Abstract
Background:
Campylobacter is recognized as a major cause of foodborne gastroenteritis in humans in many countries and may be transferred from animals to humans. The consumption of chicken meat is identified as a major cause of Campylobacter infection in humans.
Aims:
To find out the contamination rate of chicken meat with Campylobacter, the antimicrobial resistance (AMR) pattern, and the virulence-associated genes of the isolates.
Methods:
Ninety packed chicken meat from 7 main poultry slaughterhouses in Shiraz were analyzed for Campylobacter spp. isolation through microbiological methods. Specific primers were used for the identification of the Campylobacter isolates on species level by polymerase chain reaction (PCR). Antibiotic resistant profiles were determined using the disc diffusion method based on Clinical and Laboratory Standards Institute (CLSI) standards. All the isolates were screened for 7 virulence-associated genes, namely cdtA, cdtB, cdtC, cadF, pldA, cgtB, and virB11 by PCR.
Results:
Out of 90 chicken meats, 26 (28.9%) Campylobacter spp. have been isolated. Resistance to ciprofloxacin (CIP), nalidixic acid (NA), and cefixime (CFM) was observed in all the isolates. Resistance to trimethoprim/sulfamethoxazole (SXT), tetracycline (TET), ampicillin (AMP), and chloramphenicol (CHO) was 80.8%, 88.5%, 76.9%, and 30.8%, respectively. Multidrug resistance (MDR) phenotype was observed in 80.8% of the Campylobacter isolates. All the isolates were positive for cdtA, cdtB, cdtC, and cadF genes. pldA and cgtB were detected in 65.4% and 15.4% of the isolates, respectively.
Conclusion:
In this study, the presence of several virulence genes and an alarming level of MDR in Campylobacter spp. isolates were reported. Particularly, resistance to CIP and TET should be highlighted, since both are key drugs for the treatment of human campylobacteriosis.
Key Words: Campylobacter, Chickens, Meat, Resistance, Virulence
Introduction
Campylobacter species are part of the gut microbiota of livestock, domestic animals, and birds. However, they can cause gastroenteritis in infected people and are identified as one of the main causes of foodborne diseases (Barletta et al., 2013 ▶; Changkwanyeun et al., 2015 ▶; Cha et al., 2016 ▶; Facciola et al., 2017 ▶; Martin et al., 2018 ▶). In humans, handling and/or consumption of contaminated meat especially poultry was recognized as the primary source of infection. While Campylobacter jejuni is responsible for 85% of the infections in humans, Campylobacter coli accounts for most of the remainder (Giacomelli et al., 2014 ▶). Clinical presentation of Campylobacter infection in humans varies in spectrum from mild to severe diarrhea, and inflammatory bloody diarrhea (Al-Mahmeed et al., 2006 ▶). Although such infections are usually self-limiting, several complications can arise, the most important of which are bacteraemia, Guillain-Barré syndrome (GBS), reactive arthritis (RA), and inflammatory bowel syndrome (IBS) (Wagenaar, 2018 ▶). The clinical presentations of Campylobacter gastroenteritis may be modulated by several virulence factors (Zilbauer et al., 2008 ▶). The first and essential stage of pathogenesis is intestinal epithelium adherence mediated by various bacterial surface adhesins such as cadF, which encodes the outer membrane protein that interacts with fibronectin (Facciola et al., 2017 ▶). Subsequent cell damage is mediated by various cytotoxins, the most studied of which is cytolethal distending toxin (CDT) which consists of three subunits (cdtA, -B, -C) (Zilbauer et al., 2008 ▶; Facciola et al., 2017 ▶). pldA, phospholipase A, and virB11 are among the genes responsible for the expression of invasion, whereas genes wlaN and cgtB are related to the expression of GBS (Datta et al., 2003 ▶).
Currently, macrolides (mainly erythromycin (ERY) and azithromycin (AZI)) and fluroquinolones are the drugs of choice for the treatment of Campylobacter infection (Hao et al., 2016 ▶). Antimicrobial resistance (AMR) in Campylobacter spp. from human and animal samples has been reported in many countries including Iran (Kang et al., 2006 ▶; Taremi et al., 2006 ▶; Feizabadi et al., 2007 ▶; Rahimi et al., 2010 ▶; Mansouri-najand et al., 2012 ▶; Zendehbad et al., 2013 ▶; Giacomelli et al., 2014 ▶; Zendehbad et al., 2015 ▶; Cha et al., 2016 ▶). Antimicrobial resistance in food producing animals has serious implications for humans, as it may lead to the distribution of AMR through the food chain. It has been shown that the emergence of AMR in Campylobacter is strictly related to the use of antimicrobials in veterinary medicine (Aarestrup et al., 2008 ▶).
