Abstract
Objectives
Ectopic pregnancy is an important adverse pregnancy outcome that is under-surveilled. Emergency department (ED) data can help provide insight on the trends of ectopic pregnancy incidence in the United States (US).
Methods
Data from the largest US all-payer ED database, the Healthcare Cost and Utilization Project Nationwide ED Sample, were used to identify trends in the annual ratio of ED ectopic pregnancy diagnoses to live births during 2006–2013, and the annual rate of diagnoses among all pregnancies during 2006–2010. Diagnoses were identified through International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis and procedure codes and CPT codes.
Results
The overall ratio of weighted ED visits with an ectopic pregnancy diagnosis during 2006–2013 was 12.3 per 1,000 live births. This ratio increased significantly from 2006–2013, from 11.0 to 13.7 ectopic pregnancies per 1,000 live births, with no inflections in trend. The rate of ectopic pregnancy diagnoses per 1,000 pregnancies increased during 2006–2010, from 7.0 to 8.3, with no inflections in trend. Females of all age groups experienced increases, though increases were less pronounced with increasing age. All geographic regions experienced increases, with increases being most pronounced in the Northeast.
Conclusions
Our study suggests that ED ectopic pregnancy diagnoses may be increasing in the US, although the drivers of these increases are not clear. Our results highlight the need for national measures of total pregnancies, stratified by pertinent demographic variables, to evaluate trends in pregnancy-related conditions among key populations.
Keywords: Ectopic pregnancy, epidemiology
Introduction
Ectopic pregnancy is an adverse, potentially life-threatening pregnancy outcome in which a fertilized ovum implants outside the endometrial cavity. Ectopic pregnancy is associated with fallopian tube damage, pelvic inflammatory disease (PID) or salpingitis, history of tubal surgery, previous ectopic pregnancy, in utero diethylstilbestrol (DES) exposure, and history of assisted reproductive technologies (ART) [1]. The two most commonly reported sexually transmitted infections (STIs), chlamydia and gonorrhea [2], cause the majority of primary salpingitis cases [3] and a substantial portion of PID cases [4]. Ectopic pregnancy cases often present with severe symptoms, including acute pelvic pain accompanied with vaginal bleeding and lightheadedness or fainting. Because of the severity of symptoms associated with an ectopic pregnancy, many cases in the United States (US) are diagnosed in emergency departments (EDs).
Because ectopic pregnancy is not a nationally notifiable condition and there is no national surveillance infrastructure in place to monitor it, prevalence estimates of ectopic pregnancy in the US have been difficult to ascertain [5]. Previous US studies using administrative claims data have estimated that <1–2% of all pregnancies are ectopic [6–8]. Many of these studies have relied on commercial health plans data [6–8], which may underestimate the prevalence of ectopic pregnancy [9]. For example, studies using commercial health plan data do not include ectopic pregnancies among women that are Medicaid beneficiaries or self-payers [9]. It is possible that ectopic pregnancy rates may be higher in these groups as the risk factors associated with adverse pregnancy and ectopic pregnancy outcomes, such as history of STIs, may be more prevalent in such groups [2]. Additionally, comparing prevalence estimates is difficult as no uniform operationalization of ectopic pregnancy diagnoses exists; studies have identified ectopic pregnancy diagnoses using a combination of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes and/or Current Procedural Terminology (CPT) codes [6–10]. In addition, differences in denominators used for rate calculations contributes further to variability. Some reports have evaluated ectopic pregnancies among all pregnancies, while others have compared ectopic pregnancy diagnoses to deliveries or live births only [2, 6, 7]. Total pregnancies is the most inclusive denominator to evaluate ectopic pregnancy, but total pregnancy estimates are imprecise, as not all pregnancies are reliably captured and national data on all pregnancies are not currently available for recent years [11]. The nationally collected measure of live births may instead be a suitable comparator when evaluating trends in ectopic pregnancy.
To date, no study has used nationally representative ED data to enumerate trends in ectopic pregnancies. Our study uses a nationwide ED sample to identify trends in the ratio of ectopic pregnancy diagnoses to all live births during 2006–2013, providing a nationally representative estimate of this important pregnancy-related outcome.
Methods
We analyzed data from the Healthcare Cost and Utilization Project (HCUP) Nationwide Emergency Department Sample (NEDS), the largest all-payer ED database in the US [12]. Data are provided from statewide data organizations of ED visits that may or may not have resulted in a hospital admission [12]. During 2006–2013, 24–30 states participated in HCUP, and data were provided from 947 to 980 hospitals containing in the range of 25.7 to 31.0 million ED visits annually [12]. Weights are provided in the NEDS database to calculate national estimates representing up to 120–135 million ED visits annually [12]. The weights are calculated by stratifying and clustering by geographic region (primary sampling units, PSUs), hospitals within those PSUs, EDs within the hospitals, and visits within the EDs. For each ED visit, the NEDS database includes information about geographic and patient characteristics, as well as visit-specific information, such as dates, procedures, and diagnoses [12]. Race/ethnicity and laboratory result data are not included in the HCUP NEDS database. Approval from the Centers for Disease Control and Prevention’s (CDC) Institutional Review Board was not required for this study as the data are publicly available and permanently de-identified.
