Abstract
Some of the simplest and most powerful carbon-carbon bond forming strategies take advantage of readily accessible ubiquitous motifs: carbonyls and olefins. Herein, we report a fundamentally distinct mode of reactivity between carbonyls and olefins that differs from established acid-catalyzed carbonyl-ene, Prins, and carbonyl-olefin metathesis reaction paths. A range of epsilon, zeta-unsaturated ketones undergo Brønsted acid-catalyzed intramolecular cyclization to provide tetrahydrofluorene products via the formation of two new carbon-carbon bonds. Theoretical calculations and accompanying mechanistic studies suggest that this carbocyclization reaction proceeds through the intermediacy of a transient oxetane formed by oxygen atom transfer. The complex polycyclic frameworks in this product class appear as common substructures in organic materials, bioactive natural products, and recently developed pharmaceuticals.
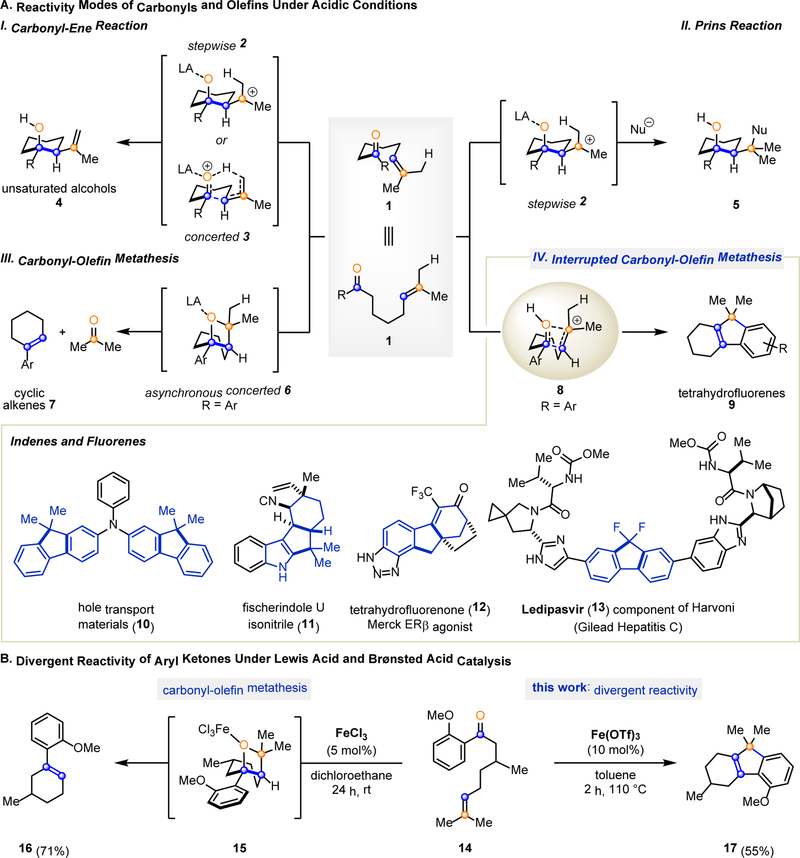
Carbonyl and olefin functionalities generally react together through carbonyl-ene (1–4, 5) or Prins (5, 6–10) pathways upon activation with strong Brønsted or Lewis acids. Both pathways can be accessed from similar substrate 1 depending on the choice of catalyst and conditions (Fig. 1A). Upon coordination to a carbonyl, Lewis acids can induce a change in polarization that enhances reactivity. Different Lewis acids can subtly alter the charge distribution in the resulting Lewis acid-substrate complex (11–14) to generate a continuum between a stepwise mechanism involving carbocationic intermediate 2 and a concerted mechanism via a 6-membered transition state 3 (I, Fig. 1A) (3). The carbonyl-ene reaction can proceed through either route to generate homoallylic alcohol 4 upon addition of an electrophilic carbonyl to an alkene with concomitant transfer of an allylic hydrogen atom. In comparison, the Prins reaction proceeds through intermediate carbocation 2 that is subsequently captured by an exogenous nucleophile to provide the corresponding alcohol 5 (II, Fig. 1A) (5). In a further modulation of the reactivity between carbonyls and olefins, we have recently reported an iron(III)-catalyzed carbonyl-olefin ring-closing metathesis reaction of substrate 1 to provide cyclohexene product 7 (III, Fig. 1A) (15). Mechanistic studies supported a concerted, asynchronous [2+2]-cycloaddition reaction that does not rely on carbocation intermediates to form oxetane 6, which subsequently fragments in an asynchronous, concerted retro-[2+2]-cycloaddition to provide the corresponding cyclopentene and -hexene metathesis products (16). In this context, Lewis acid activation opens access to intermediate oxetanes that are otherwise restricted to photochemical [2+2] cycloadditions such as the Paternὸ-Büchi reaction (17–19).