Multiple Iranian studies have reported the prevalence and AMR of Campylobacter spp. in livestock, broiler flocks, poultry, and red meat (Kang et al., 2006 ▶; Taremi et al., 2006 ▶; Rahimi et al., 2010 ▶; Ansari-Lari et al., 2011 ▶; Zendehbad et al., 2013 ▶; Zendehbad et al., 2015 ▶). Some studies have focused on the prevalence of virulence genes in Campylobacter spp. isolated from animals and/or humans (Hamidian et al., 2011 ▶; Khoshbakht et al., 2013 ▶; Ghorbanalizadgan et al., 2014 ▶; Raeisi et al., 2017 ▶). In Iran, Campylobacter spp. strains are reported as the cause of 5.4-10.8% of acute diarrhea (Feizabadi et al., 2007 ▶; Hassanzadeh and Motamedifar, 2007 ▶; Jafari et al., 2009 ▶; Ghorbanalizadgan et al., 2014 ▶).
An understanding of the Campylobacter epidemiology, including the contamination rate and AMR in animal source, is a key step in developing effective strategies for reducing both the Campylobacter infection and the likelihood of AMR in humans. The aims of this study were to find out the rate of Campylobacter spp. contamination in chicken meat, to investigate the AMR pattern and virulence-associated genes of Campylobacter isolates from chicken. Furthermore, we compared the Campylobacter isolates from chicken meat with the strains isolated from patient with diarrhea in our previous study (Aminshahidi et al., 2017 ▶) for any possible similarities of AMR and virulence associated genes. Results on the antibiotic resistance profiles of Campylobacter isolates may be used for the development of a database to be used for effective clinical treatment of the illness during outbreak of foodborne disease.
Materials and Methods
Samples
From September to November of 2016, 90 packed chickens ready for distribution in the market were purchased from 7 main poultry slaughterhouses in Shiraz, Iran. All samples were transported in cool box to Clinical Microbiology Research Center where all the experiments were carried out. We also included the Campylobacter strains of human source that have been isolated from children with acute diarrhea in our previous study (Aminshahidi et al., 2017 ▶), in order to determine and compare their virulence associated gene patterns.
Isolation and identification of Campylobacter
Sample processing was done within 2 h following the reception in our laboratory according to the International Standards Organization (ISO) standard method (EN/ISO10272-1, 2006). Briefly, 25 g of skinless chicken meat was homogenized in Stomacher 400 circulator (Seward, England) with 225 ml of Preston enrichment broth base (Himedia, India), supplemented with CAMP SELECTVIAL (MAST, UK) and 5% sheep blood in sterile plastic bags. After 24 h of incubation at 42°C under microaerophilic conditions (10% CO2, 5% O2, and 83% N2) created in an Anoxomat (MART®, Microbiology, B.V., Netherlands), 100 µL of mixture was streaked on the selective medium (Skirrow Campylobacter selective agar, Oxoid, UK(. The latter was made using Columbia agar (Biolife, Italy) as the base, to which the following was added: 7% of lysed horse blood, vancomycin, trimethoprim, and polymyxin B (Sigma, USA) with final concentrations of 0.01 g, 0.005 g, and 2500 IU per L, respectively. The plates were then incubated at 42°C for 2-3 days under the same condition.
All the Campylobacter suspected colonies after examination with Gram stain and wet smear were confirmed by 23S rRNA polymerase chain reaction (PCR), and species were identified using specific primer sets as presented in Table 1.
Table 1.