For each year during 2006–2013, we selected all ED visits among females aged 15–44 years and assessed the number of visits where an ectopic pregnancy was diagnosed using ICD-9-CM diagnosis codes (n=16), ICD-9-CM procedure codes (n=2), and CPT codes (n=8) (Table 1). Since the data were visit-based, it is possible for a person to be represented more than once if they were diagnosed with an ectopic pregnancy ≥1 time during the study period. Deduplication of visits to perform person-based analysis was not possible due to the lack of unique identifiers in the data.
Table 1.
International Classification of Diseases, 9th Revision, Clinical Modification Codes and Current Procedural Terminology Codes Used to Identify Ectopic Pregnancies in the Healthcare Cost and Utilization Project Nationwide Emergency Department Sample from 2006 to 2013
| ICD-9-CM Classification of Diseases & Injuries | |
| 633.0 | Abdominal pregnancy, Intraperitoneal pregnancy |
| 633.00 | Abdominal pregnancy without intrauterine pregnancy |
| 633.01 | Abdominal pregnancy with intrauterine pregnancy |
| 633.1 | Tubal pregnancy, Fallopian pregnancy, Rupture of (fallopian) tube due to pregnancy, Tubal abortion |
| 633.10 | Tubal pregnancy without intrauterine pregnancy |
| 633.11 | Tubal pregnancy with intrauterine pregnancy |
| 633.2 | Ovarian pregnancy |
| 633.20 | Ovarian pregnancy without intrauterine pregnancy |
| 633.21 | Ovarian pregnancy with intrauterine pregnancy |
| 633.8 | Other ectopic pregnancy, Pregnancy: cervical, combined, cornual, intraligamentous, mesometric, mural |
| 633.80 | Other ectopic pregnancy without intrauterine pregnancy |
| 633.81 | Other ectopic pregnancy with intrauterine pregnancy |
| 633.9 | Unspecified ectopic pregnancy |
| 633.90 | Unspecified ectopic pregnancy without intrauterine pregnancy |
| 633.91 | Unspecified ectopic pregnancy with intrauterine pregnancy |
| 761.4 | Ectopic pregnancy, Pregnancy: abdominal, intraperitoneal, tubal |
| ICD-9-CM Classification of Procedures | |
| 66.32 | Removal of extratubal ectopic pregnancy, Removal of: ectopic abdominal pregnancy, fetus from peritoneal or extraperitoneal cavity following uterine or tubal rupture |
| 66.69 | Salpingectomy with removal of tubal pregnancy |
| CPT | |
| 59120 | Surgical treatment of ectopic, tubal or ovarian, with abdominal salpingectomy and/or oophorectomy |
| 59121 | Surgical treatment of ectopic, tubal or ovarian, without abdominal salpingectomy and/or oophorectomy |
| 759130 | Surgical treatment of abdominal ectopic pregnancy |
| 59135 | Surgical treatment of interstitial uterine ectopic pregnancy requiring total hysterectomy |
| 59136 | Surgical treatment of interstitial uterine ectopic pregnancy with partial resection of uterus |
| 59140 | Surgical treatment of cervical ectopic pregnancy with vaginal approach |
| 59150 | Laparoscopic treatment of ectopic pregnancy without salpingectomy and/or oophorectomy |
| 59151 | Laparoscopic treatment of ectopic pregnancy with salpingectomy and/or oophorectomy |
Data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC). Analyses were performed using SURVEYFREQ procedures and were weighted to represent all ED visits nationwide during each year. The weighted number of visits with an ectopic pregnancy diagnosis among females aged 15–44 was calculated for each year during 2006–2013. In order to calculate the ratio of ectopic pregnancies to live births, we divided the annual estimates of the weighted number of ectopic pregnancy diagnoses by the number of live births in a given year. The total number of live births in the US during 2006–2013 was derived from birth certificate data (US natality files) from the CDC’s National Center for Health Statistics (NCHS); these data are publicly available for download [13]. Live births were stratified by US census region, based on the mother’s legal residence at the time of birth, as well as maternal age group. Because trends in ectopic pregnancies may be influenced by changes in the total number of pregnancies, we conducted a supplementary analysis identifying the rate of ectopic pregnancies among all pregnancies. The total number of pregnancies included live births, fetal losses, and induced abortions, and were provided directly from NCHS for the years 2006–2010. These data were not available for subsequent years and were not available by maternal age group or region.
