Abstract
Non-invasive quantitative imaging of cerebral oxygen metabolism (CMRO2) in small animal models is crucial to understand the role of oxidative metabolism in healthy and diseased brains. In this study, we developed a multimodal method combining near-infrared spectroscopy (NIRS) and MRI to non-invasively study oxygen delivery and consumption in the cortex of mouse and rat models. The term CASNIRS is proposed to the technique that measures CMRO2 with ASL and NIRS. To determine the reliability of this method, CMRO2 values were compared with reported values measured with other techniques. Also, the sensitivity of the CASNIRS technique to detect changes in CMRO2 in the cortex of the animals was assessed by applying a reduction in core temperature, which is known to reduce CMRO2. Cerebral blood flow (CBF) and CMRO2 were measured in five mice and five rats at a core temperature of 37°C followed by another measurement at 33°C. CMRO2 was 7.8 ± 1.8 and 3.7 ± 0.9 (ml/100g/min, mean ± SD) in mice and rats respectively. These values are in good agreement with reported values measured by 15O PET, 17O NMR, and BOLD fMRI. In hypothermia, we detected a significant decrease of 37% and 32% in CMRO2 in the cortex of mice and rats, respectively. Q10 was calculated to be 3.2 in mice and 2.7 in rats.
In this study we showed that it is possible to assess absolute values of metabolic correlates such as CMRO2, CBF and oxygen extraction fraction (OEF) noninvasively in living brain of mice and rats by combining NIRS with MRI. This will open new possibilities for studying brain metabolism in patients as well as the many mouse/rat models of brain disorders.
Keywords: cerebral metabolic rate for oxygen (CMRO2), cerebral blood flow (CBF), Near-infrared spectroscopy (NIRS), arterial spin labeling, MRI, hypothermia
1. Introduction
The brain is highly aerobic, using approximately 20% of the body’s total oxygen uptake. The gray matter (GM) consumes 3–4 times more oxygen than the white matter (Dunning and Wolff, 1937; Pantano et al., 1984) and has a higher mitochondrial density (Gerstl et al., 1969; Santuy et al., 2018). This requirement for oxygen means that changes in the rate of oxygen uptake, i.e., the cerebral metabolic rate of oxygen (CMRO2), should be a sensitive index of injury and disease. In addition, CMRO2 is usually tightly coupled to both metabolic rate in the brain and to cerebral blood flow (CBF). A method of monitoring all parameters simultaneously would be of great benefit for studying the regulation of oxygen delivery as well as for understanding how oxygen uptake (related to mitochondrial function) is impacted by disease.
In most neurological diseases, including multiple sclerosis (Lin and Beal, 2006; Sun et al., 1998), Alzheimer’s Disease (Ni et al., 2018) and Parkinson’s disease (Borghammer et al.,2012), abnormal oxidative metabolism is likely to occur. Consequently, both CMRO2 and CBF may be affected. These physiological parameters can be detected currently by neuroimaging modalities such as positron emission tomography (PET) (Mintun et al., 1984; Ter-Pogossian et al., 1970), functional magnetic resonance imaging (fMRI) (Hyder et al., 2001; Sicard and Duong, 2005), and 17O-NMR (Zhu et al., 2005). However, these techniques involve the use of either O2 isotopes as exogenous tracers, or complex biophysical models. In addition, technical procedures, such as intubation, and placing arterial and venous catheters, are required in these techniques and present more challenges in small animals (Cui et al., 2013). Since many of the neurological disease models are mouse or rat models, a non-invasive and relatively simple means of accurately measuring oxidative metabolism and hemodynamics in these models, would provide an important tool for exploring cerebral pathophysiology. In this paper, we propose a novel method for quantification of CMRO2, that is tailored specifically to rodent models, but also has potential for use in patients.
Based on the Fick principle, CMRO2 can be derived from the product of CBF, hemoglobin concentration in blood, and the oxygen extraction fraction (OEF) which is equal to the change in oxygen content of blood between the arterial and venous systems. To obtain these values, we need to quantify CBF, arterial oxy-hemoglobin saturation (SaO2), tissue microvascular oxyhemoglobin saturation (StO2) and total Hb (tHb) concentration in the brain. In our multimodal system, CBF is quantified using arterial spin labeling (ASL) MRI. SaO2 is quantified with a pulse oximeter. Using broadband near-infrared spectroscopy (NIRS), we quantify tHb and StO2, which can then be used to estimate venous oxygen content (see section 2.5.2).
NIRS is widely used in experimental and clinical applications to measure changes in oxygenation and hemodynamics. We showed that with a custom-built broadband NIRS system and an adapted processing pipeline based on the second differential method, it is possible to quantify the absolute concentration of dHb at any given time point in the cortex of rat models (Zhang et al., 2010). By applying an anoxia pulse method (Cooper et al., 1998), we can quantify tHb concentration and StO2. Due to the “banana” shaped path of light in the brain (Chuang et al., 2013; Johnson, 2017; Mansouri et al., 2010), we will be measuring largely from the animal GM.
The objective of the current study is to validate our proposed CMRO2 measurement technique by manipulating metabolic rate in commonly used rodent species (mouse and rat). For this, we will be applying mild hypothermia, as it is known to reduce brain CMRO2 (Klementavicius et al., 1996; Zhu et al., 2007; Zhu et al., 2005). Additionally, it has been shown that hypothermia, and also isoflurane anesthesia, induce burst suppression (BS) in the cortex, which is an EEG state associated with brain inactivation (Ching et al., 2012). This EEG pattern was found to have neuroprotective effects, achieved through the reduction of brain metabolism (Doyle and Matta, 1999). Therefore, our experimental model was to reversibly change the core temperature of the animals from 37°C to 33°C. We show that there is reasonable agreement with literature values (when measured at 37°C), and that we can reproducibly detect a decline in CMRO2 with hypothermia.
We propose the term CASNIRS to represent our technique, measuring CMRO2 using ASL and NIRS. With CASNIRS, we show that one can quantify tissue oxygenation, CBF, OEF and CMRO2 in the GM of rodent models of neurological disorders. This method could be translated to patients as well.