List of primers used
| Gene name | Primers | Annealing temperature | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| cdtA | F: CCTTGTGATGCAAGCAATC R: ACACTCCATTTGCTTTCTG |
49°C | 370 | |
| cdtB | F: CAGAAAGCAAATGGAGTGTT R: AGCTAAAAGCGGTGGAGTAT |
51°C | 620 | |
| cdtC | F: CGATGAGTTAAAACAAAAAGATA R: TTGGCATTATAGAAAATACAGTT |
48°C | 182 | |
| virB11 | F: TCTTGTGAGTTGCCTTACCCCTTTT R: CCTGCGTGTCCTGTGTTATTTACCC |
53°C | 494 | |
| iamA | F: GCGCAAAATATTATCACCC R: TTCACGACTACTATGCGG |
52°C | 518 | |
| wlaN | F: TGCTGGGTATACAAAGGTTGTG R: AATTTTGGATATGGGTGGGG |
56°C | 330 | |
| cgtB | F: TTAAGAGCAAGATATGAAGGTG R: GCSCATAGAGAACGCTACAA |
56°C | 562 | |
| pldA | F: AAGCTTATGCGTTTTT R: TATAAGGCTTTCTCCA |
45°C | 913 | |
| cadF | F: TTGAAGGTAATTTAGATATG R: CTAATACCTAAAGTTGAAAC |
42°C | 400 | |
| hipO | F: ACTTCTTTATTGCTTGCTGC R: GCCACAACAAGTAAAGAAGC |
50°C | 323 | pubmlst.org/Campylobacter |
| C. coli | F: TCAAGGCGTTTATGCTGCAC R: CCATCACTTACAAGCTTATAC |
50°C | 323 | pubmlst.org/Campylobacter |
| 23s rRNA | F: TATACCGGTAAGGAGTGCTGGAG R: ATCAATTAACCTTCGAGCACCG |
50°C | 650 | pubmlst.org/Campylobacter |
F: Forward, and R: Reverse
DNA extraction and PCR analysis for virulence-associated genes
NucleoSpin® Tissue kit (MACHEREY-NAGEL, Germany) kit was used for bacterial DNA extraction. Spectrophotometry was used at 260 and 280 nm to determine the purity and concentration of the extracted DNA (Nanodrop, nd1000, USA). All isolates were screened for 7 virulence-associated genes, including three cytolethal toxin production genes: cdtA, cdtB, cdtC; GBS associated gene: cgtB; adherence and colonization factor cadF; phospholipase pldA; and virB11 invasion factors by PCR assays. In order to identify the virulence-associated genes, all 26 Campylobacter strains isolated in this study underwent PCR using the primers listed in Table 1. In addition to Campylobacter isolates from chicken meat in this study, we also investigated virulence associated genes of the 7 Campylobacter strains isolated from children with acute diarrhea from our previous study (Aminshahidi et al., 2017 ▶).
Polymerase chain reaction was performed in the final volume of 25 µL, including 2.5 µL PCR buffer (CinnaGen, Iran), 1.5 mM of MgCl2 (CinnaGen, Iran), 1 µL of mixed dNTP 10 mM (CinnaGen, Iran), 1 µL of 10 picomol of each primer (Bioneer, South Korea), 1.25 units of Taq polymerase (CinnaGen, Iran), and 2 µL of template. The final volume of each reaction increased to 25 µL with distilled water. The solutions were then subjected to the following cycling conditions: 94°C for 5 min, 94°C for 30 s, annealing step for 30 s (the optimal annealing temperature varied depending on the gene mentioned in Table 1), 72°C for 30 s (35 cycles), and a final extension step (72°C for 8 min) in a thermal cycler (Applied Biosystem, USA; Veriti). Subsequently, 8 µL of the PCR product was subjected to gel electrophoresis (Biorad, Wide mini-sub® Cell GT, USA) employing 1.5% Agarose (Invitrogen, 16500, USA), stained by means of GelRed Nucleic Acid Gel Stain (Biotium, 41002, USA), and visualized by gel documentation (UVitec, DBT-08, UK).
Antimicrobial susceptibility testing
To determine the susceptibility of the Campylobacter isolates to antimicrobial agents, isolates were inoculated on Muller-Hinton agar with 5% horse blood. Following antibiotic discs have been used for antimicrobial susceptibility testing: ciprofloxacin (CIP, 5 µg), AZI (15 µg), ERY (15 µg), gentamicin (GEN, 10 µg), nalidixic acid (NA, 30 µg), ampicillin (AMP, 10 µg), meropenem (MRP, 10 µg), cephalothin (CEP, 30 µg), cefixime (CFM, 5 µg), cefuroxime (CXM, 30 µg), ceftriaxone (CTR, 30 µg), cefepime (FEP, 30 µg), amikacin (AMK, 30 µg), tetracycline (TET, 30 µg), chloramphenicol (CHO, 30 µg), and trimethoprim/sulfamethoxazole (SXT, 25 µg) (Rosco Neo-Sensitabs Denmark). The plates were then incubated overnight at 42°C in a microaerophilic condition. The results were interpreted according to the company’s breakpoints which are in accordance to M100-S25 document of Clinical and Laboratory Standards Institute (CLSI) standards (2015). In this study isolates are classified as multidrug resistant (MDR) if they are resistant to minimum three different classes of antimicrobial agents (Tanwar et al., 2014 ▶).