Total and annual percent changes (APCs) were estimated in (1) the ratio of ED visits with an ectopic pregnancy diagnosis to live births during 2006–2013 and (2) the rate of ED visits with an ectopic pregnancy diagnosis among all pregnancies during 2006–2010. Total percent change was calculated as the difference between the ratio or rate in 2006 compared to 2013 (or 2010), divided by the ratio or rate in 2006. Joinpoint software version 4.4.0 (National Cancer Institute, Bethesda, MD), which fits trend data to identify the log-linear model with the fewest number of inflection points, was used to identify significant trends [14]. APC was estimated by Joinpoint using the log-linear slope of trend segments between inflection points. Calculated ratio standard errors were manually provided to the Joinpoint software for modeling and were computed using the standard error of the annual estimate of the weighted number of ectopic pregnancies divided by the number of live births in a given year. Total and APCs were estimated overall, as well as by maternal age group and region of hospital.
Results
There were approximately 58 million ED visits among women aged 15–44 years during 2006–2013; the number of visits increased from 6.5 million in 2006 to 7.4 million in 2013 (Table 2). Nearly half of ED visits occurred each year during the study period in hospitals in the South (41%), 24% in the Midwest, 19% in the Northeast, and 16% in the West, with approximately 55% of visits occurring in women under 30 years of age. The proportion of ED visits among women covered by private insurance decreased through the study period, from approximately 38% in 2006 to 30% in 2013, whereas the proportion of visits among women covered by Medicaid increased from 30% in 2006 to 38% in 2013; the proportion of Medicare, self-pay, no charge, or other visits remained stable throughout the study period. However, the ratio of ED visits with an ectopic pregnancy to live births or to all pregnancies could not be determined by healthcare coverage group as natality and pregnancy data are not available stratified by healthcare coverage [11, 13].
Table 2.
Characteristics of Visits Among Women Aged 15-44 Years Visiting Emergency Departments by Selected Characteristics — Healthcare Cost and Utilization Project Nationwide Emergency Department Sample, United States, 2006–2013
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | |
|---|---|---|---|---|---|---|---|---|
| Characteristic | # (%)* | # (%)* | # (%)* | # (%)* | # (%)* | # (%)* | # (%)* | # (%)* |
| Total (row %) | 6,500,611 (11.2) | 6,763,901 (11.7) | 7,268,015 (12.6) | 7,318,387 (12.7) | 7,398,478 (12.8) | 7,266,975 (12.6) | 7,862,990 (13.6) | 7,432,550 (12.9) |
| Age group, in years | ||||||||
| 15–19 | 1,020,469 (15.8) | 1,055,976 (15.7) | 1,122,259 (15.5) | 1,146,337 (15.7) | 1,075,601 (14.6) | 1,039,215 (14.4) | 1,088,915 (13.9) | 1,000,131 (13.5) |
| 20–24 | 1,358,220 (20.9) | 1,410,693 (20.8) | 1,524,181 (21.0) | 1,556,025 (21.3) | 1,590,049 (21.6) | 1,560,783 (21.5) | 1,678,662 (21.3) | 1,573,340 (21.2) |
| 25–29 | 1,200,021 (18.4) | 1,285,930 (19.0) | 1,400,389 (19.2) | 1,408,621 (19.3) | 1,443,970 (19.5) | 1,419,146 (19.5) | 1,538,458 (19.5) | 1,457,210 (19.6) |
| 30–34 | 984,726 (15.1) | 1,032,267 (15.2) | 1,122,828 (15.4) | 1,148,030 (15.7) | 1,211,185 (16.3) | 1,223,196 (16.8) | 1,354,149 (17.2) | 1,302,989 (17.5) |
| 35–39 | 964,600 (14.8) | 997,330 (14.7) | 1,067,347 (14.6) | 1,052,671 (14.3) | 1,060,666 (14.3) | 1,020,754 (14.0) | 1,113,072 (14.1) | 1,066,270 (14.3) |
| 40–44 | 972,575 (15.0) | 981,705 (14.5) | 1,031,011 (14.2) | 1,006,703 (13.7) | 1,017,007 (13.7) | 1,003,881 (13.8) | 1,089,734 (13.8) | 1,032,610 (13.9) |
| Region of hospital | ||||||||
| Northeast | 1,217,006 (18.9) | 1,197,750 (19.0) | 1,355,810 (19.1) | 1,391,397 (19.1) | 1,281,849 (18.4) | 1,288,983 (18.5) | 1,412,044 (18.3) | 1,217,101 (18.5) |
| Midwest | 1,498,940 (23.3) | 1,528,743 (24.2) | 1,621,660 (24.1) | 1,590,284 (23.2) | 1,592,941 (24.4) | 1,517,030 (24.2) | 1,576,736 (23.4) | 1,586,015 (23.1) |
| South | 2,819,449 (41.0) | 2,968,141 (41.0) | 3,198,361 (41.0) | 3,154,528 (41.3) | 3,351,002 (41.1) | 3,205,097 (40.7) | 3,575,571 (41.8) | 3,425,007 (42.1) |
