides Rhizomes. Phytochemistry. 2003;64:997–1001. doi: 10.1016/S0031-9422(03)00462-X. [DOI] [PubMed] [Google Scholar]
- 14.Oketch-Rabah H.A., Dossaji S.F., Brøgger Christensen S., Frydenvang K., Lemmich E., Cornett K., Olsen C.E., Chen M., Kharazmi A., Theander T. Antiprotozoal Compounds from Asparagus africanus. J. Nat. Prod. 1997;60:1017–1022. doi: 10.1021/np970217f. [DOI] [PubMed] [Google Scholar]
- 15.Li X.-N., Chu C., Cheng D.-P., Tong S.-Q., Yan J.-Z. Norlignans from Asparagus cochinchinensis. Nat. Prod. Comm. 2012;7:1357–1358. doi: 10.1177/1934578X1200701027. [DOI] [PubMed] [Google Scholar]
- 16.Tsui W.Y., Brown G.D. (+)-nyasol from Asparagus cochinchinensis. Phytochemistry. 1996;43:1413–1415. doi: 10.1016/S0031-9422(96)00442-6. [DOI] [Google Scholar]
- 17.Zhang H.-J., Sydara K., Teng G.T., Cuiying M., Southavong B., Soejarto D.D., Pezzuto J.M., Fong H.H.S. Bioactive Constituents from Asparagus cochinchinensis. J. Nat. Prod. 2004;67:194–200. doi: 10.1021/np030370b. [DOI] [PubMed] [Google Scholar]
- 18.Yang C.-X., Huang S.-S., Yang X.-P., Jia Z.-J. Nor-lignans and Steroidal Saponins from Asparagus gobicus. Planta Med. 2004;70:446–451. doi: 10.1055/s-2004-818974. [DOI] [PubMed] [Google Scholar]
- 19.Shah M.A., Abdullah S.M., Khan M.A., Nasar G., Saba I. Antibacterial Activity of Chemical Constituents Isolated from Asparagus racemosus. Bangladesh J. Pharmacol. 2014;9:1–3. doi: 10.3329/bjp.v9i1.16672. [DOI] [Google Scholar]
- 20.Koorbanally C., Mulholland D.A., Crouch N.R. Norlignans and Homoisoflavanones from Two South African Drimiopsis Species (Hyacinthaceae: Hyacinthoideae) Biochem. Syst. Ecol. 2006;34:588–592. doi: 10.1016/j.bse.2005.12.011. [DOI] [Google Scholar]
- 21.Du Toit K., Elgorashi E.E., Malan S.F., Drewes S.E., Van Staden J., Crouch N.R., Mulholland D.A. Anti-Inflammatory Activity and QSAR Studies of Compounds Isolated from Hyacinthaceae Species and Tachiadenus longiflorus Griseb. (Gentianaceae) Bioorg. Med. Chem. 2005;13:2561–2568. doi: 10.1016/j.bmc.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Schwikkard S., Alqahtani A., Knirsch W., Wetschnig W., Jaksevicius A., Opara E.I., Langat M.K., Andriantiana J.L., Muholland D.A. Phytochemical Investigations of three Rhodocodon (Hyacinthaceae sensu APG II) Species. J. Nat. Prod. 2017;80:30–37. doi: 10.1021/acs.jnatprod.6b00240. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y., Xie Y.-G., Zhang Y., Li T., Li H.-L., Yan S.-K., Jin H.-Z., Zhang W.-D. New Norlignans and Flavonoids of Dysosma versipellis. Phytochem. Lett. 2016;16:75–81. doi: 10.1016/j.phytol.2016.03.001. [DOI] [Google Scholar]
- 24.Sun K., Li X., Li W., Liu J.-M., Wang J.-H., Yi S. A new nor-Lignan from the Seeds of Descurainia sophia. Nat. Prod. Res. 2006;20:519–522. doi: 10.1080/14786410500045309. [DOI] [PubMed] [Google Scholar]
- 25.Yang M., Wang C.-M., Zhang Q., Han Y.-F., Jia Z.-J. Sesquiterpenes, Lignans and other Constituents from Saussurea macrota. Pharmazie. 2004;59:972–976. doi: 10.1002/chin.200516160. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M., Dong X.P., Deng Y., Wang H., Li X.N., Song Q. A New Sesqui-Norlignan from Herpetospermum pedunculosum. Acta Pharm. Sin. 2006;41:659–661. [PubMed] [Google Scholar]