2. Methods
2.1. Study Design
The animals were anesthetized with 5% isoflurane added to a gas mixture of 70% N2 and 30% O2 and maintained at 2% isoflurane. Hair from the mice and rats head was removed using depilatory cream (Nair; Church & Dwight Co. Inc., USA) to reduce the scattering effect and improve the coupling between the optic fibers and the head. The fibers were placed on top of the head, with the posterior edge of the prism placed on the line between the two external auditory meatus (within 1mm of the interaural line). The fibers were secured by covering the area with masking tape connected to the outside of the MRI coil cradle. Fibers were spaced with a black rubber separator to keep the optimal distance constant between the source and detector fibers (4 mm for mouse and 7mm for rat) and to help reduce direct illumination between the source and detector. These separation distances, which were calculated by simulations for light propagation modelling (unpublished data), ensure that the optical signal emanates mainly from the GM of the cerebral cortex (Johnson, 2017). Heart rate, breathing rate and SaO2 were monitored by the MouseOx MRI-compatible pulse oximeter (Starr Life Sciences, USA) on the shaved left thigh of the animal. Core temperature was monitored by a rectal thermometer. The animal was placed on a heating pad in the MRI coil together with the aforementioned instruments and positioned for optimal image quality. Animals were monitored for 30 minutes to ensure a stable core temperature of 37°C was reached before data acquisition. After 30 minutes, ASL acquisition was initiated and required approximately 14 minutes. Core temperature was then reduced to 33°C (over approximately 8 minutes). When the temperature was stable (8 minutes later), ASL was repeated. Core temperature was returned to 37°C (approximately 5 minutes to return core temperature and 8 minutes to allow the physiology to stabilize). An anoxia pulse was given for 50s followed by 5 minutes of 70%N2/30%O2. The animal was removed from the MRI and a blood sample (150 μl) was taken from the tail and analysed with the blood gas analyser (Stat Profile pHOx Ultra, Nova Biomedical Corporation, USA) to quantify the hemoglobin concentration in large vessels. NIRS data were averaged over the time of the ASL for use in the CMRO2 calculations.
2.2. Animals
5 Male Wistar rats (150–200 g, 6 weeks old from Charles River, Québec, Canada), and 5 male C57BL/6J mice (21–23 g, 5–6 weeks old from Charles River, Québec, Canada) were housed and maintained in the University of Calgary Animal Care facility with a 12h light and dark cycle with access to water and food pellets ad libitum. Animal protocols were approved by the Animal Care Committee of the University of Calgary and conformed to the guidelines established by the Canadian Council of Animal Care (CCAC).
2.3. NIRS System
A custom-built continuous wave (CW) NIRS system, consisting of a broadband white light source and a spectrograph, was employed to measure the attenuation spectrum (255 wavelengths) over the range of 705 to 960nm. A single MR-compatible optic fiber with a 1000 μm core diameter and 0.39 NA (FT1000EMT, Thorlabs Inc, USA) was used to deliver the light provided by the 100-watt broadband quartz-tungsten-halogen light bulb of the fiber optic illuminator (model 77501, Oriel Instruments Inc., USA) to the animal brain. A focal lens (AC127– 019-B-ML, Thorlabs Inc, USA) was used to focus the output light onto the fiber optic. The light intensity was moderated and optimized by an iris and a shutter to ensure that the power deposition did not damage the biological tissue. A GRIN lens with a 1.80 mm diameter and 0.55 NA (#64–525, Edmund Optics, USA) and a 90°prism with a leg length of 2 mm (#45–524,TECHSPEC N-SF11, Edmund Optics, USA) were glued with a cyanoacrylate containing adhesive (Krazy Glue, Elmer’s Products, Inc, USA) to the end of the fiber to collimate the light and direct it into the tissue (Johnson, 2017). A schematic of the experimental setup is shown in Figure 1. Light coupling to the skin was enhanced by covering the prisms with a thin layer of glycerol (Bashkatov et al., 2002). A similar optic fiber was used to transport the NIR light from the mouse brain to the spectrograph (Shamrock 303i, Andor Technology Inc, Northern Ireland). Input NIR light was spread along wavelengths, using a diffraction grating, to form the NIR spectrum. Collected spectra were projected onto the CCD camera (Andor iDus) mounted on the spectrograph for digitization. The CCD camera was cooled to − 40°C to significantly reduce the dark noise contribution.
Figure 1:
Schematic design of the CASNIRS system. The system consists of the custom-built CW NIRS system and a 9.4T Bruker MRI system. Inset A shows a closeup view of the animal with the fiber optics inside the MRI coil. Inset B shows a closeup view of the optic fibers positioned on the shaved scalp of the animal. © 2019 Rindala Hachem.
2.4. Magnetic Resonance Imaging (MRI)
A 9.4 T horizontal bore MRI (Bruker Avance console, Bruker Biospin GmbH, Rheinstetten, Germany) with a 35 mm quadrature volume coil was used to non-invasively quantify absolute CBF with an ASL sequence. An axial slice was acquired around the bregma, where the optic fibers were located, using a CASL-HASTE sequence (Lei et al., 2001) with the following parameters: TR = 3000 ms, TE = 13.3 ms, FOV = 30×30 mm, matrix size = 128×128 pixels, slice thickness= 1 mm, 16 averages. Four perfusion images were collected per measurement: 2 control images and 2 tagged images, to correct for magnetization transfer. Following these images, a T1 map was obtained in the same location using a RARE-VTR sequence where TE = 10 ms, TR = 100, 500, 1000, 3000, and 7500 ms. Together, the four perfusion images and the T1 map were collected over a period of 14 minutes.
2.5. Data Analysis
2.5.1. CBF Quantification.
A perfusion map was generated from the four perfusion images and the T1 map, using a custom Matlab script (Johnson, 2017). In this map, CBF was calculated on a voxel-by-voxel basis using the following equation (Buxton, 2005; Pekar et al., 1996):
| (EQ. 1) |
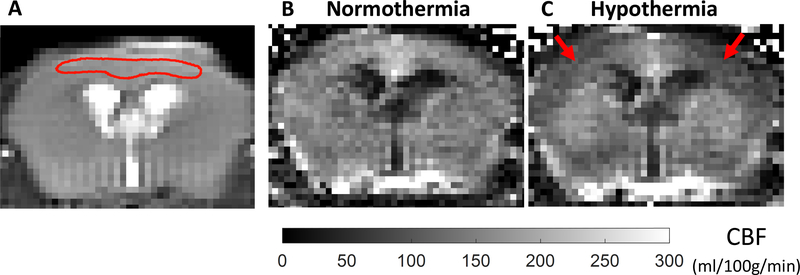
Where f is the tissue perfusion in ml/100g/min, λ = 0.9 is the blood-brain partition coefficient (Herscovitch and Raichle, 1985; Leithner et al., 2010), Mc is the average signal of the two control images, ML is the average signal of the two tagged images, α = 0.675 ± 0.044 is the spin-labelling efficiency (Johnson, 2017), and T’1 is the measured T1 value in each voxel. Individual perfusion maps were calculated for each animal. An ROI was created using a tagged image, which has more contrast, and which encompassed a region that is estimated to be similar to the region of sensitivity for NIRS. This is largely in cortical GM (Figure 2). This ROI was then used on the perfusion and T1 maps to obtain a mean ± SD (ml/100g/min) for perfusion and T1 (ms) values for each condition.