| West | 965,216 (16.8) | 1,069,267 (15.8) | 1,092,184 (15.8) | 1,182,178 (16.3) | 1,172,686 (16.1) | 1,255,865 (16.5) | 1,298,639 (16.5) | 1,204,427 (16.3) |
| Primary expected payer** | ||||||||
| Medicare | 243,849 (3.8) | 261,402 (3.9) | 289,569 (4.0) | 284,552 (3.9) | 305,793 (4.2) | 321,100 (4.4) | 363,480 (4.7) | 334,519 (4.6) |
| Medicaid | 1,939,637 (30.1) | 1,943,312 (28.8) | 2,180,756 (30.1) | 2,420,818 (33.0) | 2,574,621 (34.7) | 2,679,862 (37.0) | 2,970,542 (37.7) | 2,819,058 (37.8) |
| Private including HMO | 2,481,712 (38.4) | 2,553,109 (38.4) | 2,730,909 (37.9) | 2,570,699 (35.7) | 2,429,263 (33.5) | 2,311,178 (31.9) | 2,339,082 (30.2) | 2,212,812 (30.4) |
| Self-pay | 1,404,130 (21.4) | 1,590,159 (23.4) | 1,598,456 (22.1) | 1,580,146 (21.4) | 1,659,222 (22.1) | 1,516,847 (21.0) | 1,711,743 (21.5) | 1,615,237 (21.4) |
| No charge | 79,229 (1.2) | 73,164 (0.9) | 73,558 (0.9) | 76,077 (1.0) | 58,730 (0.8) | 59,170 (0.8) | 53,933 (0.6) | 84,649 (1.1) |
| Other | 325,752 (5.2) | 303,278 (4.6) | 350,491 (5.0) | 352,309 (4.9) | 334,823 (4.7) | 347,850 (4.8) | 410,856 (5.3) | 350,877 (4.7) |
Frequency estimates are unweighted; percent estimates are weighted using HCUP discharge weights and are representative of the reported total ED visits in the US.
Data not adding up to totals is due to missing information for indicator variable.
The overall weighted ratio of ED visits with an ectopic pregnancy diagnosis during 2006–2013 was 12.3 per 1,000 live births. This ratio increased significantly throughout the study period, with no inflections in trend, from 11.0 ectopic pregnancies per 1,000 live births in 2006 to 13.7 ectopic pregnancies per 1,000 live births in 2013 (Table 3). This represents a total percent increase of nearly 25% and an APC of 3.1% (95% CI: 2.5%, 3.7%) (Table 4). Similarly, the weighted rate of ectopic pregnancy among all pregnancies, including live births, fetal losses, and induced abortions, also increased during 2006–2010, from 7.0 ectopic pregnancies per 1,000 pregnancies in 2006 to 8.3 ectopic pregnancies per 1,000 pregnancies in 2010 (APC: 3.9%; 95% CI: 2.2%, 5.5%). No inflections in trend were observed.
Table 3.
Diagnoses of Ectopic Pregnancy per 1,000 Live Births Among Females Aged 15-44 Years by Selected Characteristics — Healthcare Cost and Utilization Project Nationwide Emergency Department Sample, United States, 2006–2013
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N* | Ratio* | N* | Ratio* | N* | Ratio* | N* | Ratio* | N* | Ratio* | N* | Ratio* | N* | Ratio* | N* | Ratio* | |
| Total | 46,666 | 11.0 | 49,342 | 11.5 | 49,774 | 11.8 | 49,332 | 12.0 | 51,184 | 12.8 | 49,619 | 12.6 | 52,307 | 13.3 | 53,717 | 13.7 |
| Region of hospital | ||||||||||||||||
| Northeast | 8,502 | 12.5 | 10,578 | 15.5 | 10,886 | 16.2 | 10,614 | 16.1 | 9,041 | 14.0 | 10,641 | 16.6 | 11,548 | 18.1 | 12,245 | 19.5 |
| Midwest | 10,083 | 11.2 | 9,811 | 10.9 | 9,853 | 11.1 | 9,472 | 10.9 | 10,772 | 12.8 | 10,443 | 12.5 | 10,317 | 12.4 | 9,916 | 12.0 |
| South | 17,991 | 11.2 | 19,235 | 11.8 | 19,780 | 12.3 | 20,413 | 13.0 | 21,497 | 14.2 | 18,891 | 12.6 | 20,676 | 13.8 | 21,902 | 14.6 |
| West | 10,089 | 9.5 | 9,717 | 9.0 | 9,255 | 8.7 | 8,833 | 8.7 | 9,873 | 10.1 | 9,644 | 10.0 | 9,766 | 10.1 | 9,654 | 10.1 |
| Age group, in years | ||||||||||||||||
| 15–19 | 3,237 | 7.4 | 3,232 | 7.3 | 3,286 | 7.6 | 3,458 | 8.4 | 3,157 | 8.6 | 2,688 | 8.2 | 2,656 | 8.7 | 2,758 | 10.1 |
| 20–24 | 10,596 | 9.8 | 10,621 | 9.8 | 11,167 | 10.6 | 11,351 | 11.3 | 11,627 | 12.2 | 11,137 | 12.0 | 11,088 | 12.1 | 11,335 | 12.6 |
| 25–29 | 13,224 | 11.2 | 13,940 | 11.5 | 14,344 | 12.0 | 13,847 | 11.9 | 14,817 | 13.1 | 14,453 | 12.8 | 15,099 | 13.4 | 15,566 | 13.9 |
| 30–34 | 10,680 | 11.2 | 12,041 | 12.5 | 11,692 | 12.2 | 11,380 | 11.9 | 12,471 | 13.0 | 12,203 | 12.4 | 13,792 | 13.6 | 14,369 | 13.9 |
| 35–39 | 7,089 | 14.2 | 7,527 | 15.1 | 7,241 | 14.8 | 7,151 | 15.1 | 7,155 | 15.4 | 7,052 | 15.2 | 7,248 | 15.3 | 7,641 | 15.8 |
| 40–44 | 1,840 | 17.4 | 1,980 | 18.8 | 2,044 | 19.3 | 2,145 | 20.3 | 1,957 | 18.3 | 2,086 | 19.2 | 2,424 | 22.1 | 2,049 | 18.7 |
Frequency estimates and ratios of ectopic pregnancy per 1,000 live births are weighted using HCUP discharge weights and are representative of the total reported ED visits in the US.
