Abstract
Dendritic cells (DCs) are specialized cells of the innate immune system that are characterized by their ability to take up, process and present antigens (Ag) to effector T cells. They are derived from DC precursors produced in the bone marrow. Different DC subsets have been described according to lineage-specific transcription factors required for their development and function. Functionally, DCs are responsible for inducing Ag-specific immune responses that mediate organ transplant rejection. Consequently, to prevent anti-donor immune responses, therapeutic strategies have been directed towards the inhibition of DC activation. In addition however, an extensive body of pre-clinical research, using transplant models in rodents and non-human primates, has established a central role of DCs in the negative regulation of alloimmune responses. As a result, DCs have been employed as cell-based immunotherapy in early phase I/II clinical trials in organ transplantation. Together with in vivo targeting through use of myeloid cell-specific nanobiologics, DC manipulation represents a promising approach for the induction of transplantation tolerance. In this review, we summarize fundamental characteristics of DCs and their roles in promotion of central and peripheral tolerance. We also discuss their clinical application to promote improved long-term outcomes in organ transplantation.
Keywords: Dendritic cells, immune tolerance, organ transplantation
Basic principles
Dendritic cells (DCs) were first identified and characterized by Steinman and Cohn in 1973–4 [1, 2]. These cells are uniquely specialized in antigen (Ag) uptake, processing and presentation, with the ability to stimulate T cell proliferation in mixed leukocyte reactions (MLR) more potently than other Ag-presenting cells (APC) [3]. They link innate and adaptive immune responses [4]. DCs are derived from committed DC precursors (pre-DCs) in the bone marrow (BM) and comprise different subsets, according to their ontogeny, tissue distribution, phenotype and function.
The main conventional DC (cDC) subsets include cDC1 and cDC2, that are defined by lineage-specific transcription factors, such as interferon regulatory factor (IRF)8, basic leucine zipper ATF-like transcription factor 3 (BATF3) and inhibitor of DNA binding 2 (ID2) (cDC1) and IRF4 and zinc finger E-box binding homeobox 2 (ZEB2) (cDC2). In addition, cell surface phenotypic markers may be used to characterize cDC1 (X-C motif chemokine receptor 1 [XCR1] and C-type lectin domain family 9 member A [Clec9a]) and cDC2 (CD172). Development of a separate subset, non-conventional plasmacytoid DCs (pDCs), depends on the transcription factor E2–2. pDCs are characterized phenotypically by the absence of myeloid Ags and the expression of CD123 (IL-3Rα). cDCs are located in lymphoid and non-lymphoid tissues and are known primarily for presenting Ags through major histocompatibility complex class II (MHC-II) and MHC-I via cross-presentation [5]. pDCs also reside in lymphoid and peripheral organs and secrete high amounts of type I interferon (IFN) upon viral infection [6].
It remains unclear whether monocyte-derived cells constitute a DC subset. Monocyte-derived cells express classical DC markers, such as CD11c and MHC-II under inflammatory conditions, and are capable of inducing T cell proliferation in vitro. Consequently, monocyte-derived cells were classified initially as monocyte-derived DCs on the basis of limited phenotypic markers and in vitro functional properties. However, while cDCs and pDCs derive from a common DC precursor (CDP) and depend on FMS-like tyrosine kinase 3 (FLT3) for their development, monocyte-derived cells arise from common monocyte progenitors and develop in response to colony-stimulating factors 1 and 2 (CSF1/2). Therefore, a recently proposed classification [7] suggests that monocyte-derived cells represent a different cell type, with overlapping DC functions.
Besides Ag capture, processing and presentation that induce T cell priming in response to non-self [8, 9], an essential role of DC subsets is to coordinate an adequate physiological response to preserve self-tolerance [10]. Removal of DCs in transgenic CD11c-CRE mice results in the development of spontaneous autoimmunity [11]. In the context of organ transplantation, depletion of CD11c-expressing myeloid cells can lead to prolonged allograft survival, suggesting that the absence of DCs prevents an efficient immune response to the transplanted organ [12]. While removal of DCs represents a potential therapeutic methodology for the induction of immune tolerance, protective immunity against infections may be compromised using myeloid cell-specific depletional approaches. As a general view, anti-donor immune responses are mediated by mature DCs expressing high levels of MHC and costimulatory molecules (CM) under inflammatory conditions, whereas immune tolerance is induced by immature, tolerogenic DCs (tolDCs). Therefore, generation of tolDCs with or without loading of donor Ag, represents a clinically applicable approach for the induction of indefinite allograft survival in comparison with procedures that deplete stimulatory DCs.
Mechanisms by which tolDCs regulate immunity
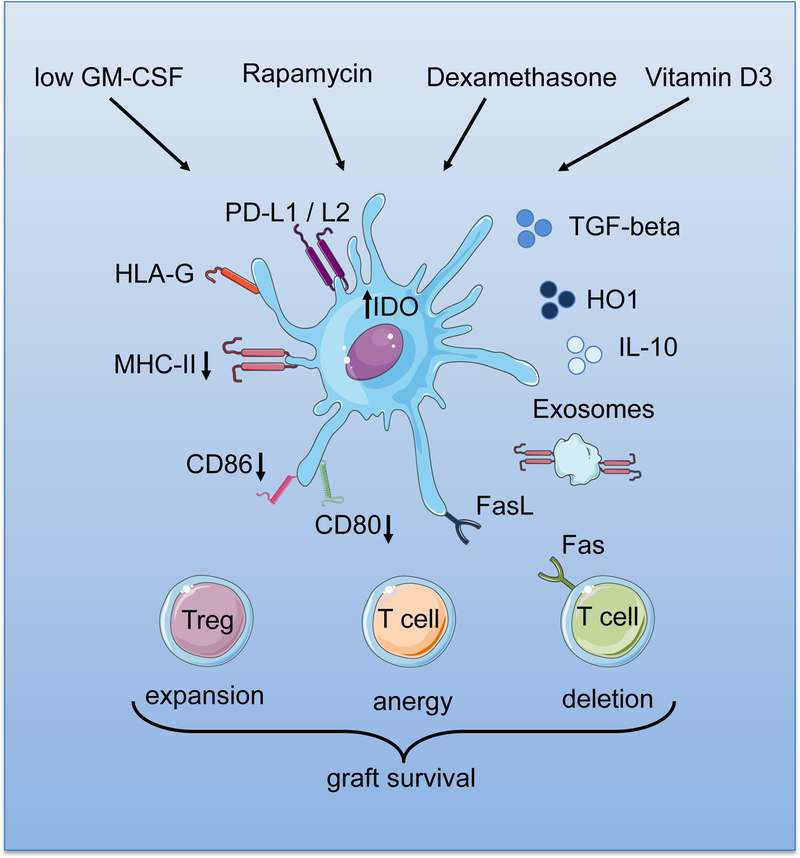
TolDCs subvert effector T cell responses via distinct mechanisms, that include the induction of T cell anergy and clonal deletion due to inadequate expression of cell surface CM [13] (Figure 1). TolDCs also induce apoptosis in naïve and memory T cells via the Fas (CD95)/FasL pathway and by elevated expression of indoleamine 2,3-dioxygenase (IDO) [14, 15]. Another important function of tolDCs is their ability to promote the induction and expansion of different subsets of regulatory lymphocytes that, in turn, promote peripheral tolerance. These regulatory cells include classical CD4+CD25hi forkhead box p3 (Foxp3+) Tregs [16], LAG-3+CD49b+CD25+Foxp3+/− T regulatory type 1 (Tr1) cells [17], CD8+ Tregs [18], regulatory B cells (Bregs) [19], and IFNγ-producing double-negative (CD3+CD4−CD8−) T cells, in both mice and humans [20, 21]. TolDCs also contribute to the development of tolerance by increased expression and release of immunomodulatory molecules. These include programed death ligand (PD-L) 1, PD-L2, human leukocyte Ag-G (HLA-G), and tumor necrosis factor (TNF)-related apoptosis-inducing ligands. Other immunosuppressive (IS) factors include IL-10, transforming growth factor beta (TGFβ), IL-27, and nitric oxide (NO) [22–25]. Heme-oxygenase (HO-1) has been shown to confer tolerogenic properties to DCs [26]. HO-1 is a rate-limiting enzyme that degrades free heme in biliverdin, carbon monoxide (CO) and Fe++, which have several anti-inflammatory and tolerogenic actions [27]. Expression of HO-1 has been shown to be a mechanism of action of tolerogenic DCs in organ transplantation [28]. These suggest that tolDCs employ different mechanisms to facilitate tolerance induction through distinct immune regulatory pathways.
Figure 1.
Conditioning factors that promote the generation of tolerogenic DC (TolDC), their cell surface characteristics, and products that regulate alloreactive T cell responses and promote graft survival/transplant tolerance.
