Abstract
Background:
Liposomes constitute a promising drug delivery vehicle, and are believed to improve drugs’ effectiveness. This study was aimed to compare antihypertensive and vascular modifying activities of liposomal and non-liposomal forms of ascorbic acid.
Methods:
Forty-nine male Sprague-Dawley rats were randomly divided into seven groups (n=7): A sham vehicle-receiving (Sham-veh), hypertensive (HTN), vehicle-receiving hypertensive (HTN-Veh), two liposomal Ascorbic acid-treated hypertensive at 50 or 100 mg/kg/day (LVC-50 and LVC-100), and two non-liposomal Ascorbic acid-treated hypertensive at 50 or 100 mg/kg/day (VC-50 and VC-100). Systolic blood pressure (SBP) and heart rate (HR) were measured weekly; after 4 weeks, dose-responses to phenylephrine (PE) in the absence and presence of nitro-L-arginine methyl ester (L-NAME), acetylcholine (Ach), and sodium nitroprusside (SNP) were obtained on aortic rings. Data were analyzed with one-way ANOVA and Duncan’s multiple range test at a P value of <0.05 using Sigmastat statistical software
Results:
Compared to the non-liposomal form, the liposomal one was associated with more prominent effects on the final SBP. Both forms of Ascorbic acid decreased SBP dose-dependently. The basal and stimulated release of Nitric Oxide (NO) was significantly recovered by both forms of Ascorbic acid. The PE maximal responses were not significantly different between the liposomal and non-liposomal groups (P=0.08). Although the Emax of Ach-relaxation response was not different in two preparation forms, Ach-relaxation response induced a lower concentration of the liposomal form of Ascorbic acid (P=0.03
Conclusion:
The liposomal Ascorbic acid exhibited relaxation activity in significantly lower concentrations. The observed effects were partly mediated by the increased basal release of NO.
Keywords: Liposomes, Ascorbic acid, Rats, Hypertension, Nitric oxide
What’s Known
It is known that high doses of the standard form of vitamin C have the ability to correct blood pressure and vascular function.
What’s New
The present study was designed to examine the biological and pharmacological effects of both standard and liposomal ascorbic acid formulations at the same doses.
Based on the results, liposomal effect was more pronounced; thus, it could be suggested that liposomal formulation could reduce the needed dose.
Introduction
Hypertension is one of the greatest world’s hearth problems due to its enormous long-term end-organ damages. One of the modifiable complications of uncontrolled hypertension is functional and structural vascular abnormalities.
Vascular endothelium effectively modulates vascular tone and structure by producing a number of vasorelaxing agents such as nitric oxide (NO), prostaglandins (PG), and endothelium-derived hyperpolarizing factors. Vasorelaxing factors could effectively protect vessel walls from extra loaded-pressure and balance the effects of potent endogenous and/or exogenous contracting factors. 1 Hypertension is associated with an imbalance between endothelial relaxing and contracting factors, leading to less production or availability of NO and, consequently, less antagonism of contracting factors. 2 We and others have shown that experimental hypertension is associated with impaired endothelial-dependent relaxation due to the acetylcholine (Ach) and enhanced vascular contraction response to the contracting agents such as phenylephrine (PE). 3 , 4 Moreover, clinical and experimental hypertension is associated with increased markers of oxidative stress. 5 - 7
The development of hypertension has been partly attributed to the increased oxidative stress; therefore, antioxidants, such as vitamin C, have been shown to have antihypertensive effects. 8 Despite the tremendous progress in the development and availability of antihypertensive medications, hypertension still remains one of the most relevant cardiovascular risk factors. Currently, various drugs, including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, beta blockers, are used to treat hypertension. However, it has also been shown that natural antioxidant substances, such as vitamin C, 8 vitamin E, 9 and herbal flavonoids such as resveratrol and oleuropein, 10 , 11 have a significant power to prevent and/or control hypertension.
Vitamin C is a micronutrient with aqueous-phase antioxidant properties. The early hypothesis of antihypertensive activity of vitamin C was introduced in 1946; 12 then, it was followed by other clinical and experimental studies. 13 , 14 There are some mechanistic studies that indicate a major role for vitamin C in maintaining normal blood pressure, function or reversing vascular abnormalities via the normal production and biological activity of endothelium-derived NO. 15
Liposomal drug delivery is an established technology system and due to its several advantages, it has obtained considerable clinical acceptance. These preparations have some typical pharmacodynamics and pharmacokinetic properties such as improvement in drug efficacy and therapeutic index, increased drug stability, reduced drug exposure to sensitive tissue, and a significant reduction in some deleterious adverse effects. These properties are responsible for its more clinical acceptance and applications. 16 Moreover, liposomal forms lead to higher plasma levels and circulating time compared to standard forms. 17
Considering antioxidant and antihypertensive activity of high doses of standard vitamin C, and superiority of liposomal dosage forms, the present study was designed to compare the antihypertensive as well as vascular modifying activities of liposomal and non-liposomal vitamin C in a rat model of two-kidney, one-clip renovascular hypertension.