Table 4.
Trends in the Ratio of Emergency Department Visits with an Ectopic Pregnancy Diagnosis Among Live Births, Females Aged 15-44 years — Healthcare Cost and Utilization Project Nationwide Emergency Department Sample, United States, 2006–2013
| % Change in Prevalence, 2006 to 2013 | Inflection Year(s) | Trend Segment | Annual % Change in Prevalence (95% CI) | |
|---|---|---|---|---|
| Overall | 24.5% | -- | 2006–2013 | 3.1 (2.5-3.7) |
| Maternal Age Group | ||||
| 15–19 | 36.5% | -- | 2006–2013 | 3.7 (1.7-5.7) |
| 20–24 | 28.6% | -- | 2006–2013 | 4.1 (2.8-5.4) |
| 25–29 | 24.1% | -- | 2006–2013 | 3.1 (2.3-3.9) |
| 30–34 | 24.1% | -- | 2006–2013 | 2.5 (1.0-4.0) |
| 35–39 | 11.3% | -- | 2006–2013 | 1.1 (0.5-1.8) |
| 40–44 | 7.5% | -- | 2006–2013 | 1.6 (−1.1-4.3) |
| Region of Hospital | ||||
| Northeast | 56.0% | -- | 2006–2013 | 4.6 (1.2-8.0) |
| Midwest | 7.1% | -- | 2006–2013 | 1.9 (0.0-3.9) |
| South | 30.4% | -- | 2006–2013 | 3.4 (1.6-5.3) |
| West | 5.9% | -- | 2006–2013 | 1.8 (−0.2-3.9) |
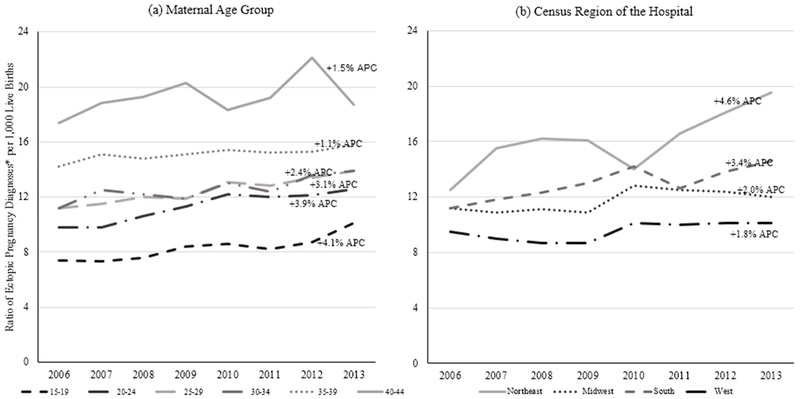
Although the ratio of ED visits due to ectopic pregnancy to live births was consistently highest among women aged 40–44 years (range: 17.4 to 18.7 per 1,000 live births), the largest total and annual percent increases were among females aged 15–19 years (total: 36.5%; APC: 3.7%; 95% CI: 1.7%, 5.7%). Females of all age groups experienced total percent increases in the ratio of ectopic pregnancy ED visits to live births; however, these increases were less pronounced with increasing age (Fig. 1a). For example, females aged 20–24 years had a total percent increase of 28.6% during the study period (95% CI: 2.8%, 5.4%) compared to 40–44 year olds who had a total percent increase of 7.5% (95% CI: −1.1%, 4.3%). Despite having the highest ratio of ectopic pregnancy to live births during the study period, females aged 40–44 years did not experience a statistically significant increase in this ratio during that time. Statistically significant increases were observed in females of all other age groups. No inflection in trends were observed for any age group.