- 27.Chen T.-H., Liau B.-C., Wang S.-Y., Jong T.-T. Isolation and Cytotoxicity of the Lignanoids from Chamaecyparis formosensis. Planta Med. 2008;74:1806–1811. doi: 10.1055/s-0028-1088325. [DOI] [PubMed] [Google Scholar]
- 28.Chien S.-C., Chang J.-Y., Kuo C.-C., Hsieh C.-C., Yang N.-S., Kuo Y.-H. Cytotoxic and Novel Skeleton Compounds from the Heartwood of Chamaecyparis obtusa var. formosana. Tetrahedr. Lett. 2007;48:1567–1569. doi: 10.1016/j.tetlet.2007.01.011. [DOI] [Google Scholar]
- 29.Hirose Y., Oishi N., Nagaki H., Nakatsuka T. The Structure of Hinokiresinol. Tetrahedr. Lett. 1965;6:3665–3668. doi: 10.1016/S0040-4039(01)89152-8. [DOI] [Google Scholar]
- 30.Nagasaki T., Yasuda S., Imai T. Immunohistochemical Localization of Agatharesinol, a Heartwood Norlignan, in Cryptomeria japonica. Phytochemistry. 2002;60:461–466. doi: 10.1016/S0031-9422(02)00141-3. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K., Ogiyama K. Phenols of Discolored Sugi (Cryptomeriajaponica D. Don) Sapwood II. Norlignans of Discolored Sugi Sapwood Collected in the Kyushu Region. Mokuzai Gakkaishi. 1985;31:28–38. [Google Scholar]
- 32.Erdtman H., Harmata J. Phenolic and Terpenoid Heartwood Constituents of Libocedrus yateensis. Phytochemistry. 1979;18:1495–1500. doi: 10.1016/S0031-9422(00)98482-6. [DOI] [Google Scholar]
- 33.Dong L.-B., He J., Wang Y.-Y., Wu X.-D., Deng X., Pan Z.-H., Xu G., Peng L.-Y., Zhao Y., Li Y., et al. Terpenoids and Norlignans from Metasequoia glyptostroboides. J. Nat. Prod. 2011;74:234–239. doi: 10.1021/np100694k. [DOI] [PubMed] [Google Scholar]
- 34.Zeng Q., Cheng X.-R., Qin J.-J., Guan B., Chang R.-J., Yan S.-K., Jin H.-Z., Zhang W.-D. Norlignans and Phenylpropanoids from Metasequoia glyptostroboides Hu et Cheng. Helv. Chim. Acta. 2012;95:606–612. doi: 10.1002/hlca.201100363. [DOI] [Google Scholar]
- 35.Zhang Y.-M., Tan N.-H., Yang Y.-B., Lu Y., Cao P., Wu Y.-S. Norlignans from Sequoia sempervirens. Chem. Biodivers. 2005;2:497–505. doi: 10.1002/cbdv.200590030. [DOI] [PubMed] [Google Scholar]
- 36.Henley-Smith P., Whiting D.A. New Norlignans of Sequoiadendron gigantea: Phytochemical Comparison with Sequoia sempervirens. Phytochemistry. 1973;15:1285–1287. doi: 10.1016/0031-9422(76)85096-0. [DOI] [Google Scholar]
- 37.Zhang Y.M., Tan N.H., Zeng G.Z., Adebayo A.H., Ji C.J. A New Norlignan from Taxodium ascendens. Fitoterapia. 2009;80:361–363. doi: 10.1016/j.fitote.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Su Z., Yuan W., Wang P., Li S. Ethnobotany, Phytochemistry, and Biological Activities of Taxodium Rich. Pharm. Crops. 2013;4:1–14. [Google Scholar]
- 39.Wang W., Zeng Y.H., Osman K., Shinde K., Rahman M., Gibbons S., Mu Q. Norlignans, Acylphloroglucinols, and a Dimeric Xanthone from Hypericum chinense. J. Nat. Prod. 2010;73:1815–1820. doi: 10.1021/np1004483. [DOI] [PubMed] [Google Scholar]
- 40.Li N., Zhu C.-C., Xiao H.-M., Wang K.-J. Norlignan Derivatives from Curculigo breviscapa. Fitoterapia. 2010;81:528–531. doi: 10.1016/j.fitote.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Chang W.-L., Lee S.-S. Norneolignan and Phenols from Curculigo capitulata. Phytochemistry. 1998;49:2133–2136. doi: 10.1016/S0031-9422(98)00379-3. [DOI] [Google Scholar]
- 42.Li N., Chen J.-J., Zhao Y.-X., Zhou J. Three New Norlignans from Curculigo capitulata. J. Asian Nat. Prod. Res. 2005;7:189–195. doi: 10.1080/1028602032000169578. [DOI] [PubMed] [Google Scholar]