Materials and Methods
Liposomal Vitamin C Preparation
The liposomal form of vitamin C was prepared in the Department of Pharmaceutics, School of Pharmacy, Shiraz University of Medical Science, Shiraz, Iran, using the following methods. 18 Briefly, ascorbic acid-sorbitol granules as the carrier were used to produce pro-liposomes using the film deposition method. Then, the granules were transferred into a flask, which was connected to a rotary evaporator under vacuum. The lipid phase (soy lecithin-cholesterol) was then added in drop to the flask until chloroform was evaporated completely. The obtained solid pro-liposome powder was passed through 35 meshes and left in a desiccator for complete drying. Liposomes were reconstituted in water, and the volume median diameter (VMD, 50% undersize) of the liposomes was evaluated by particle size analyzer (Shimadzu, SALD-2101, Japan). The amount of pro-liposome powder recovery was 95±1.2 (%). Reconstituted liposomes size was 46.6±0.2 micron.
Animals
Forty-nine male Sprague-Dawley rats, weighing 200-250 g, were obtained from Laboratory Animal Breeding Center, Shiraz University of Medical Sciences, Shiraz, Iran, and fed on standard rat chow with free access to drinking water. The animals were cared according to the national guidelines on the use of laboratory animals. All animal procedures of the present study were approved by the Institutional Animal Care and Use Committee, revised 2011. 19
Experimental Design
Animals were randomly divided into seven groups (n=7 in each), including a sham-operated assigned to receive vehicle (Sham-Veh), a 2K1C hypertensive group (HTN), a hypertensive group assigned to receive liposomes not loaded with vitamin C, as vehicle (HTN-veh), two hypertensive assigned to liposomal vitamin C (Sigma-Aldrich Chemical Co. Steinheim, North Rhine-Westphalia, Germany) at 50 (LVC-50) or 100 (LVC-100) mg/kg/day, and two hypertensive allocated to receive non-liposomal vitamin C at 50 (VC-50) or 100 (VC-100) mg/kg/day. The vehicle was composed of L-α-lecithin (3-sn -phosphatidyl choline) from soybean, which was not loaded with vitamin C. The vehicle or drugs were administered by oral gavage.
Experimental Protocol
Induction of 2K1C hypertension
Two-kidney, one clip (2K1C) renovascular hypertension was induced as previously described. 20 Briefly, animals were anesthetized with intraperitoneal injections of ketamine (60 mg/kg) and xylazine (8 mg/kg) (Alfasan; Woerden, Holland). Through a left flank incision, left renal arteries were exposed and dissected away from renal veins. Afterward, self-made solid plexiglass clips (internal diameter of 0.2-0.22 mm) were placed around the arteries. Antibiotic (penicillin) powder (Jaber Ebn-e Hayyan, Tehran, Iran) was applied to the incision sites, and the abdominal wall and skin were sutured using absorbable (catgut 3/0) and nonabsorbable (silk 3/0) suture materials, respectively. Sham-operated animals were subjected to a similar procedure, but no clip was placed around the renal arteries. Animals were then recovered from anesthesia and were kept for 4 weeks under standard condition (temperature, 22±2 ºC; relative humidity, 50%; light/dark cycle, 12 hours) with food (standard rat chow) and water ad libitum. Oral pretreatment with vehicle, liposomal or standard forms of vitamin C was started from the next day after the operation and the duration of administration was 28 days. Systolic blood pressure (SBP) and heart rate (HR) were measured weekly using noninvasive tail-cuff method (Chart 5.0 software; PowerLab 4/30, AD Instruments Inc., MA, Australia). Three consecutive blood pressure measurements, which had a difference of less than 5 mmHg, were considered as valid.
Isolated Aortic Studies
The thoracic aortas were dissected and cleaned of surrounding tissues; then, cut into small rings (3–4 mm each). The rings were mounted on hooks connected to force transducers in isolated tissue organ baths (K30, Hugo Sachs Electronik, Germany) filled with 20 ml of a physiological solution containing the following composition (mmol/L): NaCl 118, KCl 4.7, KH2PO4 1.2, CaCl2 2.5, MgSO4 1.2, NaHCO3 25, D-glucose 11.1, and bubbled constantly with 95% O2 and 5% CO2 at a pH of 7.4 and a temperature of 37 ºC. Tensions were recorded by a four-channel polygraph (model 705/1; Hugo Sachs Elektronik, Germany). The tissues were allowed to stabilize for 60 min, during which the buffer was changed every 20 min. Then, full concentration–contraction response curves to PE (Sigma-Aldrich Chemical Co. Steinheim, North Rhine-Westphalia, Germany) were performed. At the plateau response to PE, the tissues were washed twice 15 min apart. After a 30-min equilibration, each ring was contracted with PE using a concentration that caused 50% of the maximal contraction. At the plateau of contraction response to PE, concentration–relaxation response to Ach or SNP (Sigma-Aldrich Chemical Co. Steinheim, North Rhine-Westphalia, Germany) was performed. For the examination of the basal release of NO, full concentration–response to PE was performed in the absence and presence of 10-4M L-NAME (Sigma-Aldrich Chemical Co. Steinheim, North Rhine-Westphalia, Germany).21 Concentration–response curves to PE were compared using effective concentration 50 (EC50) and maximal response (Emax); concentration–response curves to Ach or SNP were compared using inhibitory concentration 50 (IC50) and Emax.