Regulatory role of DC-derived exosomes
More recently, a unique, although not cell-specific mechanism by which DCs modulate the alloimmune response has been described. Exosomes are membrane nanovesicles with a uniform shape and size described originally in the 1980s, produced by a variety of cells, such as DCs, T and B lymphocytes and macrophages [29]. While their biological function is not fully understood [30], recent findings suggest that exosomes act as non-cellular vehicles to transfer molecules between cells under homeostatic [31] and pathological conditions [32]. Exosomes display a specific pattern of molecules on their surface that reflects the type and state of activation of the cell of origin. In the case of DCs and other professional APCs, this may include MHC molecules, T cell CM, as well as adhesion molecules, indicating that DC-derived exosomes function as Ag-presenting nanovesicles (< 100 nm) [33]. While it is becoming clear that DC-derived exosomes bearing MHC molecules are effective intercellular communicators and provide activating signals that promote anti-donor immune responses [34, 35], donor-derived exosomes also participate in the induction and maintenance of peripheral T cell tolerance [36].
The tolerogenic function of exosomes was demonstrated initially in experimental oral tolerance in which exosomes released by the intestinal epithelium of rats fed with a model Ag induced specific tolerance when injected into naïve recipients [37, 38]. Around the same time, it was demonstrated that presentation of donor MHC Ags by BM-derived DC exosomes prolonged heart allograft survival in rats when administered before transplantation [39]. Interestingly, the combination of BM-derived exosomes with short-term desoxypergualin analog treatment induced Ag-specific tolerance to the graft [40]. It remains unclear whether exosomes derived from cDCs or pDCs may be better able to modulate immune reactivity to favor tolerance. However, it has been demonstrated recently that tolerance associated with microchimerism may be induced by cross-dressed cDCs and pDCs that acquire donor exosomes and upregulate immune regulatory molecules, such as PD-L1 and prolong allograft survival [41]. Moreover, spontaneous liver transplant tolerance in mice is associated with cross-dressing of host cDCs within the allograft. These cross-dressed DCs exhibit elevated levels of PD-L1 and IL-10 and markedly inhibit anti-donor T cell responses, concomitant with senescence of PD1+ TIM3+ graft-infiltrating effector T cells [42]. Based on their important roles in regulation of the alloresponse, DCs are potential targets for manipulation to achieve prolonged graft survival and transplantation tolerance. Approaches that have been used to target DCs in situ to promote transplant tolerance and its immune regulatory effects are summarized in Table 1.
Table 1.
Targeting of DC in situ to promote (transplant) tolerance
| Method | Species | Protocol | Effect | Refs |
|---|---|---|---|---|
| Vesicles | ||||
| Apoptotic cell vesicles | Mouse | i.v. injection of donor splenocytes in early apoptosis alone or with αCD154 mAb, 7 days before heart transplant | Donor specific deletion of indirectly alloreactive T cells; increase in alloreactive T regs | [123–125] |
| Immature donor DC-derived exosomes | Mouse | i.v. injection before or after heart transplant plus low close rapamycin | Donor-specific tolerance | [109] |
| Rat | Pre-transplant (heart) infusion of donor BM-derived exosomes in fully MHC-mismatched recipients | Prolongation of graft survival; decreased anti-donor T cell responses; increased anti-donor MHC II alloAb production | [39] | |
| Rat | Post-transplant infusion (x2) combined with deoxyspergualin analogue | Donor-specific tolerance; suppression of chronic rejection | [40] | |
| Rat | Caudal injection on d −7, 0 and 7 in relation to allogeneic liver transplantation ± exogenous donor-specific Tregs | Indefinite graft survival with exosome/Treg combination | [126] | |
| Antibody | ||||
| mAb directed against DC surface Ags (lectin-like receptors) | Mouse | Ag coupled to anti-CD205 mAb | Ag-specific CD8 T cell deletional tolerance | [117] |
| Mouse | Pretreatment with anti-33D1 (DCIR2) conj. with H2kd monomer in combination with αCD8-depleting Ab | Prevention of CD4 indirect alloresponses and IgG against partially MHC I-mismatched skin grafts (B6.Kd) | [127] | |
| Rhesus monkey | i.v. MD-3 anti-ICAM Ab combined with low dose rapamycin and αCD154 | Long-term survival of pig xenoislets | [128] | |
| Humanized mouse | MD-3 mAb before transplant | Xenospecific T cell tolerance; prevention of xenoislet rejection | [128] | |
| Anti-DC-A5GPR† mAb | Cynomolgus monkey | i.d. immunization with Ag fused to anti-DC-ASGPRAb every 5–6 w after flu virus | Ag-specific, IL-10 producing Tregs in vivo | [129] |
| Myeloid cell-specific nanobiologics* | Mouse | Post-transplant treatment of heart allograft recipients | Indefinite graft survival with expansion of CD4+ Tregs | [63] |
mTORi HDL treatment + CD40-TRAF6-specific nanobiologic (TRAF6i-HDL);
DC-ASGPR = DC-asialoglycoprotein
Nanoparticle-based modulation of DCs in vivo
Current clinical organ transplant management requires continuous, and typically, life-long IS drug administration. Common anti-rejection agents, including steroids and the IS pro-drugs, cyclosporine, tacrolimus and rapamycin, modulate various immune cell types non-specifically. This results in generalized IS, with associated risks of cancer development and infection [43]. Engineering nanoparticles (NP) for modulating innate immune responses in organ transplantation represents a valuable tool to avoid these side effects [44]. The potential benefits of in vivo NP-based therapeutics include improved pharmacokinetics, increased bioavailability of IS drugs, specific biodistribution to minimize systemic toxicity, protection of therapeutic molecules from enzymatic and chemical degradation, and co-delivery of multiple therapeutic agents [45–47]. While tolDC may ingest and process peptides and tolerogenic molecules in vitro without an nano-envelopment, material composition, size, shape, charge, and hydrophobicity of NP are some of the key parameters that affect the delivery of therapeutic agents to tolDC in vivo.
The use of NP for therapeutic drug delivery represents a unique approach to deliver Ags and immune modulatory agents to APCs in vivo [44], which capture and phagocytose virus-like particles in the range 50–1000 nm [45]. Delivery of Ags to APCs has been achieved through the use of monoclonal antibodies (mAbs) specific for DC receptors [48]. In this respect, development of drug-loaded NP that express mAbs on their surface represents a promising approach to deliver large immune modulatory molecules to specific APC subsets [49, 50].
Another approach to induce tolDCs is to engineer NP that provide Ag to harness the natural tolerogenic process. The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that induces tolDCs that express low levels of surface MHC and CM, and promote T cell anergy and Treg development [51]. In an elegant study, Tsai et al [52] demonstrated that stimulation of self-Ag-specific CD8+ T cells with iron oxide NP conjugated with disease-relevant peptide-MHC complexes resulted in expansion of autoregulatory memory-like T cells, and consequent suppression of autoreactive CD8+ T cell activation through killing of autoAg-presenting APCs. However, delivery of NP containing only Ags in an inflammatory microenvironment may augment the immune response. One suggested strategy to circumvent this problem is to develop NP that concurrently deliver encapsulated Ags and IS therapeutics, to recruit and modulate DCs toward a tolerogenic phenotype. The co-delivery of 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (an endogenous AHR ligand) and a T cell epitope from myelin oligodendrocyte glycoprotein (MOG)35–55 by gold NP has shown promising results in the induction of tolDCs and expansion of Tregs to suppress autoimmunity [53]. The co-administration of MHC class I Ag and apoptosis-inducing anti-Fas mAb with magnetic beads has also resulted in selective depletion of Ag-specific T cells in a murine allogeneic skin transplant model [54].
Nanocarrier-based approaches to promotion of transplant tolerance are summarized in Table 2. Transplanted mice have been treated with NP-encapsulated IS drugs, including tacrolimus, rapamycin, and mycophenolic acid, that have superior efficacy in terms of inhibitory effects on DC maturation when compared to soluble drugs [55, 56]. Recently, high-density lipoprotein (HDL) NP have been tested in transplant models. These natural, small NP exert an immune protective function through macrophage targeting [57, 58]. HDL-NPs interact preferentially with receptors that are highly expressed on myeloid cells, including ATP-binding cassette receptor A1 and scavenger receptor type B-1 [59]. This allows for targeting of the innate immune system to prevent development of graft-reactive immune responses by encapsulating rapamycin, an IS drug used in organ transplantation since 1991 [60, 61]. Besides T cell suppression and the induction of Treg, rapamycin treatment induces tolDC [61]. However, its poor water solubility and low bioavailability compromise its systemic use [62].
Table 2.