Figure 2:
Representative tagged image (A), and perfusion maps at normothermia (37°C) (B) and hypothermia (33°C) (C) acquired by the CASL-HASTE sequence in the mouse model. A representative ROI (red ROI) that was used for the analysis is shown on the tagged image (A). This ROI approximates the region of brain where NIRS data are obtained. CBF values are averaged over the corresponding ROI in the perfusion maps. There is bilateral reduction in CBF in the cortex under hypothermic conditions (Red arrows).
2.5.2. dHb and StO2 Quantification.
A Matlab algorithm (Zhang et al., 2010) based on the modified Beer-Lambert law was used to convert the attenuation light, measured by NIRS, into concentration of the chromophore dHb. The second differential spectral analysis method (Matcher et al., 1994) was employed in the algorithm to remove the scattering effect from the measured absorbance spectra. The well-defined spectral absorption feature of water at 800–850 nm and the cerebral water content, which is assumed to be 80% in adult rodents (Reinoso et al., 1997), were used to estimate the pathlength that light travels through the tissue. The wavelengths analysed for dHb were 720–810 nm. The algorithm applied multilinear regression to fit the measured attenuation spectra to the specific extinction coefficients of the pure chromophores, downloaded from the University College of London medical physics website (UCL, 2005).
The concentration of tHb in the cerebral tissue was determined using the anoxia pulse method (Cooper et al., 1998; Zhang et al., 2010), in which it is assumed that during an anoxic pulse the total hemoglobin is approximated by the total [dHb] ([tHb] = [dHb]). The anoxic pulse was obtained by switching the inspired gas to 0% O2 and 100% N2 for 50 seconds. This period was sufficient to reach steady state of dHb while minimizing the effect on other physiological parameters (Cooper et al., 1998; Zhang et al., 2010). Calculated dHb and tHb concentrations were used to determine the oxygen saturation in tissue (StO2) with the following equation:
| (EQ. 2) |
2.5.3. CMRO2 Quantification.
Based on the modified Fick principle (Tichauer et al., 2006b),
| (EQ. 3) |
Where k =1.39 (ml(O2)/g(Hb)) is a factor describing the amount of O2 bound to Hb when completely saturated (Brown et al., 2003), and [Hb] is the concentration of hemoglobin (g/l) in large vessels. Since OEF is equal to the arteriovenous oxygen difference, Eq. 3 can be rewritten as the following:
| (EQ. 4) |
SaO2 can be measured with a pulse oximeter, but SvO2 is difficult to determine noninvasively. However, it can be estimated by measuring StO2. As tissue [Hb] consists of 25% arterial [Hb] and 75% venous [Hb] (Phelps et al., 1979; Tichauer et al., 2006b), an estimate of SvO2 can be expressed as:
| (EQ. 5) |
By substituting Eq. 5 in Eq. 4, CMRO2 can be quantified by combining CBF values with NIRS and pulse oximeter data, using the following equation (Eq. 6):
| (EQ. 6) |
CBF is the average value of cerebral blood flow (ml/100g/min) calculated from the ROI in the cortex (Figure 2). SaO2 is the arterial blood oxygen saturation, measured by the MouseOx MRI-compatible pulse oximeter on the thigh of the animal, and StO2 is the tissue oxygen saturation calculated from [dHb] and [tHb] obtained with the NIRS system (Eq.2). [Hb] is the concentration of hemoglobin (g/l) in large vessels, measured from the blood sample with the blood gas analyser. R is the ratio of small to large vessel hematocrit set at 0.61 (Bereczki et al., 1993).
2.5.4. Statistical analysis.
All the measured parameters were compared during normothermia and hypothermia using a paired t-test, where p < 0.05 was used as the threshold for significance.
Data Availability.
The raw data used to calculate CMRO2 are available in PRISM Dataverse: University of Calgary’s Data Repository. More data may be available upon direct request.
3. Results
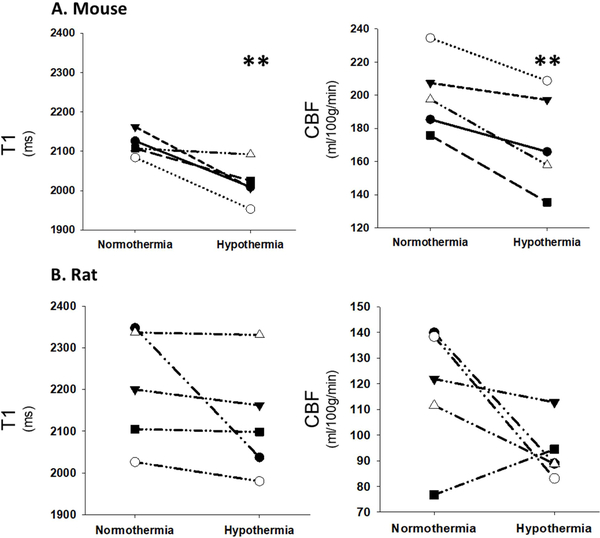
A representative mouse perfusion map is shown in Figure 2. Perfusion images and a T1 map were acquired first when the core temperature of the animal was stable at 37°C, and again after stabilizing the animal temperature at 33°C. Perfusion maps showed bilateral reduction in CBF in the cortex of the animals under hypothermic conditions (Figure 2, Table 1). A decrease in T1 values was observed as well (Table 1). Mean values of T1 and CBF were quantified from the same ROI and are presented per each mouse (Figure 3A) and rat (Figure 3B) during normothermia and hypothermia.
