Abstract
Background:
Diarrheagenic Escherichia coli (DEC) is regarded as a great public health concern all around the world causing diarrhoea which can be transmitted through food chain.
Aims:
This study aimed to determine the contamination level and exact distribution rate of DEC in food products consumed by human.
Methods:
Seven hundred and twenty samples of food from animal origin and fishes were analysed by conventional and molecular method for the presence of E. coli and two multiplex polymerase chain reaction (mPCR) for detection of DEC.
Results:
Two hundred and eighty-three E. coli isolates were detected. The classification of DEC by two multiplex PCR assay yielded 84 DEC pathotypes. Enterotoxigenic E. coli (ETEC) was detected at high rates (75%) followed by shiga-toxigenic E. coli (STEC) and enterohemorrhagic E. coli (EHEC) (each of 9.5%), enteroaggregative E. coli (EAEC) (3.5%) and atypical enteropathogenic E. coli (aEPEC) (about 2.3%). The highest number of DEC (n=26; 21.6%) was observed from beef carcasses in abattoir while the lowest number (n=7; 5.8%) was noticed from burger samples (P<0.01). Enterotoxigenic E. coli was widespread in local raw ground meat and fish surface swabs (P<0.001), EAEC (P<0.01), and EHEC (P<0.001) were only in beef carcasses swabs, STEC was more prevalent in both imported and local raw burger (P<0.01), while the isolates of aEPEC were from imported chicken carcasses (P>0.05).
Conclusion:
High DEC contamination rate that was observed is attributed to the poor hygienic practices during food processing. Therefore, a superior hygienic application is required.
Key Words: Diarrheagenic E. coli, Multiplex PCR, Public health
Introduction
Escherichia coli is considered as a main facultative anaerobic flora that occurs in human and animal intestine. This bacterium has become a highly harmful pathogen causing injurious enteric and extra-intestinal diseases if there is some type debilitation or immune-depressant diseases, or even intestinal barriers are desecrated (Kaper et al., 2004 ▶). Escherichia coli is regarded as a predominant flora of the human and animal gut which can be detected in 1 g at the rate of 9-1010 colony forming unit (CFU) (Mohammed, 2012 ▶). This is the reason why there is an increasing chance of food contamination by E. coli during animal slaughtering, food handling, storage and processing (Laury et al., 2009 ▶).
The term diarrheagenic E. coli (DEC) refers to those pathotypes that usually cause enteric infections while their pathogenicity is commonly linked to various virulence factors (Gomes et al., 2016 ▶). These factors include: intimine encoded by (eae) gene, bundle forming pilus (bfp), Shiga-like or vero-toxins (sxt), heat stable and heat labile toxins (lt and st genes with their variants) and colonization factors for enterotoxigenic E. coli (ETEC), enteroaggregative E. coli (EAEC) heat-stable enterotoxin 1 (EAST1), aggregative adherence fimbraie (AAF), regulator or transcriptional activator for the AAFs of EAEC virulence gene (aggR), pInv plasmid of enteroinvasive E. coli (EIEC) which encode invE gene for invasion and finally F1845 fimbria of diffusively adherent E. coli (DAEC), according to these factors and pathogenic features, these pathotypes are classified to six main groups including enteropathogenic E. coli (EPEC), ETEC, enterohemorrhagic E. coli (EHEC) or shiga-toxigenic E. coli E. coli (STEC), EIEC, EAEC, and DAEC (Gomes et al., 2016 ▶). The routes of DEC transmission have not been specified yet (Wang et al., 2017 ▶), but food can be regarded as a vehicle for carrying many microorganisms, and there is a potential relation between food contamination with DEC and the emergence of diarrheal episodes (Rúgeles et al., 2010 ▶). Unfortunately, no details about the relation of DEC with human diarrhea have been obtained in Iraq, except one study that was carried out in Sulaimanya city. In which a high prevalence of ETEC, EPEC, and EACE was identified in under 10 years old diarrhetic children (Arif and Salih, 2010 ▶). Recently variable molecular tools, have been well-known for recognition of DEC groups which are mostly focused on the finding of each virulence gene relating to specific pathotypes (Gomes et al., 2016 ▶). Multiplex polymerase chain reaction (mPCR) is one that were extensively used as a diagnostic method which has the capability to spot all DEC pathotypes at once in a single reaction tube with a minimum work load and materials (Vidal et al., 2005 ▶; Rúgeles et al., 2010 ▶; Fujioka et al., 2013 ▶).
Various informations are available today for the E. coli as a bacterial marker of food contamination, but till now no data in our country and scarce reports in other countries have been available on the DEC contamination level in food products of animal origin. Adequate information can be obtained by pathotyping of E. coli isolates from food products that consumed by human being and tourists coming to our area. Therefore, the aims of current study were to evaluate the exact distribution rate, to locate the source of contamination and efficient hazard evaluation of E. coli pathotypes in food types available and imported to our area.