Nanocarrier-based approaches to mediate transplant tolerance
| Nanocarrier | Drug/Agent | Model | Effect | Reference |
|---|---|---|---|---|
| PLGA-NPs | Anti-CD3 | In vivo | Prolongation of mouse heart allograft survival increased intragraft and draining lymph node Treg depletion | [49] |
| PLGA-MPs | H-2Kb-Ig dimer and anti-Fas mAb | In vivo | Prolongation of mouse skin allograft survival and depletion of Ag-specific CD8 T cells | [130] |
| PLGA-NPs | Rapamycin | In vitro | Secretion of high levels of TGF-β and very low levels of IL-10 and IL-12 by DCs | [56] |
| MPEG-PLA-NPs | Tacrolimus | In vivo | Prolongation of rat liver transplant survival | [131] |
| PEG-bl-PPS Micelle | Rapamycin and Tacrolimus | In vivo | Prolongation of mouse skin allograft survival | [132] |
| PLG-NPs | Donor Ag | In vivo | Induction of transplant tolerance in fully MHC-mismatched mouse allogeneic islet transplantation | [133] |
| PLGA-NPs | Rapamycin | In vitro | Downregulation of ICAM-1 and maintenance of an immunosuppressive cytokine milieu for DCs | [134] |
| PLGA-NPs | Mycophenolic acid | In vivo | Prolongation of mouse skin allograft survival | [135] |
| HDL-NPs | CD40-TRAF6 inhibitory and Rapamycin | In vivo | Prevention of alloreactive CD8+ T cell-mediated immunity and promotion of tolerogenic Treg cell expansion | [63] |
| PLGA-NPs | Either protein or peptide Ags and rapamycin | In vivo | Inhibition of Ag-specific CD4+ and CD8+ T cells and B cell activation while inducing Ag-specific Tregs and Bregs | [64] |
Abbreviations: HDL: high-density lipoprotein; MPEG-PLA: poly(ethyleneglycol)-poly(D,L-lactide); NPs: nanoparticles; PEG-bl-PPS: poly(ethylene glycol)-bl-poly(propylene sulfide); PLG: poly(lactide-co-glycolide); PLGA, polylactic-co-glycolic acid; TRAF6; tumor necrosis factor receptor associated factor 6
In recent work, we engineered a rapamycin HDL nanobiologic termed mTOR inhibitor (i)-HDL for the induction of organ transplant acceptance. In this study, specific myeloid-derived cell targeting allowed downregulation of the innate immune response through inhibition of pro-inflammatory mediators and CM, such as TNF-α, IL-6 and CD40, which resulted in organ transplant acceptance [63]. In a separate study, polymeric NP containing rapamycin and Ag induced durable Ag-specific immune tolerance [64]. Mechanistically, these NP were shown to generate tolDC, expand Tregs, and inhibit effector T cell activation [64, 65], suggesting that Ag-specific immune tolerance may be achieved through use of NP loaded with donor Ags.
Besides their use as drug nanocarriers, NP can help visualize and monitor events within transplanted organs. Thus, NP have been used to visualize APCs in vivo, and to assess their number, migration and functional state [66–68]. Using different NP designs and suitable detection methods, it may be possible to obtain diagnostic and prognostic information and to evaluate treatment efficacy in transplant patients [44].
DCs as cellular therapeutic agents in transplantation models
Several approaches have been adopted to generate tolDC of donor or host origin that have been adoptively-transferred to experimental allograft recipients. Their in vivo fate and function, including the role of host DCs in mediating the immune regulatory function of donor-derived (d-d) tolDCs have also been examined [24, 69, 70].
(i). Generation and testing of d-d tolDC
The concept that tolDCs might be used in transplantation as suppressors of allograft rejection was first examined >20 years ago [71, 72]. In these reports, Thomson and colleagues showed that pancreatic islet or cardiac allograft survival was prolonged when recipient animals were pre-treated iv with d-d DC progenitors expressing MHC-II, but low levels of CM [72]. These cells induced alloAg-specific T cell anergy in vitro [73]. In contrast, transfer of d-d mature DC expressing high levels of CD80 and CD86 stimulated T cell proliferation and accelerated heart allograft rejection.
Since these early studies, numerous protocols have been used to generate donor- or recipient-derived tolDCs that have been tested extensively in transplant models [24, 74–76] (Table 3). Lutz et al [77] generated d-d DCs with an immature phenotype from BM progenitors using low concentrations of GM-CSF. Compared to mature DCs generated in the presence of high concentrations of GM-CSF or GM-CSF plus IL-4, these immature DCs were weak stimulators of allogeneic and peptide-specific T cell responses, but were more effective in the presentation of native protein. Interestingly, the immature DC were resistant to maturation under inflammatory conditions, such as exposure to bacterial lipopolysaccharide (LPS), TNFα or anti-CD40 mAb, and did not increase expression of surface CM. They induced T cell unresponsiveness in vitro and in vivo, and prolonged haplotype-specific cardiac allograft survival. However, administration of in vitro-generated immature DCs had to occur at a specific time-point before transplantation (7, but not 3, 14 or 28 days pre-transplant was effective), indicating specific kinetics for tolerance induction by tolDCs. Importantly, homing to secondary lymphoid organs was found to be required to elicit the beneficial effects of ex vivo-generated tolerogenic d-d DCs on graft survival [78]. This represents a challenge for the infusion of tolDCs, since immature DCs express the chemokine receptor CCR5 that guides their migration to peripheral tissues, while CCR7 expression (by mature DCs) is required for homing to secondary lymphoid organs. A solution might be the use of semi-mature DCs that can be generated in the presence of corticosteroids. Emmer et al [79] cultured DCs in the presence of dexamethasone (dex) and matured these cells using LPS. They upregulated CD40, but expression of MHC-II and CD86 remained low. Moreover, production of pro-inflammatory IL-12 was much lower compared to mature DCs, while IL-10 production was unaffected, leading to an increased IL-10/IL-12 ratio for cells generated with dex LPS. After infusion of d-d DCs exposed to dex and LPS, responder T cells of the recipients showed donor-specific hyporesponsiveness, while fully-mismatched heart allograft survival was prolonged.
Table 3.
Promotion of indefinite organ allograft† survival in rodents by adoptive transfer of tolDC
| DC | Species | DC treatment | Additional host treatment | Route of injection (day, d) | MST | Ref |
|---|---|---|---|---|---|---|
| Donor-derived tolDC | ||||||
| MoDC | Rat | GM-CSF | TLI; ATG | iv (d14/15) | >160d | [136] |
| BMDC | Mouse | GM-CSF + IL-4 or TGFβ | Anti-CD40L mAb | iv (d −7) | >100d (40%) | [137] |
| BMDC | Mouse | Low GM-CSF | None | iv (d −7) | >100d | [77] |
| BMDC | Mouse | BM-CSF + IL-4 + NFκβ ODN + rAd CTLA4Ig | None | iv (d −7) | >100d (40%) | [90] |
| BMDC | Rat | Low GM-CSF + IL-4 | ALS | iv (d −7) | >200d§ (50%) | [138] |
| BMDC | Mouse | Low GM-CSF | Anti-CD54 mAb + CTLA4Ig | iv (d −7) | 100d | [139] |
| BMDC | Rat (kidney) | GM-CSF + IL-4 + dexamethasone | CTLA4Ig (x1) + cyclosporine | iv (d −10) | >100d | [140] |
| BMDC | Rat (liver) | GM-CSF + IL-4 | host Tregs | iv (d −7) (both tolDC and Treg) | 22d (tolDC); 30d (Treg); 42d (tolDC + Treg) vs. 8d (control) | [141] |
| Spleen DC | Mouse (skin) | Flt3L | Cyclophosphamide + T cell-depleted donor BM cells | iv (d 0) | >100d | [142] |
| BMDC | Mouse (skin) | Flt3L | CTLA4Ig + anti-CD40L; anti-NK1.1Ab | iv (d −10) | 51d (tolDC) vs. 15d (conrol) | [91] |
| Recipient-derived tolDC | ||||||
| BMDC | Rat | GM-CSF + IL-4 + donor MHC I peptide (RT1.Au) | ALS | it (d −7) | >150d | [143] |
| BMDC | Rat | GM-CSF + IL-4 + donor MHC I peptide (RT1.Au) | ALS | iv (d −7) | >200d | [82] |
| BMDC | Mouse | GM-CSF + IL-4 + RAPA + donor cell lysate | None | iv (X3) (d −10, −3, 0) | >100d | [61] |
| BMDC | Rat | GM-CSF + IL-4 | LF 15–0195* | iv | >100d | [83] |
| BMDC | Mouse | GM-CSF + IL-4 | NFκβ ODN + donor-derived lysate | iv | >100d (33%) | [144] |
| BMDC | Rat | Low GM-CSF + IL-4 | None | iv | >100d (20%) | [84] |
Heart allografts unless otherwise specified;
Deoxyspergaulin derivative;
ALS: anti-lymphocyte serum; ATG: anti-thymocyte globulin; BMDC: bone marrow-derived dendritic cells; Flt3L: fms-like tyrosine kinase 3 ligand; i.t: intra-thymic; iv: intravenous; MoDC: monocyte-derived DC; MST: mean survival time; ODN: oligodeoxyribonucleotides; rAd: recombinant adenovirus; RAPA: rapamycin; TLI: total lymphoid irradiation; Tregs: regulatory T cells
Since DC maturation depends on activation of the NFκβ pathway, Li et al [80] silenced RelB,- the primary NFκβ protein involved in DC maturation using small inhibitory (si) RNA. DC maturation was arrested, with reduced expression of MHC-II and CM, while d-d RelB-silenced DCs inhibited MLR and prevented heart allograft rejection.