Fig. 1. Chemistry of Carbonyls and Olefins.
A. Fundamental acid-catalyzed reactivity modes between carbonyls and olefins. (I) Carbonyl-ene reaction; (II) Prins reaction; (III) Carbonyl-olefin metathesis; (IV) This work: interrupted carbonyl-olefin metathesis; importance of the products accessible in this reactivity mode is showcased below. LA, Lewis acid. B. Complementary reactivity modes of aryl ketone 14 are accessible depending on the choice of iron(III)-derived Lewis acid catalyst. The use of FeCl3 leads to the formation of the carbonyl-olefin metathesis product 16, whereas Fe(OTf)3 results in tetrahydrofluorene 17.
We herein report that these fundamental acid-mediated transformations between carbonyls and olefins can be expanded to include an additional mode of reactivity (IV, Fig. 1A). This transformation resembles the carbonyl-olefin metathesis reaction in that it also proceeds via an oxetane (8); however, its fragmentation pathway is interrupted to result in the formation of an intermediate carbocation. This interrupted carbonyl-olefin metathesis path relies on Brønsted acid activation of carbonyl and olefin functionalities to yield a complex, carbocyclic framework 9 (20, 21) upon formation of two carbon-carbon bonds. Whereas this multiple bond-forming process selectively yields the tetrahydrofluorene product 9, the presence of a Brønsted acid has previously been reported to be detrimental to many Lewis acid-catalyzed carbonyl-ene and Prins reactions, resulting in undesired polymerization and isomerization of olefins (22). Furthermore, this interrupted carbonyl-olefin metathesis reaction provides access to tetrahydrofluorene product 9 in a single synthetic transformation while current strategies rely on multistep sequences and precious metal-catalyzed cycloisomerizations (23, 24). Tetrahydrofluorenes represent key structural elements in materials science (10) (25), and are ubiquitous core structures in biologically active natural products (11) (26). Additionally, tetrahydrofluorenes are found in recently developed pharmaceuticals (Merck’s ERβ agonist 12 (27) and Ledipasvir 13 (28), part of Gilead’s two-component treatment against Hepatitis C, Harvoni).
During our investigations into the iron(III)-catalyzed carbonyl-olefin metathesis reaction, we discovered a complementary mode of reactivity of aryl ketone 14 depending on the choice of iron(III) catalyst (Fig. 1B). Specifically, when 14 was converted under the optimal reaction conditions developed for carbonyl-olefin metathesis using 5 mol% iron(III) chloride (FeCl3) in dichloroethane, the desired metathesis product 16 was obtained in 71% yield. We initially postulated that iron(III) triflate (Fe(OTf)3) could function as a stronger Lewis acid catalyst to further improve the yield of the carbonyl-olefin metathesis product 16. However, subjecting the same aryl ketone 14 to 10 mol% Fe(OTf)3 resulted in the formation of a new product in 55% yield, that was identified as tetrahydrofluorene 17. We hypothesized that this new compound likely arose from an intermediate carbocation that subsequently underwent Friedel-Crafts alkylation with the pendant aromatic ring to form the tricyclic core. This outcome is in stark contrast to the reactivity observed in FeCl3-catalyzed carbonyl-olefin metathesis, which proceeds via an asynchronous, concerted mechanism and does not involve carbocations as essential intermediates. As such, this result indicated that altering the catalytic system, which we ultimately discovered to be Brønsted acid catalyzed, provides access to a distinct reaction pathway relying on carbocation intermediates. The combined importance of the tetrahydrofluorene products obtained along with the distinct reactivity observed upon Brønsted acid catalysis of aryl ketone 14 prompted us to further optimize this reaction, explore the substrate scope, and investigate the reaction mechanism of this transformation.