Results
In total, out of 90 chicken meat samples, 26 (28.9%) Campylobacter spp. were isolated; 24 isolates (96%) were C. jejuni and the other 2 (4%) isolates were C. coli.
Virulence-associated genes
Irrespective of their source of isolation, all the isolates (26 isolates of chicken meat and 7 isolates of diarrheal stool from children) were positive for cdtA, cdtB, cdtC, and cadF (Fig. 1). The cgtB gene was observed in 15.4% and 100% of Campylobacter isolates from chicken and human sources, respectively. The gene pldA was positive in all human isolates, whereas only 65.4% of chicken Campylobacter isolates had this gene. All 33 Campylobacter isolates were negative for virB11 gene.
Fig. 1.
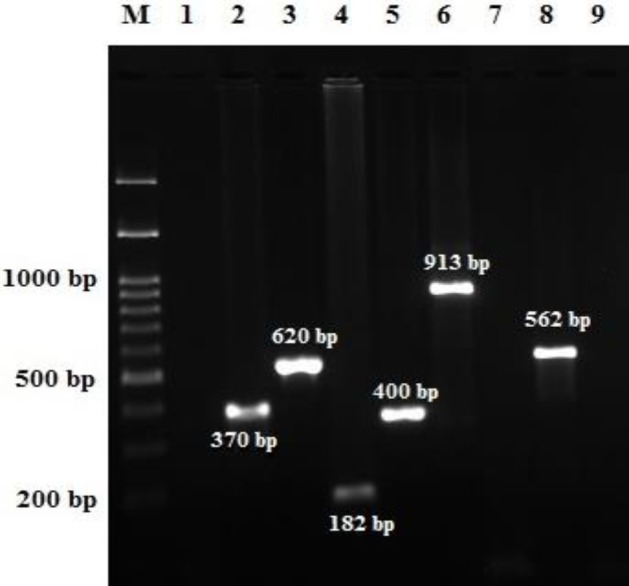
PCR for identification of virulence-associated genes of Campylobacter strains. Lane M: Ladder, 100 bps to 1.5 kb DNA, and Lanes 1-9: Campylobater jejuni strains tested for virulence genes. Lanes 1, 7, 9: Negative controls, Lane 2: Positive cdtA gene (370 bp), Lane 3: Positive cdtB gene (620 bp), Lane 4: Positive cdtC gene (182 bp), Lane 5: Positive cadF gene (400 bp), Lane 6: Positive pldA gene (913 bp), and Lane 8: Positive cgtB gene (562 bp)
Antimicrobial susceptibility testing
A total of 24 C. jejuni and 2 C. coli isolates from the chicken meat samples underwent antimicrobial susceptibility testing for 16 antibiotics from 8 antimicrobial classes. Table 2 presents the AMR profile of the 26 Campylobacter strains isolated from the chicken meat in this study.
Table 2.
Antimicrobial resistance profiles of Campylobacter strains isolated from chicken meat in Shiraz, Iran
| Antimicrobial agent |
C. jejuni
(n=24), n (%) |
C. coli
(n=2), n (%) |
|---|---|---|
| Ampicillin (AMP) | 19 (79.2) | 1 (50) |
| Trimethoprim/sulfamethoxazole (SXT) | 19 (79.2) | 2 (100) |
| Chloramphenicol (CHO) | 6 (25) | 2 (100) |
| Erythromycin (ERY) | 0 (0) | 2 (100) |
| Azithromycin (AZI) | 0 (0) | 2 (100) |
| Meropenem (MRP) | 0 (0) | 0 (0) |
| Cephalothin (CEP) | 23 (95.8) | 2 (100) |
| Cefuroxime (CXM) | 24 (100) | 1 (50) |
| Ceftriaxone (CTR) | 23 (95.8) | 1 (50) |
| Cefixime (CFM) | 24 (100) | 2 (100) |
| Cefepime (FEP) | 0 (0) | 0 (0) |
| Nalidixic acid (NA) | 24 (100) | 2 (100) |
| Ciprofloxacin (CIP) | 24 (100) | 2 (100) |
| Amikacin (AMK) | 0 (0) | 1 (50) |
| Gentamicin (GEN) | 0 (0) | 1 (50) |
| Tetracycline (TET) | 21 (87.5) | 2 (100) |
| Multidrug resistance (MDR) | 19 (79.2) | 2 (100) |
Resistance to CIP, NA, and CFM was observed in all isolates. High rates of resistance to CEP, CXM, and CTR were observed among the Campylobacter isolates, which were 96.2%, 96.2%, and 92.3%, respectively. High levels of resistance to SXT, AMP, and TET were observed among the Campylobacter isolates, which were 80.8%, 76.9%, and 88.5%, respectively. While all the C. jejuni strains were sensitive to ERY and AZI, both C. coli isolates were resistant. Evidence of resistance to MRP and FEP was obtained in Campylobacter isolates. In this study almost 80% of the isolates were MDR; C. jejuni was resistant to 4-5 classes of antimicrobials, while C. coli to 6-7 classes.