(ii). Generation and testing of host-derived tolDCs
The mammalian target of rapamycin (mTOR) pathway represents an interesting target for generation of stable, maturation-resistant tolDC. When pulsed with donor alloAg and administered a week before transplant, together with a short course of rapamycin, they promote graft infiltration by alloAg-specific Tregs and indefinite heart graft survival [61, 81].
Garrovillo et al [82] showed that intrathymic or systemic administration of immunodominant allopeptide-pulsed host thymic DCs 7 days before transplant, combined with transient anti-lymphocyte serum, resulted in permanent, donor-specific rat heart allograft survival. These results were reproduced in a more clinically-relevant model using iv injection of peptide-pulsed host BM-derived DCs. In addition, Cuturi and colleagues have studied extensively the influence of host-BM-derived tolDCs unpulsed with donor Ags (thus unable to induce host sensitization) on rodent organ allograft survival [20, 83–85]. They have shown that, in conjunction with minimal IS therapy (including use of a deoxyspergualin analog/NFKB inhibitor or anti-CD3 Ab), iv infusion of these host-derived tolDC capable of cross-presentation of donor alloAg, a day before transplant induces donor-specific Tregs and prolongs graft survival in a donor-specific fashion. It is important to highlight that, while some common immunosuppressants negatively affect the induction of Treg, IS therapy with deoxyspergualin analogs promote tolerance induction through a self-maintaining regulatory loop between TolDC and Treg [86]. Strategies using host-derived tolDC, whether or not they are pulsed with donor alloAgs, can potentially be generalized to deceased donor organ or composite tissue allotransplantation [87, 88].
(iii). Genetic modification of tolDCs
Besides exposure to pharmaceutical agents or si RNA for the generation of stable tolDCs, genetic engineering of DCs to express immunoregulatory surface molecules or cytokines has been explored. Thus, for example, BM-derived DC transfected with Fas ligand (FasL) to augment their capacity to induce apoptosis in Fas+ cells [89] inhibited T cell proliferation in MLR and induced hyporesponsiveness to alloAg in vivo. Moreover, infusion of d-d FasL-transfected DCs prolonged MHC-mismatched allograft survival.
Bonham et al [90] engineered d-d DCs to secrete cytotoxic T lymphocyte Ag 4 (CTLA4-Ig), a potent costimulation-blocking agent. These cells promoted apoptosis of activated T cells and when infused 7 days before transplant, prolonged mouse heart allograft survival. Interestingly, to prevent maturation of DCs after infection with the transducing adenoviral vector, the authors used double-stranded “decoy” oligodeoxyribonucleotides with binding sites for NFκβ, demonstrating that NFκβ antisense decoys, in conjunction with recombinant adenoviral vectors, represented a successful strategy to avoid DC maturation during the genetic engineering process.
(iv). Fate of adoptively-transferred tolDCs, and the role of host DCs in mediating the effect of d-d tolDCs
Adoptively-transferred tolDCs have been tracked by immunohistochemical staining, or fluorochrome- or radio-labeling. Host-derived, rapamycin-conditioned tolDCs labeled with PKH-67 and infused i.v. home to T cell areas of mouse secondary lymphoid tissue [61], whereas i.v.-infused indium-111-tagged tolerogenic allopeptide-primed autologous rat DC home to the spleen and liver, but not the thymus [82]. Hill et al [20] further showed that i.v.-injected PKH-26-lableled autologous tolDC established close contact with double negative T cells in spleens of rats that became tolerant to donor allografts. Yamano et al [91] observed that FITC-labeled d-d tolDC generated from mouse BM in Flt3L (but not GM-CSF) reached the thymus and spleen (but not lymph nodes) after iv injection. These cells induced both central and peripheral tolerance to donor MHC Ags and prolonged survival of donor skin grafts in NK cell-depleted and costimulation blockade-treated recipients.
While transferred d-d tolDCs may interact directly with anti-donor T cells, inducing anergy, deletion and regulation, endogenous host DC are thought to play an important role in their immunoregulatory effects [92]. Thus, in mice, infused d-d tolDCs are thought to undergo NK cell-mediated cell death and to be reprocessed by recipient DCs for presentation of donor Ag to CD4+ T cells, increasing the number of Tregs. In this concept, therapeutic donor-derived DC function as Ag-transporting cells rather than APCs to prolong allograft survival. Hence, modulating the recipient DC compartment as described above, is an alternative strategy to prolong graft survival, potentially more effectively [70, 75, 84, 93, 94].
(v). TolDCs in non-human primate (NHP) transplant studies
Pre-clinical testing of tolDCs in transplantation has been extended to NHP models. Pre-transplant (day −7) infusion of tolDC generated from donor blood monocytes in the presence of vitamin D3 and IL-10, together with minimal IS therapy (rapamycin and CTLA4Ig), was shown to prolong subsequent MHC mis-matched kidney allograft survival in rhesus macaques [95]. The rhesus d-d tolDCs expressed low MHC-II and CM, but high levels of PD-L1, and were resistant to maturation in response to pro-inflammatory cytokines. No adverse events were associated with their infusion. DC treatment reduced memory/Treg ratios in the graft recipients. More recently, the same group has addressed the influence of CTLA4-Ig on expression of the transcription factor Eomes by memory T cells in their NHP renal transplant model. The results showed that prolonged renal allograft survival achieved with d-d tolDC infusion was associated with Eomeslo CTLA4hi donor-reactive CD8+ suppressive memory T cells [96].
Of note, generation and infusion of tolDCs might not always be required to exhibit the potential of tolDCs after organ transplantation. It was shown [97] that ligation of the vitamin D receptor on DCs with 1,25-dihydroxyvitamin D(3) (VitD3) reduced expression of CM on DCs, as well as IL-12 expression and increased expression of IL-10, promoting a persistent state of DC immaturity. Adorini et al [98] treated fully-mismatched islet allografts briefly with VitD3 before transplantation. This conditioning treatment increased the percentage of CD4+CD25+ Tregs in spleen and draining lymph nodes and protected 100% of recipients from rejection.
Testing of tolDCs in clinical organ transplantation
The potential of tolDCs as a novel, adjunct induction therapy for prevention of rejection and promotion of clinical transplant tolerance has been discussed extensively in recent reviews [75, 76, 99, 100] and is an emerging approach to reduce dependence on pharmacologic IS [76, 101]. Early phase clinical trials of tolDCs in renal or liver transplantation have begun, both in Europe and the US (Table 4). Based on the therapeutic efficacy of autologous tolDCs documented in their earlier rodent allograft studies [83–85], investigators at the University of Nantes (France) have conducted a phase 1/2 (feasibility/safety) trial under the umbrella of the European consortium “The ONE Study” (www.onestudy.org), of unpulsed (no donor alloAg), autologous tolDCs, infused one day before transplant, into living donor renal transplant recipients given standard-of-care (SOC) triple drug (mycophenolic acid [MPA], steroid, tacrolimus) IS therapy (clinicaltrials.gov identifier: [69]). In this trial, the autologous, monocyte-derived tolDCs are generated in low concentration GM-CSF. The investigators postulate that following their infusion, they migrate to the graft where they capture and process d-d Ag leading to Ag-specific regulation of the host response. They also consider that use of recipient-derived tolDCs (compared with d-d tolDCs) is associated with a lower perceived risk of host sensitization, absence of NK cell-mediated killing of the infused tolDC, and suitability for application in both living- and deceased-donor transplantation. At the University of Pittsburgh (US) on the other hand, a National Institutes of Health (NIH)-supported cell dose escalation trial to test the safety of a single infusion of donor monocyte-derived tolDCs administered one week before living donor renal transplantation (Table 4) [96], together with SOC IS (MPA, steroid and tacrolimus) (), will commence in 2019. The rationale for this alternative approach, based on the extensive rodent and NHP studies, is that although the allogeneic d-d cells may not survive very long, their products are acquired by quiescent host DCs in secondary lymphoid tissue that mediate the tolerogenic effects of the infused tolDCs [92, 102].