Figure 1.
Trends in the ratio of ED visits with an ectopic pregnancy diagnosis per 1,000 live births among females aged 15–44 years by (a) maternal age group and (b) region of the hospital — Healthcare Cost and Utilization Project Nationwide Emergency Department Sample, United States, 2006–2013
*Ratios are weighted using HCUP discharge weights and are representative of the reported total ED visits in the US.
APC = annual percent change
All geographic regions experienced total percent increases in the ratio of ED visits with an ectopic pregnancy diagnosis to live births; however, these increases were most pronounced in the Northeast (Fig. 1b). The Northeast not only had the highest ratio of ectopic pregnancy diagnoses to live births throughout the study period (range: 12.5 to 19.5 per 1,000 live births), this region also experienced the largest total (56%) and annual percent increases (4.6%; 95% CI: 1.2%, 8.0%). The next highest total and annual percent increase was in the South at 30.4% (APC: 3.4%; 95% CI: 1.6%, 5.3%), followed by the Midwest (total increase: 7.1%; APC: 1.9%; 95% CI: 0.0%, 3.9%). The West experienced a total percent increase of 5.9% (APC: 1.8%; 95% CI: −0.2%, 3.9%). No infection in trends were observed for any region.
Discussion
Using nationwide ED data, this analysis provides national trends in ectopic pregnancy diagnoses during 2006–2013. During this period, the ratio of ED visits with an ectopic pregnancy diagnosis to live births significantly increased from 11.0 to 13.7 ectopic pregnancies per 1,000 live births. Some of our findings diverge from other published reports [6, 7, 8, 10]; our trend findings demonstrate that ectopic pregnancy diagnoses have increased, where other studies have found rates of ectopic pregnancy to be relatively unchanged over time. However, asymmetries, including study period, population studied, payer type, and comparator/denominator, exist among studies, making comparisons difficult [6–10].
Consistent with other studies, we found that the ratio of ectopic pregnancy to live births increased with maternal age [6, 7, 9, 10]. There are a variety of reasons that could explain the higher morbidity of ectopic pregnancy in older females (aged 40–44 years). Though up to 50% of women diagnosed with ectopic pregnancies have no identifiable risk factors [15], many risk factors, including a history of STIs, PID, tubal surgery or tubal damage, history of ectopic pregnancy, in utero DES exposure, and a pregnancy conceived by ART have been associated with ectopic pregnancy [1]. Older women may experience higher rates of ectopic pregnancy, as they have had the most time to accumulate risk factors [1]. Moreover, the higher morbidity observed in older women may be partially due to the usage of ART, as most ART procedures are performed in women aged 35 years or older [16]. Usage of ART may also partially explain our findings among women in the Northeast, as the Northeast region has the overall highest proportion of ectopic pregnancies in women aged 35–39 and 40–44 years (results not shown), and ART is more common in Northeast states [16].
Although females aged 40–44 years had the highest ratio of ectopic pregnancy to live births throughout the study period, females aged 15–19 years had the largest increases in this ratio during 2006–2013. It is unclear, however, what is driving the observed increases in younger women. If we consider the biological pathway from a STI to the development of PID and then subsequently to an ectopic pregnancy, and given that ectopic pregnancies significantly increased in this study, one would expect to see prior and/or concurrent increases in STIs and PID. Chlamydia and gonorrhea are primary causes of salpingitis [3]; however, there are limited data on the trends in chlamydia and gonorrhea incidence in the US. Although they are nationally notifiable conditions, trends in reported cases are heavily influenced by screening coverage. During 2006 to 2011, rates of diagnosed chlamydia increased among adolescent females, then decreased during 2011–2013 and rates of diagnosed gonorrhea appeared to decrease over time; however, it is unknown if trends in incidence followed similar patterns [2]. Measures of PID may provide more insight into the pathway to ectopic pregnancy for these STIs. A recent study by Kreisel et al. found that the overall percent of national ED visits due to PID decreased during 2006–2013, including significant decreases among adolescent females [17]. However, PID surveillance is also difficult, as it is based on an often imprecise clinical diagnosis and acute presentation, and does not account for cases of subclinical PID [17]. Based on these findings, it is unlikely that changes in STIs are the driver of these observed trends in ectopic pregnancy among adolescents. However, adherence to current screening recommendations (i.e., screen all sexually active young women annually for chlamydia and gonorrhea; US Prevention Services Task Force, Grade B) combined with prompt treatment and partner treatment remains important as these measures may help ensure that this vulnerable population is protected from the adverse sequelae of these infections.