- 43.Wang K.-J., Zhu C.-C., Di L., Li N., Zhao Y.-X. New Norlignan Derivatives from Curculigo capitulata. Fitoterapia. 2010;81:869–872. doi: 10.1016/j.fitote.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 44.Wang K.-J., Li N. Norlignan Derivatives from Curculigo crassifolia and their DPPH Radical Scavenging Activity. Arch. Pharm. Res. 2008;31:1313–1316. doi: 10.1007/s12272-001-2111-4. [DOI] [PubMed] [Google Scholar]
- 45.Wang K.-J., Li N., Wang H. New Acetylenic Norlignan Compounds from Rhizomes of Curculigo crassifolia. Molecules. 2008;13:1696–1701. doi: 10.3390/molecules13081696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li N., Chen J.-J., Zhou J. Four New Phenolic Compounds from Curculigo crassifolia (Hypoxidaceae) Helv. Chim. Acta. 2004;87:845–850. doi: 10.1002/hlca.200490082. [DOI] [Google Scholar]
- 47.Palazzino G., Galeffi C., Federici E., Delle Monache F., Cometa M.F., Palmery M. Benzylbenzoate and Norlignan Glucosides from Curculigo pilosa: Structural Analysis and in Vitro Vascular Activity. Phytochemistry. 2000;55:411–417. doi: 10.1016/S0031-9422(00)00256-9. [DOI] [PubMed] [Google Scholar]
- 48.Cometa M.F., Palazzino G., Galeffi C., Palmery M. Studies on Vasoconstrictor Activity of Curculigo pilosa Extracts And of its Isolated Compounds. IL Farm. 2001;56:353–356. doi: 10.1016/S0014-827X(01)01049-7. [DOI] [PubMed] [Google Scholar]
- 49.Chifundera K., Messana I., Galeffi C., De Vicente Y. Research on African Medicinal Plants -XXV- the (1R,2S) Absolute Configuration of Nyasicoside. Its Occurrence in Curculigo recurvata. Tetrahedron. 1991;47:4369–4374. doi: 10.1016/S0040-4020(01)87106-4. [DOI] [Google Scholar]
- 50.Chifundera K., Palazzino G., Messana I., Ping L., Galeffi C., Cannarsa G. Norlignan Glucosides from Curculigo recurvata. Phytochemistry. 1994;35:1343–1348. doi: 10.1016/S0031-9422(06)80122-6. [DOI] [Google Scholar]
- 51.Li N., Wang T.-M., Wang K.-J., Zhao Y.-X. Norlignans from Rhizomes of Curculigo sinensis. Helv. Chim. Acta. 2010;93:724–728. doi: 10.1002/hlca.200900286. [DOI] [Google Scholar]
- 52.Li N., Li S.-P., Wang K.-J., Yan G.-Q., Zhu Y.-Y. Novel Norlignan Glucosides from Rhizomes of Curculigo sinensis. Carbohydr. Res. 2012;351:64–67. doi: 10.1016/j.carres.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 53.Sibanda S., Ntabeni O., Nicoletti M., Galeffi C. Nyasol and 1,3(5)-diphenyl-1-pentene Related Glycosides from Hypoxis angustifolia. Biochem. Syst. Ecol. 1990;18:481–483. doi: 10.1016/0305-1978(90)90117-X. [DOI] [Google Scholar]
- 54.Laporta O., Pérez-Fons L., Mallavia R., Caturla N., Micol V. Isolation, Characterization and Antioxidant Capacity Assessment of the Bioactive Compounds Derived from Hypoxis rooperi Corm Extract (African potato) Food Chem. 2007;101:1425–1437. doi: 10.1016/j.foodchem.2006.03.051. [DOI] [Google Scholar]
- 55.Marini-Bettolo G.B., Galeffi C., Multari G., Palazzino G., Messana I. Research on African Medicinal Plants. XXVII. Interjectin a Derivative of Nyasicoside from Hypoxis interjecta and Hypoxis multiceps. Tetrahedron. 1991;47:6717–6724. doi: 10.1016/S0040-4020(01)82323-1. [DOI] [Google Scholar]