Statistical Analysis
Data, presented as mean±SEM, were analyzed using one-way analysis of variance (ANOVA). Where a significant difference was obtained with one-way ANOVA, the source of difference was located using Duncan’s multiple range test. The data were analyzed using the Sigmaplot statistical and graphical software version 11.0. (SigmaPlot. Systat Software Inc., San Jose, USA). A P value of <0.05 was considered statistically significant.
Results
Systolic Blood Pressure and Heart Rate
The weekly systolic blood pressure (SBP) and HR of all groups are shown in tables 1 and 2, respectively. Systolic blood pressure of HTN and HTN-veh groups at weeks 1 through 4 were significantly higher than those of the Sham-veh group at identical time points. Systolic blood pressures of HTN-VC50 at the second and fourth week and HTN-VC100 at weeks 2 through 4 were significantly lower than those of the HTN group. Furthermore, Systolic blood pressures of HTN-LVC50 at first and fourth weeks and HTN-LVC100 at weeks 1 through 4 were significantly lower than those of the HTN group. The SBP of HTN-LVC100 at weeks 2 through 4 were significantly lower than those of the HTN-LVC50 group. The SBP of HTN-VC100 at the first week was significantly lower than that of the HTN-VC50 group at the same time. Moreover, a comparison between liposomal and non-liposomal formulations showed that the SBP of HTN-LVC50 at the first week was significantly lower than that of the HTN-VC50 group at the identical time. Additionally, systolic blood pressures of HTN-LVC100 at weeks 2 and 4 were significantly lower than those of the HTN-VC100 group at the same time points.
Table1.
The effects of vehicle, standard, and liposomal forms of vitamin C on systolic blood pressure (mean±SEM) in all animal groups during 4 weeks of the study
| Week1 | Week2 | Week3 | Week4 | |
|---|---|---|---|---|
| Sham-veh | 117.7±3.6 | 122.5±2.9 | 121.5±2.0 | 122.2±2.5 |
| HTN | 146.1±4.1 a | 154.6±6.2 a | 162.2±7.8 a | 179.4±6.1a |
| HTN-veh | 142.6±4.1 a | 150.4±8.5 a | 149.0±6.6 a | 165.2±3.8 a |
| HTN-VC50 | 156.9±2.8 | 136.0±4.6e | 145.5±2.4 | 152.6±5.5g |
| HTN-VC100 | 137.4±3.9b | 136.1±3.5f | 135.7±1.9f | 149.5±4.3h |
| HTN-LVC50 | 128.0±3.0c, d | 150.1±3.8 | 149.3±3.1 | 154.1±3.7g |
| HTN-LVC100 | 125.0±4.7c | 112.6±3.6i,j,k | 132.0±3.3l,c | 131.5±4.0i,j,m |
Sham-veh: sham-operated group treated with vehicle; HTN: hypertensive group; HTN-Veh: hypertensive group treated with vehicle; HTN-VC50: hypertensive group treated with 50 mg/kg non-liposomal vitamin C; HTN-VC100: hypertensive group treated with 100 mg/kg non-liposomal vitamin C; HTN-LVC50: hypertensive group treated with 50 mg/kg liposomal vitamin C; HTN-LVC100: hypertensive group treated with 100 mg/kg liposomal vitamin C. Significant difference (a; P<0.001) from Sham-veh. Significant difference (c; P=0.008, e; P=0.04, f; P=0.03, g; P=0.008, h; P=0.006, j; P<0.001) from HTN. Significant difference (i; P=0.004, l; P=0.04) from HTN-LVC50. Significant difference (b; P=0.008, d; P<0.001) from HTN-VC50. Significant difference (k; P<0.001, m; P=0.03) from HTN-VC100
Table2.
The effects of vehicle, standard, and liposomal forms of vitamin C on heart rates (mean±SEM) in all animal groups during 4 weeks of the study
| Heart rate (beats/min) | ||||
|---|---|---|---|---|
| Week1 | Week2 | Week3 | Week4 | |
| Sham-veh | 404.8±6.7 | 402.0±6.8 | 401.1±6.7 | 404.5±5.4 |
| HTN | 429.0±7.6a | 443.7±5.4b | 452.1±6.5b | 456.8±8.2b |
| HTN-veh | 435.5±6.4a | 439.0±5.7b | 446.5±6.6c | 454.1±6.6b |
| HTN-VC50 | 438.3±5.9 | 435.0±6.7 | 435.7±6.1 | 433.1±8.3 |
| HTN-VC100 | 424.0±6.1 | 415.4±4.0d,l | 419.5±5.1e | 414.7±4.3f |
| HTN-LVC50 | 429.5±5.0 | 434.3±5.2 | 430.6±3.1h | 429.1±4.1g |
| HTN-LVC100 | 416.7±6.3 | 414.2±4.5i, e | 414.2±8.9j, e | 409.8±6.9i, k |
Sham-veh: sham-operated group treated with vehicle; HTN: hypertensive group; HTN-Veh: hypertensive group treated with vehicle; HTN-VC50: hypertensive group treated with 50 mg/kg non-liposomal vitamin C; HTN-VC100: hypertensive group treated with 100 mg/kg non-liposomal vitamin C; HTN-LVC50: hypertensive group treated with 50 mg/kg liposomal vitamin C; HTN-LVC100: hypertensive group treated with 100 mg/kg liposomal vitamin C. Significant difference (a; P=0.008, b; P<0.001, c; P=0.02) from Sham-veh. Significant difference (d; P=0.02, e; P=0.008, f; P=0.006, g; P=0.008, h; P=0.03, k; P<0.001) from HTN. Significant difference (i; P≤0.008, j; P≤0.03) from HTN-VC50; Significant difference (l; P=0.04) from HTN-VC50
As shown in table 2, the HR of HTN and HTN-veh groups at weeks 1 through 4 were significantly higher than those of the Sham-veh group at identical time points. Heart rates of HTN-LVC50 at weeks 3 through 4 and HTN-LVC100 at weeks 2 through 4 were significantly lower than those of the HTN group at the same time points. Moreover, HR of HTN-VC100 at weeks 2 through 4 was significantly lower than that of the HTN group. A comparison of the HR between both liposomal and non-liposomal forms either at 50 or 100 mg/kg exhibited no significant differences during the 4 weeks.