Materials and Methods
Food sampling
Overall of 720 different food samples that were collected during the period between July 2016 and March 2017 in Duhok city, Iraq, including raw ground meat, swabs from external cattle carcasses surfaces from abattoir, swabs from entire external fish surface, local and imported raw burger, local raw milk and swabs from external surfaces of frozen chicken carcasses, as shown in Table 1. The collection processes were carried out under precise hygienic condition in which 25 g of ground meat was taken from its container of daily prepared bulk meat at different restaurants and one entire burger piece from each pocket in retail shops and burger preparation factories, while 25 ml of local raw milk were collected from each bulk tank milk in yogurt manufacturing factories that receive the raw milk from most dairy yielding farms around the Duhok city. For each of the beef carcasses (abattoir), fish (retail shops) and imported chicken carcasses (food control inspection unit in Duhok city/Ministry of Health), the entire bodies surfaces were swabbed using sterile wooden or plastic swabs and directly transferred to the tubes containing 10 ml of buffered peptone water (BPW). All collected samples whether in sterile tubes or plastic bags were transported in cool condition to Microbiological Laboratory, College of Veterinary Medicine, University of Duhok and the processing of samples were carried out within 2 h after compilation.
Table 1.
Number, sources, and types of samples that were assayed in this study
| Sample source | Sample type | Sample numbers |
|---|---|---|
| Total | 720 | |
| Retail shops and restaurants | Imported and local raw burger | 120 |
| Local raw ground meat | 120 | |
| Abattoir | Beef carcasses swabs | 120 |
| Yogurt preparing factor | Local raw milk | 120 |
| Aquatic retail shop | Fish surfaces swabs | 120 |
| Food control inspection unit | Imported chicken carcasses swabs | 120 |
Escherichia coli isolation
Twenty five g or ml for either solid or liquid samples was mixed with 225 ml of BPW then directly incubated at 37°C for 18-24 h, while swabs were preserved with 10 ml BPW at the collection time and incubated upon arrival to laboratory (pre-enrichment), then 10 ml (for swab samples) or 50 ml (for other samples) of BPW were added to 50 ml of MacConkey broth (Lab M, UK) and incubated as previously mentioned (selective enrichment), 1-2 loopfulls of selective enrichment are quarter streaked on to the brilliance E. coli/coliform agar (Oxoid, UK) and incubated as mentioned previously. At least one deep purple colony per each sample was again quarter streaked onto MacConkey agar (Lab M, UK) and incubated as before. Pink colonies (lactose positive) were directly identified as E. coli by using standard biochemical tests including indol production (Lab M, UK), citrate utilization (Lab M, UK), urease enzyme negativity (Oxoid, UK) and typical reactions on triple sugar iron (TSI) agar (Lab M, UK).
DNA extraction
DNA samples were extracted according to (Nessa et al., 2007 ▶) with slight changes. From MacConkey agar 2-3 pure (similar morphology) colonies were chosen and mixed with 200 µL of sterile double distilled water in a 1.5 ml tube. The mixture was vortexed for at least 15 s and directly heated at 95°C for 10 min; the samples then cooled directly by ice, the cooled suspension was centrifuged. One hundred fifty µL supernatant was used as a template DNA for PCR. The purity and concentration of extracted DNA was examined using a nanodrop (Thermo Scientific, USA).
PCR confirmation of E. coli isolates
The presumptive recognized E. coli colonies by standard biochemical tests were directly exposed to PCR amplification of uidA gene (Table 2) that encodes for the B-glucuronidase enzyme which is common in all E. coli species. According to (Ramirez-Martinez et al., 2015), a 25 μL PCR mixture consisted of 12.5 μL of hot start premix (Genedirex, Taiwan), 1 μL of each of reverse and forward primer (10 pmol), 4 μL of sample DNA (30-100 ng/μL), the remainder was filled with nuclease free water (Qiagen, Germany). Polymerase chain reaction amplification was carried out in PCR system 9700 GeneAmp (applied bio-system, USA) with pre-PCR heating at 95°C for 5 min, subsequently exposed to 35 cycles (1 min at 94°C, 1 min at 58°C, 1 min at 72°C), and final cycle for 5 min at 72°C. The amplified product was run at 85 V for 40 min. Amplification of PCR products was confirmed in 2% agarose gel prepared with 1× Tris-acetate-EDTA )TAE( buffer and stained by red safe DNA staining solution (GeNetBio, Korea).
Table 2.