Thorough optimization involved the evaluation of various Lewis and Brønsted acids, solvents, and reaction times, and ultimately led to the following optimal reaction conditions: 5 mol% triflic acid (TfOH) at 80 ˚C in degassed benzene (see Supplementary Materials for details).
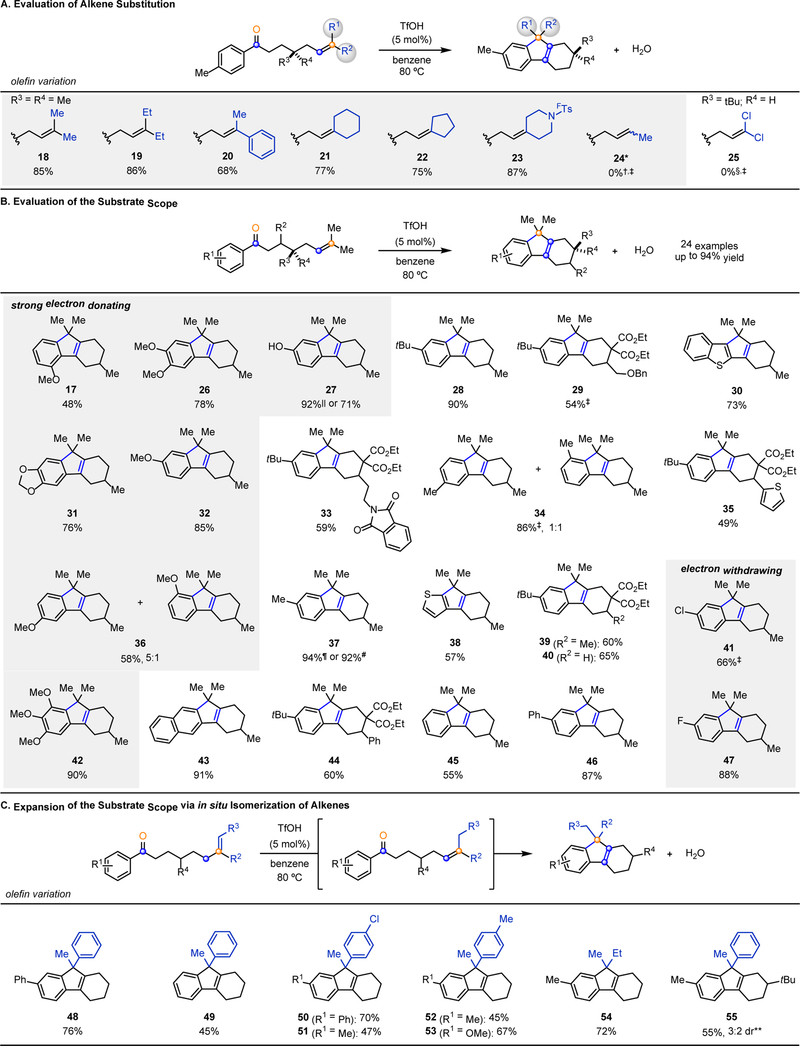
Initial efforts to explore the scope of this transformation focused on investigating the effect of varying the olefin substitution (Fig. 2A). The tri-substituted olefins 18 and 19 provided the corresponding tetrahydrofluorene product in 85% and 86% yield, respectively. Phenyl substituted olefin 20 and exocyclic olefins 21-23 proved to be viable substrates, with the latter leading to spirocyclic tetrahydrofluorene products. Specifically, piperidine 23 bearing a para-trifluoromethyl phenylsulfonylamide underwent efficient cyclization in 87% yield, demonstrating the potential for the incorporation of heteroatoms into the polycyclic scaffold. Aryl ketones 24 and 25, incorporating either a 1,2-di-substituted olefin or a 1,1-di-chlorinated olefin, failed to undergo the desired transformation, suggesting two carbon-based substituents are required to increase the nucleophilicity of the olefin.