Isolated Aortic Ring Studies
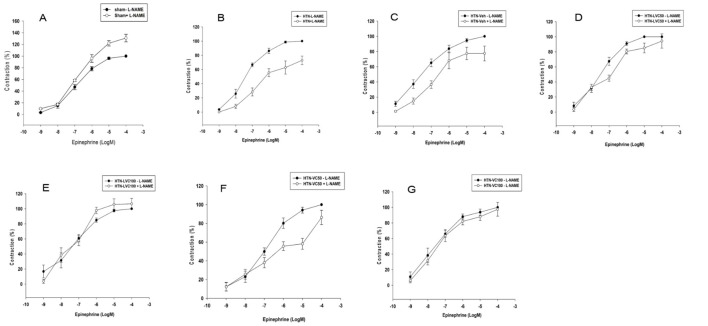
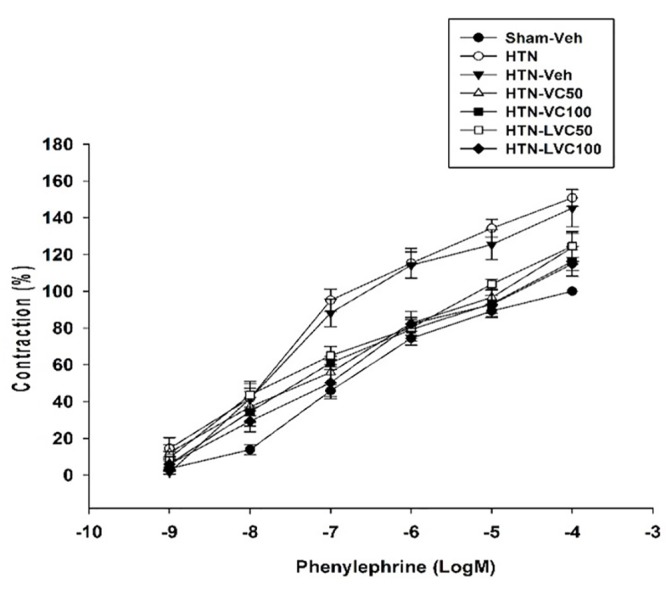
The PE concentration-response curve in the absence of L-NAME is shown in figure 1. As it shows, the Emaxs of PE concentration-responses of HTN (150.8±4.56; P<0.001) and HTN-veh (145.2±10.17; P=0.009) groups were significantly higher than that of Sham-veh group (100±0.00). The Emaxs of PE concentration-responses of HTN-VC50 (123.81±7.75; P=0.008) and HTN-VC100 (116.20±8.00; P=0.03) were significantly lower than that of the HTN group. Furthermore, the Emaxs of PE concentration-responses of HTN-LVC50 (124.5±8.00; P=0.008) and HTN-LVC100 (114.8±3.50; P=0.008) were significantly lower than that of the HTN group. There was no significant difference between the Emaxs of either liposomal or non-liposomal forms of vitamin C at each dose.
Figure1.
The figure shows thephenylephrine concentration–response curves (n=7 each) in the absence of nitro-L-arginine methyl ester (L-NAME) in aortic rings from Sham-operated vehicle-receiving (Sham-veh), hypertensive (HTN), hypertensive vehicle (HTN-veh), and 4 groups of hypertensive rats receiving 50 or 100 mg/kg/day of liposomal (HTN-LVC-50 and 100) or non-liposomal Ascorbic acid (HTN-VC50 and 100) during 4 weeks of experiment. The response (contraction %) was calculated as the percentage of Phenylephrine maximal response in the absence of L-NAME.
In the presence of L-NAME, while the Emax of Sham-veh group increased significantly (136.66±4.57; P=0.008), the Emaxs of both HTN (73.00±6.00; P<0.001) and HTN-veh (77.50±9.70; P=0.009) groups decreased significantly (figure 2A, B, and C). In contrast, compared to those in the absence of L-NAME, the Emaxs of HTN-CV50 (91.32±7.17; P=0.02), HTN-CV100 (97.50±8.88; P=0.008), HTN-LCV50 (94.30±9.46; P=0.009), and HTN-LCV100 (112.63±6.00; P<0.001) groups increased significantly during the four weeks of experiment (figure 2D, E, F, and G). There was no significant difference between the Emaxs of contraction responses to PE in the absence and presence of L-NAME in each vitamin C treated group.