Primers used for the detection of Escherichia coli and DEC pathotypes
| Genes | Pathotypes | Primer sequence (5´ to 3´) | PCR product size (bp) |
Primer Conc. (pmol/μL) |
References |
|---|---|---|---|---|---|
| Stx1 | STEC, EHEC | AGTTAATGTGGTGGCGAAGG CACCAGACAATGTAACCGC |
347 | 5 | |
| Stx2 | STEC, EHEC | TTCGGTATCCTATTCCCGG CGTCATCGTATACACAGGAG |
592 | 4 | |
| Eae | EHEC, tEPEC, aEPEC | CCCGAATTCGGCACAAGCATAAGC CCCGGATCCGTCTCGCCAGTATTCG |
881 | 5 | |
| bfpA | tEPEC | AATGGTGCTTGCGCTTGCTGC GCCGCTTTATCCAACCTGGTA |
324 | 5 | |
| aggR | EAEC | GTATACACAAAAGAAGGAAGC ACAGAATCGTCAGCATCAGC |
254 | 4 | |
| Elt | ETEC | AACGTTCCGGAGGTCTTATG CAACCTTGTGGTGCATGATG |
511 | 3 | |
| Esth | ETEC | TTCACCTTTCCCTCAGGATG ATAGCACCCGGTACAAGCAG |
172 | 4 | |
| Estp | ETEC | ACTGAATCACTTGACTCTTCA TCACAGCAGTAAAATGTGTTGT |
120 | 10 | |
| invE | EIEC | GCAGGAGCAGATCTTGAAG GAAAGGCACGAGTGACTTTC |
208 | 10 | |
| daaC | DAEC | CACTGTGGGCTCCGCGCAAGC CGGTGAGGTTCAGTGTGTAT |
418 | 10 | et al. (1999) |
| uidA | E. coli | AAAACGGCAAGAAAAAGCAG ACGCGTGGTTACAGTCTTGCG |
147 | 10 |
Stx: Shiga-like toxin, eae: Intimine, bfp: Bundle forming pilus, aggR: Transcriptional activator for EAEC virulence genes, elt: Heat labile toxin, esth and estp: Variants of heat stable toxin, invE: Protein for invasion, daaC: Dr family of adhesions (F1845), uidA: Gene encode for B-glucuronidase enzyme, STEC: Shiga-toxigenic Escherichia coli, EHEC: Enterohaemorrhagic Escherichia coli, tEPEC and aEPEC: Typical and atypical enteropathogenic Escherichia coli, EAEC: Enteroaggregative Escherichia coli, ETEC: Entertoxigenic Escherichia coli, EIEC: Enteroinvasive Escherichia coli, DAEC: Diffusely adherent Escherichia coli, and DEC: Diarrheagenic Escherichia coli
PCR detection of DEC isolates
The final PCR noticed isolates as E. coli, were subjected to multiplex PCR-DEC judgment. Details of primer sequence, sizes of PCR products and their concentration (pmol/µL or µM) for each reaction is provided in Table 2. For simplicity and for ease of reading the sizes of PCR products, two primer combination mixture groups were prepared (group 1 and 2) for classification of DEC in food samples, in which the former group was added to reaction mixture for detection of stx1, stx2, aggr, esth, and eae genes and the other next group of primers was used for the recognition of bfpA, elt, estp, daaC, and invE genes. For both primer groups, PCR reaction mixture of 50 μL were organized and composed of 25 μL hot start premix, 5-7 μL of primers mixture for each analogous genes, 4-5 μL of DNA (30-100 ng/μL) from sample and nuclease free water added up to 50 μL. In all PCR reactions, the negative control incorporated with sterile nuclease free water was used. The PCR setting program was applied according to (Fujioka et al., 2013 ▶), wherein the pre-PCR heating was for 5 min at 95°C, then 35 cycles with 1 min at 94°C, 1 min at 55°C, 1 min at 72°C and the post-PCR for 10 min at 72°C. The PCR products were run by electrophoresis at 85 V for 40 min in a 2% agarose gel prepared in 1× TAE buffer.
Special policy was applied for the classification of DEC by mPCR, as when the presence of elt and/or st variants (estp or esth) genes amplicon bands were for ETEC; stx1 and/or stx2 for STEC; either stx1 or stx2 with eae for EHEC; both eae and bfpA for typical EPEC (tEPEC); while if only eae with the absence of each of stx1, stx2, and bfpA was for atypical EPEC (aEPEC); invE for EIEC, aggr for EAEC and finally daaC for DAEC (Fujioka et al., 2013 ▶; Gomes et al., 2016 ▶).
A total of five positive standard controls DNA (Pasteur Institute of Tehran, Iran) were used in this study to optimize the PCR setting conditions. Each of these DNA carry the genes for each corresponding DEC pathotype which includes EHEC O157:H7 (caring eae, stx1, and stx2), EPEC E234869 (only eae for aEPEC), ETEC H10407 (estp and elt), Shigella flexneri (invE) for EIEC and EAEC (aggr gene). All of the above DNA controls were provided as stock (high concentration) solutions which were diluted to get an optimal concentration (30-70 ng/μL) for PCR optimization reaction.
Statistical analysis
The differences in the distribution rates of total and individual DEC pathotypes between different food samples were calculated by Chi-square test using statistical software (GraphPad Prism ver. 7.05). P-value lower than 0.05 was regarded as significant.