Fig. 2. Substrate Scope.
Conditions: Ketone (1.0 equiv.; 0.02 M), TfOH (5 mol%) in benzene at 80 °C. See supplementary materials for additional experimental details. A. Variation of the alkene substitution. B. Variation of the aryl ketone and carbon tether. C. In situ isomerization of alkenes to expand substrate scope. Footnotes: *4.3:1 mixture of E/Z alkenes. †81% of starting material 24 is recovered. ‡Percent yield and percent conversion determined by 1H-NMR using dimethyl terephthalate or mesitylene as an internal standard. §98% of starting material 25 is recovered. ||Starting material TBS protected. ¶Percent yield determined by GC using dodecane as an internal standard. #Reaction run on 10.2 mmol scale. **Percent yield and diastereomeric ratio determined by 1H-NMR using dimethyl terephthalate as an internal standard.
The optimized reaction conditions for the formation of tetrahydrofluorenes proved general for various electronically and sterically differentiated aryl ketone substrates (Fig. 2B). Electron-rich aryl ketones bearing hydroxyl, methoxy, or dioxole functionalities underwent the desired transformation in good to excellent yields (17, 26, 27, 31, 32, 36, and 42, Fig. 2B). Specifically, di- and tri-methoxy substituted aryl ketones 26 and 42 provided the desired products in 78% and 90% yield, respectively. A silyl-protected phenol underwent the desired cyclization with advantageous in situ deprotection to result in 92% yield of 27; subjection of the corresponding unprotected phenol also afforded efficient conversion to the desired tetrahydrofluorene 27 in 71% yield. Aryl ketones bearing electron-neutral tert-butyl, methyl, naphthyl, or phenyl substitution at the aromatic moiety provided the corresponding products in up to 94% yield (28, 37, 43, and 46, Fig. 2B). Electron-deficient aryl ketones bearing chlorine or fluorine substitution reacted to form 42 and 47 in 66% and 88% yield, respectively. The para-position of the aromatic subunit was thoroughly investigated and found to be widely electronically tolerant (27-29, 32, 33, 35, 37, 39-41, 44, 46, 47, Fig. 2B). Similarly, substitution at the meta- and ortho-positions of the aromatic subunit led to the desired tetrahydrofluorene products; however in the case of meta-substituted substrates, a mixture of regioisomers 34 and 36 was observed. The optimized reaction conditions also proved efficient for heteroaromatic ketones, resulting in the formation of benzothiophene 30 in 73% yield and thiophene 38 in 57% yield. Aryl ketones that contained heteroatoms distal from the reactive sites proved viable substrates for the desired transformation, resulting in the corresponding tetrahydrofluorene products in up to 59% yield (29, 33, 35, Fig. 2B). Advantageously, benzyl ether 29 and phthalimide 33 can be deprotected to provide handles for further elaboration. Tert-butyl substituted aromatic substrates bearing distinct functionalities along the carbon backbone resulted in the formation of the desired products 39, 40, and 44 in up to 65% yield, while the substrate bearing minimal substitution also proved viable providing the desired tetrahydrofluorene 45 in 55% yield. Taken together, the substrate scope suggests that the electronics of the aryl ketone and sterics on the substrate tether do not alter the efficiency of this transformation. The optimized reaction conditions also proved amenable to gram-scale synthesis of 37.
Subsequent efforts focused on further expanding the scope of this transformation to include 1,1-disubstituted alkenes that are readily accessible via Wittig olefination or hydroarylation strategies (Fig. 2C). Isomerization of these alkenes in situ under Brønsted acid-catalysis results in the corresponding 1,2,2-trisubstituted analogues that subsequently enable facile access to tetrahydrofluorene products bearing distinct substitution at the central 5-membered ring. Specifically, aryl ketones bearing electron-rich and neutral substituents proved viable substrates in the isomerization-cyclization sequence and provided the corresponding tetrahydrofluorene products in up to 76% yield (48-55, Fig. 2C). Varying the electronics of the alkene itself was tolerated with both electron-poor (50, 51) and -neutral (52, 53) styrene derivatives which underwent the desired transformation in up to 70% yield. This in situ isomerization-cyclization sequence is not only limited to terminal styrene derivatives, but also tolerated the corresponding terminal alkene bearing aliphatic substituents (54). Modest diastereoselectivity (3:2 dr) was observed for tetrahydrofluorene 55, demonstrating the potential for this mode of reactivity to be used in the development of stereoselective methods.