Table 4.
Registered clinical trials of tolDCreg or regulatory macrophages in living donor kidney or liver transplantation
| Cell type* | Organ transplant K (kidney); L (liver) | Type of trial | Target cell dose (range) | Trial ID | Recruitment status (# patients) |
|---|---|---|---|---|---|
| TolDC | |||||
| Autologous, blood monocyte-derived tolDC | K | Phase I/II | 106/kg | University of Nantes (ONE STUDY) |
Completed (11) |
| Donor blood monocyte-derived tolDC | K | Phase I | 0.5–5×106/kg (dose escalation) | University of Pittsburgh |
Recruiting (14) |
| Donor blood monocyte-derived tolDC | L | Phase I/II | 2.5–10×106/kg | University of Pittsburgh |
Recruiting (14) |
| *Regulatory macrophages (Mreg) | |||||
| Donor blood monocyte-derived regulatory macrophages | K | Phase I/II | 2.5–7.5×106/kg | University of Regensburg (ONE STUDY) |
Terminated (8) |
Administered before transplantation
In each instance, immunosuppressive therapy comprises prednisone, MPA, and tacrolimus
A first-in-human, single center, open-label, phase I/II study () to test the safety and preliminary efficacy of a single infusion of d-d tolDCs in de novo adult living donor liver transplant recipients [101] has been initiated at the University of Pittsburgh. Patients receive SOC IS (MPA, steroid and tacrolimus), without Ab induction. Good manufacturing practice (GMP) grade tolDCs are generated [103] in VitD3 and IL-10 from monocytes obtained by leukapheresis from prospective living organ donors, and infused as induction therapy into their respective recipients, one week before transplant. The tolDC dose range (2.5–10 × 106/kg) corresponds to the range for which both safety and efficacy were established in the preclinical NHP renal transplant model [95]. A half dose of MPA is administered concomitant with the tolDC infusion and until the time of transplant, to minimize any low potential risk of host sensitization. In eligible patients, determined by permissive liver function tests and (at 12 months post-transplant) a permissive liver biopsy, weaning of the remaining IS drug (tacrolimus) begins at 12 months and continues to complete withdrawal by month 24. Follow-up continues for 3 years after the last dose of IS.
Therapeutic potential of DC-derived exosomes
Exosomes derived from immature donor DCs presenting MHC-Ag complexes prolong heart allograft survival in rats, with decreased anti-donor CD4+ T cell responses [39, 40] (Table 1). Similar results have been obtained using exosomes from immature BM-derived DC in a rat intestinal transplant model, in which graft prolongation was associated with an increase in Tregs [104]. Since DC-derived exosomes exhibit immune regulatory properties in an Ag-specific manner, efforts are being made to produce and characterize clinical grade (cGMP) exosomes, that may be used as therapeutic agents [105]. As discussed above, the role of exosomes in development of tolerance versus immunity depends on the surface characteristics of the vesicles and the type and stage of activation of the cells that secrete the exosomes [106]. Additionally, the microenvironment in which the exosome interaction occurs affects the outcome of the immune response: exosomes acting in a tolerogenic milieu promote tolerance [107]. This suggests that d-d exosomes bearing MHC molecules impact the effectiveness of the immune response against non-self MHC molecules, in both vascularized and non-vascularized transplant models. Exosome-derived immune regulation may occur in secondary lymphoid tissues where cross-dressed recipient DCs present donor MHC to naïve T cells, or in the donor organ where graft-infiltrating recipient DCs acquire donor exosomes to regulate memory T cell responses. A better understanding of the regulatory interactions between DC-derived microvesicles and immune effector cells [108] will open new possibilities for optimizing and using these nanovesicles synergistically in combination with current IS agents for the induction of donor-specific immune tolerance in organ transplantation [109].
Conclusions, challenges and future prospects
Cell therapy using tolDCs of donor or host origin, or targeting of DCs in situ to promote their tolerogenicity represent emerging approaches to reduce the use of systemic pharmacologic IS in transplant patients and to promote donor-specific tolerance [44, 76, 101]. BM-derived DCs generated with GM-CSF and exhibiting immunoregulatory properties prolong allograft survival following their adoptive transfer into transplant recipients [72, 77]. These cells express DC-specific markers, including CD11c (N418) and 33D1 [110, 111]. Since 33D1 is also known as DC inhibitory receptor 2 (DCIR2), its ability to regulate Ag processing and T cell activation has been evaluated using a chimeric 33D1 mAb bearing ovalbumin (OVA). Interestingly, Ag delivered via 33D1 mAb elicited no detectable CD8 T cell responses in vitro [112]. In vivo dose-response experiments confirmed that Ag-specific CD8 T cell cell expansion after 33D1-OVA treatment was modest. This suggests that CD8−CD33D1+ (CLEC4A4/DCIR2) DCs, that correspond to cDC2, might be the main DC subset that contributes to development of tolDC. Indeed, recent reports are consistent with this hypothesis, and demonstrate that DCIR2 cDC2 promote Ag-specific activation and proliferative expansion of naturally-occurring Foxp3+ Tregs and tolerance [113, 114]. However, cDC2 are also specialized in CD4+ T cell stimulation [112, 115]. Besides, Ab targeting to DEC205 (cDC1) but not DCIR2, contributes to peripheral tolerance through the development of induced Foxp3+ Tregs under inflammatory conditions [48, 116].
Together with data showing that cDC1 contribute to homeostatic tolerance under steady-state conditions [117, 118], it remains unclear whether tolDCs represent a specific DC subset or a functional state of any particular DC subset. While strong data demonstrate that differentiation into cDC1 or cDC2 is determined within the BM at the common DC progenitor stage [119], it seems that either cDC1 or cDC2 can present Ag in vivo in a tolerogenic or immunogenic fashion [120]. The quest to identify and develop FLT3-dependent [121, 122], clinical grade human tolDCs for the induction of transplantation tolerance is ongoing [24].
Acknowledgments
Funding Sources: The authors’ work is supported by National Institutes of Health grants R01 AI 139623 (JO), R01 AI 118777, U19 AI 131453 and U01 AI 136779 (AWT)
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. The Journal of experimental medicine 1973; 137: 1142–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM, Lustig DS, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. 3. Functional properties in vivo. The Journal of experimental medicine 1974; 139: 1431–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman RM. The dendritic cell system and its role in immunogenicity. Annual review of immunology 1991; 9: 271–96. [DOI] [PubMed] [Google Scholar]
- 4.Mellman I Dendritic cells: master regulators of the immune response. Cancer Immunol Res 2013; 1: 145–9. [DOI] [PubMed] [Google Scholar]
- 5.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol 2012; 12: 557–69. [DOI] [PubMed] [Google Scholar]
- 6.Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity 2014; 40: 642–56. [DOI] [PubMed] [Google Scholar]
- 7.Guilliams M, Ginhoux F, Jakubzick C, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 2014; 14: 571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan RS, Waithman J, Bedoui S, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 2006; 25: 153–62. [DOI] [PubMed] [Google Scholar]
- 9.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 2002; 17: 211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A 2002; 99: 351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohnmacht C, Pullner A, King SB, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. The Journal of experimental medicine 2009; 206: 549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrod KR, Liu FC, Forrest LE, Parker I, Kang SM, Cahalan MD. NK cell patrolling and elimination of donor-derived dendritic cells favor indirect alloreactivity. J Immunol 2010; 184: 2329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz RH. T cell clonal anergy. Curr Opin Immunol 1997; 9: 351–7. [DOI] [PubMed] [Google Scholar]
- 14.Lu L, Qian S, Hershberger PA, Rudert WA, Lynch DH, Thomson AW. Fas ligand (CD95L) and B7 expression on dendritic cells provide counter-regulatory signals for T cell survival and proliferation. J Immunol 1997; 158: 5676–84. [PubMed] [Google Scholar]
- 15.Mellor AL, Baban B, Chandler P, et al. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol 2003; 171: 1652–5. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Dawicki W, Zhang X, Town J, Gordon JR. Tolerogenic dendritic cells induce CD4+CD25hiFoxp3+ regulatory T cell differentiation from CD4+CD25-/loFoxp3- effector T cells. J Immunol 2010; 185: 5003–10. [DOI] [PubMed] [Google Scholar]