Results
Distribution of E. coli and DEC strains among food samples
In this study, about 720 food samples from different sources were analyzed for the presence of E. coli. There were 283 (39.3%) positive samples by cultural and biochemical assays and all of them were positive by single PCR assay for uidA gene and there were no considerable differences between the two methods (Fig. 1). The highest percentages of isolated E. coli were found in the imported frozen chicken and beef carcasses (43.3% and 41.6%, respectively). However, the lowest rates were found in fish (39.1%), ground meat (38.3%), burger (37.5%) and local raw milk (35.8%). It should be noticed that insignificant differences were observed on distribution of E. coli among different food samples (Fig. 2 and Table 3; P>0.05).
Fig. 1.
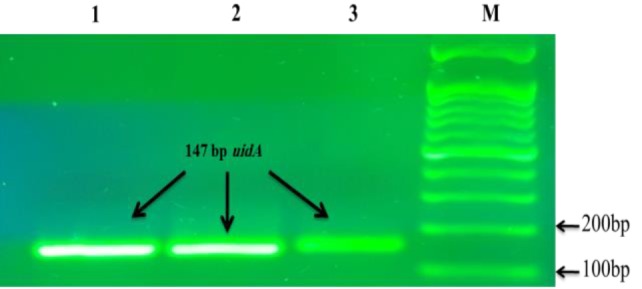
PCR confirmation of Escherichia coli isolates. Lane M: 100 bp marker (Genedirex, Tiwan), and Lanes 1-3: 147 bp positive uidA gene from samples
Fig. 2.

Distribution rates of Escherichia coli among different food samples
Table 3.
Distribution of E. coli and DEC in different samples types
| Sample type | E. coli n (%) | DEC pathotypes n (%) |
|||||
|---|---|---|---|---|---|---|---|
| Total | ETEC | EHEC | STEC | EAEC | aEPEC | ||
| Total (n=720) | 283 (39.3) | 84 (11.6) | 63 (75) | 8 (9.5) | 8 (9.5) | 3 (3.5) | 2 (2.3) |
| Beef carcasses swab (n=120) | 50 (41.6) | 26 (21.6) | 9 (34.6) | 8 (30.7) | 6 (23) | 3 (11.5) | |
| Imported chicken carcasses swabs (n=120) | 52 (43.3) | 23 (19.1) | 21 (91.3) | 2 (8.6) | |||
| Fish surfaces swabs (n=120) | 47 (39.1) | 19 (15.8) | 19 (100) | ||||
| Imported and local raw burger (n=120) | 45 (37.5) | 7 (5.8) | 5 (71.4) | 2 (28.5) | |||
| Local raw ground meat (n=120) | 46 (38.3) | 9 (7.5) | 9 (100) | ||||
| Local raw milk (n=120) | 43 (35.8) | ||||||
DEC: Diarrheagenic Escherichia coli, ETEC: Enterotoxigenic Escherichia coli, EHEC: Enterohaemorrhagic Escherichia coli, STEC: Shiga-toxigenic Escherichia coli, EAEC: Enteroaggregative Escherichia coli, and aEPEC: Atypical enteropathogenic Escherichia coli
Using two multiplex PCR assays for detection of DEC isolates among 283 E. coli isolates, 84 (29.6%) samples were found to be positive for the DEC pathotypes relevant virulence genes (Table 3). Their distribution was as follows: ETEC was the most common pathotype identified in 63 (75%) samples followed by STEC and EHEC each of 8 (9.5%) isolates, 3 (3.5%) EAEC and only 2 (2.3%) aEPEC (eae+, bfp-) isolates were recognized Enteroinvasive E. coli and DAEC pathotypes were not observed in all the food samples screened (Figs. 3A-B, Table 3).
Fig. 3.

PCR amplification of reference standard DNA control and positive virulence genes of DEC isolates from food samples. (A) Multiplex, duplex, and Monoplex PCR optimization of reference standard DNA control. Lane M: 100 bp DNA ladder (Genedirex, Taiwan), Lane 1: Mixed PCR products of all the target genes (eae, stx2, elt, stx1, aggR, invE, and estp), Lane 2: invE (208 bp), Lane 3: aggR (254 bp), Lane 4: elt (511 bp), and estp (120 bp), Lane 5: eae (881 bp), Lane 6: eae (881 bp), stx2 (592 bp), and stx1 (347 bp). (B) Multiplex PCR assay for the amplification of positive virulence genes of DEC isolates from food samples. Lane M: 50 bp DNA ladder, Lane 1: EAEC (aggR), Lane 2: ETEC (estp), Lane 3: ETEC (elt), Lane 4: ETEC (elt and estp), Lane 5: STEC (stx2), Lane 6: STEC (stx1), Lane 7: STEC (stx2 and stx1), Lane 8: aEPEC (only eae), Lane 9: EHEC (positive for eae, stx2, and stx1)
Regarding the sample source, all samples were found to be contaminated with DEC, except raw milk. The highest number of DEC (n=26; 21.6%) was observed in beef carcasses in abattoir while the lowest number (n=7; 5.8%) was recovered from burger samples (P<0.001) (Fig. 4).
Fig. 4.