- 56.Galeffi C., Multari G., Msonthi J.D., Nicoletti M., Marini-Bettolo G.B. Research on African medicinal plants – XIII. (+)-nyasicoside, a New Glucoside of Hypoxis nyasica bak. Tetrahedron. 1987;43:3519–3522. doi: 10.1016/S0040-4020(01)81644-6. [DOI] [Google Scholar]
- 57.Marini-Bettolo G.B., Nicoletti M., Messana I., Galeffi C., Msonthi J.D., Chapya W.A. Research on African Medicinal Plants – XI: Glucosides of Hypoxis nyasica bak. The Structure of Nyasoside, a New Glucoside Biologically Related to Hypoxoside. Tetrahedron. 1985;41:665–670. doi: 10.1016/S0040-4020(01)96444-0. [DOI] [Google Scholar]
- 58.Messana I., Msonthi J.D., DeVicente Y., Multari G., Galeffi C. Mononyasine A and Mononyasine B: TwoGlucosides from Hypoxis nyasica. Phytochemistry. 1989;28:2807–2809. doi: 10.1016/S0031-9422(00)98094-4. [DOI] [Google Scholar]
- 59.Nicoletti M., Pieretti S., Capasso A., Galeffi C. Analgesic Effects Induced by Hypoxoside, A norlignan Glucoside from Hypoxis spp. Phytother. Res. 1996;10:398–401. doi: 10.1002/(SICI)1099-1573(199608)10:5<398::AID-PTR860>3.0.CO;2-7. [DOI] [Google Scholar]
- 60.Galeffi C., Multari G., De Vicente Y., Messana I., Nicoletti M., Marini-Bettolo G.B. Two New Glucosides from Hypoxis obtusa: Obtuside A and obtuside B. Planta Med. 1989;55:318–320. doi: 10.1055/s-2006-962019. [DOI] [PubMed] [Google Scholar]
- 61.Cullman F., Schmidt A., Schuld F., Trennheuser M.L., Becker H. Lignans from the Liverworts Lepidozia incurvata, Chiloscyphus polyanthos and Jungermannia exsertifolia ssp. cordifolia. Phytochemistry. 1999;52:1647–1650. doi: 10.1016/S0031-9422(99)00279-4. [DOI] [Google Scholar]
- 62.Achenbach H., Gross J., Dominguez X.A., Cano G., Star J.V., Del Carmen Brussolo L., Muñoz G., Salgado F., López L. Lignans, Neolignans and Norneolignans from Krameria cystisoides. Phytochemistry. 1987;26:1159–1166. doi: 10.1016/S0031-9422(00)82370-5. [DOI] [Google Scholar]
- 63.Achenbach H., Utz W., Sanchez H.V., Touche E.M.G., Verde J.S., Dominguez X.A. Neolignans, nor-Neolignans and other Compounds from Roots of Krameria grayi. Phytochemistry. 1995;39:413–415. doi: 10.1016/0031-9422(94)00932-J. [DOI] [Google Scholar]
- 64.Achenbach H., Utz W., Usubillaga A., Rodriguez H.A. tudies on Krameriaceae. 7. Lignans from Krameria ixine. Phytochemistry. 1991;30:3753–3757. doi: 10.1016/0031-9422(91)80103-8. [DOI] [Google Scholar]
- 65.Silva S.A.S., De Castro J.C.M., Da Silva T.G., Da-Cunha E.V.L., Barbosa-Filho J.M., Da Silva M.S. Kramentosan, a New Trinorlignan from the Roots of Krameria tomentosa. Nat. Prod. Res. 2001;15:323–329. doi: 10.1080/10575630108041299. [DOI] [PubMed] [Google Scholar]
- 66.Zhu Q.-F., Wang Y.-Y., Jiang W., Qu H.-B. Three New Norlignans from Glechoma longituba. J. Asian Nat. Prod. Res. 2013;15:258–264. doi: 10.1080/10286020.2012.762640. [DOI] [PubMed] [Google Scholar]
- 67.Lacret R., Varela R.M., Molinillo J.M.G., Nogueiras C., Cisco A., Macías F.A. Tectonoelins, New Norlignans from a Bioactive Extract of Tectona grandis. Phytochem. Lett. 2012;5:382–386. doi: 10.1016/j.phytol.2012.03.008. [DOI] [Google Scholar]
- 68.Yamasaki T., Kawabata T., Masuoka C., Kinjo J., Ikeda T., Nohara T., Ono M. Two New Lignan Glucosides from the Fruit of Vitex cannabifolia. J. Nat. Med. 2008;62:47–51. doi: 10.1007/s11418-007-0184-1. [DOI] [PubMed] [Google Scholar]