- 17.Gagliani N, Magnani CF, Huber S, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 2013; 19: 739–46. [DOI] [PubMed] [Google Scholar]
- 18.Hsu SM, Mathew R, Taylor AW, Stein-Streilein J. Ex-vivo tolerogenic F4/80⁺ antigen-presenting cells (APC) induce efferent CD8⁺ regulatory T cell-dependent suppression of experimental autoimmune uveitis. Clin Exp Immunol 2014; 176: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian L, Qian C, Chen Y, et al. Regulatory dendritic cells program B cells to differentiate into CD19hiFcγIIbhi regulatory B cells through IFN-β and CD40L. Blood 2012; 120: 581–91. [DOI] [PubMed] [Google Scholar]
- 20.Hill M, Thebault P, Segovia M, et al. Cell therapy with autologous tolerogenic dendritic cells induces allograft tolerance through interferon-gamma and epstein-barr virus-induced gene 3. Am J Transplant 2011; 11: 2036–45. [DOI] [PubMed] [Google Scholar]
- 21.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol 2002; 168: 1080–6. [DOI] [PubMed] [Google Scholar]
- 22.Ilarregui JM, Croci DO, Bianco GA, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol 2009; 10: 981–91. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Shi B. Tolerogenic dendritic cells and their applications in transplantation. Cell Mol Immunol 2014; 12: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol 2007; 7: 610–21. [DOI] [PubMed] [Google Scholar]
- 25.Rémy S, Blancou P, Tesson L, et al. Carbon monoxide inhibits TLR-induced dendritic cell immunogenicity. J Immunol 2009; 182: 1877–84. [DOI] [PubMed] [Google Scholar]
- 26.Chauveau C, Remy S, Royer PJ, et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood 2005; 106: 1694–702. [DOI] [PubMed] [Google Scholar]
- 27.Blancou P, Tardif V, Simon T, et al. Immunoregulatory properties of heme oxygenase-1. Methods in molecular biology 2011; 677: 247–68. [DOI] [PubMed] [Google Scholar]
- 28.Moreau A, Hill M, Thebault P, et al. Tolerogenic dendritic cells actively inhibit T cells through heme oxygenase-1 in rodents and in nonhuman primates. FASEB journal official publication of the Federation of American Societies for Experimental Biology 2009; 23: 3070–7. [DOI] [PubMed] [Google Scholar]
- 29.Zhang QJ, Li XL, Wang D, et al. Trogocytosis of MHC-I/peptide complexes derived from tumors and infected cells enhances dendritic cell cross-priming and promotes adaptive T cell responses. PLoS One 2008; 3: e3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol 2001; 166: 3717–23. [DOI] [PubMed] [Google Scholar]
- 31.Herrera OB, Golshayan D, Tibbott R, et al. A novel pathway of alloantigen presentation by dendritic cells. J Immunol 2004; 173: 4828–37. [DOI] [PubMed] [Google Scholar]
- 32.Markey KA, Koyama M, Gartlan KH, et al. Cross-dressing by donor dendritic cells after allogeneic bone marrow transplantation contributes to formation of the immunological synapse and maximizes responses to indirectly presented antigen. J Immunol 2014; 192: 5426–33. [DOI] [PubMed] [Google Scholar]
- 33.Jiang S, Herrera O, Lechler RI. New spectrum of allorecognition pathways: implications for graft rejection and transplantation tolerance. Curr Opin Immunol 2004; 16: 550–7. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, Rojas-Canales DM, Divito SJ, et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest 2016; 126: 2805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marino J, Babiker-Mohamed MH, Crosby-Bertorini P, et al. Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci Immunol 2016; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song J, Huang J, Chen X, et al. Donor-derived exosomes induce specific regulatory T cells to suppress immune inflammation in the allograft heart. Sci Rep 2016; 7: 20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karlsson M, Lundin S, Dahlgren U, Kahu H, Pettersson I, Telemo E. “Tolerosomes” are produced by intestinal epithelial cells. Eur J Immunol 2001; 31: 2892–900. [DOI] [PubMed] [Google Scholar]
- 38.Ostman S, Taube M, Telemo E. Tolerosome-induced oral tolerance is MHC dependent. Immunology 2005; 116: 464–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peche H, Heslan M, Usal C, Amigorena S, Cuturi MC. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation 2003; 76: 1503–10. [DOI] [PubMed] [Google Scholar]
- 40.Peche H, Renaudin K, Beriou G, Merieau E, Amigorena S, Cuturi MC. Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. Am J Transplant 2006; 6: 1541–50. [DOI] [PubMed] [Google Scholar]
- 41.Bracamonte-Baran W, Florentin J, Zhou Y, et al. Modification of host dendritic cells by microchimerism-derived extracellular vesicles generates split tolerance. Proc Natl Acad Sci U S A 2017; 114: 1099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ono Y, Perez-Gutierrez A, Nakao T, et al. Graft-infiltrating PD-L1(hi) cross-dressed dendritic cells regulate antidonor T cell responses in mouse liver transplant tolerance. Hepatology 2018; 67: 1499–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chapman JR, Webster AC, Wong G. Cancer in the transplant recipient. Cold Spring Harb Perspect Med 2013; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ochando J, Braza MS. Nanoparticle-Based Modulation and Monitoring of Antigen-Presenting Cells in Organ Transplantation. Front Immunol 2017; 8: 1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith DM, Simon JK, Baker JR Jr. Applications of nanotechnology for immunology. Nat Rev Immunol 2013; 13: 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinology 2010; 151: 458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kishimoto TK, Maldonado RA. Nanoparticles for the Induction of Antigen-Specific Immunological Tolerance. Front Immunol 2018; 9: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Idoyaga J, Fiorese C, Zbytnuik L, et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest 2013; 123: 844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bahmani B, Uehara M, Jiang L, et al. Targeted delivery of immune therapeutics to lymph nodes prolongs cardiac allograft survival. J Clin Invest 2018; 128: 4770–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Etzerodt A, Maniecki MB, Graversen JH, Moller HJ, Torchilin VP, Moestrup SK. Efficient intracellular drug-targeting of macrophages using stealth liposomes directed to the hemoglobin scavenger receptor CD163. J Control Release 2012; 160: 72–80. [DOI] [PubMed] [Google Scholar]
- 51.Domogalla MP, Rostan PV, Raker VK, Steinbrink K. Tolerance through Education: How Tolerogenic Dendritic Cells Shape Immunity. Front Immunol 2017; 8: 1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai S, Shameli A, Yamanouchi J, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity 2010; 32: 568–80. [DOI] [PubMed] [Google Scholar]
- 53.Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 2012; 109: 11270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen C, He Y, Cheng K, et al. Killer artificial antigen-presenting cells deplete alloantigen-specific T cells in a murine model of alloskin transplantation. Immunol Lett 2011; 138: 144–55. [DOI] [PubMed] [Google Scholar]
- 55.Zou W, Cao G, Xi Y, Zhang N. New approach for local delivery of rapamycin by bioadhesive PLGA-carbopol nanoparticles. Drug Deliv 2009; 16: 15–23. [DOI] [PubMed] [Google Scholar]
- 56.Haddadi A, Elamanchili P, Lavasanifar A, Das S, Shapiro J, Samuel J. Delivery of rapamycin by PLGA nanoparticles enhances its suppressive activity on dendritic cells. J Biomed Mater Res A 2008; 84: 885–98. [DOI] [PubMed] [Google Scholar]
- 57.Duivenvoorden R, Tang J, Cormode DP, et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun 2014; 5: 3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thaxton CS, Rink JS, Naha PC, Cormode DP. Lipoproteins and lipoprotein mimetics for imaging and drug delivery. Adv Drug Deliv Rev 2016; 106: 116–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang XP, Amar MJ, Vaisman B, et al. Scavenger receptor-BI is a receptor for lipoprotein(a). J Lipid Res 2013; 54: 2450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shan J, Feng L, Li Y, Sun G, Chen X, Chen P. The effects of rapamycin on regulatory T cells: its potential time-dependent role in inducing transplant tolerance. Immunol Lett 2014; 162: 74–86. [DOI] [PubMed] [Google Scholar]
- 61.Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am J Transplant 2005; 5: 228–36. [DOI] [PubMed] [Google Scholar]
- 62.Napoli KL, Wang ME, Stepkowski SM, Kahan BD. Distribution of sirolimus in rat tissue. Clin Biochem 1997; 30: 135–42. [DOI] [PubMed] [Google Scholar]
- 63.Braza MS, van Leent MMT, Lameijer M, et al. Inhibiting Inflammation with Myeloid Cell-Specific Nanobiologics Promotes Organ Transplant Acceptance. Immunity 2018; 49: 819–28.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maldonado RA, LaMothe RA, Ferrari JD, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A 2015; 112: E156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kishimoto TK, Ferrari JD, LaMothe RA, et al. Improving the efficacy and safety of biologic drugs with tolerogenic nanoparticles. Nat Nanotechnol 2016; 11: 890–9. [DOI] [PubMed] [Google Scholar]