Distribution rates of total diarrheagenic Escherichia coli (DEC) isolation rate among different food samples. Different lowercase letters indicate statistically significant differences (P<0.05)
The distribution rate of individual DEC pathotypes was affected by the sample sources, because there was significant variation of each pathotype’s allocation rate between each food sample (Figs. 5A-E). Enterotoxigenic E. coli was observed in all samples except raw milk and it was more prevalent in local raw ground meat from restaurants and fish surface swabs than other sources (P<0.001), in addition all DEC from fish surfaces swabs and burger samples were of ETEC, while EAEC (P<0.01) and EHEC (P<0.001) were only in beef carcasses swabs. Shiga-toxigenic E. coli was more prevalent imported and local raw burger than other food sources (P<0.01), while the only 2 isolates of aEPEC were from imported chicken carcasses (P>0.05), (Figs. 5A-E, Table 3).
Fig. 5.
Distribution rates of individual diarrheagenic Escherichia coli (DEC) pathotypes among different food samples. (A) ETEC: Enterotoxigenic Escherichia coli, (B) EHEC: Enterohaemorrhagic Escherichia coli, (C) STEC: Shiga-toxigenic Escherichia coli, (D) EAEC: Enteroaggregative Escherichia coli, and (E) aEPEC: Atypical enteropathogenic Escherichia coli. Different lowercase letters indicates statistically significant differences (P<0.05)
Discussion
To determine the DEC contamination level in food products, to determine its relationship with diarrheal infection in children and elderly people in Duhok province, Iraq, and to distinguish among pathogenic (DEC) and non-pathogenic (normal flora) E. coli, two multiplex PCR assays were used, which had the ability to detect all virulence genes of DEC strains (Vidal et al., 2005 ▶; Fujioka et al., 2013 ▶).
High DEC contamination level of food samples was observed in this study, this is noticeably higher than DEC contamination rate by Lee et al. (2009) ▶ in Korea (1.3%), by Rúgeles et al. (2010) ▶ in Colombia (7.9%), by Canizalez-Roman et al. (2013) in Mexico (1%), by Wang et al. (2017) ▶ (6%), however, higher than this rate (43%) has been reported by Kagambèga et al. (2012a) ▶ in Burkina Faso. High DEC contamination rate in food samples that is seen in this study indicates that there is a massive warning to public health of peoples living in or tourists coming to our country.
Weanling diarrhoea between children in developing countries and traveller’s diarrhoea from infected food and water are two main conditions caused by ETEC. Therefore, in this study ETEC was the most frequent prevalent pathotype in all positive samples screened, especially from local raw ground meat from restaurants and fish, in contrast to Rúgeles et al. (2010) ▶ that fail to detect these pathotypes from food. Several reports have indicated that ETEC are the most common DEC from food products of animal origin (Lee et al., 2009 ▶; Kagambèga et al., 2012a ▶; Mohammed, 2012 ▶; Wang et al., 2017 ▶). In this study all DEC isolates from ground meat samples collected from restaurants and retail shops were of ETEC. A lower occurrence rate than this study had been seen in other studies, from Colombia that found only (4%) of the total ground meat samples contain ETEC (Amézquita-Montes et al., 2015 ▶). Enterotoxigenic E. coli that have been seen in this study may come from either contamination that happens during animal slaughtering from faecal contamination of carcasses or contamination occurred from restaurants by using contaminated grinding device or from the workers hands (Amézquita-Montes et al., 2015 ▶). All DEC from external fish surfaces were found to be ETEC, in contrast to Canizalez-Roman et al. (2013) ▶, and Wang et al. (2017) ▶ were unable to isolate ETEC from fish samples, while others confirmed the presence of ETEC in fish (Koo et al., 2012 ▶; Galal et al., 2013 ▶). An elucidation for the existence of this pathotype in fish is the accumulation of animal and human waste products in the aquatic environments of fish which contain this E. coli strains (Kambire et al., 2017 ▶) or that of unhygienic practices undertaken in retail shops (Shirke et al., 2018 ▶). There are many food products resources of ETEC as indicated by this study, this suggests that foodstuffs in our area are the main source of ETEC which is considered as the key cause of traveller diarrheal episodes in our society.
Enterohemorrhagic E. coli, STEC and EAEC were isolated at higher rates from beef carcasses swabs, although only 2 STEC isolates were detected in burger samples. Several authors suggested the presence of the above pathotypes from beef carcass sides. Lee et al. (2009) ▶ found that nearly 23% of beef carcasses were infected with EHEC, Wang et al. (2017) ▶ established that 3% out of 32 beef meat samples were STEC positive, a high rate of about 25% of beef meat was contaminated with STEC reported by Kagambèga et al. (2012b) ▶, also Canizalez-Roman et al. (2013) ▶ observed that 15% of prepared food (burger) was contaminated with STEC.