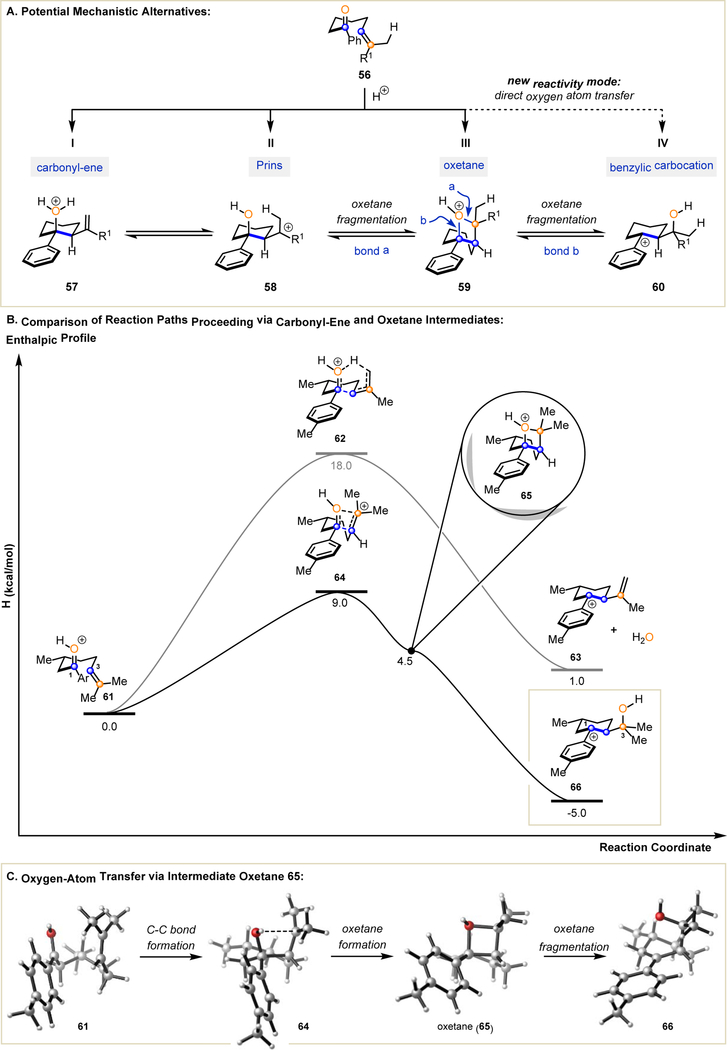
Based on the literature precedent of transformations between carbonyls and olefins, we initially considered a mechanistic hypothesis relying on a carbonyl-ene reaction to form alcohol 57 upon nucleophilic addition between the carbonyl and olefin functionalities of 56 (Fig. 3A). However, this initial mechanistic hypothesis proved inconsistent with experimental data (see Supplementary Materials for details), and other potential mechanistic alternatives were evaluated. In addition to a concerted carbonyl-ene reaction path (I, Fig. 3A), intermediate carbocation 58 could result from a nucleophilic addition between the carbonyl and olefin moieties in 56 following established Prins reactivity (II, Fig. 3A). A third alternative would be the formation of oxetane 59 in analogy to the recently established carbonyl-olefin metathesis reaction proceeding via the asynchronous, concerted formation of intermediate oxetanes (III, Fig. 3A). Intermediates 57, 58, and 59 could also interconvert in reversible transformations. Finally, Brønsted acid-catalysis of aryl ketone 56 could give rise to a fourth intermediate, benzylic carbocation 60 as the product of a direct oxygen atom transfer relying on the initial formation of oxetane 59 (IV, Fig. 3A). Unlike in carbonyl-olefin metathesis, which proceeds via a retro-[2+2]-cycloaddition of oxetane 59, this fragmentation is interrupted to result in carbocation 60 (fragmentation of bond b in 59).