Table 1:
Quantification of T1, CBF, StO2, SaO2, OEF and CMRO2 in mouse (n = 5) and rat (n = 5) models during normothermia (37°C) and hypothermia (33°C). (mean ± SD; paired t-test)
| Mice | Rats | |||
|---|---|---|---|---|
| Mean ± SD | p-value | Mean ± SD | p-value | |
| T1 (ms) | ||||
| Normothermia | 2117±29 | 0.01 | 2203±141 | 0.06 |
| Hypothermia | 2017± 50 | 2122±135 | ||
| CBF (ml/100g/min) | ||||
| Normothermia | 200.1 ± 22.6 | 0.01 | 117.7 ± 25.8 | 0.1 |
| Hypothermia | 173.0 ± 29.8 | 93.6 ± 11.5 | ||
| StO2 | ||||
| Normothermia | 0.72 ± 0.03 | < 0.001 | 0.71 ± 0.02 | 0.1 |
| Hypothermia | 0.76 ± 0.03 | 0.73 ± 0.01 | ||
| SaO2 | ||||
| Normothermia | 0.97 ± 0.01 | 0.04 | 0.95 ± 0.03 | 0.9 |
| Hypothermia | 0.95 ± 0.03 | 0.95 ± 0.02 | ||
| OEF | ||||
| Normothermia | 0.33 ± 0.05 | 0.001 | 0.32 ± 0.02 | 0.4 |
| Hypothermia | 0.25 ± 0.06 | 0.30 ± 0.02 | ||
| CMRO2 (ml/100g/min) | ||||
| Normothermia | 7.8 ± 1.8 | 0.003 | 3.7 ± 0.9 | 0.03 |
| Hypothermia | 4.9 ± 1.1 | 2.5 ± 0.1 | ||
Figure 3:
Quantification of T1 and CBF in each of the 5 mice (A) and rats (B) during normothermia (37°C) and hypothermia (33°C). Each symbol represents a different animal. Statistical analysis was performed comparing normothermia and hypothermia (** - p ≤ 0.01) using paired t-test (Table 1).
To calculate CMRO2, CBF values were combined with NIRS and pulse oximeter data, using equation 6, which is based on the modified Fick principle (Tichauer et al., 2006b). Figure 4A and 4B show representative data extracted from the recorded NIR spectra over the course of 90 minutes. The concentration of dHb (pM) (Figure 4A) was quantified from the raw NIR spectra, using the developed processing algorithm based on the second-differential method. Blue arrows in Figure 4A indicate the initiation of ASL measurements, anoxic pulse, or changing core temperature. Figure 4B shows the StO2 calculated using Equation 2. Figure 4C displays a time-series of the arterial oxygen saturation (SaO2) measured by the MouseOx pulse oximeter from the left thigh of the animal concurrently to the NIRS data collection. Upon applying the anoxic pulse, indicated by the 5th arrow (Figure 4A), the concentration of dHb started to increase sharply and remained steady when it reached the tHb concentration, while the tissue oxygen (Figure 4B) and arterial oxygen (Figure 4C) saturation dropped immediately. Following the cessation of the anoxic pulse, which lasted 50 seconds, the values of dHb, StO2, and SaO2 recovered to their baseline values.
Figure 4:
Representative data extracted from the NIR spectra and the MouseOx pulse oximeter collected from one mouse: A) The concentration of dHb (μM) quantified from the raw NIR spectra, B) The tissue O2 saturation, StO2 (%), calculated with the dHb and tHb from (A), C) The arterial O2 saturation, SaO2 (%), measured by the MouseOx pulse oximeter on the thigh. Each arrow indicates a different challenge or measurement: 1) First ASL measurement at 37°C and 30% O2, 2) Core temperature is stable at 33°C, 3) Second ASL measurement at 33°C and 30% O2, 4) Core temperature is stable at 37°C, 5) Initiation of an anoxic pulse which lasts for 50 seconds
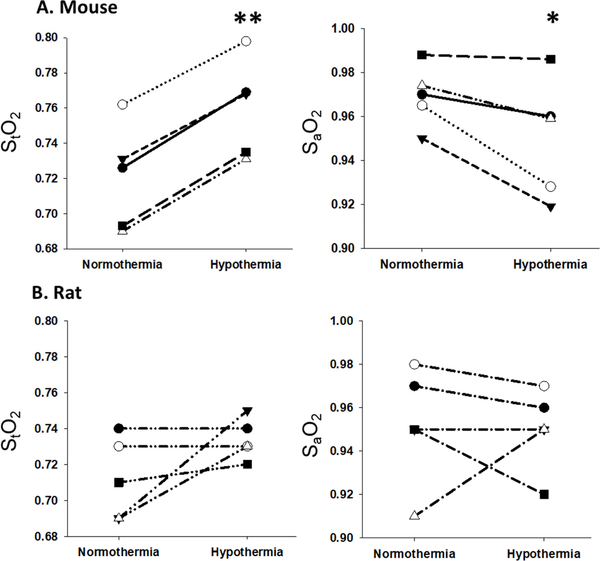
Figure 5 shows StO2 and SaO2 in each of the 10 animals. StO2 increased significantly (p < 0.001) in mice under hypothermic conditions and trended toward an increase in rats (p = 0.1), while SaO2 decreased in mice (p = 0.04) and no significant change was found in rat models. OEF and CMRO2 were quantified per each mouse (Figure 6A) and each rat (Figure 6B) during normothermia (37°C) and mild hypothermia (33°C). Table 1 summarizes the average values of these parameters in each condition. In mouse models, a significant drop of 14% in CBF (p = 0.01), 24% in OEF (p <0.01) and 37% in CMRO2 (p < 0.01) were observed. In rat models, CMRO2 declined significantly by 32% (p < 0.01), although the drop in CBF and OEF was not significant.
Figure 5:
Quantification of StO2 and SaO2 in each of the 5 mice (A) and 5 rats (B) during normothermia (37°C) and hypothermia (33°C). Each symbol represents a different animal. Statistical analysis was performed comparing normothermia and hypothermia (* - p ≤ 0.05, ** - p ≤ 0.01) using paired t-test.
Figure 6:
Quantification of OEF and CMRO2 in each of the 5 mice (A) and rats (B) during normothermia (37°C) and hypothermia (33°C). Each symbol represents a different animal. Statistical analysis was performed comparing normothermia and hypothermia (* - p ≤ 0.05, ** - p ≤ 0.01) using paired t-test (Table 1).