Enteroaggregative E. coli was also detected only in beef carcasses swabs, while Wang et al. (2017) ▶ did not segregate EAEC from beef, however, they found nearly the same rate of isolation (0.7%) in fish from local and wholesale shops. Similarly, Lee et al. (2009) ▶ did not find any EAEC, not only in beef but from all meat samples investigated (poultry, beef, and pork). Rúgeles et al. (2010) ▶ in Colombia also failed to isolate this pathotype from meat in retail shops. Kagambèga et al. (2012b) ▶ reported the presence of EAEC in beef intestine and beef meat at rates of 8% and 3%, respectively. From this point we can declare that beef meat can bear different E. coli pathotypes on its surface which may be due to the fact that cattle is regarded as the most important reservoir of these pathotypes carried in intestinal tract (Hussein and Sakuma, 2005 ▶; Stein and Katz, 2017 ▶) which can contaminate the carcasses after slaughtering and hide the removal step. There are many possible risky sources for beef carcass infectivity like hide, equipment, workers, airborne and rodents (Laury et al., 2009 ▶). The data of our study support that these pathotypes are unique to cattle and beef (Bako et al., 2017 ▶).
Only two isolates from chicken carcasses carried the eae gene but they lacked each of bfp, stx1, and stx2 genes and were selected as aEPEC (Gomes et al., 2016 ▶). Similarly Farooq et al. (2009) ▶ detected a high prevalence of aEPEC from faecal samples of healthy chicken in India and also Kagambèga et al. (2012a) ▶ isolated this pathotype from chicken meat, indicating that this pathotype may with other DEC pathotypes be present in chicken intestine and can contaminate it during the slaughtering process. Canizalez-Roman et al. (2013) ▶ found that most of the isolated EPEC from varied food samples were atypical. Recently it has been identified that the incidence rate of diarrhoea caused by aEPEC was much more than tEPEC and this is regarded as a rising diarrheal pathogen (Estrada-Garcia et al., 2009 ▶; Nunes et al., 2012 ▶; Canizalez-Roman et al., 2013 ▶). This suggests that this aEPEC identified in our study from imported chicken resembles other DEC pathotypes for causing diarrhoea.
The data of this study revealed that neither EIEC nor DAEC pathotypes were detected in all food samples screened. Similarly other reports found that these pathotypes were devoid from all food samples evaluated (Rúgeles et al., 2010 ▶; Kagambèga et al., 2012a ▶; Canizalez-Roman et al., 2013 ▶; Wang et al., 2017 ▶). Despite many reports that mentioned the isolation of EIEC from clinical diarrhea like in Mexico (Estrada-Garcia et al., 2009 ▶), in Brazil (Moreno et al., 2010 ▶) and DAEC in Iran (Abbasi et al., 2016 ▶). However until now there has been no particular food type regarded as a vehicle for EIEC and DAEC infections, here the reservoir may be the infected or the human carrier itself and the spread may occur through faecal contamination of available food and water (Croxen et al., 2013 ▶).
In current study, no DEC pathotypes were detected in any of the raw milk samples that were collected at the yogurt reparation factories regardless of high E. coli isolation rate (i.e. all isolates seem to be non pathogenic or normal flora). In contrast to Canizalez-Roman et al. (2013) ▶, dairy products were found to be the most frequent food stuff contaminated with DEC. They suggest that the presence of microorganisms in raw milk and dairy products is mostly due to the human faecal contamination at the processing point specially when artificially made with workers’ hands. Caine et al. (2014) ▶ suggested that improper hygienic procedures, using contaminated milking machines, milking utensils and the presence of animal’s faeces around the floor have a chief responsibility for the existence of E. coli in milk.
In conclusion the level of food items contaminated with DEC in this study was obviously high, this can be associated with the poor hygienic conditions during the slaughtering process, food management and storage at retail shops or cross contamination that may happen in the above processes, this poses community health hazards to the peoples living or travelling to our area, therefore absolute hygienic practices during all of these processes is recommended, which may consecutively decrease the contamination rate and diarrhoea outbreaks. This data may give a little baseline information for the regular assessment of DEC in future for food stuff in our county consumed by human being.
Acknowledgements
This study was supported by the College of Veterinary Medicine, University of Duhok, Iraq. Great thanks to general veterinary directorate, Ministry of Agriculture, of working facility. The author would like to thank Dr. S. Bouzari and Dr. H. Rahimi (Pasteur Institute of Tehran, Iran) for providing the standard DNA control. Thanks are extended to Dr. Nawzat, Nacheervan, Zeravan, Jivan, Mohammed, Alind, Rezheen, and Bayar for great supports in sample collection, statistics and improving this manuscript.