Discussion
The consumption of poultry, in particular chicken, is recognized as a major cause of Campylobacter infection in humans. Since, chicken meat is more frequently consumed by the Iranian population, the aims of this study was to determine the contamination rate of chicken meat with Campylobacter, the AMR pattern, and the virulence-associated genes profile of the isolates in Shiraz, Southwest of Iran. We have found that 28.9% of the chicken meat samples in Shiraz were contaminated with Campylobacter species.
The frequency of Campylobacter-contaminated chicken meats observed in the present study is lower than those reported in the previous studies (Taremi et al., 2006 ▶; Rahimi and Ameri, 2011 ▶) (45.5% and 43.5%, respectively). It should be noted that this variation in contamination rate between this and other studies in Iran could be related to the time of sampling, that is, during the production of chicken meat from slaughterhouses to consumers. The contamination rate presented in this study is relatively similar to a recent Canadian report where 23.5% of the retail chicken meat was contaminated with Campylobacter species (Narvaez-Bravo et al., 2017 ▶). Although Campylobacter was described as the most common cause of human bacterial gastroenteritis worldwide (Barletta et al., 2013 ▶; Changkwanyeun et al., 2015 ▶; Cha et al., 2016 ▶; Facciola et al., 2017 ▶; Martin et al., 2018 ▶), this is not the case in Iran where less than 11% of acute diarrhea was caused by Campylobacter species (Jafari et al., 2008 ▶; Jafari et al., 2009 ▶; Aminshahidi et al., 2017 ▶). One reason for such a disparity is that Iranian people usually consume completely cooked chicken in their regimen.
Research on the virulence factors of Campylobacter in animal source foods is essential for consumer safety. For this reason we have investigated the profile of 7 virulence-associated genes of Campylobacter isolates from chicken source, and their possible similarities with C. jejuni strains isolated from patients with acute diarrhea in Shiraz (Aminshahidi et al., 2017 ▶). The results of the present study revealed high prevalence of four virulence-associated genes, including three cdt genes and one cadF gene in Campylobacter isolates regardless of their origin and species. Furthermore, the virB11 gene with plasmid origin was absent in all 33 Campylobacter isolates from both sources. The product of cadF gene is an adhesion-binding protein that is involved in the process of chicken gut colonization and the invasion process of host cells (Ziprin et al., 2001 ▶; Monteville et al., 2003 ▶; Rozynek et al., 2005 ▶). The combination of cdtA, B, C genes and cadF virulence-associated genes in all the Campylobacter isolates of chicken source indicates that many strains originating from chicken meat could be potentially pathogenic for consumers. Since cgtB and pldA genes were detected in 100% of the Campylobacter isolates from children with diarrhea, in comparison to only 12.5% and 62.5% of the isolates from the chicken samples, we believe that the Campylobacter infections in the patients with diarrhea may have sources other than contaminated chicken meat.
Antimicrobial resistance, in particular MDR, is a growing, global, public health problem. These results revealed a high frequency of Campylobacter isolates resistance to fluoroquinolones (100%), TETs (88.5%), and cephalosporins (92.3%-100%). The majority of the Campylobacter isolates of this study (80.2%) were MDR; this is threatening for the chicken consumers, since poultry is recognized as the primary source of human campylobacteriosis in many industrialized countries (Kittl et al., 2011 ▶). This resistance could be linked to the extensive use of these antimicrobial agents in poultry production with lower regulations. This hypothesis is supported by the low rate of CIP resistance (37.5%) in isolates from countries with strict antimicrobial controls in animals (Kittl et al., 2011 ▶). In our study, all C. jejuni isolates, which are the predominant campylobacter species in human campylobacteriosis, were susceptible to ERY and AZI. Therefore, ERY could still be the drug of choice for empirical therapy before obtaining the results of antimicrobial susceptibility testing as many other countries (Wieczorek and Osek, 2013 ▶; Zhang et al., 2016 ▶). A similar pattern of AMR to cephalosporins, co-trimoxazole, CIP, NA, TET, macrolides, and aminoglycosides found among human (Aminshahidi et al., 2017 ▶) and chicken isolates, indicates a possible link between resistance in Campylobacter isolates from chicken and human sources.