- 69.Lou Z.-H., Li H.-M., Gao L.-H., Li R.-T. Antioxidant Lignans from the Seeds of Vitex negundo var. cannabifolia. J. Asian Nat. Prod. Res. 2014;16:963–969. doi: 10.1080/10286020.2014.929574. [DOI] [PubMed] [Google Scholar]
- 70.Haq A.-U., Malik A., Anis I., Khan S.B., Ahmed E., Ahmed Z., Nawaz S.A., Choudhary M.I. Enzymes Inhibiting Lignans from Vitex negundo. Chem. Pharm. Bull. 2004;52:1269–1272. doi: 10.1248/cpb.52.1269. [DOI] [PubMed] [Google Scholar]
- 71.Haq A.-U., Malik A., Khan M.T.H., Haq A.-U., Khan S.B., Ahmad A., Choudhary M.I. Tyrosinase Inhibitory Lignans from the Methanol Extract of the Roots of Vitex negundo Linn. and their Structure-Activity Relationship. Phytomedicine. 2006;13:255–260. doi: 10.1016/j.phymed.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Lodhi M.A., Haq A.U., Choudhary M.I., Malik A., Ahmad S. α-chymotrypsin Inhibition Studies on the Lignans from Vitex negundo Linn. J. Enzym. Inhib. Med. Chem. 2008;23:400–405. doi: 10.1080/14756360701584653. [DOI] [PubMed] [Google Scholar]
- 73.Ono M., Nishida Y., Masuoka C., Li J.-C., Okawa M., Ikeda T., Nohara T. Lignan Derivatives and A Norditerpene from the Seeds of Vitex negundo. J. Nat. Prod. 2004;67:2073–2075. doi: 10.1021/np040102t. [DOI] [PubMed] [Google Scholar]
- 74.Hu P., Li D.-H., Hu X., Li S.-G., Sai C.-M., Sun X.-C., Su T., Bai J., Wang Z.-H., Li Z.-L., et al. Lignans and Triterpenoids from Vitex negundo var. heterophylla and their Biological Evaluation. Fitoterapia. 2006;111:147–153. doi: 10.1016/j.fitote.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 75.Zheng C.J., Huang B.K., Han T., Zhang Q.Y., Zhang H., Rahman K., Qin L.P. Nitric Oxide Scavenging Lignans from Vitex negundo seeds. J. Nat. Prod. 2009;72:1627–1630. doi: 10.1021/np900320e. [DOI] [PubMed] [Google Scholar]
- 76.Nie X.-F., Yu L.-L., Tao Y., Huang J., Ding L.-Q., Feng X.-C., Jiang M.-M., Zheng L., Chen L.-X., Qiu F. Two New Lignans from the Aerial Part of Vitex negundo. J. Asian Nat. Prod. Res. 2016;18:656–661. doi: 10.1080/10286020.2016.1142975. [DOI] [PubMed] [Google Scholar]
- 77.Kawazoe K., Yutani A., Tamemoto K., Takaishi Y. Phenylnaphthalene Compounds from the Subterranean Part of Vitex rotundifolia and their Antibacterial Activity Against Methicillin-Resistant Staphylococcus aureus. J. Nat. Prod. 2001;64:588–591. doi: 10.1021/np000307b. [DOI] [PubMed] [Google Scholar]
- 78.Chérigo L., Polanco V., Ortega-Barria E., Heller M.V., Capson T.L., Cubilla Rios L. Antitrypanosomal Activity of a Novel Norlignan Purified from Nectandra lineata. Nat. Prod. Res. 2005;19:373–377. doi: 10.1080/1478641042000261950. [DOI] [PubMed] [Google Scholar]
- 79.Martini U., Zapp J., Becker H. Lignans from the Liverwort Bazzania trilobata. Phytochemistry. 1998;49:1139–1146. doi: 10.1016/S0031-9422(97)01076-5. [DOI] [Google Scholar]
- 80.Eklund P., Raitanen J.-E. 9-norlignans: Occurrence, Properties and their Semisynthetic Preparation from Hydroxy-Matairesinol. Molecules. 2019;24:220. doi: 10.3390/molecules24020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu S.-B., Wen Y., Li X.-W., Zhao Y., Zhao Z., Hu J.-F. Chemical Constituents from the Fruits of Sonneratia caseolaris and Sonneratia ovata (Sonneratiaceae) Biochem. Syst. Ecol. 2009;37:1–5. doi: 10.1016/j.bse.2009.01.002. [DOI] [Google Scholar]