References
- Abbasi, P , Kargar, M , Doosti, A , Mardaneh, J , Ghorbani-Dalini, S , Dehyadegari, MA Molecular detection of diffusely adherent Escherichia coli strains associated with diarrhea in Shiraz, Iran. Arch. Pediatr. Infect. Dis. 2016;5:e37629. [Google Scholar]
- Amézquita-Montes, Z , Tamborski, M , Kopsombut, UG , Zhang, C , Arzuza, OS , Gómez-Duarte, OG Genetic relatedness among Escherichia coli pathotypes isolated from food products for human consumption in Cartagena, Colombia. Foodborne Pathog. Dis. 2015;12:454–461. doi: 10.1089/fpd.2014.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif, SK , Salih, LIF Identification of different categories of diarrheagenic Escherichia coli in stool samples by using multiplex PCR technique. Asi. J. Medic. Sci. 2010;2:237–243. [Google Scholar]
- Bako, E , Kagambèga, A , Traore, KA , Bagre, TS , Ibrahim, HB , Bouda, SC , Bonkoungou, IJO , Kaboré, S , Zongo, C , Traore, AS , Barro, N Characterization of diarrheagenic Escherichia coli isolated in organic waste products (cattle fecal matter, manure and slurry) from cattle’s markets in Ouagadougou, Burkina Faso. Int. J. Environ. Res. Public. Health. . 2017;14:1–12. doi: 10.3390/ijerph14101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevides-Matos, N , Pieri, FA , Penatti, M , Orlandi, PP Adherence and virulence genes of Escherichia coli from children diarrhoea in the Brazilian Amazon. Braz. J. Microbiol. 2015;46:131–137. doi: 10.1590/S1517-838246120130917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine, L , Nwodo, UU , Okoh, AI , Ndip, RN , Green, E Occurrence of virulence genes associated with diarrheagenic Escherichia coli isolated from raw cow’s milk from two commercial dairy farms in the eastern Cape Province, South Africa. Int. J. Environ. Resear. Publ. Heal. . 2014;11:11950–11963. doi: 10.3390/ijerph111111950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos, LC , Vieira, MA , Trabulsi, LR , da Silva, LA , Monteiro-Neto, V , Gomes, TA Diffusely adhering Escherichia coli (DAEC) strains of fecal origin rarely express F1845 adhesin. Microbiol. Immunol. 1999;43:167–170. doi: 10.1111/j.1348-0421.1999.tb02388.x. [DOI] [PubMed] [Google Scholar]
- Canizalez-Roman, A , Gonzalez-Nuñez, E , Vidal, JE , Flores-Villaseñor, H , León-Sicairos, N Prevalence and antibiotic resistance profiles of diarrheagenic Escherichia coli strains isolated from food items in northwestern Mexico. Int. J. Food Microbiol. 2013;164:36–45. doi: 10.1016/j.ijfoodmicro.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Costa, CF , Monteiro Neto, V , Santos, BR , Costa, BR , Azevedo, A , Serra, JL , Mendes, HB , Nascimento, AR , Mendes, MB , Kuppinger, O Enterobacteria identification and detection of diarrheagenic Escherichia coli in a port complex. Braz. J. Microbiol. 2014;45:945–952. doi: 10.1590/s1517-83822014000300026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxen, MA , Law, RJ , Scholz, R , Keeney, KM , Wlodarska, M , Finlay, BB Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013;26:822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Garcia, T , Lopez-Saucedo, C , Thompson-Bonilla, R , Abonce, M , Lopez-Hernandez, D , Santos, JI , Long, KZ Association of diarrheagenic Escherichia coli pathotypes with infection and diarrhea among mexican children and association of atypical enteropathogenic E coli with acute diarrhea. J. Clin. Microbiol. 2009;47:93–98. doi: 10.1128/JCM.01166-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq, S , Hussain, I , Mir, MA , Bhat, MA , Wani, SA Isolation of atypical enteropathogenic Escherichia coli and Shiga toxin 1 and 2 producing Escherichia coli from avian species in India. Lett. Appl. Microbiol. 2009;48:692–697. doi: 10.1111/j.1472-765X.2009.02594.x. [DOI] [PubMed] [Google Scholar]
- Fujioka, M , Otomo, Y , Ahsan, CR A novel single-step multiplex polymerase chain reaction assay for the detection of diarrheagenic Escherichia coli. J. Microbiol. Method. 2013;92:289–292. doi: 10.1016/j.mimet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Galal, HM , Hakim, AS , Sohad, MD Phenotypic and virulence genes screening of Escherichia coli strains isolated from different sources in delta Egypt. Lif. Sci. J. 2013;10:352–361. [Google Scholar]
- Gomes, TA , Elias, WP , Scaletsky, IC , Guth, BE , Rodrigues, JF , Piazza, RM , Ferreira, LC , Martinez, MB Diarrheagenic Escherichia coli. Brazillian J. Microbiol. 2016;47:3–30. doi: 10.1016/j.bjm.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein, HSS , Sakuma, T Invited review: prevalence of Shiga toxin-producing Escherichia coli in dairy cattle and their products. J. Dairy Sci. 2005;88:450–465. doi: 10.3168/jds.s0022-0302(05)72706-5. [DOI] [PubMed] [Google Scholar]