Figure2.
The figure shows the phenylephrine concentration–response curves (n=7 each) in the absence (-) and presence (+) of nitro-L-arginine methyl ester (L-NAME) in aortic rings from Sham-operated vehicle-receiving (Sham-veh), hypertensive (HTN), hypertensive vehicle (HTN-veh), and 4 groups of hypertensive rats receiving 50 or 100 mg/kg/day of liposomal (HTN-LVC-50 and 100) or non-liposomal Ascorbic acid (HTN-VC50 and 100) during 4 weeks of experiment. The response (contraction %) was calculated as the percentage of Phenylephrine maximal response in the absence of L-NAME.
In the absence of L-NAME, the EC50s of PE contraction responses of HTN and HTN-veh groups were significantly lower compared to Sham-veh (table 3). There was no significant difference among the EC50s values of HTN-VC50, HTN-VC100, HTN-LVC50, and HTN-LVC100. In the presence of L-NAME, the EC50s of HTN and HTN-veh groups increased significantly. There was no significant difference among the EC50s of HTN-LVC50, HTN-LVC100, HTN-VC50, and HTN-VC100 in the presence of L-NAME (table 3).
Table3.
The effects of vehicle, standard, and liposomal forms of vitamin C on contraction and relaxation function of isolated aortic rings after 4 weeks of experiment
| PE EC50-LNAME (M) | PE EC50+LNAME (M) | Ach IC50 (M) | SNP IC50 (M) | |
|---|---|---|---|---|
| Sham-veh | -7.11±0.11 | -7.06±0.18 | -7.02±0.18 | -8.13±0.21 |
| HTN | -7.39±0.10a | -6.65±0.16c | -7.13±0.17 | -8.40±0.11 |
| HTN-veh | -7.32±0.18b | -6.79±0.16 | -7.15±0.19 | -8.37±0.23 |
| HTN-VC50 | -7.20±0.10 | -7.00±0.16 | -7.11±0.23 | -8.31±0.26 |
| HTN-VC100 | -7.00±0.13 | -7.10±0.31 | -6.38±0.10d | -8.13±0.14 |
| HTN-LVC50 | -7.40±0.05 | -7.11±0.10 | -6.94±0.17 | -8.37±0.25 |
| HTNLVC100 | -7.01±0.21 | -6.99±0.15 | -6.98±0.15e | -8.24±0.18 |
Sham-veh: sham-operated group treated with vehicle; HTN: hypertensive group; HTN-veh: hypertensive group treated with vehicle; HTN-VC50: hypertensive group treated with 50 mg/kg non-liposomal vitamin C; HTN-VC100: hypertensive group treated with 100 mg/kg non-liposomal vitamin C; HTN-LVC50: hypertensive group treated with 50 mg/kg liposomal vitamin C; HTN-LVC100: hypertensive group treated with 100 mg/kg liposomal vitamin C; PE: phenylephrine, L-NAME: nitro-L-arginine methyl ester; EC50: effective concentration 50; Ach: acetylcholine, SNP: sodium nitroprusside; IC50: inhibitory concentration 50. Significant difference (a; P=0.008, b; P=0.01) from Sham-veh. Significant difference (c; P<0.001) from HTN; Significant difference (d; P<0.001) from HTN-VC100
The Emaxs of Ach concentration responses of HTN (67.96±5.88) and HTN-Veh (62.40±5.40) groups were significantly lower than that of the Sham-veh group (85.50±4.90). The Emaxs of Ach concentration responses of HTN-LVC50 (79.06±1.20; P=0.03), HTN-LVC100 (92.27±3.50; P<0.001) and HTN-VC100 (93.69±4.00; P=0.008) were significantly higher than that of the HTN group (67.96±5.88). The Emax of Ach-relaxation response of HTN-VC100 (93.69±4.00; P=0.005) was significantly higher than that of the HTN-VC50 group (71.55±4.38). Moreover, The Emax of Ach-relaxation response of HTN-LVC100 (92.27±3.50; P=0.009) was significantly higher than that of the HTN-LVC50 group (79.06±1.20). There was no significant difference between the Emaxs of either liposomal or non-liposomal forms of vitamin C at each dose (figure 3).
Figure3.