- 82.Song M.-C., Yang H.-J., Bang M.-H., Kim D.-K., Jeong T.-S., Kim J.-P., Baek N.-I. Antioxidant and Antiatherogenic Activity of cis-Hinokiresinol from Trapa pseudoincisa. Arch. Pharm. Res. 2007;30:1392–1397. doi: 10.1007/BF02977362. [DOI] [PubMed] [Google Scholar]
- 83.Huang Y., Zeng Q., Fu J.-J., Kou Z.-J., Chang R.-J., Jin H.-Z., Zhang W.-D. Chemical Constituents from Tsoongiodendron odorum Chun. Biochem. Syst. Ecol. 2011;39:209–212. doi: 10.1016/j.bse.2011.03.002. [DOI] [Google Scholar]
- 84.Jia L., Bi Y.-F., Jing L.-L., Zhou S.-A., Kong D.-Y. Two New Compounds from Urena lobata L. J. Asian Nat. Prod. Res. 2010;12:962–967. doi: 10.1080/10286020.2010.510468. [DOI] [PubMed] [Google Scholar]
- 85.Wang B.-G., Ebel R., Wang C.-Y., Wray V., Proksch P. New Methoxylated Aryltetrahydronaphthalene Lignans and a Norlignan from Aglaia cordata. Tetrahedr. Lett. 2002;43:5783–5787. doi: 10.1016/S0040-4039(02)01180-2. [DOI] [Google Scholar]
- 86.Lee I.-S., Kim H.-J., Youn U.-J., Chen Q.-C., Kim J.-P., Ha D.T., Ngoc T.M., Min B.-S., Lee S.-M., Jung H.-J., et al. Dihydrobenzofuran Norlignans from the Leaves of Cedrela sinensis A. Juss. Helv. Chim. Acta. 2010;93:272–276. doi: 10.1002/hlca.200900180. [DOI] [Google Scholar]
- 87.Dong X.-J., Zhu Y.-F., Bao G.-H., Hu F.-L., Qin G.-W. New Limonoids and a Dihydrobenzofuran Norlignan from the Roots of Toona sinensis. Molecules. 2013;18:2840–2850. doi: 10.3390/molecules18032840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Su G., Bai R., Yu X., Cao Y., Yin X., Tu P., Chai X. Noralashinol A, a New Norlignan from Stem Barks of Syringa pinnatifolia. Nat. Prod. Res. 2016;30:2149–2153. doi: 10.1080/14786419.2016.1146886. [DOI] [PubMed] [Google Scholar]
- 89.Wang Q.-H., Huo S.R.N., Bao Y.P., Ao W.L.J. Chemical Constituents of Syringa pinnatifolia and its Chemotaxonomic Study. Chem. Nat. Compd. 2018;54:435–438. doi: 10.1007/s10600-018-2373-4. [DOI] [Google Scholar]
- 90.Zhang R.-F., Feng X., Su G.-Z., Yin X., Yang X.-Y., Zhao Y.-F., Li W.-F., Tu P.-F., Chai X.-Y. Noralashinol B, a Norlignan with Cytotoxicity from Stem Barks of Syringa pinnatifolia. J. Asian Nat. Prod. Res. 2017;19:416–422. doi: 10.1080/10286020.2017.1307188. [DOI] [PubMed] [Google Scholar]
- 91.Rischmann M., Mues R., Geiger H., Laas H.J., Eicher T. Isolation and Synthesis of 6,7-dihydroxy-4-(3,4-dihydroxyphenyl)naphthalene-2-carboxylic acid from Pellia epiphylla. Phytochemistry. 1989;28:867–869. doi: 10.1016/0031-9422(89)80132-3. [DOI] [Google Scholar]
- 92.Huang Y.-L., Chen C.-C., Hsu F.-L., Chen C.-F. Tannins, Flavonol Sulfonates, and a Norlignan from Phyllanthus virgatus. J. Nat. Prod. 1998;61:1194–1197. doi: 10.1021/np970336v. [DOI] [PubMed] [Google Scholar]
- 93.Li Y.-Z., Tong A.-P., Huang J. Two New Norlignans and a New Lignanamide from Peperomia tetraphylla. Chem. Biodivers. 2012;9:769–776. doi: 10.1002/cbdv.201100138. [DOI] [PubMed] [Google Scholar]
- 94.Li Y.-Z., Huang J., Gong X., Tian X.-Q. A novel Norlignan and a Novel Phenylpropanoid from Peperomia tetraphylla. Helv. Chim. Acta. 2007;90:2222–2226. doi: 10.1002/hlca.200790230. [DOI] [Google Scholar]
- 95.Cabanillas B.J., Le Lamer A.C., Castillo D., Arevalo J., Rojas R., Odonne G., Bourdy G., Moukarzel B., Sauvain M., Fabre N. Caffeic Acid Esters and Lignans from Piper sanguineispicum. J. Nat. Prod. 2010;73:1884–1890. doi: 10.1021/np1005357. [DOI] [PubMed] [Google Scholar]