- 66.Nahrendorf M, Zhang H, Hembrador S, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation 2007; 117: 379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pérez-Medina C, Tang J, Abdel-Atti D, et al. PET Imaging of Tumor-Associated Macrophages with 89Zr-Labeled High-Density Lipoprotein Nanoparticles. J Nucl Med 2015; 56: 1272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weissleder R, Nahrendorf M, Pittet MJ. Imaging macrophages with nanoparticles. Nat Mater 2014; 13: 125–38. [DOI] [PubMed] [Google Scholar]
- 69.Moreau A, Alliot-Licht B, Cuturi MC, Blancho G. Tolerogenic dendritic cell therapy in organ transplantation. Transplant international official journal of the European Society for Organ Transplantation 2017; 30: 754–64. [DOI] [PubMed] [Google Scholar]
- 70.Rosen SJ, Harris PE, Hardy MA. State of the Art: Role of the Dendritic Cell in Induction of Allograft Tolerance. Transplantation 2018; 102: 1603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rastellini C, Lu L, Ricordi C, Starzl TE, Rao AS, Thomson AW. Granulocyte/macrophage colony-stimulating factor-stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation 1995; 60: 1366–70. [PMC free article] [PubMed] [Google Scholar]
- 72.Fu F, Li Y, Qian S, et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86-) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation 1996; 62: 659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu L, McCaslin D, Starzl TE, Thomson AW. Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7–1dim, B7–2-) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation 1995; 60: 1539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol 2004; 4: 24–34. [DOI] [PubMed] [Google Scholar]
- 75.Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol 2011; 23: 252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marin E, Cuturi MC, Moreau A. Tolerogenic Dendritic Cells in Solid Organ Transplantation: Where Do We Stand? Front Immunol 2018; 9: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lutz MB, Suri RM, Niimi M, et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol 2000; 30: 1813–22. [DOI] [PubMed] [Google Scholar]
- 78.Garrod KR, Chang CK, Liu FC, Brennan TV, Foster RD, Kang SM. Targeted lymphoid homing of dendritic cells is required for prolongation of allograft survival. J Immunol 2006; 177: 863–8. [DOI] [PubMed] [Google Scholar]
- 79.Emmer PM, van der Vlag J, Adema GJ, Hilbrands LB. Dendritic cells activated by lipopolysaccharide after dexamethasone treatment induce donor-specific allograft hyporesponsiveness. Transplantation 2006; 81: 1451–9. [DOI] [PubMed] [Google Scholar]
- 80.Li M, Zhang X, Zheng X, et al. Immune modulation and tolerance induction by RelB-silenced dendritic cells through RNA interference. J Immunol 2007; 178: 5480–7. [DOI] [PubMed] [Google Scholar]
- 81.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol 2007; 178: 7018–31. [DOI] [PubMed] [Google Scholar]
- 82.Garrovillo M, Ali A, Depaz HA, et al. Induction of transplant tolerance with immunodominant allopeptide-pulsed host lymphoid and myeloid dendritic cells. Am J Transplant 2001; 1: 129–37. [PubMed] [Google Scholar]
- 83.Beriou G, Peche H, Guillonneau C, Merieau E, Cuturi MC. Donor-specific allograft tolerance by administration of recipient-derived immature dendritic cells and suboptimal immunosuppression. Transplantation 2005; 79: 969–72. [DOI] [PubMed] [Google Scholar]
- 84.Peche H, Trinite B, Martinet B, Cuturi MC. Prolongation of heart allograft survival by immature dendritic cells generated from recipient type bone marrow progenitors. Am J Transplant 2005; 5: 255–67. [DOI] [PubMed] [Google Scholar]
- 85.Segovia M, Louvet C, Charnet P, et al. Autologous Dendritic Cells Prolong Allograft Survival Through Tmem176b-Dependent Antigen Cross- Presentation. American Journal of Transplantation 2014; 14: 1021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Min WP, Zhou D, Ichim TE, et al. Inhibitory feedback loop between tolerogenic dendritic cells and regulatory T cells in transplant tolerance. J Immunol 2003; 170: 1304–12. [DOI] [PubMed] [Google Scholar]
- 87.Sacks JM, Kuo YR, Taieb A, et al. Prolongation of composite tissue allograft survival by immature recipient dendritic cells pulsed with donor antigen and transient low-dose immunosuppression. Plastic and reconstructive surgery 2008; 121: 37–49. [DOI] [PubMed] [Google Scholar]
- 88.Ikeguchi R, Sacks JM, Unadkat JV, et al. Long-term survival of limb allografts induced by pharmacologically conditioned, donor alloantigen-pulsed dendritic cells without maintenance immunosuppression. Transplantation 2008; 85: 237–46. [DOI] [PubMed] [Google Scholar]
- 89.Min WP, Gorczynski R, Huang XY, et al. Dendritic cells genetically engineered to express Fas ligand induce donor-specific hyporesponsiveness and prolong allograft survival. J Immunol 2000; 164: 161–7. [DOI] [PubMed] [Google Scholar]
- 90.Bonham CA, Peng L, Liang X, et al. Marked prolongation of cardiac allograft survival by dendritic cells genetically engineered with NF-kappa B oligodeoxyribonucleotide decoys and adenoviral vectors encoding CTLA4-Ig. J Immunol 2002; 169: 3382–91. [DOI] [PubMed] [Google Scholar]
- 91.Yamano T, Watanabe S, Hasegawa H, et al. Ex vivo-expanded DCs induce donor-specific central and peripheral tolerance and prolong the acceptance of donor skin grafts. Blood 2011; 117: 2640–8. [DOI] [PubMed] [Google Scholar]
- 92.Divito SJ, Wang Z, Shufesky WJ, et al. Endogenous dendritic cells mediate the effects of intravenously injected therapeutic immunosuppressive dendritic cells in transplantation. Blood 2010; 116: 2694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moreau A, Varey E, Bériou G, et al. Tolerogenic dendritic cells and negative vaccination in transplantation: from rodents to clinical trials. Front Immunol 2012; 3: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xia MJ, Shan J, Li YP, et al. Adoptive transfusion of tolerogenic dendritic cells prolongs the survival of liver allograft: a systematic review. J Evid Based Med 2014; 7: 135–46. [DOI] [PubMed] [Google Scholar]
- 95.Ezzelarab MB, Zahorchak AF, Lu L, et al. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am J Transplant 2013; 13: 1989–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ezzelarab MB, Lu L, Guo H, et al. Eomesodermin(lo) CTLA4(hi) Alloreactive CD8+ Memory T Cells Are Associated With Prolonged Renal Transplant Survival Induced by Regulatory Dendritic Cell Infusion in CTLA4 Immunoglobulin-Treated Nonhuman Primates. Transplantation 2016; 100: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A 2001; 98: 6800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem 2003; 88: 227–33. [DOI] [PubMed] [Google Scholar]
- 99.Obregon C, Kumar R, Pascual MA, Vassalli G, Golshayan D. Update on Dendritic Cell-Induced Immunological and Clinical Tolerance. Front Immunol 2017; 8: 1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thomson AW, Metes DM, Ezzelarab MB, Raich-Regue D. Regulatory dendritic cells for human organ transplantation. Transplantation reviews 2019; In press. [DOI] [PMC free article] [PubMed]
- 101.Thomson AW, Humar A, Lakkis FG, Metes DM. Regulatory dendritic cells for promotion of liver transplant operational tolerance: Rationale for a clinical trial and accompanying mechanistic studies. Hum Immunol 2018; 79: 314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morelli AE, Thomson AW. Orchestration of transplantation tolerance by regulatory dendritic cell therapy or in-situ targeting of dendritic cells. Current opinion in organ transplantation 2014; 19: 348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zahorchak AF, Macedo C, Hamm DE, Butterfield LH, Metes DM, Thomson AW. High PD-L1/CD86 MFI ratio and IL-10 secretion characterize human regulatory dendritic cells generated for clinical testing in organ transplantation. Cellular immunology 2018; 323: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang X, Meng S, Jiang H, Zhu C, Wu W. Exosomes derived from immature bone marrow dendritic cells induce tolerogenicity of intestinal transplantation in rats. J Surg Res 2011; 171: 826–32. [DOI] [PubMed] [Google Scholar]
- 105.Lamparski HG, Metha-Damani A, Yao JY, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods 2002; 270: 211–26. [DOI] [PubMed] [Google Scholar]