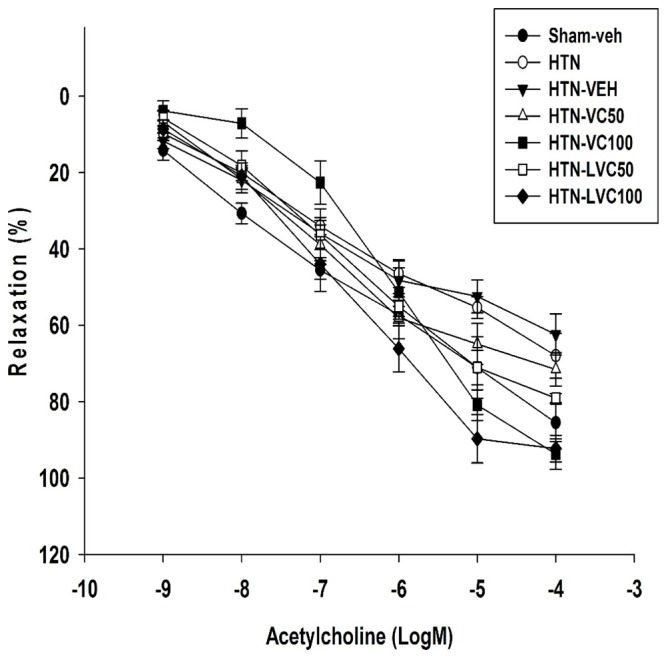
The figure shows the acetylcholine relaxation response curves (n=7 each) in aortic rings from Sham-operated vehicle-receiving (Sham-veh), hypertensive (HTN), hypertensive vehicle (HTN-veh), and 4 groups of hypertensive rats receiving 50 or 100 mg/kg/day of liposomal (HTN-LVC-50 and 100) or non-liposomal Ascorbic acid (HTN-VC50 and 100) during 4 weeks of experiment. The response (relaxation %) was calculated as the percentage of maximal responses to Acetylcholine in the control group.
There was no significant difference between EC50 of Ach relaxation responses of Sham-veh, HTN, HTN-veh, HTN-VC50, HTN-LVC50, and HTN-LVC100 (table 3). The EC50 of Ach relaxation response of HTN-VC100 was significantly higher than that of HTN (table 3). The EC50 of Ach relaxation response of HTN-LVC100 was significantly lower than that of HTN-VC100 (table 3).
There was no significant difference between Emaxs or EC50s of SNP concentration-response curves of Sham-veh, HTN-veh, HTN-LVC50, HTN-VC100, HTN-LVC50, and HTN-LVC100 groups (table 3).
Discussion
Our findings showed that the administration of both liposomal and non-liposomal forms of vitamin C led to a dose-dependent reduction in systolic blood pressure, with no significant change in HR; thus, the higher doses showed the higher blood pressure lowering effects compared to that of lower doses. As it was found, compared to non-liposomal form, the liposomal one was associated with a more prominent effect on final SBP. Treatment with Vitamin C was also associated with a significant reduction in the Emaxs of PE-induced contraction responses. The hypertension-induced hypersensitivity to PE was significantly recovered by the administration of vitamin C, either by liposomal or non-liposomal formulation. Post-L-NAME contraction response, which was markedly impaired by the chronic hypertension, was effectively improved by administration of vitamin C, dose-dependently. However, there was no significant difference between liposomal and non-liposomal forms of vitamin C regarding the later response. Although the Emax of Ach-relaxation response was not different in two preparation forms, Ach-relaxation response induced a lower concentration of the liposomal form of vitamin C (lower IC50 compared to standard form).
The present findings, which are similar to that of previous ones, 6 , 22 show a time-dependent increase in SBP and HR in untreated 2K1C animals during the 4 weeks of the experiment. There is a lot of evidence indicating that chronic hypertension negatively affects vascular function. In agreement with others and our previous reports, 1 , 3 , 11 the present study also showed that in the absence of L-NAME, the contraction response to PE increased significantly in 2K1C hypertensive aortic rings. These findings might be due to alfa-1 adrenoceptors up-regulation on hypertensive vascular smooth muscle. 23
Moreover, in this experiment, we examined both basal and stimulated release of NO on the vascular structure. In order to inspect the basal release of NO, the contraction response to PE was examined in the absence and presence of L-NAME. Enhanced contraction response to PE in the presence of L-NAME, relative to that in its absence, is an indicator of the increase in the basal release of NO and vice versa. 24 Similar to our previous reports, 3 , 11 in the presence of L-NAME, contraction response to PE was impaired in hypertensive aortic rings, which indicates impaired basal release of NO in this situation. Stimulated release of NO accessed by Ach showed a blunted relaxation response in hypertensive aortic rings. The impaired response was characterized by a decrease in Emax and an increase in IC50. 1 , 3 , 11 Such a finding indicates that in agreement with other studies, 3 , 11 chronic hypertension could strongly impair both basal and stimulated release of NO. In spite of endothelial-dependent relaxation, and as documented in other previous studies, 3 , 11 endothelial-independent relaxation response, which was tested by NO-donor agent, SNP, was not affected anyway.