- 96.Mastunaga K., Masaoki K., Shibuya Y. Imperanene, a Novel Phenolic Compound with Platelet Aggregation Inhibitory Activity from Imperata cylindrica. J. Nat. Prod. 1995;58:138–139. doi: 10.1021/np50115a022. [DOI] [PubMed] [Google Scholar]
- 97.Xiao S.-J., Lei X.-X., Xia B., Xiao H.-P., He D.-H., Fang D.-M., Qi H.-Y., Chen F., Dinga L.-S., Zhou Y. Two novel norlignans from Gymnotheca chinensis. Tetrahedr. Lett. 2014;55:2869–2871. doi: 10.1016/j.tetlet.2014.03.091. [DOI] [Google Scholar]
- 98.Feng W.-S., Li K.-K., Zheng X.-K. A New Norlignan Lignanoside from Selaginella moellendorffii Hieron. Acta Pharma. Sin. B. 2011;1:36–39. doi: 10.1016/j.apsb.2011.04.001. [DOI] [Google Scholar]
- 99.Yang G.-Y., Wang R.-R., Mu H.-X., Li Y.-K., Li X.-N., Yang L.-M., Zheng Y.-T., Xiao W.-L., Sun H.-D. Dibenzocyclooctadiene Lignans and Norlignans from Fruits of Schisandra wilsoniana. J. Nat. Prod. 2013;76:250–255. doi: 10.1021/np300745q. [DOI] [PubMed] [Google Scholar]
- 100.Mohamed K.M., Fouad M.A., Matsunami K., Kamel M.S., Otsuka H. A New Norlignan Glycoside from Cestrum diurnum L. ARKIVOC. 2007;13:63–70. [Google Scholar]
- 101.D’Abrosca B., Dellagreca M., Fiorentino A., Golino A., Minaco P., Zarrelli A. Isolation and Characterization of New Lignans from the Leaves of Cestrum parqui. Nat. Prod. Res. 2006;20:293–298. doi: 10.1080/14786410500111234. [DOI] [PubMed] [Google Scholar]
- 102.Jassbi A.R., Zare O.S., Asadollahi M., Schuman M.C. Ecological Roles and Biological Activities of Specialized Metabolites from the Genus Nicotiana. Chem. Rev. 2017;117:12227–12280. doi: 10.1021/acs.chemrev.7b00001. [DOI] [PubMed] [Google Scholar]
- 103.Liao Z., Li X., Lu Y., Shi H., Li Z., Hu Q. Norlignans from the Roots and Stems of Nicotiana tabacum and their Antitobacco Mosaic Virus Activity. Asian J. Chem. 2012;24:4895. [Google Scholar]
- 104.Yang B.-Y., Yin X., Liu Y., Sun Y., Guan W., Zhou Y.-Y., Kuang H.-X. Terpenes and Lignans from the Roots of Solanum melongena L. Nat. Prod. Res. 2019 doi: 10.1080/14786419.2018.1533828. [DOI] [PubMed] [Google Scholar]
- 105.Francielli de Oliveira P., Lopes Damasceno J., Bertanha C.S., Barbosa Araújo A.R., Mendonça Pauletti R., Tavares D.C. Study of the Cytotoxic Activity of Styrax camporum Extract and its Chemical Markers, Egonol and Homoegonol. Cytotechnology. 2016;68:1597–1602. doi: 10.1007/s10616-015-9864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pauletti P.A., Araujo A.R., Marx Young M.C., Giesbrecht A.M., Da Silva Bolzani V. Nor-lignans from the Leaves of Styrax ferrugineus (Styracaceae) with Antibacterial and Antifungal Activity. Phytochemistry. 2000;55:597–601. doi: 10.1016/S0031-9422(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 107.Min B.-S., Oh S.-R., Ahn K.-S., Kim J.-H., Lee J., Kim D.-Y., Kim E.-H., Lee H.-K. Anti-complement Activity of Norlignans and Terpenes from the Stem Bark of Styrax japonica. Planta Med. 2004;70:1210–1215. doi: 10.1055/s-2004-835853. [DOI] [PubMed] [Google Scholar]
- 108.Cao T.Q., Lee B.M., Jung Y.W., Nguyen V.T., Kim J.A., Min B.S. Cytotoxic Activity of Compounds from Styrax obassia. Nat. Prod. Comm. 2017;12:259–260. doi: 10.1177/1934578X1701200230. [DOI] [PubMed] [Google Scholar]