In conclusion, the results showed that the majority of Campylobacter isolates obtained in this study were MDR. Particularly, resistance to CIP and TET should be highlighted, since both are key drugs for the treatment of human campylobacteriosis; therefore, resistance to them presents a potential risk for public health. In addition, the similarities observed between the isolates from chicken and human sources in terms of their AMR and virulence genes in our region could indicate that many Campylobacter strains originating from chicken meat could be considered as a potential pathogenic source for consumers.
Acknowledgements
This work was supported by grant 94-6 provided by Professor Alborzi Clinical Microbiology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. We would like to express our thanks to S. Fani for her valuable assistance in editing the manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Aarestrup, FM , McDermott, PF , Wegener, HC . Transmission of antibiotic resistance from food animals to humans. In: Nachamkin, I , editor. Campylobacter. Washington D.C: ASM Press; 2008. p. 665. [Google Scholar]
- Al-Mahmeed, A , Senok, AC , Ismaeel, AY , Bindayna, KM , Tabbara, KS , Botta, GA Clinical relevance of virulence genes in Campylobacter jejuni isolates in Bahrain. J. Med. Microbiol. 2006;55:839–843. doi: 10.1099/jmm.0.46500-0. [DOI] [PubMed] [Google Scholar]
- Aminshahidi, M , Arastehfar, A , Pouladfar, G , Arman, E , Fani, F Diarrheagenic Escherichia coli and Shigella with high rate of extended-spectrum beta-lactamase production: two predominant etiological agents of acute diarrhea in Shiraz, Iran. Microb. Drug Resist. 2017;23:1037–1044. doi: 10.1089/mdr.2017.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari-Lari, M , Hosseinzadeh, S , Shekarforoush, SS , Abdollahi, M , Berizi, E Prevalence and risk factors associated with Campylobacter infections in broiler flocks in Shiraz, southern Iran. Int. J. Food. Microbiol. 2011;144:475–479. doi: 10.1016/j.ijfoodmicro.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Barletta, F , Mercado, EH , Lluque, A , Ruiz, J , Cleary, TG , Ochoa, TJ Multiplex real-time PCR for detection of Campylobacter, Salmonella, and Shigella. J. Clin. Microbiol. 2013;51:2822–2829. doi: 10.1128/JCM.01397-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha, W , Henderson, T , Collins, J , Manning, SD Factors associated with increasing campylobacteriosis incidence in Michigan, 2004-2013. Epidemiol. Infect. 2016;144:3316–3325. doi: 10.1017/S095026881600159X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha, W , Mosci, R , Wengert, SL , Singh, P , Newton, DW , Salimnia, H , Lephart, P , Khalife, W , Mansfield, LS , Rudrik, JT , Manning, SD Antimicrobial susceptibility profiles of human Campylobacter jejuni isolates and association with phylogenetic lineages. Front. Microbiol. 2016;7:589. doi: 10.3389/fmicb.2016.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changkwanyeun, R , Usui, M , Kongsoi, S , Yokoyama, K , Kim, H , Suthienkul, O , Changkaew, K , Nakajima, C , Tamura, Y , Suzuki, Y Characterization of Campylobacter jejuni DNA gyrase as the target of quinolones. J. Infect. Chemother. 2015;21:604–609. doi: 10.1016/j.jiac.2015.05.003. [DOI] [PubMed] [Google Scholar]
- CLSI . Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement. CLSI document M100-S25. 2015. [Google Scholar]
- Datta, S , Niwa, H , Itoh, K Prevalence of 11 pathogenic genes of Campylobacter jejuni by PCR in strains isolated from humans, poultry meat and broiler and bovine faeces. J. Med. Microbiol. 2003;52:345–348. doi: 10.1099/jmm.0.05056-0. [DOI] [PubMed] [Google Scholar]
- EN/ISO10272-1. Microbiology of food and animal feeding stuffs horizental method for detection and enumeraton of Campylobacter spp., Part 1: Detection method. International Organization for Standardization. Geneve, Switzerland: 2006. [Google Scholar]