It is also possible that the observed increases in the ratio of ectopic pregnancies among younger age groups may be an artifact of the denominator used for calculations. Live births decreased among young women during the study period, resulting in a smaller denominator for ratio calculations and perhaps artificially making the ratio appear to be increasing over time, if total pregnancies did not decrease at the same rate. Our secondary estimates using all pregnancies as the denominator in rate calculations addressed this issue, finding a similar statistically significant increase in the rate of ED visits with an ectopic pregnancy diagnosis per 1,000 pregnancies (for all age groups). Although we only had pregnancy data through 2010, these similar results suggest that using live births as a denominator for ratio calculations may provide comparable results to all pregnancies when evaluating population-level, all-age group trends in ectopic pregnancy.
However, there are limitations to these methods for ratio/rate calculations. The denominators used in ratio/rate calculations in this study were national annual counts of live births and all pregnancies. Live births data are limited as they do not capture changes in pregnancy rates due to population-level variations in induced abortions and fetal losses. The ratio of abortions in the US decreased during 2006–2013, from 236 to 200 abortions to 1,000 live births in 2013 [18], and the ratio of fetal deaths to 1,000 live births remained stable, from 6.1 in 2006 to 6.0 in 2013 [19]. Thus, changes in fetal loss or induced abortion rates do not explain the overall increase in ectopic pregnancy observed in our study. Pregnancy data also have limitations: not all pregnancies (e.g., missed and spontaneous abortions) are reported, data are not available stratified by age group or by key subpopulations, and data have not been collected since 2010. Instead of live births or total pregnancies, total reproductive-aged women could have been used as a denominator, but we chose not to use it as it fails to capture variations in pregnancy trends over time. Given that fetal loss and abortion rates have not increased over the study period, and that the findings of our additional analysis using all pregnancies provide the same trend results as live births, we conclude that live births are a suitable comparator for ectopic pregnancies when demonstrating population-level increases over time. However, these issues reinforce the need for more complete data on live births and all pregnancies and the need for standardized analytic methods for calculating ratio/rates of ectopic pregnancy. Future studies would benefit from these data to more precisely quantify standardized rates and evaluate trends in pregnancy-related conditions in key populations.
This study has other limitations. First, the identification of cases relies on ICD-9-CM and CPT codes, which were created for insurance billing purposes and not for surveillance. The sensitivity and specificity of these codes to accurately reflect identified ectopic pregnancies is unknown and a single prevalence estimate may be an under or over estimate of true prevalence; however, if misclassification bias remained stable over time it should have minimal effect on trend estimates. Second, because the data did not contain personal identifiers, it is possible for a person to be represented more than once (e.g., repeat visits for the same episode). As such, these analyses should be interpreted cautiously as visit-based versus person-based. Without personal identifiers, we could not evaluate whether the patient received any subsequent treatment, resulting in an inability to quantify the proportion of initial ectopic pregnancy diagnoses that were later ruled out, which may be common in the ED setting. Third, we were unable to stratify by all subpopulations of interest. Future studies would benefit from the availability of more complete data stratified by key sociodemographic variables. Fourth, these results may not be generalizable to clinical settings outside of the ED. Since some ectopic pregnancies are diagnosed and treated in an outpatient setting, our estimates of ectopic pregnancy prevalence may be an underestimate. With continued increases in ART, and thus more women likely being diagnosed with an ectopic pregnancy outside of EDs (as women with a history of ART may be more likely to be linked to obstetric/gynecological care), approximating ectopic pregnancy trends from EDs may increasingly provide an underestimate of ectopic pregnancies. Despite this potential increase in underestimation, our study still found an increase in ectopic pregnancy diagnoses over the study period. Lastly, it is also possible that patterns of health care access and utilization changed for women aged 15–44 years throughout the study period, which may have introduced variations into the trend findings. However, the study period preceded major health care utilization changes prompted by the Patient Protection and Affordable Care Act, so it is unlikely that the trends would have been impacted.
Despite the limitations, this analysis has a number of strengths. Because a heterogeneous and inclusive population seeks care in the ED and our study uses a nationwide, all-payer ED database with corresponding weights accounting for the sampling design, our results are nationally representative of all US women with a diagnosis of an ectopic pregnancy in the ED setting. Previous studies have been restricted to commercially insured populations only [6, 7], or contain data from individual US states or geographic regions [8–10]. A 2002–2013 study by Tao et al. incorporated data from both commercial and Medicaid claims databases, but the Medicaid database did not include all states and the analysis did not include populations whose health care is covered through Medicare, self-pay, or other (such as no charge and certain other government programs) [7]; such groups comprised approximately one third of our study population. It is important for maternal morbidity studies to include women from varying demographic and primary payer groups, as the risk factors associated with adverse pregnancy outcomes, such as history of STI or ART, may be more prevalent in certain groups [2, 16].
Our study, the first to use a nationwide, all-payer administrative claims database to identify changes in ectopic pregnancy, found that ectopic pregnancy diagnoses in US EDs increased during 2006–2013. Early detection and treatment of ectopic pregnancy remain important to prevent ectopic pregnancy-related morbidity. National total pregnancy measures, including live births, fetal losses, and induced abortions, should be reported and stratified by pertinent demographic variables so that trends in pregnancy-related conditions can be evaluated among key populations.