- 109.Venditti A., Frezza C., Serafini I., Pulone S., Scardelletti G., Sciubba F., Bianco A., Serafini M. Chemical Profiling of the Fruits of Styrax officinalis L. from Monti Lucretili (Latiumregion, Central Italy): Chemotaxonomy and Nutraceutical Potential. Trends Phytochem. Res. 2018;2:1–12. [Google Scholar]
- 110.Bertanha C.S., Braguine C.G., Moraes A.C.G., Gimenez V.M.M., Groppo M., Silva M.L.A., Cunha W.R., Januário A.H., Pauletti P.M. Cyclooxygenase Inhibitory Properties of nor-neolignans from Styrax pohlii. Nat. Prod. Res. 2012;26:2323–2329. doi: 10.1080/14786419.2012.671320. [DOI] [PubMed] [Google Scholar]
- 111.Timmers M.A., Guerrero-Medina J.L., Esposito D., Grace M.H., Paredes-Lopez O., García-Saucedo P.A., Lila M.A. Characterization of Phenolic Compounds and Antioxidant and Anti-Inflammatory Activities from Mamuyo (Styrax ramirezii Greenm.) fruit. J. Agric. Food Chem. 2015;63:10459–10465. doi: 10.1021/acs.jafc.5b04781. [DOI] [PubMed] [Google Scholar]
- 112.Jiao Y., Fang J., Tang S., Duan H. Penangianol A and B: Two New Norlignans from Rhizomes of Abacopteris penangiana. Chem. Nat. Compd. 2015;51:232–235. doi: 10.1007/s10600-015-1250-7. [DOI] [Google Scholar]
- 113.Mohammed M., Maxwell A.R., Ramsewaka R., Reynolds W.F. Norlignans from Pouzolzia occidentalis. Phytochem. Lett. 2010;3:29–32. doi: 10.1016/j.phytol.2009.10.008. [DOI] [Google Scholar]
- 114.Zhong C.-Q., Tao S.-H., Yi Z.-B., Guo L.-B., Xie Y.-F., Chen Y.-F. Four New Compounds from Pouzolzia zeylanica (L.) Benn. var. microphylla. Heterocycles. 2015;91:1926–1936. [Google Scholar]
- 115.Bertanha C.S., Utrera S.H., Melleiro Gimenez V.M., Groppo M., Andrade da Silva M.L., Cunha W.R., Gomes Martins C.H., Januário A.H., Mendonça Pauletti P. Antibacterial Evaluation of Styrax pohlii and Isolated Compounds. Braz. J. Pharma. Sci. 2013;49:653–658. doi: 10.1590/S1984-82502013000400004. [DOI] [Google Scholar]
- 116.Shin I.-S., Ahn K.-S., Shin N.-R., Jeon C.-M., Kwon O.-K., Chin Y.-W., Lee K., Oh S.-R. Homoegonol Attenuates the Asthmatic Responses Induced by Ovalbumin Challenge. Arch. Pharm. Res. 2014;37:1201–1210. doi: 10.1007/s12272-013-0327-8. [DOI] [PubMed] [Google Scholar]
- 117.Locke N.R., Royce S.G., Wanewright J.S., Samuel C.S., Tang M.L. Comparison of Airway Remodeling in Acute, Subacute, and Chronic Models of Allergic Airways Disease. Am. J. Resp. Cell Mol. Biol. 2007;36:625–632. doi: 10.1165/rcmb.2006-0083OC. [DOI] [PubMed] [Google Scholar]
- 118.Castro J.C.M., Silva M.S., Cortes S.F., Lemos V.S. Inhibitory Effect of the Norlignan 2-(2′-hydroxy-4′,6′-dimethoxyphenyl)-5-[(E)-propenyl]benzofuran from Krameria tomentosa on Acetylcholine-Induced Relaxation of the Rat Aorta. Planta Med. 2006;72:78–81. doi: 10.1055/s-2005-873179. [DOI] [PubMed] [Google Scholar]
- 119.Tiwari N., Mishra A., Bhatt G., Chaudhary A. Evaluation of Antistress Potential of Negundin A from Vitex negundo in Acute Stress Induced Mice. Eur. J. Med. Plants. 2015;10:1–8. doi: 10.9734/EJMP/2015/18752. [DOI] [Google Scholar]
- 120.Neufeld K., Neufeld N. Animal Feed Additive Containing Diurnoside and/or Cestrumoside. PCT Int. Appl. WO 2017129732 A1 20170803. 2017 Aug 3;
- 121.Takara K., Iwasaki H., Ujihara K., Wada K. Human Tyrosinase Inhibitor in Rum Distillate Wastewater. J. OleoSci. 2008;57:191–196. doi: 10.5650/jos.57.191. [DOI] [PubMed] [Google Scholar]