- Facciola, A , Riso, R , Avventuroso, E , Visalli, G , Delia, SA , Lagana, P Campylobacter: from microbiology to prevention. J. Prev. Med. Hyg. 2017;58:E79–E92. [PMC free article] [PubMed] [Google Scholar]
- Feizabadi, MM , Dolatabadi, S , Zali, MR Isolation and drug-resistant patterns of Campylobacter strains cultured from diarrheic children in Tehran. Jpn. J. Infect. Dis. 2007;60:217–219. [PubMed] [Google Scholar]
- Ghorbanalizadgan, M , Bakhshi, B , Kazemnejad Lili, A , Najar-Peerayeh, S , Nikmanesh, B A molecular survey of Campylobacter jejuni and Campylobacter coli virulence and diversity. Iran Biomed. J. 2014;18:158–164. doi: 10.6091/ibj.1359.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli, M , Salata, C , Martini, M , Montesissa, C , Piccirillo, A Antimicrobial resistance of Campylobacter jejuni and Campylobacter coli from poultry in Italy. Microb. Drug Resist. 2014;20:181–188. doi: 10.1089/mdr.2013.0110. [DOI] [PubMed] [Google Scholar]
- Hamidian, M , Sanaei, M , Bolfion, M , Dabiri, H , Zali, MR , Walther-Rasmussen, J Prevalence of putative virulence markers in Campylobacter jejuni and Campylobacter coli isolated from hospitalized children, raw chicken, and raw beef in Tehran, Iran. Can. J. Microbiol. 2011;57:143–148. doi: 10.1139/w10-089. [DOI] [PubMed] [Google Scholar]
- Hao, H , Ren, N , Han, J , Foley, SL , Iqbal, Z , Cheng, G , Kuang, X , Liu, J , Liu, Z , Dai, M , Wang, Y , Yuan, Z Virulence and genomic feature of multidrug resistant Campylobacter jejuni isolated from broiler chicken. Front. Microbiol. 2016;7:1605–1618. doi: 10.3389/fmicb.2016.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanzadeh, P , Motamedifar, M Occurrence of Campylobacter jejuni in Shiraz, Southwest Iran. Med. Princ. Pract. 2007;16:59–62. doi: 10.1159/000096142. [DOI] [PubMed] [Google Scholar]
- Jafari, F , Garcia-Gil, LJ , Salmanzadeh-Ahrabi, S , Shokrzadeh, L , Aslani, MM , Pourhoseingholi, MA , Derakhshan, F , Zali, MR Diagnosis and prevalence of enteropathogenic bacteria in children less than 5 years of age with acute diarrhea in Tehran children’s hospitals. J. Infect. 2009;58:21–27. doi: 10.1016/j.jinf.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Jafari, F , Shokrzadeh, L , Hamidian, M , Salmanzadeh-Ahrabi, S , Zali, MR Acute diarrhea due to enteropathogenic bacteria in patients at hospitals in Tehran. Jpn. J. Infect. Dis. 2008;61:269–273. [PubMed] [Google Scholar]
- Kang, YS , Cho, YS , Yoon, SK , Yu, MA , Kim, CM , Lee, JO , Pyun, YR Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from raw chicken meat and human stools in Korea. J. Food Prot. 2006;69:2915–2923. doi: 10.4315/0362-028x-69.12.2915. [DOI] [PubMed] [Google Scholar]
- Khoshbakht, R , Tabatabaei, M , Hosseinzadeh, S , Shekarforoush, SS , Aski, HS Distribution of nine virulence-associated genes in Campylobacter jejuni and C coli isolated from broiler feces in Shiraz, Southern Iran. Foodborne Pathog. Dis. 2013;10:764–770. doi: 10.1089/fpd.2013.1489. [DOI] [PubMed] [Google Scholar]
- Kittl, S , Kuhnert, P , Hachler, H , Korczak, BM Comparison of genotypes and antibiotic resistance of Campylobacter jejuni isolated from humans and slaughtered chickens in Switzerland. J. Appl. Microbiol. 2011;110:513–520. doi: 10.1111/j.1365-2672.2010.04906.x. [DOI] [PubMed] [Google Scholar]
- Mansouri-najand, L , Saleha, AA , Wai, SS Prevalence of multidrug resistance Campylobacter jejuni and Campylobacter coli in chickens slaughtered in selected markets, Malaysia. Trop. Biomed. 2012;29:231–238. [PubMed] [Google Scholar]
- Martin, A , Perez-Ayala, A , Chaves, F , Lora, D , Angeles Orellana, M Evaluation of the multiplex PCR Allplex-GI assay in the detection of bacterial pathogens in diarrheic stool samples. J. Microbiol. Methods. 2018;144:33–36. doi: 10.1016/j.mimet.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Monteville, MR , Yoon, JE , Konkel, ME Maximal adherence and invasion of INT 407 cells by Campylobacter jejuni requires the CadF outer-membrane protein and microfilament reorganization. Microbiology. 2003;149:153–165. doi: 10.1099/mic.0.25820-0. [DOI] [PubMed] [Google Scholar]