- 106.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014; 14: 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peinado H, Aleckovic M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18: 883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeng F, Morelli AE. Extracellular vesicle-mediated MHC cross-dressing in immune homeostasis, transplantation, infectious diseases, and cancer. Seminars in immunopathology 2018; 40: 477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li X, Li JJ, Yang JY, et al. Tolerance induction by exosomes from immature dendritic cells and rapamycin in a mouse cardiac allograft model. PLoS One 2012; 7: e44045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. The Journal of experimental medicine 1990; 171: 1753–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nussenzweig MC, Steinman RM, Witmer MD, Gutchinov B. A monoclonal antibody specific for mouse dendritic cells. Proc Natl Acad Sci U S A 1982; 79: 161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo. Science 2007; 315: 107–11. [DOI] [PubMed] [Google Scholar]
- 113.Price JD, Hotta-Iwamura C, Zhao Y, Beauchamp NM, Tarbell KV. DCIR2+ cDC2 DCs and Zbtb32 Restore CD4+ T-Cell Tolerance and Inhibit Diabetes. Diabetes 2015; 64: 3521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tabansky I, Keskin DB, Watts D, et al. Targeting DEC-205(−)DCIR2(+) dendritic cells promotes immunological tolerance in proteolipid protein-induced experimental autoimmune encephalomyelitis. Mol Med 2018; 24: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Flores-Langarica A, Cook C, Muller Luda K, et al. Intestinal CD103(+)CD11b(+) cDC2 Conventional Dendritic Cells Are Required for Primary CD4(+) T and B Cell Responses to Soluble Flagellin. Front Immunol 2018; 9: 2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Petzold C, Riewaldt J, Koenig T, Schallenberg S, Kretschmer K. Dendritic cell-targeted pancreatic beta-cell antigen leads to conversion of self-reactive CD4(+) T cells into regulatory T cells and promotes immunotolerance in NOD mice. Rev Diabet Stud 2010; 7: 47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. The Journal of experimental medicine 2002; 196: 1627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamazaki S, Dudziak D, Heidkamp GF, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol 2008; 181: 6923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schlitzer A, Sivakamasundari V, Chen J, et al. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat Immunol 2015; 16: 718–28. [DOI] [PubMed] [Google Scholar]
- 120.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol 1996; 157: 1406–14. [PubMed] [Google Scholar]
- 121.Breton G, Lee J, Zhou YJ, et al. Circulating precursors of human CD1c+ and CD141+ dendritic cells. The Journal of experimental medicine 2015; 212: 401–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee J, Breton G, Oliveira TY, et al. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. The Journal of experimental medicine 2015; 212: 385–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun E, Gao Y, Chen J, et al. Allograft tolerance induced by donor apoptotic lymphocytes requires phagocytosis in the recipient. Cell death and differentiation 2004; 11: 1258–64. [DOI] [PubMed] [Google Scholar]
- 124.Wang Z, Larregina AT, Shufesky WJ, et al. Use of the inhibitory effect of apoptotic cells on dendritic cells for graft survival via T-cell deletion and regulatory T cells. Am J Transplant 2006; 6: 1297–311. [DOI] [PubMed] [Google Scholar]
- 125.Wang Z, Shufesky WJ, Montecalvo A, Divito SJ, Larregina AT, Morelli AE. In situ-targeting of dendritic cells with donor-derived apoptotic cells restrains indirect allorecognition and ameliorates allograft vasculopathy. PLoS One 2009; 4: e4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ma B, Yang JY, Song WJ, et al. Combining Exosomes Derived from Immature DCs with Donor Antigen-Specific Treg Cells Induces Tolerance in a Rat Liver Allograft Model. Sci Rep 2016; 6: 32971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tanriver Y, Ratnasothy K, Bucy RP, Lombardi G, Lechler R. Targeting MHC class I monomers to dendritic cells inhibits the indirect pathway of allorecognition and the production of IgG alloantibodies leading to long-term allograft survival. J Immunol 2010; 184: 1757–64. [DOI] [PubMed] [Google Scholar]
- 128.Jung KC, Park CG, Jeon YK, et al. In situ induction of dendritic cell-based T cell tolerance in humanized mice and nonhuman primates. The Journal of experimental medicine 2011; 208: 2477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li D, Romain G, Flamar AL, et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. The Journal of experimental medicine 2012; 209: 109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang W, Shahzad KA, Li M, et al. An Antigen-Presenting and Apoptosis-Inducing Polymer Microparticle Prolongs Alloskin Graft Survival by Selectively and Markedly Depleting Alloreactive CD8(+) T Cells. Front Immunol 2017; 8: 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu W, Ling P, Zhang T. Toward immunosuppressive effects on liver transplantation in rat model: Tacrolimus loaded poly(ethylene glycol)-poly(d,l-lactide) nanoparticle with longer survival time. International Journal of Pharmaceutics 2014; 460: 173–80. [DOI] [PubMed] [Google Scholar]
- 132.Dane KY, Nembrini C, Tomei AA, et al. Nano-sized drug-loaded micelles deliver payload to lymph node immune cells and prolong allograft survival. Journal of Controlled Release 2011; 156: 154–60. [DOI] [PubMed] [Google Scholar]
- 133.Bryant J, Hlavaty KA, Zhang X, et al. Nanoparticle delivery of donor antigens for transplant tolerance in allogeneic islet transplantation. Biomaterials 2014; 35: 8887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Das S, Haddadi A, Veniamin S, Samuel J. Delivery of rapamycin-loaded nanoparticle down regulates ICAM-1 expression and maintains an immunosuppressive profile in human CD34+ progenitor-derived dendritic cells. Journal of Biomedical Materials Research Part A 2008; 85A: 983–92. [DOI] [PubMed] [Google Scholar]
- 135.Shirali AC, Look M, Du W, et al. Nanoparticle Delivery of Mycophenolic Acid Upregulates PD-L1 on Dendritic Cells to Prolong Murine Allograft Survival. American Journal of Transplantation 2011; 11: 2582–92. [DOI] [PubMed] [Google Scholar]
- 136.Hayamizu K, Huie P, Sibley RK, Strober S. Monocyte-derived dendritic cell precursors facilitate tolerance to heart allografts after total lymphoid irradiation. Transplantation 1998; 66: 1285–91. [DOI] [PubMed] [Google Scholar]
- 137.Lu L, Li W, Zhong C, et al. Increased apoptosis of immunoreactive host cells and augmented donor leukocyte chimerism, not sustained inhibition of B7 molecule expression are associated with prolonged cardiac allograft survival in mice preconditioned with immature donor dendritic cells plus anti-CD40L mAb. Transplantation 1999; 68: 747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.DePaz HA, Oluwole OO, Adeyeri AO, et al. Immature rat myeloid dendritic cells generated in low-dose granulocyte macrophage-colony stimulating factor prolong donor-specific rat cardiac allograft survival. Transplantation 2003; 75: 521–8. [DOI] [PubMed] [Google Scholar]
- 139.Wang Q, Zhang M, Ding G, et al. Anti-ICAM-1 antibody and CTLA-4Ig synergistically enhance immature dendritic cells to induce donor-specific immune tolerance in vivo. Immunol Lett 2003; 90: 33–42. [DOI] [PubMed] [Google Scholar]
- 140.Mirenda V, Berton I, Read J, et al. Modified dendritic cells coexpressing self and allogeneic major histocompatability complex molecules: an efficient way to induce indirect pathway regulation. Journal of the American Society of Nephrology JASN 2004; 15: 987–97. [DOI] [PubMed] [Google Scholar]
- 141.He W, Chen L, Zheng L, Luo L, Gao L. Prolonged survival effects induced by immature dendritic cells and regulatory T cells in a rat liver transplantation model. Molecular immunology 2016; 79: 92–7. [DOI] [PubMed] [Google Scholar]
- 142.Eto M, Hackstein H, Kaneko K, Nomoto K, Thomson AW. Promotion of skin graft tolerance across MHC barriers by mobilization of dendritic cells in donor hemopoietic cell infusions. J Immunol 2002; 169: 2390–6. [DOI] [PubMed] [Google Scholar]
- 143.Garrovillo M, Ali A, Oluwole SF. Indirect allorecognition in acquired thymic tolerance: induction of donor-specific tolerance to rat cardiac allografts by allopeptide-pulsed host dendritic cells. Transplantation 1999; 68: 1827–34. [DOI] [PubMed] [Google Scholar]
- 144.Tiao MM, Lu L, Tao R, Wang L, Fung JJ, Qian S. Prolongation of cardiac allograft survival by systemic administration of immature recipient dendritic cells deficient in NF-kappaB activity. Annals of surgery 2005; 241: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McCurry KR, Colvin BL, Zahorchak AF, Thomson AW. Regulatory dendritic cell therapy in organ transplantation. Transplant international official journal of the European Society for Organ Transplantation 2006; 19: 525–38. [DOI] [PubMed] [Google Scholar]
- 146.Xia MJ, Shan J, Li YP, et al. Adoptive transfusion of tolerant dendritic cells prolong the survival of renal allografts: a systematic review. J Evid Based Med 2013; 6: 250–64. [DOI] [PubMed] [Google Scholar]