Fig. 3. Mechanistic Investigations.
A. Possible mechanistic alternatives in the Brønsted acid-catalyzed formation of tetrahydrofluorenes from aryl ketones. B. DFT studies of the reaction pathway comparing carbonyl-ene reactivity to a pathway relying on a transient oxetane. C. 3D representations of intermediates and transition states.
These distinct mechanistic scenarios were subsequently investigated computationally (unrestricted B97-D density functional and 6–31+G* basis set) to determine the viability of their transition states and corresponding minimal energy pathways. The quantum chemical simulations based on the Growing String Method (29) revealed two possible reaction paths (see Supplementary Materials for computational details): i) a concerted carbonyl-ene pathway via transition state 62 following the initial mechanistic hypothesis (Fig. 3B), and ii) a single-elementary step pathway passing through transition state 64 and oxetane 65 (Fig. 3B) (30). The latter path was found to have a 9 kcal/mol lower energy barrier than the carbonyl-ene reaction, suggesting it is the preferred reaction path. This lower energy pathway yields benzylic carbocation intermediate 66 and constitutes a direct oxygen atom transfer between two carbons (C1 and C3 in 66, Fig. 3B). Electronically, conversion of 61 to 66 is enabled by an asynchronous, concerted path that is best conceptualized as two distinct transitions connected by an unstable oxetane intermediate 65. Figure 3B highlights the asynchronous nature of this path by showing the transition state 64 as the highest energy point, which forms oxetane 65 and subsequently fragments through an energetically favorable ring-opening to result in benzylic carbocation intermediate 66. The second electronic change in this reaction path is barrierless, due to the instability of the protonated oxetane 65 when compared to its fragmentation product 66.
This Brønsted acid-catalyzed mode of reactivity complements the previously established Lewis acid-catalyzed carbonyl-olefin metathesis reaction that relies on intermediate oxetanes; however, under Brønsted acid catalysis, fragmentation of the transient oxetane interrupts the carbonyl-olefin metathesis pathway and results in a new reactive intermediate, benzylic carbocation 66. As such, the transience of 65 suggests a direct oxygen atom transfer that represents a distinct reactivity mode between carbonyls and olefins to provide benzylic carbocations (Fig. 3A, pathway IV).
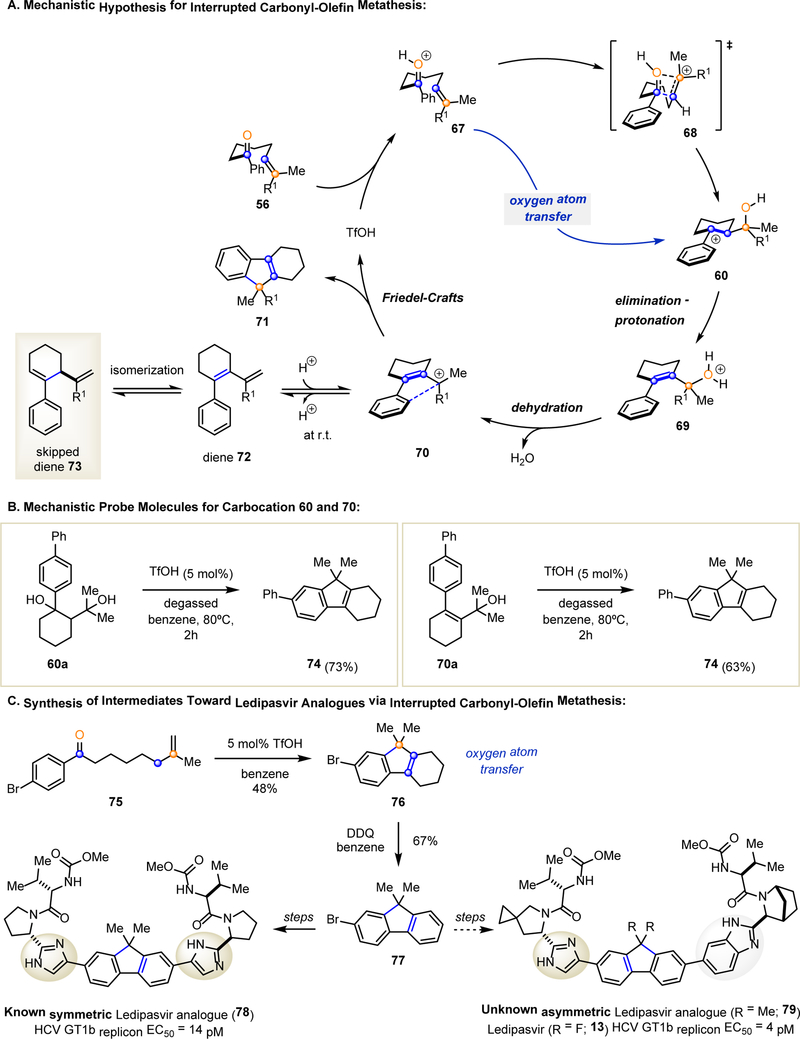
Taking into account experimental and computational results obtained, we propose the following reaction mechanism for the Brønsted acid-catalyzed interrupted carbonyl-olefin metathesis reaction (Fig. 4A). Protonation of aryl ketone 56 initiates intramolecular oxygen atom transfer via transition state 68 to form intermediate benzylic carbocation 60. Elimination and subsequent protonation of the resulting allylic alcohol provides 69 that can then undergo dehydration to produce carbocation 70. This highly stabilized allylic carbocation