- Kagambèga, A , Barro, N , Traoré, AS , Siitonen, A , Haukka, K Characterization of Salmonella enterica and detection of the virulence genes specific to diarrheagenic Escherichia coli from poultry carcasses in Ouagadougou, Burkina Faso. Foodborne Pathog. Dis. 2012a;9:589–593. doi: 10.1089/fpd.2011.1071. [DOI] [PubMed] [Google Scholar]
- Kagambèga, A , Martikainen, O , Lienemann, T , Siitonen, A , Traoré, AS , Barro, N , Haukka, K Diarrheagenic Escherichia coli detected by 16-plex PCR in raw meat and beef intestines sold at local markets in Ouagadougou, Burkina Faso. Int. J. Food Microbiol. 2012b;153:154–158. doi: 10.1016/j.ijfoodmicro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Kambire, O , Ama, AA , Yao, KM , Koffi-Nevry, R Prevalence of virulence genes associated with diarrheagenic pathotypes of Escherichia coli isolates from water, sediment, fish, and crab in Aby lagoon, Côte d’ivoire. Int. J. Microbiol. 2017;2017:9532170. doi: 10.1155/2017/9532170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper, JB , Nataro, JP , Mobley, HLT Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- Koo, HJ , Kwak, HS , Yoon, SH , Woo, GJ Phylogenetic group distribution and prevalence of virulence genes in Escherichia coli isolates from food samples in South Korea. Wor. J. Microbiol. Biotechnol. 2012;28:1813–1816. doi: 10.1007/s11274-011-0954-5. [DOI] [PubMed] [Google Scholar]
- Laury, A , Echeverry, A , Brashears, M . Fate of Escherichia coli O157:H7 in meat. In: Toldra, F , editor. Safety of meat and processed meat. (1st Edn.), Chapter 2. Lubbock, TX, USA, : Springer; 2009. pp. 31–53. [Google Scholar]
- Lee, GY , Jang, HI , Hwang, IG , Rhee, MS Prevalence and classification of pathogenic Escherichia coli isolated from fresh beef, poultry, and pork in Korea. Int. J. Food Microbiol. 2009;134:196–200. doi: 10.1016/j.ijfoodmicro.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Mohammed, MAM Molecular characterization of diarrheagenic Escherichia coli isolated from meat products sold at Mansoura city, Egypt. Food Cont. 2012;25:159–164. [Google Scholar]
- Moreno, ACR , Filho, AF , Gomes Tdo, AT , Ramos, STS , Montemor, LPG , Tavares, VC , Martinez, MB Etiology of childhood diarrhea in the northeast of Brazil: significant emergent diarrheal pathogens. Diag. Microbiol. Infect. Dis. 2010;66:50–57. doi: 10.1016/j.diagmicrobio.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Nessa, K , Ahmed, D , Islam, J , Kabir, FL , Hossain, MA Usefulness of a multiplex PCR for detection of diarrheagenic Escherichia coli in a diagnostic microbiology laboratory setting. Bang. J. Med. Microbiol. 2007;1:38–42. [Google Scholar]
- Nunes Mdo, R , Magalhães, PP , Macêdo Ada, S , Franco, RT , Penna, FJ , Mendes EN Attaching and effacing Escherichia coli and Shiga toxin-producing E coli in children with acute diarrhoea and controls in Teresina/PI, Brazil. Trans. Roy. Soc. Trop. Medic. Hyg. 2012;106:43–47. doi: 10.1016/j.trstmh.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Ramirez-Martinez, ML , Olmos-Ortiz, LM , Barajas-Mendiola, MA , Giono Cerezo, S , Avila, EE , Cuellar-Mata, P A PCR procedure for the detection of Giardia intestinalis cysts and Escherichia coli in lettuce. Lett. Appl. Microbiol. 2015;60:517–523. doi: 10.1111/lam.12402. [DOI] [PubMed] [Google Scholar]
- Rúgeles, LC , Bai, J , Martínez, AJ , Vanegas, MC , Gómez-Duarte, OG Molecular characterization of diarrheagenic Escherichia coli strains from stools samples and food products in Colombia. Int. J. Food Microbiol. 2010;138:282–286. doi: 10.1016/j.ijfoodmicro.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirke, SS , Sukham, MD , Nashad, M , Pradeep, HD , Rahman, MR An overview of adoption of hygienic practices by fish marketing personnel in selected fish markets of Port Blair city, Andaman and Nicobar Islands. Indian J. Econom. Develop. 2018;6:1–6. [Google Scholar]
- Stein, RA , Katz, DE Escherichia coli, cattle and the propagation of disease. FEMS Microbiol. Lett. 2017;364:1–11. doi: 10.1093/femsle/fnx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal, M , Kruger, E , Durán, C , Lagos, R , Levine, M , Prado, V , Toro, C , Vidal, R Single multiplex PCR assay to identify simultaneously the six categories of diarrheagenic Escherichia coli associated with enteric infections. J. Clin. Microbiol. 2005;43:5362–5365. doi: 10.1128/JCM.43.10.5362-5365.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L , Nakamura, H , Kage-Nakadai, E , Hara-Kudo, Y , Nishikawa, Y Prevalence, antimicrobial resistance and multiple-locus variable-number tandem-repeat analysis profiles of diarrheagenic Escherichia coli isolated from different retail foods. Int. J. Food Microbiol. 2017;16:44–52. doi: 10.1016/j.ijfoodmicro.2017.03.003. [DOI] [PubMed] [Google Scholar]