4. Discussion
High-field MRI was combined with broadband NIRS and a pulse oximeter to simultaneously measure CBF, OEF, and CMRO2 in the cortex of mouse and rat models. The term CASNIRS is proposed here to represent the technique measuring CMRO2 with ASL and NIRS. One type of validation for the CASNIRS method is to compare the absolute values of our data to reported values for rodent CMRO2.
The most common methods currently available to determine CBF and CMRO2 include 15O PET (Frackowiak et al., 1980) and 17O MR (Cui et al., 2013; Zhu et al., 2005). These methods involve inhalation of oxygen isotopes and tracking their consumption through their oxidative pathways or determining the washout rate of 17O-labeled water tracer introduced by a bolus injection.15O PET requires a radioactive and unstable oxygen isotope with a half-life of about two minutes (Frackowiak et al., 1980).17O MR provides a direct measure for regional CMRO2 in vivo, but it is still challenging to perform in small animals, like mouse models, due to the requirement for intubation. Although there are no direct measures for CMRO2 conducted by NIRS techniques, some NIRS approaches have been used to indirectly determine CMRO2 in piglets and humans. Previous studies (Tichauer et al., 2006a; Verdecchia et al., 2013) have measured CMRO2 in newborn piglets by combining two near-infrared techniques: diffuse correlation spectroscopy (DCS) and time resolved (TR) NIRS. However, CBF was determined in an invasive way which involved the injection of indocyanine green (ICG) as an intravascular tracer. Other studies (Elwell et al., 2005; Yoxall and Weindling, 1998) were conducted on full and pre-term infants, using spatially resolved spectroscopy to measure absolute mean cerebral oxygen saturation, and CBF by delivering a bolus of HbO2 to the brain, and then deriving CMRO2 values from these measurements. To our knowledge, no similar studies have been conducted on small animals like rats and mice.
Table 2 summarizes some values of CMRO2 from the literature, quantified by currently existing methods in mouse and rat models under different anesthesia conditions. The CMRO2 values for mice in Table 2 are fairly similar, with a mean ± SD of 6.4 ± 0.7 ml/100g/min. The rat data ranged from 3.6 ± 0.1 to 7.5 ± 0.4 ml/100g/min. These values are broadly consistent with the normothermic values measured with the multimodal CASNIRS technique (7.8 ± 1.8 and 3.7 ± 0.9 ml/100g/min for mice and rats, respectively), since they fall in the same range.
Table 2:
Values of CMRO2 (mean ± SD) from the literature, quantified by different methods in mouse and rat models under different anesthesia conditions
| Animal Model |
CMRO2 (ml/100g/min) |
Method | Anesthesia | Citation |
|---|---|---|---|---|
| Mouse | 6.3 ± 0.4 | 17O MRI | ketamine xylazine | (Zhu et al., 2013) |
| 6.5 ± 1.1 | 17O MRSI | Isoflurane | (Lou et al., 2016) | |
| 6.3 ± 0.5 | 15O PET | Isoflurane | (Temma et al., 2017) | |
| 3.6 ± 0.1 | BOLD fMRI | α-chloralose | (Hyder et al., 2000) | |
| 7.5 ± 0.4 | Morphine | |||
| Rat | 4.9 ± 0.8 | 17O MRSI | Ketamine | (Fiat and Kang, 1993) |
| 5.4 ± 0.4 | 17O MRSI | α-chloralose | (Zhu et al., 2007) | |
| 5.0 ± 0.4 | 15O microPET | α-chloralose | (Yee et al., 2006) | |
Several factors can contribute to the differences between our measured values and the ones reported in literature. This wide variability can be due to the different types of anesthesia used in different studies, as anesthesia is known to play a crucial role in altering CBF values (Nakao et al., 2001). Another reason for this difference could be the different values of core temperature in different studies. Moreover, the values presented in this study are averaged over one cortical region only. However, this limitation can be overcome by applying multifiber imaging that covers the whole brain, as used in human fNIRS, instead of the two-fibers system described here.
In order to provide another level of validation for the CASNIRS method, a mild hypothermic challenge was applied. Mild hypothermia is known to reduce GM CMRO2 (Klementavicius et al., 1996; Zhu et al., 2007; Zhu et al., 2005). Using CASNIRS, we detected a 37% and 32% decline in CMRO2 in the brain of mice and rats, respectively, when exposed to hypothermia. Using a literature value of 4.5 ml O2/100g/min at 37°C and 2.6 ml O2/100g/min at 32°C, obtained in a rat model by 17O MRSI (Zhu et al., 2007), there was a decrease of 42% in CMRO2 when the core temperature dropped by 5°C. This extrapolates to a 33.7% decline in CMRO2 with a 4°C (37– 33°C) decline in core temperate, which is a decrease in CMRO2 that is comparable to that found in our study.
An average of 44% decrease in CMRO2 has been shown previously in rat brains using direct 17O NMR detection (Zhu et al., 2007; Zhu et al., 2003). Another study has found a reduction in CMRO2 of 15–20% in mild hypothermia (Klementavicius et al., 1996). CMRO2 decreased by more than 50% when mild hypothermia (37°C-34°C) was applied to control elevated intracranial pressure in patients with head injury (Shiozaki et al., 1993). A 40% decrease in CMRO2 was reported as well in monkeys under pentobarbital anesthesia during hypothermia (33°C) (Bering JR, 1961).
Other studies have found a linear relationship between brain temperature and cerebral metabolism, reporting a change of 6–8% in metabolism per degree C temperature (Lanier, 1995). This corroborates with our data, since a change of 4 °C would change the metabolism by 24–32%.
Finally, Q10 which is an estimate for the expected change in metabolism for a 10°C change in temperature is calculated here. According to the formula: (Michenfelder and Milde, 1991), and to the values measured by CASNIRS, Q10 is 3.2 for mice and 2.7 for rats. These two Q10 values fall within the range of the reported values (2.0 – 4.4) (Klementavicius et al., 1996), and contribute to the validation of our technique. This wide variability in the reported values may be explained by the fact that total CMRO2 is comprised of basal and functional CMRO2.