undergoes a final Friedel-Crafts alkylation to form the tetrahydrofluorene product 71. This hypothesis was subsequently tested by the independent synthesis of two probe molecules, specifically, tertiary alcohols 60a and 70a (Fig. 4B). Diol 60a and allylic alcohol 70a are both able to undergo a Friedel-Crafts alkylation to provide tetrahydrofluorene product 74 upon treatment with TfOH, which supports carbocations 60 and 70 as potential intermediates. However, at lower reaction temperatures, Friedel-Crafts alkylation does not proceed and carbocation 70 is quenched via elimination to result in diene 72. Further isomerization of diene 72 provides an experimentally observed skipped diene 73 as a shunt product (see Supplementary Materials for details). Alternative pathways for the formation of skipped diene 73 were investigated computationally but were found to be higher in energy. Upon exposure to the optimized reaction conditions, skipped diene 73 re-engages in the reaction pathway to give rise to the tetrahydrofluorene product exclusively (see Supplementary Materials for experimental details).
Fig. 4. Proposed Mechanism and Application.
A. Mechanistic hypothesis for interrupted carbonyl-olefin metathesis reactions. B. Investigation of mechanistic probe molecules. C. Synthesis of intermediates to access known and new Ledipasvir analogues.
The tetrahydrofluorene products obtained in our one-step, multiple bond-forming transformation can be readily oxidized to the corresponding fluorene compounds in up to 99% yield using DDQ (see Supplementary Materials for details). The synthetic value of this cyclization/oxidation sequence has been demonstrated in the synthesis of a key fluorene intermediate towards a biologically active Ledipasvir analogue (Fig. 4C) (28). Importantly, the interrupted carbonyl-olefin metathesis reaction enables rapid entry to aromatic fluorene moieties bearing unique substitution patterns that are difficult to access with currently available synthetic methods. Specifically, symmetric and asymmetric analogues are accessible using the same fluorene core. Under the optimized reaction conditions, aryl ketone 75 yields tetrahydrofluorene 76, which upon subsequent oxidation results in fluorene 77. This intermediate (77) can be further advanced to known symmetric Ledipasvir derivative 78, with a HCV GT1b replicon EC50 value of 14 pM, or an unknown asymmetric analogue 79 (28).
The developed interrupted carbonyl-olefin metathesis reaction complements the repertoire of well-established reactions between carbonyls and olefins, and provides entry into the formation of complex, polycyclic tetrahydrofluorenes in a single synthetic step relying on TfOH as an inexpensive catalyst.
Supplementary Material
Acknowledgments. Funding:
This work was supported by the NIH/National Institute of General Medical Sciences (R01-GM118644), the David and Lucile Packard Foundation and the Alfred P. Sloan Foundation (fellowships to C.S.S.). J.R.L. and R.B.W. thank the National Science Foundation for predoctoral fellowships.