- Narvaez-Bravo, C , Taboada, EN , Mutschall, SK , Aslam, M Epidemiology of antimicrobial resistant Campylobacter spp isolated from retail meats in Canada. Int. J. Food Microbiol. 2017;253:43–47. doi: 10.1016/j.ijfoodmicro.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Raeisi, M , Khoshbakht, R , Ghaemi, EA , Bayani, M , Hashemi, M , Seyedghasemi, NS , Shirzad-Aski, H Antimicrobial resistance and virulence-associated genes of Campylobacter spp isolated from raw milk, fish, poultry, and red meat. Microb. Drug Resist. 2017;23:925–933. doi: 10.1089/mdr.2016.0183. [DOI] [PubMed] [Google Scholar]
- Rahimi, E , Ameri, M Antimicrobial resistance patterns of Campylobacter spp isolated from raw chicken, turkey, quail, partridge, and ostrich meat in Iran. Food Control. 2011;22:1165–1170. [Google Scholar]
- Rahimi, E , Ameri, M , Kazemeini, HR Prevalence and antimicrobial resistance of Campylobacter species isolated from raw camel, beef, lamb, and goat meat in Iran. Foodborne Pathog. Dis. 2010;7:443–447. doi: 10.1089/fpd.2009.0421. [DOI] [PubMed] [Google Scholar]
- Rahimi, E , Momtaz, H , Ameri, M , Ghasemian-Safaei, H , Ali-Kasemi, M Prevalence and antimicrobial resistance of Campylobacter species isolated from chicken carcasses during processing in Iran. Poult. Sci. 2010;89:1015–1020. doi: 10.3382/ps.2009-00090. [DOI] [PubMed] [Google Scholar]
- Rozynek, E , Dzierzanowska-Fangrat, K , Jozwiak, P , Popowski, J , Korsak, D , Dzierzanowska, D Prevalence of potential virulence markers in Polish Campylobacter jejuni and Campylobacter coli isolates obtained from hospitalized children and from chicken carcasses. J. Med. Microbiol. 2005;54:615–619. doi: 10.1099/jmm.0.45988-0. [DOI] [PubMed] [Google Scholar]
- Tanwar, J , Das, S , Fatima, Z , Hameed, S Multidrug resistance: an emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014 doi: 10.1155/2014/541340. Article ID 541340, 7 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taremi, M , Mehdi Soltan Dallal, M , Gachkar, L , MoezArdalan, S , Zolfagharian, K , Reza Zali, M Prevalence and antimicrobial resistance of Campylobacter isolated from retail raw chicken and beef meat, Tehran, Iran. Int. J. Food Microbiol. 2006;108:401–403. doi: 10.1016/j.ijfoodmicro.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Wagenaar, JA . OIE Manual of diagnostic tests and vaccines for terrestrial animals. (8th Edn) 2018. Infection with Campylobacter jejuni and Campylobacter coli. Chapter 9. [Google Scholar]
- Wieczorek, K , Osek, J Antimicrobial resistance mechanisms among Campylobacter. Biomed. Res. Int. 2013 doi: 10.1155/2013/340605. Article ID 340605, 12 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendehbad, B , Arian, AA , Alipour, A Identification and antimicrobial resistance of Campylobacter species isolated from poultry meat in Khorasan province, Iran. Food Control. 2013;32:724–727. [Google Scholar]
- Zendehbad, B , Khayatzadeh, J , Alipour, A Prevalence, seasonality and antibiotic susceptibility of Campylobacter spp isolates of retail broiler meat in Iran. Food Control. 2015;53:41–45. [Google Scholar]
- Zhang, T , Luo, Q , Chen, Y , Li, T , Wen, G , Zhang, R , Luo, L , Lu, Q , Ai, D , Wang, H , Shao, H Molecular epidemiology, virulence determinants and antimicrobial resistance of Campylobacter spreading in retail chicken meat in Central China. Gut. Pathog. 2016;8:48. doi: 10.1186/s13099-016-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbauer, M , Dorrell, N , Wren, BW , Bajaj-Elliott, M Campylobacter jejuni-mediated disease pathogenesis: an update. Trans. R. Soc. Trop. Med. Hyg. 2008;102:123–129. doi: 10.1016/j.trstmh.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Ziprin, RL , Young, CR , Byrd, JA , Stanker, LH , Hume, ME , Gray, SA , Kim, BJ , Konkel, ME Role of Campylobacter jejuni potential virulence genes in cecal colonization. Avian Dis. 2001;45:549–557. [PubMed] [Google Scholar]