The hypothermia intervention showed that a change in CMRO2 is detected with CASNIRS. The decline in CMRO2 was very close to published data. These data support the conclusion that this method has merit for quantifying CMRO2 in rodent models.
When a decrease in CMRO2 occurs, the hemodynamic response could be to reduce either CBF, OEF, or both. It appears that there is inter-subject variability in this response. The underlying reason is not understood, but different patterns of burst suppression induced by hypothermia may be a contributing factor. Figure 3 and Figure 6 demonstrate that some animals show different patterns of response, especially in the rat data. While it is too early to say whether this is an interspecies difference, there is a definite need for looking into the change in CMRO2 as a more robust marker, rather than the change in either CBF or OEF. It has been previously suggested that quantification of CMRO2 provides unique and additional data over quantification of either CBF or OEF alone (Tichauer et al., 2006b). Using NIRS in a pig hypoxia model, Tichauer et al. also showed that individual subjects showed variable responses in terms of OEF and CBF. This supports the conclusion that such variability occurs across species and is also likely to occur in humans.
An additional parameter that this technique can measure is StO2 in regional micro-vessels. This parameter is crucial as it represents the tissue oxygenation status. During normothermia, the StO2 value in mice was 0.72 and 0.71 in rats. In humans, StO2 was found to be 0.63 (Yang and Dunn, 2015). Any significant changes from these values would help determine if the tissue is hypoxic or hyperoxic. In the mild hypothermia condition, there was a significant increase (5.5%, p<0.001) in the StO2 of mice while in rats the value trended toward an increase (2.9%, p=0.1). This means that mild hypothermia didn’t create a hypoxic environment. In fact, there was a trend to hyperoxygenation indicating that the oxygen demand was reduced relative to the change reduction in oxygen supply (32–37% reduction in CMRO2 vs. 14–20% drop in CBF) so oxygen which was not consumed, remained in the microvessels. This is supported by the significant decline in OEF in mice. In rats, the variability of the change in OEF resulted in no significant difference in groups, but 2 of 5 subjects showed a marked decline in OEF with hypothermia.
A decrease in T1 values was also observed as the temperature was dropped. This may be expected as the T1 in ex vivo brain also declines with a reduction in temperature (Birkl et al.,2013).
The CASNIRS method has significant advantages for measuring oxygenation and CMRO2. It is non-invasive, allowing for repeated measures, does not use isotopes or ionizing radiation, and can be undertaken in any current MRI system on animals or humans.
5. Conclusions
The study was designed to determine the feasibility of a novel NIR-MRI technique to simultaneously assess absolute values of metabolic correlates including CMRO2, CBF, tHb, StO2 and OEF noninvasively in living mouse and rat cortex.
Using the CASNIRS multimodal technique we were able to non-invasively quantify absolute values and detect changes in CMRO2 in the cerebral cortex of mouse and rat models under normothermic and hypothermic conditions, with a Q10 of 3.2 for mice and 2.7 for rats.
This system provides a new tool to study cerebral physiology and pathophysiology. It is relatively simple to implement within the MRI and could be useful for future research focusing on changes in oxidative metabolism in neurological diseases. This unique technique will open new possibilities for studying brain metabolism using the many mouse and rat models of brain disease available.
Highlights.
CASNIRS measures CMRO2 non-invasively by combining ASL-MRI and NIRS
CASNIRS is demonstrated to have merit for quantifying CMRO2 in the cortex
A significant decrease in CMRO2 caused by hypothermia is detected with CASNIRS
6. Acknowledgments
This work was supported by National Institutes of Health, National Institute of Biomedical Imaging and Bioengineering (R21 EB021397); Alberta Health Solutions Team Grant (20140144); the Canadian Foundation for Innovation; and the National Sciences and Engineering Research Council, Canada (RGPIN-2015–06517).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- Bashkatov AN, Genina EA, Sinichkin YP, Tuchin VV, 2002. Influence of glycerol on the transport of light in the skin Functional Monitoring and Drug-Tissue Interaction. International Society for Optics and Photonics, pp. 144–153. 10.1117/12.469442. [DOI] [Google Scholar]
- Bereczki D, Wei L, Otsuka T, Acuff V, Pettigrew K, Patlak C, Fenstermacher J, 1993. Hypoxia increases velocity of blood flow through parenchymal microvascular systems in rat brain. J Cereb Blood Flow Metab 13, 475–486. 10.1038/jcbfm.1993.62. [DOI] [PubMed] [Google Scholar]
- Bering JR EA, 1961. Effect of body temperature change on cerebral oxygen consumption of the intact monkey. American Journal of Physiology-Legacy Content 200, 417–419. 10.1152/ajplegacy.1961.200.3.417. [DOI] [Google Scholar]
- Birkl C, Langkammer C, Haybaeck J, Ernst C, Stollberger R, Fazekas F, Ropele S, 2013. Temperature dependency of T1 relaxation time in unfixed and fixed human brain tissue. Biomedical Engineering/Biomedizinische Technik 10.1515/bmt-2013-4290. [DOI] [PubMed] [Google Scholar]
- Borghammer P, Cumming P, Østergaard K, Gjedde A, Rodell A, Bailey CJ, Vafaee MS, 2012. Cerebral oxygen metabolism in patients with early Parkinson’s disease. Journal of the neurological sciences 313, 123–128. 10.1016/j.jns.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Brown DW, Hadway J, Lee T-Y, 2003. Near-infrared spectroscopy measurement of oxygen extraction fraction and cerebral metabolic rate of oxygen in newborn piglets. Pediatric research 54, 861–867. 10.1203/01.PDR.0000090928.93045.BE. [DOI] [PubMed] [Google Scholar]
- Buxton RB, 2005. Quantifying CBF with arterial spin labeling. J Magn Reson Imaging 22, 723–726. 10.1002/jmri.20462. [DOI] [PubMed] [Google Scholar]
- Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN, 2012. A neurophysiological-metabolic model for burst suppression. Proceedings of the National Academy of Sciences 109, 3095–3100. 10.1073/pnas.1121461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CC, Chen CM, Hsieh YS, Liu TC, Sun CW, 2013. Brain structure and spatial sensitivity profile assessing by near-infrared spectroscopy modeling based on 3D MRI data. Journal of biophotonics 6, 267–274. 10.1002/jbio.201200025. [DOI] [PubMed] [Google Scholar]
- Cooper CE, Delpy DT, Nemoto EM, 1998. The relationship of oxygen delivery to absolute haemoglobin oxygenation and mitochondrial cytochrome oxidase redox state in the adult brain: a near-infrared spectroscopy study. Biochemical journal 332, 627–632. 10.1042/bj3320627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Zhu X-H, Vollmers ML, Colonna ET, Adriany G, Tramm B, Dubinsky JM, Öz G, 2013. Non-invasive measurement of cerebral oxygen metabolism in the mouse brain by ultra-high field 17O MR spectroscopy. Journal of Cerebral Blood Flow & Metabolism 33, 1846–1849. 10.1038/jcbfm.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle PW, Matta BF, 1999. Burst suppression or isoelectric encephalogram for cerebral protection: evidence from metabolic suppression studies. Br J Anaesth 83, 580–584. 10.1093/bja/83.4.580. [DOI] [PubMed] [Google Scholar]
- Dunning HS, Wolff HG, 1937. The relative vascularity of various parts of the central and peripheral nervous system of the cat and its relation to function. Journal of Comparative Neurology 67, 433–450. 10.1002/cne.900670305. [DOI] [Google Scholar]
- Elwell CE, Henty JR, Leung TS, Austin T, Meek JH, Delpy DT, Wyatt JS, 2005. Measurement of CMRO 2 in neonates undergoing intensive care using near infrared spectroscopy. Oxygen Transport to Tissue XXVI. Springer, pp. 263–268. 10.1007/0-387-26206-7_35. [DOI] [PubMed] [Google Scholar]
- Fiat D, Kang S, 1993. Determination of the rate of cerebral oxygen consumption and regional cerebral blood flow by non-invasive 17O in vivo NMR spectroscopy and magnetic resonance imaging: Part 2. Determination of CMRO2, for the rat by 17O NMR, and CMRO2, rCBF and the partition coefficient for the cat by 17O MRI. Neurological research 15, 7–22. 10.1080/01616412.1993.11740100. [DOI] [PubMed] [Google Scholar]
- Frackowiak R, Lenzi G-L, Jones T, Heather JD, 1980. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. Journal of computer assisted tomography 4, 727–736. 10.1097/00004728-198012000-00001. [DOI] [PubMed] [Google Scholar]
- Gerstl B, Eng L, Hayman R, Bond P, 1969. The lipids of mitochondria of human gray and white matter. Lipids 4, 428–434. 10.1007/bf02531018. [DOI] [PubMed] [Google Scholar]
- Herscovitch P, Raichle ME, 1985. What is the correct value for the brain-blood partition coefficient for water? Journal of Cerebral Blood Flow & Metabolism 5, 65–69. [DOI] [PubMed] [Google Scholar]
- Hyder F, Kennan RP, Kida I, Mason GF, Behar KL, Rothman D, 2000. Dependence of oxygen delivery on blood flow in rat brain: a 7 tesla nuclear magnetic resonance study. Journal of Cerebral Blood Flow & Metabolism 20, 485–498. 10.1097/00004647-200003000-00007. [DOI] [PubMed] [Google Scholar]
- Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL, 2001. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In vivo 14, 413–431. 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- Johnson TW, 2017. Measurement of brain oxygenation and metabolism in a mouse model of multiple sclerosis. Biomedical Engineering. University of Calgary; 10.11575/PRISM/26846. [DOI] [Google Scholar]
- Klementavicius R, Nemoto EM, Yonas H, 1996. The Q10 ratio for basal cerebral metabolic rate for oxygen in rats. Journal of neurosurgery 85, 482–487. 10.3171/jns.1996.85.3.0482. [DOI] [PubMed] [Google Scholar]
- Lanier WL, 1995. Cerebral metabolic rate and hypothermia: their relationship with ischemic neurologic injury. Journal of neurosurgical anesthesiology 7, 216–221. [DOI] [PubMed] [Google Scholar]
- Lei H, Grinberg O, Nwaigwe C, Hou H, Williams H, Swartz H, Dunn J, 2001. The effects of ketamine-xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain research 913, 174–179. 10.1016/s0006-8993(01)02786-x. [DOI] [PubMed] [Google Scholar]
- Leithner C, Müller S, Füchtemeier M, Lindauer U, Dirnagl U, Royl G, 2010. Determination of the brain-blood partition coefficient for water in mice using MRI. Journal of Cerebral Blood Flow & Metabolism 30, 1821–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF, 2006. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795. 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lou S, Lepak VC, Eberly LE, Roth B, Cui W, Zhu X-H, Öz G, Dubinsky JM, 2016. Oxygen consumption deficit in Huntington disease mouse brain under metabolic stress. Human molecular genetics 25, 2813–2826. 10.1093/hmg/ddw138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri C, L’Huillier J-P, Kashou NH, Humeau A, 2010. Depth sensitivity analysis of functional near-infrared spectroscopy measurement using three-dimensional Monte Carlo modelling-based magnetic resonance imaging. Lasers in medical science 25, 431–438. 10.1007/s10103-010-0754-4. [DOI] [PubMed] [Google Scholar]
- Matcher S, Cope M, Delpy D, 1994. Use of the water absorption spectrum to quantify tissue chromophore concentration changes in near-infrared spectroscopy. Physics in Medicine & Biology 39, 177. 10.1088/0031-9155/39/1/011. [DOI] [PubMed] [Google Scholar]
- Michenfelder JD, Milde JH, 1991. The relationship among canine brain temperature, metabolism, and function during hypothermia. Anesthesiology 75, 130–136. 10.1097/00000542-99107000-00021. [DOI] [PubMed] [Google Scholar]
- Mintun M, Raichle M, Martin W, Herscovitch P, 1984. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 25, 177–187. [PubMed] [Google Scholar]