Significance.
Our study is the first US study to use a nationwide, all-payer administrative claims database to identify changes in ectopic pregnancy trends. Using emergency department data, we show that ectopic pregnancy diagnoses may be increasing in the US.
Acknowledgements
Sources of Support
None declared
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflicts of Interest
None declared.
Contributor Information
Laura M. Mann, Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention; Centers for Disease Control and Prevention; 2500 Century Parkway NE, Rm 5209, MS E-33, Atlanta, GA 30329, USA.
Kristen Kreisel, Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention; 1600 Clifton Road, Atlanta, GA, USA 30329.
Eloisa Llata, Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention; 1600 Clifton Road, Atlanta, GA, USA 30329.
Jaeyoung Hong, Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention; 1600 Clifton Road, Atlanta, GA, USA 30329.
Elizabeth A. Torrone, Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention; 1600 Clifton Road, Atlanta, GA, USA 30329.
References
- 1.Ankum WM, Mol BW, Van der Veen F, & Bossuyt PM (1996). Risk factors for ectopic pregnancy: a meta-analysis. Fertility and sterility, 65(6), 1093–1099. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. (2017). Sexually transmitted disease surveillance 2016. Atlanta (GA): US Department of Health and Human Services; 2017. [Google Scholar]
- 3.Holmes K, Sparling P, Stamm W, Piot P, Wasserheit J, Corey L, & Cohen M (2007). Sexually Transmitted Diseases. New York, NY: McGraw Hill Professional. [Google Scholar]
- 4.Simms I, & Stephenson JM (2000). Pelvic inflammatory disease epidemiology: what do we know and what do we need to know?. Sexually transmitted infections, 76(2), 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zane SB, Kieke BA, Kendrick JS, & Bruce C (2002). Surveillance in a time of changing health care practices: estimating ectopic pregnancy incidence in the United States. Maternal and child health journal, 6(4), 227–236. [DOI] [PubMed] [Google Scholar]
- 6.Hoover KW, Tao G, & Kent CK (2010). Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstetrics & Gynecology, 115(3), 495–502. [DOI] [PubMed] [Google Scholar]
- 7.Tao G, Patel C, & Hoover KW (2017). Updated Estimates of Ectopic Pregnancy among Commercially and Medicaid-Insured Women in the United States, 2002–2013. Southern medical journal, 110(1), 18–24. [DOI] [PubMed] [Google Scholar]
- 8.Van Den Eeden SK, Shan J, Bruce C, & Glasser M (2005). Ectopic pregnancy rate and treatment utilization in a large managed care organization. Obstetrics & Gynecology, 105(5), 1052–1057. [DOI] [PubMed] [Google Scholar]
- 9.Stulberg DB, Cain LR, Dahlquist I, & Lauderdale DS (2013). Ectopic pregnancy rates in the Medicaid population. American journal of obstetrics and gynecology, 208(4), 274–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trabert B, Holt VL, Yu O, Van Den Eeden SK, & Scholes D (2011). Population-based ectopic pregnancy trends, 1993–2007. American journal of preventive medicine, 40(5), 556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtin SC, Abma JC, & Kost K (2010). Pregnancy rates among US women. NCHS Health E-stat. [Google Scholar]
- 12.Healthcare Cost and Utilization Project (HCUP) (2017). NEDS database documentation. Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/db/nation/neds/nedsdbdocumentation.jsp Accessed 30 March 2017.
- 13.Centers for Disease Control and Prevention (2017). Natality information live births. https://wonder.cdc.gov/natality.html Accessed 1 March 2017.
- 14.National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program (2017). Joinpoint. https://surveillance.cancer.gov/help/joinpoint Accessed 28 March 2017.
- 15.Barnhart KT (2009). Ectopic pregnancy. New England Journal of Medicine, 361(4), 379–387. [DOI] [PubMed] [Google Scholar]
- 16.Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, & Barfield WD (2017). Assisted reproductive technology surveillance—United States, 2014. MMWR Surveillance Summaries, 66(6), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreisel K, Flagg EW, & Torrone E (2018). Trends in pelvic inflammatory disease emergency department visits, United States, 2006–2013. American journal of obstetrics and gynecology, 218(1), 117–e1. [DOI] [PubMed] [Google Scholar]
- 18.Jatlaoui TC, Ewing A, Mandel MG, Simmons KB, Suchdev DB, Jamieson DJ, & Pazol K (2016). Abortion surveillance—United States, 2013. MMWR Surveillance Summaries, 65(SS-12), 1–44. [DOI] [PubMed] [Google Scholar]
- 19.MacDorman MF, & Gregory EC (2015). Fetal and perinatal mortality: United States, 2013. National vital statistics reports, 64(8), 1–24. [PubMed] [Google Scholar]