According to population-based observational studies, there is an inverse correlation between vitamin C plasma concentration and blood pressure level. 25 There is some controversy regarding the antihypertensive properties of ascorbic acid in clinical studies, which might be due to different research sample sizes, target group ages, and dose and/or duration of ascorbic acid administrations. According to Hickey and colleagues, following continuous oral administration of liposomal vitamin C, the plasma level reached approximately twice the predicted maximum level with standard form of vitamin C. 17 Accordingly, they concluded that large oral doses of vitamin C could potentially be used as a non-toxic, sustainable, therapeutic agent. 17 To the best of our knowledge, the present study is the first to compare both liposomal and non-liposomal forms of high doses vitamin C regarding chronic antihypertensive and vascular response in renovascular hypertensive rats. Our findings indicate that compared to the hypertensive group, treatment with vitamin C, in both forms and doses, could effectively decrease the SBP in a dose-dependent manner. The SBP was not different between the liposomal and non-liposomal lower doses, but in higher doses, the liposomal form of vitamin C had a greater blood pressure lowering effect compared to the non-liposomal ones. According to previous reports which compared the plasma concentration level of both standard and liposomal forms of vitamin C, 17 , 26 it could be suggested that the observed higher blood pressure lowering activity of liposomal vitamin C is due to the practice of keeping drug levels constant for longer duration, higher circulating time, and/or other liposome-forming characteristics. Moreover, our findings showed that high doses of vitamin C in both forms exert modest vascular beneficial effects as compared to the control ones. However, the present study did not find evidence demonstrating that the liposomal form of vitamin C could represent greater vascular modifying activity in untreated hypertensive aortic rings.
We also exhibited that the administration of both liposomal and non-liposomal forms of vitamin C, dose-dependently, enhanced NO basal release, which had previously been blunted in the presence of L-NAME. Enhanced NO basal release was characterized by an increased maximal contraction response to PE in the presence of L-NAME relative to the response in its absence. Moreover, the IC50 reduced significantly with the liposomal formulation. Therefore, it might be concluded that modified properties in liposomal form could help us achieve our desirable relaxation response with a significant lower concentration. In contrast, the endothelial-independent relaxation response to SNP was not affected by vitamin C treatment.
We previously reported that the 2K1C renovascular hypertension was associated with the increase in oxidative stress indicators such as malondialdehyde (MDA) and the decrease in the endogenous antioxidant capacity such as superoxide dismutase (SOD) activity. 3 , 27 The antioxidant activity of vitamin C has been fully demonstrated by several lines of evidence. 8 , 15 Therefore, the present study did not examine whether or not oxidative stress underlie the antihypertensive effects and/or vascular modifying activity of high-dose vitamin C. The findings also suggest that the use of vitamin C as a complement might lead to the reduction of blood pressure in current therapeutic protocols.
The comparison of the vascular activity of liposomal and non-liposomal forms of vitamin C, regarding EC50%, IC50%, and the maximal efficacy reported in this study, are beneficial for conducting further investigation on the formulations. However, one of the limitations of the present study was that it did not assess any relationship between dose and plasma concentration of both liposomal and non-liposomal forms of vitamin C, but doing such a measurement could provide us with important information regarding the correlation between liposome dosage form and its biological effects.
Conclusion
Our findings indicate that in spite of blood lowering activities of either liposomal or non-liposomal forms of vitamin C, the effects of the liposomal form of vitamin C on final SBP was more prominent at the end of 4 weeks of the experiment. The observed antihypertensive activity was not associated with reflex tachycardia in either form. Our vascular results exhibit that the antihypertensive activity could partly be mediated by enhancing the release of both basal and stimulated NO. Moreover, the liposomal form could help us achieve our desirable Ach-relaxation response with a significant lower concentration. Further experimental investigation is needed to clarify the relationship between the plasma level and these pharmacological effects.
Acknowledgement
The authors would like to thank Shiraz University of Medical Sciences for their financial support of the study.
Conflict of Interest: None declared.
References
- 1.Ajay M, Achike FI, Mustafa MR. Modulation of vascular reactivity in normal, hypertensive and diabetic rat aortae by a non-antioxidant flavonoid. Pharmacol Res. 2007;55:385–91. doi: 10.1016/j.phrs.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Park KH, Park WJ. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J Korean Med Sci. 2015;30:1213–25. doi: 10.3346/jkms.2015.30.9.1213. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalili A, Nekooeian AA, Khosravi MB, Fakher S. Simultaneous renal hypertension and type 2 diabetes exacerbate vascular endothelial dysfunction in rats. Int J Exp Pathol. 2012;93:210–7. doi: 10.1111/j.1365-2613.2012.00811.x. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalili A, Khosravi MB, Nekooeian AA. The effects of aqueous extract of vaccinium arctostaphylos leaves on blood pressure in renal hypertensive rats. Iran Red Crescent Med J. 2011;13:123–7. [ PMC Free Article ] [PMC free article] [PubMed] [Google Scholar]