- Nakao Y, Itoh Y, Kuang T-Y, Cook M, Jehle J, Sokoloff L, 2001. Effects of anesthesia on functional activation of cerebral blood flow and metabolism. Proceedings of the National Academy of Sciences 98, 7593–7598. 10.1073/pnas.121179898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni R, Rudin M, Klohs J, 2018. Cortical hypoperfusion and reduced cerebral metabolic rate of oxygen in the arcAβ mouse model of Alzheimer’s disease. Photoacoustics 10, 38–47. 10.1016/j.pacs.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantano P, Baron JC, Lebrun-Grandie P, Duquesnoy N, Bousser MG, Comar D, 1984. Regional cerebral blood flow and oxygen consumption in human aging. Stroke 15, 635–641. 10.1161/01.str.15.4.635. [DOI] [PubMed] [Google Scholar]
- Pekar J, Jezzard P, Roberts DA, Leigh JS Jr, Frank JA, McLaughlin AC, 1996. Perfusion imaging with compensation for asymmetric magnetization transfer effects. Magnetic resonance in medicine 35, 70–79. 10.1002/mrm.1910350110. [DOI] [PubMed] [Google Scholar]
- Phelps M, Huang S, Hoffman E, Kuhl D, 1979. Validation of tomographic measurement of cerebral blood volume with C-11-labeled carboxyhemoglobin. Journal of nuclear medicine 20, 328–334. [PubMed] [Google Scholar]
- Reinoso RF, Telfer BA, Rowland M, 1997. Tissue water content in rats measured by desiccation. Journal of pharmacological and toxicological methods 38, 87–92. 10.1016/S1056-8719(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Santuy A, Turégano-López M, Rodríguez J, Alonso-Nanclares L, DeFelipe J, Merchán-Pérez A, 2018. A quantitative study on the distribution of mitochondria in the neuropil of the juvenile rat somatosensory cortex. Cerebral Cortex 28, 3673–3684. 10.1093/cercor/bhy159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki T, Sugimoto H, Taneda M, Yoshida H, Iwai A, Yoshioka T, Sugimoto T, 1993. Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. Journal of neurosurgery 79, 363–368. 10.3171/jns.1993.79.3.0363. [DOI] [PubMed] [Google Scholar]
- Sicard KM, Duong TQ, 2005. Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage 25, 850–858. 10.1016/j.neuroimage.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Tanaka M, KoNDo S, Okamoto K, Hirai S, 1998. Clinical significance of reduced cerebral metabolism in multiple sclerosis: a combined PET and MRI study. Annals of nuclear medicine 12, 89–94. 10.1007/bf03164835. [DOI] [PubMed] [Google Scholar]
- Temma T, Yamazaki M, Miyanohara J, Shirakawa H, Kondo N, Koshino K, Kaneko S, lida H,2017. Sequential PET estimation of cerebral oxygen metabolism with spontaneous respiration of 150-gas in mice with bilateral common carotid artery stenosis. Journal of Cerebral Blood Flow & Metabolism 37, 3334–3343. 10.1177/0271678X17692815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Pogossian MM, Eichling JO, Davis DO, Welch MJ, 1970. The measure in vivo of regional cerebral oxygen utilization by means of oxyhemoglobin labeled with radioactive oxygen-15. The Journal of clinical investigation 49, 381–391. 10.1172/JCI106247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichauer KM, Brown DW, Hadway J, Lee TY, St Lawrence K, 2006a. Near-infrared spectroscopy measurements of cerebral blood flow and oxygen consumption following hypoxia-ischemia in newborn piglets. J Appl Physiol (1985) 100, 850–857. 10.1152/japplphysiol.00830.2005. [DOI] [PubMed] [Google Scholar]
- Tichauer KM, Hadway JA, Lee T-Y, Lawrence KS, 2006b. Measurement of cerebral oxidative metabolism with near-infrared spectroscopy: a validation study. Journal of Cerebral Blood Flow & Metabolism 26, 722–730. 10.1038/sj.jcbfm.9600230. [DOI] [PubMed] [Google Scholar]
- UCL M.p., 2005. Introduction to tissue spectra. Medical physics UCL website [Google Scholar]
- Verdecchia K, Diop M, Lee T-Y, Lawrence KS, 2013. Quantifying the cerebral metabolic rate of oxygen by combining diffuse correlation spectroscopy and time-resolved near-infrared spectroscopy. Journal of Biomedical Optics 18, 027007. 10.1117/1JBO.18.2.027007. [DOI] [PubMed] [Google Scholar]
- Yang R, Dunn JF, 2015. Reduced cortical microvascular oxygenation in multiple sclerosis: a blinded, case-controlled study using a novel quantitative near-infrared spectroscopy method. Scientific reports 5, 16477. 10.1038/srep16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee S-H, Lee K, Jerabek PA, Fox PT, 2006. Quantitative measurement of oxygen metabolic rate in the rat brain using microPET imaging of briefly inhaled 15O-labelled oxygen gas. Nuclear medicine communications 27, 573–581. 10.1097/01.mnm.0000220586.02591.fd. [DOI] [PubMed] [Google Scholar]
- Yoxall CW, Weindling AM, 1998. Measurement of cerebral oxygen consumption in the human neonate using near infrared spectroscopy: cerebral oxygen consumption increases with advancing gestational age. Pediatr Res 44, 283–290. 10.1203/00006450-199809000-00004. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Srinivasan S, Wu Y, Natah S, Dunn JF, 2010. A near-infrared calibration method suitable for quantification of broadband data in humans. Journal of neuroscience methods 188, 181–186. 10.1016/j.jneumeth.2010.01.037. [DOI] [PubMed] [Google Scholar]
- Zhu X-H, Chen JM, Tu T-W, Chen W, Song S-K, 2013. Simultaneous and noninvasive imaging of cerebral oxygen metabolic rate, blood flow and oxygen extraction fraction in stroke mice. Neuroimage 64, 437–447. 10.1016/j.neuroimage.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X-H, Zhang Y, Zhang N, Ugurbil K, Chen W, 2007. Noninvasive and three-dimensional imaging of CMRO2 in rats at 9.4 T: reproducibility test and normothermia/hypothermia comparison study. Journal of Cerebral Blood Flow & Metabolism 27, 1225–1234. 10.1038/sj.jcbfm.9600421. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhang Y, Ugurbil K, Chen W, 2003. 3D imaging of CMRO2 in rat brain at different temperature using high-field 17O NMR approach. Proceedings of International Society of Magnetic Resonance Medicine, Toronto 569 10.1002/mrm.24469. [DOI] [Google Scholar]
- Zhu XH, Zhang N, Zhang Y, Zhang X, Ugurbil K, Chen W, 2005. In vivo 17O NMR approaches for brain study at high field. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In vivo 18, 83–103. 10.1002/nbm.930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data used to calculate CMRO2 are available in PRISM Dataverse: University of Calgary’s Data Repository. More data may be available upon direct request.