Footnotes
Competing interests: Authors declare no competing interests.
Data and materials availability: The Supplementary Materials contain complete experimental and spectral details for all new compounds and all reactions reported herein. Crystallographic data are available free of charge from the Cambridge Crystallographic Data Centre under reference CCDC-1584828.
References
- 1.Alder K, Pascher F, Schmitz A, Berichte Dtsch. Chem. Ges. B Ser. 76, 27–53 (1943). [Google Scholar]
- 2.Clarke ML, France MB, Tetrahedron. 64, 9003–9031 (2008). [Google Scholar]
- 3.Mikami K, Shimizu M, Chem. Rev. 92, 1021–1050 (1992). [Google Scholar]
- 4.Ho C-Y, Schleicher KD, Chan C-W, Jamison TF, Synlett. 2009, 2565–2582 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snider BB, in Comp. Org. Synth, Trost BM, Fleming I, Eds. (Pergamon, Oxford, 1991), vol. 2, pp. 527–562. [Google Scholar]
- 6.Kriewitz O, Ber. 32, 57–60 (1899). [Google Scholar]
- 7.Prins HJ, Chem. Weekblad. 16, 1510–1526 (1919). [Google Scholar]
- 8.Arundale E, Mikeska LA, Chem. Rev. 51, 505–555 (1952). [Google Scholar]
- 9.Olier C, Kaafarani M, Gastaldi S, Bertrand MP, Tetrahedron. 66, 413–445 (2010). [Google Scholar]
- 10.Pastor LM, Yus M, Curr. Org. Chem. 11, 925–957 (2007). [Google Scholar]
- 11.Stephenson LM, Orfanopoulos M, J. Org. Chem. 46, 2200–2201 (1981). [Google Scholar]
- 12.Kwart H, Brechbiel M, J. Org. Chem. 47, 5409–5411 (1982). [Google Scholar]
- 13.Snider BB, Ron E, J. Am. Chem. Soc. 107, 8160–8164 (1985). [Google Scholar]
- 14.Singleton DA, Hang C, J. Org. Chem. 65, 895–899 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Ludwig JR, Zimmerman PM, Gianino JB, Schindler CS, Nature. 533, 374–379 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Ludwig JR et al. , J. Am. Chem. Soc. 139, 10832–10842 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paternò E, Gazz. Chim. Ital. 39, 237–250 (1909). [Google Scholar]
- 18.Büchi G, Inman CG, Lipinsky ES, J. Am. Chem. Soc. 76, 4327–4331 (1954). [Google Scholar]
- 19.Porco JA Jr., Schreiber SL, in Comp. Org. Synth, Trost BM, Fleming I, Eds. (Pergamon, Oxford, 1991). vol. 5, pp. 151–192. [Google Scholar]
- 20.The formation of a related tetrahydrofluorene has previously been described as a byproduct in 38% yield using catalytic Bi(OTf)3. However, the reaction was assumed to proceed following a carbonyl-ene reaction path and subsequent Friedel-Crafts alkylation. See (21).
- 21.Tremel P et al. , New J. Chem. 39, 7453–7458 (2015). [Google Scholar]
- 22.Snider BB, Acc. Chem. Res. 13, 426–432 (1980). [Google Scholar]
- 23.Lemière G et al. , Angew. Chem. Int. Ed. 45, 7596–7599 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Wilkerson-Hill SM, Lavados CM, Sarpong R, Tetrahedron. 72, 3635–3640 (2016). [Google Scholar]
- 25.Zhou A-H, Pan F, Zhu C, Ye L-W, Chem. – Eur. J. 21, 10278–10288 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Lu Z, Yang M, Chen P, Xiong X, Li A, Angew. Chem. Int. Ed. 53, 13840–13844 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Maddess ML et al. , Org. Process Res. Dev. 18, 528–538 (2014). [Google Scholar]
- 28.Link JO et al. , J. Med. Chem. 57, 2033–2046 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman PM, J. Comput. Chem. 36, 601–611 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Attempts to probe the Prins reaction pathway computationally did not support a tertiary carbocation, but instead implicated benzylic carbocation 66 via a transition state resembling an oxetane (64).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.