- 5.Naregal GV, Devaranavadagi BB, Patil SG, Aski BS. Elevation of Oxidative Stress and Decline in Endogenous Antioxidant Defense in Elderly Individuals with Hypertension. J Clin Diagn Res. 2017;11:BC09–BC12. doi: 10.7860/JCDR/2017/27931.10252. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalili A, Nekooeian AA, Khosravi MB. Oleuropein improves glucose tolerance and lipid profile in rats with simultaneous renovascular hypertension and type 2 diabetes. J Asian Nat Prod Res. 2017;19:1011–21. doi: 10.1080/10286020.2017.1307834. [DOI] [PubMed] [Google Scholar]
- 7.Nekooeian AA, Khalili A, Khosravi MB. Effects of Short-term Renovascular Hypertension and Type 2 Diabetes on Cardiac Functions in Rats. Iran J Med Sci. 2014;39:51–9. [ PMC Free Article ] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Khudairy L, Flowers N, Wheelhouse R, Ghannam O, Hartley L, Stranges S, et al. Vitamin C supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;3:CD011114. doi: 10.1002/14651858.CD011114.pub2. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuwabara A, Nakade M, Tamai H, Tsuboyama-Kasaoka N, Tanaka K. The association between vitamin E intake and hypertension: results from the re-analysis of the National Health and Nutrition Survey. J Nutr Sci Vitaminol (Tokyo) 2014;60:239–45. doi: 10.3177/jnsv.60.239. [DOI] [PubMed] [Google Scholar]
- 10.Mozafari M, Nekooeian AA, Panjeshahin MR, Zare HR. The effects of resveratrol in rats with simultaneous type 2 diabetes and renal hypertension: a study of antihypertensive mechanisms. Iran J Med Sci. 2015;40:152–60. [ PMC Free Article ] [PMC free article] [PubMed] [Google Scholar]
- 11.Nekooeian AA, Khalili A, Khosravi MB. Effects of oleuropein in rats with simultaneous type 2 diabetes and renal hypertension: a study of antihypertensive mechanisms. J Asian Nat Prod Res. 2014;16:953–62. doi: 10.1080/10286020.2014.924510. [DOI] [PubMed] [Google Scholar]
- 12.Hoitink A. Research on the influence of vitamin C administration on the human organism, in particular in connection with the working capacity. Verh Nederlands Inst Praevent. 1946;4:62–3. [Google Scholar]
- 13.Block G, Jensen CD, Norkus EP, Hudes M, Crawford PB. Vitamin C in plasma is inversely related to blood pressure and change in blood pressure during the previous year in young Black and White women. Nutr J. 2008;7:35. doi: 10.1186/1475-2891-7-35. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasdev S, Ford CA, Parai S, Longerich L, Gadag V. Dietary vitamin C supplementation lowers blood pressure in spontaneously hypertensive rats. Mol Cell Biochem. 2001;218:97–103. doi: 10.1023/a:1007234027421. [DOI] [PubMed] [Google Scholar]
- 15.Duvall WL. Endothelial dysfunction and antioxidants. Mt Sinai J Med. 2005;72:71–80. [PubMed] [Google Scholar]
- 16.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8:102. doi: 10.1186/1556-276X-8-102. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickey S, Roberts HJ, Miller NJ. Pharmacokinetics of oral vitamin C. Journal of Nutritional & Environmental Medicine. 2008;17:169–77. doi: 10.1080/13590840802305423. [DOI] [Google Scholar]
- 18.Parhizkar E, Rashedinia M, Karimi M, Alipour S. Design and development of vitamin C-encapsulated proliposome with improved in-vitro and ex-vivo antioxidant efficacy. J Microencapsul. 2018;35:301–11. doi: 10.1080/02652048.2018.1477845. [DOI] [PubMed] [Google Scholar]
- 19.Council NR. Guide for the care and use of laboratory animals. 8th edition. Washington : National Academies Press; 2011. [PubMed] [Google Scholar]
- 20.Nekooeian A, Mashhoodi T. Solid plexiglass clips to induce reproducible renal hypertension in the rat. J Pharmacol. 2007;39:25. doi: 10.4103/0253-7613.30758. [DOI] [Google Scholar]
- 21.Duarte DA, Silva KC, Rosales MA, Lopes de Faria JB, Lopes de Faria JM. The concomitance of hypertension and diabetes exacerbating retinopathy: the role of inflammation and oxidative stress. Curr Clin Pharmacol. 2013;8:266–77. doi: 10.2174/1574884711308040002. [DOI] [PubMed] [Google Scholar]
- 22.Khalili A, Khosravi MB, Nekooeian AA. The effects of aqueous extract of vaccinium arctostaphylos leaves on blood pressure in renal hypertensive rats. Iran Red Crescent Med J. 2011;13:123–7. [ PMC Free Article ] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendriks MG, Kam KL, Pijl AJ, Pfaffendorf M, van Zwieten PA. The effects of hypertension and diabetes mellitus on the vascular reactivity of resistance arteries. Blood Press. 1993;2:69–76. doi: 10.3109/08037059309077530. [DOI] [PubMed] [Google Scholar]
- 24.Wong CM, Yao X, Au CL, Tsang SY, Fung KP, Laher I, et al. Raloxifene prevents endothelial dysfunction in aging ovariectomized female rats. Vascul Pharmacol. 2006;44:290–8. doi: 10.1016/j.vph.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Moran JP, Cohen L, Greene JM, Xu G, Feldman EB, Hames CG, et al. Plasma ascorbic acid concentrations relate inversely to blood pressure in human subjects. Am J Clin Nutr. 1993;57:213–7. doi: 10.1093/ajcn/57.2.213. [DOI] [PubMed] [Google Scholar]
- 26.Pattni BS, Chupin VV, Torchilin VP. New Developments in Liposomal Drug Delivery. Chem Rev. 2015;115:10938–66. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 27.Nekooeian AA, Khalili A, Khosravi MB. Oleuropein offers cardioprotection in rats with simultaneous type 2 diabetes and renal hypertension. Indian J Pharmacol. 2014;46:398–403. doi: 10.4103/0253-7613.135951. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]